Abstract
Acute intermittent hypoxia (AIH) elicits phrenic motor plasticity via multiple distinct cellular mechanisms. With moderate AIH, phrenic motor facilitation (pMF) requires Gq protein-coupled serotonin type 2 receptor activation, ERK MAP kinase activity, and new synthesis of brain-derived neurotrophic factor. In contrast, severe AIH elicits pMF by an adenosine-dependent mechanism that requires exchange protein activated by cAMP, Akt, and mammalian target of rapamycin (mTOR) activity, followed by new tyrosine receptor kinase B protein synthesis; this same pathway is also initiated by Gs protein-coupled serotonin 7 receptors (5-HT7). Because the metabolic sensor AMP-activated protein kinase (AMPK) inhibits mTOR-dependent protein synthesis, and mTOR signaling is necessary for 5-HT7 but not 5-HT2 receptor-induced pMF, we hypothesized that spinal AMPK activity differentially regulates pMF elicited by these distinct receptor subtypes. Serotonin type 2A receptor [5-HT2A; (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride] or 5-HT7 (AS-19) receptor agonists were administered intrathecally at C4 (3 injections, 5-min intervals) while recording integrated phrenic nerve activity in anesthetized, vagotomized, paralyzed, and ventilated rats. Consistent with our hypothesis, spinal AMPK activation with 2-deoxyglucose or metformin blocked 5-HT7, but not 5-HT2A receptor-induced pMF; in both cases, pMF inhibition was reversed by spinal administration of the AMPK inhibitor compound C. Thus, AMPK differentially regulates cellular mechanisms of serotonin-induced phrenic motor plasticity.
NEW & NOTEWORTHY Spinal AMP-activated protein kinase (AMPK) overactivity, induced by local 2-deoxyglucose or metformin administration, constrains serotonin 7 (5-HT7) receptor-induced (but not serotonin type 2A receptor-induced) respiratory motor facilitation, indicating that metabolic challenges might regulate specific forms of respiratory motor plasticity. Pharmacological blockade of spinal AMPK activity restores 5-HT7 receptor-induced respiratory motor facilitation in the presence of either 2-deoxyglucose or metformin, showing that AMPK is an important regulator of 5-HT7 receptor-induced respiratory motor plasticity.
Keywords: AMPK, phrenic motor plasticity, serotonin receptors
INTRODUCTION
Multiple independent cellular mechanisms give rise to phenotypically similar phrenic motor facilitation (pMF), a persistent poststimulus increase in phrenic nerve activity. Spinal Gq protein-coupled serotonin type 2A receptor (5-HT2A) activation elicits the “Q pathway” to pMF, which requires ERK MAP kinase, tyrosine receptor kinase B (TrkB), and PKC-θ activity, and new brain-derived neurotrophic factor protein synthesis. Alternatively, spinal Gs protein-coupled serotonin 7 receptor (5-HT7) activation gives rise to pMF via the “S pathway,” which requires intracellular cAMP signaling, exchange protein activated by cAMP, Akt, and mammalian target of rapamycin complex 1 (mTORC1) activity, and new TrkB protein synthesis (11, 13, 14). Although we have learned a great deal about these distinct mechanisms of pMF, relatively little is known concerning factors that differentially regulate their expression.
Factors such as inflammation and/or energy balance may differentially regulate the Q and S pathways to pMF. For example, neuroinflammation selectively constrains Q pathway-dependent pMF (2) by a p38 MAP kinase (2, 19) and okadaic acid-sensitive protein phosphatase-dependent mechanism (Ref. 44; unpublished observations); inflammation has minimal impact on the S pathway (1). On the other hand, AMP-activated protein kinase (AMPK) regulates energy supply to maintain cellular functions and may uniquely regulate the S pathway. When activated by metabolic stress, such as severe hypoxia, AMPK inhibits mTORC1 signaling (4, 5, 7, 20, 37, 43) and regulates certain forms of hippocampal synaptic plasticity (37). On the other hand, glycolytic flux inhibition with 2-deoxyglucose (2-DG), and presumed AMPK activation, has minimal impact on Q pathway-dependent pMF following moderate acute intermittent hypoxia (27). Thus, AMPK likely regulates some, but not all, forms of phrenic motor plasticity.
Here, we test the hypothesis that AMPK activity regulates the mTORC1-dependent S pathway to pMF, but not the mTORC1-independent Q pathway (11, 13). Specifically, we tested the hypothesis that spinal AMPK activity impairs 5-HT7, but not 5-HT2A receptor-induced pMF in anesthetized, paralyzed, and ventilated rats. The Q and S pathways were induced by intrathecal (C4) injections of selective 5-HT2A or 5-HT7 receptor agonists, and AMPK activity was regulated via intrathecal injection of activators (2-DG, metformin) with/without AMPK inhibition [compound C (cC)]. We demonstrate that 2-DG and metformin both abolish 5-HT7 (not 5-HT2A) receptor-induced pMF, and this effect is reversed by cC. Differential regulation of phrenic motor plasticity may be particularly advantageous in preventing its expression during metabolic stress to minimize unnecessary energetic costs associated with protein synthesis (10, 32).
METHODS
Experimental animals.
Experiments were performed on adult (300–400 g) male Sprague Dawley rats (208A Colony, Envigo), housed in pairs and maintained on a 12:12-h light-dark cycle with access to food and water ad libitum. All experiments were approved by the University of Florida Institutional Animal Care and Use Committee and were conducted in accordance with the Animal Welfare Act, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011).
Neurophysiological experiments.
Surgical procedures and electrophysiological recording techniques were described in detail previously (3, 35). Briefly, rats were induced with 3% isoflurane in 100% O2 at 3 L/min in a 5-liter Plexiglas chamber. Rats were then transferred to a heated surgical table, and anesthesia was maintained via nose cone. The rats were tracheotomized, ventilated (~2.5 mL, 70 breaths/min; VentElite small animal ventilator; Harvard Apparatus, Holliston, MA), and bilaterally vagotomized in the midcervical region. End-tidal CO2 was monitored using a flow-through capnograph (Capnogard; Novametrix, Wallingford, CT) and maintained within a normal physiological range. The rats were then slowly converted to urethane anesthesia by reducing isoflurane by 0.5% every 3 min while urethane (2.1 g/kg, diluted in double-distilled water; Sigma-Aldrich, St. Louis, MO) was administered via tail vein catheter (24 gauge; Surflash, Somerset, NJ) with a syringe pump (6 mL/h). Based on isoflurane clearance rates (16), at least 1 h was allowed after anesthetic conversion was complete to minimize isoflurane effects on breathing. Adequate anesthetic depth was assured by the absence of a withdrawal reflex and/or blood pressure response to toe pinch. A polyethylene catheter was inserted in the right femoral artery to monitor blood pressure (Argon Pressure Transducer; DTXPlus, Plano, TX) and to draw arterial blood samples (ABL 90 Flex; Radiometer) in heparinized plastic capillary tubes (electrolyte balanced 70 IU/mL, 70 μL; Radiometer).
Rats were positioned in sternal recumbency, and muscles overlying the dorsal aspect of the cervical spinal cord were exposed through a midline incision. Muscles were separated to allow C2 laminectomy, and a silicone catheter (2 Fr, 0.6 mm OD; Access Technologies, Skokie, IL) attached to a 50-μL Hamilton syringe was inserted through a small hole in the dura mater. The catheter was advanced caudally to the C3–C4 spinal segment and stabilized in position. The left phrenic nerve was isolated and cut distally. The epineurium was gently retracted, and the central end of the phrenic nerve was recorded via saline-filled bipolar glass suction electrodes. Phrenic nerve activity was processed (10,000× amplified and band-pass filtered, 0.3–5 kHz, model 1700; A-M Systems, Sequim, WA), digitized (CED 1401; Cambridge Electronic Design, Cambridge, UK), and analyzed via Spike2 software (version 8.08; Cambridge Electronic Design). Rats were paralyzed with the nicotinic acetylcholine receptor blocker pancuronium bromide (3 mg/kg iv; Sigma-Aldrich; St. Louis, MO).
Drugs.
(±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride (5-HT2A agonist) and AS-19 (5-HT7 agonist) were purchased from Tocris Biosciences (Minneapolis, MN). The AMPK activators 2-DG and metformin were purchased from USP (Rockville, MD) and Tocris Biosciences (Minneapolis, MN), respectively; the AMPK inhibitor cC (dorsomorphin) was purchased from Tocris Biosciences. Upon arrival, drugs were diluted in 100% dimethyl sulfoxide (DMSO), separated into aliquots, and stored at −20°C. On the day of experiments, the stock vials were thawed and further diluted in artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 3 KCl, 2.5 CaCl22H2O, 1.25 MgSO4-7H2O, 1.25 KH2PO4, 25 NaHCO3, bubbled with 95% O2-5% CO2) to appropriate concentrations. Maximum DMSO concentration in the final solution was <10%, varying slightly depending on drug solubility.
Experimental protocols.
In all experiments, the inspired oxygen concentration was 60%, and the inspired CO2 concentration was adjusted for each rat to maintain end-tidal CO2 between 2 and 3 mmHg above the CO2 recruitment threshold. The apneic threshold was determined via stepwise reductions in inspired CO2 and reported as the end-tidal CO2 causing a sustained apnea (i.e., 60 s). Recruitment threshold was achieved by progressively increasing the inspired CO2 concentration and is reported as the end-tidal CO2 when phrenic activity resumed. Stable baseline phrenic nerve activity was monitored for at least 20 min, and consistency of arterial blood gas values was confirmed with multiple blood samples. The final blood sample during baseline was used as a reference for subsequent blood samples at 30, 60, and 90 min after drug injections. Experiments were analyzed only if they met the following a priori criteria: 1) within 1.5 mmHg and standard base excess (BE) within 3 meq/L of baseline values; 2) above 150 mmHg; and 3) blood pressure changes from beginning to end of experiments <30 mmHg. Only complete experiments were included for statistical analyses.
We initially verified that episodic spinal injections of 5-HT2A (100 μM, 6 μL × 3) and 5-HT7 (10 μM, 6 μL × 3) receptor agonists are sufficient to elicit consistent pMF. Rats were then pretreated (intrathecally) with 2-DG (1 mM, 10 µL) 20 min before 5-HT2A (2-DG + 5-HT2A agonist) or 5-HT7 (2-DG + 5-HT7 agonist) receptor activation. Different rat groups were pretreated (intrathecally) with metformin (2.5 or 5 mM, 10 µL) 20 min before 5-HT2A (2.5 mM Met + 5-HT2A agonist) or 5-HT7 receptor activation (5 mM Met + 5-HT7 agonist or 5 mM Met + 5-HT7 agonist). Both drugs used to activate AMPK in this study have known multitarget effects. To confirm the specificity of AMPK involvement in observed effects, cC (2 mM) was added to 2-DG and metformin solutions and administered (10 µL) 20 min before 5-HT7 receptor activation (2-DG/cC + 5-HT7 agonist and 2.5 mM Met/cC + 5-HT7 agonist, respectively). AMPK activator/inhibitor doses were chosen based on previous studies showing consistent physiological effects in response to intrathecal drug injections in the lumbar spinal cord (26).
Data analyses.
Data distribution was considered normal based on visual inspection of histograms and normal probability plots. Raw phrenic nerve activity was smoothed (0.05-s time constant) and rectified for off-line analysis. Integrated phrenic burst amplitude was averaged over 1 min at baseline and 30, 60, and 90 min after drug injection. These 1-min bins at selected times after drug injection were compared with baseline as a delta (time point − baseline) and as a percent change [percent change = (time point – baseline)/baseline × 100]. A mixed two-way ANOVA was used to evaluate overall differences on respiratory frequency and phrenic burst nerve amplitude between groups after drug injections and within groups relative to baseline. A one-way ANOVA was used to compare change in integrated phrenic burst amplitude among groups from baseline to 90 min after intrathecal drug injections. This approach complements pMF analysis as a percent change, since the absolute change in integrated phrenic burst amplitude accounts for respiratory motor output variability within and between groups during baseline, yet is minimally affected by extreme values or potential inconsistencies among group mean values. Tukey’s honestly significant difference tests were used when significant differences were indicated by the ANOVA. Values are expressed as means ± 1SD, and significant differences were considered at α < 0.05. All statistical analyses were performed in R (version 3.4.3).
RESULTS
Episodic spinal 5-HT2A and 5-HT7 receptor activation elicits pMF.
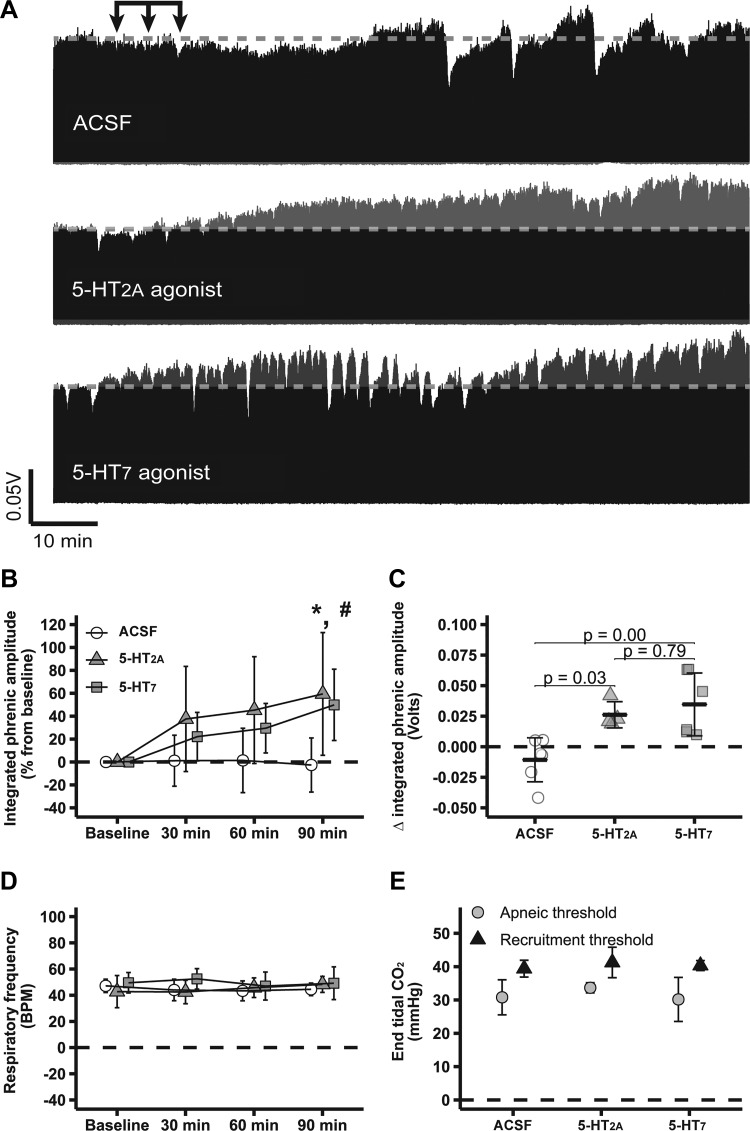
pMF following spinal 5-HT2A and 5-HT7 receptor activation has been studied extensively using similar experimental protocols (12, 13, 17, 18, 28). Figure 1A shows compressed electrophysiological recordings of integrated phrenic burst activity from representative rats showing that spinal 5-HT2A (59 ± 54%; n = 4) or 5-HT7 receptor activation alone (50 ± 31%; n = 6) causes robust pMF (Fig. 1, A and B), i.e., phrenic burst amplitude was significantly higher than baseline levels (P < 0.05), whereas no change was observed in ACSF-treated rats (−3 ± 24%; n = 6; P > 0.05). Changes in phrenic burst amplitude 90 min after intrathecal 5-HT2A (0.03 ± 0.01 volts) or 5-HT7 agonist (0.03 ± 0.03 volts) injections were higher than following ACSF (−0.01 ± 0.02 volts; P < 0.05; Fig. 1C).
Fig. 1.
Episodic spinal serotonin type 2A (5-HT2A) or serotonin 7 (5-HT7) receptor activation elicits robust phrenic motor facilitation (pMF). A: representative traces of compressed integrated phrenic burst amplitude throughout the experimental protocol. Gray broken line in each trace represents baseline phrenic burst amplitude; arrows on top indicate time of it 5-HT2A receptor agonist (n = 4), 5-HT7 receptor agonist (n = 6), or artificial cerebrospinal fluid (ACSF) injections (n = 6). B: %change in phrenic burst amplitude (vs. baseline) at 30, 60, and 90 min after last it injection. C: delta (vs. baseline) in integrated phrenic burst amplitude at 90 min after last it injection. D: respiratory frequency at baseline and 30, 60, and 90 min after last it injection. E: end-tidal CO2 level at apneic and at recruitment threshold among experimental groups. Data are presented as means ± SD. Mixed 2-way ANOVA or 1-way ANOVA was used for overall group comparison (with Tukey’s adjustment when necessary), and differences were considered significant at P < 0.05. *5-HT2A receptor agonist different from baseline. #5-HT7 receptor agonist different from baseline.
No significant changes in respiratory frequency were observed among groups or through the course of experiments (P > 0.05; Fig. 1D), consistent with the interpretation that drugs did not reach the medulla at sufficient concentrations to affect our results. Apneic and recruitment thresholds were also similar among groups (P > 0.05; Fig. 1E). , standard BE, pH, , temperature, and mean arterial pressure were maintained within the a priori specified range (Table 1).
Table 1.
Physiological variables at baseline and 30, 60, and 90 min after last it injection
| Time, min | ACSF | 5-HT2A | 5-HT7 |
|---|---|---|---|
| Baseline | 43.2 ± 3.6 | 46.7 ± 8.5 | 43.6 ± 3.1 |
| 30 | 42.9 ± 3.6 | 45.6 ± 8.5 | 43.7 ± 3.4 |
| 60 | 43.4 ± 3.1 | 43.8 ± 5.4 | 44.0 ± 3.3 |
| 90 | 42.7 ± 2.9 | 44.9 ± 9.7 | 44.4 ± 4.1 |
| Baseline | 274 ± 66 | 292 ± 26 | 303 ± 15 |
| 30 | 264 ± 65 | 290 ± 22 | 304 ± 21 |
| 60 | 264 ± 66 | 277 ± 23 | 293 ± 19 |
| 90 | 255 ± 59 | 281 ± 26 | 289 ± 17 |
| MAP, mmHg | |||
| Baseline | 129 ± 16 | 121 ± 7 | 132 ± 19 |
| 30 | 125 ± 20 | 122 ± 9 | 126 ± 21 |
| 60 | 129 ± 22 | 128 ± 12 | 131 ± 23 |
| 90 | 136 ± 17 | 130 ± 8 | 121 ± 28 |
| pH | |||
| Baseline | 7.39 ± 0.01 | 7.36 ± 0.04 | 7.40 ± 0.02 |
| 30 | 7.40 ± 0.04 | 7.41 ± 0.05 | 7.40 ± 0.03 |
| 60 | 7.39 ± 0.03 | 7.41 ± 0.03 | 7.39 ± 0.03 |
| 90 | 7.39 ± 0.04 | 7.40 ± 0.05 | 7.39 ± 0.03 |
| sBE | |||
| Baseline | 1.3 ± 1.7 | 1.0 ± 2.3 | 2.0 ± 1.5 |
| 30 | 1.7 ± 1.7 | 3.7 ± 2.3 | 2.0 ± 0.9 |
| 60 | 1.5 ± 1.3 | 3.0 ± 2.0 | 1.9 ± 0.9 |
| 90 | 0.7 ± 1.6 | 2.6 ± 2.1 | 1.6 ± 0.7 |
| Temperature, °C | |||
| Baseline | 37.4 ± 0.8 | 37.2 ± 0.4 | 37.5 ± 0.5 |
| 30 | 37.6 ± 0.8 | 37.3 ± 0.2 | 37.1 ± 0.3 |
| 60 | 37.7 ± 0.7 | 37.4 ± 0.7 | 37.3 ± 0.4 |
| 90 | 37.6 ± 0.7 | 37.2 ± 0.2 | 37.6 ± 0.3 |
ACSF, artificial cerebrospinal fluid; 5-HT2A, serotonin type 2A receptor; 5-HT7, serotonin 7 receptor; MAP, mean arterial pressure; sBE, standard base excess.
Spinal AMPK activation blocks 5-HT7-, but not 5-HT2A-induced pMF.
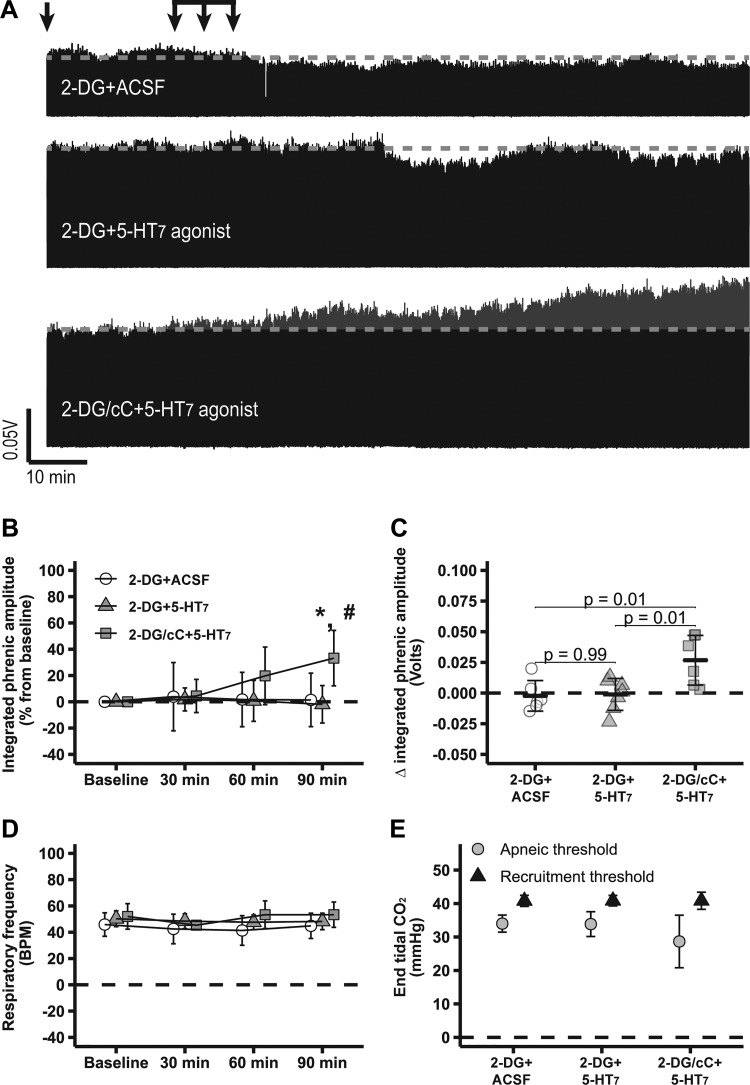
Figure 2A shows compressed electrophysiological recordings of integrated phrenic nerve burst activity from representative rats, demonstrating that intrathecal 2-DG abolishes 5-HT7-induced pMF (2-DG + 5-HT7: −2 ± 14%; n = 7), i.e., 5-HT7 receptor-induced pMF was minimal and not significantly different from baseline levels (P > 0.05), as observed with 2-DG + ACSF (1 ± 20%; n = 6; P > 0.05). cC with 2-DG restored 5-HT7-induced pMF; 2-DG/cC + 5-HT7 agonist (33 ± 21; n = 6) presented higher phrenic burst amplitudes vs. 2-DG + 5-HT7 agonist without cC or baseline levels (P < 0.05; Fig. 2, A and B). Changes in integrated phrenic burst activity 90 min after intrathecal 2-DG/cC + 5-HT7 agonist injections (0.03 ± 0.02 volts) were higher than 2-DG + 5-HT7 agonist (0.00 ± 0.01 volts) or 2-DG + ACSF (0.00 ± 0.01 volts; P < 0.05; Fig. 2C). No significant changes in respiratory frequency were observed among groups or over the course of experiments (P > 0.05; Fig. 2D). Apneic and recruitment thresholds were similar among groups (P > 0.05; Fig. 2E). , standard BE, pH, , temperature, and mean arterial pressure were maintained within the a priori specified range (Table 2).
Fig. 2.
2-Deoxyglucose (2-DG)-dependent AMP-activated protein kinase (AMPK) activation abolishes serotonin 7 receptor (5-HT7)-induced phrenic motor facilitation (pMF), which is restored by it compound C (cC). A: representative traces of compressed integrated phrenic burst amplitude throughout experimental protocol in 2-DG + artificial cerebrospinal fluid (ACSF, n = 6)-, 2-DG + 5-HT7 (n = 7)-, and 2-DG/cC + 5-HT7 (n = 6)-treated rats. Gray broken line in each trace represents baseline amplitude of phrenic burst activity, and arrows on top indicate time of it 5-HT7 receptor agonist or ACSF injections in rats pretreated with 2-DG or 2-DG plus cC. B: %change in phrenic burst amplitude (vs. baseline) at 30, 60, and 90 min after last it injection. C: delta (vs. baseline) in integrated phrenic burst amplitude at 90 min after last it injection. D: respiratory frequency at baseline and 30, 60, and 90 min after last it injection. E: end-tidal CO2 level at apneic and at recruitment threshold among experimental groups. Data are presented as means ± SD. Mixed 2-way ANOVA or 1-way ANOVA was used for overall group comparison (with Tukey’s adjustment when necessary), and differences were considered significant at P < 0.05. *2-DG/cC + 5-HT7 receptor agonist different from baseline. #2-DG/cC + 5-HT7- different from 2-DG + 5-HT7-treated rats 90 min after it drug injections.
Table 2.
Physiological variables at baseline and 30, 60, and 90 min after last it injection
| Time, min | 2-DG + ACSF | 2-DG + 5-HT2A | 2-DG + 5-HT7 | 2-DG/cC + 5-HT7 |
|---|---|---|---|---|
| Baseline | 43.9 ± 3.4 | 40.8 ± 1.7 | 43.0 ± 3.8 | 43.0 ± 2.9 |
| 30 | 43.7 ± 4.2 | 40.4 ± 2.1 | 43.8 ± 3.3 | 43.4 ± 3.4 |
| 60 | 44.6 ± 3.0 | 42.8 ± 1.3 | 43.0 ± 3.2 | 43.8 ± 3.2 |
| 90 | 44.8 ± 3.4 | 40.7 ± 1.3 | 43.5 ± 3.0 | 43.3 ± 3.7 |
| Baseline | 314 ± 20 | 304 ± 36 | 311 ± 27 | 319 ± 20 |
| 30 | 302 ± 21 | 280 ± 58 | 296 ± 23 | 319 ± 21 |
| 60 | 298 ± 42 | 282 ± 53 | 292 ± 31 | 312 ± 20 |
| 90 | 304 ± 29 | 267 ± 42 | 292 ± 35 | 305 ± 18 |
| MAP, mmHg | ||||
| Baseline | 138 ± 8 | 127 ± 28 | 122 ± 18 | 124 ± 10 |
| 30 | 136 ± 12 | 129 ± 25 | 118 ± 20 | 117 ± 13 |
| 60 | 135 ± 14 | 130 ± 24 | 120 ± 19 | 116 ± 15 |
| 90 | 133 ± 23 | 127 ± 24 | 119 ± 19 | 108 ± 17 |
| pH | ||||
| Baseline | 7.38 ± 0.02 | 7.41 ± 0.03 | 7.39 ± 0.03 | 7.39 ± 0.03 |
| 30 | 7.38 ± 0.04 | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.40 ± 0.03 |
| 60 | 7.38 ± 0.03 | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.39 ± 0.04 |
| 90 | 7.38 ± 0.02 | 7.39 ± 0.05 | 7.43 ± 0.13 | 7.39 ± 0.03 |
| sBE | ||||
| Baseline | 0.7 ± 1.1 | 1.3 ± 1.5 | 1.1 ± 1.1 | 1.2 ± 1.8 |
| 30 | 0.8 ± 1.8 | 0.9 ± 1.9 | 2.3 ± 1.0 | 1.9 ± 0.9 |
| 60 | 1.0 ± 1.3 | 1.7 ± 1.7 | 1.6 ± 1.0 | 1.8 ± 1.5 |
| 90 | 0.9 ± 1.5 | 0.6 ± 1.6 | 1.1 ± 1.4 | 1.2 ± 1.5 |
| Temperature, °C | ||||
| Baseline | 37.9 ± 0.3 | 37.3 ± 0.5 | 37.4 ± 0.4 | 37.5 ± 0.5 |
| 30 | 38.0 ± 0.4 | 37.4 ± 0.6 | 37.4 ± 0.4 | 37.2 ± 0.5 |
| 60 | 37.9 ± 0.6 | 37.4 ± 0.4 | 37.5 ± 0.4 | 37.1 ± 0.5 |
| 90 | 38.0 ± 0.6 | 37.3 ± 0.4 | 37.5 ± 0.4 | 37.2 ± 0.6 |
2-DG, 2-deoxyglucose; cC, compound C; ACSF, artificial cerebrospinal fluid; 5-HT2A, serotonin type 2A receptor; 5-HT7, serotonin 7 receptor; MAP, mean arterial pressure; sBE, standard base excess.
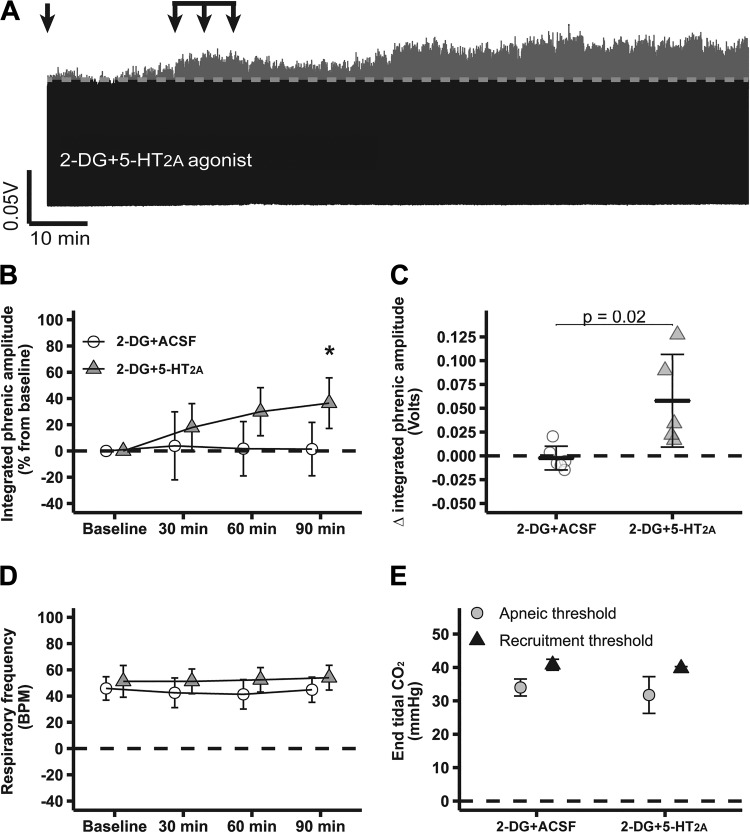
Figure 3A shows compressed electrophysiological recordings of integrated phrenic nerve burst activity from representative rats. Intrathecal 2-DG did not constrain 5-HT2A-induced pMF (2-DG + 5-HT2A: 36 ± 19; n = 5); phrenic burst amplitude in this group was higher than baseline (P < 0.05; Fig. 3, A and B), suggesting that AMPK activity selectively constrains the S, but not the Q, pathway to pMF. The change in integrated phrenic burst activity 90 min after intrathecal 2-DG + 5-HT2A agonist injections (0.06 ± 0.05 volts) was significantly higher than 2-DG + ACSF (P < 0.05; Fig. 3C). No significant changes in respiratory frequency were observed among groups or through experiments (P > 0.05; Fig. 3D). Apneic and recruitment thresholds were similar among groups (P > 0.05; Fig. 3E). , standard BE, pH, , temperature, and mean arterial pressure were maintained within the a priori specified range (Table 2).
Fig. 3.
2-Deoxyglucose (2-DG)-dependent AMP-activated protein kinase (AMPK) activation does not affect normal expression of serotonin type 2A receptor (5-HT2A)-induced phrenic motor facilitation (pMF). A: representative traces of compressed integrated phrenic burst amplitude throughout the experimental protocol in 2-DG + artificial cerebrospinal fluid (ACSF, n = 6)- and 2-DG + 5-HT2A (n = 5)-treated rats. Gray broken line in each trace represents baseline amplitude of phrenic burst activity, and arrows on top indicate time of it 5-HT2A receptor agonist or ACSF injections in rats pretreated with 2-DG. 2-DG + ACSF group is the same reported in Fig. 2. B: %change in phrenic burst amplitude (vs. baseline) at 30, 60, and 90 min after last it injection. C: delta (vs. baseline) in integrated phrenic burst amplitude at 90 min after last it injection. D: respiratory frequency at baseline and 30, 60, and 90 min after last it injection. E: end-tidal CO2 level at apneic and at recruitment threshold among experimental groups. Data are presented as means ± SD. Mixed 2-way ANOVA or 1-way ANOVA was used for overall group comparison (with Tukey’s adjustment when necessary), and differences were considered significant at P < 0.05. *2-DG + 5-HT2A different from baseline.
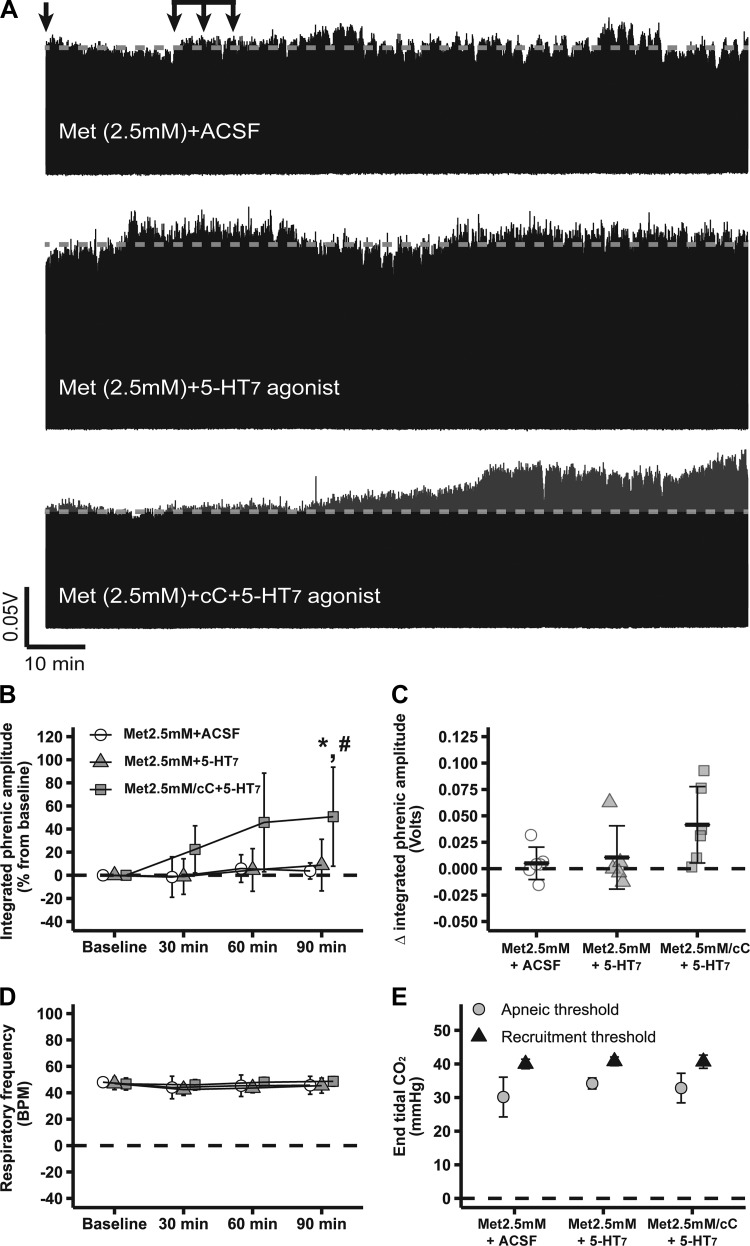
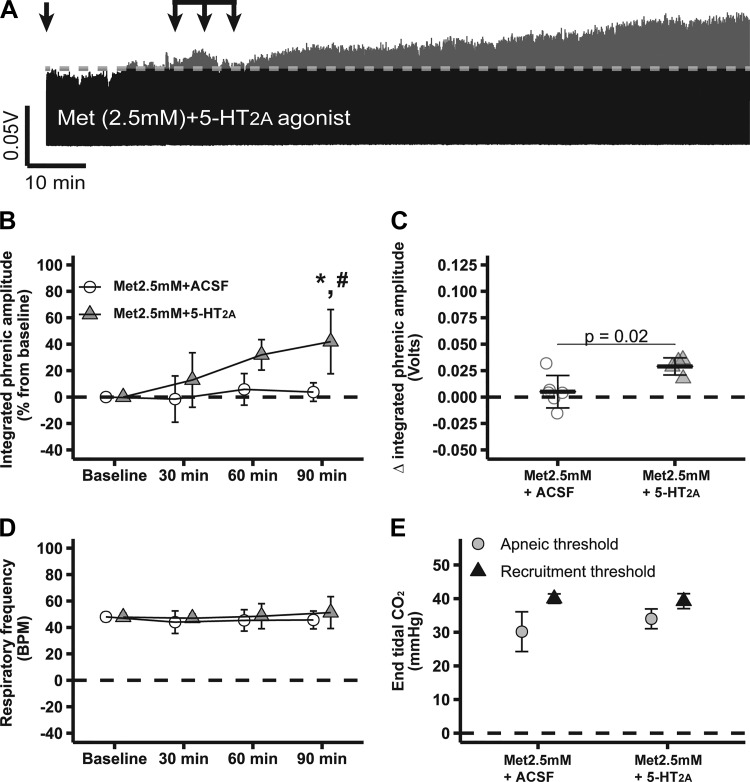
Figure 4A shows compressed recordings of integrated phrenic nerve burst activity from representative rats, demonstrating that intrathecal metformin abolished 5-HT7-induced pMF (2.5 mM Met + 5-HT7 agonist: 9 ± 22%; n = 5); phrenic burst amplitude was similar to 2.5 mM Met + ACSF (4 ± 7%; n = 6; P > 0.05) or baseline (P > 0.05). 2.5 mM Met/cC + 5-HT7 agonist (51 ± 43%; n = 6) exhibited higher phrenic burst amplitudes vs. 2.5 mM Met + ACSF or baseline (P < 0.05; Fig. 4, A and B), consistent with our findings using 2-DG (Fig. 2). Only marginal changes were observed between groups in integrated phrenic burst activity 90 min after intrathecal drug injections: 2.5 mM Met/cC + 5-HT7 agonist: 0.04 ± 0.04 volts; 2.5 mM Met + 5-HT7 agonist: 0.01 ± 0.03; 2.5 mM Met + ACSF: 0.01 ± 0.02 volts (P > 0.05; Fig. 4C). Slightly different results were obtained depending on how pMF was analyzed, which was probably due to the fact that normalization (i.e., as percent from baseline) removes baseline variability and, consequently, increases statistical power. No significant changes in respiratory frequency were observed among groups or during experiments (P > 0.05; Fig. 4D). Apneic and recruitment threshold levels were also similar among groups (P > 0.05; Fig. 4E); , standard BE, pH, , temperature, and mean arterial pressure were maintained within a priori specified range (Table 3). Because cC competitively inhibits AMPK-γ regulatory subunits with reasonable specificity (47), the observed effects of the selected AMPK activators are likely specific and account for the findings of this study.
Fig. 4.
Low-dose metformin-dependent AMP-activated protein kinase (AMPK) activation abolishes serotonin 7 receptor (5-HT7)-induced phrenic motor facilitation (pMF), which is restored by it compound C (cC). A: representative traces of compressed integrated phrenic burst amplitude throughout experimental protocol in 2.5 mM Met + artificial cerebrospinal fluid (ACSF, n = 6)-, 2.5 mM Met + 5-HT7 (n = 5)-, and 2.5 mM Met/cC + 5-HT7 (n = 6)-treated rats. Gray broken line in each trace represents baseline amplitude of phrenic burst; arrows on top indicate time of it 5-HT7 receptor agonist or ACSF injections in rats pretreated with 2.5 mM metformin or 2.5 mM metformin plus cC. B: %change in phrenic burst amplitude (vs. baseline) at 30, 60, and 90 min after last it injection. C: delta (vs. baseline) in integrated phrenic burst amplitude at 90 min after last it injection. D: respiratory frequency at baseline and 30, 60, and 90 min after last it injection. E: end-tidal CO2 level at apneic and at recruitment threshold among experimental groups. Data are presented as means ± SD. Mixed 2-way ANOVA or 1-way ANOVA was used for overall group comparison (with Tukey’s adjustment when necessary), and differences were considered significant at P < 0.05. *2.5 mM Met/cC + 5-HT7 receptor agonist different from baseline. #2.5 mM Met/cC + 5-HT7 different from 2.5 mM Met + ACSF.
Table 3.
Physiological variables at baseline and 30, 60, and 90 min after last it injection
| Time, min | Met (2.5 mM) + ACSF | Met (2.5 mM) + 5-HT2A | Met (2.5 mM) + 5-HT7 | Met (2.5 mM)/cC + 5-HT7 |
|---|---|---|---|---|
| Baseline | 45.6 ± 7.0 | 41.5 ± 3.0 | 44.0 ± 2.0 | 45.8 ± 1.3 |
| 30 | 45.4 ± 6.6 | 42.1 ± 2.2 | 44.5 ± 2.2 | 45.9 ± 1.5 |
| 60 | 45.2 ± 6.7 | 41.4 ± 1.6 | 44.1 ± 2.5 | 46.8 ± 1.5 |
| 90 | 45.6 ± 6.4 | 41.1 ± 3.8 | 43.7 ± 1.8 | 46.0 ± 1.4 |
| Baseline | 300 ± 54 | 300 ± 23 | 310 ± 26 | 310 ± 25 |
| 30 | 298 ± 37 | 297 ± 19 | 315 ± 12 | 301 ± 21 |
| 60 | 292 ± 34 | 288 ± 21 | 304 ± 17 | 293 ± 18 |
| 90 | 295 ± 34 | 284 ± 24 | 302 ± 13 | 282 ± 20 |
| MAP, mmHg | ||||
| Baseline | 129 ± 21 | 122 ± 10 | 129 ± 25 | 131 ± 16 |
| 30 | 128 ± 19 | 132 ± 12 | 123 ± 25 | 126 ± 14 |
| 60 | 128 ± 23 | 137 ± 14 | 119 ± 20 | 123 ± 17 |
| 90 | 131 ± 21 | 134 ± 13 | 114 ± 15 | 119 ± 12 |
| pH | ||||
| Baseline | 7.36 ± 0.05 | 7.40 ± 0.02 | 7.38 ± 0.02 | 7.37 ± 0.03 |
| 30 | 7.37 ± 0.04 | 7.41 ± 0.05 | 7.39 ± 0.03 | 7.37 ± 0.03 |
| 60 | 7.38 ± 0.05 | 7.42 ± 0.06 | 7.38 ± 0.02 | 7.37 ± 0.02 |
| 90 | 7.37 ± 0.04 | 7.41 ± 0.07 | 7.39 ± 0.01 | 7.36 ± 0.02 |
| sBE | ||||
| Baseline | 0.2 ± 1.6 | 0.6 ± 1.6 | 0.8 ± 1.5 | 1.2 ± 2.0 |
| 30 | 1.1 ± 1.8 | 2.6 ± 5.2 | 2.1 ± 1.4 | 0.9 ± 1.9 |
| 60 | 1.0 ± 1.4 | 2.5 ± 4.0 | 1.3 ± 1.1 | 1.8 ± 1.3 |
| 90 | 1.0 ± 0.9 | 1.0 ± 3.3 | 1.3 ± 0.9 | 0.6 ± 1.3 |
| Temperature, °C | ||||
| Baseline | 37.4 ± 0.7 | 37.7 ± 0.8 | 37.4 ± 0.5 | 37.7 ± 0.4 |
| 30 | 37.8 ± 0.4 | 37.3 ± 0.5 | 37.4 ± 0.5 | 37.6 ± 0.4 |
| 60 | 38.1 ± 0.6 | 37.3 ± 0.4 | 37.7 ± 0.1 | 37.6 ± 0.5 |
| 90 | 38.0 ± 0.6 | 37.5 ± 0.7 | 37.4 ± 0.3 | 37.6 ± 0.5 |
Met, metformin; cC, compound C; ACSF, artificial cerebrospinal fluid; 5-HT2A, serotonin type 2A receptor; 5-HT7, serotonin 7 receptor; MAP, mean arterial pressure; sBE, standard base excess.
Figure 5A shows compressed recordings of integrated phrenic nerve burst activity from representative rats, showing that intrathecal metformin did not affect 5-HT2A-induced pMF (2.5 mM Met + 5-HT2A: 42 ± 24; n = 4; P < 0.05; Fig. 5, A and B). Changes in integrated phrenic burst activity 90 min after intrathecal injections of 2.5 mM Met + 5-HT2A agonist (0.03 ± 0.01 volts) were higher than in 2.5 mM Met + ACSF rats (P < 0.05; Fig. 5C). No significant changes in respiratory frequency were observed among groups or over the course of experiments (P > 0.05; Fig. 5D). Apneic and recruitment threshold levels were similar among groups (P > 0.05; Fig. 5E). , standard BE, pH, , temperature, and mean arterial pressure were maintained within the a priori specified range (Table 3). The magnitude of 5-HT7-induced pMF (without pretreatment) was significantly greater than with 2-DG or 2.5 mM Met pretreatment; however, the magnitude of 5-HT2A-induced pMF was not affected by either 2-DG or 2.5 mM Met.
Fig. 5.
Low-dose metformin-dependent AMP-activated protein kinase (AMPK) activation does not affect normal expression of serotonin type 2A receptor (5-HT2A)-induced phrenic motor facilitation (pMF). A: representative traces of compressed integrated phrenic burst amplitude throughout the experimental protocol in 2.5 mM Met + artificial cerebrospinal fluid (ACSF, n = 6)- and 2.5 mM Met + 5-HT2A (n = 4)-treated rats. Gray broken line in each trace represents baseline amplitude of phrenic burst activity, and arrows on top indicate time of it 5-HT2A receptor agonist or ACSF injections in rats pretreated with 2.5 mM metformin. Met (2.5 mM) + ACSF group is the same reported in Fig. 2. B: %change in phrenic burst amplitude (vs. baseline) at 30, 60, and 90 min after last it injection. C: delta (vs. baseline) in integrated phrenic burst amplitude at 90 min after last it injection. D: respiratory frequency at baseline and 30, 60, and 90 min after last it injection. E: end-tidal CO2 level at apneic and at recruitment threshold among experimental groups. Data are presented as means ± SD. Mixed 2-way ANOVA or 1-way ANOVA was used for overall group comparison (with Tukey’s adjustment when necessary), and differences were considered significant at P < 0.05. *Met (2.5 mM) + 5-HT2A receptor agonist different from baseline. #Met (2.5 mM) + 5-HT2A different from 2.5 mM Met + ACSF.
No differences in phrenic burst amplitude were observed in rats treated with either 2-DG (2-DG + ACSF) or metformin (2.5 mM Met + ACSF) alone, suggesting that these drugs do not directly affect phrenic nerve activity. A higher metformin dose (5 mM) did not change phrenic burst amplitude in 5 mM Met + ACSF (−9 ± 10; n = 3) vs. baseline (P > 0.05) but prevented 5-HT7-induced pMF (5 mM Met + 5-HT7 agonist: 7 ± 25; n = 4; P > 0.05 vs. 5 mM Met + ACSF and vs. baseline). However, at this higher metformin dose, cC was ineffective at restoring 5-HT7-induced pMF in 5 mM Met/cC + 5-HT7 agonist (−12 ± 33; n = 3; P > 0.05; Fig. 6, A–C); changes in integrated phrenic nerve burst activity 90 min after drug injections were as follows: 5 mM Met/cC + 5-HT7 agonist: −0.01 ± 0.04; 5 mM Met + 5-HT7 agonist: 0.01 ± 0.02; 5 mM Met + ACSF: −0.01 ± 0.01 volts (P > 0.05). No significant changes in respiratory frequency were observed among groups or through the course of experiments (P > 0.05; Fig. 6D). Apneic and recruitment thresholds were also similar among groups (P > 0.05; Fig. 6E). , standard BE, pH, , temperature, and mean arterial pressure were maintained within the a priori specified range (Table 4). There was no significant correlation between baseline and pMF magnitude.
Fig. 6.
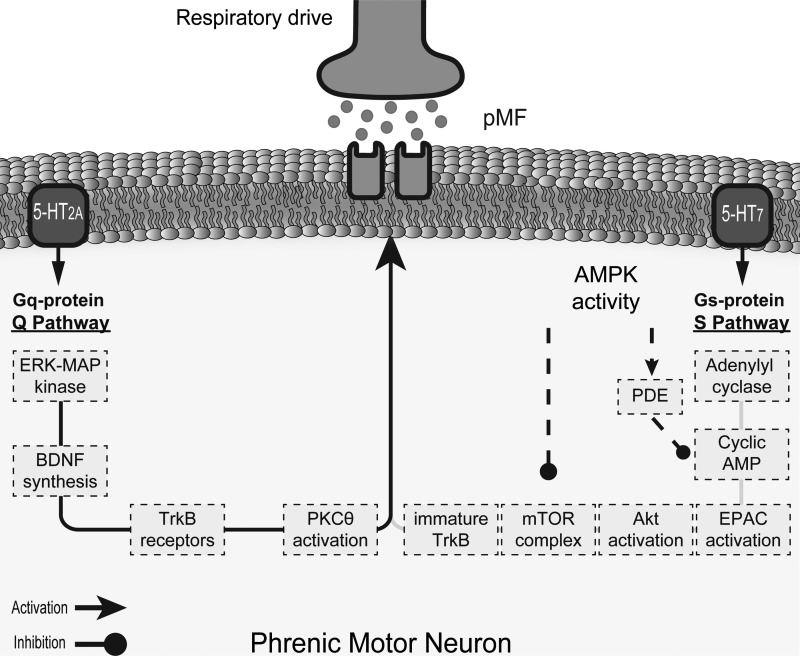
Schematic illustrating cellular mechanisms giving rise to phenotypically similar pathways to phrenic motor facilitation (pMF). Our findings indicate that spinal AMP-activated protein kinase (AMPK) activation cancels selective pathways to pMF, and possible mechanisms are suggested based on literature, such as 1) directly or indirectly (i.e., via tuberous sclerosis complex activation) AMPK-induced mammalian target of rapamycin complex inhibition (mTORC), and 2) phosphodiesterase (PDE)-induced cAMP downregulation. BDNF, brain-derived neurotrophic factor; TrkB, tyrosine receptor kinase B; EPAC, exchange protein activated by cAMP, mTOR, or mammalian target of rapamycin complex.
Table 4.
Physiological variables at baseline and 30, 60, and 90 min after last it injection
| Time, min | Met (5.0 mM) + ACSF | Met (5.0 mM) + 5-HT7 | Met (5.0 mM)/cC + 5-HT7 |
|---|---|---|---|
| Baseline | 43.3 ± 3.5 | 47.2 ± 6.3 | 45.7 ± 0.3 |
| 30 | 42.1 ± 4.4 | 47.9 ± 6.8 | 46.2 ± 1.2 |
| 60 | 43.6 ± 2.8 | 47.6 ± 5.3 | 47.1 ± 0.4 |
| 90 | 43.4 ± 3.2 | 47.7 ± 6.3 | 46.1 ± 1.3 |
| Baseline | 305 ± 13 | 312 ± 49 | 303 ± 42 |
| 30 | 281 ± 24 | 294 ± 60 | 245 ± 76 |
| 60 | 270 ± 22 | 278 ± 81 | 227 ± 49 |
| 90 | 266 ± 25 | 276 ± 72 | 223 ± 55 |
| MAP, mmHg | |||
| Baseline | 130 ± 9 | 123 ± 14 | 109 ± 33 |
| 30 | 132 ± 11 | 111 ± 31 | 114 ± 26 |
| 60 | 131 ± 10 | 113 ± 35 | 113 ± 28 |
| 90 | 128 ± 6 | 109 ± 42 | 106 ± 22 |
| pH | |||
| Baseline | 7.41 ± 0.05 | 7.37 ± 0.05 | 7.39 ± 0.01 |
| 30 | 7.41 ± 0.06 | 7.37 ± 0.04 | 7.36 ± 0.04 |
| 60 | 7.40 ± 0.04 | 7.36 ± 0.06 | 7.37 ± 0.02 |
| 90 | 7.39 ± 0.03 | 7.35 ± 0.05 | 7.38 ± 0.00 |
| sBE | |||
| Baseline | 2.4 ± 2.5 | 2.0 ± 1.1 | 2.7 ± 0.8 |
| 30 | 1.7 ± 1.7 | 2.1 ± 1.1 | 1.0 ± 3.2 |
| 60 | 1.9 ± 3.0 | 1.0 ± 1.9 | 1.6 ± 1.6 |
| 90 | 1.1 ± 2.5 | 0.6 ± 1.5 | 2.0 ± 0.6 |
| Temperature, °C | |||
| Baseline | 37.9 ± 0.7 | 37.3 ± 0.6 | 37.7 ± 0.8 |
| 30 | 37.9 ± 0.4 | 37.4 ± 0.5 | 37.9 ± 0.5 |
| 60 | 38.0 ± 0.5 | 37.3 ± 0.5 | 37.3 ± 0.4 |
| 90 | 37.7 ± 0.2 | 37.2 ± 0.6 | 37.4 ± 0.3 |
Met, metformin; cC, compound C; ACSF, artificial cerebrospinal fluid; 5-HT7, serotonin 7 receptor; MAP, mean arterial pressure; sBE, standard base excess.
DISCUSSION
The major findings of the present study are that pharmacologically induced spinal AMPK activation via 2-DG or metformin abolishes 5-HT7- but not 5-HT2A-dependent pMF. The ability of cC to restore 5-HT7 receptor-induced pMF increases confidence that 2-DG and metformin exerted their effects specifically via AMPK activation vs. unanticipated off-target effects. Thus, spinal AMPK plays an important role in selectively regulating the expression of phrenic motor plasticity. Because AMPK activity varies across the circadian cycle (42), it is possible that AMPK differentially regulates the S pathway to pMF throughout the day. Furthermore, because AMPK is activated by metabolic stress, it likely serves to shut down energetically costly mTOR-dependent protein synthesis required for S pathway expression in conditions such as severe hypoxia that challenge energy supply.
2-DG and metformin are transported into the cells through glucose transporters and organic cation proteins, respectively (33, 38). 2-DG inhibits hexokinase, the first and rate-limiting enzyme in glycolysis (24, 25, 40). In contrast, metformin inhibits complex I of the electron transport chain (34). Both drugs increase the AMP-to-ATP ratio, increasing AMP binding to the AMPK-γ regulatory subunits, leading to persistent AMPK phosphorylation and activation (38, 46). Activated AMPK stimulates ATP production and inhibits nonessential ATP-consuming functions such as protein translation via mTORC1 (39, 41). Synthesis of an immature TrkB isoform is necessary for the S pathway to pMF (14), and this synthesis requires mTORC1 activity (11, 13). Other forms of synaptic plasticity are inhibited by AMPK activity, including hippocampal long-term potentiation (37).
AMPK signaling inhibits mTORC1 activity both directly (7) and indirectly via tuberous sclerosis complex phosphorylation and activation (4, 5, 20, 43). Although mTORC1 signaling is not necessary for moderate AIH-induced Q pathway-dependent pMF, it is required for severe AIH-induced S pathway-dependent pMF (11) and 5-HT7-induced pMF (13). Thus, AMPK-dependent tuberous sclerosis complex 1/2 activation and mTORC1 inhibition are the most likely mechanism constraining 5-HT7-induced pMF although this hypothesis has not yet been tested directly. Alternatively, AMPK activates phosphodiesterases, thereby reducing cAMP concentration and downstream signaling in some cell types (21, 45). Although this is another viable explanation for the effects observed here, it is less likely since coactivation of the Q and S pathways to pMF cancels pMF expression due to upstream cross-talk inhibition (35, 36); considering that cAMP activation of protein kinase A is necessary for S to Q pathway inhibition (18, 35, 36), is it unlikely that the effects observed here resulted from phosphodiesterase activation and reduced levels of cAMP. Because neither 2-DG nor metformin affected 5-HT2A receptor-induced pMF, downstream AMPK effects on tuberous sclerosis complex 1/2 activation are a more likely explanation of effects observed in this study, since it would enable complete differentiation of AMPK effects on the S vs. Q pathways to pMF.
Recent studies from our laboratory demonstrate that moderate AIH-induced 5-HT2-dependent phrenic long-term facilitation is unaffected by systemic 2-DG (27). Conversely, 2-DG constrains respiratory metaplasticity (enhanced phrenic long-term facilitation) after 4 wk of repetitive AIH preconditioning (27). Although mechanisms underlying respiratory metaplasticity are largely unknown, a loss of cross-talk inhibition between the Q and S pathways to pMF has been postulated, leading to additive contributions from both the Q and S pathways (27). Thus, AMPK activity would eliminate the S but not Q pathway contribution to enhanced pMF.
Although AMPK plays a key role in the neural control of breathing (6, 23, 29), the present study is the first to demonstrate that spinal AMPK differentially regulates distinct mechanisms of phrenic motor plasticity. The hypoxic ventilatory response is reduced by conditional AMPK deletion in catecholaminergic cells (29). On the other hand, carotid chemoafferent neuron activity is unaffected by AMPK deletion (6, 23, 29). Thus, the relevant AMPK modulating the hypoxic ventilatory response must reside within second- and/or higher-order neurons of the carotid chemoreflex (29).
The effects of intrathecal drug injections are not restricted to phrenic motor neurons. Although accumulating evidence indicates that key molecules necessary to both 5-HT2A- and 5-HT7-induced pMF are expressed within phrenic motor neurons, the involvement of spinal interneurons and/or glial cells in mediating AMPK-dependent constraint to 5-HT7-induced pMF cannot be excluded. Both approaches used in this study to activate AMPK consistently blocked 5-HT7-induced pMF. However, cC was more effective in restoring 5-HT7-induced pMF following 2-DG vs. metformin. Metformin may lead to prolonged AMPK activation, overcoming cC effects. Alternatively, because 2.5 mM metformin (but not 5 mM) was partially restored by cC, even lower doses might be required.
In conclusion, AMPK activity constrains 5-HT7 receptor-induced (and presumably adenosine 2A receptor-induced) pMF without detectable effects on the Q pathway elicited by 5-HT2A receptor activation (Fig. 6). These findings advance our understanding of key molecules regulating the expression of the diverse signaling pathways that lead to pMF (8). It is essential to understand this and other factors regulating motor neuron plasticity as we move along a translational path, attempting to harness intermittent hypoxia to restore respiratory and nonrespiratory movements after chronic spinal injury, or with other clinical disorders that compromise movement (9, 15, 31). For example, diabetes is commonplace in those suffering from spinal cord injury; thus, many people with spinal injury are medicated with metformin (22, 30). In these individuals, the potential benefits of prolonged repetitive acute intermittent hypoxia may be undermined (see Ref. 27). However, future studies are needed to test the hypothesis that AMPK activation undermines phrenic motor plasticity in pathophysiological conditions compromising breathing, such as spinal cord injury or amyotrophic lateral sclerosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-69064 and the McKnight Brain Institute. D. P. Fields was supported by fellowships from the United Negro College Fund and the NHLBI (F30-HL-126351).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R.P. and D.P.F. performed experiments; R.R.P. analyzed data; R.R.P. and G.S.M. interpreted results of experiments; R.R.P. prepared figures; R.R.P. drafted manuscript; R.R.P., D.P.F., and G.S.M. approved final version of manuscript; D.P.F. and G.S.M. conceived and designed research; D.P.F. and G.S.M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Elisa Gonzalez-Rothi, Yasin Seven, Mia Kelly, Irawan Satriotomo, Marissa Ciesla, and Latoya Allen for their input.
REFERENCES
- 1.Agosto-Marlin IM, Nichols NL, Mitchell GS. Adenosine-dependent phrenic motor facilitation is inflammation resistant. J Neurophysiol 117: 836–845, 2017. doi: 10.1152/jn.00619.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agosto-Marlin IM, Nichols NL, Mitchell GS. Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling. J Neurophysiol 119: 2176–2185, 2018. doi: 10.1152/jn.00378.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 4.Bai L, Mei X, Shen Z, Bi Y, Yuan Y, Guo Z, Wang H, Zhao H, Zhou Z, Wang C, Zhu K, Li G, Lv G. Netrin-1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Sci Rep 7: 42288, 2017. doi: 10.1038/srep42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai L, Mei X, Wang Y, Yuan Y, Bi Y, Li G, Wang H, Yan P, Lv G. The role of netrin-1 in improving functional recovery through autophagy stimulation following spinal cord injury in rats. Front Cell Neurosci 11: 350, 2017. doi: 10.3389/fncel.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckler KJ. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch 467: 1013–1025, 2015. doi: 10.1007/s00424-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem 279: 15719–15722, 2004. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 8.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devinney MJ, Nichols NL, Mitchell GS. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J Neurosci 36: 7877–7885, 2016. doi: 10.1523/JNEUROSCI.4122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougherty BJ, Fields DP, Mitchell GS. Mammalian target of rapamycin is required for phrenic long-term facilitation following severe but not moderate acute intermittent hypoxia. J Neurophysiol 114: 1784–1791, 2015. doi: 10.1152/jn.00539.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields DP, Mitchell GS. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113, Pt A: 82–88, 2017. doi: 10.1016/j.neuropharm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114: 2015–2022, 2015. doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119: 1455–1465, 2015. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenstein LR, Hitt BA, Mazze RI. Metabolism in vitro of enflurane, isoflurane, and methoxyflurane. Anesthesiology 42: 420–424, 1975. doi: 10.1097/00000542-197504000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250: 632–643, 2013. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS. Intermittent hypoxia-induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase-dependent mechanism. J Neurosci 35: 6871–6880, 2015. doi: 10.1523/JNEUROSCI.4539-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 21.Johanns M, Lai YC, Hsu MF, Jacobs R, Vertommen D, Van Sande J, Dumont JE, Woods A, Carling D, Hue L, Viollet B, Foretz M, Rider MH. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat Commun 7: 10856, 2016. doi: 10.1038/ncomms10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazawa I, Yamaguchi T, Yamamoto M, Sugimoto T. Relationship between treatments with insulin and oral hypoglycemic agents versus the presence of vertebral fractures in type 2 diabetes mellitus. J Bone Miner Metab 28: 554–560, 2010. doi: 10.1007/s00774-010-0160-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Kang D, Martin EA, Kim I, Carroll JL. Effects of modulators of AMP-activated protein kinase on TASK-1/3 and intracellular Ca(2+) concentration in rat carotid body glomus cells. Respir Physiol Neurobiol 195: 19–26, 2014. doi: 10.1016/j.resp.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M, Savaraj N, Lane AN, Lampidis TJ. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6: 3049–3058, 2007. doi: 10.1158/1535-7163.MCT-07-0310. [DOI] [PubMed] [Google Scholar]
- 25.Kurtoglu M, Maher JC, Lampidis TJ. Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal 9: 1383–1390, 2007. doi: 10.1089/ars.2007.1714. [DOI] [PubMed] [Google Scholar]
- 26.Ling YZ, Li ZY, Ou-Yang HD, Ma C, Wu SL, Wei JY, Ding HH, Zhang XL, Liu M, Liu CC, Huang ZZ, Xin WJ. The inhibition of spinal synaptic plasticity mediated by activation of AMP-activated protein kinase signaling alleviates the acute pain induced by oxaliplatin. Exp Neurol 288: 85–93, 2017. doi: 10.1016/j.expneurol.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 27.MacFarlane PM, Vinit S, Mitchell GS. Enhancement of phrenic long-term facilitation following repetitive acute intermittent hypoxia is blocked by the glycolytic inhibitor 2-deoxyglucose. Am J Physiol Regul Integr Comp Physiol 314: R135–R144, 2018. doi: 10.1152/ajpregu.00306.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoud AD, Lewis S, Juričić L, Udoh UA, Hartmann S, Jansen MA, Ogunbayo OA, Puggioni P, Holmes AP, Kumar P, Navarro-Dorado J, Foretz M, Viollet B, Dutia MB, Marshall I, Evans AM. AMP-activated protein kinase deficiency blocks the hypoxic ventilatory response and thus precipitates hypoventilation and apnea. Am J Respir Crit Care Med 193: 1032–1043, 2016. doi: 10.1164/rccm.201508-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancini T, Mazziotti G, Doga M, Carpinteri R, Simetovic N, Vescovi PP, Giustina A. Vertebral fractures in males with type 2 diabetes treated with rosiglitazone. Bone 45: 784–788, 2009. doi: 10.1016/j.bone.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders? In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York, NY: Springer, 2007, p. 291–311. [Google Scholar]
- 32.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112: 1678–1688, 2012. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neil RG, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol 7: 388–392, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614, 2000. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perim RR, Fields DP, Mitchell GS. Cross-talk inhibition between 5-HT2B and 5-HT7 receptors in phrenic motor facilitation via NADPH oxidase and PKA. Am J Physiol Regul Integr Comp Physiol 314: R709–R715, 2018. doi: 10.1152/ajpregu.00393.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perim RR, Fields DP, Mitchell GS. Protein kinase Cδ constrains the S-pathway to phrenic motor facilitation elicited by spinal 5-HT7 receptors or severe acute intermittent hypoxia. J Physiol 597: 481–498, 2019. doi: 10.1113/JP276731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One 5: e8996, 2010. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 60: 1577–1585, 2017. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 109, Suppl 1: 17–23, 2009. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol (1985) 98: 296–306, 2005. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 41.Sanchis-Gomar F, Perez-Quilis C, Lippi G. AMP-activated protein kinase (AMPK) signaling pathway: A potential mechanism involved in PAFIYAMA syndrome? Int J Cardiol 233: 96, 2017. doi: 10.1016/j.ijcard.2016.12.142. [DOI] [PubMed] [Google Scholar]
- 42.Sardon Puig L, Valera-Alberni M, Cantó C, Pillon NJ. Circadian rhythms and mitochondria: connecting the dots. Front Genet 9: 452, 2018. doi: 10.3389/fgene.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem 280: 18717–18727, 2005. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 44.Tadjalli A, Perim RR, Satriotomo I, Santiago-Moreno J, Seven YB, Mitchell GS. LPS-induced systemic inflammation impairs phrenic long-term facilitation via okadaic acid-sensitive protein phosphatase activity. FASEB J 31: 631.1, 2017. [Google Scholar]
- 45.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ. Structural basis of AMPK regulation by small molecule activators. Nat Commun 4: 3017, 2013. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, Zhou W. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther 7: 809–817, 2008. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 47.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]