Abstract
We investigated the mechanisms involved in the development of airway hyperresponsiveness (AHR) following exposure of mice to halogens. Male mice (C57BL/6; 20–25 g) exposed to either bromine (Br2) or Cl2 (600 or 400 ppm, respectively, for 30 min) developed AHR 24 h after exposure. Nifedipine (5 mg/kg body wt; an L-type calcium channel blocker), administered subcutaneously after Br2 or Cl2 exposure, produced higher AHR compared with Br2 or Cl2 alone. In contrast, diltiazem (5 mg/kg body wt; a nondihydropyridine L-type calcium channel blocker) decreased AHR to control (air) values. Exposure of immortalized human airway smooth muscle cells (hASMC) to Br2 resulted in membrane potential depolarization (Vm Air: 62 ± 3 mV; 3 h post Br2:−45 ± 5 mV; means ± 1 SE; P < 0.001), increased intracellular [Ca2+]i, and increased expression of the calcium-sensing receptor (Ca-SR) protein. Treatment of hASMC with a siRNA against Ca-SR significantly inhibited the Br2 and nifedipine-induced Vm depolarization and [Ca2+]i increase. Intranasal administration of an antagonist to Ca-SR in mice postexposure to Br2 reversed the effects of Br2 and nifedipine on AHR. Incubation of hASMC with low-molecular-weight hyaluronan (LMW-HA), generated by exposing high-molecular-weight hyaluronan (HMW-HA) to Br2, caused Vm depolarization, [Ca2+]i increase, and Ca-SR expression to a similar extent as exposure to Br2 and Cl2. The addition of HMW-HA to cells or mice exposed to Br2, Cl2, or LMW-HA reversed these effects in vitro and improved AHR in vivo. We conclude that detrimental effects of halogen exposure on AHR are mediated via activation of the Ca-SR by LMW-HA.
Keywords: airway hyperresponsiveness, calcium-sensing receptor, diltiazem, halogens, human airway smooth muscle cells, membrane potential, nifedipine
INTRODUCTION
The halogens chlorine (Cl2) and bromine (Br2) are produced in large quantities throughout the world and used extensively in various manufacturing processes and the sanitation of drinking water. They are very reactive gases and pose significant threat to public health when released into the atmosphere in large quantities during transportation and industrial accidents, as well as acts of terrorism (51). Even brief exposures of humans to halogen gas result in cough, headache, and irritation of the eyes, nose and upper respiratory tract (reviewed in 1, 10, 26, 32, 51).
We have previously shown that exposure of C57BL/6 mice to Cl2 or Br2 in concentrations likely to be encountered in the vicinity of industrial accidents leads to acute lung injury and significant increases of airway hyper-responsiveness (AHR) in response to methacholine challenge, which persists long after the end of the exposures (2, 3, 34, 50) We also reported that exposure of immortalized human airway smooth muscle cells (hASMC) to Cl2 resulted in increased intracellular calcium [Ca2+]i due to its influx through membrane ion channels, resulting in membrane depolarization due to the activation of Ca2+ activated chloride channels (34). These changes were shown to result from the actions of low-molecular-weight hyaluronan (LMW-HA), generated by the fragmentation of high-molecular-weight hyaluronan (HMW-HA) by reactive intermediates formed by the reaction of Br2 or Cl2 with plasmalogens (4, 15, 45).
Surprisingly, the dihydropyridine Ca2+ channel blocker nifedipine did not reverse cellular membrane potential depolarization and [Ca2+]i increase following exposure to Cl2 (34). Dihydropyridine-based Ca2+ channel blockers are used to treat systemic and pulmonary hypertension; however, only 10% to 15% of patients with pulmonary hypertension benefit from the use of this class of calcium channel blockers (43, 49). This lack of efficacy has been attributed to overexpression of the calcium sensor protein (Ca-SR) in pulmonary arterial smooth muscle cells of these patients, which lead to an increase of [Ca2+]i and exacerbation of pulmonary hypertension (49). Recent studies have shown the expression of Ca-SR in human airways of asthmatics (54). Therefore, we investigated whether exposure to the halogens Cl2 and Br2 or LMW-HA activated the Ca-SR and whether this activation was responsible for the development of AHA in vivo.
Herein, we report for the first time that exposure of hASMC, as well as mouse airway smooth muscle cells in primary culture (mpASMC) to the halogens Cl2 and Br2 leads to the upregulation of the Ca-SR protein expression, which results in increased intracellular [Ca2+] and membrane depolarization. In addition, instillation of a Ca-SR antagonist (calcilytic NPS2143) post Br2 exposure returned AHR to normal level, as did diltiazem, a nondihydropyridine calcium channel blocker; on the other hand, instillation of nifedipine after Br2 exposure, exacerbated AHR and increased mortality. Finally, our results show that the effects of Br2 and Cl2, on hASMC in vitro and in mice in vivo are mediated by the fragmentation of hyaluronan, a ubiquitous cell matrix polymer found throughout the lung, including the surface of epithelial cells, which results in the production of low-molecular-weight hyaluronan (LMW-HA), which activates the Ca-SR.
MATERIALS AND METHODS
Reagents and supplies.
SmGM-2 Smooth Muscle Cell Growth Medium was purchased from Lonza (Basel, Switzerland; cat. no. CC-3182); monoclonal anti-calcium sensing receptor antibody was purchased from Sigma-Aldrich (St. Louis, MO; cat. no. C0493); Ca-SR siRNA was obtained from Fisher (Hampton, NH; cat. no. 4392420); negative control siRNA was obtained from Fisher (cat. no. 4390843); Lipofectamine RNAiMAX Transfection Reagent was purchased from Fisher (cat. no. 13778030); NPS 2143 hydrochloride was purchased from Tocris (Minneapolis, MN; cat. no. 36-261-0R); nifedipine was obtained from Sigma-Aldrich (cat. no. N7634); diltiazem hydrochloride was obtained from Sigma-Aldrich (cat. no. 1205003); Fura2-AM was obtained from TEFLabs (Austin, TX); DMEM was obtained from Gibco (Waltham, MA; cat. no. 11965); methacholine was purchased from Sigma-Aldrich (cat. no. A2251); and HBSS, DMEM/F12, and HEPES were obtained from Gibco (cat. no. 14025, 11039, 15630, respectively. High-molecular-weight hyaluronan (Yabro) was provided free by IBSA Institut Biochimique, Lugano, Switzerland; halogen gas cylinders (Cl2 and Br2) were purchased from Airgas (Birmingham, AL); mouse monoclonal anti-actin, α-smooth muscle antibody was purchased from Sigma-Aldrich (cat no. A5228); normal mouse IgG (sc-2025) was obtained from Santa Cruz Biotechnology, Dallas TX); donkey anti-mouse IgG (H+L) (highly cross-adsorbed secondary antibody Alexa Fluor 488, A-21202 and DAPI blue stain were purchased by Thermo Fisher Scientific (VECTASHIELD, H-1500, hardset antifade mounting medium with DAPI, cat no. NC9029229).
Animals.
C57BL/6 8- to 12-wk-old male (20–25 g body wt) mice were purchased from Charles River Laboratories (Wilmington, MA). All experimental procedures involving animals were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (APN20950).
Exposure to halogens.
Adult male C57BL/6 mice (8–10 wk old; 20–25 g body wt) were exposed to Br2 or Cl2 gas (600 or 400 ppm for 30 min, respectively) in cylindrical glass chambers, two at a time, as previously described (3, 6). Control mice were exposed to air in the same experimental setup as for Br2 and Cl2. After exposure, the mice were returned to their cages and had access to food and water ad libitum. All experiments were performed 24 h postexposure. All exposures were performed between 6:00 AM and 12:00 PM Tanks were replaced when the pressure in the tanks reached 500 psi. In each case, immediately following exposure, mice were returned to room air.
Measurements of airway resistance.
At 1 h postexposure, mice received subcutaneous injections of nifedipine (5 mg/kg body wt); diltiazem (5 mg/kg body wt), or vehicle (DMSO). At 24 h postexposure, mice were anesthetized with pentobarbital sodium (50 mg/kg ip.; Vortech Pharmaceuticals, Dearborn, MI) and paralyzed with pancuronium (4 mg/kg ip; Gensia Sicor Pharmaceuticals, Irvine, CA), intubated, then connected, and mechanically ventilated by a flexiVent FX system (SCIREQ, Montreal, QC, Canada). The animals were mechanically ventilated at a rate of 160 breaths per minute at a tidal volume of 0.2 mL with a positive end-expiratory pressure of 3 cm H2O, as described previously (2). The baseline value of lung resistance (R) was set via deep inhalation controlled by the system. Lungs were challenged by increased concentrations of aerosolized methacholine (0 to 40 mg/mL) applied within 10 s of the start of each measurement. Airway responses were recorded every 15 s for a consecutive 3 min following the aerosolization of methacholine. In a number of cases, flexiVent FX measurements were performed following subcutaneous injection or nifedipine and diltiazem (5 mg/kg; 1 h after Br2 exposure) or intranasal administration of Yabro (150 μg in 50 μL of saline) or calcilytic NPS2143 (1 µM, 25 µL per nostril) or their corresponding vehicles at 1 and 23 h post Br2 and Cl2 exposures.
Cell culture.
hASMC, (a generous gift from Dr. Charles W. Emala Sr., Columbia University, NY) were cultured according to Gallos et al. (17). In brief, cells were seeded in a T25 flask containing SmBM-2 medium and SmGM-2 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin mixture of antibiotics. Cells were incubated in an atmosphere of 5% CO2–95% air at 37°C.
Primary human airway smooth muscle cells (hpASMC), isolated from lung resections incidental to patient thoracic surgery at Mayo Clinic for noninfectious, focal tumors, and were provided by Dr. Prakash (Mayo Clinic, Rochester, MN). Cells were cultured, according to the protocol by Yarova et al. (54) in DMEM/F12 mixture supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin. Cells were used from passage 2 to passage 4. The cells kept their ASM morphology and the phenotype during these passages.
Primary mouse airway smooth muscle cell isolation and culture.
Primary mouse airway smooth muscle cells (mpASMC) were isolated according to the method of Lauer et al. (33). Six mice were used for each isolation. Mice were euthanized, and the tracheas were excised and placed in Ham’s F-12 supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, and kept on ice (4°C). Under a dissecting microscope, the esophagus and surrounding connective tissues were surgically removed. The tracheas were cut longitudinally to expose the lumen and then transferred to fresh Ham’s F-12 containing 0.15% pronase (Roche Applied Science), supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, and incubated at 4°C overnight. The following day, FBS was added to the tracheas (final concentration 10%) to stop the protease digestion of the tissues. The tracheas were brushed with a cotton swab to remove the remaining adherent epithelial cells, then cut into small pieces (∼30 per trachea), and transferred to a 100-cm2 tissue culture dish for attachment and outgrowth in DMEM/F-12 supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 0.25 μg/mL amphotericin B, and 10% FBS at 37°C, in a humidified atmosphere of 5% CO2-95% air. Four days later, mpASMC outgrowth was apparent, and the residual trachea pieces were removed with sterile plastic forceps. The cells were allowed to multiply for two more days, before they were lifted with trypsin-EDTA and seeded in 175-cm2 flasks and 12-mm coverslips in 12-well pates. The cultures reached confluence 3 to 5 days from seeding.
To establish that pmASMC exhibited the proper phenotype, they were fixed with 100% methanol for 5 min at room temperature and then incubated in 1% BSA in 0.1% PBS-Tween for 1 h to permeabilize the cells and block nonspecific protein. The cells were then incubated overnight at 4°C with a mouse monoclonal α-smooth muscle actin (A5228, Sigma-Aldrich, St. Louis, MO) or with mouse IgG (sc-2025, Santa Cruz Biotechnology, Dallas, TX) as a negative control. The cells then were incubated with a secondary antibody, donkey anti-mouse IgG (H+L) (highly cross-adsorbed secondary antibody, Alexa Fluor 488, A-21202, Thermo Fisher Scientific, Waltham, MA), for 1 h at room temperature. Fluorescent mounting medium was used for counterstaining with blue color to label cell nuclei (VECTASHIELD, H-1500, hardset antifade mounting medium with DAPI, NC9029229; Thermo Fisher Scientific). Images were obtained with a Nikon Eclipse Ti microscope fitted with × 20, ×40, and × 60 oil-immersion objective and Nikon Elements software (Nikon Instruments, Tokyo, Japan) (28). Experiments were performed on cells in the second passage.
Twenty-four hours before exposure to halogens, cells were seeded in six-well plates either directly or onto 12-mm diameter coverslips at a density of 5×104 cells/cm2. The plates and the coverslips were coated with collagen (0.1 mg/mL; Sigma). The following day, cells bathed in the culture medium were exposed to Cl2 or Br2 (100 ppm for 10 min) in a cylindrical glass chamber, as described previously (34). Control cells were exposed to air. At the end of the exposure, cells were transferred into a water-jacketed incubator, set at 37°C, vented with 95% air, and 5% CO2 until they were used for various measurements described below.
Measurements of membrane potential.
One hour after exposure to Br2 or air, a coverslip with hASMC was transferred to the recording chamber on the stage of an inverted microscope (Leica DM IRB, Leica) and perfused at a rate of 1 mL/min with a saline solution of the following ionic composition (in mM): 135 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 5.5 glucose, 10 HEPES, at pH 7.4 (1 N NaOH) at room temperature. Membrane potential (Vm) was measured using an Axopatch 200B (Molecular Devices, Sunnyvale, CA) in fast current-clamp conditions. In brief, after the formation of a stable giga-seal (5–10 GΩ) between the cell membrane and the pipette, the rupture of membrane patch under the pipette allowed the reading of the cell Vm. Values were recorded and stored in a computer equipped with pCLAMP software and interfaced with Digidata 1440 A (Molecular Devices). The pipette resistance used for Vm measurements varied from 1 to 2 MΩ when filled with a saline solution of the following composition in mM: 135 KCl, 10 NaCl, 2 MgCl2, 10 glucose, 0.1 EGTA, 0.2 Na2ATP, 10 HEPES, at pH 7.2 (1 N KOH).
Measurements of intracellular [Ca2+]i.
Cellular calcium imaging was performed according to a protocol described previously (34). In brief, air- or Br2-exposed hASMC cells were seeded onto 25-mm glass coverslips. The cells were incubated with Fura2-AM (8 µg) for 20 min in HBSS containing 1.8 mM CaCl2 and 25 mM HEPES at pH 7.4. Cells were then rinsed for an additional 20 min using HBSS + HEPES buffer to remove the excess of Fura2-AM. A coverslip was then transferred to the Attofluor chamber mounted on the stage of a Nikon microscope Ti80e fitted with an ×40 oil immersion objective and controlled by Nikon elements software using a computer. Changes in [Ca2+]i were reported as the change in the emission ratio R of calcium-bound Fura2 (340 nm) to calcium-unbound Fura2 (380 nm).
Western blot analysis.
hASMC, mpASMC, and hpASMC were cultured as described above, exposed to Br2 at 100 ppm for 10 min, and then returned to culture conditions in an incubator at 37°C, with a humidified atmosphere of 95% air and 5% CO2 for 24 h. Cells were lysed in RIPA buffer in the presence of protease and phosphatase inhibitors, as described recently (55). Twenty micrograms of protein was loaded in each lane of 7.5% polyacrylamide SDS-PAGE gel, then transferred to PVDF membrane for Western blot analysis using anti-Ca-SR antibody as the primary antibody (4 µg/mL), and goat anti-mouse IgG antibody, as secondary antibody (see Reagents and supplies). A β-actin antibody was used as the loading control. Blots were quantified with a PXi imagine system equipped with GeneTools software (Syngene, Cambridge, UK).
siRNA transfection.
hASMC were transfected with Ca-SR-siRNA (25 pmol) or scrambled-siRNA (25 pmol) using Lipofectamine RNAiMAX reagent. Forty-eight hours posttransfection, cells were exposed to 100 ppm Br2 for 10 min or air, and were used for various experiments.
Measurements of hyaluronan (HMW-HA and LMW-HA) in lungs.
Hyaluronan measurements in cell-free bronchoalveolar lavage fluid (BALF) were done using a commercial ELISA kit (Echelon, San Jose, CA) as per the manufacturer’s instructions (35, 39). To verify the presence of LMW-HA, concentrated and DNase/protease-pretreated BALF was run on 1% agarose gels (Lonza, Rockland, ME), along with commercially available HMW-HA (Yabro; MW ∼1,000 kDa; a generous gift from IBSA Institute Biochimique, Lugano, Switzerland), Br2 exposed HMW-HA (1.5 mg/mL Yabro; 600 ppm Br2 for 30 min and stored for 24 h at 4°C) and hyaluronan ladders, as previously described (18, 19). In some cases, 0.5 mL of BALF from mice exposed to Br2 and returned to room air for 24 h were treated with hyaluronidase (10 U/mL; Sigma Aldrich, St. Louis, MO) overnight at 37°C before agarose gel electrophoresis.
Generation of low-molecular-weight hyaluronan.
Yabro (IBSA), at 3 mg/mL or 1.5 mg/mL was exposed to 600 ppm Br2 or chlorine for 45 min to generate small fragments or low-molecular-weight hyaluronan (LMW-HA). The breakdown of HMW-HA to LMW-HA was confirmed by running the products of Yabro exposure to halogens onto an agarose gel and stained with Stains-All for visualization. The gels show distinct HMW-HA and LMW-HA bands. The molecular weight of each band is estimated from a side ladder. Cells were treated with 150 μg/mL of LMW-HA, and the final concentration was calculated using the initial Yabro concentration of 3 or 1.5 mg/mL. No attempts were made to determine the fractions in the LMW-HA, resulting from the exposure of LMW-HA to halogens.
Statistical analysis.
Data analysis and presentation were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). All data are shown as individual values and means ± SE. Statistical significance among groups was performed with ANOVA, followed by Tukey’s test adjusted for multigroup comparisons of the Students t-test when comparing two groups. P < 0.05 was considered significant.
RESULTS
Br2 depolarized hASMC and increased [Ca2+]i.
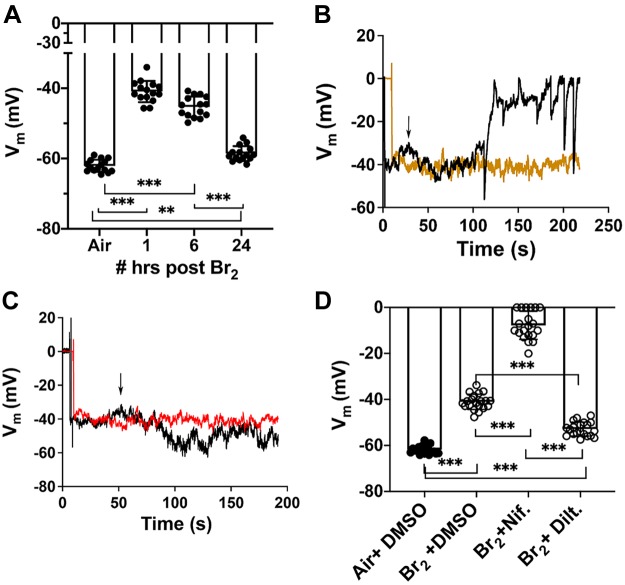
In the first set of experiments we exposed immortalized human airway smooth muscle cells (hASMC) to Br2 (100 ppm for 10 min); the medium was then removed, fresh medium was added, and cells were placed in an incubator vented with 95%O2-5% CO2 for 1–24 h prior, and Vm was measured as outlined in materials and methods section. As shown in Fig. 1A, exposure of hASMC to Br2 resulted in significant depolarization (from −60 mV to −40 mV) at 1 h postexposure, which lasted for at 24 h. Perfusion of Br2-exposed cells with 10 μM nifedipine at 1 h postexposure resulted in additional Vm depolarization (Fig. 1B). Nifedipine had no effect on air-exposed cells (data not shown). Perfusion of Br2-exposed cells with 10 μM of the nondihydropyridine L-type channel Ca+2 blocker diltiazem, at 1 h postexposure caused a significant hyperpolarization compared with Br2 (Fig. 1, C and D). However, cells were depolarized compared with the air control value. As shown in Fig. 1, B and C, perfusion with a similar concentration of vehicle (DMSO for nifedipine, saline for diltiazem) had no effect on membrane potentials.
Fig. 1.
A: exposure of human airway smooth muscle cells (hASMC) to Br2 (100 ppm for 10 min) caused membrane potential (Vm) depolarization lasting at least 24 h postexposure. Individual values and means ± 1 SE; n = 15 for each condition; ***P < 0.0001; **P = 0.002. B: effects on Vm of hASMC while perfused with 10 µM nifedipine (black trace) or DMSO (orange trace) at 1 h post-Br2 (starting at the arrow). Results of a typical experiment that was repeated at least 10 times. C: effects on Vm of hASMC while perfused with 10 µM diltiazem (black trace) or saline at 1 h after Br2 (starting at the arrow). Results are shown of a typical experiment that was repeated 10 times. D: Vm values recorded at 1–3 h after Br2 for the conditions shown in B and C. Values for nifedipine and diltiazem represent the steady-state effect obtained within 1–2 min following the perfusion with these agents. Individual values and means ± 1 SE; n = 20 for each condition; ***P < 0.0001. Statistical analysis was performed by one-way ANOVA followed by a Tukey test corrected for multiple comparisons.
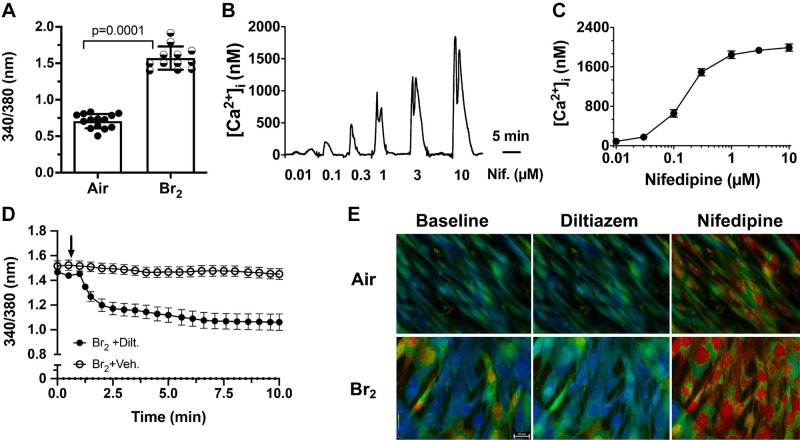
Fura2 measurements showed that exposure of hASMC to Br2 resulted in a significant increase of [Ca2+]i at 1 h postexposure (Fig. 2A). The addition of nifedipine to the bath increased [Ca2+]i in a dose-dependent manner (Fig. 2B) with an EC50 of 250 nM (Fig. 2C). On the other hand, the addition of 10 μM diltiazem to Br2-exposed cells decreased [Ca2+]. Characteristic Fura2 images for these conditions are shown in Fig. 2E. Fluorescence images were captured in black and white and the pseudo-colors were assigned based on the fluorescent intensity (red > yellow > green > blue). Neither nifedipine nor diltiazem altered [Ca2+]i in air-exposed cells (data not shown). Thus, the data shown in Figs. 1 and 2 indicate that the two L-type Ca2+ channel inhibitors had diametrically opposite effects on Vm and [Ca2+]i in immortalized human airway smooth muscle cells after Br2 exposure.
Fig. 2.
A: exposure of human airway smooth muscle cells (hASMC) to Br2 (100 ppm for 10 min) caused a significant increase in [Ca2+]i measured by Fura2 1 h postexposure. Changes in [Ca2+]i are shown as the change in the emission ratio of [Ca2+]-bound Fura2 (340 nm) to unbound (380 nm). Individual values show means ± SE; n = 14 for air and 12 for Br2. B: hASMC [Ca2+]i is shown following the addition of the indicated concentrations of nifedipine (Nif) to the bath at 1 h postexposure to Br2. Measurements were done within minutes from the addition of nifedipine. In this case, the absolute Ca2+ concentration was determined from the emission ratio R (24), using the calibration procedure based on ionophore permeabilization (42). C: dose-response of nifedipine induced peak [Ca2+]i in hASMC exposed to 100 ppm Br2 for 10 min at 1 h postexposure. Values are expressed as means ± SE; n = 5 for each nifedipine concentration. The calculated EC50 is 0.25 µM. D: treatment of hASMC with diltiazem (10 µM), 1 h postexposure to Br2, lowered [Ca2+]i. Arrow indicates the time of diltiazem addition. Diltiazem had no effect on air-treated cells (data not shown). Means ± SE; n = 8 for vehicle and 15 for diltiazem. E: pseudo-colored images showing the changes in [Ca2+]i in hASMC and 1 h postexposure to Br2 (100 ppm for 10 min) for the indicated conditions. Measurements were taken 1–2 min after addition of DMSO, diltiazem (10 µM) or nifedipine (10 µM). Fluorescence images were captured in black and white and the pseudo-colors were assigned on the basis of the fluorescent intensity (red>yellow>green>blue). The white bar in the bottom left-hand panel depicts 100 µm. Typical results repeated at least in five different experiments.
Nifedipine exacerbates halogen-induced AHR.
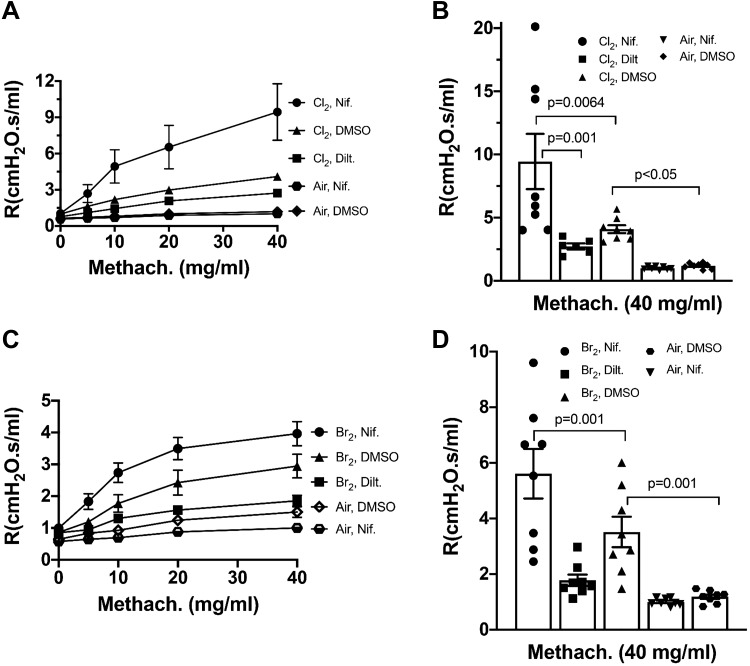
To validate the in vitro findings shown above, we exposed male C57BL/6 mice to either Br2 (600 ppm) or Cl2 (400 ppm) for 30 min, injected them with nifedipine, diltiazem (5 mg/kg body wt each), or DMSO at 1 h postexposure, and measured airway resistance at 24 h postexposure before and following challenge with increased concentrations of methacholine, using a flexiVent FX. Approximately 50% of the mice exposed to halogens and injected with 5 mg/kg nifedipine died at or before 24 h. No deaths were recorded in the other groups. Mice that had been exposed to Cl2 or Br2 exhibited robust increases of airway resistance (Fig. 3, A–D) with increased concentrations of methacholine (defined as airway hyperresponsiveness), as compared with the control values in air-treated mice. Nifedipine caused an additional increase of airway hyperresponsiviness, as compared with their corresponding halogen values, while it had no effect on air-treated mice (data not shown). On the other hand, injection of diltiazem, 5 mg/kg body wt, decreased airway hyperresponsiviness considerably (Fig. 3, A–D). No significant changes were seen in the Newtonian resistance values (Rn) for any of these groups (data not shown), indicating that the increase in airway hyperresponsiviness was due mainly to an increased resistance of lower airways.
Fig. 3.
Mice were exposed to 400 ppm Cl2 (A and B) or 600 ppm Br2 (C and D) for 30 min and returned to room air. At 1-h postexposure, the mice were injected with nifedipine (5 mg/kg body wt), diltiazem (5 mg/kg body wt), DMSO (100 µL), or saline subcutaneously. They were connected to a flexiVent FX at 24 h postexposure for measurements of airway resistance (R; prior to and after challenge with methacholine). A and C: values are means ± 1 SE. B and D: corresponding changes in airway resistance for Cl2 and Br2 exposed mice at a methacholine concentration of 40 mg/mL to enable statistical comparisons. Individual values and means ± 1 SE; number of mice in each group as follows: A and B: eight mice per group except for Cl2+Diltiazem where n = 6; C and D: n = 8 for each group. Statistical analysis for the data shown in B and D was performed by one-way ANOVA followed by the Tukey test.
Ca-SR expression is increased 24 h postexposure of mpASMC and hASMC to halogens.
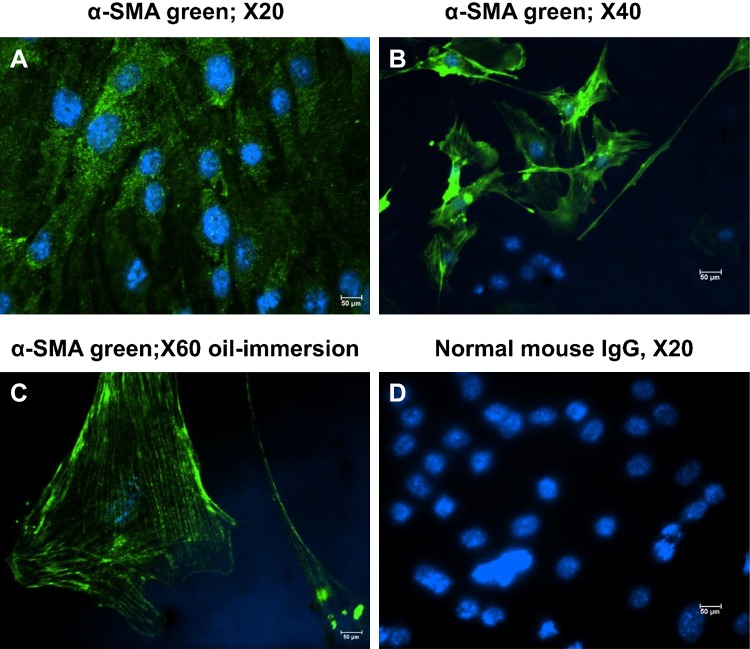
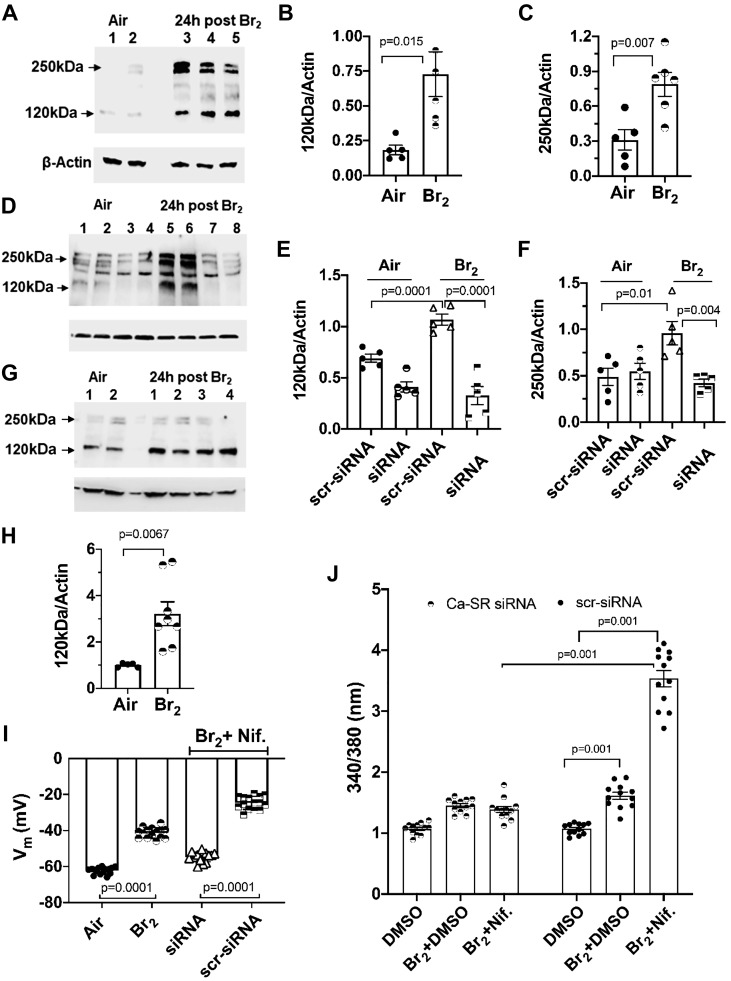
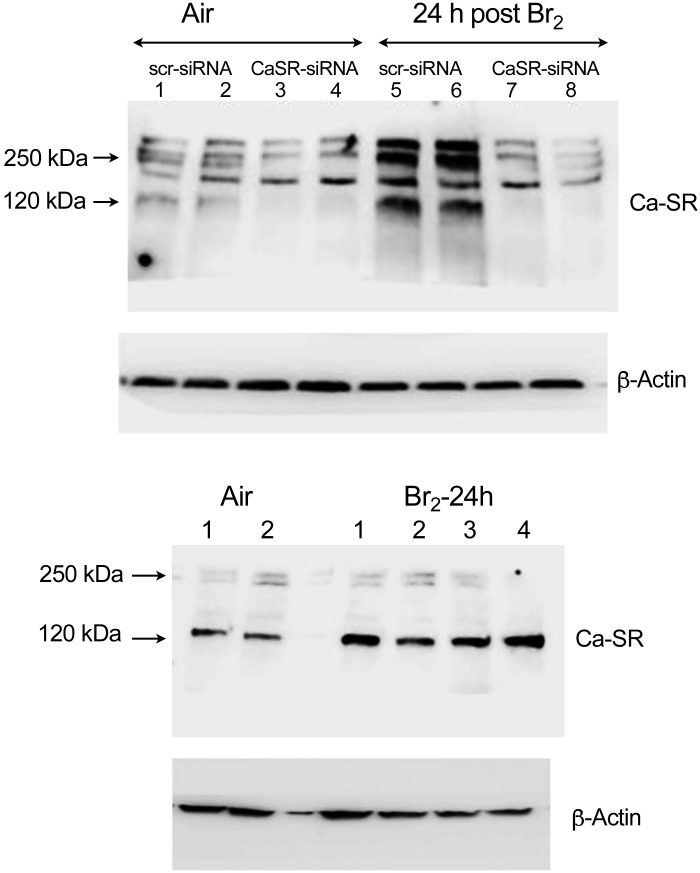
Mouse airway smooth muscle cells (mpASMC), maintained in primary culture for two passages, immunostained positive with an antibody against alpha smooth muscle actin (α-SMA) but not with nonimmune IgG (Fig. 4, A–D), indicating that they maintained their phenotype. In the next series of experiments, hASMC and mpASMC were exposed to Br2 (100 ppm for 10 min) in their original culture medium. At the end of the exposure, the culture medium was replaced with fresh medium and the cells were placed in an incubator at 37°C in an atmosphere 95% O2 and 5% CO2 for 24 h. They were then lysed and the expression of Ca-SR protein was assessed by Western blotting. Ca-SR expression in air-exposed cells was barely detectable (Fig. 5A). The expression of the Ca-SR 120 and 250 kDa bands increased by more than three-fold in hASMC, 24 h postexposure to 100 ppm Br2 for 10 min (Fig. 5, A–C). Exposure to Br2 of hASMC transfected with scrambled Ca-SR siRNA (scr-siRNA) resulted in significant increases of the Ca-SR 120 and 250 kDa bands to a degree similar to nontransfected cells (Fig. 5D). On the other hand, these two bands remained at their control values when cells were transfected with the Ca-SR siRNA (Fig. 5, D–F).
Fig. 4.
Mouse airway cells in primary culture (passage 2) were immunostained with a monoclonal antibody against α-smooth muscle actin (α-SMA) (A–C) or with an equivalent amount of mouse IgG (D), as described in materials and methods. The lens magnification is shown in each panel. Results are of a typical experiment that was repeated three times with cells from different mice.
Fig. 5.
A: characteristic Western blots of human airway smooth muscle cells (hASMC) 24 h after exposure to air (lanes 1, 2) or Br2 (100 ppm for 10 min; lanes 3, 4, 5). Cells were immunostained with a specific antibody against the calcium-sensing receptor (Ca-SR; 4 µg/mL), as described in materials and methods. The two bands at 120 and 250 kDa correspond to the monomer and dimer forms of the receptor. This experiment was repeated twice with identical results. B and C: quantification of the 120 kDa and 250 kDa bands in hASMC exposed to air or 24 h postexposure to Br2. Each point represents the digitized value of a single lane; individual values are means ± 1 SE; n = 5 for air and n = 6 for 24 h postexposure to Br2. Statistical analysis was performed with the Student’s t test. D: hASMC were transfected with a scrambled siRNA (scr-siRNA; lanes 1, 2 and 5, 6) or a Ca-SR siRNA (lanes 3, 4, and 7, 8) and exposed to air (lanes 1–4) or Br2 (100 ppm for 10 min; lanes 5–8) and returned to an incubator at 37°C vented with 95% air and 5% CO2 for 24 h. At that time, cells were processed for Western blotting analysis with a Ca-SR antibody as described in materials and methods. There was a significant increase of Ca-SR protein in Br2-exposed cells transfected with scrambled siRNA (lanes 5, 6), but not in those transfected with Ca-SR siRNA (lanes 7, 8). Each blot was repeated twice. An uncropped version of this blot is shown in Fig. 10. E and F: quantification of the 120 and 250 kDa bands separately 24 h after exposure to air or Br2. Values of individual digitized lanes and means ± SE; n = 5 for each condition. G: Western blots of primary mouse airway cells (mpASMC) exposed to air or Br2. Twenty four hours postexposure, cells were processed for Western blotting with a primary antibody against the Ca-SR protein. Each blot was repeated twice. An uncropped version of this blot is shown in Fig. 10. H: quantification of the 120 kDa bands. Individual values and means ± SE; n = 5 for each condition. I: hASMC were transfected with scrambled (scr) or Ca-SR siRNA as described in the text. Vm was measured 1 h after Br2, following the addition of nifedipine (10 µM) or vehicle (DMSO) at 30 min postexposure. Individual values and means ± 1 SE; n = 15 for each condition. Statistical analysis was performed with one-way ANOVA followed by the Tukey t test for multiple comparisons. J: hASMC were transfected with Ca-SR siRNA or a scrambled siRNA 48 h before they were exposed to Br2 (100 ppm for 10 min) or air. Thirty minutes postexposure, nifedipine (10 µM) or vehicle (DMSO) was added and [Ca2+]i was measured using Fura2 as described in the text. Individual values and means ± 1 SE; n = 10 for each condition. Statistical analysis was performed with one-way ANOVA followed by the Tukey t test for multiple comparisons.
Only the 120 kDa band (which is the monomer and thought to be the inactive form of the Ca-SR located in the cytoplasm) was observed when mpASMC were exposed to air or Br2 and its value increased significantly at 24 h post Br2 (Fig. 5, G and H). Finally, as shown in Fig. 5, I and J, silencing the Ca-SR of hASMC, by transfecting them with a Ca-SR-siRNA, reversed the effects of Br2 and nifedipine on Vm and [Ca2+]i in Br2-exposed cells.
Exposure of mice to Br2 increases the concentration of hyaluronan in the BALF.
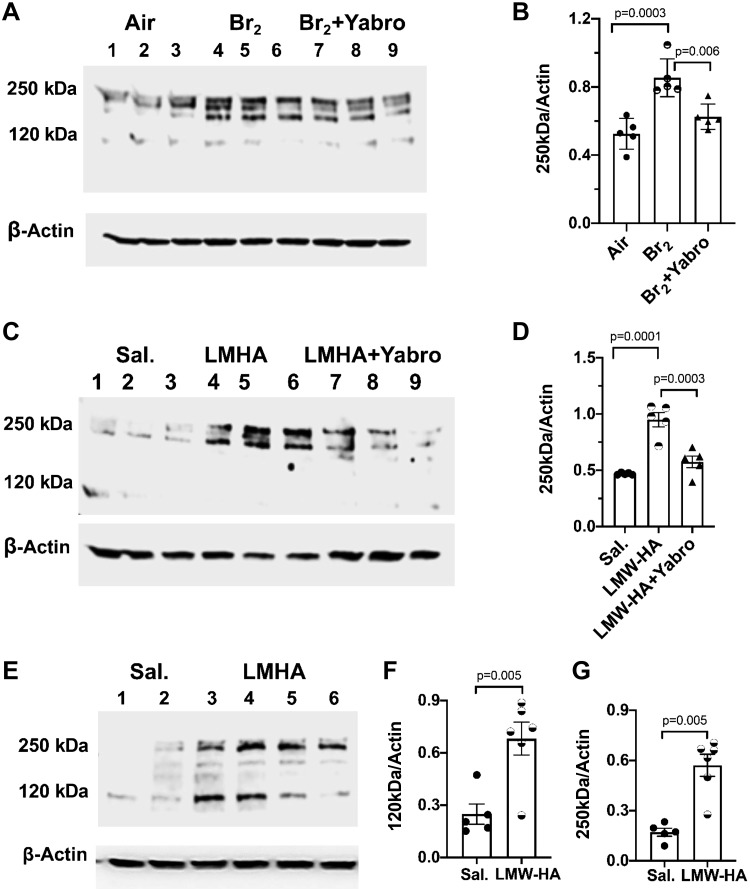
We hypothesized that the Br2-induced activation of the Ca-SR is mediated by the proinflammatory LMW-HA generated by the action of Br2 and/or HOBr on HMW-HA. As shown in Fig. 6A, hyaluronan levels (measured by ELISA) were significantly increased in the bronchoalveolar lavage fluid (BALF) of mice at 24 h after Br2 exposure. Gel electrophoresis (Fig. 6B) revealed that exposure to Br2 breaks down HMW-HA to LMW-HA; furthermore, significant levels of the proinflammatory LMW-HA were detected in the BALF of mice post Br2 exposure. We then added LMW-HA (generated by exposing HMW-HA to Br2) (Fig. 6B) on hASMC for 1 h and observed that LMW-HA caused hASMC Vm depolarization from −60 mV to −40 mV (Fig. 6C) and increased [Ca2+]i (Fig. 6D). These changes were similar to those induced following exposure of these cells to halogens.
Fig. 6.
A: concentrations of hyaluronan (HA) in bronchoalveolar lavage fluid (BALF) at 1 and 24 h postexposure to Br2 (600 ppm for 30 min), measured by ELISA. Individual values and means ± 1 SE; number of mice for each condition as follows: air = 8; 1 h and 24 h postexposure to Br2 = 9 each. One-way analysis of variance was performed followed by the Tukey t test. B, left: agar gel electrophoresis of Yabro (a form of high-molecular-weight hyaluronan; 3 mg/mL) before and following exposure to Br2. Lane 1: HA Mega-HA Ladder (Hyalose); lane 2: select-HA Hiladder; lane 3: Yabro; lane 4: Yabro exposed to Br2 (400 ppm for 30 min) and stored at 4°C for 1 or 24 h. B, right: agar gel electrophoresis of concentrated BALF from air and Br2-exposed mice. Lane 1: select-HA Hiladder; Lane 2: select-HA LoLadder. The different lanes show the appearance of BALF at the indicated times postexposure. For the last lane, BALF was obtained at 24 h postexposure to Br2 (600 ppm/30 min) and treated with hyaluronidase, which degrades HA. Characteristic blot, which was repeated five times. C: human airway smooth muscle cells (hASMC) membrane potential (Vm) measurements 24 h postincubation with 150 μg/mL LMW-HA generated as described in B. Individual values and means ± 1 SE; n = 20 each; Student’s t test. D: measurement of [Ca2+]i [as the ratio of unbound Fura2 (340 nm) to bound Fura2 (380 nm)] of hASMC loaded with Fura2 following the addition of LMW-HA or saline (indicated by the arrow). The initial fast increase in [Ca2+]i peak was followed by a decrease to a lower steady-state value that lasted as long as LMW-HA was present in the recording chamber. Individual values and means ± 1 SE; n = 8 for each condition.
LMW-HA induces Ca-SR expression in hpASMC and mpASMC.
Exposure of human airway cells from normal lungs in primary culture (hpASMC; passage 2) to Br2 (100 ppm for 10 min) or incubation with 150 µg/mL LMW-HA induced the expression of Ca-SR to the same extent 24 h later (Fig. 7, A–D). Similar changes were seen in mouse airway cells in primary cultures (mpASMC; Fig. 7, E–G). The effects of Br2 or LMW-HA on Ca-SR of hpASMC were reversed when cells were treated with HMW-HA.
Fig. 7.
A: human airway smooth muscle cells in primary culture (hpASMC, harvested from normal human lungs, cultured for 2–4 passages) were exposed to air (lanes 1–3) or Br2 (100 ppm for 10 min; lanes 4–9). At 1 h postexposure to Br2, saline (lanes 4–6) or Yabro (150 μg/mL; lanes 7–9) was added to the medium and the cells were placed in an incubator at 37°C vented with 95% air and 5% CO2 for 24 h. Western blots show immunostaining with an antibody against the calcium-sensing receptor (Ca-SR). Typical experiment of two Western blots. B: quantification of the 250 kDa band for the conditions shown. Individual values for each lane show means ± 1 SE; n = 5 for each condition. One-way ANOVA was performed followed by the Tukey t test. C: saline (lanes 1, 2, and 3) or LMW-HA (150 μg/mL; lanes 4, 5, and 6) were added into the medium of phASMC followed 1 h later by saline (lanes 4–6) or Yabro (150 μg/mL; lanes 7–9). The cells were then placed in an incubator at 37°C, vented with 95% air and 5% CO2 for 24 h, at which time they were immunostained with an antibody against the Ca-SR. D: quantification of the 250 kDa band (the active form of the Ca-SR) for the conditions shown. Individual values and means ± 1 SE; n = 5 for each condition; one-way ANOVA followed by the Tukey t test. E: pmASMCs (mouse airway smooth muscle cells in primary culture) treated with saline (lanes 1, 2) or 150 µg/mL LMW-HA (lanes 3–6) for 24 h as described above. Western blots showing immunostaining with an antibody against the Ca-SR. F and G: quantitation of the 120 kDa and 250 kDa bands of the Ca-SR. Data are individual values and means ± SE; n = 5.
HMW-HA reverses AHR induced by Br2.
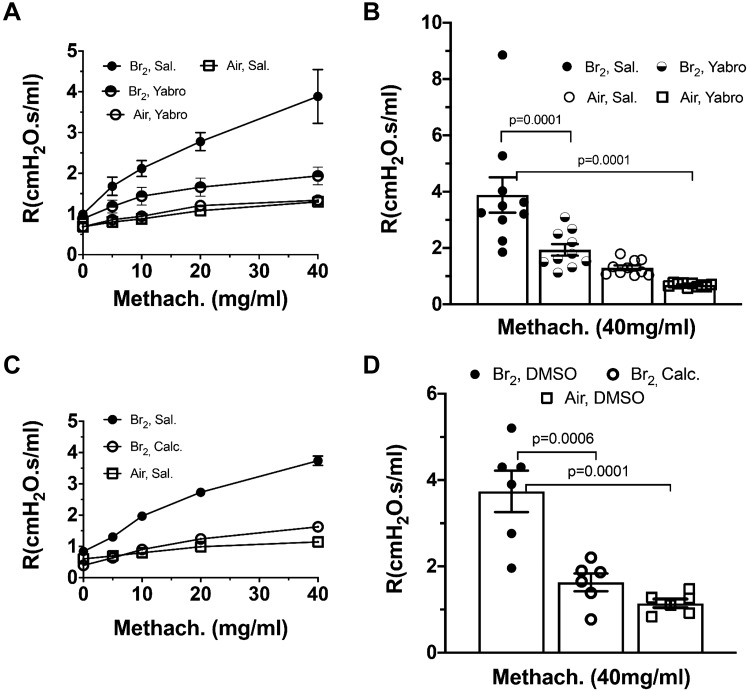
As mentioned previously, mice exposed to 600 ppm Br2 for 30 min, developed, 24 h later, increased hyperresponsiveness to aerosolized methacholine, as compared with air-exposed mice. Instillation of 50 µL of 150 μg/mL HMW-HA in the external nares at 1 h and 23 h postexposure, resulted in airway resistances that were similar to controls (Fig. 8, A and B). Similarly, instillation of the Ca-SR inhibitor (calcilytic) NPS2143, at 1 h and 23 h after Br2 exposure resulted in normal airway hyperresponsiveness (Fig. 8, C and D). These findings provide strong evidence that activation of the Ca-SR plays an important role in increased airway sensitivity to methacholine following exposure to Br2 in vivo.
Fig. 8.
A and B: mice were exposed to Br2 (600 ppm/30 min) and returned to room air; at 1 and 23 h postexposure they were instilled with high-molecular-weight hyaluronan (HMW-HA) (Yabro) (25 μL/nostril of 3 μg/μL) in saline, or saline. Exposure to Br2 induced a 4-fold increase in lung resistance; instillation of HMW-HA returned these variables to their control values. B: resistance (R) values for each group at a methacholine concentration of 40 mg/mL for statistical comparison purposes. Values are means ± 1 SE; n = 10 mice for each group; one-way ANOVA followed by the Tukey t test. C and D: mice were exposed to Br2 (600 ppm/30 min), and at 1 and 23 h postexposure they were instilled with the calcilytic NPS2143 (1 μM; 25 μl/nostril). Controls were instilled with an equivalent volume of saline. Br2 induced a 4-fold increase in lung resistance that was totally inhibited by the calcilytic NPS2143. Data are means ± SE; n = 10 for each condition; one-way ANOVA followed by the Tukey t test.
Upregulation of Ca-SR in asthmatics.
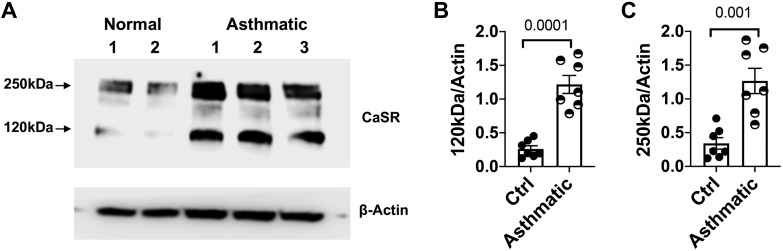
Exposure of mice to Br2 resulted in progressive injury, which resembled some of the manifestations of human asthma. To show the relevance of our findings in human disease, we isolated human airway smooth muscle cells either from lung-resected tissue from patients with either normal lung or those with asthma, cultured them to confluence, lysed them, and probed for the expression of Ca-SR using a specific antibody. As seen in (Fig. 9), both the monomer and dimer Ca-SR were at least threefold higher in asthmatics than normal subjects.
Fig. 9.
Calcium-sensing receptor (Ca-SR) expression in human primary airway smooth muscle cells (hpASMC) from normal and asthmatic patients that were cultured and used from passage 2 to 4. When confluent, cells were lysed and probed with an antibody against Ca-SR protein (A). Ca-SR expression in asthmatic patients was 5-fold higher for 120 kDa (B) and 3-fold higher for 250 kDa (C) bands (normalized for actin) than in normal patients. Individual values and means ± SE; n = 7. Statistical analysis by Student’s t test.
Figure 10 shows uncropped Western blots of hASMC and pmASMC immunostained with antibodies against the Ca-SR protein as evidence for the specificity of this antibody.
Fig. 10.
Top: uncropped version of Fig. 4D. Human airway smooth muscle cells (hASMC) were transfected with a scrambled siRNA (scr-siRNA; lanes 1, 2, 5, and 6) or a calcium-sensing receptor (Ca-SR) siRNA (lanes 3, 4, 7, and 8) and exposed to air (lanes 1–4) or Br2 (100 ppm for 10 min; lanes 5–8) and returned to an incubator at 37°C in vented with 95% air and 5% CO2 for 24 h. At that time, cells were processed for Western blotting analysis with a Ca-SR antibody as described in materials and methods. Entire gels are shown. There was a significant increase of Ca-SR protein in Br2-exposed cells transfected with scrambled siRNA (lanes 5, 6), but not in those transfected with Ca-SR siRNA (lanes 7, 8). Bottom: uncropped version of Fig. 5G. Western blots of primary mouse airway cells (mpASMC) exposed to air or Br2. Twenty-four hours postexposure, cells were processed for Western blotting with a primary antibody against the Ca-SR protein. Each blot was repeated twice.
DISCUSSION
The main findings of our study are 1) exposure of human and mouse airway smooth muscle cells in primary culture and an immortalized cell line to halogens or low-molecular-weight hyaluronan (LMW-HA) results in sustained increase of [Ca2+]i, membrane depolarization and upregulation of the Ca-SR protein; 2) pretreatment with a Ca-SR siRNA or posttreatment with high-molecular-weight hyaluronan (HMW-HA) prevents or reverses these effects; 3) exposure of mice to Br2 increases the concentrations of LMW-HA in the BALF; 4) subcutaneous injection of nifedipine resulted in increased mortality and higher levels of airway resistance following challenge with methacholine of surviving mice, while instillation of diltiazem decreased AHR to the air values, and 5) post halogen exposure administration of LMW-HA or an inhibitor of Ca-SR in mice decreased AHR to control values. While previous studies have shown that exposure of mice to Cl2 (34), HCl (55), or ozone (18) fragments HMW-HA to LMW-HA, a proinflammatory agent, and that the Ca-SR is upregulated in asthmatics and animal models of allergic asthma (54), this is the first demonstration of upregulation of the Ca-SR by LMW-HA. Furthermore, our results establish for the first time the diametrically opposite effects of the two L-type channel blockers, nifedipine and diltiazem, on AHR following oxidative lung injury.
Halogen inhalation and reaction with lung epithelial lining fluid (ELF) produce hypohalous acids that react with structures on airway cell membrane, such as plasmalogens, generating secondary long-lived lipid mediators capable of damaging distal structures (12, 15). Hyaluronan is a linear polymer formed by a repeating disaccharide structure of glucuronic acid and N-acetyl-glucosamine consisting of up to 25,000 disaccharide units (MW = 1–10 million Da). HMW-HA is a major structural component of the extracellular matrix; it promotes cell survival and has antiangiogenic properties and anti-inflammatory effects on immune cells. Some of these effects are mediated via binding to its receptors CD44, TLR2, and TLR4 (16, 19, 29–31, 47).
Hypohalous acids, as well as products released by activated neutrophils, react with HMW-HA, fractionating it to LMW-HA (34, 35, 37, 46, 55). These short fragments of hyaluronan (LMW-HA ∼300 kDa) act as endogenous innate immune ligands and promote inflammatory responses, angiogenesis, and epithelial to mesenchymal transition (19, 30, 31, 38). LMW-HA fragments stimulate cytokine production and activate the innate immune response via binding to CD44 and Toll-like receptors (TLR) signaling in an MyD-88 and NF-κB-dependent fashion, whereas HMW-HA inhibits TLR-2 signaling in vitro and in vivo (31). Binding of LMW-HA to CD44 is enhanced by inter-α-inhibitor (IαI), a serum protease inhibitor consisting of three polypeptides (7, 41); the concentration of IαI in the BAL increases considerably when blood gas permeability to plasma proteins increases (19). LMW-HA is both necessary and sufficient for the development of AHR after exposure to ozone (18, 19), ischemia-reperfusion (13), HCl (55), and various other forms of injury. LMW-HA increases vascular permeability and the lung filtration coefficient by activating RhoA and ROCK (its downstream kinase), inducing cytoskeletal reorganization, and inhibiting cell-cell contacts (36, 55). On the contrary, administration of HMW-HA protects from lung injury in ozone exposure (18), bleomycin administration (29), smoke inhalation, sepsis, and Cl2 toxicity (27, 40).
Airway smooth muscle cell contraction requires an increase of intracellular Ca2+. We found that treatment with nifedipine, a Ca2+ L-type channel inhibitor, led to increased mortality after halogen exposure, while treatment with the nondihydropyridine Ca2+ channel blocker, diltiazem, reduced AHR and mortality. Dihydropyridine derivatives, such as nifedipine and nicardipine, are used for the treatment of systemic and pulmonary artery hypertension (PAH) (49). In patients with PAH, these derivatives were found to be ineffective, and only a small fraction of the patients, 10% to 15%, benefited from their use, and in some cases, they caused complications (49). The deleterious effect of dihydropyridine derivatives in patients with PAH was linked to the overexpression of Ca-SR in pulmonary arterial smooth muscle cells (49). Evidence of Ca-SR expression in vascular smooth muscle cells was first reported by Wonneberger et al. (52). Our findings indicate that nifedipine may activate the Ca-SR in human and mouse airway smooth muscle cells but only after halogen-induced injury.
The Ca-SR was initially cloned and characterized in bovine parathyroid glands and kidneys and has well-recognized roles in sensing and regulating plasma Ca2+, particularly via modulation of parathyroid hormone secretion and actions on kidney, bone, and intestine terms of mineral Ca2+ excretion, release, and absorption (9). The human Ca-SR contains 1,078 amino acids (AA) (20) and is characterized by a large extracellular NH2 terminus of 600 AA residues, which interacts with extracellular Ca2+ ions. It has seven transmembrane-spanning domains, and a relatively large (200 AA) intracellular COOH-terminus domain that couples to G proteins and other signal transduction pathways. In addition to its well-established roles, there is increasing recognition that noncalciotropic tissues also express Ca-SR.
The expression of the Ca-SR in the respiratory system plays an important role in fetal lung growth and development by stimulating fluid secretion in the pulmonary lumen (8), lung branching, and morphogenesis in mice (14). Ca-SR is expressed in human and mouse airway smooth muscle cells and bronchiolar epithelial cells (54), and its expression was increased in the vascular airway smooth muscle of patients with PAH, asthma, and in allergen-sensitized mice (25, 53, 54). Herein, we report for the first time the induction of Ca-SR expression by halogens in human and murine smooth muscle cells, in vitro. The expression of Ca-SR in hASMC and pmASMC primary cells was increased by three- to fourfold compared with its expression in control cells. Furthermore, the fact that administration of diltiazem and of the calcilytic NPS2143, an antagonist of the Ca-SR, inhibited the Br2 and Cl2-induced AHR implies that halogens upregulated its expression in airway smooth muscle cells.
The Ca-SR is activated by extracellular calcium, magnesium, amino acids, amines, polyamines, and allergens (11, 21–23). The attachment of a trigger ion or molecule to the extracellular domain of the sensor initiates a cell-signaling cascade that leads to increased [Ca2+]i. The receptor is linked to a small G protein that triggers PLC-TP3 pathway (44, 48). In addition, the Ca-SR is known to interact with Ca2+ influx pathways although these involve non-L-type influx pathways, such as transient receptor potential channels (TRPC) and store-operated Ca2+ channels (49, 51, 53). Interestingly, L-type Ca2+ channel inhibitors such as nifedipine appear to be upstream of Ca-SR, such that they activate the Ca-SR, at least in hypertensive pulmonary artery smooth muscle (49, 53)
The mechanism by which halogens activate Ca-SR gene expression in hASMC and pmASMC in vitro and in mice in vivo had not been elucidated until now. Here, we demonstrated for the first time a central role for LMW-HA in this process by showing that LMW-HA induced the expression of Ca-SR, an effect that was inhibited by HMW-HA. HMW-HA reverses the LMW-HA Ca-SR expression by either inhibiting downstream signaling cascades or by displacing LMW-HA from is receptors.
In conclusion, our results demonstrate for the first time that activation of the Ca-SR by LMW-HA is necessary for the development of AHR following exposure to halogen gases. This finding provides new insights into the pathogenesis of halogen-induced lung injury and may suggest novel treatments of this severe disease.
GRANTS
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute of Environmental Health Sciences (NIEHS) (Grants 5UO1 ES026458 03, 3UO1 ES026458 03S1, and 5UO1 ES027697 02) to S. Matalon, the National Heart, Lung and Blood Institute (NHLBI) (Grants 2R01 HL056470-18 and 1R01 HL138402-03) to Y. S. Prakash. S. Garantziotis is supported by the Division of Intramural Research (NIEHS Grant ZIA ES102605).
DISCLOSURES
High-molecular-weight hyaluronan (Yabro) was provided free by IBSA Institut Biochimique, Lugano, Switzerland. No additional conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L., M.Y.J., J.C., S.G., Y.S.P., and S.M. conceived and designed research; A.L., Z.Y., S.D., M.Y.J., J.C., M.L., S.G., and S.M. performed experiments; A.L., Z.Y., S.D., M.Y.J., J.C., M.L., S.G., Y.S.P., and S.M. analyzed data; A.L., Z.Y., S.D., M.Y.J., J.C., M.L., S.G., Y.S.P., and S.M. interpreted results of experiments; A.L., Z.Y., S.G., and S.M. prepared figures; A.L., J.C., and S.M. drafted manuscript; A.L., J.C., S.G., Y.S.P., and S.M. edited and revised manuscript; A.L., Z.Y., S.D., M.Y.J., J.C., M.L., S.G., Y.S.P., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jeannette Eagen for technical support and Dr. Charles Emala, Sr. for providing the immortalized human airway cell line.
REFERENCES
- 1.Abara W, Wilson S, Vena J, Sanders L, Bevington T, Culley JM, Annang L, Dalemarre L, Svendsen E. Engaging a chemical disaster community: lessons from Graniteville. Int J Environ Res Public Health 11: 5684–5697, 2014. doi: 10.3390/ijerph110605684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal S, Ahmad I, Lam A, Carlisle MA, Li C, Wells JM, Raju SV, Athar M, Rowe SM, Dransfield MT, Matalon S. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight 3: e120694, 2018. doi: 10.1172/jci.insight.120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid Redox Signal 24: 99–112, 2016. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N, Louch WE, Ford DA, Doran SF, Matalon S, Dell’Italia LJ. Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats. Am J Physiol Heart Circ Physiol 316: H212–H223, 2019. doi: 10.1152/ajpheart.00652.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family–a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem 252: 339–346, 1998. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 8.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, Riccardi D. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6: 21975, 2016. doi: 10.1038/srep21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 10.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine-induced cardiopulmonary injury. Ann N Y Acad Sci 1374: 159–167, 2016. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conigrave AD. The calcium-sensing receptor and the parathyroid: past, present, future. Front Physiol 7: 563, 2016. doi: 10.3389/fphys.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J Lipid Res 59: 696–705, 2018. doi: 10.1194/jlr.M083279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldridge L, Moldobaeva A, Wagner EM. Increased hyaluronan fragmentation during pulmonary ischemia. Am J Physiol Lung Cell Mol Physiol 301: L782–L788, 2011. doi: 10.1152/ajplung.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol 586: 6007–6019, 2008. doi: 10.1113/jphysiol.2008.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res 57: 1529–1540, 2016. doi: 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J 15: 2179–2186, 2001. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- 17.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol 308: L931–L942, 2015. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [Erratum in J Biol Chem 291: 19257-8, 2016]. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20.Garrett JE, Capuano IV, Hammerland LG, Hung BC, Brown EM, Hebert SC, Nemeth EF, Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem 270: 12919–12925, 1995. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 21.Geng Y, Mosyak L, Kurinov I, Zuo H, Sturchler E, Cheng TC, Subramanyam P, Brown AP, Brennan SC, Mun HC, Bush M, Chen Y, Nguyen TX, Cao B, Chang DD, Quick M, Conigrave AD, Colecraft HM, McDonald P, Fan QR. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 5: e13662, 2016. doi: 10.7554/eLife.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerbino A, Colella M. The different facets of extracellular calcium sensors: old and new concepts in calcium-sensing receptor signaling and pharmacology. Int J Mol Sci 19: E999, 2018. doi: 10.3390/ijms19040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerbino A, Russo D, Colella M, Procino G, Svelto M, Milella L, Carmosino M. Dandelion root extract induces intracellular Ca2+ increases in HEK293 cells. Int J Mol Sci 19: E1112, 2018. doi: 10.3390/ijms19041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 25.Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol 15: 33–51, 2018. doi: 10.1038/s41574-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoyle GW, Svendsen ER. Persistent effects of chlorine inhalation on respiratory health. Ann N Y Acad Sci 1378: 33–40, 2016. doi: 10.1111/nyas.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology 15: 1131–1139, 2010. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 28.Jian MY, Liu Y, Li Q, Wolkowicz P, Alexeyev M, Zmijewski J, Creighton J. N-cadherin coordinates AMP kinase-mediated lung vascular repair. Am J Physiol Lung Cell Mol Physiol 310: L71–L85, 2016. doi: 10.1152/ajplung.00227.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 30.Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 293: 982–985, 2010. doi: 10.1002/ar.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev 91: 221–264, 2011. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam A, Vetal N, Matalon S, Aggarwal S. Role of heme in bromine-induced lung injury. Ann N Y Acad Sci 1374: 105–110, 2016. doi: 10.1111/nyas.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauer ME, Mukhopadhyay D, Fulop C, de la Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem 284: 5299–5312, 2009. doi: 10.1074/jbc.M807965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-α-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in ΔF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 1: 200–213, 2011. [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Cao Z, Zhang G, Thannickal VJ, Cheng G. Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties. Free Radic Biol Med 53: 1954–1959, 2012. doi: 10.1016/j.freeradbiomed.2012.08.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis 188: 919–926, 2003. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 40.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA. High-molecular-weight hyaluronan–a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12: R102, 2008. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malki N, Balduyck M, Maes P, Capon C, Mizon C, Han KK, Tartar A, Fournet B, Mizon J. The heavy chains of human plasma inter-alpha-trypsin inhibitor: their isolation, their identification by electrophoresis and partial sequencing. Differential reactivity with concanavalin A. Biol Chem Hoppe Seyler 373: 1009–1018, 1992. doi: 10.1515/bchm3.1992.373.2.1009. [DOI] [PubMed] [Google Scholar]
- 42.Moore ED, Becker PL, Fogarty KE, Williams DA, Fay FS. Ca2+ imaging in single living cells: theoretical and practical issues. Cell Calcium 11: 157–179, 1990. doi: 10.1016/0143-4160(90)90068-6. [DOI] [PubMed] [Google Scholar]
- 43.Nagano N, Tsutsui T. [Pharmacological characteristics of drugs targeted on calcium-sensing receptor.-properties of cinacalcet hydrochloride as allosteric modulator]. Clin Calcium 26: 839–850, 2016. [PubMed] [Google Scholar]
- 44.Nakao S, Wakabayashi S, Nakamura TY. Stimulus-dependent regulation of nuclear Ca2+ signaling in cardiomyocytes: a role of neuronal calcium sensor-1. PLoS One 10: e0125050, 2015. doi: 10.1371/journal.pone.0125050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh MW, Honavar J, Doran S, Benavides G, Darley-Usmar V, Matalon S, Ford DA, Patel RP. Chlorinated fatty acids are biomarkers and potential mediators of chlorine gas toxicity (Abstract) Free Radic Biol Med 76, Suppl 1: S165–S166, 2014. doi: 10.1016/j.freeradbiomed.2014.10.053. [DOI] [Google Scholar]
- 46.Rees MD, McNiven TN, Davies MJ. Degradation of extracellular matrix and its components by hypobromous acid. Biochem J 401: 587–596, 2007. doi: 10.1042/BJ20061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 48.Seuwen K, Boddeke HG, Migliaccio S, Perez M, Taranta A, Teti A. A novel calcium sensor stimulating inositol phosphate formation and [Ca2+]i signaling expressed by GCT23 osteoclast-like cells. Proc Assoc Am Physicians 111: 70–81, 1999. doi: 10.1046/j.1525-1381.1999.09866.x. [DOI] [PubMed] [Google Scholar]
- 49.Smith KA, Ayon RJ, Tang H, Makino A, Yuan JX. Calcium-sensing receptor regulates cytosolic [Ca2+] and plays a major role in the development of pulmonary hypertension. Front Physiol 7: 517, 2016. doi: 10.3389/fphys.2016.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a beta2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA; ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly . An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann Am Thorac Soc 14: 1060–1072, 2017. doi: 10.1513/AnnalsATS.201704-297WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol 175: 203–212, 2000. doi: 10.1007/s002320001068. [DOI] [PubMed] [Google Scholar]
- 53.Yamamura A, Yamamura H, Guo Q, Zimnicka AM, Wan J, Ko EA, Smith KA, Pohl NM, Song S, Zeifman A, Makino A, Yuan JX. Dihydropyridine Ca2+ channel blockers increase cytosolic [Ca2+] by activating Ca2+-sensing receptors in pulmonary arterial smooth muscle cells. Circ Res 112: 640–650, 2013. doi: 10.1161/CIRCRESAHA.113.300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yarova PL, Stewart AL, Sathish V, Britt RD Jr, Thompson MA, P Lowe AP, Freeman M, Aravamudan B, Kita H, Brennan SC, Schepelmann M, Davies T, Yung S, Cholisoh Z, Kidd EJ, Ford WR, Broadley KJ, Rietdorf K, Chang W, Bin Khayat ME, Ward DT, Corrigan CJ, T Ward JP, Kemp PJ, Pabelick CM, Prakash YS, Riccardi D. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med 7: 284ra60, 2015. doi: 10.1126/scitranslmed.aaa0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, Pittet JF, Aggarwal S, Garantziotis S, Song W, Matalon S. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am J Physiol Lung Cell Mol Physiol 314: L808–L821, 2018. doi: 10.1152/ajplung.00510.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]