Abstract
Significance: Chronic wounds affect millions of patients worldwide, placing a huge burden on health care resources. Although significant progress has been made in the development of wound treatments, very few advances have been made in wound diagnosis.
Recent Advances: Standard imaging methods like computed tomography, single-photon emission computed tomography, magnetic resonance imaging, terahertz imaging, and ultrasound imaging have been widely employed in wound diagnostics. A number of noninvasive optical imaging modalities like optical coherence tomography, near-infrared spectroscopy, laser Doppler imaging, spatial frequency domain imaging, digital camera imaging, and thermal and fluorescence imaging have emerged over the years.
Critical Issues: While standard diagnostic wound imaging modalities provide valuable information, they cannot account for dynamic changes in the wound environment. In addition, they lack the capability to predict the healing outcome. Thus, there remains a pressing need for more efficient methods that can not only indicate the current state of the wound but also help determine whether the wound is on track to heal normally.
Future Directions: Many imaging probes have been fabricated and shown to provide real-time assessment of tissue microenvironment and inflammatory responses in vivo. These probes have been demonstrated to noninvasively detect various changes in the wound environment, which include tissue pH, reactive oxygen species, fibrin deposition, matrix metalloproteinase production, and macrophage accumulation. This review summarizes the creation of these probes and their potential implications in wound monitoring.
Keywords: chronic wound diagnostics, luminescence, wound imaging probes, neutrophils, reactive oxygen species, pH

Ashwin Nair, PhD

Liping Tang, PhD
Scope and Significance
This review first provides an overview of existing methods to evaluate chronic wounds followed by a review of imaging modalities that have been applied in wound diagnosis. Optical imaging and new marker-specific optical imaging advances are then discussed. Review of previously studied and new markers is followed by recent advances in development of optical and luminescent imagers. This review will be relevant to basic scientists as well as clinicians who would like to delve further into imaging-based wound diagnosis.
Translational Relevance
Current wound diagnostic and evaluation methods have not kept pace with the advances in diagnostic imaging for various other illnesses. For example, optical imaging modalities are noninvasive and have already been translated in clinical applications. Using marker-specific optical imaging through the development of specific probes, which has been reviewed here, could usher in a new era of more efficient wound diagnostics.
Clinical Relevance
Chronic wounds affect millions of people worldwide. Understanding the pros and cons of current methods and specific wound-based markers will help develop more effective optical imaging techniques. Effective diagnosis of such wounds can lead to better treatments, which may lead to quicker healing and significant reduction in health care costs.
Background
Despite routine treatment, chronic wounds fail to progress through the normal healing stages in a timely manner.1 It is estimated that chronic wounds impair 1–2% of worldwide population, with over 6.5 million confirmed cases alone in the United States.2 In addition to encumbering the patient, chronic wounds have a large economic impact, costing the U.S. health care system over $20 billion dollars annually.1,2 Chronic wounds can vary dramatically, but generally fall under three categories: leg ulcers (venous/arterial deficiencies), pressure ulcers, and diabetic wounds.3 These wounds become increasingly dynamic as they can be infected with various microorganisms, which lead to complex routes of healing. In addition, many chronic wounds are found to have senescent cells that can impede healing and augment risk of infection.4 Despite all the advances in wound care over the years, diagnosing such wounds can be arduous for various reasons. For example, as wounds fail to progress through normal stage of healing, they become more differential in regard to shape, size, and the extent of skin/tissue damage, which is compounded by the absence of uniformly accepted wound healing diagnostic methods. In this article, we use “wound imaging” when describing wound imaging modalities, “wound monitoring” when exclusively using wound imaging modalities to assess wound healing process, and “wound assessment” when describing all types of methods for wound status assessment.
Current methods of chronic wound monitoring
Physical assessment
Visual observation is the most common tool for preliminary diagnosis of chronic wounds.5 Its appearance can then be documented and categorized based on wound assessment charts, such as the Bates-Jensen Wound Assessment Chart (BJWAT), Pressure Ulcer Grade Recording Charts, and Wagner Grading Scale. Wound assessment charts have been the standard choice for preliminary wound assessment. However, they lack the ability to predict whether the wound being examined is in a regenerative phase.6 This information can be crucial as it is known that wound healing in chronic wounds usually stalls at the inflammatory phase.4 Unfortunately, the inability of current diagnostic tools to predict treatment outcome or even the stage of healing makes diagnosis speculative and dependent on the expertise of the care provider.
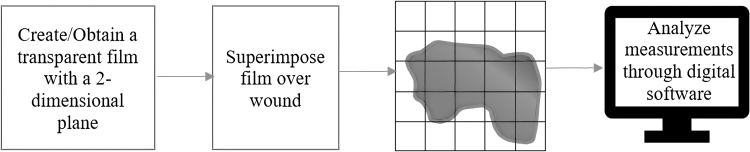
Other than visual observation, wound measurement tools exist in the form of wound photography and digital tracings due to their efficiency in two-dimensional wound assessment.7 Multidimensional approaches using wound photography and digital tracings have become particularly popular due to their noninvasive nature. They are more accurate with minimal direct interaction with the wound compared to standard linear methods that use measure tape. They have also increased the precision of wound measurements and analyses over repeated intervals.8 At the same time, digital wound tracings can quantify epithelialization and percentages of necrotic and healthy tissues through a procedure called digital planimetry as shown in Fig. 1.9 Both photography and digital planimetry offer unique methods to analyze and quantify wounds with no statistical difference in accuracy over regular intervals.10 Unfortunately, both techniques lack the ability to predict progress in wound healing process. These assessment methods have been summarized in Table 1.
Figure 1.
A brief overview of wound analysis using Digital Planimetry.
Table 1.
Comparison of wound photography and digital planimetry methods on wound size measurement
| Method | Advantages | Disadvantages | |
|---|---|---|---|
| Wound photography | Using digital photography to measure and analyze wound size and dimensions. | Nonwound contact measurement. Universally applicable. With short-term healing predictability. | Lacks long-term predictability of healing potential. |
| Digital planimetry | Tracing the wound margin with transparent films, and then manually counting the number of grid boxes filled or partially filled by the wounds or the wound area. The tracing can also be imported into computer to calculate wound area using a software. | High accuracy on wound size measurement. | Requires wound contact. Only allows two-dimensional wound measurement. |
Another popular and noninvasive wound measurement tool is the pulse oximeter that measures oxygen saturation within the patient's blood capillaries, based on infrared light absorption of oxygenated and deoxygenated hemoglobin.11 Pulse oximetry has been used for determining arterial insufficiency in patients suffering from venous leg ulcers (VLU) and those at high risk for diabetic ulcers.12 While pulse oximetry can help detect early signs of ulcers and regionalize areas with poor healing, irregular movement of the patient or failure to accommodate measurements in cases of low hemoglobin contributes to its inaccuracies.13
An emerging device, subepidermal moisture (SEM) scanner, has been used for assessing the risk of developing pressure ulcers. The device comprises an integrated sensor, which is applied to the skin with sufficient pressure to measure changes in the dielectric constant of the material contacting the sensor. This change in subepidermal electrical capacitance reading at an anatomical site (like heel and sacrum) can help predict risk for developing pressure ulcers (low risk for a change <0.6 and increased risk if change ≥0.6).14 The SEM scanner has been widely considered an objective and reliable method to assess SEM and evaluate risk of pressure ulcers.15
Microbiological and biochemical assessment of wounds
Infections can severely impair wound healing process. They can be diagnosed with several clinical tests.16,17 The most popular methods for assessing microbes on/surrounding the wound are swab tests and biopsies.16,17 Swab test can help identify the type of pathogen on the wound surface and inform the selection of a treatment plan.16 The primary reason for culturing microbes from swab tests is to isolate their colonies and uniquely identify them.17 In fact, it has been found that there is no statistical difference between the species or its frequency of isolation. In fact, deep tissue biopsies have greater sensitivity than swabbing, especially toward antibiotic-resistant isolates.18 However, both tests take about 3–5 days for any conclusive result, by when the condition of the wound would have changed significantly. Hence, there is a need for a multifaceted assessment tool to determine multiple types of bacteria simultaneously.
Biochemical analysis of wound exudate (due to blood leakages from capillaries) is another assessment technique.19 Although in small quantity, wound exudate can be collected from wound bed using syringe or specialized devices.20 Biochemical analysis of the macromolecules such as the procollagen, elastin, and hyaluronic acid in wound exudates can determine healing potential.21 Studies have shown that wound exudate analysis provides an effective way to assess the current state of the wound and offers a facet of predictability compared to many other wound assessment tests.19,22 Table 2 summarizes various microbiological and biochemical assessments discussed here.
Table 2.
Three common microbiological and biochemical wound analysis methods
| Test | Swab test | Wound biopsy | Fluid exudate analysis |
|---|---|---|---|
| Methods | Using a metal curette to collect surface colonizing bacteria for bacterial analyses. | Using the “punch” method to isolate tissue from wound bed for analyses. | Collecting wound fluid through syringe aspiration or exudate-soaked dressings for analyses. |
| Advantages | Relatively noninvasive procedure for wound infection diagnosis. | Can identify wound invading bacteria; suitable for histological analyses. | Analysis of the biomolecules to determine healing potential. |
| Disadvantages | Unable to detect invading bacteria or analyze other wound conditions. | May damage wound further. Low reproducibility due to sampling limitation. | With limited sample volume, while increasing risk to infection. |
Need for efficient diagnostic tools
With multiple limitations of current wound assessment methods, both physical and microbiological/biochemical testing,4 it is clear that there is a compelling need for fast and effective multifaceted tools that can precisely identify various pathogens infecting the wound. This tool should be able to assess wound progression in real time, informing the user whether the wound is in a state of regression or regeneration. Such a monitoring tool would help immensely by decreasing financial burden on the health care system by eliminating the need to cycle through varied protocols of treatments. In addition, the likelihood of wound regenerations should significantly increase due to faster diagnosis of wound healing and infection status.
Discussion
Current modalities for chronic wound imaging
Two-dimensional wound imaging is preferred over single-point wound assessment as the availability of more information in the former enables physicians to develop a more effective treatment plan. Imaging techniques can provide measurements of various wound parameters, including wound size, wound depth, blood flow, temperature, inflammation, and infection. Importantly, imaging databases can help improve the study of wound pathogenesis and, perhaps, help develop better and effective wound treatments. Even though this review mainly focuses on chronic wound imaging, some imaging modalities used for burn wound imaging are introduced as well. These imaging modalities are computed tomography (CT), single-photon emission computed tomography (SPECT)/CT, magnetic resonance imaging (MRI), ultrasound imaging, and terahertz (THz) spectroscopy.4
CT scan has been used to assess bone and joint pathology around the wounded region in the diagnosis of diabetic foot ulcers.23 Its high anatomic detail and resolution help illustrate osseous fragmentation and joint dislocation.24 CT angiography, one of the derivatives of CT, has been used to assess peripheral vascularization of diabetic foot ulcers due to its minimal invasiveness and three-dimensional visualization.25 However, CT scan is always considered an invasive imaging modality due to the inevitable ionizing radiation.
SPECT/CT is radiotracer-based imaging modality which uses a SPECT gamma scanner with a conventional CT scanner. With the high sensitivity 3D images provided by SPECT and high-resolution anatomical images produced by CT, SPECT/CT has been successfully used to assess regional microvascular perfusion in the lower extremities for evaluating peripheral artery disease, such as critical limb ischemia.26 Moreover, studies have shown that 99mTc-labeled perfusion radiotracers can be used in wound monitoring to reduce radiation exposure as well as to increase image quality.27 Nevertheless, high cost as well as ionizing radiation limit the application of this imaging modality in routine wound monitoring.
MRI is usually preferred over CT in soft tissue and bone pathology evaluation because of its enhanced resolution and higher sensitivity to infection.23,28 When osteomyelitis arises within chronic wounds, MRI becomes the preferred imaging modality as well as a good surgical aid.29 Despite of its high cost, MRI has been widely utilized in both diagnosis and management of diabetic foot infections.24 MR angiography, which is similar to CT angiography, has also been utilized in the evaluation of distal arterial perfusion with nonionizing radiation, but has limited spatial resolution.30
Ultrasound imaging has been widely used in wound imaging due to its safety, low cost, diverse functionality, high resolution, and real-time response. Generally, it is carried out by sending an acoustic pulse into the wound region and picking up the reflected signal with an ultrasound transducer (which can produce sound waves that bounce off body tissues and pick up echoes that are sent to a computer for image construction). With proper processing algorithms, a two-dimensional cross-sectional image is generated based on the intensity of the returned signals. Structures with small density variations such as scar tissue and fat will be visualized as dark region, while structures with significant density changes are labeled bright.31 High-frequency ultrasound, which usually produces higher resolution images with shallow penetration depth, has been utilized in dermatology,32 and in both quantitative evaluation of wound structure and assessment of wound healing status.32 Due to limited penetration depth, the utilization of ultrasound imaging is restricted to cutaneous wounds.
THz spectroscopy with its unique penetrability can analyze changes in water content between normal and diseased tissue. It has been used for both in vivo and ex vivo evaluation of burn wounds, where it detects interstitial edema and density of skin structures.33 Moreover, THz emissions have low photon energies, which make the imaging modality nonionizing and safe for clinic application.34 In burn wound assessment, THz spectroscopy imaging and MRI showed comparable sensitivity and resolution in tissue hydration gradient detection.35 It is nonionizing unlike X-ray. It can image without contacting the patient unlike ultrasound. Also, THz can penetrate deeper than other imaging techniques like near-infrared (NIR) imaging.
Ultrasound imaging is a good candidate for investigation of chronic wounds due to its lower cost, higher spatial resolution, higher safety, and lower operation time compared with CT, SPECT/CT, and MRI. However, for assessment of diabetic foot wounds and diagnosing infections, CT and MRI are more practical as they have deeper penetration depth, provide better anatomic details, and have higher sensitivity. Although THz spectroscopy has mostly been used in research, it has great potential to emerge as a prominent diagnostic technique for burn wound assessment with high sensitivity and resolution.
A general comparison between these imaging modalities is summarized in Table 3 and Fig. 2. Even though the above-mentioned conventional medical imaging modalities are occasionally used for anatomic assessment of chronic wounds, most of them are cost prohibitive and cannot be widely used for routine chronic wound diagnosis and monitoring.36 To overcome these limitations, several other imaging methods have been explored for their ability to obtain not only structural information but also functional and hemodynamic information of the wound.
Table 3.
A comparison of five different imaging modalities for chronic wound diagnosis
| Imaging modalities | Application in chronic wound imaging | Spatial resolution | Safety | Real time | Time consuming | Cost | Penetration depth |
|---|---|---|---|---|---|---|---|
| CT | Assessment of bone and joint pathology around wounded region. | High | Ionizing radiation | No | Medium | Medium | High |
| SPECT/CT | Assessment of regional microvascular perfusion in lower extremities. | High | Ionizing radiation | No | High | High | High |
| MRI | Soft tissue and bone pathology evaluation. | Low | General safe | Sometimes | High | High | High |
| Ultrasound imaging | Dermatology, assessment of wound structure and speed of flowing blood. | High | Safe | Yes | Low | Low | Low |
| THz spectroscopy | Assessment of burn wound | Medium | Safe | Sometimes | Low | Low | Medium |
CT, computed tomography; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography; THz, terahertz.
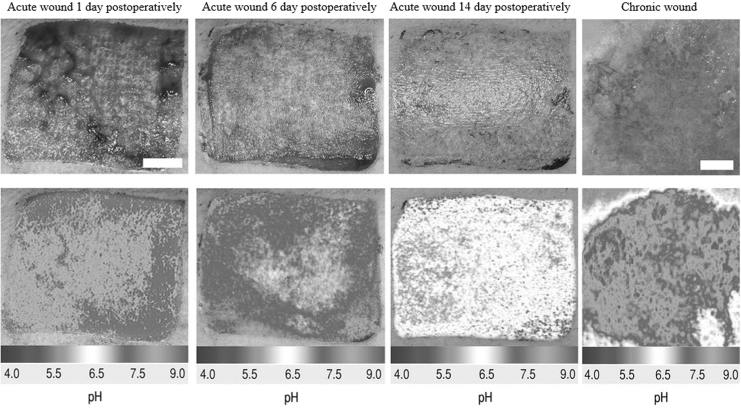
Figure 2.
Luminescence imaging of pH during cutaneous wound healing. [Image courtesy from Schreml et al. (2011). “Copyright (2010) National Academy of Sciences.”107]
New optical imaging modalities for chronic wound monitoring
Various noninvasive optical imaging modalities applied for chronic wound imaging applications include digital camera imaging, hyperspectral imaging (HSI), thermal imaging, optical coherence tomography (OCT), laser Doppler imaging (LDI), spatial frequency domain imaging (SFDI), fluorescence imaging, and NIR imaging spectroscopy.37 These methods can gather a wide spectrum of wound parameters such as blood flow, hemoglobin content, moisture level, vascular structure, collagen reestablishment, bacterial colonization, and infection.
Digital camera imaging is the most cost-effective, noninvasive, and easiest approach, which can provide relatively accurate high-resolution wound recording and size measurement. However, this technique cannot ascertain the depth or volume of the wound, which can be vital for assessing healing outcome. Hence, for wound depth measurement, the time-of-flight camera has been utilized by measuring the phase shift of reflected light waves from the wound surface.38 Along with the depth images, acquired RGB images are processed by image processing algorithms (segmentation, edge detection, color processing, active contouring, and volumetric information) to determine the surface wound size and wound boundary, and even build up a three-dimensional structure of the wound.39,40 For chronic wound imaging, the color of the wounded tissue can also be processed to determine the stage of healing.41 Due to the universal applicability of this technique, it has been used not only in routine wound diagnosis but also in clinical trials of new wound care devices.42 Although this imaging modality can estimate chronic wound healing process, such estimates still need corroboration with other chemical and biological wound information.43
HSI is a noninvasive optical imaging modality that can capture a series of images, recording the intensity of diffusely reflected light from the wounded tissue, and generate a three-dimensional data cube.44–46 Using halogen lamps or light-emitting diodes as light sources and charge-coupled device or hyperspectral camera as detectors, HSI can quantify factors including oxyhemoglobin, deoxyhemoglobin, and blood oxygen saturation, thereby reflecting the extent of wound oxygenation.47 Perfusion parameters, such as oxygenated Hemoglobin, deoxygenated Hemoglobin, and total Hemoglobin, measured by HSI has been used to distinguish burn depth (intermediate dermal, deep dermal, and full thickness) in a mice burn wound model.48 The ability of HSI to differentiate these levels of burn injury was verified by histology. By generating anatomical wound oxygen maps, this technique can evaluate healing potential of diabetic foot ulcers.49 Overall, HSI has shown unique strengths in identifying microvasculature abnormality and tissue oxygenation in chronic wounds, despite its limited imaging depth.
Thermal imaging or thermography is commonly used to measure infrared radiation emitted from the wound tissue using an infrared camera. As the emissivity is proportionally related to temperature of the object, the relative skin temperature of the wound region can be determined through a color-contoured image.50,51 Based on the fact that inflammatory reactions induce temperature elevation, thermal diffusivity of the wounded tissue can be quantified using thermal imaging to reflect the extent and stage of wound healing.52 Generally, skin temperature ranges between 31.1°C and 36.5°C. However, at the wound site, a prolonged temperature increase of at least 1.11°C could indicate the existence of infections and changes in metabolic activity.53,54 Many enzymatic activities are temperature dependent. Higher wound temperature might induce stronger enzymatic reaction, which can be used as an indicator of a prolonged inflammatory phase. On the other hand, ischemic chronic wounds may possess a lower temperature due to decreased blood flow.55 Thermal imaging can also be used to determine “burn depth” in the case of burn wounds, by taking advantage of the fact that an increased inflammatory process generates heat at the superficial burn region, while vasculature damage leads to lower temperatures at the deeper burn region compared with intact skin.56 Thermal imaging has also been developed to allow automatic generation of chromatic and temperature patterns of a wound with the acquired geometrical data.57 Moreover, by combining thermal imaging and visual appearance for wound diagnosis, a wound monitoring smartphone application has been developed to enhance traditional appearance-based wound healing monitoring as summarized in Table 4.58 Despite these exciting progresses, thermal imaging has many limitations associated with its accuracy, specificity, and sensitivity.59 In addition, water loss caused by evaporation in the wound may lead to image distortion.60
Table 4.
Recent approaches and achievements on thermal imaging research on wound monitoring
| Approaches | Achievements | Limitations | |
|---|---|---|---|
| Automatic thermal imager | An innovative and noninvasive optical imaging system that consisted of a structured light based three-dimensional optical scanner and a thermal imager. | Generate chromatic and temperature patterns of a whole wound and help in monitoring chronic wounds by measuring extension, depth and temperature distribution of the leg ulcers. | The equipment cost is high. The imaging requires a tedious and labor-intensive process. |
| Smartphone based thermal imager | A smartphone attached thermal imager which merges thermal images and visual appearance of wound. | Record wound temperature variation to calculate the wound healing index as well as wound blood flow. | The imaging could not distinguish treated wounds from untreated ones. More work is needed to further develop the imager. |
OCT is able to generate high-resolution cross-sectional images of internal microstructure of the tissue based on low-coherence interferometry.61 By monitoring structural changes such as wound dimension, epidermal migration, dermal-epidermal junction formation, vasodilation, vasoconstriction, and epithelization during wound healing process, OCT has been widely used as a noninvasive diagnostic tool in clinical applications.62–64 It has also been utilized in the assessment of wound severity, depth, and microvascular structure in burn wounds through measurements of collagen birefringence reduction and fiber orientation.65–67 Moreover, as chronic wound healing process is reliably associated with epithelialization, collagen deposition, and inflammation, OCT has a great potential for use in monitoring the progress in chronic wound healing by quantifying that information.68 Specifically, OCT angiography has been used to quantify wound area and wound contraction rate,69 while density measurements have been used to assess collagen deposition disorders.70 Ultrahigh-resolution OCT was also used to characterize the backscatter from the inflamed tissues in the wound bed in a punch biopsy injured mice model to demonstrate the suitability of OCT in monitoring cutaneous wound healing process.71 However, due to its poor tissue penetration capability, the application of OCT is limited to only cutaneous wound monitoring.
LDI is a unique imaging modality for blood flow quantification by measuring wavelength variations of reflected and scattered electromagnetic radiation after encountering moving red blood cells and static surrounding tissue.60,72 Based on the color-coded blood flow pattern, quantitative information of blood flow, perfusion, and microvasculature can be acquired by LDI in real time.73 As an imaging modality approved by the Food and Drug Administration (FDA) for burn wound depth measurement, LDI can predict burn wound treatment outcome and healing time through the vasculature and blood flow measurements.59,74,75 However, in the study of microcirculation and microvascular deformation, LDI has limited application due to the lack of accuracy and lower limb perfusion in diabetic patients.76,77
SFDI is another new noncontact optical imaging modality, which reveals optical properties of wounded tissue over a wide field by separating and quantifying absorbed and scattered incoherent monochromatic light.78 Factors such as oxygen saturation, blood volume, and water fraction can be measured with SFDI and this information can be used to interpret vascularization and infection of the wounded tissue.79 SFDI has been used to measure the wound depth and severity, and identify infection in burn wounds.80 It has also been shown to quantify tissue oxygen saturation and blood volume, which may reflect physiological changes as well as vascular occlusion in the wound.81 However, the limited scanning area makes SFDI a time-consuming and inefficient modality for imaging large wounds.
Fluorescence imaging has been utilized to detect either autofluorescence (AF) of endogenous fluorophores at the wound site (such as collagen and elastin) or topically administered exogenous fluorescent dyes. For example, nicotinamide adenine dinucleotide is usually measured at the wound site as an endogenous fluorescent marker, because it plays an important role in oxidative phosphorylation and reflects cellular metabolic activity, both of which have strong relationship with wound healing progress.82,83 Fluorescence imaging method has been applied to quantify wound size, closure extents, and gaps based on dermal fluorescence of pepsin-digestible collagen cross-links in an ex vivo human skin wound healing study.84 For exogenous fluorescence imaging process, the most widely used fluorescent dye is indocyanine green (ICG), which has been approved by the FDA for intravenous injection for imaging.85 Through intravenous injection of ICG, fluorescence imaging can be used to reveal wound depth and vascularization around the wound.86,87 As the signals are collected directly from the fluorophores at the region of interest, fluorescence imaging generally possesses high optical contrast compared with other imaging modalities.88 However, the time taken for delivery of the dye can make the process time-consuming.
NIR spectroscopy is a noninvasive modality that measures maximum light absorption wavelengths of different components, including oxygen saturation, hemoglobin content, and water content, around wound sites.89 For example, it has been used to measure burn wound depth and edema. NIR imaging can be used to quantify hemoglobin content that reflects oxygen saturation and can estimate the depth of burn wound.74,75 In addition, it has been utilized to monitor wound healing process in both preclinical animal models and human patients of burn wounds and diabetic ulcers.90–92 However, due to the potential overlap/shifting of the absorption wavelengths of various components, NIR spectroscopy can sometimes lack specificity.
Digital camera imaging, thermal imaging, and NIR spectroscopy are all simple optical imaging methods, which require plain imaging conditions. However, they share a common disadvantage of poor specificity. HSI and OCT are mainly used to image the microvasculature in chronic wounds, but their poor imaging penetration depth limits their application. SFDI requires long scanning time and fluorescence imaging requires intravenous injection of imaging agent, which make neither method practical for routine clinical application. LDI fails to detect microcirculation and microvascular deformation in diabetic patients due to low limb perfusion.
A summary of these optical imaging modalities has been listed in Table 5. The specificities and sensitivities of different imaging modalities can be found in the cited references. All the above-mentioned optical imaging modalities have their distinct operating principles and feasible applications in the field of chronic wound monitoring. Nevertheless, they all have their own limitations due to the natural property of optical light and operational obstructions. To overcome their limitations, multiple optical imaging modalities should be utilized simultaneously to increase the sensitivity of diagnosis.
Table 5.
Comparison of commonly used optical imaging modalities for wound monitoring
| Imaging modalities | Principle and application | Advantages | Disadvantage |
|---|---|---|---|
| Digital camera imaging | Optical images processed by software. | Simple, universal applicability. | Lack of physiological information. |
| Determine surface wound size, wound boundary; build 3D structure; assess the stages of chronic wounds. | |||
| Hyperspectral imaging | Capture a series of images recording the intensity of diffusely reflected light from the wounded tissue and generate a three-dimensional data cube. | Simple, suitable for microvasculature abnormality of chronic wounds. | Limited imaging depth |
| Quantify factors including oxyhemoglobin, deoxyhemoglobin, and blood oxygen saturation; measure burn wound depth; evaluate healing potential of diabetic foot ulcers. | |||
| Thermal imaging | Measure infrared radiation emitted from the wound tissue using an infrared camera. | Simple, portable, real-time imaging. | Limited accuracy, specificity, and sensitivity. |
| Quantify the extent and stage of wound healing process and measure the depth of burn wounds. | |||
| Optical coherence tomography | Generate cross-sectional images of internal microstructure of the tissue based on low-coherence interferometry. | High resolution, suitable for noninvasively monitoring wound healing processes. | Limited imaging depth. |
| Monitor wound dimension, epidermal migration, dermal-epidermal junction formation, vasodilation, vasoconstriction, and epithelization during wound healing process. | |||
| Laser Doppler imaging | Measure wavelength variations of reflected and scattered electromagnetic radiation after encountering moving red blood cells and static surrounding tissue. | Real-time imaging, suitable for burn wound monitoring. | Low accuracy, not feasible for diabetic patient with lower limb perfusion. |
| Acquire quantitative information of blood flow, perfusion, and microvasculature in real time; predict burn wound treatment outcome and healing time. | |||
| Spatial frequency domain imaging | Reveal optical properties of wounded tissue through separating and quantifying absorbed and scattered incoherent monochromatic light. | Noncontact, able to distinguish infection and noninfected wounds. | Limited scanning area, high time consuming. |
| Measure oxygen saturation, blood volume, and water fraction; interpret vascularization and infection of the wounded tissue. | |||
| Fluorescence imaging | Detect either autofluorescence of endogenous fluorophores or administered exogenous fluorescent dyes at the wound site. | High optical contrast, simple imaging process and condition. | Minimally invasive due to intravenous injection of ICG. |
| Monitor wound healing process through measuring oxidative phosphorylation and cellular metabolism activity to reveal vascularization and wound depth at the wound region. | |||
| Near-infrared spectroscopy | Measure maximum light absorption wavelengths of different components, which are associated with blood oxygen saturation, hemoglobin content, and water content. | Noninvasive, high resolution. | Lack of specificity. |
| Compute burn wound depth; quantify edema; monitor diabetic ulcers and burn wound healing process. |
ICG, indocyanine green.
Representative marker-specific optical wound imaging
Numerous markers have been investigated for their ability to monitor the complex process of wound healing. Some of these include bacterial load, microbial cytokine, DNA, matrix metalloproteinase (MMP), growth factors, immunohistochemical markers, inflammatory mediators, nitric oxide, pH, reactive oxygen species (ROS), temperature, tissue oxygenation, and transepidermal water loss from periwound skin (Table 6).93 This section summarizes developments in such marker-based imaging probe for optical wound imaging.
Table 6.
List of potential markers for wound healing monitoring
| Bacterial load/specific microbial species/biofilms | Inflammatory mediators |
| Cytokine release | Nitric oxide |
| DNA | Nutritional factors |
| Enzymes and their substrates | pH of wound fluid |
| Exposed bone/foreign body reactions | Reactive oxygen species |
| Growth factors and hormones | Temperature |
| Immunohistochemical markers | Transepidermal water loss from periwound skin |
Oxygen
Adequate blood flow and oxygen saturation at the wound site are essential for angiogenesis, collagen synthesis, and epithelialization.94 Moreover, high amount of oxygen may promote inflammatory cells to produce antimicrobial oxidants.95 In fact, hyperbaric oxygen therapy has also been shown to promote healing.96 Since there is a good relationship between tissue oxygen and wound healing status, many groups have used optical imaging modalities to measure the absolute concentrations of oxyhemoglobin and deoxyhemoglobin as their absorption spectra are distinct at NIR wavelengths, which are summarized in this study.
Moza et al. fabricated a point-of-care multiwavelength imager to derive valuable perfusion and oxygen-metric data on wounds.97 By using complementary metal-oxide-semiconductor imager to image wound at 660 and 860 nm wavelengths, hyperemic conditions can be assessed based on the reflectance ratios between these two wavelengths. Since it can quantitatively monitor and analyze tissue oxygenation in real time, this imager can also be utilized for in situ measurement of wound blood flow and assessment of wound healing progress. It achieves this by evaluating hemodynamic behavior in a time- and tissue-dependent manner, which would contribute to the treatment of pressure ulcers.
In what is one of the earliest uses of NIR imaging in VLU, Lei et al. developed a handheld NIR optical scanner to differentiate healing from nonhealing VLU based on differences in blood flow (oxygenation and deoxygenation) to the wound and its surroundings.98 The system consists of an 830-nm LED and an NIR-sensitive camera to record the diffuse reflectance images of the wound. In addition, the ability of using this approach to distinguish healing and nonhealing wounds is independent of the varying imaging and data processing conditions. Optical hemodynamic imaging of VLU and the physiological oxygenation measurements can enable prediction of the healing potential before actual reduction in wound size.
Patel et al. used the SPY® fluorescent imaging system (Novadaq), which is functionally based on ICG angiography, to prognosticate healing process of foot ulcer in critical limb ischemia.99 Through intravenous injection of ICG, real-time fluorescence images are taken to assess vascularization and tissue perfusion in patients with diabetes foot ulcer. Compared with other imaging modalities, this technique provides qualitative and quantitative real-time visual images of perfusion in the areas of interest. In fact, nonhealing patients misdiagnosed with the traditional ankle-brachial index and transcutaneous oxygen pressure methods were accurately diagnosed by ICG angiography data.99 Conclusively, ICG angiography can predict healing in nonhealing foot ulcer in critical limb ischemia patients after revascularization procedure with great sensitivity.
pH
Wound pH has significant effect on wound healing process.100 Normal wound healing comprises of overlapping, yet distinct phases, namely, hemostasis, inflammation, proliferation, and remodeling. Interruption of any of these phases can lead to chronic wounds. It is known that wounds with higher pH have lower healing rate than those with lower pH.5 In fact, chronic wound environment typically exhibits a high pH ranging from 7 to 9 and lower rate of healing compared to wounds with lower and even neutral pH.100 On the other hand, actively healing wounds with inflammatory responses are accompanied with tissue acidity.101 In concurrence with these findings, it has also been found that acidity of the wound milieu promotes wound healing process in the following ways: infection control,102 increase in antimicrobial activity,103 reduction of neutrophil elastase activity,104 release of oxygen to improve cell survival,105 reduced toxicity of bacterial end products,100 and promotion of epithelialization and angiogenesis.106 Interestingly, when a chronic wound is on track to a normal healing process, the pH of the wound environment changes from alkaline to neutral and then to acidic when healing begins.100 It is reasonable to presume that pH of the wound environment could provide invaluable insights into the state of wound healing. Some of the more substantial recent work on pH-based probes, both in vitro and in vivo, are summarized in this study.
A photoluminescence-based method for 2D pH distribution measurement was used in human wound studies (Fig. 2).107,108 The novel referenced photoluminescent sensor is based on a ratiometric imaging principle where two distinct fluorophores [FITC-AC, pH sensitive; Ru(dpp)3-PAN, pH insensitive] were bound to microparticles. These particles were then immobilized in polyurethane hydrogel on transparent foils. Based on the linear relationship between pH value and the ratio of the two fluorescence intensities, the sensor can be used to determine pH distribution map in wounds. Due to the unique property of ratiometric imaging, determination of pH value is independent of topical concentration of the microparticles. Furthermore, split-thickness skin graft donor sites were used to test pH of cutaneous wound healing in three major phases: inflammation, proliferation, and tissue remodeling. With data processing, pseudo color images can be generated to reflect local pH differences during wound healing and detected inflammatory phase in the chronic wound. Interestingly, pH was found to continuously decrease during physiological healing process.109
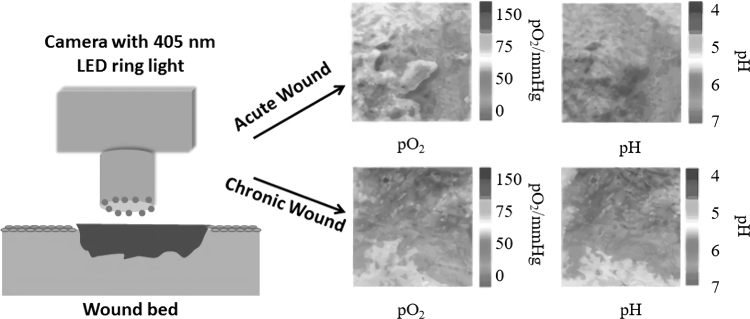
A new method for imaging both pH and oxygen simultaneously was introduced recently in which the image was acquired by a conventional digital camera as shown in the illustration (Fig. 3).110 Briefly, a sensor film, which was loaded with three dyes [pH-sensitive fluorophore isothiocyanate (FITC; λem 530 nm; signal stored in the green channel), oxygen-sensitive probe platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl) porphyrin (λem 650 nm; stored in the red channel), and reference fluorophore diphenylanthracene (DPA; λem 440 nm; stored in the blue channel)] labeled polymer microparticles, was applied on the wound. The camera worked as a rudimentary spectrometer for ratiometric imaging with one excitation wavelength and three emission wavelengths, which matched three color channels of the camera. The imaging modality offered pseudo color images that could distinguish differences between intact skin, acute wound, and chronic wound based on the pattern of pH and oxygen distribution. In vivo application showed that due to the high oxygen demand during inflammation and tissue formation during wound healing, the heterogeneous chronic wound was hypoxic with alkaline pH.110
Figure 3.
Illustration showing simultaneous imaging of pH and pO2 in wounds using a digital camera fitted with a 405 nm-LED ring light and an emission filter for photographing wounds covered in a sensor film to generate pO2 and pH maps comparing acute and chronic wounds.
Flexible pH-responsive hydrogel fibers have also been fabricated for long-term epidermal pH monitoring.111 Briefly, the pH-sensitive dye, brilliant yellow, was conjugated onto mesoporous microparticles and incorporated into hydrogel fibers using a microfluidic spinning system. The color of the hydrogel fiber changed instantly from dark red to yellow when the pH changed from alkaline to acid. Through simple digital imaging, a quantitative pH map of the hydrogel fibers and the underlying tissue was extracted. This pH-responsible hydrogel fiber device can provide on-site wound pH measurement and enable monitoring of the wound healing process through a smartphone camera.
Reactive oxygen species
ROS play a role in wound healing through multiple mechanisms. First, ROS products are secondary messengers to many immunocytes and nonlymphoid cells, which are positively involved in the repair process.112 Second, ROS can regulate angiogenesis and perfuse into the wound area by acting as intracellular signaling mediators, which activate related cytokines.113 For example, mitochondrial-derived ROS can activate LPS-mediated production of proinflammatory cytokines, such as IL-1β, IL-6, IL-18, and TNF-α.114,115 Third, as part of respiratory burst, phagocytes release ROS products, which are essential for defending against invaders.116 Finally, topical administration of Surgihoney Reactive Oxygen was used as antimicrobial agent to eradicate Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, and mixed coliforms in infected ischemic foot ulcer.117
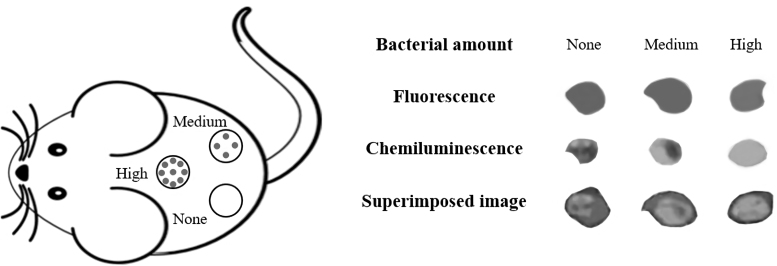
A luminescent probe—L012—was explored for its ability to assess inflammatory responses to biomaterial implants.118 L012 emits chemiluminescent signals upon reacting with ROS that contain superoxide O2−.119 Intravenously injected L012 was able to assess the extent of ROS activities and associated inflammatory responses in mice.118 However, luminescence intensity is both ROS activity and L012 concentration dependent. To eliminate the influence of L012 concentrations on ROS activity measurement, a ratiometric ROS probe was fabricated through conjugation of both ROS-sensitive chemiluminescent agents and ROS-insensitive fluorescent reference dye onto particle carriers.120 The extent of localized ROS activities was demonstrated to be proportional to the ratio of bioluminescence to reference fluorescence intensity regardless of the ROS probe concentration and tissue thickness. The study quantified this difference and found that infected wounds had almost 12 times the intensity ratios of noninfected wounds. A typical representative illustration is shown in Fig. 4. Using murine models of open wound inflammation and infection, this ratiometric probe was able to distinguish inflammatory wound from intact skin and quantify the severity of infection in real time. The results show that the ROS probes have good cell and tissue compatibility and no apparent toxicity in animals. The results of this study suggest that these ratiometric ROS-specific probes may be used to assess and detect wound inflammation and infection in real time.
Figure 4.
Illustration showing the application of ROS ratiometric probe on open infection wounds in a murine model. None, Medium, and High indicate various amounts of bacteria administered onto open wounds. The fluorescent, chemiluminescent, and superimposed images reflect the amounts of administered probes, ROS activities, and ratiometric ROS activities, respectively. The overall results show that, with the same amounts of probes administered, increase administered bacteria numbers intensify ratiometric ROS signals. ROS, reactive oxygen species.
Hypochlorous acid (HClO), a type of ROS, does play a role in the host's resistance to microbial pathogens in the innate immune response. It is produced and then released during the oxidative burst. To assess HClO activities in real time, a quinolone-based ratiometric two-photon fluorescent probe, QClO (quinolone based for ClO−), was designed and investigated in wounded tissue.121 After encountering HClO, the emission of blue wavelength from the probe decreased, while green wavelength increased. The fluorescence intensity ratios of green to blue wavelengths were proven to be positively proportional to concentrations of HClO in the measured milieu with high sensitivity. By using two-photon microscopy, the probe was capable of monitoring HClO in situ in the wounded tissues of mice, which also indicated the potential of using the probe to monitor the level of HClO production during the wound healing process.121
Bacteria
Skin is the first line of defense in the human body. Upon injury, microorganisms are much likely to get into the underlying tissue. The contaminated wound can be classified into four levels, including contamination, colonization, local infection/critical colonization, and invasive infection.122 Contamination with microorganisms may prolong the inflammatory phase of the wound, impeding its healing process. Unfortunately, as mentioned earlier, current clinical procedures used to detect wound infection—swab and biopsy—take a few days to obtain the diagnosis. Thus, there is a need for the development of a new imaging method for the detection of bacteria in wounds.
To detect the presence of bacteria, a bacteria-targeting ligand—Concanavalin A (Con A)—was used to fabricate a bacterial imaging probe. In fact, a nanoprobe (Con A-IR750) constructed by conjugating Con A and an NIR fluorescent dye (IR-750) was demonstrated to target superficial wound sites infected by luciferase transgene S. aureus and subcutaneously implanted infected catheters in mice models.123 Luciferase was the biomarker of S. aureus, while NIR fluorescent probe was fabricated to target bacteria. By overlapping the NIR and luminescent images, the study was able to show the co-localization of luminescent and fluorescent signals, which demonstrate the specificity of NIR fluorescent probe to diagnose the presence of bacteria on catheter in vivo. Since the fluorescence signal from the infected wound site can be quantified rapidly and increases proportionally with bacterial load, this optical imaging technique appears promising for clinical applications in evaluation and diagnosis of infection.
Bacterial imaging probes have also been utilized to eradicate bacteria. For example, bacterial probe has been fabricated by first labeling UBI29–41 (an antibacterial peptide fragment) with an NIR fluorescent dye (ICG02). Through in vivo targeting study, the probe was proved to have high specificity to different types of bacteria, such as S. aureus, Escherichia coli, and P. aeruginosa. Subsequently, UBI29–41, the targeting ligand of the probe, was conjugated with a bacteriostatic chloramphenicol through the linker glutaric anhydride for bacteria-targeting therapy in an S. aureus-infected mice model.124
Most recently, a handheld portable AF imaging device was developed to detect bacteria around wound site in real time. The technology is based on the light-absorbing properties of endogenously produced bacterial porphyrins, which play important roles in the metabolism of molecular oxygen and diatomic gases, as well as gene regulation.125 This study involving patients with diabetic foot ulcers found that the AF imaging device could detect various extents of bacterial load with greater accuracy and speed compared to the Levine swabbing technique. In fact, wounds that were identified as not infected by the swabbing technique were correctly determined to be infected by the AF imaging device. This imaging modality may help clinicians better diagnose infection in chronic wounds by facilitating maximum sampling of treatment-relevant pathogens in the future.
Matrix metalloproteinase
MMPs, a large family of zinc-dependent endopeptidases, are secreted by epidermal cells, macrophages, keratinocytes, and fibroblasts in mammals, influencing the wound healing process.126 Biologically, MMPs are secreted by myofibroblasts that participate in the early phase of granulation tissue formation and become fully developed in the phase of wound proliferation. MMP-1 promotes both human keratinocyte migration on fibrillar collagen127 and wound contraction as demonstrated by a study that showed reduction in dermal myofibroblast differentiation and wound contraction in MMP-2 knockout mice.128 MMP-2 can mediate platelet adhesion as well as aggregation,129 while MMP-7 is required for reepithelialization of mucosal wounds.130 MMP-8 has been shown to promote cutaneous diabetic wound healing.131 Commercially available, MMPSense 750 fast fluorescent imaging agent (PerkinElmer), has been used for imaging MMP activities in animals. The probe is optically silent upon injection and produces fluorescent signal after cleavage by disease-related MMPs, including MMP-2, -3, -7, -9, -12, and -13.132 MMPSense 750 probes were used to assess the dynamics of MMP activity in a subcutaneous animal model of infection. Significantly greater fluorescent signal was observed at the LPS treatment site compared to the phosphate-buffered saline control. The results support that wound infection might lead to the production and release of MMPs.133 Despite these exciting findings, further studies are needed to determine whether this or any other MMP imaging probe may be used to diagnose wound healing process.
Macrophage
Macrophages and their polarization have been shown to affect chronic foreign body reactions and wound healing process.134 Macrophages play different roles in wound healing processes, which include host defense, induction and resolution of inflammation, phagocytosis of apoptotic cells, promotion of cellular proliferation, and finally, tissue restoration/regeneration. On the other hand, the dysfunction of macrophage may lead to nonhealing and poorly healing wounds by eliciting excessive inflammation and fibrosis.135 To uncover the complex and dynamic macrophage responses, there has been an increased interest, in recent years, in the development of imaging probes to monitor.
Macrophages in inflamed tissues are known to have upregulated folate receptor (FR).136 By taking advantage of this unique characteristic, a study has shown that folate-conjugated NIR nanoprobes can be fabricated to target activated macrophages, enabling the detection and quantification of the extent of biomaterial-mediated inflammatory responses in vivo.137 The FR-targeting NIR nanoprobes were synthesized with an IR750 dye (a hydrophobic dye with λem at 758 nm)-encapsulated polystyrene core and a poly(N-isopropylacrylamide) shell. The surface of nanoprobes was conjugated with folic acid (or folate) as a targeting ligand. The effectiveness of the FR-targeting probes to detect macrophage responses was tested using a murine subcutaneous inflammation model.137 Intravenously injected probes quickly accumulated in the inflamed subcutaneous tissue. Interestingly, there was a good relationship between the extent of inflammatory reactions and nanoprobe-associated fluorescent intensities in tissue. The result supported that the FR-targeting NIR nanoprobes can be used to monitor and quantify the extent of macrophage recruitment within the chronic wound in real time with the aid of a fluorescence imager.
Subsequent work was carried out to develop imaging probes for simultaneously detecting polarized macrophages, including classically proinflammatory M1 cells (FR+) and alternatively proregenerative M2 cells [mannose receptor (MR)+]. For that, twin probes that can simultaneously determine M1 and M2 cells were fabricated and then characterized.138 Briefly, FR-targeting probes, which can target M1 cells, were fabricated using polyethylene glycol (PEG) linear polymer as a carrier with folic acid and Oyster 800 dye. On the other hand, MR-targeting probes, which can target M2 cells, were fabricated using PEG linear polymer as a carrier with mannose and Oyster 680 dye. In an in vivo murine model, they were able to distinguish between the infection-associated M1-dominated inflammatory response and particle implant-mediated M2-dominated regenerative response. Further studies are required to determine whether these twin probes can be used to distinguish between inflammatory and epithelialization phase, thus determining different stages of wound healing.
Neutrophils
Neutrophils are the most abundant leukocytes in the blood. Although the specific effector functions of neutrophils in wound healing responses have yet to be determined, they are the first step in host defense to tissue damage. They can protect the host from infectious threats through phagocytosis, degranulation, release of ROS, and neutrophil extracellular traps.139 Excess infiltration and activation of neutrophils at the wound site may lead to chronic inflammation and delayed wound healing process.140 It has also been reported that the peptide cinnamoyl-Phe-(D)Leu-Phe-(D)Leu-Phe (cFLFLF) has a high affinity to the formyl peptide receptor (FPR) of neutrophils. Based on this observation, an investigation was conducted to fabricate imaging probes for detecting neutrophils by targeting their FPR using cFLFLF peptide. For that, eight-arm PEG was covalently linked with NIR dye and cFLFLF peptide to produce FPR-targeting nanoprobes. The ability of the nanoprobes to detect and quantify activated neutrophils was assessed both in vitro and in vivo.141 A strong linear relationship was found between the fluorescence intensity of nanoprobes in vivo and the number of recruited neutrophils at the injured site in a murine subcutaneous inflammation model. In addition, the nanoprobes were also able to distinguish catheters with and without infection. These results confirmed the FPR-targeting nanoprobes as a powerful tool to monitor and measure the extent of neutrophil activity in real time. It is possible that, with further development, the nanoprobes may be used to monitor the neutrophil responses during wound healing process.
Fibrin
Fibrin accumulation, often accompanied with acute inflammatory responses, is an important part of wound healing process. In an open wound, platelet aggregation is usually induced by the leakage of blood from injured capillaries into the extravascular space. Subsequently, fibrin is deposited under the presence of thrombin released by the activated platelets. However, in the case of chronic wounds, tissue pressure change may lead to leakage and then accumulation of fibrin and plasma protein in the perivascular space.142
Since fibrin accumulation occurs in the early stages of acute inflammatory responses, it was assumed that fibrin imaging may be useful to detect the onset of wound healing processes. To test this hypothesis, a study used NIR-labeled fibrinogen for imaging fibrin deposition during wound healing process.143 Briefly, NIR fluorescent dye-conjugated fibrinogen was injected into the tail vein of a rat. Strong fluorescence signals were observed at the site of the wound for several days, supporting the conversion of water-soluble fibrinogen to water-insoluble fibrin at the site of injury. Overall, this study demonstrated that fluorescence imaging can be used to monitor fibrin deposition and identify wound location in vivo. Despite this early success, NIR-labeled fibrinogen imaging method cannot be used to detect chronic wounds in which fibrin has already accumulated mainly due to leakage from vasculature. To overcome this limitation, a new fibrin probe was fabricated by directly conjugating a fibrin affinity peptide (GPRPPGGSKGC with amide of C terminal) with Oyster 800 dye.144 The fibrin probe was found to accumulate at the site of localized inflammatory responses induced by subcutaneously injected particle implants. Furthermore, the study has uncovered that mast cell activation is essential to fibrin accumulation in wounds, since fibrin accumulation is significantly diminished in mice with mast cell deficiency. These findings support that the fibrin-targeting probes may be used to monitor fibrin deposition during the wound healing process.
Portable fluorescent and luminescent imager
Despite the above-mentioned exciting progress in the development of various imaging probes, there are very limited number of medical imaging modalities that can detect either fluorescent or luminescent signals for the clinical application. Some progress has been made in recent years. For example, SPY portable handheld imaging system commercially created by Novadaq has been widely used for cardiac surgery, breast reconstruction, laparoscopic cholecystectomy, and colorectal surgery for decades.145 Another low-cost digital fluorescent microscope built with commercial off-the-shelf components was developed,146 and tested for cancer cell detection. Moreover, a portable imager was developed exclusively for NIR visualization of cutaneous wounds, and is illustrated through detection of ROS activities in an excisional wound model.133
Strengths and limitations of different imaging modalities on routine wound care
For routine wound care in clinic, it is critical that wound imaging modalities should be low cost and easy operable. Based on these requirements, CT, SPECT/CT, and MRI that require long imaging time and heavy cost are not suitable for routine wound care. On the other hand, ultrasound imaging and THz spectroscopy are safe and capable of dermal wound imaging and burn wound assessment. However, since both of these imaging modalities require specific facilities as well as well-trained operators, they may provide limited utility for routine wound care. Optical imaging modalities are more appropriate for routine wound care based on these advantages—they are not expensive, require little imaging time, and have less dependence on imaging environment. It should be noted that multispectral imaging modality has become an emerging approach for routine wound care by combining multiple imaging modalities and assessing multiple factors for wound assessment at one time.147 With the development of the scale-down technology, some handheld optical imaging devices have been fabricated for evaluating wound condition in real time.
Summary
While substantial progresses have been made in the improvement of wound treatment, current wound assessment tools remain inadequate to provide real-time and fast evaluation of the wound healing status. Therefore, there is a need for the development of a new tool to assess wound healing status by measuring factors that can affect wound healing. Although several of the current nonoptical imaging modalities can be modified for wound monitoring, these methods are plagued by high equipment cost and low specificity. Based on recent developments in optical imaging for wound monitoring, this review introduces different types of optical imaging techniques and summarizes recent developments in the area of different optical imaging probes, which may greatly impact wound diagnosis. To meet the needs of the growing wound care market, there is tremendous opportunity to translate optical imaging discoveries for chronic wound diagnosis and monitoring with speed, precision, real-time responsiveness, portability, and smartphone capability. With all these advancements, it is conceivable that optical wound imaging plays a pivotal role in routine wound care in clinics in the very near future.
Take-Home Messages
Chronic wound is a significant burden on the health care system.
Current wound evaluation methods like physical evaluation are not always accurate and cannot be used to predict progress in healing.
Wound swabs and biopsies are not specific and it can take a few days to get useful information from these assessment techniques.
Medical imaging techniques like CT, SPECT/CT, MRI, and ultrasound imaging, which are commonly used for diagnosing cancer, can also be used in wound imaging.
These commonly used clinical imaging techniques are often labor intensive, costly, and subjective.
Optical imaging techniques have emerged as a potential alternative.
Fluorescence, NIR, and luminescence imaging may provide real-time assessment of the wound environment.
These imaging techniques have been modified to detect specific wound biomarkers.
New imaging tools can detect biomarkers, including various cytokines, DNA, MMPs, nitric oxide, pH, ROS, and tissue oxygenation.
Most of the imaging modalities employing these markers are bulky.
There is a need for portable imagers that can apply these new optical imaging modalities effectively and efficiently.
Abbreviations and Acronyms
- λem
emission wavelength
- AF
autofluorescence
- BJWAT
Bates-Jensen Wound Assessment Chart
- Con A
Concanavalin A
- CT
computed tomography
- FDA
Food and Drug Administration
- FPR
formyl peptide receptor
- FR
folate receptor
- HClO
hypochlorous acid
- HSI
hyperspectral imaging
- ICG
indocyanine green
- LDI
laser Doppler imaging
- LPS
Lipopolysaccharides
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- NIR
near infrared
- OCT
optical coherence tomography
- PEG
polyethylene glycol
- ROS
reactive oxygen species
- SEM
subepidermal moisture
- SFDI
spatial frequency domain imaging
- SPECT
single-photon emission computed tomography
- THz
terahertz
- VLU
venous leg ulcers
Acknowledgment and Funding Sources
This work was supported by a grant from National Institute of Health (AR064650).
Author Disclosure and Ghostwriting
Tang has a potential research conflict of interests due to a financial interest with Progenitec, Inc. A management plan has been created to preserve objectivity in research, in accordance with UTA policy. No competing financial interests exist for the other authors. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
About the Authors
Shuxin Li, BS, is a bioengineering doctoral student at the University of Texas at Arlington. His research focuses on fabricating probes and probe-loaded gauze for wound monitoring and healing. Ali H. Mohamedi is a bioengineering undergraduate student at the University of Texas at Arlington. Jon Senkowsky, MD, is a vascular surgeon with over 30 years of clinical experience in wound management. He provides care for patients with chronic wounds on a daily basis using many techniques and products for the treatment of chronic, nonhealing wounds in patients with vascular disease, venous stasis, and diabetes. Ashwin Nair, PhD, is a scientist and project leader at Progenitec, Inc., with expertise in foreign body reactions to materials and tissue regeneration techniques. Liping Tang, PhD, is a bioengineering professor at the University of Texas at Arlington. His expertise covers a broad area of biocompatibility, biomaterials, tissue engineering, inflammation imaging, infection, and stem cell therapies.
References
- 1. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen 2019;27:114–125 [DOI] [PubMed] [Google Scholar]
- 2. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 2014;7:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4:560–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–170 [DOI] [PubMed] [Google Scholar]
- 6. Barber S. A clinically relevant wound assessment method to monitor healing progression. Ostomy Wound Manage 2008;54:42–49 [PubMed] [Google Scholar]
- 7. Ahn C. Advances in wound photography and assessment methods. Adv Skin Wound Care 2008;21:85–93 [DOI] [PubMed] [Google Scholar]
- 8. Kim HM, Lowery JC, Hamill JB, Wilkins EG. Accuracy of a web-based system for monitoring chronic wounds. Telemed J E Health 2003;9:129–140 [DOI] [PubMed] [Google Scholar]
- 9. Foltynski P, Ladyzynski P, Ciechanowska A, Migalska-Musial K, Judzewicz G, Sabalinska S. Wound area measurement with digital planimetry: improved accuracy and precision with calibration based on 2 rulers. PLoS One 2015;10:e0134622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang AC, Dearman B, Greenwood JE. A comparison of wound area measurement techniques: visitrak versus photography. Eplasty 2011;11:e18. [PMC free article] [PubMed] [Google Scholar]
- 11. Jones K, Cassidy P, Killen J, Ellis H. The feasibility and usefulness of oximetry measurements in primary care. Prim Care Respir J 2003;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bianchi J, Douglas W, Dawe R, et al. Pulse oximetry: a new tool to assess patients with leg ulcers. J Wound Care 2000;9:109–112 [DOI] [PubMed] [Google Scholar]
- 13. Mardirossian G, Schneider RE. Limitations of pulse oximetry. Anesth Prog 1992;39:194. [PMC free article] [PubMed] [Google Scholar]
- 14. Harrow JJ, Mayrovitz HN. Subepidermal moisture surrounding pressure ulcers in persons with a spinal cord injury: a pilot study. J Spinal Cord Med 2014;37:719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clendenin M, Jaradeh K, Shamirian A, Rhodes SL. Inter-operator and inter-device agreement and reliability of the SEM Scanner. J Tissue Viability 2015;24:17–23 [DOI] [PubMed] [Google Scholar]
- 16. Davies CE, Hill KE, Newcombe RG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen 2007;15:17–22 [DOI] [PubMed] [Google Scholar]
- 17. Cooper R, Lawrence J. The isolation and identification of bacteria from wounds. J Wound Care 1996;5:335–340 [DOI] [PubMed] [Google Scholar]
- 18. Pellizzer G, Strazzabosco M, Presi S, et al. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb-threatening diabetic foot infection. Diabet Med 2001;18:822–827 [DOI] [PubMed] [Google Scholar]
- 19. Ono I, Gunji H, Zhang J, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns 1995;21:352–355 [DOI] [PubMed] [Google Scholar]
- 20. Aiba-Kojima E, Tsuno NH, Inoue K, et al. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen 2007;15:511–520 [DOI] [PubMed] [Google Scholar]
- 21. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care 2015;4:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cutting KF. Wound exudate: composition and functions. Br J Community Nurs 2003;8:S4–S9 [DOI] [PubMed] [Google Scholar]
- 23. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–e173 [DOI] [PubMed] [Google Scholar]
- 24. Hochman MG, Connolly C. Imaging of Infection in the Diabetic Foot. Springer: The Diabetic Foot, 2018:55–94. [Google Scholar]
- 25. Elsayed EE, Zytoon AA, Eltelwany AM. Role of computed tomography angiography and color Doppler ultrasonography in the evaluation of diabetic foot. Menoufia Med J 2018;31:508 [Google Scholar]
- 26. Chou T-H, Atway Said A, Bobbey Adam J, Sarac Timur P, Go Michael R, Stacy Mitchel R.. SPECT/CT imaging: a noninvasive approach for evaluating serial changes in angiosome foot perfusion in critical limb ischemia. Adv Wound Care 2019. [Epub ahead of print]; DOI: 10.1089/wound.2018.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soyer H, Uslu I. A patient with peripheral arterial stenosis diagnosed with lower extremity perfusion scintigraphy. Clin Nucl Med 2007;32:458–459 [DOI] [PubMed] [Google Scholar]
- 28. Smith BJ, Buchana GS, Shuler FD. A comparison of imaging modalities for the diagnosis of osteomyelitis. Marshall J Med 2016;2:84–92 [Google Scholar]
- 29. Cohen M, Cerniglia B, Gorbachova T, Horrow J. Added value of MRI to X-ray in guiding the extent of surgical resection in diabetic forefoot osteomyelitis: a review of pathologically proven, surgically treated cases. Skeletal Radiol 2019;48:405–411 [DOI] [PubMed] [Google Scholar]
- 30. Collins R, Cranny G, Burch J, et al. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess 2007;11:iii–iv, xi–xiii, 1–184. [DOI] [PubMed] [Google Scholar]
- 31. Lee KC, Dretzke J, Grover L, Logan A, Moiemen N. A systematic review of objective burn scar measurements. Burns Trauma 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohafez H, Ahmad SA, Hadizadeh M, Marhaban MH, Saripan MI. High-frequency ultrasound imaging in wound assessment: current perspectives. Pertanika J Sci Technol 2017;25:1039–1049 [Google Scholar]
- 33. Arbab MH, Winebrenner DP, Dickey TC, Chen A, Klein MB, Mourad PD. Terahertz spectroscopy for the assessment of burn injuries in vivo. J Biomed Opt 2013;18:077004. [DOI] [PubMed] [Google Scholar]
- 34. Siegel PH. Terahertz technology in biology and medicine. IEEE Trans Microw Theory and Tech 2004;52:2438–2447 [Google Scholar]
- 35. Neha B, Shijun S, James G, et al. In vivo confirmation of hydration based contrast mechanisms for terahertz medical imaging using MRI. Proc SPIE 2014;9199. DOI: 10.1117/12.2060115 [DOI] [Google Scholar]
- 36. Li WW, Arnold J. Imaging of the chronic wound and the emerging role of fluorescence microangiography. Wounds 2014;8:1–4 [Google Scholar]
- 37. Mukherjee R, Tewary S, Routray A. Diagnostic and prognostic utility of non-invasive multimodal imaging in chronic wound monitoring: a systematic review. J Med Syst 2017;41:46. [DOI] [PubMed] [Google Scholar]
- 38. Gaur A, Sunkara R, Raj ANJ, Celik T. Efficient wound measurements using RGB and depth images. Int J Biomed Eng Technol 2015;18:333–358 [Google Scholar]
- 39. Silva RH, Machado AM. A computational method for semi-automatic measurement of pressure ulcers. Wound Repair Regen 2018;26:332–339 [DOI] [PubMed] [Google Scholar]
- 40. Mukherjee R, Manohar DD, Das DK, Achar A, Mitra A, Chakraborty C. Automated tissue classification framework for reproducible chronic wound assessment. Biomed Res Int 2014;2014:851582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Treuillet S, Albouy B, Lucas Y. Three-dimensional assessment of skin wounds using a standard digital camera. IEEE Trans Med Imaging 2009;28:752–762 [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Shi L, Qin J, Yousefi S, Li Y, Wang RK. Multimodal optical imaging can reveal changes in microcirculation and tissue oxygenation during skin wound healing. Lasers Surg Med 2014;46:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wahabzada M, Besser M, Khosravani M, et al. Monitoring wound healing in a 3D wound model by hyperspectral imaging and efficient clustering. PLoS One 2017;12:e0186425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu RX, Allen DW, Huang J, et al. Developing digital tissue phantoms for hyperspectral imaging of ischemic wounds. Biomed Opt Express 2012;3:1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Calin MA, Parasca SV, Savastru D, Manea D. Hyperspectral imaging in the medical field: present and future. Appl Spectrosc Rev 2014;49:435–447 [Google Scholar]
- 47. Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt 2014;19:010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chin MS, Babchenko O, Lujan-Hernandez J, Nobel L, Ignotz R, Lalikos JF. Hyperspectral imaging for burn depth assessment in an animal model. Plast Reconstr Surg Glob Open 2015;3:e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care 2009;32:2056–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang S, Gnyawali S, Huang J, et al. Multimodal imaging of cutaneous wound tissue. J Biomed Opt 2015;20:016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hernandez-Contreras D, Peregrina-Barreto H, Rangel-Magdaleno J, Gonzalez-Bernal J. Narrative review: diabetic foot and infrared thermography. Infrared Phys Technol 2016;78:105–117 [Google Scholar]
- 52. Chanmugam A, Langemo D, Thomason K, et al. Relative temperature maximum in wound infection and inflammation as compared with a control subject using long-wave infrared thermography. Adv Skin Wound Care 2017;30:406–414 [DOI] [PubMed] [Google Scholar]
- 53. Fierheller M, Sibbald RG. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv Skin Wound Care 2010;23:369–379 [DOI] [PubMed] [Google Scholar]
- 54. Gethin G, O'Connor GM, Abedin J, et al. Monitoring of pH and temperature of neuropathic diabetic and non-diabetic foot ulcers for 12-weeks: an observational study. Wound Repair Regen 2018;26:251–256 [DOI] [PubMed] [Google Scholar]
- 55. Power G, Moore Z, O'Connor T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J Wound Care 2017;26:381–397 [DOI] [PubMed] [Google Scholar]
- 56. Renkielska A, Kaczmarek M, Nowakowski A, et al. Active dynamic infrared thermal imaging in burn depth evaluation. J Burn Care Res 2014;35:e294–e303 [DOI] [PubMed] [Google Scholar]
- 57. Barone S, Paoli A, Razionale AV. Assessment of chronic wounds by three-dimensional optical imaging based on integrating geometrical, chromatic, and thermal data. Proc Inst Mech Eng H 2011;225:181–193 [DOI] [PubMed] [Google Scholar]
- 58. Yi S, Lu M, Yee A, Harmon J, Meng F, Hinduja S. Enhance wound healing monitoring through a thermal imaging based smartphone app. In: SPIE Medical Imaging 2018: Imaging Informatics for Healthcare, Research, and Applications. Houston, TX: International Society for Optics and Photonics, 2018:105791P [Google Scholar]
- 59. Burke-Smith A, Collier J, Jones I. A comparison of non-invasive imaging modalities: infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns 2015;41:1695–1707 [DOI] [PubMed] [Google Scholar]
- 60. Wearn C, Lee KC, Hardwicke J, et al. Prospective comparative evaluation study of Laser Doppler Imaging and thermal imaging in the assessment of burn depth. Burns 2018;44:124–133 [DOI] [PubMed] [Google Scholar]
- 61. Izatt JA, Choma MA, Dhalla A-H. Theory of optical coherence tomography. In: Drexler W, Fujimoto JG, eds. Optical coherence tomography. Cham, Switzerland: Springer, 2015:65–94 [Google Scholar]
- 62. Baran U, Li Y, Choi WJ, Kalkan G, Wang RK. High resolution imaging of acne lesion development and scarring in human facial skin using OCT-based microangiography. Lasers Surg Med 2015;47:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim DW, Park TJ, Jang SJ, You S, Oh W-Y. Plasma treatment effect on angiogenesis in wound healing process evaluated in vivo using angiographic optical coherence tomography. Appl Phys Lett 2016;109:233701 [Google Scholar]
- 64. Kuck M, Strese H, Alawi SA, et al. Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing. Skin Res Technol 2014;20:1–7 [DOI] [PubMed] [Google Scholar]
- 65. Jaspers ME, Feroldi F, Vlig M, de Boer JF, van Zuijlen PP. In vivo polarization-sensitive optical coherence tomography of human burn scars: birefringence quantification and correspondence with histologically determined collagen density. J Biomed Opt 2017;22:121712. [DOI] [PubMed] [Google Scholar]
- 66. De Boer JF, Hitzenberger CK, Yasuno Y. Polarization sensitive optical coherence tomography—a review. Biomed Opt Express 2017;8:1838–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Park BH, de Boer JF. Polarization sensitive optical coherence tomography. In: Drexler W, Fujimoto J, eds. Optical Coherence Tomography. Cham, Switzerland: Springer, 2015:1055 [Google Scholar]
- 68. Lo WC, Villiger M, Golberg A, et al. Longitudinal, 3D imaging of collagen remodeling in murine hypertrophic scars in vivo using polarization-sensitive optical frequency domain imaging. J Invest Dermatol 2016;136:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deegan AJ, Wang W, Men S, et al. Optical coherence tomography angiography monitors human cutaneous wound healing over time. Quant Imaging Med Surg 2018;8:135–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ring HC, Mogensen M, Hussain AA, et al. Imaging of collagen deposition disorders using optical coherence tomography. J Eur Acad Dermatol Venereol 2015;29:890–898 [DOI] [PubMed] [Google Scholar]
- 71. Cobb MJ, Chen Y, Underwood RA, Usui ML, Olerud J, Li X. Noninvasive assessment of cutaneous wound healing using ultrahigh-resolution optical coherence tomography. J Biomed Opt 2006;11:064002. [DOI] [PubMed] [Google Scholar]
- 72. Gnyawali SC, Blum K, Pal D, et al. Retooling laser speckle contrast analysis algorithm to enhance non-invasive high resolution laser speckle functional imaging of cutaneous microcirculation. Sci Rep 2017;7:41048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fuchs D, Dupon PP, Schaap LA, Draijer R. The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol 2017;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761–769 [DOI] [PubMed] [Google Scholar]
- 75. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Andersen CA. Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg 2010;52:76S–80S [DOI] [PubMed] [Google Scholar]
- 77. Forsythe RO, Hinchliffe RJ. Assessment of foot perfusion in patients with a diabetic foot ulcer. Diabetes Metab Res Rev 2016;32:232–238 [DOI] [PubMed] [Google Scholar]
- 78. Cuccia DJ. Spatial frequency domain imaging (SFDI): a technology overview and validation of an LED-based clinic friendly device. In: SPIE MOEMS-MEMS, 2012: Emerging Digital Micromirror Device Based Systems and Applications IV. San Francisco, CA: International Society for Optics and Photonics, 2012:825405 [Google Scholar]
- 79. Thatcher JE, Squiers JJ, Kanick SC, et al. Imaging techniques for clinical burn assessment with a focus on multispectral imaging. Adv Wound Care 2016;5:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nguyen JQM, Crouzet C, Mai T, et al. Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt 2013;18:066010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yafi A, Muakkassa FK, Pasupneti T, et al. Quantitative skin assessment using spatial frequency domain imaging (SFDI) in patients with or at high risk for pressure ulcers. Lasers Surg Med 2017;49:827–834 [DOI] [PubMed] [Google Scholar]
- 82. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012;15:581–592 [DOI] [PubMed] [Google Scholar]
- 83. Quinn KP, Leal EC, Tellechea A, et al. Diabetic wounds exhibit distinct microstructural and metabolic heterogeneity through label-free multiphoton microscopy. J invest Dermatol 2016;136:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang Y, Gutierrez-Herrera E, Ortega-Martinez A, Anderson RR, Franco W. UV fluorescence excitation imaging of healing of wounds in skin: evaluation of wound closure in organ culture model. Lasers Surg Med 2016;48:678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626–634 [DOI] [PubMed] [Google Scholar]
- 86. Deka G, Wu W-W, Kao F-J. In vivo wound healing diagnosis with second harmonic and fluorescence lifetime imaging. J Biomed Opt 2012;18:061222. [PubMed] [Google Scholar]
- 87. Sen CK, Ghatak S, Gnyawali SC, Roy S, Gordillo GM. Cutaneous imaging technologies in acute burn and chronic wound care. Plast Reconstr Surg 2016;138:119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. DaCosta RS, Kulbatski I, Lindvere-Teene L, et al. Point-of-care autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: first-in-human results. PLoS One 2015;10:e0116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jayachandran M, Rodriguez S, Solis E, Lei J, Godavarty A. Critical review of noninvasive optical technologies for wound imaging. Adv Wound Care 2016;5:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Papazoglou E, Zubkov L, Zhu L, Weingarten M, Tyagi S, Pourrezaei K. Monitoring diabetic wound healing by NIR spectroscopy. In: 27th Annual International Conference of the Engineering in Medicine and Biology Society, 2005. Shanghai, China: IEEE-EMBS 2005. IEEE, 2006:6662–6664 [DOI] [PubMed] [Google Scholar]
- 91. Weingarten MS, Neidrauer M, Mateo A, et al. Prediction of wound healing in human diabetic foot ulcers by diffuse near-infrared spectroscopy: a pilot study. Wound Repair Regen 2010;18:180–185 [DOI] [PubMed] [Google Scholar]
- 92. Lei J, Rodriguez S, Jayachandran M, et al. Quantitative wound healing studies using a portable, low cost, handheld near-infrared optical scanner: preliminary sensitivity and specificity analysis. In: SPIE BIOS, 2016 Optics and Biophotonics in Low-Resource Settings II. San Francisco, CA: International Society for Optics and Photonics, 2016:96990S [Google Scholar]
- 93. Societies WUoWH. Principles of Best Practice: Diagnostics and Wounds. A Consensus Document. London, UK: Medical Education Partnership (MEP) Ltd., 2008 [Google Scholar]
- 94. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sen CK, Khanna S, Gordillo G, Bagchi D, Bagchi M, Roy S. Oxygen, oxidants, and antioxidants in wound healing: an emerging paradigm. Ann N Y Acad Sci 2002;957:239–249 [DOI] [PubMed] [Google Scholar]
- 96. Stoekenbroek R, Santema T, Legemate D, Ubbink D, Van Den Brink A, Koelemay M. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg 2014;47:647–655 [DOI] [PubMed] [Google Scholar]
- 97. Moza R, DiMaio JM, Melendez J. Deep-tissue dynamic monitoring of decubitus ulcers: wound care and assessment. IEEE Eng Med Biol Mag 2010;29:71–77 [DOI] [PubMed] [Google Scholar]
- 98. Lei J, Rodriguez S, Jayachandran M, et al. Assessing the healing of venous leg ulcers using a noncontact near-infrared optical imaging approach. Adv Wound Care 2018;7:134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Patel HM, Bulsara SS, Banerjee S, et al. Indocyanine green angiography to prognosticate healing of foot ulcer in critical limb ischemia: a novel technique. Ann Vasc Surg 2018;51:86–94 [DOI] [PubMed] [Google Scholar]
- 100. Gethin G. The significance of surface pH in chronic wounds. Wounds UK 2007;3:52 [Google Scholar]
- 101. Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int 2013;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jones EM, Cochrane CA, Percival SL. The effect of pH on the extracellular matrix and biofilms. Adv Wound Care 2015;4:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Percival SL, Thomas J, Linton S, Okel T, Corum L, Slone W. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. Int Wound J 2012;9:488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rushton I. Understanding the role of proteases and pH in wound healing. Nurs Stand 2007;21:68–74 [DOI] [PubMed] [Google Scholar]
- 105. Hunt TK, Hopf HW. Wound healing and wound infection: what surgeons and anesthesiologists can do. Surg Clin North Am 1997;77:587–606 [DOI] [PubMed] [Google Scholar]
- 106. Nagoba B, Gandhi R, Wadher B, Potekar R, Kolhe S. Microbiological, histopathological and clinical changes in chronic infected wounds after citric acid treatment. J Med Microbiol 2008;57:681–682 [DOI] [PubMed] [Google Scholar]
- 107. Schreml S, Meier RJ, Wolfbeis OS, Landthaler M, Szeimies R-M, Babilas P. 2D luminescence imaging of pH in vivo. Proc Natl Acad Sci U S A 2011;108:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schreml S, Meier RJ, Kirschbaum M, et al. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics 2014;4:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schreml S, Meier RJ, Weiß KT, et al. A s prayable luminescent pH sensor and its use for wound imaging in vivo. Exp Dermatol 2012;21:951–953 [DOI] [PubMed] [Google Scholar]
- 110. Meier RJ, Schreml S, Wang XD, Landthaler M, Babilas P, Wolfbeis OS. Simultaneous photographing of oxygen and pH in vivo using sensor films. Angew Chem Int Ed Engl 2011;123:11085–11088 [DOI] [PubMed] [Google Scholar]
- 111. Tamayol A, Akbari M, Zilberman Y, et al. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv Healthc Mater 2016;5:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dryden M, Cooke J, Salib R, Holding R, Pender SL, Brooks J. Hot topics in reactive oxygen therapy: antimicrobial and immunological mechanisms, safety and clinical applications. J Glob Antimicrob Resist 2017;8:194–198 [DOI] [PubMed] [Google Scholar]
- 113. Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 2002;9:231–238 [DOI] [PubMed] [Google Scholar]
- 114. Bulua AC, Simon A, Maddipati R, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 2011;208:519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bylund J, Björnsdottir H, Sundqvist M, Karlsson A, Dahlgren C. Measurement of respiratory burst products, released or retained, during activation of professional phagocytes. Methods Mol Biol 2014;1124:321–338 [DOI] [PubMed] [Google Scholar]
- 117. Dryden M, Milward G, Saeed K. Infection prevention in wounds with Surgihoney. J Hosp Infect 2014;88:121–122 [DOI] [PubMed] [Google Scholar]
- 118. Zhou J, Tsai Y-T, Weng H, Tang L. Noninvasive assessment of localized inflammatory responses. Free Radic Biol Med 2012;52:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zielonka J, Lambeth JD, Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic Biol Med 2013;65:1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhou J, Weng H, Huang Y, Gu Y, Tang L, Hu W. Ratiometric reactive oxygen species nanoprobe for noninvasive in vivo imaging of subcutaneous inflammation/infection. J Biomed Nanotechnol 2016;12:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mao Z, Ye M, Hu W, et al. Design of a ratiometric two-photon probe for imaging of hypochlorous acid (HClO) in wounded tissues. Chem Sci 2018;9:6035–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–96 [DOI] [PubMed] [Google Scholar]
- 123. Tang EN, Nair A, Baker DW, Hu W, Zhou J. In vivo imaging of infection using a bacteria-targeting optical nanoprobe. J Biomed Nanotechnol 2014;10:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen H, Liu C, Chen D, et al. Bacteria-targeting conjugates based on antimicrobial peptide for bacteria diagnosis and therapy. Mol Pharm 2015;12:2505–2516 [DOI] [PubMed] [Google Scholar]
- 125. Ottolino-Perry K, Chamma E, Blackmore KM, et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int Wound J 2017;14:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008;40:1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997;137:1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Toriseva MJ, Ala-aho R, Karvinen J, et al. Collagenase-3 (MMP-13) enhances remodeling of three-dimensional collagen and promotes survival of human skin fibroblasts. J Invest Dermatol 2007;127:49–59 [DOI] [PubMed] [Google Scholar]
- 129. Jurasz P, Chung AW, Radomski A, Radomski MW. Nonremodeling properties of matrix metalloproteinases: the platelet connection: Circ Res 2002;90:1041–1043 [DOI] [PubMed] [Google Scholar]
- 130. Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS One 2009;4:e6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gooyit M, Peng Z, Wolter WR, et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol 2013;9:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu Y, Bauer AQ, Akers WJ, et al. Hands-free, wireless goggles for near-infrared fluorescence and real-time image-guided surgery. Surgery 2011;149:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Peng Z, Zhou J, Dacy A, et al. Design of a portable imager for near-infrared visualization of cutaneous wounds. J Biomed Opt 2017;22:016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sheikh Z, Brooks P, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015;8:5671–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci 2005;94:2135–2146 [DOI] [PubMed] [Google Scholar]
- 137. Zhou J, Tsai Y-T, Weng H, Baker DW, Tang L. Real time monitoring of biomaterial-mediated inflammatory responses via macrophage-targeting NIR nanoprobes. Biomaterials 2011;32:9383–9390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Baker DW, Zhou J, Tsai Y-T, et al. Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta Biomater 2014;10:2945–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173. [DOI] [PubMed] [Google Scholar]
- 140. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 2016;16:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhou J, Tsai Y-T, Weng H, et al. Real-time detection of implant-associated neutrophil responses using a formyl peptide receptor-targeting NIR nanoprobe. Int J Nanomed 2012;7:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pan C-P, Shi Y, Amin K, Greenberg CS, Haroon Z, Faris GW. Wound healing monitoring using near infrared fluorescent fibrinogen. Biomed Opt Express 2010;1:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tsai Y-T, Zhou J, Weng H, Tang EN, Baker DW, Tang L. Optical imaging of fibrin deposition to elucidate participation of mast cells in foreign body responses. Biomaterials 2014;35:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mondal SB, Gao S, Zhu N, Liang R, Gruev V, Achilefu S. Real-time fluorescence image-guided oncologic surgery. Adv Cancer Res 2014;124:171–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hasan MM, Alam MW, Wahid KA, Miah S, Lukong KE. A low-cost digital microscope with real-time fluorescent imaging capability. PLoS One 2016;11:e0167863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Nouri D, Lucas Y, Treuillet S, Jolivot R, Marzani F. Colour and multispectral imaging for wound healing evaluation in the context of a comparative preclinical study. In: SPIE Medical Imaging 2013: Image Processing. Lake Buena Vista, FL: International Society for Optics and Photonics, 2013:866923 [Google Scholar]