Keywords: metabolomics, one-carbon metabolism, probiotics, secondary bile acids, trimethylamine-N-oxide
Abstract
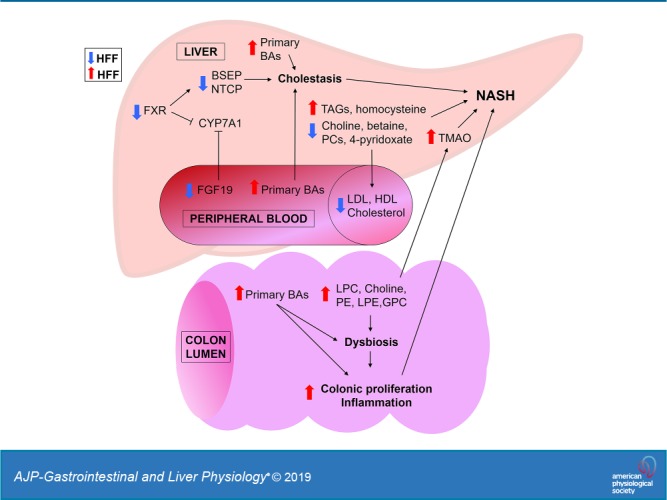
To investigate the role of bile acids (BAs) in the pathogenesis of diet-induced nonalcoholic steatohepatitis (NASH), we fed a “Western-style diet” [high fructose, high fat (HFF)] enriched with fructose, cholesterol, and saturated fat for 10 wk to juvenile Iberian pigs. We also supplemented probiotics with in vitro BA deconjugating activity to evaluate their potential therapeutic effect in NASH. Liver lipid and function, cytokines, and hormones were analyzed using commercially available kits. Metabolites, BAs, and fatty acids were measured by liquid chromatography-mass spectrometry. Histology and gene and protein expression analyses were performed using standard protocols. HFF-fed pigs developed NASH, cholestasis, and impaired enterohepatic Farnesoid-X receptor (FXR)-fibroblast growth factor 19 (FGF19) signaling in the absence of obesity and insulin resistance. Choline depletion in HFF livers was associated with decreased lipoprotein and cholesterol in serum and an increase of choline-containing phospholipids in colon contents and trimethylamine-N-oxide in the liver. Additionally, gut dysbiosis and hyperplasia increased with the severity of NASH, and were correlated with increased colonic levels of choline metabolites and secondary BAs. Supplementation of probiotics in the HFF diet enhanced NASH, inhibited hepatic autophagy, increased excretion of taurine and choline, and decreased gut microbial diversity. In conclusion, dysregulation of BA homeostasis was associated with injury and choline depletion in the liver, as well as increased biliary secretion, gut metabolism and excretion of choline-based phospholipids. Choline depletion limited lipoprotein synthesis, resulting in hepatic steatosis, whereas secondary BAs and choline-containing phospholipids in colon may have promoted dysbiosis, hyperplasia, and trimethylamine synthesis, causing further damage to the liver.
NEW & NOTEWORTHY Impaired Farnesoid-X receptor (FXR)-fibroblast growth factor 19 (FGF19) signaling and cholestasis has been described in nonalcoholic fatty liver disease (NAFLD) patients. However, therapeutic interventions with FXR agonists have produced contradictory results. In a swine model of pediatric nonalcoholic steatohepatitis (NASH), we show that the uncoupling of intestinal FXR-FGF19 signaling and a decrease in FGF19 levels are associated with a choline-deficient phenotype of NASH and increased choline excretion in the gut, with the subsequent dysbiosis, colonic hyperplasia, and accumulation of trimethylamine-N-oxide in the liver.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) represents the major cause of pediatric chronic liver pathology in the United States, resulting in annual medical costs of approximately $6 billion (122). It has been estimated that the prevalence of NAFLD extends from 0.7% in 2- to 4-yr-old infants to 17.3% in adolescents, and up to 38% in obese individuals (91, 116). The prevailing two-hit model of NAFLD suggests that insulin resistance promotes initial steatosis, which is followed by inflammation and fibrosis in response to multiple factors, including oxidative stress and mitochondrial dysfunction (25). Consequently, obesity, dyslipidemia, and type 2 diabetes have been identified as important risk factors for pediatric NAFLD (90). However, an increasing body of literature has demonstrated the development of NAFLD in a population of nonobese patients, who present a lower prevalence of hypertriglyceridemia and insulin resistance but increased hepatic inflammation and liver injury, indicating that additional factors are involved in the pathogenesis of the disease (28, 64).
In recent years, there has been a growing interest in the role of bile acids (BAs) in the development of NAFLD, due to their potential hepatotoxic effects and their ability to regulate insulin sensitivity and glucose and lipid metabolism via activation of hepatic Farnesoid-X receptor (FXR) (4). Primary BAs [e.g., cholic (CA), chenodeoxycholic (CDCA) and hyocholic (HCA) acids] are synthesized from cholesterol in the liver and conjugated with taurine (t) or glycine (g) before they are released into the small intestine. Conjugated BAs are then actively reabsorbed in the ileum and recirculated to the liver with the portal blood, preserving >95% of the BA pool (4). Small amounts of primary BAs reach the distal ileum (DI) and colon, where they are deconjugated by microbial bile salt hydrolase (BSH) and 7-dehydroxylase into secondary BAs [i.e., deoxycholic (DCA), ursodeoxycholic (UDCA), lithocholic (LCA) and hyodeoxycholic (HDCA) acids], which are then excreted in the feces, with a small fraction also entering the enterohepatic circulation (4, 114). The synthesis of BAs is regulated by a negative feedback loop via BA activation of small heterodimer partner (SHP) in the liver, which, in turn, inhibits the expression of cholesterol 7α-hydroxylase (CYP7A1), a rate-limiting enzyme in the classic BA synthesis pathway (4). In addition, FXR is activated by BAs in ileum, where it induces the expression of fibroblast growth factor (FGF) 19. FGF19 travels to the liver with the portal blood and also inhibits the CYP7A1 gene (4).
A connection between impaired FXR-FGF19 signaling and elevated circulating BA levels has been described in both child and adult populations with NAFLD (35, 50, 79). However, therapeutic interventions targeting BA signaling with FXR agonists (e.g., obeticholic acid) have produced contradictory results (4), indicating that our knowledge of BA contribution to the pathogenesis of the liver disease is not complete, in part due to the lack of animal models that recapitulate the dysregulation of BA homeostasis in NAFLD. Pigs represent an excellent translational model for liver research due to similarities between pigs and humans with respect to liver size and structure, gastrointestinal physiology, and lipoprotein profiles (99, 104). In particular, the Iberian and its direct descendant the Ossabaw pigs (65) have emerged as research models of metabolic syndrome and NAFLD because of their elevated voluntary feed intake and great lipogenic potential (58, 106). Similar to the Zucker fatty rat, Iberian pigs have a mutation in the leptin receptor gene, which has been associated with a leptin resistance phenotype characterized by hyperphagia, obesity, and increased leptin levels in comparison to other porcine breeds (33, 73). Therefore, the first goal of our study was to perform a comprehensive characterization of a new model of pediatric NAFLD in juvenile Iberian pigs, in which animals developed cholestasis and impairment of enterohepatic FXR-FGF19 signaling in response to a “Western-style diet.”
The role of the gut has become a critical question in the progression of NAFLD, since increased intestinal permeability (39, 66) and dysbiosis (8, 68) have been associated with the severity of the liver disease. Subsequently, the use of probiotics as a potential therapeutic strategy to manage NAFLD, by modulating microbiota and gut mucosa function (119), has been tested in both adult and pediatric populations with inconsistent results (2, 52, 95). Therefore, our second goal was to evaluate whether supplementation of probiotics in the diet would be an effective therapeutic strategy to attenuate the onset and progression of NAFLD in our pediatric model.
MATERIALS AND METHODS
Animals and experimental design.
Experiments were in accordance with the Institutional Animal Care and Use Committee of California State University (no. 1611), and the National Research Council Guide for the Care and Use of Laboratory Animals. Twenty-eight 13-day-old male (M) and female (F) Iberian pigs were moved into a temperature-controlled room with a 12:12-h light-dark cycle. Pigs were housed in pairs in 1.5 × 1.5-m pens, balanced for sex and weight, and randomly assigned to receive one of the four liquid diets (Tables 1 and 2): 1) control nonprobiotic (CON-N; n = 8, 4 M/4 F), 2) high-fructose, high-fat nonprobiotic (HFF-N; n = 6, 3 M/3 F), 3) CON + 6.2 × 104 colony-forming units (CFU)/mL probiotics (CON-P; n = 8, 5 M/3 F), and 4) HFF-N + 6.2 × 104 CFU/mL probiotics (HFF-P; n = 6, 3 M/3 F). The probiotic mixture contained Pediococcus acidilactici, Pediococcus pentosaceus, Lactobacillus plantarum, and Bacillus subtilis (MultiBio 3PS; BiOWiSH Technologies, Cincinnati, OH), and was selected because of its hypocholesterolemic effects (12) and in vitro BSH activity (described below). Animals were fed 45 mL/kg body wt at 6-h intervals to match the physiological volume of milk consumed by pigs during lactation. Meals were consumed entirely within 60 min. If two or more pens had leftovers during two consecutive feedings, a 10% reduction in feed supply was applied to all pens during 24 h. Room temperature was gradually decreased from 28°C (13 days of age) to 22°C (83 days of age) to maximize feed intake. To prevent diarrhea, HFF diet was gradually increased until week 5 of the study by diluting it with CON, and diets were warmed in a water bath at 27°C for 10 min before feeding. In addition, liquid diets were poured into two bowls per pen to decrease competition between pigs. The CON diet was formulated to meet all of the nutrient requirements of growing Iberian pigs, according to the National Research Council (NRC) (70) and the Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA) (26), whereas the HFF diet exceeded by 54% the NRC recommended daily energy intake (Tables 1 and 2). Percentage of kilocalories from carbohydrate, fat, and protein sources in the HFF diet, and levels of cholesterol and fructose, were based on the diet 5B4L (TestDiet, St. Louis, MO), used to induce nonalcoholic steatohepatitis (NASH) in Ossabaw pigs (58, 74). Fructose content was set at 12% to prevent osmotic diarrheas. Diets were mixed every 3 days using a 6½-gallon food blender (LAR-25LMB; Skyfood, Miami, FL) and tap water, and stored at 2–3°C in a commercial refrigerator, with aliquots taken out of the stock in every feeding. To prevent bacteria overgrowth, probiotics were mixed in the diets daily by adding 0.2 g/L of MultiBio 3PS (BiOWiSH Technologies) using a commercial agitator (Grovhac, Brookfield, WI) at 300 rpm.
Table 1.
Ingredient composition of control and high-fructose high-fat diets fed to 13-day-old pigs during 10 consecutive weeks
| Item | CON-N and CON-Pi | HFF-N and HFF-Pi |
|---|---|---|
| Whey protein concentratea | 8.50 | 9.00 |
| Fructoseb | 0.00 | 12.0 |
| Dextroseb | 6.00 | 3.50 |
| Fat Pak 80c | 3.20 | 1.75 |
| Hydrogenated lardd | 0.00 | 3.10 |
| Hydrogenated coconut oilb | 0.00 | 4.23 |
| Corn oile | 3.20 | 0.00 |
| Xanthan gumf | 0.40 | 0.40 |
| Vitamin premixg,h | 0.32 | 0.32 |
| Mineral premixg,h | 1.20 | 1.20 |
| Cholesterolg | 0.00 | 0.70 |
| Water | 77.2 | 63.8 |
Values are in percent. CON-N, control nonprobiotic; CON-P, control probiotic; HFF-N, high-fructose, high-fat nonprobiotic; HFF-P, high fructose, high fat probiotic.
80% Whey protein concentrate (Hilmar Ingredients, Hilmar, CA).
Tate & Lyle, IL.
Advanced Fat-Pak 80 (Milk Specialties Global Animal Nutrition, MN).
Armour, TX.
Healthy Brand Oil Corporation, NY.
NutraBlend, MO.
Dyets Inc., Bethlehem, PA.
Provided per kilogram vitamin premix (vitamin A: 4,409,171 IU; vitamin D-3: 661,376 IU; vitamin E: 17,637 IU; vitamin B-12: 15.4 mg; menadione: 1,764 mg; riboflavin, 3,307 mg; d-pantothenic acid, 11,023 mg; niacin, 19,841 mg; phytase, 200,000 FTU). Provided per kilogram mineral premix (Fe, 110,000 mg; Zn, 110,000 mg; Mn, 26,400 mg; Cu, 11,000 mg; I, 200 mg; Se, 200 mg).
Multibio 3PS (BiOWiSH Technologies, Cincinnati, OH). 6.2 × 104 CFU/mL of Pediococcus acidilactici, Pediococcus pentosaceus, Lactobacillus plantarum, and Bacillus subtilis.
Table 2.
Daily nutrient and metabolizable energy of control and high-fructose, high-fat diets fed to 13-day-old pigs during 10 consecutive weeks
| Item | CON-N and CON-P | HFF-N and HFF-P |
|---|---|---|
| Feed amount, L·kg BW−1·day−1 | 0.18 | 0.18 |
| Dry matter, g·kg BW−1·day−1 | 40.8 | 63.9 |
| Crude protein, g·kg BW−1·day−1 | 13.2 | 13.1 |
| Metabolizable energy, kcal·kg BW−1·day−1 | 199.3 | 301.0 |
| Carbohydrates, g·kg BW−1·day−1 | 12.8 | 30.1 |
| Ether extract, g·kg BW−1·day−1 | 11.2 | 16.2 |
| Amino acids, g·kg BW−1·day−1 | ||
| Arginine | 0.30 | 0.30 |
| Histidine | 0.27 | 0.26 |
| Isoleucine | 0.78 | 0.77 |
| Leucine | 1.44 | 1.43 |
| Lysine | 1.11 | 1.10 |
| Methionine | 0.26 | 0.26 |
| Cysteine | 0.32 | 0.32 |
| Phenylalanine | 0.41 | 0.40 |
| Tyrosine | 0.32 | 0.32 |
| Threonine | 0.76 | 0.75 |
| Tryptophan | 0.20 | 0.20 |
| Valine | 0.70 | 0.69 |
| Fatty acids, g·kg BW−1·day−1 | ||
| Caprylic | 0.00 | 0.48 |
| Capric | 0.00 | 1.07 |
| Lauric | 0.00 | 3.39 |
| Myristic | 0.07 | 1.38 |
| Palmitic | 1.78 | 2.58 |
| Stearic | 0.67 | 1.28 |
| Arachidic/eicosanoic | 0.01 | 0.06 |
| Behenic | 0.00 | 0.00 |
| Palmitoleic | 0.14 | 0.23 |
| Oleic | 3.48 | 3.88 |
| Gadoleic | 0.06 | 0.08 |
| Linoleic | 3.86 | 0.96 |
| Linolenic | 0.04 | 0.06 |
| Arachidonic | 0.02 | 0.02 |
| Cholesterol, g·kg BW−1·day−1 | 0.03 | 1.30 |
| Choline, mg·kg BW−1·day−1 | 34.20 | 33.49 |
| Calcium, g·kg BW−1·day−1 | 0.62 | 0.62 |
| Phosphorus, g·kg BW−1·day−1 | 0.43 | 0.43 |
| Fructose, g·kg BW−1·day−1 | 0.00 | 21.6 |
| Dextrose, g·kg BW−1·day−1 | 10.8 | 6.30 |
Values were calculated and expressed as fed. BW, body weight; CON-N, control nonprobiotic; CON-P, control probiotic; HFF-N, high fructose, high fat nonprobiotic; HFF-P, high-fructose, high-fat probiotic.
The first day the experimental diets were fed was considered as day 0 of the study, and pig body weight was measured every 3 days. Blood was collected on day 65 at 2-h postfeeding, and on day 70 after 8-h fasting. Animals were euthanized on day 70 using an intramuscular injection of tiletamine and zolazepam (4 mg/kg; Zoetis, Parsippany, NJ), followed by an intracardiac injection of pentobarbital sodium (0.4 mL/kg; Schering-Plough, Union, NJ). Tissue from left medial segment in the liver, proximal jejunum (PJ), DI, and colon, and luminal content from DI, cecum, and colon, was collected immediately after euthanasia and frozen in liquid nitrogen, or fixed for histological analysis. In addition, liver, heart, and kidneys were removed and weighed, and lean mass composition was calculated as previously described (10).
In vitro analysis of BSH activity in probiotics.
Each probiotic strain was subjected to whole genome sequencing (MR DNA, Shallowater, TX) with a coverage depth greater than 100×. Resulting sequences were assembled with the NGen V15 assembly software (DNASTAR, Inc., Madison, WI), subjected to rapid annotation using subsystem technology (5) and searched for homologies to BSH genes (https://www.uniprot.org/uniprot/Q06115). P. acidilactici and pentosaceus presented two copies of choloylglycine hydrolases, but one was truncated, L. plantarum had three copies, and B. subtilis had one.
To assess BA deconjugating activity in vitro, each strain was plated individually at 1:100 in 0.1% sterile peptone (Fisher Scientific, Fair Lawn, NJ) and individually streaked out on De Man, Rogosa, and Sharpe agar (GranuCult MRS, MilliporeSigma, Burlington, MA), supplemented with 0.5% sodium taurodeoxycholic acid (tDCA), as previously described (23). Plates were incubated at 37°C in aerobic and anaerobic conditions for 72 h. Colonies of Lactobacillus plantarum on MRS spiked with sodium tDCA had small precipitates and more opaque color compared with control in both aerobic and anerobic conditions, indicative of BSH activity (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.10293656). No differences in colony color, size, or precipitate were observed for Pediococcus or Bacillus strains.
Liver lipid and function parameters.
Blood was sampled from the jugular vein at 2 h postfeeding on day 65. Samples were centrifuged at 2,100 rpm for 15 min at 4°C, and plasma and serum stored in 1.5-mL tubes at −80°C. Serum lipid and liver biochemistries were analyzed by the Comparative Pathology Laboratory at the University of California, Davis (Davis, CA) using a Roche Integra 400 Plus (Roche Diagnostics, Pleasanton, CA).
Hormones, glucose, and proinflammatory cytokines in serum.
To assess fasting levels of insulin, glucose, and leptin, blood samples were collected at 8-h postfeeding on day 70 of the study. Blood was taken from the left ventricle immediately before euthanasia by using a 2-in. 14-gauge needle, and serum and plasma samples were processed as described above. Insulin, leptin, and glucose levels were analyzed by the Animal Health Diagnostic Center at Cornell University (Ithaca, NY) using a human insulin radioimmunoassay kit (MilliporeSigma, Burlington, MA), a multispecies leptin RIA kit (Millipore), and a Cobas c501 (Roche Diagnostics), respectively. Serum levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1α, and transforming growth factor (TGF)-β were analyzed by the Cytokine Core Laboratory at the University of Maryland (Baltimore, MD) using a pig-specific enzyme-linked immunosorbent assay.
Triacylglyceride content, fatty acid composition, and citrate synthase activity.
Total triacylglyceride (TAG) content and citrate synthase activity in liver tissue were determined following previously described methods (53, 100). Fatty acid composition was quantified by gas chromatography of the fatty acid methyl esters using modified previously published methods (34). Chromatographic separations and analysis were conducted on an Agilent 7890B (Agilent Technologies, Palo Alto, CA) equipped with a flame ionization detector (FID), a 7683B automatic liquid sampler (Agilent Technologies), a split/splitless injection port, a J&W DB-23 column (Agilent Technologies), and helium used as the carrier gas. The inlet temperature was set to 250°C with an injection volume of 1 μL and split ratio of 20:1. The FID temperature was set to 280°C. The column temperature was initially held at 50°C for 1 min, then increased to 175°C at 25°C/min, then to 230°C at 4°C/min, and held for 6 min. Calibration standards were generated using a Supelco 37 component FAME Mix (MilliporeSigma).
Histological analysis of liver and intestinal tissue.
To assess histological features in the liver, tissue was placed in cassettes (Macrosette; Simport Scientific, Beloeil, Quebec, Canada) and fixed in 10% neutral buffered formalin (Medline, San Ramon, CA) at room temperature, transferred to 70% ethanol at 48 h, and submitted to the Comparative Pathology Laboratory (Davis, CA) for further processing. Tissues were embedded into standard paraffin blocks, and 5-µm-thick sections were stained with hematoxylin and eosin and Masson’s trichrome. Fresh liver samples were also embedded in optimum cutting temperature compound (Sakura Finetek USA; Torrance, CA), gradually frozen in 2-methylbutane (MilliporeSigma), cooled with liquid nitrogen, and stored at −80°C. Frozen blocks were cut with a thickness of 5 µm at the Histology Core at Baylor College of Medicine (Houston, TX) and stained with Oil Red O for lipid staining. The liver stains were examined by an experienced pathologist (D.I.; Comparative Pathology Laboratory, Davis CA) who was blinded to the treatment groups. Steatosis, ballooning degeneration, Mallory-denk bodies, lobular inflammation, fibrosis, and necrosis were semiquantitatively evaluated for each pig, and a composite lesion score was calculated (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.10293674). Zonal distribution of each of these variables was also systematically annotated. NASH was defined by the presence of macrovesicular steatosis ≥ 1, hepatocellular ballooning ≥ 1, and lobular inflammatory infiltrates ≥ 1, as previously recommended (88). To determine the number of proliferative cells in the liver, Ki67 immunostaining was performed on formalin-fixed and paraffin-embedded tissue by the Histology Core at Baylor College of Medicine. Sections were pretreated using heat-mediated antigen retrieval with citrate buffer for 20 min, followed by incubation with an anti-Ki67 antibody at 1:1,000 for 15 min (Abcam, Cambridge, MA). The Ki67 antibody was detected using the Bond Polymer Refine Detection Kit (Leica Biosystems, Buffalo Grove, IL) on a Leica Bond-III automated system (Leica Biosystems). Slides were then dehydrated through a series of alcohols and xylene, and a coverslip was applied using a permanent mounting media (Thermo Fisher Scientific, Waltham, MA). To quantify Ki67+ cells, each section was divided into five quadrants, and two fields of view per quadrant were captured with a final magnification of ×200. The number of Ki67+ hepatocytes was determined by manual counting in five random visual fields (1 field per quadrant) using the multipoint tool in ImageJ software (89). Counts were averaged, and the ratio between the labeled Ki67+ (brown-stained) and total hepatocyte count was expressed as a percentage for each sample.
To assess histological features in the gut, PJ, DI, and colon were excised, open longitudinally, and gently washed in 0.9% ice-cold saline solution immediately after collection. The PJ section was immediately distal to the duodenum and ligament of Treitz, the DI section was the distal part of the small intestine immediately proximal to the cecum, the cecum was collected entirely, and the colon section was the third spiral loop. Intestinal samples were fixed and processed as described for liver tissue. Hematoxylin-eosin stains were examined using an optical microscope (Axioscop 40; Zeiss, Thornwood, NY) fitted with a camera (AxioCam MRc; Zeiss), and the images were analyzed using image analysis software (Axiovision SE64 4.7.1; Zeiss). Four fields of view per sample were captured with a final magnification of ×40, and the height and depth of every well-oriented villus and crypt, respectively, were measured in micrometers and used to calculate an average value for each intestinal segment of each pig. A villus was deemed well oriented if it was visible from the apex of the villus to the base, whereas well-oriented crypts were adjacent to villus and visible from the luminal opening to the bottom of the crypt. To determine the number of proliferative cells in the PJ, DI, and colon, Ki67 immunostaining was performed as described for liver tissue. Between 10 and 20 well-oriented crypts per slide were captured with a final magnification of ×200 using an Olympus BX53 microscope fitted with an Olympus DP73 digital camera and cellSens software (Olympus; Center Valley, PA). The total number of cells, the number of Ki67+ cells, and the number of goblet cells on each crypt were quantified with ImageJ (89). Data are presented as absolute values.
Gene expression analysis.
Total RNA was extracted using guanidinium thiocyanate-phenol-chloroform method with TRIzol (Thermo Fisher Scientific) and subsequently purified using RNeasy Mini Kit (QIAGEN, Hilden, Germany). Purity and yield of RNA were assessed with a NanoDrop 1000 (Thermo Fisher Scientific), whereas integrity was analyzed by electrophoresis in a 1.2% RNA agarose gel with 1.0 μg/mL of ethidium bromide (MilliporeSigma). The QuantiTect Reverse Transcription Kit (QIAGEN) was used to reverse transcribe 200 ng of RNA per sample, and resulting cDNA was diluted 1:6 with free RNAse water (MilliporeSigma). Quantitative PCR was performed on an ABI Prism 7500 (Applied Biosystems, Foster City, CA) using Power SYBR Green PCR Master Mix (Applied Biosystems) with the following thermal cycling conditions: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s, and a dissociation curve (95°C for 15 s, 60°C for 60 s, and 95°C for 15 s). Primer sequences are presented in Supplemental Table S2 (see https://doi.org/10.6084/m9.figshare.10293671). Primers were designed to span exon-exon junctions using the PrimerQuest Tool software (Integrated DNA Technologies, Coralville, IA) based on publicly available swine reference sequences deposited in the National Center for Biotechnology Information (70). Primer pairs were tested by PCR using GoTaq Green Master Mix (Promega, Madison, WI) on a T100 thermocycler (Bio-Rad Laboratories, Hercules, CA) with the following thermal cycling conditions: 2 min at 95°C, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, and a final extension step at 72°C for 5 min. Product length and yield were analyzed by electrophoresis in 2.5% agarose gels with 1.0 μg/mL of ethidium bromide (MilliporeSigma). Gels were analyzed using a Gel Doc XR+ system and Image Laboratory 5.0 software (Bio-Rad Laboratories). The PCR products were purified from the agarose gel using a NucleoSpin Extract II kit (Clontech Laboratories, Mountain View, CA) and sequence was verified at UC Davis DNA Sequencing Facility (Davis, CA). Efficiency of the qPCR reaction for each primer pair was calculated from a twofold five-point standard curve, according to the formula (2−1/slope − 1) × 100. Target genes were normalized by three reference genes selected with geNorm software (Supplemental Figs. S2 and S3; see https://doi.org/10.6084/m9.figshare.10293653 and https://doi.org/10.6084/m9.figshare.10293659, respectively), as previously described (110). Gene expression was calculated using the 2−ΔΔCt method (63) and is presented as fold change.
Western blot analysis.
Approximately 100 mg of liver tissue was homogenized in 800 µl of ice-cold lysis buffer containing 50 mM Tris·HCl (RPI, Mount Prospect, IL), 150 mM NaCl (Thermo Fisher Scientific) 1% NP-40 (VWR Life Sciences, Solon, OH), 0.5% sodium dodecyl sulfate (SDS, 20% solution; RPI), 50 mM β-glycerophosphate (MilliporeSigma), 10 mM EDTA (Invitrogen, Rockford, IL), 10 mM NaF (VWR Life Sciences), 1 mM sodium orthovanadate (Alfa Aesar, Ward Hill, MA), 0.1 mM PMSF (Thermo Fisher Scientific), 1 µg leupeptin (MilliporeSigma), and protease inhibitor mix (Pierce; Thermo Fisher Scientific). Protein samples were electrophoretically separated on 7–12% SDS polyacrylamide gels (PAGE; C.B.S Scientific, Del Mar, CA), transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific), and blocked with 5% bovine serum albumin. Membranes were incubated overnight with primary antibodies (Cell Signaling Technology, Danvers, MA), followed by 1-h incubation with secondary antibodies (Cell Signaling Technology). Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), and analyzed using a ChemiDoc-It Imaging System (Bio-Rad Laboratories). For normalization, immunoblots performed with anti-phospho-specific antibodies were exposed to stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with non-phospho-specific antibodies against total protein (Cell Signaling Technology). Primary antibodies used were against protein kinase B (PKB total and Ser473), microtubule-associated protein 1A/1B light chain 3A (LC3 I/II), extracellular signal-regulated kinase 1/2 (ERK1/2, total and Thr202/Tyr204), ubiquitin-binding protein p62 (P62), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Analysis of metabolites.
Metabolomics were performed on plasma, liver, and colon content samples using protein precipitation extraction with ultraperformance liquid chromatography tandem quadrupole mass spectrometry, using modified previously published methods (107). Briefly, 25 µL of plasma, and 50–60 mg of liver and colon content were added to 1.5-mL tubes before being spiked with 20-µL isotopically-labeled surrogates, followed by 750-µL chilled methanol. Samples were then vortexed 1 min before being centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was transferred to 1.5-mL high-performance liquid chromatography amber glass vials, dried by centrifugal vacuum evaporation, and reconstituted in 3:1 methanol-acetonitrile containing 100 nM of 1-cyclohexyl-ureido, 3-dodecanoic acid (MilliporeSigma). The reconstituted solution was vortexed 1 min and filtered by centrifugation at 9,500 rpm for 3 min at room temperature through a polyvinylidene fluoride membrane (Durapore PVDF, 0.1 μm; MilliporeSigma). Analyses were conducted on an Acquity UPLC I-Class system (Waters, Milford, MA) coupled with a 4000 QTRAP LC-MS/MS System (SCIEX, Concord, ON, Canada) using multiple reaction monitoring quantified with MultiQuant software (version 3.0; SCIEX). General metabolites were separated using a 150 × 2.0-mm Luna NH2 column (Phenomenex, Torrance, CA) and detected by negative ion mode electrospray ionization (5, 107). For the aminomics assay, metabolites were separated using a 150 × 2.1-mm Atlantis HILIC column (Waters) and detected by positive ion mode electrospray ionization (107). For the lipidomics assay, metabolites were separated using a 150 × 3.0-mm Prosphere HP C4 column (Grace Discovery Sciences, Columbia, MD) and detected by positive ion mode electrospray ionization (82, 107). For BA analysis, analytical targets were separated using an Acquity UPLC I-Class system (Waters) equipped with a 2.1 × 100-mm, 1.7-µm BEH C18 column (Waters) operated in negative mode electrospray ionization. Metabolite intensities were normalized to that of internal standards (Cayman Chemical, Ann Arbor, MI) added during the extraction, with 6- to 8-point calibration curves, and to sample weight to account for small variations in starting tissue. General metabolites in liver, plasma, and colon digesta were expressed as metabolite peak areas under the curve, and BAs in plasma and colon digesta were expressed in nanomoles. In addition, a hydrophobicity index (HI) for plasma and colon BA pools was calculated using the BA molar fractions, as previously described (45). Liver and plasma values are presented as absolute composition, whereas data in colon digesta were standardized to relative composition (so sample totals are 100%). We acknowledge that, due to the presence of steatosis in the liver, it is possible that there may have been some decrease in content of other hepatic metabolites due to rearrangement within the cellular matrix as fat content increased. However, because of the strong agreement between changes in plasma and liver values for key metabolites (i.e., BAs, choline, homocysteine, and 4-pyridoxate), it is likely that a fat-induced dilution effect has no impact in the interpretation of the results.
Short-chain fatty acid (SCFA) composition in cecum samples was quantified by gas chromatography using modified previously published methods (38). Chromatographic separations and analysis were conducted on an Agilent 7890A Gas Chromatograph (Agilent Technologies) equipped with a FID detector, a 7683B automatic liquid sampler (Agilent Technologies), split/splitless injection port, a ZB-WAX column (Phenomenex, Torrance, CA), and helium was used as the carrier gas. The inlet temperature was set to 250°C with an injection volume of 1 µL in splitless mode. The FID temperature was set to 280°C. The column temperature was initially 90°C, then increased to 150°C at 15°C/min, then to 170°C at 5°C/min, and finally to 250°C at 20°C/min and held at this temperature for 5 min. Identification and quantitation of the SCFAs was based on the retention times and integrated peak areas of standard compounds.
16s rRNA.
Bacterial DNA from DI, cecum, and colon was extracted in duplicates using a DNeasy PowerLyzer PowerSoil Kit (QIAGEN), according to manufacturer’s instructions, and DNA concentration and quality were measured with a NanoDrop 1000 (Thermo Fisher Scientific). Samples were submitted to a commercial laboratory (MR DNA; Shallowater, TX) for 16S rRNA gene amplification, library construction, and sequencing using paired-end 16S community sequencing on the Illumina platform (Illumina; San Diego, CA). Briefly, PCR was performed using HotStarTaq Plus Master Mix Kit (QIAGEN), 1 µL of DNA, and primers targeting the V4 hypervariable region of the 16S rRNA gene (515F forward primer: 5′-GTGYCAGCMGCCGCGGTAA-3′; 806R reverse primer: 5′-GGACTACNVGGGTWTCTAAT-3′) under the following cycle parameters: 94°C for 3 min, followed by 30 cycles of 94°C for 30 s, 53°C for 40 s, 72°C for 1 min, and a final elongation step of 72°C for 5 min. After amplification, PCR products were checked in 2% agarose gel to determine the success of amplification and the relative size of bands (~300–350 bp), and purified using AMpure XP beads (Beckman Coulter Genomics, Indianapolis, IN). A TrueSeq library preparation kit (Illumina) was used to attach the dual indexes according to manufacturer recommendations, and another round of PCR clean-up was done with AMpure XP beads (Beckman Coulter Genomics). Sequencing of the purified amplicons was performed in one run of a MiSeq (Illumina) using the paired-end 300-bp protocol following the manufacturer’s guidelines. The sequences were processed and filtered through MR DNA standard pipeline for microbiome data analysis. In summary, sequences were joined and barcodes, sequences <150 bp, sequences with ambiguous base calls, and sequences with homopolymer runs exceeding 6 bp were removed. Remaining sequences were then denoised, and operational taxonomic units (OTUs) were defined clustering at 97% similarity followed by removal of singleton sequences and chimeras. A total of 8,191,344 fragments were retained (2,737,430; 2,691,011; and 2,762,903 for DI, cecum, and colon, respectively), with an average of 97,516 fragments per sample. Final OTUs were taxonomically classified using BLASTn (71) against a curated database derived from the Ribosomal Database Project (RDP-II; Michigan State University, East Lansing, MI) and National Center of Biotechnology (70), and compiled into each taxonomic level into both counts and percentage files. Pre-processed 16S rRNA gene high-throughput files obtained from MR DNA were further processed for alpha- and beta-diversity using Quantitative Insights into Microbial Ecology (QIIME) (11). Alpha diversity indexes, including OTUs and Shannon diversity, were computed using the core_diversity_analyses.py script and a rarefaction of 50,793 OTUs. The output from the collate_alpha.py script, which is part of the workflow of the core_diversity_analyses.py script, was used to generate box plots using the compare_alpha_diversity.py script.
Predicted functional pathways.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database (55) was used to predict functional categories from the 16S rRNA gene high-throughput sequencing data using the package Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (57a). Briefly, OTUs were picked at a 97% identity threshold using the pick_closed_reference.py script against the Greengenes 13.5 database available in QIIME. Output was then uploaded to the PICRUSt server. Copy numbers were normalized using the PICRUSt server, functional predictions were determined using KEGG orthologs, and functions were then categorized at levels 2 and 3.
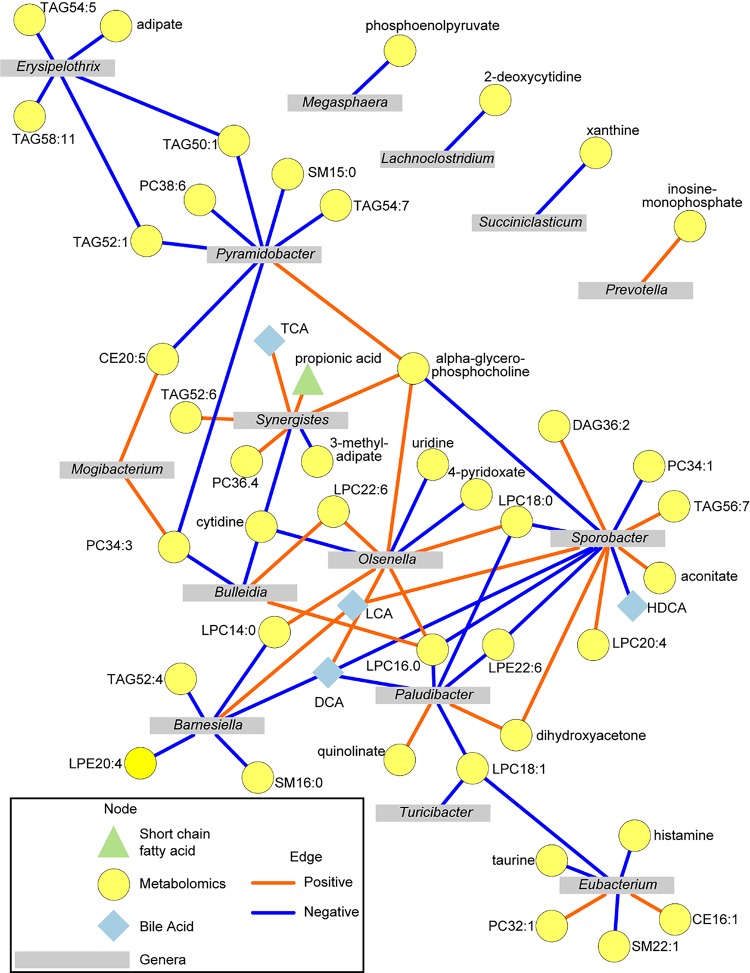
Intersection of microbiome and metabolites.
Significant association between the colon content of bacterial genera and metabolites altered by diet were assessed by Spearman’s correlations in the R Statistical Language (version 3.6.0). Relative abundance data for each metabolomics platform were combined, and samples were dropped if they had >90% of metabolite data missing. A total of six samples met these criteria and were dropped from the analysis (3 CON and 3 HFF). The remaining samples had <1% missing data across all metabolites. Missing data for these samples were imputed using the k-nearest neighbor imputation method (44, 108). Relative abundance data for microbial genera were then matched to metabolomics data. All data were then transformed to center-log ratios to minimize negative correlation bias in composition data (40). Spearman’s correlations were assessed for pairwise correlations by the rcorr() function in the Hmisc package (43). P values were extracted so that genera were the source node and metabolites were target nodes, and these data were then adjusted for multiple comparisons using the false discovery rate (FDR) procedure (6). Correlations with FDR ≤ 0.05 (n = 24) were then considered for network using Cytoscape version 3.7.1 (93). A correlation network consisting of correlations with FDR ≤ 0.001 was developed to aid in visualizing the strongest relationships between genera and metabolites altered by diet.
Statistical analysis.
Pen was considered as the experimental unit. Body weight, lean mass, relative organ weight, serum biochemistry and hormones, TAG content, gene and protein expression, and histological features were analyzed by a two-way ANOVA using a mixed model in SAS 9.2 (SAS Institute, Cary, NC) that included diet × probiotic as fixed effect, and pen nested in diet × probiotic as random effect. Analysis of protein expression data included also blot as random effect in the model to account for interblot variability. Normality of the residuals and presence of outliers were assessed, and nonnormally distributed parameters were power transformed by a parameter φ whose optimal value was estimated using the maximum likelihood method (77). Multiple comparisons were corrected with Tukey test. Data are presented as least squares means ± SE. Significant effects were considered at P ≤ 0.05. Differences in hepatic histological features between groups were analyzed by Kruskal-Wallis with Bonferroni multiple comparisons test for nonparametric data (PROC NPAR1WAY and PROC RANK; SAS).
Metabolomics data were imported into the Primer-E software (version 7; Primer-E Ltd., Plymouth, UK), log transformed into a normal distribution approximation, and analyzed with a Euclidean distance matrix. A nonparametric permutational analysis of variance (PERMANOVA; Primer-E) with diet × probiotic as fixed effects, and pen nested in diet × probiotic as random effect, was used for testing the null hypothesis of no difference between groups under a reduced model, 9,999 permutations, and type III sum of squares. Metabolomics data were further assessed by principal component analysis (PCA) to visualize group discrimination in a two-dimensional scores plot. PCA analyses were conducted in the R Statistical Language using the prcomp() function. Cube-root transformed concentrations or relative abundance data were scaled to unit variance before PCA assessment. PCA scores from components 1 and 2 were plotted and overlaid with diet and probiotic classifiers. Component 1 was assessed for group differences using ANOVA, and the total R2, F statistic, and P value from these models were reported. Identification of metabolites differentially expressed between groups was performed by a two-way ANOVA with the same random and fixed effects described above. The FDR procedure was used to adjust the reported P values.
Microbiome data were standardized to relative composition (so sample totals are 100%), imported into the Primer-E (Primer-E), square-root-transformed and analyzed with a Bray-Curtis similarity distance matrix. Hierarchical clustering analysis was carried out with a similarity profile permutation test to determine whether samples clustered together with significant similarity (P ≤ 0.05). Beta-diversity (Bray-Curtis) was determined using the beta_diversity.py script in QIIME. The output generated by the script was then used to construct principal coordinate analyses (PCoA) plots using the make_2d_plots.py script in QIIME, to visualize any grouping effects related to intestinal section, diet, and probiotics. PERMANOVA was then used to determine significant differences in beta-diversity values across the different groups of interest using the compare_categories.py script in QIIME. Values are shown in the beta-diversity PCoA plots. An analysis of similarity percentages (Primer-E Ltd.) with a cutoff value of 70% was used to select genera contributing to the overall microbiome dissimilarity between CON and HFF on each intestinal section. Diet-induced changes in genera across the gut sections were analyzed by a two-way ANOVA that included 1) diet × intestinal section as fixed effects, 2) pen nested in diet as random effect, and 3) a repeated-measurement statement with intestinal section as repeated factor, and pen nested in diet as subject. Normality of the residuals and presence of outliers were assessed as described above.
RESULTS
Results are discussed for HFF-N, HFF-P, CON-N, and CON-P groups, if the interaction between diet and probiotics was significant (P ≤ 0.05); otherwise, only the effect of diet (HFF versus CON) and/or probiotic is reported.
HFF-fed juvenile Iberian pigs developed NASH.
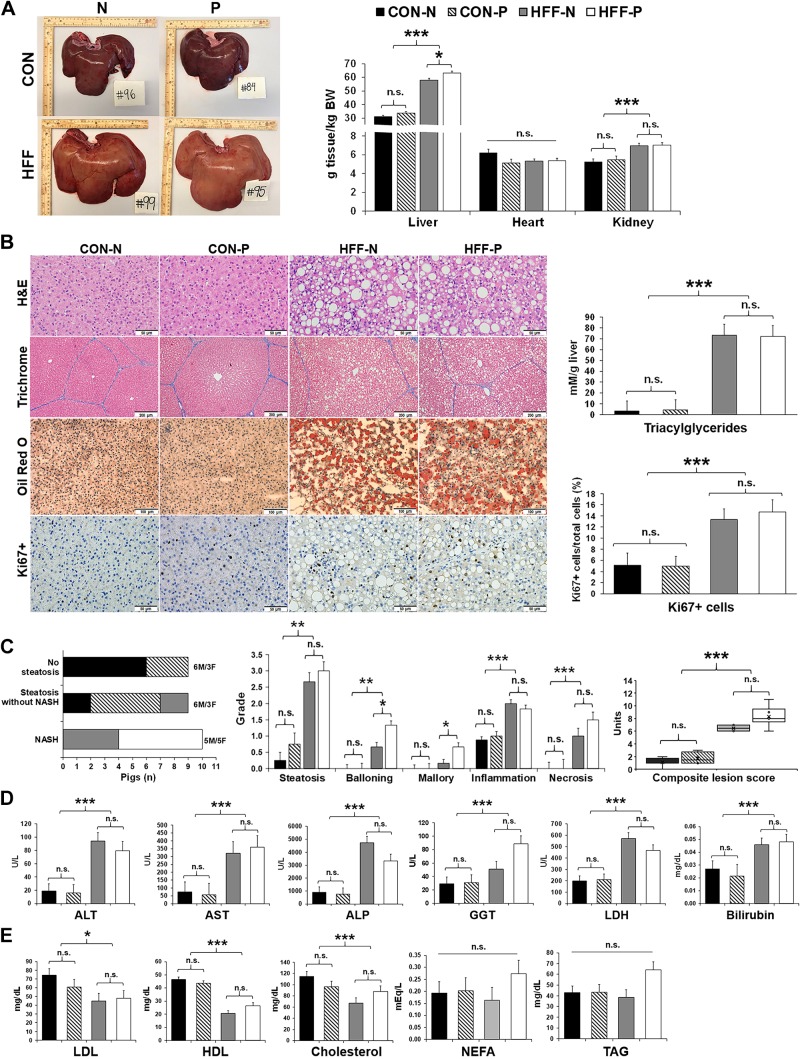
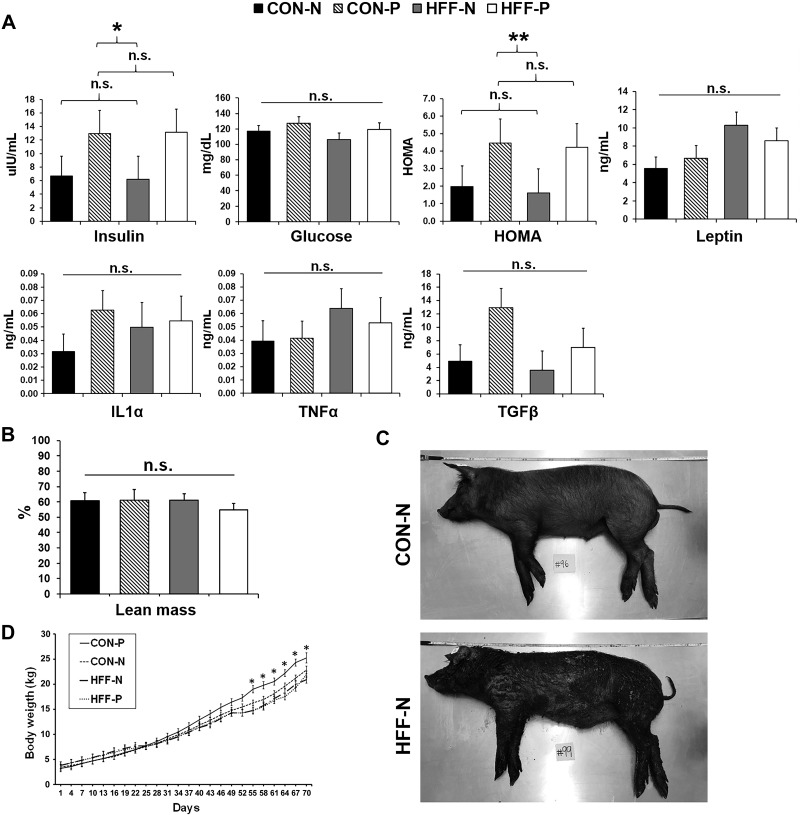
Because mature Iberian pigs nutritionally challenged with high-fat hypercaloric diets showed all features characteristic of the human metabolic syndrome (106), we used this breed to develop a diet-induced model of pediatric NASH. We found that feeding a HFF diet to 13-day-old pigs for 10 wk caused both hepato- and nephromegaly (P ≤ 0.001; Fig. 1A) and a 10-fold increase in hepatic TAG content (P ≤ 0.001; Fig. 1B). None of the pigs developed fibrosis, likely due to the short duration of the study, as a recent report in juvenile Ossabaw pigs fed an obesogenic diet showed fibrotic lesions after 16 wk (74), compared with 10 wk for the present study. Nonetheless, HFF livers had an increase in proliferative cells (P ≤ 0.001; Fig. 1B), which has been associated with fibrogenesis in mouse models of NAFLD (13). Over 80% of HFF-fed pigs (n = 10) showed histopathological lesions consistent with NASH at 83 day of age, including steatosis, hepatocellular ballooning, Mallory hyaline, lobular inflammation, and necrosis (Fig. 1C and Supplemental Table S1). In addition, 43% of CON and 17% of HFF-fed pigs (n = 9) developed steatosis without inflammation and/or cellular ballooning, and 57% of CON group (n = 9) had no lesions in the liver (Fig. 1C). Hepatic steatosis was classified as macrovesicular with centrilobular distribution in CON-N and CON-P, whereas HFF-N and HFF-P developed both macro- and microvesicular steatosis with periportal or diffuse distribution (Supplemental Table S1). Serum markers of liver injury were increased in HFF-fed pigs on day 65 (P ≤ 0.001; Fig. 1D). However, fasting levels of insulin, glucose, leptin, and proinflammatory cytokines TNF-α, TGF-β and IL-1α, as well as lean mass composition, did not differ between HFF and CON groups (Fig. 2, A–C). Similar results have been observed in choline/methionine-deficient dietary models of NASH, in which mice develop steatosis, lobular inflammation, and ballooning in the absence of other metabolic features seen in human NAFLD, including insulin resistance and dyslipidemia (49, 83, 85). A depletion in choline levels can also explain the decrease in serum levels of cholesterol (P ≤ 0.001) and low- and high-density lipoproteins (P ≤ 0.05 and P ≤ 0.001) in HFF compared with CON-fed animals (Fig. 1E). Decreased choline availability reduces the hepatic synthesis of phosphatidylcholine (PC) via the cytidine 5′-diphosphocholine pathway, which then impairs the production of very-low-density lipoproteins and export of fat and cholesterol from the liver (18, 72). Interestingly, relative liver weight, ballooning degeneration, and Mallory-denk bodies increased in HFF-P compared with HFF-N (P ≤ 0.05; Fig. 1, A and C). Furthermore, probiotics increased body weight in the CON group between days 55–70 and fasting insulin levels in HFF and CON pigs (P ≤ 0.05; Fig. 2, A and D) compared with nonprobiotic animals. Taken together, these results suggest that HFF-fed pigs developed a diet-induced pathology in the liver, which was accentuated by the supplementation of probiotics in the HFF diet.
Fig. 1.
High-fructose, high-fat (HFF) diet induced nonalcoholic steatohepatitis (NASH) in juvenile Iberian pigs. A: representative liver images from pigs fed either a control (CON) or a HFF diet, with probiotics (P) or without probiotics [nonprobiotics (N)], taken immediately after euthanasia on day 70 of the study. Feeding a HFF diet to 13-day-old Iberian pigs for 10 wk caused an enlargement of liver and kidney. B: photographs of hematoxylin-eosin (H&E) and trichrome staining of paraffin embedded sections show steatosis without fibrosis in HFF-fed pigs. Oil Red O staining of frozen liver sections demonstrated lipid droplet accumulation in HFF group, whereas Ki67+ staining showed increased cell division in HFF histological sections. Triacylglyceride (TAG) content, measured with a commercial kit, was increased in HFF livers. C: 80% of HFF-fed pigs showed histopathological changes in liver tissue consistent with NASH (left) of which hepatocellular ballooning, Mallory hyaline (middle), and the composite liver score (right) were increased by the inclusion of probiotics in the HFF diet. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and bilirubin, measured on day 65 at 2-h postfeeding, were elevated in HFF-fed pigs (D), whereas low-density lipoprotein (LDL), high-density lipoprotein (HDL), and cholesterol were decreased in HFF compared with CON (E). Values are least squares means ± SE; n, no. of pigs. P values were adjusted for multiple testing with Tukey’s post hoc test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., Nonsignificant. F, female; M, male.
Fig. 2.
High-fructose, high-fat (HFF)-fed pigs did not develop obesity or hyperinsulinemia. A: fasting levels of insulin, glucose, homeostatic model assessment (HOMA), leptin, interleukin-1α (IL-1α), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) did not differ between HFF and control (CON) on day 70 of the study. Probiotics increased insulin levels and HOMA index, which is a quantitative measure of insulin resistance. HOMA values were calculated according to the formula [fasting insulin (µU/mL) × fasting glucose (mg/dL)]/405. B: lean mass composition did not differ between groups and was calculated using the formula 100 × [8.588 + (0.465 × hot carcass weight) − (21.896 × 10th rib fat depth) + (3.005 × 10th rib loin muscle area)]/hot carcass weight. C: representative images of pigs fed CON nonprobiotic (CON-N) and HFF nonprobiotic (HFF-N) diets on day 70 immediately after euthanasia. D: CON probiotic (CON-P) pigs gained more weight than HFF-N and HFF probiotic (HFF-P) between days 55 and 70 and CON-N on days 55, 58, and 67. Values are least square means ± SE. P values were adjusted for multiple testing with Tukey’s post hoc test. *P ≤ 0.05, **P ≤ 0.01. n.s., Not significant.
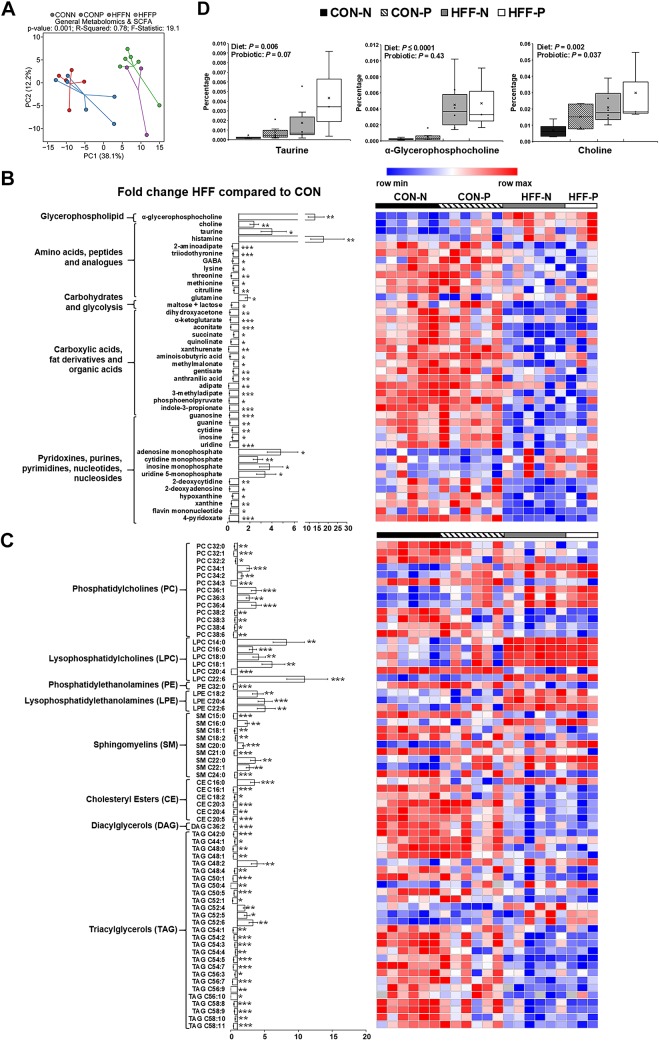
HFF diet decreased choline levels and dysregulated one-carbon and lipid metabolism in liver and plasma.
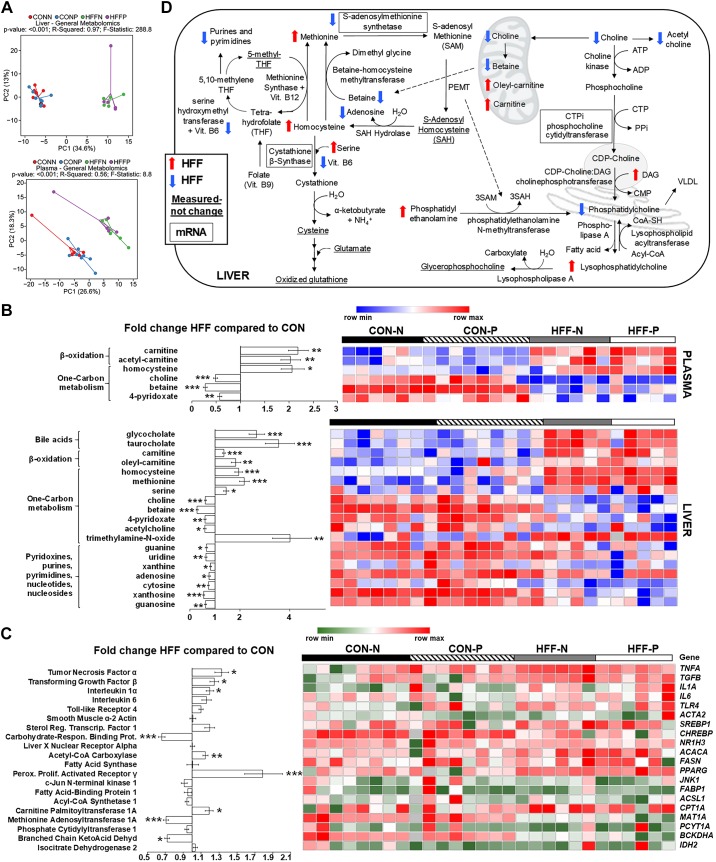
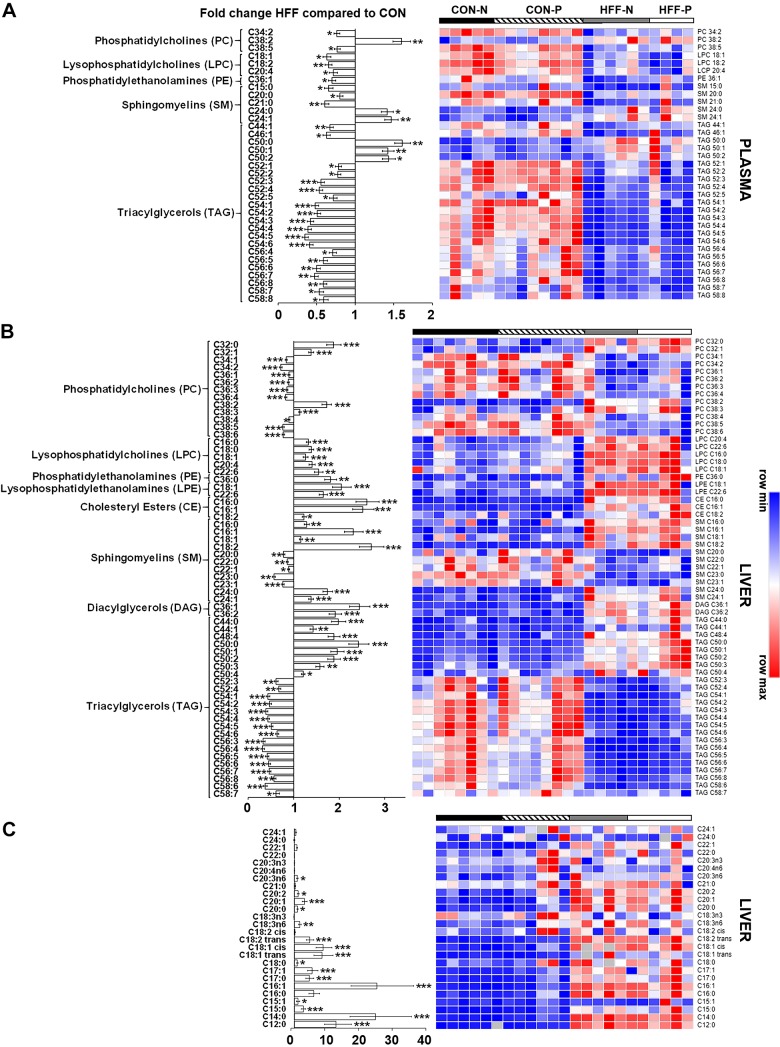
To investigate the potential mechanisms of NASH, we examined markers of inflammation and lipid metabolism using metabolomics, enzymatic assay, and qPCR. A total of 218 and 224 metabolites were detected in liver and plasma, respectively, of which 105 and 64 were different between HFF and CON groups. Multivariate analysis showed no differences between probiotic and nonprobiotic animals (Fig. 3A). Choline (P ≤ 0.001), betaine (P ≤ 0.001), and 4-pyridoxate (P ≤ 0.01) decreased in liver and plasma of HFF-fed pigs, whereas BAs gCA and tCA (P ≤ 0.001), homocysteine (P ≤ 0.001 and P ≤ 0.05), and carnitines (P ≤ 0.001 and P ≤ 0.05) increased in HFF compared with CON (Fig. 3B). These results are consistent with metabolite changes in diet-induced choline deficiency in humans and mouse models of NASH (92, 105) and suggest a dysregulation of hepatic one-carbon metabolism in response to HFF diet (Fig. 3D). Hepatic trimethylamine-N-oxide (TMAO), a metabolite associated with NASH, which is derived from gut microbial catabolism of choline (14, 15), was also higher in the HFF group (P ≤ 0.01). Phosphatidylethanolamines (PEs), lysophosphatidylethanolamines (LPEs), and lysophosphatidylcholines (LPCs) decreased in plasma (P ≤ 0.01) and increased in liver (P ≤ 0.001) of HFF-fed pigs, whereas cholesteryl esters (CEs) and diacylglycerols (DAGs) increased in liver (P ≤ 0.01; Fig. 4, A and B). Conversely, multiple PC species decreased in liver of HFF compared with CON (P ≤ 0.001; Fig. 4B), which, along with the choline and betaine depletion, suggests an impairment in hepatic PC biosynthesis (Fig. 3D). Interestingly, HFF livers had decreased TAGs with longer carbon chain and higher number of double bonds (P ≤ 0.001), and a disproportionate increase in fatty acids with carbon length C12–14 (P ≤ 0.01; Fig. 4, B and C), likely due to the elevated content of medium-chain fatty acids in the diet. Similarly, HFF diet decreased TAGs with longer carbon chain in plasma (P ≤ 0.001; Fig. 4A).
Fig. 3.
Metabolic changes in high-fructose, high-fat (HFF) livers and plasma were consistent with dysregulation of one-carbon metabolism. A: principal component analysis [principal components 1 and 2 (PC1 and PC2)] to visualize group discrimination in a two-dimensional scores plot showed a separation of HFF and control (CON) samples, but not a probiotic effect. Each point represents an individual pig, and color of point denotes diet. Group differences were assessed by nonparametric permutational analysis of variance with diet × probiotic as fixed effects and pen nested in diet × probiotic as random effect, under a reduced model, 9,999 permutations, and type III sum of squares. N, nonprobiotic; P, probiotic. B: heat map of absolute abundance of metabolites significantly altered by the diet in plasma and liver samples of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Only metabolites related to bile acids, β-oxidation, one-carbon metabolism, and nucleic acids are shown. Columns are individual pigs, and rows are log2-transformed metabolites. Blue and red represent the row minimum and maximum values, respectively. C: heat map of relative mRNA abundance of genes related to inflammation, one-carbon metabolism, de novo lipogenesis, and fat transport and β-oxidation in the liver, measured by qPCR in 83-day-old pigs. Columns are individual pigs, and rows are ΔCt values. Green and red represent the row minimum and maximum values, respectively. Fold change was calculated as 2−ΔΔCt. D: mechanistic illustration of pathways involved in hepatic one-carbon and lipid metabolism, and relevant metabolites and genes altered by the diet. Blue and red arrows represent increased and decreased expression in HFF-fed pigs compared with CON, respectively. A decrease in choline availability may have reduced the hepatic synthesis of phosphatidylcholines (PCs) via the cytidine 5′-diphosphocholine pathway, decreasing the production of lipoproteins and export of fat and cholesterol from the liver. The increase in phosphatidylethanolamines (PEs), lysophosphatidylethanolamines (LPEs), and lysophosphatidylcholines (LPCs) in HFF livers suggests also an impairment in the hepatic synthesis of PCs via phosphatidylethanolamine N-methyltransferase (PEMT) and lysophospholipid-acyltransferase pathways. Because PEMT requires three S-adenosyl methionine (SAM) molecules to convert a PE into a PC, it is possible that HFF-fed pigs became deficient in SAM. In this regard, HFF livers had increased homocysteine and methionine levels, and a decrease in SAM synthetase expression, which catalyzes the conversion of methionine into SAM. Furthermore, 4-pyridoxate, purines and pyrimidines were lower in HFF, indicative of a deficit in folate cycle intermediates, which may have impaired the remethylation of homocysteine via the methionine synthase pathway, while a decrease in betaine may have reduced substrate availability for betaine-homocysteine methyl transferase. P values were calculated by a two-way ANOVA with a mixed model and adjusted for multiple testing with Benjamin-Hochberg procedure and Tukey’s post hoc test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Fig. 4.
High-fructose, high-fat (HFF)-fed pigs had decreased phosphatidylcholines (PCs) and increased phosphatidylethanolamines (PEs), lysophosphatidylethanolamines (LPEs), and lysophosphatidylcholines (LPCs) in liver. Heat map is shown of absolute abundance of lipids significantly altered by the diet in plasma (A) and liver (B), along with individual fatty acids in liver (C) of 83-day-old pigs, with fold change and significance levels by HFF compared with control (CON). Lipidome and fatty acids analyses were performed by mass spectrometry and gas chromatography, respectively. Columns are individual pigs, and rows are log2-transformed metabolites. Blue and red represent the row minimum and maximum values, respectively. P values for each metabolite were calculated by a two-way ANOVA with a mixed model and adjusted for multiple testing with Benjamin-Hochberg procedure. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
In agreement with the histopathology results, expression of TNFA, IL1A, and TGFB was upregulated in liver tissue of HFF-fed pigs (P ≤ 0.05; Fig. 3C). The relative mRNA abundance of hepatic transcription factors and enzymes involved in de novo lipogenesis did not differ or was decreased (i.e., carbohydrate response element-binding protein, CHREBP; P ≤ 0.001) in HFF-fed pigs, except for acetyl-CoA carboxylase 1 (ACACA), which increased for HFF compared with CON (P ≤ 0.01; Fig. 3C). Conversely, metabolites and enzymes related to tricarboxylic acid cycle (i.e., fumarate and malate levels, and citrate synthase activity; P ≤ 0.05) (Supplemental Fig. S4, A–C; see https://doi.org/10.6084/m9.figshare.10293665), and gene expression of liver markers of fatty acid transport (i.e., peroxisome proliferator-activated receptor-γ, PPARG; P ≤ 0.001) and β-oxidation (i.e., carnitine palmitoyltransferase 1A, CPT1A; P ≤ 0.05), were higher in the HFF group (Fig. 3C and Supplemental Fig. S4, A–C), suggesting an increase in hepatic fat catabolism compared with CON. These results, along with the lack of obesity in HFF-fed pigs, are consistent with mouse NASH models induced by dietary choline deficiency, characterized by a hypermetabolic state with decreased lipogenesis and increased fatty acid uptake, β-oxidation, and energy expenditure (84, 85).
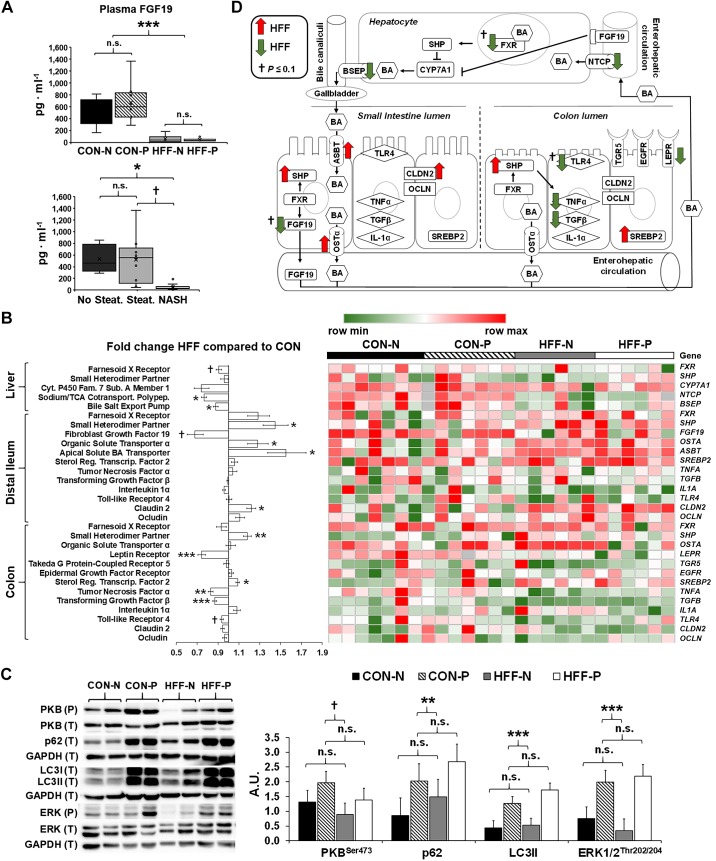
HFF-fed pigs had dysregulated FXR-FGF19 signaling and reduced FGF19 levels in plasma.
High-fat diets have been shown to increase biliary output of BAs, which activate enterohepatic FXR-FGF19 signaling to inhibit hepatic CYP7A1 transcription and reduce BA synthesis (31). To prevent cytotoxic BA accumulation, FXR also increases the transcription of bile salt export pump (BSEP) and inhibits Na+-taurocholate cotransporter (NTCP) in hepatocytes, whereas in the gut it inhibits the expression of apical sodium-dependent BA transporter (ASBT) and upregulates organic solute and steroid transporter (OSTA) (24). To investigate whether NASH was associated with a dysregulation of BA regulatory mechanisms in our pig model, we analyzed plasma levels of FGF19 by ELISA, and the expression of FXR-induced genes in DI, colon, and liver tissue by qPCR. Circulating FGF19 decreased in HFF compared with CON (P ≤ 0.001), and in NASH compared with pigs without steatosis (P ≤ 0.05; Fig. 5A). The relative quantification of mRNA in DI showed an increase in SHP, OSTA, and ASBT (P ≤ 0.05), and a decreasing trend in FGF19 expression (P ≤ 0.1) for HFF-fed pigs compared with CON, whereas FXR did not differ between groups (Fig. 5, B and D). In liver, BSEP and NTCP decreased (P ≤ 0.05), and FXR had a decreasing trend (P ≤ 0.1) in HFF compared with CON, but SHP and CYP7A1 remained unchanged between diets (Fig. 5, B and D). Together, these results suggest a lack of suppression of CYP7A1 expression associated with the uncoupling of intestinal FXR-FGF19 signaling and decreased FGF19 plasma levels in HFF-fed pigs.
Fig. 5.
High-fructose, high-fat (HFF) diet and probiotics dysregulated enterohepatic Farnesoid-X receptor (FXR)-fibroblast growth factor 19 (FGF19) signaling and markers of autophagy in the liver. A: plasma levels of FGF19, measured by ELISA on day 70 at 8-h postfeeding, decreased in HFF compared with control (CON)-fed pigs, and in nonalcoholic steatohepatitis (NASH) compared with pigs without steatosis. N, nonprobiotic; P, probiotic. B: heat map of relative mRNA abundance of genes related to the integrity, inflammation, and proliferation of the intestinal mucosa, and enterohepatic FXR-FGF19 signaling, measured by qPCR in 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Columns are individual pigs and rows are ΔCts. Green and red represent the row minimum and maximum values. Fold change calculated as 2−ΔΔCt. P values for each gene were calculated by a two-way ANOVA with a mixed model. C: representative Western blots (left) and histograms with the quantification of bands expressed as arbitrary units (A.U.; right), to assess the expression of macroautophagy-related proteins in liver tissue. Microtubule-associated protein 1A/1B light chain 3A (LC3II), ubiquitin-binding protein p62 (P62), and phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2) were significantly increased in probiotic-fed pigs, whereas phosphorylated protein kinase B (PKB) showed an increasing trend between nonprobiotic and probiotic groups. D: mechanistic illustration of genes involved in bile acid (BA)-related pathways in liver, distal ileum (DI), and colon tissue, altered by the diet. Green and red arrows represent increased and decreased expression in HFF-fed pigs compared with CON, respectively. Values in A and C are least square means ± SE. P values in A and C were adjusted for multiple testing with Tukey’s post hoc test. †P ≤ 0.1, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., Nonsignificant. ASBT, apical sodium-dependent bile acid transporter; BSEP, bile salt export pump; CLDN2, claudin-2; CYP7A1, cholesterol 7α-hydroxylase; EGFR, epidermal growth factor receptor; LEPR, leptin receptor; NTCP, Na+-taurocholate cotransporter; OCLN, occludin; OSTα, organic solute and steroid transport; SHP, small heterodimer partner; SREBP2, sterol response element-binding protein 2; TGF-β, transforming growth factor-β; TGR5, Takeda G protein-coupled receptor 5; TLR4, Toll-like receptor 4.
Previous studies have shown that mRNA expressions of FXR, SHP, FGF19, and BSEP are regulated by macroautophagy in the liver, whereby an impairment of autophagic flux reduces FXR expression and, in turn, negatively affects BA metabolism (56). To investigate deficiencies in hepatic autophagy, we quantified macroautophagy-related proteins LC3 I/II and P62, and kinases that control the activation of autophagic pathway, including PKB and ERK1/2. We did not find differences between CON and HFF livers for any of the proteins analyzed (Fig. 5C). However, PKB phosphorylation showed an increasing trend between nonprobiotic and probiotic groups (P ≤ 0.1), and LC3II, P62, and ERK1/2 were significantly increased in probiotic-fed pigs (P ≤ 0.01; Fig. 5C). Since P62 and LC3II are degraded inside the lysosomes during autophagy, the increase in both proteins suggests an impairment in the hepatic autophagic flux by probiotics (121).
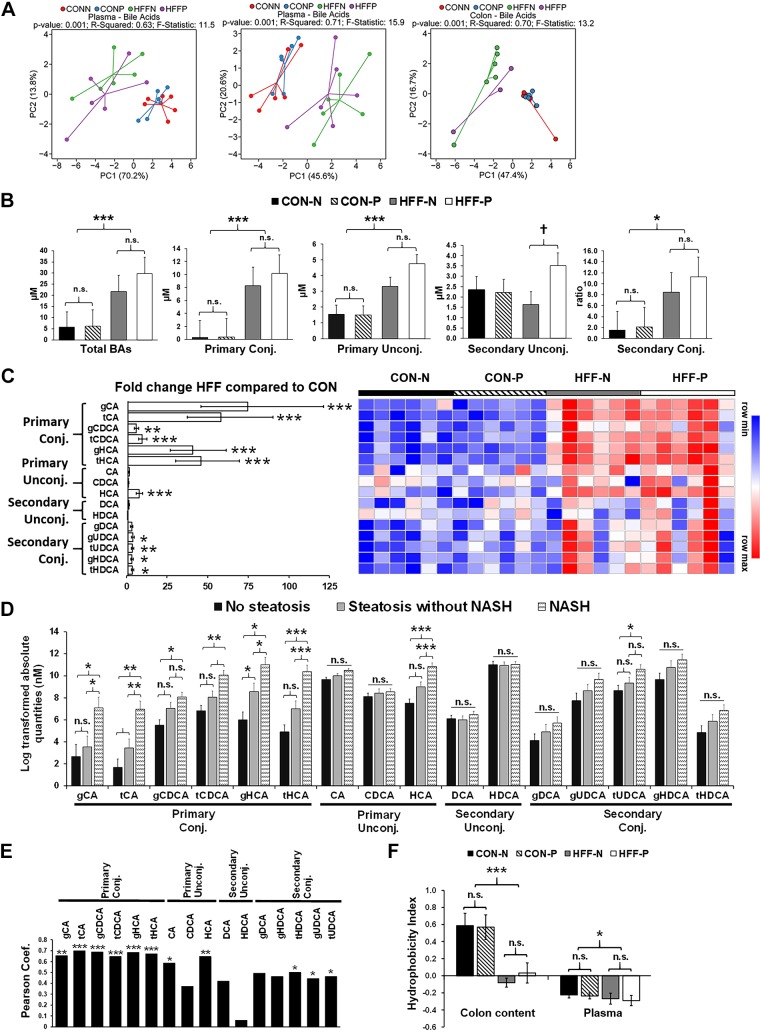
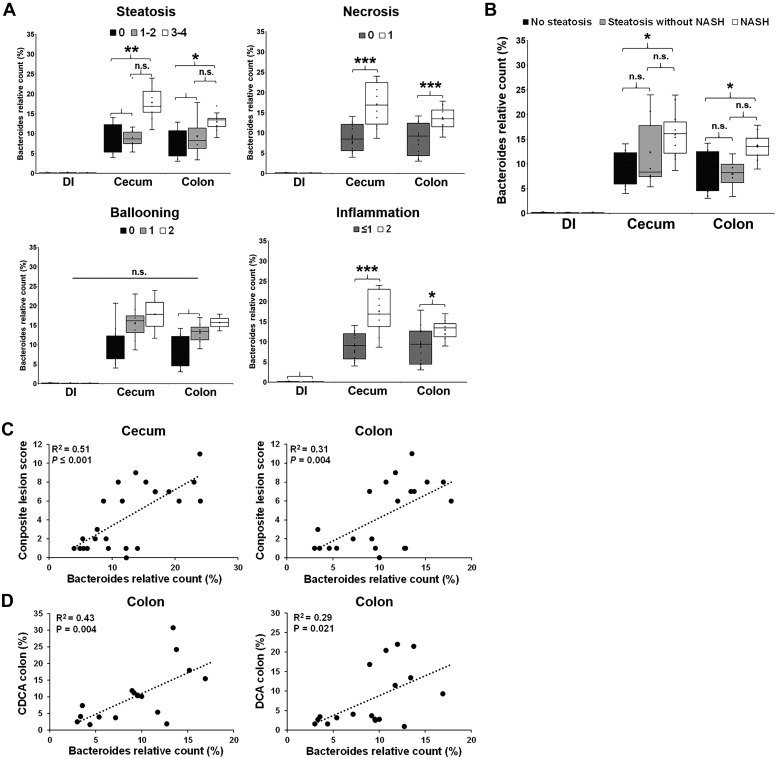
The severity of NAFLD was associated with increased BAs in blood and colon contents.
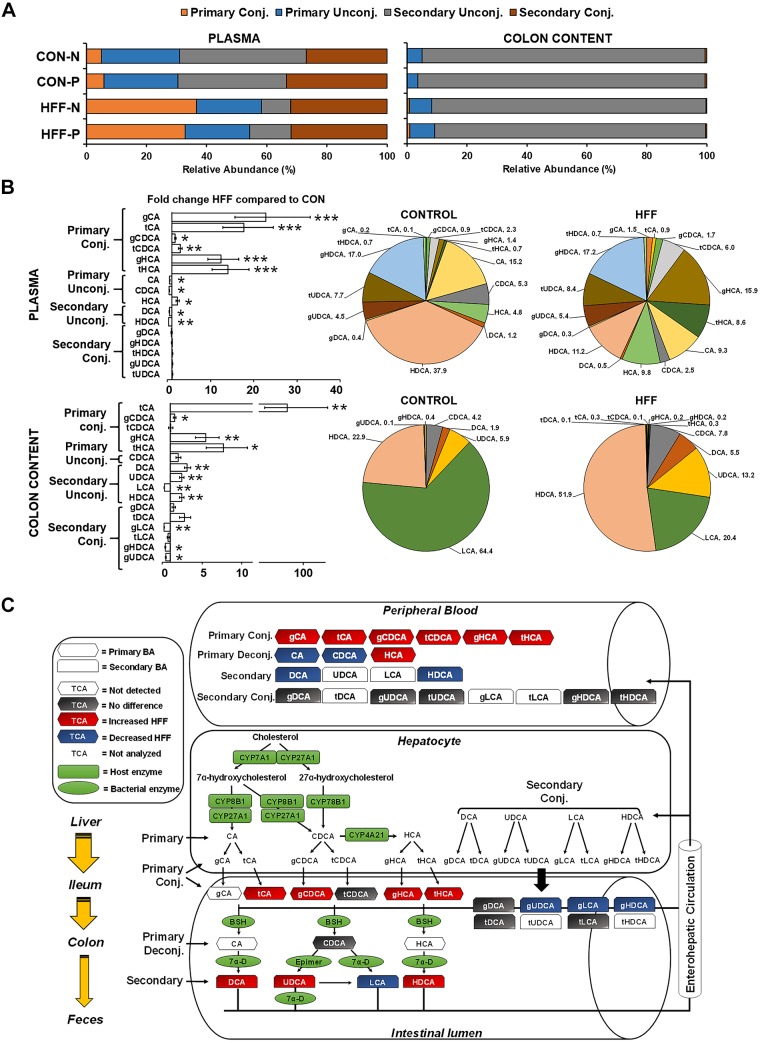
To assess whether the dysregulation of FXR-FGF19 signaling was associated with an increase in the circulating BA pool, we analyzed BAs in plasma using targeted metabolomics. Multivariate statistics revealed a significant effect of the diet (Fig. 6A), with an increase in total BAs (P ≤ 0.001) in the HFF group, driven mostly by primary conjugated and unconjugated BAs (P ≤ 0.001) and, to a lesser degree, by secondary conjugated BAs (P ≤ 0.05; Fig. 6, B and C). Probiotics did not separate samples in the PCA chart; however, secondary unconjugated BAs showed an increasing trend between HFF-N and HFF-P (P ≤ 0.1; Fig. 6B). Further analysis showed an increase in BAs in a stepwise manner from no steatosis to steatosis to NASH for tCA (P ≤ 0.01) and gHCA (P ≤ 0.05), whereas gCA and gCDCA (P ≤ 0.05), tCDCA (P ≤ 0.01), and tHCA (P ≤ 0.001) were higher in NASH compared with pigs without steatosis (Fig. 6D). In addition, most primary conjugated BAs (P ≤ 0.001), CAs (P ≤ 0.001), HCAs (P ≤ 0.001), and tHDCAs, gUDCAs, and tUDCAs (P ≤ 0.05) were positively correlated with the number of Ki67+ cells in the liver (Fig. 6E). Generally, the hepatotoxicity of BAs is related to their degree of hydrophobicity, in the order LCA > DCA > CDCA > CA > HDCA > HCA > UDCA, and unconjugated > conjugated (45). However, the HI in plasma was lower in HFF-fed pigs compared with CON (P ≤ 0.05; Fig. 6F), due to the increase in less hydrophobic species, such as UDCA and HCA, and a decrease in HDCA. BAs differ also in the potency to activate FXR (i.e., CDCA > DCA > LCA > CA) (75), so FXR signaling may depend not only on absolute levels of individual BAs, but also their relative abundance within the BA pool. Consistent with the absolute values, HFF diet increased the relative composition of primary conjugated BAs in plasma (P ≤ 0.05; Fig. 7A), whereas the percentage of most potent FXR agonists (i.e., CDCA and DCA) was lower in HFF-fed pigs compared with CON (P ≤ 0.05; Fig. 7, B and C). These findings suggest that primary conjugated BAs may play an important role in the development of NASH.
Fig. 6.
Plasma levels of primary bile acids (BAs) were increased in high-fructose, high-fat (HFF)-fed pigs and associated with the severity of liver disease. A: PCAs of absolute and relative abundance of plasma BAs, and relative abundance of colon content BAs discriminated HFF- and control (CON)-fed pigs along the first component. Data were scaled to unit variance before PCA assessment. Two-dimensional visualization of PCA scores are projected from their group centroid along components 1 and 2 (PC1 and PC2). P value, R2, and F statistic are derived from ANOVA assessed on the first principal component. Each point represents an individual pig, and color of point denotes diet. N, nonprobiotic; P, probiotic. B: total, primary, and secondary levels of BAs in plasma increased in HFF-fed pigs compared with CON. There was an increasing trend in secondary unconjugated BAs in response to probiotic supplementation. C: heat map of absolute abundance of BAs in plasma samples of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Columns are individual pigs, and rows are log2-transformed BA values. Blue and red represent the row minimum and maximum values, respectively. P values for each metabolite were calculated by a two-way ANOVA with a mixed model and adjusted for multiple testing with Benjamin-Hochberg procedure. Primary conjugated and unconjugated BAs increased in a stepwise manner from no steatosis to steatosis to nonalcoholic steatohepatitis (NASH) (D) and were positively correlated (Pearson) with the number of Ki67+ cells in liver tissue (E). F: hydrophobicity index decreased in colon digesta and plasma of HFF-fed pigs. Values in B, D, and F are least square means ± SE. P values were adjusted for multiple testing with Tukey’s post hoc test. †P ≤ 0.1, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., Nonsignificant. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; g, glycine; HCA hyocholic acid; HDCA, hyodeoxycholic; t, taurine; UDCA, ursodeoxycholic acid.
Fig. 7.
High-fructose, high-fat (HFF) diet modified the composition of the bile acid (BA) pool in plasma and colon content. A: the percentage of primary conjugated BAs increased, and secondary unconjugated BAs decreased in plasma and colon content of HFF-fed pigs. CON, control; N, nonprobiotic; P, probiotic. B: pie charts of relative abundance of individual BAs in plasma and colon content samples of 83-day-old pigs, with fold change and significance levels by HFF compared with control (CON). P values were adjusted for multiple testing with Benjamin-Hochberg procedure. C: mechanistic illustration of enterohepatic BA circulation, with red and blue indicating an increase and decrease, respectively, in relative abundance of individual BAs in HFF compared with CON. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. BSH, bile salt hydrolase; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; g, glycine; HCA hyocholic acid; HDCA, hyodeoxycholic; LCA, lithocholic acid; t, taurine; TCA, tricarboxylic acid; UDCA, ursodeoxycholic acid.
To investigate whether the increase in relative abundance of primary conjugated BAs in plasma was associated with a reduction in microbial deconjugating activity, we analyzed BAs in colonic digesta of HFF and CON pigs. Results are presented only as relative composition. Multivariate statistics revealed a significant effect of the diet but not a probiotic effect (Fig. 6A). HFF pigs had higher relative abundance of primary conjugated BAs in colon content, with a disproportionate increase in tCA (P ≤ 0.01). However, the percentage of unconjugated BA pool did not differ between groups, despite the increase in DCA, UDCA, and HDCA in HFF-fed pigs (P ≤ 0.01), due to the large decrease in LCA in HFF compared with CON group (P ≤ 0.01; Fig. 7, B and C). Interestingly, a decrease in fecal content of LCA was also found in NASH patients and associated with the severity of liver disease (68). In agreement with changes in BA levels, HI in colon digesta was significantly lower in HFF compared with CON (P ≤ 0.001; Fig. 6F). No differences were observed between CON-N and CON-P, or between HFF-N and HFF-P, animals for any of the unconjugated BAs, suggesting that the in vitro BSH activity of L. plantarum was not enough to alter the colonic BA profile or microbial BSH activity in the animals.
Taurine and PC-derived metabolites increased in colon content of HFF-fed pigs.
We next investigated changes in the composition of colonic digesta among treatments using untargeted metabolomic analysis. Multivariate statistics showed a separation between CON and HFF samples (Fig. 8A). Relative abundance of most metabolites decreased in HFF pigs (P ≤ 0.05; Fig. 8, B and C), suggesting an upregulation of enzymatic digestion and absorption to adapt to the high-fat content of the diet (9, 60, 76). Conversely, choline, glycerophosphocholine, and multiple PCs, LPCs, and LPEs were increased in HFF-fed pigs (P ≤ 0.01; Fig. 8, B and C). Given that choline content did not differ between CON and HFF diets, and bile delivers two to four times more PCs to the intestinal lumen than that supplied by the diet (16), the relative increase in colonic choline-containing metabolites in HFF-fed pigs may be derived from increased biliary secretion and subsequent phospholipid digestion in the gut. Taurine was also increased in HFF-fed pigs (P ≤ 0.05; Fig. 8B), indicative of a preferential synthesis of taurine-conjugated BAs in response to the HFF diet (29). Interestingly, when analyzed as a separate variable, we also found an increase in relative abundance of choline (P ≤ 0.01) and an increasing trend in taurine (P ≤ 0.1) in probiotic-fed pigs (Fig. 8D), suggesting an effect of probiotics in BA production and/or deconjugation, probably connected to their BSH activity (27, 57). Other metabolites increased in colon content of HFF-fed pigs were histamine (P ≤ 0.01) and several nucleotide monophosphates (P ≤ 0.05), implying intestinal inflammation (94) and cell sloughing (21).
Fig. 8.
Taurine and choline-derived phospholipids increased in colon content of high-fructose, high-fat (HFF)-fed pigs. A: PCAs of relative abundance of colon content metabolites discriminated HFF- and control (CON)-fed pigs along the first component. Data were scaled to unit variance before PCA assessment. Two-dimensional visualization of PCA scores are projected from their group centroid along components 1 and 2 (PC1 and PC2). N, nonprobiotic; P, probiotic. P value, R2, and F statistic are derived from ANOVA assessed on the first principal component. Each point represents an individual pig, and color of point denotes diet. B and C: heat map of relative abundance of metabolites significantly altered by the diet in colon digesta of 70-day-old pigs, with fold change and significance levels by HFF compared with CON. Columns are individual pigs, and rows are log2-transformed metabolite data. Blue and red represent the row minimum and maximum values, respectively. P values for each metabolite were calculated by a two-way ANOVA with a mixed model and adjusted for multiple testing with Benjamin-Hochberg procedure. D: analysis of choline as a separate variable showed a significant increase for both HFF and probiotic-fed pigs. There was also an increasing trend in taurine in response to probiotic supplementation, and both taurine and glycerophosphocholine increased in HFF compared with CON. Values are least square means ± SE. P values were adjusted for multiple testing with Tukey’s post hoc test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Colonic hyperplasia was correlated with BA levels and increased with the severity of liver disease.
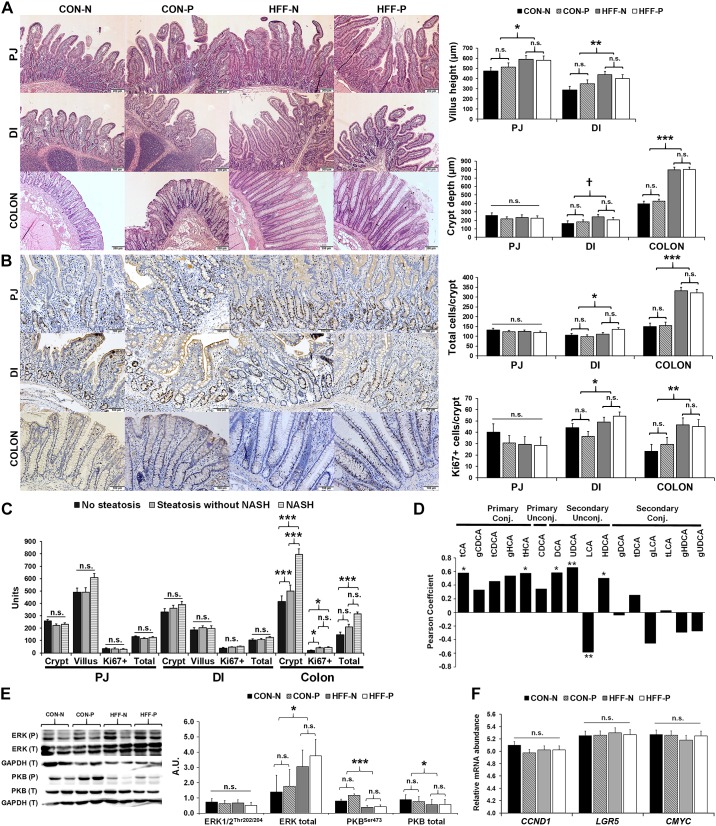
Secondary BAs (21, 102) and dietary fat (115) have been found to damage the colonic mucosa and increase intestinal permeability and inflammation, which in turn can expose the liver to gut-derived lipopolysaccharides and other bacterial endotoxins causing NASH (39, 51, 66). To investigate whether an increase in colonic secondary BAs and choline-containing phospholipids in HFF-fed pigs was associated with changes in the gut structure, we assessed the expression of proinflammatory cytokines and tight junction proteins by qPCR and histopathological changes in the intestinal mucosa. Probiotics did not have an effect on any of the parameters measured. Comparing CON and HFF groups with respect to gene expression, SHP (a marker of FXR activation) increased (P ≤ 0.01), whereas TNFA and TGFB decreased (P ≤ 0.01), and Toll-like receptor 4 (TLR4), had a decreasing trend (P ≤ 0.1), in HFF compared with CON (Fig. 5, B and D). Gene expression of tight junction proteins claudin-2 (CLDN2) and ocludin (OCLN) did not differ between groups. These results suggest the increase of FXR signaling in colon, which has been shown to stabilize the epithelium and decrease macrophage proinflammatory cytokine production (37, 97). Histological examination showed increased accumulation of lymphocytes and plasma cells in the lamina propria and submucosa of HFF compared with CON (P ≤ 0.05), but goblet cell counts per crypt did not differ between groups. We also found an increase in villi length in PJ (P ≤ 0.05) and DI (P ≤ 0.01), and over twofold increase in crypt depth in colon of HFF-fed pigs compared with CON (Fig. 9A; P ≤ 0.001), indicative of tissue regeneration as a result of chronic damage (21, 32). Active cell division was observed in all histological sections, as shown by the presence of crypt cell nuclei with clear brown staining (i.e., Ki67+ cells). Total crypt cell number and Ki67+ cells per crypt were higher in DI (P ≤ 0.05) and colon (P ≤ 0.001) of HFF-fed pigs compared with CON (Fig. 9B). Further analysis of intestinal histological features showed an increase in Ki67+ cells per crypt in DI (P ≤ 0.05), and an increase in crypt depth (P ≤ 0.001), Ki67+ cells per crypt (P ≤ 0.05), and total crypt cell number (P ≤ 0.001) in colon of NASH pigs compared with those without steatosis (Fig. 9C).
Fig. 9.
Cellular proliferation and hyperplasia increased in the intestinal mucosa of nonalcoholic steatohepatitis (NASH) pigs. A: photographs of hematoxylin-eosin staining of paraffin embedded sections showing an increase in villus height in proximal jejunum (PJ) and distal ileum (DI), and an increase in crypt depth in colon of high-fructose, high-fat (HFF)-fed pigs. B: active cell division, represented by an increase of crypt cell nuclei with clear brown staining (Ki67+ cells), was higher in DI and colon crypts of HFF-fed pigs. C: crypt depth (µm), number of Ki67+ cells, and total number of cells per crypt increased in NASH pigs compared with pigs without steatosis. D: primary conjugated and secondary unconjugated bile acids (BAs) were correlated (Pearson) with Ki67+ cells in colon crypts. E: representative Western blots (left) and histograms with the quantification of bands expressed as arbitrary units (A.U.; right), to measure the expression of proteins involved in BA-induced cell proliferation. Total extracellular signal-regulated kinase 1/2 (ERK1/2) increased, while phosphorylated (P) and total (T) protein kinase B (PKB) decreased in HFF group. F: gene expression of cyclin D1 (CCND1), leucine rich repeat containing G protein-coupled receptor 5 (LGR5), and master regulator of cell cycle entry (CMYC) did not differ between groups. Relative mRNA abundance was measured by qPCR, and reported as ΔCt values. All values are least square means ± SE. P values were adjusted for multiple testing with Tukey’s post hoc test. †P ≤ 0.1, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., Nonsignificant. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; g, glycine; HCA hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; t, taurine; UDCA, ursodeoxycholic acid.
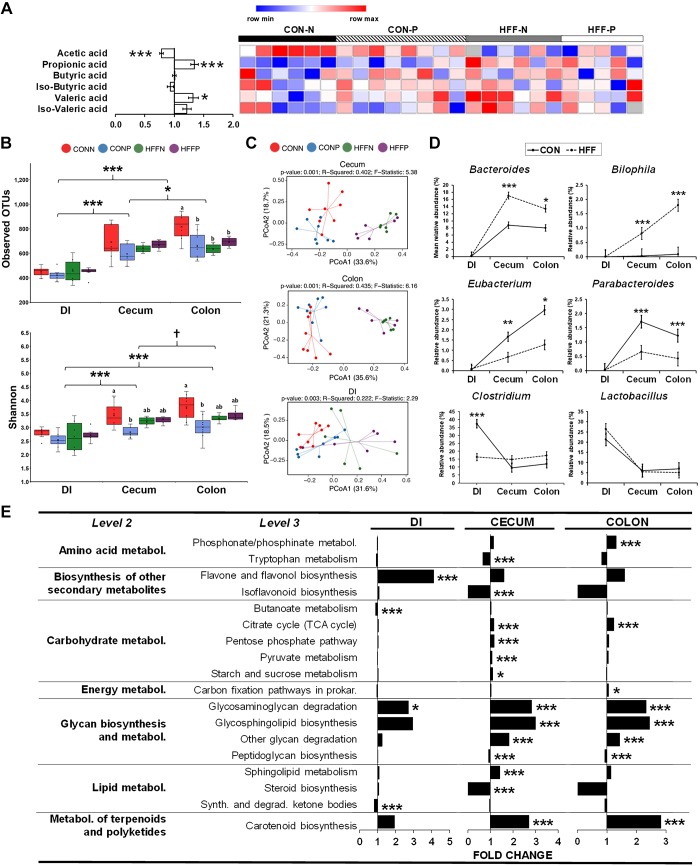
Because the number of Ki67+ colonocytes was positively correlated with both primary and secondary BAs in the colon content (P ≤ 0.05; Fig. 9D), we next examined the expression of receptors and protein kinases related to BA-induced cell proliferation in colon tissue (30). Gene expression of Takeda G protein-coupled receptor 5 (TGR5) and epidermal growth factor receptor (EGFR) did not differ between groups, and leptin receptor (LEPR) decreased in HFF compared with CON (P ≤ 0.001; Fig. 5, B and D). Additionally, quantification of ERK1/2 showed an increase in total protein expression in HFF (P ≤ 0.05), but not changes in phosphorylation, whereas total (P ≤ 0.05) and phosphorylation (P ≤ 0.001) levels of PKB decreased in HFF-fed pigs (Fig. 9E). To investigate the possibility that BAs upregulate intestinal stem cells (36), we performed qPCR for normal and cancer-related stem cell genes cyclin D1 (CCND1), leucine rich repeat containing G protein-coupled receptor 5 (LGR5), and master regulator of cell cycle entry (CMYC). However, we found no differences between the CON and HFF group (Fig. 9F). Interestingly, the expression of sterol regulatory element-binding protein 2 (SREBF2), which has been connected to lipid-induced tumor growth (117), was increased in HFF-fed pigs (P ≤ 0.05; Fig. 5, B and D). Given that SCFAs play a role in maintaining intestinal health by providing energy for colonocytes and exerting an anti-inflammatory effect (123), we also quantified acetate, propionate, butyrate, and valerate levels in cecum content by gas chromatography (Fig. 10A). Relative abundance of propionate (P ≤ 0.001) and valerate (P ≤ 0.05) increased, and acetate decreased (P ≤ 0.001) in HFF-fed pigs, whereas butyrate did not differ between groups. Because butyrate is the most potent pro-apoptotic and anti-inflammatory SCFA in the gut (46), colonic hyperplasia may not have been affected by changes in SCFAs.
Fig. 10.
High fructose, high fat (HFF) and probiotics reduced microbial diversity, but did not increase bile acid (BA)-deconjugating bacteria in cecum and colon. A: heat map of relative abundance of short-chain fatty acids (SCFAs) in colon content of 70-day-old pigs, with fold change and significance levels by HFF compared with control (CON). SCFAs were measured using gas chromatography analysis. Columns are individual pigs, and rows are log2-transformed SCFA values. Blue and red represent the row minimum and maximum values, respectively. B: observed operational taxonomic units (OTUs) and Shannon diversity index in cecum and colon decreased in CON probiotic (CON-P), HFF nonprobiotic (HFF-N), and HFF probiotic (HFF-P) compared with CON. Indexes were computed using the core_diversity_analyses.py script and a rarefaction of 50,793 OTUs. C: principal coordinate analyses ordination (PCoA) of Bray-Curtis dissimilarity matrix on OTUs from distal ileum (DI), cecum and colon. Each point represents an individual pig, and color of point denotes diet. Permutational multivariate ANOVA (PERMANOVA) results, including P value, R2, and F statistic were calculated using the compare_categories.py script available in Quantitative Insights into Microbial Ecology. D: relative counts of representative bile salt hydrolase (BSH+) genera across DI, cecum, and colon in HFF-fed pigs compared with CON. Data were analyzed by a two-way ANOVA that included diet × intestinal section as fixed effects, pen nested in diet as random effect, and a repeated measurement statement with intestinal section as repeated factor and pen nested in diet as subject. E: analysis of predicted Kyoto Encyclopedia of Genes and Genomes functional pathways showed a significant increase in level 3 categories related to carbohydrate, energy, and glycosphingolipids metabolism, and flavone and carotenoid biosynthesis in cecum and colon of HFF pigs. Values are least square means ± SE. P values were adjusted for multiple testing with Tukey’s post hoc test. Mean comparison between sections: †P ≤ 0.1, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 for; mean comparison within section: a,b P ≤ 0.05. TCA, tricarboxylic acid.
HFF and probiotics reduced microbial diversity but did not increase BA-converting bacteria in cecum and colon.
The composition of the gut microbiome determines the production of secondary BAs and, consequently, influences FXR-mediated signaling in the gut and the liver (114). To investigate whether HFF-fed pigs had an increase in intestinal bacteria with BSH capability, we performed the analysis of 16S rRNA and KEGG-predicted functional categories in DI, colon and cecum content, and correlated it with metabolomic data. A total of 4,406 OTUs were aggregated into 24 phyla and 263 genera. The number of OTUs increased in a stepwise manner from DI to cecum (P ≤ 0.001) to colon (P ≤ 0.05) and was higher in CON-N compared with CON-P, HFF-N, and HFF-P (P ≤ 0.05; Fig. 10B). Evenness was also higher in cecum and colon compared with DI (P ≤ 0.001), and increased in CON-N compared with CON-P (P ≤ 0.05; Fig. 10B). These results suggest a decrease in the hindgut microbial diversity connected to HFF and probiotics. Bacteria with BSH activity have been identified in genera belonging to the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria phyla (96); however, only Bacteroidetes was increased in cecum of HFF-fed pigs (P ≤ 0.05; Supplemental Fig. S5A; see https://doi.org/10.6084/m9.figshare.10293650). Microbial communities at genus level were separated in the PCoA charts and dendrogram between CON and HFF (Fig. 10C and Supplemental Fig. S5B). Among genera with BSH activity, Bacteroides increased in cecum (P ≤ 0.001) and colon (P ≤ 0.05) of HFF-fed pigs, whereas Clostridium in DI (P ≤ 0.001), and Eubacterium (P ≤ 0.05) and Parabacteroides (P ≤ 0.001) in colon and cecum, decreased in HFF compared with CON, and Lactobacillus did not differ between groups. (Fig. 10D and Supplemental Fig. S6; see https://doi.org/10.6084/m9.figshare.10293662). In addition, cecum and colon of HFF-fed pigs had higher relative abundance of Bilophila, which metabolizes taurine (P ≤ 0.001; Fig. 10D). These changes in bacterial populations agree with published data in NASH patients (8) and collectively suggest that the HFF diet did not cause an overall shift in microbiome toward BA metabolizing bacteria. Because Bacteroides has been associated with the severity of liver disease (8), we next investigated its relative abundance based on histological features of NASH. Relative counts of Bacteroides in cecum and colon increased with the grade of steatosis (P ≤ 0.01 and P ≤ 0.05), necrosis (P ≤ 0.001), and inflammation (P ≤ 0.001 and P ≤ 0.05) in the liver and were higher in NASH compared with pigs without steatosis (P ≤ 0.05; Fig. 11, A and B). In addition, the percentage of Bacteroides in colon and cecum was positively correlated with composite lesion score in the liver (P ≤ 0.01) and percentage of CDCA (P ≤ 0.01) and DCA (P ≤ 0.05) in colon (Fig. 11, C and D).
Fig. 11.
Bacteroides relative abundance was associated with the severity of liver disease. Relative counts of Bacteroides in cecum and colon increased with the grade of hepatic steatosis, necrosis and inflammation (A) and were higher in nonalcoholic steatohepatitis (NASH) compared with pigs without steatosis. No differences were observed in distal ileum (DI) across groups or severity of liver injury (B). The percentage of Bacteroides was positively correlated (Pearson) with hepatic composite lesion score in cecum and colon (C), and the percentage of chenodeoxycholic (CDCA) and deoxycholic acids (DCA) in colon (D). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., Nonsignificant. DI, distal ileum.
Predictive functional profiles of all intestinal sections determined that bacteria in HFF pigs might have enhanced carbohydrate metabolism in cecum (P ≤ 0.001) and carbon fixation in colon (P ≤ 0.05), suggesting an increase in energy harvesting potential (Fig. 10E). Differential representation of pathways related to lipid and glycan metabolism predicted an increase in sphingolipid metabolism in cecum (P ≤ 0.001), and glycosphingolipid biosynthesis and glycosaminoglycan degradation in both cecum and colon (P ≤ 0.001). Interestingly, sphingolipids are absent in most genera, but highly represented in Bacteroides, in which constitute essential molecular signals for bacteria survival in the intestine (3). The correlation analysis between 16S rRNA and colon metabolites revealed that HFF-induced changes in secondary BAs and choline-containing phospholipids were associated with specific compositional shifts of the gut microbiota (P ≤ 0.001; Fig. 12). Positive associations were identified between Olsenella, Synergistes, and Bulleida, and host TCA, DCA, glycerophosphocholine, PCs, and LPCs. Pyramidobacter was also positively correlated with glycerophosphocholine and negatively associated with multiple TAGs, sphingomyelins, and PCs that decreased in HFF. Similarly, Erysipelothrix, which has been associated with choline deficiency-induced fatty liver disease (98), was negatively correlated with TAGs decreased in HFF-fed pigs. Negative correlations with DCA, HDCA, PCs, LPCs, and LPEs were identified for Paludibacter, Sporobacter, Barnesiella, Turicibacter, and Eubacterium, whereas Mogibacterium, lower in HFF, was positively correlated with fats that decreased in HFF-fed pigs.
Fig. 12.
Bile acids and choline-containing phospholipids were correlated with microbiome changes in colon. Associations between identified colon content metabolites and colon bacterial genera altered by diet in 83-day-old pigs provided control nonprobiotic (CON-N), control probiotic (CON-P), high-fructose, high-fat nonprobiotic (HFF-N), and high-fructose, high-fat probiotic (HFF-P) diets. Network displays Spearman’s correlations between metabolites and genera with false discovery rate ≤ 0.001; orange and blue edges represent positive and negative correlations, respectively. Nodes shapes and colors: yellow circles represent metabolites; blue represent bile acids; green triangle represents short chain fatty acids. Rectangular nodes represent colon bacterial genera altered by diet. Genera and metabolites altered by diet were only considered for correlation network. Centered log ratio transformations were applied to relative abundance data before performing Spearman’s correlations. DCA, deoxycholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; TCA, tricarboxylic acid.
DISCUSSION
The prevalence of NAFLD in children has increased over the past decades (116), creating a need for animal models that recapitulate the features of the pediatric disease. The suitability of the Iberian breed as a translational model of pediatric NAFLD is supported by the findings in our study, in which over 80% of the HFF-fed pigs developed NASH at 12 wk of age. This study represents the earliest age of diet-induced NASH in pigs (Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.11594175) and occurred without evidence of obesity and insulin resistance. It is also notable that, unlike previous models of pediatric NASH, the pigs in the present study were fed a “Western-style diet” under controlled caloric intake conditions, and both CON and HFF diets were formulated to meet the choline and methionine requirements of the animals (Supplemental Table S4; see https://doi.org/10.6084/m9.figshare.10293677). In association with NASH, juvenile Iberian pigs developed cholestasis and dysregulation of FXR-FGF19 axis similar to that observed in child and adult NAFLD populations (50, 79). The mechanisms underlying the uncoupling of FXR-FGF19 signaling are not clear, but may involve changes in BA concentrations, phospholipids, and/or cholesterol in the liver and gut. In this regard, the secondary BA LCA, which was decreased in colon contents of HFF-fed pigs and in feces of NASH patients (68), has been shown to induce FGF19 expression by binding to the FGF19 promoter in intestinal cells independently of FXR (118). Consistent with previous studies, our findings also show that NASH was associated with cholestasis, based on the increase in plasma and hepatic levels of bilirubin and BAs and the downregulation of hepatic BSEP. In addition, the elevated concentrations of conjugated primary BA observed in plasma have been shown to promote hepatic stellate cell proliferation, inflammation, and apoptosis in previous studies (62, 103).
A key finding of this study is the dysregulation of enterohepatic BA metabolism in parallel to an increase in choline excretion in NASH pigs. Choline is an essential nutrient that is stored mostly as PCs in the liver, where they are required for the assembly/secretion of hepatic lipoproteins and solubilization of cholesterol and BAs in bile (17). Biliary PC secretion accounts for a large amount of choline expenditure, with an estimated daily amount equivalent to ∼80% of hepatic PC content (112). Dietary and biliary PCs are broken down by intestinal, pancreatic, and microbial phospholipases into LPCs and glycerophosphocholine, which are then absorbed by micelle-dependent diffusion in the duodenum and jejunum (17). Approximately 95% of biliary PCs are reabsorbed in the small intestine, but only ∼40% are returned to the liver (7, 59, 86), requiring a continuous supply of choline to maintain hepatic BA output (112). We postulate that a potential increase in bile secretory rate as a result of high-fat feeding (81, 109) and the lack of feed-back suppression of BA synthesis may have saturated the choline absorptive capacity of the intestine in HFF-fed pigs. In turn, the increased colonic excretion of choline-containing metabolites may have decreased levels of choline in blood and liver, shutting down hepatic lipoprotein production to maintain PC supply for biliary secretion. Thus our HFF-induced pediatric model results in choline depletion that likely contributes to disruption of hepatic fat metabolism and liver injury. This contrasts to the often-used mouse model of diet-induced choline deficiency that leads to NASH. Although a choline-deficient dietary model of NASH is not the best model to resemble the human condition, our finding of an apparent increase in colonic choline excretion provides a plausible explanation for the lack of positive effects of choline interventions in the treatment of pediatric NAFLD (124). Moreover, inclusion of choline supplements in high-fat diets may be detrimental for NAFLD children with impaired BA homeostasis, as they may increase the accumulation of choline-based phospholipids in the colon, thereby promoting trimethylamine synthesis (14, 15), which, in turn, can perpetuate the liver disease. Alternatively, dietary supplementation of betaine, which is absorbed by choline-independent mechanisms (20), has been shown to ameliorate NAFLD in previous clinical trials (1).
We observed a strong correlation between secondary BAs and Ki67+ cells in colon, as well as an increase in colonic hyperplasia in NASH pigs. Clinical studies have shown an increased risk of colorectal adenoma in patients with NAFLD, and colorectal cancer and NAFLD share the same risk factors, including intake of high-fat diets and elevated levels of serum and fecal BAs (47, 120). Nonetheless, conflicting results exist in the literature regarding the proinflammatory and proliferative roles of BAs in the intestinal mucosa (30, 36, 123), which may be related to their dual mechanism of action. Consistent with an increase in FXR signaling (37, 97, 111), we found the upregulation of SHP and decreased gene expression of proinflammatory markers in colon tissue of HFF-fed pigs, in the absence of changes in goblet cells and expression of tight junction proteins. Conversely, the accumulation of primary BAs in the intestinal lumen may have caused injury to the colonic mucosa, activating intestinal epithelial proliferation (21), and perhaps triggering intestinal permeability, which play an important role in NASH (39, 66). In assessing the etiology of intestinal hyperplasia, we cannot discount the effect of dietary fat (115) and fructose (41), as well as secondary metabolites produced as a result of the digestive activity of the host and microbiota. In this regard, the increase in concentration of phospholipids and primary BAs in the gut may have promoted the proliferation of Bacteroides, based on their ability to survive hostile environments by synthesizing sphingomyelins (3). Because Bacteroides was the second largest genera in the colon, its increase may explain the higher relative abundance of DCA and taurine, despite the fact that all other major BA producers were decreased or unaffected by the diet. In turn, an increase in taurine may have favored the growth of Bilophila, which, by producing hydrogen sulfide, can reduce disulfide bonds in the mucus layer of the gut epithelium, disrupting its barrier function and causing inflammation (29). Damage to the intestinal mucosa may also have been caused by the proliferation of Pyramidobacter and Synergistes, a group of bile-tolerant bacteria capable of hydrogen sulfide production and previously associated with colorectal cancer in humans, as well as by the decrease in Eubacterium, which produces butyrate with anti-inflammatory properties (80). Additionally, an increase in phospholipids and DCA may have affected negatively Barnesiella, which might in turn have detrimental health effects as this genus promotes pathogen-resistance colonization in the intestine (113). Although not related to gut integrity, proliferation of Olsenella may have increased the anaerobic catabolism of choline into trimethylamine and subsequent conversion to TMAO in the liver, which has been associated with NASH (14, 15).
The evidence that probiotics can decrease cholesterol levels has prompted a growing interest in their use as a therapeutic strategy to manage NAFLD (57). The hypocholesterolemic effect of probiotics is due in part to their BA deconjugating activity, which reduces cholesterol solubility and absorption in the gut, and increases cholesterol utilization in the liver to replace secondary BAs lost in feces (57). In this regard, probiotics decreased microbial diversity in the hindgut, and increased weight gain in the CON-P group, effects which have been connected to increased secondary BA production in antimicrobial-treated pigs (48). However, probiotic supplementation did not change colonic BA levels or BSH active genera compared with nonprobiotic groups, and increased both liver weight and hepatocellular degeneration in HFF-fed pigs. An increase in total adiposity and hepatic steatosis has also been observed in pediatric and adult populations in response to commercial probiotic VSL#3 (52, 95), whereas supplementation of Lactobacillus species in infant formula has been associated with weight gain in neonates (67). We also observed an increase in ERK1/2 activation and the impairment of autophagy in probiotic-fed pigs, which possibly contributed to the hyperinsulinemia in CON-P and HFF-P, given that insulin is degraded in hepatic autophagosomes (69), and sustained ERK1/2 signaling decreased autophagy in cell lines (19). While the increased excretion of choline may have amplified the hepatic injury in HFF-P pigs, we cannot discard a direct effect of probiotics in the liver, either by synthesis of bacterial antigens (78) or by translocation through the gut mucosa (61, 87). In this regard, Lactobacillus plantarum and VSL#3 have been shown to increase ERK1/2 signaling in intestinal cells and peritoneal macrophages in mice (22, 54). Furthermore, we identified genes encoding one copy of patatin-like phospholipase in P. acidilactici and B. subtilis, and one to two copies of lysophospholipases in L. plantarum and B. subtilis, suggesting the probiotic’s capability to break down membrane phospholipids, which has been associated with the translocation of Lactobacillus rhamnosus in mice (87).
In conclusion, our results in juvenile Iberian pigs show the earliest evidence of NASH induced by a high-fructose high-fat diet and provides a new pediatric model of NASH. This pig model of NASH is associated with dysregulation of FXR-FGF19 signaling that may have altered enterohepatic circulation of BAs and choline-containing phospholipids, leading to cholestasis, choline depletion and liver injury. Moreover, the HFF diet induced an increase secretion of biliary constituents, bile and phospholipids, that resulted in colonic cell proliferation. Given the strong association between impaired BA homeostasis and the severity of the liver disease, it is likely that interventions with FXR agonists tailored to NASH patients with lower FGF19 levels or increased fecal BA excretion may improve the therapeutic response.
GRANTS
This work was supported by California State University Agriculture Research Institute (Grants 58873 and 58913), CalPoly internal funding programs Baker/Koob, RSCA, and STRIDE, the US Department of Agriculture, Agricultural Research Service Grants 3092-51000-060-01 and 6026-51000-012-06S, the National Institutes of Health Grant DK-094616, and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338), BiOWiSH Technologies, Hilmar Ingredients and Acorn Seekers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.E., D.G.P., D.C.B., D.A.C., M.S.R., C.L.K., M.R.L.F., D.G.B., M. Maj, and R.M. conceived and designed research; G.V.H., V.A.S., M. Melnyk, M.A.B., K.A.S., M.S.E., D.C.B., R.K.F., C.I., T.M.S.-R., J.M.B., J.J.V., B.D.P., M.R.L.F., D.G.B., M. Maj, and R.M. performed experiments; G.V.H., V.A.S., M. Melnyk, R.K.F., D.A.C., J.P.S., H.G., C.I., T.M.S.-R., J.M.B., J.J.V., B.D.P., M.R.L.F., M. Maj, and R.M. analyzed data; G.V.H., V.A.S., M.A.B., K.A.S., M.S.E., D.G.P., D.C.B., R.K.F., D.A.C., H.G., C.I., M.S.R., T.M.S.-R., J.J.V., C.L.K., B.D.P., M.R.L.F., D.G.B., M. Maj, and R.M. interpreted results of experiments; G.V.H., V.A.S., T.M.S.-R., B.D.P., and R.M. prepared figures; G.V.H., V.A.S., and M. Melnyk drafted manuscript; M.A.B., K.A.S., D.G.P., D.C.B., R.K.F., D.A.C., J.P.S., H.G., C.I., M.S.R., T.M.S.-R., J.M.B., J.J.V., C.L.K., B.D.P., M.R.L.F., D.G.B., M. Maj, and R.M. edited and revised manuscript; G.V.H., V.A.S., M.A.B., K.A.S., M.S.E., D.G.P., D.C.B., R.K.F., D.A.C., J.P.S., H.G., C.I., M.S.R., T.M.S.-R., J.M.B., J.J.V., C.L.K., B.D.P., M.R.L.F., D.G.B., M. Maj, and R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Animal Science Department and Dr. Jaymie Noland for making available the Veterinary Clinic and Animal Facilities; Susan Tonik, Manuel Murga, Sergio Marsal, and Beth Reynolds for technical assistance; and Isabell Meyer, Christian Terkatz, Mieko Temple, Brooke Harbottle, Lauren Wienker, Baylee Wilhelmson, Sarah Bartak, Sarah Hershorin, Avery Emond, and Trista Noland for help during data collection.
REFERENCES
- 1.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 96: 2711–2717, 2001. doi: 10.1111/j.1572-0241.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- 2.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 39: 1276–1285, 2014. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci USA 108, Suppl 1: 4666–4671, 2011. doi: 10.1073/pnas.1001501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65: 350–362, 2017. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75, 2008. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 7.Borgström B. Phospholipid absorption. : Lipid Absorption: Biochemical and Clinical Aspects, edited by Rommel KG, Bohmer HR. London: MTP Press, 1976, p. 65–72. [Google Scholar]
- 8.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63: 764–775, 2016. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brannon PM. Adaptation of the exocrine pancreas to diet. Annu Rev Nutr 10: 85–105, 1990. doi: 10.1146/annurev.nu.10.070190.000505. [DOI] [PubMed] [Google Scholar]
- 10.Burson D. Procedures for Estimating Pork Carcass Composition (Online). https://alec.unl.edu/documents/cde/2017/livestock-management/2017-procedures-for-estimating-pork-carcass-composition-2006.pdf [1 Jan 2020].
- 11.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter RS, Huff EW, Kapur A. Compositions and Methods for Improving Human Health and Nutrition. United State Patent No. 10022409 (Online). https://patents.justia.com/patent/10022409 [1 Jan 2020].
- 13.Cast A, Kumbaji M, D’Souza A, Rodriguez K, Gupta A, Karns R, Timchenko L, Timchenko N. Liver proliferation is an essential driver of fibrosis in mouse models of nonalcoholic fatty liver disease. Hepatol Commun 3: 1036–1049, 2019. doi: 10.1002/hep4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 6: 19076, 2016. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chittim CL, Martínez Del Campo A, Balskus EP. Gut bacterial phospholipase Ds support disease-associated metabolism by generating choline. Nat Microbiol 4: 155–163, 2019. doi: 10.1038/s41564-018-0294-4. [DOI] [PubMed] [Google Scholar]
- 16.Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2: 116–127, 2010. doi: 10.3390/nu2020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Combs GF, McClung JP. Vitamin-like factors. : The Vitamins: Fundamental Aspects In Nutrition and Health (5th ed.). London: Elsevier/Academic Press, 2017, p. 453–460. [Google Scholar]
- 18.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 28: 159–165, 2012. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fénichel P, Hofman P, Poujeol P, Mograbi B. Control of the autophagy maturation step by the MAPK ERK and p38: lessons from environmental carcinogens. Autophagy 3: 57–59, 2007. doi: 10.4161/auto.3424. [DOI] [PubMed] [Google Scholar]
- 20.Craig SA. Betaine in human nutrition. Am J Clin Nutr 80: 539–549, 2004. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 21.Craven PA, Pfanstiel J, Saito R, DeRubertis FR. Relationship between loss of rat colonic surface epithelium induced by deoxycholate and initiation of the subsequent proliferative response. Cancer Res 46: 5754–5759, 1986. [PubMed] [Google Scholar]
- 22.Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med 29: 202–208, 2012. doi: 10.3892/ijmm.2011.839. [DOI] [PubMed] [Google Scholar]
- 23.Dashkevicz MP, Feighner SD. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol 55: 11–16, 1989. doi: 10.1128/AEM.55.1.11-16.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res 50: 2340–2357, 2009. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 26.De Blas C, Gasa J, Mateos GG. Necesidades Nutricionales Para Ganado Porcino: Normas FEDNA (Online). Universidad Politécnica de Madrid and Universidad Autónoma de Barcelona; http://www.fundacionfedna.org/sites/default/files/Normas%20PORCINO_2013rev2.pdf [8 Jan 2020]. [Google Scholar]
- 27.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 7: 12–18, 2014. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 28.Dela Cruz AC, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, Mills PR, Dam-Larsen S, Bjornsson ES, Haflidadottir S, Adams LA, Bendtsen F, Angulo P. Characteristics and long‐term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology 146, Suppl 1: S909, 2014. doi: 10.1016/S0016-5085(14)63307-2. [DOI] [Google Scholar]
- 29.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dossa AY, Escobar O, Golden J, Frey MR, Ford HR, Gayer CP. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol 310: G81–G92, 2016. doi: 10.1152/ajpgi.00065.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Y, Zhang F, Yuan W, Wei Y, Wei M, Zhou Y, Yang Y, Chang Y, Wu X. Hepatic cholesterol accumulation ascribed to the activation of ileum Fxr-Fgf15 pathway inhibiting hepatic Cyp7a1 in high-fat diet-induced obesity rats. Life Sci 232: 116638, 2019. doi: 10.1016/j.lfs.2019.116638. [DOI] [PubMed] [Google Scholar]
- 32.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kühl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 7: 4557–4576, 2014. [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Fígares I, Lachica M, Nieto R, Rivera-Ferre MG, Aguilera JF. Serum profile of metabolites and hormones in obese (Iberian) and lean (Landrace) growing gilts fed balanced or lysine deficient diets. Livest Sci 110: 73–81, 2007. doi: 10.1016/j.livsci.2006.10.002. [DOI] [Google Scholar]
- 34.Figueiredo IL, Claus T, Oliveira Santos Júnior O, Almeida VC, Magon T, Visentainer JV. Fast derivatization of fatty acids in different meat samples for gas chromatography analysis. J Chromatogr A 1456: 235–241, 2016. doi: 10.1016/j.chroma.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich D, Marschall HU, Lammert F. Response of fibroblast growth factor 19 and bile acid synthesis after a body weight-adjusted oral fat tolerance test in overweight and obese NAFLD patients: a non-randomized controlled pilot trial. BMC Gastroenterol 18: 76, 2018. doi: 10.1186/s12876-018-0805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, Zhu Q, Zhang T, Leblanc M, Liu S, He M, Waizenegger W, Gasser E, Schnabl B, Atkins AR, Yu RT, Knight R, Liddle C, Downes M, Evans RM. FXR regulates intestinal cancer stem cell proliferation. Cell 176: 1098–1112.e18, 2019. doi: 10.1016/j.cell.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60: 463–472, 2011. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 38.García-Villalba R, Giménez-Bastida JA, García-Conesa MT, Tomás-Barberán FA, Carlos Espín J, Larrosa M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J Sep Sci 35: 1906–1913, 2012. doi: 10.1002/jssc.201101121. [DOI] [PubMed] [Google Scholar]
- 39.Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis 46: 556–560, 2014. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Gloor GB, Reid G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can J Microbiol 62: 692–703, 2016. doi: 10.1139/cjm-2015-0821. [DOI] [PubMed] [Google Scholar]
- 41.Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, Pauli C, Morris R, Taylor S, Bosch K, Yang S, Wang Y, Van Riper J, Lekaye HC, Roper J, Kim Y, Chen Q, Gross SS, Rhee KY, Cantley LC, Yun J. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363: 1345–1349, 2019. doi: 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrell FE, Dupont C. Hmisc: Harrell Miscellaneous. R package version 4.2–0. 2019 (Online). https://cran.r-project.org/web/packages/misc/index.html [13 Jan 2020].
- 44.Hastie T, Tibshirani R, Narasimhan B, Chu G. Imputation for Microarray Data. R Package, Version 1.58.0. Bioconductor, 2019. doi: 10.18129/B9.bioc.impute. [DOI] [Google Scholar]
- 45.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res 30: 719–730, 1989. [PubMed] [Google Scholar]
- 46.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr 132: 1012–1017, 2002. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 47.Hwang ST, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Won KH, Jin W. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 25: 562–567, 2010. doi: 10.1111/j.1440-1746.2009.06117.x. [DOI] [PubMed] [Google Scholar]
- 48.Ipharraguerre IR, Pastor JJ, Gavaldà-Navarro A, Villarroya F, Mereu A. Antimicrobial promotion of pig growth is associated with tissue-specific remodeling of bile acid signature and signaling. Sci Rep 8: 13671, 2018. doi: 10.1038/s41598-018-32107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itagaki H, Shimizu K, Morikawa S, Ogawa K, Ezaki T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp Pathol 6: 2683–2696, 2013. [PMC free article] [PubMed] [Google Scholar]
- 50.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67: 1881–1891, 2018. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 51.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int J Hepatol 2014: 560620, 2014. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones RB, Alderete TL, Martin AA, Geary BA, Hwang DH, Palmer SL, Goran MI. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatr Obes 13: 705–714, 2018. doi: 10.1111/ijpo.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jouihan H. Measurement of liver triglyceride content. Bio Protoc 2: e223, 2012. doi: 10.21769/BioProtoc.223. [DOI] [Google Scholar]
- 54.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol 184: 3505–3513, 2010. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- 55.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30, 2000. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khambu B, Li T, Yan S, Yu C, Chen X, Goheen M, Li Y, Lin J, Cummings OW, Lee YA, Friedman S, Dong Z, Feng GS, Wu S, Yin XM. Hepatic autophagy deficiency compromises farnesoid x receptor functionality and causes cholestatic injury. Hepatology 69: 2196–2213, 2019. doi: 10.1002/hep.30407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res 2012: 902917, 2012. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31: 814–821, 2013. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50: 56–67, 2009. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Agellon LB, Vance DE. Phosphatidylcholine homeostasis and liver failure. J Biol Chem 280: 37798–37802, 2005. doi: 10.1074/jbc.M508575200. [DOI] [PubMed] [Google Scholar]
- 60.Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci Rep 6: 21618, 2016. doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liong MT. Safety of probiotics: translocation and infection. Nutr Rev 66: 192–202, 2008. doi: 10.1111/j.1753-4887.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z, Zhang Z, Huang M, Sun X, Liu B, Guo Q, Chang Q, Duan Z. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol 18: 112, 2018. doi: 10.1186/s12876-018-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 64.Margariti A, Deutsch M, Manolakopoulos S, Tiniakos D, Papatheodoridis GV. The severity of histologic liver lesions is independent of body mass index in patients with nonalcoholic fatty liver disease. J Clin Gastroenterol 47: 280–286, 2013. doi: 10.1097/MCG.0b013e31826be328. [DOI] [PubMed] [Google Scholar]
- 65.Mayer JJ, Brisbin IL. Wild Pigs of the United States: Their History, Morphology, and Current Status. Athens, GA: University of Georgia Press, 1991, p. 313. [Google Scholar]
- 66.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49: 1877–1887, 2009. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 67.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog 53: 100–108, 2012. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One 11: e0151829, 2016. doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda) 34: 198–215, 2019. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Research Council Nutrient Requirements of Swine (10th ed.). Washington, DC: National Academy Press, 1998, p. 110–123. [Google Scholar]
- 71.NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 44, D1: D7–D19, 2016. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 5: 3481–3495, 2013. doi: 10.3390/nu5093481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Óvilo C, Fernández A, Noguera JL, Barragán C, Letón R, Rodríguez C, Mercadé A, Alves E, Folch JM, Varona L, Toro M. Fine mapping of porcine chromosome 6 QTL and LEPR effects on body composition in multiple generations of an Iberian by Landrace intercross. Genet Res 85: 57–67, 2005. doi: 10.1017/S0016672305007330. [DOI] [PubMed] [Google Scholar]
- 74.Panasevich MR, Meers GM, Linden MA, Booth FW, Perfield JW II, Fritsche KL, Wankhade UD, Chintapalli SV, Shankar K, Ibdah JA, Rector RS. High-fat, high-fructose, high-cholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am J Physiol Endocrinol Metab 314: E78–E92, 2018. doi: 10.1152/ajpendo.00015.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 76.Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48: 278–287, 2007. doi: 10.1194/jlr.M600283-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Piepho HP. Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101: 865–869, 2009. doi: 10.2134/agronj2008.0226x. [DOI] [Google Scholar]
- 78.Plantinga TS, van Maren WW, van Bergenhenegouwen J, Hameetman M, Nierkens S, Jacobs C, de Jong DJ, Joosten LA, van’t Land B, Garssen J, Adema GJ, Netea MG. Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin Vaccine Immunol 18: 621–628, 2011. doi: 10.1128/CVI.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67: 534–548, 2018. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38: 996–1047, 2014. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redinger RN, Hermann AH, Small DM. Primate biliary physiology. X. Effects of diet and fasting on biliary lipid secretion and relative composition and bile salt metabolism in the rhesus monkey. Gastroenterology 64: 610–621, 1973. doi: 10.1016/S0016-5085(73)80133-7. [DOI] [PubMed] [Google Scholar]
- 82.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121: 1402–1411, 2011. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 40: 47–51, 2004. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res 49: 1068–1076, 2008. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res 47: 2280–2290, 2006. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Robins SJ. Recirculation and reutilization of micellar bile lecithin. Am J Physiol 229: 598–602, 1975. doi: 10.1152/ajplegacy.1975.229.3.598. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez AV, Baigorí MD, Alvarez S, Castro GR, Oliver G. Phosphatidylinositol-specific phospholipase C activity in Lactobacillus rhamnosus with capacity to translocate. FEMS Microbiol Lett 204: 33–38, 2001. doi: 10.1111/j.1574-6968.2001.tb10858.x. [DOI] [PubMed] [Google Scholar]
- 88.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 54: 344–353, 2011. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr 143: 500–505, 2003. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 91.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 118: 1388–1393, 2006. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 92.Sha W, da Costa KA, Fischer LM, Milburn MV, Lawton KA, Berger A, Jia W, Zeisel SH. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J 24: 2962–2975, 2010. doi: 10.1096/fj.09-154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smolinska S, Jutel M, Crameri R, O’Mahony L. Histamine and gut mucosal immune regulation. Allergy 69: 273–281, 2014. doi: 10.1111/all.12330. [DOI] [PubMed] [Google Scholar]
- 95.Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol 42: 1117–1119, 2008. doi: 10.1097/MCG.0b013e31816d920c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, Kalavagunta PK, Liao J, Jin L, Shang J, Li J. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 7: 9, 2019. doi: 10.1186/s40168-019-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, Moghadamrad S, Hassan M, Albillos A, Francés R, Juanola O, Spadoni I, Rescigno M, Wiest R. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol 71: 1126–1140, 2019. doi: 10.1016/j.jhep.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140: 976–986, 2011. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr 138: 397–402, 2008. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 100.Srere PA. Citrate synthase. Methods Enzymol 13: 3–11, 1969. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- 102.Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 304: G227–G234, 2013. doi: 10.1152/ajpgi.00267.2012. [DOI] [PubMed] [Google Scholar]
- 103.Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, Jansen PL, Candelaresi C, Benedetti A, Moshage H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology 128: 1042–1055, 2005. doi: 10.1053/j.gastro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 104.Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 49: 344–356, 2012. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 56: 118–129, 2012. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torres-Rovira L, Astiz S, Caro A, Lopez-Bote C, Ovilo C, Pallares P, Perez-Solana ML, Sanchez-Sanchez R, Gonzalez-Bulnes A. Diet-induced swine model with obesity/leptin resistance for the study of metabolic syndrome and type 2 diabetes. ScientificWorldJournal 2012: 510149, 2012. doi: 10.1100/2012/510149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, Tworoger SS, Wolpin BM. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 59: 1657–1667, 2013. doi: 10.1373/clinchem.2012.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics 17: 520–525, 2001. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 109.Tuchweber B, Yousef IM, Ferland G, Perea A. Nutrition and bile formation. Nutr Res 16: 1041–1080, 1996. doi: 10.1016/0271-5317(96)00104-2. [DOI] [Google Scholar]
- 110.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol 185: 409–419, 2015. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Verkade HJ, Havinga R, Shields DJ, Wolters H, Bloks VW, Kuipers F, Vance DE, Agellon LB. The phosphatidylethanolamine N-methyltransferase pathway is quantitatively not essential for biliary phosphatidylcholine secretion. J Lipid Res 48: 2058–2064, 2007. doi: 10.1194/jlr.M700278-JLR200. [DOI] [PubMed] [Google Scholar]
- 113.Volynets V, Küper MA, Strahl S, Maier IB, Spruss A, Wagnerberger S, Königsrainer A, Bischoff SC, Bergheim I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig Dis Sci 57: 1932–1941, 2012. doi: 10.1007/s10620-012-2112-9. [DOI] [PubMed] [Google Scholar]
- 114.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24: 41–50, 2016. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 115.Wargovich MJ, Eng VW, Newmark HL. Calcium inhibits the damaging and compensatory proliferative effects of fatty acids on mouse colon epithelium. Cancer Lett 23: 253–258, 1984. doi: 10.1016/0304-3835(84)90091-0. [DOI] [PubMed] [Google Scholar]
- 116.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr 162: 496–500.e1, 2013. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wen YA, Xiong X, Zaytseva YY, Napier DL, Vallee E, Li AT, Wang C, Weiss HL, Evers BM, Gao T. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death Dis 9: 265, 2018. doi: 10.1038/s41419-018-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wistuba W, Gnewuch C, Liebisch G, Schmitz G, Langmann T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J Gastroenterol 13: 4230–4235, 2007. doi: 10.3748/wjg.v13.i31.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J, Geng Y. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep 7: 45176, 2017. doi: 10.1038/srep45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang YJ, Bang CS, Shin SP, Baik GH. Clinical impact of non-alcoholic fatty liver disease on the occurrence of colorectal neoplasm: Propensity score matching analysis. PLoS One 12: e0182014, 2017. doi: 10.1371/journal.pone.0182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci 18: 1865, 2017. doi: 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64: 1577–1586, 2016. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 123.Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci 20: 1214, 2019. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zöhrer E, Alisi A, Jahnel J, Mosca A, Della Corte C, Crudele A, Fauler G, Nobili V. Efficacy of docosahexaenoic acid-choline-vitamin E in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab 42: 948–954, 2017. doi: 10.1139/apnm-2016-0689. [DOI] [PubMed] [Google Scholar]