Abstract
Psychomotor stimulants are prescribed for many medical conditions, including obesity, sleep disorders, and attention-deficit/hyperactivity disorder. However, despite their acknowledged therapeutic utility, these stimulants are frequently abused, and their use can have both short- and long-term negative consequences. Although stimulants such as amphetamines acutely elevate blood pressure, it is unclear whether they cause any long-term effects on cardiovascular function after use has been discontinued. Previous work in our laboratory has demonstrated that physiological and psychosocial stressors will produce sensitization of the hypertensive response, a heightened pressor response to a hypertensinogenic stimulus delivered after stressor exposure. Here, we tested whether pretreatment with amphetamine for 1 wk can sensitize the hypertensive response in rats. We found that repeated amphetamine administration induced and maintained sensitization of the pressor response to angiotensin II following a 7-day delay after amphetamine injections were terminated. We also found that amphetamine pretreatment altered mRNA expression for molecular markers associated with neuroinflammation and renin-angiotensin-aldosterone system (RAAS) activation in the lamina terminalis, a brain region implicated in the control of sympathetic nervous system tone and blood pressure. The results indicated amphetamine upregulated mRNA expression underlying neuroinflammation and, to a lesser degree, message for components of the RAAS in the lamina terminalis. However, we found no changes in mRNA expression in the paraventricular nucleus. These results suggest that a history of stimulant use may predispose individuals to developing hypertension by promoting neuroinflammation and upregulating activity of the RAAS in the lamina terminalis.
Keywords: amphetamine, angiotensin II, hypertension, lamina terminalis, neuroinflammation
INTRODUCTION
Neuroplasticity is an intrinsic capacity of the nervous system that allows animals to make behavioral and physiological adaptations to environmental challenges. However, in some cases, behavioral and physiological plasticity can promote disease states. Recent work from our laboratory (17, 18) has implicated a role for neuroplasticity in the etiology of essential hypertension. That is, events that generally are regarded as challenges (i.e., stressors) will produce hypertensive response sensitization (HTRS), where prior exposure to a stressor can make animals susceptible to developing greater hypertension to subsequent hypertensinogenic challenges. For example, administration of subpressor doses of angiotensin II (ANG II) or aldosterone, consumption of a high-fat diet, or social defeat exacerbate blood pressure (BP) responses to a slow-pressor dose of ANG II delivered following a delay after the stressor exposure has concluded (31–34). HTRS has been linked to neuroplasticity in the neural network that regulates sympathetic tone and BP control (17, 18).
Forebrain, hypothalamic, and hindbrain nuclei form a neural network that mediates the central regulation of BP (7, 12). The lamina terminalis (LT), located in the forebrain, connects to and modifies neural activity in the paraventricular nucleus of the hypothalamus (PVN), which, in turn, modulates hindbrain and spinal cord control over BP. Elevation of the brain renin-angiotensin-aldosterone system (RAAS) and neuroinflammation at sites in this neural circuitry has been implicated in the expression of hypertension as well as HTRS (13, 17, 18, 30). That is, experiences that produce HTRS tend to elevate the central RAAS and central proinflammatory cytokines in the LT and PVN. Therefore, prior stressor exposure may chronically upregulate the brain RAAS and induce neuroinflammation in systems that regulate BP to provide a physiological foundation for HTRS.
Psychomotor stimulants such as cocaine and amphetamine are commonly abused in substance use disorders. Furthermore, psychomotor stimulants are often prescribed for chronic treatment of attention-deficit/hyperactivity disorder. Psychomotor stimulant use acutely elevates BP (2, 28); however, whether psychomotor stimulant use has a long-term impact on BP is unclear. Reports have either found no effect of prior cocaine use on BP or only mild effects of cocaine on increasing the likelihood of hypertension (1, 3, 4). Less is known about amphetamine use and hypertension. Amphetamine use is linked to other cardiovascular diseases, including pulmonary hypertension and cardiomyopathy (2, 6, 10). One report found no association between prior amphetamine use and hypertension (1), but, given the weak effect of prior cocaine use on elevating BP (1), a larger sample size may be necessary to determine whether amphetamine use is linked to hypertension.
In the present study, we determined whether a history of amphetamine treatments sensitizes the pressor response in a model of HTRS developed in our laboratory (17, 18, 33). In this model, daily mean arterial blood pressure (MAP) and heart rate (HR) are recorded over four phases: a control period that assesses baseline MAP, an induction phase (IND), a delay phase (DEL), and an expression phase (EXP). During IND, rats are exposed to a treatment that produces a sensitized hypertensive response. Rats then are allowed a 1-wk rest (i.e., DEL) after IND to ensure recovery from pretreatment and metabolism of any drugs administered. During EXP, which then follows, rats receive a chronic slow-pressor dose of ANG II, which elicits a gradual elevation in MAP over a 2-wk period. We hypothesized that administration of amphetamine during IND would sensitize the hypertensive response produced by a slow-pressor dose of ANG II delivered during EXP. Furthermore, we determined whether a history of amphetamine treatment affected molecular markers for the central RAAS and neuroinflammation in neural systems involved in cardiovascular control. Our results indicate that a history of amphetamine exposure is sufficient to produce HTRS and is associated with neuroinflammation in the LT but not the PVN.
MATERIALS AND METHODS
Subjects.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan Teklad, Indianapolis, IN) that weighed between 275 and 300 g on arrival to the laboratory were used as subjects. Rats were maintained on a 12:12-h light-dark cycle and housed in translucent cages in a temperature- and humidity-controlled room. All rats were allowed ≥1 wk of habituation to the laboratory before experiments began. Animals had ad libitum access to filtered tap water and NIH-31 irradiated modified open formula mouse/rat diet.
Experiment timelines.
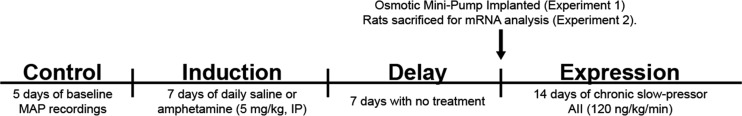
The timeline for experiments 1 and 2 is displayed in Fig. 1. In experiment 1, we determined whether daily amphetamine injection would sensitize ANG II-induced hypertension. Rats (n = 8 vehicle, n = 7 amphetamine) received a telemetry implant to record daily MAP and HR and recovered from surgery for ≥1 wk. Baseline MAP and HR were measured over 5 days to ensure stable MAP and HR recordings before IND. During IND, rats received daily injections of vehicle or amphetamine for 7 days (5 mg/kg ip, 1 injection/day; Sigma-Aldrich, St. Louis, MO). The dose of amphetamine was selected from studies (14, 35) employing daily treatments of the drug over periods ranging from 5 to 14 days to induce sensitization of the psychomotor response (i.e., a progressive increase in drug-induced locomotor behavior). Following the 1st, 3rd, 5th, and 7th injections, acute MAP and HR were recorded for 2 h in a subset of rats (n = 4 vehicle, n = 4 amphetamine). After amphetamine injections were completed, rats entered a 1-wk DEL to ensure amphetamine was metabolized and to determine whether a sensitized state induced by amphetamine was sustained. Then, at the beginning of EXP, rats were implanted with subcutaneous osmotic mini-pumps that delivered a slow-pressor dose of ANG II (120 ng·kg−1·min−1 in a volume of 0.5 µL/h; Sigma-Aldrich) over 14 days, during which time MAP and HR were recorded. Experiment 2 was conducted to determine whether the daily amphetamine injections would have an effect on molecular signaling in the LT and PVN. Rats (n = 8 saline, n = 10 amphetamine) were treated identically to experiment 1; however, telemetry devices were not implanted, rats were euthanized at the end of DEL (during the light phase), and tissues from the LT and PVN were collected. Of note, rats were euthanized at the end of DEL to examine molecular markers that presumably set the foundation for HTRS. Furthermore, during the EXP phase, MAP is altered and molecular mechanisms examined during this time point are confounded by elevated MAP.
Fig. 1.
Timeline for hypertensive response sensitization experiments. Rats underwent an induction-delay-expression (IND-DEL-EXP) protocol to examine sensitization of the pressor response induced by amphetamine injections. After a control period mean arterial pressure (MAP), rats received 7 days of amphetamine injections during IND. Rats were then allowed a 7-day DEL where they received no treatment. During EXP, rats received 14 days of chronic slow-pressor angiotensin II (AII) to induce a gradual elevation in MAP. Separate cohorts of rats were treated identically but euthanized at the end of DEL, and tissue was collected to examine mRNA expression in the lamina terminalis and paraventricular nucleus of the hypothalamus.
Surgeries.
Rats received telemetry implants (TA11PA-C40; Data Sciences International, St. Paul, MN) to record chronic MAP and HR. Briefly, rats were anesthetized with ketamine-xylazine (100 mg/kg ketamine-10 mg/kg xylazine), and the right femoral artery was isolated as described previously (33). The telemetry probe catheter was inserted into the right femoral artery, and the transmitter was implanted in a pocket along the right flank.
Before EXP, an osmotic mini-pump that released a slow-pressor dose of ANG II was implanted in a separate surgery that occurred at the end of DEL (Fig. 1). At that time, rats were anesthetized with isoflurane and an incision was made along the dorsal surface of the rat. An osmotic mini-pump (model 2002; ALZET, Cupertino, CA) was implanted subcutaneously dorsal to the spine. Rats received subcutaneous Ketofen (5 mg/kg, once per day over 3 days) after each surgery to provide analgesia.
Measurement of mRNA expression in the LT and PVN.
mRNA levels were assessed using real-time PCR (RT-PCR) as described previously (33). Briefly, RNA was isolated from LT and PVN using the TRIzol method (Invitrogen, Carlsbad, CA). RNA was reverse-transcribed using random hexamers following manufacturer’s instructions (Applied Biosystems, Foster City, CA). The primers used for RT-PCR are presented in Table 1. cDNA was amplified and analyzed using a C1000 thermocycler system (Bio-Rad, Hercules, CA). Changes in mRNA expression levels were normalized to GAPDH levels and calculated using the comparative cycle threshold method. Results are expressed as relative fold change in arbitrary units (mean fold change ± SE).
Table 1.
Primer sequences for real-time PCR
| Gene | Forward Primer | Reverse Primer | Product Size, bp |
|---|---|---|---|
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 |
| Renin | CTGCCACCTTGTTGTGTGAG | ACCTGGCTACAGTTCACAACG | 154 |
| AGT | TCCCTCGCTCTCTGGACTTA | AAGTGAACGTAGGTGTTGAAA | 209 |
| ACE1 | GTGTTGTGGAACGAATACGC | CCTTCTTTATGATCCGCTTGA | 187 |
| AT1R | CTCAAGCCTGTCTACGAAAATGAG | GTGAATGGTCCTTTGGTCGT | 188 |
| MR | GCCCGGCAAATCTCAACAACTCAA | TTAGGGAAAGGAACGTCGTGAGCA | 235 |
| NOX2 | CAAGATGGAGGTGGGACAGT | GCTTATCACAGCCACAAGCA | 170 |
| IL-1β | AGCAACGACAAAATCCCT GT | GAAGACAAACCGCTTTTCCA | 209 |
| IL-6 | GCCTATTGAAAATCTGCTCTGG | GGAAGTTGGGGTAGGAAGGA | 160 |
| TNF-α | GCCGATTTGCCACTTCATAC | AAGTAGACCTGCCCGGACTC | 209 |
| CD11b | TTACCGGACTGTGTGGACAA | AGTCTCCCACCACCAAAGTG | 239 |
ACE1, angiotensin-converting enzyme 1; AGT, angiotensinogen; AT1R, angiotensin II type I receptor; CD11b, cluster of differentiation molecule 11b; IL-1β, interleukin-1β; IL-6 interleukin-6; MR, mineralocorticoid receptor; NOX2, NADPH oxidase 2; TNF-α, tumor necrosis factor-α.
Data analysis.
MAP and HR were assessed once every hour over a 24-h period and averaged to yield daily MAP and HR data. On days that rats received amphetamine or saline injections, MAP and HR measurements were excluded from daily MAP and HR calculations for the 6 h following injection. Weekly means of MAP and HR were calculated by averaging daily mean MAP and HR. Day and night differences in MAP and HR were calculated by averaging light- and dark-phase MAP and HR (the mean of hourly MAP and HR over a 12-h period). For acute MAP and HR recordings following amphetamine or saline injection, MAP and HR were collected every 10 s and averaged over 10-min time periods for 2 h after injection.
A two-way repeated-measures ANOVA was used to assess differences in daily MAP and acute MAP following amphetamine injections. We also assessed whether MAP and HR differed between the light and dark phases of the light cycle using a three-way repeated-measures ANOVA. Newman-Keuls post hoc tests were conducted on data of the acute effects of amphetamine on MAP and HR. Šídák multiple comparison test was used for post hoc analysis of daily MAP and HR to control for the increased likelihood of a type 1 error that could be observed from the large data set obtained from daily recordings. Daily MAP and HR data were also blocked into phases of testing (e.g., MAP and HR during the control phase, induction phase, delay phase, 1st week of the expression phase, and the 2nd week of the expression phase) and analyzed using a two-way repeated-measures ANOVA or Student’s t tests when pre hoc hypotheses predicted differences between saline and amphetamine rats would only be observed during a specific phase. Student’s t tests were conducted for all mRNA analyses.
RESULTS
Amphetamine pretreatment sensitizes MAP to slow-pressor ANG II.
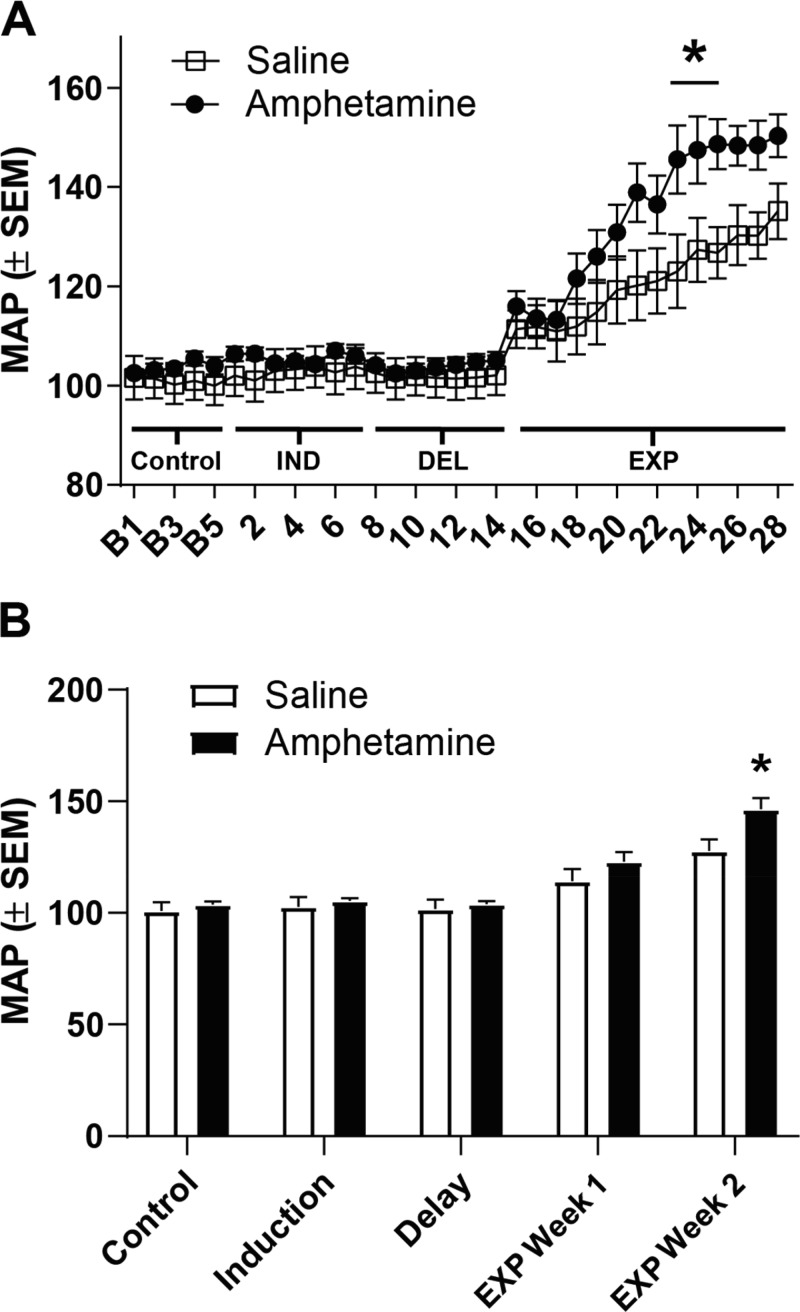
Figure 2A displays average daily MAP during control MAP recordings, IND (days 1–7), DEL (days 8–14), and EXP (days 15–28). Analyses revealed that there was a main effect of day [F(32, 416) = 35.97, P < 0.0001] and a treatment-by-day interaction [F(32, 416) = 2.301, P < 0.0001] and no main effect of treatment [F(1, 13) = 3.066, P = 0.10]. A post hoc test indicated that MAP in the amphetamine group was significantly elevated above that of saline rats on days 23–25. Furthermore, amphetamine-pretreated rats showed a more rapid elevation in MAP during EXP relative to saline rats (P < 0.05). That is, amphetamine-pretreated rats exhibited a significant elevation in MAP beginning on day 18 compared with day 1 of MAP recordings, whereas saline-pretreated rats exhibited a significant elevation beginning on day 20. We examined MAP blocked by phase (control, IND, DEL, and EXP phases; Fig. 2B). As we hypothesized that MAP would be sensitized during EXP, the 1st and last weeks of MAP data were analyzed separately. There were no significant differences between saline- and amphetamine-treated rats during the 1st week of EXP [t(13) = 1.219, P > 0.05]. However, there was a significant elevation in MAP during the 2nd week of EXP [t(13) = 2.558, P < 0.05]. Finally, we analyzed whether the light and dark phase of the cycle impacted MAP using a three-way ANOVA. No significant main effects or interactions of the light cycle were found (all P values > 0.88).
Fig. 2.
Amphetamine pretreatment elicits hypertensive response sensitization (HTRS). Rats that received a history of amphetamine during induction (IND) exhibited elevated mean arterial pressure (MAP) to a slow-pressor dose of angiotensin II during an expression phase (EXP) displayed in A. Control MAP during recordings 1–5 (B1–B5) was used to assess that MAP was equivalent across groups at baseline. Amphetamine was injected on days 1–7 (IND), and the delay period (DEL) occurred between days 8 and 14. On day 15, EXP began. A significant elevation in MAP in amphetamine-pretreated rats was observed on days 23–25 compared with saline-pretreated rats (*P < 0.05), demonstrating HTRS. Furthermore, MAP was blocked across experiment phases (B). We analyzed the difference between MAP during EXP week 1 and EXP week 2 as we hypothesized MAP would be elevated during EXP. A significant elevation in MAP was found during the 2nd week of EXP (*P < 0.05).
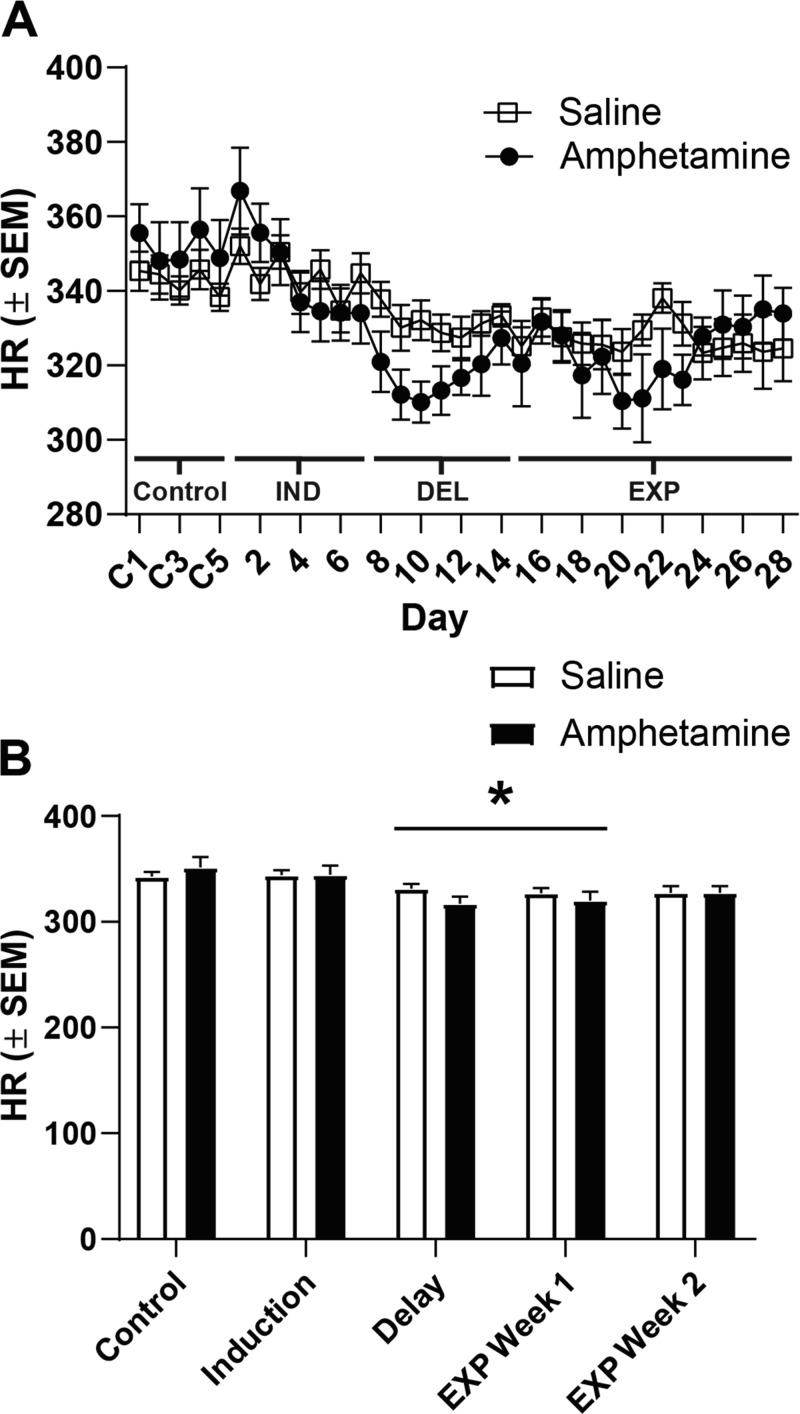
Average daily HR was also measured over baseline, IND, DEL, and EXP (Fig. 3). Analyses revealed a main effect of day [F(3.231, 42.00) = 11.73, P < 0.0001] and a treatment-by-day interaction [F(32, 416) = 2.811, P < 0.0001] but no main effect of treatment [F(1, 13) = 0.1524, P = 0.70]. Post hoc tests failed to reveal any significant differences between saline and amphetamine groups; however, when data were blocked by the experimental phase (control, IND, DEL, and EXP), there was a significant decrease in HR across phases (Fig. 3B; P < 0.05). That is, independent of treatment history, rats exhibited a decrease in HR during DEL and week 1 of EXP relative to baseline and induction phases, however, HR during EXP week 2 did not significantly differ from any other phase. Furthermore, we analyzed HR data across light and dark phases using a three-way ANOVA. We found a significant day-by-light phase interaction [(F(32, 832) = 3.700, P < 0.0001], and post hoc tests found that HR tended to be elevated during the dark phase in both saline- and amphetamine-pretreated rats. However, no interactions between treatment and light phase were observed (P > 0.4).
Fig. 3.
Amphetamine pretreatment did not impact heart rate (HR). HR across recording days is displayed in A. No significant differences across groups were observed over testing. However, it was found that HR decreased in both saline- and amphetamine-treated rats during the delay (DEL) and expression (EXP) week 1 phases compared with the control and induction (IND) phase when data were blocked by phase (B; *P < 0.05 vs. control and IND). C1–C5, control recordings 1–5.
Tolerance develops to amphetamine’s acute actions on MAP and HR.
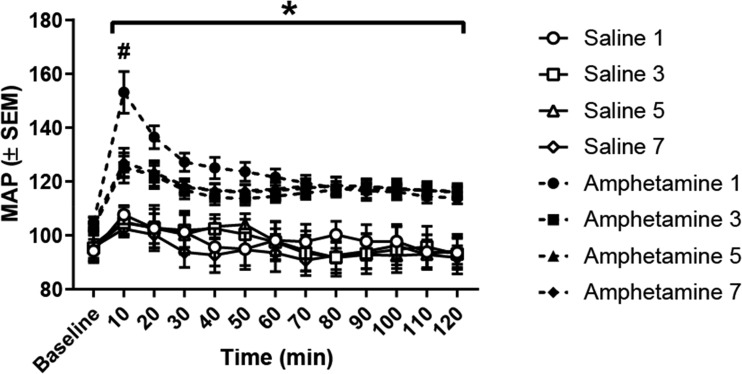
During IND, we investigated how amphetamine injections impact both MAP and HR for the 2 h following injection. Figure 4 displays MAP to acute amphetamine or saline injection on IND days 1, 3, 5, and 7. There was a significant main effect of time [F(11, 264) = 34.51, P < 0.0001], treatment [F(7, 24) = 7.178, P < 0.0001], and a treatment-by-time interaction [F(77, 264) = 3.128, P < 0.0001]. Post hoc tests found that amphetamine treatment elevated MAP across all time points (P < 0.05); however, rats that received their first amphetamine injection exhibited a greater increase in MAP at the 10-min time point compared with all other groups, including rats that received their third, fifth, and seventh amphetamine injections (P < 0.05). That is, amphetamine-treated rats exhibited an attenuated elevation in MAP by the third amphetamine injection. These data confirm that amphetamine injection elevated MAP and demonstrate that tolerance develops to the robust elevation in MAP observed within the 1st 10 min after amphetamine injection.
Fig. 4.
Acute mean arterial pressure (MAP) at baseline and following amphetamine or saline injection. All amphetamine injections elevated MAP across all time points compared with all saline injections (*P < 0.05). However, the 1st amphetamine injection significantly elevated MAP above all other groups (#P < 0.05). This robust elevation in MAP was not observed during any subsequent amphetamine injections, indicating that rats developed tolerance to the robust hypertensive response elicited by amphetamine.
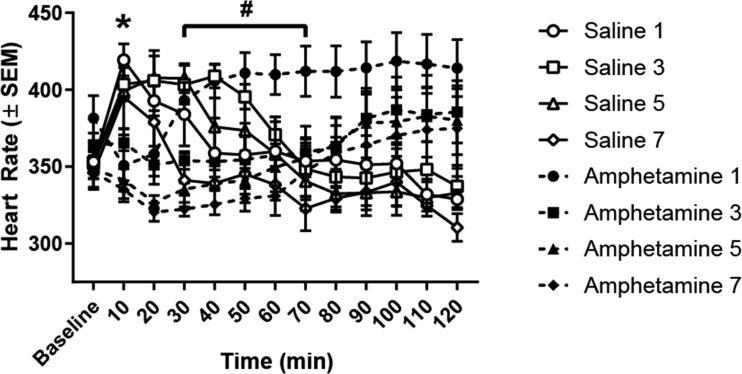
Figure 5 displays HR to acute amphetamine or saline injections. An ANOVA revealed significant main effects of time [F(11, 264) = 2.045, P < 0.05], treatment [F(7, 24) = 2.529, P < 0.05], and a treatment-by-time interaction [F(77, 264) = 4.790, P < 0.0001]. Post hoc tests found that at the 10-min time point amphetamine-treated rats exhibited lower HR than saline-treated rats (P < 0.05), however, HR in saline-treated rats decreased over time after injection and group differences were abolished. It is possible that the stress of receiving an intraperitoneal injection caused a rapid increase in HR in saline-treated rats, which dissipated over time. In contrast, amphetamine-treated rats likely exhibited an initial decrease in HR as a baroreflex to accommodate the increase in MAP caused by amphetamine injection. Over the 30- to 70-min period, rats receiving an amphetamine injection for the first time exhibited an elevation in HR relative to rats receiving amphetamine for the seventh time (P < 0.05), a finding that indicates that tolerance developed to tachycardia elicited by amphetamine injection during this period.
Fig. 5.
Acute heart rate (HR) at baseline and following amphetamine or saline injection. Initially, amphetamine lowered HR relative to saline rats across every injection day at the 10-min time point (*P < 0.05). However, this effect rapidly dissipated such that saline and amphetamine rats failed to differ at any other time point. Rats that received amphetamine injection for the 1st time exhibited a significant elevation in HR above rats that received amphetamine for the 7th time (#P < 0.05, amphetamine 1 vs. amphetamine 7), indicating that rats exhibit reduced HR over repeated amphetamine injections.
A history of amphetamine elevates molecular markers of neuroinflammation and the RAAS in the LT but not the PVN.
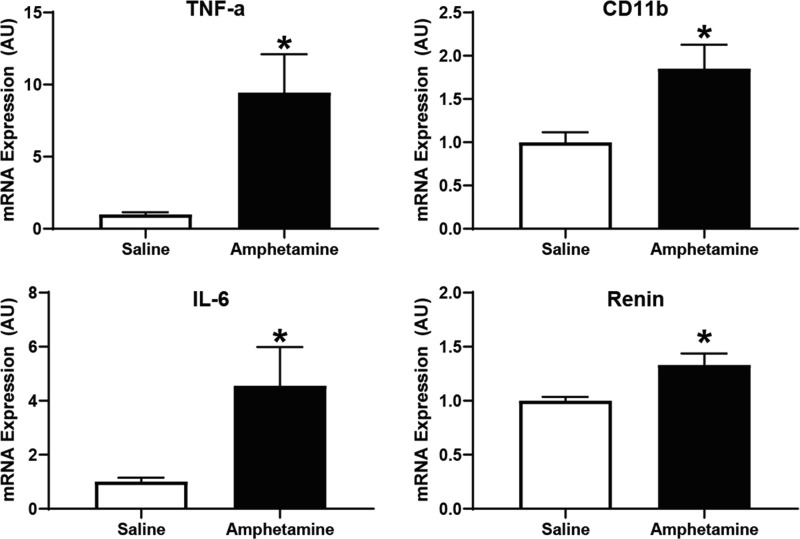
Separate cohorts of rats were treated with seven daily injections of amphetamine or saline during IND. The rats were then euthanized after 1 wk at the end of DEL, LT and PVN tissues were collected, and RT-PCR was conducted to examine molecular changes in mRNA expression. We found molecular alterations in the LT that indicate activation of neuroinflammation and the central RAAS. Specifically, as presented in Fig. 6, we found elevated mRNA expression of tumor necrosis factor-α [TNF-α; t(16) = 2.823, P < 0.05], interleukin-6 [IL-6; t(16) = 2.199, P < 0.05], and cluster of differentiation molecule 11b [CD11b; t(16) = 2.597, P < 0.05], all of which are molecular markers that indicate elevation in central cytokines or microglia activation (13, 27, 30). Furthermore, message for renin was elevated in the LT [t(16) = 2.682, P < 0.05], supporting the possibility that the central RAAS was engaged. However, many markers of the RAAS in the LT were not found to be significantly different from those of saline-treated rats (Table 2).
Fig. 6.
Amphetamine pretreatment during induction elevated molecular markers underlying neuroinflammation and the renin-angiotensin-aldosterone system in the lamina terminalis. Rats were euthanized at the end of the delay period, brain tissue was collected, and mRNA expression was examined using real-time PCR. A significant elevation in mRNA expression of tumor necrosis factor-α (TNF-a), cluster of differentiation molecule 11b (CD11b), interleukin-6 (IL-6), and renin was observed in amphetamine-pretreated rats [*P < 0.05; units expressed in arbitrary units (AU)].
Table 2.
Nonsignificant changes in LT mRNA expression
| Marker | Difference ± SE, AU | P Value |
|---|---|---|
| AGT | 0.4192 ± 0.2089 | 0.062 |
| ACE1 | 1.220 ± 0.6468 | 0.0774 |
| AT1 | 0.6603 ± 0.3133 | 0.0512 |
| MR | 0.4355 ± 0.2600 | 0.1134 |
| NOX2 | 0.4832 ± 0.2518 | 0.073 |
| IL-1β | 0.3738 ± 0.2376 | 0.1352 |
mRNA expression that was nonsignificantly altered in the lamina terminalis (LT). Difference in mRNA expression (amphetamine – saline rats) is displayed in arbitrary units (AU) along with P values. Many markers of the renin-angiotensin-aldosterone system were not significantly elevated by amphetamine pretreatment. ACE1, angiotensin-converting enzyme 1; AGT, angiotensinogen; AT1, angiotensin II receptor type 1; IL-1β, interleukin-1β; MR, mineralocorticoid receptor; NOX2, NADPH oxidase 2.
mRNA expression in the PVN was also examined, and data are presented in Table 3. We found no significant differences in mRNA expression for any of the markers tested in the PVN, suggesting that neither neuroinflammation nor the central RAAS is upregulated in the PVN at the end of DEL. These findings indicate that amphetamine has unique effects on molecular signaling in the LT that do not occur in the PVN.
Table 3.
Nonsignificant changes in PVN mRNA expression
| Marker | Difference ± SE, AU | P Value |
|---|---|---|
| Renin | −0.06422 ± 0.08847 | 0.4783 |
| AGT | −0.1663 ± 0.1216 | 0.1903 |
| ACE1 | −0.2422 ± 0.1306 | 0.0823 |
| AT1 | −0.1094 ± 0.2290 | 0.6394 |
| MR | −0.08719 ± 0.1232 | 0.4895 |
| NOX2 | −0.2412 ± 0.2288 | 0.3075 |
| CD11b | −0.3328 ± 0.1770 | 0.0784 |
| TNF-α | −0.08678 ± 0.3540 | 0.8094 |
| IL-1β | −0.1908 ± 0.2752 | 0.4981 |
| IL-6 | −0.1093 ± 0.2349 | 0.648 |
mRNA expression that was nonsignificantly elevated in the paraventricular nucleus (PVN). Difference in mRNA expression (amphetamine – saline rats) is displayed in arbitrary units (AU) along with P values. All molecular markers examined were not significantly elevated by amphetamine pretreatment. ACE1, angiotensin-converting enzyme 1; AGT, angiotensinogen; AT1, angiotensin II receptor type 1; CD11b, cluster of differentiation molecule 11b; IL-1β, interleukin-1β; IL-6 interleukin-6; MR, mineralocorticoid receptor; NOX2, NADPH oxidase 2; TNF-α, tumor necrosis factor-α.
DISCUSSION
The present experiments examined whether amphetamine pretreatment elicited HTRS and whether pretreatment altered molecular markers for neuroinflammation and the RAAS in brain areas involved in cardiovascular control. We found that amphetamine pretreatment during IND was sufficient to elicit HTRS and elevate molecular markers underlying neuroinflammation and the RAAS in the LT but not the PVN. These data support a role for amphetamine in predisposing the development of hypertension when exposed to subsequent challenges (stressors). Furthermore, they suggest that amphetamine induces neuroinflammation and upregulation of the RAAS, which have been linked to the pathogenesis of hypertension (17).
Psychomotor stimulants such as amphetamines are often abused in individuals with substance use disorders. Amphetamine use has been linked to cardiovascular diseases (2, 10), but it is unclear whether amphetamine use drives hypertension (1). Furthermore, psychomotor stimulants such as methylphenidate are commonly prescribed to treat attention-deficit/hyperactivity disorder, and these treatments acutely elevate BP (19, 28). Here, we found that amphetamine pretreatment was sufficient to evoke HTRS that was expressed weeks after the last amphetamine treatment (Fig. 2). It is unlikely that HTRS observed in amphetamine-pretreated rats was due to altered locomotor behavior. Others have shown that rats with a history of amphetamine injections exhibit normal locomotor behavior 1 wk after amphetamine treatments concluded (29). Our finding supports the possibility that a history of amphetamine use can make individuals more susceptible to developing hypertension. As such, amphetamine use may elicit long-term negative consequences on the cardiovascular system. These data warrant additional study on whether a history of stimulant use in humans has long-lasting effects that increase the probability of developing hypertension.
The present findings support prior literature indicating that amphetamine and ANG II likely share common physiological mechanisms. For example, repeated amphetamine injection induces water and salt intake, effects that are commonly elicited by ANG II (26). Furthermore, ANG II receptor type 1 blockade attenuates sensitized locomotor activity elicited by repeated amphetamine injections and alters neuronal activation elicited by amphetamine (15, 23, 24). Interestingly, amphetamine injection followed by a delay period appears to desensitize salt appetite, urine sodium excretion, and plasma renin induced by intracerebroventricular ANG II in mice (5). Here, we show that amphetamine can potentiate the pressor response to ANG II, providing additional evidence for shared neural substrates between the consequences of amphetamine action and ANG II.
Amphetamine treatment elevated MAP over the 2-h period of recording after injection while lowering HR. Amphetamine-treated rats exhibited lower HR relative to saline-treated animals. This difference in postinjection HR was likely due, in part, to an increase in HR in saline-treated rats as a result of the stress of receiving an injection and, in part, due to activation of a baroreceptor reflex-mediated decrease in HR in the amphetamine-treated rats. In other words, amphetamine reflexively lowered HR in response to the rapid rise in arterial pressure produced by increased peripheral resistance from amphetamine. Interestingly, there were relatively quick adaptations to the cardiovascular effects of repeated amphetamine injection. Although amphetamine elevated MAP after each injection, tolerance developed to the effects of amphetamine on MAP such that rats showed less of an increase in MAP on the third, fifth, and seventh injections. Furthermore, rats exhibited reduced HR over repeated amphetamine injections. That is, from the 30- to 70-min period, rats receiving amphetamine for the seventh time exhibited a decrease in HR relative to rats receiving amphetamine for the first time (Fig. 5). One intriguing possibility is that the tolerance to repeated amphetamine may be caused by enhanced baroreceptor reflex function over repeated amphetamine injections. However, we did not directly assess the baroreceptor reflex in the present experiments, but this would be a fruitful approach in future work. It is important to note that tolerance developed to the acute effects of amphetamine on MAP and HR, but amphetamine pretreatment ultimately sensitized the long-term pressor response to chronic slow-pressor ANG II delivered beginning 1 wk after the cessation of amphetamine injections. It is likely that different mechanisms govern tolerance to the amphetamine-induced acute pressor response and the later expression of HTRS, which is likely to involve neuroplasticity in the neural network controlling sympathetic tone and BP (17, 18).
A neural network composed of forebrain, midbrain, and hindbrain nuclei regulates cardiovascular control (7, 12, 17, 18). One component of this network is the LT, which is located in the forebrain and contains circumventricular organs that are sensitive to osmolarity and the concentration of circulating hormones such as ANG II and aldosterone (16). The LT projects to the PVN, which, in turn, projects to hindbrain and spinal cord structures involved in cardiovascular regulation. We hypothesized that neuroplasticity within structures in this network mediates HTRS as this network is integral to the regulation of MAP and HR. In the present study, we examined whether mRNA underlying neuroinflammation and the central RAAS were upregulated after amphetamine pretreatment in the LT and PVN. We found that markers for neuroinflammation, including TNF-α, CD11b, and IL-6, were elevated along the LT, but not the PVN, at the end of DEL in amphetamine-pretreated rats. This implicates a distinct role for neuroinflammation in LT structures in driving HTRS. TNF-α and IL-6 are both proinflammatory cytokines that have been linked to hypertension (13, 30), whereas CD11b is an indicator of microglia activation (27). Microglia activation has also been linked to the pathogenesis of hypertension (13, 30). It should be noted that tissue samples were collected during the light phase when brain inflammatory markers tend to be elevated (8, 9) and it is possible that tissue collection during the day may have pronounced effects on LT markers of neuroinflammation. However, we failed to find differences in circadian MAP, suggesting that it is unlikely circadian variations in neuroinflammation impacted MAP in our study. We also found elevated message for renin, indicating that the central RAAS is upregulated in the LT in amphetamine-pretreated rats. However, message for other indicators of the central RAAS failed to be significantly increased after amphetamine injection (although some indicators trended toward significance; Table 2). Importantly, we failed to find significant increases in mRNA expression in the PVN for all mRNAs investigated (Table 3). Taken together, the results indicate that HTRS induced by amphetamine pretreatment is primarily driven by neuroinflammation in the LT but not the PVN. Future work targeting neuroinflammation in the LT may abolish amphetamine-induced HTRS. HTRS may also be partially driven by upregulation of the RAAS in the LT as we found some evidence for elevations in message of the central RAAS after amphetamine pretreatment. However, it should be noted that we did not investigate whether proteins are elevated from amphetamine pretreatment.
Based on our findings, we propose a model where neuroinflammation increases excitatory tone in the LT, which, in turn, promotes downstream activation of PVN neurons to drive elevated MAP (20). Neuroinflammation induced by TNF-α has been linked to elevated excitatory neuronal tone (21). Specifically, neuroinflammation downregulates astrocyte glutamate transporter-1 (GLT-1) to decrease glutamate uptake from synapses (11, 21, 22). Neuroinflammation also augments glutamatergic N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors while decreasing GABA receptors to strengthen the excitatory drive of neurons. In our study, amphetamine pretreatment appeared to produce a relatively long-lasting elevation in LT neuroinflammation. It is likely that LT neuroinflammation provides a mechanism for HTRS and markers for neuroinflammation remain elevated during the EXP phase to elicit HTRS.
We previously found that many treatments that induce HTRS elevate RAAS and neuroinflammation in the PVN, however, these molecular markers in the PVN remained unchanged from amphetamine pretreatment. It is possible that amphetamine pretreatment alters traditional mechanisms of neural plasticity in the PVN such as glutamatergic NMDA and AMPA receptors, however, additional studies are needed to test this hypothesis. Furthermore, the central RAAS and neuroinflammation in the PVN may not have been elevated in the present experiment because tolerance developed to the acute effects of amphetamine on MAP.
Our present findings lay the foundation for future research on amphetamine’s effects on HTRS. One direction would be to examine other dose and injection protocols of amphetamine. The dose of amphetamine used in the present experiment is relatively high compared with therapeutic doses of psychomotor stimulants that are used clinically and the dose is higher than the amount of amphetamine rats self-administer per hour (25). However, this dose has been used to study translationally relevant aspects of psychomotor stimulant addiction in rats (14). In the clinic, psychomotor stimulants are prescribed at low doses for long periods to young people that may be undergoing critical periods in their development. Examining whether a chronic low dose of amphetamine in adolescent rats impacts HTRS is an exciting future direction. Furthermore, future work will test our proposed model by examining levels of GLT-1, GABA, NMDA, and AMPA receptors in the LT and whether antagonizing glutamatergic systems will prevent HTRS elicited by amphetamine.
Perspectives and Significance
The present findings lend further support to the role of neuroplasticity as a critical factor in some forms of hypertension. With the use of an IND-DEL-EXP model, we have shown that a variety of stressors are capable of driving HTRS. For example, pretreatment with subpressor doses of ANG II or aldosterone, social defeat, maintenance on a high-fat diet, and amphetamine pretreatment are all sufficient to elicit HTRS (17, 18, 31–34). Many of these treatments also chronically elevate molecular markers of the central RAAS and neuroinflammation in the LT or in the PVN. This indicates that neuroplasticity within central nervous system structures involved in cardiovascular control is sensitive to internal and environmental stressors. In other words, it is likely that evolutionary selection pressures ensured that neural networks that control MAP and HR can undergo long-term changes mediated via neuroplasticity to control MAP. HTRS may have been beneficial in environments where repeated exposure to stressors would elicit elevations in MAP to allow for rapid redistribution of cellular resources to meet repeated environmental challenges. However, HTRS is probably maladaptive for modern-day humans where a sensitized pressor response is likely to result in cardiovascular disease.
GRANTS
This work was supported by the NIH Grants HL-14388, HL-98207, MH-80241, and HL-139575.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.W.H. and A.K.J. conceived and designed research; S.W.H., T.G.B., F.G., and B.X. performed experiments; S.W.H. and B.X. analyzed data; S.W.H., B.X., and A.K.J. interpreted results of experiments; S.W.H. prepared figures; S.W.H. drafted manuscript; S.W.H., B.X., and A.K.J. edited and revised manuscript; S.W.H., T.G.B., F.G., B.X., and A.K.J. approved final version of manuscript.
REFERENCES
- 1.Akkina SK, Ricardo AC, Patel A, Das A, Bazzano LA, Brecklin C, Fischer MJ, Lash JP. Illicit drug use, hypertension, and chronic kidney disease in the US adult population. Transl Res 160: 391–398, 2012. doi: 10.1016/j.trsl.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med 170: 214–219, 1999. [PMC free article] [PubMed] [Google Scholar]
- 3.Braun BL, Murray DM, Sidney S. Lifetime cocaine use and cardiovascular characteristics among young adults: the CARDIA study. Am J Public Health 87: 629–634, 1997. doi: 10.2105/AJPH.87.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brecklin CS, Gopaniuk-Folga A, Kravetz T, Sabah S, Singh A, Arruda JAL, Dunea G. Prevalence of hypertension in chronic cocaine users. Am J Hypertens 11: 1279–1283, 1998. doi: 10.1016/S0895-7061(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 5.Casarsa BS, Marinzalda MÁ, Marchese NA, Paz MC, Vivas L, Baiardi G, Bregonzio C. A previous history of repeated amphetamine exposure modifies brain angiotensin II AT1 receptor functionality. Neuroscience 307: 1–13, 2015. doi: 10.1016/j.neuroscience.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest 130: 1657–1663, 2006. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 7.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 45: 171–179, 2015. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonken LK, Weil ZM, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun 34: 159–163, 2013. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Frishman WH, Del Vecchio A, Sanal S, Ismail A. Cardiovascular manifestations of substance abuse: part 2: alcohol, amphetamines, heroin, cannabis, and caffeine. Heart Dis 5: 253–271, 2003. doi: 10.1097/01.hdx.0000080713.09303.a6. [DOI] [PubMed] [Google Scholar]
- 11.Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology 76: 276–286, 2014. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 13.Haspula D, Clark MA. Neuroinflammation and sympathetic overactivity: mechanisms and implications in hypertension. Auton Neurosci 210: 10–17, 2018. doi: 10.1016/j.autneu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Jaber M, Cador M, Dumartin B, Normand E, Stinus L, Bloch B. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger RNA levels and Fos immunoreactivity in rat striatal neurons. Neuroscience 65: 1041–1050, 1995. doi: 10.1016/0306-4522(94)00537-F. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Zhu R, Bu Q, Li Y, Shao X, Gu H, Kong J, Luo L, Long H, Guo W, Tian J, Zhao Y, Cen X. Brain renin-angiotensin system blockade attenuates methamphetamine-induced hyperlocomotion and neurotoxicity. Neurotherapeutics 15: 500–510, 2018. doi: 10.1007/s13311-018-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AK, Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol 14: 750–766, 2018. doi: 10.1038/s41581-018-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AK, Zhang Z, Clayton SC, Beltz TG, Hurley SW, Thunhorst RL, Xue B. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1309–R1325, 2015. doi: 10.1152/ajpregu.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaman MG, Atalay F, Tufan AE, Erdogan A. Pulmonary arterial hypertension in an adolescent treated with methylphenidate. J Child Adolesc Psychopharmacol 20: 229–231, 2010. doi: 10.1089/cap.2009.0095. [DOI] [PubMed] [Google Scholar]
- 20.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 21.Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014: 861231, 2014. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omrani A, Melone M, Bellesi M, Safiulina V, Aida T, Tanaka K, Cherubini E, Conti F. Up-regulation of GLT-1 severely impairs LTD at mossy fibre–CA3 synapses. J Physiol 587: 4575–4588, 2009. doi: 10.1113/jphysiol.2009.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paz MC, Assis MA, Cabrera RJ, Cancela LM, Bregonzio C. The AT1 angiotensin II receptor blockade attenuates the development of amphetamine-induced behavioral sensitization in a two-injection protocol. Synapse 65: 505–512, 2011. doi: 10.1002/syn.20868. [DOI] [PubMed] [Google Scholar]
- 24.Paz MC, Marchese NA, Cancela LM, Bregonzio C. Angiotensin II AT1 receptors are involved in neuronal activation induced by amphetamine in a two-injection protocol. Biomed Res Int 2013: 534817, 2013. doi: 10.1155/2013/534817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickens R. Self-administration of stimulants by rats. Int J Addict 3: 215–221, 1968. doi: 10.3109/10826086809042896. [DOI] [Google Scholar]
- 26.Rowland N, Antelman SM, Kocan D. Elevated water intake in rats treated chronically with amphetamine: drinking in excess of need? Appetite 2: 51–66, 1981. doi: 10.1016/S0195-6663(81)80036-0. [DOI] [PubMed] [Google Scholar]
- 27.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem 281: 14971–14980, 2006. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol 21: 92–95, 2006. doi: 10.1007/s00467-005-2051-1. [DOI] [PubMed] [Google Scholar]
- 29.Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2: 249–255, 1974. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- 30.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension 67: 163–170, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue B, Yu Y, Wei SG, Beltz TG, Guo F, Felder RB, Johnson AK. Stress-induced sensitization of angiotensin II hypertension is reversed by blockade of angiotensin-converting enzyme or tumor necrosis factor-α. Am J Hypertens 32: 909–917, 2019. doi: 10.1093/ajh/hpz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 59: 459–466, 2012. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 60: 1023–1030, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, Tarazi FI, Campbell A, Baldessarini RJ. GABAB receptors: altered coupling to G-proteins in rats sensitized to amphetamine. Neuroscience 101: 5–10, 2000. doi: 10.1016/S0306-4522(00)00344-4. [DOI] [PubMed] [Google Scholar]