Keywords: hepatic progenitor cells, liver fibrosis, liver repair, macrophages, stellate cells
Abstract
Hepatic ischemia-reperfusion (I/R) is a major complication of liver resection, trauma, and liver transplantation; however, liver repair after I/R in diseased liver has not been studied. The present study sought to determine the manner in which the fibrotic liver repairs itself after I/R. Liver fibrosis was established in mice by CCl4 administration for 6 wk, and then liver I/R was performed to investigate liver injury and subsequent liver repair in fibrotic and control livers. After I/R, fibrotic liver had more injury compared with nonfibrotic, control liver; however, fibrotic liver showed rapid resolution of liver necrosis and reconstruction of liver parenchyma. Marked accumulation of hepatic stellate cells and macrophages were observed specifically in the fibrotic septa in early reparative phase. Fibrotic liver had higher numbers of hepatic stellate cells, macrophages, and hepatic progenitor cells during liver recovery after I/R than did control liver, but hepatocyte proliferation was unchanged. Fibrotic liver also had significantly greater number of phagocytic macrophages than control liver. Clodronate liposome injection into fibrotic mice after I/R caused decreased macrophage accumulation and delay of liver recovery. Conversely, CSF1-Fc injection into normal mice after I/R resulted in increased macrophage accumulation and concomitant decrease in necrotic tissue during liver recovery. In conclusion, fibrotic liver clears necrotic areas and restores normal parenchyma faster than normal liver after I/R. This beneficial response appears to be directly related to the increased numbers of nonparenchymal cells, particularly phagocytic macrophages, in the fibrotic liver.
NEW & NOTEWORTHY This study is the first to reveal how diseased liver recovers after ischemia-reperfusion (I/R) injury. Although it was not completely unexpected that fibrotic liver had increased hepatic injury after I/R, a novel finding was that fibrotic liver had accelerated recovery and repair compared with normal liver. Enhanced repair after I/R in fibrotic liver was associated with increased expansion of phagocytic macrophages, hepatic stellate cells, and progenitor cells.
INTRODUCTION
Liver cancer is one of the most common malignancies in the world, and liver resection is an important treatment strategy that provides a potential cure (43). Specifically, hepatocellular carcinoma develops under the condition of chronical liver injury, mainly caused by hepatitis B, hepatitis C, and nonalcoholic steatohepatitis, and patients with hepatocellular carcinoma commonly suffer from chronical liver damage, liver fibrosis, or cirrhosis (9). Although liver resection can be curative, the mortality rate after liver resection in cirrhosis patients has been reported to be higher than in patients without underlying liver disease (4). One contributing factor may be the high susceptibility of the cirrhotic liver to hepatic ischemia-reperfusion (I/R) injury, which is induced by the vascular occlusion during the surgery (17). Liver I/R injury is a major complication during complex liver surgery, liver transplantation, and trauma and is mediated by an inflammatory response, culminating in the recruitment of activated leukocytes and subsequent hepatocellular damage (21). Although the impact of I/R injury on the fibrotic liver has been studied (10, 28, 37), the mechanisms by which the fibrotic liver repairs and regenerates after I/R has not been studied.
Patients with liver fibrosis have been shown to exhibit poor liver regeneration and postoperative liver function after liver resection, which is correlated to the extent of fibrosis (29). Rodent models of liver fibrosis also revealed impaired hepatocyte proliferation after partial hepatectomy (25, 31). However, partial hepatectomy models do not contain injury components, such as I/R, which are often present in a number of clinical scenarios. Liver recovery after I/R is characterized by clearance of dead tissue and its replacement with functional liver parenchyma. Our laboratory previously reported that the expansion of macrophages and hepatic stellate cells is observed during liver recovery after I/R injury (23). Macrophages have several functions for liver recovery, including phagocytosis, promoting angiogenesis, and inducing a fibrotic response (46). Specifically, after hepatic I/R, the inhibition of macrophage recruitment by blockade of VEGFR1 or BLT1 resulted in impaired hepatocyte proliferation and delayed liver recovery (33, 34). Macrophages that phagocytose hepatocyte debris also upregulate Wnt3a expression, which results in differentiation of hepatic progenitor cells to hepatocytes (5). On the other hand, hepatic stellate cells may differentiate into myofibroblasts and contribute to liver repair by producing extracellular matrix after I/R (22). Human samples after acute liver failure also demonstrated stellate cell activation surrounding ductular progenitor cell proliferation (7). Those reports show that nonparenchymal cells contribute to liver repair and regeneration after hepatic injury. Liver fibrosis is a consequence of chronic liver injury and a repeated wound-healing process, and macrophage and stellate cell accumulation is well known to occur in fibrotic liver (8, 36). In this study, we sought to determine how the fibrotic liver repairs itself in response to the clinically relevant injury, hepatic I/R. Surprisingly, we found that the fibrotic liver clears and resolves necrotic liver tissue significantly faster than normal liver and that this is likely due to the presence and accumulation of significantly higher numbers of hepatic stellate cells, progenitor cells, and macrophages.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice (aged 8–12 wk) from Jackson Laboratory (Bar Harbor, ME) were used in these experiments. To create the liver fibrosis model, mice were treated with intraperitoneal injections of 0.8 mL/kg carbon tetrachloride (CCl4) (Sigma-Aldrich, St. Louis, MO) twice weekly for 6 wk (39). Control mice had mineral oil injection for the same duration. Hepatic I/R was performed 3 days after the last injection. This project was approved by the University of Cincinnati Animal Care and Use Committee and complied with the National Institutes of Health guidelines.
Hepatic I/R injury model.
Partial hepatic ischemia was performed as described previously (27). Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg ip). A midline laparotomy was performed, and an atraumatic clip was used to interrupt blood supply to the left and middle lobes of the liver. After 90 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Mice were euthanized after the indicated periods of reperfusion, and blood and liver samples were taken for analysis. The left lobe was used for the histological analysis of an ischemic liver. For some experiments, mice were injected intraperitoneally with liposomes containing clodronate or containing a vehicle control (FormuMax Scientific, Sunnyvale, CA) 24 h and 72 h after surgery. In other experiments, mice were injected subcutaneously with 0.75 μg/g CSF1-FC (13a; Bio-Rad, Hercules, CA) or PBS as a vehicle every day starting after 24 h of surgery.
Blood analysis.
Blood was obtained by cardiac puncture. Measurement of serum alanine amino transferase (ALT), as an index of hepatocellular injury, was made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina), according to the manufacturer’s instructions.
Histological analysis.
Liver tissues were fixed in 10% neutral-buffered formalin, processed and then embedded in paraffin for light microscopy. Sections were stained with hematoxylin and eosin (H&E) for histological examination and processed further for immunostaining. For immunohistochemical staining, sections had antigen retrieval, and nonspecific binding was blocked with hydrogen peroxide, avidin/biotin, and serum. Sections were then incubated with primary antibodies against PCNA (sc-56; Santa Cruz Biotechnology, Dallas, TX), Desmin (ab15200; Abcam, Cambridge, MA), CK7 (ab181598; Abcam), Ly6G (MCA2387; Bio-Rad), F4/80 (ab6640; Abcam), IRF5 (ab181553; Abcam), and CD163 (ab182422; Abcam). Sections were washed and incubated with secondary antibodies followed by horseradish peroxidase. Immunoreactive proteins were detected by diaminobenzidine. Sections were also stained with Sirius Red for determination of collagen deposition. Quantitative morphometric analysis of staining was performed with histologic sections at low power using ImageJ. Desmin staining area and F4/80 staining area were expressed as percentage of total area examined, as described previously (2). Evaluation of hepatocyte PCNA immunostaining was performed on the basis of the percentage of positive nuclei of 400–600 hepatocytes from the five highest positive fields at high power (×400) and was expressed as hepatocyte PCNA-labeling index. The number of progenitor cells was assessed by calculating the average number of CK7-positive progenitor cells/reactive ductules that do not form lumina from five fields at high power (×200), as described previously (20).
RT-PCR analysis.
Total RNA was extracted from liver tissue using TRIzol (Invitrogen, Carlsbad, CA). cDNA was generated through the reverse transcription of 2 μg of RNA using high-capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA). Samples were incubated at 25°C for 10 min, 37°C for 120 min, 85°C for 5 min to inactivate the reverse transcriptase and then cooled at 5°C for 5 min. Four microliters of diluted cDNA samples were used for quantitative two-step PCR using SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Each sample was analyzed in duplicate. 18S was used as a housekeeping control gene. Threshold cycles were automatically calculated by the iCycler iQ real-time detection system. Threshold cycle values were normalized to the housekeeping control (18S) to give relative genomic equivalence. Primers used were as follows: HGF sense: CAGGAACAGGGGCTTTACGT and antisense: CAAGAACTTGTGCCGGTGTG; EGF sense: CCATGCTCGATGCGTTTCAG and antisense: AGACAGCCACCACCATGATG, and 18S sense: AGTCCCTGCCCTTTGTACACA and antisense: GATCCGAGGGCCTCAAAC.
Flow cytometry analysis.
Isolation of hepatic cell fraction was performed using a liver dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolated cells were counted with a cell counter (Beckman Coulter). The cells that were CD45 positive and F4/80 positive were gated, and analysis of the expression of IRF-5 and CD163 and Ly6c in F4/80-positive leukocytes was performed by flow cytometry with the Attune flow cytometer (Life Technologies, Foster City, CA). The following antibodies were used: CD45 (Clone 30-F11; BD Biosciences, San Jose, CA), F4/80 (Clone BM8; Biolegend, San Diego, CA), IRF-5 (Clone 903430; R&D, Minneapolis, MN), CD163 (Clone TNKUPJ; Invitrogen, Carlsbad CA), Ly6C (563011, BD Biosciences). The cells that were CD45 positive and F4/80 positive were gated, and a phagocytosis assay was performed with pHrodo Green Escherichia coli BioParticles (Invitrogen) by flow cytometry.
Statistical analysis.
All data are expressed as the means ± SE. Data were analyzed with a one-way ANOVA with subsequent Student’s t test. Differences were considered significant when P < 0.05.
RESULTS
Fibrotic liver has accelerated liver repair after I/R.
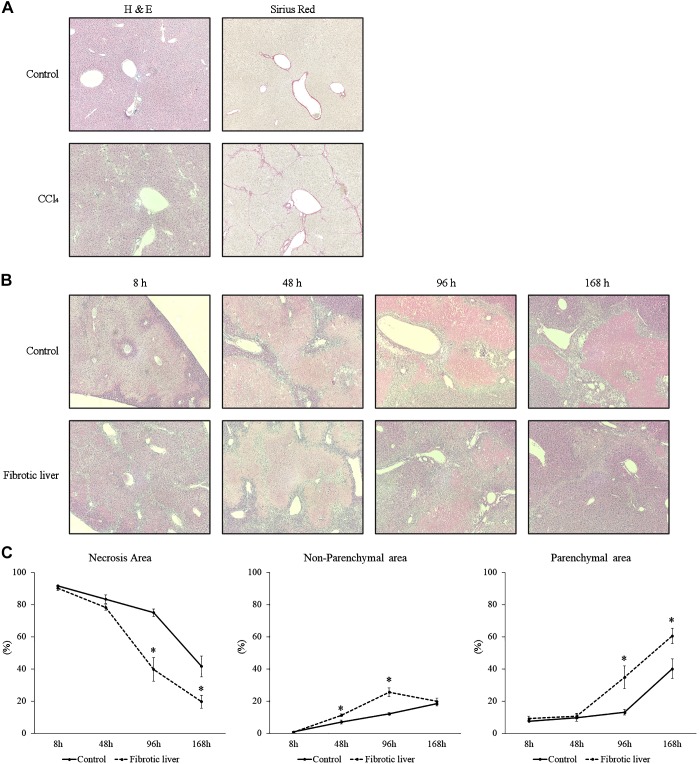
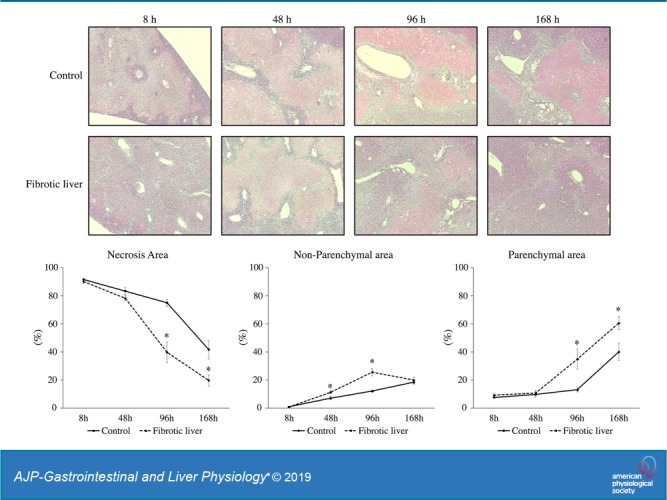
To investigate liver recovery and regeneration after I/R in fibrotic liver, we established liver fibrosis in mice with administration of CCl4. To confirm the established liver fibrosis, liver sections were stained with H&E and Sirius red (Fig. 1A). While control liver did not have fibrotic change, CCl4 administration resulted in bridging fibrosis among portal areas which was comparable with METAVIR scores F2 or F3 (Fig. 1A) (3). Then we performed hepatic I/R and analyzed liver injury 8 h after reperfusion by measuring serum levels of ALT and H&E staining of postischemic liver. Fibrotic liver had significantly higher serum ALT levels than control liver at baseline and 8 h after I/R (Table 1). However, 48 h after I/R, serum ALT in mice with fibrotic livers were significantly lower than those with nonfibrotic livers (Table 1). We then analyzed livers histologically over a 168-h time course after I/R (Fig. 1B) and assessed the necrosis area, the parenchymal area (normal liver structure), and the nonparenchymal area (areas of nonparenchymal cells) (Fig. 1C). When livers were analyzed histologically, the percentage of necrotic area 8 h after I/R was not different between fibrotic liver and control liver (Fig. 1C), but nonparenchymal cells were observed along the areas of bridging fibrosis in the fibrotic livers (Fig. 1B). We also observed that nonparenchymal area was detected around the portal area and around the border of areas of necrosis at 48 h and 96 h after I/R in control livers and that fibrotic livers had significantly more nonparenchymal area 48 and 96 h after I/R (Fig. 1, B and C). This increase in nonparenchymal cells in the fibrotic liver was associated with a significant reduction in necrotic area and an increase in parenchymal area 96 and 168 h after I/R (Fig. 1C), suggesting that increases in nonparenchymal cells may assist in the clearance of necrotic liver and facilitating liver repair.
Fig. 1.
Liver recovery after ischemia-reperfusion (I/R) in fibrotic liver. A: liver histology after carbon tetrachloride (CCl4) injection was assessed by staining with hematoxylin and eosin and Sirius red. Mice receiving CCl4 suffered liver fibrosis. Original magnification is ×200. B: liver histology was assessed by staining liver sections with hematoxylin and eosin. Original magnification is ×100. C: necrosis area, parenchymal area (normal liver structure), and nonparenchymal area (areas of nonparenchymal cells) in the liver after I/R was measured. Data are expressed as means ± SE with n = 4–7 mice per group. *P < 0.05 compared with control.
Table 1.
Serum ALT level after I/R
| Before Operation | 8 Hours | 48 Hours | 96 Hours | 168 Hours | |
|---|---|---|---|---|---|
| Control | 2.4 ± 0.8 | 6,967.7 ± 495.3 | 172.8 ± 28.8 | 18.2 ± 2.9 | 8.0 ± 2.8 |
| Fibrotic liver | 13.7 ± 2.7* | 8,886.1 ± 390.2* | 78.9 ± 16.8* | 17.3 ± 1.5 | 16.3 ± 11.7 |
Data are expressed as means ± SE with n = 4–7 mice per group. While fibrotic liver had significantly higher serum amino transferase (ALT) levels than control liver at baseline and 8 h after ischemia-reperfusion (I/R), serum ALT in mice with fibrotic livers were significantly lower than those with nonfibrotic livers 48 h after I/R.
P < 0.05, compared with control.
Increased liver repair in fibrotic liver after I/R is not associated with increased hepatocyte proliferation.
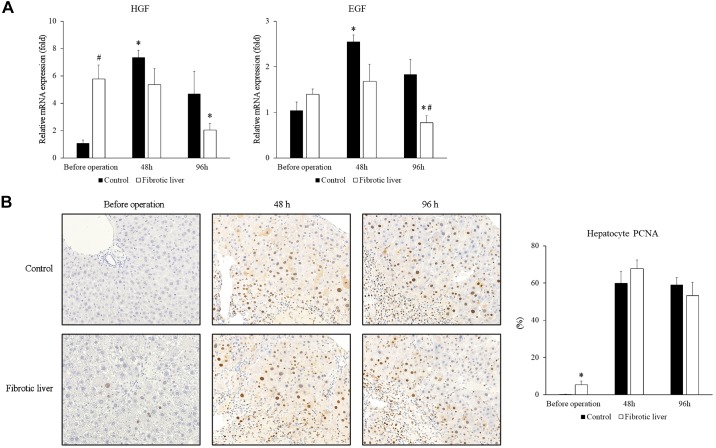
Because we found that the fibrotic liver had accelerated liver repair after I/R injury, we sought to clarify whether this was accomplished by increased hepatocyte proliferation. HGF and EGF are major growth factors for hepatocytes, so we examined the mRNA expression of HGF and EGF in the postischemic liver. Interestingly, the fibrotic liver had significantly higher HGF mRNA expression before I/R injury (Fig. 2A). The mRNA expression of both HGF and EGF increased 48 h after I/R in control liver (Fig. 2A). In fibrotic liver, HGF and EGF mRNA expression did not increase 48 h after I/R, but HGF and EGF mRNA expression was decreased 96 h after I/R (Fig. 2A). Next, we analyzed hepatocyte proliferation by staining liver sections with PCNA. While fibrotic liver had significantly higher hepatocyte proliferation before the operation than control, we observed no differences in hepatocyte proliferation between control liver and fibrotic liver at both 48 h and 96 h after I/R (Fig. 2B). These data suggest that the increased liver repair observed in fibrotic livers is not mediated by increased hepatocyte proliferation.
Fig. 2.
Hepatocyte proliferation after ischemia-reperfusion (I/R). A: liver mRNA expression of HGF and EGF after I/R in control and fibrotic livers was measured by RT-PCR. Data are expressed as means ± SE with n = 4 mice per group. *P < 0.05 compared with before operation; #P < 0.05 compared with control. B: hepatocyte proliferation was measured by staining liver sections with PCNA and calculating the percentage of PCNA positive hepatocytes. Original magnification is ×400. Data are expressed as means ± SE with n = 4 mice per group. *P < 0.05 compared with control.
Increased numbers of nonparenchymal cells in fibrotic liver during recovery after I/R.
It is well known that nonparenchymal cells, including hepatic stellate cells, hepatic progenitor cells, and macrophages are increased after liver injury, including fibrosis and I/R, and contribute both to liver pathology and to liver repair (8, 11, 13, 23) We next investigated the involvement of hepatic stellate cells, hepatic progenitor cells, and macrophages in the accelerated liver repair in fibrotic livers after I/R.
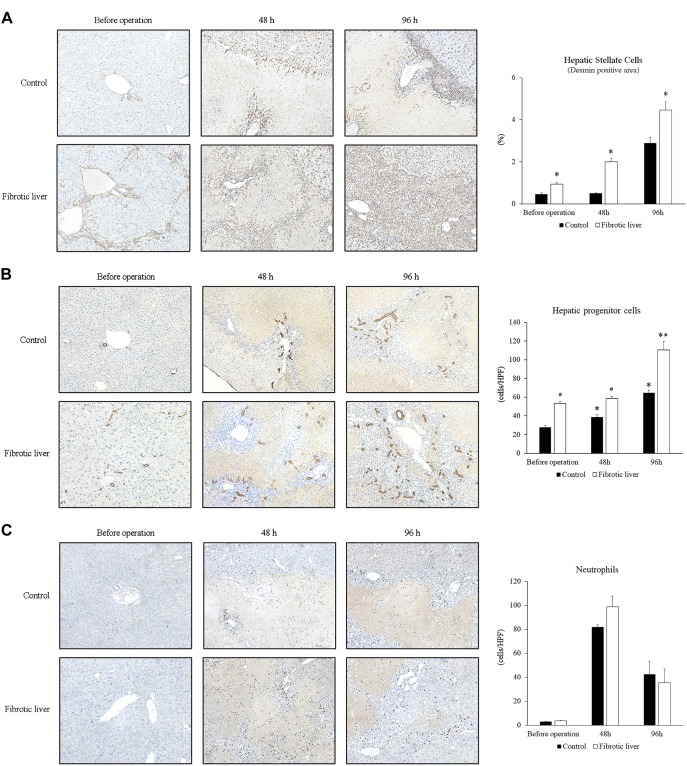
We have previously shown that hepatic stellate cells are an important component of liver recovery after I/R, which includes transient fibrosis as a component of liver repair after I/R (22, 23). We analyzed hepatic stellate cells in liver sections by staining with desmin. Similar to our previous report (23), the number of stellate cells increased around portal areas 48 h after I/R, and by 96 h after I/R, cells were abundant at the border of necrotic areas in control livers (Fig. 3A). In fibrotic liver, stellate cells were observed along the fibrotic septa before operation. After I/R injury in fibrotic livers, increased numbers of stellate cells could be seen along the areas of bridging fibrosis 48 h after I/R, and by 96 h after I/R, stellate cells were observed in all areas of liver injury (Fig. 3A). The percentage of desmin-positive areas was significantly higher in fibrotic liver than in control liver (Fig. 3A).
Fig. 3.
Changes in nonparenchymal cells after ischemia-reperfusion (I/R). A: expansion of hepatic stellate cells after I/R. Hepatic stellate cells in the liver were identified by staining with desmin. Stellate cells accumulated at the border of necrotic areas after I/R in control livers. In fibrotic liver, stellate cells were observed along the fibrotic septa before operation. Increased numbers of stellate cells could be seen along the areas of bridging fibrosis at 48 h after I/R, and stellate cells were observed in all areas of liver injury at 96 h. The percentage of desmin-positive area was significantly higher in fibrotic liver than in control liver. Original magnification is ×200. Data are expressed as means ± SE with n = 4 mice per group. *P < 0.05 compared with control. B: expansion of hepatic progenitor cells after I/R. Hepatic progenitor cells in the liver were identified by staining liver sections with CK7. Although CK7-positive progenitor cells/reactive ductules, which were observed singly or in irregular strings without lumens, were seen around the portal veins at the boundary of necrotic areas in control liver, extensive distribution of CK7-positive progenitor cells/reactive ductules were observed in the large areas of nonparenchymal cells after I/R in fibrotic liver. The number of hepatic progenitor cells was significantly increased after I/R in control liver, and fibrotic liver had significantly more hepatic progenitor cells than control liver. Original magnification is ×200. Data are expressed as means ± SE with n = 4 mice per group. *P < 0.05 compared with before operation. #P < 0.05 compared with control. C: no change in neutrophil accumulation. Neutrophils were identified by staining liver sections for Ly6G. The number of neutrophils were not different between control and fibrotic liver groups. Data are expressed as means ± SE with n = 4 mice per group.
The ductular reaction, which includes the expansion of hepatic progenitor cells, is also a component of both liver fibrosis and liver repair and regeneration (11, 12, 40, 45). To assess liver progenitor cell expansion in control and fibrotic liver after I/R, we stained liver sections with CK7. In control liver, CK7-positive cholangiocytes and CK7-positive progenitor cells were detected around portal veins before I/R injury (Fig. 3B). During liver recovery after I/R, CK7-positive progenitor cells/reactive ductules, which were observed singly or in irregular strings without lumens, were seen around the portal veins at the boundary of necrotic areas 96 h after I/R (Fig. 3B). In contrast, in fibrotic liver, CK7-positive progenitor cells/reactive ductules were not restricted to portal areas before operation and, after I/R, extensive distribution of CK7-positive progenitor cells/reactive ductules were observed in the large areas of nonparenchymal cells at 48 h and 96 h after I/R (Fig. 3B). When we counted the number of CK7-positive hepatic progenitor cells, we found the number of progenitor cells increased after I/R in both control and fibrotic livers and that fibrotic liver had significantly more progenitor cells than control liver (Fig. 3B).
Because neutrophils may affect not only liver injury but also the reparative response after injury (44), we investigated the accumulation of neutrophils in the liver during liver repair after I/R by staining liver sections with Ly6G. There was no difference in the number of Ly6G-positive neutrophils in the liver at 48 h and 96 h after I/R between control liver and fibrotic liver (Fig. 3C).
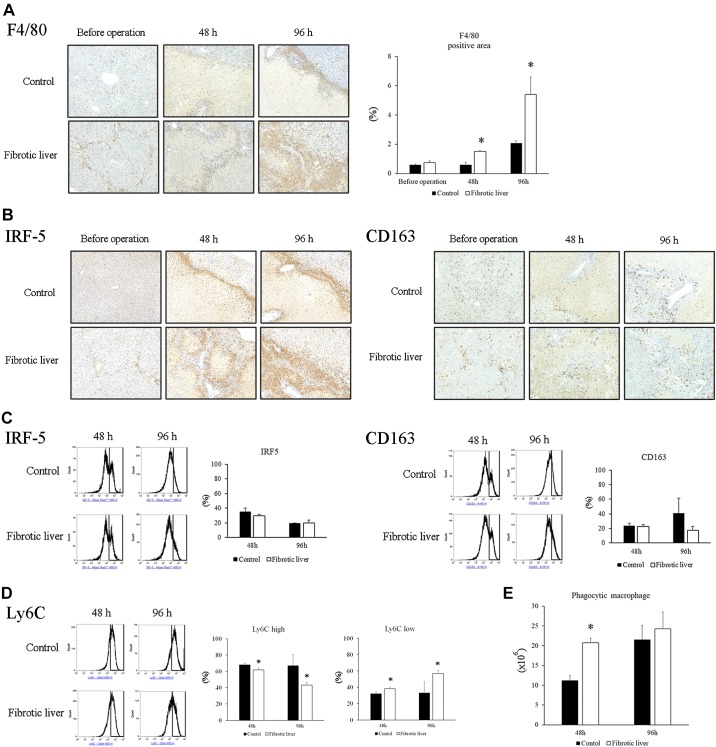
Liver accumulation of macrophages is another commonality between liver fibrosis and liver repair (46). To determine their potential contribution to the increased liver repair observed in fibrotic livers after I/R, we measured macrophage accumulation by staining for F4/80, a pan-macrophage marker. In control livers, macrophages were present around portal areas 48 h after I/R and were found around the borders of necrotic areas 96 h after I/R (Fig. 4A). In contrast, in fibrotic livers, macrophages were observed along the fibrotic septa, as well as sinusoids before I/R (Fig. 4A). After I/R injury, there were increased numbers of macrophages along the areas of bridging fibrosis 48 h after I/R, and by 96 h after I/R, macrophages were observed in areas of necrosis (Fig. 4A). The percentage of F4/80-positive area was significantly higher in fibrotic liver than control liver at 48 h and 96 h after I/R (Fig. 4A).
Fig. 4.
Accumulation of macrophages after ischemia-reperfusion (I/R). A: macrophages in the liver were identified by staining liver sections with F4/80. Macrophages accumulated at the border of necrotic areas after I/R in control livers. In fibrotic liver, macrophages were observed along the fibrotic septa before operation. There were increased numbers of macrophages along the areas of bridging fibrosis 48 h after I/R, and macrophages were observed in areas of liver injury at 96 h. F4/80-positive area in the liver had significantly higher in the fibrotic liver than control liver after I/R. Original magnification is ×200. Data are expressed as means ± SE with n = 4 mice per group. *P < 0.05 compared with control. B: M1 macrophages and M2 macrophages were identified by staining for IRF-5 and CD163, respectively. Both IRF5-positive and CD163-positive macrophages accumulated in the nonparenchymal area after I/R. C: percentage of IRF5-positive and CD163-positive macrophages in the liver after I/R was measured by flow cytometry. The cells with CD45-positive and F4/80-positive were gated and stained with IRF5 or CD163. There was no difference in the percentage of IRF5- or CD163-positive macrophages between fibrotic liver and control liver. Data are expressed as means ± SE with n = 3 mice per group. D: Ly6Clo low macrophages in the liver after I/R was analyzed by flow cytometry. The percentage of Ly6Clo macrophages in the liver at 48 h and 96 h after I/R was significantly higher in the fibrotic liver than in the control liver. Data are expressed as means ± SE with n = 3 mice per group. D: phagocytic activity of macrophages after I/R was determined by flow cytometry. The number of phagocytic macrophages in the liver at 48 h after I/R was significantly higher in the fibrotic liver than in the control liver. Data are expressed as means ± SE with n = 3 mice per group. *P < 0.05 compared with control.
We next examined the phenotype of intrahepatic macrophages after I/R. Liver sections were stained with IRF5 and CD163 to determine M1 and M2 macrophages, respectively. Immunostaining showed that both IRF5-positive macrophages and CD163-positive macrophages are present in increasing numbers in both control liver and fibrotic liver at 48 h and 96 h after I/R (Fig. 4B). Flow cytometric analysis of mononuclear cells isolated from livers showed that there was no difference in the percentage of cells expressing either IRF5 or CD163 between control and fibrotic liver at 48 h and 96 h after I/R (Fig. 4C). Next, we analyzed Ly6C expression on the intrahepatic macrophages. Flow cytometric analysis revealed that fibrotic liver had a significantly higher percentage of Ly6Clo macrophages than control liver at 48 h and 96 h after I/R (Fig. 4D). Ly6Clo macrophages have been shown to contribute to tissue repair after I/R in other model systems (15, 19, 32), so these results suggest that Ly6Clo reparative macrophages may play an important role in the recovery from I/R injury in the fibrotic liver.
Finally, we assessed the phagocytic activity of hepatic macrophages after I/R by flow cytometry. The number of phagocytic F4/80-positive cells in fibrotic liver was significantly higher 48 h after I/R than control liver, but there was no significant difference at 96 h (Fig. 4E). These results show that there is an increase in the phagocytic activity of macrophages in the fibrotic liver that occurs during the initial phases of liver repair, which could contribute to clearance of necrotic cells and tissue repair.
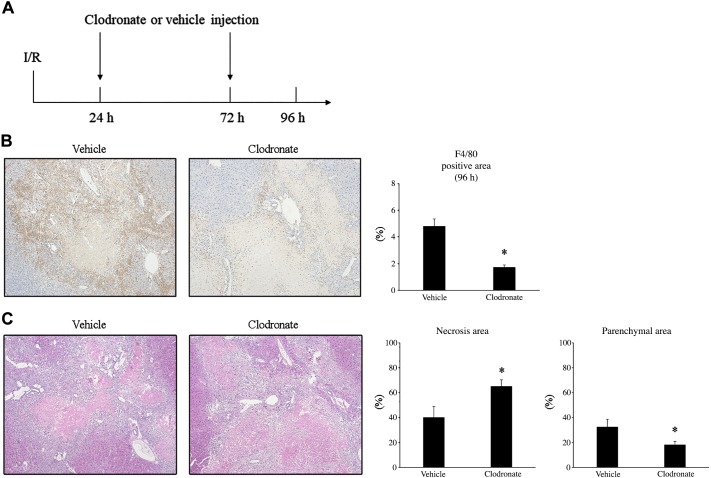
Attenuation of macrophage accumulation delays liver repair after I/R.
Because the increased liver repair that we observed in fibrotic livers was not associated with increased hepatocyte proliferation, but correlated with increased numbers of nonparenchymal cells and macrophage phagocytic activity, we sought to explore the role of macrophages as a potential mechanism of the enhanced liver repair in the fibrotic liver. To do this, we performed I/R in fibrotic mice and injected the mice with liposomes containing vehicle or clodronate intraperitoneally 24 h and 72 h after surgery (Fig. 5A). Clodronate liposome injection resulted in a significant decrease in the accumulation of F4/80-positive macrophages in the liver at 96 h after I/R (Fig. 5B). Clodronate liposome injection also significantly delayed the clearance of necrotic liver tissue 96 h after I/R (Fig. 5C). These data suggest that the increased liver repair observed in fibrotic liver after I/R may be mediated, at least in part, by increased macrophage accumulation and phagocytic clearance of dead tissue.
Fig. 5.
The effect of macrophage depletion on liver repair of fibrotic liver after I/R. A: mice with fibrotic liver underwent ischemia-reperfusion (I/R) and got injected with clodronate liposome at 24 h and 72 h after surgery. B: macrophages in the liver were identified by staining liver sections with F4/80. Clodronate liposome injection decreased the accumulation of macrophages at 96 h after I/R. Original magnification is ×100. C: liver necrosis and parenchymal area were assessed by hematoxylin-and-eosin staining, and necrosis area was calculated. Clodronate liposome injection treatment delayed the clearance of necrotic tissue and liver regeneration after I/R. Original magnification is ×100. Data are expressed as means ± SE with n = 4–5 mice per group. *P < 0.05 compared with control.
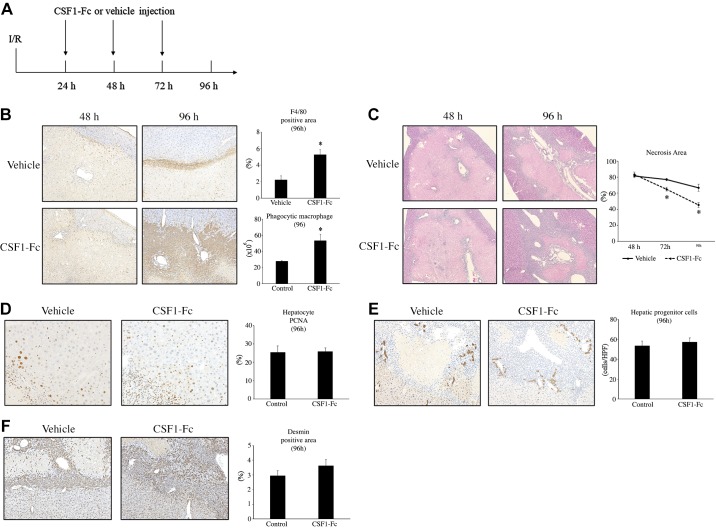
Induction of macrophage accumulation promotes liver repair after I/R.
To determine whether induction of macrophage accumulation in the liver after injury could enhance liver repair after I/R, we performed I/R in otherwise normal mice and injected the mice with vehicle or CSF1-Fc subcutaneously every day for 3 days starting 24 h after surgery (Fig. 6A). CSF1-Fc administration has previously been shown to induce macrophage recruitment to the liver (13a). CSF1-Fc treatment resulted in a significant increase in the accumulation of F4/80-positive macrophages and phagocytic macrophages in the liver by 96 h after I/R (Fig. 6B). CSF1-Fc treatment also significantly decreased necrosis area 96 h after I/R (Fig. 6C). Treatment with CSF1-Fc did not influence hepatocyte proliferation (Fig. 6D), progenitor cell expansion (Fig. 6E), or stellate cell accumulation (Fig. 6F) after I/R. These data provide further supportive evidence that macrophage accumulation and phagocytic activity may contribute to liver repair in the fibrotic liver.
Fig. 6.
The effect of increased macrophage accumulation on liver repair after ischemia-reperfusion (I/R). A: normal mice underwent I/R and got injected with CSF1-Fc every day starting 24 h after surgery. B: macrophages in the liver were identified by staining liver sections with F4/80. CSF1-Fc treatment increased the accumulation of macrophages at 96 h after I/R. Original magnification is ×200. Flow cytometric analysis revealed that CSF-Fc treatment also increased phagocytic macrophages in the liver at 96 h after I/R. C: liver necrosis was assessed by hematoxylin-and-eosin staining, and necrosis area was calculated. CSF1-Fc treatment decreased the necrosis area after I/R. Original magnification is ×100. D: hepatocyte proliferation was determined by PCNA staining. CSF1-Fc treatment did not influence hepatocyte proliferation after I/R. Original magnification is ×400. E: hepatic progenitor cells were identified by CK7 staining. CSF1-Fc treatment did not change the number of hepatic progenitor cells after I/R. F: hepatic stellate cells were identified by desmin staining. CSF1-Fc treatment did not affect the accumulation of hepatic stellate cells after I/R. Original magnification is ×200. For all panels, data are expressed as means ± SE with n = 4–7 mice per group. *P < 0.05 compared with control.
DISCUSSION
Liver repair after I/R injury is critical to restore proper liver function. Our laboratory previously reported that liver repair and regeneration after I/R involves the expansion of hepatic stellate cells and macrophages at the boundaries of necrotic regions of liver (23); however, the recovery of the diseased liver after I/R has not been well studied. Using liver fibrosis as a model of underlying disease, we found that fibrotic livers suffered augmented liver injury after I/R. This finding is consistent with previous reports that liver cirrhosis results in low antioxidative capacity and poor blood flow to the liver, both of which cause susceptibility to I/R injury (24, 26, 37). Interestingly, despite the exaggerated liver injury, we found that the fibrotic liver had an accelerated reparative response compared with normal liver. Our data suggest that the increased numbers of nonparenchymal cells, particularly macrophages, that were present in the liver as a component of the fibrotic response, may be responsible for the rapid repair after I/R injury.
Interestingly, a previous study has shown that fibrotic livers subjected to partial hepatectomy have reduced liver regeneration secondary to a severe fibrogenic response (25). Although hepatectomy results in the loss of both functional and physical mass, liver I/R results only in the loss of functional mass, and it presents an additional challenge for the host in terms of dead liver tissue that must be cleared, remodeled, and replaced with functional tissue. In our model, we saw no difference in hepatocyte proliferation between normal and fibrotic liver after I/R. However, we did observe significantly more hepatic stellate cells, hepatic progenitor cells, and macrophages in the fibrotic liver after I/R, which suggests that the accumulation/expansion of nonparenchymal cells as part of the fibrotic response provides the necessary cells to more rapidly clear and repair the dead tissue resulting from I/R injury.
Macrophages play an important role in the elimination of dead tissue after injury (16). Ly6Clo macrophages possess anti-inflammatory functions and contribute to promote liver repair, while Ly6Chi macrophages are a proinflammatory phenotype (42, 47). The current study revealed that reparative Ly6Clo macrophages were predominant in fibrotic liver and may contribute to liver recovery after I/R. Furthermore, we did observe significantly more macrophages in the injured fibrotic liver than in normal liver, as well as an increased number of phagocytic macrophages in the fibrotic liver. These findings provide strong evidence that the increased number of phagocytic macrophages contributed significantly to the reduced necrotic area and improved repair in fibrotic livers. This concept is further supported by our experiments using clodronate liposome to reduce macrophage accumulation after I/R injury. We also assessed the effect of macrophage induction on liver repair after I/R. When we subjected normal mice to I/R injury and injected them with CSF1-Fc, the number of macrophages in the liver were significantly increased, similar to the fibrotic liver. Also similar to the fibrotic liver, CSF1-Fc treatment had no effect on hepatocyte proliferation, yet there was a significant decrease in necrotic area, suggesting increased clearance of dead tissue. CSF1-Fc also had no effect on the numbers of hepatic progenitor cells and hepatic stellate cells, whereas the fibrotic liver showed increased numbers of these cells.
Hepatic progenitor cells represent an alternative pathway of liver regeneration. While hepatocytes can respond to multiple signals that result in hepatocyte proliferation, when functional liver mass is massively diminished and/or regenerative capacity of hepatocyte impaired, progenitor cells can proliferate and transdifferentiate to hepatocytes to help restore liver function (14). Our observation that fibrotic liver had an increase in parenchymal area after I/R in the absence of a corresponding increase in hepatocyte proliferation suggests that the repair was independent of hepatocyte proliferation. We did, however, observe an increase in the number of hepatic progenitor cells during the reparative response after I/R in fibrotic livers. This is consistent with the current understanding that expansion of these cells occurs during liver repair and regeneration after severe acute liver injury (20, 38). Furthermore, our finding that expansion of hepatic progenitor cells in fibrotic livers after I/R occurred in nonportal areas suggests that these progenitor cells may contribute to liver repair, as has been described previously (6, 11, 40, 41, 48).
Hepatic stellate cells are central not only to the development of liver fibrosis, but also to liver repair and regeneration after injury (1). In both cases, they produce not only matrix components, but also modulate the viability and activity of adjacent hepatocytes, progenitor cells, and macrophages (18, 30, 35). We previously demonstrated that after I/R injury, stellate cells proliferate and contribute to liver repair (22). The current study shows augmented stellate cell expansion in fibrotic liver, which may be caused by proliferation of stellate cells contributing to, and in the areas of, the bridging fibrosis. Stellate cell proliferation/expansion in the fibrotic liver, therefore, might support the rapid liver repair observed after I/R through interactions with colocated progenitor cells and macrophages.
In conclusion, the current study shows that the fibrotic liver recovers more quickly than normal liver after I/R injury. This response occurred despite the fibrotic liver having a higher degree of liver injury induced by I/R. The accelerated reparative response in fibrotic liver was associated with increased numbers of nonparenchymal cells, hepatic stellate cells, hepatic progenitor cells, and macrophages. A higher number of the latter were phagocytic, which likely contributed to enhanced removal of dead tissue and replacement with functional liver mass.
GRANTS
This work was supported in part by National Institutes of Health Grant DK-56029 (to A. B. Lentsch).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K., C.C.C., and A.B.L. conceived and designed research; T.K., R.M.S., and H.S.G. performed experiments; T.K., R.M.S., and H.S.G. analyzed data; T.K., C.C.C., and A.B.L. interpreted results of experiments; T.K. prepared figures; T.K., R.M.S., and A.B.L. drafted manuscript; T.K., C.C.C., and A.B.L. edited and revised manuscript; T.K., R.M.S., H.S.G., C.C.C., and A.B.L. approved final version of manuscript.
REFERENCES
- 1.Bansal MB. Hepatic stellate cells: fibrogenic, regenerative or both? Heterogeneity and context are key. Hepatol Int 10: 902–908, 2016. doi: 10.1007/s12072-016-9758-x. [DOI] [PubMed] [Google Scholar]
- 2.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest 87: 292–303, 2007. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 3.Bedossa P; The French METAVIR Cooperative Study Group . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20: 15–20, 1994. doi: 10.1002/hep.1840200104. [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 191: 38–46, 2000. doi: 10.1016/S1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 5.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579, 2012. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. J Clin Invest 123: 1867–1873, 2013. doi: 10.1172/JCI66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dechêne A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A, Dries V, Odenthal M, Gerken G, Friedman SL, Canbay A. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 52: 1008–1016, 2010. doi: 10.1002/hep.23754. [DOI] [PubMed] [Google Scholar]
- 8.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005. doi: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132: 2557–2576, 2007. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Ercolani G, Ravaioli M, Grazi GL, Cescon M, Del Gaudio M, Vetrone G, Zanello M, Pinna AD. Use of vascular clamping in hepatic surgery: lessons learned from 1260 liver resections. Arch Surg 143: 380–387, 2008. doi: 10.1001/archsurg.143.4.380. [DOI] [PubMed] [Google Scholar]
- 11.Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143: 1564–1575.e7, 2012. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte ‘buds’ in cirrhosis from intrabiliary stem cells. J Hepatol 39: 357–364, 2003. doi: 10.1016/S0168-8278(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 13.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 39: 1477–1487, 2004. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 13a.Gow DJ, Sauter KA, Pridans C, Moffat L, Sehgal A, Stutchfield BM, Raza S, Beard PM, Tsai YT, Bainbridge G, Boner PL, Fici G, Garcia-Tapia D, Martin RA, Oliphant T, Shelly JA, Tiwari R, Wilson TL, Smith LB, Mabbott NA, Hume DA. Characterisation of a novel Fc conjugate of macrophage colony-stimulating factor. Mol Ther 22: 1580-1592, 2014. doi: 10.1038/mt.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilgenkrantz H, Collin de l’Hortet A. Understanding liver regeneration: from mechanisms to regenerative medicine. Am J Pathol 188: 1316–1327, 2018. doi: 10.1016/j.ajpath.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6C high monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114: 1611–1622, 2014. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol 84: 1410–1421, 2008. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JH, Kang KJ, Kang Y, Lee IS, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transpl 14: 980–988, 2008. doi: 10.1002/lt.21467. [DOI] [PubMed] [Google Scholar]
- 18.Kalinichenko VV, Bhattacharyya D, Zhou Y, Gusarova GA, Kim W, Shin B, Costa RH. Foxf1 +/− mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology 37: 107–117, 2003. doi: 10.1053/jhep.2003.50005. [DOI] [PubMed] [Google Scholar]
- 19.Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, Kuboki E, Yagita H, Uchida K, Nitta K, Tanaka M. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol 26: 896–906, 2015. doi: 10.1681/ASN.2014020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int 26: 1225–1233, 2006. doi: 10.1111/j.1478-3231.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 21.Konishi T, Lentsch AB. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr 17: 277–287, 2017. doi: 10.3727/105221617X15042750874156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi T, Schuster RM, Lentsch AB. Liver repair and regeneration after ischemia/reperfusion injury is associated with prolonged fibrosis. Am J Physiol Gastrointest Liver Physiol 316: G323–G331, 2019. doi: 10.1152/ajpgi.00154.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi T, Schuster RM, Lentsch AB. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 314: G471–G482, 2018. doi: 10.1152/ajpgi.00153.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koruk M, Aksoy H, Akçay F, Onuk MD. Antioxidant capacity and nitric oxide in patients with hepatic cirrhosis. Ann Clin Lab Sci 32: 252–256, 2002. [PubMed] [Google Scholar]
- 25.Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183: 182–194, 2013. doi: 10.1016/j.ajpath.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurokawa T, Nonami T, Harada A, Nakao A, Sugiyama S, Ozawa T, Takagi H. Effects of prostaglandin E1 on the recovery of ischemia-induced liver mitochondrial dysfunction in rats with cirrhosis. Scand J Gastroenterol 26: 269–274, 1991. doi: 10.3109/00365529109025041. [DOI] [PubMed] [Google Scholar]
- 27.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and kupffer cells Hepatology 27: 1172–1177, 1998. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 28.Li SQ, Liang LJ, Huang JF, Li Z. Hepatocyte apoptosis induced by hepatic ischemia-reperfusion injury in cirrhotic rats. Hepatobiliary Pancreat Dis Int 2: 102–105, 2003. [PubMed] [Google Scholar]
- 29.Miyazaki S, Takasaki K, Yamamoto M, Tsugita M, Otsubo T. Liver regeneration and restoration of liver function after partial hepatectomy: the relation of fibrosis of the liver parenchyma. Hepatogastroenterology 46: 2919–2924, 1999. [PubMed] [Google Scholar]
- 30.Mochizuki A, Pace A, Rockwell CE, Roth KJ, Chow A, O’Brien KM, Albee R, Kelly K, Towery K, Luyendyk JP, Copple BL. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1α-dependent manner by modulating macrophage phenotype in mice. J Immunol 192: 3847–3857, 2014. doi: 10.4049/jimmunol.1303195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg 248: 821–828, 2008. doi: 10.1097/SLA.0b013e31818584c7. [DOI] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohkubo H, Ito Y, Minamino T, Eshima K, Kojo K, Okizaki S, Hirata M, Shibuya M, Watanabe M, Majima M. VEGFR1-positive macrophages facilitate liver repair and sinusoidal reconstruction after hepatic ischemia/reperfusion injury. PLoS One 9: e105533, 2014. doi: 10.1371/journal.pone.0105533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohkubo H, Ito Y, Minamino T, Mishima T, Hirata M, Hosono K, Shibuya M, Yokomizo T, Shimizu T, Watanabe M, Majima M. Leukotriene B4 type-1 receptor signaling promotes liver repair after hepatic ischemia/reperfusion injury through the enhancement of macrophage recruitment. FASEB J 27: 3132–3143, 2013. doi: 10.1096/fj.13-227421. [DOI] [PubMed] [Google Scholar]
- 35.Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE. Hepatic stellate cells’ involvement in progenitor-mediated liver regeneration. Lab Invest 90: 1199–1208, 2010. doi: 10.1038/labinvest.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 109: E3186–E3195, 2012. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhoden EL, Pereira-Lima L, Kalil AN, Lucas ML, Mauri M, Menti E, Rhoden CR, Pereira-Lima J, Zettler CG, Belló-Klein A. Effects of ischemia and reperfusion on oxidative stress in hepatic cirrhosis induced by carbon tetrachloride in rats. Kobe J Med Sci 46: 171–180, 2000. [PubMed] [Google Scholar]
- 38.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis 23: 385–396, 2003. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 39.Scholten D, Trebicka J, Liedtke C, Weiskirchen R. The carbon tetrachloride model in mice. Lab Anim 49, Suppl: 4–11, 2015. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
- 40.Shin S, Upadhyay N, Greenbaum LE, Kaestner KH. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology 148: 192–202.e3, 2015. doi: 10.1053/j.gastro.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stueck AE, Wanless IR. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology 61: 1696–1707, 2015. doi: 10.1002/hep.27706. [DOI] [PubMed] [Google Scholar]
- 42.Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage phenotype in liver injury and repair. Scand J Immunol 85: 166–174, 2017. doi: 10.1111/sji.12468. [DOI] [PubMed] [Google Scholar]
- 43.Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Gasbarrini A, Sacco R, Foschi FG, Missale G, Morisco F, Svegliati Baroni G, Virdone R, Cillo U; Italian Liver Cancer (ITA.LI.CA) Group . Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 62: 617–624, 2015. doi: 10.1016/j.jhep.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358: 111–116, 2017. doi: 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- 45.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 146: 349–356, 2014. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 46.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, Fu L, Tian C, Yang J, He F, Tang L. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun 10: 1076, 2019. doi: 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H, Rogler LE, Teperman L, Morgan G, Rogler CE. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology 45: 716–724, 2007. doi: 10.1002/hep.21557. [DOI] [PubMed] [Google Scholar]