This cohort study uses data from 41 European cancer registries to estimate the incidence and survival of patients with conjunctival melanoma in Europe.
Key Points
Question
What are the incidence and survival of patients with conjunctival melanoma in Europe?
Findings
In this cohort study of 724 patients with conjunctival melanoma from population-based data of 41 cancer registries, the overall incidence was 0.46 cases per 1 000 000 person-years. Overall, 5-year relative survival was 83.5% and varied among European geographic areas, with the highest in the UK and Ireland (89.1%) and lowest in Southern Europe (65.7%).
Meaning
Geographical differences in survival indicate room for outcome improvement in Southern, Northern, and Eastern European countries.
Abstract
Importance
Conjunctival melanoma (CM) is a rare ocular tumor. Estimates of incidence and survival of patients with CM are important to researchers and policy makers.
Objective
To estimate incidence and survival of patients with CM in Europe.
Design, Setting, and Participants
This population-based cohort study used data from 41 European cancer registries adhering to the RARECAREnet project. All individuals diagnosed as having malignant CM from January 1995 to December 2007 coded according to the International Classification of Diseases for Oncology, Third Edition codes C69.0 (conjunctiva) and 8720-8780 (melanoma) were included. Analysis began March 2019.
Main Outcomes and Measures
Trend estimates for incidence and for 5-year relative survival (the ratio of the measured survival of patients to the expected survival in the general population for the same country, age, sex, and calendar year). Crude, age-standardized, and bayesian incidence rates were calculated. Five-year relative survival was calculated by the Ederer II method with the cohort and period approach.
Results
A total of 724 patients 15 years or older (512 [70.7%] were 55 years or older; 366 [50.6%] were female) were analyzed with an overall crude incidence of CM (per 1 000 000 person/y) of 0.46 (95% CI, 0.42-0.49). Crude incidence was similar in men and women (0.48; 95% CI, 0.44-0.54 and 0.46; 95% CI, 0.41-0.51, respectively) and increased with age. Age-standardized incidence increased over time only in men and was the highest in Norway and the Netherlands (more than 0.70). Only 1 case in 14 years was estimated to occur in Iceland vs about 20 cases per year in large countries such as France and Germany. Percentage of 5-year survival (83.5 overall; 95% CI, 78.6-87.3) was not different between adult and elderly patients but showed large geographical disparities across European regions (range, 66-89) and improved markedly in male patients (from 76 in 1995-1998 to 86 in 2003-2007, with a difference of 10.2 [95% CI, 1.3-19.2]; P < .05) becoming similar to that of women in the last period.
Conclusions and Relevance
Although these data are only available through 2007 and based on registries not uniformly covering the European population, the study provides the first Europe-wide estimates of the incidence and relative survival of patients with CM using population-based data. Geographical differences in survival indicate room for outcome improvement in Southern, Northern, and Eastern European countries.
Introduction
Conjunctival melanoma (CM) is a rare ocular malignant neoplasm, appearing as a variably pigmented, vascularized mass at any site of the conjunctiva. Conjunctival melanoma has been referred to as one of the most dreaded and unpredictable ocular tumors.1 Local recurrence after therapy is high, up to 50% at 10 years.2,3,4,5 Regional and distant metastases are reported in one-fourth of cases after 10 years since diagnosis, and the more common sites are lymph node (cervical, preauricular, parotid, and submandibular), lungs, liver, skin, brain, and adrenals.2
Kaštelan et al6 have recently summarized incidence studies on CM, which varied from 0.1 to 0.9 per 1 000 000 person-years in 14 studies spanning different periods and conducted in various geographic areas. Three studies found increasing overall incidence, and 2 found an increase only in male individuals. Hu et al7 investigated the incidence of CM in 168 patients using the Surveillance, Epidemiology, and End Results database, finding a crude incidence rate (IR) of 0.53 per 1 000 000 person-years. This study was conducted in the United States and was designed on racial differences and found that rates for black individuals were 3 times lower than for white individuals. Studies in Denmark and Sweden8,9,10,11,12,13 found that the 5- and 10-year relative survival for patients with CM remained stable for both men (83%; 95% CI, 69%-98% and 70%; 95% CI, 51%-89%, respectively) and women (93%; 95% CI, 79%-108% and 82%; 95% CI, 60%-105%, respectively).
Although a relatively large number of studies has been conducted on the epidemiology of this tumor, they were generally single-country studies so the number of cases was limited by its rarity.7 To update and expand the knowledge on the incidence of CM in different European regions and survival of affected patients, we conducted a study using population-based data from cancer registries adhering to the RARECAREnet project.14
Methods
We included data on malignant CM available from 41 registries included in the RARECAREnet project as identified by the International Classification of Diseases for Oncology, Third Edition topography code C69.0 (conjunctiva) and morphology codes 8720 to 8780 (melanoma). The ethics committee of the IRCCS National Cancer Institute in Milan, Italy, approved the project and stated that no patient-informed consent was needed for anonymized and aggregated registry-based data. Crude and age-standardized IRs were calculated per 1 000 000 person per year in the whole period (January 1995 to December 2007) by age, sex, country, area, and quadrennials periods (1995-1998, 1999-2002, 2003-2007). The European standard population was used for age-standardized IRs. This was the longest and the most recent period for which incidence and 5-year survival data are available for most cancer registries participating in the study.
Because CM is an extremely rare tumor, we used a bayesian method introduced by Botta et al15 to obtain country-level estimates of IRs, using the European IR as an informative prior to merge with country-specific observed data. The aim of this analysis was to improve IR estimates when data were sparse, such as in small countries or countries with few registries providing data, and provide a comprehensible indicator that represents the expected time in years needed to observe 1 new case in the specific country.
To remove the mortality due to causes other than cancer, which can vary widely by country, we estimated 5-year relative survival, as the ratio of the measured survival of patients with cancer to the expected survival in a comparable group from the general population. Crude relative survival by age, sex, area, and quadrennials periods was estimated using the complete cohort approach by the Ederer II method15 from registry-specific population life tables stratified by age, sex, and calendar year. To include also the most recently diagnosed patients in survival analysis, time trends of crude relative survival were also estimated by the period approach, considering 3 follow-up periods: 1996 to 1999 (cohorts diagnosed from January 1, 1992, to December 31, 1999), 2000 to 2003 (cohorts diagnosed from January 1, 1996, to December 31, 2003), and 2004 to 2007 (cohorts diagnosed from January 1, 2000, to December 31, 2007). The statistical significance for trends were based on the z test. Analysis began March 2019. P values were 2-sided, and the significance threshold was less than .05.
A sensitivity analysis by geographic areas was conducted to explore potential misclassification of cancers extending to peribulbar tissues by including topographic codes different from C69.0 (conjunctiva): C69.6 (orbit) and C69.8 (overlapping lesion of eye and adnexa). The use of the code C69.9–not otherwise specified topographies varied widely across geographic areas, and we decided not to include this code in our sensitivity analyses because previous studies found it was used specifically for large uveal melanomas in some geographic areas, particularly in the UK.19 Additional methodological details on the RARECAREnet project are available in the broader framework of the EUROCARE-5 study on cancer survival in Europe.17
Results
Population
Overall, 714 patients with CM were recorded from 1995 to 2007 in Europe, including 46 in Northern Europe (2 registries), 313 in the UK and Ireland (5 registries), 206 in Central Europe (10 registries), 47 in Southern Europe (16 registries), and 102 in Eastern Europe (8 registries).
The histologic verification rate was complete (100%) in Northern and Eastern Europe and was almost complete in Central Europe (99%), Southern Europe (97.9%), and the UK and Ireland (96.5%).
Incidence
Overall crude IR (per 1 000 000 person-years) was 0.46 (95% CI, 0.42-0.49). The Table shows annual crude IR and their 95% CI by sex, age class, and geographical area. Conjunctival melanoma incidence significantly increased with age and was similar between male and female individuals.
Table. Number of Patients, Crude Incidence, and 5-Year Relative Survival With 95% CI Overall by Age, Sex, Geographic Area, 1995-2007.
| Variable | Crude incidence rate per 1 000 000 person-years | 5-y Relative survival | ||
|---|---|---|---|---|
| Patients, No. | Incidence rate (95% CI) | Patients, No. | % (95% CI) | |
| Overall | 714 | 0.46 (0.42-0.49) | 707 | 83.46 (78.61-87.3) |
| Age, y | ||||
| 15-54 | 209 | 0.20 (0.18-0.23) | 207 | 88.38 (82.25-92.49) |
| 55-74 | 309 | 0.87 (0.77-0.97) | 305 | 80.26 (73.34-85.56) |
| ≥75 | 196 | 1.57 (1.36-1.81) | 195 | 82.74 (65.47-91.88) |
| Sex | ||||
| Male | 355 | 0.48 (0.44-0.54) | 352 | 81.85 (74.67-87.17) |
| Female | 359 | 0.46 (0.41-0.51) | 355 | 85.08 (77.85-90.1) |
| Area | ||||
| Northern Europe | 46 | 0.92 (0.77-1.23) | 44 | 76.45 (53.48-89.12) |
| UK and Ireland | 313 | 0.47 (0.42-0.52) | 312 | 89.09 (80.61-94.00) |
| Central Europe | 206 | 0.64 (0.56-0.74) | 204 | 83.68 (74.14-89.93) |
| Southern Europe | 47 | 0.41 (0.30-0.55) | 46 | 65.74 (44.03-80.69) |
| Eastern Europe | 102 | 0.28 (0.23-0.34) | 101 | 76.84 (62.29-86.36) |
Age-standardized rates were lower than the corresponding crude rates (0.42 vs 0.46 overall) owing to the younger age distribution of the European standard population. Age-standardized IRs were 0.81 (95% CI, 0.59-1.09) in Northern Europe, 0.40 (95% CI, 0.36-0.45) in the UK and Ireland, 0.59 (95% CI, 0.51-0.68) in Central Europe, 0.35 (95% CI, 0.26-0.47) in Southern Europe, and 0.27 (95% CI, 0.22-0.33) in Eastern Europe. An overall upward trend in incidence was found from 0.40 (95% CI, 0.34-0.46) in 1995 to 1998 to 0.43 (95% CI, 0.38-0.48) in 2003 to 2007. This increase was not significant (P = .19) but resulted from a marked and significant (P = .02) increase from 0.41 (95% CI, 0.33-0.50) to 0.53 (95% CI, 0.45-0.61) in men and a stable trend from 0.39 (95% CI, 0.32-0.47) to 0.34 (95% CI, 0.29-0.41) in women.
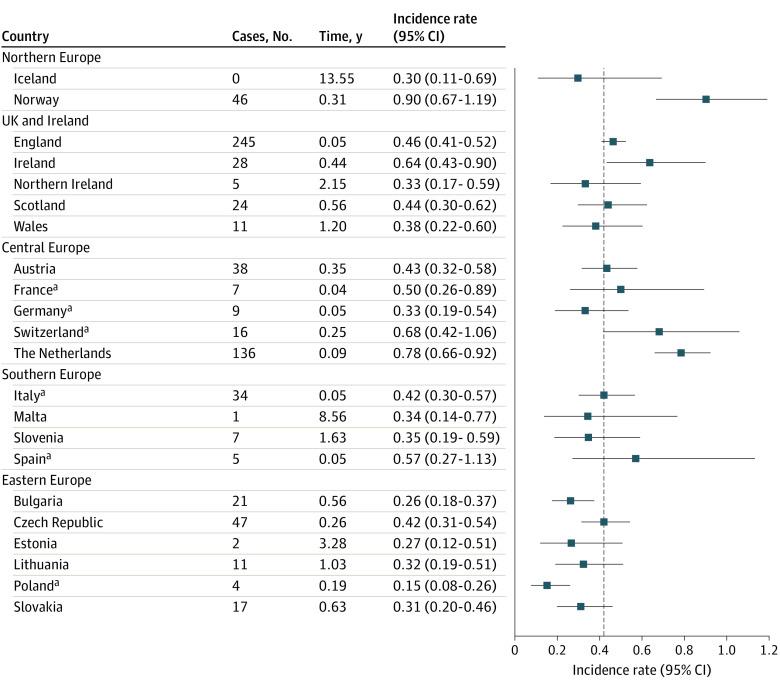
Age-standardized bayesian incidence estimates for all countries are plotted by region in Figure 1, together with the number of cases in each country and the time expected to observe 1 case (expressed in years and calculated with bayesian IRs and country-level person-year). As expected, bayesian estimates are smoothed toward the European average for countries with very sparse data, such as Iceland, Estonia, Malta, and Poland. Considering our bayesian estimates of time expected to observe 1 case, we found that 7 of 22 countries (31.8%) are expected to have less than 1 case per year (Estonia, Iceland, Lithuania, Malta, Slovenia, Northern Ireland, and Wales), with Iceland projected to observe 1 new case every 13 years. No association between standardized IRs and registry latitude was observed (eFigure in the Supplement).
Figure 1. Bayesian Incidence Rates and 95% CI With the Number of Cases for Each Registry and Time, Expected to Observe 1 Case in Each Country.
aNo country-level coverage was available. The registries providing data are shown in eTable 1 in the Supplement.
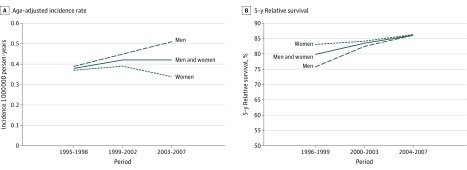
Figure 2 presents trends in age-standardized IRs and 5-year relative survival across 3 study periods overall and by sex. An upward overall trend in incidence was found from 0.37 (95% CI, 0.34-0.46) in 1995 to 1998 to 0.43 (95% CI, 0.38-0.48) in 2003 to 2007. This increase was the result of a marked, but still not significant, increase from 0.41 (95% CI, 0.33-0.50) to 0.53 (95% CI, 0.45-0.61) in men and of a stable trend from 0.39 (95% CI, 0.32-0.47) to 0.34 (95% CI, 0.29-0.41) in women.
Figure 2. Temporal Trends of Age-Adjusted Incidence Rate and 5-Year Relative Survival in Europe Across 1995-1998, 1999-2002, and 2003-2007.
The sensitivity analysis with potentially misclassified codes (eTable in the Supplement) found an age-tastandardized incidence of 0.57 (95% CI, 0.53-0.61) including sites C69.6 and C69.8 (orbit and overlapping lesion of eye and adnexa, total cases 973). The increase in incidence due to potential misclassification of CM, ascribed to the invasion of adjacent structures (sites C69.6 and C69.8), was about 20% to 30% for all geographic areas except for Eastern Europe (90%), where 5 of 8 registries more than doubled the number of cases.
Survival
The Table presents 5-year relative survival calculated for 707 patients, excluding 7 cases detected with death certificate only or incidental autoptic cases, by age, sex, and European areas. Overall, 5-year relative survival was 83.5% (95% CI, 78.6%-87.3%). Overall, 5-year relative survival was 83.5% (95% CI, 78.6%-87.3%). Relative survival was slightly higher for younger patients with respect to the older patients, 88.4% (95% CI, 82.2%-92.5%) in those younger than 55 years vs 80.3% (95% CI, 73.4%-85.6%) in individuals aged 55 to 74 years and 82.7% (95% CI, 65.5%-91.9%) in individuals 75 years and older. It was also slightly higher for women (85.1% [95% CI, 77.8%-90.1%]) than for men (81.9% [95% CI, 74.7%-87.2%]). None of these differences was statistically significant, with borderline significance of the difference between the 2 youngest age classes. Comparing geographical regions, relative survival was 76.5% (95% CI, 53.5%-89.1%) in Northern Europe, 89.1% (95% CI, 80.6%-94.0%) in the UK and Ireland, 83.7% (95% CI, 74.1%-89.9%) in Central Europe, 65.7% (95% CI, 44.0%-80.7%) in Southern Europe, and 76.8% (95% CI, 62.3%-86.4%) in Eastern Europe. The same analysis including melanoma arising in the periocular tissue, thus including the International Classification of Diseases for Oncology, Third Edition topography codes 68.8 and 69.6, reduced 5-year survival in all the regions particularly in Eastern Europe from 76.8% to 62.4%.
Time trends in relative survival for the 3 study periods (Figure 2B) showed a linear increase from 79.9% (95% CI, 70.0%-86.8%) in the first period to 86.0% (95% CI, 77.8%-91.3%) in the third period, with a difference of 6.1%. A large female vs male advantage, estimated as 7.3% (95% CI, −10% to 24.7%) in 1996 to 1999, was progressively reduced to 0 in 2004 to 2007.
Discussion
In this study, we have provided Europe-wide estimates of CM incidence and relative survival in a large population-based sample collected by 41 cancer registries adhering to RARECAREnet, based on cases diagnosed during 1995 to 2007. We found, even if not significant owing to the low number of cases, some interesting variability in incidence and survival in Europe.
Incidence rates of CM significantly with age, and no difference in crude rates between sexes was recorded. However, when adjusting by age, the incidence of CM showed an upward trend in men and remained stable in women, resulting in a 50% higher risk for men from 2003 to 2007. The overall CM incidence varied in Europe from 0.28 in Southern Europe to 0.90 per 1 000 000 person-years in Northern Europe. Theoretically, geographical variation may depend on the prevalence of risk factors, eg, sun exposure or blue eye color. Interestingly, the highest CM incidence estimates were obtained for Norway, Switzerland, and the Netherlands, the same 3 countries with the highest incidence of skin melanoma in Europe.14 Moreover, Eastern European countries had the lowest rates of both conjunctival and skin melanoma. However, we cannot exclude that the differences are partly due to diagnostic pathology skills and incomplete or vague diagnosis, which has been seen to be an issue in rare cancer diagnosis.18 We expect these sources of variation are minimized in RARECAREnet because strict rules for pathology interpretation are required for registries that adhere to this network. Our sensitivity analysis including sites C69.6 and C69.8 (orbit and overlapping lesion of eye and adnexa) showed a potential for topographic misclassification in Eastern Europe if CM cases extending to periocular regions are not attributed to the conjunctival site. The inclusion of these topographic codes markedly increased the incidence in the Eastern area, reaching the average European value and reduced survival. Therefore, we can conclude that part of the difference in incidence may be due to a country’s pathology services skills. Other factors, such as long-term exposure to solar radiation in recreational (golfing, fishing, boating) or occupational activities (construction and farming) are suggested as risk factors and explain the high incidence in Norway and in the Netherlands (>0.7) in our study. Other studies found a higher CM frequency in men than in women, which was not found in our analysis. Interestingly, in our study, incidence increased only in the male population, resulting in a higher incidence for men than for women from 2003 to 2007.
Loss of cases due to migration might also be a potential source of bias in this study. However, UK and Nordic registries have national coverage, and the effect of migration in the period should be very small. Moreover, European populations are known to be much more geographically stable than in the US.
A small improvement of 5-year relative survival was seen during the period considered, but, unexpectedly, it was more marked for men. This could be due to a late-stage presentation in men at the beginning of the study period because differences in treatment according to sex are unlikely. Interestingly, no difference in 5-year survival was found in the population 50 years or older, suggesting that therapies are well tolerated in elderly individuals. There was a large variation in relative survival between geographic regions. The European geographical variation in survival was also reported for all rare cancers together, with age-adjusted and case-mix–adjusted 5-year relative survival varying from more than 50% in Northern Europe to less than 40% in Eastern Europe, with corresponding values of 59.6% and 45% for all cancers combined.20,21 One may speculate that investment in the health care system may explain part of survival variation, and actually 5-year survival, adjusted by age when possible, is one of the most succinct indicators of cancer control performance.18,20
Strengths and Limitations
Strengths of our study were the large sample size and its Europe-wide coverage, as well as the adoption of an agreed protocol between registries for standardized definition of the variables and centralization of the data check and analysis.17 As we are dealing with an exceptionally rare cancer, we used a bayesian approach taking advantage of the European estimate from our large database to obtain more realistic, especially in small countries with sparse data, country-specific incidence estimates.15
A limitation of our population-based study consists in the limited number of variables collected that cannot provide explanation of the observed trends. Among these, ethnicity is not recorded, but, in 168 CM cases in the US, Hu et al7 found the incidence of CM varies widely according to ethnicity with a white individual–to–black individual incidence ratio of 2.6:1, which is much less than that of uveal melanoma and cutaneous melanoma and is similar to that of mucosal melanoma.22 For this reason, and considering that the study period is relatively short, we believe that the effect of our failure to account for changes of the ethnic profile of the European population is less than what we found in studies on uveal melanoma.17
A further limitation, common to all the population-based estimates at the European level, is that the analyzed data set is not geographically representative of the European population. For example, more than one-third of cases were recorded in England. This is because cancer registries did not uniformly cover the European population.
The estimates of incidence obtained from UK and Ireland registries are very close to the average of all European regions (Figure 1), and we expect little bias in European estimates. On the other hand, 5-year relative survival was the highest in the UK and Ireland, and this could lead to some overestimation of the overall survival. Country-specific estimates of relative survival are meaningful to policy makers and researchers investigating heterogeneity in the health care of this rare tumor. Our study cannot investigate whether differences in survival reflect higher standards of care, but referral for management in specialized centers was suggested to improve outcomes.18
Conjunctival melanoma is a rare tumor and 5 cases or less were recorded in 6 registries; however, 4 of these registries had national coverage, eg, in Iceland where no cases were recorded in the study period; thus, we expect underreporting to be unlikely. We used bayesian methods and informative priors to obtain robust estimates of incidence with sparse data.15
Kaštelan et al6 have recently summarized incidence on CM in 14 studies, of which 3 reported increasing incidences with time, with 2 additional studies finding an increase only in men. A potential explanation for these differences is the study conduction in a different geographic area and period. For example, Yu et al23 identified 206 newly diagnosed patients with CM from 1973 to 1999 using the Surveillance, Epidemiology, and End Results program, finding the estimated biannual percent change was significantly elevated for white men (estimated biannual percent change: 11.2; 95% CI, 6.3-16.3) but not for white women (estimated biannual percent change: 0.3; 95% CI, 4.1-4.9). In a study conducted in Europe, Isager et al8,9 reported on incidence and survival of ocular melanoma in Denmark, including 115 cases of CM. During the 55-year study period, the overall CM incidence is similar to ours (0.5 cases per 1 000 000 person-years), but it increased during the longer studied period. In the same study, the 5- and 10-year relative survival for the conjunctiva was 83% and 70% for men and 93% and 82% for women, respectively. The same consideration applies to the study by Triay et al,10 who reported time trends in all patients with CM from 1960 to 2005 in Sweden, identified through the Swedish Cancer Registry. They found age-standardized incidence increased significantly in men (n = 89) from 0.10 cases per 1 000 000 person-years to 0.74 cases per 1 000 000 person-years (P = .001) and in women (n = 81) from 0.06 cases per 1 000 000 person-years to 0.45 cases per 1 000 000 person-years (P = .007). This study could analyze clinical data and found that, during the period of study, tumors became smaller and thinner at the time of diagnosis and increasingly arose from parts of the conjunctiva exposed to ultraviolet radiation, concluding that increasing incidence was partly explained by earlier diagnosis. The role of sunlight exposure could not be investigated on our study, but it could explain the larger increase in incidence found in men compared with women, possibly owing to professional exposure in men performing outdoor work.23 Of interest, however, we found no association between incidence and the latitude of the registry.
Few studies have investigated survival of patients with CM. Kujala et al24 reported a slightly higher 10-year melanoma-related death for patients with CM (39%) compared with patients with uveal melanoma (32%). On the contrary, Mahendraraj et al25 used the Surveillance, Epidemiology, and End Results database and included 649 patients with CM finding 5-year cancer-specific survival was slightly better for CM compared with uveal melanoma (73% vs 70%). In Europe, we also reported a better outcome, after 5 years since diagnosis, for CM: 84% (95% CI, 79%-87%) vs 71% (95% CI, 70%-72%) for uveal melanoma.26 The progress of survival can be due to early diagnosis, treatment, or both. We do not have standardized information on stage and treatment, but we can supply some indications about these factors from the analysis of conditional survival. Because survival was almost complete in the first year of diagnosis and because deaths are very few, we can assume that delayed diagnosis is not an issue because delayed diagnosis is commonly believed to increase early mortality. In any case, the improvement in survival is modest in our study period, which is consistent with the lack of standard treatments with proven efficacy on metastatic disease in patients with CM. Clinical trials are currently ongoing to further evaluate the role of targeted therapies, as well as immunotherapies.5,25,27
Recently, a multicenter, international study yielded evidence that the CM staging system in the eighth edition of the American Joint Committee on Cancer staging manual can be used to accurately defined metastasis at diagnosis and survival after metastases.28 Interestingly, this study found no evidence that metastasis mortality is associated with age or sex, adjusting for clinical staging.
Conclusions
In conclusion, we have reported the first estimate of the incidence and relative survival of patients with CM in Europe, to our knowledge, using population-based data from cancer registries adhering to the RARECAREnet project. We found variation in incidence as well as in 5-year survival in the European population, indicating that both risk factors and skill in the diagnosis of this rare cancer, as well as timely access to centralized specialist clinical centers are responsible for the variation. Actually, in 6 European countries, centralization of uveal melanoma treatment reached one of the highest levels compared with centralization of the other rare cancers.18 A Dutch study by Brouwer et al,29 concluded that the initial treatment at a large referral center improves clinical outcome and that patients should be referred as soon as possible. The low success of treatments required large international collaboration for clinical studies. In Europe, the European Reference Networks for rare diseases was created in response to these needs. Networking is the most appropriate answer to the issues pertaining to rare cancers. The Joint Action of Rare Cancers is another major European initiative that will help the mission of the European Reference Networks. Actually, the Joint Action of Rare Cancers objectives are to prioritize rare cancers in the agendas of the European Union member states and to optimize the functioning of the European Reference Networks, providing operational solutions and professional guidance in quality of care, epidemiology, research, education, and state of the art definition on prevention, diagnosis, and treatment of rare cancers. The performance and clinical usefulness of this population-based study could be improved by collecting key important clinical variables at cancer registries, for example stage at diagnosis and treatment center. The collection of data could also be improved using electronic resources that ensure better communication and connect primary care ophthalmologists, specialist centers responsible for treatment and diagnosis, pathologists, and cancer registries.
eFigure. Standardized incidence rates of conjunctival melanoma in European Registries adhering to the RARECAREnet project (y-axis) against the latitude of each registry (x-axis); the marker for each registry is specific to its geographic area. Years of diagnosis between 1995 and 2007
eTable. Sensitivity analysis adding sites 69.6 and 69.8, corresponding to periocular tissues, to age standardized incidence rates and their corresponding 95% confidence intervals (95%CI) calculation
eAppendix.
References
- 1.Seregard S. Conjunctival melanoma. Surv Ophthalmol. 1998;42(4):321-350. doi: 10.1016/S0039-6257(97)00122-7 [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118(2):389-95.e1, 2. doi: 10.1016/j.ophtha.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 3.Shildkrot Y, Wilson MW. Conjunctival melanoma: pitfalls and dilemmas in management. Curr Opin Ophthalmol. 2010;21(5):380-386. doi: 10.1097/ICU.0b013e32833b7aab [DOI] [PubMed] [Google Scholar]
- 4.Kao A, Afshar A, Bloomer M, Damato B. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23(2):117-125. doi: 10.1177/107327481602300205 [DOI] [PubMed] [Google Scholar]
- 5.Vora GK, Demirci H, Marr B, Mruthyunjaya P. Advances in the management of conjunctival melanoma. Surv Ophthalmol. 2017;62(1):26-42. doi: 10.1016/j.survophthal.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaštelan S, Gverović Antunica A, Beketić Orešković L, Salopek Rabatić J, Kasun B, Bakija I. Conjunctival melanoma: epidemiological trends and features. Pathol Oncol Res. 2018;24(4):787-796. doi: 10.1007/s12253-018-0419-3 [DOI] [PubMed] [Google Scholar]
- 7.Hu DN, Yu G, McCormick SA, Finger PT. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol. 2008;145(3):418-423. doi: 10.1016/j.ajo.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 8.Isager P, Engholm G, Overgaard J, Storm H. Uveal and conjunctival malignant melanoma in denmark 1943-97: observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006;13(2):85-96. doi: 10.1080/09286580600553330 [DOI] [PubMed] [Google Scholar]
- 9.Isager P, Østerlind A, Engholm G, et al. Uveal and conjunctival malignant melanoma in Denmark, 1943-97: incidence and validation study. Ophthalmic Epidemiol. 2005;12(4):223-232. doi: 10.1080/09286580591000836 [DOI] [PubMed] [Google Scholar]
- 10.Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009;93(11):1524-1528. doi: 10.1136/bjo.2009.157933 [DOI] [PubMed] [Google Scholar]
- 11.Larsen AC. Conjunctival malignant melanoma in Denmark. Epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol. 2016;94(8):842. doi: 10.1111/aos.13207 [DOI] [PubMed] [Google Scholar]
- 12.Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94(5):463-470. doi: 10.1111/aos.13007 [DOI] [PubMed] [Google Scholar]
- 13.Larsen AC, Dahmcke CM, Dahl C, et al. A retrospective review of conjunctival melanoma presentation, treatment, and outcome and an investigation of features associated with BRAF mutations. JAMA Ophthalmol. 2015;133(11):1295-1303. doi: 10.1001/jamaophthalmol.2015.3200 [DOI] [PubMed] [Google Scholar]
- 14.European Network of Cancer Registries ENCR factsheets: skin melanoma. Published April 2015. Accessed December 4, 2019. https://www.encr.eu/sites/default/files/factsheets/ENCR_Factsheet_Malignant_Melanoma_2015.pdf
- 15.Botta L, Capocaccia R, Trama A, et al. ; RARECAREnet Working group . Bayesian estimates of the incidence of rare cancers in Europe. Cancer Epidemiol. 2018;54:95-100. doi: 10.1016/j.canep.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Ederer F, Heise H The effect of eliminating deaths from cancer on general population survival rates, methodological note. End Results and Mortality Trends in Cancer. National Cancer Institute; 1961. [Google Scholar]
- 17.Rossi S, Baili P, Capocaccia R, et al. ; EUROCARE-5 Working Group . The EUROCARE-5 study on cancer survival in Europe 1999-2007: database, quality checks and statistical analysis methods. Eur J Cancer. 2015;51(15):2104-2119. doi: 10.1016/j.ejca.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Gatta G, Capocaccia R, Botta L, et al. ; RARECAREnet working group . Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18(8):1022-1039. doi: 10.1016/S1470-2045(17)30445-X [DOI] [PubMed] [Google Scholar]
- 19.Virgili G, Gatta G, Ciccolallo L, et al. ; EUROCARE Working Group . Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114(12):2309-2315. doi: 10.1016/j.ophtha.2007.01.032 [DOI] [PubMed] [Google Scholar]
- 20.Baili P, Di Salvo F, Marcos-Gragera R, et al. ; EUROCARE-5 Working Group . Age and case mix-standardised survival for all cancer patients in Europe 1999-2007: results of EUROCARE-5, a population-based study. Eur J Cancer. 2015;51(15):2120-2129. doi: 10.1016/j.ejca.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 21.Gatta G, Trama A, Capocaccia R; RARECARENet Working Group . Epidemiology of rare cancers and inequalities in oncologic outcomes. Eur J Surg Oncol. 2019;45(1):3-11. doi: 10.1016/j.ejso.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 22.Mallone S, De Vries E, Guzzo M, et al. ; The RARECARE WG . Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48(8):1167-1175. doi: 10.1016/j.ejca.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003;135(6):800-806. doi: 10.1016/S0002-9394(02)02288-2 [DOI] [PubMed] [Google Scholar]
- 24.Kujala E, Tuomaala S, Eskelin S, Kivelä T. Mortality after uveal and conjunctival melanoma: which tumour is more deadly? Acta Ophthalmol. 2009;87(2):149-153. doi: 10.1111/j.1755-3768.2008.01369.x [DOI] [PubMed] [Google Scholar]
- 25.Mahendraraj K, Shrestha S, Lau CS, Chamberlain RS. Ocular melanoma-when you have seen one, you have not seen them all: a clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973-2012). Clin Ophthalmol. 2017;11:153-160. doi: 10.2147/OPTH.S120530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RARECAREnet. On line analysis. Accessed February 24, 2020. http://www.rarecarenet.eu/analysis.php
- 27.Blum ES, Yang J, Komatsubara KM, Carvajal RD. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30(1):29-32, 34-43, 48. [PubMed] [Google Scholar]
- 28.Jain P, Finger PT, Damato B, et al. ; American Joint Committee on Cancer Ophthalmic Oncology Task Force . Multicenter, international assessment of the eighth edition of the American Joint Committee on Cancer Cancer Staging Manual for Conjunctival Melanoma. JAMA Ophthalmol. Published online June 6, 2019. doi: 10.1001/jamaophthalmol.2019.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwer NJ, Marinkovic M, van Duinen SG, Bleeker JC, Jager MJ, Luyten GPM. Treatment of conjunctival melanoma in a Dutch referral centre. Br J Ophthalmol. 2018;102(9):1277-1282. doi: 10.1136/bjophthalmol-2017-311082 [DOI] [PubMed] [Google Scholar]
- 30.Damato B, Coupland SE. An audit of conjunctival melanoma treatment in Liverpool. Eye (Lond). 2009;23(4):801-809. doi: 10.1038/eye.2008.154 [DOI] [PubMed] [Google Scholar]
- 31.Charbotel B, Fervers B, Droz JP. Occupational exposures in rare cancers: a critical review of the literature. Crit Rev Oncol Hematol. 2014;90(2):99-134. doi: 10.1016/j.critrevonc.2013.12.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Standardized incidence rates of conjunctival melanoma in European Registries adhering to the RARECAREnet project (y-axis) against the latitude of each registry (x-axis); the marker for each registry is specific to its geographic area. Years of diagnosis between 1995 and 2007
eTable. Sensitivity analysis adding sites 69.6 and 69.8, corresponding to periocular tissues, to age standardized incidence rates and their corresponding 95% confidence intervals (95%CI) calculation
eAppendix.