Abstract
Tendon injuries are a common clinical condition with limited treatment options. The cellular components of the innate immune system, such as neutrophils and macrophages, have been studied in tendon injuries. However, the adaptive immune system, comprising specialized lymphocytes, plays an important role in orchestrating the healing of numerous tissues, but less is known about these cells in tendon healing. To gain a greater understanding of the biological processes that regulate tendon healing, we determined how the cellular components of the adaptive and innate immune system respond to a tendon injury using two-month-old male mice. We observed that lymphatic vasculature is present in the epitenon and superficial regions of Achilles tendons, and that the lymphatics drain into the popliteal lymph node. We then created an acute Achilles tenotomy followed by repair, and collected tendons and popliteal lymph nodes 1, 2, and 4 wk after injury. Tendon injury resulted in a robust adaptive immune cell response that followed an initial innate immune cell response in tendons and lymph nodes. Monocytes, neutrophils, and macrophages initially accumulated at 1 wk after injury in tendons, while dendritic cells and CD4+ T cells peaked at 2 wk after injury. B cells and CD8+ T cells progressively increased over time. In parallel, immune cells of the popliteal lymph node demonstrated a similarly coordinated response to the injury. These results suggest that there is an adaptive immune response to tendon injury, and adaptive immune cells may play a role in regulating tendon healing.
NEW & NOTEWORTHY While the innate immune system, consisting of macrophages and related hematopoietic cells, has been studied in tendon injury, less is known about the adaptive immune system. Using a mouse model of Achilles tendon tenotomy and repair, we observed an adaptive immune cell response, consisting of CD4+ and CD8+ T cells, and B cells, which occur through 4 wk after tendon injury. This response appeared to be coordinated by the draining popliteal lymph node.
Keywords: B cells, lymph nodes, lymphocytes, T cells, tenocytes
INTRODUCTION
Tendon injuries, which often occur due to excess mechanical loading, are a common clinical disorder in musculoskeletal medicine (23, 38). A scar is often formed at the site of tendon tears, which can impair tissue biomechanical properties and overall mobility (16). The innate immune system, consisting, in part, of macrophages, neutrophils, and other related hematopoietic cell types, has been studied in different models of tendon tear and repair, and is thought to play a role in the formation and remodeling of scar tissue (38). In acute tendon injuries, there is a rapid and transient accretion of neutrophils and proinflammatory M1 macrophages, followed by a slow and persistent accumulation of anti-inflammatory M2 macrophages (22, 35, 38). While numerous studies have documented the role of the innate immune system in tendon injuries, less is known about the potential roles of T cells, B cells, and other components of the adaptive immune system in tendon healing and scar formation.
Emerging evidence has indicated that both the adaptive and innate immune systems work along with tissue-resident cells to coordinate tissue repair and fibrosis (15, 20). Upon peripheral tissue injury, signals such as cytokines and cells, travel via lymphatic vessels to the draining lymph node to stimulate the T and B cells there to initiate an adaptive immune response (4, 20). With immune responses, the draining lymph node undergoes hypertrophy, reflecting both increased lymphocyte trafficking to and proliferation in lymph nodes (4). While recent studies in humans have identified that the adaptive immune system is activated in chronic degenerative tendinopathies (25), less is known about whether the adaptive immune response plays a role in frank tendon tears. Additionally, the lymphatic system, which allows for the recovery of interstitial fluid from tissue and regulates the immune cell response to an injury (4, 32), has not been well studied in the context of tendon injuries. Since further understanding of the role of the adaptive immune response may help to identify potential therapeutic interventions to improve outcomes for patients with tendon injuries, our objective was to study the adaptive immune cell and lymph node response to an acute tendon injury and repair. We hypothesized that an adaptive immune cell response would be present after an acute tendon injury and repair, along with a corresponding lymph node response. To test this hypothesis, we identified the location of the lymphatic vasculature in Achilles tendons and draining lymph nodes, and then performed an acute Achilles tenotomy and repair in mice. Tendons and lymph nodes were collected 1, 2, and 4 wk after the surgical intervention. Flow cytometry and immunofluorescence staining of tissue sections were performed using markers of innate and adaptive immune cells in parallel with measurement of changes in expression of genes with known roles in immune cell recruitment, extracellular matrix (ECM) composition, and tenogenesis.
METHODS
Animals.
This study was approved by the Hospital for Special Surgery/Weill Cornell Medical College/Memorial Sloan Kettering Cancer Center, Institutional Animal Care and Use Committee (IACUC; protocol 2017-0035). All experiments were conducted in accordance with our IACUC protocol and were consistent with the U.S. Public Health Service “Policy on the Humane Care and Use of Laboratory Animals”. Two-month-old male C57BL/6J mice (strain 000664; Jackson Laboratories, Bar Harbor, ME) and transgenic mice that express green fluorescent protein (GFP) under the control of a 4-kb segment of the scleraxis promoter (ScxGFP) (28) were used in this study. This age was selected to be reflective of early adulthood in humans, as the cells within rodent tendons have low proliferation rates and are mostly differentiated at this point, but the ECM is still actively being synthesized at a low rate (1, 8, 13, 24). Mice were housed under specific pathogen-free conditions and provided with ad libitum access to food and water.
Surgeries.
To identify the lymph node that drains the Achilles tendon, mice (n = 4) were deeply anesthetized with 2% isoflurane, and the skin overlying the lower limb was shaved and cleaned with 4% chlorhexidine. Approximately 20 µL of 2% Evan’s Blue Dye (EBD; Sigma Aldrich, St. Louis, MO) was injected into the peritendinous space of the Achilles tendons of mice. The EBD was allowed to drain into the lymphatics and draining lymph node for 15 min, and the mice were then euthanized with carbon dioxide followed by cervical dislocation. The skin overlying the lower limb was carefully dissected from the mice to visualize the popliteal lymph nodes.
A separate cohort of mice underwent an Achilles tenotomy and repair, using techniques modified from a previous study (27, 35). To ensure consistency between samples, a single surgeon performed tenotomy and repair procedures. Mice were deeply anesthetized with 2% isoflurane and placed in a prone position. The skin overlying the surgical site was shaved and cleaned with 4% chlorhexidine. A midline skin incision was made on the posterodistal aspect of the hindlimb, and the paratenon was reflected. A full-thickness tenotomy was completed in the midsubstance of the Achilles tendon followed by immediate two-strand repair using a modified Kessler technique with 6-0 Prolene suture (Ethicon, Somerville, NJ). The skin was then closed with interrupted sutures using 6-0 Prolene suture (Ethicon). The plantaris tendon was left intact. Buprenorphine (0.05 mg/kg; Reckitt, Parsippany, NJ) was administered for analgesia during the postoperative recovery period. Weight-bearing and cage activity were allowed after surgery, and mice were closely monitored thereafter for signs of pain or distress. Surgical interventions were well tolerated.
To be reflective of early, middle, and late stages of tendon healing, at either 1, 2, or 4 wk after tenotomy and repair (n = 12 mice per time point), mice were euthanized by exposure to carbon dioxide followed by cervical dislocation, and Achilles tendons were harvested for flow cytometry, gene expression, and histology. All tendons and lymph nodes from injured animals in this study had grossly apparent intact tendon repairs. Popliteal lymph nodes were also harvested for flow cytometry. Achilles tendons and popliteal lymph nodes were also collected from control mice that did not undergo surgical repair. The Achilles tendons of an additional set of uninjured ScxGFP mice (n = 4) were collected, as described above, for experiments to visualize the presence of the lymphatic vasculature in tendons. Bilateral tissues were combined for flow cytometry and gene expression analysis.
We also sought to determine the specificity of the immune cell response to the tenotomy and not the skin injury alone. We performed sham surgeries (n = 2 mice per time point) in which a skin incision was performed, no manipulation occurred to the tendon, and the skin was immediately closed as described above. Tissues were harvested at 1 and 2 wk after surgery.
Histology.
Histology was performed as previously described (11, 18). Achilles tendons were placed in 30% sucrose solution for 1 h and then snap frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and stored at −80°C until use. Tissues were sectioned at a thickness of 10 µm in a cryostat. For the immunolocalization of the lymphatic vasculature in the tendon, slides were fixed in 4% paraformaldehyde, permeabilized in 0.2% Triton X-100, and blocked with 5% goat serum. Slides were incubated with primary antibodies against CD31 (1:100, cat. no. 550274, BD, San Diego, CA) to label endothelial cells and LYVE-1 (1:200, cat. no. 14044380, Thermo Fisher Scientific) and podoplanin (1:300, cat. no. 127408, BioLegend, San Diego, CA) to label the lymphatic vasculature (17). To visualize the immune cell response after injury in the tendon, tissues were fixed in ice-cold acetone for 10 min, blocked with 5% goat serum for 1 h, and stained with biotinylated antibodies against CD11b to identify myeloid cells (1:1,000, cat. no. 101204, BioLegend), CD3 to identify T cells (1:250, cat. no. ab5690; Abcam), and FITC-conjugated antibodies against B220 to identify B cells (1:100, cat. no. 103206; BioLegend). Secondary antibodies conjugated to Alexa Fluor 405 (1:400, cat. no. ab175671; Abcam), Alexa Fluor 555 (1:400, cat. no. A21428; Thermo Fisher Scientific), or Alexa Fluor 647 (1:400, cat. no. 405510, BioLegend; 1:500, cat. no. A21245; Thermo Fisher Scientific) or streptavidin conjugated to Alexa Fluor 647 (1:500, S32357; Invitrogen) were used to detect primary antibodies that were not already conjugated to a fluorophore. Nuclei were stained with DAPI (1:500; Sigma Aldrich). High-resolution images were captured with an Eclipse Ni-E microscope (Nikon, Melville, NY).
Flow cytometry.
Freshly harvested tendons and lymph nodes were prepared for flow cytometry, as modified from previous studies (18, 33). Tissues were finely minced and then digested in type II collagenase (616 U/mL; Worthington Biochemical, Lakewood, NJ), DNase I (80 µg/mL; Sigma Aldrich), and EDTA (20 µL/mL; Thermo Fisher Scientific) for 40 min at 37°C. Cell suspensions were subsequently triturated with glass pipettes and passed through 70-µm filters to remove undigested debris. Total cell counts were determined using a Z Series Coulter Counter (Beckman Coulter, Indianapolis, IN).
Cells were labeled with fluorophore-conjugated antibodies from BioLegend, including antibodies against B220 (cat. no. 103206), CD3 (cat. no. 100330), CD4 (cat. no. 100434), CD8 (cat. no. 100730), CD11b (cat. no. 101233), CD11c (cat. no. 117324), CD64 (cat. no. 139304), Ly6C (cat. no. 128014), and MHC II (cat. no. 116415). Cells were then fixed and permeabilized with Cytofix (BD) and then labeled with antibodies against intracellular IgG (cat. no. 405308) to detect intracellular IgG that are high in plasma cells (18). Gating strategies are based on our previously published work (2, 14, 18). Although the use of intracellular staining precluded the use of DAPI to assess dead cells, parallel samples that were not fixed, but were labeled with CD45 (cat. no. 103127) and DAPI (cat. no. 422801) demonstrated that 95% of tendon and lymph node CD45+ cells were DAPI−, indicating that they were viable cells. Cells were analyzed using a FACSCanto (BD) and FlowJo software (version 10; Tree Star, Ashland, OR).
Gene expression.
RNA isolation and gene expression were performed as modified from previous reports (6, 9, 36). Achilles tendons were placed in TRIzol Reagent (Life Technologies, Carlsbad, CA) in tubes containing 1.5-mm zirconium beads (Benchmark Scientific, Sayreville, NJ), processed in a shaking bead mill for two cycles of 3 min, and then snap frozen and stored at −80°C. Samples were thawed on ice, and RNA was isolated using MaXtract high-density columns (Qiagen, Valencia, CA), followed by further purification with Zymogen IC spin columns (Zymo Research, Irvine, CA). RNA was treated with DNase I (Qiagen) and quantified using a Nanodrop system (Thermo Fisher Scientific). Samples with A260/A280 ≥ 1.8 were used in downstream analysis. Approximately 100 ng of RNA was reverse transcribed into cDNA with iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Equal amounts of cDNA were amplified in a CFX96 real-time thermal cycler (Bio-Rad) using SsoFast Universal SYBR Green Supermix (Bio-Rad). Target gene expression was normalized to the stable housekeeping gene Ppid using the 2−ΔCt method. Primer sequences are provided in Table 1.
Table 1.
Primer sequences used for quantitative PCR
| Gene | Description | Forward Primer | Reverse Primer | Size (bp) | Reference Sequence |
|---|---|---|---|---|---|
| Ccl2 | Chemokine (C-C motif) ligand 2 | CCTGCTGCTACTCATTCACCA | ATTCCTTCTTGGGGTCAGCA | 147 | NM_011333.3 |
| Col1a1 | Collagen, type I, α1 | ACTGCAACATGGAGACAGGTCAGA | ATCGGTCATGCTCTCTCCAAACCA | 128 | NM_007742.4 |
| Col4a1 | Collagen, type IV, α1 | GGCAGGTCAAGTTCTAGCGT | TGGCCTGATGTTGGTAACCC | 106 | NM_009931.2 |
| Cxcl12 | Chemokine (C-X-C motif) ligand 12 | CGGTTCTTCGAGAGCCACAT | GGGTCAATGCACACTTGTCT | 125 | NM_013655 |
| Mrc1 | Mannose receptor, C type 1 (CD206) | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC | 132 | NM_008625.2 |
| Ppid | Peptidylprolyl isomerase D (cyclophlin D) | AGTGAAGATGTCCCACGCAT | CCACGTCAAAGAAGACTCGC | 74 | NM_026352.3 |
| Scx | Scleraxis | CCTTCTGCCTCAGCAACCAG | GGTCCAAAGTGGGGCTCTCCGTGACT | 156 | NM_198885.3 |
| Tnmd | Tenomodulin | TGTACTGGATCAATCCCACTCT | GCTCATTCTGGTCAATCCCCT | 114 | NM_022322.2 |
Statistics.
Data are presented as means ± SD. For normally distributed data, differences between groups were tested using a one-way ANOVA followed by Bonferroni post hoc sorting. Data that was not normally distributed was tested using a Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Statistical analyses were conducted using Prism software (version 8.0, GraphPad, La Jolla, CA). Differences were considered statistically significant when P < 0.05. Data from sham surgery mice are provided for illustrative purposes and not directly included in statistical evaluations.
RESULTS
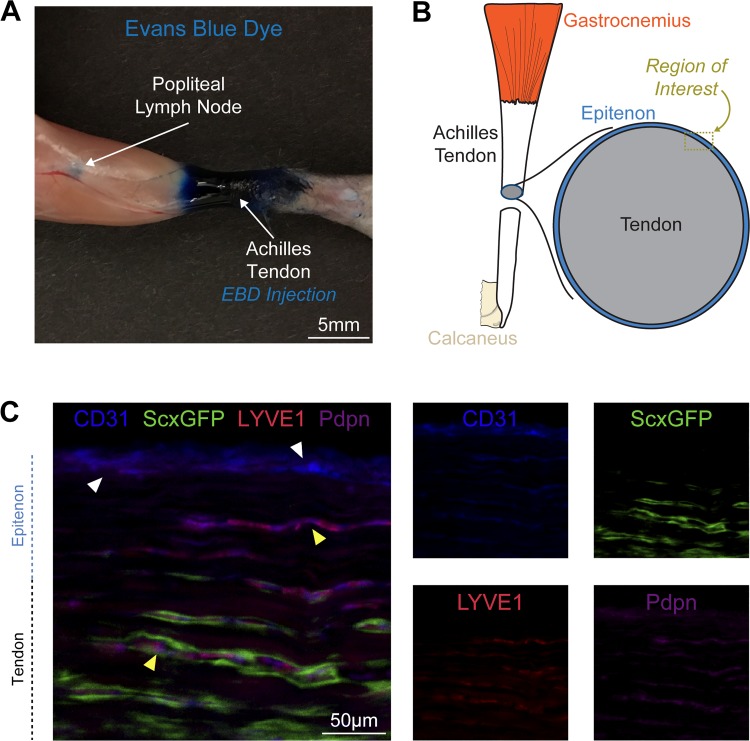
We first sought to confirm that the Achilles tendon and surrounding tissue drained to the popliteal lymph node. EBD was injected into the peritendinous space of the Achilles tendon, and 15 min later, a visible collection of EBD was found in the popliteal lymph node (Fig. 1A). After determining that the Achilles tendon drains to the popliteal lymph node, we identified the location of the lymphatic vasculature in Achilles tendons using ScxGFP mice, which express GFP in scleraxis-expressing tenocytes. We observed lymphatic vessels, which jointly express the endothelial cell marker CD31, the lymphatic endothelial cell marker podoplanin, and lymphatic capillary marker LYVE1 in the epitenon that surrounds Achilles tendons (Fig. 1, B and C). The lymphatic vessels that enter into the superficial tendon were flanked by scleraxis-expressing tenocytes (Fig. 1C). The lymphatic vessels were also in close proximity to the blood vasculature, as identified by CD31 vessels that did not contain LYVE1 or podoplanin (Fig. 1C).
Fig. 1.
Lymph node drainage and lymphatic vessel location in tendon. A: posterior view of the lower limb demonstrating that 15 min after Evans blue dye (EBD) injection into the Achilles peritendinous region, there is an accumulation of EBD in the popliteal lymph node. B: overview of the region of interest for C, which contains the epitenon and superficial tendon regions. C: histology demonstrating the presence of blood vasculature, as indicated by CD31 signal in the absence of podoplanin and LYVE1 (white arrowheads), and lymphatic vasculature (yellow arrowheads), as indicated by CD31 with combined podoplanin and LYVE1 signal, with adjacent scleraxis-expressing tenocytes. Each channel is also shown individually in smaller panels. Blue, CD31; red, LYVE1; magenta, podoplanin; green, scleraxis-GFP. Scale bar: 50 µm.
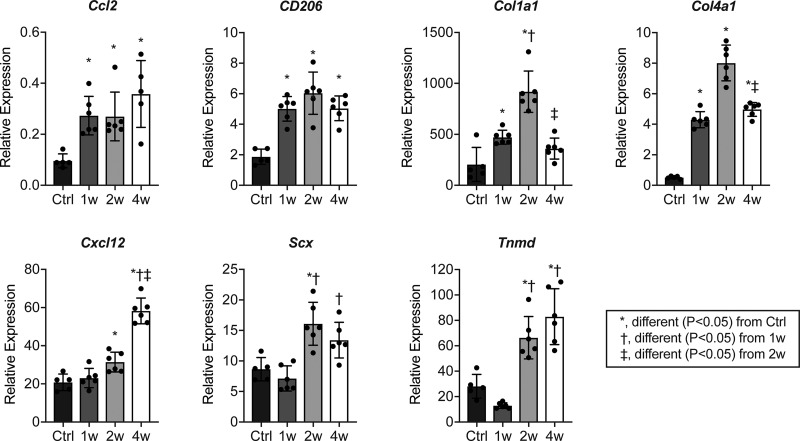
Following the studies that identified the lymphatic drainage from the Achilles tendon, we then determined innate and adaptive immune cell populations in tendons through 4 wk after tenotomy and repair. There was an initial upregulation in the ECM genes Col1a1 and Col4a1 1 wk after tenotomy and repair, with a peak observed at 2 wk (Fig. 2). The tenogenic marker genes Scx and Tnmd were elevated beginning at 2 wk after injury (Fig. 2). There was also an initial upregulation in the monocyte chemoattractant gene Ccl2 at 1 wk, while Cxcl12, which can promote the trafficking of multiple immune cell populations, including T- and B-cells, was not upregulated until 2 wk after injury (Fig. 2). The M2 macrophage marker, CD206, peaked at 2 wk and remained elevated at 4 wk (Fig. 2).
Fig. 2.
Expression of genes involved in immune cell recruitment, extracellular matrix composition, and tenogenesis after Achilles tenotomy and repair. Values are presented as means ± SD. Differences between groups tested using a one-way ANOVA (α = 0.05) followed by Bonferroni post hoc sorting. *Significantly different, P < 0.05, from control (Ctrl). †Significantly different, P < 0.05, from 1 wk. ‡Significantly different, P < 0.05 from 2 wk. n ≥ 3 mice per time point.
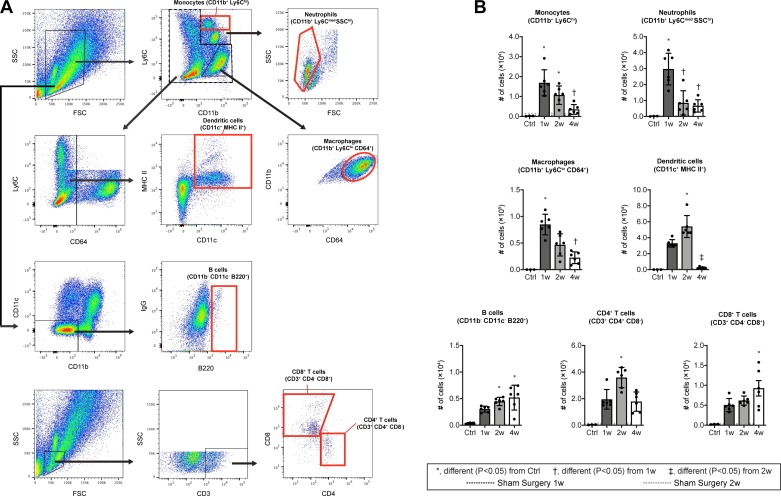
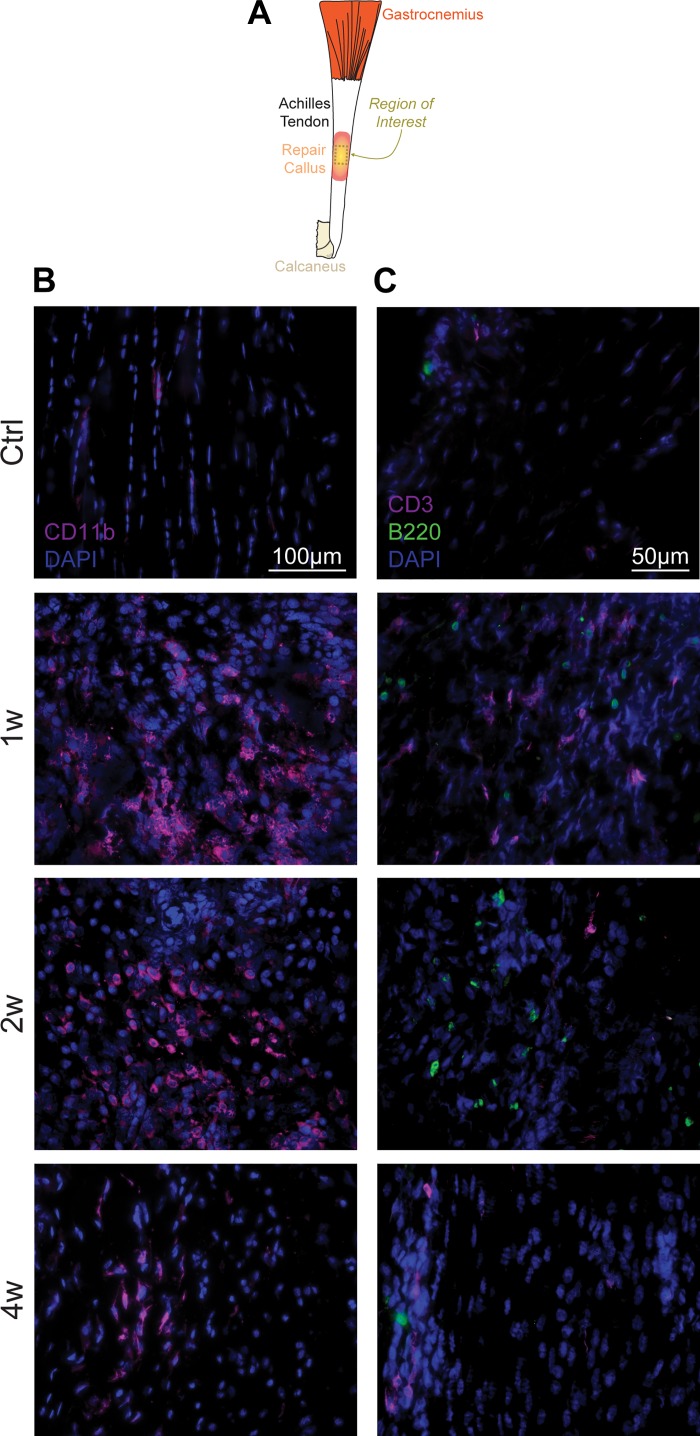
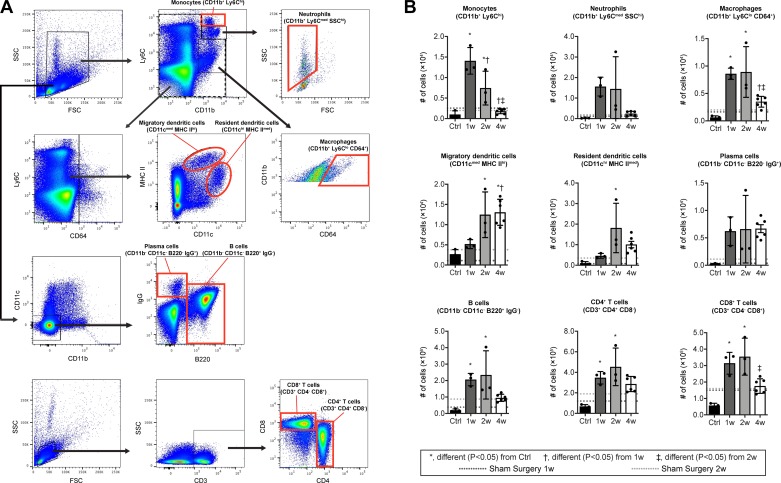
Consistent with the idea of early recruitment of innate cells, there was a marked accumulation of CD11b+/Ly6Chi monocytes and CD11b+/Ly6Cmed/SSChi neutrophils at 1 wk after injury, followed by their precipitous decline (Fig. 3, A and B). CD11b+/Ly6Clo/CD64+ macrophages were similarly transiently increased (Fig. 3, A and B), presumably reflecting both the differentiation of accumulated monocyte-derived macrophages and the proliferation of resident macrophages. The initial accumulation of these innate cells was followed by an accumulation of CD4+ T cells (CD3+/CD4+/CD8−) and CD11c+ MHC II+ antigen-presenting cells likely composed mostly of dendritic cells at 2 wk after injury, while B cells (CD11b−/CD11c−/B220+) and CD8+ T cells (CD3+/CD4-/CD8+) demonstrated a progressive increase over time (Fig. 3, A and B). The abundance of immune cell populations in sham surgery mice was similar to the noninjured controls (Fig. 3B). We observed similar qualitative results in histology experiments of control and tenotomy groups (Fig. 4A), evaluating the presence of innate (Fig. 4B) and adaptive (Fig. 4C) markers in injured tendons.
Fig. 3.
Flow cytometry of immune cell populations in Achilles tendons after tenotomy and repair. A: dot plot demonstrating gating strategies. B: quantification of populations of monocytes (CD11b+/Ly6Chi), neutrophils (CD11b+/Ly6Cmed/SSChi), macrophages (CD11b+/Ly6Clo/CD64+), dendritic cells (CD11c+/MHC II+), B cells (CD11b−/CD11c−/B220+), CD4+ T cells (CD3+/CD4+/CD8−), and CD8+ T cells (CD3+/CD4−/CD8+) from Achilles tendons. Mean values of sham-operated mice at 1wk (dark gray) or 2 wk (light gray) are shown as horizontal dotted lines but are not directly included in the statistical model. Differences between groups tested were obtained using a one-way ANOVA (α = 0.05) followed by Bonferroni post hoc sorting, as well as the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. *Significantly different, P < 0.05, from control (Ctrl). †Significantly different, P < 0.05, from 1 wk. ‡Significantly different, P < 0.05, from 2 wk. n ≥ 3 mice per control or surgical time point.
Fig. 4.
Location of immune cells in injured tendons. A: overview of the region of interest for B and C, which is taken from the midsubstance of the Achilles tendon. In the groups that underwent a tenotomy and repair, this region was in the callus that formed in the repaired area. B: myeloid cells, as indicated by CD11b signal, in control tendons, and tendons either 1 wk (1w), 2 wk (2w), or 4 wk (4w) after tenotomy and repair. Blue, nuclei (DAPI); magenta, CD11b. Scale bar for all panels equals 100 µm. C: T cells and B cells, as indicated by CD3 and B220 signal, respectively, in control tendons, and tendons either 1w, 2w, or 4w after tenotomy and repair. Blue denotes nuclei (DAPI); magenta denotes CD3; green denotes B220. Scale bar for all panels equals 50 µm. Images are reflective of n ≥ 4 samples per time point.
Finally, we evaluated changes in innate and adaptive cell populations of popliteal lymph nodes after tenotomy and repair, and we observed lymph node hypertrophy with a similar pattern of innate cell accumulation followed by adaptive cell accumulation. There was a pronounced accumulation of monocytes and macrophages 1 wk after injury, while no differences in neutrophils or plasma cells (CD11b−/CD11c−/B220−/IgG+) were observed (Fig. 5, A and B). Migratory (CD11cmed/MHC IIhi) and resident (CD11chi/MHC IImed) dendritic cells showed a more gradual accumulation, starting at 1 wk and peaking at 2 wk. The bulk of the lymph node hypertrophy reflected the accumulation of CD4+ and CD8+ T cells and B cells, which reached plateau numbers by 1 wk but were maintained through the second week (Fig. 5, A and B). Plasma cells, the differentiated effector B cells that secrete antibodies, were not statistically different across time points but showed a trend to increase at 4 wk (P = 0.058). The injury to the skin and surrounding connective tissue by itself is expected to cause an immune cell response, which would be apparent in draining lymph nodes, and as expected, the lymph nodes of sham surgery mice displayed some response to the skin injury (Fig. 5B). However, the magnitude of response for most immune cell populations was generally much greater in the tenotomy groups than the sham-operated mice at 1w and 2w (Fig. 5B). Overall, the lymph node hypertrophy and presence of plasma cells support the idea that the draining lymph node is responding to the tendon injury.
Fig. 5.
Flow cytometry of immune cell populations in popliteal lymph nodes after Achilles tenotomy and repair. A: dot plot demonstrating gating strategies. B: quantification of populations of monocytes (CD11b+/Ly6Chi), neutrophils (CD11b+/Ly6Cmed/SSChi), macrophages (CD11b+/Ly6Clo/CD64+), migratory dendritic cells (CD11cmed/MHC IIhi), resident dendritic cells (CD11chi/MHC IImed), plasma cells (CD11b−/CD11c−/B220−/IgG+), B cells (CD11b−/CD11c−/B220+/IgG−), CD4+ T cells (CD3+/CD4+/CD8−), and CD8+ T cells (CD3+/CD4−/CD8+) from popliteal lymph nodes. Mean values of sham operated mice at 1 wk (1w; dark gray) or 2 wk (2w; light gray) are shown as horizontal dotted lines but are not directly included in the statistical model. Differences between groups tested using a one-way ANOVA (α = 0.05) followed by Bonferroni post hoc sorting, as well as Kruskal-Wallis test followed by Dunn’s multiple comparisons: *Significantly different, P < 0.05, from control (Ctrl). †Significantly different, P < 0.05, from 1w. ‡Significantly different, P < 0.05, from 2w. n ≥ 3 mice per control or surgical time point.
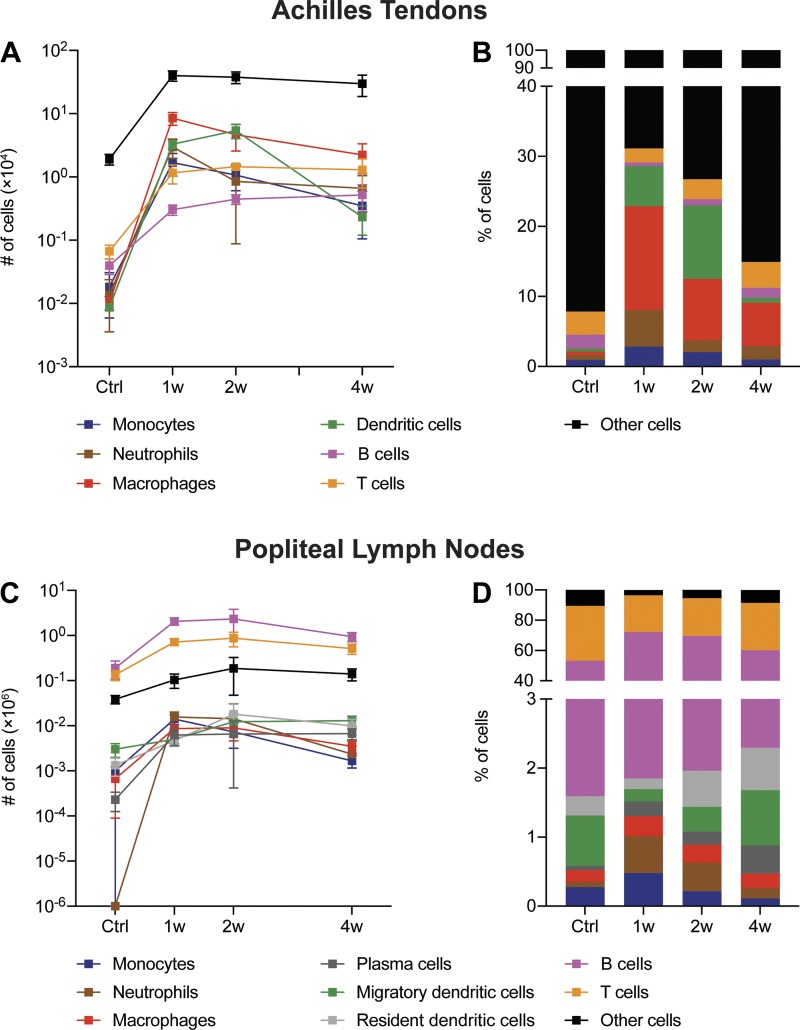
In addition to the individual cell populations presented in Figs. 3B and 5B, Fig. 6 provides a summary of major cell types across all control and tenotomy time points.
Fig. 6.
Summary of immune cell changes in Achilles tendons and lymph nodes after Achilles tenotomy and repair. The absolute number (A) and percentage (B) of monocytes, neutrophils, macrophages, dendritic cells, B cells, T cells, and other cells from Achilles tendons in control tendons and tendons 1 wk (1w), 2 wk (2w), or 4 wk (4w) after tenotomy and repair are shown. The absolute number (C) and percentage (D) of monocytes, neutrophils, macrophages, plasma cells, migratory dendritic cells, resident dendritic cells, B cells, T cells, and other cells from popliteal lymph nodes in control tendons and tendons 1w, 2w, or 4w after tenotomy and repair are shown.
DISCUSSION
Changes in innate immune cell populations have been studied in tendon injuries (22, 35, 38), but less is known about adaptive immune cell responses. In the current study, we sought to characterize the adaptive immune cell response in tendons and tendon-draining lymph nodes to tendon injury and repair. In addition to corroborating innate immune cell data from previous tenotomy studies, we identified a robust adaptive immune cell response after tenotomy and repair in both the tendon and the tendon-draining popliteal lymph node that generally peaks following the innate immune cell response. Maximal T- and B-cell accumulation in the popliteal lymph node occurred slightly earlier than in the tendon, consistent with the paradigm that T- and B-cells are initially stimulated and activated by antigen flowing from the affected tissue and then traffic to the affected tissue to carry out their effector functions (7). These results provide novel insight into the facets of the immune system that are involved in the response to tendon injury and provide a basis for future studies to interrogate their function in tendon injury and healing, with the long-term goal of potentially manipulating adaptive immunity to improve tendon healing.
Using a similar Achilles tenotomy and repair model and time points in rats, we previously demonstrated that M1 macrophages accumulate within 1 wk of tendon injury, while M2 macrophages start appearing in appreciable numbers by the 2nd wk (35). Although we did not sort macrophages into subtypes, the results in the current study generally follow similar trends in markers of macrophage abundance that we observed in rats (35). Supporting our observations from flow cytometry, the monocyte attractant gene Ccl2 (15, 20) was also upregulated 1 wk after injury, and the M2 marker CD206 (35) was elevated at 2 wk. Macrophages also appear to directly modulate tendon healing. Depleting macrophages reduces markers of inflammation and improves mechanical properties of tendons at 1 and 2 wk after tendon injury (4a), although since M2 macrophages do not begin to accumulate until 2 wk after injury, it is possible that an early reduction in inflammation may result in incomplete healing and reduced long-term functional outcomes. Much still remains to be determined about how different macrophage populations interact with other cell types in tendon to modulate tissue repair.
The adaptive immune system is known to play an important role in tissue pathology in disease states, but its role in healing of various musculoskeletal injuries is an emerging area of study (20), and even less is known about lymphocytes in tendon healing. There is also cross-talk between the innate and adaptive immune systems during tissue repair (15). A previous study in rats subjected to an Achilles tendon tear with or without Botox treatment evaluated macrophage and T-cell changes through 10 days after injury, and identified a response from adaptive immune cells, but conclusions from this study are limited by lack of data on absolute cell numbers, the absence of analysis of uninjured animals, and the lack of statistical analysis of the effect of time on cell accumulation (3).
In the current study, we observed the presence of lymphatic vasculature in the superficial regions of tendon, as indicated by the presence of the lymphatic markers LYVE1 and podoplanin (17), similar to previous observations in rat Achilles tendons (37). We also report a small population of tissue-resident adaptive immune cells, as well as an induction of Cxcl12 expression, which promotes circulating lymphocyte chemotaxis and tissue entry (15, 20).
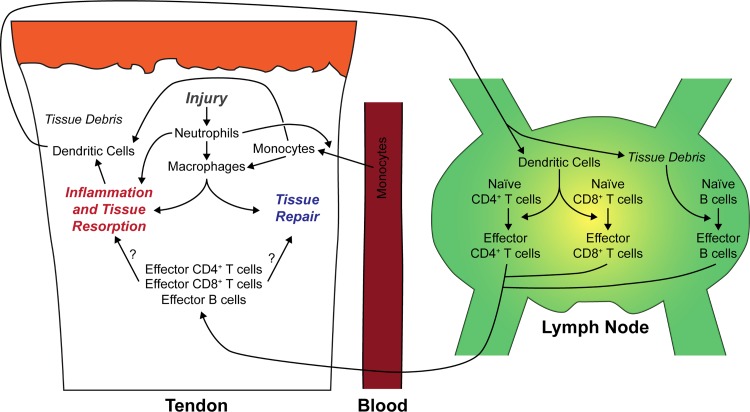
On the basis of results from the current study, previous studies evaluating the innate immune response in acutely injured tendons (4a, 22, 35, 38), and our understanding of the regulation of adaptive immune cells in other tissue types (4, 15, 20, 21, 29, 34), we have developed a model of immune cell population changes in the context of healing following a tendon tear (Fig. 7). Upon injury, there is an accumulation of neutrophils, which initiate the inflammatory response and begin to recruit circulating monocytes to the tendon. Monocytes can then differentiate into macrophages and dendritic cells, both of which are able to process antigen from the injured site and present antigen fragments to lymphocytes. The macrophage response through 2 wk after injury is dominated by a population of proinflammatory M1 macrophages, which are gradually replaced by an anti-inflammatory and tissue regenerative M2 macrophage population. Dendritic cells, which are stimulated by local inflammation, begin to migrate from the tendon and, likely, surrounding tissues, such as skin and fascia to the draining lymph node within days, where they prime naïve CD4+ and CD8+ T cells. In addition, even before high-quantity dendritic cell migration from the tendon, soluble antigens and cytokines from the injured tendon flow via the lymphatics to the draining lymph node, which contributes to the rapid accumulation of T and B cells. The activated effector CD4+ and CD8+ T cells then leave the lymph node and migrate to the tendon, where they likely help to orchestrate the resolution of inflammation and tissue repair through coordination of tenocyte and other cell activities. Antibody-secreting plasma cells, differentiated from naïve B cells in the lymph node, may also migrate to the tendon to modulate inflammation or repair. Further studies are necessary to more precisely identify the roles of the different lymphocyte populations and the role of the draining lymph nodes in tendon healing.
Fig. 7.
Summary of immune cell changes after tendon injury. We present an overview of the proposed changes in immune cell abundance and interactions in the context of tendon injury and repair.
There are several limitations to this study. We only evaluated tendons through 4 wk after injury, and we expect immune cells continue to play a role in tissue inflammation and repair beyond this point. There are likely also more pronounced changes in early innate immune cells, such as neutrophils, that occur earlier than 1 wk. Although we measured total macrophage abundance after tenotomy and repair, we did not assess M1 and M2 macrophage markers, but did measure the M2 marker CD206 by qPCR. We only evaluated changes in young adult mice, and the immune response and regenerative capacity are likely to differ as animals age. While tendon mechanics and transcriptional profiles are similar between male and female mice (31), there are known to be sex-based differences in immune cell biology (30), and as we only included male mice in this study, additional experiments evaluating the role of sex would provide additional insight into the biology of tendon repair. Our study focused on the popliteal lymph nodes, as these are in closest proximity to the site of injury. However, it is likely that the inguinal and paraaortic lymph nodes, which drain the popliteal lymph node (12), are also involved in the response to a tendon injury. We did not evaluate changes in tendon mechanics or how loading and unloading impacts immune cell populations, and integrating this could further inform the functional role of the innate and adaptive immune systems in tendon healing. Despite these limitations, we feel that this study provides novel insight into the adaptive immune system during tendon healing.
Tendon tears remain a challenging clinical condition (23, 38). Even with improvements in surgical interventions and rehabilitation protocols, many patients continue to have impaired mobility, and up to one-third of athletes are unable to return to play after tendon rupture (16, 38, 39). Inflammation plays an important role in the response of tendons to injury, and studies that have used broad anti-inflammatory agents such as NSAIDs often show an initial improvement but long-term deficit in tissue healing (23). To improve upon this, regenerative strategies for the management of chronic tendon injuries have mostly focused on the delivery of stem cells and growth factors to sites of injured tendon tissue to begin the healing process (5, 10). As such, successful tendon repair requires a fundamental understanding of the inflammatory cascade within musculoskeletal tissue in response to injury. The formation of new tendon tissue relies on a complex interplay between the infiltrating immune cell populations in the epitenon and the resident tenocytes. While the utilization of stem cells and growth factors has been associated with early promising results in animal studies (26), the main limitation of this treatment approach is the bulk administration of these biological agents, without consideration of normal spatial and temporal variations within the acute inflammatory response. In the current study, the presence of adaptive immune cells, such as T and B cells, was demonstrated at the repair site of an injured Achilles tendon at least 1 mo after the tenotomy and repair were performed. This finding is consistent with the persistent inflammatory cell infiltrates seen on biopsies taken from the Achilles tendons of symptomatic patients with chronic tendinopathy several months after the initial injury (25). Little is known about the contribution of T and B cells to the progression of chronic tendon injuries, but since the immune system plays a critical role in the repair of many different tissue types throughout the body, targeting components of the adaptive immune response and the downstream signaling pathways should be considered in any regenerative therapy for tendon injuries. This highlights the need for additional experiments focused on depletion of adaptive immune cells or inhibition of their inflammatory cytokines to determine the extent in which tendon healing can be augmented.
GRANTS
This work was supported by National Institutes of Health Grants R01-AR063649 and R01-AI079178.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.N., T.M.L., L.M.M., S.W., S.A.R., T.T.L., and C.L.M. conceived and designed research; A.C.N., T.M.L., L.M.M., S.W., J.B.S., and N.P.D. performed experiments; A.C.N., T.M.L., L.M.M., J.B.S., N.P.D., K.B.S., S.A.R., T.T.L., and C.L.M. analyzed data; A.C.N., T.M.L., L.M.M., N.P.D., K.B.S., S.A.R., T.T.L., and C.L.M. interpreted results of experiments; A.C.N., T.M.L., L.M.M., T.T.L., and C.L.M. prepared figures; A.C.N., T.M.L., L.M.M., K.B.S., T.T.L., and C.L.M. drafted manuscript; A.C.N., T.M.L., L.M.M., K.B.S., T.T.L., and C.L.M. edited and revised manuscript; A.C.N., T.M.L., L.M.M., S.W., J.B.S., N.P.D., K.B.S., S.A.R., T.T.L., and C.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The ScxGFP mice in this report were kindly provided by Dr. Ronen Schweitzer of Shriners Childrens Hospital of Portland. We acknowledge technical assistance from Mr. Alex Piacentini at the Hospital for Special Surgery, and useful discussions with Dr. Dragos Dasoveanu, and Dr. Lionel Ivashkiv at the Hospital for Special Surgery.
REFERENCES
- 1.Bechshøft CL, Schjerling P, Bornø A, Holm L. Existence of life-time stable proteins in mature rats. Dating of proteins’ age by repeated short-term exposure to labeled amino acids throughout age. PLoS One 12: e0185605, 2017. doi: 10.1371/journal.pone.0185605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benahmed F, Chyou S, Dasoveanu D, Chen J, Kumar V, Iwakura Y, Lu TT. Multiple CD11c+ cells collaboratively express IL-1β to modulate stromal vascular endothelial growth factor and lymph node vascular-stromal growth. J Immunol 192: 4153–4163, 2014. doi: 10.4049/jimmunol.1301765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomgran P, Blomgran R, Ernerudh J, Aspenberg P. A possible link between loading, inflammation and healing: Immune cell populations during tendon healing in the rat. Sci Rep 6: 29824, 2016. doi: 10.1038/srep29824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasoveanu DC, Shipman WD, Chia JJ, Chyou S, Lu TT. Regulation of lymph node vascular-stromal compartment by dendritic cells. Trends Immunol 37: 764–777, 2016. doi: 10.1016/j.it.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.de la Durantaye M, Piette AB, van Rooijen N, Frenette J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res 32: 279–285, 2014. doi: 10.1002/jor.22504. [DOI] [PubMed] [Google Scholar]
- 5.de Vos RJ, van Veldhoven PLJ, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull 95: 63–77, 2010. doi: 10.1093/bmb/ldq006. [DOI] [PubMed] [Google Scholar]
- 6.Disser NP, Sugg KB, Talarek JR, Sarver DC, Rourke BJ, Mendias CL. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J 33: 12680–12695, 2019. doi: 10.1096/fj.201901503R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girard J-P, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12: 762–773, 2012. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 8.Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL. A distinct transition from cell growth to physiological homeostasis in the tendon. eLife 8: e48689, 2019. doi: 10.7554/eLife.48689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinstein M, Dingwall HL, Shah RR, Capellini TD, Galloway JL. A robust method for RNA extraction and purification from a single adult mouse tendon. PeerJ 6: e4664, 2018. doi: 10.7717/peerj.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guevara-Alvarez A, Schmitt A, Russell RP, Imhoff AB, Buchmann S. Growth factor delivery vehicles for tendon injuries: Mesenchymal stem cells and platelet-rich plasma. Muscles Ligaments Tendons J 4: 378–385, 2014. doi: 10.32098/mltj.03.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol (1985) 117: 1287–1291, 2014. doi: 10.1152/japplphysiol.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods 332: 170–174, 2008. doi: 10.1016/j.jim.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J 27: 2074–2079, 2013. doi: 10.1096/fj.12-225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez CJ, Yang X, Ji G, Niu Y, Sethuraman AS, Koressel J, Shirley M, Fields MW, Chyou S, Li TM, Luna M, Callahan RL, Ross FP, Lu TT, Brito IL, Carli AV, Bostrom MPG. Disruption of the gut microbiome increases the risk of periprosthetic joint infection in mice. Clin Orthop Relat Res 477: 2588–2598, 2019. doi: 10.1097/CORR.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater 53: 13–28, 2017. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 21: 228–237, 2012. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong L-L, Yang N-Z, Shi L-H, Zhao G-H, Zhou W, Ding Q, Wang M-H, Zhang Y-S. The optimum marker for the detection of lymphatic vessels. Mol Clin Oncol 7: 515–520, 2017. doi: 10.3892/mco.2017.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Dasoveanu DC, Chyou S, Tzeng T-C, Rozo C, Liang Y, Stohl W, Fu Y-X, Ruddle NH, Lu TT. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity 42: 719–730, 2015. doi: 10.1016/j.immuni.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Tan J, Martino MM, Lui KO. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol 9: 585, 2018. doi: 10.3389/fimmu.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol 3: eaat1604, 2018. doi: 10.1126/sciimmunol.aat1604. [DOI] [PubMed] [Google Scholar]
- 22.Marsolais D, Côté CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res 19: 1203–1209, 2001. doi: 10.1016/S0736-0266(01)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Mead MP, Gumucio JP, Awan TM, Mendias CL, Sugg KB. Pathogenesis and management of tendinopathies in sports medicine. Transl Sports Med 1: 5–13, 2018. doi: 10.1002/tsm2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30: 606–612, 2012. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millar NL, Murrell GAC, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol 13: 110–122, 2017. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 26.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol 11: 223–233, 2015. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 27.Palmes D, Spiegel HU, Schneider TO, Langer M, Stratmann U, Budny T, Probst A. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J Orthop Res 20: 939–946, 2002. doi: 10.1016/S0736-0266(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 28.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236: 1677–1682, 2007. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 29.Rankin LC, Artis D. Beyond host defense: emerging functions of the immune system in regulating complex tissue physiology. Cell 173: 554–567, 2018. doi: 10.1016/j.cell.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav 88: 95–105, 2017. doi: 10.1016/j.yhbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Sarver DC, Kharaz YA, Sugg KB, Gumucio JP, Comerford E, Mendias CL. Sex differences in tendon structure and function. J Orthop Res 35: 2117–2126, 2017. doi: 10.1002/jor.23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz N, Chalasani MLS, Li TM, Feng Z, Shipman WD, Lu TT. Lymphatic function in autoimmune diseases. Front Immunol 10: 519, 2019. doi: 10.3389/fimmu.2019.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shipman WD, Chyou S, Ramanathan A, Izmirly PM, Sharma S, Pannellini T, Dasoveanu DC, Qing X, Magro CM, Granstein RD, Lowes MA, Pamer EG, Kaplan DH, Salmon JE, Mehrara BJ, Young JW, Clancy RM, Blobel CP, Lu TT. A protective Langerhans cell-keratinocyte axis that is dysfunctional in photosensitivity. Sci Transl Med 10: eaap9527, 2018. doi: 10.1126/scitranslmed.aap9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sîrbulescu RF, Boehm CK, Soon E, Wilks MQ, Ilieş I, Yuan H, Maxner B, Chronos N, Kaittanis C, Normandin MD, El Fakhri G, Orgill DP, Sluder AE, Poznansky MC. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen 25: 774–791, 2017. doi: 10.1111/wrr.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res 32: 944–951, 2014. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugg KB, Markworth JF, Disser NP, Rizzi AM, Talarek JR, Sarver DC, Brooks SV, Mendias CL. Postnatal tendon growth and remodeling require platelet-derived growth factor receptor signaling. Am J Physiol Cell Physiol 314: C389–C403, 2018. doi: 10.1152/ajpcell.00258.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tempfer H, Kaser-Eichberger A, Korntner S, Lehner C, Kunkel N, Traweger A, Trost A, Strohmaier C, Bogner B, Runge C, Bruckner D, Krefft K, Heindl LM, Reitsamer HA, Schrödl F. Presence of lymphatics in a rat tendon lesion model. Histochem Cell Biol 143: 411–419, 2015. doi: 10.1007/s00418-014-1287-x. [DOI] [PubMed] [Google Scholar]
- 38.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res 33: 832–839, 2015. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trofa DP, Miller JC, Jang ES, Woode DR, Greisberg JK, Vosseller JT. Professional athletes’ return to play and performance after operative repair of an Achilles tendon rupture. Am J Sports Med 45: 2864–2871, 2017. doi: 10.1177/0363546517713001. [DOI] [PubMed] [Google Scholar]