Abstract
Medial and lateral entorhinal cortices convey spatial/contextual and item/object information to the hippocampus, respectively. Whether the distinct inputs are integrated as one cognitive map by hippocampal neurons to represent location and the objects therein, or whether they remain as parallel outputs, to be integrated in a downstream region, remains unclear. Principal, or complex spike bursting, neurons of hippocampus exhibit location-specific firing, and it is likely that the activity of “place cells” supports spatial memory/navigation in rodents. Consistent with cognitive map theory, the activity of CA1 hippocampal neurons is also critical for nonspatial memory, such as object recognition. However, the degree to which CA1 neuronal activity represents the associations of object-context or object-in-place memory is not well understood. Here, the contributions of mouse CA1 neuronal activity to object recognition memory and the emergence of object-place conjunctive representations were tested using in vivo recordings and functional inactivation. Independent of arena configuration, CA1 place fields were stable throughout testing and object-place representations were not identified in CA1, although the number of fields per cell increased during object sessions, and few object-related firing CA1 neurons (nonplace) were recorded. The results of the inactivation studies confirmed the significant contribution of CA1 neuronal activity to object recognition memory when a delay of 20 min, but not 5 min, was imposed between encoding and retrieval. Together, our results confirm the delay-dependent contribution of the CA1 region to object memory and suggest that object information is processed in parallel with the ongoing spatial mapping function that is a hallmark of hippocampal memory.
NEW & NOTEWORTHY We developed variations of the object recognition task to examine the contribution of mouse CA1 neuronal activity to object memory and the degree to which object-context conjunctive representations are formed during object training. Our results indicate that, within the CA1 region, object information is processed in a parallel but delay-dependent manner, with ongoing spatial mapping.
Keywords: CA1, contextual information, memory, muscimol, object recognition
INTRODUCTION
A fundamental feature of the mammalian hippocampal system is its ability to rapidly store and retrieve memories of events or experiences, such as associating a spatial location, the items encountered there, and the time when the event occurred (Rolls 2007, 2018; Tse et al. 2007). Such one-trial associations are likely critical for episodic memory and temporal order memory. This view of the hippocampal role in memory is also congruent with the theory that the hippocampus serves as a cognitive map: that is, a cognitive map representing locations where relevant items or objects were encountered and where specific events occurred within a contextual or spatial reference frame (O’Keefe and Nadel 1978). The hippocampus receives two parallel but interrelated streams of input: spatial information from the medial entorhinal cortex and information about external cues, items, and objects from the lateral entorhinal cortex (Knierim et al. 2014; Lisman 2007; van Strien et al. 2009). Potentially, associations between object and place representations (i.e., object-in-context or object-in-place) could be formed within the hippocampus proper during or after specific experiences, and then those conjunctive representations should be reflected in the pattern of activity conveyed back to neocortex from the CA1 region. Individual CA1 and CA3 neurons of hippocampus represent place by firing at distinct rates when a rodent occupies different spatial locations (O’Keefe and Dostrovsky 1971; Wilson and McNaughton 1993). The firing patterns of CA1 neurons have also been shown to represent configural associations of odor-in-location (Komorowski et al. 2009) and object-in-location (Burke et al. 2011; Deshmukh and Knierim 2013; Manns and Eichenbaum 2009). It should be noted that object- and object location-related firing has been recorded from both medial and lateral entorhinal cortical neurons (Høydal et al. 2019; Tsao et al. 2013), complicating the notion of convergence of place and object information in hippocampus. Additional studies are needed to define how such object coding in the hippocampus and entorhinal cortices relates to object recognition memory.
The hippocampus and its associated regions are essential for spatial and nonspatial aspects of episodic memory in mammals (Clark et al. 2000; Eichenbaum et al. 2007; Morris et al. 1982; Pena et al. 2014; Riedel et al. 1999; Squire et al. 2004). The rodent hippocampus, and the CA1 region in particular, contributes to object recognition (OR) memory, under specific testing conditions (Broadbent et al. 2010; Clark et al. 2000; Cohen et al. 2013; de Lima et al. 2006; Hammond et al. 2004; Stackman et al. 2016; Tuscher et al. 2018; for a review see Cohen and Stackman 2015), but not under other testing conditions (Mumby et al. 2002, 2005; Winters et al. 2004). Selective posttraining blockade of protein synthesis in the CA1 region impairs consolidation of OR memory (Cohen et al. 2013; Fu et al. 2007; Furini et al. 2015; Rossato et al. 2007), and the recognition of an object induces significant changes in protein expression in the CA1 region in mice (von Ziegler et al. 2018). Moreover, exploration of novel objects, as compared with familiar objects, increases the firing rates of CA1 pyramidal neurons and increases glutamate efflux from dorsal hippocampus (Cohen et al. 2013). These task-induced changes in hippocampal physiology may be directly related to the encoding of object-place conjunctive representations that support event memories of the “object-explored-within-a-familiar-context.”
In the standard version of the OR task, a rodent explores two identical novel objects in a familiar arena during a sample session. The rodent is then returned to the same arena at a later time for the test session, in which a novel object has replaced one of the original objects. Temporary inactivation of CA1 neuronal activity before or after the sample session, or just before the test session, abolishes object memory encoding and/or consolidation and retrieval of object memory in mice (Cohen et al. 2013). It remains unclear whether hippocampal-dependent object memory is guided by object-context conjunctive representations formed by modifications of CA1 place cell activity as the object memory is encoded or consolidated. We previously reported that CA1 place cell activity established before object memory training was not altered by subsequent OR performance (Cohen et al. 2013), suggesting that object and context information may be dissociable, or that these processes involve functionally distinct hippocampal output streams. It has also been argued that object memory impairments observed following CA1 inactivation result from disturbing recognition of the spatial or contextual attributes of the behavioral task; a view consistent with a hippocampal-dependent object-in-context memory (Bussey et al. 2000; Good et al. 2007; Mumby et al. 2002; Save et al. 1992; Winters et al. 2004). If so, then one might expect that alterations of the context in which the OR task is performed may change the sensitivity of CA1 place cells to OR task-induced remapping or change the sensitivity of OR task performance to CA1 inactivation.
In the present study, we used modifications of the standard OR task to examine whether object-place conjunctive representations emerged within CA1 neuronal activity over a time course consistent with behavioral performance. When a rodent is placed into the arena for the sample session of the OR task, presumably it devotes some time to exploring a bit of the arena floor and walls to determine that the arena is familiar. We propose that if the process of recognizing the arena as a familiar context was made easier, such as by including a prominent polarizing cue inside the arena (Kentros et al. 2004), then this might improve the chance that the novel objects encountered during the sample session would become associated with that familiar context. In turn, this might promote the emergence of object-place or object-context conjunctive representations within CA1 neuronal activity. The current experiments examined OR task performance of mice in a high-walled arena under three different cue conditions to test the contribution of CA1 neuronal activity and, in parallel, the stability of CA1 place cell firing fields before, during, and after OR sample and test sessions. The different arena cue conditions were designed to increase the recognition of the context as being familiar. The results revealed that postsample inactivation of CA1 neuronal activity impaired object discrimination when mice were tested 24 h or 20 min, but not 5 min, after sample, supporting a delay-dependent involvement of CA1 in object memory (Baker and Kim 2002; Clark et al. 2000; Cohen et al. 2013; Hammond et al. 2004; Stackman et al. 2016). In vivo recordings revealed that CA1 place cells did not remap during the exploration of objects introduced during the OR sample or test sessions (denoted as test). Stability of place fields was positively correlated with OR performance when a 20-min, but not a 5-min, delay was imposed, suggesting a deferred recruitment of CA1 neurons to object memory, which is consistent with the delay-dependent deficit in object memory after CA1 inactivation. Lastly, we report evidence of object-related firing of nonbursting CA1 neurons in mice during object exploration, similar to a recent report in rats (Deshmukh and Knierim 2013). Taken together, our functional inactivation and neuronal recording findings provide compelling evidence that the CA1-dependent object memory formed during the OR task is not supported by the establishment of “object-in-context” representations by CA1 neurons. The data are consistent with the view that, within the relatively short timeframe of the OR task sessions, object and spatial/contextual information are processed in parallel within CA1 and are likely integrated downstream of the CA1.
MATERIALS AND METHODS
Mice and Surgery
Male C57BL/6J mice (7–10 wk old; Jackson Laboratories) were used as subjects in these experiments. Our initial rationale for restricting our subject sample to male mice reflected concern regarding estrous cyclicity influences on hippocampal behavior and neurophysiology in female mice. However, Bettis and Jacobs (2012) reported a lack of sex difference in object recognition among adult C57BL/6J mice when test objects are sufficiently different, and results from recent studies conducted in our laboratory indicate the lack of sex difference in object discrimination after weak or strong object memory training (B. Hindman and R.W. Stackman, unpublished results). These findings suggest that the results we report below from analyses of male C57BL/6J mice would also reflect performance and neurophysiology of female C57BL/6J mice.
Mice were housed one or four per cage with ad libitum access to food and water. All procedures were conducted in accordance with NIH guidelines and were approved by the Florida Atlantic University’s Institutional Animal Care and Use Committee before the initiation of experiments. Surgical implantation of guide cannulae (n = 75) or tetrodes (n = 20) was completed when all mice were 8–11 wk old, at least 1 wk after acclimatization to the vivarium. For the inactivation experiments, mice were randomly assigned to experimental group and investigators were blind to treatment during behavior sessions and data analyses.
Intrahippocampal Cannulation and Microinfusion
Mice were implanted with chronic bilateral guide cannulae (Plastics One, Inc., Roanoke, VA) above the CA1 region of dorsal hippocampus (A/P −2.0 mm, M/L ±1.5 mm, D/V −1.1 mm from bregma; corresponding to intermediate CA1). The guide cannulae were mounted to the skull using skull screws (000-120, Antrim) and cold-curing dental acrylic (ColdPac, Chicago, IL). Each mouse was administered buprenorphine (0.5 mg/kg ip) after surgery and triple antibiotic ointment was applied to the wound. Behavioral testing began 7–10 days later to permit postoperative recovery. Each mouse received a “mock infusion” each day for the 2 days before the actual intrahippocampal microinfusion, 20 min before or immediately after the arena habituation sessions, to acclimate the mice to the microinfusion procedure. The mock infusion procedure involved a brief restraint of the mouse, during which the protective cap and dummy internal cannula were removed and dummy infusion cannulae were inserted into each guide cannula. These dummy infusion cannulae did not project beyond the tip of the implanted guide cannulae. Once the infusion cannulae were inserted, the mouse was released into an empty polycarbonate mouse cage for the 3-min duration of the “infusion.” Each mouse was again briefly restrained to remove the infusion cannulae and replace the dummy internal cannulae and protective cap and was then returned to the home cage. For the actual microinfusions, mice received bilateral (0.35 µl/side, 0.334 µl/min) intrahippocampal muscimol (1 µg/µl in 0.9% saline, Tocris) or saline immediately after the sample session. However, for the Conventional OR protocol when a 5-min delay was imposed between the sample and test, intra-CA1 saline or muscimol was infused 20 min before the sample since the intersession delay was too short to ensure CA1 neuronal silencing during object memory processing. The procedures for the actual bilateral microinfusion followed that described above for the mock infusion; however, this time the inserted infusion cannulae penetrated 1 mm beyond the tip of the guide cannulae to achieve bilateral intrahippocampal infusion.
Tetrode and Recording Microdrive Construction and Implantation
Recording drives contained four tetrodes mounted to a microdrive device. Tetrodes were constructed from 25-µm diameter Nichrome wires (California Fine Wire, Grover Beach, CA) twisted together. For one mouse, the design of the tetrode array was modified to accommodate a microinfusion guide cannula (constructed from 18 G metal tubing). Each tetrode array was implanted directly above the intermediate CA1 region of the right dorsal hippocampus, A/P −2.0 mm, M/L +1.5 mm, D/V −1.1 mm from bregma for all mice of the place cell recording studies, and for one mouse in which object-specific cell recordings were conducted. Tetrodes were implanted at A/P −2.0 mm, M/L +1.2, or 1.15 mm, D/V −1.1 mm from bregma in the other three mice from which recordings were made of the three cells that demonstrated object-specific firing. The tetrode array was anchored to the skull using miniature stainless-steel screws (000-120, Antrim) and cold-curing dental acrylic (ColdPac, Chicago, IL). Each mouse was administered buprenorphine (0.5 mg/kg ip) after surgery, and triple antibiotic ointment was applied to the wound. Mice were individually housed following surgery to protect the microelectrode. Beginning 7 days after surgery, mice were habituated to the recording arena and tetrodes slowly lowered (~5–20 µm/day) until CA1 neuronal activity was detected. To confirm the time course of the neuronal silencing effect of locally infused muscimol on CA1 neurons, mice remained in their home cages throughout the experiment. Baseline activity was recorded in 5-min intervals before, during, and for 5.5 h after the drug infusion.
Novel Object Recognition Task and Protocols
The apparatus consisted of one or two open-top, high-walled square arenas made of white acrylonitrile-butadine-styrene (ABS, each: 37.5 × 37.5 × 50 cm). For all inactivation and in vivo recording experiments, each mouse was habituated to one of the arenas for two 10-min sessions. Then, each mouse received one sample session and one test session in the habituated (i.e., familiar) arena. During the sample, each mouse was returned to the familiar arena that now contained two identical novel objects (stainless steel cabinet leveling feet, each attached to a Plexiglas base, 4.2 cm diameter and 6.0 cm tall). The two objects were positioned on the arena floor 2 cm from opposite corners (NW and SE). For the inactivation studies, each mouse was removed from the arena upon accumulating 30 s of exploration of each object, or 38 s of either object — a criterion determined by previous studies to allow for strong novel object preference 24 h following the sample session (Cohen et al. 2013; Cohen and Stackman 2015). This sample object exploration criterion was imposed to ensure that all mice were matched for sample session performance. The data from two mice that failed to reach the sample session exploration criteria were removed from the analyses. For the in vivo CA1 recording studies, each mouse was permitted a total of 10-min exploration during the sample sessions; however, four of the mice did not reach the sample object exploration criterion and their data were removed from further analyses. During the test session presented 5 min, 20 min, or 24 h later (inactivation studies only), the familiar arena contained one of the familiar objects and one novel object (plastic toy gorilla or metal spring attached to a Plexiglas base). For the inactivation studies, the test session duration lasted 5 min. A 10-min test session was implemented for the recording studies to ensure that neuronal activity represented adequate sampling of the arena floor (see Fig. 1B for protocol schematics). The objects and the arena floor and walls were cleaned with 10% ethanol after each session to limit odor cues. For the inactivation studies, all behavioral testing data was digitally acquired by the EthoVision XT (Noldus Inc., Leesburg, VA) software package. For all studies, object exploration was scored off-line from the digital video files by experimenters that were blind to the treatment condition of the mice. Object memory was inferred from the discrimination ratio, calculated for each mouse by subtracting the time spent exploring the familiar object from the time spent exploring the novel object and dividing the result by the total time spent exploring both objects. Discrimination ratio scores range from −1 to 1, with positive scores indicating novel object preference, while a ratio = 0 indicating chance performance or a lack of preference for one object over another.
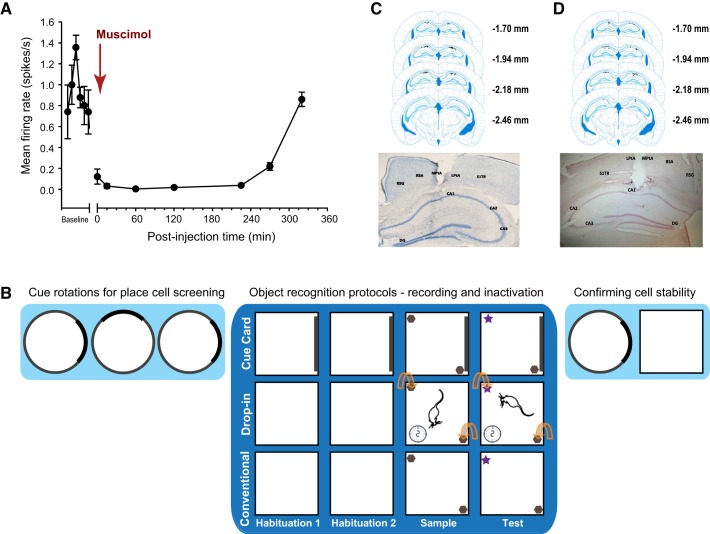
Fig. 1.
A: mean ± SE normalized firing frequency of CA1 hippocampal neurons before, during and at multiple time points after a local infusion of muscimol. Time 0 = the start of the infusion of muscimol. B: schematic depicting the in vivo recording and functional inactivation protocols. The darker blue background highlights the specific sessions for the three different object recognition (OR) protocols. Top: Cue Card OR, white square arena with dark cue card on one wall throughout all sessions. Middle: Drop-In OR, white square arena (no polarizing cue) where the mouse explored the empty arena for 2 min, after which the objects were placed into the arena in the positions indicated by the curved arrows. Bottom: conventional OR, same white square arena as in Drop-In OR, except that objects were present from the beginning upon mice entering the arena for the object sessions. Mice who received bilateral intrahippocampal infusion of saline or muscimol (i.e., inactivation studies) completed only the sessions in the darker blue section of the figure, while mice implanted with microdrive/tetrode arrays (i.e., in vivo recording studies) completed the lighter blue cue card rotations before, and immediately after, behavioral testing in the OR protocols (while place cells were recorded), to confirm cell stability and unit isolation. C, top: representative placement of the unilateral tetrode arrays implanted over the CA1 region of the right dorsal hippocampus for all mice (black dots). Bottom: representative photomicrograph of tetrode tip location in the dorsal hippocampus. D, top: representative bilateral intrahippocampal infusion sites within the CA1 region of the dorsal hippocampus for the Cue Card OR experiment (black dots). These sites are representative of the infusion locations for all of the inactivation experiments. Bottom: characteristic photomicrograph of the intrahippocampal microinfusion site into the CA1. Abbreviations in photomicrographs in C and D: CA1, CA2, and CA3, respective pyramidal cell fields of the hippocampus; DG, dentate gyrus; LPtA, lateral parietal association cortex; MPtA, medial parietal association cortex; RSA, retrosplenial agranular cortex; RSG, retrosplenial granular cortex; S1TR, primary somatosensory cortex trunk region.
Recording Apparatus and Protocol
During daily screening or recording sessions, each mouse was briefly restrained to mate the implanted microdrive/tetrodes assembly to the recording cable headstage containing 16 unit gain operational amplifiers. Unit activity was then monitored while mice moved freely about the floor of the high-walled cylinder or square arenas. The positions of two light-emitting diodes (one red and one green) on the headstage and mouse behavior were acquired by CinePlex video tracking system (Plexon Inc., Dallas, TX). Neuronal activity (amplified 10,000×, filtered at 150–8,000 Hz and digitized at 40 kHz) was simultaneously recorded with a 16-channel MAP system (Plexon Inc., Dallas, TX). Spikes were discriminated and analyzed by manual and automatic sorting algorithms based on waveform characteristics using Offline Sorter software (Plexon v3.2.1). The putative CA1 pyramidal neurons were classified using the following criteria: 1) low baseline firing rate (< 15 Hz) and irregular firing pattern; 2) dominant short interspike interval (3–10 ms) by histograms showing a characteristic peak at 3–5 ms followed by a rapid exponential decay; and 3) a waveform latency of >300 µs. The putative interneurons were classified as having relatively narrow waveforms (<250 µs) and high firing rates (>5 Hz), and the interspike interval histograms exhibited a later peak and a much slower decay compared with putative pyramidal neurons. All interneurons were excluded from further analysis. Only units with clear boundaries and less than 0.5% of spike intervals within a 1-ms refractory period were included in the analyses.
Prior to OR testing of tetrode-implanted mice, the responses of putative place cells were recorded during a sequence of three 10-min cylinder sessions in which the cue card was positioned at the standard 3 o’clock position, again after rotation of the cue card 90° counterclockwise to the 12 o’clock position, and for a third time after the cue card was rotated back to its standard position (see Fig. 1B). These sessions were conducted to verify that the cue card exerted the expected stimulus control over the place cell’s place field (Muller and Kubie 1987). The rationale being that if the place cells responded as expected to the local cue card, then we would be more certain that responses to the subsequent novel objects would not be spurious or reflecting a lack of attention to the cues available (Kentros et al. 2004; Muzzio et al. 2009a, 2009b), but rather legitimately reflecting the responses of place cells during the encoding of an object-in-place memory. If the expected pattern of results from the cue card rotation sessions was achieved, then the place cells were recorded during OR testing, which included two 10-min square arena habituation sessions, a sample session, and a test session. Following OR testing, two additional sessions were run to confirm cell and electrode stability. Only CA1 neurons that exhibited complex spike activity had place fields that rotated positions accordingly as expected during the cue card rotations and achieved our criterion for place field stability (r > 0.6 for the pixel-by-pixel cross-correlation comparison of the place × firing rate maps for arena Habituations 1 and 2 during testing) were included in all subsequent analyses. These inclusion criteria were imposed as such to establish that the CA1 neurons were stable and appropriately responsive to distal cues before assessing the influence of novel objects on the firing properties of these neurons and on quantitative measures of their spatial firing characteristics. Although these criteria could be viewed as being very strict, we adhered to this approach in light of prior reports that CA1 place cells in mice are considerably less stable under context conditions that are relatively cue poor (Kentros et al. 2004; Muzzio et al. 2009a, 2009b). Therefore, we felt it was important to first establish the stability of the CA1 place cells in our mice before determining whether those cells exhibited rate remapping or spatial remapping during or after the interaction of the mice with novel objects.
Recording Object-Specific Activity
Hippocampal activity was recorded throughout multiple sessions in various arenas before and after the introduction of objects. All recording sessions were 5–10 min long and contained either no objects, one object, or two objects of similar size as those used during the OR task. Multiple different arena configurations and objects were used during these CA1 recording sessions (an opaque cylindrical arena was used throughout; yet, in some sessions an inverted black ice bucket was positioned in the middle of the arena floor to create a circular track, and various intramaze cues were used to alter the arena geometry). Once activity of a single neuron was clearly observed to be associated with the location of an object, subsequent analyses were conducted to determine whether that same cell’s object-related activity had been present during previous recording sessions.
Data Analyses
For simultaneous muscimol infusion while recording CA1 neuronal activity, firing rates for all cells were normalized before statistical analysis. For the inactivation studies, the discrimination ratio and latency (in seconds) to reach the sample object exploration criterion scores of the vehicle- and muscimol-treated mice were compared using two-tailed Student’s t tests. Significant findings were further evaluated by calculating Cohen’s d values to assess effect sizes and sample power. Sorted CA1 pyramidal neuron spikes were combined with the position coordinates provided by Plexon CinePlex Editor and place × firing rate maps were constructed using NeuroExplorer (version 3.266, Nex Technologies, Littleton, MA). To relate the head position of the mouse to spatial location, color-coded firing rate maps were plotted. The arena space was discretized into a matrix of 32 × 32 pixels and spikes associated with each location determined. Pixels with occupancy less that 100 ms were considered unvisited and not counted. Pixels associated with unvisited locations were assigned white color for the purposes of the place × firing rate maps. The place × firing rate maps were exported to MATLAB R2011b (version 7.13.0.564), and smoothed using an adaptive binning technique (Skaggs et al. 1996). Stability of place × firing rate maps was determined by computing a pixel-by-pixel Pearson cross-correlation analysis between the place × firing rate maps of two recording sessions. Measures of spatial coherence (Muller and Kubie 1989) and spatial information content (Skaggs et al. 1993) were also computed for each place cell using custom-written MATLAB scripts. Spatial coherence was measured by calculating the Z transform of the correlation between the firing rates in each pixel and the average firing rates of the eight nearest-neighbor pixels. Spatial information content was calculated by subdividing the training arena into a 32 × 32 pixelated grid in the same manner as described for the generation of place × firing rate maps. The amount of information that each bin generated by the grid conveys about the location of the animal was calculated using the formula: ∑ Pi (Ri/R) log2 (Ri/R), where Pi is the probability of occupancy in bin i, Ri is the average firing rate for bin i, and R is the overall mean firing rate.
Multifactor repeated-measures ANOVAs were used to examine the influence of respective protocols, recording sessions, and intersession delays on place field stability using parametric statistics in SigmaPlot 11.0 (Systat Software, Inc. SigmaPlot for Windows). Autocorrelograms of interspike intervals, perievent histograms, and basic firing properties of the object-location cells were obtained using NeuroExplorer (version 3.266, Nex Technologies, Littleton, MA) following cluster cutting and the construction of place × firing rate maps, as described above. For the inactivation and recording studies, mice that did not achieve sufficient exploration were removed from all further analyses (n = 4).
Histology
At the conclusion of behavioral testing and in vivo recordings, each mouse was deeply anesthetized with 5% isoflurane. Tetrode-implanted mice were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde before brain dissection. Brains of the mice from functional inactivation studies were dissected and placed in 4% paraformaldehyde. All brains were cryoprotected, then sectioned at 50 µm using a sliding microtome (Leica SM2010) with an automatically controlled freezing stage (Physitemp Instruments, Clifton, NJ). Cannulae and tetrode placements were confirmed by examination of cresyl violet-stained sections under a light microscope. The data for any mice that were determined to have inappropriate placement (n = 6) were excluded from the analyses (for representative photomicrographs depicting correct placement of cannulae and tetrode, see Fig. 1, C and D, respectively).
RESULTS
Time Course of Muscimol-Induced Suppression of CA1 Neuronal Activity
Neuronal activity was recorded from CA1 neurons (n = 6) in a freely moving mouse before and after muscimol, a GABAA agonist, was infused (0.335 µl at a concentration of 1 µg/µl) into the CA1 region of the dorsal hippocampus ipsilateral and adjacent to the tetrode array. Neuronal activity was recorded at eight postinfusion time points for 5-min epochs approximately every 45–105 min. Local infusion of muscimol induced a rapid decline in the firing rates of CA1 neurons, and the suppression of firing lasted for several hours (Fig. 1A). A Friedman repeated-measures ANOVA on ranks analysis of neuronal firing rates yielded a significant main effect of time [χ2(39) = 181.76, P < 0.01]. By the 320–324 min postinjection time bin, firing rates had recovered to a level equivalent to the firing pretreatment [t(29) = 1.412, not significant (n.s.)]. The rapid onset and duration of muscimol’s effect on mouse CA1 neurons is consistent with that recently reported in rats (Bonnevie et al. 2013).
Overall Analyses of Single-Unit Activity
A total of 112 pyramidal neurons were recorded from tetrode arrays positioned in the intermediate CA1 region of the right dorsal hippocampus (Fig. 1C). Recordings were conducted as mice actively explored inside a cylindrical arena containing a polarizing cue. Of the total, 67 CA1 neurons exhibited complex spike activity and stable location-specific firing during repeated recording sessions and therefore were classified as place cells. Place cell stability and stimulus control of the local cue card (Bostock et al. 1991; Knierim et al. 1995; Muller et al. 1987; Taube et al. 1990) were evaluated during three consecutive 10-min cue card rotation sessions (i.e., standard, 90° rotation, standard) conducted within the cylindrical arena. Place cells that met the criteria for stability (see materials and methods) were next recorded during one of the OR protocol conditions (see Fig. 1B). Upon completion of recordings during OR testing, two additional 10-min recording sessions were conducted to confirm place cell stability (Fig. 1B). Four neurons recorded from distal CA1 were found to exhibit object-related firing that was independent of location; three of these were object-related neurons (Supplemental Fig. S1d; see Supplemental Fig. S1 at https://doi.org/10.6084/m9.figshare.11482281).
Overall Analyses of Object Recognition Behavior and CA1 Place Cell Activity Across All OR Protocols
The results of the in vivo recording and functional inactivation experiments are organized below according to respective OR protocol (i.e., Cue Card, Drop-In, and Conventional). All three protocols comprised a total of four 10-min recording sessions: Habituation 1, Habituation 2, Sample, and Test. For the studies involving functional inactivation of CA1, sample session object exploration criteria were imposed to ensure that all mice were matched for object exploration (see materials and methods). To ensure that much of the arena floor surface was sampled (necessary for proper comparison of the place × firing rate maps), the sample session object exploration criteria were not imposed for the mice of the in vivo recording studies. For most of the functional inactivation studies, a 24-h delay was imposed between the sample and test session, consistent with our prior studies. However, for the functional inactivation studies using the Conventional OR protocol, a delay of either 5 or 20 min was imposed between sample and test sessions to permit appropriate comparisons of OR behavior between the functional inactivation and in vivo recording studies.
Influence of OR protocol on task performance.
A one-factor (OR protocol) ANOVA on discrimination ratio scores for the intra-CA1 saline-treated mice of the functional inactivation studies yielded no significant main effect of OR protocol [F(4, 43) = 0.14, n.s., Fig. 2], and the intra-CA1 saline-treated mice performed significantly above chance during the test session [t(47) = 17.41, P < 0.01, see Fig. 2]. These results suggest that the different cue configurations of the testing arena did not affect OR task performance. Similar analysis of object recognition behavior revealed that mice exhibited significant novel object preference during recordings of CA1 place cells; discrimination ratios were significantly above chance [t(19) = 3.97; P < 0.01; mean ± SE = 0.22 ± 0.06]. Additionally, the recording and inactivation (saline-treated) mice performed similarly. A two-factor (protocol, delay) ANOVA on discrimination ratio yielded no significant main effect of protocol [F(2, 19) = 2.20, n.s.], or delay [F(1, 19) = 0.35, n.s.], and no protocol-by-delay interaction [F(2, 19) = 0.76, n.s.]. A three-factor (protocol, delay, session) ANOVA on object exploration during sample and test yielded no significant main effect of protocol, delay, session, and no interaction between the three factors [protocol: F(2, 39) = 0.72, n.s.; delay: F(1, 39) = 0.00, n.s.; session: F(1, 39) = 0.00, n.s.; interaction: F(2, 39) = 0.06, n.s.]. These results imply a remarkable consistency in discrimination behavior across the different arena configurations used in the three distinct OR protocols.
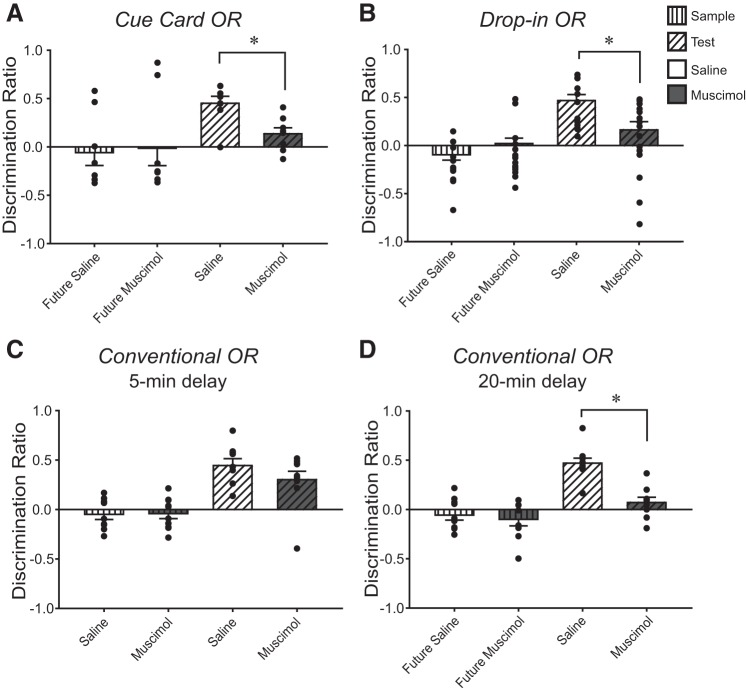
Fig. 2.
A–D: mean ± SE discrimination ratios for object sessions in the Cue Card object recognition (OR), Drop-In OR, and Conventional OR protocols (5-min and 20-min delays). White and gray bars of each graph represent the vehicle- and muscimol-treated groups, respectively. Within the bars, vertical lines represent object discrimination during sample, while diagonal lines indicate object discrimination during the test. Object exploration during sample was equivalent across the treatment groups and mice did not discriminate between the two identical sample session objects. Significant differences between groups were found for the test (all, except Conventional OR protocol, 5-min delay), and these are designated with an asterisk. *P < 0.05 versus vehicle group. Each mouse received bilateral 0.335-µl microinfusion of vehicle or muscimol (1 µg/µl) immediately after acquiring sample exploration criterion (see materials and methods), or in the 5-min delay of the Conventional OR protocol, the treatments were administered 20-min before sample.
Cue Card OR protocol.
Here, the square arena contained a prominent cue card covering ~67% of the east wall during all Cue Card OR sessions (41.9 cm × 26.1 cm piece of gray ABS, Fig. 1B). With the cue card, the arena resembled those typical of place cell recording studies (Kentros et al. 2004; Muller and Kubie 1987; Muzzio et al. 2009b). The rationale for including a highly salient cue was that this might facilitate self-localization or spatial orientation during the OR sample and test sessions and might increase recognition of the familiar arena. We reasoned that the cue card might thereby enhance the sensitivity of CA1 place cells to the presence of novel objects and permit testing whether object memory would remain sensitive to CA1 inactivation under enhanced cue conditions.
or behavior following ca1 inactivation.
Histological analyses of infusion cannula tracks for this and all other intra-CA1 studies described verified that local infusions (0.335 µl/side) were restricted to the CA1 region of dorsal hippocampus (Fig. 1D). Previously, we reported that bilateral intra-CA1 fluorophore-conjugated muscimol diffused within the mouse CA1 region without spreading to the CA3 or dentate gyrus (Cohen et al. 2013; Stackman et al. 2012, 2016). The present studies used identical implant coordinates and the same volume of muscimol as in our previous reports. Therefore, we assume a comparable CA1-restricted distribution of muscimol in the studies involving local infusion.
Latency to acquire the object exploration criteria during the Cue Card OR sample session was similar between the future treatment groups (n = 16/treatment) [vehicle 498 s ± 30 s, muscimol 448 s ± 40 s; t(14) = 1.00, n.s.]. Mice received bilateral intra-CA1 infusions of vehicle or muscimol immediately after the Cue Card OR sample session. Discrimination ratio scores, computed from object exploration 24 h later during the Cue Card OR test session, were significantly lower for the postsample muscimol group compared with those of the postsample vehicle group [t(14) = 3.33, P < 0.01, d = 1.67, Fig. 2A]. Despite the differences in discrimination, total object exploration was similar between the postsample treatment groups [vehicle 52 s ± 4 s, muscimol 46 s ± 4 s; t(14) = 1.10, n.s.]. These results indicate that the consolidation of object memory acquired during the Cue Card OR protocol is dependent upon CA1 neuronal activity, consistent with previous studies (Cohen et al. 2013; de Lima et al. 2006). The findings also suggest that the inclusion of a salient polarizing cue, which presumably heightens recognition of the arena as familiar, did not reduce the sensitivity of object memory to CA1 inactivation.
in vivo ca1 neuronal recordings.
Mice with CA1 tetrode microdrives (mice: n = 7) spent equivalent time exploring the objects during the Cue Card OR sample (62 s ± 6 s) and test (55 s ± 5 s) sessions [t(7) = 0.35, n.s.]. Recordings yielded 23 place cells with place fields exhibiting high average stability (r = 0.77) between all sessions and delays. Specifically, place field locations were not observed to undergo angular or linear rotations in response to the presence of objects (Fig. 4A). A two-factor (session, delay) ANOVA on place field stability scores yielded no significant main effect of delay [F(1, 67) = 0.15, n.s.], session [F(2, 78) = 0.14, n.s.] or the interaction of session by delay [F(2, 67) = 0.51, n.s.; Fig. 3A]. These results are consistent with our previous finding of minimal influence of novel objects on mouse CA1 place fields (Cohen et al. 2013).
Fig. 4.
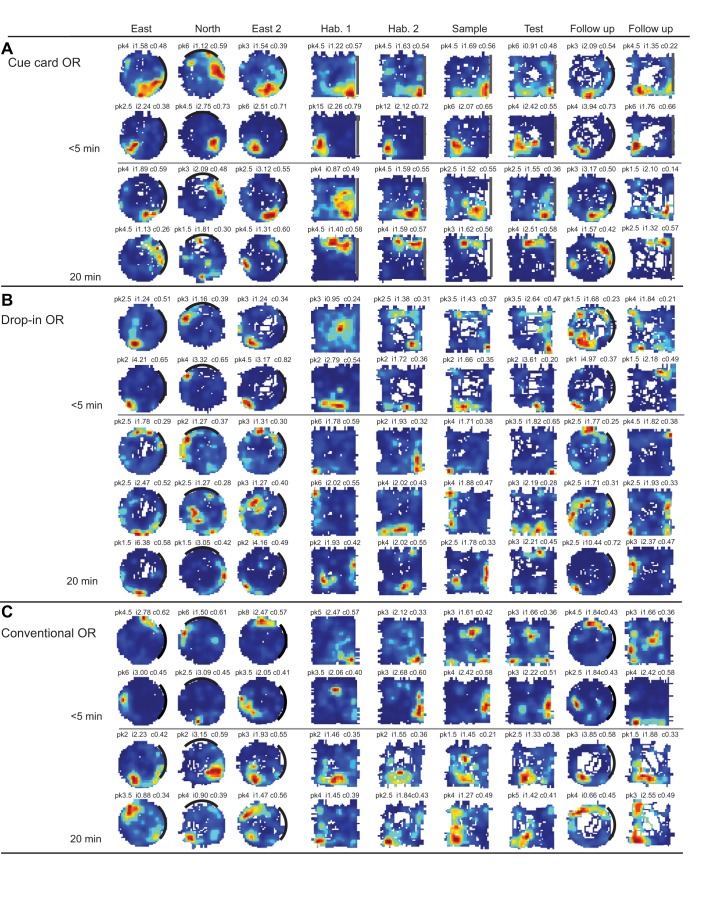
Representative place × firing rate maps of individual CA1 place cells recorded during each of the three object recognition (OR) protocols. For each map, firing rate is represented by the color-coded pixels, with red referring to the location(s) where the cell discharged at its highest rate, and blue referring to the visited locations where the cell was silent. White pixels represent areas of the arena that were unvisited by the given mouse. Each row represents the location-specific firing properties of a given CA1 neuron recorded across the nine 10-min sessions of a respective protocol. The label above the top row of maps identifies the respective recording sessions. The numbers on the left and above each place × firing rate map represent the average in-field firing rate for that cell (pk), information content (i), and spatial coherence (c). A: representative place × firing rate maps from two mice tested in the Cue Card OR protocol with an intersession delay of 5-min and 20-min, respectively. B: same as in A, but for two mice tested in the Drop-In OR protocol. C: same as in A, but for two mice tested in the Conventional OR protocol. Representative place × firing maps for “object cells” can be found in Supplemental Fig. S1 at https://doi.org/10.6084/m9.figshare.11482281.
Fig. 3.
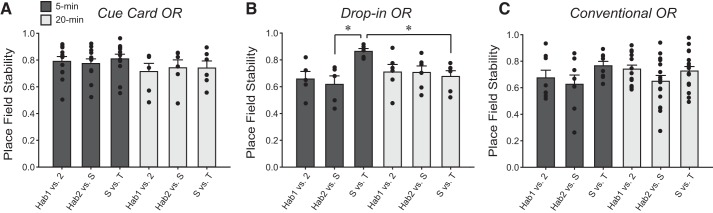
A–C: mean ± SE place × firing rate map correlation (0–1) calculated between two adjacent recording sessions for Cue Card OR, Drop-In OR, and Conventional OR protocols, respectively. Calculated correlations between Habituation 1 and Habituation 2 sessions is represented by “Hab1 versus 2”; correlation between Habituation 2 and sample is represented by “Hab 2 versus S”; and the correlation between sample and test object sessions is represented by “S versus T.” Darker colored bars indicate a 5-min delay between recording sessions whereas lighter colored bars represent a 20-min delay between adjacent sessions. *P < 0.01. OR, object recognition.
Drop-In OR Protocol.
Here, the arena lacked the polarizing cue card (Fig. 1B); thus, conditions were typical of those in which OR is often assessed (Cohen and Stackman 2015). In this protocol, each mouse first explored the familiar, otherwise empty arena for 2 min, and then the experimenter introduced the respective objects by hand to initiate the Drop-In OR sample or test session. Place cell activity was not recorded during this initial 2-min arena exploration period, since preliminary studies revealed that there was not sufficient sampling of the arena floor by the mice during this initial period to accurately determine place field locations. No attempt was made to limit the mouse’s view of the objects as they were lowered into the arena. All other aspects of the protocol were as described above for the Cue Card OR protocol. The Drop-In OR protocol was designed to promote the ability of the mice to recognize the arena as familiar, before encoding a memory of the objects, whether associated with that of the arena or orthogonal to it.
or behavior following ca1 inactivation.
Latency to acquire the sample object exploration criteria during the Drop-In OR sample session was similar between the future treatment groups [vehicle 356 s ± 41 s, muscimol 439 s ± 40 s; t(17) = −1.46, n.s.] indicating that all mice exhibited equivalent motivation to explore the objects. During the Drop-In OR test session 24 h later, the postsample muscimol-treated mice failed to exhibit a strong preference for the novel object. The discrimination ratio scores of the postsample muscimol group were significantly lower than those of the postsample vehicle group [t(17) = 2.77, P = 0.01, d = 1.51, Fig. 2B], while there was no significant difference in total object exploration between the treatment groups [vehicle 57 s ± 9 s, muscimol 43 s ± 7 s; t(17) = 1.25, n.s.]. These results indicate that under the modified conditions of the Drop-In OR protocol, mice encode a CA1-dependent object memory, which may be distinct from the previously consolidated and retrieved memory of the context.
in vivo ca1 neuronal recordings.
Mice with CA1 tetrodes (mice: n = 6) spent equivalent time exploring objects during the Drop-In OR sample (70 s ± 8 s) and test (79 s ± 8 s) sessions [t(6) = −0.41, n.s.]. Recordings yielded 19 place cells, and cross-correlation analyses of place × firing rate maps across all sessions returned a mean stability score of 0.64. Further analysis revealed that the mean stability score computed for recorded sessions pairs was 0.68 when a 5-min intersession delay was imposed between object sessions, and was 0.61 when the intersession delay was 20 min (Fig. 3B). Although stability of firing rate maps was lower when the longer delay was imposed between Drop-In OR sample and test sessions, the difference was not statistically significant. A two-factor (session pair, delay) ANOVA on stability correlation measures yielded a significant main effect of Drop-In OR session [F(2, 56) = 4.23, P = 0.02], but no significant main effect of delay [F(1, 56) = 2.69, n.s.] or interaction of session by delay [F(2, 56) = 0.90, n.s.]. The results of the corresponding post hoc analysis indicated that place field stability was higher between Drop-In OR sample and test sessions than between Habituations 1 and 2.
During specific Drop-In OR sessions, the place fields of all cells recorded from four mice rotated by 90° from original position. This result suggests that, during the Drop-In OR protocol, place cells were anchored to the relatively ambiguous square geometry of the arena. Figure 4B (see bottom row labeled 20-min delay) depicts one case in which the place field appears to undergo misorientation between object sessions. The otherwise feature-limited arena of the Drop-In OR protocol may have promoted the misorientation of the arena map retrieved before the introduction of objects. We did not consider these cognitive map misorientations to be a form of remapping since all place fields of a given mouse rotated in unison and to the same degree, so as to continually represent the same arena location. Cross-correlation analysis revealed that, when the misoriented maps were manually rotated to their expected orientation, correlation measures were greater than expected by chance. The subsequent insertion of objects into the arena, while place fields were misoriented, may have led the mice to perceive that a familiar object had been placed into a novel location. However, object discrimination performance in the Drop-In OR protocol was equivalent to that observed in the other OR protocols. It should be clear that the disambiguation of geometrically equivalent spatial locations was not necessary for successful object discrimination in this object recognition memory task, given that this training protocol appears to promote encoding of an object-in-context memory (Cohen et al. 2013). Therefore, it may not be surprising that novel object preference was observed in mice while place cell firing rate maps were misoriented. These results, along with the absence of misorientation during the Cue Card OR protocol, indicate that place fields likely anchor to the cue card.
Conventional OR Protocol.
This protocol employed the same arena and stimuli as in the Drop-In OR protocol and those typically used to study OR in rodents (Clark et al. 2000; Cohen et al. 2013; Cohen and Stackman 2015; Fernandez et al. 2008). All behavioral sessions were conducted in the white, otherwise feature-limited arenas, as described above for the Drop-In OR protocol, and the sample and test sessions began immediately upon placement of the mouse into the arena which already contained the two objects. This protocol served as a control against which to compare the effects of behavior and CA1 neuronal activity of the mice tested in the Cue Card OR and Drop-In OR protocols.
or behavior following ca1 inactivation (5-min delay).
Naïve mice received intra-CA1 infusions of vehicle or muscimol 20 min before the sample session to ensure silencing of CA1 neuronal activity during the encoding and consolidation of object memory, consistent with our previous report (Cohen et al. 2013). Latency to acquire the sample session object exploration criteria in the Conventional OR protocol was equivalent between the treatment groups [vehicle 523 s ± 32 s, muscimol 503 s ± 33 s; t(17) = 0.42, n.s.]. During the test session presented 5 min after the Conventional OR sample session, discrimination ratio scores of presample vehicle and muscimol groups were similar [t(17) = 1.30, n.s., Fig. 2C] and were significantly greater than chance [t(9) = 3.62, P < 0.01 and t(8) = 6.49, P < 0.01, respectively]. These results indicate that silencing of CA1 neuronal activity before the OR sample session did not affect the novel object preference of the mice when the test session was presented 5 min after the sample session. Importantly, both groups spent comparable time exploring the objects during the test session [vehicle 44 s ± 3 s, muscimol 48 s ± 5 s; t(17) = −0.66, n.s.]. Given our results shown in Fig. 1A, indicating that intra-CA1 muscimol depressed the activity of pyramidal neurons for over 3 h, the infusion of muscimol before the sample session would have silenced CA1 neuronal activity over the entire OR protocol, from the sample session, over the course of the 5-min delay, and then throughout the test session.
or behavior following ca1 inactivation (20-min delay).
Latency to acquire the Conventional OR sample session object exploration criteria was similar between the future treatment groups [vehicle 500 s ± 47 s, muscimol 436 s ± 48 s; t(17) = 0.95, n.s.]. Mice received intra-CA1 infusions of vehicle or muscimol immediately after acquiring the sample object exploration criteria. During the Conventional OR test session presented 20 min after the sample session, discrimination ratio scores of the postsample muscimol group were significantly lower than those of the postsample vehicle group [t(17) = 5.41, P < 0.01, d = 2.49, Fig. 2D], although total object exploration times were equivalent [vehicle 49 s ± 5 s, muscimol 45 s ± 6 s; t(17) = 0.57, n.s.]. These findings suggest that CA1 neuronal activity is critical for object memory consolidation when a 20-min delay is imposed between the Conventional OR sample and test sessions. We previously reported similar findings from an experiment using identical postsample hippocampal inactivation strategies, but with a 24-h delay imposed between object memory encoding and retrieval (Cohen et al. 2013). As in the present study, those mice achieved sample object exploration criterion in similar times [vehicle 469 s ± 42 s, and muscimol 459 s ± 36 s; t(21) = 1.93, n.s.], yet, during the test session 24 h later, the discrimination ratios of the postsample muscimol-treated mice were significantly lower than those of the postsample vehicle-treated mice [t(21) = 5.93, P < 0.01] (Cohen et al. 2013). An analysis of test session behavior following a 24-h delay (from our previous report) to that of the present 20-min delay results revealed that object discrimination was equivalent between the two treatment conditions [saline: t(20) = 0.92, n.s.; muscimol: t(18) = 0.95, n.s.]. Along with the results from the Conventional OR 5-min delay experiment, the findings are consistent with the view that the hippocampus plays a delay-dependent role in object memory (Clark et al. 2000; Hammond et al. 2004).
in vivo ca1 neuronal recordings.
Mice with CA1 tetrodes (mice: n = 7) spent equivalent time exploring the objects during the Conventional OR sample (72 s ± 7 s) and test (68 s ± 6 s) sessions [t(7) = 0.22, n.s.]. Recordings of CA1 neuronal activity during baseline sessions in the cylinder and empty arena yielded 25 place cells with an overall average stability measure of 0.69. Analyses of sample versus test session place × firing rate maps yielded mean stability scores of 0.68 when a 5-min delay was imposed between sessions and a score of 0.70 when a 20-min delay was imposed between sessions (Fig. 3C). A two-factor (session, delay) ANOVA on stability scores yielded no significant main effect of delay [F(1, 89) = 0.06, n.s.], session [F(2, 89) = 3.07, n.s.], or interaction between the two factors [F(2, 89) = 0.66, n.s.]. All place × firing rate maps from one mouse were observed to rotate 90° clockwise between the two arena habituation sessions (Fig. 4C, 5-min delay, second row). This misorientation may be a result of the diminished availability of salient polarizing cues within the arena during arena habituation sessions. Cross-correlation analysis revealed that, when the misoriented maps were manually rotated to their expected orientation, correlation measures were greater than expected by chance. This finding is consistent with the misorientation observed in the Drop-In OR protocol; however, similar misorientations of firing rate maps were not observed during the sample or test sessions. It may be that the presence of objects within the arena provided sufficient spatial information to anchor the spatial reference frame.
Additional Analyses
Number of place fields and average in-field firing rates.
Analyses revealed that the number of place fields per cell changed over the course of the recording sessions for the three OR protocols, while the average in-field firing rate was not influenced by object exploration or discrimination (Table 1). A three-factor (protocol, delay, session: Cylinder 1, Habituation 1, Sample, and Test) ANOVA on the number of place fields per CA1 neuron yielded a significant effect of session [F(3, 264) = 4.38, P < 0.01], with post hoc Bonferroni multiple comparisons test revealing a significant increase in place field number during the Sample and Test compared with Habituation 1. The analysis also yielded a significant protocol × delay interaction [F(2, 264) = 4.01, P = 0.01), but nonsignificant main effects of protocol, delay, and the interactions of protocol × delay × session, protocol × session, and delay × session. Consistent with others (Burke et al. 2011), we observed an increase in place field number per cell specific to object sessions, independent of intersession delay. A three-factor (protocol, delay, session) ANOVA conducted on average in-field firing rates of place cells (Table 1) yielded no significant main effect of protocol, session, delay, or protocol × delay and no significant interactions between the factors.
Table 1.
Firing characteristics of mouse CA1 place cells during object recognition tasks
| Protocol | Measure | Delay | Cylinder 1 | Arena Hab_1 | Sample | Test |
|---|---|---|---|---|---|---|
| Cue Card OR |
fields/cell | <5 | 1.13 | 1.12 | 1.20 | 1.20 |
| 20 | 1.00 | 1.20 | 1.50 | 1.40 | ||
| overall | 1.08 ± 0.06 | 1.17 ± 0.09 | 1.32 ± 0.10 | 1.28 ± 0.09 | ||
| proportion >1 | 0.16 | 0.18 | 0.25 | 0.24 | ||
| average in-field firing rate (Hz) | <5 | 4.87 | 2.70 | 4.47 | 3.90 | |
| 20 | 3.60 | 3.00 | 3.80 | 3.30 | ||
| overall | 4.36 ± 0.37 | 2.82 ± 0.65 | 4.20 ± 0.53 | 3.66 ± 0.35 | ||
| stability (versus Arena Hab_1) | <5 | 0.73 | 0.70 | |||
| 20 | 0.68 | 0.68 | ||||
| Drop-In OR |
fields/cell | <5 | 1.22 | 1.22 | 1.22 | 1.56 |
| 20 | 1.20 | 1.20 | 1.20 | 1.40 | ||
| overall | 1.21 ± 0.10 | 1.21 ± 0.10 | 1.21 ± 0.10 | 1.47 ± 0.14 | ||
| proportion >1 | 0.14 | 0.18 | 0.28 | 0.26 | ||
| average in-field firing rate (Hz) | <5 | 3.56 | 2.72 | 3.56 | 3.11 | |
| 20 | 2.45 | 2.95 | 3.20 | 4.35 | ||
| overall | 2.97 ± 0.54 | 2.84 ± 0.44 | 3.37 ± 0.53 | 3.76 ± 0.72 | ||
| stability (versus Arena Hab_1) | <5 | 0.56 | 0.55 | |||
| 20 | 0.52 | 0.49 | ||||
| Conventional OR |
fields/cell | <5 | 1.17 | 1.17 | 1.50 | 1.25 |
| 20 | 1.12 | 1.18 | 1.25 | 1.13 | ||
| overall | 1.14 ± 0.07 | 1.18 ± 0.07 | 1.36 ± 0.12 | 1.18 ± 0.10 | ||
| proportion >1 | 0.09 | 0.10 | 0.18 | 0.13 | ||
| average in-field firing rate (Hz) | <5 | 3.38 | 3.29 | 2.63 | 2.54 | |
| 20 | 3.16 | 4.12 | 4.44 | 3.65 | ||
| overall | 3.25 ± 0.32 | 3.77 ± 0.45 | 3.66 ± 0.60 | 3.18 ± 0.40 | ||
| stability (versus Arena Hab_1) | <5 | 0.59 | 0.58 | |||
| 20 | 0.65 | 0.63 |
Values are means ± SE. Cylinder 1, the initial recording session in the cylindrical arena with the cue card in the 3 o'clock position; Arena Hab_1, the first recording session in the square arena; OR, object recognition.
Quantitative measures of CA1 place cell activity.
analyses of place field stability.
A three-factor (session pair: Habituation 1 versus Habituation 2, Habituation 2 versus Sample, Sample versus Test; delay; protocol) ANOVA, performed on the place field stability scores of the 67 CA1 cells recorded, yielded a significant main effect of protocol [F(2, 214) = 9.62, P < 0.01]. Post hoc Holm-Sidak multiple comparisons tests indicated that the mean place field stability scores for the Cue Card OR protocol were significantly higher than those of the Drop-In and Conventional OR protocols. The ANOVA also yielded a significant main effect of session pair [F(2, 214) = 6.20, P < 0.01], with corresponding post hoc tests indicating that place field stability of Sample versus Test was significantly higher than that of Habituation 1 versus Habituation 2 or Habituation 2 versus Sample. Place field stability of Habituation 1 versus Habituation 2 was equivalent to that of Habituation 2 versus Sample. The ANOVA also yielded a significant main effect of delay [F(1, 214) = 3.93, P < 0.05], but no significant interaction between any of the main factors [F(4, 214) = 0.77, n.s.].
A two-factor (protocol, delay) ANOVA on the Sample versus Test place field stability scores to assess the specific effects of objects within the arena yielded a significant main effect of delay [F(1, 71) = 7.91, P < 0.01], but no significant main effect of protocol [F(2, 71) = 0.99, n.s.] or of a protocol × delay interaction [F(2, 71) = 0.55, n.s.]. A two-factor (delay, session pair: Habituation 1 versus Habituation 2, Habituation 2 versus Sample) ANOVA was performed on the place field stability scores to determine whether differences in intersession delay had an effect on place field stability before the consolidation and retrieval of object memory. The analysis yielded nonsignificant main effects of delay [F(1, 139) = 1.01, n.s.], session pair [F(1, 139) = 0.12, n.s.], and delay × session pair interaction [F(1, 139) = 0.81, n.s.].
analyses of spatial coherence.
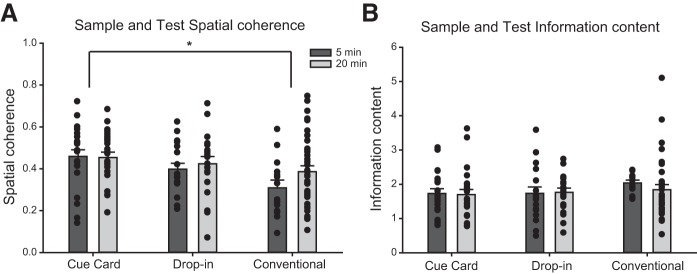
A two-factor (OR protocol: Cue Card, Drop-In, Conventional; delay: 5-min, 20-min) ANOVA on spatial coherence measures from the final place cell screening session in the cylinder (East2) yielded a nonsignificant main effects of protocol [F(2, 66) = 1.31, n.s.], delay [F(1, 66) = 0.05, n.s.], and the interaction of protocol × delay [F(2, 66) = 0.92, n.s.]. Thus, the analyses confirmed that there were no differences in place cell spatial coherence before initiating the respective OR protocols. A three-factor (OR protocol: Cue Card, Drop-In, Conventional; session: Habituation 1, Habituation 2, Sample, Test; delay: 5-min, 20-min) ANOVA on spatial coherence measures yielded significant main effects of protocol [F(2, 236) = 7.59, P < 0.01] and OR protocol × delay [F(2, 236) = 4.02, P = 0.02], but nonsignificant main effects of session [F(3, 236) = 0.36, n.s.] and delay [F(1, 236) = 0.73, n.s.]. The corresponding post hoc analyses indicated that place cell spatial coherence measures during the Cue Card OR protocol were significantly higher than those of the Conventional OR protocol. Furthermore, the post hoc analyses of the OR protocol × delay interaction revealed that, for both the Cue Card OR and Conventional OR protocols, the spatial coherence measures of the 5-min delay condition were significantly higher than that of the 20-min delay condition (Fig. 5A). Finally, a three-factor (OR protocol: Cue Card, Drop-In, Conventional; session: Sample, Test; delay: 5-min, 20-min) ANOVA was performed on the spatial coherence calculated for each cell recorded during the object sessions to determine whether the specific objects present during sample and test affected place cell spatial coherence. The analysis yielded a significant main effect of OR protocol only [F(2, 122) = 5.765, P < 0.01], with the corresponding post hoc tests indicating that spatial coherence was again significantly higher in the Cue Card protocol as compared with the Conventional protocol, but no different than in the Drop-In protocol. This pattern of results implies that the spatial coherence of place fields during OR testing is greatly affected by the presence of the polarizing cue card. Furthermore, these effects appear to be delay dependent, dissipating when a longer (i.e., 20-min) delay was imposed between behavioral sessions. The absence of a session effect suggests that, within the timeframe of the OR task object sessions, CA1 place cell activity does not shift to represent a conjunctive object-context association. However, it is possible that the spatial coherence measure is not sufficiently sensitive to detect OR-induced changes in place cell activity.
Fig. 5.
A: spatial coherence across the three protocols at both delays calculated from place fields during sample and test. *P < 0.01. B: spatial information content of place fields across protocols at both delays obtained from sample and test.
analyses of spatial information content.
A two-factor (OR protocol: Cue Card, Drop-In, Conventional; delay: 5-min, 20-min) ANOVA on information content measures from the final place cell screening session in the cylinder (East2) yielded a nonsignificant main effect of OR protocol [F(2, 66) = 0.35, n.s.], delay [F(1, 66) = 0.20, n.s.], and protocol × delay [F(2, 66) = 0.77, n.s.]. Thus, as for spatial coherence, spatial information content measures of CA1 place cells were equivalent before object sessions. A three-factor (OR protocol: Cue Card, Drop-In, Conventional; session: Habituation 1, Habituation 2, Sample, Test; and delay: 5-min, 20-min) ANOVA on information content measures yielded a significant interaction of OR protocol × delay [F(2, 289) = 3.75, P = 0.03], but nonsignificant main effects of OR protocol [F(2, 236) = 2.48, n.s.], delay [F(1, 236) = 0.31, n.s.], and session [F(3, 236) = 0.28, n.s.]. Post hoc multiple comparisons tests indicated that the information content measures of the Drop-In OR protocol were significantly higher than those of the Cue Card OR protocol, but only when a 20-min delay was imposed between sessions (Fig. 5B). Given that the place field stability scores of the Drop-In OR protocol were significantly lower than those of Cue Card OR, this inverse relationship between spatial coherence and information content for the Drop-In OR protocol was expected. Lastly, a three-factor (OR protocol: Cue Card, Drop-In, Conventional; session: Sample, Test; and delay: 5-min, 20-min) ANOVA was performed to investigate the changes in information content resulting from the presence of a novel object (in a familiar location) during the test. The analysis yielded nonsignificant main effects of OR protocol [F(2, 122) = 1.68, n.s.], delay [F(1, 122) = 0.40, n.s.], session [F(1, 236) = 0.65, n.s.], or the interaction between the factors [F(1, 236) = 0.97, n.s.].
ca1 place field stability predicts or discrimination ratio.
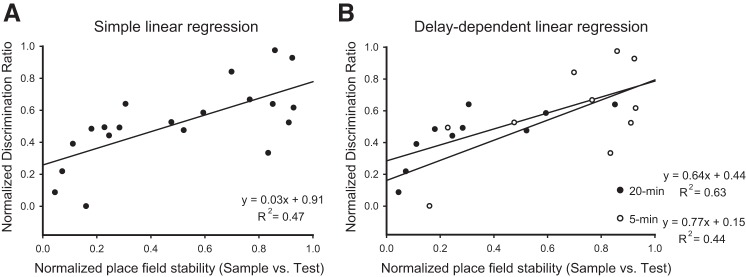
The stability of CA1 place fields between sample and test sessions correlated moderately with the discrimination scores for each mouse (Pearson’s r = 0.62, n = 20, P < 0.01; Fig. 6A). Further analysis to determine the degree of variance in discrimination ratio explained by place field stability yielded a significant linear regression [F(1, 18) = 16.03, P < 0.01], with R2 = 0.47. This regression equation indicated that a mouse’s predicted discrimination ratio was 0.03x + 0.91 depending on the calculated correlation between object sessions. Furthermore, a significant linear regression was found between the normalized place field stability scores and discrimination ratio when a 5-min delay was imposed between sessions [F(1, 8) = 6.18, P = 0.03], with R2 = 0.44 (see Fig. 6B), indicating that a mouse’s predicted discrimination ratio was 0.77x + 0.15 depending on the calculated correlation between object sessions. For those sessions in which a 20-min delay was imposed between sessions, a stronger relationship between normalized place cell stability scores and discrimination ratio emerged [F(1, 8) = 13.43, P < 0.01], with R2 = 0.63 (Fig. 6B), indicating that a mouse’s predicted discrimination ratio was 0.64x + 0.44 depending on the calculated correlation between object sessions. Although significant relationships between the measures emerged, there appears to be a stronger linear relationship detected when a 20-min intersession delay was imposed compared with a 5-min delay between sessions.
Fig. 6.
A: linear regression analysis illustrates the positive relationship between the normalized discrimination ratio and the normalized place field stability averaged for each mouse across object recognition protocols. B: regression analysis and representative plot demonstrate the positive relationship between the normalized discrimination ratio and the normalized place field stability averaged over each mouse by delay.
object-related firing.
Four CA1 neurons exhibited a similar pattern of object-related but place-independent firing properties (see Supplemental Fig. S1a; for this and all other plots within Supplemental Fig. S1, see https://doi.org/10.6084/m9.figshare.11482281). One neuron fired at its peak rate when the mouse actively explored objects during OR testing, while simultaneously recorded place cells maintained consistent location-specific firing (Supplemental Fig. S1b). Three other object-related firing cells were subsequently identified from distal CA1 under different experimental conditions (Supplemental Fig. S1c and see materials and methods). The activity of all four object-related firing cells was not influenced by the location of objects, nor did these cells fire at above background rates when objects were not present in the arena (see Supplemental Fig. S1b for representative place × firing rate maps plotted for the object-related firing cells). Supplemental Fig. S1b, right, depicts perievent time histograms. Autocorrelograms of interspike intervals computed from these four neurons with object-related firing indicated a lack of complex spike activity and instead suggest a more consistent spiking pattern (Supplemental Fig. S1d). Further analyses suggest that the average in-field firing frequency of these “object cells” increased over the course of daily recording sessions, while maintaining a baseline firing frequency of 0 Hz when no objects were present. A one-factor (time) repeated-measures ANOVA conducted on the Z-scored average in-field firing frequency of each of the four “object cells” recorded over several days (Supplemental Fig. S1, d–f), yielded a significant main effect of time [F(2, 5) = 37.72, P < 0.01]. A similar one-factor (time) repeated-measures ANOVA on average in-field firing frequency of four randomly chosen place cells (3 were recorded simultaneously with “object cells”; Supplemental Fig. S1, e and f) yielded a nonsignificant effect of time [F(2, 4) = 0.94, n.s.].
DISCUSSION
The hippocampal circuit enables the rapid, one-trial association of contextual or spatial information with specific item or feature information (Day et al. 2003; Rolls 2018). As mice explore novel objects during the sample session of the spontaneous object recognition task, the hippocampal circuitry theoretically enables the rapid association of contextual or spatial information together with the specific details about the objects explored (O'Reilly and Norman 2002; Rudy and Sutherland 1995; Sutherland and Rudy 1989). For the present studies, we examined the contribution of CA1 neuronal activity to the rapid acquisition of object-in-context memory and the degree to which task-induced changes in CA1 neuronal activity reflected the rapid, one-trial acquisition of object-in-context memory. We hypothesized that arena cue or context conditions may influence the degree to which CA1 neuronal activity is necessary for object memory in the OR task as suggested by Rudy (2009) and the sensitivity of CA1 neurons to undergo object memory-induced changes in place fields. These experiments yielded five main findings. First, we confirmed that CA1 neuronal activity was critical for successful object discrimination, regardless of the contextual information afforded by each of three distinct OR protocols. That is, inactivation of CA1 neurons following the sample session consistently impaired subsequent preference of the mice to explore the novel object during the test presented 20 min or 24 h later. These results provide further support to a growing literature that object memory depends upon the integrity of the hippocampus, and the CA1 region specifically (Clark et al. 2000; Cohen et al. 2013; de Lima et al. 2006; Hammond et al. 2004; Mumby et al. 2002; Stackman et al. 2016; for a review see Cohen and Stackman 2015). Second, we determined that novel object preference was spared after inactivation of CA1 neuronal activity, when a 5-min delay was imposed between the sample and test session. This result indicates that object memory is likely guided by a hippocampal-independent working memory-like process during the first few minutes after the object information is encoded during the sample session. This finding is consistent with previous reports (Clark et al. 2000; Hammond et al. 2004), and we suggest that this hippocampal-independent memory may involve the perirhinal cortex (Ainge et al. 2006; Mumby et al. 2005; Oliveira et al. 2010; Winters and Bussey 2005; Winters et al. 2004). Third, CA1 place cell fields were found to be remarkably stable during and after exploration of novel objects in the arena regardless of the three distinct OR conditions of the testing arena. Furthermore, measures of place cell spatial coherence and spatial information content were remarkably consistent throughout the OR protocols, indicating that place cells were largely unaffected by the presence of novel objects within the arena. However, we found no support for object exploration-induced alteration in CA1 place fields. Thus, our results provide no evidence that CA1 place cells shift their spatial firing correlates to an object-in-context conjunctive representation within the time window of the sample and test sessions of the standard OR task. Fourth, we found a strong positive correlation between OR discrimination ratio and the stability of place fields between the OR sample and test, irrespective of OR protocol. Considering this correlation together with the other main findings, it is compelling to suggest that both discrimination ratio and place field stability are strong indicators of the fidelity of hippocampal function. Fifth, a subset of CA1 neurons was found to discharge at peak rates when the mice were actively exploring objects but remained silent when objects were absent. Although limited by number of cells, our analyses suggest that the firing properties of the nonplace, object-responsive cells were quite distinct from that of the characteristic complex spike firing patterns of CA1 and CA3 place cells.
Object Discrimination Is Influenced by CA1 Neuronal Activity
A hippocampal-dependent object-in-context memory forms after relatively limited exploration of a novel object by rodents (Clark et al. 2000, 2002; Cohen et al. 2013; Tuscher et al. 2018). An object-independent of context memory is formed after extended training in a modified OR task, and this memory is also dependent upon CA1 neuronal activity (Cohen et al. 2013). We recently reported that the inactivation of mouse CA1 neurons disrupted the retrieval of object memory and of spatial memory — to an equivalent degree (Stackman et al. 2016), supporting the view that the dorsal hippocampus contributes as much to event memory for items/objects, as it does to spatial memory. Our inactivation/behavioral results revealed that, regardless of arena configuration as dictated by the three distinct OR protocols, inactivation of CA1 neuronal activity impaired object recognition memory regardless of the presence of a polarizing cue (Cue Card) or whether time was provided for the mice to retrieve the context memory before exploring the objects (Drop-In). Presumably, during the Drop-In protocol (no polarizing cue available) the mice would have had the time to retrieve the map for the familiar arena, before encoding a memory of the introduced sample objects. In this manner, the memory of the context and that of the objects would be encoded and retrieved independently or at distinct time points during the task. If, as many have proposed, nonspatial object recognition is supported by cortical regions, like the perirhinal cortex (Ainge et al. 2006; Mumby et al. 2005; Winters et al. 2004), then intra-CA1 muscimol should not impair object recognition in a version of the task designed to explicitly dissociate the object and context memories. Our finding that intra-CA1 muscimol impaired object memory in the Drop-In protocol is consistent with our previous report that CA1 is required for the retrieval of object-dissociated-from-context-memory (Cohen et al. 2013). Interestingly, object discrimination performance of the respective groups of saline-treated mice was essentially equivalent across all three OR protocols, indicating that arena cue conditions did not affect encoding, consolidation, or retrieval of object memory. However, the collective results of the present study indicate that, regardless of the critical contribution of CA1 neurons to both object and spatial memory, we found no evidence to support the idea that conjunctive representations of object-context or object-location are rapidly formed within the CA1 during the OR sample session. Thus, these results extend the evidence supporting the contribution of CA1 neuronal activity to object memory processes.
Delay-Dependent Processing of Object Memory by CA1 Neurons
Previous findings have suggested that the rodent hippocampus processes object memory in a delay-dependent manner (Clark et al. 2000; Hammond et al. 2004). In the present set of studies, inactivation of CA1 neuronal activity failed to impair object discrimination when a 5-min delay was imposed between the encoding and retrieval of object memory. However, when a 20-min delay was imposed between encoding and retrieval, object discrimination was significantly impaired. Reconciling this pattern of results with our earlier reports and those of others suggests that there is a time window <20-min before the hippocampus becomes engaged in object memory processes. Thus, when the test session is presented following a brief delay (e.g., 5-min), a short-term or working memory-like process must support object discrimination behavior of mice. These results imply that object memory can be successfully encoded and then retrieved a short time later, all while CA1 neuronal activity is depressed. The spared OR task performance at 5 min after the sample session in the CA1-inactivated mice may reflect a working memory-like process mediated by other regions, such as the perirhinal cortex. The lack of effect of hippocampal perturbation on object recognition memory when a short delay is imposed between the sample and test session is consistent with previous reports (Clark et al. 2000; Hammond et al. 2004; Langston et al. 2010; Mumby et al. 2005). Interestingly, place fields of CA1 neurons recorded during the Drop-In OR protocol were found to be significantly more stable between the sample and test sessions when a 5-min intersession delay was imposed, compared with when a 20-min delay was imposed (Fig. 2D). These results support the time window described above and suggest that, at the 5-min delay, CA1 neurons had not yet been recruited to process object-related information. It is also possible that the increase in place field stability observed for Sample versus Test compared with that of the Habituation 1 versus Habituation 2 and Habituation 2 versus Sample reflects the additional spatial information afforded by the presence of objects. Conversely, place field stability observed for Sample versus Test was essentially equivalent to that observed for Habituation 1 versus Habituation 2 and Habituation 2 versus Sample when a 20-min intersession delay was imposed. If the CA1 neurons were processing object information at both delays, a comparable increase in place field stability would be expected in the Conventional and Drop-In OR protocols (no polarizing cue available), at the 20-min delay. The stability measures obtained from place cell recordings during the Cue Card OR protocol were significantly higher than those of the Conventional and Drop-In OR protocols, so any additional spatial information afforded by objects may have been masked by this ceiling effect.
Measures of CA1 place field stability between sample and test were found to be positively correlated with OR discrimination ratio measures, and this correlation was significantly influenced by the delay imposed between sessions. These results suggest that after a time window of <20-min CA1 neuronal function contributes to object memory retrieval and supports the view that spatial/contextual and object information processing both depend on CA1 neuronal activity. The weaker correlation between the place field stability and discrimination ratio at the 5-min delay suggests that CA1 place cells do not yet experience competing interference, which further supports a delay-dependent role of CA1 in object memory. Together, the findings suggest that CA1 is not actively engaged in processing object information until a postsample time delay between 5 and 20 min has elapsed and also imply that CA1 processing of spatial and object information occurs simultaneously but independently within the hippocampal circuit.
Conjunctive Object-in-Context Representations Do Not Emerge in CA1 During the OR Task
Rodents can rapidly acquire information about novel objects encountered in an otherwise familiar arena during an OR sample session, and that object memory is retained over delays of 24 or 48 h (Clark et al. 2000; Cohen et al. 2013; Forwood et al. 2005; Haijima and Ichitani 2012; Hammond et al. 2004; Mumby et al. 2005; Stackman et al. 2016; Winters et al. 2004). Therefore, one might predict that CA1 neuronal activity recorded during and after the sample session would reveal evidence that those preexisting place field maps of the familiar arena would be rapidly modified to reflect the object experience therein. An alternative prediction was that the place field maps would remain stable during the OR task, and a representation of the objects would be encoded as an independent output from CA1. OR is often tested in a feature-limited arena lacking spatial cues, and therefore one might expect that the subsequent presence of objects in the same arena may affect retrieval or reconsolidation of the place map of the familiar arena. Previously, we reported that CA1 place cells recorded in our Conventional feature-limited arena during the sample and test sessions were remarkably unaffected by exploration of the to-be-remembered objects (Cohen et al. 2013). One interpretation might be that this place field stability may have arisen from the mice using the location of the objects as spatial references, which enhanced stability of the maps of the feature-limited arena. Therefore, we designed two modified OR protocols to facilitate recognition of the arena as being familiar, with the assumption that this would allow increased attention to be paid to the novel objects and, in turn, either permit the modification of the preexisting place map or enable the object information to be processed separately from contextual information. Analyses of place × firing rate map stability consistently revealed strong positive correlations between maps across all three OR protocols, indicating that location by firing rate maps were largely unaffected by object exploration. Coupled with the lack of influence of OR performance on place cell measures of spatial coherence or information content, these results provide robust evidence that the spatial mapping function of hippocampal CA1 neurons is not altered by the processing of object memory. Stability of CA1 place fields was significantly higher during the Cue Card protocol compared with Drop-In and Conventional protocols (see Fig. 3). However, behavioral performance was equivalent across the three OR protocols for the respective groups of intra-CA1 saline-treated mice and mice from which CA1 place cells were recorded. Thus, the significant increase in place field stability in the Cue Card protocol was not accompanied by an enhancement in OR behavioral performance.
Occasionally, in the absence of the cue card, such as during the Drop-In and Conventional protocols, all place fields for a given mouse were observed to rotate in unison by 90° increments compared with earlier sessions, suggesting a misorientation of the arena representation (cognitive spatial map). The presence of an orienting cue (e.g., the Cue Card OR protocol), or having the objects already in the arena at the start of the sample and test sessions, as in the Conventional OR protocol, may have prevented the expression of such map misorientations. Place fields likely anchor to the most salient environmental feature available, whether it be to a cue card or to the arena geometry. The misorientations of the place field maps observed between object sessions of the Drop-In protocol may reflect the mice anchoring the retrieved arena map to an arbitrary reference in the empty arena and then maintaining that misorientation even after the placement of objects into perceived novel locations within the arena. Importantly, misorientations of CA1 place fields had no impact on CA1-dependent object discrimination behavior. The stability of mouse CA1 place cell activity during the three distinct OR protocols is consistent with previous recording studies of rats and mice (Burke et al. 2011; Cohen et al. 2013; Cressant et al. 1997; Deshmukh and Knierim 2013).
Recordings of CA1 gamma oscillations and place cell activity in rats engaged in object recognition and object-in-place memory tasks revealed a significant increase in fast, but not slow, gamma power in CA1 and the spiking rate of CA1 place cells during an object-in-place test session reflecting exploration of the novel object location (Zheng et al. 2016). However, such a change in fast gamma power was not found for the exploration of the novel object during a novel object task (Zheng et al. 2016) that was similar to the OR task protocols used in the present study. This lack of effect might be interpreted as consistent with the results of the present study; however, the behavioral results reported by Zheng et al. (2016) indicate that the rats failed to exhibit a significant increase in exploration of the novel object over the familiar object in their novel object test condition. Thus, it is difficult to draw conclusions about their reported lack of influence of the novel object task on CA1 place cell activity and fast and slow gamma oscillations. More recently, Trimper et al. (2017) reported that slow gamma power in the dentate gyrus and CA3 was significantly elevated during the exploration of novel objects presented on a circular track, as compared with that during the exploration of familiar objects. Additional studies will be needed to understand whether neuronal spiking of dentate gyrus, CA3, and CA1 neurons differentially represent spatial and object information during distinct phases of object memory.
Object-Specific Activity of CA1 Neurons
Support for nonspatial information processing by rodent CA1 neurons comes from reports of object-specific activity recorded from neurons in the distal and medial subregions of CA1, which receive direct input from the perirhinal and lateral entorhinal cortices (Amaral and Witter 1995; Naber et al. 1999), which primarily convey item-related information (Graves et al. 2012; Jacobs and Schenk 2003; Knierim et al. 2014; van Strien et al. 2009). However, recent reports also revealed that medial and lateral entorhinal cortical neurons code for object-related experiences (Høydal et al. 2019; Tsao et al. 2013). Future studies will be needed to determine the degree to which conjunctive representations of place and item information by entorhinal cortical neurons emerges during or after initial exploration of novel objects during the OR task. Our recording studies focused on determining whether the activity of CA1 place cells, determined to be stable in the otherwise empty, familiar arena, modified their activity to represent object-in-place or object-in-context during or after the sample session. Based on our inclusion criteria (see materials and methods), CA1 neurons that were silent during the preliminary cue card rotation sessions were not analyzed during the subsequent sample and test sessions. However, in reviewing all tetrode channels after completing data collection, we did identify an interesting nonplace correlate during object recording sessions (i.e., sample and test). Specifically, we recorded a subset of CA1 neurons with firing modulated by the exploration of objects independent of spatial location. When the mouse was at the location of objects, these neurons fired at higher rates, and in a manner unlike hippocampal complex-spiking pyramidal neurons. The activity of these nonplace CA1 neurons was often recorded from the same tetrodes that the activity of place cells was recorded simultaneously. CA1 neurons have been reported to fire when the rat is in close proximity to items or barriers within specific contexts, but these cells exhibit complex spike discharge patterns; likely reflecting a place cell subtype (Rivard et al. 2004). Interestingly, our unique CA1 cells fired exclusively at objects, regardless of the object identity, possibly indicating that their activity represents a partial and incomplete representation of the object, much like a place field represents a subregion within a specific context. Future studies could test whether the activity of a given object-specific cell fires concurrently for object identity or is dependent upon input from the lateral entorhinal cortex or perirhinal cortex. Analyses revealed that the peak firing rates of object-related CA1 neurons increased across several object exposures, an experience-dependent effect that has not been observed during repeated recordings of place cells (see Supplemental Fig. S1e). That is, hippocampal place cells tend to maintain a variable, firing rate independent of objects (Burke et al. 2011, and see Supplemental Fig. S1f). Previous studies report that rats trained in paired context/location-specific item-reward associations exhibit changes in CA1 and CA3 complex spike activity, and repeated training resulted in increased item-location firing frequencies (Komorowski et al. 2009); however, only complex-spiking cells were reported. Notably, throughout training, there were no reported differences in the firing properties of stable place cells. Therefore, it is plausible that item-location activity is reflected in the firing of object-specific neurons, which modulate the spatial map over time or across training. Taken together, our reported object-specific activity provides electrophysiological evidence for CA1 object information processing and lends support to the view that spatial and nonspatial information is processed in parallel within the CA1 of hippocampus.
Conclusions
Our data indicate that, on the timescale at which CA1-dependent object memory is encoded and retrieved during the object recognition task, the firing properties and spatial mapping of CA1 neurons are not altered. Our results suggest that following a sufficient postsample session delay, object memory is processed concurrently with spatial information in the CA1 region of hippocampus. The lack of influence of OR task performance on place cell firing properties implies that place cells do not code for object information. Additionally, CA1 place field maps established during OR arena habituation sessions were not altered during subsequent recording sessions in which objects were present (i.e., sample and test), implying that the recorded place cell activity did not reflect object-in-context representations. However, silencing CA1 activity led to significant impairments in each of the three OR protocols, in a delay-dependent manner. Thus, the pattern of results suggests a conundrum — CA1 neuronal activity is critical for object memory and spatial localization, yet during the OR task object and spatial information may be processed in parallel via distinct output streams from the CA1. This interpretation is consistent with the parallel map theory (Jacobs and Schenk 2003), which states that integrated object-context representations may be extrahippocampal (Chang and Huerta 2012) and more specifically attributed to the postrhinal cortex (Furtak et al. 2012) or subiculum. Interestingly, two distinct pyramidal cell types, referred to as bursting and regular spiking, have been characterized within the CA1 and subiculum from acute hippocampal slice recordings. Graves et al. (2012) concluded that these distinct cell types process different types of information in parallel, with the bursting cells receiving inputs from the medial entorhinal cortex regarding spatial information and the regular spiking cells receiving direct projections from the lateral entorhinal cortex regarding nonspatial information. Accordingly, our object-specific neuronal activity may reflect that of regular spiking (nonbursting) pyramidal cells since these neurons did not exhibit complex spike activity, a hallmark characteristic of CA1 place cells. Given that inactivation of CA1 neuronal activity impaired OR (at sufficiently long delays), and place maps remained stable throughout the task, we conclude that it is not an object-in-context memory that is impaired when CA1 neuronal function is suppressed, but rather a subset of regular spiking object information processing CA1 neurons that are responsible for the OR impairments. Accordingly, these results demonstrate a compelling delay-dependent, context-independent parallel role of CA1 in rodent object memory.
GRANTS
This project was supported in part by a grant from the National Institute of Mental Health (MH086591) to R.W.S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.N.A., S.J.C., and R.W.S. conceived and designed research; H.N.A. and S.J.C. performed experiments; H.N.A., S.J.C., and R.W.S. analyzed data; H.N.A., S.J.C., and R.W.S. interpreted results of experiments; H.N.A., S.J.C., and R.W.S. prepared figures; H.N.A., S.J.C., and R.W.S. drafted manuscript; S.J.C. and R.W.S. edited and revised manuscript; H.N.A., S.J.C., and R.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alcira H. Munchow for expert assistance with stereotaxic surgeries and behavioral testing, Dr. Gongliang Zhang for advice regarding the in vivo electrophysiological recording protocols, and Brittany Crafton for assistance conducting MATLAB analyses of place cell activity. We thank Daniel Wilson for offering advice on conducting analyses with MATLAB, and we are grateful to Drs. Isabel Muzzio and Alexander Keinath for providing several MATLAB scripts critical for the analyses. We also thank Drs. Rodney Murphey, Robert Vertes, and Elan Barenholtz for advice and lively discussions of the interpretation of the hippocampal recording results, and for critiques of an earlier draft of this manuscript.
REFERENCES
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res 167: 183–195, 2006. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: The Rat Nervous System, edited by Paxinos G. San Diego, CA: Academic Press Inc, 1995, p. 443–493. [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem 9: 58–65, 2002. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettis T, Jacobs LF. Sex differences in object recognition are modulated by object similarity. Behav Brain Res 233: 288–292, 2012. doi: 10.1016/j.bbr.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. Grid cells require excitatory drive from the hippocampus. Nat Neurosci 16: 309–317, 2013. doi: 10.1038/nn.3311. [DOI] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193–205, 1991. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem 17: 5–11, 2010. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety AR, Wallace JL, Barnes CA. The influence of objects on place field expression and size in distal hippocampal CA1. Hippocampus 21: 783–801, 2011. doi: 10.1002/hipo.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res 111: 187–202, 2000. doi: 10.1016/S0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Chang EH, Huerta PT. Neurophysiological correlates of object recognition in the dorsal subiculum. Front Behav Neurosci 6: 46, 2012. doi: 10.3389/fnbeh.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. J Neurosci 22: 4663–4669, 2002. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860, 2000. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdóttir HN, Stackman RW Jr. The rodent hippocampus is essential for nonspatial object memory. Curr Biol 23: 1685–1690, 2013. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285: 105–117, 2015. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Muller RU, Poucet B. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci 17: 2531–2542, 1997. doi: 10.1523/JNEUROSCI.17-07-02531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Langston R, Morris RG. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424: 205–209, 2003. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Luft T, Roesler R, Schröder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett 405: 142–146, 2006. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus 23: 253–267, 2013. doi: 10.1002/hipo.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152, 2007. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28: 8660–8667, 2008. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15: 347–355, 2005. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience 144: 1186–1192, 2007. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Furini CR, Myskiw JC, Schmidt BE, Zinn CG, Peixoto PB, Pereira LD, Izquierdo I. The relationship between protein synthesis and protein degradation in object recognition memory. Behav Brain Res 294: 17–24, 2015. doi: 10.1016/j.bbr.2015.07.038. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron 76: 976–988, 2012. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci 121: 218–223, 2007. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, Spruston N. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 76: 776–789, 2012. [Erratum in Neuron 77: P375, 2013]. doi: 10.1016/j.neuron.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijima A, Ichitani Y. Dissociable anterograde amnesic effects of retrosplenial cortex and hippocampal lesions on spontaneous object recognition memory in rats. Hippocampus 22: 1868–1875, 2012. doi: 10.1002/hipo.22021. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82: 26–34, 2004. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Høydal ØA, Skytøen ER, Andersson SO, Moser MB, Moser EI. Object-vector coding in the medial entorhinal cortex. Nature 568: 400–404, 2019. doi: 10.1038/s41586-019-1077-7. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Schenk F. Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychol Rev 110: 285–315, 2003. doi: 10.1037/0033-295X.110.2.285. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42: 283–295, 2004. doi: 10.1016/S0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15: 1648–1659, 1995. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci 369: 20130369, 2014. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci 29: 9918–9929, 2009. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER. The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res 215: 275–291, 2010. doi: 10.1016/j.bbr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets. Prog Brain Res 163: 615–625, 2007. doi: 10.1016/S0079-6123(07)63033-7. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem 16: 616–624, 2009. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683, 1982. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951–1968, 1987. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The firing of hippocampal place cells predicts the future position of freely moving rats. J Neurosci 9: 4101–4110, 1989. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB Jr. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci 7: 1935–1950, 1987. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9: 49–57, 2002. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus 15: 1050–1056, 2005. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- Muzzio IA, Kentros C, Kandel E. What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. J Physiol 587: 2837–2854, 2009a. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio IA, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott LF, Kandel ER. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus [Errata in PLoS Biol 8: 10.1371/annotation/5e28240c-6186-43eb-b319-79c391d9468a and 10.1371/annotation/d0e5ef6f-d08b-474a-8fde-aeebeee7369d]. PLoS Biol 7: e1000140, 2009b. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopez da Silva FH. Perirhinal cortex input to the hippocampus in the rat: evidence for parallel pathways, both direct and indirect. A combined physiological and anatomical study. Eur J Neurosci 11: 4119–4133, 1999. doi: 10.1046/j.1460-9568.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, UK: Clarendon Press, 1978. [Google Scholar]
- O’Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends Cogn Sci 6: 505–510, 2002. doi: 10.1016/S1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17: 155–160, 2010. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena RR, Pereira-Caixeta AR, Moraes MF, Pereira GS. Anisomycin administered in the olfactory bulb and dorsal hippocampus impaired social recognition memory consolidation in different time-points. Brain Res Bull 109: 151–157, 2014. doi: 10.1016/j.brainresbull.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2: 898–905, 1999. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Rivard B, Li Y, Lenck-Santini PP, Poucet B, Muller RU. Representation of objects in space by two classes of hippocampal pyramidal cells. J Gen Physiol 124: 9–25, 2004. doi: 10.1085/jgp.200409015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn Mem 14: 714–731, 2007. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Res 373: 577–604, 2018. doi: 10.1007/s00441-017-2744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14: 36–46, 2007. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem 16: 573–585, 2009. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus 5: 375–389, 1995. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci 106: 447–456, 1992. doi: 10.1037/0735-7044.106.3.447. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An Information-Theoretic Approach to Deciphering the Hippocampal Code, edited by Hanson SJ, Cowan JD, Giles CL. San Mateo, CA: Morgan Kaufmann, 1993, p. 1030–1037. Advances in Neural Information Processing Systems. [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6: 149–172, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 27: 279–306, 2004. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stackman RW Jr, Cohen SJ, Lora JC, Rios LM. Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiol Learn Mem 133: 118–128, 2016. doi: 10.1016/j.nlm.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW Jr, Lora JC, Williams SB. Directional responding of C57BL/6J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. J Neurosci 32: 10211–10225, 2012. doi: 10.1523/JNEUROSCI.4868-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: the role of the hippocampal formation in learning, memory and amnesia. Psychobiology 17: 129–144, 1989. doi: 10.3758/BF03337828. [DOI] [Google Scholar]
- Taube JS, Muller RU, Ranck JB Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10: 436–447, 1990. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimper JB, Galloway CR, Jones AC, Mandi K, Manns JR. Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep 21: 2419–2432, 2017. doi: 10.1016/j.celrep.2017.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser MB, Moser EI. Traces of experience in the lateral entorhinal cortex. Curr Biol 23: 399–405, 2013. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science 316: 76–82, 2007. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Fortress AM, Frick KM. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156: 103–116, 2018. doi: 10.1016/j.nlm.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10: 272–282, 2009. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- von Ziegler LM, Selevsek N, Tweedie-Cullen RY, Kremer E, Mansuy IM. Subregion-specific proteomic signature in the hippocampus for recognition processes in adult mice. Cell Rep 22: 3362–3374, 2018. doi: 10.1016/j.celrep.2018.02.079. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science 261: 1055–1058, 1993. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25: 52–61, 2005. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24: 5901–5908, 2004. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Hwaun E, Colgin LL. Fast gamma rhythms in the hippocampus promote encoding of novel object-place pairings. eNeuro 3: ENEURO.0001-16.2016, 2016. doi: 10.1523/ENEURO.0001-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]