Keywords: bile acid metabolism, bile acid therapies, farnesoid X receptor, alcoholic and nonalcoholic fatty, liver diseases, Takeda G protein-coupled receptor 5
Abstract
Bile acid synthesis is the most significant pathway for catabolism of cholesterol and for maintenance of whole body cholesterol homeostasis. Bile acids are physiological detergents that absorb, distribute, metabolize, and excrete nutrients, drugs, and xenobiotics. Bile acids also are signal molecules and metabolic integrators that activate nuclear farnesoid X receptor (FXR) and membrane Takeda G protein-coupled receptor 5 (TGR5; i.e., G protein-coupled bile acid receptor 1) to regulate glucose, lipid, and energy metabolism. The gut-to-liver axis plays a critical role in the transformation of primary bile acids to secondary bile acids, in the regulation of bile acid synthesis to maintain composition within the bile acid pool, and in the regulation of metabolic homeostasis to prevent hyperglycemia, dyslipidemia, obesity, and diabetes. High-fat and high-calorie diets, dysbiosis, alcohol, drugs, and disruption of sleep and circadian rhythms cause metabolic diseases, including alcoholic and nonalcoholic fatty liver diseases, obesity, diabetes, and cardiovascular disease. Bile acid-based drugs that target bile acid receptors are being developed for the treatment of metabolic diseases of the liver.
INTRODUCTION
Emerging research in the last two decades has identified bile acids as important nutrient sensors and metabolic integrators that play a critical role in maintaining metabolic homeostasis. Bile acids are physiological detergents needed for absorption of dietary fat, steroids, and lipid-soluble vitamins, and are also signal molecules and endogenous ligands that activate nuclear farnesoid X receptor (FXR) and membrane Takeda G protein-coupled receptor 5 (TGR5, i.e., G protein-coupled bile acid receptor-1) (22). Bile acid signaling through these two major bile acid receptors plays a critical role in integrating glucose, lipid, and energy metabolisms (25, 27). Bile acid synthesis is the most significant pathway for the catabolism of cholesterol and is responsible for the daily output of ~90% cholesterol in the body (166). Primary bile acids synthesized in the liver are secreted into bile, stored in the gallbladder, and released to the gastrointestinal tract after food intake. Bile acids are reabsorbed mostly in the ileum and are transported back to the liver via portal blood circulation to regulate bile acid synthesis and homeostasis. The enterohepatic circulation of bile acids is an important physiological mechanism for maintaining whole body glucose, lipid, and energy homeostasis to prevent hyperglycemia, dyslipidemia, and obesity, and it protects against inflammatory metabolic diseases of the digestive and cardiovascular systems (27). The bile acid-mediated integration of metabolic regulation is very complex and not completely understood. Most studies of bile acid metabolism and regulation rely on using genetically modified mice fed various high-fat diets to induce obesity, insulin resistance, and fatty liver disease. The study of bile acid metabolism in animal models has been translated to therapeutic treatment of obesity, diabetes, and nonalcoholic fatty liver disease in human patients (106). This review will provide an update on the recent advances in understanding the role and mechanism of bile acid receptor signaling in metabolic regulation and pathogenesis of fatty liver diseases and advances in bile acid-based drug therapies for metabolic diseases of the liver. Most references cited were published from 2000 to 2020, and older original articles can be found in the review articles cited.
BILE ACID SYNTHESIS AND METABOLISM
Bile Acid Synthesis in the Liver: Classic and Alternative Pathways
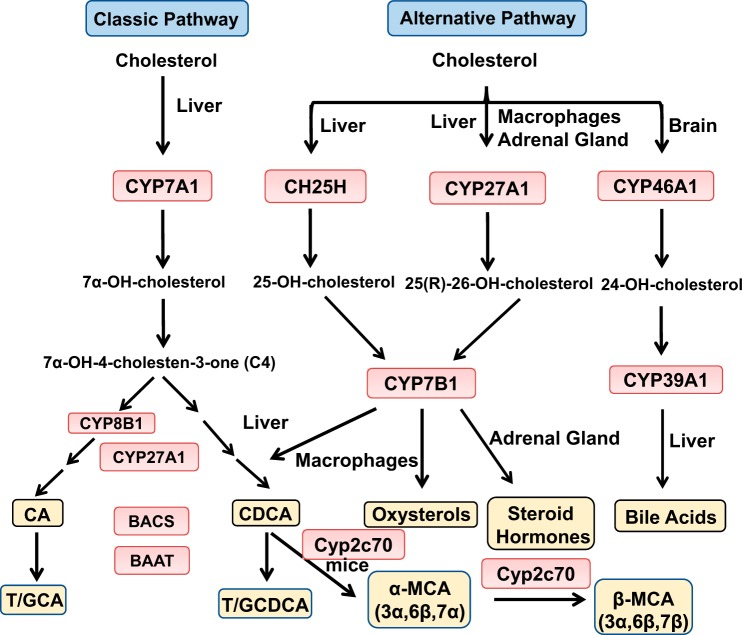
Bile acid synthesis is remarkably similar between humans and mice, but bile acid composition and pool size vary markedly between rodents and humans (27). These differences may be due to differential utilization of the two bile acid synthesis pathways, the anatomic difference of the gastrointestinal tract, the gut microbiota, and the enterohepatic circulation of bile acids. There are 2 major bile acid synthesis pathways catalyzed by 17 enzymatic reactions. The classic bile acid synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1), the only rate-limiting enzyme to synthesize the two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), in the human liver (Fig. 1). CYP7A1 converts cholesterol to 7α-hydroxycholesterol, which is then converted to 7α-hydroxy-4-cholesten-3-one (C4) by a hydroxy steroid dehydrogenase. C4 is the common precursor for synthesis of CA and CDCA. Serum C4 levels reflect the rate of bile acid synthesis and are used as a biomarker for the rate of bile acid synthesis in the liver. Sterol 12α-hydroxylase (CYP8B1) is a branching enzyme that hydroxylates C4 and produces CA. Without CYP8B1, C4 is converted to CDCA. Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes a steroid side-chain oxidation in preparation for a peroxisomal β-oxidation reaction that cleaves a propionyl-CoA to form cholyl-CoA and chenodeoxycholyl-CoA, respectively (Fig. 1). Bile salt CoA-synthase (BACS) activates recycled CA and CDCA, and bile acid intermediates in hepatocytes to cholyl-CoA and chenodeoxycholyl-CoA, which are then conjugated to the amino acids taurine (T) or glycine (G) by bile acid-CoA-to-amino acid N-acyltransferase (BAAT), forming T/G-CA and T/G-CDCA.
Fig. 1.
Bile acid synthesis in the liver. The classic bile acid synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1). Sterol 12α-hydroxylase (CYP8B1) is involved in synthesis of cholic acid (CA), and mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid side-chain oxidation. CA and chenodeoxycholic acid (CDCA) are the two major primary bile acids synthesized in the human liver. Bile acid-CoA synthase (BACS) catalyzes the addition of a CoA group and then bile acid-CoA-to-amino acid transferase (BAAT) adds taurine (T) or glycine (G) to form T/G-conjugated bile acids for secretion in bile. The alternative bile acid synthesis pathway is initiated by hydroxylation of the steroid side chain, followed by steroid ring modifications. In the liver, cholesterol is hydroxylated by sterol 25-hydroxylase and CYP27A1 to form 25-hydroxycholesterol and 27-hydroxycholesterol [also named 25(R)-26-hydroxycholesterol], respectively, which are then hydroxylated at the 7α-position by oxysterol 7α-hydroxylase (CYP7B1). CYP27A1 and CYP7B1 are widely expressed in macrophages, adrenal glands, and other tissues. In the brain, sterol 24-hydroxylase (CYP46A1) converts cholesterol to 24-hydroxycholesterol, which is 7α-hydroxylated by sterol 7α-hydroxylase (CYP39A1) in the liver. In mice, CDCA is converted to α-muricholic acid (MCA) and β-MCA by Cyp2c70.
The alternative bile acid synthesis pathway is initiated by CYP27A1 and converts cholesterol to 27-hydroxycholesterol [also known as 25(R)-26-hydroxycholesterol] and 3β-hydroxy-5-cholestenoic acid, which are then hydroxylated at the 7α position by oxysterol 7α-hydroxylase (CYP7B1) (Fig. 1). CYP27A1 and CYP7B1 are widely expressed in extrahepatic tissues, including steroidogenic tissues and macrophages. The most abundant circulating oxysterols are 24-hydroxycholesterol, 25-hydroxycholesterol, and 27-hydroxycholesterol (88, 167). These oxysterols can be transported to the liver and are converted to CDCA and CA. In the brain, sterol 24-hydroxylase (CYP46A1) forms 24-hydroxycholesterol, which is converted to 7α, 24-dihydroxycholesterol by sterol 7α-hydroxylase (CYP39A1) in the liver. Cholesterol also can be hydroxylated at the C25 position to form 25-hydroxycholesterol by sterol-25-hydroxylase, a noncytochrome P450 enzyme, followed by CYP7B1 to form 7α, 25-dihydroxycholesterol (88). Specific to rodents, Cyp2c70 converts CDCA to α-muricholic acid (MCA) and β-MCA (185).
The classic bile acid synthesis pathway initiated by CYP7A1 accounts for the majority of bile acid synthesis (>90%) in humans (40). The alternative bile acid synthesis pathway initiated by CYP27A1 may be the predominant bile acid synthesis pathway in the neonate. After weaning, CYP7A1 is expressed, and the classic bile acid synthesis pathway dominates to synthesize bile acids. It was thought that the alternative pathway only synthesized CDCA; however, recent studies in humans and mice deficient of key genes in bile acid synthesis revealed that the alternative pathway also can synthesize CA (44). In a human patient with a CYP7A1 gene mutation, CA and deoxycholic acid (DCA) were present in stool, indicating that the alternative pathway also produces CA (154). In mice, the classic and alternative bile acid synthesis pathways may contribute equally to produce a bile acid pool containing nearly equal amounts of T-CA and T-MCAs (derived from CDCA). In Cyp7a1 deficient mice, the bile acid pool size decreases, and bile acid synthesis switches to the alternative pathway to produce more MCA, but CA still accounts for ~32% of total bile acids in the bile (44). In Cyp7b1-deficient mice, bile acid pool size, serum cholesterol, and triglycerides are maintained, but 25 and 27-hydrocholesterol levels are increased compared with those in wild-type mice (114). Cyp8b1-deficient mice have increased Cyp7a1 expression and bile acid pool size and are resistant to Western diet-induced obesity and steatosis (8, 21, 91, 134). In Cyp27a1-deficient mice, Cyp7a1 expression is induced, but bile acid pool size is reduced by ~50%, and increased cholesterol and fatty acid synthesis contribute to hepatomegaly and hypertriglyceridemia (159). Interestingly, double deficiency of Cyp7a1 and Cyp27a1 in mouse livers resulted in reduced bile acid pool but unaltered bile acid composition in the liver, serum, gallbladder, and intestine (164). Overexpression of Cyp7a1 in mice enlarged the bile acid pool and protected mice from Western high-fat diet-induced obesity and insulin resistance while maintaining cholesterol homeostasis (111, 112). Thus, the classic and alternative bile acid synthesis pathways can compensate each other to produce bile acids and alter bile acid composition and pool size but still maintain bile acid and cholesterol homeostasis.
CYP27A1 is highly expressed in macrophages and metabolizes cholesterol to 27-hydroxycholesterol, which has been shown to activate liver X receptor α (LXRα) (53). 27-hydroxycholesterol is a selective-estrogen receptor modulator (193). Activation of estrogen receptor and LXRα by 27-hydroxycholesterol promoted estrogen receptor-dependent growth and LXR-dependent metastasis in a mouse model of breast cancer (126). CYP7B1 catalyzes 7α-hydroxylation of 25-hydroxycholesterol and 27-hydroxycholesterol to convert oxysterols to bile acids in hepatocytes. A 10-wk-old boy with mutation of the CYP7B1 gene presented with severe neonatal cholestasis, cirrhosis, and liver failure, suggesting a critical role for CYP7B1 in the regulation of oxysterol metabolism in extrahepatic tissues and macrophages as well as the importance of the alternative pathway for detoxification of oxysterols and synthesis of bile acids in newborns (88). It was thought that the classic pathway is highly regulated, whereas the alternative pathway is constitutively active. More recent studies show that the alternative pathway can be regulated by steroidogenic acute regulatory protein D1-mediated transport of cholesterol to mitochondria, which lack cholesterol. Transport of cholesterol into mitochondria for 27-hydroxylation by CYP27A1 is the rate-limiting step for the alternative pathway (157). These studies support the theory that the alternative pathway plays a key role in anti-inflammation and lipogenesis. Interestingly, a recent study in mice reported that cholesterol synthesis is increased and Cyp7b1 is induced to convert cholesterol to bile acids as an adaptive response to thermogenesis (206). Thus, the alternative pathway can be induced to produce bile acids for the promotion of energy metabolism in brown adipose tissues.
Bile Acid Transformation in the Gut
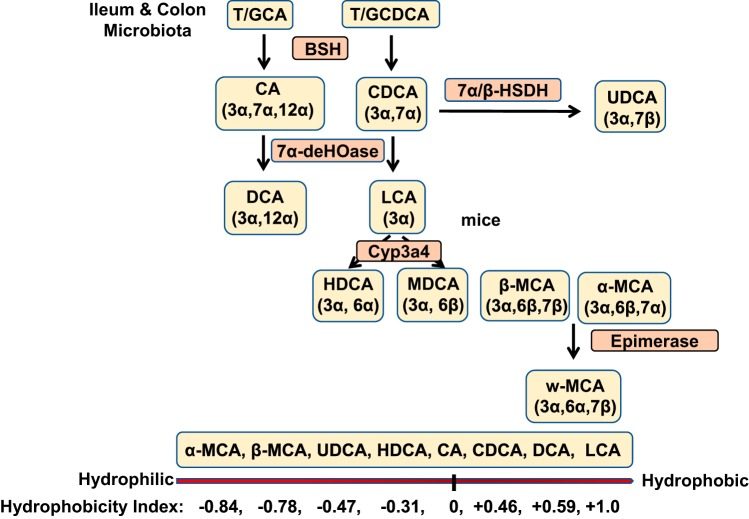
Bile acids stored in the gallbladder are secreted into the intestinal tract after each meal and are reabsorbed in the ileum. In the colon, a portion of T/G-CA and T/G-CDCA is deconjugated to CA and CDCA by bacterial bile salt hydrolase (BSH); then, bacterial 7α-dehydroxylase removes a 7α-hydroxyl group from CA and CDCA to form the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), respectively (Fig. 2).
Fig. 2.
Bile acid transformation in the gut. The primary bile acids are transformed to secondary bile acids by gut bacteria. Bile salt hydroxylase (BSH) deconjugates glycine (G)/taurine (T)-conjugated bile acids, then bacterial 7α-dehydroxylase removes a 7α-HO group from chloric acid (CA) and chenodeoxycholic acid (CDCA) to form deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. CDCA is converted to ursodeoxycholic acid (UDCA) by 7β-hydroxysteroid dehydrogenase (7βHSDH). UDCA can be converted to LCA by 7β-dehydroxylase. In mouse liver, CDCA and UDCA are converted to α-muricholic acid (MCA) and β-MCA by Cyp2c70, and β-MCA is converted to ω-MCA by 6β-epimerase by the gut bacteria. CDCA also can be converted to hyocholic acid by 7α-epimerase in human and pig livers. In mice, LCA can be hydroxylated to hyodeoxycholic acid (HDCA) and murideoxycholic acid (MDCA). DCA and LCA can by rehydroxylated to CA and CDCA, respectively, in mouse liver. Bile acid hydrophobicity index is shown at the bottom.
DCA is reabsorbed into the colon and is reconjugated and secreted into blood circulation (20%–25% of the bile acid pool), whereas LCA is circulated to the liver, reconjugated, and sulfated for excretion into urine. Human gut bacterial 3α, 7α, and 12α-hydroxysteroid dehydrogenases (HSDHs) epimerize the α-hydroxyl groups of bile acids to carbonyl groups to form 3-oxo, 7-oxo, and 12-oxo-bile acids, respectively. Then, the carbonyl groups in oxo-bile acids are converted to β epimers, iso-bile acids, and epi-bile acids by 3β, 7β, and 12β-HSDHs. The 7α-hydroxy-bile acids have higher bactericidal activity than oxo-bile acids and β-epimers. In humans, 7β-HSDH epimerizes the 7α-hydroxyl group of CDCA to a 7β-HO group, forming ursodeoxycholic acid (UDCA), increasing the solubility and decreasing the toxicity of the bile acid pool. Mouse α-MCA and β-MCA are epimerized to ω-MCA in the gut, further increasing solubility for portal circulation or fecal excretion. In humans and pigs, CDCA can be converted to the more hydrophilic hyocholic acid by 6α-hydroxylase in the liver. In rodents, LCA can be 6α or 6β-hydroxylated to hyodeoxycholic acid, and murideoxycholic acid, respectively, by Cyp3a4 (Fig. 2). It is noteworthy that mice, but not humans, can rehydroxylate DCA and LCA to CA and CDCA, respectively, in the liver by a recently identified enzyme Cyp2a12 (73). Thus, in mice, most bile acids are CA (50%) and MCAs (50%), with very little DCA and CDCA, whereas in humans, CA (40%), CDCA (40%) and DCA (20%) are the major bile acids in the bile acid pool. The number of HO groups, the location of the HO groups on the steroid ring (C6 or C7), and stereo-configuration (α- or β-epimer) determine the hydrophobic and hydrophilic properties of bile acids. In general, 7β- and 6β-hydroxy-bile acids are more soluble than their respective 7α- and 6α-hydroxy-bile acids (Fig. 2). Bile acid hydrophobicity is determined by the order of their retention in liquid chromatography. The hydrophobicity index of CA is designated 0, and CA is more hydrophilic than CDCA (+0.46), DCA (+0.59), and LCA (+1.0), the most hydrophobic bile acid. α-MCA (−0.84) and β-MCA (−0.78) are more hydrophilic than UDCA (−0.47), hyodeoxycholic acid (−0.31) and CA (Fig. 2).
Bile Acid Pool Size and Composition
Bile acid pool size and bile acid composition are maintained by bile acid feedback regulation via the gut-to-liver axis (23, 27, 47). Alteration of the bile acid pool size and/or composition may indicate impairment in bile acid synthesis because of liver injury, obstruction of bile ducts, or inflammation. Bile acid pool size comprises the total bile acid content in the liver, serum, intestine, and gallbladder. Bile acid pool size and composition vary during fasting and postprandial states, and composition varies in serum, liver, gallbladder (bile), intestine (ileum and colon), and feces. Bile acid composition in the liver and gallbladder most closely represent the newly synthesized bile acids and recirculated bile acids in the pool. Most bile acids in the liver and gallbladder are taurine-conjugated (95%) in mice. The bile acid pool in many mammalian species, such as mice, rats, and dogs, contains predominantly taurine conjugates, whereas the bile acid pool in humans, hamsters, and rabbits contains about two-thirds glycine conjugates and one-third taurine conjugates (72). Taurine-conjugated bile acids are less toxic than their corresponding glycine-conjugated and unconjugated bile acids. The species-dependent preference for using glycine or taurine for bile acid conjugation may be due to substrate specificity of BAAT and the availability of taurine and glycine.
Most serum bile acids are unconjugated CA, DCA, and MCAs in mice and CA, DCA, and CDCA in humans. The ileum contains more conjugated bile acids than unconjugated bile acids, whereas in the colon and feces the predominant bile acid (>95%) is unconjugated DCA (95%) in mice and humans. Increased bile acid pool size may not always imply increased synthesis, as increased intestinal absorption or decreased fecal excretion can also increase the pool size. Decreased bile acid pool size can result from reduced bile acid synthesis or biliary secretion, and/or reduced intestinal bile acid reabsorption or increased fecal bile acid secretion. During cholestasis, bile acids accumulate in hepatocytes, and biliary bile acid secretion is reduced, resulting in reduced total bile acid pool size.
BILE ACID SIGNALING IN BILE ACID HOMEOSTASIS
Bile acids activate FXR, which plays a central role in the regulation of bile acid synthesis and the enterohepatic circulation of bile acids to maintain bile acid homeostasis (reviewed in Ref. 108). FXR is highly expressed in the gastrointestinal tract and acts as a bile acid sensor to mediate bile acid feedback inhibition of bile acid synthesis and maintain very low levels of bile acids in hepatocytes to prevent liver injury and cholestasis. Bile acids differ widely in their efficacy in activation of FXR: among bile acids, CDCA is the most potent endogenous FXR agonist, whereas CA is a weak FXR agonist (200).
FXR Regulation of Bile Acid Synthesis
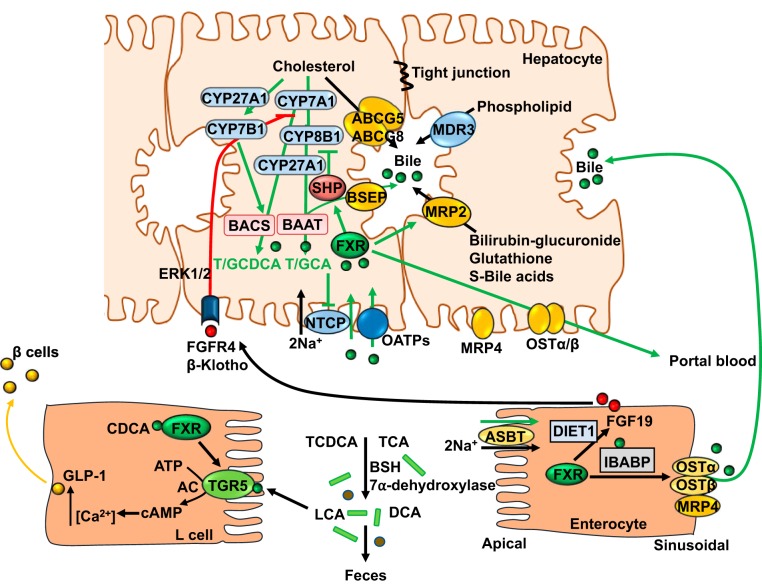
Two FXR-dependent pathways have been proposed to mediate bile acid feedback inhibition of bile acid synthesis (Fig. 3). Activation of FXR by bile acids induces the negative nuclear receptor small heterodimer partner (SHP) to inhibit transcription of the Cyp7a1 and Cyp8b1 genes in hepatocytes (24, 36, 65, 168, 219). In 1995, Pandak et al. (131) reported that intraduodenal transfusion of taurocholate inhibited Cyp7a1 enzyme activity and mRNA in biliary-diverted rats, but an intravenous infusion did not, suggesting that TCA might induce an intestinal factor in the ileum to regulate bile acid synthesis in the liver. Ten years later, fibroblast growth factor 15 (FGF15) was identified as an intestinal FXR-regulated hormone that activates hepatic membrane FGF receptor 4 (FGFR4)/β-Klotho complex to inhibit Cyp7a1 and Cyp8b1 gene transcription via activation of cJun of the mitogen-activated protein kinase (MAPK) pathway (77). FGF15-deficient mice have increased Cyp7a1 mRNA and protein levels, supporting the notion that the gut-to-liver axis plays a critical role in bile acid feedback regulation. FGF15 is expressed in mouse intestines but is not detected in mouse serum and is not expressed in mouse liver (51, 90), whereas the human ortholog fibroblast growth factor 19 (FGF19) can be detected in human serum and hepatocytes. In human primary hepatocytes, bile acids induce FGF19 to activate extracellular signal-regulated kinase 1/2 (ERK1/2) of the MAPK pathway and inhibit CYP7A1 gene transcription without involving SHP (180) (Fig. 3). In human ileal explants, bile acids strongly induced FGF19 transcript and protein expression (217). FGF19 levels are increased in patients with extrahepatic cholestasis and are correlated to reduced CYP7A1 mRNA levels in human hepatocytes (170). Another study reports that variations in the DIET1 gene may influence FGF19 levels in patients with bile acid diarrhea (101). The Diet1 locus was identified in B6By mice, which are resistant to diet-induced hypercholesterolemia and atherosclerosis (143). Diet1 is expressed in the enterocytes, and its expression levels parallel FGF15, indicating that Diet1 may regulate bile acid synthesis by an unknown mechanism (196). It has been reported that hepatic FXR/SHP preferentially inhibits Cyp8b1 gene, whereas intestinal FGF15/liver FGFR4 signaling preferentially inhibits Cyp7a1 gene transcription (97). Bile acid concentration is much higher in the ileum and colon (in millimolars) than that in the liver (in micromolars). It is likely that bile acid activation of intestinal FXR/FGF19 signaling to activate the hepatic FGFR4 pathway is the major physiological mechanism for bile acid feedback regulation of bile acid synthesis. When bile acid concentrations increase in the liver during cholestasis, the FXR/SHP pathway may be activated to inhibit bile acid synthesis and prevent bile acid reabsorption from portal blood. In addition, bile acids induce the proinflammatory cytokines TNFα and IL-1β in macrophages/Kupffer cells to activate Toll-like receptor 4 in hepatocytes and inhibit CYP7A1 and CYP8B1 via ERK1/2/JNK signaling (116). Furthermore, post-transcriptional regulation of CYP7A1 mRNA stability may also regulate CYP7A1 expression. The liver expresses multiple CYP7A1 mRNA species with difference lengths of the 3′ untranslated region, which contains multiple AUUU sequence motifs for the destabilization of mRNA (113). A recent study reports that activation of FXR induces an RNA binding protein ZFP36L1, which binds to the 3′ untranslated region to destabilize CYP7A1 mRNA (188).
Fig. 3.
Farnesoid X receptor (FXR) regulation of bile acid homeostasis. In hepatocytes, activation of FXR induces small heterodimer partner (SHP) to inhibit cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CYP8B1) gene transcription. FXR induces bile salt expert pump (BSEP) to efflux bile acids into bile. ATP-binding cassette (ABC) G5/ABCG8 heterodimer effluxes cholesterol, whereas multidrug resistant (MDR) 3 effluxes phospholipids into bile. Bile acids, cholesterol, and phospholipids form mixed micelles. In the intestinal lumen, gut bacterial bile salt hydrolase (BSH) deconjugates taurine (T)/glycine (G) chenodeoxycholic acid (CDCA) and T/G cholic acid (CA) and bacterial 7α-dehydroxylase removes a 7α-HO group to form deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. Bile acids are reabsorbed into enterocytes via apical sodium-dependent bile acid transporter (ASBT), which is inhibited by bile acids. In enterocytes, FXR induces ileum bile acid binding protein (IBABP) and organic solute transporter heterodimer (OST) α and OSTβ to efflux bile acids into portal blood circulation. DIET1 is coexpressed with FGF15 in enterocytes and modulates FGF15 levels, bile acid secretion, and bile acid pool size. FXR also induces FGF19, which binds to hepatic FGF receptor (FGFR) 4/β-Klotho complex to activate ERK1/2 and inhibit CYP7A1 gene transcription. FXR and Takeda G protein-coupled receptor 5 (TGR5) are coexpressed in enteroendocrine L cells; FXR induces TGR5 to activate cAMP and intracellular Ca2+ to secrete glucagon-like peptide-1 (GLP-1), which stimulates insulin secretion from pancreatic β cells. AC, adenylyl cyclase; BAAT, bile acid-CoA-to-amino acid transferase; BACS, bile salt CoA-synthase; CYP27A1, sterol 27-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; MRP, MDR-related protein; NTCP, sodium/taurocholate cotransporting polypeptide.
FXR Regulation of Bile Acid Homeostasis
FXR plays a key role in the regulation of bile acid enterohepatic circulation and transport from liver to intestine (Fig. 3). In hepatocytes, FXR induces expression of bile salt export pump (BSEP) (ATP-binding cassette transporter B11), which effluxes bile acids into bile. FXR also induces ATP-binding cassette transporter (ABC) G5 and ABCG8 to efflux cholesterol and multidrug resistant transporter 3 (MDR3 or ABCB4) to efflux phospholipids into bile. MDR-related protein 2 (MRP2 or ABCC2) effluxes bilirubin, glutathione, glucuronate, and sulfate conjugates of bile acids into bile. Bile acids, cholesterol, and phospholipids form mixed micelles to concentrate bile acids and to prevent cholesterol precipitation in the gallbladder bile. On the sinusoidal membrane, bile acids inhibit sodium/taurocholate cotransporting polypeptide (NTCP) and organic anion transporting peptides. Thus, FXR acts as a bile acid sensor to maintain low intrahepatic bile acid concentration and to prevent cholestatic liver injury. In the ileum, bile acids are efficiently reabsorbed into enterocytes via apical sodium-dependent bile acid transporter (SLC10A2). FXR inhibits apical sodium-dependent bile acid transporter but induces ileum bile acid binding protein, which binds and transports bile acids across enterocytes to the sinusoidal membrane, where FXR induces organic solute transporter α and β heterodimer (OSTα and OSTβ, respectively) (SLC51A and SLC51B) and MRP4 to secrete bile acids into portal circulation (Fig. 3). OSTα, OSTβ, and MRP4 are also expressed in the sinusoidal membrane of hepatocytes to secrete bile acids into portal blood. Thus, FXR also acts as a sensor to control bile acid reabsorption and secretion in portal blood to prevent accumulation of bile acids in enterocytes and protect intestinal barrier function. Portal circulation of bile acids from intestine to hepatocytes, in which sinusoidal sodium/taurocholate cotransporting polypeptide reabsorbs bile acids from portal blood, completes the enterohepatic circulation of bile acids.
BILE ACID SIGNALING IN METABOLIC REGULATION
Bile Acids in the Gut-Liver-Brain Axis and Circadian Rhythms
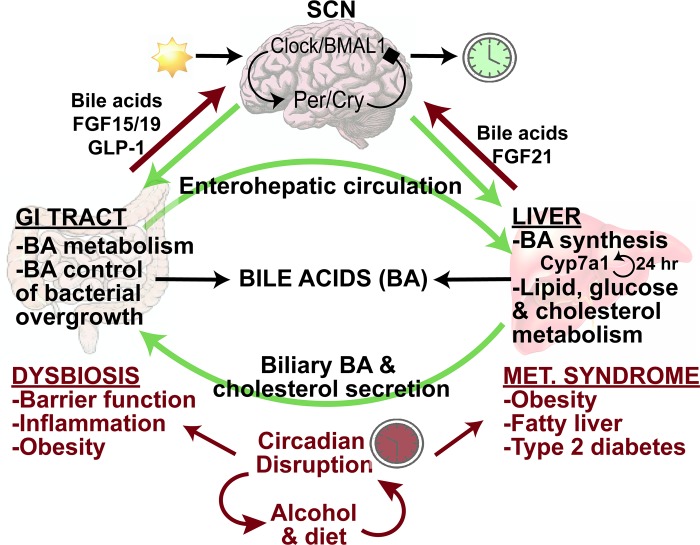
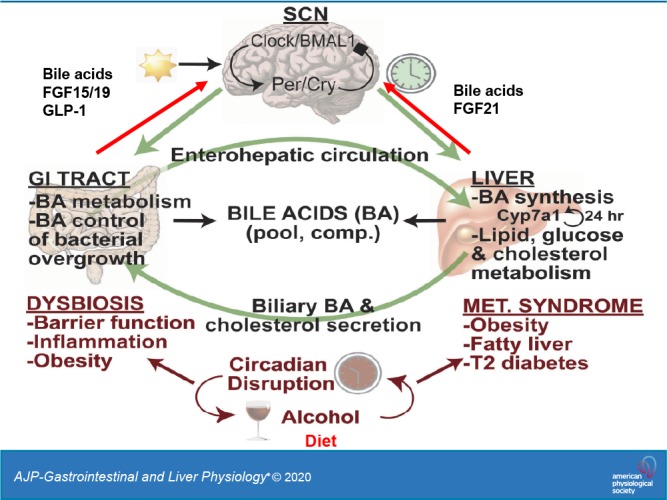
Figure 4 illustrates the central role of bile acids in the gut-liver-brain axis to regulate metabolic homeostasis. The enterohepatic circulation of bile acids maintains the bile acid pool. In the liver, bile acids regulate lipid, glucose, and cholesterol metabolism, whereas in the gastrointestinal tract, bile acids control bacterial overgrowth, and gut bacteria regulate bile acid metabolism to control bile acid composition and pool size (45).
Fig. 4.
Bile acids (BAs) in the gut-liver-brain axis and circadian rhythms. Circadian rhythms are generated and maintained by the suprachiasmatic nucleus (SCN) via rhythmic transcription and translation of core clock genes. Clock and Bmal1 induce Period (Per) and Cryptochrome (Cry), which feed back to inhibit Clock/Bmal1. All peripheral organs have molecular clocks that are synchronized to the SCN to regulate rhythms in lipid, glucose, and cholesterol metabolism, as well as cholesterol 7α-hydroxylase (CYP7A1) expression in the liver. In the gastrointestinal (GI) tract, gut bacteria metabolize bile acids, which control bacterial overgrowth. Dysbiosis, caused by circadian disruption, alcohol, or high-fat diets, impairs intestinal barrier function and causes inflammation. Dysbiosis also contributes to obesity, fatty liver, and type 2 diabetes mellitus. In the gut-to-brain axis, glucagon-like peptide-1 (GLP-1), FGF19, and bile acids mediate signaling cross-talk between the gut and brain, and FGF21 and bile acids mediate signaling cross-talk between the brain and liver.
Circadian rhythms refer to physiological processes that occur with a repeating period of ~24 h. Circadian rhythms are generated and maintained by the suprachiasmatic nucleus (SCN), located in the hypothalamus, and ensure that internal physiology is appropriately synchronized to the external environment. In the SCN, protein products of the core clock genes Clock and Bmal1 heterodimerize and induce the transcription of additional clock genes Period (Per) and Cryptochrome (Cry). Per and Cry proteins feed back to negatively regulate Clock and BMAL1 proteins, and the Per/Cry complex is eventually phosphorylated by casein kinase, marking it for degradation. The nuclear receptors Rev-erbα and retinoic acid receptor-related orphan receptor α participate in an accessory feedback loop to further regulate Bmal1 transcription. These feedback loops take ~24 h to complete and are synchronized by daily retinal photic input signals. The SCN also directs the neural and hormonal signals that synchronize the peripheral clocks that exist in nearly all tissues (45).
In the liver, rhythms are synchronized or altered by hormones, fasting and feeding, and diet (27, 46). Clock and Bmal1 regulate the clock-controlled gene D-site binding protein, whereas Rev-erbα regulates the transcription of the bile acid synthesis genes Cyp7a1 and Cyp8b1, the gluconeogenesis genes phosphoenolpyruvate kinase and glucose-6-phosphatase, and the lipid metabolism genes sterol regulatory element binding protein 1c (Srebp1c), fatty acid synthase, and acetyl-CoA carboxylase.
Bile acid synthesis exhibits a distinct diurnal rhythm, peaking at night and reaching a nadir at midday in mice (45). There are few studies on rhythms in bile acid synthesis in humans. In one study, bile acid synthesis (assayed by serum C4) showed two peaks, at 1:00 PM and 9:00 PM, declining at night and returning to the baseline the next morning (56). The postprandial rise of bile acid synthesis corresponds to the timing of food intake and is regulated by FGF19, which precedes the decline in bile acid synthesis (57, 117). Dysregulation of bile acid synthesis affects triglyceride and cholesterol homeostasis and contributes to obesity, fatty liver disease, type 2 diabetes, and metabolic syndrome, which is a collection of five metabolic phenotypes: hyperglycemia, hyperlipidemia, hypertension, insulin resistance, and obesity. Circadian disruption, diet, or alcohol drinking can cause dysbiosis (alteration of the gut microbiota that negatively impacts host health), impaired intestinal barrier function (leaky gut), inflammatory bowel diseases, fatty liver disease, diabetes, and obesity. Bile acids and circulating FGF19 levels vary widely in humans (56, 117). Bile acid synthesis is reduced in women compared with men and is correlated to serum triglyceride levels (57). Interestingly, a recent study of healthy men showed asynchronous rhythms of circulating conjugated and unconjugated bile acids (2). Bile acid synthesis is predominant in the daytime, whereas cholesterol is synthesized at night. Serum-conjugated bile acids peak after food intake, and the subsequent increase of FGF19 reduces bile acid synthesis and conjugated bile acids. Unconjugated bile acids exhibit one peak from night to early morning and are not rhythmic.
Circulating fibroblast growth factor 21 (FGF21) varies greatly in healthy individuals, with no correlation to age, gender, body mass index, serum lipid levels, or glucose levels (58). Increased free fatty acids during fasting activate peroxisome proliferator-activated receptor (PPAR) α and PPARγ coactivator protein-1α (PGC-1α) to stimulate FGF21 production in the liver and adipose tissue. As an adaptation to prolonged fasting, increased FGF21 stimulates glucose and energy metabolism in mice to maintain metabolic homeostasis, independent of insulin (78, 150). FGF21 reduces serum triglycerides by stimulating energy metabolism in adipose tissue (172) and inhibits mouse mammalian target of rapamycin complex 1 (mTORC1) signaling to improve insulin sensitivity and reduce hepatic steatosis (63, 211). FGF21 induces PGC-1α to stimulate fatty acid oxidation, ketogenesis, and energy metabolism during adaptive starvation (150), and in the brain, regulates circadian behavior, physical activity, and nutrient preference for carbohydrates, fats, and protein, and it reduces sweet and alcohol preference in mice (10, 186).
Bile Acid Regulation of Lipid Metabolism and Inflammation
Activation of FXR has been shown to improve glucose and lipid metabolism and reduce inflammation in diabetes (54, 64, 108, 153, 190). The role and mechanism of bile acid signaling in lipid lowering and glycemic control are not completely understood. Deficiency of Fxr in mice increased hepatic triglycerides, serum triglycerides, and cholesterol and promoted a proatherogenic lipid profile, suggesting that FXR regulates lipid and lipoprotein metabolism (178). Activation of mouse FXR inhibits SREBP-1c-mediated lipogenesis via induction of the FXR-SHP pathway, inhibiting LXR induction of SREBP-1c and the lipogenic genes fatty acid synthase, acetyl-CoA carboxylase, and stearoyl-CoA desaturase-1 (204). FXR activation also inhibits glucose-induced carbohydrate response element binding protein, which regulates glycolysis genes (17). However, in mice, transgenic overexpression of Cyp7a1 increased the bile acid pool, increased T-CDCA, and reduced T-MCAs, indicating activation of hepatic FXR signaling although none of the FXR target genes in lipogenesis were repressed (112). These mice were resistant to diet-induced obesity and diabetes. Interestingly, Cyp7a1-transgenic mice have increased cholesterol synthesis, but bile acid and cholesterol homeostasis were maintained by increasing biliary secretion of bile acids, cholesterol, and lipids (109). These studies showed that increasing bile acid synthesis stimulated SREBP-2-mediated cholesterol synthesis and reduced SREBP-1c-mediated lipogenesis by shunting acetyl-CoA from lipogenesis to cholesterol synthesis, resulting in reduced lipogenesis. FXR has been shown to induce human peroxisome proliferator-activated receptorα (PPARα), which stimulates fatty acid oxidation (145). Activation of FXR induces apolipoprotein (Apo) CII and ApoA5 but inhibits ApoA1 and ApoCIII, ultimately activating lipoprotein lipase in VLDL particles to reduce serum triglycerides (29). Free fatty acids mobilized from adipose tissue during fasting and starvation are transported to hepatocytes for synthesis of triglycerides, which are assembled in VLDL particles for secretion, transport, and distribution to muscle and other tissues for energy metabolism. Increased hepatic free fatty acid metabolism and VLDL secretion can cause lipotoxicity and induce endoplasmic reticulum (ER) stress and reactive oxidizing species (ROS).
The effects of bile acids on energy metabolism may be mainly due to activation of TGR5 by secondary bile acids (T/LCA and T/DCA). TGR5 is widely expressed in the epithelial cells of the intestine, gallbladder, and liver sinusoid, and in the Kupffer cells but not in hepatocytes. TGR5 activates adenylyl cyclase (AC) to convert ATP to cAMP, which induces protein kinase A to activate cAMP response element binding protein (CREB), which is involved in various cAMP signaling pathways. In muscle and brown adipose tissues, TGR5 induces deiodinase type 2 (DIO2), which converts thyroid hormone thyroxine to 3,5,3′-triiodothyronine and stimulates energy metabolism in the mitochondria (203). TGR5 also promotes adipose tissue browning, induces uncoupling proteins, and activates PPARα and PGC-1α to increase mitochondrial oxidative phosphorylation and energy metabolism and to prevent obesity and diabetes (50, 141, 148, 183). In the mouse intestine, activation of TGR5 stimulates glucagon-like peptide-1 (GLP-1) secretion from intestinal enteroendocrine L cells to increase insulin secretion from pancreatic β-cells and to increase glucose tolerance (Fig. 3) (189). TGR5 is also anti-inflammatory via inhibition of NF-κB-mediated proinflammatory cytokine production (202), induction of nitric oxide (NO) production to reduce monocyte adhesion in vascular endothelial cells (93), induction of endothelial NO synthase (eNOS) in liver sinusoidal endothelial cells (92), and inhibition of atherosclerosis by reducing macrophage inflammation and lipid loading (147).
Bile Acid Regulation of Glucose Metabolism
Upon feeding, bile acids are secreted from the gallbladder, and hepatic bile acid synthesis is derepressed. In the postprandial state, bile acids stimulate insulin signaling and improve glucose metabolism. In the late postprandial state, bile acids activate FXR to induce FGF19, which stimulates glycogen synthesis and lowers fasting plasma glucose independent of insulin (96). FGF15/19 also inhibits hepatic gluconeogenic gene expression by inhibiting the CREB-PGC-1α pathway (149). An early study reported that FXR bound to and activated the promoter of phosphoenolpyruvate carboxykinase (PEPCK), and FXR agonism induced PEPCK mRNA and glucose output in human and rat hepatocytes and mouse liver (181). In contrast, a later study reported that bile acid activation of FXR inhibited gluconeogenic gene expression by inducing SHP, which inhibits hepatocyte nuclear factor 4α and forkhead box O1 activation of PEPCK and reduces serum glucose in wild-type mice. SHP also inhibits growth hormone-mediated induction of gluconeogenesis by inhibiting signal transducer and activator of transcription 5 signaling (95). Fxr−/− mice developed fatty liver disease, had elevated serum free fatty acids and glucose, and were insulin resistant and hyperglycemic (118). Furthermore, FXR agonism improved hyperglycemia, enhanced insulin signaling in adipocytes, and improved insulin resistance in ob/ob and db/db mice (16, 220). In complete contrast, it was reported later that whole body Fxr-deficient mice had improved glucose homeostasis and adipose tissue insulin sensitivity, whereas hepatic insulin sensitivity did not change, and hepatic steatosis was aggravated (153). Further, this study showed that liver-specific Fxr−/− mice were not protected from diet-induced obesity and insulin resistance, suggesting FXR-independent regulation of glucose metabolism and obesity (153). It has been reported that both FXR and TGR5 are expressed in pancreatic β cells, and bile acid signaling through these two receptors may directly regulate insulin synthesis, secretion and sensitivity, and glucose metabolism (41, 99, 158, 171, 189).
INTESTINAL FXR IN LIVER METABOLISM
The implication of intestinal FXR in liver metabolism was first reported by Sayin et al. (169) in their study of germ-free mice and Fxr −/− mice and by Li et al. (105) in the study of intestine-specific-Fxr−/− mice. Analysis of bile acid composition revealed that T-MCA levels and bile acid pool size were reduced in conventionally raised mice compared with germ-free mice (169). This study identified T-αMCA and T-βMCA as intestinal FXR antagonists and confirmed the role of the gut microbiota in FXR-mediated inhibition of bile acid synthesis. High-fat diet-fed mice treated with tempol, an antioxidant, had reduced Lactobacillus and BSH activity to increase Tβ-MCA, which antagonized intestinal FXR, reduced FGF15, and derepressed bile acid synthesis to improve diet-induced obesity (DIO) and diabetes (105). Furthermore, they showed that intestine-specific-Fxr−/− mice were resistant to DIO, indicating that intestinal FXR signaling plays a critical role in the modulation of metabolic disease (80, 81). These studies also discovered that intestinal FXR induces ceramide synthesis and increases serum and liver ceramide concentrations (209). Ceramides cause ER stress and mitochondrial oxidative stress and inhibit insulin signaling in hepatocytes (64). Paradoxically, the intestine-selective FXR agonist fexaramine altered serum bile acid composition, promoted adipose tissue browning, and also reduced DIO in mice (43). It was shown that fexaramine treatment altered the gut microbiota by increasing the abundance of the LCA-producing gut bacteria Acetatifactor and Bacteroides (135). These bacteria have both 7α and 7β-dehydroxylase activity to convert CDCA and UDCA to LCA. LCA and DCA activate TGR5 to stimulate GLP-1 secretion from L cells and improve insulin sensitivity. TGR5 activation also promotes adipose tissue browning and energy metabolism to reduce weight in obese diabetic mice (135). These studies demonstrate that intestinal bile acid-FXR signaling plays a critical role in the regulation of liver metabolism.
FXR and TGR5 Signal Cross-talk in Enteroendocrine Cells
FXR and TGR5 are colocalized in enteroendocrine L cells (Fig. 3). Activation of FXR has been shown to induce Tgr5 gene transcription via an FXR binding site located in the Tgr5 gene promoter (138). LCA and DCA levels are high in the colon to activate enteroendocrine TGR5. Glucose is transported into L cells via sodium glucose transporter 1 and produces cAMP from ATP, which activates CREB. CREB induces prohormone convertase 1/3 to cleave preproglucagon to GLP-1. cAMP also stimulates Ca2+ secretion from the mitochondria to the cytosol and inhibits the K+ATP channel, depolarizing the membrane potential and stimulating GLP-1 secretion from L cells (138). GLP-1 stimulates insulin secretion from pancreatic β cells to improve insulin sensitivity and promote adipose tissue browning and energy metabolism to reduce weight (Fig. 3).
BILE ACID SIGNALING IN LIVER METABOLISM DURING FASTING TO FEEDING TRANSITION
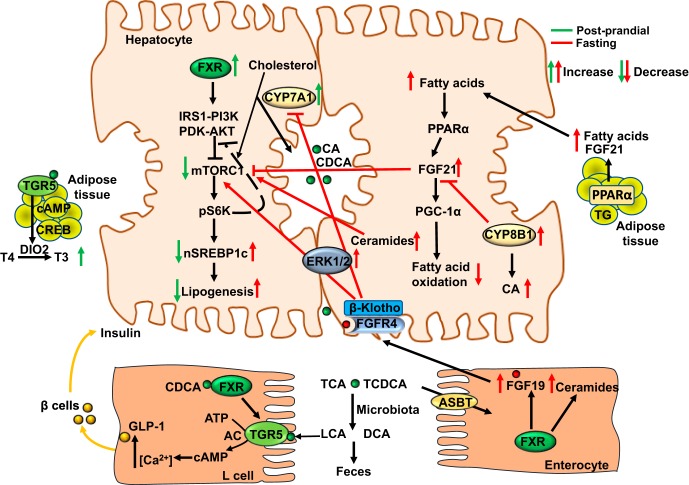
Bile acids facilitate nutrient absorption and act as nutrient sensors that regulate metabolism during fasting and feeding. Among all bile acids, cholic acid has the lowest critical micelle concentration (50 μM) and is the most efficacious bile acid in the absorption of dietary cholesterol and fat. Fasting and feeding rapidly alter the peak expression of the Cyp7a1 gene and the rhythmicity of bile acid synthesis, pool size and composition, and hepatic metabolism in mice (26, 45, 46) and humans (48). Feeding rapidly induces CYP7A1 but inhibits CYP8B1 expression, increasing bile acid synthesis and aiding in nutrient absorption during the postprandial state (Fig. 5).
Fig. 5.
Nutrient regulation of bile acid synthesis and hepatic metabolism. Feeding rapidly induces cholesterol 7α-hydroxylase (CYP7A1) but inhibits sterol 12α-hydroxylase (CYP8B1) expression to increase bile acid synthesis and aid in nutrient absorption during the postprandial state. Stimulating bile acid synthesis induces hepatic autophagy via insulin/AKT signaling to inhibit the mammalian target of rapamycin complex 1 (mTORC1). Farnesoid X receptor (FXR) activates insulin receptor substrate 1-AKT (protein kinase B) signaling to inhibit mTORC1/pS6K-signaling to promote nuclear translocation of sterol regulatory element binding protein 1c (nSREBP-1c) and lipogenesis. During the postprandial state, glucose is transported to enterocytes via the sodium-glucose-cotransporter 2. Chenodeoxycholic acid (CDCA) activates FXR in enteroendocrine L cells to induce Takeda G protein-coupled receptor 5 (TGR5) signaling and stimulate glucose-induced glucagon-like peptide-1 (GLP-1) secretion via increased intracellular cAMP and Ca2+. GLP-1 promotes insulin secretion from pancreatic β cells and increases insulin sensitivity. Activation of TGR5 in brown adipose tissue stimulates energy metabolism and the conversion of thyroxine to 3,5,3′-triiodothyronine. In the late post-prandial state to postabsorptive state, intestinal FXR induces FGF19, which activates hepatic FGF receptor (FGFR) 4-ERK1/2 signaling to inhibit bile acid synthesis. FGF19 regulates glucose metabolism, glycogen synthesis, and protein synthesis when insulin levels are decreased. In the intestine, activation of FXR induces ceramide synthesis. Ceramides activate mTORC1 signaling to induce SREBP-1c-mediated lipogenesis and induce ER stress and ROS to cause insulin resistance. During prolonged fasting or starvation, CYP7A1 expression and bile acid synthesis is reduced, but CYP8B1 is induced to increase chloric acid (CA) synthesis and deoxycholic acid (DCA) content in the colon, promoting ceramide synthesis and SREBP-1c-mediated lipogenesis. Increasing free fatty acids activate peroxisome proliferator-activated receptor (PPAR) α and PPARγ coactivator protein-1α (PGC-1α) to stimulate FGF21 production in liver and adipose tissue. As an adaptation to prolonged fasting to maintain metabolic homeostasis, increased FGF21 stimulates glucose and energy metabolism independent of insulin. FGF21 reduces serum triglycerides by stimulating energy metabolism in adipose tissue. FGF21 also inhibits mTORC1 signaling to improve insulin sensitivity and reduce hepatic steatosis. Green arrows indicate changes in the postprandial state and red arrows indicate changes in the fasting state. ABST, apical sodium-dependent bile acid transporter AC, adenylyl cyclase; CREB, cAMP response element binding protein; DIO2, deiodinase type 2; IRS1, insulin-resistant substrate 1; LCA, lithocholic acid; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; TG, triglycerides.
Stimulating bile acid synthesis reduces hepatic cholesterol and induces hepatic autophagy via insulin/AKT signaling and inhibition of mTORC1 (201). Autophagy is a highly conserved lysosomal pathway that is programmed to maintain lipid and metabolic homeostasis and cellular function (30). mTORC1 is regulated by cellular nutrients and energy to stimulate the anabolic pathways that consume ATP and inhibit the catabolic pathways that produce energy. In the postprandial state, FXR activates insulin-AKT signaling to inhibit mTORC1/pS6P-signaling, which is known to promote nuclear translocation of SREBP-1c and lipogenesis, thus inhibiting de novo lipogenesis in the postprandial state. CDCA activates FXR in enteroendocrine L cells to induce TGR5 expression and signaling, increase intracellular cAMP and Ca2+, and stimulate glucose-induced GLP-1 secretion, whereas activation of TGR5 in brown adipose tissue stimulates energy metabolism.
In the late postprandial state to the postabsorptive state, intestinal FXR induces FGF19, which activates hepatic FGFR4-ERK1/2 signaling to inhibit bile acid synthesis (180). In mice, Fgf15 regulates glucose metabolism and glycogen and protein synthesis when insulin levels are decreased (96). In the mouse intestine, activation of FXR induces ceramide synthesis (209), which causes insulin resistance in nonalcoholic fatty liver disease (NAFLD) in both rodents and humans (19, 55, 64, 214) via mTORC1 signaling, SREBP-1c-mediated lipogenesis, ER stress, and ROS.
During prolonged fasting and starvation, CYP7A1 expression and bile acid synthesis is reduced, but CYP8B1 is induced to increase CA synthesis and thus increase DCA in the colon, promoting ceramide synthesis and SREBP-1c-mediated lipogenesis.
INTERACTIONS BETWEEN BILE ACIDS AND GUT MICROBIOTA
Gut Microbiota
The human gut microbiome is composed of trillions of bacteria that belong to four major phyla: Firmicutes (60%), Bacteroidetes (22%), Actinobacteria (17%) and Proteobacteria (1%) (76). Recent metagenomic studies of the human gut microbiome revealed over 15,000 genomes and genomic blueprints (4, 133). Firmicutes are butyrate-producing bacteria, whereas Bacteroidetes degrade carbohydrates. The increase in the ratio of Firmicutes to Bacteroidetes commonly seen in patients with obesity allows gut microbes to extract energy more efficiently from high-fat diets, thus increasing adiposity. Animal-based diets alter the human gut microbiota by increasing the bile-tolerant bacteria Bilophila wadsworthia and decreasing Firmicutes (32). High-saturated-fat diets induce TCA to increase the expansion of Biophila wadsworthia and promote proinflammatory responses and inflammatory bowel disease in mice (38). Gut bacterial diversity is reduced in liver diseases; patients with NASH and NAFLD have significantly higher levels of Bacteroidetes and lower Firmicutes compared with heathy control subjects (122, 175). In advanced fibrosis, Gram-negative Proteobacteria is significantly increased, suggesting that Gram-negative bacteria might contribute to liver fibrosis (115). Patients with cirrhosis have increased fecal Enterobacteriaceae (a pathobiont) but reduced Ruminococcaceae, Lachonospiraceae, and Blautia compared with control subjects (89).
The reciprocal relationship between the gut microbiota and bile acids has been recognized for over half a century. In humans, CDCA is positively correlated to Enterobacteriaceae abundance, DCA is positively correlated to Ruminococcaceae, and LCA/CDCA positively correlated to Blautia (89). DCA and LCA are strong bactericides that control gut bacterial growth and the community of gut bacteria (microbiota). Gut microbes, in turn, deconjugate primary bile acids and convert them to secondary bile acids and thus control bile acid composition and the circulating bile acid pool via intestinal FXR/Fgf15 (FGF19) signaling (169). A study of germ-free and conventionally raised wild-type and Fxr−/− mice revealed that the gut microbiota promoted weight gain and hepatic steatosis by altering bile acid composition and reducing pool size via FXR signaling (132). In humans, a BSH-active probiotic increased bile acids and FGF19 (119). Gut bacteria harvest nutrients and energy from food ingested by the host and produce metabolites that affect host metabolism (76, 198). Diet, drugs, antibiotics, alcohol, and toxic bacterial metabolites disrupt the gut bacteria community and have negative effects on host metabolism (dysbiosis) and contribute to diseases including obesity, type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD), hepatocellular carcinoma (HCC), inflammatory bowel diseases (IBDs), and atherosclerosis (28, 76, 85, 139, 144, 160, 194, 218).
Bacterial BSH and 7α-dehydroxylase
Bacterial BSH and 7α-dehydroxylase play critical roles in the regulation of bile acid metabolism and homeostasis, and alteration of their activity significantly impacts host metabolism and health (82, 85, 100, 161). BSH is abundantly expressed in the human gut and is expressed in a broad spectrum of bacteria such as the Gram-positive genera Clostridium, Enterococcus, Listeria, and Lactobacillus (all of which belong to the phylum Firmicutes) and the Gram-negative genus Bacteroides, whereas 7α-dehydroxylase is restricted to a limited number of low abundant anaerobes (<1% of intestinal bacteria) (161). The multiple steps of 7α-dehydroxylation are catalyzed by enzymes encoded on a cluster of bile acid-inducible (bai) genes (162). The majority of the Bai-expressing bacteria are classified in the Ruminococcaceae (90%) family. The Bai gene operon (Bai A–J) in Clostridiales has been sequenced, and the enzymes encoded by the bai genes were characterized. The baiE gene encodes bile acid 7α-HSDH, and the baiI gene may encode 7β-HSDH.
Expression of a cloned BSH enzyme in the gastrointestinal tract of mice significantly altered serum bile acid species and transcription of the key genes involved in lipid, cholesterol, and circadian metabolism and gastrointestinal homeostasis (86). Interestingly, high expression of BSH activity in conventionally raised mice significantly reduced weight gain, plasma cholesterol, and liver triglycerides. A metagenome-wide association study of the gut microbiota in patients with T2DM reported increased Lactobacillus abundance (156). Reducing Lactobacillus by the antioxidant tempol or Bacteroides fragilis by metformin improves DIO in mice and in patients with T2DM (105, 182). Compared with those found in healthy control subjects, Firmicutes-derived BSH and 7α-dehydroxylase and HSDH genes are reduced in patients with ulcerative colitis and T2DM but not in patients with Crohn’s disease (100).
BILE ACID METABOLISM IN NONALCOHOLIC FATTY LIVER DISEASE
The global epidemic of obesity has increased the prevalence of T2DM, NAFLD, and cardiovascular disease. NAFLD is a significant complication of obesity and T2DM, and an independent risk factor for cardiovascular disease. NAFLD has become the most common chronic liver disease, affecting ~30% of the US population. NAFLD is a spectrum of liver diseases ranging from simple hepatic steatosis to progressive nonalcoholic steatohepatitis (NASH) with or without fibrosis and cirrhosis. Simple steatosis is reversible, but 30% of patients with NAFLD progress to NASH, which affects ~15% of the US population. NASH is a chronic liver disease with inflammation, macrovascular ballooning, macrophage infiltration, and fibrosis. NASH fibrosis can progress to cirrhosis and HCC in ~0.5% of patients with NAFLD (5, 216). HCC is the sixth most common cancer, and the rate of liver cancer death has increased rapidly, becoming the second most lethal cancer after pancreatic cancer (197). The pathogenesis and progression from simple hepatic steatosis to NASH are not completely understood. Many factors, including lipotoxicity, cigarette smoking, insulin resistance, cholesterol, and reactive oxidizing species (ROS), have been implicated in NASH progression. Patients with NASH have increased de novo lipogenesis, which causes accumulation of toxic lipids, including saturated fatty acids, lysophosphatidylcholine (LPC), free cholesterol, and ceramides, that can directly cause ER stress, apoptosis, liver inflammation, and injury (94). ER stress induces de novo lipogenesis and impaired hepatic lipid and glucose homeostasis (7).
Bile acids play a critical role in the control of metabolic disease, obesity, T2DM, and NAFLD (20). Patients with NAFLD have increased CA, CDCA, and bile acid synthesis and an increased ratio of primary bile acids to secondary bile acids (122). A more recent study reports that patients with NASH have increased circulating conjugated primary bile acids (especially G/T-CA and T-CDCA), an increased ratio of conjugated CA to CDCA, and decreased secondary bile acids (155). It is not clear whether increased and altered serum bile acids are the causes or consequences of NASH pathogenesis. In mice, it was shown that accumulation of toxic bile acids in hepatocytes can activate ER stress, de novo lipogenesis, and progression of hepatic steatosis to NASH (67, 70, 94). In patients with obesity and insulin resistance, serum 12α-hydroxylated bile acid levels are increased (66), and insulin and glucose have been implicated in the regulation of bile acid synthesis (107, 110). Metabolic phenotypes in diabetes, hyperinsulinemia, and hyperglycemia induce Cyp8b1 expression and increase serum cholesterol and 12α-hydroxylated bile acids in mice (137). A recent study reported that glucose-6-phosphate induces Cyp8b1 expression via glucose induction of carbohydrate response element binding protein (74). Increasing CA synthesis increases dietary cholesterol absorption and reduces biliary cholesterol secretion. Deficiency of Cyp8b1 in mice increases T-MCAs, which antagonize intestinal FXR to reduce FGF15 and increase bile acid synthesis and pool size. These mice are protected from diet-induced obesity and insulin resistance, hepatic steatosis, dyslipidemia, and atherosclerosis (21, 91, 134, 179). On the other hand, overexpression of Cyp8b1 increases SREBP-1c-mediated lipogenesis, dyslipidemia, and insulin resistance (136).
BILE ACID METABOLISM IN ALCOHOL-ASSOCIATED LIVER DISEASE
Alcohol-associated liver disease (AALD) is a major cause of chronic liver disease, is associated with ~50% of deaths caused by liver disease, and is the primary reason for one-third of liver transplants. AALD accounts for about half of the deaths caused by liver cirrhosis (59). Alcohol is a high-calorie, high-carbohydrate substance, and chronic alcohol consumption may lead to hepatic steatosis and progress to inflammation, fibrosis, cirrhosis, and HCC. Ethanol is metabolized by alcohol dehydrogenase and CYP2E1 to acetaldehyde, which is then converted to acetate by aldehyde dehydrogenase. Acetate is a precursor for the synthesis of fatty acids and glucose. Alcohol metabolism increases the cellular NADH/NAD+ ratio, which inhibits fatty acid oxidation and stimulates lipogenesis (215). Ethanol metabolism generates aldehydes that produce reactive oxidizing species and promote ER stress and DNA and protein adducts, causing liver injury, intestinal barrier dysfunction, and innate immune and adaptive responses. Alcohol increases gut permeability to release bacterial endotoxins and lipopolysaccharides (LPS) from the gut lumen to the liver, activating Toll-like receptor 4 signaling and causing inflammation and liver injury in AALD (184). There are several established alcohol research models, and each model may differentially affect the gut microbiome. However, in general, alcohol increases intestinal bacteria overgrowth of endotoxin-producing Gram-negative bacteria and causes gut microbial dysbiosis, which is considered to be the major cause of AALD progression to cirrhosis. In humans, chronic alcohol consumption was associated with long-term changes in colonic bacterial composition (125).
Active alcohol use in patients with cirrhosis is associated with a significant increase of the secondary bile acids compared with abstinent alcoholic cirrhosis and AALD (87). Patients with alcoholism have increased serum bile acids, cholestatic liver injury, and bile acid pool size and have reduced biliary bile flow and reduced fecal excretion. However, the role of bile acids in AALD is controversial and not understood. It has been reported that chronic or binge alcohol feeding induced Cyp7a1 and enlarged the bile acid pool in mice (18). In rats, alcohol feeding significantly reduced taurine-conjugated bile acids in the liver and gastrointestinal tracts, increased expression of bile acid synthesis and efflux genes, and decreased bile acid uptake transporter (210). Chronic ethanol feeding in mice induced the expression of bacteria expressing cholylglycine hydrolase, increased unconjugated bile acids, lowered FGF15, and increased Cyp7a1 (69). These effects and symptoms of alcoholic liver disease were reduced with antibiotics or administration of an FXR-specific agonist (69). These studies suggest that alcohol stimulates bile acid synthesis to increase bile acid pool size and cause cholestatic liver injury. In contrast, chronic plus binge alcohol feeding has been shown to reduce Cyp7a1 expression but increase the bile acid pool by increasing intestinal bile acid reabsorption in wild-type mice, whereas it exacerbates liver injury in Cyp7a1−/− mice (39). A recent analysis of the serum metabolome of patients with alcoholism identified increased serum levels of GCDCA, TCDCA, GCA, and TCA, which were positively correlated with AALD progression (213). Interestingly, this study found that liver CYP7A1 levels were decreased but that CYP7B1 levels were increased in AALD. They also found that serum GLP-1 and FGF21 levels but not FGF19 levels were increased in patients who drank heavily. Another recent study of patients with AALD found that alcohol drinking decreased hepatic bile acid synthesis but increased serum bile acids and FGF19 levels, which were positively correlated to the severity of AALD (13). This study also confirmed that bile acid synthesis is reduced in patients with AALD and not increased as previously thought by many investigators. There is no effective drug therapy for AALD. Several recent studies reported that FXR and TGR5 agonists ameliorated liver injury, steatosis, and inflammation in mouse models of binge and prolonged alcohol feeding (79, 207). These studies suggest that bile acid homeostasis is dysregulated in AALD, and modulation of bile acid signaling and/or the gut microbiota might be promising strategies for treating alcoholic hepatitis.
BILE ACID-BASED THERAPIES FOR FATTY LIVER DISEASES
Bile acid-based therapies targeting FXR and TGR5 were first developed to treat rare cholestatic liver diseases, including primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC). PBC is an acquired chronic cholestasis associated with autoimmune destruction of the small bile ducts, portal infiltration, and fibrosis. PSC is associated with liver injury and fibrosis of both intrahepatic and extrahepatic bile ducts, resulting in biliary strictures and obstructed bile flow. These bile acid drug therapies have been extended to clinical trials for NASH and AALD and are based on the anti-inflammatory effects of the activation of FXR and TGR5 signaling in liver, adipose tissue, and intestine in mouse models of diet-induced obesity, insulin resistance, and NAFLD. Targeting FXR and TGR5 signaling has been shown to improve hepatic bile acid metabolism and lipid and glucose homeostasis to prevent progression of NASH in clinical trials. Both activation and antagonism of intestinal FXR alleviate diet-induced obesity and diabetes. Table 1 summarizes bile acid-based therapies for NAFLD.
Table 1.
Bile acid-based therapies
| Treatment | |
|---|---|
| Bile acids | |
| CA (Cholbam) and CDCA (Chenodiol) | Treats inborne errors of bile acid synthesis; dissolves gallstones |
| UDCA (Ursodiol®, Actigall) | Dissolves gallstones; reduces bile acid cytotoxicity |
| NorUDCA | PSC |
| Bile acid sequestrants | |
| Cholestyramine, Colesevelam | Dissolves gallstones; reduces bile acid pool and stimulates bile acid synthesis; decreases FGF19; activates TGR5/GLP-1 and improves glycemic control; stimulates thermogenesis in BAT; BAD |
| FXR agonists: anti-inflammation, cholestasis, NASH fibrosis, diabetes, obesity | |
| OCA (OCALIVA) | PBC and BAD |
| Fexaramine (intestine-restricted FXR agonist) | stimulates adipose tissue browning, stimulates GLP-1 secretion, improves insulin sensitivity and reduces weight |
| FXR antagonists (intestine) | |
| GUDCA, Gly-MCA | PBC; reduces weight, improves diabetes |
| FGF19 analog | |
| NGM282 | anti-inflammation and fibrosis; NASH |
| Nonsteroidal FXR agonists | |
| Cilofexor, Tropifexor | PBC and NASH |
BAD, bile acid diarrhea; BAT, brown adipose tissue; CA, cholic acid; CDCA, chenodeoxycholic acid; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; Gly, glycine; GUDCA, glyco-ursodeoxycholic acid; MCA, muricholic acid; NASH, nonalcoholic steatohepatitis; OCA, obeticholic acid; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TGR5, Takeda G protein-coupled receptor 5; UDCA, ursodeoxycholic acid.
FXR Agonists
Obeticholic acid (OCA) (OCALIVA, 6α-ethyl-CDCA, EC50 = 0.099 μM) improved NASH scores in human patients with PBC who had inadequate responses to UDCA and in patients with T2DM and NASH without cirrhosis (71, 98, 123, 128, 129). However, an unwanted side effect of OCA is increased serum LDL cholesterol and decreased HDL cholesterol, and pruritus is a common side effect of bile acid therapy (142). OCA increased FGF19 in the gallbladder to reduce emptying, decrease bile acid synthesis, and increase bile acid hydrophobicity and cholesterol saturation index in patients with gallstone disease (1). OCA has been shown to improve NASH (128), and the third-phase clinical trial of OCA for treatment of NASH with fibrosis has shown significant improvement of fibrosis by one stage. Nonsteroidal FXR ligands have been developed for clinical trials for liver diseases (60). Cilofexor (GS-9674 derived from GW4064) has been shown to improve cholestasis and liver injury in a phase two clinical trial of PSC (191), and the non-bile acid FXR agonist tropifexor (LJN452) reversed fibrosis and reduced hepatic steatosis and triglycerides in mice (192) and is currently in phase 2B trials for the treatment of NASH and PBC in humans (140). Recently, OCA has been used to treat bile acid diarrhea (BAD), possibly by stimulating FGF19 (75, 101, 199). BAD results from excessive colonic bile acids caused by ileal resection, inflammatory injury, and inflammatory bowel diseases (IBDs) (14).
FXR Antagonists
Most recent studies show that FXR antagonism may have therapeutic value for treating NAFLD. UDCA (Ursodiol, Actigall), a weak FXR antagonist, has been shown to reduce ER stress and restore glucose homeostasis in a mouse model of T2DM (130). UDCA stimulated bile acid synthesis by reducing FGF19 and depleting hepatic LDL cholesterol but induced liver stearoyl-CoA desaturase-1 to increase liver triglycerides in a small trial of patients with NAFLD and morbid obesity (124). The antidiabetic drug metformin reduces Bacteroides fragilis and increases G-UDCA in patients with diabetes in 3 days (182), suggesting that metformin may alter the gut microbiota to antagonize intestinal FXR via UDCA and improve metabolism during diabetes.
TGR5 Agonists
The TGR5-selective agonist INT-777 (6α-ethyl-23(S)-methyl-cholic acid, EC50 = 0.82 μM) stimulates adipose tissue thermogenesis and energy metabolism, reduces inflammation (141), and stimulates GLP-1 secretion and insulin sensitivity but does not affect serum lipids (138). INT-777 may have anti-inflammasome effects in pancreatic acinar cells and may also reduce neuroinflammation (104, 208). A derivative of UDCA, BAR501 (6β-3α, 7β-dihydroxy-5β-cholan-24-ol), has been shown to reduce lipid deposition and vascular injury in DIO mice and regulate natural killer T cells during hepatitis (9, 173). Another selective TGR5 agonist, RDX8940, improved hepatic steatosis and insulin sensitivity with minimal systemic effects in Western diet-fed mice (49). However, activation of TGR5 may also induce unwanted side effects, including inhibition of gallbladder emptying and gallstone formation (195) and change of heart rate and blood pressure (146).
Dual FXR and TGR5 Agonists
The bile acid derivative 6α-ethyl-3α, 7α, 23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt (INT-767) is an FXR (EC50 = 0.03 μM) and TGR5 (EC50 = 0.63 μM) dual agonist. INT-767 is a more potent FXR agonist than OCA and is in a phase one clinical trial for NASH. INT-767 reduced expression of classic pathway bile acid synthesis genes but induced those in the alternative pathway to decrease TCA and increase T-MCA in mice (138). INT-767 induced intestinal FXR expression and intracellular Ca2+ and cAMP activity to stimulate GLP-1 secretion and improve glucose and lipid metabolism in DIO mice. BAR502, a nonsteroidal dual FXR and TGR5 agonist, promotes white adipose tissue browning and reverses high-fat diet-induced hepatic steatosis and fibrosis in mice (15).
FGF19 and FGF21
Drugs targeted to the prevention of the progression of simple steatosis to NASH are being developed. Serum FGF19 levels increase after bariatric surgery and are associated with improvement of glycemic control and diabetes remission in patients with obesity and diabetes, suggesting that targeting FXR and FGF19 may have therapeutic potential for treating NASH and HCC (11, 61). However, as a growth factor, FGF19 increases tumorigenesis when highly expressed at nonphysiological levels (221). A nontumorigenic FGF19 derivative, NGM282, reduced Cyp7a1 expression, bile acid pool size, and hepatic inflammation and improved NASH fibrosis in 12 wk of clinical trials (68). FGF21 reduces serum triglycerides by stimulating lipolysis in white and brown adipose tissues in mice (172). A long-lasting FGF21 analog, PF-05231023, has been shown to reduce body weight and improve lipid profile in patients with T2DM (187). FGF21 analogs are currently in clinical trials for obesity and T2DM.
Bile Acid Sequestrants
Bile acid sequestrants are a class of drugs that bind negatively charged bile acids in the intestine to prevent bile acid reabsorption and reduce bile acid pool size. This results in reduced FGF15 and increased CYP7A1 gene transcription and hepatic bile acid synthesis. Cholestyramine and colestipol are classic bile acid sequestrants used to treat cholesterol gallstone disease and hypercholesterolemia in human patients. Colesevelam and colestimide are second-generation bile acid sequestrants that have been shown to improve glycemic control in T2DM (50a). Bile acid sequestrants may increase secondary bile acids in the colon to stimulate TGR5-mediated secretion of GLP-1 and TGR5-mediated thermogenesis in brown adipose tissue and beiging of white adipose tissue (151, 174). By increasing bile acid synthesis to reduce hepatic cholesterol, bile acid sequestrants increase hepatic LDL-cholesterol uptake, reduce serum cholesterol, and protect against cardiovascular disease. Bile acid sequestrants also have been used to treat BAD and IBD (Crohn’s disease) by reducing intestinal bile acid reabsorption (14, 205). However, bile acid sequestrants have the unwanted side effects of increasing serum triglycerides and dyslipidemia.
Probiotics
Targeting the gut microbiota using probiotics and nondigestible fermentable carbohydrates (prebiotics, fermentable dietary fibers, and microbiota-accessible carbohydrates) has been shown to improve health and metabolic diseases (34, 194). Probiotics are live microorganisms that may provide benefits for HCC, NASH, and IBD by modifying the gut microbiota (12). Lactobacillus with active BSH activity in yogurt reduces serum cholesterol in human trials (83, 84). Probiotic VSL 3 induces BSH activity to promote bile acid deconjugation and increase bile acid synthesis by inhibiting the FXR-Fgf15 axis, thus increasing fecal bile acid excretion to improve insulin signaling and protecting against NASH in mice (35, 120). Bifidobacterium and Lactobacillus are commonly used as probiotics to improve intestinal barrier function (121). Akkermansia, Eubacterium and Faecalibacterium also have beneficial effects for diabetes and Crohn’s disease (31, 42, 176). Clostridia and Bacteroides are associated with intestinal infection but are used as probiotics for patients with colitis (6, 165). Bacteroides fragilis is a commensal bacterium that alters the gut microbiome, induces TGR5/GLP-1, and promotes adipose tissue browning to reduce weight. Bacteroides acidifaciens improves obesity and insulin sensitivity by increasing GLP-1 and decreasing intestinal dipeptidyl-4 in mice (212). This effect is apparently mediated through activation of TGR5 signaling. However, these over-the-counter probiotics have not been tested rigorously for their claimed beneficial metabolic effects and safety. A recent exploratory study shows that supplementation with Akkermansia muciniphila improved gut-barrier function and insulin sensitivity and reduced plasma total cholesterol in patients with T2DM who were overweight (37). This proof-of-concept study shows supplementation of Akkermansia muciniphila is safe and well tolerated to improve metabolic parameters.
Bariatric Surgery
Bariatric surgeries, such as Roux-en-Y gastric bypass and vertical sleeve gastrectomy, are the most effective treatments for reducing weight (11). In patients with T2DM, serum FGF19 levels are lower, whereas serum FGF21 levels are higher. Many studies demonstrate that bariatric surgery increases serum bile acids and FGF19 levels, which are associated with rapid improvement of insulin resistance in patients with obesity (11, 127, 152, 163, 177). Interestingly, serum FGF19 in individuals with obesity increased after bariatric surgery but not after conventional diet-induced weight loss (62). Serum FGF19 levels coincide with increased serum bile acids and GLP-1 following bariatric surgery (1a). The mechanisms by which bile acids improve glycemic control following gastric bypass may involve FXR signaling in glucose metabolism and/or TGR5 signaling in energy metabolism and insulin sensitivity. The greater increases in unconjugated and glycine-conjugated primary bile acids after duodenal switch compared with gastric bypass suggest that a constitutively increased bile acid pool may be due to shorter enterohepatic circulation of bile acids, which alters the gut microbiome and metabolites to improve the metabolism. Another recent study of serum bile acid profiles following gastric bypass and duodenal switch surgeries over 5 yr showed substantial increase of total serum bile acid concentration, likely via activation of both FXR and TGR5 signaling (163). Both primary and secondary bile acid levels are higher in subjects with insulin resistance and obesity and are positively correlated to insulin-resistance markers, and sleeve gastrectomy reduces total conjugated and unconjugated CA and increases LCA (33). This finding may suggest that increasing LCA can stimulate TGR5/GLP-1 signaling to improve glycemic control after sleeve gastrectomy. A systemic review and meta-analysis showed that bariatric surgery completely resolved NAFLD in patients with obesity (103). Thus, alteration of the gut microbiota and bile acid metabolism may be a major contribution to the metabolic benefits of bariatric surgery.
CONCLUSIONS AND FUTURE PERSPECTIVES
Significant progress in basic research of bile acid metabolism and signaling has been made in the last three decades. The roles and mechanisms of bile acid signaling in the pathophysiology of metabolic diseases have been unveiled with the use of genetic and diet-induced mouse models of cholestasis, diabetes, and obesity. Current trends in liver research are focused on the pathogenesis of NAFLD, especially the progression of nonfibrotic NASH to fibrotic NASH, cirrhosis, and HCC. Importantly, basic liver research has been translated to bile acid-based drug therapies for the treatment of NASH, diabetes, and obesity; although some bile acid derivatives may induce unwanted side effects, such as pruritus and cardiovascular and gallbladder-emptying effects. FXR agonists have been successfully approved for treating PSC and PBC, which are rare liver diseases. Many bile acid-based FXR agonists and nonsteroidal FXR agonists are in clinical trials, and it is anticipated that FXR agonists will be approved for treating NASH fibrosis in the near future. Dysbiosis appears to be a significant risk factor for the pathogenesis of diabetes, obesity, and NAFLD. Directly targeting gut dysbiosis with dietary management and probiotic and prebiotic supplements may be a potential strategy for treating liver-related disease. However, further studies are needed to identify serum bile acids, metabolites, and gut species as markers for diagnosis, treatment, and prevention of diabetes, obesity, NASH, and AALD.
GRANTS
This work is supported by National Institutes of Health Grants R01-DK-44441 and R37-DK-58379.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and J.M.F. conceived and designed research; J.C. analyzed data; J.M.F. interpreted results of experiments; J.C. prepared figures; J.C. and J.M.F. drafted manuscript; J.C. and J.M.F. edited and revised manuscript; J.C. and J.M.F. approved final version of manuscript.
REFERENCES
- 1.Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab 100: E1225–E1233, 2015. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Al-Dury S, Wahlström A, Panzitt K, Thorell A, Ståhlman M, Trauner M, Fickert P, Bäckhed F, Fändriks L, Wagner M, Marschall HU. Obeticholic acid may increase the risk of gallstone formation in susceptible patients. J Hepatol 71: 986–991, 2019. doi: 10.1016/j.jhep.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khaifi A, Straniero S, Voronova V, Chernikova D, Sokolov V, Kumar C, Angelin B, Rudling M. Asynchronous rhythms of circulating conjugated and unconjugated bile acids in the modulation of human metabolism. J Intern Med 284: 546–559, 2018. doi: 10.1111/joim.12811. [DOI] [PubMed] [Google Scholar]
- 4.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD. A new genomic blueprint of the human gut microbiota. Nature 568: 499–504, 2019. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 16: 411–428, 2019. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 6.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500: 232–236, 2013. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 7.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56: 952–964, 2012. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, Chan RB, Wang L, Haeusler RA. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am J Physiol Endocrinol Metab 313: E121–E133, 2017. doi: 10.1152/ajpendo.00409.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biagioli M, Carino A, Fiorucci C, Marchianò S, Di Giorgio C, Roselli R, Magro M, Distrutti E, Bereshchenko O, Scarpelli P, Zampella A, Fiorucci S. GPBAR1 functions as gatekeeper for liver NKT cells and provides counterregulatory signals in mouse models of immune-mediated hepatitis. Cell Mol Gastroenterol Hepatol 8: 447–473, 2019. doi: 10.1016/j.jcmgh.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19: 1147–1152, 2013. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozadjieva N, Heppner KM, Seeley RJ. Targeting FXR and FGF19 to treat metabolic diseases-lessons learned from bariatric surgery. Diabetes 67: 1720–1728, 2018. doi: 10.2337/dbi17-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandi G, De Lorenzo S, Candela M, Pantaleo MA, Bellentani S, Tovoli F, Saccoccio G, Biasco G. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis 38: 231–240, 2017. doi: 10.1093/carcin/bgx007. [DOI] [PubMed] [Google Scholar]
- 13.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Coulter S, Liddle C, Ling L, Rossi SJ, DePaoli AM, Loomba R, Mehal WZ, Fouts DE, Lucey MR, Bosques-Padilla F, Mathurin P, Louvet A, Garcia-Tsao G, Verna EC, Abraldes JG, Brown RS Jr, Vargas V, Altamirano J, Caballería J, Shawcross D, Stärkel P, Ho SB, Bataller R, Schnabl B. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol 69: 396–405, 2018. doi: 10.1016/j.jhep.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilleri M. Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 9: 332–339, 2015. doi: 10.5009/gnl14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carino A, Cipriani S, Marchianò S, Biagioli M, Santorelli C, Donini A, Zampella A, Monti MC, Fiorucci S. BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Sci Rep 7: 42801, 2017. doi: 10.1038/srep42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 281: 11039–11049, 2006. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 17.Caron S, Huaman Samanez C, Dehondt H, Ploton M, Briand O, Lien F, Dorchies E, Dumont J, Postic C, Cariou B, Lefebvre P, Staels B. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol Cell Biol 33: 2202–2211, 2013. doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanda D, Kim Y-H, Li T, Misra J, Kim D-K, Kim JR, Kwon J, Jeong W-I, Ahn S-H, Park T-S, Koo SH, Chiang JY, Lee CH, Choi HS. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS One 8: e68845, 2013. doi: 10.1371/journal.pone.0068845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594, 2012. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152: 1679–1694.e3, 2017. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Chevre R, Trigueros-Motos L, Castaño D, Chua T, Corlianò M, Patankar JV, Sng L, Sim L, Juin TL, Carissimo G, Ng LFP, Yi CNJ, Eliathamby CC, Groen AK, Hayden MR, Singaraja RR. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. FASEB J 32: 3792–3802, 2018. doi: 10.1096/fj.201701084RR. [DOI] [PubMed] [Google Scholar]
- 22.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang JY. Recent advances in understanding bile acid homeostasis. F1000 Res 6: 2029, 2017. doi: 10.12688/f1000research.12449.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang JY, Chen W, Zheng M, Wu Z, Kimmel R, Stroup D. Bile acids and FXR represses cholesterol 7A-hydroxylase (CYP7A1), sterol 12A-hydroxylase (CYP8B1) and sterol 27-hydroxylase (CYP27A1), but not oxysterol 7A-hydroxylase (CYP7B1) gene transcription (Abstract) Gastroenterology 118: A1006, 2000. doi: 10.1016/S0016-5085(00)86175-2. [DOI] [Google Scholar]
- 25.Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res 1: 3–9, 2017. doi: 10.1016/j.livres.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr 18: 71–87, 2018. doi: 10.3727/105221618X15156018385515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang JYL, Ferrell JM. Bile acids as metabolic regulators and nutrient sensors. Annu Rev Nutr 39: 175–200, 2019. doi: 10.1146/annurev-nutr-082018-124344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O’Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 3: 186–202, 2012. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, Fruchart JC, Gonzalez FJ, Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology 125: 544–555, 2003. doi: 10.1016/S0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 30.Czaja MJ, Ding WX, Donohue TM Jr, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131–1158, 2013. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K; MICRO-Obes Consortium . Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436, 2016. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 32.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol Spectr 5: 5, 2017. doi: 10.1128/microbiolspec.BAD-0019-2017. [DOI] [PubMed] [Google Scholar]
- 35.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Reports 7: 12–18, 2014. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 36.del Castillo-Olivares A, Campos JA, Pandak WM, Gil G. The role of alpha1-fetoprotein transcription factor/LRH-1 in bile acid biosynthesis: a known nuclear receptor activator that can act as a suppressor of bile acid biosynthesis. J Biol Chem 279: 16813–16821, 2004. doi: 10.1074/jbc.M400646200. [DOI] [PubMed] [Google Scholar]
- 37.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25: 1096–1103, 2019. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.De Vuono S, Ricci MA, Nulli Migliola E, Monti MC, Morretta E, Boni M, Ministrini S, Carino A, Fiorucci S, Distrutti E, Lupattelli G. Serum bile acid levels before and after sleeve gastrectomy and their correlation with obesity-related comorbidities. Obes Surg 29: 2517–2526, 2019. doi: 10.1007/s11695-019-03877-6. [DOI] [PubMed] [Google Scholar]
- 39.Donepudi AC, Ferrell JM, Boehme S, Choi HS, Chiang JYL. Deficiency of cholesterol 7α-hydroxylase in bile acid synthesis exacerbates alcohol-induced liver injury in mice. Hepatol Commun 2: 99–112, 2018. doi: 10.1002/hep4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duane WC, Javitt NB. 27-hydroxycholesterol: production rates in normal human subjects. J Lipid Res 40: 1194–1199, 1999. [PubMed] [Google Scholar]
- 41.Düfer M, Hörth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, Oberwinkler J, Lukowski R, Gonzalez FJ, Krippeit-Drews P, Drews G. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes 61: 1479–1489, 2012. doi: 10.2337/db11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 21: 159–165, 2015. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrell JM, Boehme S, Li F, Chiang JY. Cholesterol 7α-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid Res 57: 1144–1154, 2016. doi: 10.1194/jlr.M064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrell JM, Chiang JY. Circadian rhythms in liver metabolism and disease. Acta Pharm Sin B 5: 113–122, 2015. doi: 10.1016/j.apsb.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrell JM, Chiang JY. Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell Mol Gastroenterol Hepatol 1: 664–677, 2015. doi: 10.1016/j.jcmgh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrell JM, Chiang JYL. Understanding bile acid signaling in diabetes: from pathophysiology to therapeutic targets. Diabetes Metab J 43: 257–272, 2019. doi: 10.4093/dmj.2019.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiamoncini J, Yiorkas AM, Gedrich K, Rundle M, Alsters SI, Roeselers G, van den Broek TJ, Clavel T, Lagkouvardos I, Wopereis S, Frost G, van Ommen B, Blakemore AI, Daniel H. Determinants of postprandial plasma bile acid kinetics in human volunteers. Am J Physiol Gastrointest Liver Physiol 313: G300–G312, 2017. doi: 10.1152/ajpgi.00157.2017. [DOI] [PubMed] [Google Scholar]
- 49.Finn PD, Rodriguez D, Kohler J, Jiang Z, Wan S, Blanco E, King AJ, Chen T, Bell N, Dragoli D, Jacobs JW, Jain R, Leadbetter M, Siegel M, Carreras CW, Koo-McCoy S, Shaw K, Le C, Vanegas S, Hsu IH, Kozuka K, Okamoto K, Caldwell JS, Lewis JG. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am J Physiol Gastrointest Liver Physiol 316: G412–G424, 2019. doi: 10.1152/ajpgi.00300.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 30: 570–580, 2009. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 50a.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab 12: 384–392, 2010. doi: 10.1111/j.1463-1326.2009.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24: 2050–2064, 2010. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 276: 38378–38387, 2001. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 54.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60: 463–472, 2011. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 55.Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis 12: 98, 2013. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gälman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 129: 1445–1453, 2005. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Gälman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med 270: 580–588, 2011. doi: 10.1111/j.1365-2796.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 58.Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8: 169–174, 2008. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gege C, Hambruch E, Hambruch N, Kinzel O, Kremoser C. Nonsteroidal FXR ligands: current status and clinical applications. Handb Exp Pharmacol 256: 167–205, 2019. doi: 10.1007/164_2019_232. [DOI] [PubMed] [Google Scholar]
- 61.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care 36: 1859–1864, 2013. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, Valentí V, Salvador J, Giralt M, Villarroya F, Frühbeck G. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr 36: 861–868, 2017. doi: 10.1016/j.clnu.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 63.Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H, Gao J, Chen X, Han Y, Liang Q, Ye D, Shi L, Chin YE, Wang Y, Xiao H, Guo F, Liu Y, Zang M, Xu A, Li Y. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 64: 425–438, 2016. doi: 10.1002/hep.28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez FJ, Jiang C, Patterson AD. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151: 845–859, 2016. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517–526, 2000. doi: 10.1016/S1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 66.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 62: 4184–4191, 2013. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han J, Murthy R, Wood B, Song B, Wang S, Sun B, Malhi H, Kaufman RJ. ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice. Diabetologia 56: 911–924, 2013. doi: 10.1007/s00125-012-2809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, Banerjee R, Jaros MJ, Owers S, Baxter BA, Ling L, DePaoli AM. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. In press. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, Alnouti Y, Fouts DE, Stärkel P, Loomba R, Coulter S, Liddle C, Yu RT, Ling L, Rossi SJ, DePaoli AM, Downes M, Evans RM, Brenner DA, Schnabl B. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 67: 2150–2166, 2018. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henkel AS. Unfolded protein response sensors in hepatic lipid metabolism and nonalcoholic fatty liver disease. Semin Liver Dis 38: 320–332, 2018. doi: 10.1055/s-0038-1670677. [DOI] [PubMed] [Google Scholar]
- 71.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148: 751–61.e8, 2015. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res 51: 226–246, 2010. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honda A, Miyazaki T, Iwamoto J, Hirayama T, Morishita Y, Monma T, Ueda H, Mizuno S, Sugiyama F, Takahashi S, Ikegami T. Regulations of bile acid metabolism in mouse models with hydrophobic bile acid composition. J Lipid Res 61: 54–69, 2020. doi: 10.1194/jlr.RA119000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoogerland JA, Lei Y, Wolters JC, de Boer JF, Bos T, Bleeker A, Mulder NL, van Dijk TH, Kuivenhoven JA, Rajas F, Mithieux G, Haeusler RA, Verkade HJ, Bloks VW, Kuipers F, Oosterveer MH. Glucose-6-phosphate regulates hepatic bile acid synthesis in mice. Hepatology 70: 2171–2184, 2019. doi: 10.1002/hep.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hvas CL, Ott P, Paine P, Lal S, Jørgensen SP, Dahlerup JF. Obeticholic acid for severe bile acid diarrhea with intestinal failure: a case report and review of the literature. World J Gastroenterol 24: 2320–2326, 2018. doi: 10.3748/wjg.v24.i21.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikegami T, Honda A. Reciprocal interactions between bile acids and gut microbiota in human liver diseases. Hepatol Res 48: 15–27, 2018. doi: 10.1111/hepr.13001. [DOI] [PubMed] [Google Scholar]
- 77.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5: 415–425, 2007. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Iracheta-Vellve A, Calenda CD, Petrasek J, Ambade A, Kodys K, Adorini L, Szabo G. FXR and TGR5 agonists ameliorate liver injury, steatosis, and inflammation after binge or prolonged alcohol feeding in mice. Hepatol Commun 2: 1379–1391, 2018. doi: 10.1002/hep4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 125: 386–402, 2015. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, Liu Y, Gavrilova O, Patterson AD, Gonzalez FJ. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 6: 10166, 2015. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 105: 13580–13585, 2008. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones ML, Martoni CJ, Parent M, Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107: 1505–1513, 2012. doi: 10.1017/S0007114511004703. [DOI] [PubMed] [Google Scholar]
- 84.Jones ML, Martoni CJ, Tamber S, Parent M, Prakash S. Evaluation of safety and tolerance of microencapsulated Lactobacillus reuteri NCIMB 30242 in a yogurt formulation: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol 50: 2216–2223, 2012. doi: 10.1016/j.fct.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Joyce SA, Gahan CG. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol 30: 120–127, 2014. doi: 10.1097/MOG.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 86.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci USA 111: 7421–7426, 2014. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, Nittono H, Ridlon JM, Fuchs M, Gurley EC, Wang Y, Liu R, Sanyal AJ, Gillevet PM, Bajaj JS. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol 306: G929–G937, 2014. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kakiyama G, Marques D, Takei H, Nittono H, Erickson S, Fuchs M, Rodriguez-Agudo D, Gil G, Hylemon PB, Zhou H, Bajaj JS, Pandak WM. Mitochondrial oxysterol biosynthetic pathway gives evidence for CYP7B1 as controller of regulatory oxysterols. J Steroid Biochem Mol Biol 189: 36–47, 2019. doi: 10.1016/j.jsbmb.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 89.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 58: 949–955, 2013. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katafuchi T, Esterházy D, Lemoff A, Ding X, Sondhi V, Kliewer SA, Mirzaei H, Mangelsdorf DJ. Detection of FGF15 in plasma by stable isotope standards and capture by anti-peptide antibodies and targeted mass spectrometry. Cell Metab 21: 898–904, 2015. doi: 10.1016/j.cmet.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, Groen AK, Verchere CB, Singaraja RR, Hayden MR. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes 64: 1168–1179, 2015. doi: 10.2337/db14-0716. [DOI] [PubMed] [Google Scholar]
- 92.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45: 695–704, 2007. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 93.Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol 33: 1663–1669, 2013. doi: 10.1161/ATVBAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- 94.Kim JY, Garcia-Carbonell R, Yamachika S, Zhao P, Dhar D, Loomba R, Kaufman RJ, Saltiel AR, Karin M. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell 175: 133–145.e15, 2018. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim YD, Li T, Ahn S-W, Kim DK, Lee JM, Hwang SL, Kim YH, Lee CH, Lee IK, Chiang JY, Choi HS. Orphan nuclear receptor small heterodimer partner negatively regulates growth hormone-mediated induction of hepatic gluconeogenesis through inhibition of signal transducer and activator of transcription 5 (STAT5) transactivation. J Biol Chem 287: 37098–37108, 2012. doi: 10.1074/jbc.M112.339887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331: 1621–1624, 2011. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56: 1034–1043, 2012. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, Vincent C, Rust C, Parés A, Mason A, Marschall HU, Shapiro D, Adorini L, Sciacca C, Beecher-Jones T, Böhm O, Pencek R, Jones D; Obeticholic Acid PBC Monotherapy Study Group . A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 67: 1890–1902, 2018. doi: 10.1002/hep.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar DP, Asgharpour A, Mirshahi F, Park SH, Liu S, Imai Y, Nadler JL, Grider JR, Murthy KS, Sanyal AJ. Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet alpha cells to promote glucose homeostasis. J Biol Chem 291: 6626–6640, 2016. doi: 10.1074/jbc.M115.699504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Labbé A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn’s, ulcerative colitis and type 2 diabetes metagenomes. PLoS One 9: e115175, 2014. doi: 10.1371/journal.pone.0115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JM, Ong JR, Vergnes L, de Aguiar Vallim TQ, Nolan J, Cantor RM, Walters JRF, Reue K. Diet1, bile acid diarrhea, and FGF15/19: mouse model and human genetic variants. J Lipid Res 59: 429–438, 2018. doi: 10.1194/jlr.M078279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 17: 1040–1060.e11, 2019. doi: 10.1016/j.cgh.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 104.Li B, Yang N, Li C, Li C, Gao K, Xie X, Dong X, Yang J, Yang Q, Tong Z, Lu G, Li W. INT-777, a bile acid receptor agonist, extenuates pancreatic acinar cells necrosis in a mouse model of acute pancreatitis. Biochem Biophys Res Commun 503: 38–44, 2018. doi: 10.1016/j.bbrc.2018.05.120. [DOI] [PubMed] [Google Scholar]
- 105.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4: 2384, 2013. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J, Dawson PA. Animal models to study bile acid metabolism. Biochim Biophys Acta Mol Basis Dis 1865: 895–911, 2019. doi: 10.1016/j.bbadis.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li T, Chanda D, Zhang Y, Choi HS, Chiang JYL. Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. J Lipid Res 51: 832–842, 2010. doi: 10.1194/jlr.M002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66: 948–983, 2014. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 58: 1111–1121, 2013. doi: 10.1002/hep.26427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem 287: 1861–1873, 2012. doi: 10.1074/jbc.M111.305789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JY. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53: 996–1006, 2011. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 52: 678–690, 2010. doi: 10.1002/hep.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li YC, Wang DP, Chiang JY. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. J Biol Chem 265: 12012–12019, 1990. [PubMed] [Google Scholar]
- 114.Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem 275: 16536–16542, 2000. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- 115.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 25: 1054–1062.e5, 2017. [Erratum in: Cell Metab 30: 607, 2019.] doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lou G, Ma X, Fu X, Meng Z, Zhang W, Wang YD, Van Ness C, Yu D, Xu R, Huang W. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One 9: e93567, 2014. doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 260: 530–536, 2006. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 118.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116: 1102–1109, 2006. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martoni CJ, Labbé A, Ganopolsky JG, Prakash S, Jones ML. Changes in bile acids, FGF-19 and sterol absorption in response to bile salt hydrolase active L. reuteri NCIMB 30242. Gut Microbes 6: 57–65, 2015. doi: 10.1080/19490976.2015.1005474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mencarelli A, Cipriani S, Renga B, Bruno A, D’Amore C, Distrutti E, Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One 7: e45425, 2012. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol 10: 185, 2019. doi: 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One 11: e0151829, 2016. doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145: 574–82.e1, 2013. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 124.Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, Hoesel B, Fauler G, Stojakovic T, Einarsson C, Marschall HU, Trauner M. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol 62: 1398–1404, 2015. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 302: G966–G978, 2012. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342: 1094–1098, 2013. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nemati R, Lu J, Dokpuang D, Booth M, Plank LD, Murphy R. Increased bile acids and FGF19 after sleeve gastrectomy and Roux-en-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes Surg 28: 2672–2686, 2018. doi: 10.1007/s11695-018-3216-x. [DOI] [PubMed] [Google Scholar]
- 128.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385: 956–965, 2015. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group . A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 375: 631–643, 2016. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 130.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pandak WM, Heuman DM, Hylemon PB, Chiang JY, Vlahcevic ZR. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology 108: 533–544, 1995. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 132.Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, Greiner TU, Perkins R, Bäckhed F. Microbiota-induced obesity requires farnesoid X receptor. Gut 66: 429–437, 2017. doi: 10.1136/gutjnl-2015-310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P, Collado MC, Rice BL, DuLong C, Morgan XC, Golden CD, Quince C, Huttenhower C, Segata N. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 176: 649–662.e20, 2019. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patankar JV, Wong CK, Morampudi V, Gibson WT, Vallance B, Ioannou GN, Hayden MR. Genetic ablation of Cyp8b1 preserves host metabolic function by repressing steatohepatitis and altering gut microbiota composition. Am J Physiol Endocrinol Metab 314: E418–E432, 2018. doi: 10.1152/ajpendo.00172.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68: 1574–1588, 2018. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pathak P, Chiang JYL. Sterol 12α-hydroxylase aggravates dyslipidemia by Activating the ceramide/mTORC1/SREBP-1C pathway via FGF21 and FGF15. Gene Expr 19: 161–173, 2019. doi: 10.3727/105221619X15529371970455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pathak P, Li T, Chiang JY. Retinoic acid-related orphan receptor α regulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J Biol Chem 288: 37154–37165, 2013. doi: 10.1074/jbc.M113.485987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem 292: 11055–11069, 2017. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes. Postgrad Med J 92: 286–300, 2016. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 140.Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, Loomba R, Ratziu V, Kochuparampil J, Fischer L, Vaidyanathan S, Anstee QM. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp Clin Trials 88: 105889, 2020. doi: 10.1016/j.cct.2019.105889. [DOI] [PubMed] [Google Scholar]
- 141.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A, Pastorini E, Schoonjans K, Auwerx J. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem 52: 7958–7961, 2009. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- 142.Pencek R, Marmon T, Roth JD, Liberman A, Hooshmand-Rad R, Young MA. Effects of obeticholic acid on lipoprotein metabolism in healthy volunteers. Diabetes Obes Metab 18: 936–940, 2016. doi: 10.1111/dom.12681. [DOI] [PubMed] [Google Scholar]
- 143.Phan J, Pesaran T, Davis RC, Reue K. The Diet1 locus confers protection against hypercholesterolemia through enhanced bile acid metabolism. J Biol Chem 277: 469–477, 2002. doi: 10.1074/jbc.M107107200. [DOI] [PubMed] [Google Scholar]
- 144.Piglionica M, Cariello M, Moschetta A. The gut-liver axis in hepatocarcinoma: a focus on the nuclear receptor FXR and the enterokine FGF19. Curr Opin Pharmacol 43: 93–98, 2018. doi: 10.1016/j.coph.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol 17: 259–272, 2003. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 146.Piotrowski DW, Futatsugi K, Warmus JS, Orr ST, Freeman-Cook KD, Londregan AT, Wei L, Jennings SM, Herr M, Coffey SB, Jiao W, Storer G, Hepworth D, Wang J, Lavergne SY, Chin JE, Hadcock JR, Brenner MB, Wolford AC, Janssen AM, Roush NS, Buxton J, Hinchey T, Kalgutkar AS, Sharma R, Flynn DA. Identification of tetrahydropyrido[4,3-d]pyrimidine amides as a new class of orally bioavailable TGR5 agonists. ACS Med Chem Lett 4: 63–68, 2013. doi: 10.1021/ml300277t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 14: 747–757, 2011. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54: 1263–1272, 2011. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 13: 729–738, 2011. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858, 2009. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol 304: G371–G380, 2013. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153: 3613–3619, 2012. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, Dorchies E, Daoudi M, Lestavel S, Gonzalez FJ, Oresic M, Cariou B, Kuipers F, Caron S, Staels B. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60: 1861–1871, 2011. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110: 109–117, 2002. doi: 10.1172/JCI0215387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67: 534–548, 2018. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60, 2012. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 157.Ren S, Hylemon PB, Marques D, Gurley E, Bodhan P, Hall E, Redford K, Gil G, Pandak WM. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology 40: 910–917, 2004. doi: 10.1002/hep.1840400421. [DOI] [PubMed] [Google Scholar]
- 158.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta 1802: 363–372, 2010. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Repa JJ, Lund EG, Horton JD, Leitersdorf E, Russell DW, Dietschy JM, Turley SD. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem 275: 39685–39692, 2000. doi: 10.1074/jbc.M007653200. [DOI] [PubMed] [Google Scholar]
- 160.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 4: 382–387, 2013. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7: 22–39, 2016. [Erratum in: Gut Microbes 7: 262, 2016.] doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 163.Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, Gulseth HL, Thorsby PM, Vincent RP, Engström M, Olbers T, Mala T. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis 13: 1544–1553, 2017. doi: 10.1016/j.soard.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 164.Rizzolo D, Buckley K, Kong B, Zhan L, Shen J, Stofan M, Brinker A, Goedken M, Buckley B, Guo GL. Bile acid homeostasis in a cholesterol 7α-hydroxylase and sterol 27-hydroxylase double knockout mouse model. Hepatology 70: 389–402, 2019. doi: 10.1002/hep.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974–977, 2011. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 167.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta 1529: 126–135, 2000. doi: 10.1016/S1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 168.Sanyal S, Båvner A, Haroniti A, Nilsson LM, Lundåsen T, Rehnmark S, Witt MR, Einarsson C, Talianidis I, Gustafsson JA, Treuter E. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc Natl Acad Sci USA 104: 15665–15670, 2007. doi: 10.1073/pnas.0706736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235, 2013. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49: 1228–1235, 2009. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 171.Schittenhelm B, Wagner R, Kähny V, Peter A, Krippeit-Drews P, Düfer M, Drews G. Role of FXR in β-cells of lean and obese mice. Endocrinology 156: 1263–1271, 2015. doi: 10.1210/en.2014-1751. [DOI] [PubMed] [Google Scholar]
- 172.Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J, Scheja L. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab 23: 441–453, 2016. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Sepe V, Renga B, Festa C, D’Amore C, Masullo D, Cipriani S, Di Leva FS, Monti MC, Novellino E, Limongelli V, Zampella A, Fiorucci S. Modification on ursodeoxycholic acid (UDCA) scaffold. discovery of bile acid derivatives as selective agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1). J Med Chem 57: 7687–7701, 2014. doi: 10.1021/jm500889f. [DOI] [PubMed] [Google Scholar]
- 174.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol 298: G419–G424, 2010. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 175.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 16: 375–381, 2017. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 176.Sheng L, Jena PK, Liu HX, Hu Y, Nagar N, Bronner DN, Settles ML, Bäumler AJ, Wan YY. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J 32: 6371–6384, 2018. doi: 10.1096/fj.201800370R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, Laakso M, Gylling H, Patti ME, Auwerx J, Pihlajamäki J. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg 22: 1473–1480, 2012. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 179.Slätis K, Gåfvels M, Kannisto K, Ovchinnikova O, Paulsson-Berne G, Parini P, Jiang ZY, Eggertsen G. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res 51: 3289–3298, 2010. doi: 10.1194/jlr.M009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49: 297–305, 2009. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF, Burris TP. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 146: 984–991, 2005. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- 182.Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Wang X, Gonzalez FJ, Jiang C. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 24: 1919–1929, 2018. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Svensson PA, Olsson M, Andersson-Assarsson JC, Taube M, Pereira MJ, Froguel P, Jacobson P. The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem Biophys Res Commun 433: 563–566, 2013. doi: 10.1016/j.bbrc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148: 30–36, 2015. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, Krausz KW, Wolf CR, Henderson CJ, Gonzalez FJ. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res 57: 2130–2137, 2016. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Müller CP, Tang H, Mangelsdorf DJ, Goodwin B, Kliewer SA. FGF21 regulates sweet and alcohol preference. Cell Metab 23: 344–349, 2016. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, Brenner MB, Trimmer JK, Gropp KE, Chabot JR, Erion DM, Rolph TP, Goodwin B, Calle RA. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 23: 427–440, 2016. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 188.Tarling EJ, Clifford BL, Cheng J, Morand P, Cheng A, Lester E, Sallam T, Turner M, de Aguiar Vallim TQ. RNA-binding protein ZFP36L1 maintains posttranscriptional regulation of bile acid metabolism. J Clin Invest 127: 3741–3754, 2017. doi: 10.1172/JCI94029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis 28: 220–224, 2010. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 191.Trauner M, Gulamhusein A, Hameed B, Caldwell S, Shiffman ML, Landis C, Eksteen B, Agarwal K, Muir A, Rushbrook S, Lu X, Xu J, Chuang JC, Billin AN, Li G, Chung C, Subramanian GM, Myers RP, Bowlus CL, Kowdley KV. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 70: 788–801, 2019. doi: 10.1002/hep.30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X, Bao D, Zoll J, Kim Y, Groessl T, McNamara P, Seidel HM, Molteni V, Liu B, Phimister A, Joseph SB, Laffitte B. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem 60: 9960–9973, 2017. doi: 10.1021/acs.jmedchem.7b00907. [DOI] [PubMed] [Google Scholar]
- 193.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13: 1185–1192, 2007. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 194.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 361: k2179, 2018. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HR Jr, Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398: 423–430, 2006. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vergnes L, Lee JM, Chin RG, Auwerx J, Reue K. Diet1 functions in the FGF15/19 enterohepatic signaling axis to modulate bile acid and lipid levels. Cell Metab 17: 916–928, 2013. doi: 10.1016/j.cmet.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Villanueva A. Hepatocellular carcinoma. N Engl J Med 380: 1450–1462, 2019. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 198.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24: 41–50, 2016. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 199.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther 41: 54–64, 2015. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 200.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553, 1999. doi: 10.1016/S1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 201.Wang Y, Ding Y, Li J, Chavan H, Matye D, Ni HM, Chiang JY, Krishnamurthy P, Ding WX, Li T. Targeting the enterohepatic bile acid signaling induces hepatic autophagy via a CYP7A1-AKT-mTOR axis in mice. Cell Mol Gastroenterol Hepatol 3: 245–260, 2016. doi: 10.1016/j.jcmgh.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54: 1421–1432, 2011. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 204.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113: 1408–1418, 2004. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther 39: 923–939, 2014. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]
- 206.Worthmann A, John C, Rühlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, Fischer M, Dandri M, Kremoser C, Scheja L, Franke A, Shaul PW, Heeren J. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23: 839–849, 2017. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 207.Wu W, Zhu B, Peng X, Zhou M, Jia D, Gu J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun 443: 68–73, 2014. doi: 10.1016/j.bbrc.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 208.Wu X, Lv YG, Du YF, Hu M, Reed MN, Long Y, Suppiramaniam V, Hong H, Tang SS. Inhibitory effect of INT-777 on lipopolysaccharide-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Prog Neuropsychopharmacol Biol Psychiatry 88: 360–374, 2019. doi: 10.1016/j.pnpbp.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 209.Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, Wang T, Takahashi S, Anitha M, Krausz KW, Patterson AD, Gonzalez FJ. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66: 613–626, 2017. doi: 10.2337/db16-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z, Zeisel SH, Jia W. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J 27: 3583–3593, 2013. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol 10: 104–116, 2017. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 213.Yang Z, Kusumanchi P, Ross RA, Heathers L, Chandler K, Oshodi A, Thoudam T, Li F, Wang L, Liangpunsakul S. Serum metabolomic profiling identifies key metabolic signatures associated with pathogenesis of alcoholic liver disease in humans. Hepatol Commun 3: 542–557, 2019. doi: 10.1002/hep4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yki-Järvinen H. Ceramides - a cause of insulin resistance in NAFLD in both murine models and humans. Hepatology. In press. doi: 10.1002/hep.31095. [DOI] [PubMed] [Google Scholar]
- 215.You M, Arteel GE. Effect of ethanol on lipid metabolism. J Hepatol 70: 237–248, 2019. doi: 10.1016/j.jhep.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15: 11–20, 2018. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 217.Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, Williamson C, Walters JRF. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol 304: G940–G948, 2013. doi: 10.1152/ajpgi.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang L, Xie C, Nichols RG, Chan SH, Jiang C, Hao R, Smith PB, Cai J, Simons MN, Hatzakis E, Maranas CD, Gonzalez FJ, Patterson AD. Farnesoid x receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems 1: e00070-16, 2016. doi: 10.1128/mSystems.00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J Biol Chem 276: 41690–41699, 2001. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 220.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA 103: 1006–1011, 2006. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 74: 3306–3316, 2014. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]