Abstract
Phrenic long-term facilitation (LTF) is a sustained increase in phrenic motor output occurring after exposure to multiple (but not single) hypoxic episodes. Ampakines are a class of drugs that enhance AMPA receptor function. Ampakines can enhance expression of neuroplasticity, and the phrenic motor system is fundamentally dependent on excitatory glutamatergic currents. Accordingly, we tested the hypothesis that combining ampakine pretreatment with a single brief hypoxic exposure would result in phrenic motor facilitation lasting well beyond the period of hypoxia. Phrenic nerve output was recorded in urethane-anesthetized, ventilated, and vagotomized adult Sprague-Dawley rats. Ampakine CX717 (15 mg/kg iv; n = 8) produced a small increase in phrenic inspiratory burst amplitude and frequency, but values quickly returned to predrug baseline. When CX717 was followed 2 min later by a 5-min exposure to hypoxia (n = 8; ~45 mmHg), a persistent increase in phrenic inspiratory burst amplitude (i.e., phrenic motor facilitation) was observed up to 60 min posthypoxia (103 ± 53% increase from baseline). In contrast, when hypoxia was preceded by vehicle injection (10% 2-hydroxypropyl-β-cyclodextrin; n = 8), inspiratory phrenic bursting was similar to baseline values at 60 min. Additional experiments with another ampakine (CX1739, 15 mg/kg) produced comparable results. We conclude that pairing low-dose ampakine treatment with a single brief hypoxic exposure can evoke sustained phrenic motor facilitation. This targeted approach for enhancing respiratory neuroplasticity may have value in the context of hypoxia-based neurorehabilitation strategies.
NEW & NOTEWORTHY A single brief episode of hypoxia (e.g., 3–5 min) does not evoke long-lasting increases in respiratory motor output after the hypoxia is concluded. Ampakines are a class of drugs that enhance AMPA receptor function. We show that pairing low-dose ampakine treatment with a single brief hypoxic exposure can evoke sustained phrenic motor facilitation after the acute hypoxic episode.
Keywords: ampakine, neuroplasticity, respiratory
INTRODUCTION
Exposure to acute intermittent hypoxia triggers sustained increases in respiratory motor output that are sustained well beyond the hypoxia exposure. This response is termed long-term facilitation (LTF), and the underlying molecular mechanisms have been comprehensively studied (Fuller and Mitchell 2017; Gonzalez-Rothi et al. 2015; Navarrete-Opazo and Mitchell 2014). While many intermittent hypoxia paradigms are reported in the literature (Navarrete-Opazo and Mitchell 2014), a unifying theme is that respiratory LTF requires repeated episodes of hypoxia interspersed with periods of reoxygenation (Devinney et al. 2013; Gonzalez-Rothi et al. 2015). Thus a single episode of hypoxia does not evoke sustained increases in respiratory motor output (Baker and Mitchell 2000; Wilkerson et al. 2018). This was shown by Baker and Mitchell (2000), who directly compared the effects of episodic vs. continuous hypoxia on phrenic motor output and concluded that LTF required an episodic pattern of exposure.
Ampakines are a class of synthetic pharmacological compounds designed to enhance α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated glutamatergic currents (Arai and Kessler 2007; Lynch 2006). In vitro studies confirm that ampakines are not AMPA receptor agonists, nor do they impact NMDA or kainite receptors directly (Arai and Kessler 2007), but rather they are allosteric modulators of AMPA receptor activity (Arai et al. 1996, 2002, 2004). Acute delivery of ampakines can augment long-term potentiation in the hippocampus (Radin et al. 2016), and chronic ampakine exposure can elevate expression of neurotrophic factors in the central nervous system (Radin et al. 2018; Simmons et al. 2009). Furthermore, ampakines can be potent respiratory stimulants, particularly during conditions of low respiratory motor output (ElMallah et al. 2015; Lorier et al. 2010; Oertel et al. 2010; Ogier et al. 2007; Ren et al. 2009, 2013).
We previously reported that ampakine CX717, administered before three hypoxic exposures, can potentiate respiratory motor LTF recorded from the hypoglossal nerve of anesthetized mice (Turner et al. 2016). This finding indicates an interaction between the mechanisms activated by ampakines and those triggered by repeated hypoxia exposure (Turner et al. 2016, 2018). In the present study, we examined the impact of ampakine pretreatment on phrenic motor output following a single episode of hypoxia. We hypothesized that ampakine pretreatment followed by a single brief hypoxic exposure would result in phrenic motor facilitation lasting well beyond the period of hypoxia. The hypothesis was tested by using an adult rat model in which arterial carbon dioxide was maintained in a narrow isocapnic window throughout the recording procedures. The results support the hypothesis and suggest that this targeted approach for enhancing respiratory neuroplasticity may have value in the context of hypoxia-based neurorehabilitation strategies (Gonzalez-Rothi et al. 2015).
MATERIALS AND METHODS
Animals.
For all experiments, we used adult male Sprague-Dawley rats (Colony 206; ENVIGO Laboratories). Rats were housed in pairs in a controlled environment at the University of Florida (12:12 light-dark cycle, 22°C, 20–30% relative humidity) with food and water available ad libitum. All procedures and protocols described were approved by the University of Florida Institutional Animal Care and Use Committee and are in accordance with National Institutes of Health guidelines. To test the primary hypothesis that ampakine pretreatment followed by hypoxia would result in phrenic motor facilitation, we studied three treatment groups of rats: ampakine CX717 alone (n = 8, age = 11 ± 2 wk, body weight = 331 ± 32 g), ampakine CX717 followed by hypoxia (n = 8, age = 11 ± 2 wk, body weight = 317 ± 34 g), or sham treatment (vehicle) followed by hypoxia (n = 8, age = 11 ± 2 wk, body weight = 318 ± 28 g).
Preparation of ampakines.
Ampakines CX717 and CX1739 were kindly provided by RespireRx in powder form. The ampakines were mixed in 10% 2-hydroxypropyl-β-cyclodextrin (HPCD) solution (Sigma) at 5 mg/ml and stored in 1.5-mL aliquots at −18°C. Aliquots were thawed and allowed to warm to room temperature before use on the day of each experiment. The ampakines were used at a concentration (15 mg/kg) that can stimulate breathing in neuromuscular disease (ElMallah et al. 2015) and enhance intermittent hypoxia-induced LTF of hypoglossal inspiratory motor output in anesthetized mice (Turner et al. 2016). HPCD solution (10% HPCD in 0.45% saline solution) was used for sham (vehicle) injection. The HPCD was stored at 4°C and allowed to warm to room temperature before use on the day of each experiment.
Phrenic nerve recordings.
Rats were studied using established in vivo electrophysiology methods, the fundamentals of which were recently described (Streeter et al. 2017). Rats were anesthetized with 3% isoflurane (in 100% O2), and a tail vein catheter was placed for intravenous delivery of urethane and fluids. Rats were placed on an electric heating pad on medium heat and maintained under 1% isoflurane as urethane (1.7 g/kg iv; 0.7 g/mL in distilled water) was slowly infused (6 mL/h; Harvard Apparatus syringe pump) until a stable plane of anesthesia was achieved. Rats were then transferred to a heated surgical station where core body temperature was maintained at 37 ± 0.2°C (model 700 TC-1000; CWE). The trachea was cannulated, rats were pump-ventilated (rodent ventilator 683; Harvard Apparatus), and a bilateral vagotomy was performed. Inspired air comprised 65% O2, 1–3% CO2 to maintain end-tidal CO2 between ~45 and 50 mmHg, and nitrogen for the remainder (Capnogard; Respironics, Inc.). Tracheal pressure was continuously monitored, and lungs were periodically hyperinflated (~1/h, 2–3 breaths) via expiratory line occlusions. A femoral arterial catheter was placed to monitor blood pressure and to enable sampling of arterial blood for measurement of pH and partial pressure of oxygen () and carbon dioxide () using an ABL90 FLEX (Radiometer).
With a dorsal approach, the left and right phrenic nerves were isolated and cut distally. The epineurium was removed at the distal end of the nerve to facilitate suction electrode recordings. Animals received the neuromuscular paralytic pancuronium bromide (2.5 mg/kg iv; Hospira) to prevent respiratory muscle contraction. A continuous intravenous infusion (1 mL/h) of a 1:4 solution (8.4% sodium bicarbonate-lactated Ringer’s solution) was maintained during the experiment. Adequate depth of anesthesia was verified before the start of each experimental protocol by assessing arterial blood pressure response to toe pinch. Urethane supplements (0.2-mL bolus, 0.7 g/mL) were given in the event of toe-pinch response. Bilateral phrenic nerve output was recorded using custom-made bipolar suction electrodes filled with 0.9% saline. Compound action potentials were amplified (×10 kHz; Grass Instruments P511), analog bandpass filtered (3 Hz–3 kHz), digitized [16 bit, 25,000 samples/s per channel; Power1401, Cambridge Electronic Design (CED)], and integrated (time constant = 20 ms) with Spike2 software (CED).
Primary experiments.
After recording of a baseline period where phrenic nerve activity was stable for at least 20 min, rats received one of the following three treatments (n = 8 per group): 1) ampakine alone (CX717, 15 mg/kg iv), 2) ampakine followed by hypoxia (12.5% inspired O2), or 3) vehicle (10% HPCD, matched to CX717 volume, intravenous) followed by hypoxia. The CX717 was dissolved at 5 mg/mL in 10% HPCD solution.
In preliminary studies, we observed that the acute effects of CX717 on phrenic motor output are evident rapidly following the intravenous infusion, with a modest increase in burst amplitude observed after ~2 min. Our a priori goal was to provide the acute hypoxic exposure during the period associated with the peak effects of CX717. Accordingly, hypoxic exposures (5-min duration) were initiated 2 min after the ampakine or sham infusions. Recordings were maintained for 60 min following each treatment, after which each animal was exposed to another 5-min episode of hypoxia, followed by 5 min of recovery back to baseline. At that time, a “maximal” chemoreceptor challenge was achieved by briefly stopping the mechanical ventilation until apnea occurred. Arterial blood samples were obtained at baseline, during the first episode of hypoxia, and at the 20-, 40-, and 60-min time points.
Additional experiments.
In an additional cohort of CX717-treated animals (n = 4 per group; same 3 experimental groups as primary experiments), phrenic nerve recordings were extended to 90 min following the hypoxic episode, with an additional blood sample taken at the 90-min time point. These experiments were conducted separately and were analyzed separately from the larger cohort of CX717-treated rats. In an additional set of experiments, we assessed the effects of intravenous infusion of another low-impact ampakine, CX1739. In these experiments, we studied administration of CX1739 alone (n = 4) and CX1739 followed by a single episode of hypoxia (n = 4). In the CX1739 experiments we also recorded phrenic motor output for 90 min following the hypoxic exposure. Animals in the CX1739 supplemental experiments were slightly older (16.2 ± 4.2 wk) and weighed more (384 ± 30 g) compared with animals in the CX717 treatment groups.
Data analyses.
All data were collected using Spike2.v8 software (CED), and statistical analyses were performed using GraphPad Prism 7. Integrated phrenic nerve burst amplitude is reported as absolute amplitude (arbitrary units, a.u.) and normalized to baseline output. The phrenic inspiratory burst frequency is expressed as bursts per minute. Heart rate is expressed as beats per minute, and mean arterial pressure (MAP, mmHg) was calculated as [systolic blood pressure + (2 × diastolic blood pressure)]/3.
The acute response to ampakine or HPCD was averaged over the third minute after the start of infusion. Data from time points where blood draw occurred were averaged over the 1-min period before arterial blood draw. Data from the final hypoxia challenge were averaged over the third minute after the onset of hypoxia. The peak response to maximal chemoreceptor activation was measured from the largest single burst amplitude that occurred during the ventilator-off challenge (usually the last burst before apnea).
Age, weight, amplitude, frequency, heart rate and mean arterial pressure (MAP) at baseline, and response to hypoxia (end of protocol) and maximal chemoreceptor activation (brief cessation of ventilator support) were statistically compared between groups using one-way analysis of variance (ANOVA). The responses to hypoxia associated with treatments (CX717 + hypoxia and HPCD + hypoxia) were compared using a Student’s unpaired t test. At the 90-min time point, one-way ANOVA was used to compare groups with each other, and individual group means were also compared with zero (baseline) using a one-sample Student’s t test. The acute responses to CX717 or HPCD were compared with each other and with zero (baseline) using a one-sample Student’s t test. Two-way repeated measures ANOVA was used to compare amplitude, frequency, heart rate, MAP, and blood gasses between the three treatment groups over 60 min. When appropriate, individual multiple comparisons were made using Tukey’s post hoc test. Statistical significance was assumed if P ≤ 0.05. Initial evaluation of the data showed no statistical differences (or any identifiable trends) between responses recorded in the left and right phrenic nerves at any time during the experiment. Therefore, subsequent statistical analysis and presentation of phrenic burst amplitude focused on left phrenic nerve. In all graphs, individual data points are shown and horizontal lines indicate the mean ± SD
RESULTS
Baseline data and arterial blood gases.
There were no differences in phrenic nerve burst amplitude, frequency, heart rate, or MAP between the three experimental groups [i.e., CX717, CX717 + hypoxia, and vehicle (HPCD) +hypoxia] at baseline (Table 1). Additionally, baseline , , and pH were similar between groups (Table 2). Within each group, and were stable over the course of the experimental protocols (other than the expected reduction in during hypoxia). All rats were well oxygenated with values > 200 mmHg during the baseline and posthypoxia recording periods. Compared across groups, at 20 min posthypoxia was higher in the CX717 group (310 ± 21 mmHg) vs. the CX717 + hypoxia group (256 ± 41 mmHg). The arterial pH was stable over the course of the experimental protocol in all groups, although a slight reduction was noted in the CX717 + hypoxia group (7.315 ± 0.031) at 60 min compared with CX717 (7.32 ± 0.033) and HPCD (7.32 ± 0.057) (Table 2).
Table 1.
Average phrenic nerve inspiratory burst amplitude, inspiratory burst frequency, heart rate, and mean arterial pressure during the baseline recording period
| CX717 | CX717 + Hypoxia | HPCD + Hypoxia | |
|---|---|---|---|
| Right burst amplitude, a.u. | 0.009 ± 0.005 | 0.007 ± 0.005 | 0.006 ± 0.005 |
| Left burst amplitude, a.u. | 0.008 ± 0.005 | 0.004 ± 0.002 | 0.006 ± 0.004 |
| Burst frequency, bursts/min | 48.8 ± 5.8 | 49.1 ± 7.2 | 47.8 ± 5.1 |
| Heart rate, beats/min | 438.4 ± 19.7 | 433.7 ± 16.6 | 430.2 ± 22.9 |
| Mean arterial pressure, mmHg | 136.7 ± 18.3 | 132.9 ± 19.7 | 138.3 ± 24.1 |
Data are means ± SD. There were no statistically significant differences in these parameters between treatment groups (all P = 0.1774). a.u., Arbitrary units; HPCD, 2-hydroxypropyl-β-cyclodextrin.
Table 2.
Arterial blood gas values from primary experiments with CX717
| , mmHg | , mmHg | pH | |
|---|---|---|---|
| CX717 | |||
| Baseline | 46.3 ± 2.5 | 316.4 ± 24.5 | 7.34 ± 0.03 |
| 20 min | 46.8 ± 2.1 | †310.3 ± 20.6 | 7.32 ± 0.02 |
| 40 min | 46.2 ± 2.7 | 314.6 ± 20.9 | 7.32 ± 0.02 |
| 60 min | 46.3 ± 1.9 | 316.1 ± 23.8 | 7.32 ± 0.03 |
| 90 min | 47.1 ± 1.8 | 337.2 ± 18.5 | 4.34 ± 0.03 |
| CX717 + Hypoxia | |||
| Baseline | 47.2 ± 1.8 | 282.2 ± 29.5 | 7.34 ± 0.02 |
| Hypoxia 1 | 46 ± 2.1 | 45.5 ± 0.07 | 7.33 ± 0.03 |
| 20 min | 46.8 ± 1.9 | 273.1 ± 40.1 | 7.32 ± 0.03 |
| 40 min | 46.5 ± 1.9 | 304.3 ± 32.1 | 7.32 ± 0.02 |
| 60 min | 47.4 ± 2.1 | 327.1 ± 40.5 | *7.31 ± 0.03 |
| 90 min | 46.7 ± 1.8 | 345.5 ± 29.2 | 7.32 ± 0.01 |
| HPCD + Hypoxia | |||
| Baseline | 47.2 ± 2.5 | 310.4 ± 41.4 | 7.32 ± 0.05 |
| Hypoxia 1 | 48.1 ± 9.1 | 45.8 ± 1.4 | 7.31 ± 0.07 |
| 20 min | 46.8 ± 3.2 | 290.4 ± 67.5 | 7.31 ± 0.05 |
| 40 min | 47.1 ± 4.5 | 319.6 ± 56.1 | 7.31 ± 0.04 |
| 60 min | 46.7 ± 2.1 | 311.1 ± 35.4 | 7.32 ± 0.06 |
| 90 min | 47.1 ± 3.2 | 357.1 ± 47.2 | 7.35 ± 0.03 |
Values are means ± SD of arterial partial pressures of carbon dioxide () and oxygen () and pH. There were no statistically significant differences between the treatment groups during the baseline recording condition (all P = 0.3554). All groups have n = 8 rats except for the 90-min data, which are included as a separate assessment (e.g., Fig. 4C) and have n = 4 rats per group.
P < 0.05, significant difference compared with baseline within that particular treatment group.
P < 0.05, significant difference compared with CX717 + hypoxia treatment group.
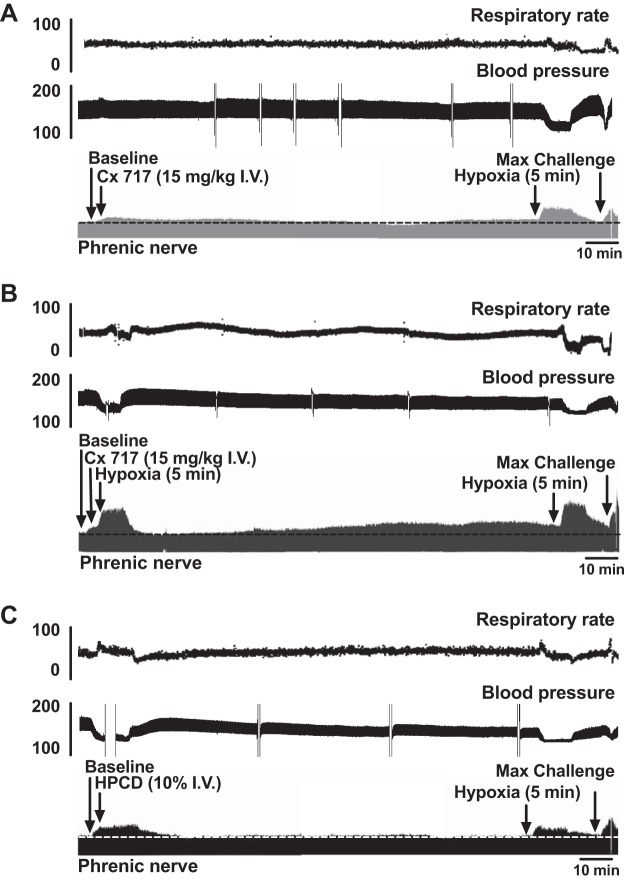
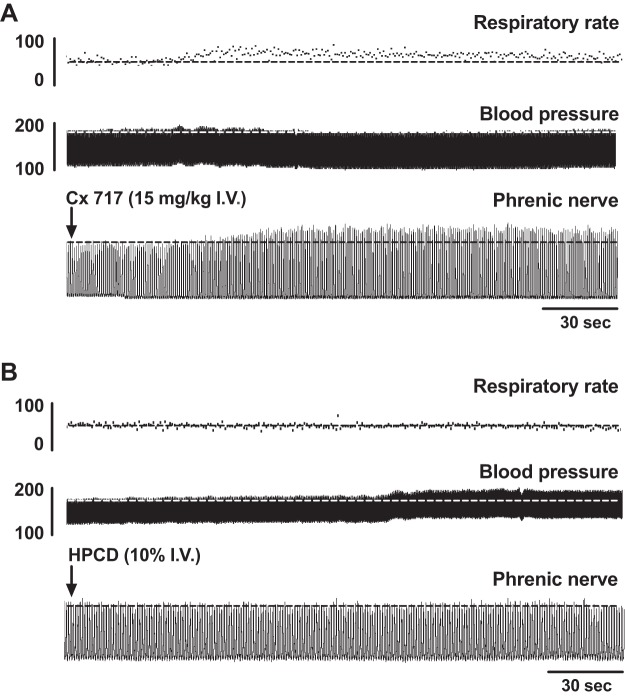
Acute impact of CX717.
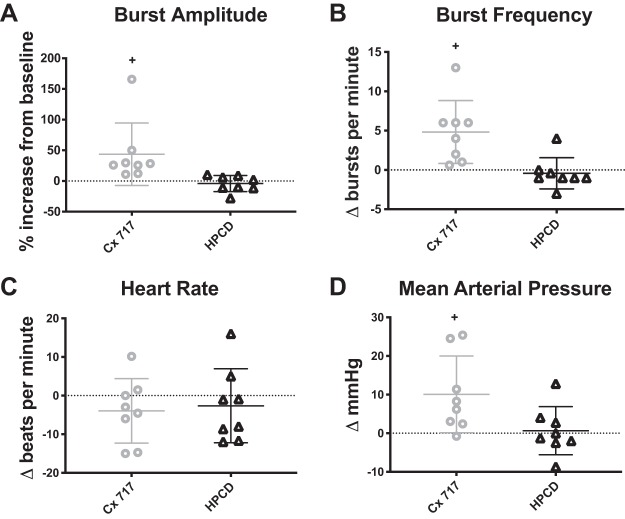
Figure 1 shows example recordings from each of the experimental protocols: CX717 application alone (A), CX717 + hypoxia (B), and HPCD + hypoxia (C). The compressed traces allow for visualization of changes in burst amplitude (bottom trace), burst frequency (top trace), and arterial blood pressure (middle trace) over an entire experimental protocol. As is evident in Fig. 1A, CX717 produced a rapid, transient increase in phrenic motor amplitude that peaked ~2 min after the start of intravenous infusion. An expanded view of this time point is shown in Fig. 2A for CX717 and Fig. 2B for HPCD. Figure 3 shows the group data, and it is evident that intravenous delivery of HPCD alone had no discernable acute impact on phrenic burst amplitude (−4 ± 13% baseline, P = 0.4029) or frequency (−2 ± 1 ∆bursts/min, P = 0.5648), heart rate (−2 ± 9 ∆beats/min, P = 0.4623), or MAP (0.6 ± 6 ∆mmHg, P = 0.7729). In contrast, intravenous CX717 caused an acute increase in phrenic nerve burst amplitude (43 ± 50% baseline, P = 0.0456), burst frequency (5 ± 4 ∆bursts/min, P = 0.0113), and MAP (10 ± 9 ∆mmHg, P = 0.0241) (Fig. 3). CX717 did not have a statistically significant acute impact on heart rate (−4 ± 8 ∆beats/min, P = 0.2214).
Fig. 1.
Representative data from each experimental group. A: CX717 alone. B: CX717 followed by a single 5-min episode of hypoxia. C: vehicle injection 2-hydroxypropyl-β-cyclodextrin (HPCD) followed by a single 5-min episode of hypoxia. For each group, compressed records of instantaneous phrenic inspiratory burst frequency (top), arterial blood pressure (middle), and the phrenic neurogram (bottom) are shown at baseline, for 90 min following respective treatments, and during a single 5-min episode of hypoxia and maximum chemoreceptor activation (max challenge) at the end of each experiment (indicated by arrows).
Fig. 2.
Representative data illustrating the acute impact of ampakine delivery on phrenic motor output. Traces show that intravenous (I.V.) CX717 (A) caused an acute increase in phrenic nerve inspiratory burst amplitude. In contrast, vehicle injection (2-hydroxypropyl-β-cyclodextrin, HPCD; B) shows no apparent impact on phrenic output. Also depicted are arterial blood pressure and inspiratory burst rate.
Fig. 3.
Acute respiratory and cardiovascular effects of CX717 vs. vehicle (2-hydroxypropyl-β-cyclodextrin, HPCD). Peak effects of intravenous CX717or HPCD were measured 2 min after the start of the intravenous infusion. CX717 alone resulted in a significant increase in phrenic burst amplitude (CX717, P = 0.0467; HPCD, P = 0.4092; A), inspiratory burst frequency (CX717, P = 0.0010; HPCD, P = 0.5648; B), and mean arterial pressure (CX717, P = 0.0397; HPCD, P = 0.7729; D) but had no discernable impact on heart rate (CX717, P = 0.2214; HPCD, P = 0.4635; C). There was no acute impact of HPCD on any of these parameters. Individual data points are shown, and horizontal lines indicate means ± SD. +P < 0.05, statistically significant change from baseline conditions.
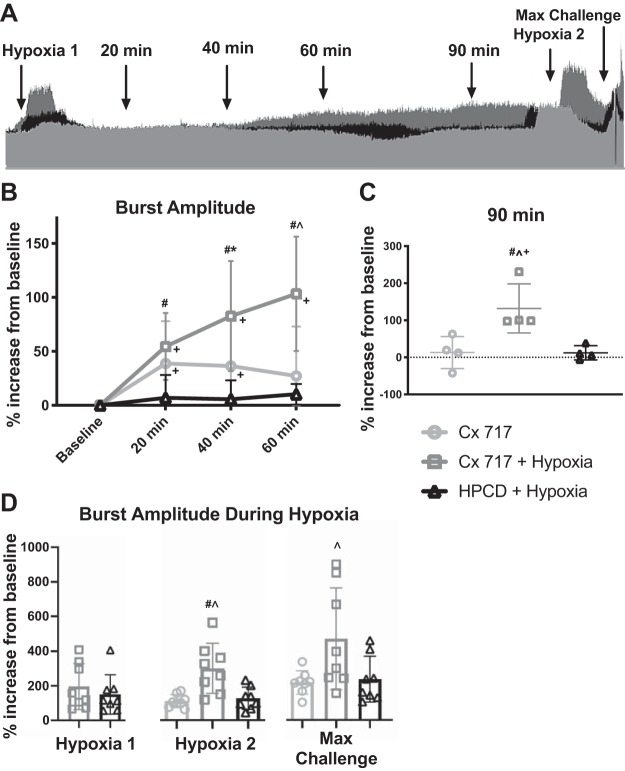
Facilitation of inspiratory phrenic burst amplitude.
When representative phrenic neurograms from each group are superimposed (Fig. 4A), it is clear that the combination of CX717 and a single hypoxic episode (dark gray trace) produced a sustained increase in phrenic inspiratory burst amplitude compared with CX717 alone (light gray trace) or vehicle (HPCD) coupled with hypoxia (black trace). Evaluation of the group data using two-way ANOVA revealed an interaction between time and phrenic burst amplitude [F(6,63) = 8.419, P < 0.0001]. Figure 4B shows that phrenic burst amplitudes were similar at 20, 40, and 60 min posthypoxia in the vehicle group, with no indication of facilitation. Delivery of CX717 alone resulted in a modest but sustained increase in phrenic burst amplitude that was higher than baseline values at both the 20-min (P = 0.0042) and 40-min time points (P = 0.0081). However, phrenic burst amplitude returned to baseline at 60 min post-CX717. The group data confirmed that the combination of CX717 + hypoxia resulted in phrenic motor facilitation. Thus phrenic inspiratory burst amplitude at 20 (P < 0.0001), 40 (P < 0.0001), and 60 min posthypoxia (P < 0.0001) were all increased relative to baseline. Furthermore, at both 40 (P = 0.0185) and 60 min posthypoxia (P < 0.0001), the CX717 + hypoxia group showed statistically increased phrenic burst amplitude compared with both of the other groups.
Fig. 4.
Pretreating with ampakine enables a single episode of hypoxia to trigger sustained facilitation of phrenic inspiratory burst amplitude. A: superimposed phrenic neurograms from each treatment group highlight the effect of CX717+ hypoxia (dark gray) on the amplitude of phrenic motor output compared with CX717alone (light gray) and 2-hydroxypropyl-β-cyclodextrin (HPCD) + hypoxia (black). Dotted line represents baseline amplitude. Arrows indicate points used for data analysis. B: average change in phrenic burst amplitude in the 3 experimental groups. Data from 90 min posttreatment, which were collected in a separate cohort, are shown in C. CX717 + hypoxia resulted in sustained increases in phrenic bursting that were 100% greater than baseline by 60 min. At the 90-min time point, average amplitude was significantly different in the CX717 + hypoxia group compared with the other groups and was significantly different from baseline. D: average change in burst amplitude from baseline during chemoreceptor challenge. Pretreatment with CX717 did not alter the amplitude of phrenic bursts during subsequent hypoxia (hypoxia 1) compared with the HPCD + hypoxia treatment group. After 60 min, the increase in burst amplitude during a second hypoxia challenge (hypoxia 2) as well as the maximal chemoreceptor challenge (max challenge) were elevated in the CX717+ hypoxia group. Individual data points are shown, and horizontal lines indicate means ± SD. #P < 0.05, significant difference between CX717 + hypoxia and HPCD + hypoxia. *P < 0.05, significant difference between CX717 and HPCD + hypoxia. ^P < 0.05, significant difference between CX717 and CX717 + hypoxia. +P < 0.05, significant difference compared with 0 (baseline).
Data from the additional n = 4 rats per group that were evaluated at 90 min posthypoxia are presented in Fig. 4C [1-way ANOVA: treatment effect, F(2,9) = 8.628, P = 0.0081]. These supplemental experiments demonstrated that phrenic motor facilitation in the CX717 + hypoxia group persisted at 90 min (P = 0.0281, 132 ± 66% baseline). However, burst amplitude had returned toward baseline after 90 min in the CX717-alone (P = 0.5898, 13 ± 43% baseline) and vehicle + hypoxia groups (P = 0.2893, 12 ± 19% baseline).
To determine if the combination of CX717 + hypoxia produced a sustained (e.g., >60 min) impact on the ability to increase phrenic bursting during chemoreceptor stimulation, we compared peak burst amplitudes during the two hypoxic challenges (i.e., immediately postampakine vs. 60 min later) and a maximal chemoreceptor challenge. Figure 4D shows that there was no difference in the phrenic burst amplitude response to a single hypoxic episode immediately following intravenous infusion of CX717 or HPCD (unpaired t test: P = 0.9046). However, when evaluated 60 min later, the response to hypoxia was increased in the CX717 + hypoxia group compared with the other two groups [1-way ANOVA: treatment, F(2,21) = 6.155, P = 0.0079]. A similar effect was seen in the response to the maximal chemoreceptor challenge, recorded a few minutes after the second hypoxic response [1-way ANOVA: treatment, F(2,21) = 4.368, P = 0.0259].
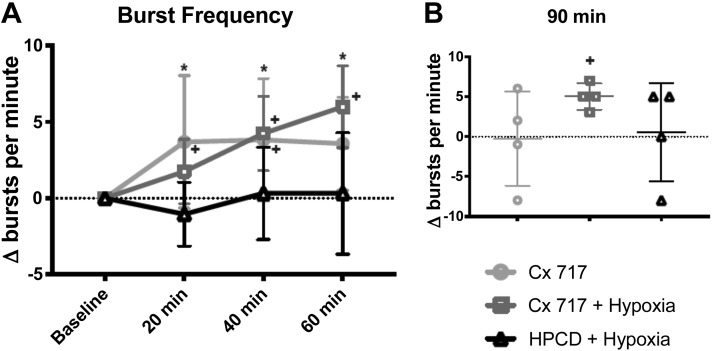
Phrenic burst frequency.
Figure 5A shows the effects of each treatment on phrenic burst frequency across 60 min. Frequency was similar between groups during the baseline recordings [Table 1; 1-way ANOVA: F(5,42) = 1.087, P = 0.3817]; therefore, the results are presented as change in burst frequency from the baseline value. Comparison of burst frequency between the three treatment groups revealed a statistical interaction between time and “Δbaseline” [bursts/min; 2-way ANOVA: interaction, F(6,63) = 3.568, P = 0.0042]. Figure 5 shows that HPCD injection followed by hypoxia did not impact phrenic burst frequency over the 60 min following hypoxia. In contrast, CX717 caused phrenic burst frequency to be elevated (vs. baseline) at the 20-min (P = 0.0073), and 40-min (P = 0.0052) time points. In the CX717 + hypoxia group, burst frequency was elevated compared with that at both baseline and in the HPCD + hypoxia group at the 40- and 60-min time points. In the CX717 group, the largest increase from baseline occurred at the 20-min time point (3.7 ± 4.3 ∆bursts/min), whereas in the CX717 + hypoxia group, the largest increase occurred at the 60-min time point (6 ± 2.6 ∆bursts/min).
Fig. 5.
Impact of ampakine CX717 on phrenic nerve burst frequency. A: average change in inspiratory phrenic burst frequency (Δbursts/min) from baseline after CX717 alone, CX717+ hypoxia, and 2-hydroxypropyl-β-cyclodextrin (HPCD + hypoxia). Data from 90 min posttreatment, which were collected in a separate cohort, are shown in B. Burst frequency was not significantly different at any time point in the HPCD + hypoxia group but was increased from baseline at 20 and 40 min after CX717 alone and at 40 and 60 min after CX717+ hypoxia. At 90 min, there was no significant difference in frequency between groups; however, in the CX717 + hypoxia group, frequency was significantly different from baseline. *P < 0.05, HPCD + hypoxia is significantly different from both other groups. +P < 0.05, significant difference compared with 0 (baseline).
Data for the additional experimental animals evaluated at 90 min posthypoxia are presented in Fig. 5B. At this time point, the burst frequency in the CX717 + hypoxia group remained significantly elevated compared with the baseline value (5.0 ± 1.6 ∆bursts/min, P = 0.0088). This value was not, however, statistically different from that for CX717 alone (−0.25 ± 5.9 ∆bursts/min) or HPCD + hypoxia (0.5 ± 6.1 ∆bursts/min) [1-way ANOVA: F(2,9) = 1.286, P = 0.3227].
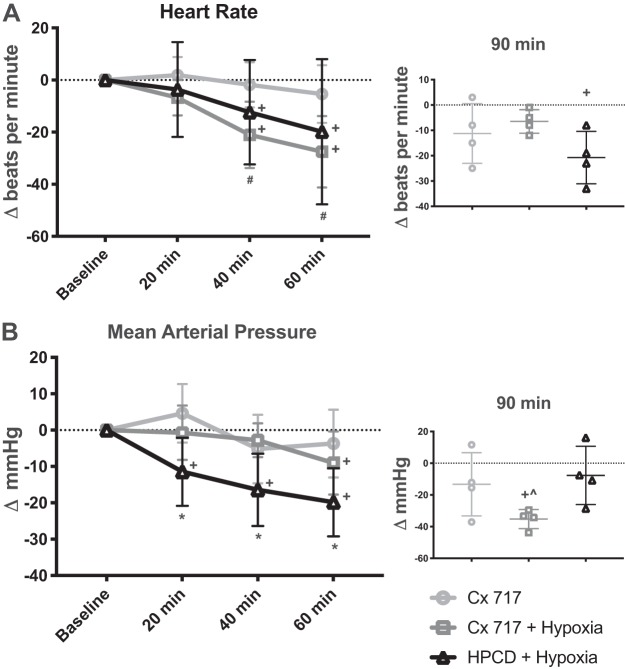
Heart rate and blood pressure.
Figure 6A shows heart rate over the course of the experimental protocol; two-way ANOVA indicated a significant interaction between time and treatment [F(6,63) = 2.423, P = 0.0359]. There was no change in heart rate from baseline at any time point following treatment with CX717 without hypoxia. In contrast, hypoxia exposure was associated with a progressive reduction in heart rate over time. Both the CX717 + hypoxia and HPCD + hypoxia groups showed reductions in heart rate during the 60 min that followed hypoxia exposure (Fig. 6A). At both 40 and 60 min posttreatment, the average heart rate of the CX717 group was significantly greater than that of both the CX717 + hypoxia group and the HPCD + hypoxia group. At 90 min (Fig. 6A, inset), there were no significant differences between treatment groups [1-way ANOVA: F(2,9) = 2.362, P = 0.1498]. The average heart rate in the HPCD + hypoxia group was significantly different from baseline values at the 90-min time point (−21 ± 10 ∆beats/min, P = 0.0278). The moderate declines noted in the other two groups did not reach statistical significance relative to the baseline values (CX717: −11 ± 12 ∆beats/min, P = 0.1523; CX717 + hypoxia: −6 ± 4 ∆beats/min, P = 0.0683).
Fig. 6.
Effects of ampakine CX717 on heart rate and mean arterial pressure. A: average change in heart rate (Δbeats/min) from baseline after CX717 alone, CX717+ hypoxia, and 2-hydroxypropyl-β-cyclodextrin (HPCD + hypoxia). Data from 90 min posttreatment, which were collected in a separate cohort, are shown in inset. There was no significant change in heart rate from baseline with CX717 alone; however, heart rate was lower than baseline at 40 and 60 min after CX717+ hypoxia and HPCD + hypoxia. At the 90-min time point, there were no differences between groups; however, the HPCD + hypoxia group was significantly different from baseline. B: average change in mean arterial pressure (ΔmmHg) from baseline. There was no significant decrease from baseline in mean arterial pressure in the CX717 group; however, average mean arterial pressure was decreased at 60 min after CX717 + hypoxia and at all time points after HPCD + hypoxia. At the 90-min time point, CX717 + hypoxia was significantly different from both other groups and significantly different from baseline. #P < 0.05, CX717 is significantly different from both other groups. *P < 0.05, HPCD + hypoxia is significantly different from both other groups. ^P < 0.05, CX717 + hypoxia is significantly different from both other groups. +P < 0.05, significant difference compared with 0 (baseline).
Figure 6B shows the mean arterial pressure (MAP) over the course of the experimental protocol in each of the three experimental treatment groups. Analyses of these data using two-way ANOVA revealed a significant interaction between time and treatment [F(6,63) = 3.86, P = 0.0024]. The MAP was stable after CX717 alone and did not statistically change over the course of the protocol. In contrast, both groups that were exposed to hypoxia showed eventual declines in MAP. This occurred at 20 min posthypoxia in the HPCD group and at 60 min posthypoxia in the CX717 group (Fig. 6B). In the subset of experiments in which recordings were extended to 90 min (Fig. 6B, inset), the average MAP had declined below baseline values in all groups. The CX717 + hypoxia rats showed the largest decline at 90 min (−35 ± 6 ∆mmHg, P = 0.0014 vs. baseline) compared with rats treated with CX717 alone (−13 ± 19 ∆mmHg, P = 0.2744) or HPCD + hypoxia (−7 ± 18 ∆mmHg, P = 0.4635).
Supplemental experiments using CX1739.
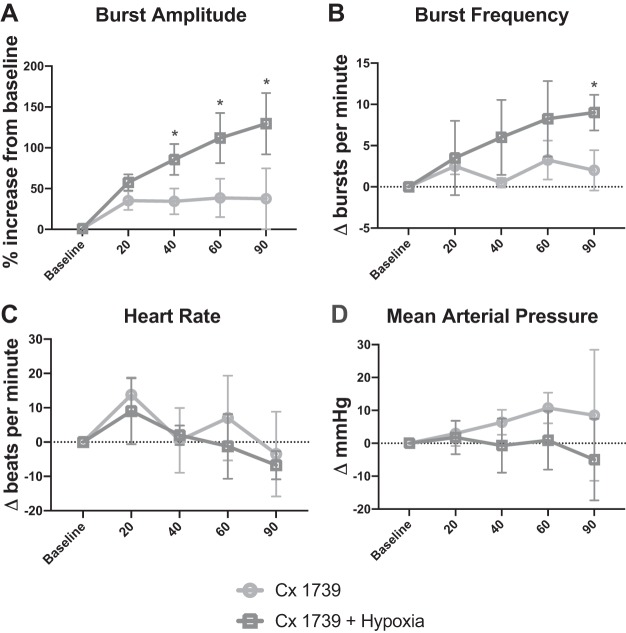
Another low-impact ampakine, CX1739, was studied in a supplemental set of experiments (n = 4 rats per group). The results of these additional experiments are shown in Fig. 7 and are qualitatively similar to those of the CX717 experiments. In regards to changes in phrenic inspiratory burst amplitude, there was a statistical interaction between treatment (i.e., CX1739 + hypoxia vs. CX1739 alone) and time posttreatment [F(4,39) = 11.464, P < 0.001; Fig. 7A]. The burst amplitude was increased in the CX1739 + hypoxia group at the 40-, 60-, and 90-min time points (P < 0.05 vs. CX1739 alone). Evaluation of inspiratory burst frequency also revealed a statistical interaction between time and treatment [F(4,39) = 4.049, P = 0.012; Fig. 7B]. In the CX1739 + hypoxia group, relative to CX1739 alone, the increase in burst frequency (∆baseline) tended to be greater at 40 (P = 0.081) and 60 min posthypoxia (P = 0.054) and was statistically greater at 90 min (P = 0.007). Changes in heart rate [F(1,31) = 0.528, P = 0.495; Fig. 7C] and mean arterial blood pressure [F(1,39) = 2.55, P = 0.161; Fig. 7D] were not different between the two treatment groups. Additionally, , , and pH were all stable over the course of the experimental protocols and did not differ between the CX1739 and CX1739 + hypoxia treatment groups (Table 3).
Fig. 7.
Results of supplemental experiments using ampakine CX1739. Phrenic nerve inspiratory burst amplitude was increased in rats receiving CX1739 + hypoxia vs. CX1739 alone (A). Inspiratory burst frequency also tended to be increased in the CX1739 + hypoxia group (B). Heart rate (C) and arterial blood pressure (D) were similar between groups. *P < 0.05, different from CX1739 alone.
Table 3.
Arterial blood gas values from supplemental experiments with ampakine CX1739
| , mmHg | , mmHg | pH | |
|---|---|---|---|
| CX1739 | |||
| Baseline | 46.4 ± 2.4 | 325.3 ± 23.7 | 7.35 ± 0.03 |
| 20 min | 46.4 ± 2.1 | †318.3 ± 13.7 | 7.35 ± 0.04 |
| 40 min | 45.3 ± 2.3 | 312.0 ± 21.8 | 7.35 ± 0.03 |
| 60 min | 45.9 ± 2.7 | 319.5 ± 14.6 | 7.35 ± 0.28 |
| 90 min | 46.1 ± 2.3 | 319.3 ± 12.1 | 7.35 ± 0.03 |
| CX1739 + Hypoxia | |||
| Baseline | 46.8 ± 2.2 | 298.3 ± 28.6 | 7.34 ± 0.01 |
| Hypoxia 1 | 48.6 ± 2.8 | *48.7 ± 7.6 | 7.33 ± 0.01 |
| 20 min | 47.3 ± 2.8 | *213.3 ± 31.9 | 7.33 ± 0.02 |
| 40 min | 46.9 ± 2.7 | 298.3 ± 23.1 | 7.33 ± 0.02 |
| 60 min | 48.5 ± 2.7 | 295.8 ± 27.3 | 7.33 ± 0.02 |
| 90 min | 48.7 ± 2.6 | 283.3 ± 42.6 | 7.32 ± 0.02 |
Values are means ± SD of arterial partial pressures of carbon dioxide () and oxygen () and pH. Statistical evaluation of these data showed that there were no statistically significant differences between the treatment groups during the baseline recording condition (all P = 0.7044). Both groups have n = 8 rats except for the 90-min data, which are included as a separate assessment and have n = 4 rats per group.
P < 0.05, significant difference compared with baseline within that particular treatment group.
P < 0.05, significant difference compared with CX1739 + hypoxia treatment group.
DISCUSSION
These experiments show that pretreatment with a low-impact ampakine can enable a single episode of hypoxia to produce a large and sustained facilitation of phrenic nerve inspiratory burst amplitude. The consensus from the respiratory neuroplasticity literature is that multiple episodes of hypoxia are required to evoke sustained phrenic motor facilitation (Gonzalez-Rothi et al. 2015; Mitchell and Johnson 2003; Satriotomo et al. 2016). This response typically manifests as a persistent (e.g., minutes to hours) increase in phrenic nerve inspiratory burst amplitude following the exposure to acute intermittent hypoxia. Our data suggest that ampakines can reduce the number of hypoxia episodes necessary to evoke mechanisms that can trigger sustained increases in phrenic motor output. This concept is consistent with studies indicating that ampakines can augment the capacity for synaptic plasticity in other experimental models (Baudry et al. 2012; Rex et al. 2006) and raises the possibility of coupling ampakines with moderate hypoxia for purposes of neurorehabilitation (Gonzalez-Rothi et al. 2015).
Selection of ampakines used in the current experiments.
Ampakines are a family of compounds that act as positive allosteric modulators of the AMPA-type glutamate receptors. While ampakines have no direct agonistic properties, when bound, glutamatergic transmission through the AMPA receptor is enhanced (Arai and Kessler 2007). Ampakines are divided into two general classes: type I, considered to be “high impact,” and type II, considered to be “low impact” (Arai and Kessler 2007). While both types enhance the amplitude of the AMPA-mediated current, high-impact ampakines additionally prolong the current by delaying channel closing. Because of this, high-impact ampakines are considered to have a poor safety profile due to the potential to cause seizure-like activity. Low-impact ampakines retain the ability to act as positive allosteric AMPA receptor modulators, but the receptor kinetics differ by a shorter decay time constant (Arai and Kessler 2007). Low-impact ampakines have not been observed to produce seizures and are well tolerated in humans. Both high- and low-impact ampakines can facilitate long-term potentiation, but the high-impact form may also promote long-term depression (Arai and Kessler 2007).
For the current study, we used two low-impact ampakines, CX717 and CX1739. The primary hypothesis that ampakine pretreatment followed by a single brief hypoxic exposure could produce phrenic motor facilitation was tested using CX717. We chose CX717 because it has passed through initial clinical studies in humans (Boyle et al. 2012; Oertel et al. 2010; Wesensten et al. 2007) and prior rodent studies have shown that CX717 can enhance the hypoglossal motor response to three episodes of hypoxia (Turner et al. 2016). A supplemental set of experiments were conducted using another low-impact ampakine, CX1739, and were undertaken after the initial CX717 trials were finished. These experiments were conducted to determine if the phrenic motor facilitation was uniquely triggered by CX717 or if another low-impact ampakine could produce a similar response.
Acute impact of ampakines on respiratory motor output.
Prior reports indicate that low doses of low-impact ampakines have very modest or no impact on breathing in animals (ElMallah et al. 2015; Ren et al. 2012, 2013, 2015) or humans (Oertel et al. 2010) under conditions in which breathing is not impaired in any way (i.e., no hypoventilation is present). For example, a study of 16 healthy human men (age 27 yr, SD = 5) showed that breathing was indistinguishable following administration of ampakine CX717 (1,500 mg) or a placebo (Oertel et al. 2010), suggesting no impact of the CX717 on respiratory output. In studies of healthy adult 129SVE mice, CX717 (15 mg/kg) produced no significant changes in phrenic or XII nerve output, or ventilation (ElMallah et al. 2015). Turner et al. (2016) also reported little impact of CX717 on hypoglossal nerve inspiratory bursting in adult 129SVE mice. In the present study, we observed that CX717 alone (i.e., without hypoxia) had a small and transient impact on respiratory motor output. The 15 mg/kg dose in the current studies evoked an ~20% increase in phrenic nerve burst amplitude that returned to baseline (preampakine) values within 20 min. In the supplemental experiments, we found that 15 mg/kg of another low-impact ampakine, CX1739, also produced a small increase in phrenic nerve inspiratory burst amplitude. Collectively, these results are consistent with the prior reports indicating that low doses of low-impact ampakines have relatively little impact on respiratory motor output in healthy adult mammals (ElMallah et al. 2015; Oertel et al. 2010; Ren et al. 2012, 2013, 2015).
In contrast, if respiratory insufficiency is present, ampakines can have a much stronger impact on breathing. One prominent example is the impact of ampakines following opioid overdose (Greer and Ren 2009). Opioids have direct actions on brain stem respiratory rhythm, generating neurons as well as chemosensory neurons that influence the respiratory rhythm. Thus opioid overdose can produce irregular breathing and fatal apneas (Dahan et al. 2018). Following opioid overdose in animal models, administration of low-impact ampakines including CX717 can restore inspiratory frequency with little to no effect on inspiratory burst amplitudes or tidal volume (Ren et al. 2012, 2013). In a murine model of Rett syndrome with a phenotype that includes reduced inspiratory rate (Mecp2 null mice), ampakine CX5456 treatment can restore inspiratory burst frequency to values indistinguishable from those in wild-type mice (Ogier et al. 2007). Thus multiple published studies have shown that ampakines can increase the rate of breathing during conditions associated with hypoventilation. Conversely, in an animal model with hypoventilation and considerably impaired phrenic-diaphragm neuromuscular function, the Pompe disease mouse (Gaa−/−; DeRuisseau et al. 2009), ampakine CX717 had very little impact on inspiratory rate (ElMallah et al. 2015). However, CX717 was able to evoke considerable increases in phrenic and hypoglossal nerve inspiratory burst amplitudes as well as inspiratory tidal volume.
When breathing is impaired, the relative ability of ampakines to impact the rate of breathing as compared with the amplitude of efferent respiratory bursts (or tidal volume) may relate to the mechanisms that are responsible for hypoventilation. For example, the aforementioned Pompe mouse model shows extensive pathology in motoneurons with evidence for phrenic motoneuron loss (DeRuisseau et al. 2009), and ampakines robustly increased inspiratory burst amplitude and tidal volume. Conversely, hypoventilation following opioid overdose (Ren et al. 2012, 2013) is almost certainly occurring due to suppression of premotor inputs to respiratory motoneurons, and under these conditions ampakines robustly stimulate respiratory rate. In the current study, at 60 min posttreatment, we observed an ~4–5 burst/min increase in respiratory rate in both the CX717 and CX717 + hypoxia treatment groups. In contrast, only the CX717 + hypoxia treatment resulted in a sustained increase in inspiratory burst amplitude recorded in the phrenic nerve, reaching approximately twofold baseline values at 60 min (e.g., Fig. 4). Thus the ampakine treatment did impact respiratory rate, but coupling of ampakines with hypoxia was required to produce sustained facilitation of inspiratory phrenic burst amplitude.
Ampakines and respiratory neuroplasticity.
Respiratory neuroplasticity has been defined as a persistent change in the respiratory neural control system based on prior experience. Thus the acute respiratory stimulation induced by ampakines (e.g., Fig. 2) is not plasticity, but rather shows the immediate impact of the drug on respiratory motor control. In contrast, the apparent interaction between low-dose ampakine treatment and acute hypoxia exposure, manifest as a sustained increase in phrenic motor output, is an example of respiratory neuroplasticity. The current proof-of-concept study was not designed to address the mechanisms by which ampakines interact with hypoxia-induced signaling. At this time, our working hypothesis is that the ability of ampakines to enhance hypoxia-induced phrenic motor plasticity derives primarily (although not necessarily exclusively) from actions on phrenic motoneurons in the mid-cervical spinal cord. In this regard, lessons learned from prior studies of hypoxia-induced respiratory plasticity may shed light on this question and provide direction for future experiments. Work from Gordon Mitchell and others has established that acute intermittent hypoxia elicits long-term facilitation of phrenic burst amplitude via mechanisms that require episodic spinal serotonin (5-HT) release (Kinkead et al. 2001), serotonin receptor (type 2) activation (Baker-Herman and Mitchell 2002; Fuller et al. 2001; Kinkead and Mitchell 1999), ERK MAP kinase activity (Hoffman et al. 2012; Wilkerson and Mitchell 2009), and new synthesis of brain-derived neurotrophic factor (BDNF) (Baker-Herman et al. 2004) and activation of its high-affinity receptor, tyrosine kinase B (TrkB) (Baker-Herman et al. 2004). This cellular cascade is referred to as the Q pathway to phrenic motor facilitation since Gq proteins are associated with the initiating (5-HT2) metabotropic receptor (Dale-Nagle et al. 2010). The Q pathway can also be induced with intrathecal serotonin receptor agonist injections (MacFarlane et al. 2011). A potential point of convergence between the Q pathway and the impact of ampakines may be upregulation of BDNF. Independent laboratories confirm that ampakines can increase BDNF expression in neurons (Kramár et al. 2012; Lauterborn et al. 2000, 2003; Ogier et al. 2007; Simmons et al. 2009, 2011). Since BDNF/TrkB signaling is an essential component of the Q pathway to phrenic motor facilitation, a logical hypothesis is that ampakines enhance phrenic motor plasticity by increasing the potential for BDNF/TrkB signaling within phrenic motoneurons. In turn, BDNF/TrkB signaling regulates NMDA receptor subunit phosphorylation, trafficking, and currents (Caldeira et al. 2007; Carvalho et al. 2008).
Significance.
Recent studies have shown that repeated brief exposures to hypoxia can have therapeutic benefits after neurologic injury (Gonzalez-Rothi et al. 2015; Trumbower et al. 2012). For example, in humans with complete spinal cord injury (SCI), acute intermittent hypoxia can elicit sustained increases in volitional muscle strength and electromyogram activity (Trumbower et al. 2012). Additionally, intermittent hypoxia paradigms can evoke sustained increases in minute ventilation in people with chronic SCI (Sankari et al. 2015; Tester et al. 2014). To date, the neurorehabilitative work done with acute intermittent hypoxia after SCI has all utilized repeated hypoxic episodes (Gonzalez-Rothi et al. 2015). The current data show that pretreatment with ampakine can enable a single episode of hypoxia to trigger sustained facilitation of phrenic motor output, at least in spinally intact rats. Accordingly, pairing low-dose and low-impact ampakines with brief hypoxia exposure may have value in the context of neurorehabilitation paradigms. Specifically, ampakines may provide a pharmacological tool to boost the impact of intermittent hypoxia paradigms and/or reduce the number of hypoxic exposures needed to trigger beneficial neuroplasticity. Current protocols are designed to avoid the well-documented negative effects of chronic intermittent hypoxia (Navarrete-Opazo and Mitchell 2014), and adjunctive ampakine therapy could provide a further “safety margin” by reducing the total number of hypoxic exposures. We recommend that future studies evaluate the impact of low-impact ampakines on respiratory motor function after acute and chronic SCI, with and without hypoxia exposure.
Is it possible to translate use of low-dose ampakines to human use, particularly in the context of augmenting respiratory neuroplasticity? As demonstrated in initial early stage clinical trials related to cognitive and affective disorders, ampakines are metabolically stable, readily cross the blood-brain barrier, and produce minimal side effects at therapeutic doses (Doraiswamy and Xiong 2006; Oertel et al. 2010; Porrino et al. 2005; Wesensten et al. 2007). Ampakine CX717 has been given to humans without any reported adverse events (Boyle et al. 2012; Oertel et al. 2010; Wesensten et al. 2007). In clinical studies focusing on cognitive function (Boyle et al. 2012; Wesensten et al. 2007) and opiate-induced respiratory depression (Oertel et al. 2010), CX717 doses ranged from 100 to 1,500 mg, with efficacy found at 1,000–1,500 mg. The effective dose in these prior studies was ~13–20 mg/kg for a 75-kg human. Thus considerable evidence shows that it is feasible and safe to give low-dose, low-impact ampakines to humans, and the dose used in the current studies (15 mg/kg) is within the range previously used in human trials.
GRANTS
This work was supported by National Institutes of Health Grants 1T32HL134621-01A1 (to L. B. Wollman), K99HL143207-01 (to K. A. Streeter), F32NS095620-01 (to K. A. Streeter), and 1R01HL139708-01A1 (to D. D. Fuller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.B.W., K.A.S., and D.D.F. conceived and designed research; L.B.W. performed experiments; L.B.W. and D.D.F. analyzed data; L.B.W., K.A.S., and D.D.F. interpreted results of experiments; L.B.W. prepared figures; L.B.W. drafted manuscript; L.B.W., K.A.S., and D.D.F. edited and revised manuscript; L.B.W., K.A.S., and D.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Arnold Lippa and Richard Purcell of RespireRx for providing the ampakines that were used in these studies. We are grateful to Dr. John Greer for initial discussions regarding ampakines and control of breathing.
REFERENCES
- Arai A, Kessler M, Rogers G, Lynch G. Effects of a memory-enhancing drug on dl-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther 278: 627–638, 1996. [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets 8: 583–602, 2007. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Rogers G, Lynch G, Kessler M. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther 303: 1075–1085, 2002. doi: 10.1124/jpet.102.040360. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience 123: 1011–1024, 2004. doi: 10.1016/j.neuroscience.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis 47: 210–215, 2012. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk DJ. Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep. J Psychopharmacol 26: 1047–1057, 2012. doi: 10.1177/0269881111405353. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci 35: 208–219, 2007. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 153, Suppl 1: S310–S324, 2008. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M. Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology 128: 1027–1037, 2018. doi: 10.1097/ALN.0000000000002184. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH Jr, Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA 106: 9419–9424, 2009. doi: 10.1073/pnas.0902534106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Xiong GL. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert Opin Pharmacother 7: 1–10, 2006. doi: 10.1517/14656566.7.1.1. [DOI] [PubMed] [Google Scholar]
- ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, Greer JJ, Fuller DD. Stimulation of respiratory motor output and ventilation in a murine model of pompe disease by ampakines. Am J Respir Cell Mol Biol 53: 326–335, 2015. doi: 10.1165/rcmb.2014-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS. Respiratory neuroplasticity—Overview, significance and future directions. Exp Neurol 287: 144–152, 2017. doi: 10.1016/j.expneurol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006, 2001. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119: 1455–1465, 2015. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol 168: 153–157, 2009. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 130: 207–218, 2001. doi: 10.1016/S1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol Regul Integr Comp Physiol 277: R658–R666, 1999. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging 33: 708–719, 2012. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci 20: 8–21, 2000. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther 307: 297–305, 2003. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Funk GD, Greer JJ. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS One 5: e8766, 2010. doi: 10.1371/journal.pone.0008766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol 6: 82–88, 2006. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307: R1181–R1197, 2014. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lötsch J. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther 87: 204–211, 2010. doi: 10.1038/clpt.2009.194. [DOI] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci 27: 10912–10917, 2007. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol 3: e299, 2005. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin DP, Johnson S, Purcell R, Lippa AS. Effects of chronic systemic low-impact ampakine treatment on neurotrophin expression in rat brain. Biomed Pharmacother 105: 540–544, 2018. doi: 10.1016/j.biopha.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Radin DP, Zhong S, Purcell R, Lippa A. Acute ampakine treatment ameliorates age-related deficits in long-term potentiation. Biomed Pharmacother 84: 806–809, 2016. doi: 10.1016/j.biopha.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110: 1364–1370, 2009. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J Appl Physiol (1985) 113: 1004–1011, 2012. doi: 10.1152/japplphysiol.00752.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ. Ampakines enhance weak endogenous respiratory drive and alleviate apnea in perinatal rats. Am J Respir Crit Care Med 191: 704–710, 2015. doi: 10.1164/rccm.201410-1898OC. [DOI] [PubMed] [Google Scholar]
- Ren J, Lenal F, Yang M, Ding X, Greer JJ. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 118: 1437–1445, 2013. doi: 10.1097/ALN.0b013e318291079c. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol 96: 677–685, 2006. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Bascom AT, Riehani A, Badr MS. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. J Appl Physiol (1985) 119: 1183–1193, 2015. doi: 10.1152/japplphysiol.00088.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Nichols NL, Dale EA, Emery AT, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience 322: 479–488, 2016. doi: 10.1016/j.neuroscience.2016.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Mehta RA, Lauterborn JC, Gall CM, Lynch G. Brief ampakine treatments slow the progression of Huntington’s disease phenotypes in R6/2 mice. Neurobiol Dis 41: 436–444, 2011. doi: 10.1016/j.nbd.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc Natl Acad Sci USA 106: 4906–4911, 2009. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel S, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD. Intermittent hypoxia enhances functional connectivity of midcervical spinal interneurons. J Neurosci 37: 8349–8362, 2017. doi: 10.1523/JNEUROSCI.0992-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189: 57–65, 2014. doi: 10.1164/rccm.201401-0089LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Turner S, Streeter KA, Greer J, Mitchell GS, Fuller DD. Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respir Physiol Neurobiol 256: 4–14, 2018. doi: 10.1016/j.resp.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, ElMallah MK, Hoyt AK, Greer JJ, Fuller DD. Ampakine CX717 potentiates intermittent hypoxia-induced hypoglossal long-term facilitation. J Neurophysiol 116: 1232–1238, 2016. doi: 10.1152/jn.00210.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesensten NJ, Reichardt RM, Balkin TJ. Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med 78: 937–943, 2007. doi: 10.3357/ASEM.2055.2007. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JER, Devinney M, Mitchell GS. Intermittent but not sustained moderate hypoxia elicits long-term facilitation of hypoglossal motor output. Respir Physiol Neurobiol 256: 15–20, 2018. doi: 10.1016/j.resp.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]