Abstract
In males, obesity increases sympathetic nerve activity (SNA), but the mechanisms are unclear. Here, we investigate insulin, via an action in the arcuate nucleus (ArcN), and downstream neuropathways, including melanocortin receptor 3/4 (MC3/4R) in the hypothalamic paraventricular nucleus (PVN) and dorsal medial hypothalamus (DMH). We studied conscious and α-chloralose-anesthetized Sprague-Dawley rats fed a high-fat diet, which causes obesity prone (OP) rats to accrue excess fat and obesity-resistant (OR) rats to maintain fat content, similar to rats fed a standard control (CON) diet. Nonspecific blockade of the ArcN with muscimol and specific blockade of ArcN insulin receptors (InsR) decreased lumbar SNA (LSNA), heart rate (HR), and mean arterial pressure (MAP) in OP, but not OR or CON, rats, indicating that insulin supports LSNA in obese males. In conscious rats, intracerebroventricular infusion of insulin increased MAP only in OP rats and also improved HR baroreflex function from subnormal to supranormal. The brain sensitization to insulin may elucidate how insulin can drive central SNA pathways when transport of insulin across the blood-brain barrier may be impaired. Blockade of PVN, but not DMH, MC3/4R with SHU9119 decreased LSNA, HR, and, MAP in OP, but not OR or CON, rats. Interestingly, nanoinjection of the MC3/4R agonist melanotan II (MTII) into the PVN increased LSNA only in OP rats, similar to PVN MTII-induced increases in LSNA in CON rats after blockade of sympathoinhibitory neuropeptide Y Y1 receptors. ArcN InsR expression was not increased in OP rats. Collectively, these data indicate that obesity increases SNA, in part via increased InsR signaling and downstream PVN MC3/4R.
Keywords: arcuate nucleus, insulin, obesity, paraventricular nucleus, sympathetic nerve activity
INTRODUCTION
The prevalence of obesity continues to increase worldwide, despite the well-established association of numerous metabolic and cardiovascular diseases. An early event upon initiation of a high-fat diet (HFD) and obesity development is an increase in sympathetic nerve activity (SNA) (4, 50), which can lead to hypertension, particularly in males (14, 26). However, the mechanistic links between obesity and sympathoexcitation are incompletely understood.
While leptin has received considerable attention (9, 28), the role of another sympathoexcitatory metabolic hormone, insulin, remains uncertain. A recent report that intracerebroventricular infusion of insulin increases basal and baroreflex control of SNA and heart rate (HR) dramatically more in anesthetized obese than lean male rats provides indirect support for a role of insulin (60). However, whether endogenous insulin contributes to basal sympathoexcitation in obese subjects is unclear. Therefore, because insulin acts in the arcuate nucleus (ArcN) to increase SNA (16, 44), we determined if nonspecific blockade of the ArcN with muscimol or select blockade of ArcN insulin receptors (InsR) with an InsR antagonist (S961) decreases SNA in anesthetized obese, but not lean, male rats.
The downstream neuropathways by which insulin increases basal SNA with obesity require clarification. Injection of insulin into the ArcN increases SNA via two major neuronal types that influence SNA: insulin suppresses the activity of inhibitory neuropeptide Y (NPY) neurons (17) and activates excitatory proopiomelanocortin (POMC) neurons (74). These two cell types project to several hypothalamic sites, but two nuclei, in particular, have been highlighted as key in obesity-induced sympathoexcitation and hypertension: the paraventricular nucleus (PVN) (19) and the dorsomedial hypothalamus (DMH) (65). PVN presympathetic neurons receive direct inputs from ArcN NPY neurons and POMC neurons, the latter of which release α-melanocyte-stimulating hormone (α-MSH), which binds to melanocortin receptor type 4 receptors (MC4R) (62). Importantly, tonic NPY inhibition normally prevents sympathoexcitation induced by α-MSH (62), but we recently showed that obesity suppresses NPY inhibition of SNA in the PVN (60). Therefore, this disinhibition may permit POMC neurons and α-MSH inputs to the PVN and/or DMH to contribute to basal sympathoexcitation, as well as enhanced responses to insulin, in obese individuals. Indeed, acute or chronic intracerebroventricular administration of SHU9119, which blocks MC4R, reverses obesity-induced hypertension and decreases SNA (7, 25), yet the specific sites of action of α-MSH are unknown (for review see Ref. 23). Therefore, we also tested the hypothesis that PVN and/or DMH α-MSH contributes to basal SNA by determining if blockade of PVN or DMH MC4R with SHU9119 decreases SNA in obese, but not lean, male rats.
Finally, an important role for insulin in obese humans has been disputed, because intravenous insulin infusion increases muscle SNA (MSNA) less in awake obese than lean subjects (70, 73). However, as previously discussed (14, 42, 73), obesity decreases transport of insulin across the blood-brain barrier (BBB) (8, 32, 33, 66), which would limit the impact of further increments in plasma insulin in obese individuals with already elevated plasma insulin levels. Yet, if the brain were sensitized, insulin could still contribute to obesity-induced sympathoexcitation and hypertension. To test this hypothesis, in conscious obese and lean rats, we infused insulin intracerebroventricularly, to bypass the BBB, and measured changes in mean arterial pressure (MAP) and autonomic function.
METHODS
Animals
Male (n = 154, not including “middle” rats) Sprague-Dawley rats (Charles River Laboratories) were housed individually in a temperature-controlled (22 ± 2°C) room with a 12:12-h light-dark cycle. Food and water were provided ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Health and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University.
Diet-Induced Obesity Rat Model
At ~8 wk of age, male rats (~250 g body wt) were placed on a purified moderately HFD [32% kcal as fat; Purina LabDiet 571R (5001 with 10% lard) or Research Diets D16030908] or a low-fat control (CON) diet (13.5% kcal as fat; LabDiet 5001 or Research Diets D16030911). HFD-fed rats diverge into two populations: those with high weight gain [obesity-prone (OP)] and those with weight gain similar to rats fed the low-fat CON diet [obesity-resistant (OR)]. Consistent with significant previous work (11, 24, 38, 46, 60, 76), after ~4 wk on the diet, rats in the top tertile of weight gain were defined as OP and those in the bottom tertile as OR; the “middle” tertile of rats was not studied.
Hypothalamic Nanoinjections in Anesthetized Rats
Surgery.
Anesthesia was induced and maintained with 2–5% isoflurane in 100% oxygen. A rectal thermistor and heating pad were used to maintain body temperature at 37 ± 1°C. A tracheal tube and femoral arterial and venous catheters were implanted, and stainless steel electrodes were placed around the lumbar sympathetic nerve, as previously described (59, 60). We measured lumbar SNA (LSNA), because the lumbar nerve is particularly sensitive to insulin (whereas others, e.g., the renal or adrenal, are unresponsive) (49, 74). The rat was then placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA), and, following a midline incision, a hole was burred in the skull near the midline in preparation for hypothalamic PVN, DMH, or ArcN nanoinjections. After completion of surgery, isoflurane anesthesia was slowly withdrawn over 30 min, and a continuous intravenous infusion of α-chloralose (Sigma-Aldrich, St. Louis, MO) was initiated and continued for the duration of the experiment. Lean (CON and OR) rats received a 50 mg/kg loading dose over 30 min followed by a 25 mg·kg−1·h−1 maintenance dose; OP rats received a dose equivalent to the weight of a lean rat at a similar age (60). Throughout the experiment, artificial ventilation with 100% oxygen was maintained, and respiratory rate and tidal volume were adjusted to maintain expired CO2 at 3.5–4.5%. Anesthetic depth was regularly confirmed by the lack of a pressor response to a foot or tail pinch; if necessary, additional α-chloralose was administered. After completion of surgery and the α-chloralose loading dose, rats were allowed to stabilize for ≥60 min before experimentation. After experimentation, all rats were killed with an overdose of pentobarbital, and the postmortem nerve activity served as a measure of background noise.
Hypothalamic nanoinjections.
Nanoinjections into the PVN, DMH, and ArcN were conducted with single-barreled glass micropipettes (20- to 40-μm tip outer diameter), as described previously (60). Briefly, with a flat skull and using bregma and the dorsal surface of the dura as zero, micropipettes were positioned using the following coordinates: 1.7–1.9 mm caudal, 0.5 mm lateral, and 7.3–7.5 mm ventral for the PVN, 3.5–3.7 mm caudal, 0.3 mm lateral, and 9.5–9.7 mm ventral for the ArcN, and −3.2 mm caudal, 0.5 mm lateral, and −8.3 to –8.8 mm ventral for the DMH. All nanoinjections (60 nL for PVN and DMH and 30 nL for ArcN) were made bilaterally, with ∼2 min between injections, and each injection was conducted over ~5–10 s using a pressure injection system (Pressure System IIe, Toohey Company, Fairfield, NJ). In some animals, before nanoinjection of drugs, artificial cerebrospinal fluid (aCSF) was injected into the ArcN, DMH, or PVN as vehicle control. At the conclusion of the experiment, the same pipette and coordinates used for experimental nanoinjections were used to inject fluorescent microbeads or 2.5% Alcian blue into the PVN, DMH, or ArcN. The brain was removed and placed in 4% paraformaldehyde for ≥48 h. A cryostat was used to cut the hypothalamus into 25-μm sections, which were mounted on gelatin-coated glass microscope slides. Correct placements were confirmed using a standard anatomical atlas (54).
Experimental protocols.
After a 60-min equilibration period and collection of baseline data, one of the following experiments was performed in OP, OR, and CON rats. 1) To determine if insulin actions in the ArcN support SNA and MAP in obese rats, muscimol (30 pmol in 30 nL; Tocris Bioscience, Minneapolis, MN), the InsR antagonist S961 (100 ng in 30 nL; Novo Nordisk, Bagsvaerd, Denmark) (53, 58), or aCSF was nanoinjected bilaterally into the ArcN, as previously described (16, 17), and measurements of MAP, HR, and LSNA were continued for 10–90 min. Note that the muscimol dose is relatively small (39) to avoid the nonspecific effects of leakage across the ventral ventricular surface. We previously documented that the above-mentioned dose of S961 blocks SNA responses to moderate doses of insulin in the ArcN (61). 2) To determine if PVN or DMH MC4R support SNA and MAP in obese rats, muscimol (60 pmol in 60 nL) or the MC4R antagonist SHU9119 (30 pmol in 60 nL; Tocris Bioscience) was injected into the PVN or DMH, as described above. In some rats, muscimol was injected into the DMH ≥1 h following injection of SHU9119. Because leptin (62) and insulin (67) each increase SNA in part by activating PVN ionotropic glutamate receptors [iGluR; which may interact with α-MSH (62)], we also tested the effect of bilateral nanoinjections of kynurenate (KYN, 2.7 nmol in 60 nL; Tocris Bioscience) into the PVN in another set of CON, OR, and OP rats. 3) To determine if activation of PVN MC4R increases SNA more in obese rats, the MC4R agonist melanotan II (MTII, 60 pmol in 60 nL; Tocris Bioscience) was injected bilaterally into the PVN, and measurements were continued for 30 min.
Data acquisition.
Pulsatile arterial pressure and MAP, heart rate (HR), and raw LSNA were continuously recorded throughout the experiment with Grass amplifiers (model 79D, Grass Instrument, Quincy, MA) and a data acquisition and analysis system (model MP100, Biopac Systems, Santa Barbara, CA) sampling at 2,000 Hz. SNA was band-pass-filtered (100–3,000 Hz) and amplified (×10,000). The SNA signal was then rectified, integrated in 1-s bins, and, in each rat, corrected for postmortem background activity. SNA was normalized to the baseline (or control) SNA, which was defined as the average of the 30-s period before the hypothalamic nanoinjections, and expressed as percentage of control.
Drugs.
Muscimol (18, 74) (1 mM) and SHU9119 (59, 74) (0.5 mM in aCSF with 10% DMSO) were obtained from Tocris Bioscience and the InsR antagonist S961 (100 ng in 30 nL) from Novo Nordisk (53, 58). Drugs for nanoinjection were dissolved in aCSF [in mM: 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3, 1.3 Na2HPO4, and 2 dextrose (pH 7.4)].
Intracerebroventricular Insulin Infusion in Conscious Male Rats
Conscious male rats were studied to confirm that obesity amplifies the effects of insulin observed in anesthetized animals (60). In addition to monitoring MAP and HR, we also assessed baroreflex control of HR, which is impaired by obesity and was shown to be improved in anesthetized OP rats by intracerebroventricular infusion of insulin (60).
Surgery.
For all surgeries, rats were anesthetized with 2% isoflurane in 100% oxygen, and aseptic technique was used. Rats received a single intramuscular injection of 30,000 U of penicillin G (Hanford’s United States Veterinary Products) 10–15 min before the incision and codeine (1 mg/100 mL) in their drinking water for 3 days before the procedure.
Intracerebroventricular cannula implantation.
After ~3.5 wk on the diets, the rats were weighed, anesthetized, and placed in a stereotaxic apparatus (David Kopf) with the incisor bar at −3.5 mm. A 23-gauge 16-mm-long sterile guide cannula was implanted from vertical into the lateral ventricle with the following coordinates relative to bregma: −1 mm anteroposterior, +1.4 mm lateral from the midline, and −4.2 mm dorsoventral. The intracerebroventricular cannula was then fixed in place with dental cement, and rats were kept in individual cages postoperatively. The position of the cannula was verified postmortem by dye injection through the cannula and the presence of dye throughout the cerebroventricular system.
Catheter implantation.
At 5 days following the intracerebroventricular cannula implantation, the rats were anesthetized, and an arterial catheter (PE 50) was inserted through a small inguinal incision into the femoral artery and advanced into the distal abdominal aorta. In addition, two venous catheters (PE 10) were inserted into the femoral vein and advanced into the distal inferior vena cava. The catheters were tunneled subcutaneously and exteriorized between the scapulae. Catheter patency was maintained by flushing with heparin saline (100 U/mL) at least three times per week. At least 5 days of recovery were allowed before experimentation.
Drugs.
Insulin (human, 100 U/mL) was obtained from Novo Nordisk (catalog no. U-100, Novolin). aCSF [in mM: 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3, and 1.3 Na2HPO4 (pH 7.4)] was filtered before use.
Experimental protocol.
Experiments were conducted 5 days after the last surgery, when the rats had been on their respective diets for 4–6 wk. Baseline HR baroreflex curves were obtained as described previously (76). Then an intracerebroventricular infusion of a maximal dose of insulin [100 µU/min at 0.6 µL/min (5, 55)] commenced and was continued for the duration of the experiment. The dose was chosen based on prior dose-response studies (51) and because we wanted to compare this study with a previous report in anesthetized rats (60). After 1 h, a second baroreflex curve was generated.
Baroreflex curve generation.
Complete baroreflex function curves were produced using well-established, previously published methodology (5, 76) in conscious rats while they remained in their home cage. Briefly, arterial pressure was increased and decreased using separate slow intravenous infusions of increasing doses of phenylephrine and nitroprusside, respectively, with each ramp in pressure conducted in a random fashion and completed in ~3–5 min. Blood pressure and HR were allowed to return to basal levels before another ramp was initiated; therefore, the construction of a complete curve could take up to 1 h. The data were collected using a Biopac MP100 data acquisition and analysis system sampling at 1,000 Hz.
Measurement of ArcN InsR
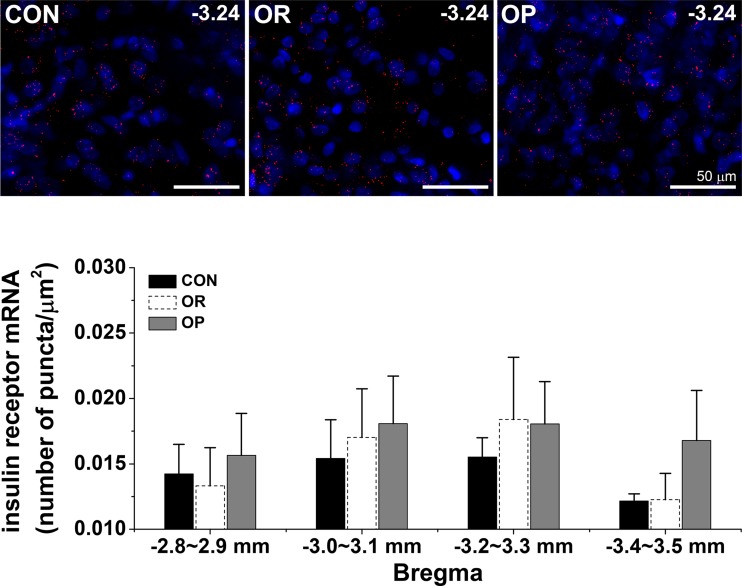
To test if insulin actions in the ArcN in obese rats involve an increase in InsR expression, InsR mRNA was quantified in the ArcN using fluorescent in situ hybridization (FISH; RNAScope) in CON, OR, and OP rats, as previously described (60). Briefly, male CON, OR, and OP rats (4–6 wk HFD) were deeply anesthetized and perfused transcardially with physiological saline followed by ice-cold 4% paraformaldehyde in 0.1 M sodium phosphate buffer. After brains were removed and sectioned (15–20 μm), FISH was performed using the RNAScope Fluorescent Multiplex Kit (catalog no. 320850, ACDBio, San Francisco, CA) according to the manufacturer’s instructions. The InsR ACDBio probe was Rattus norvegicus InsR (406421; accession no. NM_017071.2; target region 431–1287). Generally, brains from a set of CON, OR, and OP male rats were processed in parallel. Sections were imaged on a Zeiss ApoTome2 on AxioImager with a ×20/0.8 Plan APO objective; the InsR fluorophore was detected using ATTO 550. Images were analyzed using Zen 2 (blue edition) software (Zeiss, Jena, Germany) according to the following protocol. The lowest threshold that eliminated signal in the negative control was determined to set the initial threshold for the signal with absorbance at 555 nm (A555). The ArcN region of interest (ROI) was outlined, and the threshold was readjusted slightly, if needed, so that individual puncta representing only InsR mRNA within the ROI were counted. For each ArcN section, one side was analyzed as follows. The total area of the arcuate ROI was determined, and the number of InsR mRNA puncta within the ROI was counted and summed. If both sides of the ArcN were quantified, the data were averaged. Then, for each animal, the ratio of InsR to arcuate area was calculated in four regions of the ArcN (mm from bregma: 2.8–2.9, 3.0–3.1, 3.2–3.3, and 3.4–3.5), and data from two sections per region were averaged. The data were compared between groups using two-way repeated-measures ANOVA. In Fig. 9, to ensure a visible InsR mRNA signal, the signal was optimized equally in all images in Zen 2.
Fig. 9.
Obesity does not alter arcuate nucleus (ArcN) insulin receptor (InsR) expression. Top: representative ventral ArcN images of InsR at bregma level −3.24 from a set of control (CON), obesity-resistant (OR), and obesity-prone (OP) rats (n = 3–4 per group). Red puncta are InsR; blue is DAPI nuclear stain. Bottom: ArcN InsR expression did not differ significantly between CON, OR, and OP rats.
Data Analysis
One-way ANOVA and the post hoc Newman-Keuls test were used to test for between-group (OP, OR, and CON) differences in baseline values. The between-group effects of intracerebroventricular insulin infusion on basal or baroreflex values in conscious rats were determined using two-way repeated-measures ANOVA and the post hoc Newman-Keuls test; the absolute value baroreflex gain is depicted in insets in the figures. For experiments involving hypothalamic nanoinjections, between-group differences in the time course were first tested using a two-way ANOVA for repeated measures. If this analysis revealed a significant F value, the maximum (or minimum) change in LSNA, HR, and MAP was identified and compared between groups using two-way ANOVA and the Newman-Keuls test. Data are expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Insulin Supports SNA in Obese Males via a Neuropathway that Includes the ArcN and the PVN
Effects of obesity.
The HFD increased body weight more in OP than OR or CON rats, without altering basal MAP or HR (Table 1).
Table 1.
Effects of HFD on basal values in anesthetized rats
| CON (n = 37) |
OR (n = 35) |
OP (n = 42) |
|
|---|---|---|---|
| MAP, mmHg | 104 ± 3 | 104 ± 2 | 110 ± 2 |
| HR, beats/min | 357 ± 6 | 360 ± 7 | 368 ± 7 |
| Body wt, g | |||
| Baseline | 266 ± 2 | 264 ± 2 | 268 ± 2 |
| 4 wk HFD | 438 ± 5 | 425 ± 5 | 490 ± 5* |
| ∆ | 173 ± 5 | 161 ± 5 | 212 ± 3* |
Values are means ± SE; n, number of rats. HFD, high-fat diet; MAP, mean arterial pressure; HR, heart rate; CON, control; OR, obesity-resistant; OP, obesity-prone. Baseline body weight was not obtained in 5 CON, 3 OR, and 5 OP rats.
P < 0.05 vs. CON and OR.
ArcN insulin supports LSNA and MAP in OP rats.
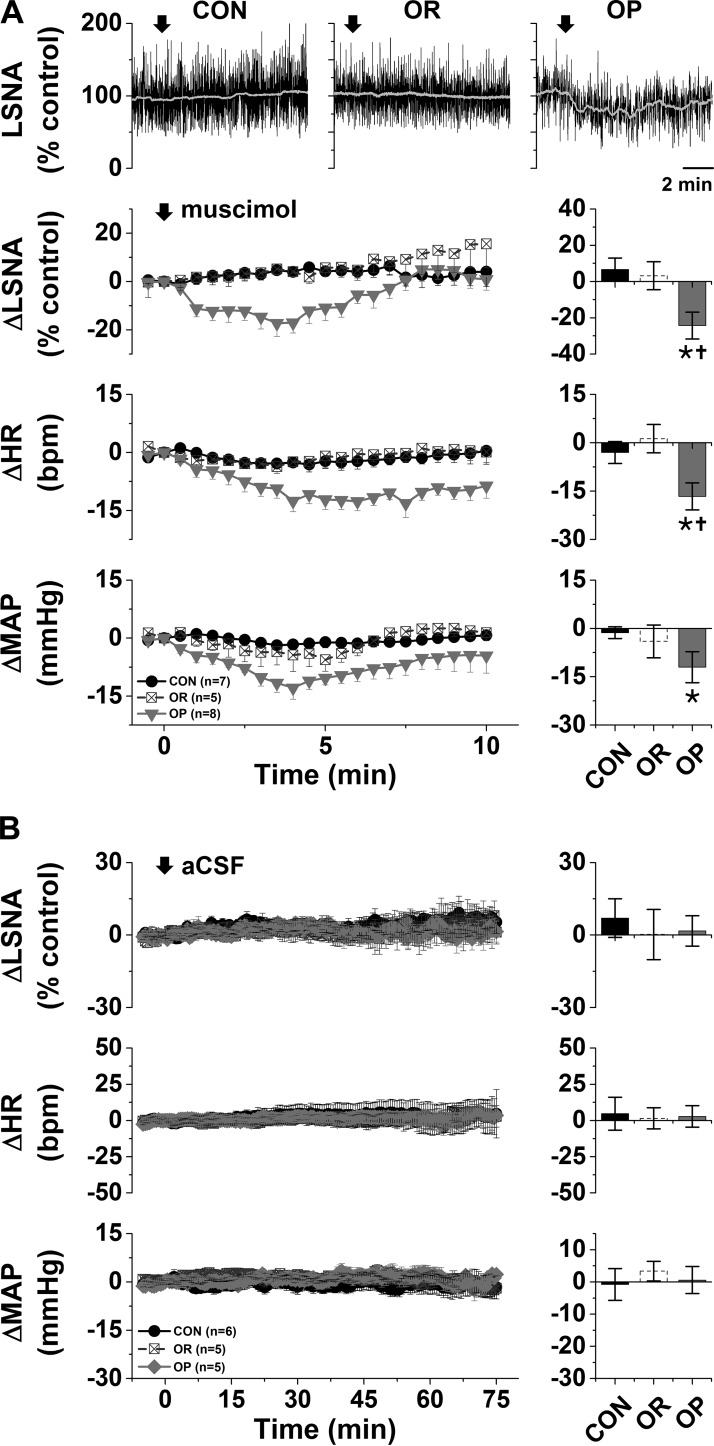
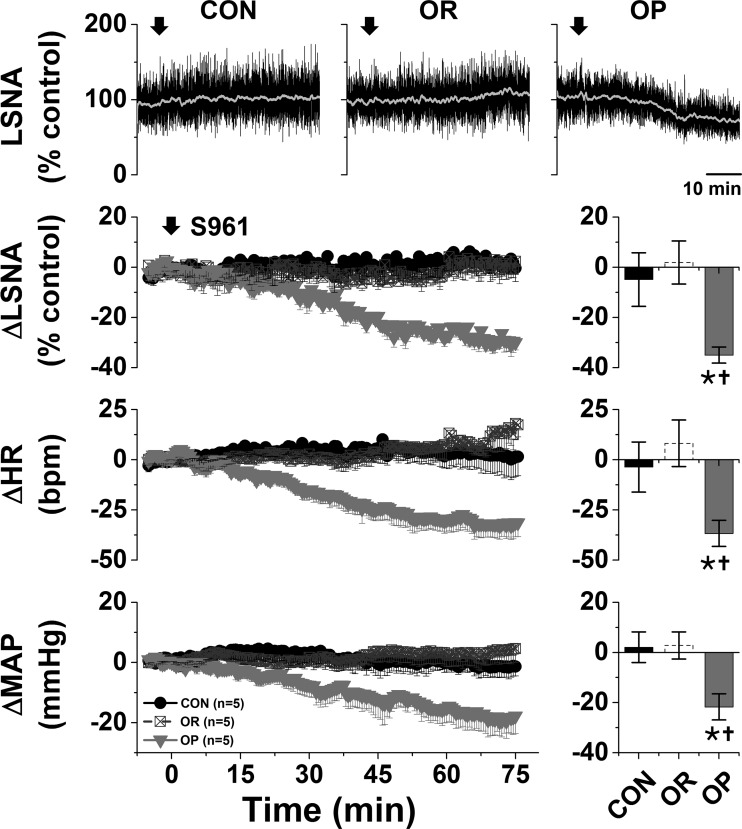
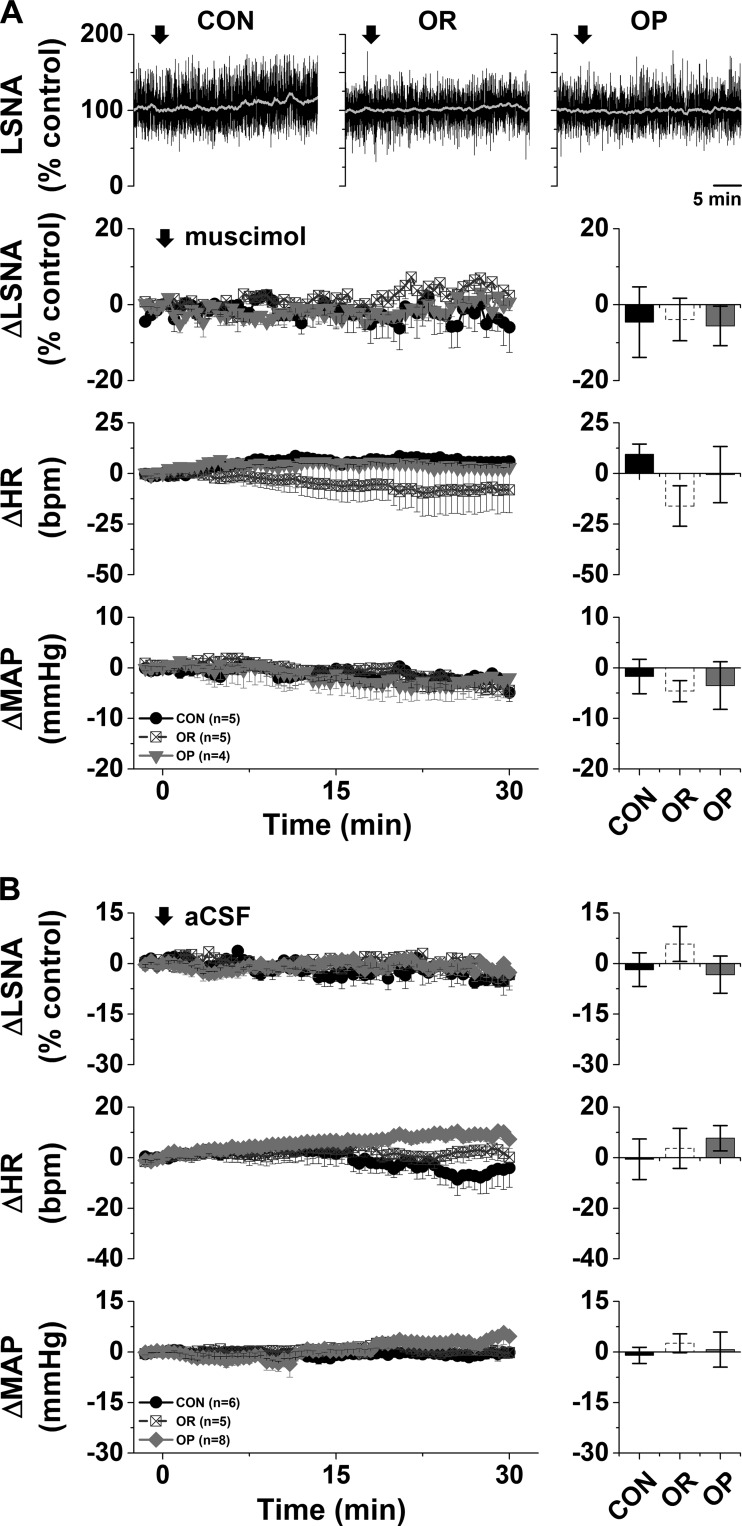
Bilateral nanoinjection of muscimol into the ArcN had no effects in either CON or OR rats but significantly decreased LSNA (−24 ± 7%), MAP (−15 ± 3 mmHg), and HR (−17 ± 4 beats/min) in OP rats (Fig. 1A). The decreases were abruptly initiated but transient, usually lasting for <10 min (Fig. 1A), and likely due to the low dose of muscimol. Blockade of ArcN InsR also decreased LSNA (−35 ± 3%), MAP (−22 ± 5 mmHg), and HR (−37 ± 5 beats/min) in OP, but not OR or CON, rats (Fig. 2). In contrast to nanoinjection of muscimol into the ArcN, the responses took ∼30 min to develop, and values remained suppressed for ≥90 min. In contrast, aCSF injections into the ArcN had no significant effects in any group (Fig. 1B). Collectively, these data indicate that the ArcN, in part via a slowly reversible action of insulin, supports LSNA, HR, and MAP in obese male rats.
Fig. 1.
A: blockade of the arcuate nucleus (ArcN) with muscimol decreases lumbar sympathetic nerve activity (LSNA), heart rate (HR), and mean arterial pressure (MAP) in obesity-prone (OP, n = 8), but not obesity-resistant (OR, n = 5) or control (CON, n = 7), rats. Top: representative traces of LSNA. Bottom: grouped data showing time course of changes (left) and maximal changes (right) in LSNA (at 3.7 ± 0.5 min), HR (at 5.9 ± 0.6 min), and MAP (at 4.7 ± 0.4 min). Two-way repeated-measures ANOVA revealed significant group (P = 0.01), time (P < 0.0001), and interaction (P = 0.01) for LSNA; significant group (P < 0.01), time (P < 0.0001), and interaction (P < 0.0001) for HR; and significant group (P < 0.05) and time (P < 0.0001), but not interaction (P = 0.11), for MAP. *P < 0.05 vs. baseline (0); †P < 0.05 vs. OR and CON. B: nanoinjections of artificial cerebrospinal fluid (aCSF) into the ArcN had no effects on LSNA, HR, or MAP in OP (n = 5), OR (n = 5), or CON (n = 6) rats.
Fig. 2.
Blockade of the arcuate nucleus with the insulin receptor antagonist S961 decreases lumbar sympathetic nerve activity (LSNA), heart rate (HR), and mean arterial pressure (MAP) in obesity-prone (OP, n = 5), but not obesity-resistant (OR, n = 5) and control (CON, n = 5), rats. Top: representative LSNA traces. Bottom: grouped data showing time course of changes (left) and maximal changes (right) in LSNA (at 67.5 ± 4.0 min), HR (at 72.7 ± 1.3 min), and MAP (at 66.3 ± 5.1 min). Two-way repeated-measures ANOVA revealed significant group (P = 0.005), time (P < 0.0001), and interaction (P < 0.0001) for LSNA; significant group (P < 0.005) and interaction (P < 0.0001), but not time, for HR; and significant group (P < 0.05), time (P < 0.01), and interaction (P = 0.0002) for MAP. *P < 0.05 vs. baseline (0); †P < 0.05 vs. OR and CON.
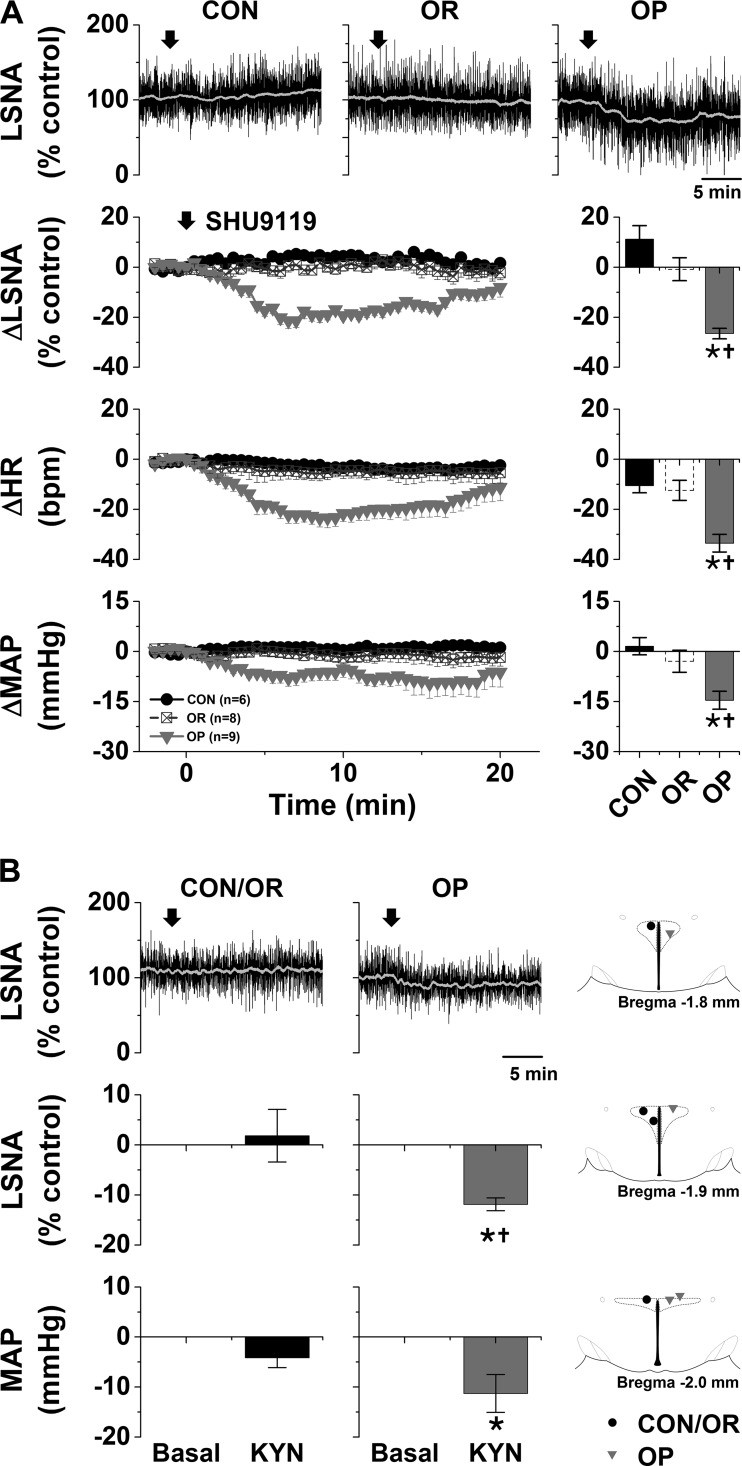
PVN MC4R and iGluR.
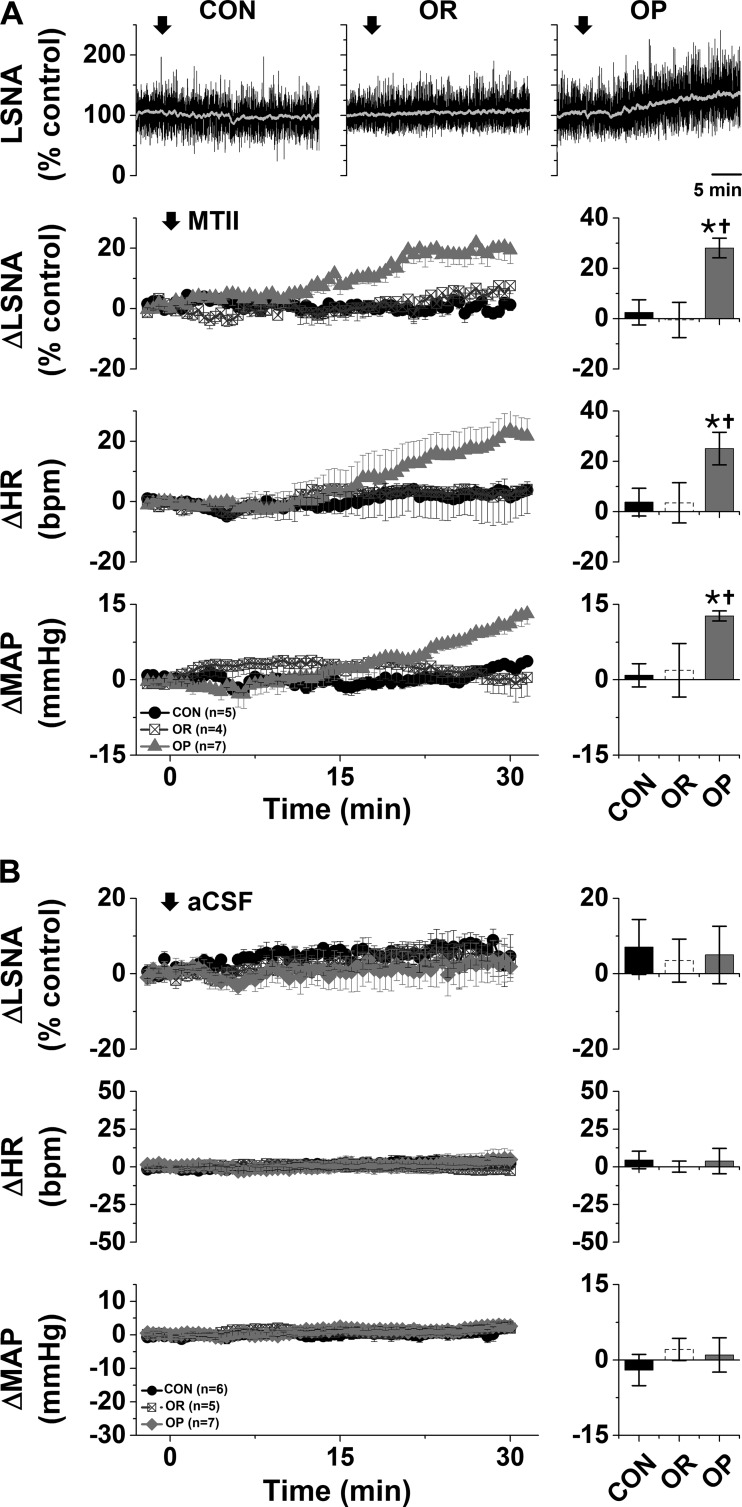
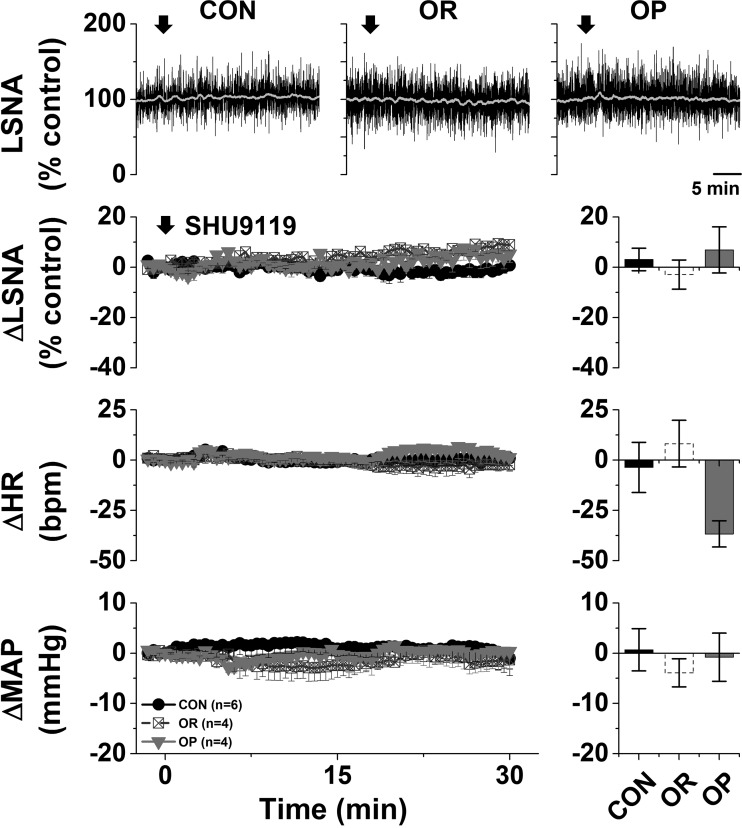
Previous studies demonstrated that nonspecific blockade of the PVN with muscimol decreases LSNA, HR, and MAP in male rats fed a HFD for 12 wk compared with CON rats (19). In preliminary experiments, we found that nanoinjection of muscimol into the PVN also decreased (P < 0.05) LSNA (−19 ± 6% of control), HR (−15 ± 5 beats/min), and MAP (−24 ± 6 mmHg) in OP (n = 3; HFD 4–6 wk), but not CON, rats (62). More selective local blockade of PVN melanocortin receptor 3/4 (MC3/4R) with SHU9119 or iGluR with KYN also elicited rapid decreases in LSNA, HR, and MAP only in OP rats (Fig. 3). On the other hand, nanoinjections of the MC4R agonist MTII into the PVN slowly increased these variables (Fig. 4A) in OP, but not OR or CON, rats. Tonic PVN NPY sympathoinhibition is completely lost in OP rats (60), and in OP rats the increases in LSNA (28 ± 4% of control), HR (25 ± 7 beats/min), and MAP (13 ± 1 mmHg) following nanoinjection of MTII into the PVN were similar to the increases in CON rats after blockade of PVN NPY receptor Y1 (Y1R) (62): 38 ± 9% of control LSNA, 33 ± 7 beats/min HR, and 11 ± 2 mmHg MAP (not significant by Student’s t test). Again, nanoinjection of aCSF into the PVN was without effects (Fig. 4B). Therefore, the PVN, via tonic activation of MC4R and iGluR, is another hypothalamic nucleus that supports LSNA in male obese rats.
Fig. 3.
A: blockade of the paraventricular nucleus (PVN) with SHU9119 decreases lumbar sympathetic nerve activity (LSNA), heart rate (HR), and mean arterial pressure (MAP) in obesity-prone (OP, n = 9), but not obesity-resistant (OR, n = 8) and control (CON, n = 6), rats. Top: representative LSNA traces. Bottom: grouped data showing time course of changes (left) and maximal changes (right) in LSNA (at 7.4 ± 1.0 min), HR (at 9.6 ± 1.7 min), and MAP (at 11.5 ± 2.1 min). Two-way repeated-measures (RM) ANOVA revealed significant group (P = 0002), time (P < 0.0001), and interaction (P < 0.0001) for LSNA; significant group (P < 0.05), time (P < 0.01), and interaction (P < 0.0001) for HR; and significant group (P < 0.05), but not time or interaction, for MAP. *P < 0.05 vs. baseline (0); †P < 0.05 vs. OR and CON. B, left: nanoinjections of kynurenate (KYN) into the PVN decreased LSNA and MAP in OP (n = 4), but not OR (n = 2) or CON (n = 2), rats. HR was not altered in any group (data not shown). *P < 0.05 vs. basal; †P < 0.05 vs. CON/OR (by 2-way RM ANOVA). B, right: histological maps showing injection sites in OP and OR/CON rats.
Fig. 4.
A: nanoinjection of melanotan II (MTII) into the paraventricular nucleus (PVN) increases lumbar sympathetic nerve activity (LSNA), heart rate (HR), and mean arterial pressure (MAP) in obesity-prone (OP, n = 7), but not obesity-resistant (OR, n = 4) and control (CON, n = 5), rats. Top: representative LSNA trace. Bottom: grouped data showing time course of changes (left) and maximal changes (right) in LSNA (at 26.1 ± 2.1 min), HR (at 27.1 ± 1.8 min), and MAP (at 28.6 ± 0.5 min). Two-way repeated-measures (RM) ANOVA revealed significant group (P < 0.01), time (P < 0.0001), and interaction (P < 0.0001) for LSNA; significant time (P < 0.0001) and interaction (P < 0.0001), but not group, for HR; and significant time (P < 0.0001) and interaction (P < 0.0001), but not group, for MAP. *P < 0.05 vs. baseline (0); †P < 0.05 vs. OR and CON. B: nanoinjections of artificial cerebrospinal fluid (aCSF) into the PVN had no effects on LSNA, HR, or MAP in OP (n = 7), OR (n = 5), or CON (n = 6) rats (by 2-way RM ANOVA).
DMH MC4R.
In contrast to the PVN, neither nonspecific blockade of the DMH with muscimol, select blockade of DMH MC3/4R, nor aCSF altered LSNA or HR in any group (Figs. 5 and 6). Blockade of the DMH with muscimol (but not SHU9119 or aCSF) decreased MAP (ANOVA, time effect, P < 0.001), but the hypotensive response did not differ between groups (Fig. 5). These results do not support a role for the DMH (and its stimulation by α-MSH) in support of LSNA, HR, or MAP in OP male rats.
Fig. 5.
A: nanoinjections of muscimol into the dorsal medial hypothalamus (DMH) had no effects on lumbar sympathetic nerve activity (LSNA) or heart rate (HR) in obesity-prone (OP, n = 4), obesity-resistant (OR, n = 5), or control (CON, n = 5) rats. Nanoinjections of muscimol [but not SHU9119 or artificial cerebrospinal fluid (aCSF)] into the DMH decreased MAP (ANOVA, time effect, P < 0.001), but the hypotensive response did not differ between groups. B: nanoinjections of aCSF into the DMH had no effects on LSNA, HR, or MAP in OP (n = 8), OR (n = 5), or CON (n = 6) rats.
Fig. 6.
Nanoinjections of SHU9119 into the dorsal medial hypothalamus had no effects on lumbar sympathetic nerve activity (LSNA), heart rate (HR), or mean arterial pressure (MAP) in obesity-prone (OP, n = 4), obesity-resistant (OR, n = 4), or control (CON, n = 6) rats.
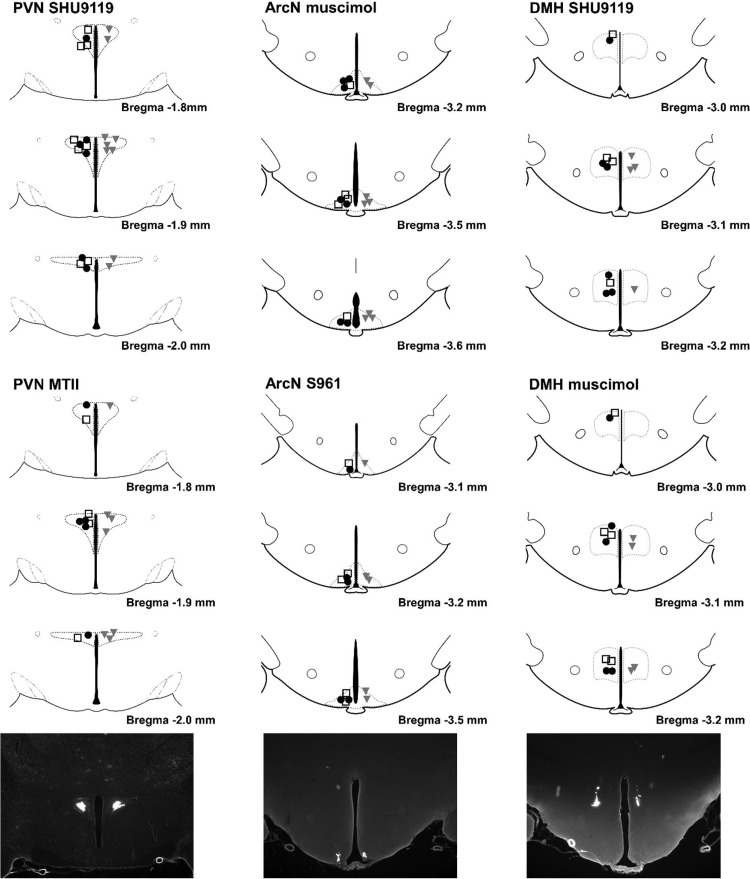
Histological verification of nanoinjections.
A map of the hypothalamus summarizes ArcN, PVN, and DMH injection sites (Fig. 7).
Fig. 7.
Top: summary of paraventricular nucleus (PVN), arcuate nucleus (ArcN), and dorsal medial hypothalamus (DMH) nanoinjection sites. ●, Control (CON); □, obesity-resistant (OR); solid gray triangles, obesity-prone (OP). MTII, melanotan II. [Histological maps were hand drawn with the Paxinos and Watson atlas (54) used as a guide.] Bottom: representative injection sites in the PVN (left), ArcN (middle), and DMH (right), visualized with fluorescent microbeads.
Intracerebroventricular Insulin Infusion Increases MAP and Improves Baroreflex Control of HR in Obese, but not Lean, Male Conscious Rats
Effects of obesity.
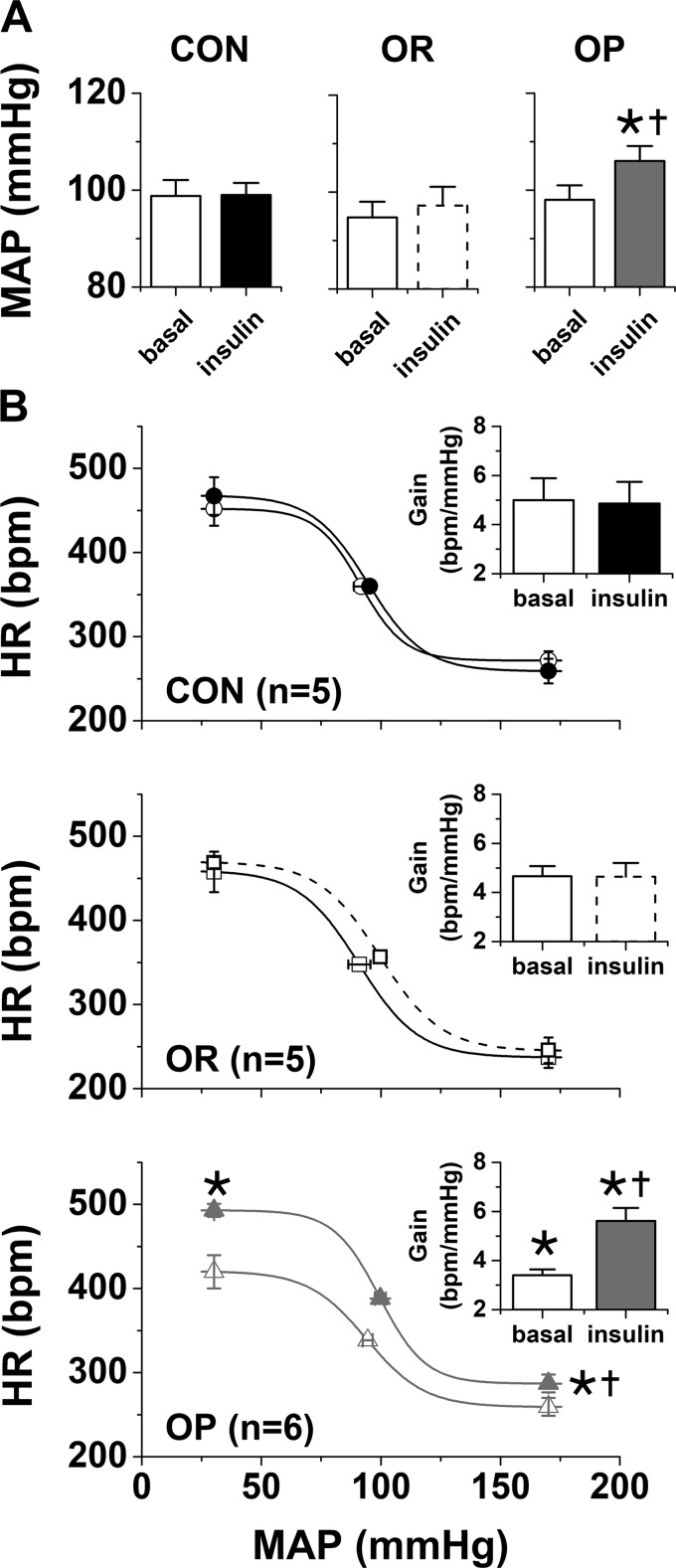
As in rats studied in the anesthetized state (Table 1), after ∼4 wk on the HFD, conscious OP rats (475 ± 8 g, n = 6) weighed more than OR (399 ± 6 g, n = 5) or CON (406 ± 6 g, n = 5) rats (P < 0.05). However, neither MAP nor HR was significantly different between groups (Table 2, Fig. 8). Baroreflex gain was depressed in OP rats (Fig. 8); no other baroreflex parameters were significantly different (Fig. 8, Table 2).
Table 2.
Effect of intracerebroventricular insulin infusion on HR and baroreflex parameters in conscious OP, OR, and CON rats
| OP (n = 6) |
OR (n = 5) |
CON (n = 5) |
||||
|---|---|---|---|---|---|---|
| Baseline | Insulin | Baseline | Insulin | Baseline | Insulin | |
| Maximum, beats/min | 417 ± 17 | 500 ± 12* | 459 ± 24 | 470 ± 7 | 452 ± 20 | 468 ± 18 |
| Minimum, beats/min | 259 ± 11 | 286 ± 7*† | 237 ± 12 | 245 ± 15 | 272 ± 11 | 259 ± 15 |
| Range, beats/min | 162 ± 13 | 206 ± 10 | 222 ± 23 | 225 ± 13 | 181 ± 29 | 209 ± 20 |
| BP50, mmHg | 95 ± 2 | 99 ± 2 | 91 ± 5 | 100 ± 5 | 91 ± 3 | 95 ± 2 |
| Width, mmHg | 11.7 ± 1.2 | 9.7 ± 0.8 | 12.5 ± 1.7 | 12.8 ± 1.5 | 9.2 ± 0.9 | 11.6 ± 1.4 |
| HR, beats/min | 322 ± 7 | 349 ± 10 | 324 ± 15 | 339 ± 14 | 331 ± 6 | 341 ± 15 |
Values are means ± SE; n, number of rats. HR, heart rate; CON, control; OR, obesity-resistant; OP, obesity-prone; BP50, arterial pressure at the midpoint of HR range.
P < 0.05 vs. basal;
P < 0.05 vs. CON and OR.
Fig. 8.
Intracerebroventricular insulin infusion increases mean arterial pressure (MAP, A) and baroreflex control of heart rate (HR, B) in conscious obesity-prone (OP, n = 6), but not obesity-resistant (OR, n = 5) and control (CON, n = 5), rats. A: baseline MAP was not different between groups. In OP, but not OR or CON, rats, intracerebroventricular insulin increased MAP. B: HR baroreflex gain [absolute values (insets)] was lower in OP than CON or OR rats. Intracerebroventricular insulin increased baroreflex gain only in OP rats to levels higher than in OR or CON rats. OP, open and closed gray triangles, bars, and lines; OR, open solid squares and bars and solid and dashed black lines; CON, open and closed solid circles and bars. *P < 0.05 vs. OR and CON under the same condition; †P < 0.05 vs. baseline (by 2-way repeated-measures ANOVA).
Intracerebroventricular insulin infusion in OP, OR, and CON rats.
Insulin infusion did not significantly alter HR in any group (Table 2); however, it increased MAP in the OP rats to levels higher than in OR or CON animals (Fig. 8). Insulin infusion also increased HR baroreflex gain and the minimum baroreflex HR in OP rats to levels beyond those in OR or CON rats (Fig. 8, Table 2); no other sigmoidal parameters changed significantly. In contrast, intracerebroventricular insulin infusion failed to influence baroreflex control of HR in CON or OR rats (Fig. 8, Table 2). These results indicate that, as in anesthetized rats (60), obesity sensitizes the brain to the pressor and autonomic actions of insulin.
ArcN InsR expression.
As noted previously (45), InsR expression was abundant and did not vary significantly from the rostral to caudal ArcN (−2.8 to −3.4 mm from bregma) (Fig. 9). Moreover, ArcN InsR expression in OP rats did not differ significantly from that in OR and CON rats (Fig. 9).
DISCUSSION
The purpose of the present study was to test if insulin contributes to basal sympathoexcitation in obese males and if this action includes downstream engagement of α-MSH-receptive neurons in the PVN and/or DMH. The important new findings are as follows. 1) Nonspecific or select blockade of ArcN InsR decreases LSNA and MAP in OP, but not OR or CON, rats. 2) Blockade of PVN, but not DMH, MC4R also decreases LSNA, HR, and MAP in male OP rats. 3) Activation of PVN MC4R increases LSNA (HR and MAP) in OP, but not OR/CON, rats. 4) Intracerebroventricular insulin infusion increases MAP and baroreflex control of HR more in conscious OP than OR or CON rats. 5) ArcN InsR expression does not differ between OP, OR, and CON rats. Collectively, these findings suggest that, in obese males, endogenous insulin acts in the ArcN to increase SNA via a neuronal pathway that includes PVN MC4R.
The possibility that insulin contributes to obesity-induced sympathoexcitation and hypertension was proposed decades ago (36), and several reports have indirectly supported this hypothesis. 1) Obesity produces insulin resistance and elevated insulin levels, which are sympathoexcitatory in humans and animals (3, 16, 37). 2) In humans, insulin resistance and norepinephrine spillover are related, and weight loss decreases norepinephrine spillover only in insulin-resistant subjects (69). 3) In obese rabbits, intracerebroventricular administration of an InsR antagonist decreases MAP [without altering renal SNA (RSNA)] (41). 4) Intracerebroventricular infusion of insulin increases LSNA dramatically more in obese male rats (60). We now provide direct evidence for insulin’s sympathoexcitatory role in obesity. More specifically, nonselective inhibition of the ArcN decreased LSNA only in OP rats. While the ArcN is the sole central site at which insulin increases SNA (16, 44), this result could also be explained by the actions of other sympathoexcitatory factors, such as leptin (29) and chronic inflammation (71). However, we further showed that blockade of ArcN InsR also dramatically decreased LSNA, HR, and MAP in OP rats. Therefore, we conclude that the ArcN, via an action of insulin, is a major contributor to obesity-induced sympathoexcitation. The finding that the decreases in SNA (HR and MAP) following ArcN InsR blockade in OP rats took several minutes to develop suggests that insulin’s actions are mediated by cellular signaling (and, possibly, genomic) mechanisms, which cannot be rapidly reversed.
In obese humans, systemic insulin infusion does not produce further increments in MSNA, which has led to the conclusion that insulin does not contribute to obesity-induced sympathoexcitation (70, 73). However, as previously discussed (14, 42, 73), this negative result is likely due to reduced transport of insulin across the BBB, which may even lower insulin levels in the brain or cerebrospinal fluid (8, 32, 33, 66). However, the sympathoexcitatory response to intracerebroventricular infusion of insulin (which bypasses the BBB) is markedly amplified in anesthetized OP rats (60), and, as shown here, intracerebroventricular administration of insulin increases MAP in conscious obese, but not lean, male rats. Thus, insulin appears capable of contributing to obesity-induced sympathoexcitation and hypertension, despite reduced BBB transport and brain insulin levels, because of brain sensitization to insulin.
We next investigated downstream neuropathways and mechanisms. ArcN sympathoexcitatory POMC neurons (21, 47) [and sympathoinhibitory NPY neurons (63)] project heavily to the PVN and DMH. Moreover, insulin increases SNA via activation of MC3/4R (74) and iGluR (67) in the PVN. The present finding that blockade of PVN MC3/4R or iGluR decreased LSNA, HR, and MAP only in OP rats highlight PVN MC4R and iGluR as other sources of obesity-induced sympathoexcitation, likely due in part to actions of insulin in the ArcN.
As we reported previously (62), PVN injection of a low dose of the α-MSH agonist MTII does not alter LSNA in lean rats, unless tonic NPY inhibition of PVN presympathetic nerves is eliminated by prior administration of a NPY Y1R antagonist. A key finding in the present study was that nanoinjection of MTII into the PVN did increase LSNA in OP rats, and this response was the same as the MTII-induced increase in LSNA in CON rats after blockade of PVN NPY Y1R (62). Because tonic PVN NPY sympathoinhibition is absent in OP rats (60), this equivalency of the LSNA responses to MTII has several potential implications. 1) The responsiveness of only OP rats to MTII at this early stage is likely due solely to the loss of tonic PVN NPY inhibition, not increased PVN MC4R expression or signaling (Fig. 10). 2) While it is likely that the rostral ventrolateral medulla (RVLM) contributes to HFD-induced sympathoexcitation (68), at this early stage the mechanism does not appear to involve amplification of excitatory inputs downstream of ArcN POMC neurons to the RVLM but, instead, transmission of upstream excitation. 3) If the nodes downstream from the ArcN, in the PVN and RVLM, are not sites of amplification, then the greatly enhanced sympathoexcitatory response to intracerebroventricular infusion of insulin in male OP rats (60) or the pressor response to intracerebroventricular administration of insulin only in conscious OP rats (Fig. 8) is most likely explained by enhanced ArcN responsiveness to insulin (Fig. 10). However, InsR expression throughout the ArcN did not differ between groups, in agreement with previous studies of OP rats that quantified InsR expression in a block of ArcN tissue (20) or InsR expression in ArcN NPY neurons (60); therefore, the sensitization likely involves events downstream of InsR, specifically, the induced cellular signaling pathways. The Rahmouni laboratory has shown that intracerebroventricular infusion of insulin increases the activity of various sympathetic nerves via different signaling pathways, as deduced by the ability of intracerebroventricular administration of either phosphoinositide 3-kinase or MAPK signaling inhibitors to block the SNA effects of intracerebroventricular insulin infusion (57). However, given the widespread inhibition of signaling in this study, the specific ArcN InsR cellular signaling mechanisms that mediate insulin-induced increases in LSNA in lean animals remain unclear. Moreover, whether or how this signaling becomes enhanced with obesity will be challenging to unravel, since, overall, insulin or leptin signaling in the ArcN becomes impaired with obesity (22, 56).
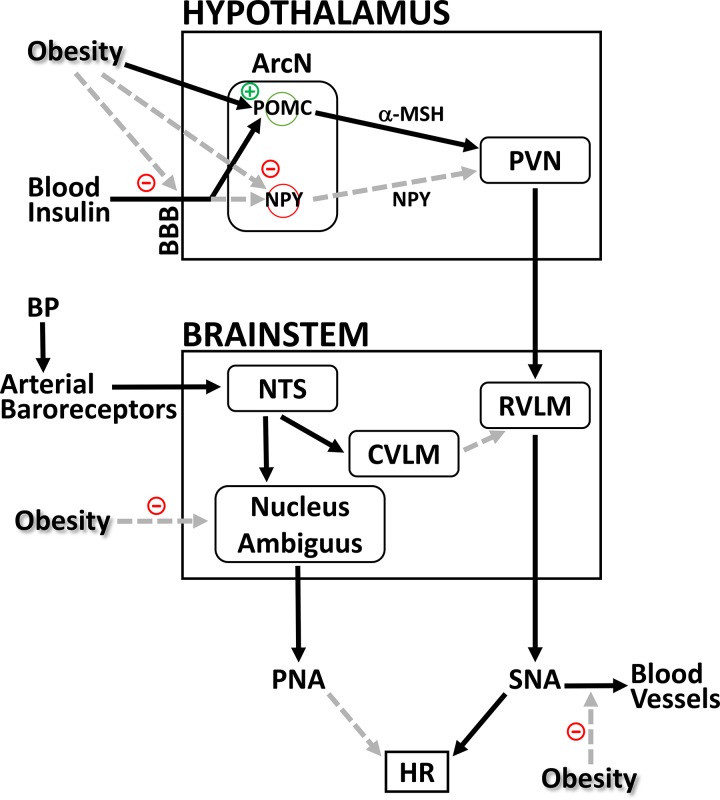
Fig. 10.
Hypothesis to explain how insulin can contribute to increased basal sympathetic nerve activity (SNA) and elicit augmented lumbar SNA (LSNA) responses (60) early in obesity development, without normalizing impaired heart rate (HR) baroreflex gain or producing hypertension. Black arrows represent excitatory inputs; gray dashed arrows represent inhibitory inputs. The brain site at which obesity impairs medullary control of parasympathetic nerve activity (PNA) is unknown. ArcN, arcuate nucleus; BBB, blood-brain barrier; BP, blood pressure; CVLM, caudal ventrolateral medulla; α-MSH, α-melanocyte-stimulating hormone; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; POMC, proopiomelanocortin; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla; SNA, sympathetic nerve activity. See text for details.
Other sites or sources of sympathoexcitation in obese males deserve discussion. First, in agreement with a previous study in obese rabbits (40), the present results do not support a role for the DMH: neither nonselective inhibition nor selective MC3/4R blockade in the DMH lowered SNA, HR, or MAP in OP rats. This finding conflicts with another report that acute blockade or deletion of leptin receptors in the DMH lowers systolic blood pressure in obese mice (65). However, as previously discussed in detail (12), in this study DMH injection volumes were large and likely also influenced nearby hypothalamic sites, such as the ArcN, ventromedial hypothalamus (VMH), or lateral hypothalamus. In studies of the VMH in obese rabbits, Lim and colleagues (40) reported results parallel to ours: VMH injections of α-MSH increased renal SNA and MAP, and blockade of VMH MC3/4R with SHU9119 (or blockade of leptin receptors) decreased RSNA and MAP only in HFD rabbits. Interestingly, the rat VMH is nearly devoid of α-MSH inputs (fibers) and MC4R (6, 21, 31, 34, 48). Thus the role of the VMH in rabbits may reflect a species difference; in support of this notion, mice, unlike rats, express moderate levels of MC4R in the VMH (43). Alternatively, VMH neurons have long dendrites that extend into a neuron-poor “shell” around the VMH, and it has been proposed that POMC (α-MSH) fibers may form synapses and excite VMH neurons in this intermediate zone (27). If so, then obesity-induced increases in SNA may be driven by a network of hypothalamic sites via the actions of both insulin (ArcN via the PVN) and leptin (ArcN and VMH via the PVN).
It is important to emphasize that we investigated OP rats fed a diet relatively low in fat (32%) for only 4–6 wk. The rats were not hypertensive. This finding is consistent with telemetric studies, which document that increases in MAP were not detected for several weeks (>9–12 wk after beginning a HFD) (11, 15). Thus our results address mechanisms in the developmental stage of obesity-induced hypertension and reinforce the idea that the central engagement of sympathoexcitatory pathways is an early event (4, 13, 50). Why, then, does blood pressure not increase in parallel? Obese, but normotensive, humans exhibit reduced vascular sensitivity to norepinephrine (1), which may explain in part why not all obese humans are chronically hypertensive. Therefore, one possibility is that the vasculature (or the kidney) is not responding optimally to the increased SNA. Alternatively, the appearance of hypertension may depend on the delayed excitation of sympathetic nerves other than the lumbar. While we focused on LSNA, as it is preferentially stimulated by insulin (57, 74) and increases early in obesity development (50), likely due to an action of insulin (41) (Fig. 2), whether insulin activates other sympathetic nerves at this early stage in OP rats remains to be determined. However, as obesity is sustained, central sensitization to the sympathoexcitatory effects of insulin and leptin could grow (41) and engage additional hypothalamic sites, such as the VMH or DMH, and other sympathetic nerves in parallel with sensitization of downstream sympathoexcitatory nodes, such as the PVN and RVLM (30, 35). As a result, an increase in RSNA could activate the renin-angiotensin system and promote fluid retention, which would synergize with greater increases in LSNA to raise MAP. Thus, increases in MAP may only occur when vascular or renal adrenergic mechanisms become receptive to, or are engaged by, elevations in SNA.
The present results in conscious rats confirm that obesity impairs HR baroreflex gain (2, 46, 52, 72, 76). The mechanism involves suppression of cardiac parasympathetic and sympathetic nerve activity (46, 72); however, specific brain sites and mediators have not been identified. HR baroreflex gain and insulin sensitivity are well correlated in conscious CON, OR, and OP rats (76), suggesting that a factor that contributes to or is associated with obesity-induced insulin resistance is involved. In the present study, intracerebroventricularly infused insulin failed to influence the HR baroreflex in CON and OR rats, similar to observations in conscious female rats (5) and young men (75). These data indicate that the stimulatory effects of insulin on the HR baroreflex are maximal in normal conscious subjects. In contrast, in OP rats, intracerebroventricular insulin infusion increased HR baroreflex gain from subnormal to supranormal. These findings not only confirm brain sensitization to insulin in conscious obese rats, but they also suggest that, despite sensitization, brain insulin actions are insufficient to counteract the action of obesity to suppress baroreflex function.
How can insulin contribute to baseline drive of SNA in OP rats (despite low brain insulin levels) without maintaining or enhancing baroreflex control of HR? The answer likely rests on the fact that baroreflex control of HR involves both the sympathetic and parasympathetic nervous systems (Fig. 10). Normally, a fall in BP decreases the tonic activity of arterial baroreceptors, which decreases glutamatergic drive of barosensitive neurons in the nucleus tractus solitarius. Via connections to the nucleus ambiguus, which houses cardiac parasympathetic preganglionic neurons, this leads to a decrease in parasympathetic nerve activity (PNA). Simultaneously, neurons in the RVLM are disinhibited, which increases SNA. In obese animals, the PNA component is markedly suppressed; indeed, electrical stimulation of baroreceptor afferents fails to decrease HR in sympathetically blocked OP rats (46). On the other hand, insulin appears to influence (increase) sympathetic, not parasympathetic, control of the heart (10, 64). Therefore, despite central sensitization to insulin, any improvement in the sympathetic component is unable to overcome profound suppression of PNA with obesity, and baroreflex control of HR remains suppressed.
Perspectives and Significance
The present results document, for the first time, that early in obesity development, insulin contributes to increased drive of LSNA via sympathoexcitatory sites and neuronal pathways that include ArcN InsR and PVN MC4R. The data also confirm in conscious animals that obesity sensitizes the brain to the pressor effects of insulin (60), which helps explain how insulin can drive LSNA when BBB transport and brain levels may be reduced in obese subjects (8, 32, 33, 66). The sensitization does not appear to involve an upregulation of PVN MC4R or ArcN InsR expression or downstream circuits but, instead, may engage amplified signaling in the ArcN. These data underscore the clinical urgency in treating insulin resistance and diabetes in obese individuals, given its potential contribution to sympathoexcitation and, ultimately, the hypertensive state.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-088552 and HL-128181 and American Heart Association Grants 09GRNT2060630, 12GRNT11550018, and 15POST23040042. Use of the Advanced Light Microscopic Core was funded in part by National Institute of Neurological Disorders and Stroke Grant P30 NS-061800 (S. Aicher, Principal Investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S., D.Z., P.A.C., and V.L.B. conceived and designed research; Z.S., D.Z., and P.A.C. performed experiments; Z.S., D.Z., P.A.C., and V.L.B. analyzed data; Z.S., D.Z., P.A.C., and V.L.B. interpreted results of experiments; Z.S. and V.L.B. prepared figures; Z.S. and V.L.B. edited and revised manuscript; Z.S., D.Z., P.A.C., and V.L.B. approved final version of manuscript; V.L.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lauge Schaffer (Novo Nordisk) for the gift of the insulin receptor antagonist S961. We are grateful for the technical assistance provided by Jennifer Wong, Nicole Pelletier, and Alyssa Bonillas.
REFERENCES
- 1.Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension 52: 687–695, 2008. doi: 10.1161/HYPERTENSIONAHA.107.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002. doi: 10.1161/01.CIR.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 60: 163–171, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 5.Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in conscious rats: role of brain insulin. Hypertension 57: 283–288, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci 19: RC26, 1999. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzel B, Lim K, Davern PJ, Burke SL, Armitage JA, Head GA. Central proopiomelanocortin but not neuropeptide Y mediates sympathoexcitation and hypertension in fat fed conscious rabbits. J Hypertens 34: 464–473, 2016. doi: 10.1097/HJH.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 8.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D Jr, Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci 36: 627–633, 1985. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 9.Bell BB, Rahmouni K. Leptin as a mediator of obesity-induced hypertension. Curr Obes Rep 5: 397–404, 2016. doi: 10.1007/s13679-016-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkelaar M, Eekhoff EM, Simonis-Bik AM, Boomsma DI, Diamant M, Ijzerman RG, Dekker JM, ’t Hart LM, de Geus EJ. Effects of induced hyperinsulinaemia with and without hyperglycaemia on measures of cardiac vagal control. Diabetologia 56: 1436–1443, 2013. doi: 10.1007/s00125-013-2848-6. [DOI] [PubMed] [Google Scholar]
- 11.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 289: R181–R186, 2005. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- 12.Brooks VL. F1000 Prime Dissenting Opinion on [Simonds SE et al., Cell 2014 159(6):1404–1416]. [ February 2015]. doi: 10.3410/f.725266063.793503746. [DOI]
- 13.Brooks VL, Osborn JW. High-fat food, sympathetic nerve activity, and hypertension: danger soon after the first bite? Hypertension 60: 1387–1388, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks VL, Shi Z, Holwerda SW, Fadel PJ. Obesity-induced increases in sympathetic nerve activity: sex matters. Auton Neurosci 187: 18–26, 2015. doi: 10.1016/j.autneu.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll JF, Zenebe WJ, Strange TB. Cardiovascular function in a rat model of diet-induced obesity. Hypertension 48: 65–72, 2006. doi: 10.1161/01.HYP.0000224147.01024.77. [DOI] [PubMed] [Google Scholar]
- 16.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589: 1643–1662, 2011. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassaglia PA, Shi Z, Brooks VL. Insulin increases sympathetic nerve activity in part by suppression of tonic inhibitory neuropeptide Y inputs into the paraventricular nucleus in female rats. Am J Physiol Regul Integr Comp Physiol 311: R97–R103, 2016. doi: 10.1152/ajpregu.00054.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassaglia PA, Shi Z, Li B, Reis WL, Clute-Reinig NM, Stern JE, Brooks VL. Neuropeptide Y acts in the paraventricular nucleus to suppress sympathetic nerve activity and its baroreflex regulation. J Physiol 592: 1655–1675, 2014. doi: 10.1113/jphysiol.2013.268763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Cham JL, Badoer E. High-fat feeding alters the cardiovascular role of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 298: R799–R807, 2010. doi: 10.1152/ajpregu.00558.2009. [DOI] [PubMed] [Google Scholar]
- 20.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 21.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 22.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 23.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, Perez de Lara CE, Hall JE. Role of the brain melanocortins in blood pressure regulation. Biochim Biophys Acta Mol Basis Dis 1863: 2508–2514, 2017. doi: 10.1016/j.bbadis.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35: 1009–1015, 2000. doi: 10.1161/01.HYP.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 25.Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. J Hypertens 28: 1466–1470, 2010. doi: 10.1097/HJH.0b013e328339f20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 27.Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci 28: 5433–5449, 2008. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 108: 808–812, 2011. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber DA, Schreihofer AM. Exaggerated sympathoexcitatory reflexes develop with changes in the rostral ventrolateral medulla in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 311: R243–R253, 2016. doi: 10.1152/ajpregu.00085.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobowitz DM, O’Donohue TL. α-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA 75: 6300–6304, 1978. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 33.Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 49: 2790–2792, 2006. doi: 10.1007/s00125-006-0409-y. [DOI] [PubMed] [Google Scholar]
- 34.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 35.Konno S, Hirooka Y, Kishi T, Sunagawa K. Sympathoinhibitory effects of telmisartan through the reduction of oxidative stress in the rostral ventrolateral medulla of obesity-induced hypertensive rats. J Hypertens 30: 1992–1999, 2012. doi: 10.1097/HJH.0b013e328357fa98. [DOI] [PubMed] [Google Scholar]
- 36.Krieger DR, Landsberg L. Mechanisms in obesity-related hypertension: role of insulin and catecholamines. Am J Hypertens 1: 84–90, 1988. doi: 10.1093/ajh/1.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Landsberg L. Pathophysiology of obesity-related hypertension: role of insulin and the sympathetic nervous system. J Cardiovasc Pharmacol 23, Suppl 1: S1–S8, 1994. doi: 10.1097/00005344-199423001-00002. [DOI] [PubMed] [Google Scholar]
- 38.Levin BE, Strack AM. Diet-induced obesity in animal models and what they tell us about human obesity. In: Neurobiology and Obesity, edited by Harvey J and Withers DJ. Cambridge, UK: Cambridge University Press, 2008, p. 164–195. [Google Scholar]
- 39.Li DP, Pan HL. Role of γ-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626, 2007. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 40.Lim K, Barzel B, Burke SL, Armitage JA, Head GA. Origin of aberrant blood pressure and sympathetic regulation in diet-induced obesity. Hypertension 68: 491–500, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07461. [DOI] [PubMed] [Google Scholar]
- 41.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension 61: 628–634, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 42.Limberg JK, Curry TB, Prabhakar NR, Joyner MJ. Is insulin the new intermittent hypoxia? Med Hypotheses 82: 730–735, 2014. doi: 10.1016/j.mehy.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23: 7143–7154, 2003. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 304: H1538–H1546, 2013. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks JL, Li M, Schwartz M, Porte D Jr, Baskin DG. Effect of fasting on regional levels of neuropeptide Y mRNA and insulin receptors in the rat hypothalamus: an autoradiographic study. Mol Cell Neurosci 3: 199–205, 1992. doi: 10.1016/1044-7431(92)90039-5. [DOI] [PubMed] [Google Scholar]
- 46.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol 302: H2083–H2091, 2012. doi: 10.1152/ajpheart.01200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci 7: 19, 2013. doi: 10.3389/fnins.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mezey E, Kiss JZ, Mueller GP, Eskay R, O’Donohue TL, Palkovits M. Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, α-melanocyte-stimulating hormone and β-endorphin (ACTH, α-MSH, β-END) in the rat hypothalamus. Brain Res 328: 341–347, 1985. doi: 10.1016/0006-8993(85)91046-7. [DOI] [PubMed] [Google Scholar]
- 49.Morgan DA, Balon TW, Ginsberg BH, Mark AL. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol Regul Integr Comp Physiol 264: R423–R427, 1993. doi: 10.1152/ajpregu.1993.264.2.R423. [DOI] [PubMed] [Google Scholar]
- 50.Muntzel MS, Al-Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension 60: 1498–1502, 2012. doi: 10.1161/HYPERTENSIONAHA.112.194886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 267: R1350–R1355, 1994. doi: 10.1152/ajpregu.1994.267.5.R1350. [DOI] [PubMed] [Google Scholar]
- 52.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J 74: 1379–1383, 2010. doi: 10.1253/circj.CJ-09-0960. [DOI] [PubMed] [Google Scholar]
- 53.Paranjape SA, Chan O, Zhu W, Horblitt AM, Grillo CA, Wilson S, Reagan L, Sherwin RS. Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am J Physiol Endocrinol Metab 301: E978–E983, 2011. doi: 10.1152/ajpendo.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2007. [Google Scholar]
- 55.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 51: 514–520, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 56.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55: 862–868, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 57.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schäffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 376: 380–383, 2008. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- 59.Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen. J Physiol 593: 1633–1647, 2015. doi: 10.1113/jphysiol.2014.284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Z, Cassaglia PA, Pelletier NE, Brooks VL. Sex differences in the sympathoexcitatory response to insulin in obese rats: role of neuropeptide Y. J Physiol 597: 1757–1775, 2019. doi: 10.1113/JP277517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Z, Hansen KM, Bullock KM, Morofuji Y, Banks WA, Brooks VL. Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats. J Physiol 597: 4087–4100, 2019. doi: 10.1113/JP278282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension 66: 1034–1041, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest 127: 2868–2880, 2017. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siani A, Strazzullo P, Giorgione N, De Leo A, Mancini M. Insulin-induced increase in heart rate and its prevention by propranolol. Eur J Clin Pharmacol 38: 393–395, 1990. doi: 10.1007/BF00315583. [DOI] [PubMed] [Google Scholar]
- 65.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr, Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416, 2014. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte D Jr, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of obese Zucker rats. Endocrinology 121: 1611–1615, 1987. doi: 10.1210/endo-121-5-1611. [DOI] [PubMed] [Google Scholar]
- 67.Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol 113: 1302–1309, 2015. doi: 10.1152/jn.00764.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- 69.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 90: 5998–6005, 2005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 70.Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ, McGrane MT, Mariani JA, Socratous F, Chopra R, Esler MD, Schlaich MP, Lambert EA. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr 89: 27–36, 2009. doi: 10.3945/ajcn.2008.26299. [DOI] [PubMed] [Google Scholar]
- 71.Valdearcos M, Xu AW, Koliwad SK. Hypothalamic inflammation in the control of metabolic function. Annu Rev Physiol 77: 131–160, 2015. doi: 10.1146/annurev-physiol-021014-071656. [DOI] [PubMed] [Google Scholar]
- 72.Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ Jr. Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol 269: H629–H637, 1995. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 73.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 93: 2365–2371, 1994. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao D, McCully BH, Brooks VL. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J Pharmacol Exp Ther 343: 206–213, 2012. doi: 10.1124/jpet.112.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]