Abstract
Neurophysiological studies suggest that when decisions are made between concrete actions, the selection process involves a competition between potential action representations in the same sensorimotor structures involved in executing those actions. However, it is unclear how such models can explain situations, often encountered during natural behavior, in which we make decisions while were are already engaged in performing an action. Does the process of deliberation characterized in classical studies of decision-making proceed the same way when subjects are deciding while already acting? In the present study, human subjects continuously tracked a target moving in the horizontal plane and were occasionally presented with a new target to which they could freely choose to switch at any time, whereupon it became the new tracked target. We found that the probability of choosing to switch increased with decreasing distance to the new target and increasing size of the new target relative to the tracked target, as well as when the direction to the new target was aligned (either toward or opposite) to the current tracking direction. However, contrary to our expectations, subjects did not choose targets that minimized the energetic costs of execution, as calculated by a biomechanical model of the arm. When the constraints of continuous tracking were removed in variants of the task involving point-to-point movements, the expected preference for lower cost choices was seen. These results are discussed in the context of current theories of nested feedback control, internal models of forward dynamics, and high-dimensional neural spaces.
NEW & NOTEWORTHY Current theories of decision-making primarily address how subjects make decisions before executing selected actions. However, in our daily lives we often make decisions while already performing some action (e.g., while playing a sport or navigating through a crowd). To gain insight into how current theories can be extended to such “decide-while-acting” scenarios, we examined human decisions during continuous manual tracking and found some intriguing departures from how decisions are made in classical “decide-then-act” paradigms.
Keywords: action selection, biomechanics, decision-making, manual tracking, reaching movements
INTRODUCTION
In our daily lives, we are faced with a wide variety of decision-making scenarios. Some decisions are purely abstract, such as choosing which courses to take during one’s university studies. These can be described as choices between representations of the costs and benefits of different predicted outcomes in a common currency of subjective utility (Levy and Glimcher 2012; Padoa-Schioppa 2011; Rangel et al. 2008). Other decisions are more mundane and concrete, such as choosing where to sit when entering a classroom (Cisek and Pastor-Bernier 2014). Such “embodied decisions” can be thought of as involving selection between different potential actions, or what Gibson (1979) called “affordances.” In that scenario, the potential actions are directly specified by sensory information about the geometry of the world, and selection between them can take place through a biased competition between internal representations of potential movements (Cisek 2007; Erlhagen and Schöner 2002; Gold and Shadlen 2007), which may be biased by their required effort (Cos et al. 2011; Morel et al. 2017; Shadmehr et al. 2016). Psychological and neurophysiological experiments on decision-making often combine aspects of both of these types of decisions by asking subjects to make a choice, about a percept or an estimate of value, and then report it with an action, such as a reaching movement or a saccade. Numerous studies have shown that when the actions used to report different choices are known to the subject ahead of time, the deliberation process is reflected in the sensorimotor regions responsible for guiding the movement, i.e., in the reaching network for reach decisions (Andersen and Cui 2009; Christopoulos et al. 2015, 2018; Cisek and Kalaska 2005; Cui and Andersen 2007; Klaes et al. 2011; Pastor-Bernier and Cisek 2011; Pesaran et al. 2008; Scherberger and Andersen 2007; Thura and Cisek 2014; Westendorff et al. 2010) and in the oculomotor network for saccade choices (Ditterich et al. 2003; Gold and Shadlen 2000, 2007; Huk and Shadlen 2005; McPeek and Keller 2002; Platt and Glimcher 1999; Roitman and Shadlen 2002). This makes sense from an ecological perspective, which suggests that the brain evolved first and foremost to govern our interaction with the environment (e.g., selecting where to sit) and only much later elaborated its mechanisms toward abstract decision-making scenarios (e.g., selecting a university curriculum) (Cisek and Kalaska 2010; Cisek and Pastor-Bernier 2014; Engel et al. 2013; Pezzulo and Castelfranchi 2009; Pezzulo and Cisek 2016).
Nevertheless, even studies explicitly aimed at understanding how the brain selects between concrete actions have not fully addressed the complexities of real embodied decisions. In particular, they have primarily used what one may call “decide-then-act” paradigms, in which subjects are completely motionless during deliberation and make a movement only after committing to their final choice. Such tasks have led to the development of a diverse class of models that suggest decisions are made when neural activity selective for a given act reaches a threshold, at which time movement is initiated (Bogacz et al. 2006; Carland et al. 2016; Cisek et al. 2009; Hanes and Schall 1996; Laming 1968; Mazurek et al. 2003; Ratcliff 1978; Ratcliff and McKoon 2008; Stone 1960; Thura et al. 2012; Usher and McClelland 2001).
However, in our daily lives we often make decisions while we are already moving, such as when navigating through a crowd of students all struggling to get to different classrooms on time. In this scenario, each person is already performing an action, continuously adjusting it through feedback, all the while remaining sensitive to new potential options that may present themselves. The decision is between continuing to perform the current action and switching to a new one, and requires one to continuously weigh the relative desirability of available options. If our theories of the neural mechanisms of embodied decision-making are to apply to natural behavior in the real world, they should be able to address these kinds of “decide-while-acting” scenarios. This presents a challenge to models that describe the transition between deliberation and commitment as the crossing of a neural threshold (Bogacz et al. 2006; Carland et al. 2016; Cisek et al. 2009; Hanes and Schall 1996; Laming 1968; Mazurek et al. 2003; Ratcliff 1978; Ratcliff and McKoon 2008; Stone 1960; Thura et al. 2012; Usher and McClelland 2001) or entering an attractor (Amari 1977; Cisek 2006; Grossberg 1973; Wang 2002), because whatever group of cells is responsible for the ongoing action must already be past its threshold (or the system must already be within its attractor). Nevertheless, the system as a whole must still be capable of specifying alternative options and implementing a process of deliberation between continuing the current action versus switching to another. Furthermore, if a decision unfolds within the same brain regions that control ongoing actions (Cisek 2007; Erlhagen and Schöner 2002; Gold and Shadlen 2007; Klaes et al. 2012), then how can one deliberate about switching without interfering with the ongoing action?
Recent studies have examined situations in which reaching movements are initiated before decision commitment is complete. These have shown that deliberation influences the trajectory, at least during the early part of the action, and can even be used as a window into cognitive processes (Chapman et al. 2010; Farmer et al. 2007; Gallivan et al. 2018; Gallivan et al. 2011; McKinstry et al. 2008; Song and Nakayama 2008, 2009; Wood et al. 2011). Other studies have shown that subjects can be externally induced to change their trajectory choices by a physical perturbation applied during a movement (Nashed et al. 2014). However, to our knowledge, no study has examined how a subject who is already committed and fully engaged in performing some action can voluntarily deliberate about switching to an alternative action without interfering with the ongoing movement. It is this type of scenario that is most challenging for current models.
In this study, we investigated decision-making during ongoing action control through behavioral experiments in human subjects performing a planar manual task. Our goal was to test which factors shown to influence choices during standard decide-then-act paradigms influence choices during a decide-while-acting paradigm. To maintain precise control over the kinematic and kinetic variables of interest, we asked subjects to continuously track a target with their hand while other potential choice targets were presented, and subjects were free to either continue tracking the current target or switch to the new one. Tracking direction and choice target placement were designed to independently control spatial factors such as target distance, direction, and size, and kinetic factors such as biomechanical cost (in terms of average muscle torque). To provide a link to standard decide-then-act paradigms, we also tested subjects in a discontinuous version of the task in which all movements were point to point, as well as in a standard delayed reach decision task. Based on previous studies (Cos et al. 2011, 2012; Morel et al. 2017), we predicted that subjects would show preferences for switching to near targets rather than far ones, to large targets rather than small ones, to targets well aligned with the current movement direction, and to directions incurring lower biomechanical costs. Some of these results have previously appeared in abstract form (Michalski and Cisek 2017; Michalski et al. 2018).
MATERIALS AND METHODS
Subjects and Apparatus
Twenty-two right-handed subjects (7 men, 15 women) participated in the study. They had no known neurological disorders and had normal or corrected-to-normal vision, and all were naive about the purpose of these experiments. They all provided written informed consent before the experimental session was initiated and received a payment of $25 per session for their participation. The protocol was approved by the Human Research Ethics Committee of the Faculté de Médicine, Université de Montréal.
The task apparatus consisted of a 91-cm × 61-cm digitizing tablet (GTCO Calcomp IV, Columbia, MD) in the horizontal plane and a half-silvered mirror suspended 16 cm above and parallel to the digitizer. Visual stimuli were projected onto the mirror by an LCD monitor suspended 16 cm above, producing the illusion that the targets lie on the plane of the digitizing tablet. Subjects used their right hand to make movements using a digitizing stylus whose position was sampled at 125 Hz with a spatial resolution of 0.013 cm. Subjects were seated in front of the task apparatus with their right shoulder aligned to the center of the screen and with their right arm resting in a sling supporting it just above the elbow. The sling was 107 cm long and was positioned so that the anchor point was approximately directly above the subjects’ elbow when they held the pen in the center of the screen.
Behavioral Tasks
Continuous tracking task.
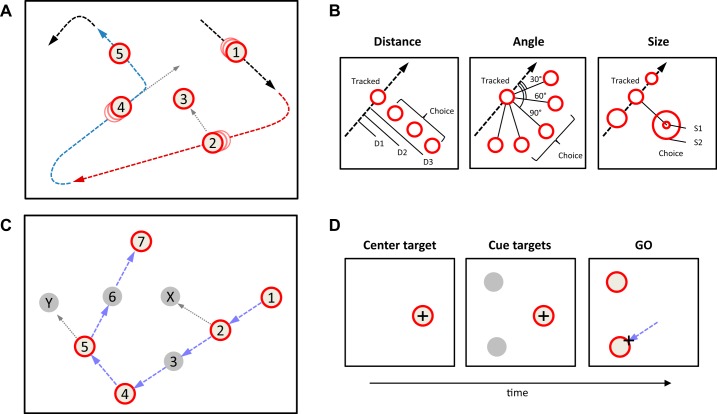
Each experimental session consisted of an average of 80 “runs” of continuous tracking for about a minute each (Fig. 1A). At the start of each run, a luminous target (white with red border, 1-cm radius) is projected on a black background. When the stylus enters the target, it begins to move, accelerating over 1 s to a constant speed of 6 cm/s that is maintained as long as the stylus is within the circle. This “tracked target” moves in a straight line until it reaches the edge of the workspace, where it gradually changes direction (either clockwise or counterclockwise in a path along the circumference of a 1.5-cm-radius circle) until it begins to head in a new direction toward the central region of the screen. While the tracked target is passing through the central region, the subject is presented with a stationary “choice target” that remains available for 1,400 ± 200 ms. The subject can choose to ignore this choice target and continue tracking the tracked target; we call this a “no-switch” trial. Alternatively, the subject can choose to move the stylus into the choice target, whereupon the abandoned tracked target disappears while the choice target accelerates over 1 s to move at a constant speed in the same direction as the trajectory of the hand as it enters, and thus becomes the new tracked target. We call this a “switch” trial. The time at which the cursor starts to move toward the choice target (i.e., switching time) was defined as the first time (between choice appearance and tracked target exit) at which the distance to the target began consistently decreasing at a rate of more than 3 cm/s. This time was obtained by starting at the moment the cursor exited the currently tracked target (i.e., when the rate of decrease had already exceeded the 3 cm/s threshold) and going backward in time until we found a time point at which the rate of decrease in distance to the choice target dropped below 3 cm/s. The interval between choice target appearance and the switching time is defined as the “switch reaction time” (SRT).
Fig. 1.
Behavioral tasks. A: an example schematizing the continuous tracking task. The subject’s hand (dashed line) follows a “tracked target” (white circle with a red border), which moves around the screen at 6 cm/s. In the actual display, only the circle and a cross indicating hand position are visible. When the tracked target is at point 2, a “choice target” appears (circle 3), offering a potential new movement (gray dotted arrow), but the subject ignores it and continues to track the current target. At point 4, a new choice target appears (circle 5) and the subject switches to it, so it now becomes the new tracked target. Individual “trials” are defined by the colored segments, each of which presents the subject with a single decision scenario, and dotted gray lines indicate the options not taken. B: three variations of the continuous tracking task. In the “distance block,” the choice target always appears in a direction orthogonal to the current tracking direction, but at 5 different distances (only 3 shown). In the “angle block,” the target always appears at a distance of 4.8 cm, but at 1 of 5 angles with respect to the current tracking direction. In the “size block,” the tracked target is gradually shrinking and the choice target always appears orthogonal to the current tracking direction and at a distance of 4.8 cm, but can be either larger or smaller than the currently tracked target. C: the discontinuous tracking task. The tracked target (red circle) jumps by 4.8 cm every 900 ms, and the subject tracks it with point-to-point movements. At time 1, the next target (2) is displayed along with 2 gray circles (3 and X), foreshadowing future targets. These turn red (not shown) 900 ms after the subject moves into target 2. Now the subject can make a choice and, in this example, chooses to go to target 3, which is “aligned” with the previous point-to-point movement. At time 5, the subject chooses to go to target 6, which is “unaligned” with the previous point-to-point movement. D: the replay task. The task is broken into individual trials, each starting when the subject places the cursor in the red circle, at which time 2 cues appear (gray circles). After 900 ms they turn red, indicating the GO signal, when the subject can freely choose either target. Importantly, the placement of the start and target circles is a replay of choice scenarios previously experienced when performing the discontinuous tracking task (in this example, from time 2 in C).
The task continues in this fashion so that the subject is always either continuously tracking the current target or switching to a new choice target. Whenever the currently tracked target reaches the edge of the screen, we define this moment as the end of a “trial” and the beginning of the next trial. Each continuous “run” consists of several trials without interruptions for ~1 min, after which the subjects get an opportunity to briefly rest before starting the next run. Subjects are given a 5-min period to practice the task, after which they are instructed to keep going for blocks of 30 min.
Within each trial, the position at which the choice target appears depends on the type of block type currently being performed (Fig. 1B). Pilot studies determined that in all three block types, the mean SRT was ~500 ms. The choice target’s position is thus determined on the basis of where the tracked target will be 500 ms after the choice target appears. In the “distance block” (Fig. 1B, left), the choice target appears perpendicular to the direction of motion at one of five possible distances (2.4, 4.8, 7.2, 9.6, or 12 cm). In the “angle block” (Fig. 1B, center), the choice target appears at a 4.8-cm distance, at one of five possible angles (30°, 60°, 90°, 120°, or 150°) relative to the direction of motion. In the “size block” (Fig. 1B, right), the tracked target is shrinking in size from its initial radius (e.g., 1 cm for the first target of a run) to a minimum radius of 0.6 cm at a rate of 0.3 mm/s. Each choice target appears perpendicular to the direction of motion and at 4.8 cm, but its radius is a value between 0.6 and 1.2 cm. Thus the choice target is sometimes larger and sometimes smaller than the currently tracked target. If the choice target is entered, it becomes the new tracked target and immediately begins to shrink in size, until it is abandoned in favor of a new choice target or the run ends. If the tracked target reaches a minimum radius of 0.6 cm, it stops shrinking but continues to move around the screen.
In all conditions, subjects were instructed to follow the motion of the tracked target and to stay within the white circle as long as possible, but they were allowed to freely choose to switch to a different target if one appeared. They were explicitly told that the choice whether to switch or not was completely up to them and that as long as they were tracking a target, it did not matter whether it was the old or new one. While it may seem that there is no reason for a subject to ever switch, in fact they did so quite often, allowing us to quantify the influence of the various factors that we manipulated (distance, size, etc.).
Discontinuous tracking task.
This task is conceptually similar to the continuous tracking task, except that it involves a series of point-to-point movements (Fig. 1C) instead of smooth continuous tracking. When the stylus moves into the “tracked” target, there is a 900-ms delay and then the target disappears while a new target appears 4.8 cm away. That target remains available until the subject enters it, and then after 900 ms it also disappears and another new target appears. Thus the subject makes a series of point-to-point movements approximately every 900 ms. Tracked targets are presented in the same direction as the last movement unless the edge of the screen is reached, at which point they turn around. After the edge of the screen is reached, there are at least two jumps before a choice scenario (“trial”) begins (although these are not depicted in Fig. 1C). At the start of each choice scenario, when the new tracked target location is presented, two dim cues also appear simultaneously, indicating the future choices. One dim cue represents the position of the next tracked target, 4.8 cm away from the current tracked target and in the same direction as the previous movement. The second dim cue is positioned where the alternative target will be, 4.8 cm away from the current tracked target and in one of four directions with respect to the workspace (45°, 135°, 225°, or 315°, where 0° is to the right). The angle of separation between the two dim cues was forced to be between 45° and 135° and in most cases was in the 60–120° range. Once the stylus reaches the tracked target, the dim cues are replaced with white targets with red borders, representing the next “tracked target” (aligned with previous motion) and the “choice target” (unaligned with previous motion), and the subject is free to choose to move to either of these. Subjects are required to wait for the targets to turn red, which can be considered equivalent to a “GO signal” in standard delayed reaching tasks (Kalaska and Crammond 1995).
We presented the dim cues to make both of the future target positions equally predictable well ahead of the time the subject would have to make their choice. This was motivated by pilot studies in which no such dim cues were presented, and subjects were just shown two white circles with red borders on entering the tracked target. In that scenario, one of the circles was always in a highly predictable location (4.8 cm away and aligned with the previous movement) while the other could be in one of many locations, and we found that subjects showed an overwhelming preference to choose the predictable target. Thus, because we wanted to study the influence of factors other than target predictability, such as biomechanical costs, we chose to make the position of both targets fully and equally predictable by presenting the dim cues ahead of the time of the choice.
Replay task.
In the discontinuous tracking task, subjects make decisions in the context of a sequence of movements. To compare these to decisions made outside of the context of a sequence, we presented subjects with a “replay” of the decision scenarios they encountered in the discontinuous tracking task, using the same spatial targets but in separate independent trials, each similar to classic instructed delay reaching tasks. In the replay task (Fig. 1D), the subject starts each trial by moving the cursor into an initial target, and then two gray choice targets appear 4.8 cm from the initial one. After 900 ms, the targets turn white (with a red border), indicating the GO signal, and the subject then moves the cursor to one of the two choices, ending the trial. In the 67% of subjects that performed the discontinuous task before the replay task, the positions of the initial target and the two choice targets were taken from the decision scenarios encountered during the discontinuous tracking task. Thus these trials recreated the same decision scenarios that the subject faced in the discontinuous tracking task in terms of spatial locations and angular separations, but in a shuffled order and without the element of continuity between decisions. In 33% of subjects, the first block of the replay task was run before any blocks of discontinuous tracking, using the discontinuous session of a previous subject to determine target placements. Because behavior was the same in these replay blocks as those based on the subject’s own performance (i.e., there was no effect of block order), we analyze all of these together.
Biomechanical Modeling
For each trial, we used a biomechanical model to estimate the net torque produced by muscles during a period from 500 ms before target onset to 1,000 ms after target onset. The model was built using the SimMechanics package within the SIMULINK simulation environment in MATLAB. The upper arm and forearm + hand limb segments were modeled as two thin rods with uniform mass distribution and average lengths and weight (males: upper arm, 30.9 cm, 2.1 kg; forearm + hand, 29.1 cm, 1.7 kg; females: upper arm, 28.6 cm, 1.7 kg; forearm + hand, 25.8 cm, 1 kg) (Nikolova and Toshev 2007). The two limb segments were joined at the elbow with 1 rotational degree of freedom, and the proximal upper arm segment was joined to a static body with 1 rotational degree of freedom. The model was constrained to a two-dimensional (2-D) horizontal plane.
The recorded positions of the stylus were interpolated at 100 Hz using a 2-D spline and filtered at 20 Hz with a low-pass Butterworth filter (9th order) with zero delay. Velocity was computed using a five-point differentiation routine, and then both position and velocity were upsampled to 1,000 Hz with linear interpolation and again low-pass filtered at 20 Hz. Using inverse kinematics equations for a planar arm model, we calculated the angular position of each joint through time and then passed them through an inverse dynamics model (SimMechanics) to calculate the muscle torques produced at the shoulder and elbow joints.
We calculated the sum of the absolute muscle torques produced at both joints, averaged over a period of time meant to capture the cost of switching versus continuing. For switch trials, the average torque was calculated from 100 ms before the time of the switch to 100 ms after the cursor entered the choice target. For no-switch trials, average torque was calculated from 400 ms after choice target onset (this corresponds to ~100 ms before the average time that subjects normally switch to a choice target, which is 500 ms as noted above) until the moment when the cursor reached the edge of the screen (before a change in tracking direction). Note that because no-switch trials involved a straight movement at a nearly constant speed, and thus nearly constant torque, the average torque calculation was not sensitive to the duration of the tracking or the precise window that was used. In discontinuous trials, average torque was calculated between the onset and offset of movement, each detected as 5% of peak movement speed. About 5.3% of trials in which onset and offset could not be clearly detected were excluded from this analysis.
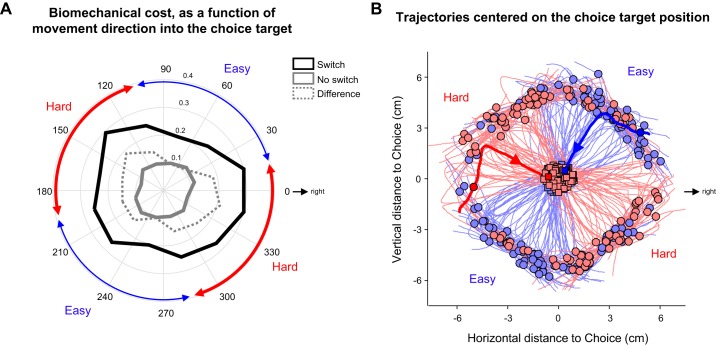
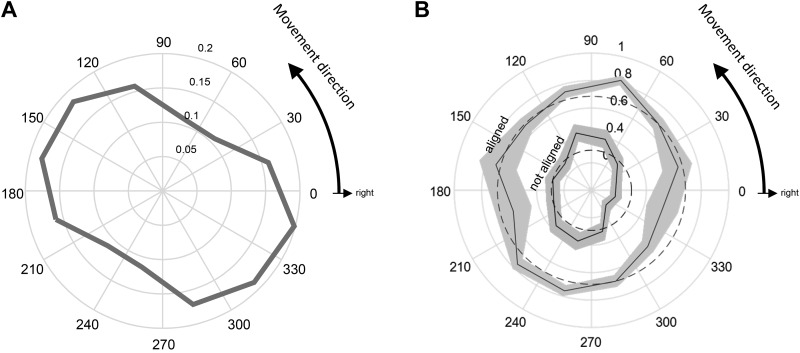
As described in results, we found that some switches of direction during continuous tracking were more biomechanically costly than others, and this strongly depended on the angle from the cursor to the choice target (measured counterclockwise, where 0° is to the right). Consequently, we classified as “hard” those trials in which that angle was either between 286° and 15° or between 106° and 195°, and as “easy” those in which the angle was between 16° and 105° or between 196° and 285° (see Fig. 3A).
Fig. 3.
A: average cost of switching (solid black line, n = 796 trials) vs. continuing to track (solid gray line, n = 1478), in trials where the choice target appeared at 90° to the current tracking direction. Cost is expressed in N·m, averaged from choice target appearance until the time it was entered (switch trials) or until the screen edge was reached (no-switch trials), and plotted as a function of the movement direction (in absolute angles with respect to the workspace) to the choice target. Dotted gray line indicates the difference. Note that switching toward a choice target at 150° or 330° is more costly than switching to one at 60° or 240°. Hence, we define trials in which the choice target direction was in the range of angles marked in red as biomechanically “hard” (n = 1,067) and those in which the choice target direction was in the range marked in blue as biomechanically “easy” (n = 1,207). B: sample segments of trajectories from switch trials, all aligned to the location of the choice target. Each segment begins 200 ms before the choice appears (circle) and ends when the cursor enters the target (square). Segments are color coded according to the direction to the choice target, as defined in A (blue: easy, n = 114; red: hard, n = 104). We include trials in which the choice target appeared 4.8 cm away at 90° from the current tracking direction. Two example trials are highlighted.
Analyses of Choice Preferences
In the distance block of the continuous tracking task, we quantified the effect of distance by calculating the proportion of switch choices for each choice target distance, separately for easy and hard trials, and fit these with a sigmoidal curve described as
where X is the distance to the choice target and a and b are the slope and the mean of the sigmoid, respectively. If distance has an effect, then we expect this sigmoid to have a negative slope. To test for significance of the distance effect, we computed 1,000 sigmoids by randomly resampling the data (with replacement) across all subjects, and if 97.5% of the distribution of parameter a was negative, we considered the effect of distance to be significant at P < 0.05.
A similar approach was used to examine effects in the size block, except that in this case, X was defined as the difference between the choice target diameter and the tracked target diameter, trials were grouped into nine bins according to X, and we tested for values of parameter a that were greater than zero (i.e., more switching to choice targets that are larger than the currently tracked target).
As described in results, for the angle block, subject choice preference curves were nonmonotonic and so could not be fitted with sigmoidal functions. Consequently, we fit the data with a second-order polynomial described as Pswitch(X) = a + bX + cX2, where X is the angle between the current tracking direction and the direction to the choice target. An angle effect was considered significant if the resampled distribution for either parameter b or c was different from zero.
To test for the effect of biomechanical costs in all three blocks (distance, angle, and size), we computed a distribution of the difference in the area under the curve (AUC) between biomechanically easy versus hard trials. This was done using a sigmoidal curve for distance and size blocks and a polynomial curve for the angle block. Next, to test for the significance of this difference, we constructed a distribution of 1,000 differences after randomly resampling (with replacement) within each trial type. If zero lay outside the 95% confidence interval of this distribution, then the effect of biomechanical costs was considered significant at P < 0.05.
To test whether the proportion of switch choices depends on the current tracking direction or the direction to the choice target, we subdivided the circle into 30° bins, computed the proportion of switch choices within each bin, and performed a χ2 test to see if the switch choices were equally distributed across the bins. Results were considered significant at P < 0.05.
RESULTS
Behavior in the Continuous Tracking Task
Eleven subjects performed the continuous tracking task, completing 747 trials on average (range 533–1,013). Five of these subjects also participated in some pilot studies, but their behavior in the final paradigm was no different than that of the remaining subjects, so their data were included. Figure 2 shows an excerpt of the cursor trajectory from an example session. During the trial highlighted in blue, the subject was moving to the upper right when a choice target (red circle) appeared 4.8 cm to the left of the trajectory. The subject switched to this target after a 486-ms switch reaction time (SRT), whereupon it became the new tracked target and the task continued.
Fig. 2.
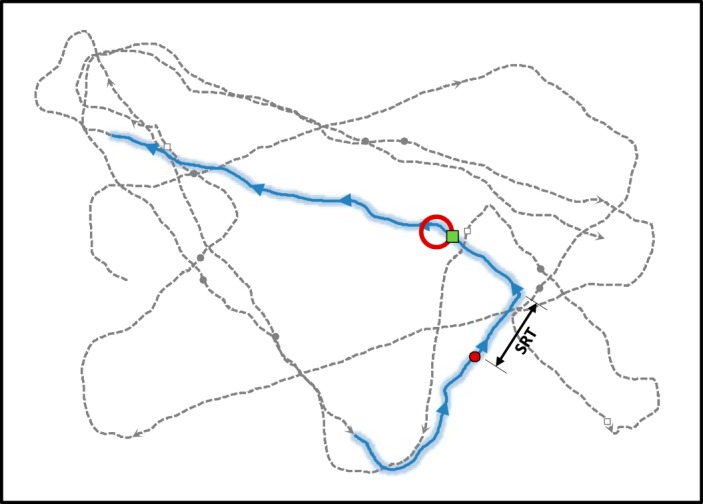
Hand trajectory (dashed gray line) from an example run of the continuous tracking task (“distance” block). A single trial is highlighted in blue, with arrowheads indicating movement direction. The choice target (red circle) appeared at the moment the subject was at the position indicated by the red dot. After a short “switch reaction time” (SRT), the subject abandoned the tracked target, switched direction, and entered the choice target at the point indicated by the green square. Gray circles and open squares indicate analogous events in other trials within the same continuous run.
Across all subjects, the average SRT was 498 ms (SD ±168 ms), but it varied between the different blocks. SRTs were fastest in the angle block (median 457 ms) and slowest in the size block (median 500 ms) (Mann–Whitney U test, P < 0.05). Within each block, the differences between conditions (closer vs. farther, small vs. large angle, smaller vs. bigger radius) were negligible, although they reached significance in a few cases (e.g., fastest SRT when the choice target was 2.4 cm away).
To examine whether presentation of a choice target and subsequent deliberation had any impact on movement kinematics, we examined the velocity and curvature of trajectories in trials in which a choice target was presented but subjects did not switch. The tangential velocity of tracking movements stayed close to the 6 cm/s speed of the tracked target (6.04 ± 1.85 cm/s, mean ± SD). During trials in which subjects did not switch to the choice target, there was a 2.8% decrease in average velocity in a window 250–350 ms after choice target appearance (to a mean velocity of 5.844 cm/s). This was significant when averaged across trials (P = 0.0138), but it was not consistent in individual trials. Indeed, of the 1,302 trials tested, the velocity 250–350 ms after choice appearance was significantly slower than in the 100 ms before the choice appearance in 637 (48%) trials, but it was significantly faster in 586 (45%) trials. Thus we conclude that the slight reduction apparent in the average is not indicative of any consistent effect of deliberation processes on movement velocity. There also was no consistent effect of deliberation processes on the curvature of the trajectory, which remained straight throughout no-switch trials.
The calculated biomechanical cost of switching versus continuing strongly depended on the current tracking direction (Fig. 3A). Switching to a transverse direction after moving in a sagittal direction was more costly than switching from transverse to sagittal directions. Consequently, we classified specific choice scenarios as easy or hard depending on the angle between the cursor and the choice target (see materials and methods). Figure 3B shows excerpts of trajectories (all aligned on the position of the choice target) from individual trials in which a subject performed an easy or hard switch during the distance block of the task, with choice targets at a distance of 4.8 cm. Across blocks, SRTs were not significantly different to biomechanically easy than to hard choice targets (median 485 vs. 489 ms; Mann–Whitney U test, P = 0.761).
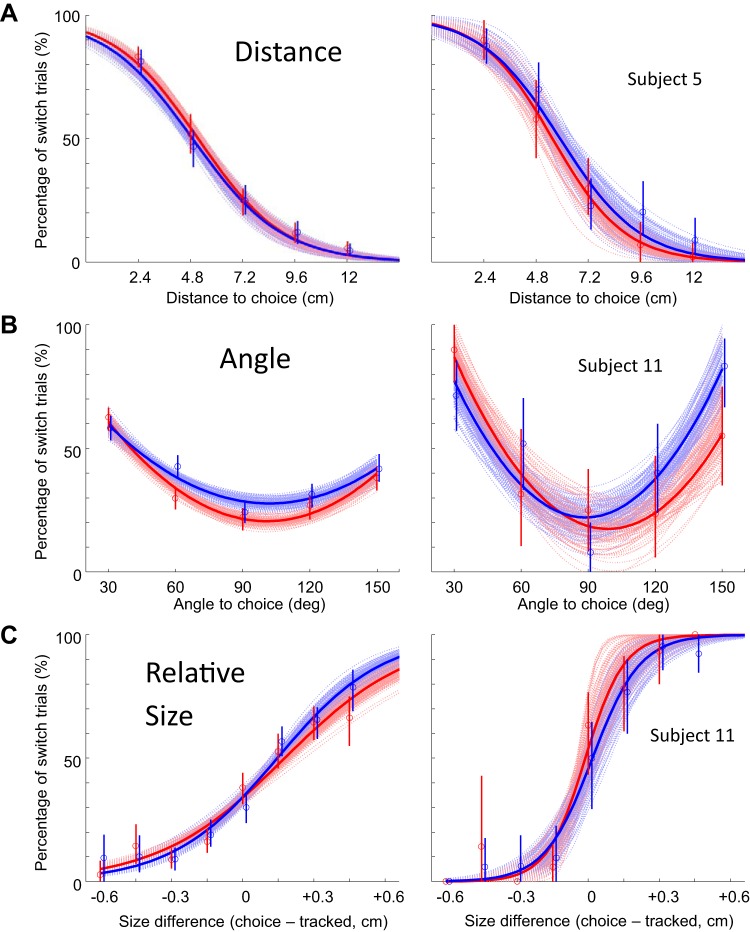
Figure 4 shows the percentage of switch trials when subjects were faced with different kinds of choices. In the distance block (Fig. 4A), subjects exhibited the expected preference for choice targets that were close over choice targets that were distant (a < 0; resampling test, P < 0.001; see materials and methods). However, there was no difference in the percentage of switch trials to a choice target that was biomechanically easy versus one that was hard (AUC easy − hard = 0; resampling test, P > 0.170).
Fig. 4.
Percentage of switch choices, as a function of different task variables in each block of the continuous tracking task. A–C show data for all subjects (left) and for an example subject (right). A: switch choice percentage as a function of target distance (left: 5 subjects, n = 2,319 trials; right: 1 subject, n = 557 trials). B: switch choice percentage as a function of target angle with respect to current tracking direction (left: 11 subjects, n = 3,676 trials; right: 1 subject, n = 237 trials). C: switch choice percentage as a function of the radius of the choice target relative to the tracked target (choice minus tracked, in cm) (left: 11 subjects, n = 2,973 trials; right: 1 subject, n = 320 trials). Red, hard trials; blue, easy trials. Each panel shows a fit to the data (thick line) as well as 100 fits obtained after the data were resampled with replacement (thin curves). Vertical lines indicate confidence intervals calculated by resampling.
In the angle block (Fig. 4B), subjects switched more often when the choice target was closely aligned with the current tracking trajectory (30° or 60°) than when it was orthogonal (90°), and interestingly, they also chose to switch more often to targets oriented at large angles (120° and 150°) than at 90°. In other words, they tended to prefer choice targets in directions that lay along the current movement direction (even backward) over choice targets oriented orthogonally (c > 0; resampling test, P < 0.001). At the group level, there was also a mild preference for biomechanically easy choices (AUC easy − hard > 0; P = 0.002).
Finally, in the size block (Fig. 4C), subjects chose to switch to the choice target more often when it was larger than the currently tracked target (a > 0; resampling test, P < 0.001). The point of subjective equality (when the switch choice was made 50% of the time) differed for individual subjects, but at the group level it averaged out to 0.15 cm. These choice preferences were not significantly different between biomechanically easy and hard trials (AUC easy −hard = 0; P > 0.201).
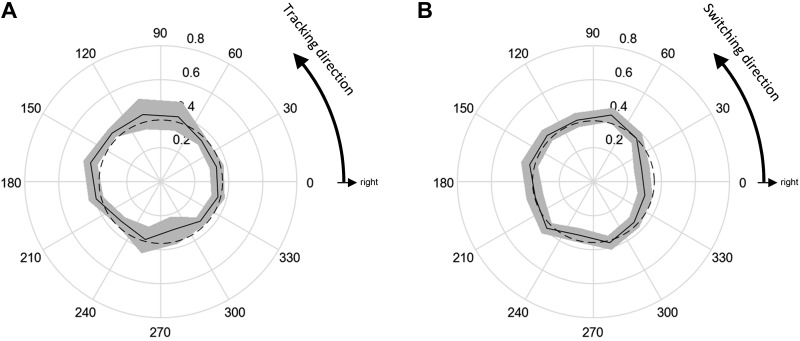
Subject choice preferences were remarkably similar across directions. For example, the probability of switching to a choice target at 90° to the currently tracked direction was ~35% across all tracking directions. When the percentage of switch trials was plotted as a function of the current tracking direction (Fig. 5A), there was a small but significant deviation from uniformity (χ2 test, P = 0.0171). Notably, however, when the percentage of switch trials was plotted as a function of the direction to the choice target, it was not significantly different from uniformity (Fig. 5B; χ2 test, P = 0.207). This was surprising because it contrasts with the anisotropy of the biomechanical costs of switching, shown in Fig. 3A.
Fig. 5.
Percentage of switch choices made to the choice target during the continuous tracking task (solid lines) compared with a uniform circle (dashed lines). Shaded areas indicate the 95% confidence interval computed using the Clopper-Pearson method. A: the percentage is plotted as a function of the ongoing tracking direction (in absolute angles with respect to the workspace) at the time the choice target appeared. B: the percentage is plotted as a function of the direction to the choice target. In both A and B, only trials where the choice target was oriented at 90° to the current tracking direction are included (n = 5,971 trials).
Behavior in the Discontinuous Tracking Task
Fifteen subjects performed the discontinuous tracking task, completing 407 trials on average (range 334–594). Because there was a GO signal in this task, and movements occurred on a 900-ms rhythm, reaction times were much shorter (~250 ms) than the SRTs in the continuous tracking task. This is presumably because the precues allowed the subjects to make their choice well ahead of time, as in classic instructed delay tasks with a GO signal. On average, RTs were slightly faster when subjects chose the aligned target than when they chose the unaligned target (median 245 vs. 261ms; Mann–Whitney U test, P < 0.001).
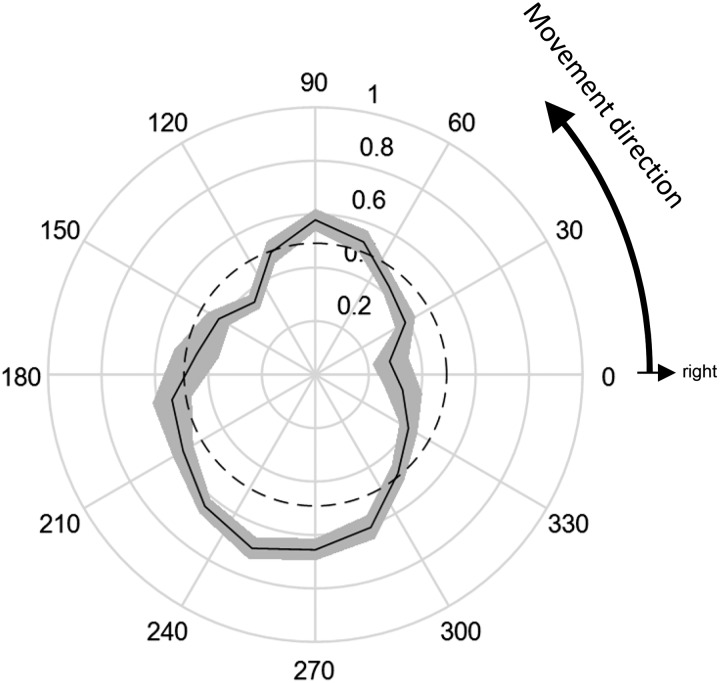
Figure 6A shows the biomechanical cost of point-to-point movements during the discontinuous tracking task as a function of movement direction. As expected, this has an ellipsoidal shape that is similar in orientation to the cost of making a switch in the continuous tracking task (Fig. 3A, black). Figure 6B shows the percentage of choices made during the discontinuous tracking task as a function of the direction to the chosen target for targets that were aligned or unaligned with the previous movement. First, note that although subjects made a full stop between each point-to-point movement, the percentage of choices is higher toward targets aligned with the previous point-to-point movement than to targets that are not aligned (χ2 test, P < 1 × 10−100). Furthermore, the pattern is significantly nonisotropic, with more choices made to targets in sagittal directions than in transverse directions both when the target is aligned (χ2 test, P = 0.012) and when it is unaligned (χ2 test, P = 2.68 × 10−12). This is consistent with the pattern of biomechanical costs shown in Fig. 6A. In other words, in the discontinuous tracking task, subjects exhibited a preference for targets aligned to their previous movement, as well as a smaller but significant preference for targets in directions of lower biomechanical costs.
Fig. 6.
Data from the discontinuous tracking task. A: calculated cost (in N·m) of moving to a target as a function of the direction to that target (n = 3,019 trials). Note that, similarly to Fig. 3A, the plot implies that there is a greater cost in moving to a target oriented at 150° or 330°. B: outer lines indicate the probability of selecting a target that is aligned to the previous movement, as a function of the direction to that target (n = 2,159 trials). Inner lines show the probability of selecting a target that is not aligned with the previous movement, as a function of the direction to that target (n = 860 trials). Solid lines indicate means, shaded areas indicate confidence intervals (computed using the Clopper-Pearson method), and dashed circles indicate a uniform distribution.
Behavior in the Replay Task
All of the subjects who performed the discontinuous tracking task also performed the replay task. As in the discontinuous tracking task, subjects were given a predictable GO signal, and consequently their RTs were short (~200 ms), slightly shorter than in the discontinuous tracking task (Mann–Whitney U test, P = 0.0072).
To examine the effect of biomechanical costs outside of the context of a sequence of movements, we replayed (in a random order) the choice scenarios that subjects previously experienced in the discontinuous tracking task, this time in a standard design of individual and independent point-to-point reach decision trials. As shown in Fig. 7, subjects chose targets in the biomechanically easier sagittal directions significantly more often than targets in the biomechanically harder transverse directions (χ2 test, P = 0.0100). There was also a slight but significant preference for targets toward the body (χ2 test, P = 0.0032).
Fig. 7.
Data from the replay task. Probability of selecting a target is plotted as a function of the movement direction to that target (n = 4,523 trials). Solid line indicates the mean, shaded area indicates confidence intervals (computed using the Clopper-Pearson method), and dashed circle indicates a uniform distribution.
Comparison of Biomechanical Costs of Tracking, Switching, and Point-to-Point Movements
As shown above (Fig. 4), in the continuous tracking task, we found that choice preferences were strongly influenced by target distance, angle, and relative size, but not by the biomechanical cost of movements. This is in contrast to our findings in the other tasks (Fig. 6B and Fig. 7), in which choices were significantly biased toward the biomechanically easier movements. One potential reason for this could be the different torque demands of performing continuous tracking versus point-to-point movements. To examine this question, we compared the distribution of biomechanical costs (average arm muscle torque; see materials and methods) of three kinds of trials: point-to-point movements during the discontinuous tracking task, no-switch trials in the continuous tracking task, and switch trials in the continuous tracking task.
Figure 8 shows the resulting distributions. In solid black and gray lines are the histograms from switch and no-switch trials, respectively, during the continuous tracking task. In the dashed line is a histogram of the biomechanical costs from the discontinuous tracking task. To prevent biasing the distribution to the lower cost choices that subjects tended to prefer (Fig. 6B), this distribution was calculated using an equal number of 100 randomly sampled movements from each 30° bin of movement directions.
Fig. 8.
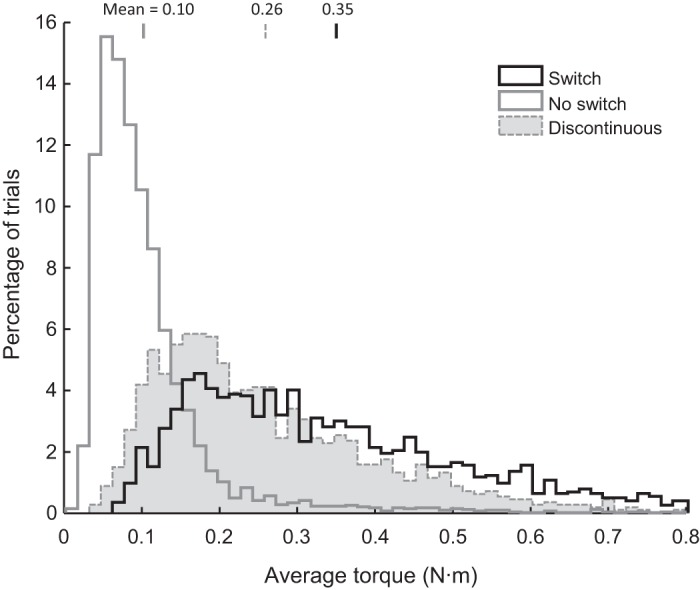
Distributions of biomechanical costs for different kinds of movements (in N·m), computed as the sum of the absolute shoulder and elbow muscle torques averaged across time (see materials and methods). Black histogram shows continuous tracking trials when the subject switched to the choice target (n = 2,144 trials). Gray histogram shows continuous tracking trials when the choice target was ignored (n = 3,827 trials). Dashed histogram represents the discontinuous tracking task (n = 6,108), with data resampled 100 times from each 30° bin of directions, to avoid biases caused by the anisotropy of subject choices. Small vertical lines above the plot indicate the means of each distribution.
As expected, the average cost of switching movements is higher than the cost of continued tracking. Furthermore, the cost of the movements made when switching targets is similar to, but slightly higher than, the cost of point-to-point movements during the discontinuous task (Mann–Whitney U test, P < 1 × 10−36). This could be partially attributed to the fact that movements made when switching tended to be faster than movements made during discontinuous tracking and partially to the necessity to slow down before changing direction. Most importantly, however, one cannot conclude that subjects ignore biomechanical costs during continuous tracking (Fig. 4) because those costs are so low as to be negligible, since a similar range of biomechanical costs does appear to influence their decisions during the discontinuous tracking task (Fig. 6B).
DISCUSSION
Neurophysiological studies conducted over the last few decades often show decision-related modulations of neural activity in many brain regions commonly associated with the execution of movements (for reviews, see Andersen and Cui 2009; Cisek and Kalaska 2010; Gold and Shadlen 2007). Even when choices are about perceptual discriminations or reward value comparisons, as long as the response actions are known, the decision process appears to engage neural activity in the regions associated with those actions, e.g., saccade regions for eye movements (Bennur and Gold 2011; Ditterich et al. 2003; Gold and Shadlen 2000, 2007; Huk and Shadlen 2005; McPeek and Keller 2002; Platt and Glimcher 1999; Roitman and Shadlen 2002), reach regions for decisions about arm movement (Andersen and Cui 2009; Christopoulos et al. 2015, 2018; Cisek and Kalaska 2005; Cui and Andersen 2007; Klaes et al. 2011; Pastor-Bernier and Cisek 2011; Pesaran et al. 2008; Scherberger and Andersen 2007; Thura and Cisek 2014; Westendorff et al. 2010), or grasp regions for decisions about grip types (Baumann et al. 2009). Behavioral studies reveal congruent results: if decisions between reach choices must be made quickly, sometimes even after movement begins, then reach trajectories often start in between the targets, as if two motor “plans” or “goals” are being mixed (Chapman et al. 2010; Gallivan et al. 2018; Gallivan et al. 2016; Wood et al. 2011; but see Haith et al. 2015).
However, in many natural situations, humans and other animals must make decisions while they are already engaged in complex activity and cannot allow those decisions to interfere with the ongoing action. For example, while running away from a fox, a rabbit can consider a variety of escape routes that may reveal themselves as the chase unfolds. However, while it deliberates about these possibilities, it must not allow the deliberation to interfere with ongoing foot placement, obstacle avoidance, etc. Models suggesting that decisions unfold within the circuits controlling action (Cisek 2007; Cisek and Kalaska 2010; Erlhagen and Schöner 2002; Gold and Shadlen 2007; Klaes et al. 2012) must confront this challenge: how can decisions unfold in the same neural system controlling an action without interfering with that action?
In this study, we sought to examine what kinds of factors bear on decisions made during ongoing manual tracking behavior. Our long-term goal is to examine whether models of action selection developed on the basis of standard decide-then-act paradigms can generalize to situations in which decisions must be made while already acting. This question is relevant both to models described at a behavioral level (Busemeyer and Townsend 1993; Cisek et al. 2009; Ratcliff 1978; Ratcliff and McKoon, 2008) as well as to models of the neural mechanisms (Amari 1977; Cisek 2006; Grossberg 1973; Mazurek et al. 2003; Wang 2002), especially ones that define commitment as the crossing of an initiation threshold or falling into an attractor. To characterize the constraints for such models, the specific goal of this study was to determine which of the factors that influence choices during standard decide-then-act tasks also influence choices during decide-while-acting tasks. In particular, we looked for the influence of target distance, target size, target direction with respect to current movement, and the relative biomechanical cost of switching versus continuing to track the target.
Although there was no explicit reason for subjects to switch to the choice target, they in fact did so quite often. This allowed us to quantify how the probability of switching varied as a function of the kinematic and kinetic factors that we manipulated. As expected, we found that subjects chose to switch to a new target more often when it was close to the current tracking target and less often when it appeared far away (Fig. 4A). Also as expected, subjects preferred to switch to targets that were larger in size than the currently tracked target, although they did sometimes switch when it was slightly smaller (Fig. 4C). These results are in agreement with previous studies on free-choice reaching tasks (Cos et al. 2011, 2012, 2014; Morel et al. 2017) as well as with a recent study showing both size and distance preferences in human subjects performing a “foraging task” involving reaching movements to targets on a plane (Diamond et al. 2017).
Somewhat more surprising was the pattern of choices as a function of the angle between the choice target and the current tracking movement (Fig. 4B): subjects tended to switch often when the angle was small, least often when it was orthogonal, and then again slightly more often when the choice was behind the tracked target, requiring a movement in a nearly opposite direction. One possible explanation for this result implicates the recruitment of muscle synergies (d’Avella and Bizzi 2005; Domkin et al. 2002; Tresch and Jarc 2009). A tracking movement requires the activation of agonist muscles that move the arm in the tracked direction as well as some engagement of antagonist muscles that stabilize the cursor within the target and ensure accurate velocity matching (Engel and Soechting 2000). Thus a synergy of muscles acting both along and against the current movement vector are already engaged and controlled during manual tracking. By contrast, muscle groups that act orthogonally to the current movement vector are not active. It is possible that switching from a currently used synergy to an orthogonally acting one incurs some additional costs that reduce the desirability of targets in the orthogonal direction. It would be interesting to explore this possibility using analyses of muscle activity, but that was beyond the scope of the current study.
The most surprising result of our study, however, was the lack of a consistent influence of the biomechanical costs of movement on the decision to switch. Although an orthogonal turn from a movement at 45° to one at 135° required nearly 50% more muscle torque than the opposite orthogonal turn (Fig. 3A), the observed choice preferences did not reflect that cost. The only case of a significant difference in choice preferences was a slight preference for the biomechanically easy target when averaged across all subjects in the angle block, primarily due to trials in which the choice target appeared at 60° (Fig. 4B). In general, however, it does not appear that subjects made choices that minimized biomechanical costs. This is surprising given prior evidence that subjects can take biomechanical costs into account when selecting different point-to-point reaching movements (Cos et al. 2011, 2012), and do so within 200 ms of target presentation and well before movement onset (Cos et al. 2014). It is also surprising given the growing theoretical motivation and empirical evidence that classical economic choices and energetic aspects of motor control may be treated by unified mechanisms aimed at maximizing a common measure of utility (Carland et al. 2019; Morel et al. 2017; Shadmehr et al. 2016; Yoon et al. 2018).
This surprising result could potentially be explained if the differences in torque requirements were simply too small to be relevant to our subjects. As shown in Fig. 8, however, the average torques of switch trials were comparable and, indeed, slightly higher than those encountered during the discontinuous task, in which clear preferences for movements with lower biomechanical cost were seen (Fig. 6B). Nevertheless, it is possible that while biomechanical costs during continuous tracking were not negligible, their influence was dwarfed by all of the other factors that together determine a subject’s behavioral success. In one of our previous studies of manual choices in a decide-then-act paradigm (Cos et al. 2012), we found that the influence of biomechanical cost was strongest when subjects had the fewest constraints on their movement trajectory. In particular, the influence of biomechanics was strongest when choosing between wide, easy-to-hit targets without the requirement to stop in the chosen one. When the size of the targets was reduced, the effect of biomechanics was smaller. When subjects were instructed to stop in the target, the effect of biomechanics was reduced still further (although it was never completely absent). In other words, as subjects faced additional constraints in the movements they had to perform, the relative influence of biomechanical costs on their choice behavior was reduced. In the continuous tracking task studied here, subjects face still more constraints: they have to keep both the position and velocity of their hand matched to the position and velocity of the tracked target (Engel and Soechting 2000; Miall et al. 1993), requiring simultaneous visual tracking (Danion and Flanagan 2018; Mather and Putchat 1983). Success in the task is defined as meeting these constraints, whereas the choice to switch is completely free and arbitrary, so the energy expended may be less important. Indeed, when the demands of manual tracking are eased, as in the discontinuous tracking and replay tasks, biomechanical influences become stronger and more consistent with minimization of effort (Figs. 6B and 7).
Another interesting observation is that the switch reaction times of our subjects performing the continuous tracking task (median 490 ms for a choice target at 90°) are in the normal range of reaction times for simple decisions in tasks where the target and GO signal are presented simultaneously (and thus do not allow preparation in advance). This suggests that subjects do not need substantial time to “disengage” from their current action so that they can plan a new one (although switching to a new target did take longer than the ~230-ms latency to adjust one’s trajectory when a tracked target unpredictably changes direction; see Engel and Soechting 2000). Nevertheless, the presentation of a potential choice during the ongoing tracking action did not appear to interfere with the performance of that action. While a slight tendency for tangential velocity to decrease after choice target presentation appeared when averaged across trials (see results), this was not consistent in individual trials.
Importantly, the choices facing our subjects were not about switching between different kinds of activity (e.g., saccade vs. reach) but were always about different movements made with the same effector, their right arm. Therefore, if the selection, planning, and control of reaching is governed by activity on a map of potential actions (Cisek 2006; Erlhagen and Schöner 2002; Klaes et al. 2012), then our task forces the activity on that map to simultaneously control an ongoing movement while representing an alternative potential action. But if that alternative potential action competes with the ongoing movement, then how can it not interfere with its execution?
Two hypotheses seem plausible. First, it is possible that the competition between actions takes place in neural circuits that are separate from those controlling the ongoing movement. For example, ongoing movement control may be governed by primary motor and somatosensory cortex, which together comprise a tightly integrated “inner” circuit straddling the central sulcus (Bullock et al. 1994; Crammond and Kalaska 2000; Johnson et al. 1996; Jones et al. 1978; Kalaska et al. 1989; Pandya and Yeterian 1985). In contrast, action selection may unfold independently in an “outer” circuit that includes the dorsal premotor cortex and medial intraparietal area (Andersen and Cui 2009; Crammond and Kalaska 2000; Johnson et al. 1996; Westendorff et al. 2010; Wise et al. 1997). Alternatively, all of these regions could govern ongoing control while target selection instead takes place in an abstract space of outcomes represented in still more rostral regions, including dorsolateral prefrontal cortex and other frontal lobe areas (Padoa-Schioppa 2011). In both cases, activity related to selection would need to be transmitted to sensorimotor circuits only at the time the new action was to be initiated. This does not explain, however, why biomechanical costs influence decision-making processes in decide-then-act situations. One potential explanation could be that when subjects make decisions while stationary, sensorimotor circuits play a role in computing the biomechanical costs of potential movements and this information is transmitted back to the circuit involved in selection (Lepora and Pezzulo 2015). As long as such sensorimotor circuit activity remained below the threshold for causing changes in muscle activity, it would not interfere with ongoing postural maintenance. In contrast, during ongoing movement, sensorimotor circuits might simply be too “busy” with the task of online control to provide information about biomechanical costs to decision-making processes.
A second hypothesis is made possible by the high dimensionality of the space spanned by the millions of neurons in all of these regions. High dimensionality implies that any given movement can be redundantly specified and identically controlled by a very wide variety of neural activity patterns that define an “output-potent” subspace. Similarly, there are many combinations of activity patterns that do not influence a given motor action, and these define an “output-null” subspace for that action (Kaufman et al. 2014). In mathematical terms, the two subspaces are orthogonal, as recently suggested for motor cortical populations controlling different arms (Ames and Churchland 2019). However, different activity patterns within a given action’s output-null subspace can lie closer or farther from the set of neural activity patterns that make up an output-potent subspace for controlling a different action. This means that the decision to switch could unfold as a shift of the neural activity pattern, always within the null subspace orthogonal to the ongoing action (or the ongoing maintenance of posture), yet moving increasingly toward the subspace of a new action being considered. Only when the neural activity pattern crosses over into the output-potent subspace of the new action does a switch in behavior occur. If the subspace in which deliberation takes place in decide-then-act tasks is quite different from that in which it takes place during an ongoing action, this might help to explain why in our study biomechanical costs did not appear to influence choices during ongoing action despite influencing choices when the initial state is stationary (Figs. 6 and 7; Cos et al. 2011). Note that the first hypothesis is a really special case of the second hypothesis; it is a particular case in which the two subspaces involve largely distinct neural populations.
Another explanation, not exclusive of the others, is that biomechanical costs of multiple potential actions can be computed by a cerebellar forward model during decide-then-act tasks (Bastian 2006; Pasalar et al. 2006), but that during decide-while-acting tasks, the circuit is too busy in controlling the ongoing action to predict the costs of a potential switching movement. This would predict that the cerebellum can represent multiple potential actions when an effector is at rest, but once a given action begins, then only that one action can be processed.
Finally, an alternative interpretation of our results is that they are less related to whether deliberation occurs when the hand is at rest versus when moving and more indicative of differences in the kinds of variables required for controlling the different tasks we have explored. As noted above, continuous tracking requires subjects to control their velocity to match that of the tracked target while maintaining the hand within a specific target location. Perhaps biomechanical costs are simply not very important in the face of such constraints. Furthermore, tracking movements may engage a different subset of cells from those primarily involved in point-to-point movements, such as switching to a new target. Perhaps cells involved in tracking are less sensitive to variables closely related to biomechanical costs (e.g., acceleration) than cells involved in point-to-point movements.
Answering such questions motivates future studies involving neural recordings in animals trained to perform tasks such as the continuous tracking task. Although to our knowledge this type of paradigm has not yet been attempted in monkeys, potential insights may be found in data from studies in other species and other conditions. For example, studies in cats have examined situations in which the animal must choose, during ongoing locomotion, which forelimb to use to step over an obstacle (Drew and Marigold 2015). These studies suggest that cells in parietal cortex estimate the animal’s position with respect to the obstacle, while cells in motor cortex primarily contribute to the execution of the stepping movements and their modifications. In contrast, cells in premotor cortex appear related both to the execution of the gait modification as well as to the selection of the limb that will be used to step over it (Nakajima et al. 2019). Importantly, many premotor cells exhibit a gradual increase of discharge rate several steps before the gait modification. This is seen both in cells that are limb independent and in cells specific to a given forelimb, but no changes in electromyographic (EMG) activity are observed until the final moment of gait modification. It therefore appears that at least in the cat locomotion system, it is possible for cells putatively involved in execution to also exhibit decision-related activity, even during ongoing actions. By analogy, for the primate reaching system, one might therefore predict that during continuous tracking, cells in dorsal premotor cortex tuned to the direction of the choice target will begin to increase their activity as the subject is deliberating, and then either increase even more during switch trials or fall back to baseline in no-switch trials. Ultimately, neurophysiological studies of decide-while-acting paradigms will be required to shed light on these interesting questions.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council Discovery Grants RGPIN/05245 (to P. Cisek) and RGPIN/05408 (to A. Green).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M., A.M.G., and P.C. conceived and designed research; J.M. performed experiments; J.M. and P.C. analyzed data; J.M., A.M.G., and P.C. interpreted results of experiments; J.M. and P.C. prepared figures; J.M. and P.C. drafted manuscript; J.M., A.M.G., and P.C. edited and revised manuscript; J.M., A.M.G., and P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christophe Martin for assistance in the implementation of the biomechanical modeling.
REFERENCES
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern 27: 77–87, 1977. doi: 10.1007/BF00337259. [DOI] [PubMed] [Google Scholar]
- Ames KC, Churchland MM. Motor cortex signals for each arm are mixed across hemispheres and neurons yet partitioned within the population response. eLife 8: e46159, 2019. doi: 10.7554/eLife.46159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron 63: 568–583, 2009. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16: 645–649, 2006. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Baumann MA, Fluet MC, Scherberger H. Context-specific grasp movement representation in the macaque anterior intraparietal area. J Neurosci 29: 6436–6448, 2009. doi: 10.1523/JNEUROSCI.5479-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci 31: 913–921, 2011. doi: 10.1523/JNEUROSCI.4417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev 113: 700–765, 2006. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Bullock D, Cisek P, Grossberg S. A neural model of voluntary movement and proprioception (Abstract). Soc Neurosci Abstr 20: 1405, 1994. [Google Scholar]
- Busemeyer JR, Townsend JT. Decision field theory: a dynamic-cognitive approach to decision making in an uncertain environment. Psychol Rev 100: 432–459, 1993. doi: 10.1037/0033-295X.100.3.432. [DOI] [PubMed] [Google Scholar]
- Carland MA, Marcos E, Thura D, Cisek P. Evidence against perfect integration of sensory information during perceptual decision making. J Neurophysiol 115: 915–930, 2016. doi: 10.1152/jn.00264.2015. [DOI] [PubMed] [Google Scholar]
- Carland MA, Thura D, Cisek P. The urge to decide and act: implications for brain function and dysfunction. Neuroscientist 25: 491–511, 2019. doi: 10.1177/1073858419841553. [DOI] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wood DK, Milne JL, Culham JC, Goodale MA. Reaching for the unknown: multiple target encoding and real-time decision-making in a rapid reach task. Cognition 116: 168–176, 2010. doi: 10.1016/j.cognition.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Christopoulos VN, Bonaiuto J, Kagan I, Andersen RA. Inactivation of parietal reach region affects reaching but not saccade choices in internally guided decisions. J Neurosci 35: 11719–11728, 2015. doi: 10.1523/JNEUROSCI.1068-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos VN, Kagan I, Andersen RA. Lateral intraparietal area (LIP) is largely effector-specific in free-choice decisions. Sci Rep 8: 8611, 2018. doi: 10.1038/s41598-018-26366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–9770, 2006. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362: 1585–1599, 2007. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45: 801–814, 2005. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cisek P, Pastor-Bernier A. On the challenges and mechanisms of embodied decision-making. Philos Trans R Soc Lond B Biol Sci 369: 20130479, 2014. doi: 10.1098/rstb.2013.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Puskas GA, El-Murr S. Decisions in changing conditions: the urgency-gating model. J Neurosci 29: 11560–11571, 2009. doi: 10.1523/JNEUROSCI.1844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos I, Bélanger N, Cisek P. The influence of predicted arm biomechanics on decision making. J Neurophysiol 105: 3022–3033, 2011. doi: 10.1152/jn.00975.2010. [DOI] [PubMed] [Google Scholar]
- Cos I, Duque J, Cisek P. Rapid prediction of biomechanical costs during action decisions. J Neurophysiol 112: 1256–1266, 2014. doi: 10.1152/jn.00147.2014. [DOI] [PubMed] [Google Scholar]
- Cos I, Medleg F, Cisek P. The modulatory influence of end-point controllability on decisions between actions. J Neurophysiol 108: 1764–1780, 2012. doi: 10.1152/jn.00081.2012. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84: 986–1005, 2000. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron 56: 552–559, 2007. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci USA 102: 3076–3081, 2005. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion FR, Flanagan JR. Different gaze strategies during eye versus hand tracking of a moving target. Sci Rep 8: 10059, 2018. doi: 10.1038/s41598-018-28434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Wolpert DM, Flanagan JR. Rapid target foraging with reach or gaze: The hand looks further ahead than the eye. PLoS Comput Biol 13: e1005504, 2017. doi: 10.1371/journal.pcbi.1005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditterich J, Mazurek ME, Shadlen MN. Microstimulation of visual cortex affects the speed of perceptual decisions. Nat Neurosci 6: 891–898, 2003. doi: 10.1038/nn1094. [DOI] [PubMed] [Google Scholar]
- Domkin D, Laczko J, Jaric S, Johansson H, Latash ML. Structure of joint variability in bimanual pointing tasks. Exp Brain Res 143: 11–23, 2002. doi: 10.1007/s00221-001-0944-1. [DOI] [PubMed] [Google Scholar]
- Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol 33: 25–33, 2015. doi: 10.1016/j.conb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Engel AK, Maye A, Kurthen M, König P. Where’s the action? The pragmatic turn in cognitive science. Trends Cogn Sci 17: 202–209, 2013. doi: 10.1016/j.tics.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Engel KC, Soechting JF. Manual tracking in two dimensions. J Neurophysiol 83: 3483–3496, 2000. doi: 10.1152/jn.2000.83.6.3483. [DOI] [PubMed] [Google Scholar]
- Erlhagen W, Schöner G. Dynamic field theory of movement preparation. Psychol Rev 109: 545–572, 2002. doi: 10.1037/0033-295X.109.3.545. [DOI] [PubMed] [Google Scholar]
- Farmer TA, Cargill SA, Spivey MJ. Gradiency and visual context in syntactic garden-paths. J Mem Lang 57: 570–595, 2007. doi: 10.1016/j.jml.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, Wolpert DM, Flanagan JR. Decision-making in sensorimotor control. Nat Rev Neurosci 19: 519–534, 2018. doi: 10.1038/s41583-018-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, Wood DK, Milne JL, Ansari D, Culham JC, Goodale MA. One to four, and nothing more: nonconscious parallel individuation of objects during action planning. Psychol Sci 22: 803–811, 2011. doi: 10.1177/0956797611408733. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Logan L, Wolpert DM, Flanagan JR. Parallel specification of competing sensorimotor control policies for alternative action options. Nat Neurosci 19: 320–326, 2016. doi: 10.1038/nn.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. The Ecological Approach to Visual Perception. Boston, MA: Houghton Mifflin, 1979. [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature 404: 390–394, 2000. doi: 10.1038/35006062. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Contour enhancement, short term memory, and constancies in reverberating neural networks. Stud Appl Math 52: 213–257, 1973. doi: 10.1002/sapm1973523213. [DOI] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. Hedging your bets: intermediate movements as optimal behavior in the context of an incomplete decision. PLoS Comput Biol 11: e1004171, 2015. doi: 10.1371/journal.pcbi.1004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Huk AC, Shadlen MN. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J Neurosci 25: 10420–10436, 2005. doi: 10.1523/JNEUROSCI.4684-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181: 291–347, 1978. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DAD, Hyde ML, Prud’homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. doi: 10.1523/JNEUROSCI.09-06-02080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex 5: 410–428, 1995. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. Cortical activity in the null space: permitting preparation without movement. Nat Neurosci 17: 440–448, 2014. doi: 10.1038/nn.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes C, Schneegans S, Schöner G, Gail A. Sensorimotor learning biases choice behavior: a learning neural field model for decision making. PLoS Comput Biol 8: e1002774, 2012. doi: 10.1371/journal.pcbi.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes C, Westendorff S, Chakrabarti S, Gail A. Choosing goals, not rules: deciding among rule-based action plans. Neuron 70: 536–548, 2011. doi: 10.1016/j.neuron.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Laming D. Information Theory of Choice Reaction Time. New York: Wiley, 1968. [Google Scholar]
- Lepora NF, Pezzulo G. Embodied choice: how action influences perceptual decision making. PLoS Comput Biol 11: e1004110, 2015. doi: 10.1371/journal.pcbi.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol 22: 1027–1038, 2012. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather JA, Putchat C. Parallel ocular and manual tracking responses to a continuously moving visual target. J Mot Behav 15: 29–38, 1983. doi: 10.1080/00222895.1983.10735287. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex 13: 1257–1269, 2003. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- McKinstry C, Dale R, Spivey MJ. Action dynamics reveal parallel competition in decision making. Psychol Sci 19: 22–24, 2008. doi: 10.1111/j.1467-9280.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Superior colliculus activity related to concurrent processing of saccade goals in a visual search task. J Neurophysiol 87: 1805–1815, 2002. doi: 10.1152/jn.00501.2001. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav 25: 53–63, 1993. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Michalski J, Cisek P. Deciding while acting—an investigation of decision-making during ongoing action control. Program No. 405.17 2017 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2017. [Google Scholar]
- Michalski J, Green AM, Cisek P. An investigation of reach decision preferences during ongoing action control. Program No. 401.03 2018 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2018. [Google Scholar]
- Morel P, Ulbrich P, Gail A. What makes a reach movement effortful? Physical effort discounting supports common minimization principles in decision making and motor control. PLoS Biol 15: e2001323, 2017. doi: 10.1371/journal.pbio.2001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Fortier-Lebel N, Drew T. Premotor cortex provides a substrate for the temporal transformation of information during the planning of gait modifications. Cereb Cortex 29: 4982–5008, 2019. doi: 10.1093/cercor/bhz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci 34: 1769–1780; Rapid online, 2014. doi: 10.1523/JNEUROSCI.3063-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova GS, Toshev YE. Estimation of male and female body segment parameters of the Bulgarian population using a 16-segmental mathematical model. J Biomech 40: 3700–3707, 2007. doi: 10.1016/j.jbiomech.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annu Rev Neurosci 34: 333–359, 2011. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Cerebral Cortex, edited by Peters A, Jones EG. New York: Plenum, 1985, vol. 4, p. 3–61. [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9: 1404–1411, 2006. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- Pastor-Bernier A, Cisek P. Neural correlates of biased competition in premotor cortex. J Neurosci 31: 7083–7088, 2011. doi: 10.1523/JNEUROSCI.5681-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: 406–409, 2008. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Castelfranchi C. Thinking as the control of imagination: a conceptual framework for goal-directed systems. Psychol Res 73: 559–577, 2009. doi: 10.1007/s00426-009-0237-z. [DOI] [PubMed] [Google Scholar]
- Pezzulo G, Cisek P. Navigating the affordance landscape: feedback control as a process model of behavior and cognition. Trends Cogn Sci 20: 414–424, 2016. doi: 10.1016/j.tics.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature 400: 233–238, 1999. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9: 545–556, 2008. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychol Rev 85: 59–108, 1978. doi: 10.1037/0033-295X.85.2.59. [DOI] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20: 873–922, 2008. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci 27: 2001–2012, 2007. doi: 10.1523/JNEUROSCI.4274-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol 26: 1929–1934, 2016. doi: 10.1016/j.cub.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Target selection in visual search as revealed by movement trajectories. Vision Res 48: 853–861, 2008. doi: 10.1016/j.visres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Hidden cognitive states revealed in choice reaching tasks. Trends Cogn Sci 13: 360–366, 2009. doi: 10.1016/j.tics.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Stone M. Models for choice reaction time. Psychometrika 25: 251–260, 1960. doi: 10.1007/BF02289729. [DOI] [Google Scholar]
- Thura D, Beauregard-Racine J, Fradet CW, Cisek P. Decision making by urgency gating: theory and experimental support. J Neurophysiol 108: 2912–2930, 2012. doi: 10.1152/jn.01071.2011. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81: 1401–1416, 2014. doi: 10.1016/j.neuron.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 19: 601–607, 2009. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher M, McClelland JL. The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev 108: 550–592, 2001. doi: 10.1037/0033-295X.108.3.550. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36: 955–968, 2002. doi: 10.1016/S0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- Westendorff S, Klaes C, Gail A. The cortical timeline for deciding on reach motor goals. J Neurosci 30: 5426–5436, 2010. doi: 10.1523/JNEUROSCI.4628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Wood DK, Gallivan JP, Chapman CS, Milne JL, Culham JC, Goodale MA. Visual salience dominates early visuomotor competition in reaching behavior. J Vis 11: 16, 2011. doi: 10.1167/11.10.16. [DOI] [PubMed] [Google Scholar]
- Yoon T, Geary RB, Ahmed AA, Shadmehr R. Control of movement vigor and decision making during foraging. Proc Natl Acad Sci USA 115: E10476–E10485, 2018. [Erratum in Proc Natl Acad Sci USA 115: E11884, 2018.] doi: 10.1073/pnas.1812979115. [DOI] [PMC free article] [PubMed] [Google Scholar]