Abstract
A wide range of spinal cord levels (cervical 8–thoracic 6) project to the stellate ganglia (which provides >90% of sympathetic supply to the heart), with a peak at the thoracic 2 (T2) level. We hypothesize that despite the proximity of the lesions, high thoracic spinal cord injuries (i.e., T2–3 SCI) do not closely mimic the hemodynamic responses recorded with cervical SCI (i.e., C6–7 SCI). To test this hypothesis, rats were instrumented with an intra-arterial telemetry device (Data Sciences International PA-C40) for recording arterial pressure, heart rate, and locomotor activity as well as a catheter within the intraperitoneal space. After recovery, rats were subjected to complete C6–7 spinal cord transection (n = 8), sham transection (n = 4), or T2–3 spinal cord transection (n = 7). After the spinal cord transection or sham transection, arterial pressure, heart rate, and activity counts were recorded in conscious animals, in a thermoneutral environment, for 20 s every minute, 24 h/day for 12 consecutive weeks. After 12 wk, chronic reflex- and stress-induced cardiovascular and hormonal responses were compared in all groups. C6–7 rats had hypotension, bradycardia, and reduced physical activity. In contrast, T2–3 rats were tachycardic. C6–7 rats compared with T2–3 and spinal intact rats also had reduced cardiac sympathetic tonus, reduced reflex- and stress induced cardiovascular responses, and reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Thus injuries above and below the peak level (T2) of spinal cord projections to the stellate ganglia have remarkably different outcomes.
NEW & NOTEWORTHY Twelve consecutive weeks of resting hemodynamic data as well as chronic reflex- and stress-induced cardiovascular, autonomic, and hormonal responses were compared in spinal intact and C6–7 and T2–3 spinal cord-transected rats. C6–7 rats compared with T2–3 and spinal intact rats had reduced cardiac sympathetic tonus, reduced reflex- and stress-induced cardiovascular responses, and reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Thus injuries above and below the peak level (T2) of spinal cord projections to the stellate ganglia have remarkably different outcomes.
Keywords: blood pressure regulation, parasympathetic nervous system, sympathetic nervous system, tetraplegia
INTRODUCTION
Consider the importance of sparing a few spinal levels on the quality of life for an individual living with a spinal cord injury (SCI). As an example, a SCI at cervical 4 (C4) would likely result in paralysis of the arms and hands, the individual may have trouble breathing and coughing on their own, and speaking could be difficult (53). This C4 level of SCI may also require 24-hours-a-day personal care.
In contrast, with a SCI at cervical 8 (C8), the individual should be able move their arms and grasp and release objects and should be able to breathe and speak normally (53). Importantly, the individual with a C8 SCI should be able to do most activities of daily living independently and may be able to drive an adapted vehicle (53). Thus injury at the transition zones of projections to the diaphragm and arms have remarkably different outcomes, and sparing four spinal levels dramatically alters the quality of life (53).
In this context, a wide range of spinal cord levels [C8–thoracic 6 (T6)] project to the stellate ganglia (which provides >90% of sympathetic supply to the heart), with a peak at the T2 level. Specifically, 92% of retrograde-labeled nerves from the heart have their origins in the stellate ganglion (44, 45). A complete SCI between C8 and T6 reduces sympathetic activity below and increases sympathetic activity above the level of the injury (26, 34). For example, a SCI at T5 results in loss of descending sympathetic drive to the vasculature innervated by sympathetic preganglionic neurons (SPNs) below the level of the injury (26, 39). The loss of sympathetic drive to the vasculature below the level of the injury results in hypotension and a marked arterial baroreflex-mediated increase in sympathetic outflow to the heart. High cardiac sympathetic outflow, following T5 SCI, promotes calcium overload (52), cardiac injury, left ventricular dysfunction, and ST segment elevation (40). The associated cardiac injury promotes nerve growth factor (NGF) production that stimulates sympathetic sprouting and hyperinnervation of the heart (26, 29, 31). Thus a SCI above the midthoracic sympathetic outflow is critical to the development of cardiac sympathetic dysfunction.
However, the higher the level of the SCI, the greater the degree of sympathetic dysfunction (18, 36, 38). In this context, Mathias and colleagues (37) documented reduced resting catecholamine levels in individuals with cervical SCIs compared with able-bodied individuals and individuals with paraplegia. Similarly, Krum and associates (25) reported reduced plasma norepinephrine levels and reduced arterial blood pressure in individuals with cervical SCI compared with able-bodied individuals. Accordingly, the clinical sequelae of reduced sympathetic activity below and enhanced sympathetic activity above the level of the injury critically depends on the site of the injury, with a transition zone at T2.
High thoracic SCI (i.e., T2–3) spares upper limb function and some (C8–T2) supraspinal control of SPNs to the heart. Thus T2–3 SCI decentralizes practically all SPNs since it has been suggested that these injuries spread (secondary cascade) at least one or two segments rostral within the cord (12, 34, 55). The remaining T1 segment is responsible for <10% of postganglionic cardiac innervation (56). In contrast, cervical SCI (i.e., C6–7) disconnects supraspinal control to all SPNs. Furthermore, body mass and levels of physical activity are influenced by the level of the injury. Our goal was to emulate the postinjury experience in humans in preclinical models of high thoracic and cervical SCIs. We hypothesize that despite the proximity of the lesions, high thoracic SCIs (i.e., T2–3 SCI) do not closely mimic the hemodynamic responses recorded with cervical injuries (i.e., C6–7 SCI) (34). The results are expected to document that high thoracic lesions are not applicable to cervical lesions when examining cardiovascular consequences. The magnitude of this difference will document a critical need for a chronic preclinical model of cervical SCI. Accordingly, we provide a direct comparison between T2–3 paraplegic and C6–7 tetraplegic SCI models and reveal novel cardiovascular insights.
MATERIALS AND METHODS
Animals and General Experimental Procedures
Experimental procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were conducted in 19 male Sprague-Dawley rats starting at 10 wk of age and continuing for 15 wk. Rats were instrumented with an intra-arterial telemetry device (Data Sciences International PA-C40) for recording arterial pressure, heart rate, and locomotor activity as well as a catheter within the intraperitoneal space. After recovery, rats were subjected to complete C6–7 spinal cord transection (n = 8), sham transection (n = 4), or T2–3 spinal cord transection (n = 7). After the spinal cord transection or sham transection, animals were housed separately in standard rat cages (~13,350 cm3) in a dedicated animal room where ambient temperature was maintained at the thermoneutral zone for rats between 28° and 32°C (35, 57). Entry into the animal room was restricted to the investigators, ensuring that the animals were not unnecessarily disturbed. Arterial pressure, heart rate, and activity counts were recorded in conscious animals for 20 s every minute, 24 h/day for 12 consecutive weeks.
Subsequently, five additional experimental procedures were performed (described below and in Ref. 34) over 3 additional weeks. Thus these five additional experiments occurred between 12 and 15 wk after injury. For these experiments, conscious unrestrained rats were studied in their home cages (~13,350 cm3). Temperature within the cage was monitored and maintained near the thermoneutral zone for rats of ~28–32°C (35, 57) with the use of a Pelonis fan-forced heater and thermostat.
Surgical Procedures
Two survival surgeries were performed on each animal. Before each surgery, animals received atropine (0.05 mg/kg). In addition, multimodal preemptive analgesia was achieved with a local infiltration of bupivacaine (diluted to 0.25%, 5 mg/kg) and lidocaine (diluted to 0.5%, 5 mg/kg) around the incision site (sc) and the administration of a nonsteroidal anti-inflammatory agent (carprofen, 10 mg/kg ip). Animals were anesthetized initially with 3–5% isoflurane and intubated. Subsequently, animals were supported by a ventilator delivering isoflurane at 1–3%. Carprofen and buprenorphine (0.05 mg/kg) were administered for 3 days postoperatively. To avoid infections, the antibiotic cefazolin (10 mg/kg) was administered preoperatively and for 3 days postoperatively.
First surgical procedure.
Under aseptic conditions, a catheter from a telemetry device (Data Sciences International PA-C40) was inserted into the left carotid artery and advanced into the descending aorta for recording arterial pressure. Subsequently, a catheter was placed in the intraperitoneal space for the infusion of fluids and drugs. The catheter was exteriorized on the dorsal aspect of the neck.
All animals remained on the feedback-based temperature control system and ventilator until recovered from the anesthesia. Once the animals regained consciousness, they were placed in a “rodent recovery cage” (Intensive Care Unit; ThermoCare, Paso Robles, CA). Animals were returned to the housing room when they fully recovered from the anesthesia and gained the ability to maintain an upright body position and body temperature. At least 14 days were allowed for recovery. During the recovery and recording periods, the rats were provided with supplemental enrichment treats (Bio-Serv, Flemington, NJ), handled, weighed, and acclimatized to the laboratory and investigators.
Second surgical procedure.
After the recovery period and after the animals returned to their presurgical weight, the second surgical procedure was performed. The animals were anesthetized as described above and subjected to a complete C6–7, T2–3, or sham spinal cord transection. Specifically, the spinous process and laminae of the 6th cervical vertebra were carefully removed, exposing the 6–7th cervical spinal cord segment. The 2nd–3rd thoracic spinal cord segment is readily seen without a laminectomy. The spinal cord was subsequently transected between C6 and C7 or between T2 and T3 with a microknife (Fine Science 10316-14) and Vannas spring scissors (Fine Science 15000-08). Spinal cord transection between C6 and C7 eliminates supraspinal control of all SPNs. Spinal cord transection between T2 and T3 preserves supraspinal control to some SPNs. The extensiveness of the spinal cord transection was established by visual inspection of the transection site. Indistinguishable procedures were followed for the sham-transected rats, except that the spinal cord was not transected. The completeness and location of similar injuries have been confirmed by fast-spin echo MRI (34). In the present study, all injuries were confirmed by visual inspection at autopsy.
Injury reproducibility is an important characteristic of experimental models of SCI because it limits the variability in outcome measures. Although spinal cord contusion injuries may be the most clinically relevant model, since most injuries in humans occur in a similar manner, this model has a greater variability in outcome measures and spared descending systems compared with the complete spinal cord transection model (3). Therefore, to enhance injury reproducibility we used the complete spinal cord transection model (7, 8, 26–32, 34, 48–50).
Acute postoperative care for spinal cord-injured animals.
Immediately after spinal cord transection, animals present with flaccid paralysis and loss of sensation below the level of the lesion. This poses a major challenge to the injured animal that requires 24-h assistance with basic needs including eating, drinking, and micturition. This specialized care continues until the animal can eat and drink on its own as well as the return of reflexive micturition. Typically, this period lasts 7–10 days after transection. The animals were also provided with novel, nutritionally complete, gel diets (Bio-Serv) and fruit to encourage eating. In addition, with a syringe, the animals were hand fed liquid diets such as BOOST nutritional drink or diluted gel diets (Bio-Serv) several times a day. Spinal transection also impairs micturition reflexes. Therefore, the urinary bladder was voided by manual compression every 4 h, 24 h/day, 7 days/wk. Once automatic voidance was observed, manual compression continued 4 times a day to completely empty any residual urine that might pose a risk of infections.
Body temperature regulation is a major challenge for animals, and maintaining a thermoneutral environment was critical for recovery. Therefore, animals were housed in a dedicated animal room where ambient temperature was maintained at the thermoneutral zone for rats between 28°C and 32°C (35, 57).
Finally, injured animals were handled several times daily to passively move joints and massage muscles below the level of the lesion. These procedures prevented pressure sores and contractures. Sham-operated animals were also housed in a thermoneutral environment and handled daily and received nutritional supplements.
Experimental Procedures
Arterial pressure, heart rate, and activity counts were recorded in conscious animals for 20 s every minute, 24 h/day for 12 consecutive weeks. Subsequently, five additional experimental procedures were performed as described in a recent investigation of rats with C6–7 transection (34). For these experiments, conscious, unrestrained rats were studied in their home cages (∼13,350 cm3). The temperature within the cage was monitored and maintained near the thermoneutral zone for rats of ~28–32°C (35, 57) with the use of a Pelonis fan-forced heater with thermostat.
Experimental procedure 1: resting sympathetic activity (cardiac sympathetic tonus) and resting sympathetic support of arterial blood pressure.
On the day of the experiment, rats were brought into the laboratory and studied in their standard home cage. Temperature within the cage was maintained within the thermoneutral zone for rats. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, the heart rate and arterial pressure responses to cardiac autonomic sympathetic and parasympathetic blockade (β1-adrenergic and muscarinic-cholinergic receptor blockade, respectively) were determined. Drug doses for the sympathetic and parasympathetic antagonists were calculated relative to the animal’s body weight on each experimental day. Cardiac muscarinic-cholinergic receptor blockade was achieved by infusion of the nonspecific muscarinic-cholinergic receptor antagonist atropine methylbromide [methylatropine (MA) 3 mg/kg] through the intraperitoneal catheter. Because the heart rate response to MA reached its peak in 10–15 min, this time interval was standardized before the heart rate measurement. Cardiac β1-adrenergic receptor blockade was achieved by infusion of the specific β1-adrenergic receptor antagonist metoprolol (MT, 10 mg/kg) through the intraperitoneal catheter. MT was infused 15 min after MA, and again the heart rate response was measured after 15 min. Intrinsic HR (HRI) was the HR after complete cardiac autonomic blockade (muscarinic-cholinergic and β1-adrenergic receptor blockades). Sympathetic tonus was calculated as HRM − HRI, where HRM is heart rate after muscarinic-cholinergic receptor blockade (33). Finally, hexamethonium chloride [a nicotinic ACh (NN) receptor antagonist (20 mg/kg)] was given to abolish ganglionic transmission and calculate the sympathetic support of blood pressure (SSBP). SSBP was calculated as the reduction in blood pressure in response to ganglionic blockade. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 2: resting angiotensin support of blood pressure.
On an alternate day, animals were treated identically as described above except that the order of cardiac autonomic blockade was reversed. After cardiac blockade, captopril, an angiotensin-converting enzyme (ACE) inhibitor (1 mg/kg), was given to block the production of angiotensin and calculate the angiotensin support of blood pressure (ASBP). ASBP was calculated as the reduction in blood pressure in response to ACE inhibition. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 3: reflex-induced cardiovascular responses.
On an alternate day, rats were brought into the laboratory and studied in their standard home cage. Temperature within the cage was maintained within the thermoneutral zone for rats. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, one dose of sodium nitroprusside (SNP) was infused through the intraperitoneal catheter (0.3 mg/kg for the sham-transected spinal intact rats and 0.05 mg/kg for the transected rats). The mean peak reduction in mean arterial pressure and increase in heart rate in response to bolus injections of SNP were measured for the three groups of rats. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 4: stress-induced cardiovascular responses.
On an alternate day, rats were brought into the laboratory and studied in their standard home cage. Temperature within the cage was maintained within the thermoneutral zone for rats. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, each rat was placed within a standard rat restrainer. The steady-state arterial pressure and heart rate responses were continuously recorded for 20 min. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 5: cardiovascular response to upright tilt.
On an alternate day, rats were brought into the laboratory and studied in their standard home cage. Temperature within the cage was maintained within the thermoneutral zone for rats. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, each rat was gently physically moved from the horizontal to the 90° upright position and manually held in this position for 1 min. The arterial pressure and heart rate responses were continuously recorded for 1 min. At the end of the experiment, the animals were returned to their housing facilities.
Data Analysis
All physiological recordings were sampled at 2 kHz, and the data are expressed as means ± SE. A two-way ANOVA with repeated measures was used for each of the following comparisons between spinal intact, paraplegic, and tetraplegic rats (group): 1) body weight before and each week for 12 consecutive weeks, 2) daily heart rate over 12 wk, 3) daily arterial blood pressure over 12 wk, 4) daily activity over 12 wk, 5) hemodynamic responses to restrainer stress, and 6) hemodynamic responses to upright tilt. Significant interactions allowed for further intergroup comparisons with a Student–Newman–Keuls post hoc analysis.
Box and whisker plots were generated for body weight (Fig. 1B), heart rate (Fig. 2B), arterial pressure (Fig. 3B), and physical activity (Fig. 4B) averaged over the 12 wk, cardiac sympathetic tonus (Fig. 5), SSBP and ASBP pressure (Fig. 6), hemodynamic responses to restrainer stress averaged over 20 min (Fig. 7, C and D), reflex-induced cardiovascular responses (Fig. 8), and hemodynamic responses to upright tilt averaged over 1 min (Fig. 9, C and D). The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The bold center line in each box plot represents the median value. A one-way ANOVA was used to determine differences in the data used to generate the box and whisker plots between spinal intact, paraplegic, and tetraplegic rats. Significance allowed for further intergroup comparisons with a Student–Newman–Keuls post hoc analysis.
Fig. 1.
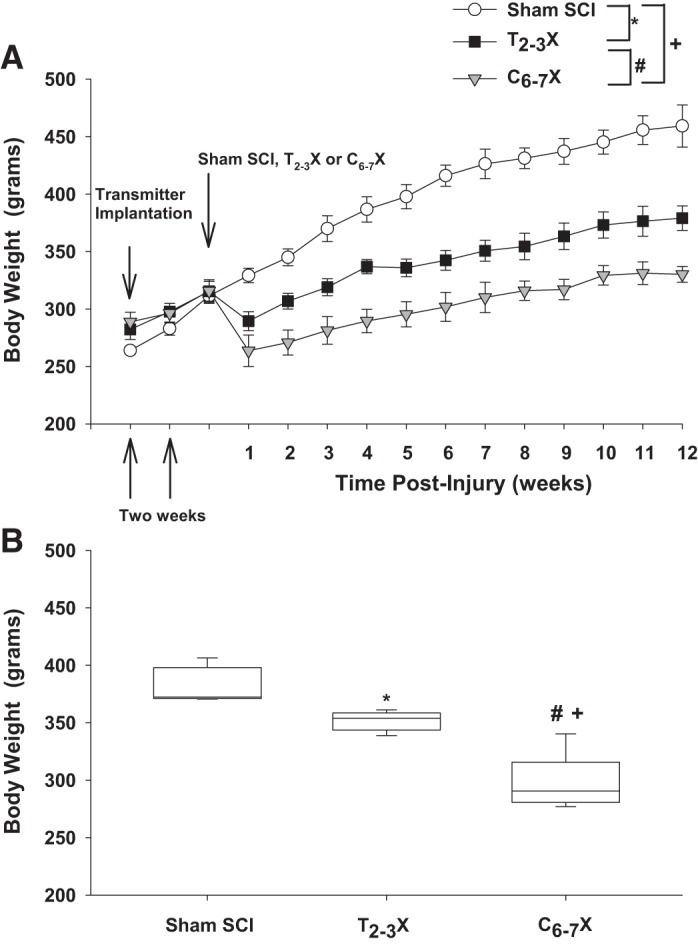
A: body weight 2 wk before the spinal cord injury (SCI) and each week for 12 consecutive weeks for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. B: box and whiskers plots for body weight averaged over the 12 wk. The central box represents the values from the 1st and 3rd quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
Fig. 2.
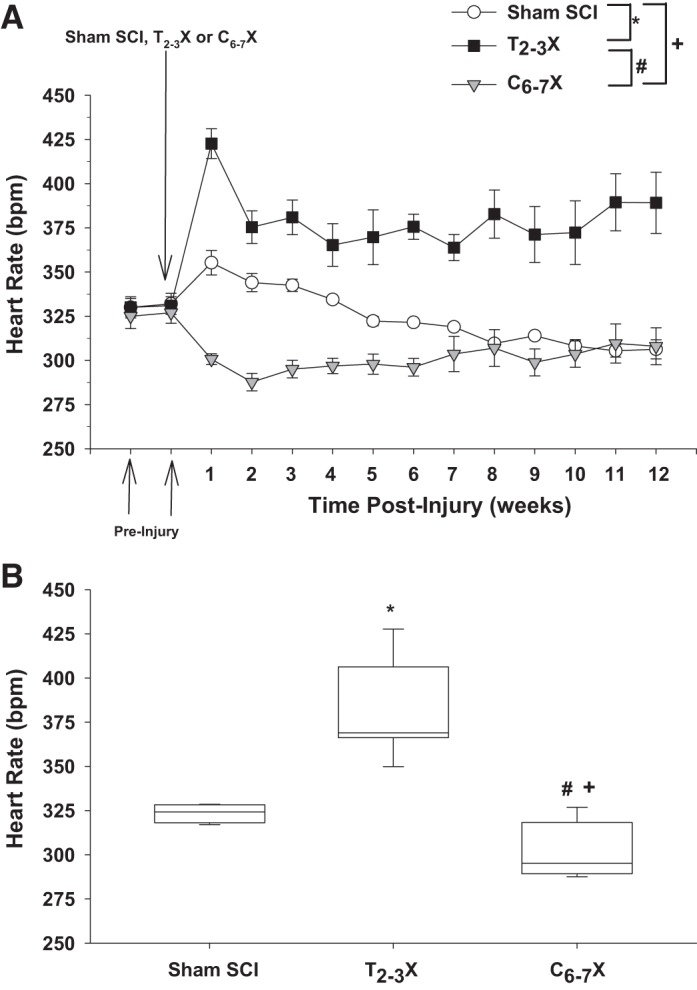
A: heart rate [beats/min (bpm)] 2 wk before the spinal cord injury (SCI) and each week for 12 consecutive weeks for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. B: box and whiskers plots for heart rate averaged over the 12 wk. The central box represents the values from the 1st and 3rd quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
Fig. 3.
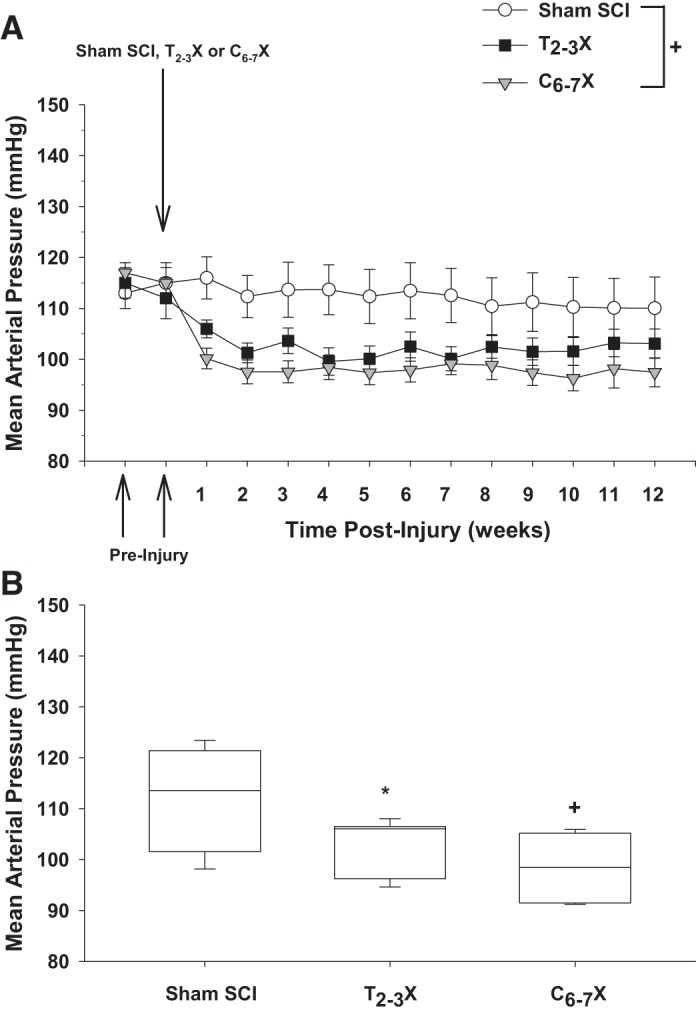
A: arterial pressure 2 wk before the spinal cord injury (SCI) and each week for 12 consecutive weeks for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. B: box and whiskers plots for arterial pressure averaged over the 12 wk. The central box represents the values from the 1st and 3rd quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. Mean arterial pressure was significantly lower in tetraplegic rats compared with spinal intact rats. *P < 0.05, Sham SCI vs. T2–3X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
Fig. 4.
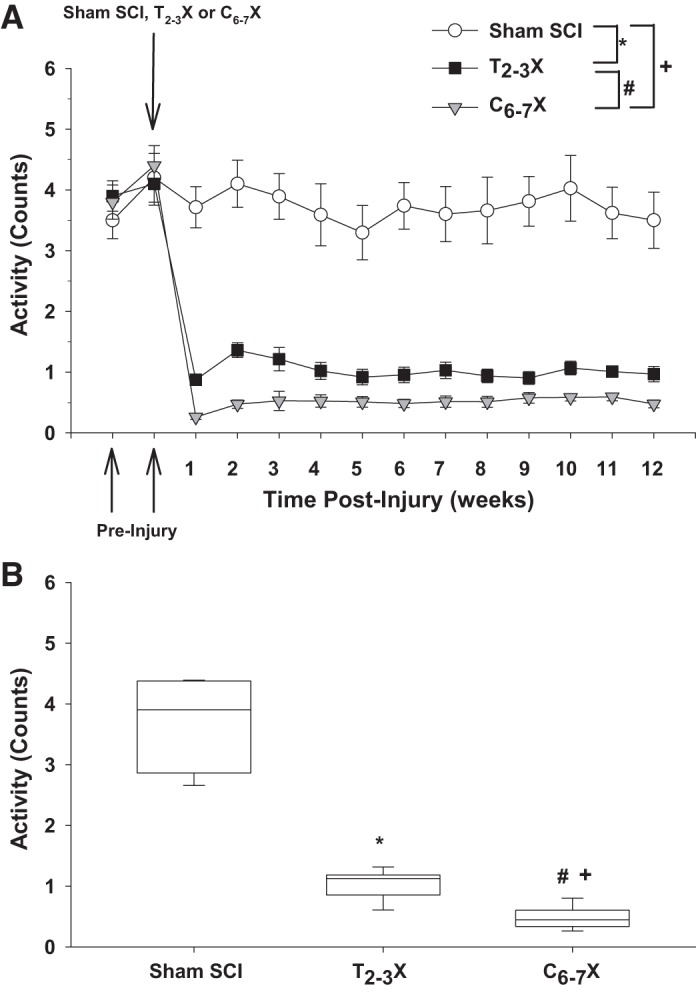
A: locomotor activity 2 wk before the spinal cord injury (SCI) and each week for 12 consecutive weeks for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. B: box and whiskers plots for locomotor activity averaged over the 12 wk. The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
Fig. 5.
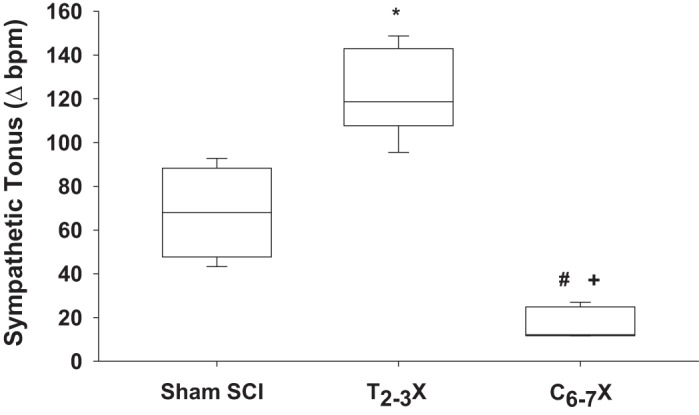
Box and whiskers plots for cardiac sympathetic tonus [beats/min (bpm)] in spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Cardiac sympathetic tonus was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. In contrast, cardiac sympathetic tonus in paraplegic rats was significantly elevated above spinal intact rats. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
Fig. 6.
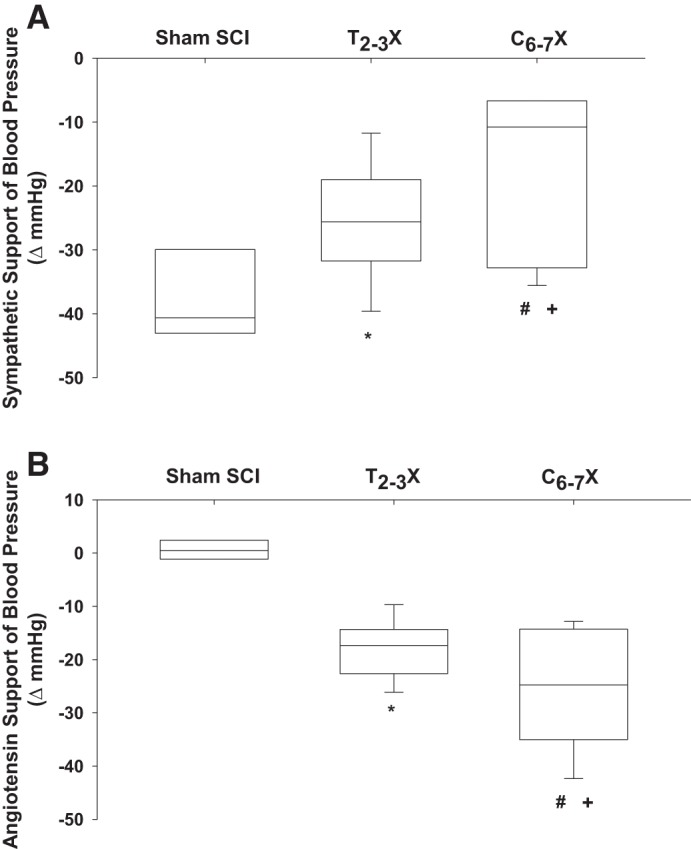
Box and whiskers plots for sympathetic (A) and angiotensin (B) support of blood pressure for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Paraplegic rats had a reduced sympathetic support of blood pressure and a higher angiotensin support of blood pressure compared with spinal intact rats. Furthermore, the sympathetic support of blood pressure was lower in tetraplegic rats compared with paraplegic and spinal intact rats. Tetraplegic rats had an enhanced reliance on angiotensin to maintain arterial blood pressure compared with spinal intact and paraplegic rats. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
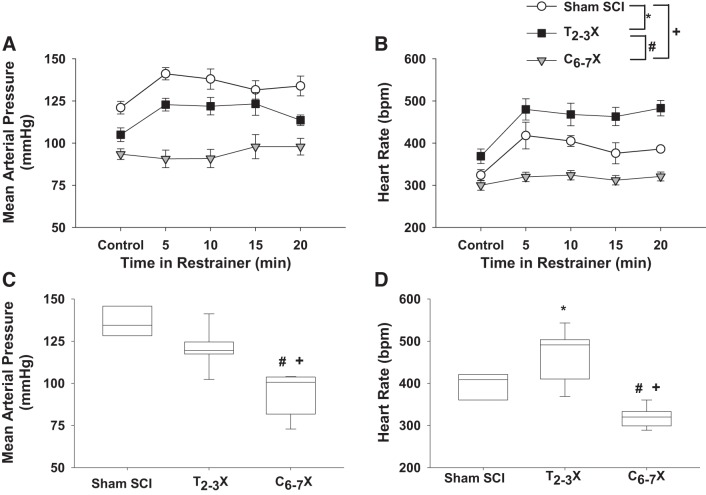
Fig. 7.
A and B: changes in arterial pressure (A) and heart rate [beats/min (bpm); B] in response to 20 min of restrainer stress for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. C and D: box and whiskers plots averaged over the 20 min. The blood pressure response to restrainer stress was significantly lower in paraplegic rats compared with spinal intact rats, whereas the heart rate response was significantly higher in paraplegic rats compared with spinal intact rats. In contrast, tetraplegic rats had virtually no arterial pressure or heart rate responses to restrainer stress compared with spinal intact and paraplegic rats. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05 Sham SCI vs. C6–7X, group effect.
Fig. 8.
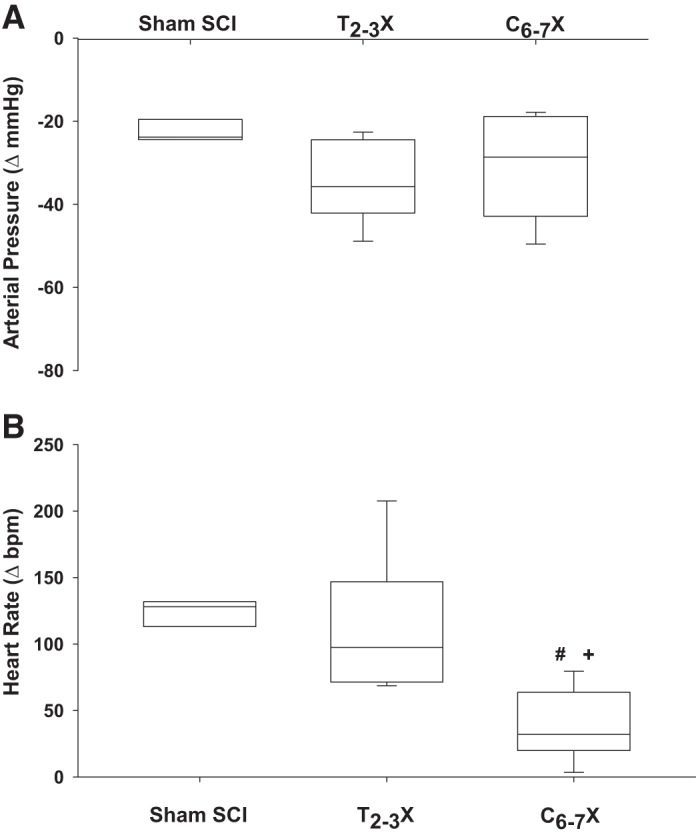
Box and whiskers plots for reflex-induced arterial pressure (A) and heart rate [beats/min (bpm); B] responses to a single dose of sodium nitroprusside for spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Although there were no differences in the arterial pressure responses between groups, tetraplegic rats had a significantly lower reflex heart rate response compared with paraplegic and spinal intact rats. #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05, Sham SCI vs. C6–7X, group effect.
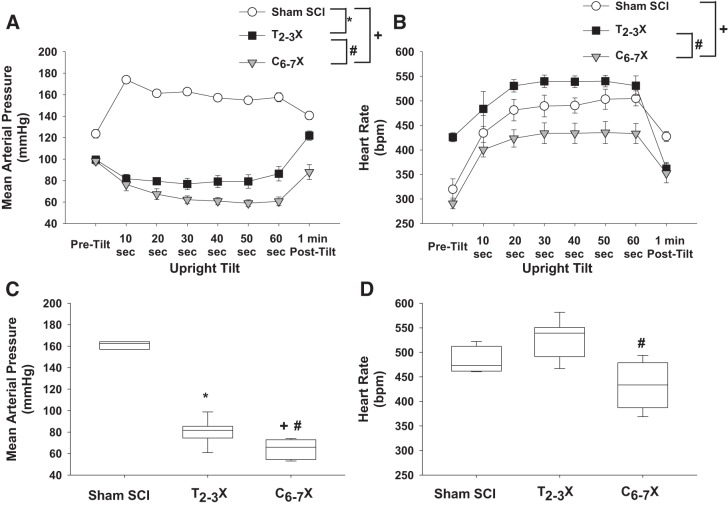
Fig. 9.
A and B: arterial pressure (A) and heart rate [beats/min (bpm); B] responses before upright tilt (Pre-tilt), during upright tilt, and at 1 min after upright tilt (Post-tilt) for t spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. C and D: box and whiskers plots averaged over 1 min. In Sham SCI rats, upright tilt increased blood pressure. In contrast, upright tilt decreased blood pressure in paraplegic rats, and the decrease was significantly greater in tetraplegic rats. Although upright tilt increased heart rate in all groups, the response was significantly lower in tetraplegic rats compared with paraplegic and spinal intact rats. *P < 0.05, Sham SCI vs. T2–3X, group effect; #P < 0.05, T2–3X vs. C6–7X, group effect; +P < 0.05 Sham SCI vs. C6–7X, group effect.
RESULTS
Body weight (weekly average) before and each week for 12 consecutive weeks for the spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats is presented in Fig. 1A. Although all rats had steady weight gain over 12 wk, body weight after C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. Body weight was significantly lower in paraplegic (T2–3X) rats compared with spinal intact rats. Box and whiskers plots of body weight averaged over the 12 wk are shown in Fig 1B. The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. Despite the significantly lower body weight in paraplegic and tetraplegic rats, all rats appeared healthy, grooming and eating and drinking regularly.
Figure 2 presents heart rate (weekly average) before and each week for 12 consecutive weeks for the spinal intact (Sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Heart rate after C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. Loss of supraspinal control of sympathetic preganglionic neurons (SPNs) projecting to the heart resulted in a profound bradycardia in tetraplegic rats. In contrast, heart rate in T2–3X paraplegic rats remained elevated above that of spinal intact rats for the entire 12 wk. Box and whiskers plots of heart rate averaged over the 12 wk are shown in Fig. 2B. The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value. Note that recordings were made via telemetry, in a dedicated room, 24 h per day and 7 days per week with ambient temperature maintained at the neutral temperature of rats [~29°C (35, 57),].
Mean arterial pressure (weekly average) before and each week for 12 consecutive weeks for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats is presented in Fig. 3. Mean arterial pressure after C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact rats. Box and whiskers plots of mean arterial pressure averaged over the 12 wk are shown in Fig. 3B. The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value.
Figure 4 presents locomotor activity (weekly average) before and each week for 12 consecutive weeks for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Locomotor activity after C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. Locomotor activity after T2–3 transection was significantly lower in paraplegic rats compared with spinal intact rats. Box and whiskers plots of locomotor activity averaged over the 12 wk are shown in Fig. 4B. The central box represents the values from the first and third quartiles (25th–75th percentiles). The vertical lines (whiskers) denote minimum and maximum values. The center line in each box plot represents the median value.
After the 12 wk of recording hemodynamic and locomotor parameters 24 h/day and 7 days/wk in the temperature-controlled room, all rats were studied in the laboratory. A direct comparison between spinal intact, T2–3 paraplegic, and C6–7 tetraplegic rats documented distinctly different responses depending on the level of the injury.
For example, Fig. 5 presents box and whiskers plots of cardiac sympathetic tonus for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Cardiac sympathetic tonus following C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. Loss of supraspinal control of SPNs projecting to the heart resulted in a profoundly reduced cardiac sympathetic tonus in tetraplegic rats. In contrast, cardiac sympathetic tonus in T2–3 paraplegic rats was significantly elevated above spinal intact rats.
SSBP (Fig. 6A) and ASBP (Fig. 6B) for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats are presented as box and whiskers plots in Fig. 6. SSBP after C6–7 transection was significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. SSBP after T2–3 transection was significantly lower in paraplegic rats compared with spinal intact rats. ASBP after C6–7 transection was significantly higher in tetraplegic rats compared with spinal intact and paraplegic rats. ASBP after T2–3 transection was significantly higher in paraplegic rats compared with spinal intact rats.
Blood pressure and heart rate responses to restrainer stress for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats are presented in Fig. 7, A and B. Box and whiskers plots of blood pressure and heart rate responses to restrainer stress averaged over the 20 min are presented in Fig. 7, C and D. The blood pressure and heart rate responses to restrainer stress following C6–7 transection were significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats. The blood pressure response to restrainer stress following T2–3 transection was significantly lower in paraplegic rats compared with spinal intact rats, whereas in contrast the heart rate response was significantly higher in paraplegic rats compared with spinal intact rats.
Figure 8 presents box and whiskers plots of the blood pressure (Fig. 8A) and heart rate (Fig. 8B) responses to a single dose of SNP for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats. Reflex responses to reductions in blood pressure after C6–7 transection were significantly lower in tetraplegic rats compared with spinal intact and paraplegic rats.
Arterial pressure and heart rate responses to upright tilt for the spinal intact (sham SCI), paraplegic (T2–3X), and tetraplegic (C6–7X) rats are presented in Fig. 9, A and B. Box and whiskers plots of the arterial pressure and heart rate responses to upright tilt averaged over 1 min are shown in Fig. 9, C and D. The responses to upright tilt were significantly different in tetraplegic compared with paraplegic rats. Note the profound tachycardiac response in the paraplegic rats. Furthermore, note that the heart rate response in the tetraplegic rats did not reach the resting heart rate observed in the paraplegic rats.
DISCUSSION
As shown by published data from the National Spinal Cord Injury Statistical Center, the annual incidence of SCI in the United States alone is ~17,730 each year (42a). Approximately 59.9% of these injuries result in tetraplegia (47.6% incomplete, 12.3% complete). Although the incidence of complete cervical SCI is low, public health needs are reflected not only by how many people have a particular condition but also by the “burden of condition”—the impact of a health condition as measured by mortality, morbidity, financial cost, and other indicators (19). Different conditions can impose vastly different kinds of burdens. Some conditions may cause premature death, whereas other chronic conditions may cause long-term disability and impose great emotional and monetary toll for patients, family members, and society (19).
Disease incidence and prevalence may be unrelated to societal and personal burden. However, mortality and years of life lost correlate with societal burden. Disability-adjusted life years—a measure that estimates the equivalent number of healthy years lost because of disability or early death—are important indicators of societal and personal burden (41, 42).
In this context, the average remaining years of life for individuals with SCI have not improved since the 1980s and remain significantly below life expectancies of persons without SCI. For example, an individual without a SCI at 20 yr of age has 60.6 average remaining years of life. In contrast, with an injury at 20 yr of age, an individual with paraplegia has 46.6 average remaining years of life and an individual with low tetraplegia has 40.9 average remaining years of life (42a). Finally, disease burden is not the only factor in setting societal priorities. We, as a society, must also be committed to supporting research into rare conditions, which affect a smaller component of the population. This approach will also result in findings that can have implications for the cause and treatment of a variety of diseases and can be applied across several fields.
SCI causing paraplegia or tetraplegia results in paralysis and sensory impairment as well as autonomic dysfunction that triggers compromised cardiovascular, bowel, bladder, and sexual activity in humans and rodents. Accordingly, the reluctance to perform experiments on preclinical spinal cord-injured models is understandable. The challenges of animal care as well as the emotional concern for injured animals can be difficult to overcome. We are keenly aware of and sensitive to these issues. Accordingly, our spinal cord-injured animals are treated with the same care and respect we provide to humans with SCI (13–15). This is a critical consideration because addressing autonomic losses and cardiovascular consequences will greatly improve the quality of life for individuals and families living with paraplegia and tetraplegia. As stated profoundly by Christopher Reeve, “Spinal cord injury is a ferocious assault on the body that leaves havoc in its wake. Paralysis is certainly part of its legacy, but there are other equally devastating consequences including autonomic dysfunction: compromised cardiovascular, bowel, bladder and sexual function. Treatment and cures for these losses would greatly improve the quality of life for all of us living with spinal cord injury” (46). Moreover, individuals with SCI prioritize the recovery of autonomic functions, such as cardiovascular, sexual, and bowel and bladder control, above the ability to walk (2).
It is important to note that cervical SCI is unique. During the first 7–10 days after injury, the animals required intervention (bladder expression, feeding, and movement) every 4 h. Maintaining body temperature was also critical. After 10 days, the rats no longer required feeding; however, manual bladder compression continued 4 times/day to completely empty any residual urine that might risk infections. With these efforts, we have achieved a <10% mortality rate. When problems occurred, they appeared to be due to our failure to adequately maintain the rat’s body temperature.
Although no behavioral testing was performed, all rats with tetraplegia had complete paralysis of the forepaws and were unable to grasp objects. Although the rats could not grip objects, they could hold objects between their forepaws without closing the paw. The rats would support themselves on their shoulders and hold food between their forepaws to eat. The rats could also move their forelimbs (elbow flexion) and had complete shoulder movement that allowed them to move modestly within the cage and groom their head and face. All rats with paraplegia had complete control of the forepaws and were able to grasp objects. This allowed them to move easily within the cage and groom their head and face.
Loss of supraspinal control to all sympathetic preganglionic neurons (SPNs) projecting to the heart and vasculature, as well as reduced body mass and physical activity, resulted in a profound bradycardia and hypotension (Figs. 2 and 3), reduced cardiac sympathetic tonus (Fig. 5), reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure (Fig. 6), and reduced stress (Fig. 7)- and reflex (Fig. 8)-induced sympathetic responses. The profound bradycardia altered cardiac autonomic tone and enhanced reliance on angiotensin in rats with tetraplegia are expected to alter cardiac electrophysiology and increase the susceptibility to a variety of arrhythmias. Specifically, mammalian heart rate and cardiac electrophysiology are profoundly influenced by the sympathetic and parasympathetic divisions of the autonomic nervous system. The renin-angiotensin aldosterone system also has a profound effect on cardiac electrophysiology by altering membrane ion channels (4, 23), suppression of the sarcoplasmic reticulum Ca2+-ATPase pump (22) or the ryanodine receptor (17, 58), and disruption of cell-to-cell coupling through modification of gap junctional conductance. Accordingly, the profound bradycardia and reliance on angiotensin in rats with tetraplegia are expected to alter cardiac electrophysiology and increase the susceptibility to a variety of arrhythmias. This expectation merits future consideration.
Rats with high thoracic SCI (T2–3X), which preserves supraspinal control of some cardiac SPNs projecting to the heart, had tachycardia, enhanced cardiac sympathetic tonus, enhanced reflex- and stress-induced sympathetic responses, as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Similar results were previously recorded in rats with midthoracic SCI (26, 28, 50). Thus high thoracic and midthoracic SCI resulted in high cardiac sympathetic activity. This may be mediated by the loss of sympathetic drive to the vasculature below the level of the injury that results in hypotension and a marked arterial baroreflex-mediated increase in sympathetic outflow to the heart. Specifically, loss of descending sympathetic drive to the vasculature innervated by SPNs, which exit the spinal cord below the level of transection, results in hypotension and thus a dramatic reflex-mediated increase in sympathetic outflow to the heart, the pathways of which are preserved above the level of the transection. These levels of sympathetic outflow have been reported to be high enough to establish a setting where NGF is produced and induces sympathetic sprouting and hyperinnervation of the heart (26, 29, 31).
It is important to note that in addition to the differences in supraspinal control of SPNs in rats with high thoracic versus cervical SCI, we observed markedly different body masses and levels of physical activity between groups. It seems likely that these effects contributed to the differences in autonomic function. Specifically, the thermic effects of food, the composition of the food (e.g., salt), and the postabsorptive responses to feeding have an influence on sympathetic function. The uncontrolled consumption of the available diet may have led to a differential of sympathetic outflow between groups.
In addition, the vascular architecture below the level of the lesion undergoes significant remodeling after SCI. Importantly, these vascular adaptations are proportional to changes in body composition (51). Plasma volume may also be reduced as a consequence of this vascular remodeling (11, 21). Finally, it is necessary to consider that leptin, secreted by adipose tissue, has an effect on humoral and sympathetic function.
The use of a weight-based dose of pharmacological agents may also have contributed to the results. Specifically, variations in body mass between animals and groups led to differences in the absolute concentration of agent delivered. Because of receptor plasticity and other postinjury consequences, this can lead to changes in the observed responses.
Limitations
A major limitation of this study is the lack of direct sympathetic recordings even though sympathetic control of cardiovascular function is a major focus of the study. Although we assessed differences in blood pressure and heart rate in response to several pharmacological and behavioral maneuvers as a surrogate of sympathetic recordings, it is possible that the hemodynamic responses do not reflect autonomic responses in these models. Specifically, the profound changes in cardiovascular control following SCI may confound the relationships between cardiac sympathetic outflow and hemodynamic responses. It is important to note, however, that investigators have used a variety of indirect approaches to assess “sympathetic tone,” including indirect measures such as pharmacological or surgical blockade of autonomic ganglia (24, 59). Others have evaluated the range of fluctuations of blood pressure (blood pressure variability) over time (43). Many others have recorded the changes in the frequency components of heart rate or systolic blood pressure variability (1, 47) or measured total or regional norepinephrine spillover via the use of radiotracer dilution technology (16). Each of these methods provides important clues about sympathetic function; however, none can substitute for a direct recording of the activity within the sympathetic nerves that control various autonomic effector organs. However, methods of direct recording of sympathetic outflow over 12 wk are not currently possible.
It is important to note that during the initial weeks after SCI individuals tend to lose weight because of hypercatabolism (5), muscle atrophy, and decreased basal metabolic rate. Energy requirements for physical activity also decline because of relatively sedentary lifestyles. However, without diet adjustment to new metabolic requirements after SCI, energy intake quickly exceeds energy requirements, resulting in weight gain (20). In fact, based on clinical observations, individuals with SCI tend to become obese during the initial 12 mo after SCI (9). Crane and colleagues document a significant increase in body weight after new SCI despite a reduction in activity (10). These dramatic changes in body composition, caloric intake, and physical activity may confound interpretation of the results.
Finally, whenever possible both male and female subjects should be studied. We did not study female rats because of cost restrictions associated with this initial long-term study and the fact that 80% of all SCIs occur in males (6). However, the failure to study females should be corrected in future studies.
Conclusions
High-fidelity recordings, via radiotelemetry, of intravascular arterial blood pressure, heart rate, and physical activity (20 s every minute during 12 consecutive weeks) document that C6–7 tetraplegic rats had hypotension, bradycardia, and reduced physical activity. In contrast, T2–3 paraplegic rats were tachycardiac. C6–7 tetraplegic rats compared with T2–3 paraplegic and spinal intact rats also had reduced cardiac sympathetic tonus, reduced reflex- and stress-induced sympathetic responses, and reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure. In sharp contrast, T2–3 paraplegic rats compared with spinal intact rats had enhanced cardiac sympathetic tonus, enhanced reflex- and stress-induced sympathetic responses, as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Thus injuries above and below the peak level (T2) of spinal cord projections to the stellate ganglia have remarkably different outcomes, and sparing a few spinal levels dramatically alters autonomic control of the heart.
SCI resulting in tetraplegia is a devastating, life-changing insult causing paralysis and sensory impairment as well as autonomic dysfunction that triggers compromised cardiovascular, bowel, bladder, and sexual activity (34). Life becomes a battle for independence as even routine bodily functions and the smallest activity of daily living become a major challenge (34). Accordingly, there is a critical need for a chronic preclinical model of tetraplegia. Despite this need, virtually all preclinical investigations induce lesions in the thoracic region (34). This study documents that thoracic lesions are not applicable to cervical lesions when examining autonomic dysfunction and cardiovascular consequences.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-122223.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L.L. and S.E.D. conceived and designed research; H.L.L. and S.E.D. performed experiments; H.L.L. and S.E.D. analyzed data; H.L.L. and S.E.D. interpreted results of experiments; H.L.L. and S.E.D. prepared figures; H.L.L. and S.E.D. drafted manuscript; H.L.L. and S.E.D. edited and revised manuscript; H.L.L. and S.E.D. approved final version of manuscript.
REFERENCES
- 1.Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput 44: 1031–1051, 2006. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139: 244–256, 1996. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 4.Caballero R, Gómez R, Moreno I, Nuñez L, González T, Arias C, Guizy M, Valenzuela C, Tamargo J, Delpón E. Interaction of angiotensin II with the angiotensin type 2 receptor inhibits the cardiac transient outward potassium current. Cardiovasc Res 62: 86–95, 2004. doi: 10.1016/j.cardiores.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord 44: 82–91, 2006. doi: 10.1038/sj.sc.3101818. [DOI] [PubMed] [Google Scholar]
- 6.Christopher Reeve Paralysis Foundation Spinal Cord Injury (Online). https://www.christopherreeve.org/living-with-paralysis/health/causes-of-paralysis/spinal-cord-injury [28 January 2020].
- 7.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T5 spinal rats. Am J Physiol Heart Circ Physiol 282: H1566–H1570, 2002. doi: 10.1152/ajpheart.00733.2001. [DOI] [PubMed] [Google Scholar]
- 8.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002. doi: 10.1152/ajpheart.00253.2002. [DOI] [PubMed] [Google Scholar]
- 9.Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma 25: 419–423, 1985. doi: 10.1097/00005373-198505000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Crane DA, Little JW, Burns SP. Weight gain following spinal cord injury: a pilot study. J Spinal Cord Med 34: 227–232, 2011. doi: 10.1179/2045772311Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehabil 87: 688–696, 2006. doi: 10.1016/j.apmr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 12.DeVeau KM, Martin EK, King NT, Shum-Siu A, Keller BB, West CR, Magnuson DS. Challenging cardiac function post-spinal cord injury with dobutamine. Auton Neurosci 209: 19–24, 2018. doi: 10.1016/j.autneu.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCarlo SE. Effect of arm ergometry training on wheelchair propulsion endurance of individuals with quadriplegia. Phys Ther 68: 40–44, 1988. doi: 10.1093/ptj/68.1.40. [DOI] [PubMed] [Google Scholar]
- 14.DiCarlo SE. Improved cardiopulmonary status after a two-month program of graded arm exercise in a patient with C6 quadriplegia. A case report. Phys Ther 62: 456–459, 1982. doi: 10.1093/ptj/62.4.456. [DOI] [PubMed] [Google Scholar]
- 15.DiCarlo SE, Supp MD, Taylor HC. Effect of arm ergometry training on physical work capacity of individuals with spinal cord injuries. Phys Ther 63: 1104–1107, 1983. doi: 10.1093/ptj/63.7.1104. [DOI] [PubMed] [Google Scholar]
- 16.Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol Endocrinol Metab 247: E21–E28, 1984. doi: 10.1152/ajpendo.1984.247.1.E21. [DOI] [PubMed] [Google Scholar]
- 17.Flesch M, Schiffer F, Zolk O, Pinto Y, Stasch JP, Knorr A, Ettelbrück S, Böhm M. Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca2+-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens 15: 1001–1009, 1997. doi: 10.1097/00004872-199715090-00011. [DOI] [PubMed] [Google Scholar]
- 18.Frankel HL, Michaelis LS, Golding DR, Beral V. The blood pressure in paraplegia. I. Paraplegia 10: 193–200, 1972. doi: 10.1038/sc.1972.32. [DOI] [PubMed] [Google Scholar]
- 19.Gillum LA, Gouveia C, Dorsey ER, Pletcher M, Mathers CD, McCulloch CE, Johnston SC. NIH disease funding levels and burden of disease. PLoS One 6: e16837, 2011. doi: 10.1371/journal.pone.0016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury—a retrospective study. Spinal Cord 44: 92–94, 2006. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- 21.Houtman S, Oeseburg B, Hopman MT. Blood volume and hemoglobin after spinal cord injury. Am J Phys Med Rehabil 79: 260–265, 2000. doi: 10.1097/00002060-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ju H, Scammel-La Fleur T, Dixon IM. Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol 28: 1119–1128, 1996. doi: 10.1006/jmcc.1996.0103. [DOI] [PubMed] [Google Scholar]
- 23.Kaibara M, Mitarai S, Yano K, Kameyama M. Involvement of Na+-H+ antiporter in regulation of L-type Ca2+ channel current by angiotensin II in rabbit ventricular myocytes. Circ Res 75: 1121–1125, 1994. doi: 10.1161/01.RES.75.6.1121. [DOI] [PubMed] [Google Scholar]
- 24.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 25.Krum H, Louis WJ, Brown DJ, Jackman GP, Howes LG. Diurnal blood pressure variation in quadriplegic chronic spinal cord injury patients. Clin Sci (Lond) 80: 271–276, 1991. doi: 10.1042/cs0800271. [DOI] [PubMed] [Google Scholar]
- 26.Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus, and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009. doi: 10.1152/ajpheart.01286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lujan HL, DiCarlo SE. Fundamental hemodynamic mechanisms mediating the response to myocardial ischemia in conscious paraplegic mice: cardiac output versus peripheral resistance. Physiol Rep 5: e13214, 2017. doi: 10.14814/phy2.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007. doi: 10.1152/ajpheart.01019.2007. [DOI] [PubMed] [Google Scholar]
- 29.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol (1985) 113: 1332–1341, 2012. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lujan HL, Janbaih H, DiCarlo SE. Structural remodeling of the heart and its premotor cardioinhibitory vagal neurons following T5 spinal cord transection. J Appl Physiol (1985) 116: 1148–1155, 2014. doi: 10.1152/japplphysiol.01285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol 299: R985–R995, 2010. doi: 10.1152/ajpregu.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lujan HL, Palani G, Peduzzi JD, DiCarlo SE. Targeted ablation of mesenteric projecting sympathetic neurons reduces the hemodynamic response to pain in conscious, spinal cord-transected rats. Am J Physiol Regul Integr Comp Physiol 298: R1358–R1365, 2010. doi: 10.1152/ajpregu.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lujan HL, Rivers JP, DiCarlo SE. Complex and interacting influences of the autonomic nervous system on cardiac electrophysiology in conscious mice. Auton Neurosci 201: 24–31, 2016. doi: 10.1016/j.autneu.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lujan HL, Tonson A, Wiseman RW, DiCarlo SE. Chronic, complete cervical6-7 cord transection: distinct autonomic and cardiac deficits. J Appl Physiol (1985) 124: 1471–1482, 2018. doi: 10.1152/japplphysiol.01104.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29: 413–420, 2014. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 36.Mathias C, Frankel H. Autonomic Disturbances in Spinal Cord Lesions. Oxford, UK: Oxford Univ. Press, 1992. [Google Scholar]
- 37.Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Spalding JM. Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Circ Res 39: 204–208, 1976. doi: 10.1161/01.RES.39.2.204. [DOI] [PubMed] [Google Scholar]
- 38.Mathias CJ, Frankel HL. The cardiovascular system in tetraplegia and paraplegia. In: Spinal Cord Trauma, Handbook of Clinical Neurology, edited by Vinken P, Bruyn GV, Klara HL. Amsterdam: Elsevier Science, 1992, p. 435–456. [Google Scholar]
- 39.Moffitt JA. Editorial Focus: role for neural growth factor in autonomically driven arrhythmogenesis? Focus on: “Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization”. Am J Physiol Regul Integr Comp Physiol 299: R983–R984, 2010. doi: 10.1152/ajpregu.00524.2010. [DOI] [PubMed] [Google Scholar]
- 40.Morita S, Inokuchi S, Yamagiwa T, Aoki H, Nakagawa Y, Yamamoto I. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation after central spinal cord injury: a case report. J Emerg Med 39: 301–304, 2010. doi: 10.1016/j.jemermed.2007.10.086. [DOI] [PubMed] [Google Scholar]
- 41.Morrow RH, Bryant JH. Health policy approaches to measuring and valuing human life: conceptual and ethical issues. Am J Public Health 85: 1356–1360, 1995. doi: 10.2105/AJPH.85.10.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray CJ. Rethinking DALYs. In: The Global Burden of Disease, edited by Murray CJ, Lopez AD. Cambridge, MA: Harvard School of Public Health on behalf of the World Health Organization and World Bank, 1996, p. 1–98. [Google Scholar]
- 42a.National Spinal Cord Injury Statistical Center Spinal Cord Injury Facts and Figures at a Glance. Birmingham, AL: Univ. of Alabama at Birmingham, 2019. [Google Scholar]
- 43.Parati G, Ochoa JE, Salvi P, Lombardi C, Bilo G. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Diabetes Care 36, Suppl 2: S312–S324, 2013. doi: 10.2337/dcS13-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardini BJ, Lund DD, Schmid PG. Innervation patterns of the middle cervical-stellate ganglion complex in the rat. Neurosci Lett 117: 300–306, 1990. doi: 10.1016/0304-3940(90)90681-X. [DOI] [PubMed] [Google Scholar]
- 45.Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst 28: 193–201, 1989. doi: 10.1016/0165-1838(89)90146-X. [DOI] [PubMed] [Google Scholar]
- 46.Reeve C. Dedication. In: Autonomic Dysfunction after Spinal Cord Injury, Progress in Brain Research, edited by Weaver L, Polosa C.. Amsterdam: Elsevier Science, 2005, p. ix. [Google Scholar]
- 47.Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50: 477–487, 2013. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 48.Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens 25: 349–358, 2003. doi: 10.1081/CEH-120023544. [DOI] [PubMed] [Google Scholar]
- 49.Rodenbaugh DW, Collins HL, DiCarlo SE. Paraplegia differentially increases arterial blood pressure related cardiovascular disease risk factors in normotensive and hypertensive rats. Brain Res 980: 242–248, 2003. doi: 10.1016/S0006-8993(03)02982-2. [DOI] [PubMed] [Google Scholar]
- 50.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003. doi: 10.1152/ajpheart.00319.2003. [DOI] [PubMed] [Google Scholar]
- 51.Rowley NJ, Dawson EA, Hopman MT, George KP, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc 44: 844–849, 2012. doi: 10.1249/MSS.0b013e31823f6887. [DOI] [PubMed] [Google Scholar]
- 52.Sharov VG, Galakhin KA. [Myocardial changes after spinal cord injuries in humans and experimental animals]. Arkh Patol 46: 17–20, 1984. [PubMed] [Google Scholar]
- 53.Shepherd C. Understand Spinal Cord Injury: What You Should Know About Spinal Cord Injury and Recovery (Online). http://www.spinalinjury101.org/files/understanding-spinal-cord-injury.pdf [28 January 28, 2020].
- 55.Squair JW, Liu J, Tetzlaff W, Krassioukov AV, West CR. Spinal cord injury-induced cardiomyocyte atrophy and impaired cardiac function are severity dependent. Exp Physiol 103: 179–189, 2018. doi: 10.1113/EP086549. [DOI] [PubMed] [Google Scholar]
- 56.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 57.Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287: R391–R396, 2004. doi: 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 58.Tokuhisa T, Yano M, Obayashi M, Noma T, Mochizuki M, Oda T, Okuda S, Doi M, Liu J, Ikeda Y, Yamamoto T, Ohkusa T, Matsuzaki M. AT1 receptor antagonist restores cardiac ryanodine receptor function, rendering isoproterenol-induced failing heart less susceptible to Ca2+-leak induced by oxidative stress. Circ J 70: 777–786, 2006. doi: 10.1253/circj.70.777. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]