Abstract
Group 1 pulmonary hypertension (PH), i.e., pulmonary arterial hypertension (PAH), is associated with a metabolic shift favoring glycolysis in cells comprising the lung vasculature as well as skeletal muscle and right heart. We sought to determine whether this metabolic switch is also detectable in circulating platelets from PAH patients. We used Seahorse Extracellular Flux to measure bioenergetics in platelets isolated from group 1 PH (PAH), group 2 PH, patients with dyspnea and normal pulmonary artery pressures, and healthy controls. We show that platelets from group 1 PH patients exhibit enhanced basal glycolysis and lower glycolytic reserve compared with platelets from healthy controls but do not differ from platelets of group 2 PH or dyspnea patients without PH. Although we were unable to identify a glycolytic phenotype unique to platelets from PAH patients, we found that platelet glycolytic metabolism correlated with hemodynamic severity only in group 1 PH patients, supporting the known link between PAH pathology and altered glycolytic metabolism and extending this association to ex vivo platelets. Pulmonary artery pressure and pulmonary vascular resistance in patients with group 1 PH were directly associated with basal platelet glycolysis and inversely associated with maximal and reserve glycolysis, suggesting that PAH progression reduces the capacity for glycolysis even while demanding an increase in glycolytic metabolism. Therefore, platelets may provide an easy-to-harvest, real-time window into the metabolic shift occurring in the lung vasculature and represent a useful surrogate for interrogating the glycolytic shift central to PAH pathology.
Keywords: bioenergetics, glycolysis, metabolism, platelets, pulmonary arterial hypertension
INTRODUCTION
Pulmonary hypertension (PH) is diagnosed on the basis of elevated pulmonary artery pressure, and it can be separated into five distinct groups based on differing pathophysiology, clinical presentation, hemodynamic characteristics, and response to treatment (34). Group 1 PH, i.e., pulmonary arterial hypertension (PAH), is characterized by progressive pulmonary microvascular remodeling, which increases pulmonary vascular resistance (PVR), leading to elevated pulmonary artery pressure and ultimately right heart failure (17, 38). Meanwhile, patients with group 2 PH, i.e., PH due to left heart disease (LHD), experience an increase in pulmonary artery pressure due to backward transmission of an increase in left atrial pressure (40).
Pulmonary vascular remodeling in PAH is the result of hyperproliferation and resistance to apoptosis at all layers of the pulmonary vasculature, which leads to thickening of arterioles and formation of plexiform lesions (2, 11, 15, 20). Vascular remodeling in PAH is associated with a metabolic shift from mitochondrial oxidative phosphorylation to glycolytic metabolism similar to the Warburg effect observed in tumor cells (29, 46). For example, increased glucose uptake in PAH lungs can be detected in vivo using [18F]fluoro-2-deoxyglucose positron emission tomography (PET) scanning (47). In addition, vascular cells cultured in vitro from PAH lungs show altered bioenergetics and abnormal mitochondria (8, 47). Therapies targeting this glycolytic shift can ameliorate disease in rodent PAH models (8, 24, 48). Furthermore, preliminary data using the pyruvate dehydrogenase kinase inhibitor dichloroacetate show promise in genetically susceptible PAH patients (25).
The metabolic shift in PAH is not restricted to the pulmonary vasculature. As PAH progresses, the right heart undergoes a metabolic shift that can be detected with [18F]fluoro-2-deoxyglucose PET (1, 22, 37), and skeletal muscle exhibits decreased expression of oxidative enzymes and increased expression of glycolytic enzymes (23). Although PET provides a broad visual representation of overall glucose uptake, in-depth metabolic analysis requires tissue. The ideal tissues (lung vasculature and right ventricle) are not regularly obtained since biopsies entail unnecessary risks in PH, and therefore, most tissue analysis is limited to end-stage disease, when lungs can be harvested upon transplant or death. Therefore, great interest is placed in finding surrogate tissues or cells that allow for metabolic investigation during PAH progression.
Circulating platelets are easy to collect, are an abundant source of functional mitochondria, and have been used as vehicles to study metabolic processes in several diseases (3, 6, 7, 10, 26, 30, 31, 35, 39, 45). In addition, platelets have been identified as a surrogate for muscle cell bioenergetics in healthy individuals (9). Furthermore, platelets have special relevance in PAH, as the disease is associated with quantitative and qualitative platelet defects (4, 16). In separate studies, Nguyen et al. (27) found an increased mitochondrial reserve capacity and basal glycolysis of platelets from patients with PAH and an increased mitochondrial reserve capacity in patients with group 2 PH (28) compared with healthy controls. It remains unclear whether these abnormalities in PH platelet metabolism are unique or common to respiratory patients without PH, since platelet metabolism is sensitive to factors that include physical activity, smoking, asthma, pulmonary embolism, and acute coronary syndrome (13, 14, 21, 32, 36, 42, 43, 45).
We hypothesized that platelets from PAH patients show a metabolic shift toward glycolysis and that platelet glycolytic parameters correlate with hemodynamic severity. We used Seahorse Extracellular Flux to investigate platelet bioenergetics in PAH patients (group 1 PH), LHD patients (group 2 PH), patients with dyspnea but no PH upon right heart catheterization (RHC), and healthy controls. We show that although platelets from group 1 PH patients have a distinctive glycolytic phenotype compared with healthy controls, this phenotype is not unique to PAH, as it is also seen in platelets from group 2 PH and patients with dyspnea and no PH. However, we also show that platelet glycolysis correlated with hemodynamic severity only in group 1 PH patients, supporting the idea that PAH pathology involves a metabolic switch that is detectable in the periphery and positioning platelets as a real-time window into this metabolic shift.
MATERIALS AND METHODS
Study subjects.
This study was approved by the Cleveland Clinic Institutional Review Board (study nos. 08-309 and 06-245). Written informed consent was obtained. We included consecutive age and BMI-matched patients who underwent right heart catheterization (RHC) and had the diagnosis of PH group 1, PH group 2, or dyspnea with normal hemodynamics from July 2018 to May 2019. Group 2 PH included both the isolated post-capillary and the combined pre- and post-capillary PH forms. Two PH experts reviewed the information available and agreed on the PH etiology based on the proceedings of the 6th World Symposium in PH (34).
Right heart catheterization.
Patients underwent at least 4 h of fasting before RHC. The procedure was performed by a single operator (A. R. Tonelli) in the outpatient setting, using only local anesthesia (5 mL of 2% lidocaine). Oxygen (O2) supplementation was provided to patients in whom the resting pulse oxygen saturation () was < 90%. Right atrial pressure (RAP), mean pulmonary artery pressure (mPAP), and pulmonary artery wedge pressure (PAWP) were recorded in the supine position, with the pressure transducer located at the midthoracic line (4th intercostal space). Cardiac output (CO) was measured by thermodilution and indirect Fick methodology. We calculated the cardiac index (CO/body surface area) and PVR ([mPAP-PAWP]/CO).
Platelet isolation.
Ten milliliters of blood were collected from the distal port of the pulmonary artery catheter (mixed venous blood) in patients and obtained via peripheral blood draw in healthy controls. Whole blood was collected in EDTA-coated vacutainers (BD Biosciences) and processed at room temperature (RT) within 8 h of collection based on Kramer et al. (18). Blood from healthy controls was collected between patient RHCs such that the timing of blood processing between controls and patients was not biased. Blood tubes were centrifuged for 15 min (150 g, RT, no brake). Platelet-rich plasma (PRP) was placed into a 15-mL conical tube with 100 nM prostaglandin E1 (PGE1; Sigma) to prevent aggregation and pelleted for 15 min (650 g, RT). The pellet was washed with 10 mL of Hanks’ Balanced Salt solution (with no phenol red, CaCl2, or MgCl2) supplemented with 100 nM PGE1 and counted via turbidimetry according to Walkowiak et al. (41). Briefly, PRP was diluted 1:10, and 360 µL was added in triplicate to a 96-well clear, flat-bottom plate (Corning). Absorbance was measured at 800 nm and platelet number was calculated according to the formula determined by Walkowiak et al. (41).
Seahorse Extracellular Flux.
The washed platelets were pelleted for 15 min (650 g, RT) and resuspended at a concentration of 2.5 × 108 platelets/mL in Seahorse media (Seahorse XF Assay Medium containing Glutamax; Agilent no. 102365-100, pH 7.4, 0.22-µm filtered, supplemented with 10 mM glucose and 1 mM sodium pyruvate) containing 100 nM PGE1. A 200-µL platelet solution was added to each well of a Seahorse XF24e plate previously coated with 1.12 µg Cell-Tak (Corning) for a final count of 5 × 107 platelets/well. The cell plate was centrifuged (300 g, 1 s, RT, no brake), the orientation of the cell plate was reversed, and it was centrifuged again (200 g, 1s, RT, no brake). Three-hundred microliters of Seahorse media without 100 nM PGE1 was added to each well for a final volume of 500 µL. The cell plate was placed in a non-CO2 incubator at 37°C for ≤1 h before assay.
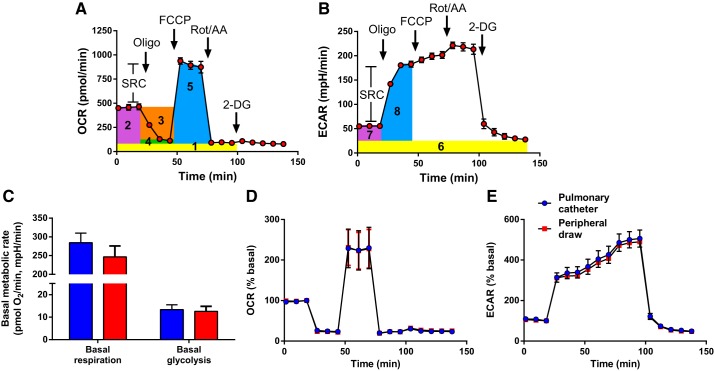
To measure platelet bioenergetics, the cell plate was equilibrated in the XF24e, and three basal measurements were followed by sequential injections of oligomycin (1µM), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; 0.6µM), a combination of rotenone (1 µM) and antimycin A (1 µM), and 2-deoxy-d-glucose (2-DG; 50 mM), all from Sigma. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were recorded for 24 min after each of the first three injections and 40 min following 2-DG (Fig. 1, A and B). The last measurement following each injection was used to calculate standard metrics of bioenergetics illustrated in Fig. 1. Rotenone/antimycin A-insensitive OCR was considered nonmitochondrial respiration, and 2-DG-insensitive ECAR was considered nonglycolytic acidification. Basal respiration was calculated by subtracting nonmitochondrial respiration from basal OCR. Basal glycolysis was calculated by subtracting nonglycolytic acidification from basal ECAR. All other parameters are presented as percent of basal by normalizing OCR and ECAR to the basal reading.
Fig. 1.
Representative data from Seahorse Extracellular Flux Assay. A and B: platelets were seeded at 50 × 106/well, and graphs show means ± SD of 4 replicate wells of oxygen consumption rate (OCR; A) and extracellular acidification rate (ECAR; B) from 1 female dyspnea patient. Arrows show sequential injections of oligomycin, FCCP, rotenone/antimycin A, and 2-deoxy-d-glucose (2-DG). Height of rectangles illustrates how we calculated metabolic parameters. In A, yellow 1 is nonmitochondrial respiration, purple 2 is basal respiration, orange 3 is ATP-linked respiration, green 4 is proton leak, and blue 5 is maximal respiration. Bar between maximal and basal respiration represents spare reserve capacity (SRC) of respiration. In B, yellow 6 is nonglycolytic acidification, purple 7 is basal glycolysis, and blue 8 is maximal glycolysis. Bar between maximal and basal glycolysis represents SRC of glycolysis. C–E: platelet metabolism is not altered by the collection techniques used in this study.
To ensure a robust basal ECAR signal (>20 mpH/min for the XF24e) given that platelet metabolism is highly oxidative with low basal glycolysis (19), we seeded 50 million platelets/well. If platelets consumed all oxygen from the media after injection of FCCP, these assays were excluded from analysis of maximal and spare reserve respiration, as the results would not be accurate. This resulted in a smaller sample size for analyses involving maximal and spare reserve respiration (n = 9 group 1, n = 11 group 2, n = 9 dyspnea, and n = 15 healthy controls).
Statistical analysis.
Continuous data are presented as means ± SD or SE, as specified. Categorical data are summarized as discrete values and percentages (n, %). Comparisons among all groups were conducted using one-way ANOVA or Kruskal-Wallis test when appropriate. Post hoc comparisons between group 1 PH and the other groups were conducted using Dunnett’s or Dunn’s multiple-comparisons test, depending on data distribution. Categorical variables were compared using Chi-Square and Fisher’s exact test. Correlation analyses were conducted by Pearson or Spearman correlation test as appropriate. All statistical analyses were two-tailed and considered statistically significant when α < 0.05, using the statistical package GraphPad Prism 6 (GraphPad Software Inc.).
RESULTS
Age affects basal glycolysis of platelets.
Our study contained a total of 60 subjects, including 15 patients with group 1 PH (PAH), 15 patients with group 2 PH (LHD), 14 patients with dyspnea and no PH upon RHC, and 16 healthy controls with no history of pulmonary or cardiovascular disease (Table 1). All groups were BMI matched, but healthy controls were significantly younger than patients (1-way ANOVA P < 0.0001, Dunnett’s test group 1 PH vs. HC P < 0.001; Table 1). No metrics of mitochondrial respiration correlated with age. However, age was positively correlated with basal glycolysis (Pearson r = 0.77, P = 0.002) but not with maximal or reserve glycolysis. Neither use of supplemental O2 nor treatment with anticoagulants was associated with any platelet metabolic parameter.
Table 1.
Patient demographics and clinical characteristics
| Group 1 PH, means ± SD or n (%) | Group 2 PH, means ± SD or n (%) | Dyspnea (No PH), means ± SD or n (%) | Healthy, means ± SD or n (%) | ANOVA P Value | |
|---|---|---|---|---|---|
| n | 15 | 15 | 14 | 16 | |
| Age, yr | 63.7 ± 16.2 | 66.6 ± 14.6 | 52.3 ± 12.0 | 46.6 ± 11.0‡ | <0.0001 |
| BMI, kg/m2 | 26.5 ± 5.1 | 30.3 ± 3.8 | 29.8 ± 8.5 | 26.7 ± 5.8 | 0.24 |
| Female sex | 10 (67) | 13 (87) | 12 (86) | 12 (71) | 0.50 |
| Caucasian race | 14 (93) | 13 (87) | 13 (93) | 14 (82) | 0.69 |
| Supplemental O2 | 4 (27) | 3 (20) | 0 | 0* | 0.04 |
| NYHA class III/IV | 9 (60) | 6 (40) | 3 (21) | 0.19 | |
| RAP, mmHg | 8.3 ± 2.7 | 10.5 ± 3.9 | 4.8 ± 1.9* | <0.001 | |
| mPAP, mmHg | 45.5 ± 11.9 | 36.7 ± 11.3* | 16.4 ± 2.8§ | <0.0001 | |
| PAWP, mmHg | 13.1 ± 3.3 | 19.9 ± 3.2§ | 9.3 ± 2.4* | <0.0001 | |
| PVR (Wood units) | 7.0 ± 4.6 | 3.5 ± 2.3† | 1.3 ± 0.5§ | <0.0001 | |
| CI, L·min−1·m2 | 3.3 ± 1.5 | 3.2 ± 1.3 | 3.0 ± 0.6 | 0.93 | |
| Diffusing capacity (DLco %predicted) | 45.8 ± 23.1 | 60.9 ± 20.4 | 81.3 ± 24.9‡ | 0.002 | |
| FVC (%predicted) | 80.3 ± 19.0 | 78.1 ± 19.3 | 88.6 ± 15.5 | 0.28 | |
| FEV1 (%predicted) | 72.6 ± 18.0 | 72.9 ± 20.6 | 84.4 ± 19.7 | 0.20 | |
| FEV1/FVC | 68.8 ± 7.8 | 70.4 ± 10.0 | 75.2 ± 13.0* | 0.024 | |
| Etiology of PAH | |||||
| Connective tissue disease | 6 (40) | ||||
| Idiopathic | 4 (27) | ||||
| Liver disease | 2 (13) | ||||
| Heritable | 1 (7) | ||||
| Congenital heart disease | 1 (7) | ||||
| Pulmonary veno-occlusive disease | 1 (7) | ||||
| Medication use | |||||
| Anticoagulant | 10 (67) | 8 (53) | 7 (50) | 0.63 | |
| PDE5 inhibitor | 8 (53) | 2 (13) | 0* | 0.002 | |
| Endothelin receptor antagonist | 5 (33) | 2 (13) | 0* | 0.047 | |
| Riociguat | 4 (27) | 1 (7) | 1 (7) | 0.19 | |
| Prostacyclin analog | 2 (13) | 1 (7) | 0 | 0.49 | |
| Smoking history | |||||
| Former | 9 (60) | 7 (47) | 1 (7)† | 1 (6)† | 0.001 |
| Current | 0 | 0 | 1 (7) | 0 | 0.16 |
| Comorbidities | |||||
| Diabetes mellitus | 1 (7) | 2 (13) | 2 (14) | 0.78 | |
| Chronic obstructive pulmonary disease | 3 (20) | 2 (13) | 1 (7) | 0.60 | |
| Scleroderma | 5 (33) | 0* | 1 (7) | 0.02 | |
| Rheumatoid arthritis | 0 | 1 (7) | 2 (14) | 0.43 |
Values are means ± SD. CI, cardiac index; DLco, carbon monoxide diffusion capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE5, phosphodiesterase 5; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure.
P < 0.05,
P < 0.01,
P < 0.001, and
P < 0.0001
vs. group 1 PH.
Platelets from group 1 PH patients exhibit increased maximal and reserve respiration.
Platelets from patients with group 1 PH showed increased maximal (Dunnett’s test P = 0.022) and reserve (Dunnett’s test P = 0.035) respiration compared with healthy controls but did not differ from platelets of patients with group 2 PH or dyspnea without PH (Table 2), suggesting that the phenotype of increased maximal and reserve respiration is not unique to group 1 PH or even to PH but possibly a consequence of conditions associated with dyspnea. Because collection methods were categorically different between all patients (pulmonary catheter during RHC) and all controls (peripheral blood draw), we experimentally confirmed that our collection techniques had no effect on platelet metabolism (Fig. 1, C–E). Finally, there were no differences in platelet basal respiration, ATP-linked respiration, or proton leak between group 1 PH and the other groups (Table 2).
Table 2.
Platelet mitochondrial metabolism
| Group 1 PH | Group 2 PH | Dyspnea (No PH) | Healthy | ANOVA P Value | |
|---|---|---|---|---|---|
| Basal, pmol/min | 434 ± 93 | 381 ± 162 | 402 ± 120 | 473 ± 106 | 0.372 |
| ATP-linked, %basal | 78 ± 2 | 75 ± 6 | 76 ± 5 | 77 ± 4 | 0.137 |
| Maximal, %basal | 180 ± 30 | 168 ± 47 | 168 ± 47 | 138 ± 38* | 0.030 |
| Reserve, %basal | 97 ± 34 | 88 ± 47 | 90 ± 48 | 56 ± 39* | 0.037 |
| Proton leak, %basal | 5 ± 3 | 5 ± 4 | 3 ± 4 | 5 ± 3 | 0.289 |
Values are means ± SD. PH, pulmonary hypertension;
P < 0.05 vs. group 1 PH, Dunnett’s test.
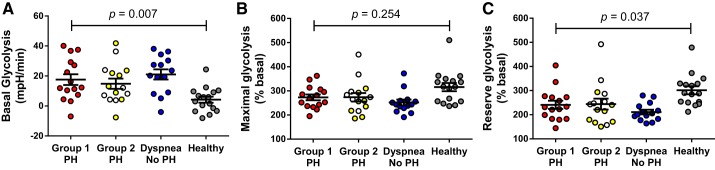
Platelets from group 1 PH patients exhibit enhanced basal glycolysis and lower reserve glycolysis.
Platelets from group 1 PH patients showed increased basal glycolysis compared with healthy controls (Dunnett’s test P = 0.007; Fig. 2A) but did not differ from platelets of patients with group 2 PH or dyspnea without PH, suggesting that this glycolytic phenotype is not unique to group 1 PH or even to PH. Because basal glycolysis was positively correlated with age and patients were significantly older than healthy controls, the enhanced basal glycolysis in patients versus healthy controls could be explained partly by age. Maximal glycolysis did not significantly differ between platelets from group 1 PH and healthy controls (Kruskal-Wallis test P = 0.029, Dunn’s test P = 0.254; Fig. 2B). Reserve glycolysis was lower in platelets from group 1 PH patients compared with healthy controls (Dunn’s test P = 0.037; Fig. 2C) but not different from platelets of group 2 PH or dyspnea patients with no PH. Of note is that neither maximal nor reserve glycolysis correlated with age.
Fig. 2.
Platelet glycolytic metabolism in patients with pulmonary hypertension (PH), patients with dyspnea, and healthy controls. Basal glycolysis (A) was increased, whereas reserve glycolysis (B) was decreased in group 1 PH platelets (n = 15, 10 females) compared with healthy control platelets (n = 16; 12 female) but did not differ from platelets of group 2 PH (n = 15; 13 female) or dyspnea patients with no PH (n = 14, 12 female). C: reserve glycolysis is the difference between basal and maximal glycolysis, which also trended lower in group 1 PH vs. healthy control platelets. Group 2 PH includes patients with isolated post-capillary PH (○) and combined pre- and post-capillary PH (yellow circles). Graphs show means ± SE. P values were calculated using 1-way ANOVA, with post hoc Dunnett’s test between group 1 PH and healthy controls (basal glycolysis) and Kruskal-Wallis test with post-hoc Dunn’s test between group 1 PH and healthy control (maximal and reserve glycolysis).
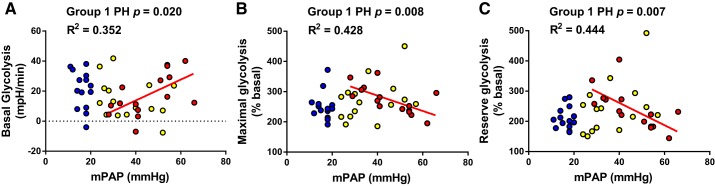
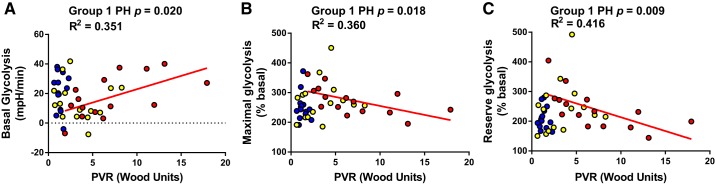
Platelet glycolytic metabolism correlated with mPAP and PVR only in group 1 PH patients.
We tested whether glycolytic parameters measured via Seahorse Extracellular Flux correlated with hemodynamic severity. Correlations between platelet glycolysis and hemodynamics were observed in group 1 PH patients but not group 2 PH or dyspnea patients with normal hemodynamics. In group 1 PH, mPAP was directly associated with basal platelet glycolysis (Pearson r = 0.593, P = 0.020) and inversely associated with maximal (Pearson r = −0.654, P = 0.008) and reserve platelet glycolysis (Spearman r = −0.666, P = 0.007; Fig. 3). Similarly, PVR was directly associated with basal platelet glycolysis (Pearson r = −0.593, P = 0.020) and inversely associated with maximal (Pearson r = −0.60, P = 0.018) and reserve platelet glycolysis (Pearson r = −0.645, P = 0.009; Fig. 4). Platelet glycolysis did not correlate with RAP, PAWP, or CI in any group (data not shown).
Fig. 3.
Pulmonary artery pressure correlated with platelet glycolytic metabolism only in group 1 pulmonary hypertension (PH). Mean pulmonary artery pressure (mPAP) correlated with platelet basal (A), maximal (B), and reserve (C) glycolysis in group 1 PH (red; n = 15, 10 females) but not group 2 PH (yellow; n = 15, 13 females) or dyspnea with no PH (blue; n = 14, 12 female). P and r2 values calculated using Pearson correlation analysis (basal, maximal glycolysis) or Spearman correlation analysis (reserve glycolysis).
Fig. 4.
Pulmonary vascular resistance (PVR) correlated with platelet glycolytic metabolism only in group 1 pulmonary hypertension (PH). PVR correlated with platelet basal (A), maximal (B), and reserve (C) glycolysis in group 1 PH (red; n = 15, 10 females) but not group 2 PH (yellow; n = 15, 13 female) or dyspnea with no PH (blue; n = 14, 12 females). P and r2 values calculated using Pearson correlation analysis.
Platelet glycolytic metabolism differs between group 1 and 2 PH patients when stratified by mPAP and PVR.
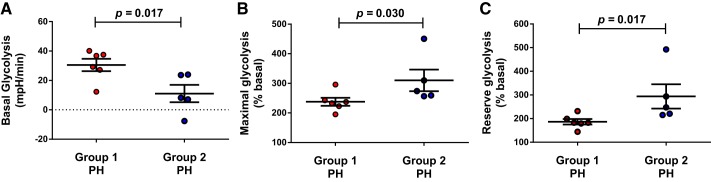
We stratified all PH patients using this study’s median PVR of 4.15 Wood units and median mPAP of 41 mmHg. Platelet glycolysis from PH patients with both mPAP and PVR greater than the study median differed between group 1 PH (n = 6) and group 2 (n = 5; Fig. 5). In patients with mPAP and PVR above the median, basal glycolysis was greater (Mann-Whitney test P = 0.017), whereas maximal glycolysis (Mann-Whitney test P = 0.030) and reserve glycolysis (Mann-Whitney test P = 0.017) were lower in platelets from group 1 compared with group 2 PH patients.
Fig. 5.
Platelet glycolysis differed between group 1 and 2 pulmonary hypertension (PH) patients when stratified by hemodynamics. Group 1 and 2 PH patients with both mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) above the study median were compared. Platelet basal glycolysis (A) was higher, whereas platelet maximal glycolysis (B) and reserve glycolysis (C) were lower in group 1 PH (n = 6, 5 female) vs. group 2 PH (n = 5, 4 females, 5 combined pre- and post-capillary PH). Graphs show means ± SE. P values calculated using Mann-Whitney tests.
DISCUSSION
In this study, we investigated whether platelets from group 1 PH patients exhibit a distinct metabolic phenotype characterized by a shift toward glycolytic metabolism. Because platelet metabolism is sensitive to many factors, including smoking (36), physical activity (13, 43), asthma (42, 45), acute coronary syndromes (21), remote ischemia (14), and pulmonary embolism (32), we compared group 1 PH platelets with not only healthy controls but also group 2 PH and with dyspnea patients who had normal hemodynamics upon RHC but share broad respiratory symptomology and reduction in physical activity. We found that platelet metabolism was abnormal in patients with group 1 PH compared with healthy controls, but the same abnormalities were present in platelets from group 2 PH and patients who underwent RHC due to dyspnea yet had normal hemodynamics (Table 2 and Fig. 2). Platelets from group 1 PH, group 2 PH, and dyspnea patients all showed increased basal glycolysis. However, because basal glycolysis was directly associated with age and patients were significantly older than healthy controls, this difference could be explained by age. Indeed, defects in platelet mitochondria (9, 33, 44) and an increase in basal glycolysis (12) have been associated with aging. However, platelets from group 1 PH, group 2 PH, and dyspnea patients also showed decreased glycolytic reserve, increased maximal respiration, and increased respiratory reserve compared with healthy control platelets, none of which were correlated with age and thus are possibly a consequence of conditions associated with dyspnea, such as a decrease in physical activity.
Although in our study it was not possible to use platelet bioenergetics as a specific biomarker of PAH since the platelet glycolytic signature could be found in other cardiorespiratory conditions, we showed that platelet glycolysis correlated with hemodynamic severity only in group 1 PH patients but not group 2 PH or patients with dyspnea (Figs. 3 and 4). Stratifying groups 1 and 2 PH patients based on the study median PVR (4.15 Wood units) and mPAP (41 mmHg) revealed that platelet glycolysis was elevated in PH group 1 patients compared with group 2 PH when strictly considering subjects with mPAP and PVR above the median (Fig. 5). The associations between platelet glycolysis and hemodynamic severity unique to group 1 PH suggest that a glycolytic switch is indeed associated with PAH pathogenesis and can be detected in platelets ex vivo. Given these findings, although platelet bioenergetics may not be useful as a specific biomarker, they may be a useful addition to risk stratification in PAH.
We identified a positive correlation between both mPAP and PVR with basal platelet glycolysis only in group 1 PH patients (Figs. 3 and 4). This relationship is consistent with the known glycolytic shift of pulmonary vasculature during PAH that has been described in vitro using pulmonary artery endothelial and smooth muscle cells cultured from PAH lungs and using [18F]fluoro-2-deoxyglucose PET scanning of the lung in vivo (8, 47). However, in our study, platelets did not undergo a corresponding inhibition of mitochondrial oxidative phosphorylation as seen in cultured PAH pulmonary artery endothelial and smooth muscle cells (8, 47). Although this difference may be cell specific, it is also possible that platelets measured immediately ex vivo are able to capture an important metabolic feature that is lost by passaging cells in culture. If so, the glycolytic shift in vivo may have less to do with ATP production than with intermediates produced via glycolysis or a parallel pathway such as the pentose phosphate pathway (PPP) or the hexosamine biosynthetic pathway (HBP), which has been implicated in PAH (5).
We also identified glycolytic changes in platelets over the course of PAH that have not yet been identified in cultured PAH cells and that are not currently measurable in vivo by [18F]fluoro-2-deoxyglucose PET scanning. We found a negative correlation between both mPAP and PVR with maximal platelet glycolysis (Figs. 3 and 4). Because the glycolytic reserve is the difference between maximal and basal glycolysis, the increased basal and decreased maximal glycolysis also resulted in a negative correlation between mPAP and PVR with platelet glycolytic reserve (Figs. 3 and 4). Therefore, even as platelets exhibit an increased rate of glycolysis with PAH progression, their maximal capacity for glycolysis is reduced, compromising the ability to dynamically upregulate glycolysis in the event of metabolic stress. The result is that at later stages of PAH, just as platelets apparently require more glucose flux through glycolysis and/or parallel pathways such as the PPP or HBP (as evidenced by increased basal glycolysis seen in this study), their capacity for doing so (maximal and spare reserve capacity) is reduced. It is possible that the glycolytic switch in PAH is a compensatory mechanism to supply intermediates provided by such pathways, and final stages of the disease occur only when the cellular ability to increase glycolysis fails to keep up with metabolic demand.
Our study was limited by a relatively small sample size and the use of group 1 PH patients that were not treatment-naïve. Further investigation is needed to understand whether platelet bioenergetics is modified by the use of PH-specific therapies. We were not able to address the underlying mechanism of platelet metabolic dysfunction in this study. However, because metabolic alterations are found not only in platelets but in vascular and muscle cells (1, 23), the mechanism does not seem to be mechanical, requiring passage through lung lesions. Finally, we did not compare group 1 PH with groups 3–5. Despite these limitations, we identified correlations between platelet glycolysis and hemodynamics that were unique to group 1 PH compared with group 2 PH and patients with dyspnea. Furthermore, only hemodynamic measures that could be associated directly with pulmonary vascular remodeling (mPAP and PVR) were correlated with platelet glycolysis in group 1 PH. Neither PAWP (which is not associated with PAH pathology) nor metrics of heart performance (CI and RAP) were correlated with platelet glycolysis.
Conclusions.
Platelets from group 1 PH patients showed a distinct glycolytic phenotype compared with platelets from healthy controls but could not be distinguished from age and BMI-matched patients with group 2 PH or dyspnea with normal hemodynamics, indicating that other components of poor respiratory health contribute to the platelet glycolytic signature. Platelet glycolysis was correlated with hemodynamic severity only in group 1 PH patients, supporting the idea that PAH involves a metabolic switch that is detectable in the periphery. Whereas mPAP and PVR were directly associated with basal glycolysis in platelets from group 1 PH, they were indirectly associated with maximal and reserve glycolysis, suggesting that PAH progression reduces glycolytic capacity even while demanding an increase in glycolytic metabolism. Although we were unable to resolve a unique glycolytic profile in platelets as a biomarker of group 1 PH, our data show that platelets do undergo a distinct metabolic switch as PAH progresses and may, therefore, provide insight into PAH pathology during disease progression.
GRANTS
This work was supported by NIH Grant R01-HL-130209 (R. A. Dweik).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.M., A.R.T., and R.A.D. conceived and designed research; R.E.M., A.A., C.A.M., B.E.B., J.E.N., and A.R.T. performed experiments; R.E.M., A.A., and A.R.T. analyzed data; R.E.M., K.S.A., A.R.T., and R.A.D. interpreted results of experiments; R.E.M. prepared figures; R.E.M. drafted manuscript; R.E.M., K.S.A., A.R.T., and R.A.D. edited and revised manuscript; R.E.M., K.S.A., A.A., C.A.M., B.E.B., J.E.N., A.R.T., and R.A.D. approved final version of manuscript.
REFERENCES
- 1.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 3: 144–152, 2013. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JGN, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 3.Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes 120: 248–251, 2012. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, Dweik RA. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 302: L512–L520, 2012. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes JW, Tian L, Heresi GA, Farver CF, Asosingh K, Comhair SAA, Aulak KS, Dweik RA. O-linked β-N-acetylglucosamine transferase directs cell proliferation in idiopathic pulmonary arterial hypertension. Circulation 131: 1260–1268, 2015. doi: 10.1161/CIRCULATIONAHA.114.013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazzichi L, Giannaccini G, Betti L, Fabbrini L, Schmid L, Palego L, Giacomelli C, Rossi A, Giusti L, De Feo F, Giuliano T, Mascia G, Bombardieri S, Lucacchini A. ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia. Clin Biochem 41: 1084–1090, 2008. doi: 10.1016/j.clinbiochem.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shachar D, Bonne O, Chisin R, Klein E, Lester H, Aharon-Peretz J, Yona I, Freedman N. Cerebral glucose utilization and platelet mitochondrial complex I activity in schizophrenia: A FDG-PET study. Prog Neuropsychopharmacol Biol Psychiatry 31: 807–813, 2007. doi: 10.1016/j.pnpbp.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 9.Braganza A, Corey CG, Santanasto AJ, Distefano G, Coen PM, Glynn NW, Nouraie SM, Goodpaster BH, Newman AB, Shiva S. Platelet bioenergetics correlate with muscle energetics and are altered in older adults. JCI Insight 5: 128248, 2019. doi: 10.1172/jci.insight.128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, Novelli EM, Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood 123: 2864–2872, 2014. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 146: 389–397, 1995. [PMC free article] [PubMed] [Google Scholar]
- 12.D’Aurelio M, Merlo Pich M, Catani L, Sgarbi GL, Bovina C, Formiggini G, Parenti Castelli G, Baum H, Tura S, Lenaz G. Decreased Pasteur effect in platelets of aged individuals. Mech Ageing Dev 122: 823–833, 2001. doi: 10.1016/S0047-6374(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 13.de Lucas RD, Caputo F, Mendes de Souza K, Sigwalt AR, Ghisoni K, Lock Silveira PC, Remor AP, da Luz Scheffer D, Guglielmo LG, Latini A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J Sports Sci 32: 22–30, 2014. doi: 10.1080/02640414.2013.797098. [DOI] [PubMed] [Google Scholar]
- 14.Dezfulian C, Taft M, Corey C, Hill G, Krehel N, Rittenberger JC, Guyette FX, Shiva S. Biochemical signaling by remote ischemic conditioning of the arm versus thigh: Is one raise of the cuff enough? Redox Biol 12: 491–498, 2017. doi: 10.1016/j.redox.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duong HT, Comhair SA, Aldred MA, Mavrakis L, Savasky BM, Erzurum SC, Asosingh K. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ 1: 475–486, 2011. doi: 10.4103/2045-8932.93547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herve P, Humbert M, Sitbon O, Parent F, Nunes H, Legal C, Garcia G, Simonneau G. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med 22: 451–458, 2001. doi: 10.1016/S0272-5231(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J Vis Exp 85: 51301, 2014. doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2: 206–210, 2014. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, Hansen KC, Moldovan R, McKeon BA, Frid M, Kumar S, Li H, Liu H, Caánovas A, Medrano JF, Thomas MG, Iloska D, Plecitá-Hlavatá L, Ježek P, Pullamsetti S, Fini MA, El Kasmi KC, Zhang Q, Stenmark KR. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation 134: 1105–1121, 2016. [Erratum in Circulation 139: e1, 2019]. 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Farré AJ, Zamorano-Leon JJ, Azcona L, Modrego J, Mateos-Cáceres PJ, González-Armengol J, Villarroel P, Moreno-Herrero R, Rodríguez-Sierra P, Segura A, Tamargo J, Macaya C. Proteomic changes related to “bewildered” circulating platelets in the acute coronary syndrome. Proteomics 11: 3335–3348, 2011. doi: 10.1002/pmic.201000708. [DOI] [PubMed] [Google Scholar]
- 22.Lundgrin EL, Park MM, Sharp J, Tang WHW, Thomas JD, Asosingh K, Comhair SA, DiFilippo FP, Neumann DR, Davis L, Graham BB, Tuder RM, Dostanic I, Erzurum SC. Fasting 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc 10: 1–9, 2013. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malenfant S, Potus F, Fournier F, Breuils-Bonnet S, Pflieger A, Bourassa S, Tremblay È, Nehmé B, Droit A, Bonnet S, Provencher S. Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J Mol Med (Berl) 93: 573–584, 2015. doi: 10.1007/s00109-014-1244-0. [DOI] [PubMed] [Google Scholar]
- 24.McMurtry MS, Bonnet S, Wu X, Dyck JRB, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95: 830–840, 2004. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 25.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR, Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: eaao4583, 2017. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 26.Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J Alzheimers Dis 27: 483–490, 2011. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen QL, Corey C, White P, Watson A, Gladwin MT, Simon MA, Shiva S. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. JCI Insight 2: e91415, 2017. doi: 10.1172/jci.insight.91415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen QL, Wang Y, Helbling N, Simon MA, Shiva S. Alterations in platelet bioenergetics in Group 2 PH-HFpEF patients. PLoS One 14: e0220490, 2019. doi: 10.1371/journal.pone.0220490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 115: 148–164, 2014. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 30.Prestia FA, Galeano P, Martino Adami PV, Do Carmo S, Castaño EM, Cuello AC, Morelli L. Platelets Bioenergetics Screening Reflects the Impact of Brain Aβ Plaque Accumulation in a Rat Model of Alzheimer. Neurochem Res 44: 1375–1386, 2019. doi: 10.1007/s11064-018-2657-x. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets 20: 588–593, 2009. [Erratum in Platelets 21: 84, 2010]. 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 32.Rezania S, Puskarich MA, Petrusca DN, Neto-Neves EM, Rondina MT, Kline JA. Platelet hyperactivation, apoptosis and hypercoagulability in patients with acute pulmonary embolism. Thromb Res 155: 106–115, 2017. doi: 10.1016/j.thromres.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Shi C, Guo K, Yew DT, Yao Z, Forster EL, Wang H, Xu J. Effects of ageing and Alzheimer’s disease on mitochondrial function of human platelets. Exp Gerontol 43: 589–594, 2008. doi: 10.1016/j.exger.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53: 1801913, 2019. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjövall F, Morota S, Asander Frostner E, Hansson MJ, Elmér E. Cytokine and nitric oxide levels in patients with sepsis—temporal evolvement and relation to platelet mitochondrial respiratory function. PLoS One 9: e97673, 2014. [Erratum in PLoS One 9: e103756, 2014]. doi: 10.1371/journal.pone.0097673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PR, Cooper JM, Govan GG, Harding AE, Schapira AHV. Smoking and mitochondrial function: a model for environmental toxins. Q J Med 86: 657–660, 1993. doi: 10.1093/qjmed/86.10.657. [DOI] [PubMed] [Google Scholar]
- 37.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab 19: 558–573, 2014. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 188: 365–369, 2013. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Shively C, Andrews RN, Neth B, Keene CD, Mintz A, Craft S, Molina AJA. Blood-based bioenergetic profiling reflects differences in brain bioenergetics and metabolism. Oxid Med Cell Longev 2017: 1–9, 2017. doi: 10.1155/2017/7317251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JSR, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 62, Suppl: D100–D108, 2013. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Walkowiak B, Kȩsy A, Michalec L. Microplate reader—a convenient tool in studies of blood coagulation. Thromb Res 87: 95–103, 1997. doi: 10.1016/S0049-3848(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 42.Winnica D, Corey C, Mullett S, Reynolds M, Hill G, Wendell S, Que L, Holguin F, Shiva S. Bioenergetic Differences in the Airway Epithelium of Lean Versus Obese Asthmatics Are Driven by Nitric Oxide and Reflected in Circulating Platelets. Antioxid Redox Signal 31: 673–686, 2019. doi: 10.1089/ars.2018.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu LH, Chang SC, Fu TC, Huang CH, Wang JS. High-intensity interval training improves mitochondrial function and suppresses thrombin generation in platelets undergoing hypoxic stress. Sci Rep 7: 4191, 2017. doi: 10.1038/s41598-017-04035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Shi C, Li Q, Wu J, Forster EL, Yew DT. Mitochondrial dysfunction in platelets and hippocampi of senescence-accelerated mice. J Bioenerg Biomembr 39: 195–202, 2007. doi: 10.1007/s10863-007-9077-y. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Cardenes N, Corey C, Erzurum SC, Shiva S. Platelets from asthmatic individuals show less reliance on glycolysis. PLoS One 10: e0132007, 2015. doi: 10.1371/journal.pone.0132007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol 1: 357–372, 2011. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 104: 1342–1347, 2007. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang YL, Zhang R, Shen YF, Huang KY, He YY, Zhao JH, Jing ZC. 3-Bromopyruvate Attenuates Experimental Pulmonary Hypertension via Inhibition of Glycolysis. Am J Hypertens 32: 426–432, 2019. doi: 10.1093/ajh/hpy191. [DOI] [PubMed] [Google Scholar]