Keywords: metabolic syndrome, microbiota, non-alcoholic fatty liver diseases, small intestine, vitamin D
Abstract
A lack of sunlight exposure, residence in the northern latitudes, and dietary vitamin D insufficiency are coprevalent with metabolic syndrome (MetS), Type 2 diabetes (T2D), and nonalcoholic fatty liver diseases (NAFLD), implying a potential causality and underlying mechanism. Whether vitamin D supplementation or treatment can improve these disorders is controversial, in part, because of the absence of large-scale trials. Experimental investigations, on the other hand, have uncovered novel biological functions of vitamin D in development, tumor suppression, and immune regulation, far beyond its original role as a vitamin that maintained calcium homeostasis. While the large intestine harbors massive numbers of microbes, the small intestine has a minimal quantity of bacteria, indicating the existence of a gating system located in the distal region of the small intestine that may restrain bacterial translocation to the small intestine. Vitamin D receptor (VDR) was found to be highly expressed at the distal region of small intestine, where the vitamin D signaling promotes innate immunity, including the expression of α-defensins by Paneth cells, and maintains the intestinal tight junctions. Thus, a new hypothesis is emerging, indicating that vitamin D deficiency may impair the intestinal innate immunity, including downregulation of Paneth cell defensins, leading to bacterial translocation, endotoxemia, systemic inflammation, insulin resistance, and hepatic steatosis. Here, we review the studies for vitamin D for innate immunity and metabolic homeostasis, and we outline the clinical trials of vitamin D for mitigating MetS, T2D, and NAFLD.
VITAMIN D, MORE THAN A VITAMIN
Having been discovered almost 100 years ago, and named as the fourth vitamin (54), vitamin D in many ways does not fit the classic definition for vitamins, since humans are able to synthesize it, given sufficient sunlight exposure. Differing biochemically and differentiating itself from other vitamins, which often serve as coenzymes for metabolic reactions, vitamin D is more like a steroid hormone that regulates gene expression through nuclear receptor (vitamin D receptor, VDR). As a consequence of evolution and migration from Africa to the northern latitudes with clothing covering their bodies, humans gradually developed mechanisms for de novo synthesis of vitamin D (18). To cope with insufficient sunshine exposure in current societies worldwide, dairy products are often fortified with vitamin D. Thus, vitamin D has become a micronutrient, and 10–20 µg/daily (400–800 IU) is recommended in many countries. Determination of the vitamin D status in the body is often assessed by measuring the plasma 25(OH)D3. The 25(OH)D3 has a half-life of ~3 wk, while the biological active component, 1,25(OH)2VD is unstable, and its half-life is measured in hours. The definition of vitamin D deficiency or insufficiency is a matter of debate; but the plasma 25(OH)VD3 levels at 50–75 nM is generally regarded as a healthy condition, as defined by the Institute of Medicine (IOM) and Endocrine Society (ES) (1, 37). Vitamin D insufficiency (plasma 25OHVD3 at 25–50 nM) and deficiency (25OHVD3<25 nM) are overwhelmingly prevalent worldwide. Vitamin D deficiency or insufficiency is a global health issue that afflicts more than 1 billion people worldwide (37). Recent studies showed that in Europe, vitamin D deficiency (<30 nmol/L) and insufficiency (<50 nmol/L) account for 13% and 40% of the general population, respectively (67). Public health agencies in many countries, including National Academy of Medicine in the United States, increased the recommendation of daily value of vitamin D (RDA) to 600 IU for people 1–70 yr-old, and 800 IU for 71+ yr-old (5).
In response to UVB exposure and through light-catalyzed reactions, the ring of 7-dehyroxyl-cholesterol is broken and converted to secosteroid, as cholecalciferol or vitamin D3 (Fig. 1). It is estimated that 20 min of direct sunlight exposure may synthesize 400–800 IU VD3. Otherwise, fortified milk or vitamin D-containing foods, such as fish and yolk, are the major source of vitamin D. In sunlight, an equivalent secosteroid can be made in plants, for example, mushrooms, called vitamin D2 (ergocalciferol), which has undistinguishable biological functions as VD3. Vitamin D supplements (VD2 or VD3) are widely accepted in developed countries. Calcitriol, 1,25(OH)2VD3, the biologically active component of vitamin D, is synthesized by two steps of hydroxylation that occurs sequentially in the liver (CYP2R1/CYP27A1 for 25-hydroxylation) and kidney (CYP27B1 for 1α-hydroxylation). Turnover of calcitriol takes ~5 to 8 h in humans through hydroxylation at position-24 of the secosteroid (CYP24A1) followed by oxidation to vitamin D acid for excretion in the urine. Calcitriol, similar to the steroid hormones, assumes its biological functions mainly through the vitamin D receptor (VDR), a typical nuclear receptor and transcriptional factor, which binds at particular DNA response elements called vitamin D response elements (VDRE) for gene expression in genome-wide scales and epigenetic determination (42).
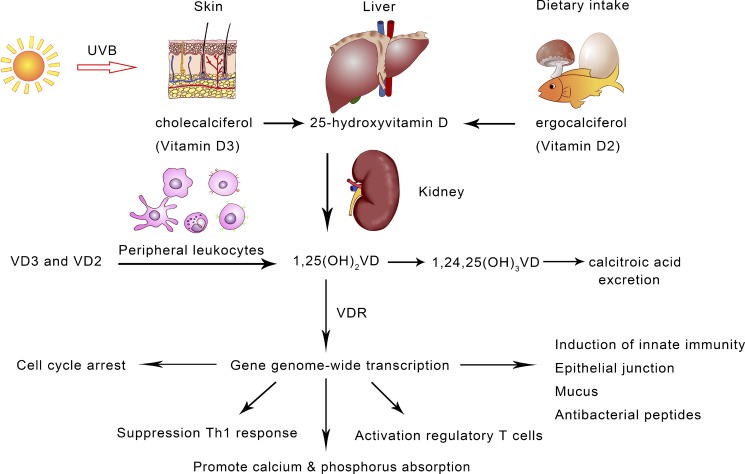
Fig. 1.
Vitamin D biosynthesis and major biological functions. With exposure to sunlight, animals, including humans and certain plants, can synthesize vitamin D through light reactions in the epithelial cells. As the consequence of urbanization and a lack of sunlight exposure, vitamin D has been regarded as a micronutrient in supplements and dairy products. The biologically active vitamin D, 1,25(OH)2VD, also known as calcitriol, is synthesized sequentially in the liver and kidney. In the course of evolution with regular exposure to sunlight, humans and other terrestrial animals have developed the vitamin D-dependent metabolisms, mediated through vitamin D receptor (VDR) and signaling for transcriptional control at a genome-wide scale. In addition to promoting calcium/phosphorus adsorption in the small intestine, vitamin D exerts pleiotropic functions. It is known that vitamin D signaling maintains immune homeostasis through suppression of adaptive immunity and promoting innate immunity.
The prevalence of vitamin D deficiency overlaps with the incidence of Type 2 diabetes (T2D), metabolic syndrome (MetS), and nonalcoholic fatty liver disease (NAFLD). Metabolic diseases, including obesity, T2D, MetS, and NAFLD, often share common etiology basis, such as systemic inflammation, insulin resistance, cardiovascular disorders, and hepatic steatosis. These metabolic diseases also have common causes, such as excessive calorie intake and insufficient physical activity. On the other hand, a lack of sunlight exposure is often linked to obesity and MetS, suggesting the contribution of vitamin D deficiency. Many epidemiological surveys clearly demonstrate an association of vitamin D deficiency with T2D, MetS, and NAFLD. For example, a cohort study in the European Prospective Investigation with 9,671 incident T2D cases found that the plasma total 25(OH)D levels were inversely associated with the incidence of T2D (97). A cross-sectional study from the United States found a significant inverse relationship between 1,25(OH)2D (n = 1,048) and 25(OH)D (n = 2,096) concentrations, with high triglyceride and low high-density lipoprotein levels (11). A report from the National Health and Nutrition Examination Surveys 2003–2006 concluded that higher 25(OH)D and dietary vitamin D intake are associated with reduced prevalence of metabolic syndrome (51). The association of vitamin D deficiency and metabolic risk was also reported in Chinese adults with prediabetes (81). One study of 4,364 postmenopausal Korean women demonstrated that an increased serum 25(OH)D level was associated with low incidence of elevated blood pressure, elevated TGs, and reduced HDL-C (22). Vitamin D levels are inversely associated with the adolescent obesity and T2D (30). One study of people living in Northern Finland (latitude 65°N) noted that nearly 80% of the subjects had low vitamin D levels, which were associated with a high prevalence of MetS, while the subjects with vitamin D supplementation had a significantly lower incidence of MetS (59). A recent meta-analysis has suggested a significant reduction in alkaline phosphatase after vitamin D supplementation in NAFLD patients (52). Low 25(OH)vitamin D levels are associated with the presence of NAFLD, and VDR expression in the liver is negatively associated with the severity of liver histology in nonalcoholic steatohepatitis (NASH) (8, 9). A study of biopsy-proven NAFLD found that serum 25OHD3 levels and the VDR gene single nucleotide polymorphism (SNP) were significantly and independently associated with the severity of liver fibrosis (3).
Despite the public enthusiasm and apparently strong association between vitamin D deficiency and these metabolic diseases, it is still unclear whether vitamin D deficiency contributes or enhances the metabolic syndrome and NAFLD. Although clinical surveys and trials have sometimes given contradictory results, the experimental models based on animal studies have clearly demonstrated the critical roles of vitamin D signaling in physiological functions beyond its roles in calcium and phosphorus homeostasis (18). More well-designed human studies and trials are needed. In this review, we focus on vitamin D functions in innate immunity for antagonizing the MetS, T2D, and NAFLD. Additional studies for the association of vitamin D (VD) status with MetS, T2D, and NAFLD are summarized in Table 1.
Table 1.
Clinical investigation for the association of vitamin D status with metabolic syndrome, T2D, and NAFLD
| First Author | Study Design | n | Participants | Vitamin D Measure | Efficacy Measurement | Conclusion |
|---|---|---|---|---|---|---|
| Ceglia et al. (20) | Cross-sectional | 2,161 | Adults with prediabetes | Weight and 25OHD at baseline | Body weight, BMI, waist circumference, and adipose tissue distribution | Inversely associated |
| Krul-Poel et al. (45) | Cross-sectional | 241 | Dutch patients with Type 2 diabetes | 50,000 IU vitamin D once a month for 6 mo | Glycemic control | No significant association with health-related quality of life |
| Naderpoor et al. (60) | Cross-sectional | 120 | Healthy volunteers with no history of liver disease | Serum 25OHD concentration and liver enzymes | Liver enzymes (GGT, ALT, and ALP) | No association |
| Park et al. (63) | Cross-sectional | 7,514 | Healthy Korean adults | Serum 25OHD levels and visceral fat mass | NAFLD was diagnosed by using abdominal ultrasonography | Vitamin D status was associated with NAFLD in men but not in women. |
| Zhai et al. (95) | Cross-sectional | 5,066 | Adults in 16 areas of East China | 25OHD levels, plasma glucose and lipid profile and insulin resistance | NAFLD evaluation by liver ultrasonography | A significantly higher prevalence of NAFLD in men with lower vitamin D |
| Wang et al. (87) | Cross-sectional | 4,161 | 2,700 men with a mean age of 53 yr and 1,461 postmenopausal women over 55 | 25OHD and sex hormone binding globulin SHBG levels | The degree of fatty liver based on ultrasonography following guidelines of the American Diabetes Association | The combined association of low SHBG and low vitamin D was much higher in moderate-severe NAFLD |
ALP, alkaline phosphatase; ALT, amino transferase; GGT, glutamyl transpeptidase; NAFLD, nonalcoholic fatty liver disease; SHBG, sex hormone-binding globulin; T2D, Type 2 diabetes.
Vitamin D suppresses inflammation and Th1 response. Decades of work have shown that vitamin D plays critical roles in modulation of adaptive and innate immunity (56). For example, the vitamin D metabolite 1,25(OH)2VD3 can inhibit T-helper 1 (TH1)- and enhance TH2-cell responses. It also decreases TH17 cell differentiation, with reciprocal upregulation of forkhead box protein 3 (FOXP3)+ regulatory T (TReg) cells and T regulatory type 1 (TR1) cells (99). 1,25(OH)2VD3 also inhibits the proliferation of B cells and their differentiation into antibody-secreting cells. 1,25(OH)2VD3 modulates dendritic cell functions by impairing their maturation to suppress Th1 cells (64). Thus, VD signaling contributes to immune homeostasis by suppression of adaptive immunity. It has been known for centuries that sunlight exposure and fish liver oil could mitigate tuberculosis. Vitamin D as an immune adjuvant can suppress Mycobacterium tuberculosis grown in macrophages through Toll-like receptor (TLR) activation (48). The gastrointestinal tract is the major tissue in humans that expresses VDR in high quantity, suggesting physiological roles of vitamin D signaling in the innate immunity of the gut. Intestinal tissues of vitamin D3-deficient mice displayed increased inflammatory cell infiltrates, as well as significantly higher gene transcript levels of inflammatory mediators, such as TNF-α, IL-1β, IL-6, TGF-β, IL-17A, and IL-17F, as well as the antimicrobial peptide REG3γ (16). One study identified the inhibitory effects of vitamin D on LPS-stimulated inflammatory response in human blood monocytes and noticed that both 1,25OH2D3 and 25OHD3 dose dependently inhibited LPS-induced p38 phosphorylation through induction of MAPK phosphatase-1 (MKP-1) in monocytes/macrophages (96).
Oral immune tolerance for food antigens and microbes from the gut is mediated by intestinal immunity, which is mediated, in part, by the peripheral mucosal iTreg cells (induced T-regulatory cells) and tolerogenic antigen-presenting cells in the gut-associated lymphoid tissue. Vitamin D signaling contributes significantly to the immune tolerance in many ways. For example, in a colitis animal model, mice carrying VDR deletion in gut epithelial cells (Villin-Cre-VDR) developed more severe colitis than VDR control mice, characterized by more robust T1 and T17 responses, with greater increases in mucosal IFN-γ, IL-17, and IFN-γ IL-17 T cells (34). The functions of VD in immune tolerance were further demonstrated by clinical studies in which providing human subjects a high dose of VD supplement promoted peripheral TReg (13, 69).
Vitamin D promotes intestinal innate immunity and barrier functions. The intestinal mucosal barrier through its physical, biochemical, and immune machineries constitutes the first barrier in the gut. First, mucus separates the hydrolytic luminal contents from the intestinal epithelium (83). The mucus consists of a highly glycosylated hydrated gel (mucin) that is secreted by Goblet cells (G cells). The mucus also works as a molecular lubricator, preventing large food particles from injuring the epithelial cell layer, while allowing small molecules to pass through. Mucin 2 coding gene, muc2, has VDRE cis-elements in its proximal promoter, and dietary vitamin D depletion downregulated muc2 expression and disruption of the mucus lining (76). In animal models, VD signaling was found to regulate mucin gene expression, and dietary vitamin D deficiency led to impaired mucus and increased permeability. Tight junctions in the lateral sides of intestinal epithelial cells are critical for innate immunity. Work on animal models has demonstrated that vitamin D deficiency, either dietary depletion of VD or VDR genetic knockout (KO), can significantly downregulate mucin genes, as well as tight junction genes, such as ZO1, occludin, and claudin (76). In dextran sulfate sodium (DSS)-induced colitis model, VDR KO mice were much more susceptible for intestinal injury than the wild-type mice (43). From in vitro cell culture, it was known that calcitriol could promote the tight junctions and improve the impermeability for bacterial endotoxin infiltration (4). Intestinal bacterial translocation to the liver is often found in chronic liver disease patients, in part, due to loss of the intestinal barrier. On the basis of the rat model of liver disease, one found that 1,25(OH)2D3 could improve the cirrhosis through increased intestinal tight junction protein expression and barrier exclusion (88).
The gut is an active site of vitamin D-targeting tissue, while Cyp27B1, a critical enzyme for 1α-hydroxylation of 25OHVD was found expressed in the gut, suggesting potential paracrine of the VD signaling for resident immune and intestinal epithelial cells (2). Animal work showed that vitamin D deficiency altered intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis (71). The vitamin D-deficient mice suffered greater bacterial translocation, including the mesenteric lymph nodes, spleen, and liver.
One study showed that pigs subjected to antigen exposure together with the addition of 1α,25(OH)2D3 could enhance the antigen-specific IgA-response and activate the lymphocytes in the GALT tissues (84). Innate lymphoid cells (ILC) are critical for protective immunity and mucosal barrier in the gut, and a study showed that vitamin D receptor signaling regulates gut ILC3. VDR deletion led to reduction in colonic ILC3 populations at steady state and impaired ILC3 responses following Citrobacter rodentium infection, resulting in substantial increases in intestinal bacterial growth and mouse mortality (35)
The innate response is often initiated when foreign microbes are recognized by pattern recognition receptors (PRR), a group of sensor proteins on the cellular membrane or inside cells, which recognize components that are common for the microorganisms. The PRR of the innate immune cells, including dendritic cells (DCs), macrophages, monocytes, neutrophils, and epithelial cells in the small intestine, can recognize two categories of targeting components: the pathogen-associated molecular patterns (PAMPs), which are derived from the foreign microbes or antigens; and damage-associated molecular patterns (DAMPs), which are derived from the host’s damaged or dead components. Among the PRR superfamily are 1) the TLR, located either in the plasma membrane or in the cytoplasm; 2) the cytosolic receptors, such as NOD-like receptors and RIG (retinoic acid-inducible genes)-like receptors; and 3) inflammasomes that make matured IL-1β and IL-18. Vitamin D signaling was found to suppress the expression of PRR to prevent the host from excessive immune reactions for tissue homeostasis (41). It is well known how VD signaling upregulates the gene expression through VDR/VDRE-promoted transcription initiation, while it is less known how VD signaling suppresses the genes for inflammation.
Vitamin D promotes the Paneth cell defensins to restrain microbial growth in the small intestine (Fig. 2). A remarkable function of innate immunity in the gut is the production antimicrobe peptides (AMPs) that can directly suppress or kill the microbes in the lumen of the small intestine. In the small intestine, Paneth cells (PCs), located at the bottom of crypts of Lieberkühn, are the major source of lumen-secreted antimicrobial components, including α‐defensins along with lysozyme and phospholipase A2. Paneth cells are exclusively found throughout the intestinal epithelium, and there are a greater number of PCs in the distal small intestine compared with the proximal portions. Most of defensin peptides made by innate immune cells, such as neutrophils and NK cells, are secreted into the bloodstream in an endocrine fashion, while α-defensin 5 (DEFA5) and α-defensin 6 (DEFA6) (cryptdins for mice), produced by Paneth cells, are secreted exclusively into the lumen of the small intestine. Defensins are 3–4 kDa, small conserved amphipathic peptides rich in cationic and hydrophobic amino acid residues. α-Defensins have two peptide chains with six conserved cysteine residues to form three pairs of disulfide bonds. In Paneth cells, prodefensins are proteolytically processed and activated by matrix metalloproteinase-7 (MMP7) (6). In sensing of bacterial antigens, Paneth cells can quickly release the defensins (7). Lacking VD signaling, either dietary vitamin D depletion or VDR-KO can downregulate the expression of MMP7 and α-defensin 5 in Paneth cells for gut dysbiosis (77). Autophagy was found to be essential for Paneth cells to sense bacterial invasion and secrete antibacterial peptides (12, 15). One study found that vitamin D through induction of autophagy controlled expression of cathelicidin, a protein that has direct antimicrobial activity (93). Using transgenic mice to express human DEFA5, authors of one study showed reduction of gut Firmicutes, and concomitantly promoted the relative abundance of Bacteroidetes. However, MMP7 KO mice, presumably lacking of α-defensin maturation and activation, had opposite consequences, which collectively demonstrated the critical roles of α-defensins from Paneth cells in maintaining intestinal eubiosis (73). Likewise, the impaired expression of the Paneth cell-specific defensins has been found in obese human subjects (36).
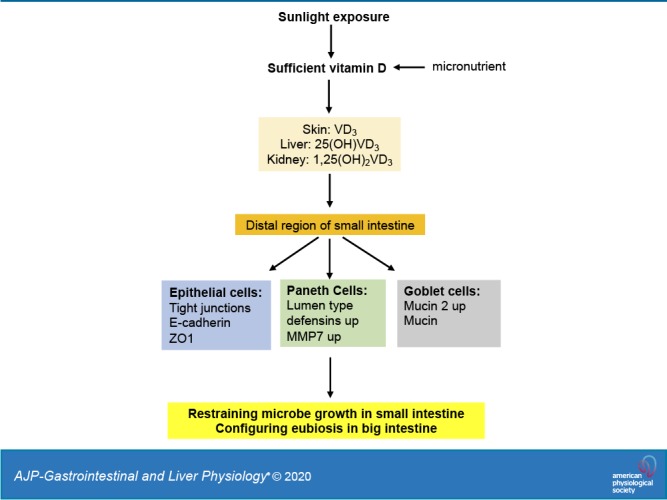
Fig. 2.
Vitamin D in modulating intestinal innate immunity to determine gut microbiota. While the large intestine harbors massive number of microbes, the small intestine has a minimal level of the microbiome, indicating the existence of a gating system at the distal region of the ileum, which restrains the translocalization of gut microbes into the small intestine. The vitamin D receptor is highly expressed at the end of small intestine, and vitamin D signaling can upregulate the Paneth cell defensins that restrain bacterial growth in the small intestine. Moreover, vitamin D signaling also upregulates the tight junctions of intestinal epithelial cells and mucin expression in the Goblet cells for innate immune homeostasis. Conversely, vitamin D deficiency, which is often associated with Type 2 diabetes and nonalcoholic fatty liver disease, may lead to gut dysbiosis and endotoxemia for systemic inflammation, insulin resistance, and metabolic disorders.
Vitamin D regulates gut innate immunity in the pattern recognition receptor (PRR) pathways. PRRs are essential for the innate immune system. PRRs are cellular sensors, which detect molecules typical for the pathogen types. PRRs in the intestinal epithelial cells can identify two classes of molecules: pathogen-associated molecular patterns (PAMPs) from microbial pathogens and damage-associated molecular patterns (DAMPs) from damaged tissues. PRRs also initiate antigen-specific adaptive immune response and release of inflammatory mediators. There are membrane-bound PRRs, including Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as cytoplasmic PRRs, such as NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs). An in vitro study showed that calcitriol decreased TLR-induced cytokine production and enhanced cytokine levels induced by muramyl dipeptide (MDP), the NOD2 ligand. However, calcitriol inhibited Mo-D-TLR-induced cytokines, but costimulation of NOD2 resulted in increased IL-10 and IL-23 (25). In another in vitro experiment, calcitriol was found to stimulate expression of NOD2/CARD15/IBD1 gene and protein in primary human monocytic and epithelial cells, whereas NOD2 was often downregulated in inflammatory bowel diseases (89). It was shown that calcitriol can suppress TLR4-mediated inflammation through induction of IκB, a cellular inhibitor IKK pathway (28). Likewise, vitamin D downregulates proinflammatory cytokine response to Mycobacterium tuberculosis through PRR, while inducing protective cathelicidin production (41).
Vitamin D, through epigenetic remodeling, regulates intestinal physiology. Approximately 3% of the mouse and human genomes are regulated directly or indirectly by vitamin D signaling (19). VDR, functioning as heterodimer with retinoid X receptor (RXR) as VDR/RXR, can directly bind to specific genomic sequences on the promoter regions of target genes, called vitamin D response elements (or VDREs), which can further recruit transcription factors, cotranscriptional factors, or cosuppressors to either activate or suppress specific gene transcription. The nature of VDR binding to specific genes relies on cell types and their existing epigenetic code, namely, the chromatin modifications, such as methylation and acetylation. Furthermore, VD-VDR binding may also impact the local epigenetic modeling that may lead to various consequences, such as cell cycle, immune function, cell proliferation and differentiation, growth regulation, and tumor suppression. For example, VDR along with histone modification enzymes may be involved in acetylation, deacetylation, methylation, and demethylation, of different epigenetically regulated target genes (94). Vitamin D changes the status of DNA methylation and histone modifications, resulting in the activation of tumor suppressors and inhibition of oncogenes. In addition, vitamin D activates the expression of tumor-suppressing miRNAs, which contribute to the tumor-suppressive activity (78). Epigenetic studies showed that 1,25(OH)2D3 can regulate thousands of genes at genome-wide scale, and ~2,000 to 8,000 VDR binding sites can be detected following activation by 1,25(OH)2D3 stimulation in a cell-type-dependent manner. On the other hand, large amounts of VDR are persistently bound in chromatin independent of 1,25(OH)2D3 binding (65). Moreover, there are reports showing how 1,25(OH)2D3 regulates transcription via multiple enhancers at distal regions of the targeting genes.
Nutritional abnormalities are common in patients with gastroparesis, a disorder that may affect gastric motility and may delay emptying. One report showed that low 25(OH)VD levels is associated with slow gastric emptying (40). Clinical trials are under way to examine the influence of vitamin D on the effects of epigenetic aging (21). A recent report found that neuroprotective effects of vitamin D for high-fat diet and palmitic acid-induced enteric neuronal loss in the mice (47).
SUNLIGHT EXPOSURE AND VITAMIN D IN MODULATING GUT MICROBIOTA
Whether sunlight exposure or vitamin D can modulate gut microbiota is an intriguing topic. In zoology and at an ecogeographical scale, Bergmann’s Rule states that the body size of the warm-blooded animals is related to the local temperature and sunlight at various latitudes (55). One study found a positive correlation between Firmicutes and northern latitudes, and a negative correlation between Bacteroidetes, showing that variation of gut microbial composition is consistent with the pattern expected by Bergmann’s rule (79). Importantly, this finding also implies that sunlight exposure may impact on gut microbiota and the body’s metabolic capacity and homeostasis. It is well known that a high abundance of Firmicutes and a low abundance of Bacteroidetes in gut microbiome are often related to obese and metabolic syndromes. It is known that Firmicutes promote energy harvesting (82). Using conditional knockout mice, one group found that a lack of vitamin D signaling in intestinal epithelial cells resulted in gut dysbiosis and exacerbated drug-induced inflammatory bowel diseases (92). In the VDR KO mice, Lactobacillus was depleted in the fecal stool, whereas Clostridium and Bacteroides were enriched (38).
Using animal models, we demonstrated the importance of vitamin D signaling in maintaining gut microbiota in an eubiosis state through functioning Paneth cells (76). In particular, we found VDR is highly expressed in the distal region of small intestine where the VDR levels are ~1,000-fold greater than that in the liver. VDR is abundantly expressed in the cells of crypts of Lieberkühn, the location of the Paneth cells. We found that vitamin D signaling is critical for maintaining the high-level expression of α-defensin 5, and MMP7, a converting enzyme for pro-α-defensin. Conversely, dietary depletion of vitamin D or VDR knockout resulted in downregulation of α-defensins and MMP7, as well as tight junction proteins in the ileum. Likewise, in an animal model of high-fat feeding, we found that sufficient vitamin D in the standard chow (AIN93), by which the VD levels are equivalent to the daily value of diet for humans, could significantly suppress the metabolic disorders featured by partial restoration of insulin sensitivity, reduction of plasma triglycerides, improved glucose tolerance, and attenuation of hepatic steatosis. Importantly, vitamin D deficiency or VDR knockout in the animal models exacerbated gut dysbiosis, showing increased abundance of Firmicutes and decreased Bacteroidetes. In particular, in mice in C57/BL6 background, Helicobacter hepaticus, a pathogenic species was substantially increased in the small intestine, while Akkermansia muciniphila, a symbiotic, was downregulated in the mice fed the vitamin D-depleted chow or in VDR knockout mice. Conversely, oral administration of synthetic human DEFA5 was able to suppress Helicobacter hepaticus and restore Akkermansia muciniphila in the gut. A major signature of gut dysbiosis is the increased Firmicutes and downregulation of Bacteroidetes, while how that contributes to metabolic diseases is less understood. Most of Bacteriodetes are Gram-negative bacteria, and their endotoxin, lipopolysaccharide (LPS), is a prominent ligand as PAMP for PRR activation and inflammation. Chronic infection and systemic inflammation are the key driving force for insulin resistance. One study showed that subcutaneous infusion of LPS could produce hyperglycemia and insulinemia and fatty liver in the mice (17). Decreased Bacteroidetes, presumably due to their death, may release LPS to drive systemic inflammation, which is a key driving force for insulin resistance and metabolic disorders. We found that oral administration of cholestyramine, polycationic resins, could sufficiently deplete gut endotoxin and improve insulin resistance and metabolic disorders and fatty liver in the high-fat feeding animal models (98). Another study showed that probiotics Lactobacillus rhamnosus and Lactobacillus plantarum were able to increase VDR protein expression in both mouse and human intestinal epithelial cells, while probiotic treatment also enhanced numbers of Paneth cells (91). Cyp27b1 (Cyp) knockout mice have impaired synthesis of 1,25-hydroxylation of VD, and in these mice, dysbiosis appeared. Giving 1,25(OH)2D3 to the Cyp KO mice decreased the colitis severity and suppressed Helicobacteraceae in the feces (62). A genome-wide association analysis (GWAS) in 1,812 individuals identified the variation in levels of vitamin D receptor among other host factors influencing the gut microbiota, and nongenetic and genetic factors, each accounting for ~10% of the variation in gut microbiota (86). A meta-analysis showed that 12 of 14 human studies found association of vitamin D deficiency and altered gut microbiota (90).
VITAMIN D INTERVENTION FOR METABOLIC SYNDROME AND NAFLD
While compelling data from epidemiological surveys show that vitamin D deficiency is associated with the incidence of MetS, T2D, and NAFLD, controlled clinical trials have emerged testing causality and efficacy. One controlled and double-blind intervention was conducted to test the impact of vitamin D on insulin resistance by administering 4,000 IU vitamin D3 (n = 42) or placebo (n = 39) daily for 6 mo to the T2D patients with vitamin D deficiency. With the treatment, the serum 25OHD3 increased significantly from 21 to 75 nmol/l with supplementation and significant improvements in insulin sensitivity were achieved (85). Another trial showed that daily supplementation of vitamin D for 12 wk, along with moderate endurance physical activity, significantly increased 25OHD3 concentration and induced a significant reduction in lipid profile in metabolic syndrome patients (26). Similarly, for postmenopausal women with VD deficiency (n = 80), supplementation with 1,000 IU vitamin D3 for 9 mo was associated with a reduction in the MetS risk profile, showing a reduction of hypertriglyceridemia and hyperglycemia (27). Elevation of plasma von Willebrand factor is associated with increased risk of metabolic syndrome and stroke. Data from 14 clinical trials that contained a total of 1,253 participants, and one meta-analysis found that vitamin D supplementation (≤4,000 IU/day) could significantly decrease von Willebrand factor (vWF) (80). Polycystic ovary syndrome (PCOS) is often related to metabolic syndrome, such as obesity and T2D. One trial showed that 50,000 IU vitamin D supplementation every other week for 8 wk had beneficial effects on insulin metabolism and lipid profile in PCOS patients.
The large-scale clinical trials using vitamin D on T2D are emerging. For example, a total of 2,423 participants with pre-T2D-diabetic conditions underwent randomization (1,211 participants to the vitamin D group, 4,000 IU/daily, and 1,212 to the placebo group). By month 24, while the serum 25-hydroxyvitamin D level in the vitamin D group was significantly improved to the placebo group, the incidence of progression to T2D stage did not change significantly (68). In a small scale, randomized, placebo-controlled study, 47 patients of T2D were given vitamin D at a dose of 1,000 U/day for 12 mo. Metabolic parameters, including fasting glucose, lipid profile, HbA1C, insulin, hs-CRP, 25 OH vitamin D, adiponectin and leptin, HOMA-IR, and central aortic augmentation index (AI) were evaluated (14). The results showed improved AI, while other parameters did not change statistically. Additional information regarding the clinical interventions using VD supplementation for MetS and NAFLD is summarized in Table 2.
Table 2.
Clinical intervention with vitamin D for metabolic syndrome, T2D, and NAFLD
| First Author | Study Design | n | Participants | Intervention | Goals and Primary Outcomes |
|---|---|---|---|---|---|
| Soric et al. (75) | Randomized, interventional study (NCT00985361) | 37 | Type 2 diabetes patients with hemoglobin A1C >7%, aged 21 to 75 yr | Vitamin D3 2,000 IU tablets daily for 3 mo | Hemoglobin A1c N.A. |
| Breslavsky et al. (14) | Randomized, interventional study (NCT01854463) | 47 | Type 2 diabetes patients | 25-hydroxy vitamin D 1,000 IU daily for 12 mo | Glycemic control status Significant decrease in AI during 1 yr of treatment |
| Kampmann et al. (39) | Randomized, interventional study (NCT00812578) | 16 | Diabetes patients with 25(OH)D <50 nmol/L | 8 tablets cholecalciferol equivalent to 280 µg for 2 wk and 4 tablets equivalent to 140 µg for 10 wk | Insulin secretion and insulin resistance Not improve insulin resistance, blood pressure, inflammation or HbA1c, but might increase insulin secretion in patients with established Type 2 diabetes |
| Krul-Poel et al. (44) | Randomized double-blind placebo-controlled trial (NTR3154) |
300 | T2D patients receiving lifestyle advice, metformin, or sulfonylurea derivatives | placebo or 50,000 IU vitamin D3 at monthly intervals | change in glycated hemoglobin level between baseline and six months N.A. |
| Sadiya et al. (72) | Randomized controlled trial (NCT02101151) |
87 | T2DM patients with obesity | In Phase 1 the vitamin D arm received 6,000 IU vitamin D3/day (3 mo) followed by Phase 2 with 3,000 IU vitamin D3/day. During follow up (phase 3), both of the arms were unblinded and supplemented with 2,200 IU vitamin D3/day for another 6 mo. | Anthropometric measures High daily dose of vitamin D3 we did not achieve target levels of serum-25(OH)D above 75 nmol/L |
| Gulseth et al. (32) | Randomized controlled trial (NCT00992797) |
62 | T2DM patients with VD deficiency | 400,000 IU with an additional 200,000 IU D3 if serum 25(OH)D was <100 nmol/L after 4 wk | Euglycemic hyperinsulinemic clamp and intravenous glucose tolerance test IVGTT No improvement of change insulin sensitivity or insulin secretion |
| Rezagholizadeh et al. (70) | Randomized controlled trial | 40 | T2DM patients | 4000 IU vitamin D3 for 2 mo | Fasting blood glucose FBG, HbA1c, and insulin Increase serum anti-inflammatory adipokine SFRP5 but decrease serum pro-inflammatory Wnt5a and TNF-α |
| Harris et al. (33) | Randomized controlled trial | 22 | Overweight subjects | 60,000 IU monthly vitamin D3 for 16 wk | Endothelial function by low-mediated dilation Testing Improving vascular endothelial function in African-American adults |
| Larsen et al. (46) | Randomized controlled trial (NCT01166165) |
112 | Hypertension patients | 3,000 IU daily cholecalciferol for 20 wk | Central blood pressure measurement No improvement of blood pressure |
| Martins et al. (53) | Randomized controlled trial | 130 | Overweight and obese subjects | 100,000 IU vitamin D3 monthly for 3 mo | Adipocyte cytokine expression and arterial stiffness Increased serum 25(OH)D levels, decreased intact PTH level and the levels of select inflammatory and oxidative stress mediators of arterial stiffness. Longer term prospective studies are warranted to evaluate the effect of high dose vitamin D supplementation on arterial stiffness. |
| Pilz et al. (66) | Randomized controlled trial (NCT02136771) |
188 | Patients with hypertension | 2,800 IU vitamin D3 daily for 8 wk | 24-h systolic blood pressure Not significant |
| de Courten et al. (24) | Randomized, interventional study (NCT02112721) | 50 | Nondiabetic patients with 25(OH)D <50 nmol/L, aged 18 to 60 yr | Each participant was given an initial dose of 100,000 IU of vitamin D3. Thereafter, participants took 4,000 IU daily for a period of 16 wk. |
Insulin sensitivity measure using euglycemic glucose clamp N.A. |
| Forouhi et al. (29) | Randomized controlled trial | 340 | Patients with prediabetes | 100,000 IU vitamin D2 or D3 monthly for 4 mo | HbA1c Short-term supplementation with vitamin D2 or D3 had no effect on HbA1c. |
| Moreira-Lucas et al. (57) | Randomized controlled trial | 71 | Patients with prediabetes with VD deficiency | 28,000 IU weekly vitamin D3 for 24 wk | 2-h 75-g oral glucose tolerance test No improvement in oral glucose tolerance |
| Mousa et al. (58) | Randomized controlled trial | 65 | Overweight or obese subjects with VD deficiency | A bolus oral dose of 100,000 IU cholecalciferol followed by 4,000 IU cholecalciferol/day or a matching placebo for 16 wk | Change in insulin resistance by IVGTT No effects |
| Niroomand et al. (61) | Randomized controlled trial | 162 | Prediabetes patients with VD deficiency | 50,000 IU weekly for 3 mo | Fasting plasma glucose FPG/ 2-h oral glucose tolerance test OGTT In patients with prediabetes and hypovitaminosis D, high-dose vitamin D improves insulin sensitivity and decreases risk of progression toward diabetes. |
| Farag et al. (26) | Randomized controlled trial | 70 | Metabolic syndrome patients | Vitamin D 2,000 IU daily for 12 wk with or without endurance physical activity | Lipid profiles (cholesterol, LDL-C, HDL-C) Daily supplementation of vitamin D for 12 wk, along with moderate-endurance physical activity, significantly increases vitamin D concentration and induces a significant reduction in lipid profile in metabolic syndrome patients. |
| Sharifi et al. (74) | Randomized controlled trial | 53 | NAFLD patients | 50,000 IU every other week for 4 mo | Serum ALT levels, hs-CRP, HOMA-IR, TNF-α, malondialdehyde (MDA), total antioxidant capacity, and TGF-β improved vitamin D status and led to amelioration in serum hs-CRP and MDA in patients with NAFLD. |
| Barchetta et al. (10) | Randomized controlled trial | 65 | NAFLD patients with T2DM | 2000 IU cholecalciferol daily over 24 wk | The reduction of MRI-measured hepatic fat fraction, change in transaminase/CK18-M30/P3NP levels and fatty liver index did not improve hepatic steatosis or metabolic/cardiovascular parameters. |
| Lorvand et al. (49) | Randomized controlled trial | 73 | NAFLD patients with vitamin D defficiency | 25 μg calcitriol with hypocaloric diet | The grade of fatty liver disease by liver fat accumulation may have positive effects on lipid profile, liver enzyme tests, and insulin sensitivity during a weight-loss program. |
| Lorvand et al. (50) | Randomized controlled trial | 120 | NAFLD, overweight patients | 25 μg calcitriol with or without 500 mg calcium for 12 wk | Lipid profile and glucose measures, Liver enzyme tests, and ultrosonography Calcium plus calcitriol supplementation for 12 wk may be potentially effective for biochemical parameters in NAFLD patients. |
| Dabbaghmanesh et al. (23) | Randomized controlled trial | 91 | NAFLD patients | 50,000 IU vitamin D3 (cholecalciferol) pearl or 0.25 mg calcitriol weekly for 3 mo | Serum ALT, AST, and GGT No beneficial effects |
| Geier et al. (31) | Randomized controlled trial | 22 | NASH patients | 2,100 IU vitamin D over 48 wk | Change in ALT level from baseline to the end of treatment Significant improvement of serum ALT levels |
AI, augmentation index; ALT, amino transferase; AST, aspartate aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; GGT, glutamyl transpeptidase; HDL-C, high-density lipoprotein-cholesterol; hs-CRP, high-sensitivity C-reactive protein; IU, international unit; IVGTT, intravenous glucose tolerance test; LDL-C, low-density lipoprotein-cholesterol; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OGTT, oral glucose tolerance test; P3NP, procollagen type III NH2-terminal peptide; T2D, Type 2 diabetes; T2DM, Type 2 diabetes mellitus.
CONCLUSIONS AND PERSPECTIVE
As terrestrial animals, humans have evolved relying on the sunlight, which not only provides the warmth, but initiates the synthesis of vitamin D to regulate metabolic homeostasis in many ways. With human migration to northern latitudes, we have to depend on vitamin D as a micronutrient through supplementation. Vitamin D regulates the genome on a wide scale. As an immune adjuvant, vitamin D suppresses adaptive immunity but upregulates innate immunity. In particular, VDR is highly expressed in the gut epithelial cells, including Paneth cells to regulate α-defensins to restrain microbial overgrowth in the small intestine. Epidemiological surveys show an association of the prevalence of vitamin D deficiency and obesity, metabolic syndrome, T2D, and NAFLD, indicating potential linkages. Evidence from animal studies demonstrate that lacking VD or VD signaling pathways impairs gut innate immunity, leading to gut dysbiosis and low-grade systemic inflammation, a key driving force for insulin resistance and metabolic disorders. Evidence is emerging that vitamin D supplementation improves metabolic syndrome, although the impact on NAFLD is not known. Large-scale and controlled trials are needed to address the role of VD in these metabolic disorders (76).
GRANTS
The work was supported by the Natural Science Foundation of China Grants 31571165 and 31771288 to Y.-P. Han, the National Institutes of Health (Bethesda, MD) Grants P01CA163200, P50 AA011999, and P01DK-098108 to S. J. Pandol, and the Sichuan Science and Technology Program (No. 2017TJPT0013) and Public Health and Clinical Center of Chengdu Scientific Research Project (Grant 2018K01) to Y. L. Zeng.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Z. and M.L. conceived the review; Liwei Pan, S.G., D.L., L. Z., Y. L, Lisha Pan, S. X., R. Z., C. Z., P. W., and L.G helped literature search and analysis; Y.C. prepared figures; Y.-P.H. drafted manuscript; and M.N., S.J.P., and Y.-P.H. edited and revised manuscript.
REFERENCES
- 1.Abbott-Johnson WJ, Kerlin P, Abiad G, Clague AE, Cuneo RC. Dark adaptation in vitamin A-deficient adults awaiting liver transplantation: improvement with intramuscular vitamin A treatment. Br J Ophthalmol 95: 544–548, 2011. doi: 10.1136/bjo.2009.179176. [DOI] [PubMed] [Google Scholar]
- 2.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4: 80–90, 2008. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai T, Atsukawa M, Tsubota A, Koeda M, Yoshida Y, Okubo T, Nakagawa A, Itokawa N, Kondo C, Nakatsuka K, Masu T, Kato K, Shimada N, Hatori T, Emoto N, Kage M, Iwakiri K. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dig Liver Dis 51: 1036–1042, 2019. doi: 10.1016/j.dld.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis 210: 1296–1305, 2014. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson SA. [The new dietary reference intakes from the Institute of Medicine for calcium and vitamin D]. Perspect Infirm 8: 5, 2011. [PubMed] [Google Scholar]
- 6.Ayabe T, Satchell DP, Pesendorfer P, Tanabe H, Wilson CL, Hagen SJ, Ouellette AJ. Activation of Paneth cell α-defensins in mouse small intestine. J Biol Chem 277: 5219–5228, 2002. doi: 10.1074/jbc.M109410200. [DOI] [PubMed] [Google Scholar]
- 7.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 8.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between nonalcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 9: 85, 2011. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A, Morini S, Cavallo MG. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology 56: 2180–2187, 2012. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- 10.Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C, Bertoccini L, Cimini FA, Panimolle F, Catalano C, Baroni MG, Cavallo MG. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med 14: 92, 2016. doi: 10.1186/s12916-016-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bea JW, Jurutka PW, Hibler EA, Lance P, Martínez ME, Roe DJ, Sardo Molmenti CL, Thompson PA, Jacobs ET. Concentrations of the vitamin D metabolite 1,25(OH)2D and odds of metabolic syndrome and its components. Metabolism 64: 447–459, 2015. doi: 10.1016/j.metabol.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bel S, Hooper LV. Secretory autophagy of lysozyme in Paneth cells. Autophagy 14: 719–721, 2018. doi: 10.1080/15548627.2018.1430462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bock G, Prietl B, Mader JK, Höller E, Wolf M, Pilz S, Graninger WB, Obermayer-Pietsch BM, Pieber TR. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: a randomized controlled trial. Diabetes Metab Res Rev 27: 942–945, 2011. doi: 10.1002/dmrr.1276. [DOI] [PubMed] [Google Scholar]
- 14.Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr 32: 970–975, 2013. doi: 10.1016/j.clnu.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW IV. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One 10: e0141770, 2015. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 18.Carlberg C. Nutrigenomics of Vitamin D. Nutrients 11: 676, 2019. doi: 10.3390/nu11030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlberg C, Seuter S. A genomic perspective on vitamin D signaling. Anticancer Res 29: 3485–3493, 2009. [PubMed] [Google Scholar]
- 20.Ceglia L, Nelson J, Ware J, Alysandratos KD, Bray GA, Garganta C, Nathan DM, Hu FB, Dawson-Hughes B, Pittas AG; Diabetes Prevention Program Research Group . Association between body weight and composition and plasma 25-hydroxyvitamin D level in the Diabetes Prevention Program. Eur J Nutr 56: 161–170, 2017. doi: 10.1007/s00394-015-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J Gerontol A Biol Sci Med Sci 74: 91–98, 2019. doi: 10.1093/gerona/gly223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chon SJ, Yun BH, Jung YS, Cho SH, Choi YS, Kim SY, Lee BS, Seo SK. Association between vitamin D status and risk of metabolic syndrome among Korean postmenopausal women. PLoS One 9: e89721, 2014. doi: 10.1371/journal.pone.0089721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabbaghmanesh MH, Danafar F, Eshraghian A, Omrani GR. Vitamin D supplementation for the treatment of non-alcoholic fatty liver disease: A randomized double blind placebo controlled trial. Diabetes Metab Syndr 12: 513–517, 2018. doi: 10.1016/j.dsx.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 24.de Courten B, Mousa A, Naderpoor N, Teede H, de Courten MP, Scragg R. Vitamin D supplementation for the prevention of type 2 diabetes in overweight adults: study protocol for a randomized controlled trial. Trials 16: 335, 2015. doi: 10.1186/s13063-015-0851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dionne S, Calderon MR, White JH, Memari B, Elimrani I, Adelson B, Piccirillo C, Seidman EG. Differential effect of vitamin D on NOD2- and TLR-induced cytokines in Crohn’s disease. Mucosal Immunol 7: 1405–1415, 2014. doi: 10.1038/mi.2014.30. [DOI] [PubMed] [Google Scholar]
- 26.Farag HAM, Hosseinzadeh-Attar MJ, Muhammad BA, Esmaillzadeh A, Hamid El Bilbeisi A. Effects of vitamin D supplementation along with endurance physical activity on lipid profile in metabolic syndrome patients: A randomized controlled trial. Diabetes Metab Syndr 13: 1093–1098, 2019. doi: 10.1016/j.dsx.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira PP, Cangussu L, Bueloni-Dias FN, Orsatti CL, Schmitt EB, Nahas-Neto J, Nahas EAP. Vitamin D supplementation improves the metabolic syndrome risk profile in postmenopausal women. Climacteric 23: 24–31, 2020. doi: 10.1080/13697137.2019.1611761. [DOI] [PubMed] [Google Scholar]
- 28.Fitch N, Becker AB, HayGlass KT. Vitamin D [1,25(OH)2D3] differentially regulates human innate cytokine responses to bacterial versus viral pattern recognition receptor stimuli. J Immunol 196: 2965–2972, 2016. doi: 10.4049/jimmunol.1500460. [DOI] [PubMed] [Google Scholar]
- 29.Forouhi NG, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, Rickard AP, Boucher BJ, Chowdhury TA, Griffiths CJ, Greenwald SE, Griffin SJ, Hitman GA. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab 18: 392–400, 2016. doi: 10.1111/dom.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu J, Han L, Zhao Y, Li G, Zhu Y, Li Y, Li M, Gao S, Willi SM. Vitamin D levels are associated with metabolic syndrome in adolescents and young adults: The BCAMS study. Clin Nutr 38: 2161–2167, 2019. doi: 10.1016/j.clnu.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Geier A, Eichinger M, Stirnimann G, Semela D, Tay F, Seifert B, Tschopp O, Bantel H, Jahn D, Marques Maggio E, Saleh L, Bischoff-Ferrari HA, Müllhaupt B, Dufour JF. Treatment of non-alcoholic steatohepatitis patients with vitamin D: a double-blinded, randomized, placebo-controlled pilot study. Scand J Gastroenterol 53: 1114–1120, 2018. doi: 10.1080/00365521.2018.1501091. [DOI] [PubMed] [Google Scholar]
- 32.Gulseth HL, Wium C, Angel K, Eriksen EF, Birkeland KI. Effects of vitamin D supplementation on insulin sensitivity and insulin secretion in subjects with Type 2 diabetes and vitamin D deficiency: a randomized controlled trial. Diabetes Care 40: 872–878, 2017. doi: 10.2337/dc16-2302. [DOI] [PubMed] [Google Scholar]
- 33.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens 24: 557–562, 2011. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, Chen Y, Bissonnette M, Li YC. Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology 159: 967–979, 2018. doi: 10.1210/en.2017-00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He L, Zhou M, Li YC. Vitamin D/vitamin D receptor signaling is required for normal development and function of group 3 innate lymphoid cells in the gut. iScience 17: 119–131, 2019. doi: 10.1016/j.isci.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodin CM, Verdam FJ, Grootjans J, Rensen SS, Verheyen FK, Dejong CH, Buurman WA, Greve JW, Lenaerts K. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J Pathol 225: 276–284, 2011. doi: 10.1002/path.2917. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930, 2011. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 38.Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, Sun J. Lack of Vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther 37: 996–1009.e7, 2015. doi: 10.1016/j.clinthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Kampmann U, Mosekilde L, Juhl C, Moller N, Christensen B, Rejnmark L, Wamberg L, Orskov L. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, β-cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency—a double-blind, randomized, placebo-controlled trial. Metabolism 63: 1115–1124, 2014. doi: 10.1016/j.metabol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Kedar A, Nikitina Y, Henry OR, Abell KB, Vedanarayanan V, Griswold ME, Subramony C, Abell TL. Gastric dysmotility and low serum vitamin D levels in patients with gastroparesis. Horm Metab Res 45: 47–53, 2013. doi: 10.1055/s-0032-1337913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoo AL, Chai LY, Koenen HJ, Oosting M, Steinmeyer A, Zuegel U, Joosten I, Netea MG, van der Ven AJ. Vitamin D3 down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 55: 294–300, 2011. doi: 10.1016/j.cyto.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355: 446–449, 1992. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 44.Krul-Poel YH, van Wijland H, Stam F, ten Boekel E, Lips P, Simsek S. Study protocol: a randomised placebo-controlled clinical trial to study the effect of vitamin D supplementation on glycemic control in type 2 diabetes mellitus SUNNY trial. BMC Endocr Disord 14: 59, 2014. doi: 10.1186/1472-6823-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krul-Poel YH, Westra S, van Wijland HJ, Stam F, Lips P, Pouwer F, Simsek S. Vitamin D status and health-related quality of life in patients with Type 2 diabetes. Diabet Med 33: 300–306, 2016. doi: 10.1111/dme.12834. [DOI] [PubMed] [Google Scholar]
- 46.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens 25: 1215–1222, 2012. doi: 10.1038/ajh.2012.111. [DOI] [PubMed] [Google Scholar]
- 47.Larsson S, Voss U. Neuroprotective effects of vitamin D on high fat diet- and palmitic acid-induced enteric neuronal loss in mice. BMC Gastroenterol 18: 175, 2018. doi: 10.1186/s12876-018-0905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 49.Lorvand Amiri H, Agah S, Mousavi SN, Hosseini AF, Shidfar F. Regression of non-alcoholic fatty liver by vitamin D supplement: a double-blind randomized controlled clinical trial. Arch Iran Med 19: 631–638, 2016. [PubMed] [Google Scholar]
- 50.Lorvand Amiri H, Agah S, Tolouei Azar J, Hosseini S, Shidfar F, Mousavi SN. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin Nutr 36: 1490–1497, 2017. doi: 10.1016/j.clnu.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Maki KC, Fulgoni VL III, Keast DR, Rains TM, Park KM, Rubin MR. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab Syndr Relat Disord 10: 363–372, 2012. doi: 10.1089/met.2012.0020. [DOI] [PubMed] [Google Scholar]
- 52.Mansour-Ghanaei F, Pourmasoumi M, Hadi A, Ramezani-Jolfaie N, Joukar F. The efficacy of vitamin D supplementation against nonalcoholic fatty liver disease: a meta-analysis. J Diet Suppl 10.1080/19390211.2019.1624671. [DOI] [PubMed] [Google Scholar]
- 53.Martins D, Meng YX, Tareen N, Artaza J, Lee JE, Farodolu C, Gibbons G, Norris K. The effect of short-term vitamin D supplementation on the inflammatory and oxidative mediators of arterial stiffness. Health (Irvine Calif) 6: 1503–1511, 2014. doi: 10.4236/health.2014.612185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCollum EV, Simmonds N, Ernestine BJ, and Shipley PG. Study on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 53: 293–311, 1922. [PubMed] [Google Scholar]
- 55.Meiri S, Dayan T, Simberloff D, Grenyer R. Life on the edge: carnivore body size variation is all over the place. Proc Biol Sci 276: 1469–1476, 2009. doi: 10.1098/rspb.2008.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8: 685–698, 2008. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira-Lucas TS, Duncan AM, Rabasa-Lhoret R, Vieth R, Gibbs AL, Badawi A, Wolever TM. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes Metab 19: 133–141, 2017. doi: 10.1111/dom.12794. [DOI] [PubMed] [Google Scholar]
- 58.Mousa A, Naderpoor N, de Courten MP, Teede H, Kellow N, Walker K, Scragg R, de Courten B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. Am J Clin Nutr 105: 1372–1381, 2017. doi: 10.3945/ajcn.117.152736. [DOI] [PubMed] [Google Scholar]
- 59.Mutt SJ, Jokelainen J, Sebert S, Auvinen J, Järvelin MR, Keinänen-Kiukaanniemi S, Herzig KH. Vitamin D status and components of metabolic syndrome in older Subjects from Northern Finland (Latitude 65°North). Nutrients 11: 1229, 2019. doi: 10.3390/nu11061229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naderpoor N, Mousa A, de Courten M, Scragg R, de Courten B. The relationship between 25-hydroxyvitamin D concentration and liver enzymes in overweight or obese adults: Cross-sectional and interventional outcomes. J Steroid Biochem Mol Biol 177: 193–199, 2018. doi: 10.1016/j.jsbmb.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res Clin Pract 148: 1–9, 2019. doi: 10.1016/j.diabres.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr 143: 1679–1686, 2013. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park D, Kwon H, Oh SW, Joh HK, Hwang SS, Park JH, Yun JM, Lee H, Chung GE, Ze S, Park JH, Bae Y, Lee A. Is vitamin D an independent risk factor of nonalcoholic fatty liver disease?: a cross-sectional study of the healthy population. J Korean Med Sci 32: 95–101, 2017. doi: 10.3346/jkms.2017.32.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penna G, Adorini L. 1 α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164: 2405–2411, 2000. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 65.Pike JW, Meyer MB, Benkusky NA, Lee SM, St. John H, Carlson A, Onal M, Shamsuzzaman S. Genomic determinants of vitamin D-regulated gene expression. Vitam Horm 100: 21–44, 2016. doi: 10.1016/bs.vh.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, Treiber G, Drechsler C, Ó Hartaigh B, Obermayer-Pietsch B, Schwetz V, Aberer F, Mader J, Scharnagl H, Meinitzer A, Lerchbaum E, Dekker JM, Zittermann A, März W, Tomaschitz A. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension 65: 1195–1201, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05319. [DOI] [PubMed] [Google Scholar]
- 67.Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, Grant WB, Pludowski P, Hiligsmann M, Trummer C, Schwetz V, Lerchbaum E, Pandis M, Tomaschitz A, Grübler MR, Gaksch M, Verheyen N, Hollis BW, Rejnmark L, Karras SN, Hahn A, Bischoff-Ferrari HA, Reichrath J, Jorde R, Elmadfa I, Vieth R, Scragg R, Calvo MS, van Schoor NM, Bouillon R, Lips P, Itkonen ST, Martineau AR, Lamberg-Allardt C, Zittermann A. Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol (Lausanne) 9: 373, 2018. doi: 10.3389/fendo.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M; D2d Research Group . Vitamin D supplementation and prevention of Type 2 diabetes. N Engl J Med 381: 520–530, 2019. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, Pieber TR. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J 12: 136–139, 2010. [PubMed] [Google Scholar]
- 70.Rezagholizadeh F, Keshavarz SA, Djalali M, Rad EY, Alizadeh S, Javanbakht MH. Vitamin D3 supplementation improves serum SFRP5 and Wnt5a levels in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Int J Vitam Nutr Res 88: 73–79, 2018. doi: 10.1024/0300-9831/a000509. [DOI] [PubMed] [Google Scholar]
- 71.Ryz NR, Lochner A, Bhullar K, Ma C, Huang T, Bhinder G, Bosman E, Wu X, Innis SM, Jacobson K, Vallance BA. Dietary vitamin D3 deficiency alters intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 309: G730–G742, 2015. doi: 10.1152/ajpgi.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadiya A, Ahmed SM, Carlsson M, Tesfa Y, George M, Ali SH, Siddieg HH, Abusnana S. Vitamin D3 supplementation and body composition in persons with obesity and type 2 diabetes in the UAE: A randomized controlled double-blinded clinical trial. Clin Nutr 35: 77–82, 2016. doi: 10.1016/j.clnu.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11: 76–82, 2010. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 47: 70–80, 2014. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 75.Soric MM, Renner ET, Smith SR. Effect of daily vitamin D supplementation on HbA1c in patients with uncontrolled type 2 diabetes mellitus: a pilot study. J Diabetes 4: 104–105, 2012. doi: 10.1111/j.1753-0407.2011.00164.x. [DOI] [PubMed] [Google Scholar]
- 76.Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, Luo M, Sun Q, Cai L, Lai Y, Xiao Z, Duan Z, Zheng S, Wu G, Hu R, Tsukamoto H, Lugea A, Liu Z, Pandol SJ, Han YP. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front Physiol 7: 498, 2016. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy 12: 1057–1058, 2016. doi: 10.1080/15548627.2015.1072670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun M, Guo B. Vitamin D and the epigenetic machinery in colon cancer. Curr Med Chem 24: 888–897, 2017. doi: 10.2174/0929867324666161117155325. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki TA, Worobey M. Geographical variation of human gut microbial composition. Biol Lett 10: 20131037, 2014. doi: 10.1098/rsbl.2013.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabrizi R, Akbari M, Lankarani KB, Heydari ST, Kolahdooz F, Asemi Z. The effects of vitamin D supplementation on endothelial activation among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 15: 85, 2018. doi: 10.1186/s12986-018-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian LQ, Shi WQ, Zhou Y, Zhang YW, Zhang ML. The association of serum vitamin D deficiency and metabolic risk factors in Chinese adults with prediabetes: a cross-sectional study. J Nutr Sci Vitaminol (Tokyo) 65: 211–218, 2019. doi: 10.3177/jnsv.65.211. [DOI] [PubMed] [Google Scholar]
- 82.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 83.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 84.Van Der Stede Y, Verfaillie T, Cox E, Verdonck F, Goddeeris BM. 1α,25-dihydroxyvitamin D3 increases IgA serum antibody responses and IgA antibody-secreting cell numbers in the Peyer’s patches of pigs after intramuscular immunization. Clin Exp Immunol 135: 380–390, 2004. doi: 10.1111/j.1365-2249.2003.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr 103: 549–555, 2010. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, Hübenthal M, Koch M, Schwarz K, Rimbach G, Hübbe P, Pan WH, Sheibani-Tezerji R, Häsler R, Rosenstiel P, D’Amato M, Cloppenborg-Schmidt K, Künzel S, Laudes M, Marschall HU, Lieb W, Nöthlings U, Karlsen TH, Baines JF, Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet 48: 1396–1406, 2016. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang N, Zhai H, Zhu C, Li Q, Han B, Chen Y, Zhu C, Chen Y, Xia F, Lin D, Lu Y. Combined association of vitamin D and sex hormone binding globulin with nonalcoholic fatty liver disease in men and postmenopausal women: a cross-sectional study. Medicine (Baltimore) 95: e2621, 2016. doi: 10.1097/MD.0000000000002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang PF, Yao DH, Hu YY, Li Y. Vitamin D improves intestinal barrier function in cirrhosis rats by upregulating heme oxygenase-1 expression. Biomol Ther (Seoul) 27: 222–230, 2019. doi: 10.4062/biomolther.2018.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem 285: 2227–2231, 2010. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr 58: 2895–2910, 2019. [DOI] [PubMed] [Google Scholar]
- 91.Wu S, Yoon S, Zhang YG, Lu R, Xia Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol 309: G341–G349, 2015. doi: 10.1152/ajpgi.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G, Sun J. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 64: 1082–1094, 2015. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6: 231–243, 2009. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol 24: 128–147, 2010. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhai HL, Wang NJ, Han B, Li Q, Chen Y, Zhu CF, Chen YC, Xia FZ, Cang Z, Zhu CX, Lu M, Lu YL. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: a cross-sectional study (Survey on prevalence in East China for metabolic diseases and risk factors (SPECT-China)). Br J Nutr 115: 1352–1359, 2016. doi: 10.1017/S0007114516000386. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127–2135, 2012. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng JS, Imamura F, Sharp SJ, van der Schouw YT, Sluijs I, Gundersen TE, Ardanaz E, Boeing H, Bonet C, Gómez JH, Dow C, Fagherazzi G, Franks PW, Jenab M, Kühn T, Kaaks R, Key TJ, Khaw KT, Lasheras C, Mokoroa O, Mancini FR, Nilsson PM, Overvad K, Panico S, Palli D, Rolandsson O, Sieri S, Salamanca-Fernández E, Sacerdote C, Spijkerman AMW, Stepien M, Tjonneland A, Tumino R, Butterworth AS, Riboli E, Danesh J, Langenberg C, Forouhi NG, Wareham NJ. Association of plasma vitamin D metabolites with incident type 2 diabetes: EPIC-InterAct case-cohort Study. J Clin Endocrinol Metab 104: 1293–1303, 2019. doi: 10.1210/jc.2018-01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu A, Chen J, Wu P, Luo M, Zeng Y, Liu Y, Zheng H, Zhang L, Chen Z, Sun Q, Li W, Duan Y, Su D, Xiao Z, Duan Z, Zheng S, Bai L, Zhang X, Ju Z, Li Y, Hu R, Pandol SJ, Han YP. Cationic polystyrene resolves nonalcoholic steatohepatitis, obesity, and metabolic disorders by promoting eubiosis of gut microbiota and decreasing endotoxemia. Diabetes 66: 2137–2143, 2017. doi: 10.2337/db17-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, Gyimesi E, Hajas A, Zeher M, Szegedi G, Bodolay E. Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol 39: 490–497, 2010. doi: 10.3109/03009741003781951. [DOI] [PubMed] [Google Scholar]