Abstract
Hemoglobins (Hbs) of crocodilians are reportedly characterized by unique mechanisms of allosteric regulatory control, but there are conflicting reports regarding the importance of different effectors, such as chloride ions, organic phosphates, and CO2. Progress in understanding the unusual properties of crocodilian Hbs has also been hindered by a dearth of structural information. Here, we present the first comparative analysis of blood properties and Hb structure and function in a phylogenetically diverse set of crocodilian species. We examine mechanisms of allosteric regulation in the Hbs of 13 crocodilian species belonging to the families Crocodylidae and Alligatoridae. We also report new amino acid sequences for the α- and β-globins of these taxa, which, in combination with structural analyses, provide insights into molecular mechanisms of allosteric regulation. All crocodilian Hbs exhibited a remarkably strong sensitivity to CO2, which would permit effective O2 unloading to tissues in response to an increase in metabolism during intense activity and diving. Although the Hbs of all crocodilians exhibit similar intrinsic O2-affinities, there is considerable variation in sensitivity to Cl− ions and ATP, which appears to be at least partly attributable to variation in the extent of NH2-terminal acetylation. Whereas chloride appears to be a potent allosteric effector of all crocodile Hbs, ATP has a strong, chloride-independent effect on Hb-O2 affinity only in caimans. Modeling suggests that allosteric ATP binding has a somewhat different structural basis in crocodilian and mammalian Hbs.
Keywords: adaptation, allostery, blood, oxygen transport, reptile
INTRODUCTION
Among vertebrate hemoglobins (Hbs), those of crocodilians are renowned for their unique allosteric mechanism for regulating O2-binding affinity. In most vertebrates, the O2 affinity of Hb is tightly controlled by changes in the red blood cell (RBC) concentration of allosteric cofactors such as organic phosphates, chloride ions, and CO2, each of which bind to specific (nonheme) sites on the Hb and stabilize the low-affinity T state relative to the high-affinity R state. Changes in the RBC concentrations of these cofactors shift the allosteric T↔R equilibrium of the Hb, thereby modulating O2 uptake and delivery. In contrast to the Hbs of other jawed vertebrates, those of crocodilians are reportedly unique in that O2 affinity is responsive to changes in the RBC concentration of bicarbonate ions but not to changes in the concentration of molecular CO2 (2, 44). Responsiveness to bicarbonate ions has been documented for hagfish Hbs (15), but it is not a property of other vertebrate Hbs that have been examined to date.
In crocodilian Hbs, the affinity-reducing effect of bicarbonate should enhance O2 unloading from the blood when plasma bicarbonate levels are elevated, which may occur as a result of metabolic compensation to respiratory acidosis, as during diving. The bicarbonate sensitivity of crocodilian Hb has also been suggested to ensure O2 delivery to sustain the increase in metabolism associated with digestion (61). Alligators, for example, experience a postprandial “alkaline tide” as the secretion of gastric acid produces an elevation of plasma bicarbonate (10, 11). However, the alkalinization of the blood is ameliorated by a postprandial elevation of Pco2 due to hypoventilation, so changes in blood-O2 affinity during digestion may not be very dramatic (7).
The sensitivity to bicarbonate ions and insensitivity to CO2 was originally described for the Hb of a single crocodilian species, the spectacled caiman (Caiman crocodilus) (2). However, subsequent studies on the Hbs of American alligator (Alligator mississippiensis) and dwarf caiman (Paleosuchus palpebrosus) have provided evidence for a direct CO2 effect (26, 59), which suggests the possibility of carbamino formation from the reaction between CO2 and the NH2 termini of the α- and β-chain Hb subunits. In addition to discrepancies in the literature regarding the allosteric effects of CO2-binding, some studies have concluded that crocodilian Hbs are insensitive to RBC organic phosphates (2, 3, 44), whereas others have demonstrated that the Hbs of at least some crocodilian species are sensitive to organic phosphates and chloride ions (60, 61). Currently, it is not possible to draw general conclusions about the roles of CO2 and anions in the allosteric regulation of crocodilian Hbs because studies to date have used different experimental conditions and different study species.
Here, we present the first comparative analysis of functional properties of Hb in a phylogenetically diverse set of crocodilian species. We report functional properties and mechanisms of allosteric regulation in the Hbs of 13 species belonging to the two major families Crocodylidae and Alligatoridae. We also report amino acid sequences for the α- and β-globins of these taxa, in combination with structural modeling analyses to provide insights into molecular mechanisms underlying the unusual allosteric properties of crocodilian Hbs.
MATERIALS AND METHODS
Animals and Blood Collection
Venous blood (∼3 mL) was drawn through direct puncture of the postoccipital sinus of nonanesthetized adult crocodiles and was collected in 5-mL syringes containing heparin as anticoagulant. Sex was not determined. Animals were housed in the Crocodile Zoo (Eskilstrup, Falster, Denmark) and were manually restrained for the 3–5 min required for blood sampling. Blood was sampled from a single specimen of each of 13 species belonging to the two most speciose families of extant crocodilians. Species in the family Alligatoridae included American alligator (Alligator mississippiensis), Chinese alligator (A. sinensis), broad-snouted caiman (Caiman latirostris), yacaré caiman (C. yacare), black caiman (Melanosuchus niger), and smooth-fronted caiman (Paleosuchus trigonatus). Species in the family Crocodylidae included American crocodile (Crocodylus acutus), Philippine crocodile (C. mindorensis), Nile crocodile (C. niloticus), New Guinea crocodile (C. novaeguineae), saltwater crocodile (C. porosus), Cuban crocodile (C. rhombifer), and Siamese crocodile (C. siamensis). All animal procedures were conducted according to the Danish Law for Animal Experimentation and were approved by the Danish Animal Experiments Inspectorate under permit no. 2018-15-0201-01507.
Hematological Traits
For all species, blood hematocrit (Hct; the %volume of RBC) was measured in duplicate after centrifugation at 13,000 g for 3 min in capillary tubes. Whole blood hemoglobin concentration ([Hb]) was measured by mixing blood aliquots with the Drabkin’s reagent (Sigma-Aldrich, St. Louis, MO). Mean corpuscular hemoglobin concentration (MCHC; mM heme) was calculated as ([Hb]/Hct) × 100.
cDNA Cloning and Sequencing
To characterize structural variation of crocodilian Hbs, we cloned and sequenced the adult-expressed α- and β-type globin genes from each of the 13 species mentioned above. We extracted total RNA from whole blood (∼30 µL) using the RNeasy kit (Qiagen, Valencia, CA), and we amplified full-length cDNAs of adult-expressed globin genes using a OneStep RTPCR kit (Qiagen). We designed paralog-specific primers (Table 1) using 5′- and 3′-UTR sequences from annotated globin genes in the genome assemblies of Crocodylus porosus, Gavialis gangeticus, and Alligator mississippiensis (16, 21). We cloned reverse transcription (RT)-PCR products into pCR4-TOPO vector using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA), and we sequenced at least five clones per gene in each individual to recover both alleles. This enabled us to determine full diploid genotypes for major adult-expressed globin genes in each species. All new sequences were deposited in GenBank under the accession nos. MN905601-MN905626.
Table 1.
Reverse transcriptase-PCR primers used to amplify adult-expressed α- and β-type globin genes of 13 crocodilian species
| Genes | Primer Names | Sequences (5′ to 3′) |
|---|---|---|
| α | Croc_HBA_FOR1 | GTGGCTGTCACTGCRCRTCTGCAABCATG |
| α | Croc_HBA_FOR2 | GGGTACCAGGGCTGGTGGCTGTCACTGC |
| α | Croc_HBA_REV1 | CTGGCTGGCGGCTCGGAGCCCAGCCGGGC |
| β | Croc_HBB_FOR1 | GATGCTYTAAAACAACTCCAGGACTCYTCAC |
| β | Croc_HBB_REV1 | AGCAGCATCTTTTTGTGGTGTGCTTCCCTC |
| β | Croc_HBB_REV2 | GCACCCAGCGGTGCCCAGGAGGAAGCAG |
Phylogenetic Analysis
Phylogenetic relationships among the α- and β-type globin sequences of crocodilians were estimated using maximum likelihood (ML). The analyses were based on an alignment of amino acid sequences from a total of 15 species representing each of the three extant crocodilian families (Crocodylidae, Gavilidae, and Alligatoridae). The alignment included sequences obtained from each of the 13 species that we sampled as well as publicly available sequences from Gavialis gangeticus and Paleosuchus palpebrosus. As outgroups for the analysis of α-type globins, we used sequences from the full repertoire of α-type globin genes in representative birds and turtles. As outgroups for the analysis of β-type globins, we used paralogous sequences from the full repertoire of intact β-type globin genes in Crocodylus porosus, Gavialis gangeticus, Alligator mississippiensis, and Alligator sinensis as well as adult β-globin genes from the green sea turtle (Chelonia mydas). Globin gene nomenclature follows Hoffmann et al. (21).
Sequence alignments were performed using the program MAFFT version 7.304 (28), as implemented in the following server: http://mafft.cbrc.jp/alignment/server/. ML analyses were run using IQ-Tree ver. 1.5.5 (40) in the implementation of the program on the IQ-Tree web server (55). Statistical support for the nodes of each estimated tree was evaluated with 1,000 pseudoreplicates of the ultrafast bootstrap procedure (36).
Examination of RBC IsoHb Composition
Hb solutions were prepared by lysis of RBC after blood centrifugation and RBC washing with 0.9% NaCl (25). Organic phosphates were removed from the Hb solutions, yielding purified “stripped” Hb, by gel filtration on PD-10 columns (GE Healthcare) in 10 mM HEPES, pH 7.6, and 0.5 mM EDTA, after NaCl (0.2 M final concentration) was added to the samples to facilitate phosphate removal. Hb concentration (heme, mM) and lack of heme oxidation were obtained from the absorption at 575 nm (15.37 mM−1 cm−1) and 541 nm (14.37 mM−1 cm−1) of the oxy derivative (56). Hb samples were stored in aliquots (>1 mM heme) at −80°C. For each sample, we tested for the presence of multiple Hb isoforms by means of thin-layer polyacrylamide gel isoelectrofocusing (pH range 3–9), using PhastSystem (GE Healthcare). Isoelectric points (pI) of Hb bands were calculated from linear regression of standard pI markers run in parallel (8, 24, 47).
Mass Spectrometry
To quantify NH2-terminal acetylation of the α- and β-chain subunits of crocodilian Hb, we conducted tandem mass spectrometry (MS/MS) analyses on samples from six species: Crocodylus siamensis, Crocodylus porosus, Alligator mississippiensis, Melanosuchus niger, Paleosuchus trigonatus, and Caiman yacare. Native Hbs were separated in a mini-protean precast 4–20% SDS PAGE gel (Bio-Rad, Hercules, CA) and were subsequently stained with Coomassie brilliant blue-G. The stained bands were excised and processed for in-gel tryptic digestion (51), and the eluted peptides were then analyzed using a Thermo Orbitrap Fusion Lumos Tribrid (Thermo Scientific) mass spectrometer in data-dependent acquisition mode. Peptides were identified by searching MS/MS data against a customized reference database that contained adult-expressed globin genes of all crocodilian species used in the experiments on Hb function as well as the complete globin gene repertoires of Crocodylus porosus, Gavialis gangeticus, and Alligator mississippiensis. The reference database also included an avian αD-globin sequence to confirm results of a previous comparative genomic analysis, which indicated that the ortholog of this gene was deleted in the common ancestor of modern crocodilians (21). The search was set up for full tryptic peptides with a maximum of two missed cleavage sites. Acetylation of α- and β-chain NH2 termini, carbamino formation, and the oxidation of methionines was included as variable modifications, and the carbamidomethylation of cysteines was set as fixed modification. The precursor mass tolerance threshold was set as 10 ppm, and maximum fragment mass error was set at 0.02 Da. Qualitative analysis was performed using PEAKS X software. The significance threshold of the ion score was calculated based on a false discovery rate of ≤1%. Assuming an equimolar ratio for the α- and β-chain subunits (1:1) in tetrameric Hb, we measured the relative fractions of acetylated and unacetylated (free) NH2 termini for each subunit type.
Oxygen Equilibrium Curves
O2 equilibrium curves of purified Hb from each species were measured using a thin-layer modified diffusion chamber technique (8, 38, 39, 54, 58). The custom-made chamber was connected to a Cary 60 UV-Vis spectrophotometer equipped with fiber optic probes (Agilent Technologies) and to a programmable Gas Mixing System (Loligo Systems, Viborg, Denmark) for mixing ultrapure N2 and O2 to generate discrete oxygen tension (Po2) values. At each Po2 step, the O2 saturation (Y) was obtained from the relative absorption change at 415 nm. To determine the effect of chloride and ATP on Hb oxygenation, O2 equilibrium curves were measured in 0.1 M HEPES buffer and 0.5 mM EDTA, pH 7.2, 25°C, at 0.3 mM heme in the absence (stripped) and presence of 0.1 M KCl and 0.45 mM ATP (corresponding to an ATP/Hb tetramer ratio of 6.0), added separately and in combination. Although crocodilian RBCs contain several types of organic phosphates, ATP is the one present at highest concentrations in RBCs from adult crocodilians (1, 46) and was therefore chosen to characterize its effect on Hb oxygenation. O2 affinity (expressed as P50, the Po2 at half saturation) and Hill’s cooperativity coefficient (n) were obtained by fitting the sigmoidal Hill equation Y = Po2n/(Po2n + P50n) to the saturation data (Y vs. Po2) using nonlinear regression (4–6 saturation data in each curve).
CO2 Effect
The sensitivity of individual Hbs to CO2 was tested in 0.1 M HEPES buffer and 0.5 mM EDTA, pH 7.2, at 25°C and 0.3 mM heme by using the modified diffusion chamber set at a constant Po2 (i.e., the individual P50 measured previously for each sample). After equilibration to 50% O2 saturation, 1% CO2 was added to the gas mixture while still delivering a constant Po2, and the decrease in O2 saturation was recorded. Human HbA (Sigma-Aldrich) was used in control experiments. The pH of samples equilibrated with 1% CO2 was measured using an InLab Micro pH electrode (Mettler, Toledo, OH) and did not change appreciably.
Computational Modeling of Hb Structure
To gain insights into the structural origin of allosteric effects, we performed atomistic classical molecular dynamics simulations, which have been widely employed in investigations of protein dynamics and ligand-protein interactions (22). These simulations rely on parameterized potential energy surfaces called force fields.
Starting structures.
We performed homology-based modeling of A. mississippiensis deoxy Hb with MODELER software (57). As a template, we used the 2.5 Å resolution X-ray structure of human deoxy Hb complexed with 2,3-diphosphoglycerate (DPG), obtained from the Research Collaboratory for Structural Bioinformatics database (https://www.rcsb.org, PDB entry code 1b86) (48). Standard protonation states at physiological pH were assigned to all ionizable residues (Asp, Glu, Lys, and Arg), whereas protonation of His residues was assigned on the basis of the hydrogen-bond pattern with neighboring residues. In particular, proximal heme-bound His (HF8) protonation was chosen to be in the Nδ position, since this is the protonation state that allows coordination to the iron. Acetyl groups were added to the β-chain NH2 termini according to the internal coordinates of the Amber topology files, as determined using the LEaP software in the AmberTools package. ATP was incorporated into the deoxyHb structure in the conformation described in Gronenborn et al. (18), with phosphate groups manually superimposed on those of DPG. Finally, complete starting structures were immersed in an 86 Å truncated octahedral box of TIP3P water molecules (27). Starting structures were subjected to two cycles of energy minimization. Only water molecules were relaxed during the first 500 steps to avoid unfavorable contacts with the complex; sidechains (including acetyl groups) and ATP were included during the subsequent 1,000 steps.
Classical molecular dynamics simulations.
Molecular dynamics simulations were performed using the PMEMD module of the Amber16 package. The Amber ff14SB force field (32) was used for all amino acid residues, whereas heme parameters were derived from Marti et al. (34) (developed and tested in subsequent studies; see Refs. 5, 6, 9, and 37), and ATP parameters were obtained from Meagher et al. (35). Simulations were performed using periodic boundary conditions and Ewald sums to treat long-range electrostatic interactions (13). The SHAKE algorithm (49) was used to keep bonds involving hydrogen atoms at their equilibrium length, and the Langevin thermostat (31, 63) and Berendsen barostat (4) were used to control the system temperature and pressure, respectively. Thermalization involved a 45-ps heating to 100 K in the NTV ensemble with a harmonic restraint (weight = 50 kcal/mol·Å2) applied to every atom in the complex, followed by a 400-ps heating to 300 K in the NTP ensemble with a lighter restraint weight (15 kcal/mol·Å2). Structures were then equilibrated for 500 ps at 300 K with restraints (weight 5 kcal/mol·Å2) applied only to the backbone and ATP atoms so that the system could relax the sidechains and reach stable density. Production MD runs consisted of 100-ns trajectories in the NVT ensemble. Because T state Hb may not be a stable structure in MD simulations (14, 23), and because the alligator Hb structure was built from homology modeling, we performed production dynamics that imposed a 5 kcal/mol Å2 harmonic restraint on all α-carbons except for β-chain residues 1–3. This retained the protein in the desired conformation while conferring the NH2 termini with the flexibility required for ligand interactions.
RESULTS AND DISCUSSION
Hematology
All examined crocodilian species exhibited low hematocrits and Hb concentrations (Table 2), consistent with previous studies (45), and reflecting the low blood-O2 carrying capacities of reptiles.
Table 2.
Hematological traits and oxygenation properties of stripped, purified Hbs (on 0.1 M HEPES, pH 7.2, 25°C) for 13 species of crocodilians
| Hematological Traits |
Oxygen Affinity and Cooperativity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hct, % | Blood [Hb] (mM heme) | MCHC (mM heme) | P50 (Torr) | ± SE | n50 | ± SE | r2 | |
| Alligatoridae | ||||||||
| Alligator mississipiensis | 25 | 2.37 | 9.48 | 2.84 | 0.02 | 2.14 | 0.04 | 0.9999 |
| Alligator sinensis | 15 | 1.29 | 8.60 | 2.60 | 0.04 | 2.41 | 0.10 | 0.9995 |
| Melanosuchus niger | 12 | 1.02 | 8.50 | 3.01 | 0.03 | 1.79 | 0.04 | 0.9999 |
| Paleosuchus trigonatus | 10 | 0.89 | 8.90 | 4.73 | 0.08 | 1.49 | 0.04 | 0.9997 |
| Caiman latirostis | 20 | 1.73 | 8.65 | 2.97 | 0.03 | 1.56 | 0.03 | 0.9998 |
| Caiman yacare | 20 | 1.52 | 7.60 | 3.04 | 0.05 | 1.60 | 0.05 | 0.9998 |
| Crocodylidae | ||||||||
| Crocodylus acutus | 13 | 1.57 | 12.08 | 2.55 | 0.04 | 2.29 | 0.09 | 0.9996 |
| Crocodylus mindorensis | 17 | 1.69 | 9.94 | 2.54 | 0.04 | 2.23 | 0.09 | 0.9995 |
| Crocodylus niloticus | 16 | 1.81 | 11.31 | 2.56 | 0.05 | 2.14 | 0.12 | 0.9992 |
| Crocodylus novaeguineae | 21 | 2.00 | 9.52 | 2.54 | 0.04 | 2.12 | 0.08 | 0.9996 |
| Crocodylus porosus | 19 | 1.70 | 8.95 | 2.48 | 0.03 | 2.25 | 0.08 | 0.9996 |
| Crocodylus rhombifer | 23 | 2.07 | 9.00 | 2.60 | 0.05 | 2.28 | 0.11 | 0.9994 |
| Crocodylus siamensis | 21 | 1.86 | 8.86 | 2.40 | 0.06 | 1.85 | 0.11 | 0.9991 |
MCHC, mean corpuscular hemoglobin (Hb) concentration P50, the partial pressure of O2 at which Hb is 50% saturated (1 Torr = 133 Pa); n50, Hill’s cooperativity coefficient.
Sequence Variation
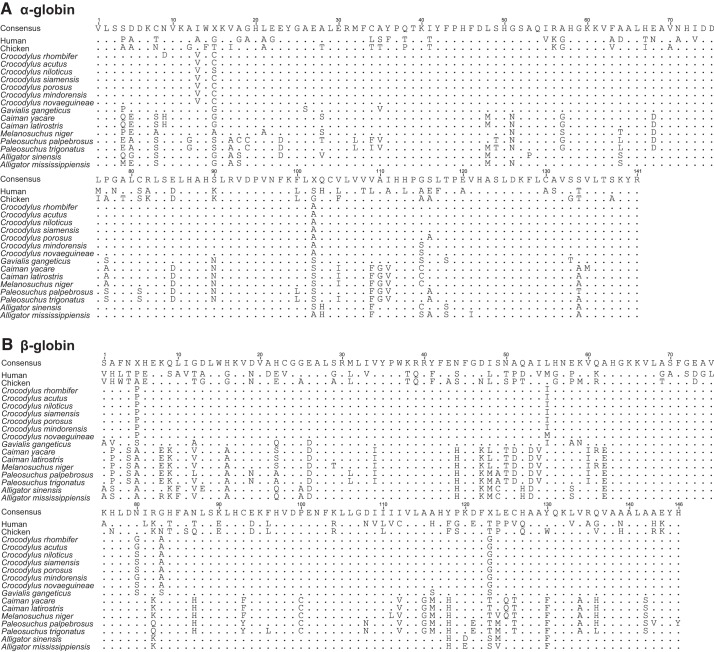
Sequencing of globin cDNAs revealed that all examined crocodilian species express single α- and β-type globins in adult RBCs. Although the genome assembly of Chinese alligator (Alligator sinensis) indicates that this species possesses duplicate copies of the adult β-globin gene (21), we recovered a single cDNA sequence from this species corresponding to the same HBB-T4 gene that encodes the β-chain of adult Hb in all other crocodilians. Alignment of α- and β-globin sequences revealed very little amino acid variation within Crocodylidae and somewhat more extensive variation within Alligatoridae (Fig. 1). This is consistent with differences in the time scale of diversification; phylogenetic analyses suggest that all extant species in the genus Crocodylus descend from a common ancestor that existed 10–15 mya in the mid-Miocene, whereas extant caiman and alligator species (comprising the family Alligatoridae) descend from a far more ancient common ancestor that existed ∼60–70 mya in the late Cretaceous or early Paleogene (41).
Fig. 1.
Alignment of amino acid sequences for the adult-expressed α- (A) and β-type (B) globin genes of crocodilians, with homologous sequences from human (Homo sapiens) and chicken (Gallus gallus) included for comparison.
Given that the Nile crocodile (Crocodylus nicotilus) has figured prominently in previous studies of structure-function relationships of crocodilian Hbs (29, 43, 44), it is worth noting that our amino acid sequence of the adult β-globin of this species (based on independently cloned and sequenced cDNAs) differs from the originally reported sequence (30) at seven sites. At these discrepant sites, our C. niloticus sequence possesses the same amino acid as the adult β-globins of all other Crocodylus species, leading us to suspect that the discrepancies in the sequence reported by Leclercq et al. (30) represent artifacts of peptide sequencing.
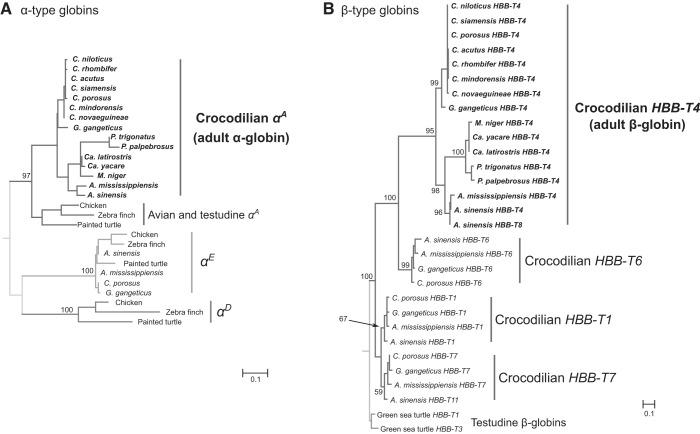
Phylogenetic Relationships
Within the α- and β-globin subfamilies, estimated phylogenetic relationships among paralogous genes of crocodilians and representative sauropsid outgroups (Fig. 2) are consistent with results of previous studies (19–21). Adult-expressed β-globins of crocodilians (HBB-T4) are nested within a clade containing three other β-type crocodilian globins (HBB-T1, HBB-T6, and HBB-T7), all of which are monophyletic relative to those of other amniotes (e.g., HBB-T1 and HBB-T3 of turtles; Fig. 2B). This finding is consistent with results of previous phylogenetic reconstructions and analyses of conserved synteny, which revealed that the set of tandemly linked crocodilian β-type globin genes represents products of multiple rounds of lineage-specific duplication (21). Finally, branching relationships of orthologous adult-expressed globins within the α- and β-globin subfamilies (Fig. 2) are consistent with expected relationships among representatives of different crocodilian families and genera (41).
Fig. 2.
Maximum likelihood phylogenies of crocodilian α- (A) and β-type (B) globins, based on amino acid sequences. Orthologous and/or paralogous sequences from other sauropsid taxa are used as outgroups. Support values for relevant nodes are shown as bootstrap percentages.
Hb Isoform Composition
Consistent with the cDNA sequencing results, isoelectric focusing (IEF) analyses revealed that all crocodilian species expressed a single adult Hb isoform (Fig. 3). All species in the family Crocodylidae, along with A. mississippiensis, M. niger, C. latirostris, and C. yacare, expressed single Hbs with a pI of ∼7.1, whereas A. sinensis and P. trigonatus expressed single Hbs with slightly lower pIs (∼6.2 and ∼6.4, respectively; Fig. 3). Mass spectrometry experiments confirmed that, unlike members of all other sauropsid lineages (12, 42, 53, 54), crocodilians do not express an adult Hb isoform that incorporates products of the αD-globin gene.
Fig. 3.
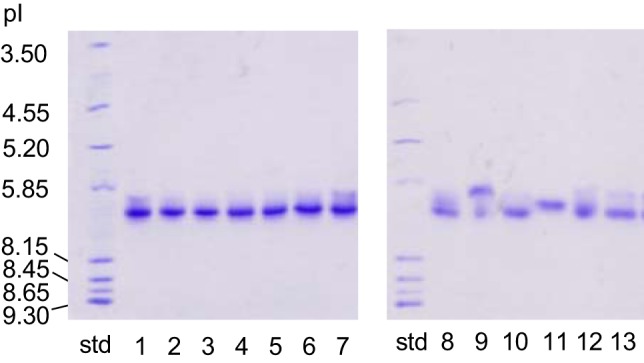
Isoelectric focusing gels (pH 3–9) indicating the presence of single hemoglobin (Hb) isoforms in red blood cells of adult crocodilians. Lanes are labeled as follows: Std, isoelectric point (pI) markers. Left (Crocodilyde): 1, Crocodylus acutus; 2, C. mindoriensis; 3, C. niloticus; 4, C. novaeguineae; 5, C. porosus; 6, C. rhombifer; 7, C. siamensis. Right (Alligatoridae): 8, Alligator mississippiensis; 9, A. sinensis; 10; Melanosuchus niger; 11, Paleosuchus trigonatus; 12, Caiman latirostris; 13, C. yacare.
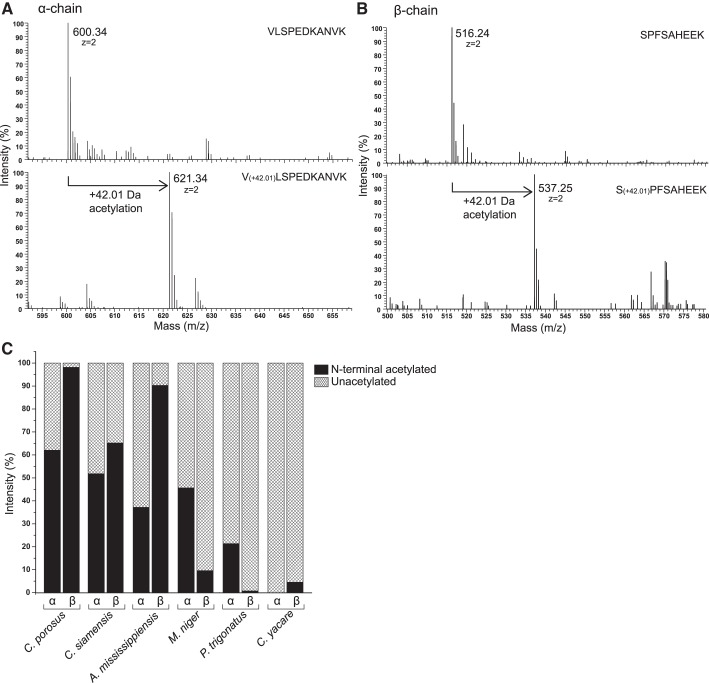
NH2-Terminal Acetylation
Previous studies of select crocodilian species reported that the NH2 termini of the β-chain subunits are acetylated (50, 59). Because the free NH2 termini of the α- and β-chain Hb subunits play key roles in the allosteric binding of Cl− ions, organic phosphates, and CO2 (via carbamino formation), it is important to determine the pervasiveness of NH2-terminal acetylation of crocodilian Hbs. Acetylation of NH2-terminal globin peptides results in a mass increase of +42.01 Da, which can be readily detected by a shift in mass spectra (Fig. 4, A and B) (33, 62). The MS/MS results indicated considerable variation among species in the extent of N-terminal acetylation (Fig. 4C). The α-chain NH2 termini of Hb from Caiman yacare were completely free, whereas the percentage of acetylated NH2 termini for the remaining species ranged from ∼20 to ~60%. Acetylation of β-chain NH2 termini ranged from ∼60 to ∼100% in all examined species of Crocodylus and Alligator (which possess residues β1Ser-β2Ala and β1Ala-β2Ser, respectively) and was <10% in the three examined caiman species (all of which possess β1Ser-β2Pro) (Figs. 1B and 4).
Fig. 4.
NH2-terminal acetylation of crocodilian hemoglobins (Hbs). A: MALDI-MS spectra of the α-chain subunit of Hb from Melanosuchus niger, illustrating how acetylation of the –NH2 terminus can be detected as a 42.01-Da increase in mass of the NH2-terminal peptide VLSPEDKANVK. B: MALDI-MS spectra of the β-chain subunit from M. niger, illustrating how acetylation of the NH2 terminus can be detected as a 42.01-Da increase in mass of the NH2-terminal peptide SPFSAHEEK. C: estimated extent of NH2-terminal acetylation for the α- and β-chain subunits of adult Hbs from 6 representative crocodilian species.
Oxygenation Properties of Crocodilian Hbs
The Hbs of all species exhibited cooperative O2 binding and relatively high intrinsic O2 affinities, with P50’s of stripped Hbs ranging between 2.40 and 4.73 Torr (25°C, pH 7.2) (Table 2). Hb-O2 affinities measured under identical conditions were almost identical among all members of Crocodylidae (2.52 Torr on average) and varied more within Alligatoridae (Table 2). These data indicate that crocodilian Hbs have a high intrinsic affinity (i.e., low P50) in the absence of anions. P50 values of stripped Hbs are highly consistent with those reported for Paleosuchus palpebrosus (2.69 Torr, pH 7.4, 25°C) (59) and for both A. mississippiensis and Caiman crocodilus (∼3 Torr, pH 7.1, 25°C) (60). However, our data are not consistent with previously reported P50 values for the stripped hemolysate of C. porosus by Bauer et al. (2) (6.3 Torr, pH 7.2, 25°C). This discrepancy is most likely explained by the fact that the experiments of Bauer et al. (2) were performed using chloride-containing buffer solutions, which markedly affects Hb-O2 affinity (see below).
The Hbs of all examined crocodilian species exhibited a remarkably strong sensitivity to CO2. When the Hb solution was maintained at a constant pH and a constant Po2, yielding an O2 saturation of ∼50%, the addition of 1% CO2 (Pco2 = 7.52 Torr) in the gas mixture caused a pronounced decrease in the O2 saturation (Fig. 5). This effect was much more pronounced than that observed in human HbA (Fig. 5). A strong effect of CO2 on Hb-O2 affinity was previously reported for C. porosus hemolysate (3) and was later ascribed to a specific sensitivity to bicarbonate ions as a unique allosteric feature of crocodilian Hbs (2). A marked CO2 effect on Hb-O2 affinity has also been reported for A. mississippiensis (26) and P. palpebrosus (59), but these studies did not exclude the possible contribution of CO2-derived carbamino formation at the unprotonated NH2 termini of the globin chains in conjunction with the allosteric binding of bicarbonate ions. In the case of A. mississippiensis, carbamino formation could be expected to disproportionately involve CO2 binding to the NH2 termini of the α-chains rather than the β-chains due to differences in the prevalence of NH2-terminal acetylation (∼40 vs. ∼90%, respectively; Fig. 4C). Our experiments did not allow us to determine whether the allosteric mechanism underlying the observed decrease in O2 saturation (Fig. 5) stems from a direct effect of carbamino formation (CO2 binding) or an indirect effect of bicarbonate formed during CO2 hydration, but we are currently examining these possibilities using a kinetic approach (15).
Fig. 5.
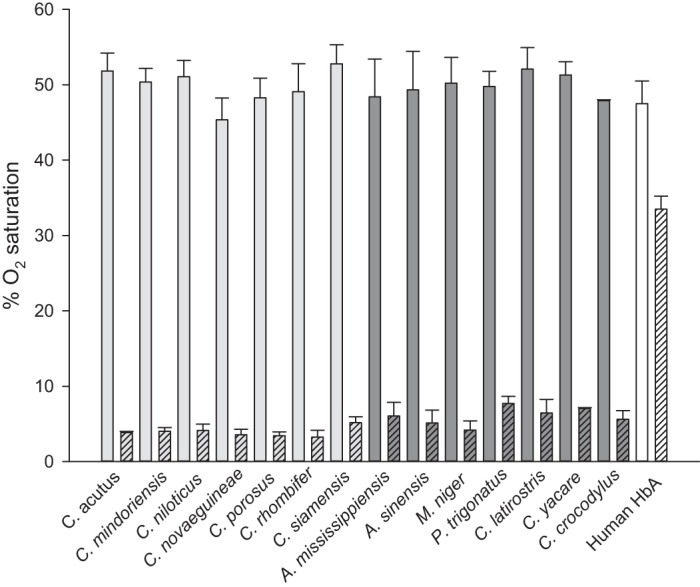
Effect of 1% CO2 on the O2 saturation of crocodilian hemoglobins (Hbs). Data are expressed as means and SD of replicate measurements (n = 4, except for C. porosus, M. niger, and C. crocodylus, where n = 3) in 0.1 M HEPES, pH 7.2, 25°C, at a constant Po2 (approximating the P50 of each Hb) in the absence (open bars) and presence (hatched bars) of 1% CO2 in the gas mixture. Light gray bars, Crocodylidae; dark gray bars, Alligatoridae; open bars, human HbA.
Although crocodilian Hbs exhibited similar intrinsic O2 affinities (Table 2), similar sensitivities to CO2 (Fig. 5), and similar Bohr effects (3, 59, 60), we found considerable variation in their sensitivities to Cl− ions and ATP (Fig. 6). For detailed experimental measurements of anion sensitivity, we selected two representative species of Crocodylidae and four representative species of Alligatoridae that exhibited variation in Hb isoelectric point and intrinsic O2 affinity (Table 2). These experiments revealed that the Hbs of all species exhibited a marked increase in P50 upon addition of Cl− ions, with the Hbs of C. porosus, C. siamensis, and A. mississippiensis showing a larger P50 shift compared with those of M. niger, P. trigonatus, and C. yacare (Fig. 6). These results indicate that chloride is a major anionic allosteric effector of crocodilian Hbs. In contrast, the individual effect of ATP on Hb-O2 affinity, expressed as a shift in P50, was small in C. porosus, C. siamensis, and A. mississippiensis and was similar to the effect of chloride in the three examined caiman species M. niger, P. trigonatus, and C. yacare (Fig. 6). For all species, simultaneous addition of ATP and chloride reduced Hb-O2 affinity to the same extent as chloride alone, indicating that chloride binds Hb more strongly than ATP. These data are in good agreement with previous reports that Hbs of C. crocodylus, A. mississippiensis, and P. palpebrosus exhibit measurable ATP sensitivity only in the absence of chloride (59, 60), although absolute values of P50 reported here are slightly lower due to different experimental conditions. Whereas chloride appears to be a potent allosteric effector of all crocodile Hbs, ATP has a strong, chloride-independent effect on Hb-O2 affinity only in caimans (Table 2 and Fig. 6).
Fig. 6.
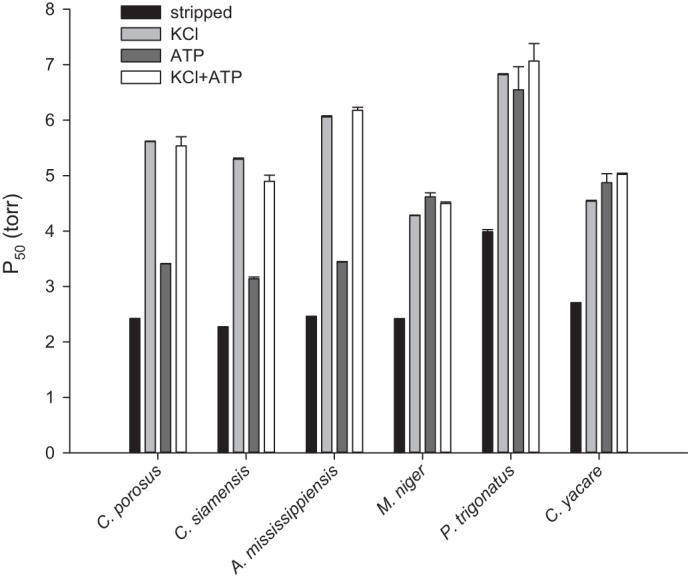
Effect of Cl− ions and ATP alone and in combination on the O2 affinity (P50) of purified crocodilian Hbs. Data are expressed as means and SE of O2 equilibrium curve fitting, as described in materials and methods. Conditions: 0.1 M HEPES, pH 7.2, 25°C, 0.1 M KCl, and 0.45 mM ATP.
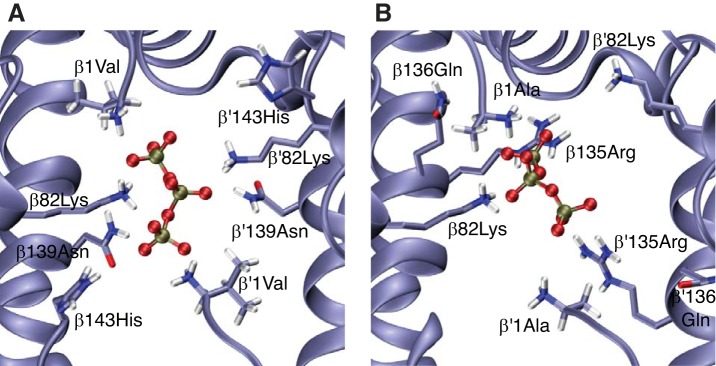
Insights into Structure-Function Relationships
We performed homology modeling and molecular dynamics simulations to gain insight into the possible structural basis of allosteric binding of ATP to crocodilian Hb. Consistent with previous studies of DPG binding (52), our modeling results indicate that ATP binding to human deoxy Hb involves interactions with residues in the central cavity of the Hb tetramer, specifically with β1Val, β82Lys, β139Asn, and, to a lesser extent, β2His and β143His (Fig. 7A). By contrast, in the absence of NH2-terminal acetylation, ATP-binding to deoxy Hb of A. mississippiensis involves a bilateral interaction with β135Arg in addition to β1Ala and β82Lys (Fig. 7B). This finding for alligator Hb is consistent with previous modeling results for avian Hb, which indicated that β135Arg plays an important role in the allosteric binding of organic phosphates (17). The observed difference in ATP sensitivity between caimans and all other crocodilians may stem largely from differences in the extent of NH2-terminal acetylation (Fig. 4C). When the β-chain NH2 termini of alligator Hb are acetylated, molecular dynamics simulations indicate that the phosphate groups of ATP do not interact with the NH2-terminal acetyl group, and the interaction with β135Arg is sporadic. Because the β135Arg residues preferentially interact with the NH2-terminal acetyl group of the same subunit, ATP is predicted to bind between the β82Lys residues of the opposing β-chains (Fig. 8). In addition to the effects of NH2-terminal acetylation, the difference in ATP-sensitivity between the Hbs of caimans and those of other crocodilians may stem from the effects of amino acid substitutions that alter the orientation of the β-chain NH2 termini (caimans differ from all other crocodilians at 3–4 of the first 5 β-chain residues; Fig. 1B) or that otherwise alter the distribution of charged residues in the central cavity.
Fig. 7.
Predicted binding of ATP in the central cavity between the β1-and β2-chains of human and crocodilian hemoglobins (Hbs) in the T-state. ATP bound to human HbA (A) and Alligator mississippiensis Hb (B) with free (unacetylated) β-chain NH2 termini. For clarity, only the ATP phosphate groups are depicted.
Fig. 8.
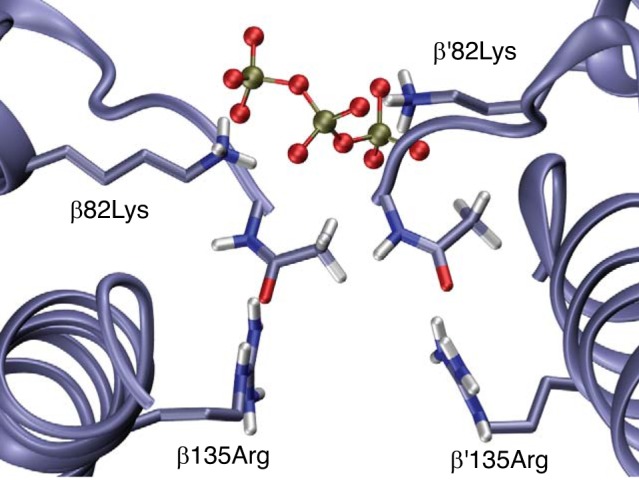
Representative snapshot of molecular dynamics simulations of ATP binding to Alligator mississippiensis hemoglobin (Hb) with acetylated β-chain NH2 termini. This frame illustrates how the ATP phosphates may bind between the β82Lys residues of the opposing β chains when the β135Arg residues preferentially engage in an intra-subunit interaction with the NH2-terminal acetyl groups of β-subunits. For clarity, only the ATP phosphate groups are depicted.
Further insights into the structural basis of the CO2 effect of crocodilian Hb will require additional experimental and computational work, but it is worth noting that the Hbs of all crocodilians exhibit highly uniform responses to CO2 (Fig. 5) despite the considerable variation among species in the accessibility of the α- and β-chain free NH2 termini for carbamino formation (Fig. 4C). This suggests that the observed allosteric effect of CO2 may involve oxygenation-linked CO2 binding to other sites on the protein, or it may be an indirect effect of bicarbonate binding.
Perspectives and Significance
This investigation provides the first broad comparative overview of the functional and structural characteristics of crocodilian Hbs. We confirm that CO2 is a strong allosteric regulator of Hb-O2 affinity, and it may involve a novel mechanism that is at least partly independent of NH2-terminal carbamino formation, an aspect that we are currently investigating. A strong sensitivity to CO2 would enable Hb to effectively unload O2 in response to increased activity or respiratory acidosis, when blood bicarbonate levels and Pco2 are both elevated (7, 26). Given the high intrinsic O2-affinity of crocodilian Hbs, the allosteric effect of CO2 may also play an important physiological role in maintaining blood-O2 affinity in a range that is conducive to efficient O2 delivery to respiring tissues.
Contrary to some previous reports, our results also reveal that crocodilian Hbs are sensitive to ATP as well as Cl− ions and that ATP sensitivity is especially pronounced in caiman Hbs. Interestingly, the structural basis of oxygenation-linked phosphate binding to crocodilian Hbs appears to be quite distinct from that described for human HbA. Continued research on crocodilian Hbs holds much promise for broadening our understanding of structural and functional mechanisms of allosteric regulatory control and the functional consequences of posttranslational modifications such as NH2-terminal acetylation.
GRANTS
This research was supported by grants to A. Fago and T. Wang from the Independent Research Fund Denmark, Natural Sciences (4181-00094 to A. Fago), and to J. F. Storz from the National Institutes of Health (HL-087216), the National Science Foundation (OIA-1736249 and IOS-1927675), and the Fulbright Foreign Scholar Program in Argentina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.F. and J.F.S. conceived and designed research; A.F., C.N., M.P., F.G.H., T.W., S.I.D., F.M.I., M.A.M., D.A.E., and J.F.S. performed experiments; A.F., C.N., M.P., F.G.H., S.I.D., F.M.I., M.A.M., D.A.E., and J.F.S. analyzed data; A.F., M.P., F.G.H., R.E.W., S.I.D., F.M.I., M.A.M., D.A.E., and J.F.S. interpreted results of experiments; A.F., C.N., M.P., F.G.H., S.I.D., F.M.I., M.A.M., and J.F.S. prepared figures; A.F. and J.F.S. drafted manuscript; A.F., M.P., F.G.H., T.W., R.E.W., S.I.D., F.M.I., M.A.M., D.A.E., and J.F.S. edited and revised manuscript; A.F., C.N., M.P., F.G.H., T.W., R.E.W., S.I.D., F.M.I., M.A.M., D.A.E., and J.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elin E. Petersen, Marie Skou Pedersen, Per G. Henriksen, Heidi Meldgaard, Claus Wandborg, Rasmus Buchanan from Aarhus University and the staff from the Crocodile Zoo, Eskilstrup, Denmark, for assistance in blood sampling and in laboratory work. We thank Vikas Kumar (Mass Spectrometry and Proteomics Core Facility, University of Nebraska Medical Center) for assistance with the MS/MS data analysis and Anthony Signore for helpful suggestions.
REFERENCES
- 1.Bartlett GR. Phosphate compounds in vertebrate red blood cells. Am Zool 20: 103–114, 1980. doi: 10.1093/icb/20.1.103. [DOI] [Google Scholar]
- 2.Bauer C, Forster M, Gros G, Mosca A, Perrella M, Rollema HS, Vogel D. Analysis of bicarbonate binding to crocodilian hemoglobin. J Biol Chem 256: 8429–8435, 1981. [PubMed] [Google Scholar]
- 3.Bauer C, Jelkmann W. Carbon dioxide governs the oxygen affinity of crocodile blood. Nature 269: 825–827, 1977. doi: 10.1038/269825a0. [DOI] [PubMed] [Google Scholar]
- 4.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys 81: 3684–3690, 1984. doi: 10.1063/1.448118. [DOI] [Google Scholar]
- 5.Bidon-Chanal A, Marti MA, Estrin DA, Luque FJ. Exploring the nitric oxide detoxification mechanism of Mycobacterium tuberculosis truncated haemoglobin N. In: Self-Organization of Molecular Systems: From Molecules and Clusters to Nanotubes and Proteins, edited by Russo N, Antonchenko VY, and Kryachko ES. Dordrecht, The Netherlands: Springer, 2009, p. 33–47. [Google Scholar]
- 6.Bringas M, Petruk AA, Estrin DA, Capece L, Martí MA. Tertiary and quaternary structural basis of oxygen affinity in human hemoglobin as revealed by multiscale simulations. Sci Rep 7: 10926, 2017. doi: 10.1038/s41598-017-11259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busk M, Overgaard J, Hicks JW, Bennett AF, Wang T. Effects of feeding on arterial blood gases in the American alligator Alligator mississippiensis. J Exp Biol 203: 3117–3124, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Cadiz L, Bundgaard A, Malte H, Fago A. Hypoxia enhances blood O2 affinity and depresses skeletal muscle O2 consumption in zebrafish (Danio rerio). Comp Biochem Physiol B Biochem Mol Biol 234: 18–25, 2019. doi: 10.1016/j.cbpb.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Capece L, Estrin DA, Marti MA. Dynamical characterization of the heme NO oxygen binding (HNOX) domain. Insight into soluble guanylate cyclase allosteric transition. Biochemistry 47: 9416–9427, 2008. doi: 10.1021/bi800682k. [DOI] [PubMed] [Google Scholar]
- 10.Coulson RA, Hernandez T. Alligator metabolism studies on chemical reactions in vivo. Comp Biochem Physiol B 74: 1–175, 1983. doi: 10.1016/0305-0491(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 11.Coulson RA, Hernandez T, Dessauer HC. Alkaline tide of the alligator. Proc Soc Exp Biol Med 74: 866–869, 1950. doi: 10.3181/00379727-74-18072. [DOI] [PubMed] [Google Scholar]
- 12.Damsgaard C, Storz JF, Hoffmann FG, Fago A. Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am J Physiol Regul Integr Comp Physiol 305: R961–R967, 2013. doi: 10.1152/ajpregu.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darden T, York D, Pedersen L. Particle mesh Ewald: An N.Log(N) method for Ewald sums in large systems. J Chem Phys 98: 10089–10092, 1993. doi: 10.1063/1.464397. [DOI] [Google Scholar]
- 14.El Hage K, Hédin F, Gupta PK, Meuwly M, Karplus M. Valid molecular dynamics simulations of human hemoglobin require a surprisingly large box size. eLife 7: e35560, 2018. doi: 10.7554/eLife.35560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fago A, Malte H, Dohn N. Bicarbonate binding to hemoglobin links oxygen and carbon dioxide transport in hagfish. Respir Physiol 115: 309–315, 1999. doi: 10.1016/S0034-5687(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 16.Green RE, Braun EL, Armstrong J, Earl D, Nguyen N, Hickey G, Vandewege MW, St John JA, Capella-Gutiérrez S, Castoe TA, Kern C, Fujita MK, Opazo JC, Jurka J, Kojima KK, Caballero J, Hubley RM, Smit AF, Platt RN, Lavoie CA, Ramakodi MP, Finger JW Jr, Suh A, Isberg SR, Miles L, Chong AY, Jaratlerdsiri W, Gongora J, Moran C, Iriarte A, McCormack J, Burgess SC, Edwards SV, Lyons E, Williams C, Breen M, Howard JT, Gresham CR, Peterson DG, Schmitz J, Pollock DD, Haussler D, Triplett EW, Zhang G, Irie N, Jarvis ED, Brochu CA, Schmidt CJ, McCarthy FM, Faircloth BC, Hoffmann FG, Glenn TC, Gabaldón T, Paten B, Ray DA. Three crocodilian genomes reveal ancestral patterns of evolution among archosaurs. Science 346: 1254449, 2014. doi: 10.1126/science.1254449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grispo MT, Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem 287: 37647–37658, 2012. doi: 10.1074/jbc.M112.375600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gronenborn AM, Clore GM, Brunori M, Giardina B, Falcioni G, Perutz MF. Stereochemistry of ATP and GTP bound to fish haemoglobins. A transferred nuclear overhauser enhancement, 31P-nuclear magnetic resonance, oxygen equilibrium and molecular modelling study. J Mol Biol 178: 731–742, 1984. doi: 10.1016/0022-2836(84)90249-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann FG, Storz JF. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol 24: 1982–1990, 2007. doi: 10.1093/molbev/msm127. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol Biol Evol 27: 1126–1138, 2010. doi: 10.1093/molbev/msp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann FG, Vandewege MW, Storz JF, Opazo JC. Gene turnover and diversification of the α- and β-globin gene families in sauropsid vertebrates. Genome Biol Evol 10: 344–358, 2018. doi: 10.1093/gbe/evy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingsworth SA, Dror RO. Molecular dynamics simulation for all. Neuron 99: 1129–1143, 2018. doi: 10.1016/j.neuron.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hub JS, Kubitzki MB, de Groot BL. Spontaneous quaternary and tertiary T-R transitions of human hemoglobin in molecular dynamics simulation. PLOS Comput Biol 6: e1000774, 2010. doi: 10.1371/journal.pcbi.1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janecka JE, Nielsen SSE, Andersen SD, Hoffmann FG, Weber RE, Anderson T, Storz JF, Fago A. Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J Exp Biol 218: 2402–2409, 2015. doi: 10.1242/jeb.125369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jendroszek A, Malte H, Overgaard CB, Beedholm K, Natarajan C, Weber RE, Storz JF, Fago A. Allosteric mechanisms underlying the adaptive increase in hemoglobin-oxygen affinity of the bar-headed goose. J Exp Biol 221: jeb185470, 2018. doi: 10.1242/jeb.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen FB, Wang T, Jones DR, Brahm J. Carbon dioxide transport in alligator blood and its erythrocyte permeability to anions and water. Am J Physiol Regul Integr Comp Physiol 274: R661–R671, 1998. doi: 10.1152/ajpregu.1998.274.3.R661. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys 79: 926–935, 1983. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 28.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780, 2013. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komiyama NH, Miyazaki G, Tame J, Nagai K. Transplanting a unique allosteric effect from crocodile into human haemoglobin. Nature 373: 244–246, 1995. doi: 10.1038/373244a0. [DOI] [PubMed] [Google Scholar]
- 30.Leclercq F, Schnek AG, Braunitzer G, Stangl A, Schrank B. Direct reciprocal allosteric interaction of oxygen and hydrogen carbonate sequence of the haemoglobins of the Caiman (Caiman crocodylus), the Nile crocodile (Crocodylus niloticus) and the Mississippi crocodile (Alligator mississippiensis). Hoppe Seylers Z Physiol Chem 362: 1151–1158, 1981. [PubMed] [Google Scholar]
- 31.Loncharich RJ, Brooks BR, Pastor RW. Langevin dynamics of peptides: the frictional dependence of isomerization rates of N-acetylalanyl-N'-methylamide. Biopolymers 32: 523–535, 1992. doi: 10.1002/bip.360320508. [DOI] [PubMed] [Google Scholar]
- 32.Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11: 3696–3713, 2015. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nat Biotechnol 21: 255–261, 2003. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 34.Marti MA, Crespo A, Capece L, Boechi L, Bikiel DE, Scherlis DA, Estrin DA. Dioxygen affinity in heme proteins investigated by computer simulation. J Inorg Biochem 100: 761–770, 2006. doi: 10.1016/j.jinorgbio.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Meagher KL, Redman LT, Carlson HA. Development of polyphosphate parameters for use with the AMBER force field. J Comput Chem 24: 1016–1025, 2003. doi: 10.1002/jcc.10262. [DOI] [PubMed] [Google Scholar]
- 36.Minh BQ, Nguyen MAT, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 30: 1188–1195, 2013. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadra AD, Martí MA, Pesce A, Bolognesi M, Estrin DA. Exploring the molecular basis of heme coordination in human neuroglobin. Proteins 71: 695–705, 2008. doi: 10.1002/prot.21814. [DOI] [PubMed] [Google Scholar]
- 38.Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol 32: 978–997, 2015. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natarajan C, Hoffmann FG, Weber RE, Fago A, Witt CC, Storz JF. Predictable convergence in hemoglobin function has unpredictable molecular underpinnings. Science 354: 336–339, 2016. doi: 10.1126/science.aaf9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274, 2015. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oaks JR. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution 65: 3285–3297, 2011. doi: 10.1111/j.1558-5646.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- 42.Opazo JC, Hoffmann FG, Natarajan C, Witt CC, Berenbrink M, Storz JF. Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression. Mol Biol Evol 32: 871–887, 2015. doi: 10.1093/molbev/msu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perutz MF. Species adaptation in a protein molecule. Mol Biol Evol 1: 1–28, 1983. [DOI] [PubMed] [Google Scholar]
- 44.Perutz MF, Bauer C, Gros G, Leclercq F, Vandecasserie C, Schnek AG, Braunitzer G, Friday AE, Joysey KA. Allosteric regulation of crocodilian haemoglobin. Nature 291: 682–684, 1981. doi: 10.1038/291682a0. [DOI] [PubMed] [Google Scholar]
- 45.Pough FH. Blood-oxygen transport and delivery in reptiles. Am Zool 20: 173–185, 1980. doi: 10.1093/icb/20.1.173. [DOI] [Google Scholar]
- 46.Rapoport S, Guest GM. Distribution of acid-soluble phosphorus in the blood cells of various vertebrates. J Biol Chem 138: 269–282, 1941. [Google Scholar]
- 47.Revsbech IG, Tufts DM, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF, Fago A. Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J Exp Biol 216: 4264–4271, 2013. doi: 10.1242/jeb.091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard V, Dodson GG, Mauguen Y. Human deoxyhaemoglobin-2,3-diphosphoglycerate complex low-salt structure at 2.5 Å resolution. J Mol Biol 233: 270–274, 1993. doi: 10.1006/jmbi.1993.1505. [DOI] [PubMed] [Google Scholar]
- 49.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of Cartesian equations of motion of a system with constraints - molecular dynamics of N-alkanes. J Comput Phys 23: 327–341, 1977. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 50.Schafer W, Braunitzer G, Stangl A. Direct allosteric interaction of oxygen and bicarbonate: N-acetyl-alanyl-seryl-phenylalanine, N-terminal sequence of the beta-chains of the hemoglobins of Nile crocodile (Crocodylus niloticus) and Mississippi crocodile (Alligator mississippiensis). Zeitschrift Naturforschung C Biosci 36: 902–903, 1981. [PubMed] [Google Scholar]
- 51.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1: 2856–2860, 2006. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 52.Storz JF. Hemoglobin: Insights into Protein Structure, Function, and Evolution. Oxford, UK: Oxford University Press, 2019. [Google Scholar]
- 53.Storz JF, Hoffmann FG, Opazo JC, Sanger TJ, Moriyama H. Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J Exp Biol 214: 575–581, 2011. doi: 10.1242/jeb.050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storz JF, Natarajan C, Moriyama H, Hoffmann FG, Wang T, Fago A, Malte H, Overgaard J, Weber RE. Oxygenation properties and isoform diversity of snake hemoglobins. Am J Physiol Regul Integr Comp Physiol 309: R1178–R1191, 2015. doi: 10.1152/ajpregu.00327.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44: W232–W235, 2016. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Assendelft OW. Spectrophotometry of hemoglobin derivatives. Assen, The Netherlands: Royal Vangorcum, Ltd, 1970. [Google Scholar]
- 57.Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics 54: 5.6.1–5.6.37, 2016. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol (1985) 72: 1611–1615, 1992. doi: 10.1152/jappl.1992.72.4.1611. [DOI] [PubMed] [Google Scholar]
- 59.Weber RE, Fago A, Malte H, Storz JF, Gorr TA. Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin. Am J Physiol Regul Integr Comp Physiol 305: R300–R312, 2013. doi: 10.1152/ajpregu.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber R, White F. Chloride-dependent organic phosphate sensitivity of the oxygenation reaction in crocodilian hemoglobins. J Exp Biol 192: 1–11, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Weber RE, White FN. Oxygen binding in alligator blood related to temperature, diving, and “alkaline tide”. Am J Physiol Regul Integr Comp Physiol 251: R901–R908, 1986. doi: 10.1152/ajpregu.1986.251.5.R901. [DOI] [PubMed] [Google Scholar]
- 62.Witze ES, Old WM, Resing KA, Ahn NG. Mapping protein post-translational modifications with mass spectrometry. Nat Methods 4: 798–806, 2007. doi: 10.1038/nmeth1100. [DOI] [PubMed] [Google Scholar]
- 63.Xiang TX, Liu F, Grant DM. Generalized Langevin equations for molecular dynamics in solution. J Chem Phys 94: 4463–4471, 1991. doi: 10.1063/1.460602. [DOI] [Google Scholar]