Abstract
Objective: Epidermal CD34+ stem cells located in the hair follicle (HF) bulge area are capable of inducing HF neogenesis and enhancing wound healing after transplantation. In this study, we observed CD34+ cells derived from blood directly participate in dermal regeneration during full-thickness excisional wound healing.
Approach: We isolated and in vitro expanded a subset of hematopoietic stem cell (HSC)-like precursor cells from the peripheral blood of adult mice with the surface markers: CD34+, leucine rich repeat containing G protein-coupled receptor 5 (LGR5)+, CD44+, c-kit+, lineage negative (lin−), and E-cadherin−. These blood-derived precursor cells (BDPCs), can be further differentiated into epithelial-like cells (eBDPCs) and secret fibroblast growth factor 9 (Fgf9) protein.
Result: When transplanted into full-thickness skin wounds, eBDPC treatment produced accelerated healing and enhanced skin structure regeneration with less dermal scar formation. Also, HF neogenesis (HFN) was observed with incorporation of labeled BDPCs in the wound area.
Innovation:
Nondermal-derived CD34+ cells (BDPCs) from the adult unmobilized peripheral blood are capable of in vitro expansion and differentiation.
Successful establishment of an in vitro technical platform for BDPCs expansion and differentiation.
The in vitro expanded and differentiated epithelial-like cells (eBDPCs) enhance wound healing and directly contribute to skin regeneration and HFN.
Conclusion: BDPCs isolated and expanded from adult peripheral blood may provide a possible new cell-based treatment strategy for HF neogenesis and skin wound regeneration.
Keywords: stem/precursor cell, CD34+ cells, wound heal, scar, skin regeneration, hair follicle neogenesis

H. Peter Lorenz, MD
Introduction
Stem cells play an essential role in wound healing and tissue regeneration. Studies have shown that repair of skin wounds depends on the activation of a pool of dedicated epidermal stem cells residing in the basal keratinocyte layer and hair follicle (HF) bulge.1,2 The epidermal stem/precursor cells retain the homeostasis of the epidermal layer, HFs, and sweat glands, as well as play an important role in wound healing.3–5 These epidermal stem cells express well-recognized markers CD34, K14, K15, leucine rich repeat containing G protein-coupled receptor 5 (LGR5), LGR6, and Sca-1.6,7 A group of CD34 positive (CD34+) epidermal stem cells in the HF bulge are reported to be responsible for hair cycling and may enhance the regeneration of the new epidermis postinjury.7 Previous studies found that transplantation of epidermal CD34+ stem cells into skin defects result in the regeneration of epidermis, HFs, and sebaceous glands.8
HF neogenesis (HFN) is presumed to be induced by epidermal CD34+ stem cells through two principal mechanisms: cell–cell contact and cytokine regulation.9 Although epidermal CD34+ cells are widely studied and demonstrated to be critical during wound healing and HFN, the functional mechanism remains unclear.8,10,11 Recent studies report that dermal gamma delta T lymphocytes (γδ T) could induce HFN.11 Gay et al. discovered that dermal γδ T cells secreted fibroblast growth factor 9 (Fgf9) could induce HF neogenesis during wound repair.10
Noncutaneous stem cells from bone marrow, umbilical cord, and peripheral blood are also found to participate in the wound healing process.12,13 Scientists observed that donor cells could replace some keratinocytes and persist in the epidermis for years posthuman bone marrow transplantation.14 Other findings from bone marrow transplantation are donor fibroblast-like cell populations from both hematopoietic and mesenchymal lineages present in the recipient's dermis and the amount of these cells increases during skin wound repair process.15 The human clinical studies have suggested a close association between skin repair and bone marrow precursor cells.
Nonepidermal stem cells participate in the wound healing process. For example, CD34-enriched blood mononuclear cells injected into the ischemic limbs of diabetic mice showed a significant improvement in blood flow and rapid wound healing.16,17 However, whether blood-derived CD34+ precursor cells can induce HFN and enhance local tissue regeneration has not been reported. We sought to address this question by purifying and expanding blood-derived CD34+ precursor cells (BDPCs), transplanting, and tracking their fate in the wounds. Our results demonstrate that enriched and expanded CD34+ BDPCs from adult peripheral blood could be induced to transdifferentiate into epithelial-like cells (eBDPCs) and secrete Fgf9 protein in vitro. After transplantation into skin wounds, eBDPCs can remarkably enhance the wound healing rate, reduce collagen scar formation, and generate HFN.
Clinical Problem Addressed
A full-thickness wound has injury to all layers of the skin structure. Normal healing in mammalian skin results in fibrotic scar with dense collagen and extracellular matrix (ECM) lacking epidermal appendages such as HFs and sweat glands.18,19 Wounded tissue cannot restore preinjured skin architecture and function. Excessive scarring may cause later need for scar revision surgery as a consequence of functional and cosmetic compromise. Nonhealing wounds in the diabetic patient or patient subject to venous stasis ulcers are a significant burden for the health care system.
Nonhealing wounds affect >3–6 million people in the United States and result in an estimated $25 billion health care burden annually.20 Current treatments for nonhealing wounds are expensive and met with limited success. Stem cell therapy is crucial for physiological tissue renewal and skin regeneration after injury.9
Materials and Methods
Isolation and expansion BDPCs
Unmobilized peripheral blood was collected from L2G Friend leukemia virus B (FVB)-Tg (CAG-luc, enhanced green fluorescent protein) or L2G85Chco/J adult mice (Jackson Laboratory, Bar Harbor, ME). Red blood cells (RBCs) were depleted by lysis buffer (8.3 g/L NH4Cl, 1 g/L KHCO3, 3.7 g/L EDTA), at (10:1) in a 50 mL tube containing whole blood as previously reported.21 The pellet was centrifuged at 3,000g for 15 min at 4°C. The cell suspension was treated with 1:4.4 dilution of Optiprep™ Density Gradient Medium (Sigma-Aldrich, St. Louis, MO) to deplete platelets and yielded a density of 1.063 for the collection of mononuclear cells. The cell pellet was resuspended in phosphate-buffered saline (PBS) for further flow cytometry analysis or resuspended in the culture medium (minimum essential medium, α-MEM, with 20% fetal bovine serum [FBS], 1 × antibiotic-antimycotic, 20 mg gentamicin) for in vitro studies.
Alpha mouse liver 12 (AML12) hepatic cells (ATCC, Manassas, VA) were mitotically inactivated with 30 mg/L mitomycin C for 2 h then inoculated into six-well plates in DMEM/F12 supplemented with 10% FBS. Inactivated AML12 cells adhered to the bottom of the wells and were ∼80% confluent in 16 h. The collected cell suspension in α-MEM was placed evenly into the upper chambers of transwell plates (24-mm insert; Corning, Corning, NY). Thus, the collected blood cells were separated from the AML12 cells by the transwell membrane (0.4-μm pore size). The culture medium was changed every other day.
Enrichment and characterization of CD34+ cells
Two weeks after coculture, the CD34+ cell fraction was enriched by magnetic-activated cell sorting (MACS; Miltenyi Biotec, Inc., San Diego, CA). In brief, trypsinized cells were incubated with an anti-CD34 (rat) antibody for 30 min, followed by incubation with anti-rat magnetic beads for 30 min, at 4°C. The mixed cells, in 500 μL of separation buffer, were applied onto a MACS Column. The enriched and expanded cells were analyzed for CD34 positivity as well as other surface markers: CD45, CD44, CD29, CD38, CD3, Lin, spinocerebellar ataxia type 1 (Sca-1), thymocyte antigen 1 (Thy 1).1, c-kit, and CD41 (antibodies are listed in Table 1) by flow cytometry analysis. Immunoglobulin G (IgG) isotype was used as a negative control. Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Table 1.
Antibodies
| Name | Sources | Working Dilution |
|---|---|---|
| B-catenin | Santa Cruz Biotechnology, Dallas, TX | 200 |
| CD3 | BD Biosciences, San Jose, CA | 50 |
| CD4 | Santa Cruz Biotechnology, Dallas, TX | 100 |
| CD8 | Santa Cruz Biotechnology, Dallas, TX | 100 |
| CD34 | Biolegend, San Diego, CA | 100 |
| CD38 | EMD Millipore, Billerica, MA | 50 |
| CD41 | Biolegend, San Diego, CA | 100 |
| CD44 | Biolegend, San Diego, CA | 100 |
| CD105 | BD Biosciences, San Jose, CA | 100 |
| CD117 (c-kit) | Biolegend, San Diego, CA | 100 |
| EpCAM | Santa Cruz Biotechnology, Dallas, TX | 200 |
| fgf9 | Santa Cruz Biotechnology, Dallas, TX | 200 |
| LGR5 | Santa Cruz Biotechnology, Dallas, TX | 100 |
| lin | BD Biosciences, San Jose, CA | 50 |
| pan-keratin | Novus Biologicals, Littleton, CO | 250 |
| Sca-1 | BD Biosciences, San Jose, CA | 100 |
| TCR | Biolegend, San Diego, CA | 100 |
| Twist | Santa Cruz Biotechnology, Dallas, TX | 100 |
Fgf9, fibroblast growth factor 9; LGR5, leucine rich repeat containing G protein-coupled receptor 5; Sca1, spinocerebellar ataxia type 1; TCR, T cell receptor.
Immunofluorescent staining
In brief, rehydrated paraffin- or fixed frozen-tissue sections at 5-μm in thickness were blocked with 1% normal horse serum for 30 min. The sections were stained with specific primary antibodies and fluorescence-conjugated secondary antibodies with adequate PBS wash. The samples were mounted with DAPI (4,6-diamino-2-phenylindole dihydrochloride; Vector Laboratories, Burlingame, CA) containing sealant. Mouse IgG and rabbit IgG antibodies were used as negative controls. The stained sections were viewed with a Leica DMRA microscope.
Enzyme-linked immunosorbent assay for cell secreted fibroblast growth factor 9 protein
In vitro expanded BDPCs were cultured with α-MEM without serum for 2 days. Cell culture supernatants with soluble Fgf9 were collected, and Fgf9 was quantified by enzyme-linked immunosorbent assay (ELISA) (Fgf9 ELISA kit; Abcam) according to the manufacturer's protocol. The Fgf9 concentrations are calculated at per 104 cells at a given time.
Epithelial-related gene expression profile
Total RNA was extracted from the expanded BDPCs using Trizol reagent (Invitrogen, Carlsbad, CA). Synthesis of cDNA was completed using a SuperScript II reverse transcriptase (Life Technologies, Carlsbad, CA). Then, reverse transcription polymerase chain reaction (RT-PCR) was completed using 1,000 ng template cDNA with PCR Master Mix (Promega, Madison, WI). Table 2 lists the primer sequences for the targeted genes.
Table 2.
Primers for polymerase chain reaction
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | ACGGCTACCACATCCAAGGA | CAATTACAGGGCCTCGAAAGAG |
| eGFP | GCAAGCTGACCCTGAAGTTCATC | TCACCTTGATGCCGTTCTTCTG |
| HNF4α | TCTTCTTTGATCCAGATGCC | GGTCGTTGATGTAATCCTCC |
| MMP9 | CTTGAAGTCTCAGAAGGTGG | GGCTTTGTCTTGGTACTGG |
| Notch | GACAACTCCTACCTCTGCTTATGCC | TTACTGTTGCACTCGTTGACCTCG |
| Occludin | ACCTGATGAATTCAAACCCA | GTGAAGAATTTCATCTTCCGG |
| Snail | CCACTGCAACCGTGCTTTT | CACATCCGAGTGGGTTTGG |
| Vimentin | AGCAGTATGAAAGCGTGGCT | CTCCAGGGACTCGTTAGTGC |
| Wnt 1 | GGCTGCAGTGACAACATCGA | AAGGTTCATGAGGAAGCGTAGGT |
| Wnt 2b | TCTGTCTATCTTGGGCATTCTG | TTCCTTCGCTATGTGATGTTTC |
| Wnt2 | ACCTTCCTCTACCCTCAATCCT | TCACTCAGCCTCCTAAATCCAT |
| Wnt3 | TGCATCCGCTCTGACACTTAATAC | TCCAGAACAGGGCCAAAGAT |
| Wnt4 | GCCGGGCACTCATGAATC | CCCGCATGTGTGTCAAGATG |
| Wnt5a | GCTCGGGTGGCGACTTC | AATATTCCAATGGGCTTCTTCATG |
| Wnt7a | CCGCGCTATCATACTTCTCCTT | CCGCAGCCGATAATCGCATA |
| Wnt7b | CGCTACGGCATCGACTTTTCT | TCTGCCCGCCTCATTGTTG |
| Wnt9b | GGGTGTGTGTGGTGACAATCT | GGTCCTTGCTTCCTCTCTTG |
| Wnt10a | AGAACGCTTCTCTAAGGACTTTCTG | AGTCGGGAGATTTCTCAAAGTAGAC |
eGFP, enhanced green fluorescent protein; HNF, hepatocyte nuclear factor; Wnt, wingless/integrated.
Preparation of the fibrin gel and the 3-D CD34 cell construct
Fibrin gels were fabricated with fibrinogen and thrombin with a final concentration of fibrinogen at 12.5 mg/mL and thrombin at 2.5 U/mL plus CaCl2 (final concentration: 45 mM).
Then, 200 μL of the mixture was placed into each well of a 24-well plate. The solution formed a fibrin gel for 2 h at 37°C. The eBDPCs (5 × 105 in 2 mL α-MEM culture medium) were seeded on the fibrin gel in each well for one night. The eBDPCs were confluent, and the eBDPCs/vehicle construct were ready for transplantation.
Creation and treatment of full-thickness skin wound
All animal care and procedures were approved by the Stanford University Administrative Panel of Laboratory Animal Care. Thirty wild-type FVB mice (male, 10 weeks) received 6 mm in diameter full-thickness excision wounds on the mid-dorsal skin by using a biopsy punch (Acuerm, Inc., Fort Lauderdale, FL) under isoflurane anesthesia.
A donut-shaped splint (inner diameter of 10 mm; outer diameter of 14 mm) was placed around the wound area and fixed with eight interrupted 6-0 nylon sutures (Ethilon Nylon Suture; Ethicon LLC., Cornelia, GA) to prevent contraction. Then the premade eBDPCs (500,000 cells/each wound)/vehicle constructs (250 μL volume for each) was scooped out of the single well of the 24-well plate and transplanted onto the wound bed of each mouse (n = 10), respectively. Fibrin gel without eBDPCs was transplanted to the sham control mice (n = 10). The wound beds of mice (n = 10) were treated with saline as the nontherapy control. A trimmed semiocclusive dressing (Tegaderm Film 9506 W; 3 M Health Care, St. Paul, MN) was applied circumferentially around the trunk of each animal.
Wound healing analysis
Photographic images of wounds were taken every day using a digital camera (Sony Cyber-shot® DSC-TX 7, New York, NY) from a fixed distance. The ratio of wound closure area was determined by dividing the actual wound area by the original wound area. The wound was considered completely closed when the wound area was grossly equal to 0.
The mice were euthanized by CO2 asphyxiation on day 25 post-treatment. The wounds and adjacent tissues (about 2.5 cm2) were harvested. Half of each wound tissue sample was snap-frozen in liquid nitrogen. The remaining half was fixed in 10% (v/v) buffered formaldehyde for paraffin embedding. Paraffin slides were sliced into 5 μm in thickness and stained with hematoxylin and eosin to examine local cell degeneration and inflammation. Masson's trichrome stains show collagen and connective tissues. Histological parameters were used to assess re-epithelialization, dermal regeneration, fibrous deposition, and inflammation. The regeneration of skin appendages was assessed by counting the number of HFs or sebaceous glands in the wound bed. Some paraffin sections were further processed for immunohistochemistry (IHC) or immunofluorescence (IF) labeling.
Statistical analysis
Histological images were analyzed to calculate wound closure rate and percentage of scar area by using image analysis software (ImageJ; National Institutes of Health, Bethesda, MD). Collagenous matrix deposition in the wound was determined by red region (cellular structure area) divided by blue region (collagenous matrix deposition) using the split color channel method with the measure setting in ImageJ. Statistical analysis was performed using a paired two-tailed Student's t-test to compare two groups. A value of p < 0.05 was considered statistically significant.
Results
Characterization of BDPCs
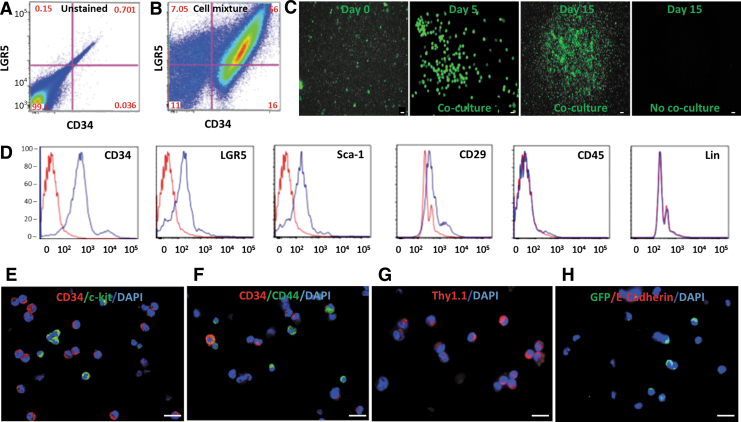
Freshly isolated cells from the peripheral blood of adult L2G mice demonstrated a highly heterogeneous cell mixture. More than 60% of the cells were double positive for surface markers CD34 and LGR5 after depletion of erythrocytes and platelets (Fig. 1A, B). The mixed green fluorescent protein (GFP)+ cell fraction rapidly expanded during coculture with the inactivated AML12 cell line. However, cells not in the coculture system did not expand (Fig. 1C).
Figure 1.
Isolation and characterization of CD34+ BDPCs. (A, B) Freshly isolated cells from the peripheral blood of an L2G adult mouse present a heterogeneous cellular mixture with >60% of cells co-expressing surface markers CD34 and LGR5 by flow cytometric analysis. (C) GFP+ cells from L2G mice rapidly expanded when cocultured with AML12 cells on day 5 and 15 in comparison with the cells on day 0. However, GFP expression in L2G cells without coculture ceased by day 15. (D–H) Flow cytometry analysis and immunofluorescent staining demonstrate that the majority of the BDPCs express surface markers of CD34, LGR5, Sca-1, CD29, c-kit, CD44, and Thy1.1, but are negative for CD45, lineage markers (lin) and E-cadherin. Scale bar = 5 μm. AML12, alpha mouse liver 12; BDPC, blood-derived CD34+ precursor cells; GFP, green fluorescent protein; LGR5, leucine rich repeat containing G protein-coupled receptor 5; Sca-1, spinocerebellar ataxia type 1; Thy1, thymocyte antigen 1.
The phenotypic analysis of expanded BDPCs demonstrated that the cells expressed surface markers characteristic of stem cells and were negative for lymphocyte lineages. Flow cytometry analysis and immunofluorescent staining demonstrated that the majority of the expanded BDPCs expressed stem cell markers CD34, LGR5, Sca-1, c-kit, CD44, CD29, and Thy1.1. However, the leukocyte lineage markers, such as CD45 and Lin, were all negative (Fig. 1D–G). E-cadherin was also negative (Fig. 1H).
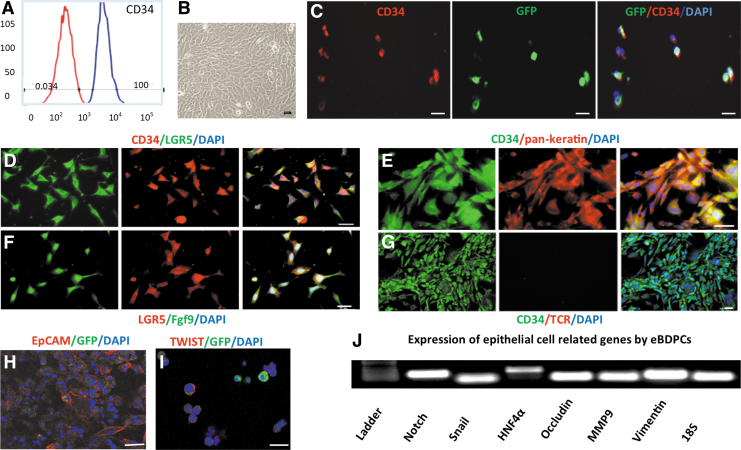
Enrichment of expanded BDPCs
Expanded BDPCs were enriched for CD34+ cells with MACS to reach 100% purity (Fig. 2A). BDPCs cocultured with inactivated AML12 cells had pronounced morphological changes, including increased cytoplasmic volume, flat and elongated shape, and decreased nuclei to cytoplasm ratio (Fig. 2B). Co-expression of CD34 and GFP confirmed that the enriched cells were L2G mouse-derived cells (Fig. 2C). Besides continued co-expression of LGR5 (Fig. 2D), the CD34+-enriched BDPCs expressed typical epithelial markers: pan-keratin and epithelial cell adhesion molecule (EpCAM) (Fig. 2E, H). They also expressed Fgf9 (Fig. 2F). However, the expanded BDPCs were negative for T cell receptor (TCR) antibody, a specific marker for γδ T lymphocytes (Fig. 2G). Twist protein expressed in the CD34+-enriched BDPCs (Fig. 2I). PCR analysis demonstrated expression of epithelial transition-related genes, such as Notch, Snail, HNF4α, Occludin, MMP9, and Vimentin (Fig. 2J). Hence, these CD34+ BDPCs are designated epithelial-like BDPCs (eBDPCs).
Figure 2.
Enrichment and epithelial-like differentiation of CD34+ BDPCs. (A) After MACS, enriched CD34+ cells achieved near 100% purity in 10 days of expansion. (B) BDPCs cocultured with AML12 cells demonstrate the morphological changes, including increased volume, a flat and elongated shape, and a decreased nuclei to cytoplasm ratio. (C) Co-expression of CD34 and GFP confirms that the enriched cells retain GFP expression. (D) Immunofluorescent double staining demonstrates that enriched BDPCs co-express CD34 and LGR5. (E) The CD34+ cells express epithelial marker pan-keratin. (F) LGR5 positive cells synthesize and express Fgf9. (G) The epithelial-like differentiated BDPCs (eBDPCs) are negative for TCR antibody, a specific marker for γδ T lymphocytes. (H) Clear surface expression of epithelial cell marker EpCAM on the cellular membrane of cultured eBDPCs. (I) Twist expressed in eBDPCs. (J) PCR analysis reveals the active expression of epithelial transition-related genes, such as Notch, Snail, HNF4α, Occludin, MMP9, and Vimentin. Scale bar = 10 μm. EpCAM, epithelial cell adhesion molecule; Fgf9, fibroblast growth factor 9; HNF, hepatocyte nuclear factor; MACS, magnetic-activated cell sorting; TCR, T cell receptor.
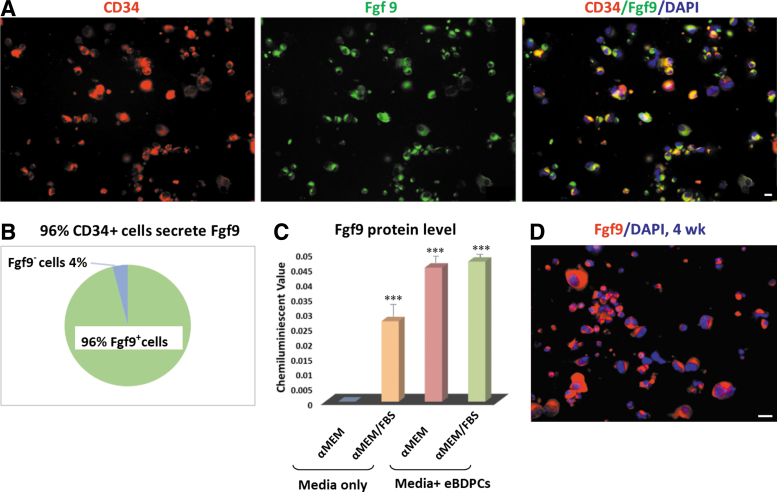
Fgf9 in vitro testing
The eBDPC cells were shown to synthesize and secrete Fgf9 protein (Fig. 3A). The majority (>96%) of eBDPCs expressed Fgf9 protein after 2 weeks of coculture with inactivated AML12 cells (Fig. 3B). A significant increase in Fgf9 expression in eBDPC culture medium with or without FBS was shown by ELISA (FBS, p < 0.001, Fig. 3C). IF staining demonstrated that the Fgf9 protein was primarily located in the cytoplasm of eBDPCs (Fig. 3D).
Figure 3.
eBDPCs express Fgf9 protein in vitro. The eBDPCs synthesize and secrete Fgf9 protein. (A) The co-expression of CD34 (red fluorescent) and Fgf9 protein (green fluorescent) in eBDPCs in 2 weeks of coculture. (B) More than 96% of CD34+ cells express Fgf9 protein among the total eBDPCs. (C) ELISA shows a dramatic increase of Fgf9 protein level in media collected from eBDPC culture dish. The addition of fetal bovine serum did not influence the level of Fgf9 in the supernatant. (***p < 0.001) (D) Fgf9 protein mainly located in the cytoplasm and increases over time (red). Scale bar = 10 μm. ELISA, enzyme-linked immunosorbent assay.
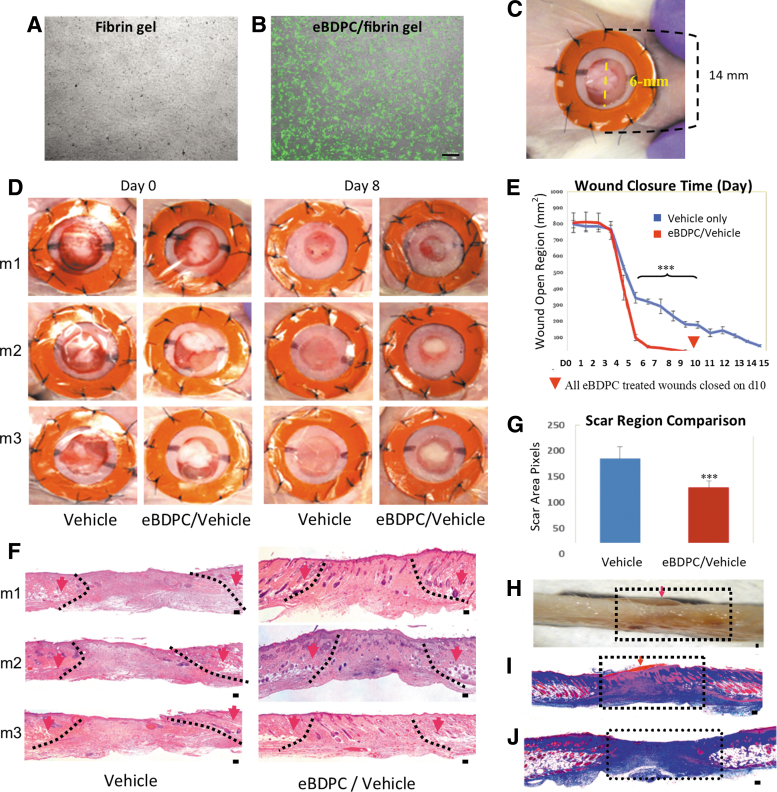
In vivo wound analysis
The GFP+ eBDPCs grew robustly to confluency in the culture dish after seeding in the gel (Fig. 4A, B). Silicone splints fixed around the wounds effectively minimized wound contraction (Fig. 4C). Thus, wound closure was largely dependent upon dermal and epidermal cells merging from either wound edge and the eBDPC/vehicle constructs. An initial undifferentiated CD34+ control did not show any significant influence on wound healing (data not shown).
Figure 4.
eBDPC treatment enhances wound healing. (A) Fibrin gel without cells in culture dish (B) eBDPCs (GFP+) present at confluent growth on the fibrin gel in culture under UV microscope before transplantation. (C) A 6 mm full-thickness excision wound was created with a donut-shaped splint fixed around the wound to inhibit contraction. (D) Morphological images showed that eBDPC treatment significantly accelerated the wound healing rate. (E) Cumulative image analysis indicated a significant improvement (***p < 0.01) in wound closure time on days 3, 5, 7, and 9 in eBDPC-treated wounds compared with the vehicle-treated control wounds. All eBDPC-treated wounds closure time is day 10 (red arrow head shows). (F, G) Histological evaluation (H&E) of samples collected on day 25 demonstrate that eBDPC-treated wounds healed with markedly less scar region (***p < 0.01) and almost full regeneration of the epidermis and dermis layers. Within the wound boundary (defined by the disrupted adipose and muscle tissues), eBDPC-treated wounds showed enhanced re-epithelialization and more cellular activities compared with that of vehicle-treated animals. (H) A sagittal section from the healing area of eBDPC/vehicle (day 25) demonstrates that the residual construct of cell/gel is still covering the entire wound region (arrow). Many cellular structures are observed (in scarlet red) migrating from the top of cell/gel construct (arrow) down into the wound area. (I) Masson trichrome staining of the same region as previously shown (J) The vehicle-treated wound (day 25) demonstrated a large area of nonstructural collagen mass in the healing area. Scale bar = 50 μm.
The eBDPC-treated wounds exhibited markedly reduced closure time, scar area, and fibrous tissue deposition. Morphological image analysis revealed that eBDPC treatment resulted in wound closure as early as day 3 postwounding and became more evident on day 8 (Fig. 4D) with statistically significant improvement in wound healing on days 3, 5, 7, and 9 compared with the vehicle-treated group. All eBDPC-treated wounds closed by day 10, whereas vehicle-treated wounds did not close until day 15 (Fig. 4E). Histological evaluation on all the samples from the wound area collected on day 25 after surgery showed that eBDPC-treated wounds healed with little to none scar formation. The wound areas recovered a nearly normal epidermal and dermal layer histological structure. The eBDPC-treated wound beds showed a more significant amount of cellular regeneration than that of the vehicle-only treated wounds (Fig. 4F). The wound surface areas of the eBDPC treatment were significantly smaller compared with the vehicle-treated wounds (Fig. 4G). Trichrome staining showed many HF-like structures from the top of the eBDPC/vehicle composite were migrating into the wound area (Fig. 4H, I). In contrast, the vehicle-treated wounds formed a large area of nonstructural collagen deposition (Fig. 4J). The disrupted subdermal adipose and smooth muscle tissues were used to define the edges for all wound analysis.
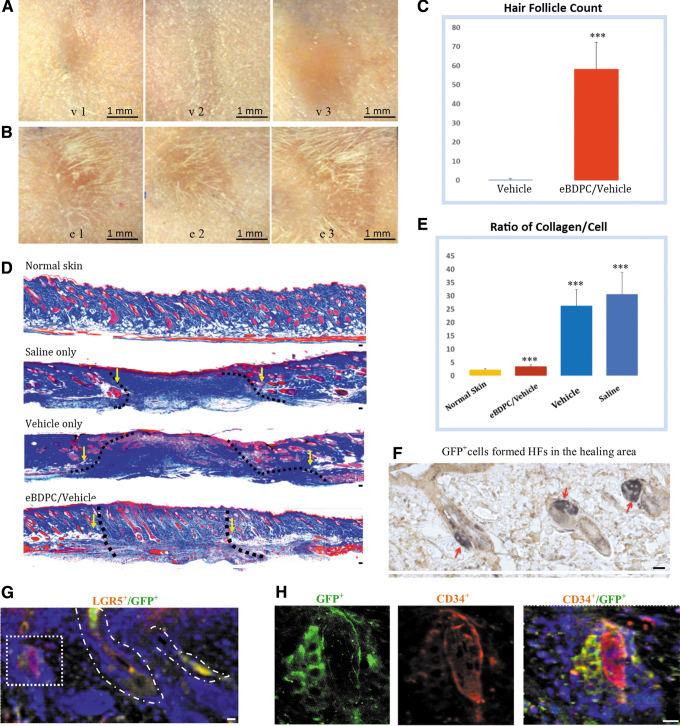
Hair follicle neogenesis
The unique discovery of this study is the regeneration of HF in the healing region of the full-thickness skin wounds treated with eBDPCs. Daily photographs showed that all the vehicle-treated wounds demonstrated normal scar formation without hair growth (Fig. 5A, v1–v3). However, gross new hairs were present in each eBDPC-treated wound area, either along the edge or in the center (Fig. 5B, e1–e3). HF counts showed a marked increase in eBDPC/vehicle-treated wounds compared with vehicle-treated wounds (Fig. 5C). To investigate the amount of collagen in the wound tissue by Masson's trichrome staining, blue-stained structures represent collagen deposition and connective tissue matrix. Tissues stained with red, including cells and cellular structures such as HFs, sebaceous glands, and blood vessels (Fig. 5D). Normal unwounded skin of FVB mice demonstrates an ordered distribution of epidermal appendages in a reticular ECM in the dermis. Saline-treated wound healed with a large densely packed amount of collagenous protein filling the wound gap with neither tissue structure nor HFs. Vehicle-treated wound also formed a large area of dense collagen deposition. The vehicle, fibrin gel caused inflammatory cell infiltration adjacent to the treatment region. Analysis of the ratio of collagen/cellular structure in the wound area demonstrated that eBDPC-treated wounds had significantly reduced fibrous tissue and increased numbers of HFs (Fig. 5E). The GFP antibody was used to identify whether eBDPCs were incorporated into the local healing tissue. In the eBDPC-treated wounds, GFP+ cells were observed in the HF bulge and sebaceous gland of pilosebaceous units (Fig. 5F). Some of the CD34+ cells in the wound HFs co-expressed GFP (Fig. 5G, H).
Figure 5.
HFN post-eBDPC treatment. (A) The wound healing area of the vehicle-treated wounds (upper panel, v1–v3) healed with large or contracted scar region without hair formation. (B) The eBDPC-treated wound area (lower panel, e1–e3) demonstrates hair growing around the wound edge or in the center of the wound region. (C) HF count showed increased new HFN in the wound area on the histological section (***p < 0.001). (D) With trichrome staining, the normal skin of FVB mouse demonstrates an intact clearly layered cellular structures. Saline-treated wound healing shows a large amount of collagen mass lacking cellular structures filling the wound gap. Vehicle-treated wound also shows a large area of collagen deposit. The fibrin gel caused some inflammatory cell infiltration in the wound area. The eBDPC-treated mice show a reduction in fibrous tissue deposits, and a high density of HFs formed in the scar area. The disrupted subdermal adipose and smooth muscle tissues are used to define the edges of all wounds (yellow arrows and dotted line). (E) The graph shows a significant lower collagen deposit in the eBDPC treatment group (***p < 0.01). (F) IF staining shows GFP+ cells (green) in HFs within the wound healing area. (G) GFP+ cells co-express LGF5 (red) in the HFs of the wound area. (H) Two HFs are seen in the wound area. Both HFs are CD34+, but only the one on the left strongly expresses GFP indicating that the newly formed HF is from transplanted cells. Scale bar = 20 μm except for specifically labeled. FVB, Friend leukemia virus B; HFs, hair follicles; IF, immunofluorescence.
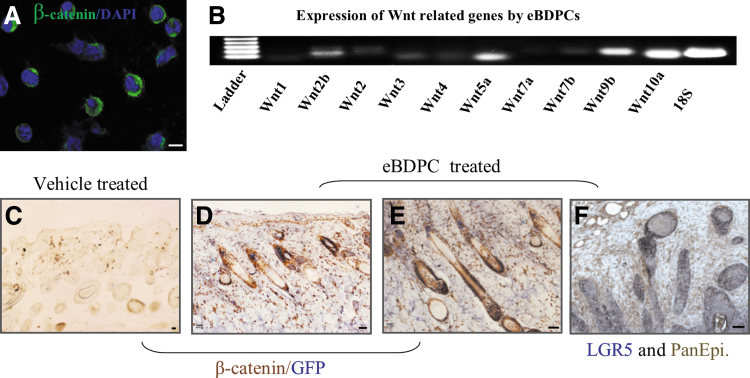
Beta-catenin (β-catenin), an important wingless/integrated (Wnt) family member, showed strong expression in eBDPCs during the epithelial-like differentiation in vitro (Fig. 6A) and during HF regeneration in vivo. Specifically, Wnts, including Wnt2, Wnt5a, Wnt9b, and Wnt10a, were shown by RT-PCR analysis to be actively expressed in eBDPCs during their transition process from peripheral blood to regenerate skin in the wound healing environment (Fig. 6B). In vivo tracking of the transplanted eBDPCs showed that Wnt and beta-catenin were actively expressed in the wound bed and adjacent region as well as in cells that co-express with GFP or Pan-epithelial marker in HFs (Fig. 6C–F).
Figure 6.
Wnt genes are activated in the process of wound healing and HFN. (A) The eBDPCs expressed β-catenin during the epithelial-like differentiation in vitro. (B) The active expression of several genes within the Wnt family, such as Wnt 2, Wnt 2b, Wnt 3, Wnt 4, Wnt5a, Wnt 7b, Wnt9b, and Wnt10a was demonstrated by RT-PCR in eBDPCs in vitro. (C–E) β-catenin (brown) positively expresses in the HFs within the area of wound regeneration (day 25). GFP+ cells (gray/blue) present mostly in the HFs by double immunostaining. (F) LGR5 (gray) co-express with pan-epithelial antigen (brown). Scale bar = 10 μm. RT-PCR, reverse transcription polymerase chain reaction; Wnt, wingless/integrated.
Discussion
Characterization of BDPCs
The CD34 surface marker is associated with primitive hematopoietic stem cells (HSCs) as well as a major marker for HF stem cells.11,14 It is hypothesized that LGR5 is a HF stem cell marker expressed in a multipotent stem cell population to give rise to new HFs.22 Co-expression of CD34 and LGR5 in most of the BDPCs isolated from peripheral blood prompted the investigation of these cells ability to induce HF regeneration during the wound healing process.
Data from flow cytometry and IF analysis showed that the expanded BDPCs are CD34+/LGR5+/CD105+/CD44+/CD73/c-kit+/Sca-1+/Thy1.1+/CD45−/lineage negative (lin−). The lin− cell population from bone marrow and blood was reported to contain different stem cell precursors.23 Expanded BDPCs are within the lin− population, which supports a precursor feature of the cells. BDPCs positively express CD44, CD73, CD105, Thy1.1 and Sca-1 and negatively express leukocyte marker CD45. This marker expression panel is characteristic of mesenchymal stem cells (MSCs). The BDPCs may be a group of very early or primitive precursor cells derived from adult peripheral blood. The negativity for Lin and CD45 also demonstrates that these BDPCs are not lymphocytes.
In vitro expansion of BDPCs
Previous studies have shown primitive HSCs isolated from the bone marrow possess the ability to differentiate into distinct cell types, including skin tissue cells.24 However, the differentiation process from hematopoietic to nonhematopoietic tissue is rather slow. The cells need to adapt to a foreign environment and a significant challenge to HSC expansion.25 The liver is the primary site of hematopoiesis during mammalian prenatal development. The hepatic hematopoiesis is proved to persist into adulthood.26–28 In the liver, hepatocytes are the primary parenchymal cells and execute an essential metabolic function. A coculture system with AML12 cells was established to explore whether the hepatic environment may be beneficial to BDPCs proliferation and differentiation. The results show that CD34+ BDPCs can be induced to proliferate rapidly and differentiate into epithelial-like cells in coculture with AML12 cells within 2 weeks. After enrichment through MACS, CD34+ cells reached 100% purity in the expanded BDPC group.
Epithelial differentiation of BDPCs
Epithelial cells are stationary and characterized by an apical-basal polarity, tight junctions, and the expression of cell–cell adhesion markers. In contrast, primitive epidermal stem cells are without cell–cell contacts, invade through the ECM, and express markers such as vimentin, fibronectin, Twist, and Snail.29,30
BDPCs are likely early primitive stem cells. Although BDPCs are CD34+, which usually represents the cells with a hematopoietic origin, they are also positive for most MSC markers. Furthermore, eBDPCs demonstrated increased epithelial-related gene activity as well as specific primitive markers, including vimentin, Twist, and Snail, during coculture with hepatocytes.
Mesenchymal–epithelial transition (MET) is a biological process that marks the transition from motile spindle-shaped mesenchymal cells to polarized epithelial cells representing a profound gene expression reprogramming.31 The hepatocyte nuclear factor (HNF) 4α is a crucial master factor for MET,32,33 acting as a positive regulator of epithelial gene transcription and direct repressor of mesenchymal genes. Both BDPCs and eBDPCs strongly express HNF4α, consistent with the transition of primitive stem cells toward differentiated epithelial cells. Epithelialized BDPCs highly expressed transcriptional regulators, such as Snail, and downstream regulatory molecules such as β-catenin, targeting several epithelial genes, which suggest BDPCs pass through a similar transcriptional pathway as mesenchymal stem cells during early differentiation toward an epithelial phenotype in the hepatic environment.
EpCAM is a transmembrane glycoprotein mediating cell-to-cell adhesion in epithelia.34 Occludin is also an essential protein in cell–cell tight junction and commonly function in most epithelial tissues.35 Occludin was strongly expressed in eBDPCs. Keratin 5 is a manifested marker for epithelial stem cells in addition to LGR5. The eBDPCs expressed EpCAM and pan-keratin, which include keratin 5 and 8. The expression of these markers identifies eBDPCs that have completed epithelial differentiation.
Matrix metalloproteinases (MMPs) display a crucial regulatory function during the wound-healing process, including inflammation, angiogenesis, and remodeling.36 Wounds treated with eBDPCs showed significantly increased MMP9 expression during repair, suggesting an alteration of MMP activity is a possible mechanism supporting reduced scar formation.
Wounds treated with eBDPCs demonstrated a significantly accelerated wound closure rate. Furthermore, the quality of the repair was markedly improved. The eBDPC-treated wounds presented a nearly normal pattern and density of dermal ECM fiber bundles that was rapidly restored after wound closure. Lastly, eBDPC treatment resulted in the restoration of a nearly normal HF density and pattern in the wound recovering region. Transplanted GFP+ cells were found in the HF bulge and some sebaceous glands in the wound area suggesting that incorporation of either eBDPCs or their daughter cells was essential for HF repair during the wound healing process.
Fgf9 and HFN
FGF is a large group of potent mitogens and chemoattractants that are activated during the wound healing process.37 It is recently discovered that Fgf9, secreted by epithermal γδ T lymphocytes, can induce and mediate HFN in skin wounds of adult mice.10
The results demonstrate that eBDPCs derived from adult peripheral blood synthesize and secrete Fgf9 in vitro. When transplanted into full-thickness excisional wounds, eBDPCs enhanced wound healing and participated in HFN. This result is consistent with the discovery that Fgf9 positive cells participate in HF regeneration or neogenesis (HFN). A previous study found that peripheral blood γδ T lymphocytes in humans produce Fgf9 protein.17 However, BDPCs or eBDPCs are not lymphocytes, confirmed by negative expression of surface markers for lymphocytes, such as CD3, CD4, CD8a, CD45, and lin. The further data also showed that BDPCs did not express TCR protein, which is a specific marker for γδ T lymphocytes. This study suggests that BDPCs are CD34+ HSC-like precursor cells with similar surface markers to HF epidermal stem cells and a potential recruitment source for HFN during damaged tissue regeneration.
Gay shows that Fgf9 amplifies Wnt signal pathway activity in wound regeneration and that dermal γδ T cells initiate an Fgf9/Wnt pathway feedback loop for HF regeneration. This activation induces further expression of Fgf9 that serves to perpetuate and amplify Wnt activation. The activities of Wnt/β-catenin signal pathway were investigated to better understand the molecular mechanism behind eBDPCs induction of HFN and epidermal appendage regeneration. Wnt's increased activity at an early coculture stage in vitro and β-catenin strong expression in eBDPCs during the epithelial-like differentiation and during HF regeneration in vivo demonstrate the significant role of Wnt in wound healing. We hypothesize that Wnt gene activation leads to a critical role during HFN. During differentiation into epithelial-like cells, BDPCs highly expressed LGR5, Twist, and Notch, and play key roles in the Wnt pathway.38,39 This observation lends further support to the contention that the Wnt signal pathway is actively involved.
In this study, CD34+ cells from adult peripheral blood were found to induce HFN and accelerate wound closure in mouse full-thickness skin wounds. The results are consistent with previous findings that showed the use of bone marrow stem cells to treat skin wounds.40–42 This study is also consistent with the observation that fluorescence-activated cell sorting-sorted CD34+ stem cells were shown to regrow sebaceous glands in nude mice skin grafts.43,44
The eBDPCs are attracted to the HF bulge where they support, augment, and perform epidermal stem cell functions during repair of the HF. Accelerated ECM repair rate and standard dermal architecture restoration in the dermis demonstrate the regulatory effects of eBDPCs. We hypothesize that BDPCs in the adult peripheral blood may function as a “rescue stem cell.” The BDPCs location within the circulating blood supports the advantage for a more rapid localization to the site of injury to initiate the regenerative process. The future challenge is to define the molecular mechanisms of eBDPCs associated with HFN and wound regeneration. The BDPCs isolated and expanded from adult peripheral blood may provide a new cell-based treatment strategy for HF neogenesis and skin wound healing.
Innovation
In this study, we describe a group of hematopoietic stem cell (HSC)-like precursor cells from the peripheral blood of adult mice with the surface markers: CD34+, LGR5+, CD44+, c-kit+, lin−, and E-cadherin−. The cells, termed “blood-derived precursor cells” (BDPCs), can be further differentiated into epithelial-like cells (eBDPCs) and secrete Fgf9 protein in vitro. Accelerated healing, enhanced skin structure regeneration, and less dermal scar formation were observed after transplantation of eBDPCs into full-thickness skin wounds. It was demonstrated that eBDPCs directly participate in HFN and enhance dermal regeneration.
Key Findings
Nondermal-derived CD34+ cells from the adult unmobilized peripheral blood are capable of directly participating in skin wound regeneration.
An in vitro technical platform for these cells' expansion and differentiation was successfully established.
The in vitro expanded and differentiated epithelial-like cells (eBDPCs) greatly enhanced wound healing and directly contributing to skin regeneration and HFN.
Abbreviations and Acronyms
- γδ T
gamma delta T lymphocyte
- α-MEM
minimum essential medium
- AML12
alpha mouse liver 12
- BDPC
blood-derived CD34+ precursor cells
- eBDPC
epithelial-like cells
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- EpCAM
epithelial cell adhesion molecule
- FBS
fetal bovine serum
- Fgf9
fibroblast growth factor 9
- FVB
Friend leukemia virus B
- GFP
green fluorescent protein
- HF
hair follicle
- HFN
hair follicle neogenesis
- HNF
hepatocyte nuclear factor
- IF
immunofluorescence
- IgG
immunoglobulin G
- LGR5
leucine rich repeat containing G protein-coupled receptor 5
- lin−
lineage negative
- MACS
magnetic-activated cell sorting
- MET
mesenchymal–epithelial transition
- MMP9
matrix metallopeptidase 9
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription polymerase chain reaction
- Sca-1
spinocerebellar ataxia type 1
- TCR
T cell receptor
- Thy1
thymocyte antigen 1
- Wnt
wingless/integrated
Acknowledgments and Funding Sources
We thank Marty Bigos for his help with the flow cytometry sorting and analysis. Dr. K.J. Huang helped to make the fibrin gel. We highly appreciate the comments and opinions from Dr. Michael E. Friduss, especially his professional experience with the clinical problem addressed. We give special thanks to Dr. Stacy Townsend. Her broad biotechnology background and excellent proofreading skills significantly improved the revision. This study was supported by research funding from the National Institutes of Health (NIH) grant R01 GM087609 (to H.P.L.), a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (to H.P.L.), and the Hagey Laboratory for Pediatric Regenerative Medicine.
Authors' Contributions
S.L. designed and performed the experiments; M.H. participated in the experimental design and performance. Hu also performed major data analysis and article writing. H.P.L. planned and coordinated the project and edited the article. All the authors edited and approved the final article.
Author Disclosure and Ghostwriting
All the authors edited and approved the final article. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article. All authors have concurred with the submission, and none of the data have been previously reported or are under consideration for publication elsewhere. S.L. and M.H. work at APstem Therapeutics, Inc., which has licensed the IP of this BDPC technology from Stanford University, Office of Technology licensing. The authors are trying to work out therapies for future patients. H.P.L. has no competing financial interests to declare.
About the Authors
Shaowei Li, MD, CTO, APstem Therapeutics, Inc. has expertise in wound healing and stem cell research. Li used to work at Stanford University with Dr. Lorenz when the work of this article was done. Min Hu, MD, PhD, CEO, APstem Therapeutics, Inc. is a former senior scientist at Stanford University and is an expert in stem cell R&D and cell therapies. Hu used to work at Stanford University with Dr. Lorenz when the work of this article was done. H. Peter Lorenz, MD, is a professor of surgery, plastic and reconstructive, Stanford University Medical Center.
References
- 1. Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med 2014;20:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci 2015;16:25476–25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 2012;12:170–180 [DOI] [PubMed] [Google Scholar]
- 4. Purba TS, Haslam IS, Poblet E, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays 2014;36:513–525 [DOI] [PubMed] [Google Scholar]
- 5. Sorice S, Rustad KC, Li AY, Gurtner GC. The role of stem cell therapeutics in wound healing: current understanding and future directions. Plast Reconstr Surg 2016;138(3 Suppl):31S–41S [DOI] [PubMed] [Google Scholar]
- 6. Miller SJ. CD34, stem cells, and the skin. Arch Dermatol 1994;130:624–626 [PubMed] [Google Scholar]
- 7. Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 2003;40:368–377 [DOI] [PubMed] [Google Scholar]
- 8. Zhu B, Nahmias Y, Yarmush ML, Murthy SK. Microfluidic isolation of CD34-positive skin cells enables regeneration of hair and sebaceous glands in vivo. Stem Cells Transl Med 2014;3:1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm 2017;2017:5217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gay D, Kwon O, Zhang Z, et al. Fgf9 from dermal gamma delta T cells induces hair follicle neogenesis after wounding. Nat Med 2013;19:916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010;10:467–478 [DOI] [PubMed] [Google Scholar]
- 12. Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 2012;1:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddi AS, Ende N. The use of human umbilical cord blood for wound healing, burns, and brain injury in combat zones. Mil Med 2011;176:361–363 [DOI] [PubMed] [Google Scholar]
- 14. Korbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002;346:738–746 [DOI] [PubMed] [Google Scholar]
- 15. Fathke C, Wilson L, Hutter J, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ismail AM, Abdou SM, Aty HA, et al. Autologous transplantation of CD34 bone marrow-derived mononuclear cells in management of non-reconstructable critical lower limb ischemia. Cytotechnology 2016;68:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chotinantakul K, Dechsukhum C, Dejjuy D, Leeanansaksiri W. Enhancement of wound closure in diabetic mice by ex vivo expanded cord blood CD34+ cells. Cell Mol Biol Lett 2013;18:263–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science 2014;346:941–945 [DOI] [PubMed] [Google Scholar]
- 19. Barnes LA, Marshall CD, Leavitt T, et al. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care 2018;7:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 2017;34:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S, Huang KJ, Wu JC, et al. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med 2015;4:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 2008;40:1291–1299 [DOI] [PubMed] [Google Scholar]
- 23. Hubin F, Humblet C, Belaid Z, et al. Murine bone marrow stromal cells sustain in vivo the survival of hematopoietic stem cells and the granulopoietic differentiation of more mature progenitors. Stem Cells 2005;23:1626–1633 [DOI] [PubMed] [Google Scholar]
- 24. Ramanauskaite G, Kaseta V, Vaitkuviene A, Biziuleviciene G. Skin regeneration with bone marrow-derived cell populations. Int Immunopharmacol 2010;10:1548–1551 [DOI] [PubMed] [Google Scholar]
- 25. Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci 2012;1266:138–150 [DOI] [PubMed] [Google Scholar]
- 26. Muench MO, Beyer AI, Fomin ME, et al. The adult livers of immunodeficient mice support human hematopoiesis: evidence for a hepatic mast cell population that develops early in human ontogeny. PLoS One 2014;9:e97312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garg V, Garg H, Khan A, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142:505–512 e1. [DOI] [PubMed] [Google Scholar]
- 28. Newsome PN. SOS liver damage; calling all haematopoietic stem cells. Liver Int 2014;34:1–3 [DOI] [PubMed] [Google Scholar]
- 29. Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev Biol 2016;409:129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moldovan L, Anghelina M, Kantor T, et al. A module of human peripheral blood mononuclear cell transcriptional network containing primitive and differentiation markers is related to specific cardiovascular health variables. PLoS One 2014;9:e95124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pattabiraman DR, Bierie B, Kober KI, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 2016;351:aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takaishi M, Tarutani M, Takeda J, Sano S. Mesenchymal to epithelial transition induced by reprogramming factors attenuates the malignancy of cancer cells. PLoS One 2016;11:e0156904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Santangelo L, Marchetti A, Cicchini C, et al. The stable repression of mesenchymal program is required for hepatocyte identity: a novel role for hepatocyte nuclear factor 4alpha. Hepatology 2011;53:2063–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denzel S, Maetzel D, Mack B, Eggert C, Barr G, Gires O. Initial activation of EpCAM cleavage via cell-to-cell contact. BMC Cancer 2009;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kyoko OO, Kono H, Ishimaru K, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One 2014;9:e98016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res 2016;2016:2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yildirimer L, Thanh NT, Seifalian AM. Skin regeneration scaffolds: a multimodal bottom-up approach. T Biotechnol 2012;30:638–648 [DOI] [PubMed] [Google Scholar]
- 38. Hsu HC, Liu YS, Tseng KC, Tan BC, Chen SJ, Chen HC. LGR5 regulates survival through mitochondria-mediated apoptosis and by targeting the Wnt/beta-catenin signaling pathway in colorectal cancer cells. Cell Signal 2014;26:2333–2342 [DOI] [PubMed] [Google Scholar]
- 39. Yeh JR. A Wnt inhibitor with a twist. Chem Biol 2011;18:1518–1520 [DOI] [PubMed] [Google Scholar]
- 40. Mehanni SS, Ibrahim NF, Hassan AR, Rashed LA. New approach of bone marrow-derived mesenchymal stem cells and human amniotic epithelial cells applications in accelerating wound healing of irradiated albino rats. Int J Stem Cells 2013;6:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wan J, Xia L, Liang W, Liu Y, Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res 2013;2013:647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther 2011;11:1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 2014;344:1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu CP, Polak L, Rocha AS, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 2012;150:136–150 [DOI] [PMC free article] [PubMed] [Google Scholar]