Abstract
Tandem pore domain acid-sensitive K+ (TASK) channels are present in cardiac tissue; however, their contribution to cardiac pathophysiology is not well understood. Here, we investigate the role of TASK-1 and TASK-3 in the pathogenesis of cardiac dysfunction using both human tissue and mouse models of genetic TASK channel loss of function. Compared with normal human cardiac tissue, TASK-1 gene expression is reduced in association with either cardiac hypertrophy alone or combined cardiac hypertrophy and heart failure. In a pressure overload cardiomyopathy model, TASK-1 global knockout (TASK-1 KO) mice have both reduced cardiac hypertrophy and preserved cardiac function compared with wild-type mice. In contrast to the TASK-1 KO mouse pressure overload response, TASK-3 global knockout (TASK-3 KO) mice develop cardiac hypertrophy and a delayed onset of cardiac dysfunction compared with wild-type mice. The cardioprotective effects observed in TASK-1 KO mice are associated with pressure overload-induced augmentation of AKT phosphorylation and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression, with consequent augmentation of cardiac energetics and fatty acid oxidation. The protective effects of TASK-1 loss of function are associated with an enhancement of physiologic hypertrophic signaling and preserved metabolic functions. These findings may provide a rationale for TASK-1 channel inhibition in the treatment of cardiac dysfunction.
NEW & NOTEWORTHY The role of tandem pore domain acid-sensitive K+ (TASK) channels in cardiac function is not well understood. This study demonstrates that TASK channel gene expression is associated with the onset of human cardiac hypertrophy and heart failure. TASK-1 and TASK-3 strongly affect the development of pressure overload cardiomyopathies in genetic models of TASK-1 and TASK-3 loss of function. The effects of TASK-1 loss of function were associated with enhanced AKT phosphorylation and expression of peroxisome proliferator-activated receptor-γ coactivator-1 (PGC-1) transcription factor. These data suggest that TASK channels influence the development of cardiac hypertrophy and dysfunction in response to injury.
Keywords: cardiac aging, cardiac function, cardiac hypertrophy, knockout mouse model, TASK channels
INTRODUCTION
Tandem pore domain acid-sensitive K+ (TASK) channels are members of a highly diverse group of ion channels, called two pore-forming domain potassium (K2P) channels, that contribute to background potassium current (22). TASK-1 and TASK-3, two members of the TASK channel family, were identified nearly two decades ago and are characterized by their responsiveness to physiologic levels of extracellular acidification (19, 53). Sensitivity to acidification is conferred by protonation of a histidine residue in the first extracellular loop of TASK-1 and TASK-3 (44, 53). In addition to pH responsiveness, TASK-1 and TASK-3 produce multiple physiologic responses through their ability to form either channel homodimers (37) or channel heterodimers (10). Although both TASK-1 and TASK-3 channels were initially described as producing an outward rectifying “leak” current, recent work has shown that TASK channels and other K2P channels can be voltage activated and influence resting membrane potential (57). The influence of TASK channels on a variety of physiologic responses is suggested by their presence in carotid bodies (30), the central nervous system (65), pulmonary smooth muscle cells (25), and cardiac tissue (32). These early findings have sparked a growing interest in the role of TASK channels in human physiology.
In addition to their diverse tissue distribution and responsiveness to acidification, TASK channel activity is further modulated by endosomal trafficking and hormonal/chemical stimuli (22). TASK-1 plasma membrane expression is regulated by trafficking of TASK-1 channels into and out of the endoplasmic reticulum by respectively binding to the 14-3-3 and coatomer subunit-β (β-COP) cytoplasmic proteins (48). Apart from trafficking, G protein-coupled receptor (GPCR) and heterotrimeric protein subunit Gαq (Gq) activation is known to influence TASK channel function (8, 70). Endogenous TASK channel activity in the central nervous system (42, 43) and adrenal glands (12) is, respectively, either enhanced or reduced by ligand-mediated activation of Gq-coupled receptors. Receptor activation is thought to influence TASK activity through the following mechanisms: 1) direct Gq binding to TASK channels (8), 2) phospholipase C-mediated phosphatidylinositol 4,5‐bisphosphate (PIP2; 13, 38) and diacylglycerol (DAG; 70) second messenger generation, and 3) downstream Rho kinase (61) and Src kinase activation (46). In addition to hormonal modulation, TASK channels are influenced by chemical stimuli such as hypoxia. Hypoxic stimulation of carotid body glomus cells (6) and pulmonary artery smooth muscle cells (25) enhances cellular depolarization by increasing resting membrane potential, which is influenced by reduced TASK channel activity (49). These data, linking neurohormonal activation and tissue oxygenation to TASK channel function, suggest a potential role for TASK channels in cardiovascular disease.
TASK channels are present in cardiac tissue, although our understanding of their function in cardiac physiology is evolving. Early studies revealed both TASK-1 (19, 32, 52) and TASK-3 (31, 54) expression in both rodent and human cardiac tissue. Subsequent studies of either indirect blockade of TASK-1 current (4) or genetic loss of TASK-1 (18) revealed the influence of TASK-1 on cellular automaticity and action potential duration. These electrophysiologic effects have relevance in the development of human atrial fibrillation. Among patients with atrial fibrillation, both the loss (36) and gain (59) of TASK-1 function is thought to promote atrial arrhythmogenesis. Apart from arrhythmia, human studies have identified TASK-1 as a contributor to the pathogenesis of pulmonary hypertension. In 2013, a form of inherited idiopathic pulmonary hypertension was found to be associated with TASK-1 loss-of-function mutations (39). Follow-up animal studies have suggested that alterations in TASK-1 function both are causal in the development of, and can be targeted to treat, pulmonary hypertension (3), although these findings appear to be inconsistent across model systems (33). Although these studies have shed light on the effects of TASK channels on pulmonary vasculature and atrial electrophysiology, little is known about the effect of TASK channels on left ventricular function.
Recent work from our group has identified a key role for the K2P channel two-pore domain potassium channel TREK-1 (TREK-1) on cardiac morphology and function after hypertensive injury (2). Genetic loss of TREK-1 enhances pressure overload-induced cardiac hypertrophy but preserves ventricular function through alterations in mitogen-activated protein kinase signaling (2). Given the similarities in structure and anatomic distribution between TREK and TASK channels, our prior work raises the possibility that TASK-1 and TASK-3 may also play a significant role in the mammalian response to cardiac injury and the development of heart failure. Here we investigate the effect of genetic loss of TASK-1 and TASK-3 on the development of pressure overload-induced cardiomyopathy. We identify these channels as important modulators in left ventricular function, cardiac metabolism, and the development of pathologic hypertrophy.
MATERIALS AND METHODS
Experimental animals.
Eight- to twelve-week-old control C57BL/6 wild-type (WT), TASK-1 global knockout (TASK-1 KO), and TASK-3 global knockout (TASK-3 KO) mice were used for this study. TASK-1 and TASK-3 KO mice were generously provided by Dr. Douglas Bayliss (University of Virginia, Charlottesville, VA; 16). All animal experiments performed for this study were conducted according to approved protocols and animal welfare regulations of the Institutional Animal Care and Use Committee at Duke University Medical Center (Durham, NC).
Generation of pressure overload and serial echocardiography.
Pressure overload in mice was induced using transverse aortic constriction (TAC) using methods previously described (2, 56). Serial echocardiography was performed on conscious mice from all groups with a Vevo 2100 high-resolution imaging system (VisualSonics). Mice underwent either a sham or a TAC procedure in a nonrandomized fashion. Echocardiograms were analyzed in an unblinded manner by two independent readers, and both readings were averaged. The κ-value for interobserver variability was 0.785 ± 0.048 (mean ± SE).
Pressure-volume loop analysis.
In vivo pressure-volume (P-V) analysis was performed as previously described (1). Parallel conductance volume (Vp) was determined by 10-μL injection of 15% saline into the right jugular vein to determine blood pool conductance. The derived Vp was used to correct the P-V loop data. Data were recorded digitally at 1,000 Hz and analyzed with pressure-volume analysis software (PVAN data analysis software version 3.3; Millar Instruments) as previously described (1).
Human cardiac tissue acquisition and tissue repository.
Human myocardial tissue samples used for this study were procured from the Duke Human Heart Repository. The collection and use of tissues for this study were approved by the Duke University Health System Institutional Review Board. Human myocardium was acquired from the left ventricular (LV) free wall of explanted ischemic failing or nonischemic failing hearts following cardiac transplantation. Nonfailing left ventricular tissue was acquired from donors whose hearts were not utilized for transplant. All myocardial samples were obtained from the anterolateral LV free wall, immediately flash-frozen in liquid nitrogen, and stored at −80°C.
Immunoblot analysis.
Tissue samples were homogenized in Nonidet P-40 (NP-40) lysis buffer containing 20 mM Tris (pH 7.4), 137 mM NaCl, 1% NP-40, 20% glycerol, 10 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 mM NaF, 2.5 mg/mL aprotinin, 2.5 mg/mL and leupeptin. Protein concentrations were assayed with Bio-Rad (Hercules, CA) protein assay reagent, and 80–100 μg protein were denatured by heating at 95°C for 5 min before resolving by SDS-PAGE. The following dilutions of primary antibody were used: phospho-AKT (Cell Signaling Technology), 1:1,000; total AKT (Cell Signaling Technology), 1:1000; phospho-ERK1/2 (Cell Signaling Technology), 1:1,000; total ERK1/2 (Millipore), 1:1,000; phospho-p38α (Cell Signaling Technology), 1:1,000; and total p38α (Cell Signaling Technology), 1:1,000. Detection was performed by an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). Densitometric analysis was performed with Bio-Rad Fluor-S MultiImager software.
Gene expression analysis.
At study termination, hearts were snap-frozen in liquid nitrogen and stored at –80°C. RNA samples were prepared from left ventricular tissue and were extracted using RNeasy Microarray Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions. mRNA expression analysis of selected genes shown in Fig. 5 was performed by real-time quantitative reverse transcription-PCR using TaqMan probes (Applied Biosystems) with a Bio-Rad CFX96 machine. The mRNA levels of TASK-1 and TASK-3 were quantified to confirm that these genes were successfully knocked out in heart tissue. To evaluate the effects of TASK-1 and TASK-3 on heart fibrosis, mRNA levels of selected genes shown in Supplemental Fig. S3 [collagen type I α2-chain (Col1a2), Col3a1, and periostin (Postn); see https://doi.org/10.6084/m9.figshare.11326355] were quantified. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was selected as the endogenous control gene because of its stable level of expression. Relative gene expression was quantified according to 2−∆∆Ct (where Ct is threshold cycle). The following is a listing of the TaqMan primer sets utilized in experiments: Gapdh, Mm99999915; peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a), Mm01208835_m; carnitine palmitoyltransferase 1B (Cpt1b), Mm00487191_g1; acyl-CoA dehydrogenase medium chain (Acadm), Mm01323360_g1; peroxisome proliferator-activated receptor-α (Ppara), Mm00440939_m1; estrogen-related receptor-α (Esrra), Mm00433143_m1; nuclear respiratory factor 1 (Nrf1), Mm01135606_m1; potassium two-pore domain channel subfamily K member 2 (Kcnk2), Mm01323942_m; Postn, Mm00450111_m1; Col3a1, Mm01254476_m1; Col1a2, Mm00483888_m1; Kcnk3, Mm04213388_s1; Kcnk9, Mm02014295_s1; human GAPDH, Hs02786624_g1; human KCNK3, Hs00605529_m1; and human KCNK9, Hs04397239_s1.
Fig. 5.
Transcription factor and fatty acid oxidation cardiac gene expression with acute pressure overload. Average estrogen-related receptor-α (ERRα; A), nuclear receptor factor 1 (NRF1; B), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; C), peroxisome proliferator-activated receptor-α (PPARα; D), carnitine palmitoyltransferase 1 (CPT1; E), and medium-chain acyl-CoA dehydrogenase (MCAD; F) gene expression in cardiac left ventricular samples from wild-type and tandem pore domain acid-sensitive K+ channel 1 (TASK-1) global knockout (KO) mice after 3 days of Sham or transverse aortic constriction (TAC). Data are represented as fold change from Sham of the same genotype. Error bars represent means ± SE; n = no. of mice. Statistical comparisons with Sham, which has a theoretical mean of 1, were made with a one-sample two-tailed t-test, *P < 0.05 vs. Sham. Comparisons between TAC groups only were made using an unpaired two-tailed t-test, †P < 0.05 vs. wild-type TAC. Mean raw ΔCt values (where Ct is threshold cycle; test Ct value − housekeeping gene Ct value) ± SE. Comparisons between ΔCt values were made using a two-way ANOVA with Bonferroni’s test for multiple comparisons.
Cardiomyocyte isolation.
Left ventricular cardiomyocytes were isolated using a modified procedure described previously (64). In brief, heparin was injected intraperitoneally and euthanized after 5 min. The hearts were quickly harvested and kept in ice-cold Ca2+-free solution A, consisting of (in mM) 130 NaCl, 5.4 KCl, 2 NaHCO3, 1.2 MgSO4, 10 HEPES, and 15 glucose (pH 7.35). Aorta was cannulated and perfused in a Langendorff system. Hearts were initially perfused with solution A for 5 min to remove the blood, followed by solution B, comprised of solution A plus 10 mM 2,3-butanedione monoxime for an additional 8 min. Hearts were then perfused with 35 µM Ca2+ digestion buffer, comprised of solution B plus 35 µM CaCl2, 0.13 mg/mL protease XIV (Sigma-Aldrich), 0.2 mg/mL collagenase type II (Worthington, Freehold, NJ), and 1 mg/mL bovine serum albumin (BSA). After 5 min, the Ca2+ concentration was gradually increased to 1.6 μM by gradually adding 100 µM Ca2+ buffer, comprised of 100 µM solution B, plus 5 mg/mL BSA, every 5 min. After the hearts were palpably flaccid, the hearts were minced, filtered through a 250-µm mesh, and centrifuged (600 rpm, 3 min). The formed pellet was resuspended with solution D, comprised of solution A plus 1.6 mM Ca2+) and stored at room temperature for 1 h before the contractility measurement. This isolation method can result in a yield of 50–60% of calcium-tolerant cardiomyocytes from adult hearts.
Measurement of cardiomyocyte contractility.
To measure the in vitro cellular contractility, a video-based edge detection system (IonOptix, Dublin, Ireland) was employed to continuously measure the fresh isolated cardiomyocyte contractility function, including basal performance and in response to 2 µM isoproterenol (Sigma-Aldrich). Fresh isolated cardiomyocytes were plated on a cell stimulation chamber with a field electrode, in-line heater, and temperature probe to administer the temperature. The cells met the following criteria for the contractility evaluation: 1) rod shape and defined cell edges, 2) stable contractility at the 1-Hz pacing rate and 10 V for 15 min, 3) cell length between 100 and 120 µm, and 4) no contractility without stimulation. The basal contractility data were collected with a representative and reproducible contractile phenotype before isoproterenol was added. To evaluate the effects of isoproterenol on cardiomyocytes from distinct genotypes, isoproterenol was added to the cell stimulation chamber and incubated for 3 min at 37°C.
Statistical analysis.
Data are summarized as means ± SE. Statistical analysis was performed with the GraphPad Prism software package, version 6.0 (GraphPad). In human and mouse cardiac tissue gene expression studies, statistical comparisons with “Normal,” or the Sham condition, which has a theoretical mean of 1, were made with a one-sample t-test. Comparisons between two groups were made using a two-tailed t-test. When >2 groups were compared, a Shapiro–Wilk normality test was performed to assess for a normal distribution. If the parameters passed the normality test, a one-way ANOVA with Bonferroni’s multiple comparisons test was used. When serial measurements between two groups were compared, a two-way repeated measures ANOVA with Bonferroni’s multiple comparisons test was used. A value of P < 0.05 was considered statistically significant. Errors bars reflect means ± SE.
RESULTS
TASK channels are associated with cardiac hypertrophy and heart failure.
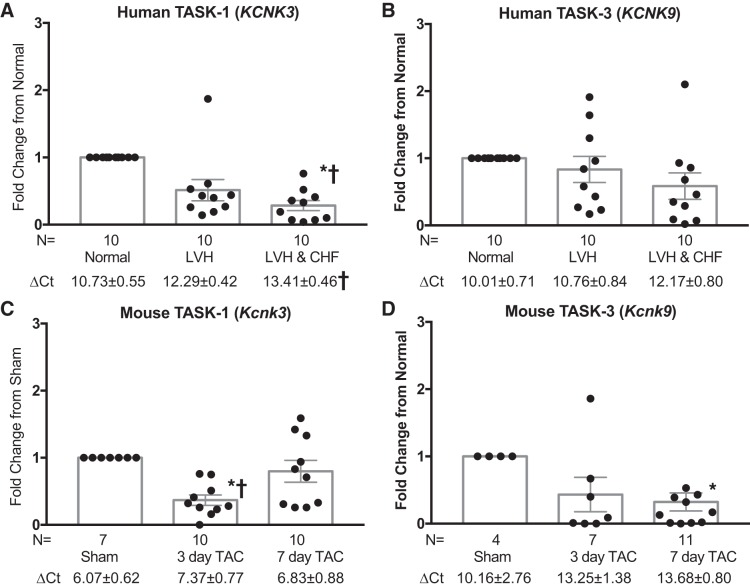
Prior studies show that both heart failure in animal models (58, 69) and atrial fibrillation in humans (60) are associated with a decline in TASK-1 gene expression. To assess whether left ventricular TASK channels are associated with human heart failure, we measured TASK-1 (KCNK3) and TASK-3 (KCNK9) gene expression in human and murine heart tissue samples. Left ventricular cardiac tissue was obtained from 1) normal subjects with neither left ventricular hypertrophy (LVH) nor congestive heart failure (CHF), 2) subjects with hypertension and LVH but no CHF, or 3) patients with CHF and LVH. TASK-1 gene expression was significantly reduced in subjects with LVH and CHF compared with normal subjects (Fig. 1A). A similar trend toward reduced TASK-3 gene expression with LVH and with both LVH and CHF was seen, although these differences were not statistically significant (Fig. 1B). In a mouse pressure overload model induced by TAC, wild-type mice had a significant reduction in TASK-1 and TASK-3 expression after TAC compared with Sham (Fig. 1, C and D). Of note, TASK-1 and TASK-3 gene expression was quantified in wild-type, TASK-1 KO, and TASK-3 KO mice. There was no TASK-3 and TASK-1 gene expression in TASK-3 KO and TASK-1 KO mice, respectively, confirming the knockout of these channels (Supplemental Fig. S1, B and C; see https://doi.org/10.6084/m9.figshare.11324507). Additionally, TREK-1 gene expression, another two-pore domain channel found in cardiac tissue, was largely unchanged after pressure overload in wild-type, TASK-1 KO, and TASK-3 KO mice (Supplemental Fig. S1A). There were no significant differences in TASK-1 channel expression in TASK-3 KO mice undergoing pressure overload and, conversely, TASK-3 channel expression in TASK-1 KO mice (Supplemental Fig. S1, B and C). Diastolic function in TASK-1 KO mice was similar to that of wild-type mice (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.11325569). These data suggest that left ventricular TASK-1 function is reduced in hypertensive cardiomyopathies in both humans and mice, whereas TASK-3 function appears to be reduced in mice alone.
Fig. 1.
Human and mouse tandem pore domain acid-sensitive K+ (TASK) channels are associated with cardiac hypertrophy and heart failure. TASK-1 (A and C) and TASK-3 (B and D) gene expression in human and murine heart tissues. Human tissue (A and B) was obtained from subjects 1) without cardiac hypertrophy or heart failure (Normal), 2) with cardiac hypertrophy but without heart failure [left ventricular hypertrophy (LVH)], and 3) with both cardiac hypertrophy and heart failure [left ventricular hypertrophy and congestive heart failure (LVH & CHF)]. Murine tissue (C and D) was harvested from mice 1) without transverse aortic constriction (TAC) treatment (Sham), 2) 3 days after TAC, and 3) 7 days after TAC. KCNK3 and KCNK9, potassium two-pore domain channel subfamily K members 3 and 9, respectively; n = no. of mice or human tissue samples. Data are represented as fold change from Normal or Sham condition. Statistical comparisons with Normal, which has a theoretical mean of 1, were made with a one-sample t-test, *P < 0.05 vs. Normal or Sham. An unpaired t-test was employed to compare the differences between different conditions, †P < 0.05 vs. LVH or 7-day TAC. Mean raw ΔCt values (where Ct is threshold cycle; test Ct value minus housekeeping gene Ct value) ± SE. Comparisons between ΔCt values were made using a one-way ANOVA with Bonferroni’s test for multiple comparisons, †P < 0.05 vs. Normal or Sham.
TASK-1 KO mice are resistant to pressure overload cardiomyopathy.
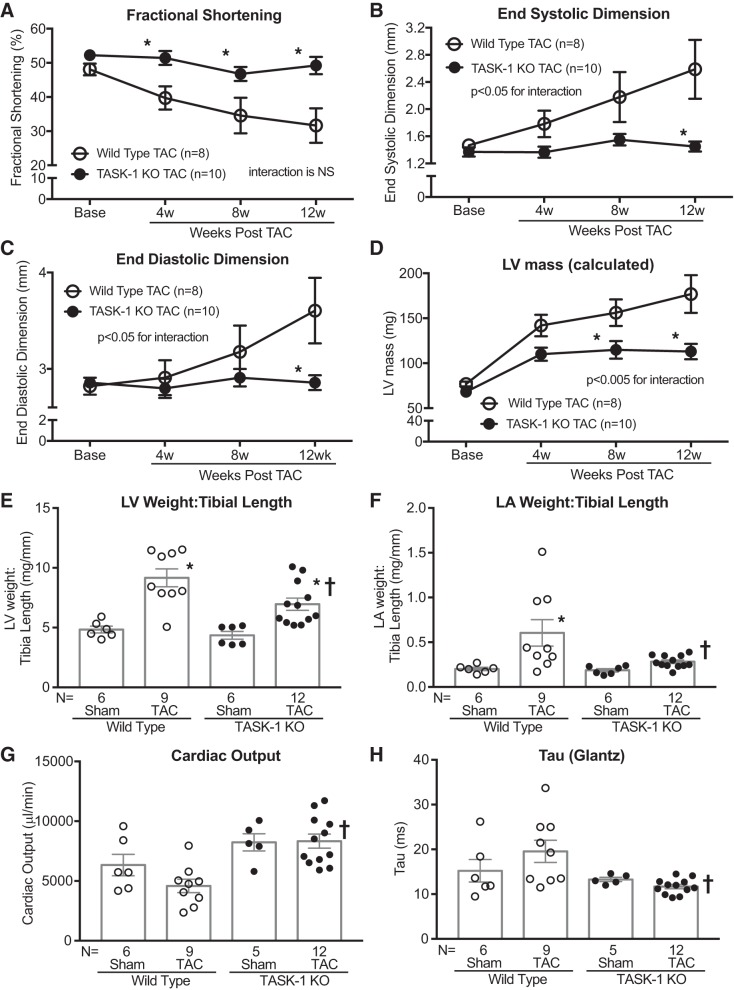
TASK-1 and TASK-3 channels are present in both human and mouse cardiac tissue (19, 21), and in humans both of these TASK channels have a high degree of sequence identity to corresponding mouse channels (69). To address whether TASK-1 is causal in the development of cardiac hypertrophy and failure, we tested whether the genetic loss of TASK-1 in mice (TASK-1 KO) affected cardiac hypertrophy development after pressure overload. In response to pressure overload, induced by TAC, TASK-1 KO mice demonstrate a preservation of systolic function, as evidenced by 1) higher fractional shortening (Fig. 2A) and mean rate-corrected velocity of circumferential shortening (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.11327201) and 2) lower end-systolic dimension (Fig. 2B) compared with wild-type mice. Moreover, TASK-1 KO mice developed significantly less ventricular dilation (Fig. 2C), concentric hypertrophy (Fig. 2D), and calculated LV mass (Supplemental Table S1) compared with wild-type mice after TAC. These noninvasive echocardiography findings were supported by heart weight data, which demonstrate a relative diminution of left ventricular weights and left atrial weights in TASK-1 KO mice compared with wild type after 12 wk of TAC (Fig. 2, E and F, and Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.11327363). Of note, right ventricle and right atrial weights were not significantly different between genotypes (Supplemental Table S2).
Fig. 2.
Tandem pore domain acid-sensitive K+ channel 1 (TASK-1) global knockout (KO) mice are resistant to pressure overload cardiomyopathy. Serial echocardiographic measurements of average %fractional shortening [(end-diastolic dimension – end-systolic dimension)/end-diastolic dimension × 100; A], average end-systolic dimension (mm; B), average end-diastolic dimension (mm; C), and average calculated LV mass (mg; D) in TASK-1 KO and wild-type mice at baseline (Base) and up to 12 wk after transverse aortic constriction (TAC). Error bars reflect means ± SE; n = no. of mice. Statistical comparisons between wild-type TAC and TASK-1 KO TAC data were made using two-way repeated measures ANOVA. The P values for the interaction between genotype and weeks post-TAC are shown. Comparisons between genotypes at each time point were made using Bonferroni’s test for multiple comparisons, *P < 0.05 vs. wild-type TAC at each time point. NS, not significant. Average left ventricular (LV) weight-to-tibia length ratio (E) and average left atrial (LA) weight-to-tibia length ratio (F) after Sham or TAC in wild-type and TASK-1 KO mice. Invasively assessed average cardiac output (μL/min; G) and time constant of left ventricular isovolumic relaxation (τ) calculated by the Glantz method (ms; H) after Sham or TAC in wild-type and TASK-1 KO mice. Statistical comparisons between groups in both chamber weights and invasive hemodynamics were made using one-way ANOVA with Bonferroni’s test for multiple comparisons, *P < 0.05 vs. wild-type Sham, †P < 0.05 vs. wild-type TAC.
To further assess the effect of TASK-1 on cardiac function after TAC, we performed pressure-volume loop analyses on wild-type and TASK-1 KO mice after 12 wk of Sham or TAC. Both wild-type and TASK-1 KO mice had similar degrees of pressure overload from TAC as measured by transstenotic or TAC gradient (54 ± 6.8 vs. 46 ± 6.0 mmHg, respectively; Table 1). In contrast to the wild-type TAC response, TASK-1 KO mice had preserved cardiac systolic function after TAC, specifically stroke volume (wild type 13 ± 1.6 vs. TASK-1 KO 20.0 ± 1.6 μL), cardiac output (wild type 4,588 ± 561 vs. TASK-1 KO 8,331 ± 587 μL/min), and stroke work (wild type 1,427 ± 254 vs. TASK-1 KO 2,621 ± 268 mmHg·μL; Fig. 2G and Table 1). Similar to systolic function, diastolic function was also preserved in TASK-1 KO mice after TAC (Fig. 2H and Table 1). Load-independent measures of contractility [end-systolic pressure-volume relationship (ESPVR), maximum slope of quadratic ESPVR (E′max), and maximal elastance (Emax)] and compliance [end-diastolic pressure-volume relationship (EDPVR)] were largely similar between genotypes (Table 1). Similar to the in vivo hemodynamic assessment of contractility, in vitro isolated LV cardiomyocyte contractility in wild-type and TASK-1 KO mice exposed to 4 wk of TAC was no different after treatment with either vehicle or isoproterenol (Supplemental Fig. S5; see https://doi.org/10.6084/m9.figshare.11325983). Of note, LV systolic blood pressures and arterial elastance were largely similar between genotypes, suggesting that the loss of TASK-1 did not have an effect on blood pressure and peripheral vascular tone. These data show that the absence of TASK-1 has a protective effect against pressure overload-induced cardiomyopathy.
Table 1.
TASK-1 global knockout TAC experiment hemodynamics
| Wild Type |
TASK-1 KO |
|||
|---|---|---|---|---|
| Sham | TAC | Sham | TAC | |
| n | 6 | 8–9 | 5 | 12 |
| TAC gradient, mmHg | NA | 54 ± 6.8 | NA | 46 ± 6.0 |
| Heart rate, beats/min | 397 ± 35 | 362 ± 24 | 443 ± 19 | 415 ± 24 |
| LVESP, mmHg | 131 ± 18 | 164 ± 15 | 125 ± 11 | 171 ± 11 |
| LVEDP, mmHg | 7.3 ± 2.2 | 6.8 ± 0.8 | 5.4 ± 0.4 | 5.9 ± 0.7 |
| LVESV, μL | 15 ± 3.0 | 27 ± 4.5 | 18 ± 3.4 | 13 ± 3.2* |
| LVEDV, μL | 31 ± 3.9 | 40 ± 3.6 | 37 ± 3.9 | 34 ± 2.8 |
| Arterial elastance, mmHg/μL | 8.6 ± 1.8 | 14.0 ± 2.5 | 6.6 ± 0.8 | 8.0 ± 0.93* |
| Systolic function parameters | ||||
| Stroke volume, μL | 16 ± 1.9 | 13 ± 1.6 | 19 ± 1.9 | 20.0 ± 1.6* |
| Ejection fraction, % | 55 ± 7.9 | 36 ± 7.5 | 52 ± 6.1 | 65.0 ± 6.6* |
| Cardiac output, μL/min | 6,334 ± 894 | 4,588 ± 561 | 8,232 ± 722 | 8,331 ± 587* |
| Stroke work, mmHg·μL | 1,555 ± 306 | 1,427 ± 254 | 1,672 ± 214 | 2,621 ± 268* |
| dP/dtmax, mmHg/s | 10,142 ± 1,978 | 6,718 ± 735 | 9,359 ± 570 | 9,289 ± 592 |
| ESPVR linear, mmHg/μL | 5.0 ± 0.8 | 6.1 ± 1.0 | 5.2 ± 1.4 | 5.9 ± 0.6 |
| ESPVR quadratic E′max, mmHg/μL | 14.0 ± 4.6 | 12.0 ± 1.9 | 13.0 ± 2.0 | 16.0 ± 1.3 |
| PRSW, mmHg | 52.0 ± 6.2 | 67.0 ± 8.5 | 54.0 ± 7.2 | 83.0 ± 6.3 |
| dP/dtmax vs. EDV, mmHg·s−1·μL−1 | 280 ± 120 | 201 ± 33 | 284 ± 85 | 225 ± 36 |
| Emax, mmHg/μL | 11.0 ± 3.7 | 9.2 ± 1.5 | 11.0 ± 3.4 | 9.7 ± 0.8 |
| Diastolic function parameters | ||||
| dP/dtmin, mmHg/s | 8,101 ± 1,645 | −6,137 ± 713 | −7,547 ± 900 | −8,479 ± 521 |
| τ (Weiss), ms | 9.1 ± 1.0 | 11.0 ± 0.9 | 8.6 ± 0.6 | 7.3 ± 0.4* |
| τ (Glantz), ms | 15.0 ± 2.5 | 20.0 ± 2.5 | 13.0 ± 0.5 | 12.0 ± 0.5* |
| τ (log), ms | 7.4 ± 0.8 | 9.2 ± 0.9 | 6.8 ± 0.2 | 6.0 ± 0.3* |
| dV/dtmin, μL/s | −431 ± 45 | −349 ± 46 | −564 ± 49 | −576 ± 64* |
| EDPVR linear, mmHg/μL | 0.30 ± 0.06 | 0.39 ± 0.10 | 0.23 ± 0.06 | 0.27 ± 0.04 |
| β-Coefficient | 0.11 ± 0.04 | 0.13 ± 0.03 | 0.06 ± 0.01 | 0.08 ± 0.01 |
Values are means ± SE; n = no. of mice. Average load-dependent and load-independent hemodynamic parameters obtain invasive pressure-volume loop analyses in wild-type and tandem pore domain acid-sensitive K+ channel 1 (TASK-1) global knockout (KO) mice that underwent either a sham procedure (Sham) or pressure overload using transverse aortic constriction (TAC). dP/dtmax and dP/dtmin, maximum and minimum dP/dt, respectively; dV/dtmin, minimum dV/dt; EDPVR, end-diastolic pressure-volume relationship; EDV, end-diastolic volume; Emax, maximal elastance; E′max, maximum slope of quadratic end-systolic pressure-volume relationship (ESPVR); LVEDP, left ventricular end-diastolic pressure; LVEDV, left ventricular end-diastolic volume; LVESP, left ventricular end-systolic pressure; LVESV, left ventricular end-systolic volume; NA, not applicable; PRSW, preload recruitable stroke work; τ, time constant of left ventricular isovolumic relaxation. Statistical comparisons between TAC gradients were made using a 2-tailed unpaired t-test, and all others were made using a 1-way ANOVA with Bonferroni’s test for multiple comparisons.
P < 0.05 vs. wild-type TAC.
TASK-3 KO delays pressure overload cardiomyopathy.
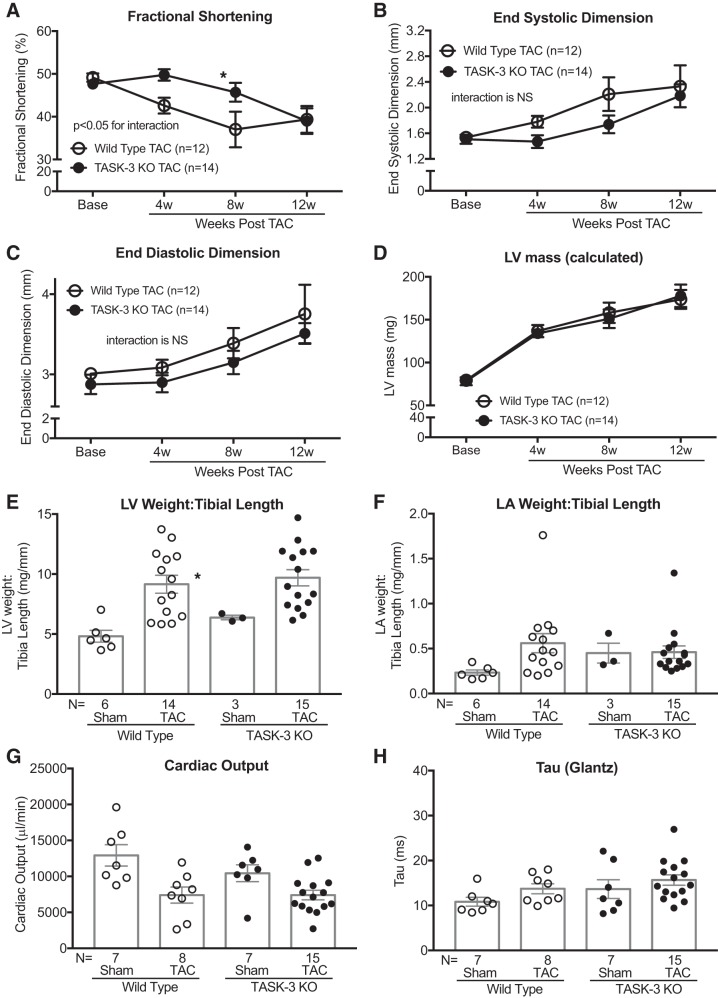
TASK-1 and TASK-3 channels have significant sequence similarities and are known to form functional heterodimers in cardiac tissue (10, 54); however, the role of TASK-1 and TASK-3 heterodimers in cardiac function is not known. To investigate whether the loss of TASK-3 alone or the combined loss of TASK-1 and TASK-3 affects cardiac function, we measured cardiac function and morphology in TASK-3 KO and TASK-1/3 double KO (DKO) mice in two complementary cardiac injury models: TAC and aging. At baseline, TASK-3 KO mice show enhanced wall thickness (posterior wall thickness of wild type 0.91 ± 0.02 mm vs. TASK-3 KO 1.10 ± 0.04 mm) and concentric remodeling (relative wall thickness of wild type 0.62 ± 0.03 vs. TASK-3 KO 0.76 ± 0.05), whereas cardiac function was largely similar to that of wild-type mice (Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.11327615). In response to TAC, TASK-3 KO mice have a relative improvement in systolic function at 4 and 8 wk that dissipates by 12 wk post-TAC as determined by serial echocardiography (Fig. 3, A and B, and Supplemental Table S3). Additionally, wild-type and TASK-3 KO mice showed largely similar TAC-induced LV dilation and cardiac hypertrophy (Fig. 3, C and D, and Supplemental Table S3). These echocardiographic findings were supported by largely similar cardiac chamber weights after exposure to either the Sham or TAC condition (Fig. 3, E and F, and Supplemental Table S4; see https://doi.org/10.6084/m9.figshare.11327822). Fibrosis gene transcription was largely similar in TASK-1 and TASK-3 KO mice compared with wild type (Supplemental Fig. S3) suggesting that the fibrotic response was largely similar between these genotypes. Hemodynamic measures of both load-dependent and load-independent systolic and diastolic function were largely similar between wild-type and TASK-3 KO mice after 12 wk TAC (Fig. 3, G and H, and Table 2). These data suggest that TASK-3 has an effect on basal cardiac morphology and a minor effect on the progression of TAC-induced cardiomyopathy.
Fig. 3.
Tandem pore domain acid-sensitive K+ channel 3 (TASK-3) global knockout (KO) mice develop a delayed pressure overload cardiomyopathy. Serial echocardiographic measurements of average %fractional shortening [(end-diastolic dimension – end-systolic dimension)/end-diastolic dimension × 100; A], average end-systolic dimension (mm; B), average end-diastolic dimension (mm; C), and average calculated LV mass (mg; D) in TASK-3 KO and wild-type mice at baseline (Base) and up to 12 wk after transverse aortic constriction (TAC). Error bars reflect means ± SE; n = no. of mice. Statistical comparisons between wild-type TAC and TASK-3 KO TAC data were made using two-way repeated measures ANOVA. The P values for the interaction between genotype and weeks post-TAC are shown. Comparisons between genotypes at each time point were made using Bonferroni’s test for multiple comparisons, *P < 0.05 vs. wild-type TAC at each time point. NS, not significant. Average left ventricular (LV) weight-to-tibia length ratio (E) and average left atrial (LA) weight-to-tibia length ratio (F) after Sham or TAC in wild-type and TASK-3 KO mice. Invasively assessed average cardiac output (μL/min; G) and time constant of left ventricular isovolumic relaxation (τ) calculated by the Glantz method (ms; H) after Sham or TAC in wild-type and TASK-3 KO mice. Statistical comparisons between groups in both chamber weights and invasive hemodynamics were made using one-way ANOVA with Bonferroni’s test for multiple comparisons, *P < 0.05 vs. wild-type Sham.
Table 2.
TASK-3 global knockout TAC experiment hemodynamics
| Wild Type |
TASK-3 KO |
|||
|---|---|---|---|---|
| Sham | TAC | Sham | TAC | |
| n | 7 | 8 | 7 | 13–15 |
| TAC gradient, mmHg | NA | NA | 67 ± 12.0 | 77 ± 8.7 |
| Heart rate, beats/min | 503 ± 35 | 397 ± 15* | 520 ± 36 | 402 ± 15* |
| LVESP, mmHg | 105 ± 9 | 188 ± 14* | 103 ± 13 | 172 ± 12* |
| LVEDP, mmHg | 13.0 ± 2.8 | 5.3 ± 1.9 | 11.0 ± 1.3 | 13.0 ± 1.5 |
| LVESV, μL | 11.0 ± 2.8 | 26.0 ± 6.1 | 33.0 ± 5.3 | 35.0 ± 5.4 |
| LVEDV, μL | 36.0 ± 3.4 | 46.0 ± 7.2 | 54.0 ± 6.8 | 55.0 ± 5.3 |
| Arterial elastance, mmHg/μL | 3.9 ± 0.5 | 13.0 ± 4.0 | 5.2 ± 0.6 | 11.0 ± 1.9 |
| Systolic function parameters | ||||
| Stroke volume, μL | 25 ± 1.6 | 19 ± 3.1 | 21 ± 3.1 | 19 ± 2.2 |
| Ejection fraction, % | 72 ± 5.2 | 46 ± 7.4* | 40 ± 3.9* | 39 ± 5.3 |
| Cardiac output, μL/min | 2,928 ± 1,487 | 7399 ± 1,111* | 10,440 ± 1,171 | 7,407 ± 670 |
| Stroke work, mmHg·μL | 1,992 ± 122 | 2,550 ± 488 | 1,712 ± 438 | 2,133 ± 206 |
| dP/dtmax, mmHg/s | 8,309 ± 859 | 9,032 ± 714 | 7,573 ± 1,079 | 7,771 ± 472 |
| ESPVR linear, mmHg/μL | 4.2 ± 0.8 | 5.5 ± 1.2 | 5.3 ± 2.1 | 7.2 ± 1.8 |
| ESPVR quadratic E′max, mmHg/μL | 11.0 ± 1.5 | 13.0 ± 2.4 | 15.0 ± 4.1 | 14.0 ± 3.5 |
| PRSW, mmHg | 61.0 ± 5.5 | 59.0 ± 8.9 | 51.0 ± 6.9 | 54.0 ± 6.2 |
| dP/dtmax vs. EDV, mmHg·s−1·μL−1 | 177 ± 42 | 187 ± 45 | 194 ± 48 | 145 ± 20 |
| Emax, mmHg/μL | 7.5 ± 0.9 | 8.9 ± 2.0 | 9.9 ± 2.6 | 10.0 ± 2.5 |
| Diastolic function parameters | ||||
| dP/dtmin, mmHg/s | −8,580 ± 475 | −9,677 ± 737 | −7,162 ± 915 | −7,485 ± 525 |
| τ (Weiss), ms | 6.4 ± 0.6 | 7.9 ± 0.5 | 8.0 ± 0.6 | 8.9 ± 0.4 |
| τ (Glantz), ms | 11.0 ± 1.0 | 14.0 ± 1.1 | 14.0 ± 2.1 | 16.0 ± 1.2 |
| τ (log), ms | 5.3 ± 0.5 | 6.8 ± 0.5 | 5.9 ± 0.7 | 7.3 ± 0.4 |
| dV/dtmin, μL/s | −764 ± 59 | −493 ± 72 | −713 ± 86 | −649 ± 78 |
| EDPVR linear, mmHg/μL | 0.18 ± 0.04 | 0.43 ± 0.06* | 0.38 ± 0.04 | 0.46 ± 0.06 |
| β-Coefficient | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.01 |
Values are means ± SE; n = no. of mice. Average load-dependent and load-independent hemodynamic parameters obtain invasive pressure-volume loop analyses in wild-type and tandem pore domain acid-sensitive K+ channel 3 (TASK-3) global knockout (KO) mice that underwent either a sham procedure (Sham) or pressure overload using transverse aortic constriction (TAC). dP/dtmax and dP/dtmin, maximum and minimum dP/dtmin, respectively; dV/dtmin, minimum dV/dt; EDPVR, end-diastolic pressure-volume relationship; EDV, end-diastolic volume; Emax, maximal elastance; E′max, maximum slope of quadratic end-systolic pressure-volume relationship; ESPVR, end-systolic pressure-volume relationship; LVEDP, left ventricular end-diastolic pressure; LVEDV, left ventricular end-diastolic volume; LVESP, left ventricular end-systolic pressure; LVESV, left ventricular end-systolic volume; PRSW, preload recruitable stroke work; τ, time constant of left ventricular isovolumic relaxation. Statistical comparisons between TAC gradients were made using a 2-tailed unpaired t-test, and all others were made using a 1-way ANOVA with Bonferroni’s test for multiple comparisons.
P < 0.05 vs. wild-type TAC.
TASK-1 influences AKT phosphorylation and peroxisome proliferator-activated receptor-γ coactivator-1α expression.
Key signaling mechanisms involving mitogen-activated protein kinases (MAPKs) and AKT have been shown to be critical regulators of the pressure overload response and hypertrophic growth (26). To address the mechanism by which TASK channels influence hypertrophic growth, we tested relative levels of phosphorylation of AKT, ERK1/2, and p38α at 3 and 7 days after TAC. After TAC, TASK-1 KO mice developed a rise in AKT phosphorylation, often associated with adaptive or physiologic cardiac hypertrophy (17), that was significantly greater than observed in wild-type or TASK-3 KO mice 3 days after TAC (Fig. 4). Phosphorylated p38a signaling was largely similar among the genotypes, whereas TASK-1 KO demonstrated enhanced ERK phosphorylation compared with wild type (Supplemental Fig. S4; see https://doi.org/10.6084/m9.figshare.11326748). Similar to AKT and ERK, the coactivation of the transcription factor peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is enhanced in physiologic cardiac hypertrophy (66). Both PGC-1α and peroxisome proliferator-activated receptor-α (PPARα) gene expression is depressed in wild-type mice after TAC; the expression of PPARα was decreased in TASK-1 KO mice after TAC, whereas the expression of PGC-1α is preserved (Fig. 5, C and D). Other known PGC-1α transcriptional coactivators, estrogen-related receptor (ERRa) and nuclear receptor factor 1 (NRF-1), were upregulated in TASK-1 KO mice post-TAC versus wild-type mice post-TAC (Fig. 5, A and B). Fatty acid oxidation-specific genes [carnitine palmitoyltransferase 1 (CPT1) and medium-chain acyl-CoA dehydrogenase (MCAD)] were depressed in wild-type mice after TAC, whereas the expression was greater in TASK-1 KO mice after TAC versus wild-type mice after TAC and preserved compared with wild-type Sham mice (Fig. 5, E and F), suggesting that deficiency of TASK-1 maintained the energy substrate utilization. These data show that the cardioprotective effects of TASK-1 loss of function are associated with both enhanced AKT phosphorylation and preserved PGC-1α transcription factor and fatty acid oxidation gene expression, which are associated with adaptive hypertrophy and preservation of cardiac function.
Fig. 4.
Tandem pore domain acid-sensitive K+ channel 1 (TASK-1) global knockout (KO) mice have enhanced cardiac AKT phosphorylation. Immunoblot analysis results showing the phosphorylation of AKT in cardiac left ventricular samples obtained from wild-type, TASK-1 KO, and TASK-3 KO mice that have undergone a Sham procedure (Sh) or 3 days post-transverse aortic constriction (TAC) procedure (3D) and 7 days post-TAC procedure (7D). Average phosphorylation (phosphorylated protein/total protein) is expressed as fold change from wild-type Sham. Quantified average phosphorylation is shown at in panel at top, and representative Western blots are shown in panel at bottom. Error bars reflect means ± SE; n = no. of mice. Statistical comparisons were made using two-way repeated measures ANOVA. The P values for the interaction between genotype and days post-TAC are shown. Comparisons between genotypes at each time point were made using Bonferroni’s test for multiple comparisons, *P < 0.05 vs. wild type, †P < 0.05 vs. TASK-3 at each time point.
TASK-1’s effect on cardiac energetics and fatty acid oxidation.
We measured the functional outcomes of fatty acid oxidation-specific gene expression using two complementary approaches. First, we assessed measurements of cardiac energetics using pressure-volume loop analysis. Cardiac efficiency, which quantifies the ratio of cardiac work to myocardial oxygen consumption (34, 68), declines in wild-type mice after TAC but is maintained in TASK-1 KO mice after TAC (Fig. 6A). Potential energy is increased in wild-type mice after TAC and is relatively unchanged in TASK-1 and TASK-3 mice after TAC, suggesting that TAC causes less available energy to be used for cardiac work in wild-type mice alone (Fig. 6B). Pressure-volume area (PVA), which is directly related to myocardial oxygen consumption, is not significantly different among the genotypes; however, there is a trend toward increased PVA in wild-type mice and decreased PVA in TASK-1 KO mice after TAC (Fig. 6C). Second, we measured long-chain acylcarnitine expression by mass spectrometry in wild-type and TASK-1 KO mice after 7 days of Sham or TAC. Both C16 and C18 long-chain acylcarnitine expression was enhanced in TASK-1 KO mice after TAC (Fig. 6D), suggesting enhanced fatty acid oxidation in TASK-1 KO mice after TAC. These findings support the proposition that TASK-1 influences both cardiac energetics and fatty acid oxidation and are functional outcomes of both enhanced AKT and PGC-1α activity in TASK-1 KO mice after TAC.
Fig. 6.
Tandem pore domain acid-sensitive K+ channel 1 (TASK-1) effect on cardiac energetics and fatty acid oxidation. Average cardiac efficiency (A), average potential energy (B), and average pressure-volume area (C) were calculated from pressure-volume loops obtained on wild-type, TASK-1 global knockout (KO), and TASK-3 KO mice after 12 wk of either Sham or transverse aortic constriction (TAC) procedure. Error bars reflect means ± SE; n = no. of mice. Statistical comparisons were made using two-way repeated measures ANOVA. Statistically significant P values for the interaction between genotype and treatment conditions are shown. Comparisons between genotypes at each time point were made using Bonferroni’s test for multiple comparisons, *P < 0.05 vs. Sham state, †P < 0.05 vs. wild-type TAC, and ‡P < 0.05 vs. TASK-3 KO TAC. Heat map (D) showing C16 and C18 long-chain acylcarnitine in wild-type (WT) and TASK-1 KO mice after 7 days of either Sham or TAC procedure. Enhanced long-chain acylcarnitine expression is observed in the TASK-1 KO hearts after TAC. Scale bar indicates acylcarnitine concentration in μM. For this experiment, the following numbers of mice were used: n = 8 wild-type Sham, n = 8 wild-type TAC, n = 6 TASK-1 KO Sham, and n = 3 TASK-1 KO TAC.
DISCUSSION
There is a growing body of literature recognizing an association between TASK channel function and heart failure; however, the mechanistic relationship between these entities is unclear. Here we show that human TASK-1 channel gene expression is depressed in failing human hearts. In genetic loss-of-function studies, TASK-1 KO mice are protected from pressure overload-induced cardiomyopathy. Specifically, TASK-1 KO mice have a relative abrogation in concentric left ventricular hypertrophy development and preservation of systolic and diastolic function compared with wild-type mice. Mechanistically, we show that the loss of TASK-1 both enhances AKT phosphorylation and preserves fatty acid oxidation-specific gene expression (CPT1 and MCAD), which are hallmarks of adaptive or physiologic hypertrophy. Functionally, these changes are associated with preserved cardiac energetics and long-chain acylcarnitine expression and suggest that the protective effect of TASK-1 loss of function is through improved cardiac metabolic function.
Physiologic cardiac hypertrophy describes a distinct form of cardiac remodeling that is associated with preserved cardiac function and is linked with activation of the serine/threonine protein kinase, AKT (66). The loss of both AKT and upstream phosphatidylinositol 3-kinase (PI3K) function has been shown to hinder the development of exercise-induced physiologic hypertrophy (17, 41). Conversely, PI3K gain of function, upstream of AKT, protects against the development of pressure overload cardiomyopathy (41). Although these studies suggest that cardiac AKT activation is largely beneficial, the beneficial effects of AKT activation are dependent on timing. Prolonged AKT activation induces severe cardiac hypertrophy and dysfunction in genetic mouse models, whereas short-term AKT activation induces physiologic cardiac hypertrophy (63). In light of these studies, the short-term rise in AKT phosphorylation, stimulated within the first 7 days after pressure overload injury, appears to mitigate cardiac damage (7, 45). This compensatory rise in AKT phosphorylation, previously shown to be dependent on G protein β-γ subunit binding to the catalytic domain of PI3K (45), is heightened in pressure-overloaded TASK-1 KO mice. This finding in TASK-1 KO mice suggests a molecular process whereby, under conditions of pressure overload, TASK-1 inhibits AKT phosphorylation. This relationship is hinted at by recent work showing that AKT overexpression inhibits TASK-1 and TASK-3 current by enhancing TASK channel endocytosis, thus highlighting cross talk between PI3K-AKT pathways and TASK channel function (55). However, the precise molecular signaling mechanism linking TASK channel and AKT function is unknown and will need to be assessed in future studies.
Both cardiac hypertrophy and dysfunction are often driven by systemic hypertension, which is robustly associated with TASK channel function. Genetic variations in human TASK-1 have been associated with hypertension in European, Hispanic, Asian, and African American populations in genome-wide association studies (23, 24, 29, 35). The association between TASK-1 function and systemic hypertension is thought to be mediated by alterations in circulating aldosterone, demonstrated in both human populations (40) and preclinical models. Early preclinical studies have suggested that female TASK-1 KO mice have increased serum aldosterone due to perturbations in adrenal gland zonation (27). These zonation abnormalities were not demonstrated in a follow-up study, which showed that the loss of TASK-1 and TASK-3 function enhanced resting membrane potentials and membrane depolarization in the aldosterone-producing portion of the adrenal gland, called the adrenal zona glomerulosa, resulting in increased aldosterone secretion (16). Additionally, aldosterone secretion is influenced by TASK-3-like channels within the mitochondrial membrane of adrenal glomerulosa cells, where they modulate mitochondrial membrane potentials and enhance aldosterone release (71). Elevated serum aldosterone levels have known cardiovascular consequences, including 1) increased systemic blood pressures, 2) increased circulating blood volume, and 3) cardiac diastolic dysfunction. Although we did not directly measure serum aldosterone levels, our data suggest that the effect of TASK channels on aldosterone secretion alone does not explain our findings. First, invasively measured systolic blood pressures [left ventricular end-systolic pressure (LVESP)], end-diastolic volumes [left ventricular end-diastolic volume (LVEDV)], and measures of early [time constant of left ventricular isovolumic relaxation (τ)] and late diastolic filling (EDPVR) in sham-treated TASK-1 KO and TASK-3 KO mice were not significantly different from those of wild-type mice (Tables 1 and 2). Second, sham-treated TASK-1 and TASK-3 KO mice have left atrial and left ventricular weights that are similar to wild type, further supporting that systemic hypertension is not present in these mice (Supplemental Tables S2 and S4). Our findings are consistent with prior work showing that TASK-1 KO mice in a basal state do not exhibit either cardiac hypertrophy or concentric remodeling (18). We did not identify sex-specific differences in cardiac hypertrophy or diastolic function in TASK-1 or TASK-3 KO mice compared with wild type (Supplemental Fig. S2). Our findings suggest that both TASK-1 and TASK-3 channels influence the cardiac response to pressure overload independent of systemic hypertension.
Emerging studies have identified TASK channels as regulators of metabolic function, although the relevance of these metabolic effects on cardiac function is not well understood. Similar to genetic association studies investigating hypertension, genome-wide association studies have suggested a link between body weight and TASK channels (28). Mechanistically, TASK channels have been found to regulate both thermogenesis and glucose metabolism through distinct molecular signaling mechanisms. TASK-1 is highly expressed in pancreatic α- and β-cells (5) and has distinct effects on both pancreatic cell types. Pancreatic α-cells produce the hormone glucagon, which promotes gluconeogenesis and glycogenolysis, under the control of intracellular calcium flux that is inhibited by TASK-1 channel activity (14). Similarly, TASK-1 channels influence pancreatic β-cells’ secretion of insulin through their effect on resting membrane potentials (15), further supporting a key role of TASK channels in glucose metabolism. Apart from altering glucose metabolism, TASK channels are now known to be key regulators of thermogenesis and are present in brown adipose tissue. Human brown adipose tissue expresses high levels of TASK-1, which promotes differentiation of white adipose to brown adipose tissue (62). Mechanistic studies have revealed that TASK-1 current inhibits brown adipose cell membrane depolarization and intracellular calcium entry, which hinders adrenergically mediated thermogenesis and lipolysis (9, 51). Consequently, the loss of TASK-1 function results in enhanced lipolysis and reduced body weight (9). Recent published studies show that TASK channels in intestinal afferent neurons affect appetite and satiety (50). In our present study, we show that the loss of TASK-1 is associated with the preservation of CPT1 and MCAD gene expression, which serves as a surrogate for fatty acid oxidation. These data raise the intriguing possibility that TASK channels may affect cardiac injury response through their influence of metabolic function. Insulin receptor activation and resultant AKT activation, seen in TASK-1 KO mice after TAC, are known molecular signaling events in the development of physiologic hypertrophy (26). Future studies will be needed to assess how TASK channels influence the activation of AKT and whether TASK-1 channels in adipose or pancreatic tissue provide a layer of extracardiac protection from cardiac injury.
In this study, we show an association between TASK-1 gene expression and the development of human heart failure. In both the human and mouse tissue, TASK-1 gene expression decreases in hypertrophied/nonfailing human heart tissue and within 3 days following pressure overload. However, TASK-1 gene expression is further depressed in hypertrophied/failing human heart tissue, whereas gene expression rises, 7 days after pressure overload. Our experimental data suggest that the global loss of TASK-1 is cardioprotective; thus our interpretation is that the initial reduction in TASK-1 in early pressure overload and human LVH reflects a compensatory response to injury. Whereas this compensation response is abrogated in wild-type mice, it remains present in the human tissue. Although conjecture, this difference may be explained by differences in 1) mouse versus human cardiac metabolic responses or 2) the type of cardiac injury (pressure overload in mice vs. multiple causes of injury in humans). In a pressure overload mouse model, the loss TASK-1 function inhibits the development of cardiac hypertrophy and preserves cardiac function. These findings suggest that TASK channels may influence cardiac signaling either by their electrophysiologic properties or through more indirect involvement in AKT activation, such as cross talk with GPCR and PI3K-mediated pathways. Our study provides a theoretical framework to target TASK channels therapeutically for cardiac dysfunction. However, there remain unanswered questions. There are a number of distinct mechanisms underlying pressure overload hypertrophy (47) that we do not fully interrogate in this paper, which raises alternative possibilities of how TASK-1 may influence pressure overload cardiomyopathy. Additionally, we used global TASK channel KO mice in this study to investigate the effects of TASK channels on cardiac function. Given the effects of these channels on noncardiac organ function, targeted disruption of TASK channels in cardiac tissue using conditional KO mice would help to address this possibility. Future studies investigating the influence of TASK current on PI3K pathways will be needed to address this gap in our understanding.
GRANTS
This work was supported by the National Institutes of Health Grant 1K08-HL-125905-01 (to D. M. Abraham).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M.A. conceived and designed research; J.H., O.I., and D.M.A. performed experiments; M.A.M., O.I., and D.M.A. analyzed data; W.D. and D.M.A. interpreted results of experiments; D.M.A. prepared figures; D.M.A. drafted manuscript; W.D., J.H., M.A.M., O.I., and D.M.A. edited and revised manuscript; D.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Duke Human Heart Repository for supplying the cardiac specimens used in this study. Additionally, we thank the Duke Cardiovascular Physiology Core and the Mandel Center for Hypertension and Atherosclerosis for support in all mouse cardiovascular phenotyping. We thank Daniel Raftis for insightful comments and manuscript edits. We appreciate Dr. Howard Rockman’s mentorship and comments on this manuscript.
REFERENCES
- 1.Abraham D, Mao L. Cardiac pressure-volume loop analysis using conductance catheters in mice. J Vis Exp 103: e52942, 2015. doi: 10.3791/52942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham DM, Lee TE, Watson LJ, Mao L, Chandok G, Wang HG, Frangakis S, Pitt GS, Shah SH, Wolf MJ, Rockman HA. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J Clin Invest 128: 4843–4855, 2018. doi: 10.1172/JCI95945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antigny F, Hautefort A, Meloche J, Belacel-Ouari M, Manoury B, Rucker-Martin C, Péchoux C, Potus F, Nadeau V, Tremblay E, Ruffenach G, Bourgeois A, Dorfmüller P, Breuils-Bonnet S, Fadel E, Ranchoux B, Jourdon P, Girerd B, Montani D, Provencher S, Bonnet S, Simonneau G, Humbert M, Perros F. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation 133: 1371–1385, 2016. doi: 10.1161/CIRCULATIONAHA.115.020951. [DOI] [PubMed] [Google Scholar]
- 4.Barbuti A, Ishii S, Shimizu T, Robinson RB, Feinmark SJ. Block of the background K+ channel TASK-1 contributes to arrhythmogenic effects of platelet-activating factor. Am J Physiol Heart Circ Physiol 282: H2024–H2030, 2002. doi: 10.1152/ajpheart.00956.2001. [DOI] [PubMed] [Google Scholar]
- 5.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 123: 1275–1284, 2013. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol 157: 55–64, 2007. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ 14: 1060–1062, 2007. doi: 10.1038/sj.cdd.4402095. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein α-subunits. Proc Natl Acad Sci U S A 103: 3422–3427, 2006. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Zeng X, Huang X, Serag S, Woolf CJ, Spiegelman BM. Crosstalk between KCNK3-mediated ion current and adrenergic signaling regulates adipose thermogenesis and obesity. Cell 171: 836–848.e13, 2017. doi: 10.1016/j.cell.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czirják G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem 277: 5426–5432, 2002. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- 12.Czirják G, Fischer T, Spät A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol 14: 863–874, 2000. doi: 10.1210/me.14.6.863. [DOI] [PubMed] [Google Scholar]
- 13.Czirják G, Petheo GL, Spät A, Enyedi P. Inhibition of TASK-1 potassium channel by phospholipase C. Am J Physiol Cell Physiol 281: C700–C708, 2001. doi: 10.1152/ajpcell.2001.281.2.C700. [DOI] [PubMed] [Google Scholar]
- 14.Dadi PK, Luo B, Vierra NC, Jacobson DA. TASK-1 potassium channels limit pancreatic α-cell calcium influx and glucagon secretion. Mol Endocrinol 29: 777–787, 2015. doi: 10.1210/me.2014-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadi PK, Vierra NC, Jacobson DA. Pancreatic β-cell-specific ablation of TASK-1 channels augments glucose-stimulated calcium entry and insulin secretion, improving glucose tolerance. Endocrinology 155: 3757–3768, 2014. doi: 10.1210/en.2013-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A 105: 2203–2208, 2008. [Erratum in Proc Natl Acad Sci U S A 105: 13696, 2008.] doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 18.Donner BC, Schullenberg M, Geduldig N, Hüning A, Mersmann J, Zacharowski K, Kovacevic A, Decking U, Aller MI, Schmidt KG. Functional role of TASK-1 in the heart: studies in TASK-1-deficient mice show prolonged cardiac repolarization and reduced heart rate variability. Basic Res Cardiol 106: 75–87, 2011. doi: 10.1007/s00395-010-0128-x. [DOI] [PubMed] [Google Scholar]
- 19.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellinghaus P, Scheubel RJ, Dobrev D, Ravens U, Holtz J, Huetter J, Nielsch U, Morawietz H. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J Thorac Cardiovasc Surg 129: 1383–1390, 2005. doi: 10.1016/j.jtcvs.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 23.Franceschini N, Carty CL, Lu Y, Tao R, Sung YJ, Manichaikul A, Haessler J, Fornage M, Schwander K, Zubair N, Bien S, Hindorff LA, Guo X, Bielinski SJ, Ehret G, Kaufman JD, Rich SS, Carlson CS, Bottinger EP, North KE, Rao DC, Chakravarti A, Barrett PQ, Loos RJ, Buyske S, Kooperberg C. Variant discovery and fine mapping of genetic loci associated with blood pressure traits in Hispanics and African Americans. PLoS One 11: e0164132, 2016. doi: 10.1371/journal.pone.0164132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganesh SK, Chasman DI, Larson MG, Guo X, Verwoert G, Bis JC, Gu X, Smith AV, Yang ML, Zhang Y, Ehret G, Rose LM, Hwang SJ, Papanicolau GJ, Sijbrands EJ, Rice K, Eiriksdottir G, Pihur V, Ridker PM, Vasan RS, Newton-Cheh C, Raffel LJ, Amin N, Rotter JI, Liu K, Launer LJ, Xu M, Caulfield M, Morrison AC, Johnson AD, Vaidya D, Dehghan A, Li G, Bouchard C, Harris TB, , et al. Effects of long-term averaging of quantitative blood pressure traits on the detection of genetic associations. Am J Hum Genet 95: 49–65, 2014. doi: 10.1016/j.ajhg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res 93: 957–964, 2003. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- 26.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 27.Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J 27: 179–187, 2008. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoggart CJ, Venturini G, Mangino M, Gomez F, Ascari G, Zhao JH, Teumer A, Winkler TW, Tšernikova N, Luan J, Mihailov E, Ehret GB, Zhang W, Lamparter D, Esko T, Macé A, Rüeger S, Bochud PY, Barcella M, Dauvilliers Y, Benyamin B, Evans DM, Hayward C, Lopez MF, Franke L, Russo A, Heid IM, Salvi E, Vendantam S, Arking DE, Boerwinkle E, Chambers JC, Fiorito G, Grallert H, Guarrera S, , et al. Novel approach identifies SNPs in SLC2A10 and KCNK9 with evidence for parent-of-origin effect on body mass index. PLoS Genet 10: e1004508, 2014. doi: 10.1371/journal.pgen.1004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, Kelly TN, Saleheen D, Lehne B, Leach IM, Drong AW, Abbott J, Wahl S, Tan ST, Scott WR, Campanella G, Chadeau-Hyam M, Afzal U, Ahluwalia TS, Bonder MJ, Chen P, Dehghan A, Edwards TL, Esko T, Go MJ, Harris SE, Hartiala J, Kasela S, Kasturiratne A, Khor CC, Kleber ME, Li H, Yu Mok Z, Nakatochi M, Sapari NS, , et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet 47: 1282–1293, 2015. doi: 10.1038/ng.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Kang D, Martin EA, Kim I, Carroll JL. Effects of modulators of AMP-activated protein kinase on TASK-1/3 and intracellular Ca2+ concentration in rat carotid body glomus cells. Respir Physiol Neurobiol 195: 19–26, 2014. doi: 10.1016/j.resp.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem 275: 9340–9347, 2000. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol Heart Circ Physiol 277: H1669–H1678, 1999. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa MG, Reynolds JO, Wehrens XH, Bryan RM Jr, Pandit LM. Hemodynamic and pathologic characterization of the TASK-1−/− mouse does not demonstrate pulmonary hypertension. Front Med (Lausanne) 4: 177, 2017. doi: 10.3389/fmed.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation 115: 918–927, 2007. doi: 10.1161/CIRCULATIONAHA.106.660639. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Kim YK, Dorajoo R, Li H, Lee IT, Cheng CY, He M, Sheu WH, Guo X, Ganesh SK, He J, Lee J, Liu J, Hu Y, Rao DC, Tsai FJ, Koh JY, Hu H, Liang KW, Palmas W, Hixson JE, Han S, Teo YY, Wang Y, Chen J, Lu CH, Zheng Y, Gui L, Lee WJ, Yao J, Gu D, Han BG, Sim X, Sun L, Zhao J, , et al. Genome-wide association study meta-analysis of long-term average blood pressure in East Asians. Circ Cardiovasc Genet 10: e001527, 2017. doi: 10.1161/CIRCGENETICS.116.001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang B, Soka M, Christensen AH, Olesen MS, Larsen AP, Knop FK, Wang F, Nielsen JB, Andersen MN, Humphreys D, Mann SA, Huttner IG, Vandenberg JI, Svendsen JH, Haunsø S, Preiss T, Seebohm G, Olesen SP, Schmitt N, Fatkin D. Genetic variation in the two-pore domain potassium channel, TASK-1, may contribute to an atrial substrate for arrhythmogenesis. J Mol Cell Cardiol 67: 69–76, 2014. doi: 10.1016/j.yjmcc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Lopes CM, Zilberberg N, Goldstein SA. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem 276: 24449–24452, 2001. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- 38.Lopes CM, Rohács T, Czirják G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol 564: 117–129, 2005. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Trégouët DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 369: 351–361, 2013. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manichaikul A, Rich SS, Allison MA, Guagliardo NA, Bayliss DA, Carey RM, Barrett PQ. KCNK3 variants are associated with hyperaldosteronism and hypertension. Hypertension 68: 356–364, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110α) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A 104: 612–617, 2007. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci 23: 6460–6469, 2003. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A 97: 3614–3618, 2000. doi: 10.1073/pnas.97.7.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton MJ, O’Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflügers Arch 445: 577–583, 2003. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- 45.Naga Prasad SV, Esposito G, Mao L, Koch WJ, Rockman HA. Gβγ-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J Biol Chem 275: 4693–4698, 2000. doi: 10.1074/jbc.275.7.4693. [DOI] [PubMed] [Google Scholar]
- 46.Nagaraj C, Tang B, Bálint Z, Wygrecka M, Hrzenjak A, Kwapiszewska G, Stacher E, Lindenmann J, Weir EK, Olschewski H, Olschewski A. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J 41: 85–95, 2013. doi: 10.1183/09031936.00211811. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15: 387–407, 2018. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 48.O’Kelly I, Butler MH, Zilberberg N, Goldstein SAN. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell 111: 577–588, 2002. doi: 10.1016/S0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- 49.Olschewski A, Veale EL, Nagy BM, Nagaraj C, Kwapiszewska G, Antigny F, Lambert M, Humbert M, Czirják G, Enyedi P, Mathie A. TASK-1 (KCNK3) channels in the lung: from cell biology to clinical implications. Eur Respir J 50: 1700754, 2017. doi: 10.1183/13993003.00754-2017. [DOI] [PubMed] [Google Scholar]
- 50.Park SJ, Yu Y, Wagner B, Valinsky WC, Lomax AE, Beyak MJ. Increased TASK channel-mediated currents underlie high-fat diet induced vagal afferent dysfunction. Am J Physiol Gastrointest Liver Physiol 315: G592–G601, 2018. doi: 10.1152/ajpgi.00335.2017. [DOI] [PubMed] [Google Scholar]
- 51.Pisani DF, Beranger GE, Corinus A, Giroud M, Ghandour RA, Altirriba J, Chambard JC, Mazure NM, Bendahhou S, Duranton C, Michiels JF, Frontini A, Rohner-Jeanrenaud F, Cinti S, Christian M, Barhanin J, Amri EZ. The K+ channel TASK1 modulates β-adrenergic response in brown adipose tissue through the mineralocorticoid receptor pathway. FASEB J 30: 909–922, 2016. doi: 10.1096/fj.15-277475. [DOI] [PubMed] [Google Scholar]
- 52.Putzke C, Wemhöner K, Sachse FB, Rinné S, Schlichthörl G, Li XT, Jaé L, Eckhardt I, Wischmeyer E, Wulf H, Preisig-Müller R, Daut J, Decher N. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc Res 75: 59–68, 2007. doi: 10.1016/j.cardiores.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 53.Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem 275: 16650–16657, 2000. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- 54.Rinné S, Kiper AK, Schlichthörl G, Dittmann S, Netter MF, Limberg SH, Silbernagel N, Zuzarte M, Moosdorf R, Wulf H, Schulze-Bahr E, Rolfes C, Decher N. TASK-1 and TASK-3 may form heterodimers in human atrial cardiomyocytes. J Mol Cell Cardiol 81: 71–80, 2015. doi: 10.1016/j.yjmcc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Rinné S, Kiper AK, Schmidt C, Ortiz-Bonnin B, Zwiener S, Seebohm G, Decher N. Stress-kinase regulation of TASK-1 and TASK-3. Cell Physiol Biochem 44: 1024–1037, 2017. doi: 10.1159/000485402. [DOI] [PubMed] [Google Scholar]
- 56.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A 88: 8277–8281, 1991. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schewe M, Nematian-Ardestani E, Sun H, Musinszki M, Cordeiro S, Bucci G, de Groot BL, Tucker SJ, Rapedius M, Baukrowitz T. A non-canonical voltage-sensing mechanism controls gating in K2P K+ channels. Cell 164: 937–949, 2016. doi: 10.1016/j.cell.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt C, Wiedmann F, Langer C, Tristram F, Anand P, Wenzel W, Lugenbiel P, Schweizer PA, Katus HA, Thomas D. Cloning, functional characterization, and remodeling of K2P3.1 (TASK-1) potassium channels in a porcine model of atrial fibrillation and heart failure. Heart Rhythm 11: 1798–1805, 2014. doi: 10.1016/j.hrthm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabó G, Kallenbach K, Karck M, Borggrefe M, Biliczki P, Ehrlich JR, Baczkó I, Lugenbiel P, Schweizer PA, Donner BC, Katus HA, Dobrev D, Thomas D. Upregulation of K2P3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 132: 82–92, 2015. doi: 10.1161/CIRCULATIONAHA.114.012657. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt C, Wiedmann F, Zhou XB, Heijman J, Voigt N, Ratte A, Lang S, Kallenberger SM, Campana C, Weymann A, De Simone R, Szabo G, Ruhparwar A, Kallenbach K, Karck M, Ehrlich JR, Baczkó I, Borggrefe M, Ravens U, Dobrev D, Katus HA, Thomas D. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy. Eur Heart J 38: 1764–1774, 2017. doi: 10.1093/eurheartj/ehw559. [DOI] [PubMed] [Google Scholar]
- 61.Seyler C, Duthil-Straub E, Zitron E, Gierten J, Scholz EP, Fink RH, Karle CA, Becker R, Katus HA, Thomas D. TASK1 (K2P3.1) K+ channel inhibition by endothelin-1 is mediated through Rho kinase-dependent phosphorylation. Br J Pharmacol 165: 1467–1475, 2012. doi: 10.1111/j.1476-5381.2011.01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, Nedergaard J, Sidossis LS, Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21: 389–394, 2015. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M, Ohte N, Wang ZM, Williams DL Jr, Little WC, Cheng CP. Altered inotropic response of endothelin-1 in cardiomyocytes from rats with isoproterenol-induced cardiomyopathy. Cardiovasc Res 39: 589–599, 1998. doi: 10.1016/S0008-6363(98)00166-7. [DOI] [PubMed] [Google Scholar]
- 65.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505, 2001. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 25: 1012–1026, 2017. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westerhof N. Cardiac work and efficiency. Cardiovasc Res 48: 4–7, 2000. doi: 10.1016/S0008-6363(00)00176-0. [DOI] [PubMed] [Google Scholar]
- 69.Wiedmann F, Schulte JS, Gomes B, Zafeiriou MP, Ratte A, Rathjens F, Fehrmann E, Scholz B, Voigt N, Müller FU, Thomas D, Katus HA, Schmidt C. Atrial fibrillation and heart failure-associated remodeling of two-pore-domain potassium (K2P) channels in murine disease models: focus on TASK-1. Basic Res Cardiol 113: 27, 2018. doi: 10.1007/s00395-018-0687-9. [DOI] [PubMed] [Google Scholar]
- 70.Wilke BU, Lindner M, Greifenberg L, Albus A, Kronimus Y, Bünemann M, Leitner MG, Oliver D. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat Commun 5: 5540, 2014. doi: 10.1038/ncomms6540. [DOI] [PubMed] [Google Scholar]
- 71.Yao J, McHedlishvili D, McIntire WE, Guagliardo NA, Erisir A, Coburn CA, Santarelli VP, Bayliss DA, Barrett PQ. Functional TASK-3-like channels in mitochondria of aldosterone-producing zona glomerulosa cells. Hypertension 70: 347–356, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08871. [DOI] [PMC free article] [PubMed] [Google Scholar]