Abstract
During auditory perception, neural oscillations are known to entrain to acoustic dynamics but their role in the processing of auditory information remains unclear. As a complex temporal structure that can be parameterized acoustically, music is particularly suited to address this issue. In a combined behavioral and EEG experiment in human participants, we investigated the relative contribution of temporal (acoustic dynamics) and nontemporal (melodic spectral complexity) dimensions of stimulation on neural entrainment, a stimulus-brain coupling phenomenon operationally defined here as the temporal coherence between acoustical and neural dynamics. We first highlight that low-frequency neural oscillations robustly entrain to complex acoustic temporal modulations, which underscores the fine-grained nature of this coupling mechanism. We also reveal that enhancing melodic spectral complexity, in terms of pitch, harmony, and pitch variation, increases neural entrainment. Importantly, this manipulation enhances activity in the theta (5 Hz) range, a frequency-selective effect independent of the note rate of the melodies, which may reflect internal temporal constraints of the neural processes involved. Moreover, while both emotional arousal ratings and neural entrainment were positively modulated by spectral complexity, no direct relationship between arousal and neural entrainment was observed. Overall, these results indicate that neural entrainment to music is sensitive to the spectral content of auditory information and indexes an auditory level of processing that should be distinguished from higher-order emotional processing stages.
NEW & NOTEWORTHY Low-frequency (<10 Hz) cortical neural oscillations are known to entrain to acoustic dynamics, the so-called neural entrainment phenomenon, but their functional implication in the processing of auditory information remains unclear. In a behavioral and EEG experiment capitalizing on parameterized musical textures, we disentangle the contribution of stimulus dynamics, melodic spectral complexity, and emotional judgments on neural entrainment and highlight their respective spatial and spectral neural signature.
Keywords: arousal, auditory perception, EEG, emotion, neural oscillations, temporal envelope
INTRODUCTION
Cortical neural oscillations are assumed to play a functional role in perception and cognition. Low-frequency oscillations in the delta-theta range (<10 Hz) in particular are known to play a mechanistic role in sensory selection, by sampling input signals and structuring the temporal activity of sensory cortices (Ghitza 2012; Giraud and Poeppel 2012; Luo and Poeppel 2007; Riecke et al. 2018; Schroeder and Lakatos 2009; VanRullen 2016). When rhythmic inputs, such as speech or music, are perceived, these cortical oscillations tend to become coupled with the slow modulations present in the stimulus’ temporal envelope (which mirrors how sound intensity fluctuates over time). This coupling phenomenon is broadly termed “neural entrainment” (Ding and Simon 2014; Haegens and Zion-Golumbic 2018; Rimmele et al. 2018). Neural entrainment has been primarily studied in the context of speech perception, during which it occurs principally at the syllabic and prosodic rates (Doelling et al. 2014; Vander Ghinst et al. 2016; Zion Golumbic et al. 2013). It was shown to be modulated by the spectro-temporal fine structure of the acoustic input (Ding et al. 2014) as well as by intelligibility (Gross et al. 2013; Peelle et al. 2013). However, the relative contribution of low-level auditory and high-level cognitive processes to neural entrainment, and the functional role of low-frequency oscillations in the processing of sensory information, are current matters of debate (Ding and Simon 2014; Kösem and van Wassenhove 2017; Steinmetzger and Rosen 2017).
Like speech, music has a rhythmic temporal structure (Ding et al. 2017), with systematic patterns of note onsets, accents, and grouping (Patel 2008). A recent study pointed to similar entrainment properties during music perception (Doelling and Poeppel 2015). Yet, contrary to speech, perceptual analysis of musical pitch sequences occurs preferentially in the right auditory cortex (Zatorre et al. 1994, 2002), which is known to operate at a slower oscillatory regime (Giraud et al. 2007; Morillon et al. 2012) and to have a better sensitivity to slow acoustic temporal modulations than its left counterpart (Abrams et al. 2008; Gross et al. 2013). Importantly, music has the capacity to carry strong expressive content, inducing feelings and thoughts (Juslin and Västfjäll 2008), and music-induced emotions arise from the functional interaction between subcortical areas, such as the striatum and amygdala and auditory cortical areas (Liégeois-Chauvel et al. 2014; Salimpoor et al. 2013, 2015). Besides, previous research revealed that musical expressions can be accurately predicted using a limited set of low-level acoustic cues, including average and variability values of temporal features (e.g., sound level, tempo, articulation, attack velocity), as well as spectral content (Juslin and Laukka 2004). Based on these findings, we hypothesized that neural entrainment to musical stimuli is, like for speech, sensitive to acoustic dynamics (temporal factor), but that it is also modulated by a nontemporal dimension of stimulation: music spectral complexity.
To investigate these issues, we conducted a combined behavioral and EEG experiment in which participants were asked to listen to short musical excerpts and to assess their emotional reaction through self-reported valence and arousal ratings. We used a generative music algorithm designed to synthesize original musical textures with defined expressive content, such as happiness or sadness or a mixture of the two (Beller 2018). To control for the spectral complexity of musical excerpts and to disentangle the impact of music spectral complexity on neural entrainment from the one of temporal modulations, we generated control versions of the original melodies (hereafter called “neutral stimuli”), with preserved temporal envelopes but reduced complexity in terms of pitch, harmony, and pitch variation. For the sake of brevity, in the following, we simply use the term “complexity” to refer to these spectral aspects of melodic complexity. Of note, in the present research, neural entrainment was operationally defined as the temporal coherence between the temporal envelope of the musical excerpt and the concomitantly recorded EEG activity. Ultimately, this design allows us to investigate whether and how music complexity (original versus neutral stimuli) and neural entrainment impact behavior (participants’ emotional ratings).
MATERIALS AND METHODS
This study is composed of a preliminary behavioral experiment, whose aim was to validate our stimuli and design, and a subsequent EEG experiment, in which the exact same material and behavioral task were used.
Participants
Eight healthy volunteers participated in the preliminary behavioral experiment (6 men; mean age 20 ± 4 yr; age range 18–30 yr) and twenty other healthy volunteers in the combined behavioral and EEG experiment (11 men; mean age 23 ± 3.8 yr; age range 18–32 yr). All participants were right-handed and had normal audition (<20 dB loss per ear and frequency band between 125 and 8,000 Hz), verified with an audiogram test before the experiment. They provided written, informed consent before the experiment, which was approved by the local ethic committee.
Emotion-Based Musical Synthesizer
Stimuli were generated using the Emotion-Based Musical Synthesizer (EBMS; Beller 2018), a computer music tool written in the Max programming language (Cycling ‘74, San Francisco, CA). EBMS algorithmically generates original musical textures of arbitrary duration, which are meant to convey either a pure emotion (happy/sad/fearful/angry) or a mixture thereof (i.e., a linear combination of the above). In more details, the algorithm generates simple pseudorandom sequences of notes, in the MIDI format (Musical Instrument Digital Interface; a technical standard for musical notation and communication), whose musical parameters can be controlled along parameters known to correspond to stereotypical musical features of emotional expressions (Juslin and Laukka 2004): speed (happy: high, sad: low), intensity (happy: high, sad: low), pitch (happy: high, sad: low), mode (happy: major, sad: minor), pitch, and intensity variation (happy: large, sad: small). Since its creation, EBMS was used in several contemporary music pieces and art installations (Dorsen 2015), but, to the best of our knowledge, it is the first time it is used for experimental research.
Original Stimuli
Twenty-one 8-s expressive piano stimuli were generated using EBMS (see examples on Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.11568999). All excerpts were composed of two monophonic lines: one melodic line played on the right-hand/high register of the piano, and one individual-note accompaniment line played on the left-hand/low register of the piano. Excerpts were generated in the major/minor keys of B, C, and F in equal proportions and were selected from the algorithm’s random output so that they sounded as different as possible from one another. We restricted the set space of emotions to two dimensions (over the four offered by EBMS) to maximize the recognition of emotions. Melodies were thus generated using parameters meant to express a mixture of happiness and sadness. The relative percentages of these two “emotions” in the mixtures were set based on informal testing.
Neutral Stimuli
In addition to the 21 expressive excerpts generated with EBMS, we manually generated 21 matched (in terms of acoustic envelope) “neutral” versions of the same stimuli, using the following procedure. We considered that the neutral version of an originally expressive melody composed of various pitches is simply the repetition of a unique pitch of the same instrument timbre, with all other acoustical features (intensity and rhythm) being matched. Taking advantage of the MIDI format, for each excerpt, we extracted the first note played in the melodic line and replaced all subsequent pitches in the line with this first pitch (for original stimuli meant to express either happiness or sadness only, all pitches in the melodic line were replaced with the pitch of the key in which the original excerpt was generated). Similarly, we replaced the accompaniment/left-hand line of each excerpt by the repetition of a single note two octaves lower than the one selected at the right hand. The neutral version thus contains one single pitch class, doubled two octaves apart. Importantly, while this procedure drastically reduced the spectral complexity of the excerpts linked to pitch, harmony, and pitch variation, it preserved the original excerpt’s temporal envelope (Fig. 1). Our design thus separates temporal and nontemporal dimensions of musical excerpts.
Fig. 1.
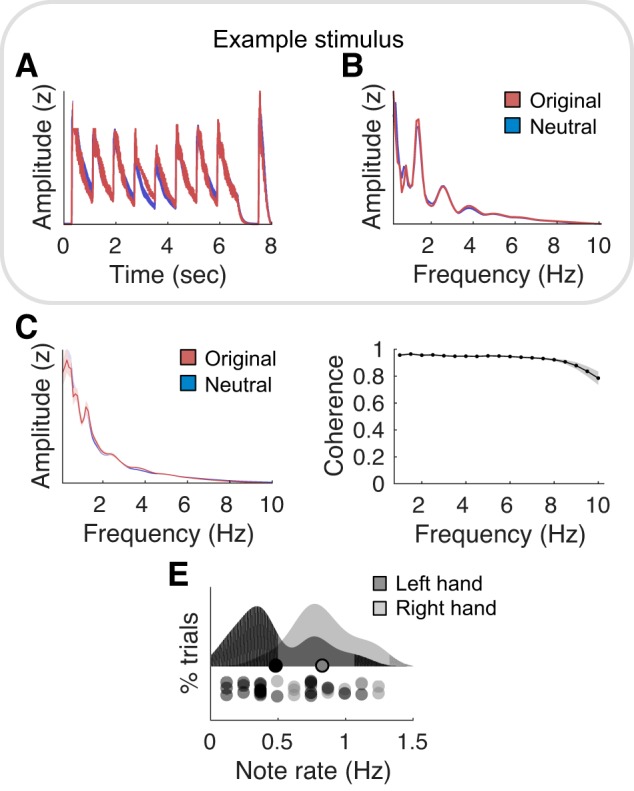
Melodic stimuli. A: amplitude envelope of a representative original expressive melody, together with its neutral melody counterpart. Neutral versions of original melodies (composed of various pitches) are simply the repetition of a unique pitch of the same instrument timbre, with all other acoustical features (intensity and rhythm) being matched. B: normalized modulation spectrum of the acoustic temporal envelope of the melodies depicted in A. C: normalized averaged modulation spectrum of the 21 original and neutral melodies. D: coherence computed between the acoustic temporal envelopes of the original and their matched neutral melodies, estimated across all melodies between (1–10) Hz. Shaded error bars indicate SE. E: distribution of the note rate, i.e., the number of acoustic events per second (with chords counting for one event), across melodies for each of the two monophonic lines (left and right hands). Circles on the distributions depict means for each monophonic line. Circles under the distributions depict individual pieces.
Experimental Setup
During both the behavioral and EEG experiments, participants were seated in front of a computer interface coded in Python using the PsychoPy module (Peirce 2007). Acoustical stimuli were presented binaurally through Sennheiser HD650 headphones during the preliminary behavioral experiment and through Sennheiser CX 300-II earphones during the EEG experiment, at comfortable level (around 60-dB sound pressure level). EEG was recorded using a BrainProduct actiCHamp system (Brain Products GmbH, Germany) with 64 electrodes (international 10–20 localization system), a sampling rate of 500 Hz, and high-pass (cutoff frequency: 0.06 Hz) and low-pass (cutoff frequency: 140 Hz) online filters. One additional electrode was placed on the left side of the nose and set as reference. Impedances were kept under 10 kΩ. To reduce the amount of eye movements during the EEG recording, participants were instructed to look at an on-screen fixation cross during stimulus presentation. Eye movements were monitored with two sets of electromyography bipolar electrodes (BIP2AUX adapter) placed above and below the left eye and at the outer canthi of both eyes, which were bipolarized off-line to yield vertical and horizontal electro-ocular activity (electrooculography), respectively. EEG recordings and stimulus presentation were synchronized using Cedrus StimTracker.
Experimental Design
The goal of the EEG experiment was to investigate whether and how neural entrainment is modulated by the complexity of the musical material used, in binaural diotic listening condition. Each of the 21 original and 21 neutral melodies was presented once, in random order. Overall, these manipulations resulted in two experimental conditions (“original” and “neutral”). The experiment lasted ~30 min.
Behavioral Task
In each trial, participants first listened to the excerpt. After the offset of the sound, participants were asked to appraise the emotional content of the excerpt by first rating its perceived arousal and then its perceived valence. Ratings were acquired on two separate visual analogic scales (with 20 steps) going from “not emotional” to “highly emotional” for arousal, and from “negative” to “positive” for valence. Participants were specifically instructed to use the entire scales, i.e., that they should rate the recognized emotional content of the excerpt relative to the set of melodies presented during the experiment. Once the answers were submitted, the next trial started.
Data Analysis
Acoustic preprocessing.
To estimate the temporal envelope of each melody, the sound signal (synthesized with a piano sound using the MIDI output of EBMS) was decomposed into 32 narrow frequency bands using a cochlear model, and the absolute value of the Hilbert transform was computed for each of these narrowband signals. The broadband temporal envelope resulted from the summation of these absolute values and was used as the acoustic signal for all subsequent stimulus-brain coherence analyses (Chandrasekaran et al. 2009).
EEG preprocessing.
Preprocessing of EEG data was performed following the standard procedure of Brainstorm (Tadel et al. 2011). Briefly, electrical artifacts were removed using low-pass filtering (at 40 Hz), slow drifts using high-pass filtering (at 0.3 Hz), and eye blink artifacts using source signal projections through principal component analysis.
Stimulus-brain coherence.
To quantify the neural entrainment induced by the acoustic signal, we computed the stimulus-brain coherence, a measure that quantifies in the spectral domain the synchronization between the acoustic envelope and the EEG neural signal. Importantly, coherence (in contrast to phase-locking value) considers both phase and amplitude estimates in the measurement of the relationship between the two signals (Lepage and Vijayan 2017). Importantly, this metric captures the similarity in the dynamics between two signals and is blind to their actual overall amplitude. Coherence was computed for each participant and experimental condition (“original” and “neutral”) according to the following steps. The acoustic and EEG signals were resampled at 128 Hz and segmented into 8-s epochs. In accordance with prior studies on neural entrainment, we restricted our analyses to the low-frequency range [(1–10) Hz; e.g., Ding and Simon 2014]. A time-frequency decomposition was performed by estimating the complex-valued wavelet transform of both acoustic and EEG signals in this frequency range (0.5-Hz resolution) using a family of Morlet wavelets [central frequency of 1 Hz, time resolution (full width at half maximum) of 3 s, i.e., three cycles], with a zero-padding approach to minimize boundary effects. These complex-valued signals were then separated into phase angle and amplitude information, and the epochs belonging to the same experimental conditions were concatenated. Finally, for each participant and condition, stimulus-brain coherence was computed over time, which here corresponds to the time duration of all concatenated trials (t ranging from 1 to n), as follows (Lepage and Vijayan 2017):
| (1) |
where Cc,f corresponds to the coherence estimated at EEG channel c and frequency f (from 1 to 10 Hz), and , respectively, are the amplitude and phase angle of the acoustic signal, and and , respectively, are the amplitude and phase angle of the EEG signal.
The EEG power response spectrum was additionally computed. The modulus of the complex-valued EEG signals obtained as described above was squared to obtain EEG power estimates, transformed in decimal logarithmic units (so that they follow a Gaussian distribution), baselined with the 2-s period preceding each trial, and finally averaged over time to obtain the spectrum per channel and frequency [between (1–10) Hz].
We also computed the acoustic temporal modulation spectrum (i.e., the spectrum of the temporal envelope) by averaging over time the modulus of the complex-valued signals obtained as described above.
Finally, we computed the coherence between the acoustic envelopes of the original and their matched neutral melodies, to estimate the similarity (both in terms of phase and amplitude) between the two categories of melodies, by applying the same routine as described above.
For the specific generalized linear mixed model (GLMM) regression analyses (see below), stimulus-brain coherence was reestimated per stimulus, i.e., per trial, using a leave-one-trial-out (LOTO) approach (Gluth and Meiran 2019). With this method, the stimulus-brain coherence value of one particular trial is estimated by taking the difference between the parameter estimate for the complete data set and for the data set with one omitted trial (corresponding to the trial of interest). The parameter estimate for the complete data set was then added to each stimulus’ estimate to center them around the mean. This method is particularly suited for data sets with low signal-to-noise ratios, such as EEG data.
Statistical Procedures
All analyses were performed at the single-subject level before applying standard nonparametric two-sided paired statistical tests at the group level. Behavioral data were submitted to Wilcoxon and Friedman tests. Where applicable, categorization of stimuli by emotional arousal into high- and low-arousing stimuli was based on a median split (on the 42 stimuli). Stimulus-brain coherence and EEG power measures were submitted to cluster-based permutation statistics (1,000 permutations; α-cluster level = 0.005), computed across electrodes and frequencies (Maris and Oostenveld 2007). Finally, to estimate the robustness of our results, we present behavioral results from two independent data sets (preliminary and main experiments) and present neural results at both the group and single-subject levels (Smith and Little 2018).
Generalized linear mixed model.
A GLMM regression analysis was performed to characterize the extent to which neural entrainment was impacted by stimulus spectral complexity (i.e., original versus neutral condition) and emotional arousal ratings. To do so, stimulus-brain coherence (as estimated with the LOTO procedure) was analyzed using GLMMs, with condition (original/neutral) and arousal ratings used as predictors, and participant number and file tag (one value per original/neutral pair) used as random factors. We report P values estimated from hierarchical model comparisons using likelihood ratio tests (Gelman and Hill 2007), and only present models that satisfy the assumption of normality (validated by visually inspecting the plots of residuals against fitted values) and statistical validation (significant difference with the nested null model). To test for main effects, we compared models with and without the fixed effect of interest. To test for interactions, we compared models, including fixed effects versus models including fixed effects, and their interaction.
Causal mediation analyses.
A model-based causal mediation analysis (CMA) was performed to determine whether spectral complexity manipulation (i.e., original versus neutral condition) had a direct effect on neural entrainment or whether neural entrainment was mediated by participants’ arousal rating. Analysis was performed using the Mediation package for CMA analysis of the R software (Tingley et al. 2014).
RESULTS
In this study, we investigated cortical neural entrainment to music. We focused on whether neural entrainment is modulated by the spectral complexity of the musical excerpt (original versus neutral condition) and investigated whether and how complexity and neural entrainment impacted behavior (participants’ emotional ratings). To this end, EEG was recorded while participants listened to and rated the emotional content of original melodies specially designed to express a mixture of happiness and sadness, as well as to their neutral versions, having preserved temporal envelopes but reduced complexity in terms of pitch, harmony, and pitch variation (see materials and methods). The behavioral effects of our manipulation were first investigated in a preliminary perceptual experiment.
Acoustic and Behavioral Validation
Concerning the acoustic stimuli, we first confirmed that original and neutral melodies were well matched in terms of loudness and rhythm. To this end, we compared the temporal envelope of each original melody to the one of its neutral version through a threefold approach. First, we verified that the envelopes of both types of melodies nearly perfectly correlated over time (mean Pearson r2 = 0.85; see Fig. 1A for a representative example). Second, we computed the spectrum of the temporal envelope (hereafter called “modulation spectrum”) of original and neutral melodies, which reflect how fast sound intensity fluctuates over time. We confirmed that they had the exact same modulation spectrum profile (see Fig. 1B for a representative example and Fig. 1C for the average over the 21 original and neutral melodies; mean Pearson r2 = 0.96; paired t tests over frequencies: all P values > 0.4, after false discovery rate correction for multiple comparison). Third, we computed the coherence between the acoustical envelopes of the original and their matched neutral melodies, to estimate the similarity (both in terms of phase and amplitude) of their respective envelopes and confirmed that they were highly similar in the (1–10) Hz range (mean coherence C = 0.93; Fig. 1D). Finally, for each stimulus and each of its two monophonic lines (melodic line played on the right-hand/high register of the piano, or left-hand/low register of the piano, see materials and methods), we calculated the note rates, i.e., the number of acoustic events per second, with chords counting for one event (Fig. 1E). This analysis shows that the acoustic events occur at a maximum rate of 1.3 Hz.
We then validated that original and neutral melodies elicited different emotional reactions in the intended direction, in both the preliminary and main experiments. Arousal was significantly reduced for neutral, compared with original melodies (Wilcoxon signed-rank test: preliminary experiment: Z = 2.38, P = 0.017; EEG experiment: Z = 3.88, P < 0.001; Fig. 2, top). There was also a trend for neutral melodies to induce more negative emotions than original melodies (preliminary experiment: Z = 1.68, P = 0.09; EEG experiment: Z = 3.32, P < 0.001; Fig. 2, bottom). Incidentally, we also controlled that the original melodies with a higher percentage of happiness (see materials and methods) were rated more positively in terms of valence than original melodies with a higher percentage of sadness (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.11568906).
Fig. 2.
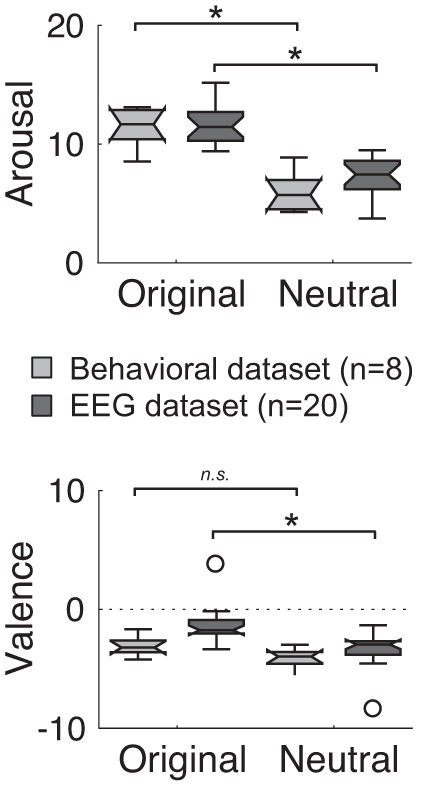
Behavioral results. Box plots of arousal and valence behavioral judgments during the preliminary behavioral (n = 8; light gray box plots) and the EEG (n = 20; dark gray box plots) experiments. Circles depict outliers. *Significant differences (P < 0.05). n.s., Nonsignificant differences.
Stimulus-Brain Coherence
To capture the general spectral profile of neural entrainment induced by our set of melodies, we averaged stimulus-brain coherence across all trials, including original and neutral melodies (Fig. 3A). Overall, coherence peaked at four frequencies (~1, 2.5, ~3.5, and 5 Hz), and this pattern was not simply due to an increase of EEG power at these frequencies (Supplemental Fig. S3A; see https://doi.org/10.6084/m9.figshare.11569008). The topographical maps estimated at those four frequencies showed that neural entrainment was maximal in parietal and temporal electrodes at 1 Hz, whereas it was maximal in fronto-central electrodes for the other peak frequencies (Fig. 3B). Moreover, our data revealed that the stimulus-brain coherence spectrum pattern (quantifying the synchronization of the acoustic envelope and neural oscillations) globally matched the acoustic modulation spectrum pattern, over the frequency range under study [(1–10) Hz], with peaks at ~1, 2.5, ~3.5, and 5 Hz (Fig. 3C). However, the 1- and 5-Hz peaks were considerably magnified in the coherence compared with the acoustic modulation spectrum.
Fig. 3.
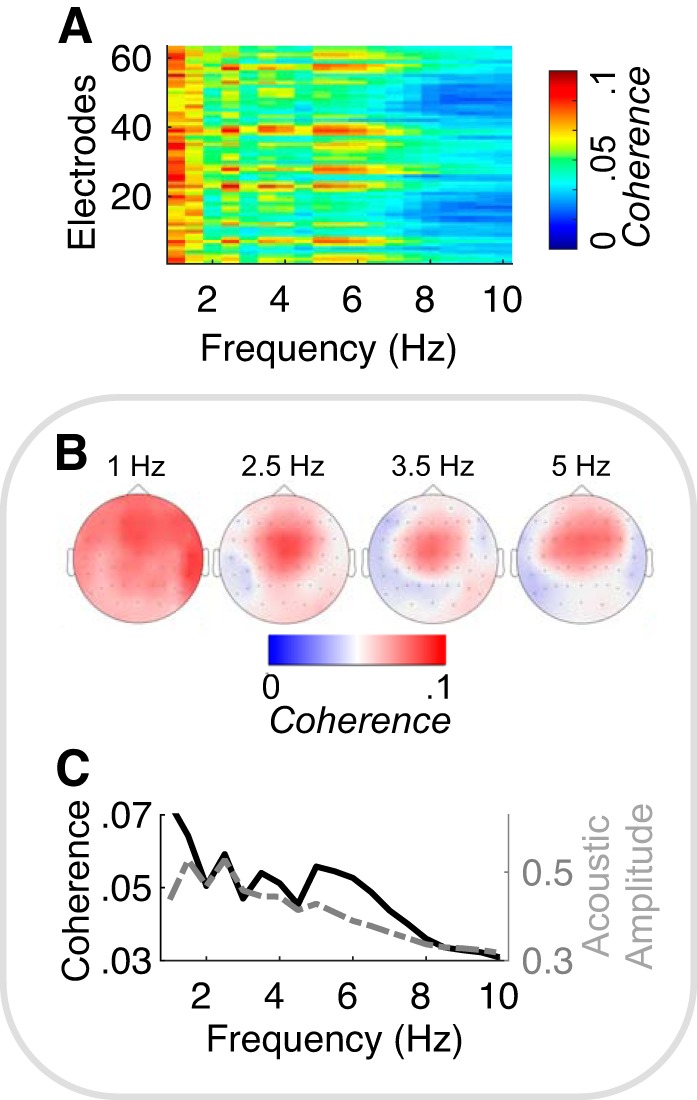
Neural entrainment to melodic stimuli. A: stimulus-brain coherence, computed between the acoustic temporal envelope and the 64 EEG neural signals, estimated between (1–10) Hz across all (original and neutral) melodies. B: topographical map of coherence estimated at 1, 2.5, 3.5, and 5 Hz. C: comparison of the acoustic modulation spectrum and the stimulus-brain coherence spectrum averaged across EEG channels. Please notice that, for visualization purposes, the exponents of the power-law-like distribution (1/f decay) of the two spectra were normalized.
Modulation of Neural Entrainment by Music Spectral Complexity
To assess the impact of complexity of the musical material on neural entrainment, we contrasted stimulus-brain coherence measures computed for original versus neutral melodies (Fig. 4A). A selective effect of spectral complexity on neural entrainment was observed on a single spectro-spatial cluster, at (5–6) Hz on fronto-central electrodes (Fig. 4, B and C): stimulus-brain coherence was significantly stronger for original melodies than for neutral melodies at 5 Hz only in this cluster of electrodes. Incidentally, EEG power was not significantly different between original and neutral melodies (Supplemental Fig. S3B; see https://doi.org/10.6084/m9.figshare.11569008). It is particularly striking that this effect of enhanced coherence for expressive melodies was not only present at the group level, but at the individual level for 16 out of 20 participants (Fig. 4D). Because original and neutral melodies were constructed to have the same modulation spectrum, and thus the same rhythmic properties (see Fig. 1D), the stronger stimulus-brain coherence at 5 Hz for expressive melodies cannot be attributed to rhythmic differences between the two categories of stimuli. Nor can it be attributed directly to pitch variations over time in the expressive excerpts, since none of the stimuli had a note rate of 5 Hz (see Fig. 1E). Incidentally, in a control analysis, we estimated a subdimension of spectral complexity: the variance of pitches (in cents) over the 8-s excerpts, defined as the mean of the deviation of each pitch from the average. We analyzed this contribution to the entrainment effect using a GLMM analysis and found no significant impact of pitch variance on neural entrainment (P = 0.21). Therefore, spectral complexity could not be reduced to pitch variance in our paradigm.
Fig. 4.
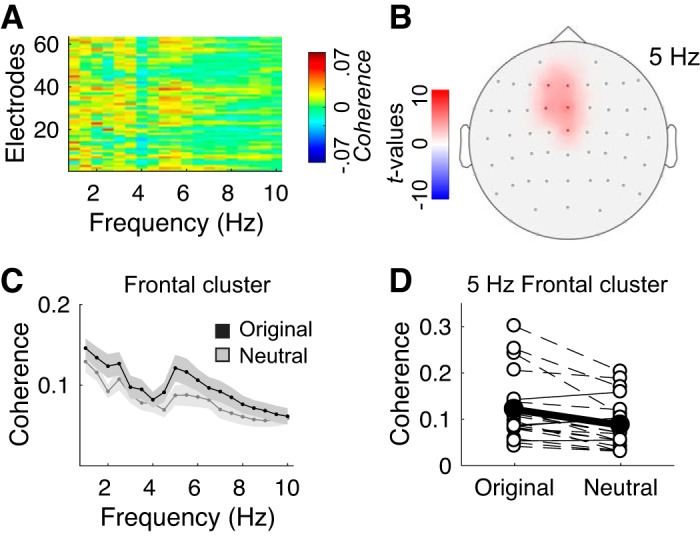
Effect of melodic spectral complexity on neural entrainment. A: contrast between stimulus-brain coherence estimates in original versus neutral conditions (original > neutral), computed across melodies for each EEG channel between (1–10) Hz. B: topographical map of the nonparametric cluster permutation statistical analysis of the coherence contrast, computed across channels and frequencies (α-cluster level = 0.005). Significant differences are observed at (5–6) Hz in a frontal cluster composed of channels F1|Fz|FC1|FCz|Cz. C: detail of the coherence spectrum of the original and neutral conditions, estimated in the frontal cluster. Shaded error bars indicate SE. D: individual coherence estimates at 5 Hz in the frontal cluster. Open circles indicate individual estimates. Dashed lines indicate participants showing increased coherence in the original vs. neutral condition. Solid circles and line indicate group average.
Modulation of Neural Entrainment with Arousal Ratings
A median split of arousal ratings was performed for each participant [(0–20) rating scale: high 14 ± 2.2 versus low 4.8 ± 2.6 arousal ratings; Wilcoxon signed-rank test: Z = 3.92, P < 0.001]. Stimulus-brain coherence at 5 Hz, on the frontal-central cluster (see Fig. 4B), was significantly stronger for high-arousing stimuli than for low-arousing stimuli (Z = 2.46, P = 0.014). This result reveals that high- and low-arousing stimuli are associated with different coherence values, with stronger neural entrainment for high-arousing stimuli.
Relationship Between Melodic Complexity, Arousal, and Neural Entrainment
Because all of the above analyses are only correlational, they do not allow us to conclude on the causal links between melodic complexity, arousal, and neural entrainment. In particular, it remains to be investigated whether the modulation of neural entrainment by music complexity reflects an acoustic or a higher-order emotional/cognitive level of processing. In other words, at this stage, we cannot conclude on whether the difference of coherence at (5–6) Hz on fronto-central electrodes is due to the intrinsic acoustic characteristics of original stimuli (compared with neutral) or to the recognized emotional load (reflected in the arousal ratings).
To study the relative effect of spectral complexity (i.e., original versus neutral condition) and emotional arousal ratings on neural entrainment (extracted from the spectro-spatial cluster, see Fig. 4B), we conducted GLMM analyses. First, we compared models containing either “condition” or “arousal” with their corresponding nested null model (i.e., first layer in hierarchical GLMM analysis). We found that both condition [Χ2(9) = 7.0, P = 0.008] and arousal [Χ2(9) = 4.2, P = 0.04] significantly improved the model’s ability to predict the variance of the coherence measure. Next, we compared the model, including both condition and arousal to models containing only one of these two factors (i.e., second hierarchical layer). We observed that condition significantly improved the model’s performance [Χ2(10) = 4.0, P = 0.04], whereas arousal did not [Χ2(10) = 1.2, P = 0.27]. Overall, this suggests that the variance of coherence explained by arousal at the first level of the GLMM is a subset of the one explained by condition. This finding supports the link between spectral complexity and arousal previously reported, showing that arousal ratings were significantly different for neutral compared with original melodies (Fig. 2).
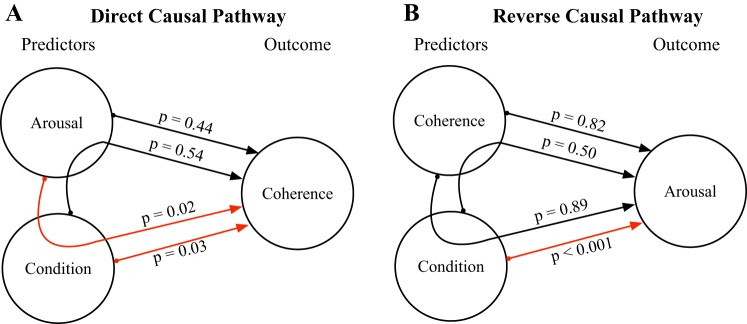
To further investigate the link between spectral complexity (i.e., original versus neutral condition), emotional arousal rating, and neural entrainment, we conducted model-based causal mediation analyses. First, we tested whether the previously evidenced link between arousal and coherence was mediated by condition (Fig. 5A). Although no significant average direct effect (ADE) from arousal to coherence was found (ADE = 2.51e-05, P = 0.44), there was a significant average causal mediation effect (ACME) mediated by condition (ACME = 2.52e-05, P = 0.02). Similarly, we tested whether the effect of condition on coherence was mediated by arousal. A significant ADE from condition to coherence (ADE = 3.76e-04, P = 0.03) was present, but no significant mediation by arousal was found (ACME = 5.06e-05, P = 0.54). These analyses suggest that the effect of arousal ratings on neural entrainment is indirect, that is to say that it is significantly mediated by the spectral complexity of stimuli (original versus neutral). Finally, we tested the reverse causal pathway by considering arousal as the outcome of the model (Fig. 5B). A significant ADE from condition to arousal was present (ADE = 2.17, P < 0.001), but no significant mediation by coherence was found (ACME = −0.0024, P = 0.50). Moreover, the effect of coherence on arousal was not significant (ADE = −1.4847, P = 0.82; ACME = −0.0928, P = 0.89).
Fig. 5.
Results of the causal mediation analysis. A: direct causal pathway: results from the causal mediation analysis when considering stimulus-brain coherence as an outcome and arousal/condition as either the treatment or the mediator. B: reverse causal pathway: results from the causal mediation analysis when considering arousal as an outcome and condition/stimulus-brain coherence as either the treatment or the mediator. Red arrows represent significant pathways.
In short, these analyses further demonstrate that the complexity of the musical material has a direct impact on neural entrainment and reveal that spectral complexity mediates the observed relationship between emotional arousal ratings and neural entrainment.
DISCUSSION
In this study, we pushed forward the idea that cortical neural oscillations play a functional role in the processing of auditory information, not only in speech but also in the less studied context of music perception. In particular, we hypothesized that neural entrainment to musical stimuli is sensitive to acoustic dynamics (temporal factor), but that it is also modulated by a nontemporal dimension of stimulation: music spectral complexity.
Slow oscillations are known to entrain to the dominant rhythm (tempo) of pure tone sequences (Morillon and Baillet 2017), speech streams (Peelle et al. 2013), or musical stimuli (Doelling and Poeppel 2015; Doelling et al. 2019). The present study significantly extends this account by showing that neural oscillations also entrain to more variable acoustic fluctuations, since the pattern of neural entrainment (i.e., stimulus-brain coherence spectrum) mirrors the complex pattern of the averaged acoustic modulation spectrum of the stimuli, with peaks at ~1, 2.5, ~3.5, and 5 Hz (Fig. 3). This highlights the fine-grained nature of this coupling mechanism, which robustly captures the temporal dynamics of acoustic stimuli.
Critically, our study also demonstrates that neural entrainment to music is also impacted by nontemporal manipulations, which is in line with previous studies in the speech domain or focusing on pure tone stimulation (reviewed in Ding and Simon 2014; Haegens and Zion-Golumbic 2018; Rimmele et al. 2018). Here, we investigated the contribution of spectral complexity of a musical excerpt to neural entrainment and show that cortical oscillations become more closely coupled with slow fluctuations in the music signal when the melody is expressive (in the sense of more harmonically complex), compared with its envelope-matched neutral version, confirming our hypothesis. In particular, we observed a selective effect of melodic spectral complexity on neural entrainment in the theta range (5 Hz). This result cannot be explained by differences in the temporal structure of our stimuli, since both the comparison of the modulation spectra of original and their matched neutral melodies (reflecting how fast sound intensity fluctuates over time) and the estimation of the coherence between their respective temporal envelopes ruled out the possibility of a selective difference of amplitude at 5 Hz across stimuli. The sole difference between original and neutral stimuli is in their spectral content: the acoustical manipulation was accomplished by creating monotonic versions of the original stimuli without changing the overall temporal envelope, in such a way that complexity in terms of pitch, harmony, and pitch variance was reduced to its minimum. In other words, this manipulation was more complex than mere changes in pitch over time (note rate), or pitch variance over the excerpt. Hence, this result provides new evidence that not only the temporal information (acoustic dynamics), but also the spectral information of acoustic stimuli critically contributes to neural entrainment (Ding et al. 2014; Henry and Obleser 2012; Lakatos et al. 2013; Zoefel and VanRullen 2015). Importantly, although our neutral stimuli consisting of one pitch only are a drastic control, the observed frequency-specific effect of spectral complexity on neural entrainment is hardly compatible with habituation/adaptation responses relative to repetitive/predictable stimuli (Pérez-González and Malmierca 2014), since the coherence measure reflects only the similarity in the dynamics between two signals (and not their actual overall amplitude). Furthermore, the present data suggest that a peak in the acoustic modulation spectrum of the melodies is not a sufficient condition to observe an effect of spectral complexity on neural entrainment. Indeed, coherence peaked at frequencies that were also present in the stimuli themselves, but the effect of spectral complexity on neural entrainment was found only at 5 Hz. That the effect is selective to the theta range could then be due to specific constraints of the neural (perceptual or attentional) structures involved in the processing of acoustic properties more complex than the note rate or pitch variance, but this remains to be further investigated. In particular, recent work highlighted a temporal coding preference of the auditory system for two distinct sampling regimes: theta (4–7 Hz) and gamma (30–45 Hz) ranges. This means that perceptual information tends to be sampled in parallel at these specific timescales, which is reflected in a preferential neural entrainment at theta and gamma rates during perception of auditory streams with a wide range of acoustic dynamics (Giroud et al. 2019; Teng et al. 2017). The present result speaks in favor of more engagement of the slower (theta) timescale during perception of original than during perception of neutral excerpt. This timescale has been shown to be implicated in the processing of abstract global (versus local) acoustic patterns (Teng et al. 2016) and could be mobilized here to deal with the higher perceptual complexity of original stimuli.
With the present setup, we could also investigate the relationships between music complexity, neural entrainment, and behavior (through participants’ emotional ratings) to disentangle whether the entrainment effect observed in the theta band reflects a difference of processing at the auditory level (low-level acoustic processing) and/or at a higher emotional level. As mentioned, the strength of this neural entrainment could scale with nontemporal perceptual complexity (see e.g., Folta-Schoofs et al. 2014), which would, in turn, impact auditory or attentional processes (e.g., Lakatos et al. 2013). Or it could scale with the strength of emotional engagement, reflecting the functional interaction between subcortical (striatum, amygdala) and auditory cortical areas (Liégeois-Chauvel et al. 2014; Salimpoor et al. 2013, 2015). Yet these two factors of perceptual complexity and emotional appraisal could also be functionally related, as shown in one recent study of musical emotional processing, in which judgments of negative valence were found to be related to the perceptual ambiguity/complexity in the auditory scene (Bonin et al. 2016). Our data show that, although high- and low-arousing stimuli are associated with different levels of neural entrainment, spectral complexity of the musical material mediates this observed relationship. Therefore, if there are some acoustical attributes of the stimuli that increase the stimulus-brain coupling phenomenon and some attributes that make them more arousing, our study demonstrates that these attributes only partly overlap. In summary, this study suggests that the selective effect of complexity on neural entrainment mostly reflects an auditory processing that should be distinguished from higher-order emotional levels of processing.
On the whole, this study provides fundamental insights into mechanisms underlying auditory information processing in the context of music perception. The present results also suggest that neural entrainment does not directly underpin emotional judgment.
Conclusion
Overall, this study highlights that cortical neural activity closely entrains to complex acoustic temporal modulations but is also sensitive to nontemporal manipulations. In particular, increasing music spectral complexity, in terms of pitch, harmony, and pitch variation, enhances neural entrainment. This effect is frequency specific (5 Hz) and could reflect specific constraints of the neural processes involved. Importantly, while spectral complexity modulates both neural entrainment and emotional arousal ratings, neural entrainment reflects an auditory level of processing independent from higher-order emotional processes. In the end, these results speak against the idea that neural entrainment only reflects acoustic temporal modulations (see also Breska and Deouell 2017; Rimmele et al. 2018) and reveal its intricate nature in a nonspeech context. If neural entrainment reflects the quality of sensory information, being in first instance an instrument of sensory selection, that is to say a gain control modulatory mechanism (Cravo et al. 2013; Nobre and van Ede 2018; Schroeder and Lakatos 2009), neural entrainment also constitutively reflects the content of sensory information, corresponding to a cortical processing stage at which multiple (spectrotemporal) acoustic features are synthetized into a whole auditory object (Ding et al. 2014; Poeppel et al. 2008; Shamma et al. 2011).
GRANTS
This work was supported by ERC CREAM (3355336) to J.J.A. Data were collected at the Centre Multidisciplinaire des Sciences Comportementales Sorbonne Université-INSEAD. B.M. is supported by grants ANR-16-CONV-0002 (ILCB), ANR-11-LABX-0036 (BLRI) and the Excellence Initiative of Aix-Marseille University (A*MIDEX).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.W., P.A., J.-J.A., and B.M. conceived and designed research; I.W., P.A., and B.M. performed experiments; I.W., P.A., and B.M. analyzed data; I.W. and B.M. interpreted results of experiments; I.W., P.A., and B.M. prepared figures; I.W. and B.M. drafted manuscript; I.W., P.A., J.-J.A., and B.M. edited and revised manuscript; I.W., P.A., J.-J.A., and B.M. approved final version of manuscript.
REFERENCES
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J Neurosci 28: 3958–3965, 2008. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller G. EBMS—Emotion-Based Musical Synthesizer (Online). www.gregbeller.com/2013/02/ebms/ [22 May 2018].
- Bonin TL, Trainor LJ, Belyk M, Andrews PW. The source dilemma hypothesis: perceptual uncertainty contributes to musical emotion. Cognition 154: 174–181, 2016. doi: 10.1016/j.cognition.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Breska A, Deouell LY. Neural mechanisms of rhythm-based temporal prediction: delta phase-locking reflects temporal predictability but not rhythmic entrainment. PLoS Biol 15: e2001665, 2017. doi: 10.1371/journal.pbio.2001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran C, Trubanova A, Stillittano S, Caplier A, Ghazanfar AA. The natural statistics of audiovisual speech. PLOS Comput Biol 5: e1000436, 2009. doi: 10.1371/journal.pcbi.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo AM, Rohenkohl G, Wyart V, Nobre AC. Temporal expectation enhances contrast sensitivity by phase entrainment of low-frequency oscillations in visual cortex. J Neurosci 33: 4002–4010, 2013. doi: 10.1523/JNEUROSCI.4675-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Chatterjee M, Simon JZ. Robust cortical entrainment to the speech envelope relies on the spectro-temporal fine structure. Neuroimage 88: 41–46, 2014. doi: 10.1016/j.neuroimage.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Patel AD, Chen L, Butler H, Luo C, Poeppel D. Temporal modulations in speech and music. Neurosci Biobehav Rev 81, Pt B: 181–187, 2017. doi: 10.1016/j.neubiorev.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Cortical entrainment to continuous speech: functional roles and interpretations. Front Hum Neurosci 8: 311, 2014. doi: 10.3389/fnhum.2014.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelling K, Arnal L, Ghitza O, Poeppel D. Acoustic landmarks drive delta-theta oscillations to enable speech comprehension by facilitating perceptual parsing. Neuroimage 85: 761–768, 2014. doi: 10.1016/j.neuroimage.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelling KB, Assaneo MF, Bevilacqua D, Pesaran B, Poeppel D. An oscillator model better predicts cortical entrainment to music. Proc Natl Acad Sci USA 116: 10113–10121, 2019. doi: 10.1073/pnas.1816414116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelling KB, Poeppel D. Cortical entrainment to music and its modulation by expertise. Proc Natl Acad Sci USA 112: E6233–E6242, 2015. doi: 10.1073/pnas.1508431112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsen A. Talk about a piece of work: a group self-interview. Drama Rev 59: 133–148, 2015. doi: 10.1162/DRAM_a_00501. [DOI] [Google Scholar]
- Folta-Schoofs K, Wolf OT, Treue S, Schoofs D. Perceptual complexity, rather than valence or arousal accounts for distracter-induced overproductions of temporal durations. Acta Psychol (Amst) 147: 51–59, 2014. doi: 10.1016/j.actpsy.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York: Cambridge University Press, 2007. [Google Scholar]
- Ghitza O. On the role of theta-driven syllabic parsing in decoding speech: intelligibility of speech with a manipulated modulation spectrum. Front Psychol 3: 238, 2012. doi: 10.3389/fpsyg.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Kleinschmidt A, Poeppel D, Lund TE, Frackowiak RS, Laufs H. Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron 56: 1127–1134, 2007. doi: 10.1016/j.neuron.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci 15: 511–517, 2012. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud J, Trébuchon A, Schön D, Marquis P, Liégeois-Chauvel C, Poeppel D, Morillon B. Asymmetric sampling in human auditory cortex reveals spectral processing hierarchy. bioRxiv 581520, 2019. doi: 10.1101/581520. [DOI] [PMC free article] [PubMed]
- Gluth S, Meiran N. Leave-One-Trial-Out, LOTO, a general approach to link single-trial parameters of cognitive models to neural data. eLife 8: e42607, 2019. doi: 10.7554/eLife.42607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Hoogenboom N, Thut G, Schyns P, Panzeri S, Belin P, Garrod S. Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol 11: e1001752, 2013. doi: 10.1371/journal.pbio.1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Zion-Golumbic E. Rhythmic facilitation of sensory processing: a critical review. Neurosci Biobehav Rev 86: 150–165, 2018. doi: 10.1016/j.neubiorev.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Henry MJ, Obleser J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proc Natl Acad Sci USA 109: 20095–20100, 2012. doi: 10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juslin PN, Laukka P. Expression, perception, and induction of musical emotions: a review and a questionnaire study of everyday listening. J New Music Res 33: 217–238, 2004. doi: 10.1080/0929821042000317813. [DOI] [Google Scholar]
- Juslin PN, Västfjäll D. Emotional responses to music: the need to consider underlying mechanisms. Behav Brain Sci 31: 559–575, 2008. doi: 10.1017/S0140525X08005293. [DOI] [PubMed] [Google Scholar]
- Kösem A, van Wassenhove V. Distinct contributions of low- and high-frequency neural oscillations to speech comprehension. Lang Cogn Neurosci 32: 536–544, 2017. doi: 10.1080/23273798.2016.1238495. [DOI] [Google Scholar]
- Lakatos P, Musacchia G, O’Connel MN, Falchier AY, Javitt DC, Schroeder CE. The spectrotemporal filter mechanism of auditory selective attention. Neuron 77: 750–761, 2013. doi: 10.1016/j.neuron.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage KQ, Vijayan S. The relationship between coherence and the phase-locking value. J Theor Biol 435: 106–109, 2017. doi: 10.1016/j.jtbi.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Liégeois-Chauvel C, Bénar C, Krieg J, Delbé C, Chauvel P, Giusiano B, Bigand E. How functional coupling between the auditory cortex and the amygdala induces musical emotion: a single case study. Cortex 60: 82–93, 2014. doi: 10.1016/j.cortex.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Luo H, Poeppel D. Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron 54: 1001–1010, 2007. doi: 10.1016/j.neuron.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Morillon B, Baillet S. Motor origin of temporal predictions in auditory attention. Proc Natl Acad Sci USA 114: E8913–E8921, 2017. doi: 10.1073/pnas.1705373114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon B, Liégeois-Chauvel C, Arnal LH, Bénar C-G, Giraud AL. Asymmetric function of theta and gamma activity in syllable processing: an intra-cortical study. Front Psychol 3: 248, 2012. doi: 10.3389/fpsyg.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, van Ede F. Anticipated moments: temporal structure in attention. Nat Rev Neurosci 19: 34–48, 2018. doi: 10.1038/nrn.2017.141. [DOI] [PubMed] [Google Scholar]
- Patel AD. Science & music: talk of the tone. Nature 453: 726–727, 2008. doi: 10.1038/453726a. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Gross J, Davis MH. Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex 23: 1378–1387, 2013. doi: 10.1093/cercor/bhs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods 162: 8–13, 2007. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González D, Malmierca MS. Adaptation in the auditory system: an overview. Front Integr Nuerosci 8: 19, 2014. doi: 10.3389/fnint.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Idsardi WJ, van Wassenhove V. Speech perception at the interface of neurobiology and linguistics. Philos Trans R Soc Lond B Biol Sci 363: 1071–1086, 2008. doi: 10.1098/rstb.2007.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecke L, Formisano E, Sorger B, Başkent D, Gaudrain E. Neural entrainment to speech modulates speech intelligibility. Curr Biol 28: 161–169.e5, 2018. doi: 10.1016/j.cub.2017.11.033. [DOI] [PubMed] [Google Scholar]
- Rimmele JM, Morillon B, Poeppel D, Arnal LH. Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn Sci 22: 870–882, 2018. doi: 10.1016/j.tics.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340: 216–219, 2013. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Zald DH, Zatorre RJ, Dagher A, McIntosh AR. Predictions and the brain: how musical sounds become rewarding. Trends Cogn Sci 19: 86–91, 2015. doi: 10.1016/j.tics.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32: 9–18, 2009. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma SA, Elhilali M, Micheyl C. Temporal coherence and attention in auditory scene analysis. Trends Neurosci 34: 114–123, 2011. doi: 10.1016/j.tins.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Little DR. Small is beautiful: in defense of the small-N design. Psychon Bull Rev 25: 2083–2101, 2018. doi: 10.3758/s13423-018-1451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetzger K, Rosen S. Effects of acoustic periodicity and intelligibility on the neural oscillations in response to speech. Neuropsychologia 95: 173–181, 2017. doi: 10.1016/j.neuropsychologia.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Tian X, Poeppel D. Testing multi-scale processing in the auditory system. Sci Rep 6: 34390, 2016. doi: 10.1038/srep34390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Tian X, Rowland J, Poeppel D. Concurrent temporal channels for auditory processing: oscillatory neural entrainment reveals segregation of function at different scales. PLoS Biol 15: e2000812, 2017. doi: 10.1371/journal.pbio.2000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw 59: 1–38, 2014.26917999 [Google Scholar]
- Vander Ghinst M, Bourguignon M, Op de Beeck M, Wens V, Marty B, Hassid S, Choufani G, Jousmäki V, Hari R, Van Bogaert P, Goldman S, De Tiège X. Left superior temporal gyrus is coupled to attended speech in a cocktail-party auditory scene. J Neurosci 36: 1596–1606, 2016. doi: 10.1523/JNEUROSCI.1730-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R. Perceptual cycles. Trends Cogn Sci 20: 723–735, 2016. doi: 10.1016/j.tics.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci 6: 37–46, 2002. doi: 10.1016/S1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919, 1994. doi: 10.1523/JNEUROSCI.14-04-01908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion Golumbic EM, Ding N, Bickel S, Lakatos P, Schevon CA, McKhann GM, Goodman RR, Emerson R, Mehta AD, Simon JZ, Poeppel D, Schroeder CE. Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party”. Neuron 77: 980–991, 2013. doi: 10.1016/j.neuron.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B, VanRullen R. Selective perceptual phase entrainment to speech rhythm in the absence of spectral energy fluctuations. J Neurosci 35: 1954–1964, 2015. doi: 10.1523/JNEUROSCI.3484-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]