Abstract
Matrix metalloproteinases (MMPs) are proteolytic enzymes that break down extracellular matrix (ECM) components and have shown to be highly active in the myocardial infarction (MI) landscape. In addition to breaking down ECM products, MMPs modulate cytokine signaling and mediate leukocyte cell physiology. MMP-2, -7, -8, -9, -12, -14, and -28 are well studied as effectors of cardiac remodeling after MI. Whereas 13 MMPs have been evaluated in the MI setting, 13 MMPs have not been investigated during cardiac remodeling. Here, we measure the remaining MMPs across the MI time continuum to provide the full catalog of MMP expression in the left ventricle after MI in mice. We found that MMP-10, -11, -16, -24, -25, and -27 increase after MI, whereas MMP-15, -17, -19, -21, -23b, and -26 did not change with MI. For the MMPs increased with MI, the macrophage was the predominant cell source. This work provides targets for investigation to understand the full complement of specific MMP roles in cardiac remodeling.
NEW & NOTEWORTHY To date, a number of matrix metalloproteinases (MMPs) have not been evaluated in the left ventricle after myocardial infarction (MI). This article supplies the missing knowledge to provide a complete MI MMP compendium.
Keywords: extracellular matrix, fibroblast, macrophage, matrix metalloproteinase, myocardial infarction
INTRODUCTION
In response to myocardial infarction (MI), the myocardium responds by undergoing a repair process that starts with a robust inflammatory response and ends with scar formation (11, 13). Wound healing ranges from formation of a stable scar to progression to heart failure (11, 13). During the inflammatory response, necrotic myocytes and damaged extracellular matrix (ECM) components from the ischemic area are enzymatically broken down. Removal is governed by proteases, in particular the matrix metalloproteinases (MMPs). ECM breakdown provides the platform on which new ECM is deposited to form the infarct scar (11, 13).
The goal of this study was to evaluate all MMPs that had not previously been evaluated in the left ventricle after MI. To date, MMP-1A, -1B, -2, -3, -7, 8, -9, -12, -13, -14, and -28 have been identified in myocardium and examined in MI mice (6, 16–19, 23, 26, 27, 33, 37). In addition, all four tissue inhibitors of metalloproteinases (TIMPs) have been evaluated in MI (5, 16, 22, 41, 44). We hypothesized that MMP-10, -11, -15, -16, -17, -19, -23A, -23B, -24, -25, -26, and -27 may change in protein expression during MI remodeling.
METHODS
Tissue collection.
The samples used for immunoblotting and multiplex immunohistochemistry were previously collected from other projects and included in the mouse Heart Attack Research Tool (mHART) database and tissue bank (10). All animal procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. The mice used were wild-type C57BL/6J 3- to 6-mo-old mice (3 males and 3 females for each time point). MI was induced by permanently occluding the coronary artery according to the Guidelines for Experimental Models of Ischemia and Infarction and as previously described, and mice were given buprenorphine (0.5 mg/kg) before the surgery (9, 20, 25, 37, 48). Echocardiography was performed under isoflurane euthanasia using the Vevo 2100 (Visual Sonics, Toronto, ON, Canada) as previously described and outlined in the Guidelines for Measuring Cardiac Physiology in Mice (9, 20, 30, 37). Heart rates were >400 beats/min and were not different among groups (ANOVA P = 0.47). The left ventricle (LV) was sliced into three sections. The apex and base sections were separated into remote left ventricle control (LVC) and infarct (LVI; including border) zones and snap-frozen for immunoblotting. The mid-papillary section was fixed in zinc-formalin and paraffin-embedded for histological evaluation. Infarct area was measured by staining with 1% 2,3,5-triphenyltetrazolium chloride and calculating the volume percentage of LV that was infarcted.
Immunoblotting.
Immunoblotting was performed according to the published guidelines (3). For first-pass assessment, the samples for each time were pooled together to allow the complete time course of remote and infarct region groups to all be run on one gel. By densitometry, MI time points that showed peak expression were selected for individual variability analysis. Total protein (10 µg) samples were run on 4–12% Criterion XT Bis-Tris precast gels (Bio-Rad, Hercules, CA) and were transferred onto Trans-Blot Turbo Transfer Pack Nitrocellulose Membranes (Bio-Rad). The membranes were stained with Peirce Reversible Protein Stain Kit for nitrocellulose membranes (Thermo Scientific, Rockford, IL), and blots were normalized to their total membrane stain (20). Total membrane stains are shown in Supplemental Fig. S1 (Supplemental material for this article can be found online at https://doi.org/10.6084/m9.figshare.10271102). Membranes were blocked with Blotting Grade Blocker (Bio-Rad) in a 5.0% triphosphate buffer solution and incubated overnight with the primary antibody at 4°C against the MMP (Table 1), followed by incubation at room temperature for 1 h with appropriate secondary antibodies. MMP-9 was incubated with the secondary antibody anti-goat IgG (1:5,000 dilution, Cat. No. PI-9500; Vector Laboratories), and all other MMPs were incubated with anti-rabbit IgG (1:5,000 dilution, Cat. No. PI-1000, Vector Laboratories). The antibodies used all recognized mouse MMPs and showed specificity for that MMP. The membrane-type MMPs are all activated by furin intracellularly, and as such only the active forms were present on the membrane (21, 45). Homogenized spleen and thyroid tumor samples from mice were used as positive controls. Chemiluminescence visualization was conducted by incubating the membrane with Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare) for 5 min and visualizing the blot with an ImageQuant LAS 4000 and ImageQuantTL V8.1 software (GE Healthcare).
Table 1.
MMP antibody list
| MMP | Company/Cat. No. | Dilution |
|---|---|---|
| MMP-8 | Abcam/ab81286 | 1:1,000 |
| MMP-9 | R&D Systems/AF909 | 1:1,000 |
| MMP-10 | MyBiosource/MBS2027749 | 1:1,000 |
| MMP-11 | MyBiosource/MBS94409414 | 1:1,000 |
| MMP-15 | MyBiosource/MBS2520010 | 1:1,000 |
| MMP-16 | MyBiosource/MBS9130916 | 1:1,000 |
| MMP-17 | MyBiosource/MBS2528233 | 1:1,000 |
| MMP-19 | MyBioSource/MB57044316 | 1:1,000 |
| MMP-21 | Epitomics 195511 | 1:1,000 |
| MMP-23B | MyBioSource/MBS2422084 | 1:2,000 |
| MMP-24 | MyBioSource/MBS9202434 | 1:1,000 |
| MMP-25 | MyBioSource/MBS126850 | 1:1,000 |
| MMP-26 | MyBioSource/MBS8236784 | 1:500 |
| MMP-27 | MyBiosource/MBS2517647 | 1:1,000 |
MMP, matrix metalloproteinase.
Multiplex immunohistochemistry imaging.
MI LV sections (n = 6) fixed in 10% zinc-buffered formalin, paraffin-embedded, and sectioned at 5 μm were used from the mHART biobank (10). MI day 1 LV was used for MMP-10, MMP-11, MMP-16, and MMP-24. MI day 7 LV was used for MMP-25, and MI day 3 LV was used for MMP-27. Naïve LV sections (n = 6) were compared against each respected MMP. Sections were stained with specific MMP antibody (1:100; Table 1), as described previously (38, 39). Neutrophils were stained with a neutrophil-specific antibody (1:100, ab21595; Abcam, Cambridge, UK). Macrophages were stained with a macrophage-specific antibody (1:100, CL8943AP; Cedarlane, Burlington, ON, Canada). Cell nuclei were stained with 4′,6-diamidino-2phenylindole (DAPI). Each MMP antibody, neutrophil antibody, and macrophage antibody was conjugated to FITC, Cy3, and Cy5 fluorophores (Opal 520, Opal 620, and Opal 690; Perkin-Elmer, Waltham, MA). Images were acquired at ×40 using the Mantra Quantitative Pathology Imaging System (Perkin-Elmer). Five random fields were chosen within the infarct region for analysis. Total MMP, MMP + neutrophil, and MMP + macrophage staining were quantified as percent area of the field using inForm cell analysis (Perkin-Elmer).
MMP mRNA expression in MI macrophages and fibroblasts.
To further validate cell source for MMPs and TIMPs, we evaluated MMP mRNA expression in previously collected transcriptomic data sets from macrophages and fibroblasts isolated from the infarct region on MI days 1, 3, and 7 and compared with day 0 no MI LV (38, 39).
Statistical analysis.
Data were analyzed according to the recommendations of the Statistical Considerations in Reporting Cardiovascular Research and are presented as means ± SE for echocardiography and SD for all other data (28). Statistical analysis was performed using GraphPad Prism 7 software. Echocardiography and necropsy variables were compared using one-way ANOVA with Newman-Keuls posttest. Comparisons between two groups were made using unpaired t-test or Mann-Whitney test. MMP-25 was compared with infarct wall thinning by Pearson correlation. Statistical significance was set at P < 0.05.
RESULTS
Proof of MI was obtained using echocardiography.
As shown in Fig. 1, the mice displayed LV dilation by dimensions, impaired myocyte contractility by fractional shortening, and infarct wall thinning. Infarct sizes ranged from ∼40 to 50%, and lung mass increased, indicating the development of pulmonary edema.
Fig. 1.
Myocardial infarction (MI) confirmation. Echocardiography on day 0 before MI and on days 1, 3, 5, 7, 14, and 28 after MI revealed increases in left ventricle (LV) end systolic (A) and diastolic (B) dimensions (mm) and decreases in fractional shortening (%; C) and LV infarct wall thickness (mm; D). E: infarct size was determined by 1% 2,3,5-triphenyltetrazolium chloride staining to determine the %total LV that was infarcted. F: lung weight (dry; mg) increased beginning at MI day 7; n = 6 (3 males/3 females) for each time point. Comparisons were made by 1-way ANOVA with Newman-Keuls post-test. *P < 0.05 vs. day 0.
Rigor and reproducibility assessment.
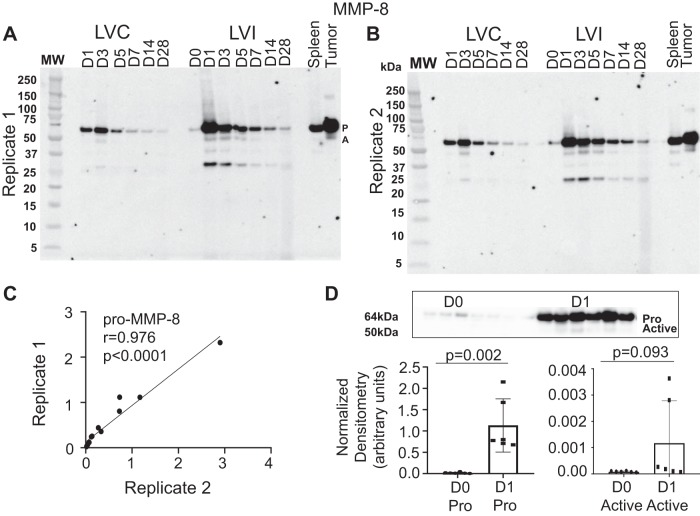
To assess the rigor and reproducibility of our immunoblotting, both operators performed immunoblotting for MMP-8 and MMP-9, which was then compared with previous findings (17, 46). MMP-8 and -9 increased following MI, with the greatest increase in expression of the pro form occurring on day 1 and the active form occurring on day 3. The interperson variation ratios for MMP-8 (Fig. 2) and MMP-9 (Fig. 3) were excellent. Therefore, our results were highly consistent between operators in both technique and analysis and were consistent with past reports (6, 14).
Fig. 2.
Variability assessment of matrix metalloproteinase (MMP)-8 immunoblotting. A: time course immunoblot replicate 1. B: time course immunoblot replicate 2. C: replicate 1 compared with replicate 2 showing the interperson/variation ratio. D: normalized densitometry for pro (P) and active (A) MMP-8 on day 0 (D0) 0 and day 1 (D1); n = 6 (3 males/3 females) for each time point. D0 to myocardial infarction (MI) D1 comparisons were made by t-test. LVC, left ventricle control; LVI, left ventricle infarct.
Fig. 3.
Variability assessment of matrix metalloproteinase (MMP)-9 immunoblotting. A: time course immunoblot replicate 1 (top) and replicate 2 (bottom). B: replicate 1 compared with replicate 2 showing the interperson/variation ratios for pro (P) MMP-9 (top) and active (A) MMP-9 (bottom). D, day; LVC, left ventricle control; LVI, left ventricle infarct.
MMP changes with MI.
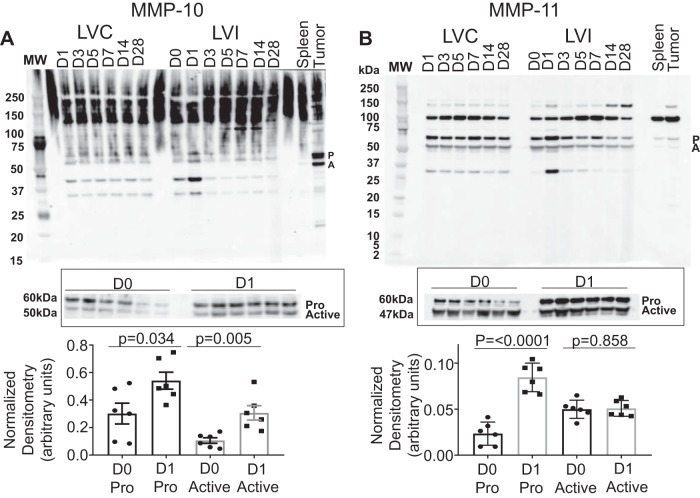
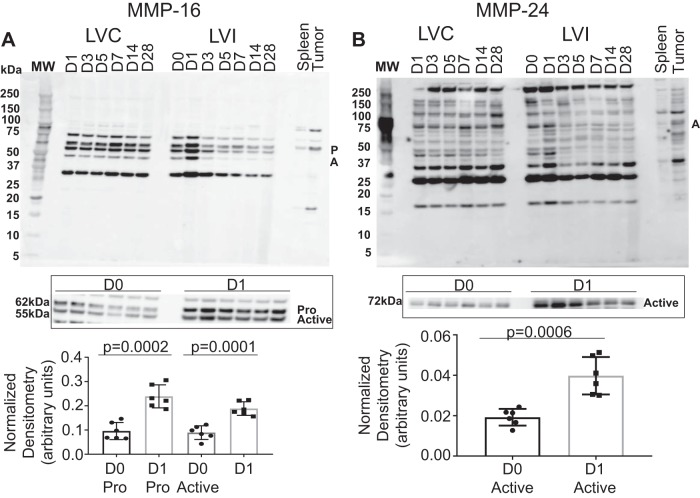
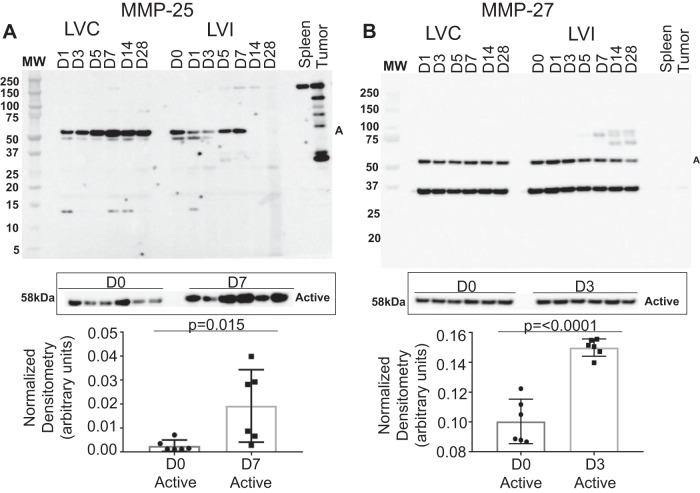
MMP-10, -11, -15, -16, -17, -19, -21, -23B, -24, -25, -26, and -27 were evaluated in the post-MI LV. MMP-10 and -11 (Fig. 4), -16 and -24 (Fig. 5), and -25 and -27 (Fig. 6) were all upregulated with MI. MMP-15 and MMP-17 (Supplemental Fig. S2), as well as MMP-19, MMP-21, MMP-23B, and MMP-26 (Supplemental Fig. S3), were not different over the MI time course. MMP-10 and MMP-11 increased with the highest level of expression in the pro form on MI day 1; the increase in the pro form was not matched with an increase in the active form. MMP-16 increased with the highest level of expression on MI day 1 in both the pro and active forms. MMP-24 exists only in active form and had the highest level of expression on MI day 1. MMP-25 exists only in the active form as well and had the greatest level of expression on MI day 7. By regression analysis, MMP-25 correlated negatively with wall thickness (r = −0.67, P = 0.02), indicating that MMP-25 was highest in infarcts that were the thinnest. MMP-27 has only an active form and had the greatest level of expression on MI day 3.
Fig. 4.
Matrix metalloproteinase (MMP)-10 and MMP-11 myocardial infarction (MI) expression. A: pooled time course expression of MMP-10 (top) and individual analysis from day 0 (D0) to MI day 1 (D1) expressed as densitometry normalized to total membrane stain. B: pooled time course expression of MMP-11 (bottom) and individual analysis from D0 to MI D1 expressed as densitometry normalized to total membrane stain; n = 6 (3 males/3 females) for each time point. D0 to MI D1 comparisons were made by t-test. A, active; LVC, left ventricle control; LVI, left ventricle infarct; P, pro.
Fig. 5.
Matrix metalloproteinase (MMP)-16 and MMP-24 myocardial infarction (MI) expression. A: pooled time course expression of MMP-16 (top) and individual analysis from day 0 (D0) to MI day 1 (D1) expressed as densitometry normalized to total membrane stain. B: pooled time course expression of MMP-24 (bottom) and individual analysis from D0 to MI D1 expressed as densitometry normalized to total membrane stain; n = 6 (3 males/3 females) for each time point. D0 to MI D1 comparisons were made by t-test. A, active; LVC, left ventricle control; LVI, left ventricle infarct; P, pro.
Fig. 6.
Matrix metalloproteinase (MMP)-25 and MMP-27 myocardial infarction (MI) expression. A: pooled time course expression of MMP-25 (top) and individual analysis from day 0 (D0) to MI day 7 (D7) expressed as densitometry normalized to total membrane stain. B: pooled time course expression of MMP-27 (bottom) and individual analysis from D0 to MI day 3 (D3) expressed as densitometry normalized to total membrane stain; n = 6 (3 males/3 females) for each time point. D0 to MI D1 comparisons were made by t-test. A, active; LVC, left ventricle control; LVI, left ventricle infarct.
MMP immunohistochemistry.
Immunohistochemistry was performed on LV sections using MMP-specific antibodies on day 0 and MI day 1 for MMP-10, -11, -16, and -24, on day 0 and MI day 7 for MMP-25, and on day 0 and MI day 3 for MMP-27 (Table 2). Total MMP-10 did not change in the LV on day 1, with MMP-10 localized within macrophages or neutrophils not changing. MMP-11 increased on MI day 1, and whereas localization within the macrophages did not change, MMP-11 within the neutrophil decreased. Total MMP-16 increased, with MMP-16 localized within macrophages but not neutrophils. Total MMP-24 increased with MI, and MMP-24 localized within macrophages increased and MMP-24 within neutrophils did not change. Total MMP-25 increased, with MMP-25 localizing within macrophages increasing and MMP-25 within neutrophils not changing. Total MMP-27 increased but did not increase in macrophages or neutrophils. These results indicate that a strong proportion of MI MMP protein expression is localized to the macrophage. These results are also consistent with MMP expression in MI neutrophils being low due to degranulation of vesicles to release the MMPs.
Table 2.
Immunohistochemistry analysis
| Total | Macrophage Localized | Neutrophil Localized | |
|---|---|---|---|
| MMP-10 | ↔ | ↔ | ↔ |
| MMP-11 | ↑ | ↔ | ↓ |
| MMP-16 | ↑ | ↑ | ↔ |
| MMP-24 | ↑ | ↑ | ↔ |
| MMP-25 | ↑ | ↑ | ↔ |
| MMP-27 | ↑ | ↔ | ↔ |
MMP, matrix metalloproteinase. Total as well as macrophage and neutrophil localized MMP expression was quantified using multiplex imaging of day 0 and myocardial infarction (MI) day 1 (for MMP-10, -11, -16, and -24), day 7 (MMP-25), or day 3 (MMP-27) infarct region. Sample sizes are n = 6 (3 males and 3 females) for each group, and groups were compared by Student’s t-test. ↑Increased or ↓decreased compared with day 0 (P < 0.05). ↔, no change.
MMP expression in MI macrophages and fibroblasts by RNAseq.
Transcriptomics were previously performed on LV macrophages isolated from LV on day 0 and MI days 1, 3, and 7 (results summary in Table 3, with full individual results in Supplemental Table S1) (38). Of the 24 MMPs evaluated, 17 showed differential expression in infarct macrophages. MMP-8, -9, and -25 show prominent increases in gene expression on MI day 1, matching the immunoblotting results. For MMP-10, -11, -16, -24, and -27, immunoblotting showed increased expression, and macrophage expression showed no change or decreases in gene expression. This indicates that the increases in MMP-10, -11, -16, -24, and -27 shown by immunoblotting were not due to macrophage as a source, or there was a mismatch between macrophage gene and protein expression.
Table 3.
MI macrophage MMP expression
| Gene | ANOVA P Value | Newman-Keuls Multiple-Comparisons Test |
|---|---|---|
| Mmp1b | 0.026 | D0–D7 (↑); D1–D7(↑); D3–D7(↑) |
| Mmp2 | 0.002 | D0–D1 (↓); D0–D3 (↓) |
| Mmp3 | 0.023 | D0–D3(↑) |
| Mmp8 | <0.0001 | D0–D1(↑); D0–D3(↑); D1–D3(↑); D1–D7(↓); D3–D7(↓) |
| Mmp9 | 0.017 | D0–D1(↑); D1–D3(↓); D1–D7(↓) |
| Mmp10 | 0.723 | ↔ |
| Mmp11 | 0.033 | D0–D1(↓); D0–D3(↓) |
| Mmp12 | <0.0001 | D0–D1(↑); D0–D3(↑);D0–D7(↑); D1–D3(↓); D1–D7(↓) |
| Mmp13 | <0.001 | D0–D3(↓); D0–D7(↓); D1–D3(↓); D1–D7(↓) |
| Mmp14 | <0.001 | D0–D1(↑); D0–D3(↑); D0–D7(↑) |
| Mmp15 | 0.009 | D0–D1(↓); D0–D3(↓); D0–D7(↓) |
| Mmp16 | 0.283 | ↔ |
| Mmp17 | 0.374 | ↔ |
| Mmp19 | <0.001 | D0–D1(↑); D0–D3(↑); D0–D7(↑); D1–D3(↓); D1–D7(↓) |
| Mmp21 | 0.372 | ↔ |
| Mmp23 | 0.004 | D0–D1(↓); D0–D3(↓); D1–D7(↑); D3–D7(↑) |
| Mmp24 | 0.203 | ↔ |
| Mmp25 | 0.011 | D0–D1(↑); D1–D3(↓); D1–D7(↓) |
| Mmp27 | 0.411 | ↔ |
| Mmp28 | 0.051 | ↔ |
| Timp1 | <0.0001 | D0–D1(↑); D0–D3(↑); D0–D7(↑); D1–D3(↓); D1–D7(↓); D3–D7(↓) |
| Timp2 | <0.0001 | D0-D1(↓); D0–D3(↓); D1–D3(↑); D1–D7(↑); D3–D7(↑) |
| Timp3 | 0.005 | D0–D1(↓); D0–D3(↓); D0–D7(↓) |
| Timp4 | 0.004 | D0–D1(↓); D0–D3(↓); D0–D7(↓) |
Arrows denote direction of change. D, day; Mmp, matrix metalloproteinase; Timp, tissue inhibitor of metalloproteinase. Sample sizes are n = 4 males for each time [D0 and myocardial infarction (MI) D1, D3, and D7]. ↑Increased or ↓decreased compared with day 0 (P < 0.05); ↔no change.
Transcriptomics were previously performed on LV fibroblasts isolated from LV on day 0 and MI days 1, 3, and 7 (results summary in Table 4, with full individual results in Supplemental Table S2) (39). Of the 24 MMPs evaluated, five showed differential expression in infarct macrophages. MMP-16 shows prominent increases in gene expression on MI day 3 in fibroblasts, whereas immunoblotting for LV MMP-16 showed increased expression on day 1. There was no difference or no change in expression of MMP-10, -11, -16, -24, and -27 in fibroblasts. This indicates that the increases in MMP-10, -11, -16, -24, and -27 shown by immunoblotting were not due to fibroblasts as a source, or there was a mismatch between fibroblast gene and protein expression.
Table 4.
MI fibroblast MMP expression
| Gene | ANOVA P Value | Newman-Keuls Multiple-Comparisons Test |
|---|---|---|
| Mmp1a | 0.730 | ↔ |
| Mmp1b | 0.325 | ↔ |
| Mmp2 | 0.061 | ↔ |
| Mmp3 | 0.010 | D0–D3(↓); D0–D7(↓); D1–D3(↓); D1–D7(↓) |
| Mmp8 | 0.587 | ↔ |
| Mmp9 | 0.561 | ↔ |
| Mmp10 | 0.007 | D1–D3(↓); D1–D7(↓) |
| Mmp11 | 0.650 | ↔ |
| Mmp12 | 0.071 | ↔ |
| Mmp13 | 0.187 | ↔ |
| Mmp14 | 0.003 | D0–D3(↑); D0–D7(↑); D1–D3(↑); D1–D7(↑) |
| Mmp15 | 0.221 | ↔ |
| Mmp16 | 0.044 | D1–D3(↑) |
| Mmp17 | 0.003 | D0–D3(↑); D0–D7(↑); D1–D3(↑); D1–D7(↑) |
| Mmp19 | 0.779 | ↔ |
| Mmp21 | 0.722 | ↔ |
| Mmp23 | 0.053 | ↔ |
| Mmp24 | 0.090 | ↔ |
| Mmp25 | 0.244 | ↔ |
| Mmp27 | 0.157 | ↔ |
| Mmp28 | 0.841 | ↔ |
| Timp1 | 0.029 | D0–D7(↑); D1–D7(↑) |
| Timp2 | 0.260 | ↔ |
| Timp3 | 0.001 | D0–D1(↓); D0–D3(↑); D0–D7(↑); D1–D3(↑); D1–D7(↑) |
| Timp4 | 0.203 | ↔ |
D, day; Mmp, matrix metalloproteinase; Timp, tissue inhibitor of metalloproteinase. Sample sizes are n = 3 males for each time [D0 and myocardial infarction (MI) D1, D3, and D7]. ↑Increased or ↓decreased compared with day 0 (P < 0.05); ↔no change.
DISCUSSION
The goal of this study was to evaluate the MMP family members that had not been previously examined in the MI LV. We validated our immunoblotting approach using MMP-8 and MMP-9 as controls and evaluated MMP-10, -11, -15, -16, -17, -19, -21 -23B, -24, -25, -26, and -27 on day 0 and MI days 1, 3, 5, 7, 14, and 28. The most salient findings of this study were that 1) MMP-10, -11, -16, -24, -25, and -27 are newly identified MMPs increased after MI by immunoblotting; 2) MMP-15, -17, -19, -21, -23B, and -26 did not change with MI from initiation through MI day 28; and 3) the macrophage was the predominant source of the MMPs by immunohistochemistry and by transcriptomic analyses. Previously, information on expression of 13 MMPs has been reported. We add to the literature information on an additional 14 MMPs to complete the MMPs in the MI LV compendium.
MMP-8 and -9 were evaluated as positive control experiments, as both have previously been shown to increase after MI (6). We showed excellent reproducibility both between the duplicate evaluations (performed by A. R. Kaminski and E. T. Moore) and with the literature. In our study, MMP-10 and -11 increased on MI day 1 in the infarcted zone. Although MMP-10 is not constitutively expressed in healthy tissue, it is heavily secreted by macrophages during acute inflammation in damaged or infected tissue (36). During infection with Pseudomonas aeruginosa, MMP-10 is responsible for mitigating the inflammatory actions of M1 macrophages as well as activating M2 anti-inflammatory macrophages (36). In cardiac tissue, TIMP-4 regulates MMP-10 during the process of LV remodeling, and in end-stage heart failure patients myocardial samples revealed a positive correlation between upregulation of MMP-10 and LV dilation, indicating that MMP-10 influenced ECM structure (47). Inhibition of MMP-10, accordingly, may help to prevent LV dilation. Substrates of MMP-11 include collagen IV and VI, fibronectin, α2-macroglobulin, and insulin-like growth factor-binding protein 1 (1). MMP-11 is regulated in M2 macrophages compared more so than M1 macrophages (14). Immunohistochemistry showed no difference in MMP-11 localized in macrophages and a decrease in neutrophils, whereas total MMP-11 increased, suggesting that neutrophils and macrophages are not the major source of MMP-11 in MI. High MMP-11 activity on MI day 1 in M1 macrophages suggests that MMP-11 may be involved in pro-inflammatory signaling or ECM turnover, which are hallmarks of the inflammatory phase.
MMP-16, also known as MT3-MMP, had highest expression on MI day 1 in both the pro and active forms, which suggests that it may have an active role in early myocardial remodeling. MMP-16 may have an indirect role through its activation of MMP-9 and, more importantly, MMP-2 (31, 49). MMP-2 cleaves collagen I, IV, V, VII, and X, laminin, aggrecan, fibronectin, and tenascin (4). This widespread degradative effect upon multiple extracellular matrix substrates suggests that MMP-16 alone may have a more influential role in directing LV remodeling than previously predicted.
MMP-24, also known as MT5-MMP, is a membrane-type MMP expressed in the nervous system (2). Importantly, MMP-24 cleaves MMP-2, which degrades type IV collagen to break down basement membranes (4, 32). MMP-24 has been evaluated as a potential therapy for Alzheimer’s disease due to regulatory effects on amyloid precursor protein metabolism (2). Because MMP-24 was highest on day 1 after MI, its role to activate MMP-2 is likely important in cardiac remodeling (15).
MMP-25, also known as MT6-MMP, is a membrane-type MMP expressed in lung, spleen, and leukocytes (43). MMP-25 substrates include fibrin, fibronectin, collagen I, and collagen IV (12). In the context of MI, pro-inflammatory N1 neutrophils contribute to LV wall thinning after MI by generating large amounts of MMP-12 and MMP-25 (34). MMP-25 showed highest expression on MI day 7 and independently tracked with infarct wall thinning on MI day 7. Therefore, MMP-25 may be relevant to neutrophil prolongation of pro-inflammation.
MMP-27 has a unique COOH-terminal extension, causing it to be retained in the endoplasmic reticulum (8). MMP-27 immunolocalizes with CD45, CD163, and CD206 in macrophages in the cycling human endometrium (7). CD163 and CD206 are expressed by M2 macrophages (7). In our study, MMP-27 was increased in its active form (58 kDa) on MI day 3, when macrophages are undergoing a metabolic shift and upregulating genes associated with M2 macrophages (38). Whether or not MMP-27 plays a role in phenotypic shift of macrophages has not been examined.
By immunohistochemistry, MMP-11, -16, -24, -25, and -27 increased with MI. These results are consistent with the immunoblotting results. Whereas MMP-10 increased by immunoblotting, a similar increase by immunohistochemistry was not observed. By transcriptomics, the macrophage was not a predominant source of MMP-10, whereas cardiac fibroblasts showed decreased expression with MI. This indicates that the immunoblotting results likely reflect the combination of effects across cell types. Transcriptomics, immunoblotting, and immunohistochemistry combined confirmed increased MMP-11, -16, -24, and -25 in the MI LV and indicated that macrophages were the predominant source of these MMPs.
Further studies on the MMPs that were differentially expressed are needed to better understand the roles of each MMP as well as their functional relationships to each other. There is a need to follow up on the MMPs elevated after MI to determine their specific cause and effect relationship in the left ventricle. MMPs have a number of roles in the myocardium, including processing of extracellular matrix substrates, inflammatory mediators, and growth factors, and the implications of these findings need to be determined (11, 18, 24). In particular, the role of the substrate fragments generated by these MMP should be investigated, as substrate proteolysis is known to release active biopeptides that influence MI remodeling (29). In addition to clarifying mechanisms of action of these MMPs in the mouse myocardium after MI, evaluating whether these MMPs also are elevated in humans after MI would provide a translational link to our results.
In conclusion, we add to the current knowledge base that MI yields increased expression of MMP-10, -11, -16, -24, -25, and -27 in the infarct region. The summary of our current knowledge of MMP expression after MI in mice is provided in Table 5.
Table 5.
Summary of our current knowledge of MMP expression after MI in mice
| Studied Previously? | Activity post-MI | Peak Protein Expression Day (Pro/Active) |
|
|---|---|---|---|
| MMP-1A* MMP-1B |
Yes (23) | ↑ | 7 |
| MMP-2 | Yes (16) | ↑ | 7 |
| MMP-3 | Yes (16) | ↑ | 4 |
| MMP-7 | Yes (27) | ↑ | 7 |
| MMP-8 | N | ↑ | 1/3 |
| MMP-9 | Yes (6, 16–18, 26, 37) | ↑ | 1/3 |
| MMP-10 | No | ↑ | 1/1 |
| MMP-11 | No | ↑ | 1/0 |
| MMP-12 | Yes (19) | ↑ | 1–7 |
| MMP-13 | Yes (16) | ↑ | Time course not performed |
| MMP-14 | Yes (16) | ↑ | Time course not performed |
| MMP-15 | No | ↔ | NA |
| MMP-16 | No | ↑ | 1/1 |
| MMP-17 | No | ↔ | NA |
| MMP-19 | No | ↔ | NA |
| MMP-20† | No | NA | NA |
| MMP-21† | No | ↔ | NA |
| MMP-23A MMP-23B |
No | ↔ | No pro/1 |
| MMP-24 | No | ↑ | No pro/1 |
| MMP-25 | No | ↑ | No pro/7 |
| MMP-26 | No | ↔ | 3/28 |
| MMP-27 | No | ↑ | No pro/3 |
| MMP-28 | Yes (33) | ↑ | High expression in myocytes at baseline; goes up (in macrophages) |
| TIMP-1 | Yes (5, 16) | ↑ | 1 |
| TIMP-2 | Yes (41) | ↑ | 1 |
| TIMP-3 | Yes (44) | ↓ | 1 |
| TIMP-4 | Yes (22) | ↓ | 1 |
Results from this study are indicated with boldface. NA, not applicable. ↑Increased or ↓decreased compared with day 0 (P < 0.05); ↔no change.
GRANTS
We acknowledge funding from the National Institutes of Health under Award Nos. HL-075360, HL-129823, and HL-137319 and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development under Award Nos. 5I01BX000505. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.K., E.T.M., E.R.F., and M.L.L. conceived and designed research; A.R.K., E.T.M., M.J.D., F.M.V., and E.R.F. performed experiments; A.R.K., E.T.M., M.J.D., F.M.V., E.R.F., and M.L.L. analyzed data; A.R.K., E.T.M., M.J.D., F.M.V., and M.L.L. interpreted results of experiments; A.R.K., E.T.M., M.J.D., and E.R.F. prepared figures; A.R.K., E.T.M., and M.J.D., and M.L.L. drafted manuscript; A.R.K., E.T.M., M.J.D., F.M.V., E.R.F., and M.L.L. edited and revised manuscript; A.R.K., E.T.M., M.J.D., F.M.V., E.R.F., and M.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Dr. Lindsey is a Stokes-Shackleford Professor at University of Nebraska Medical Center.
REFERENCES
- 1.Arcidiacono B, Chiefari E, Laria AE, Messineo S, Bilotta FL, Britti D, Foti DP, Foryst-Ludwig A, Kintscher U, Brunetti A. Expression of matrix metalloproteinase-11 is increased under conditions of insulin resistance. World J Diabetes 8: 422–428, 2017. doi: 10.4239/wjd.v8.i9.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, Py NA, Bernard A, Bauer C, Charrat E, Moschke K, Seiki M, Vignes M, Lichtenthaler SF, Checler F, Khrestchatisky M, Rivera S. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci 73: 217–236, 2016. doi: 10.1007/s00018-015-1992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caley MP, Martins VL, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 4: 225–234, 2015. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.e1–1101.e8, 2006. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation 111: 1800–1805, 2005. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cominelli A, Gaide Chevronnay HP, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Mol Hum Reprod 20: 767–775, 2014. doi: 10.1093/molehr/gau034. [DOI] [PubMed] [Google Scholar]
- 8.Cominelli A, Halbout M, N’Kuli F, Lemoine P, Courtoy PJ, Marbaix E, Tyteca D, Henriet P. A unique C-terminal domain allows retention of matrix metalloproteinase-27 in the endoplasmic reticulum. Traffic 15: 401–417, 2014. doi: 10.1111/tra.12149. [DOI] [PubMed] [Google Scholar]
- 9.DeLeon-Pennell KY, Iyer RP, Ero OK, Cates CA, Flynn ER, Cannon PL, Jung M, Shannon D, Garrett MR, Buchanan W, Hall ME, Ma Y, Lindsey ML. Periodontal-induced chronic inflammation triggers macrophage secretion of Ccl12 to inhibit fibroblast-mediated cardiac wound healing. JCI Insight 2: e94207, 2017. doi: 10.1172/jci.insight.94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLeon-Pennell KY, Iyer RP, Ma Y, Yabluchanskiy A, Zamilpa R, Chiao YA, Cannon PL, Kaplan A, Cates CA, Flynn ER, Halade GV, de Castro Brás LE, Lindsey ML. The Mouse Heart Attack Research Tool 1.0 database. Am J Physiol Heart Circ Physiol 315: H522–H530, 2018. doi: 10.1152/ajpheart.00172.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog Mol Biol Transl Sci 147: 75–100, 2017. doi: 10.1016/bs.pmbts.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.English WR, Velasco G, Stracke JO, Knäuper V, Murphy G. Catalytic activities of membrane-type 6 matrix metalloproteinase (MMP25). FEBS Lett 491: 137–142, 2001. doi: 10.1016/S0014-5793(01)02150-0. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal 8: 1907–1939, 2006. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 14.Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One 7: e42507, 2012. doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol 109: 424, 2014. doi: 10.1007/s00395-014-0424-y. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 288: H149–H158, 2005. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 17.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol 100: 109–117, 2016. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H190–H198, 2016. doi: 10.1152/ajpheart.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer RP, Patterson NL, Zouein FA, Ma Y, Dive V, de Castro Brás LE, Lindsey ML. Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int J Cardiol 185: 198–208, 2015. doi: 10.1016/j.ijcard.2015.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang T, Nagase H, Pei D. Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network. Cancer Res 62: 675–681, 2002. [PubMed] [Google Scholar]
- 22.Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285: 24487–24493, 2010. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, de Boer OJ, Ploegmaker H, Teeling P, Daemen MJ, de Winter RJ, van der Wal AC. Granulocytes in coronary thrombus evolution after myocardial infarction–time-dependent changes in expression of matrix metalloproteinases. Cardiovasc Pathol 25: 40–46, 2016. doi: 10.1016/j.carpath.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Lindsey ML. Assigning matrix metalloproteinase roles in ischaemic cardiac remodelling. Nat Rev Cardiol 15: 471–479, 2018. doi: 10.1038/s41569-018-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113: 2919–2928, 2006. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. J Am Coll Cardiol 66: 1364–1374, 2015. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, van Mil A, Aguor EN, Siddiqi S, Vrijsen K, Jaksani S, Metz C, Zhao J, Strijkers GJ, Doevendans PA, Sluijter JP. MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J Cell Mol Med 16: 2379–2386, 2012. doi: 10.1111/j.1582-4934.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llano E, Pendás AM, Freije JP, Nakano A, Knäuper V, Murphy G, López-Otin C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res 59: 2570–2576, 1999. [PubMed] [Google Scholar]
- 33.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110: 51–61, 2016. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchenko GN, Marchenko ND, Strongin AY. The structure and regulation of the human and mouse matrix metalloproteinase-21 gene and protein. Biochem J 372: 503–515, 2003. doi: 10.1042/bj20030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, McGuire JK, Gharib SA, Parks WC. Stromelysin-2 (MMP10) Moderates Inflammation by Controlling Macrophage Activation. J Immunol 197: 899–909, 2016. doi: 10.4049/jimmunol.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meschiari CA, Jung M, Iyer RP, Yabluchanskiy A, Toba H, Garrett MR, Lindsey ML. Macrophage overexpression of matrix metalloproteinase-9 in aged mice improves diastolic physiology and cardiac wound healing after myocardial infarction. Am J Physiol Heart Circ Physiol 314: H224–H235, 2018. doi: 10.1152/ajpheart.00453.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113: 26, 2018. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ 2nd, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 114: 6, 2019. doi: 10.1007/s00395-019-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573, 2006. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Ramani R, Nilles K, Gibson G, Burkhead B, Mathier M, McNamara D, McTiernan CF. Tissue inhibitor of metalloproteinase-2 gene delivery ameliorates postinfarction cardiac remodeling. Clin Transl Sci 4: 24–31, 2011. doi: 10.1111/j.1752-8062.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, Simmer JP. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res 78: 743–750, 1999. doi: 10.1177/00220345990780030601. [DOI] [PubMed] [Google Scholar]
- 43.Soria-Valles C, Gutiérrez-Fernández A, Osorio FG, Carrero D, Ferrando AA, Colado E, Fernández-García MS, Bonzon-Kulichenko E, Vázquez J, Fueyo A, López-Otín C. MMP-25 Metalloprotease Regulates Innate Immune Response through NF-κB Signaling. J Immunol 197: 296–302, 2016. doi: 10.4049/jimmunol.1600094. [DOI] [PubMed] [Google Scholar]
- 44.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 45.Timoshenko OS, Gureeva TA, Kugaevskaya EV, Solov’eva NI. [Membrane type 1 matrix metalloproteinase (MT1-MMP) and the regulators of its activity as invasive factors in squamous cell cervical carcinomas]. Biomed Khim 60: 683–688, 2014. doi: 10.18097/PBMC20146006683. [DOI] [PubMed] [Google Scholar]
- 46.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 114: 1020–1027, 2006. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 47.Wei Y, Cui C, Lainscak M, Zhang X, Li J, Huang J, Zhang H, Zheng Z, Hu S. Type-specific dysregulation of matrix metalloproteinases and their tissue inhibitors in end-stage heart failure patients: relationship between MMP-10 and LV remodelling. J Cell Mol Med 15: 773–782, 2011. doi: 10.1111/j.1582-4934.2010.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamilpa R, Zhang J, Chiao YA, de Castro Brás LE, Halade GV, Ma Y, Hacker SO, Lindsey ML. Cardiac wound healing post-myocardial infarction: a novel method to target extracellular matrix remodeling in the left ventricle. Methods Mol Biol 1037: 313–324, 2013. doi: 10.1007/978-1-62703-505-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang WL, Chen YF, Meng HZ, Du JJ, Luan GN, Wang HQ, Yang MW, Luo ZJ. Role of miR-155 in the regulation of MMP-16 expression in intervertebral disc degeneration. J Orthop Res 35: 1323–1334, 2017. doi: 10.1002/jor.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]