Abstract
Klotho- and beclin 1-driven autophagy extends life. We examined the role of beclin 1 in modifying acute kidney injury (AKI) and whether beclin 1 mediates Klotho’s known renoprotective action in AKI. AKI was induced by ischemia-reperfusion injury in mice with different levels of autophagy activity by genetic manipulation: wild-type (WT) mice with normal beclin 1 expression and function, mice with normal beclin 1 levels but high activity through knockin of gain-of-function mutant beclin 1 (Becn1F121A), mice with low beclin 1 levels and activity caused by heterozygous global deletion of beclin 1 (Becn1+/−), or mice with extremely low beclin 1 activity from knockin of the mutant constitutively active beclin 1 inhibitor Bcl-2 (Bcl2AAA). Klotho was increased by transgenic overexpression (Tg-Kl) or recombinant Klotho protein administration. After ischemia-reperfusion injury, Becn1F121A mice (high autophagy) had milder AKI and Becn1+/− and Bcl2AAA mice (low autophagy) had more severe AKI than WT mice. Tg-Kl mice had milder AKI, but its renoprotection was partially attenuated in Becn1+/−;Tg-Kl mice and was significantly reduced, although not completely abolished, in Bcl2AAA;Tg-Kl mice. Recombinant Klotho protein conferred more renoprotection from AKI in WT mice than in Becn1+/− or Bcl2AAA mice. Klotho reduced beclin 1/Bcl-2 protein complexes and increased autophagy activity, but this effect was less prominent in mice or cells with Bcl2AAA. Transfected Bcl2AAA or Becn1F123A decreased or increased autophagy activity and rendered cells more susceptible or more resistant to oxidative cytotoxicity, respectively. In conclusion, beclin 1 confers renoprotection by activating autophagy. Klotho protects the kidney partially via disruption of beclin 1/Bcl-2 interactions and enhancement of autophagy activity.
Keywords: acute kidney injury, autophagy, Bcl-2, beclin 1, ischemia-reperfusion, Klotho
INTRODUCTION
Acute kidney injury (AKI) is a syndrome with sudden loss of renal function after exposure to renal insults. The failure to maintain homeostasis causes the accumulation of waste products (1, 9, 76) and damages remote organs such as the brain, heart, and lungs (26, 57). Patients with AKI have high short-term and long-term morbidity and mortality (1, 22, 44). Even when they survive and recover from AKI, the long-term outcome is far from benign, with substantial risk of progression to chronic kidney disease (CKD) and end-stage renal disease (6, 9, 21). The poor short-term and long-term outcomes are partially caused by lack of efficient and/or specific therapy.
Klotho was originally identified as, and proposed to be, an aging suppressor (41). Transmembrane Klotho is a coreceptor for the mineral-regulating hormone fibroblast growth factor (FGF)23 (42, 74). The ectodomain of Klotho protein is shed by a disintegrin and metalloproteinase domain-containing protein (ADAM)10/17 into the circulation (10, 31) and exerts both FGF23-dependent and -independent actions (4, 11, 35, 51, 55). We have previously shown that Klotho protects the kidney against both ischemic and nephrotoxic injury (33, 59, 68, 70) and the AKI-to-CKD progression (30, 68). We have recently shown that Klotho upregulates autophagy activity in the kidney (68), but whether Klotho’s actions on autophagy mediate its renoprotective effect has not been elucidated.
Autophagy is an evolutionarily conserved degradation system beneficial for all cells (19, 40). Perturbed autophagy renders cells more susceptible to noxious stimuli and accelerates aging (20, 34). Normal autophagy activity in the kidney protects the kidney from several renal insults (23, 47, 50, 53, 63), although this notion has been debated for long time (12). Beclin 1 protein encoded by the Becn1 gene is a central regulator of autophagy in mammalian cells (64). Beclin 1 acts at the initiation stage of autophagy as part of a lipid kinase complex that stimulates the formation of the isolation membrane, a double-membrane structure that engulfs cytoplasmic material to form autophagosomes. Normal levels and normal function of beclin 1 are requisite for diverse biological processes including immunity, development, tumor suppression, lifespan extension, and protection against certain tissue degeneration and damage induced by a variety of insults, including AKI (7, 25, 37, 38, 45). However, a recent study has shown that beclin 1 haplodeficiency reduced kidney injury superimposed on a murine chronic obstructive lung disease model, which questions the protective role of autophagy activity in kidney injury (58). There is a need to definitely study the effect of high and low beclin 1 activity on kidney damage.
Bcl-2 is a negative regulator of beclin 1 and acts by directly binding to beclin 1 and inhibiting its activity (24, 61). To explore the role of the beclin 1/Bcl-2 complex in the modulation of the kidney’s susceptibility to injury, genetically manipulated mouse lines were used (Table 1). Global knockin of a substitution mutation (F121A) in the BH3 domain of mouse becn1 (Becn1F121A, BK) confers gain of function of beclin 1 (66) by disrupting binding of beclin 1 to its negative regulator Bcl-2 and consequently upregulating autophagy activity without alteration of beclin 1 protein levels in multiple mouse tissues (24, 62, 66, 75). The F121A mutation increases lifespan and rescues the short life in an Alzheimer’s disease model (66) and in Klotho hypomorphic mice (20).
Table 1.
Characterization of autophagy activities in different mouse lines
| Mouse Lines |
||||
|---|---|---|---|---|
| Bcl2AAA | Becn1+/− | WT | Becn1F121A | |
| Genetic background | ||||
| Before cross-mating | 129x1; C57BL/6J hybrid | C57BL/6J | 129sv | 129sv; C57BL/6J hybrid |
| After >10 generations of cross-mating with WT 129sv mice | 129sv; 129x1; C57BL/6J hybrid | 129sv; C57BL/6J hybrid | 129sv | 129sv; C57BL/6J hybrid |
| Autophagy activity | ||||
| Baseline | Normal | ↓ | Normal | ↑ |
| After IRI vs. the response to IRI in WT mice | ↓ | ↓ | ↑ | ↑ |
| Beclin 1 protein expression | Normal | ↓ | Normal | Normal |
| Beclin 1/Bcl-2 complex after IRI | ↓ | ↓ | ↑ | ↑↑ |
IRI, ischemia-reperfusion injury; WT, wild-type; ↓, decrease; ↑, increase.
Another mouse line has global knockin of mutant Bcl-2 lacking three phosphorylation sites (Bcl2AAA), which leads to sustained formation of the beclin 1/Bcl-2 complex blocking the beclin 1 response to autophagy induction (20, 24). Interestingly, Bcl2AAA mice have normal autophagy activity at baseline but fail to respond to autophagy stimulator-induced disruption of the beclin 1/Bcl-2 complex (20, 24).
Finally, to examine the role of beclin 1 in ischemia-reperfusion injury (IRI)-induced AKI, we used a heterozygous global Becn1 haploinsufficient (Becn1+/−) mouse line with normal beclin 1 function but reduced beclin 1 levels (64), and we compared the severity of kidney damage induced by IRI and the renoprotection by modulation of autophagy in all three mouse lines. Furthermore, we explored if Klotho protection against IRI-induced AKI is associated with and mediated by modulation of beclin 1/Bcl-2 complex-dependent autophagy activity.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used for immunoblot analysis and/or immunohistochemistry: mouse monoclonal antibody against β-actin (Sigma-Aldrich, St. Louis, MO), rabbit polyclonal anti-active caspase-3 (Sigma-Aldrich), mouse monoclonal anti-beclin 1 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-Bcl-2 (Santa Cruz Biotechnology), rat monoclonal anti-Klotho antibody (KM2076, TransGenics, Kobe, Japan), rabbit polyclonal anti-light chain (LC)3 antibody (Novus Biologicals, Littleton, CO), goat polyclonal anti-neutrophil gelatinase-associated lipocalin (NGAL) antibody (R&D Research, Minneapolis, MN), and mouse monoclonal anti-p62/ sequestosome-1 (SQSTM1) antibody (Novus Biologicals). Secondary antibodies coupled to horseradish peroxidase for immunoblot analysis or to FITC, Alexa Fluor Cy5, and SYTO 61 far red fluorescent nuclear acid for immunohistochemistry were purchased from Molecular Probes/Invitrogen (Molecular Probes, Eugene, OR).
Cell culture and plasmids.
A subtype of the opossum kidney cell line (OKP; a proximal tubule cell line with parathyroid hormone receptor) was a gift from Dr. Judith Cole (University of Memphis, Memphis, TN). OKP cells were maintained in high-glucose DMEM as previously described (5, 68). The rationale for utilization of this cell line is that these cells are well characterized and widely used for the study of physiology and pathophysiology of proximal tubules (5, 68). In vitro studies were conducted in at least four independent experiments. Each experiment was performed in triplicate for each experimental condition.
OKP cells were treated with H2O2 (200 nM) and/or recombinant Klotho (400 pM) for 24 h. Cell lysates were subjected to immunoblot analysis (5, 68). To assess autophagy activity, a green fluorescent protein (GFP)-LC3 fusion plasmid kindly provided by Dr. Noboru Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) was transiently transfected into OKP cells (Lipofectamine 2000, Invitrogen, Carlsbad, CA). One day after transfection, cells were treated with H2O2 and/or recombinant Klotho protein for 24 h followed by immunocytochemistry to determine autophagy activity. Cells were fixed and stained with SYTO 61 (Life Technologies, Eugene, OR). The number of LC3 puncta was quantified with confocal microscopy by counting at least 50 cells/sample (5, 68). Cell culture media were harvested for measurements of lactate dehydrogenase (LDH) release, a cell injury marker, with the LDH Cytotoxicity Detection Kit (TaKaRa Bio, Mountain View, CA) following established protocols (5).
Additional plasmids including human wild-type (WT) and mutant beclin 1F123A (61) and mouse WT and triple mutant Bcl-2 at T69A, S70A, and S87A (AAA) (61, 79), which were kindly provided by Dr. Beth Levine (University of Texas Southwestern Medical Center), were transiently transfected into OKP cells to evaluate autophagy activity and cell susceptibility to H2O2.
Mice and genotyping.
All animal work was conducted strictly following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. WT 129 S1/SVlmJ (129sv) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Transgenic GFP-LC3 reporter mice (C57BL/6J genetic background) (68) were kindly provided by Dr. Noboru Mizushima (Tokyo Medical and Dental University); homozygous Becn1F121A knockin (BK) mice (66), heterozygous global Becn1 deletion (Becn1+/−) mice (64), and homozygous Bcl2AAA knockin (Bcl2AAA) mice (24) were provided by Dr. Beth Levine (Table 1); and transgenic mice with Klotho overexpression (Tg-Kl) (129sv;B6 hybrid background) (41, 43, 68) were provided by Dr. Makoto Kuro-o (Jichi Medical University).
All mice were housed in a temperature-controlled room (22.0 ± 0.2°C) with a 12:12-h light-dark cycle and given ad libitum access to tap water and allowed free access to standard rodent chow (Teklad 2016, Harlan, Madison, WI). Usually, 10- to 12-wk-old mice were used (with equal numbers of male and female mice) unless specifically indicated. The following mouse lines were cross-mated with WT 129sv mice for at least 10 generations and genotyped with standard PCR protocols described in previous publications: Tg-Kl mice (41, 43, 68), GFP-LC3 mice (68), BK mice (66), Becn1+/− mice (64), and Bcl2AAA mice (24). Becn1+/−;Tg-Kl and Bcl2AAA;Tg-Kl mouse lines were generated by crossing Becn1+/− mice or Bcl2AAA mice with Tg-Kl mice, respectively. Several new mouse lines including BK;GFP-LC3 mice, Becn1+/−;GFP-LC3 mice, Bcl2AAA;GFP-LC3 mice, and Tg-Kl;GFP-LC3 mice were generated when BK, Becn1+/−, Bcl2AAA or Tg-Kl mice were cross-mated with LC3 reporter mice (GFP-LC3), respectively, and confirmed by genotyping PCR.
Ten mice (5 male and 5 female mice, 10–12 wk old) were used in each group. Because there was high mortality of Bcl2AAA mice after IRI based on our preliminary experiments, 20 mice (equal numbers of male and female mice) were used for AKI experiments including Bcl2AAA or Bcl2AAA;Tg-kl mouse lines.
IRI-induced AKI.
Bilateral IRI-induced AKI was conducted with previously established methods (33). Our preliminary experiments showed that 30–40 min of ischemia induced subtle kidney injury in BK mice. In the present study, we prolonged ischemia to 45 min and were able to induce appreciable kidney damage, allowing us to test the potential effect of Klotho on BK mice. To preserve uniformity, we used 45-min ischemia to induce AKI in all mouse lines.
Autophagy analyses.
Autophagy activity was evaluated by measurement of autophagy activity with determination of LC3 I-to-II conversion, p62 protein levels, and LC3 puncta in the kidney. For assessment of in vivo autophagy activity in kidney tissue, mice were fasted overnight followed by treatment with either PBS or chloroquine (50 mg/kg) for 4 h, as previously published (5, 20). Mouse organs were fixed by cardiac perfusion with cold 4% paraformaldehyde (PFA) in PBS, and tissues were collected and processed for frozen sectioning as previously described (20). The total number of GFP-LC3 puncta was counted per 2,500-μm2 area (>15 randomly chosen fields were used per mouse), and the average value for each tissue for each mouse was determined by an observer blinded to genotype and treatment. Kidney sections of the cortex and outer medulla were imaged using a ×63 objective on a Carl Zeiss 510 Meta confocal laser microscope (Carl Zeiss Microscopy, Munich, Germany).
For assessment of in vitro autophagy levels in cultured cells, OKP cells were transiently transfected with GFP-LC3 and treated per research design stated. Cells were fixed and imaged using a ×63 objective on a Carl Zeiss 510 Meta confocal laser microscope. The number of GFP-LC3 puncta per cell in GFP-LC3-positive cells was counted (≥50 randomly chosen GFP-positive cells per sample per condition) (68).
Blood and kidney sample collection.
At 48 h after IRI or sham operation, mice were anesthetized with isoflurane, and blood was drawn through the retroorbital venous sinus and collected in heparinized tubes. The heparinized plasma was separated and stored at −80°C for chemistry experiments. While mice were under anesthesia, the kidneys were isolated and sliced. One slice was fixed with 4% PFA and embedded in paraffin blocks for histological and immunohistological experiments; the remaining renal tissues were snap frozen in liquid N2 and stored at −80°C for RNA or protein extraction.
Plasma chemistry was analyzed using a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis, Rochester, NY). Plasma and urine creatinine concentrations were measured using a P/ACE MDQ Capillary Electrophoresis System and photodiode detector (Beckman-Coulter, Fullerton, CA) at 214 nm (33, 68).
Recombinant Klotho protein supplementation.
Klotho protein containing the ectodomain of mouse αKlotho (amino acids 31–982) with COOH-terminal V5 and 6xHis tags was generated and purified in our laboratory as previously described (30, 33). For in vivo experiments, recombinant Klotho protein (0.1 mg/kg body wt) or vehicle (normal saline) was administered intraperitoneally immediately after the initiation of reperfusion.
Kidney histopathology.
Kidney tissues were fixed in 4% PFA for 16 h at 4°C, and 4-μm sections of paraffin-embedded kidney tissues were stained with hematoxylin and eosin as well as periodic acid-Schiff. Kidney histology was examined and photographed by a histopathologist blinded to the experimental protocols. We used a semiquantitative pathological scoring system for the evaluation of kidney histological changes based on the literature, with minor modifications (5). The images were reviewed for four types of changes: inflammatory cell infiltration in the tubulointerstitium, nuclear changes in renal tubules (nuclear fading, shrinkage, fragment, or lysis), brush-border membrane detachment, and renal tubular casts at ×40 magnification. Each item was scored from 0–4 points based on percent involvement (<25%, 25–50%, 50–75%, or >75%, respectively). There was a maximum of 16 points in each field. We randomly examined 10 fields each in the cortical zone and outer medullar zone at ×40, and the range of final scores was 0–160.
Immunohistochemistry and immunoblot analysis.
Four-micrometer sections of Tissue-Tek-embedded kidneys were subjected to immunohistochemistry using established protocols (30, 68). Total kidney lysate covering all kidney zones and 40 μg protein of kidney lysate were solubilized in Laemmli’s sample buffer and subjected to SDS-PAGE as previously described (30, 69). After proteins were transferred to PVDF membranes, the membranes were immunoblotted with different primary antibodies and with β-actin as a loading control. Signals were visualized using the ECL kit (Perkin-Elmer LAS, Boston, MA).
Quantitative RT-PCR.
Total RNA was extracted using the RNAeasy kit (Qiagen, Germantown, MD) from mouse kidneys. cDNA was generated with oligo-dT primers using the SuperScript III First Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol. The primers for mouse Klotho and cyclophilin used for quantitative PCR and polymerization conditions have been documented in previous publications (30, 32). Data are expressed as amplification cycle number (Ct) as and were normalized to cyclophilin and compared with controls.
Immunoprecipitation.
Beclin 1/Bcl-2 coimmunoprecipitation was done following the previously published protocol (20). Briefly, frozen tissues were homogenized in ice-cold lysis buffer (25 mM HEPES, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100) containing protease and phosphatase inhibitors for 30 min at 4°C. Lysates were centrifuged (16,000 g at 4°C for 30 min) to remove cellular debris, and supernatants were precleared with 60 μL protein G-Sepharose fast-flow beads (GE Healthcare Bio-Science, Uppsala, Sweden) for 2 h and incubated with Bcl-2 antibody or beclin 1 antibody for 4 h followed by the addition of protein G agarose and incubation in a shaker overnight at 4°C. Immunocomplexes were washed five times, resuspended twice in SDS-PAGE loading buffer, boiled for 5 min, and subjected to immunoblot analysis for beclin 1 and Bcl-2.
Statistical analyses.
Data are expressed as means ± SD. Individual values were plotted. Analysis was performed with SigmaPlot software (Systat Software, San Jose, CA). As appropriate, statistical analysis was performed using an unpaired Student’s t test or one-way or two-way ANOVA followed by a Student-Newman-Keuls post hoc test when applicable. P values of ≤0.05 were considered statistically significant.
RESULTS
Longer renal ischemia induces more severe kidney injury in WT mice.
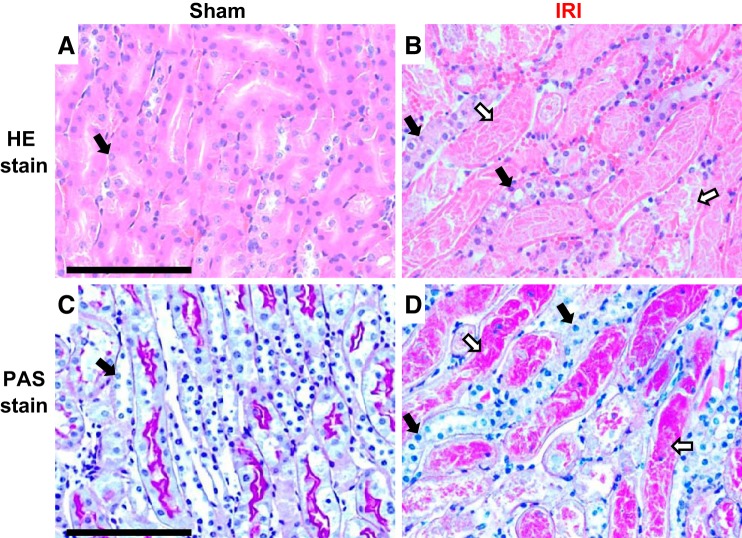
As discussed above, we used 45 min of ischemia to test the effect of Klotho on BK mice because we knew that BK mice are resistant to 30–40 min of ischemia. We used a longer ischemia time to induce severe kidney damage but not high mortality. Finally, after 45 min of ischemia, all WT mice were weak but alive at 48 h. Histologically, many necrotic changes were noted in hematoxylin and eosin-stained kidney sections (Fig. 1, A and B) and in periodic acid-Schiff-stained (Fig. 1, C and D) kidney sections. Many renal tubules lost epithelial cells and were left with naked tubular basement membranes. Many nuclei disappeared in renal tubular epithelial cells. All of these changes are consistent with necrosis in WT mice. Therefore, we used 45 min of ischemia to induce AKI in all mouse lines.
Fig. 1.
Kidney morphology in mice after ischemia-reperfusion injury (IRI). Wild-type mice were subjected to sham (A and C) or 45-min ischemia (B and D) operations. Two days after surgery, mice were euthanized, and kidneys were harvested for the evaluation of kidney morphological changes with hematoxylin and eosin (HE) staining (A and B) and periodic acid-Schiff (PAS) staining (C and D). Scale bar = 100 μm. The closed arrow depicts normal or nearly normal renal tubules; the open arrow depicts necrotic changes in renal tubules, including loss of cell nuclei, detached cell debris, and protein casts.
High autophagy activity from gain of function of beclin 1 confers renal protection in an IRI model.
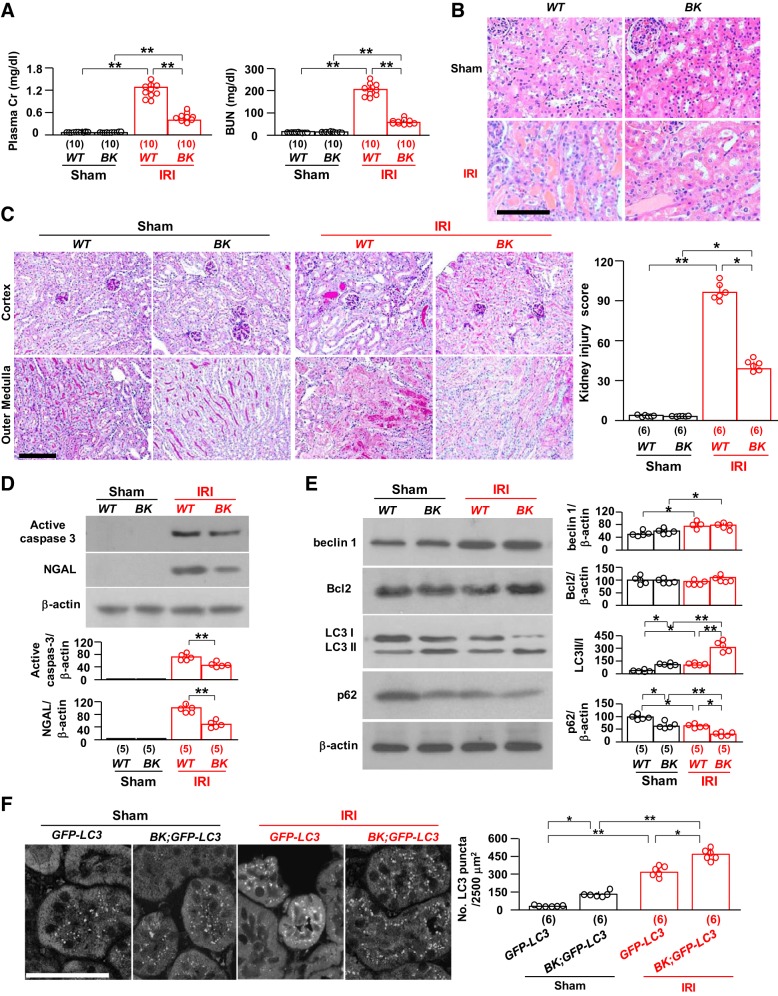
We first used BK mice, which have high autophagy activity because of knockin of gain-of-function mutant beclin 1 (Table 1) (20, 66, 73), to examine whether high autophagy activity protects the kidney from IRI. BK mice had milder kidney damage with lower plasma creatinine and blood urea nitrogen (BUN) than WT mice (Fig. 2A). There was less renal tubular damage with fewer protein casts and less tubular necrosis (Fig. 2, B and C), and there were lower levels of renal active caspase-3 (an apoptotic marker) and NGAL (a kidney injury marker) (Fig. 2D) in BK mice than in WT mice after IRI. There were higher LC3 II-to-I ratios, decreased levels of p62, and increased numbers of LC3 puncta both at baseline and after IRI in BK mice compared with WT mice (Fig. 2, D and E), indicating that BK mice had higher autophagy activity both at baseline and after IRI compared with WT mice. Thus, higher autophagy activity from constitutively high beclin 1 function is associated with less renal injury.
Fig. 2.
Gain-of-function beclin 1 (Becn1) mutation protects the kidney from ischemia-reperfusion injury (IRI). BK mice and their wild-type (WT) littermates were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, blood was drawn, and kidneys were harvested for A–E. A: kidney function. Left, plasma creatinine (Cr); right, blood urea nitrogen (BUN). B: kidney histology with hematoxylin and eosin staining. Scale bar = 100 μm. C: kidney histology with periodic acid-Schiff (PAS) staining. Left, representative images of PAS staining. Scale bar = 100 μm. Right, semiquantitative assessment of kidney injury score on PAS-stained sections in each group. D: kidney damage markers. Top, representative immunoblots for active caspase-3 and neutrophil gelatinase-associated lipocalin (NGAL) protein expression in total kidney lysates. Bottom, summary of all immunoblots from each group. E: kidney autophagic markers. Left, representative immunoblots for beclin 1, Bcl-2, light chain (LC)3, and p62 in total kidney lysates. Right, summary of all immunoblots from each group. F: green fluorescent protein (GFP)-LC3 mice and BK;GFP-LC3 mice were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, and kidneys were harvested. Left, representative microscopic images of GFP-LC3 puncta in the kidneys of GFP-LC3 mice and BK;GFP-LC3 mice by immunohistochemistry. Scale bar = 50 μm. Right, quantitative analysis of the number of GFP-LC3 puncta in renal tubules. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
Low beclin 1 levels and function from heterozygous beclin 1 deletion render the kidney more susceptible to ischemic injury.
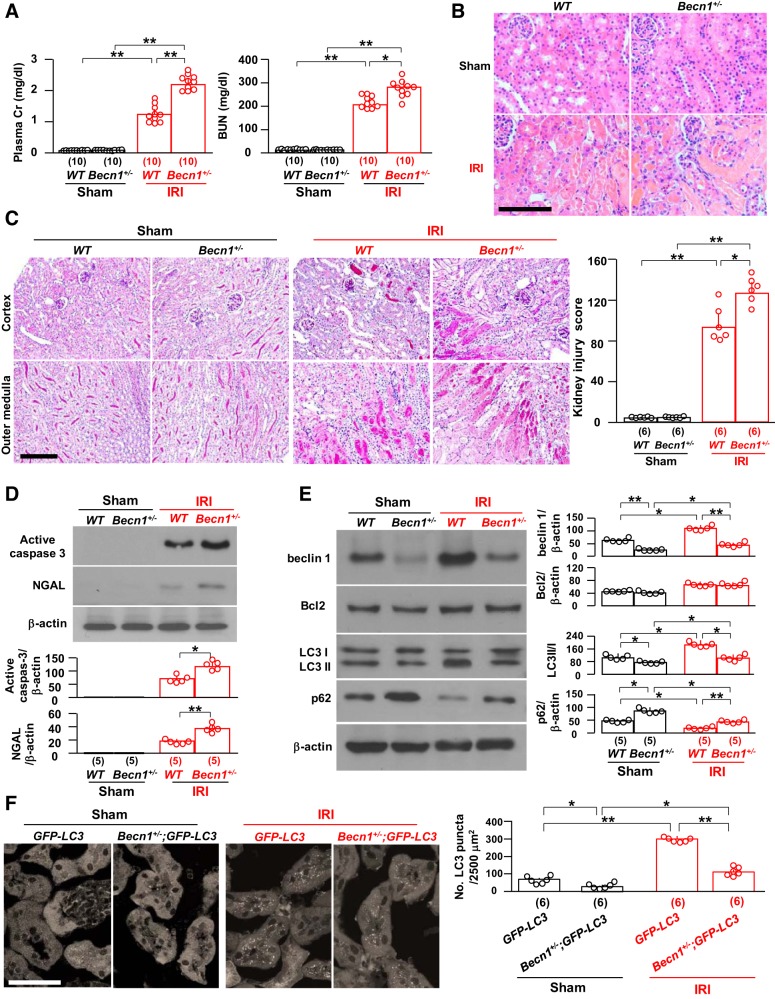
Next, we explored the opposite condition with two mouse models to see whether low beclin 1 function renders the kidney more vulnerable to IRI. Currently, there is no specific assay to directly determine beclin 1 function; we used the change in autophagy activity based on the LC3 II-to-I ratio, p62, and LC3 puncta to reflect its downstream function. The first model used is heterozygous global beclin 1 deletion (Becn1+/−) that is a haploinsufficient state (Table 1); homozygous global beclin 1 deletion is embryonically lethal (64, 80). At baseline, Becn1+/− mice had normal body weight, kidney weight (not shown), histology, plasma creatinine, and BUN (Fig. 3, A–C) compared with WT mice. After IRI, Becn1+/− mice had higher plasma creatinine and BUN (Fig. 3A) and more severe kidney pathology, as demonstrated by massive casts in renal tubules and tubular epithelial cell necrosis (Fig. 3C), compared with WT mice. There was higher expression of renal active caspase-3 and NGAL (Fig. 3D) in Becn1+/− mice than in WT mice after the induction of ischemia-reperfusion. At baseline, Becn1+/− mice had lower autophagy activity compared with WT mice but did not have notably higher apoptosis (Fig. 3, D–F). After IRI, Becn1+/− mice had upregulation of autophagy activity in the kidney above the level before IRI, but the elevation was still lower than in WT mice after IRI (Fig. 3, E and F), suggesting that the autophagy response to IRI in Becn1+/− mice is retained but not suppressed. There were higher levels of apoptosis and NGAL (Fig. 3, D–F) after IRI in Becn1+/− mice compared with WT mice, implying that haploinsufficient beclin 1 downregulates autophagy activity and, consequently, contributes to more severe kidney damage induced by IRI.
Fig. 3.
Loss of one allele of beclin 1 (Becn1) renders the kidney more susceptible to ischemia-reperfusion injury (IRI). Becn1+/− mice and their wild-type (WT) littermates were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, blood was drawn, and kidneys were harvested for A–E. A: kidney function. Left, plasma creatinine (Cr); right, blood urea nitrogen (BUN). B: representative images of kidney histology with hematoxylin and eosin staining. Scale bar = 100 μm. C: kidney histology with periodic acid-Schiff (PAS) staining. Left, representative images of PAS staining. Scale bar = 100 μm. Right, semiquantitative assessment of kidney injury score on PAS-stained sections in each group. D: kidney damage markers. Top, representative immunoblots for active caspase-3 and neutrophil gelatinase-associated lipocalin (NGAL) protein expression in total kidney lysates. Bottom, summary of all immunoblots from each group. E: kidney autophagic markers. Left, representative immunoblots for beclin 1, Bcl-2, light chain (LC)3, and p62 in total kidney lysates. Right, summary of all immunoblots from each group. F: green fluorescent protein (GFP)-LC3 mice and Becn1+/−;GFP-LC3 mice were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, and kidneys were harvested. Left, representative microscopic images of GFP-LC3 punctas in the kidneys of GFP-LC3 mice and Becn1+/−;GFP-LC3 mice by immunohistochemistry. Scale bar = 50 μm. Right, quantitative analysis of the number of GFP-LC3 puncta in renal tubules. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
Low beclin 1 function from mutant Bcl2AAA renders the kidney more susceptible to ischemic injury.
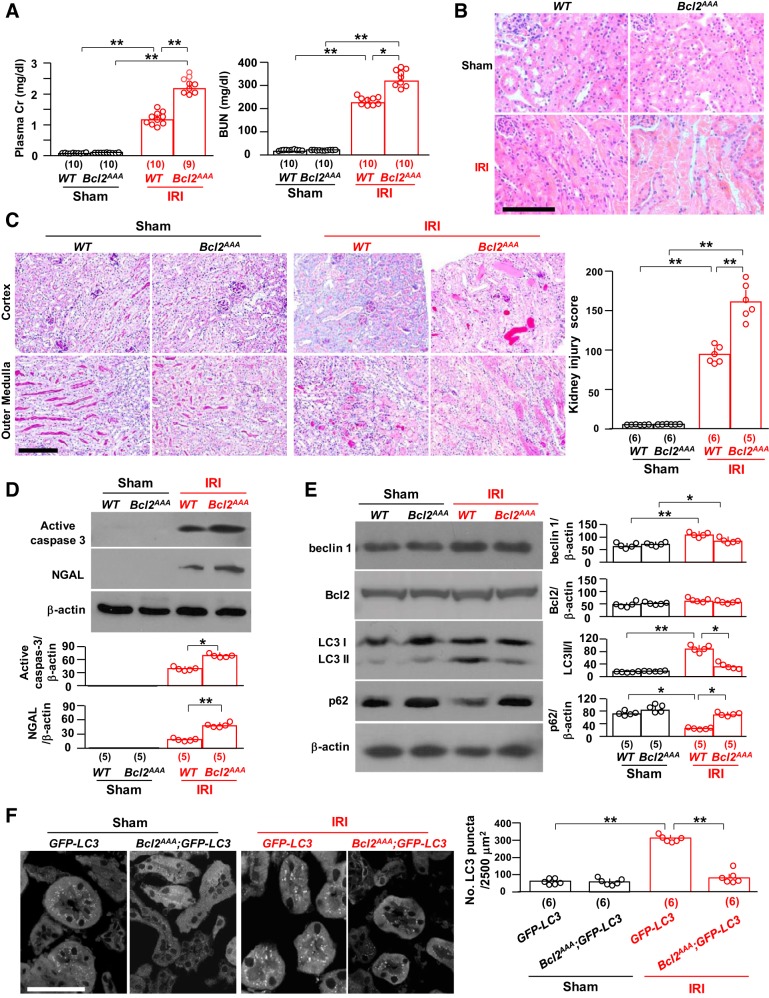
The second mouse model of low beclin 1 function was homozygous knockin of mutant Bcl2AAA (Table 1) (24). This triple mutation of three conserved phosphorylation residues in the nonstructured loop region of mouse Bcl-2 to alanines (Bcl2AAA) impairs stimulus-induced disruption of the beclin 1/Bcl-2 complex (24). Basically, beclin 1 in this mouse line is sequestered by dominant mutant Bcl-2 and is not able to be released after exposure to autophagy stimuli. At baseline, Bcl2AAA mice had normal renal function and histology (Fig. 4, A–C) with normal levels of apoptosis and autophagy activity (Fig. 4, D–F), which is consistent with previous observations (24, 60, 61, 79). Thus, this mouse line is suitable to study if stabilization of beclin 1/Bcl-2 dampens the kidney’s defense ability against ischemic injury.
Fig. 4.
Loss of phosphorylation sites in Bcl-2 (Bcl2AAA) stabilizes the beclin 1/Bcl-2 complex and renders the kidney more susceptible to ischemia-reperfusion injury (IRI). In A–D, Bcl2AAA mice and their wild-type (WT) littermates were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, blood was drawn, and kidneys were harvested. A: kidney function. Left, plasma creatinine (Cr); right, blood urea nitrogen (BUN). B: kidney histology with hematoxylin and eosin staining. Representative images are shown. Scale bar = 100 μm. C: kidney histology with periodic acid-Schiff (PAS) staining. Left, representative microscopic images of PAS staining. Scale bar = 100 μm. Right, semiquantitative assessment of kidney injury score based on PAS-stained sections in each group. D: kidney damage markers. Top, representative immunoblots for active caspase-3 and neutrophil gelatinase-associated lipocalin (NGAL) proteins in total kidney lysates. Bottom, summary of all immunoblots from each group. E: kidney autophagic markers. Left, representative immunoblots for beclin 1, Bcl-2, light chain (LC)3, and p62 in total kidney lysates. Right, summary of all immunoblots from each group. F: green fluorescent protein (GFP)-LC3 mice and Bcl2AAA;GFP-LC3 mice were subjected to IRI or sham operations. Two days after surgery, mice were euthanized, and kidneys were harvested. Left, representative microscopic images of GFP-LC3 punctas in the kidneys of GFP-LC3 mice and Bcl2AAA;GFP-LC3 mice by immunohistochemistry. Scale bar = 50 μm. Right, quantitative analysis of the number of GFP-LC3 puncta in renal tubules. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test and significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
After the induction of IRI, 40% of Bcl2AAA mice died within 2 days, whereas all of their WT littermates were alive. Among the survivors, there were significantly higher levels of plasma creatinine and BUN (Fig. 4A), more massive kidney structural destruction (Fig. 4, B and C), and higher renal active caspase-3 and NGAL (Fig. 4D) in Bcl2AAA mice than in WT mice, indicating that mutant Bcl-2 rendered the kidneys more vulnerable to IRI. Unlike Becn1+/− mice, Bcl2AAA mice had relatively normal autophagy activity in the kidney at baseline but lower autophagy activity after IRI compared with WT mice (Fig. 4, E and F), which is consistent with a previous study (61). This demonstrates that the mutant Bcl2AAA that avidly sequesters beclin 1 also blocks IRI-induced autophagy upregulation, which renders the kidney more susceptible to ischemic injury.
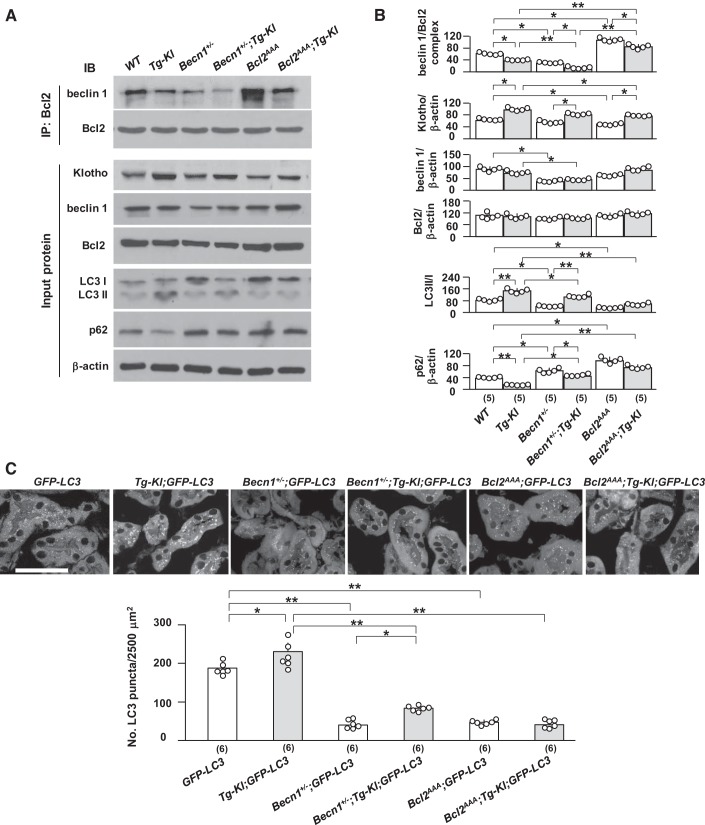
Klotho modulates the interaction of beclin 1/Bcl-2 and upregulates autophagy activity.
To determine whether Klotho protects the kidney from IRI through modification of beclin 1 levels, beclin 1 activity (determined by autophagy activity or the beclin 1/Bcl-2 complex) in the kidney, we used a transgenic mouse line overexpressing Klotho (Tg-Kl), which has higher levels of Klotho in the kidney and plasma (33, 43) and is relatively resistant to renal IRI (33, 68). As expected, Tg-Kl mice had lower levels of plasma creatinine, milder histopathological changes (Fig. 5, A–C), and lower active caspase-3 and NGAL (Fig. 5D) compared with WT mice after IRI. Interestingly, the increase in plasma creatinine and kidney damage in doubly manipulated Becn1+/−;Tg-Kl mice was less than that in Becn1+/− mice but still greater than that in Tg-Kl mice (Fig. 5, A–C). Similarly, levels of active caspase-3 and NGAL expression in the kidney were lower in Becn1+/−;Tg-Kl mice than that in Becn1+/− mice but higher than that in Tg-Kl mice (Fig. 5, A–C). The changes in biochemistry and kidney histology support the theory that Klotho-associated renoprotection is attenuated but not completely abrogated by the loss of one copy of beclin 1.
Fig. 5.
High Klotho is less effective in attenuating ischemia-reperfusion injury in mice with low beclin 1 activity. Transgenic mice overexpressing Klotho (Tg-Kl mice) and their wild-type (WT) littermates, Becn1+/− and chimeric mice with Tg-Kl (Becn1+/−;Tg-Kl), and Bcl2AAA mice and chimeric mice with Tg-Kl (Bcl2AAA;Tg-Kl) were subjected to IRI operations. Two days after surgery, mice were euthanized, blood was drawn, and kidneys were harvested. A: kidney function and plasma creatinine (Cr). B: kidney histology with hematoxylin and eosin staining. Scale bar = 100 μm. C: kidney histology with periodic acid-Schiff (PAS) staining. Top, representative microscopic images of PAS staining. Scale bar = 100 μm. Bottom, semiquantitative assessment of kidney injury score based on PAS stain in each group. D: kidney damage markers. Top, representative immunoblots for active caspase-3 and neutrophil gelatinase-associated lipocalin (NGAL) proteins in total kidney lysates. Bottom, summary of all immunoblots from each group. E: Klotho expression and autophagic markers in the kidney. Left, representative immunoblots for Klotho, beclin 1, Bcl-2, light chain (LC)3, and p62 in total kidney lysates. Right, summary of all immunoblots from each group. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test and significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
After IRI, Bcl2AAA;Tg-Kl mice had 20% mortality, whereas Bcl2AAA mice had 35% mortality (P < 0.05). Additionally, Bcl2AAA;Tg-Kl mice had modestly, but statistically and significantly, lower plasma creatinine (Fig. 5A), fewer kidney pathological changes (Fig. 5, B and C), and lower renal expression of active caspase-3 and NGAL (Fig. 5D) compared with Bcl2AAA mice, indicating that high Klotho decreased mouse death but did not completely prevent it. High Klotho still attenuated kidney injury induced by IRI in the presence of the stable beclin 1/Bcl-2 complexes and lower autophagy activity, but the magnitude of renoprotection was significantly blunted, indicating that autophagy is one of downstream factors for Klotho’s renoprotection. Moreover, there were higher ratios of LC3 II to I and lower levels of p62 expression in the kidneys of Becn1+/−;Tg-Kl mice compared with Bcl2AAA;Tg-Kl mice after IRI, although there were relatively normal levels of Klotho in the kidneys of Becn1+/−;Tg-Kl and Bcl2AAA;Tg-Kl mice after IRI compared with sham WT mice (Fig. 5F), suggesting that Klotho upregulation of autophagy activity is deficient in Bcl2AAA;Tg-Kl mice and therefore renders the kidneys more susceptible to ischemic injury compared with those in Tg-Kl mice. Notably, lower Klotho expression was found in the kidneys of Becn1+/− and Bcl2AAA mice compared with WT mice after IRI. Similarly, Klotho levels were lower in Becn1+/−;Tg-Kl and Bcl2AAA;Tg-Kl mice compared with Tg-Kl mice after IRI (Fig. 4D); lower Klotho may result from more severe kidney destruction induced by IRI.
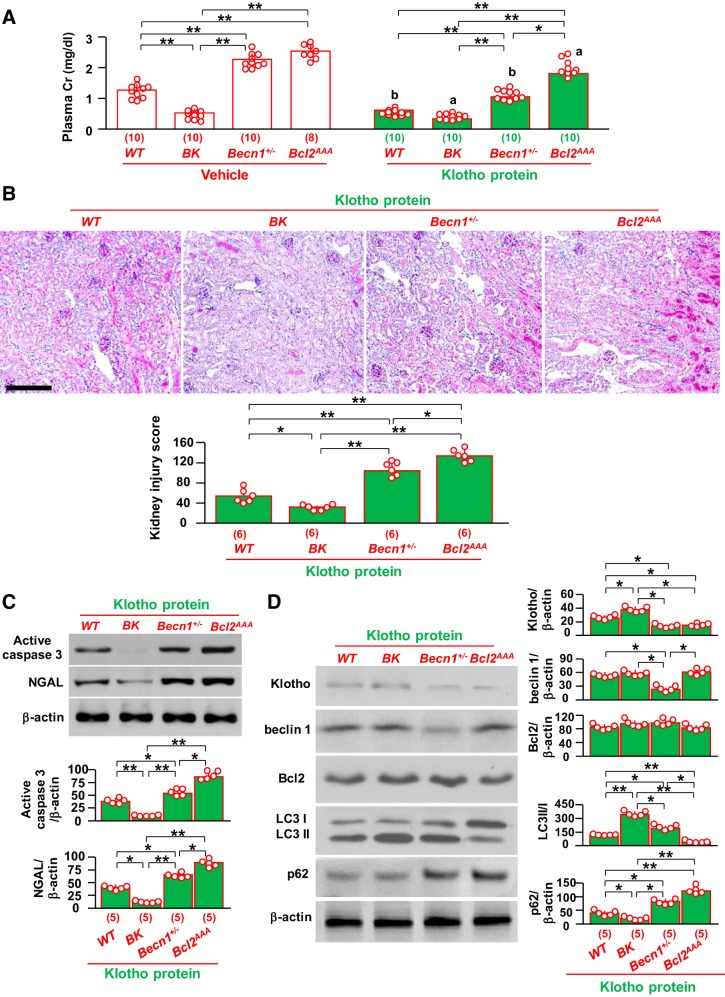
Renoprotection by administered recombinant Klotho protein is reduced in Bcl2AAA mice with lower autophagy activity.
Because Tg-Kl mice have both high levels of circulating soluble Klotho, higher renal transmembrane Klotho, and, in fact, widespread ectopic Klotho expression (33, 43), we explored whether soluble Klotho protein in the circulation is the important mediator of renoprotection. Another reason for this experiment was to test the feasibility of recombinant Klotho therapy. We injected purified recombinant Klotho protein intraperitoneally immediately after IRI into the four different mouse genotype groups with different levels of autophagy: BK, WT, Becn1+/−, and Bcl2AAA. Recombinant Klotho protein injection significantly decreased plasma creatinine levels compared with vehicle in all four genotypes (Fig. 6A). Vehicle-injected Bcl2AAA mice had 40% mortality, whereas only 25% of Klotho-injected Bcl2AA mice died (P < 0.05). None of the mice from the other three genotypes died after either vehicle or Klotho injection. After Klotho injection, the reduction of plasma creatinine was greater in WT mice (66%) than in either BK mice (43%) or Bcl2AAA mice (30%) (Fig. 6A). Parallel to the reduction of plasma creatinine, less alteration of kidney histology and lower renal active caspase-3 and NGAL protein expression were found in WT mice and BK mice after Klotho injection (Fig. 6, B and C). Interestingly, the renoprotection by recombinant Klotho protein was attenuated in both Becn1+/− and Bcl2AAA mice (Fig. 6, A–C). Bcl2AAA mice had statistically worse kidney dysfunction (Fig. 6A), more severe histological damage (Fig. 6B), and higher active caspase-3 and NGAL protein levels in the kidneys (Fig. 6C) than Becn1+/− mice. Moreover, we found lower ratios of LC3 II to I and higher p62 protein levels in the kidneys of Bcl2AAA mice than in those of Becn1+/− mice. Renal Klotho determination by immunoblot analysis represents the total amount of exogenous and endogenous Klotho because we were not able to distinguish the two. However, exogenous Klotho protein in the kidney is undetectable 48 h after a single injection (31), so Klotho protein in the kidney after 48 h of Klotho injection shown in Fig. 5D should be primarily endogenous Klotho. As expected, BK mice had higher levels of Klotho protein in the kidney after IRI compared with the other three lines, which supports the theory that low renal Klotho is the result of severe kidney damage induced by IRI. On the other hand, levels of Klotho protein in the kidney were similar between Becn1+/− and Bcl2AAA mice after Klotho injection, but levels of autophagy activity were lower in Bcl2AAA mice than in Becn1+/− mice (Fig. 6D), indicating that Klotho administration protects the kidney less efficiently from IRI through less efficient upregulation of autophagy activity in Bcl2AAA mice than in Becn1+/− mice.
Fig. 6.
Exogenous Klotho protein is less effective in attenuating ischemia-reperfusion injury in mice with low beclin 1 activity. BK, Becn1+/−, and Bcl2AAA mice and wild-type (WT) littermates were subjected to ischemia-reperfusion followed by an immediate one-time intraperitoneal injection of recombinant Klotho protein (0.1 mg/kg body wt). Two days after surgery, mice were euthanized, blood was drawn, and kidneys were harvested. A: plasma creatinine (Cr). Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. aP < 0.05 and bP < 0.01 between vehicle- and Klotho-injected groups of the same genotype of mice. B: kidney histology of Klotho-injected acute kidney injury (AKI) mice. Top, representative microscopic images of periodic acid-Schiff (PAS) staining. Scale bar = 200 μm. Bottom, semiquantitative assessment of kidney injury score based on PAS stain in each group. C: kidney damage markers in Klotho-injected mice. Top, representative immunoblots for active caspase-3 and neutrophil gelatinase-associated lipocalin (NGAL) proteins in total kidney lysates. Bottom, summary of all immunoblots from each group. D: changes in autophagic markers in kidneys of Klotho-injected AKI mice. Left, representative immunoblots for Klotho, beclin 1, Bcl-2, light chain (LC)3, and p62 in total kidney lysates. Right, summary of all immunoblots from each group. Data are expressed as means ± SD, and statistical significance was evaluated by one-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups for B–D. Sample numbers in each group are presented in brackets underneath the corresponding bars.
Higher Klotho levels are associated with less beclin 1/Bcl-2 interaction in the kidney.
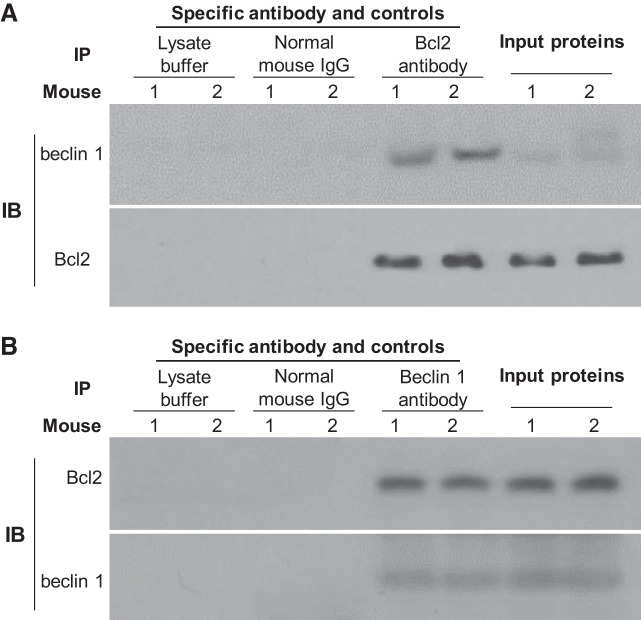
We have previously shown higher Klotho upregulates autophagy activity in the kidneys (68), but the molecular mechanism of Klotho’s effect on autophagy activity is still unclear. Because the beclin 1/Bcl-2 complex is one of key regulators of autophagy activity (20, 25, 61, 66), we examined this complex in the kidneys of transgenic mice overexpressing Klotho. Similar to findings in our previous study (20), coimmunoprecipitation showed that the detected beclin 1/Bcl-2 complex is specific because beclin 1/Bcl-2 complexes were not detected when lysate buffer or normal mouse IgG were used to replace primary anti-Bcl-2 antibody (Fig. 7A). We also successfully pulled down the beclin 1/Bcl-2 complex with anti-beclin 1 antibody and confirmed that this complex is specific and contains both beclin 1 and Bcl-2 protein (Fig. 7B).
Fig. 7.
Coimmunoprecipitation of the beclin 1/Bcl-2 complex in the kidney with anti-Bcl-2 or anti-beclin 1 antibodies. A: total kidney lysates from two normal wild-type (WT) mice were immunoprecipitated with mouse anti-Bcl-2 antibody or normal mouse IgG or lysate buffer and then immunoblotted with beclin 1 and Bcl-2 antibodies, respectively. Immunoblots (IBs) for beclin 1 and Bcl-2 protein in input proteins were shown. B: total kidney lysates from two normal WT mice were immunoprecipitated with mouse anti-beclin 1 antibody or normal mouse IgG or lysate buffer and then immunoblotted with beclin 1 and Bcl-2 antibodies, respectively. IBs for beclin 1 and Bcl-2 protein in input proteins were shown. Three independent experiments were repeated.
With the coimmunoprecipitation assay, we found more beclin 1/Bcl-2 complex in the kidneys of Bcl2AAA;Tg-Kl mice than in those of Tg-Kl mice, but less than in those of Bcl2AAA mice (Fig. 8A). Surprisingly, there was lower renal Klotho protein in Bcl2AAA mice and even in Bcl2AAA;Tg-Kl mice compared with Tg-Kl mice. Although we do not fully understand why or how Klotho protein was downregulated with lower autophagy activity, the reduction is definitely not because of kidney injury per se because the renal function and morphology were similar to WT mice at baseline (Fig. 4, A and C). Whether abnormal epithelial cell metabolism or a systemic unknown suppressor inhibit Klotho expression remains to be explored. This may be an example of a vicious cycle in which low renal Klotho might further contribute to more beclin 1/Bcl-2 complex formation. As expected, high and low levels of beclin 1/Bcl-2 interaction are associated with decreased or increased autophagy activity, respectively (Fig. 8, B and C).
Fig. 8.
There was decrease in the beclin 1/Bcl-2 complex in the kidneys of Tg-Kl mice. Tg-Kl mice and their wild-type (WT) littermates; Becn1+/− and chimeric mice with Tg-Kl (Becn1+/−;Tg-Kl) as well as Bcl2AAA mice and chimeric mice with Tg-Kl (Bcl2AAA;Tg-Kl) were fasted for 24 h. At the final 4 h, mice were intraperitoneally injected with chloroquine once. Twenty-four hours after starvation initiation, mice were euthanized, and kidneys were harvested for experiments. A: beclin 1/Bcl-2 complex and autophagic status in the kidney. Top, immunoprecipitation (IP) with Bcl-2 antibody followed by immunoblots (IBs) for beclin 1 and Bcl-2. Bottom, representative immunoblots for Klotho, beclin 1, Bcl-2, light chain (LC)3, and p62 in total input proteins. B: summary of the changes in markers from all immunoblots. C: green fluorescent protein (GFP)-LC3 puncta in the kidneys by immunohistochemistry. Top, representative microscopic images of LC3 puncta. Scale bar = 50 μm. Bottom, quantitative analysis of the number of GFP-LC3 punctas in renal tubules. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test and significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
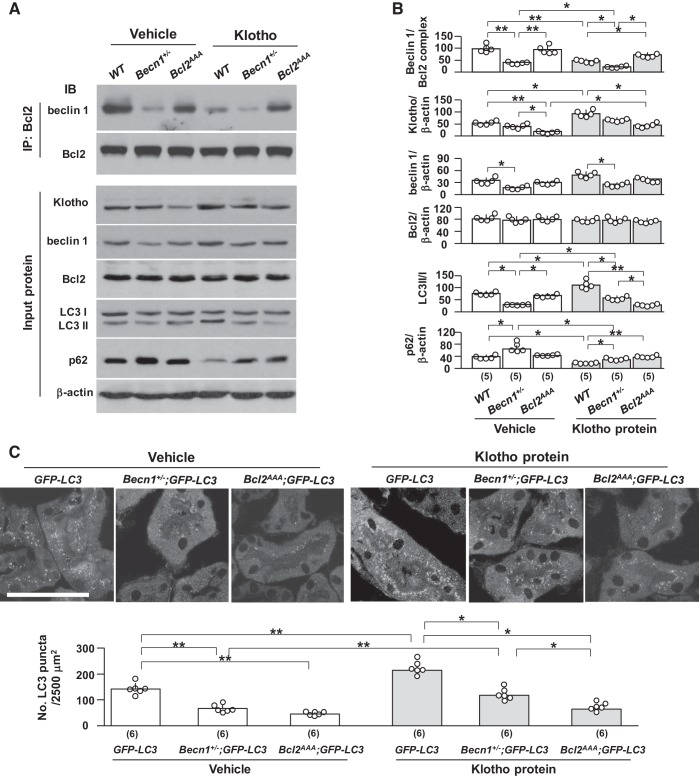
To further study if exogenous recombinant Klotho has similar biological action as that in the Tg-Kl mouse, we injected recombinant soluble Klotho protein into WT, Becn1+/−, and Bcl2AAA mice and examined the levels of beclin 1/Bcl-2 interaction and autophagy activity markers in the kidneys. In our pilot study, we found that a single injection of Klotho protein at 0.1 mg/kg body wt significantly increased autophagy activity in the kidneys of WT 129sv mice (data not shown). Thus, we used this dose for the subsequent experiments. Similar to our findings in Bcl2AAA;Tg-Kl mice (Fig. 8A), renal Klotho protein, which might be a combination of endogenous and exogenous Klotho determined by immunoblot analysis, was reduced in Bcl2AAA mice (Fig. 9A) after a single dose of Klotho injection, suggesting that low renal Klotho in Bcl2AAA mice might be the result of acceleration of exogenous Klotho degradation or reduced endogenous Klotho expression caused by as-yet-unidentified factors. Becn1+/− mice had a trend toward low renal Klotho after Klotho injection, but it was not significant.
Fig. 9.
Recombinant Klotho protein decreases the beclin 1/Bcl-2 complex in the kidney. Becn1+/− mice, Bcl2AAA mice, and their wild-type (WT) littermates were intraperitoneally injected with recombinant Klotho protein or vehicle (normal saline). After Klotho or vehicle injection, mice were subjected to starvation for 24 h, and at 20 h after starvation initiation, mice were intraperitoneally injected with chloroquine. At 24 h, mice were euthanized and kidneys were harvested. A: beclin 1/Bcl2 complex and autophagic status in the kidneys. Top, immunoprecipitation (IP) with Bcl-2 followed by immunoblots (IBs) for beclin 1 and Bcl-2. Bottom, representative IBs for Klotho, beclin 1, Bcl-2, light chain (LC)3, and p62 in total input proteins. B: summary of the changes in markers from all immunoblots. C: green fluorescent protein (GFP)-LC3 puncta in the kidneys by immunohistochemistry. Top, representative microscopic images of LC3 puncta. Scale bar = 50 μm. Bottom, quantitative analysis of the number of GFP-LC3 puncta in renal tubules. Data are expressed as means ± SD, and statistical significance was evaluated by two-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
Klotho less efficiently reduced the complex and upregulated autophagy activity markers (increase in the LC3 II-to-I ratio and decline in p62 and LC3 punctas) in Bcl2AAA and Becn1+/− mice compared with WT mice (Figs. 8, A–C, and 9, A–C). However, low renal Klotho may directly downregulate autophagy activity in Bcl2AAA mice. To address this possibility, a cell culture model was used.
We also noted that Becn1+/− mice with 50% beclin 1 protein retained a capacity, albeit lower, to upregulate autophagy activity in response to recombinant Klotho compared with WT mice (Fig. 9, A–C). Therefore, a normal amount of functional beclin 1 is important for mediating Klotho’s effect on autophagy activity, and the balance between free beclin 1 and Bcl-2-bound complex is a key modulator to autophagy activity.
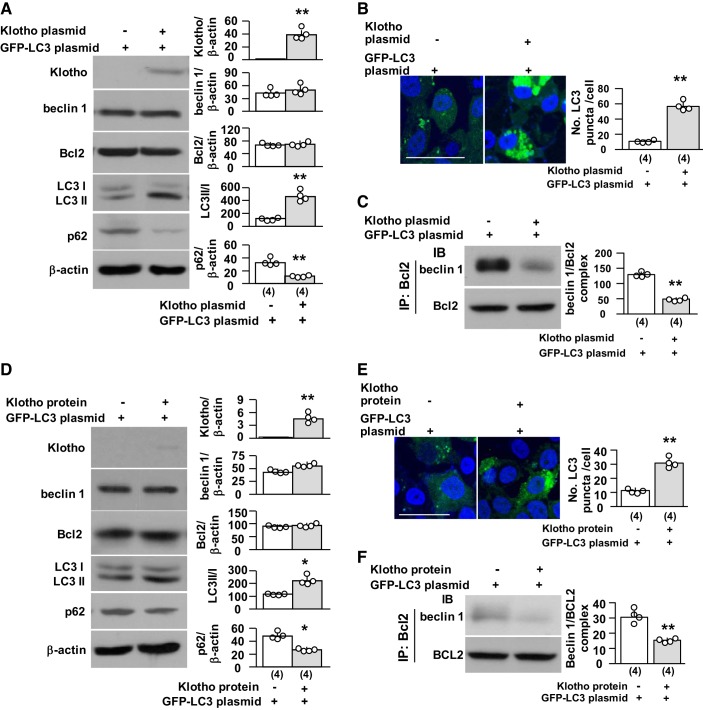
Klotho reduces beclin 1/Bcl-2 interactions in vitro.
We have previously shown that Klotho decreases beclin 1/Bcl-2 interactions and upregulates autophagy activity in HeLa cells in vitro (68). To determine whether Klotho directly modulates the beclin 1/Bcl-2 complex in renal tubules, we examined the complex and autophagy activity in an opossum kidney proximal tubular cell line (OKP cells) transfected with full-length membrane Klotho. We used Lipofectamine 2000, which typically reaches ~60–70% transfection efficiency in OKP cells (data not shown). Transfected cells had higher levels of Klotho and ratios of LC3 II to I, lower p62 protein levels, and no change in beclin 1 and Bcl-2 protein levels, indicating that upregulation of autophagy activity (Fig. 10, A and B) by Klotho is not mediated by changes in beclin 1 or Bcl-2 protein expression. Instead, levels of the beclin 1/Bcl-2 complex were reduced in Klotho-transfected cells compared with vector-transfected cells (Fig. 10C), suggesting that Klotho-induced upregulation of autophagy activity is, at least in part, due to a decline in levels of the beclin 1/Bcl-2 complex.
Fig. 10.
Klotho disrupts the beclin 1/Bcl-2 complex in renal tubular cell line. In A–C, opossum kidney clone P (OKP) cells, an opossum renal proximal tubular cell line, were transiently transfected with green fluorescent protein (GFP)-light chain (LC)3 plasmids with Klotho or empty vector for 2 days. Each experiment was repeatedly conducted four times independently, in triplicate for each experimental condition. A: immunoblots for Klotho and autophagic markers in OKP cells. Left, representative immunoblots for Klotho, beclin 1, Bcl-2, LC3, and p62. Right, summary of all immunoblots in total cell lysates. B: GFP-LC3 puncta in OKP cells. Left, representative immunofluorescent microscopic images. Scale bar = 50 μm. Right, summary of quantitation of LC3 puncta numbers. C: beclin 1/Bcl-2 complex in OKP cells. Left, representative blots for beclin 1 and Bcl-2 to detect the beclin 1/Bcl-2 complex by immunoprecipitation (IP) with Bcl-2. Right, summary of all immunoblots. In D–F, OKP cells were transiently transfected with GFP-LC3 plasmids. Twenty-four hours after transfection, cells were incubated with recombinant Klotho or vehicle for another 24 h. D: immunoblots for Klotho and autophagic markers in OKP cells. Left, representative immunoblot for Klotho, beclin 1, Bcl-2, LC3, and p62. Right, summary of all immunoblots in total cell lysates. E: GFP-LC3 puncta in OKP cells. Left, representative immunofluorescent microscopic images. Scale bar = 50 μm. Right, summary of quantitation of LC3 puncta numbers. F: beclin 1/Bcl-2 complex in OKP cells. Left, representative blots for beclin 1 and Bcl-2 to detect the beclin 1/Bcl-2 complex by IP with Bcl-2. Right, summary of all immunoblots. Data are expressed as means ± SD, and statistical significance was evaluated by one-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
We next incubated OKP cells with recombinant Klotho. Klotho protein was only weakly detected in Klotho-treated cell lysates, suggesting that the added Klotho remained primarily in the extracellular space or loosely associated with the cell membrane. Klotho did not change beclin 1 and Bcl-2 protein levels. However, the beclin 1/Bcl-2 complex was reduced, and autophagy activity was upregulated in Klotho-treated cells (Fig. 10, D–F). Klotho-upregulated autophagy activity is likely attributable to decreased levels of the beclin 1/Bcl-2 complex.
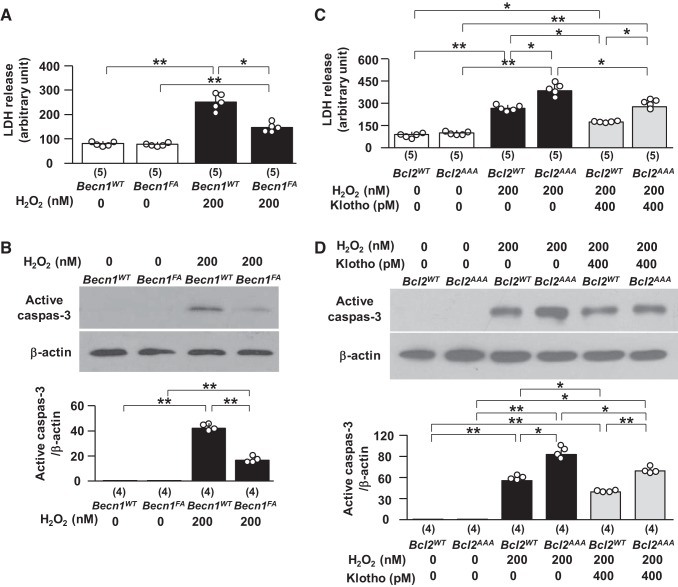
Levels of the beclin 1/Bcl-2 complex modify cells’ susceptibility to oxidative injury in vitro.
Our in vivo experiments showed less kidney injury induced by IRI in BK mice (Fig. 2) and Tg-Kl mice (Fig. 5) with less beclin 1/Bcl-2 interaction (Figs. 8 and 9) and showed more severe kidney injury induced by IRI in Bcl2AAA mice (Fig. 4) with more beclin 1/Bcl-2 interaction (Fig. 8 and 9). To define whether the beclin 1/Bcl-2 complex determines cell susceptibility to oxidative stress, we treated cells with different levels of the beclin 1/Bcl-2 complex with H2O2 and found less LDH release in culture media (Fig. 11A) and less active caspase-3 protein in cell lysates (Fig. 11B) in cells transfected with Becn1F123A plasmid compared with Becn1WT, suggesting that lower levels of the beclin 1/Bcl-2 complex render cells more resistant to H2O2. Conversely, cells transfected with Bcl2AAA plasmid had higher LDH release in culture media and more active caspase-3 in cell lysates (Fig. 11, C and D). Recombinant Klotho protein was able to ameliorate the H2O2-induced elevation of LDH and active caspase-3 in cells transfected with Bcl2AAA compared with cells without Klotho treatment (Fig. 11, C and D), but Klotho’s cytoprotection was attenuated compared with cells transfected with Bcl2WT because the reduction of LDH by Klotho was 34.2% in Bcl2WT cells but only 25.6% in Bcl2AAA cells (P < 0.05; Fig. 11C). Therefore, the level of the beclin 1/Bcl-2 complex controls the release of free beclin 1 and consequently modulates autophagy activity and determines cell sensitivity to oxidation-induced cell injury.
Fig. 11.
Beclin 1 reduces susceptibility to H2O2-induced cytotoxicity in opossum kidney clone P (OKP) cells. In A and B, OKP cells were transiently transfected with human Becn1WT or human Becn1F123A (Becn1FA). One day after transient transfection, cell injury was induced with H2O2 or vehicle for 1 day. A: the lactate dehydrogenase (LDH) concentration in culture media was measured. B: immunoblots (IBs) for apoptotic markers in OKP cells. Top, representative IBs for active caspase-3 expression. Bottom, summary of all IBs in total cell lysates. In C and D, OKP cells were transiently transfected with human Bcl2AAA or Bcl2WT. One day after transient transfection, cell injury was induced with H2O2 or vehicle in the presence of Klotho or vehicle for 1 day. C: the LDH concentration in culture media was measured. D: IBs for apoptotic markers in OKP cells. Top, representative blots for active caspase-3 expression. Bottom, summary of all IBs in total cell lysates. Data are expressed as means ± SD, and statistical significance was evaluated by one-way ANOVA followed by a Student-Newman-Keuls post hoc test; significance was accepted when *P < 0.05 and **P < 0.01 between two groups. Sample numbers in each group are presented in brackets underneath the corresponding bars.
DISCUSSION
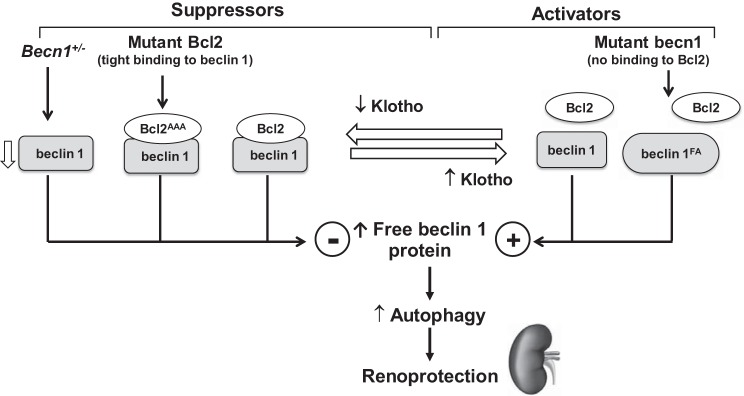
This study has three principal important findings. First, high beclin 1 function is protective against IRI-induced AKI through escalation of autophagy activity. Second, the renoprotection conferred by beclin 1 is dependent on both absolute levels of beclin 1 protein and the amount of beclin 1 that is not sequestered by the inhibitory protein Bcl-2. Third, the renoprotective effect of Klotho is, in part, mediated through the induction of dissociation of the beclin 1/Bcl-2 complex and activation of autophagy activity (Fig. 12).
Fig. 12.
Proposed molecular mechanism behind beclin 1-dependent Klotho’s renoprotection: interplay of Klotho, beclin 1, and Bcl-2 in renoprotection. The finely tuned balance between suppressors and activators controls the release of free beclin 1 protein and determines intracellular autophagy activity. On the right, high Klotho and gain-of-function mutation of beclin 1 (beclin 1FA) disrupt negative regulatory beclin 1/Bcl-2 complex, release more beclin 1 from the complex, upregulate autophagy activity, and protect the kidney against ischemic injury. On the left, three negative factors reduce free beclin 1 in the cells through distinct mechanisms. Heterozygous beclin 1 knockout (Becn1+/−) mice have only 50% beclin 1 expression, whereas loss-of-function mutation of Bcl-2 (Bcl2AAA) and Klotho deficiency stabilize the beclin 1/Bcl-2 complex, reduce free beclin 1, downregulate autophagy in the cell, and render the kidney more susceptible to ischemic injury.
Autophagy is essential for the maintenance of cellular homeostasis by degrading and recycling cellular components. Low autophagy activity is associated with kidney injury (1, 5, 23, 37, 53, 63, 69). In both in vivo and in vitro settings, deletion of autophagy related 5 and 7 (Atg5 and Atg7, respectively) sensitizes kidneys or renal tubules to ischemic cytotoxicity, hypoxic cytotoxicity, cisplatin cytotoxicity, or sepsis-induced kidney injury (27, 36, 37, 53). The present study focused on key protein beclin 1, which is a central regulator of autophagy in mammalian cells (80). The antiapoptotic protein Bcl-2 inhibits autophagy by interacting with beclin 1 (60, 61). Upon the activation of beclin 1 in response to cellular challenges, such as nutrient starvation and oxidative stress, beclin 1 is dissociated from the beclin 1/Bcl-2 complex to allow the induction and assembly of autophagosomes (24, 61, 62, 66, 79). Reduced levels of beclin 1 or stabilization of the beclin 1/Bcl-2 complex diminishes beclin 1 activity, which inhibits autophagy activity and is associated with a variety of diseases (7, 14, 20, 24, 25, 46, 62, 66).
To address whether beclin 1 is a renoprotective protein, we induced AKI in the recently generated and characterized mouse model of a global knockin of a substitution mutation in the BH3 domain of mouse beclin 1 at F121A (BK mice) with low beclin 1/Bcl-2 complex (20, 24, 62, 66). These mice had higher autophagy activity in multiple tissues at baseline without alteration of basic endogenous beclin 1 expression (20, 66, 75). BK mice had milder AKI compared with WT mice, and in vitro experiments showed that gain-of-function mutant beclin 1 protects cells from oxidative cytotoxicity. In addition, beclin 1 haploinsufficient mice had more severe AKI. We conclude that beclin 1 is a renoprotective protein and that normal beclin 1 activity is required for defense against IRI. Lower autophagy activity in proximal tubules was reported to be associated with less renal fibrosis in unilateral ureteric obstruction mice with conditional knockout of Atg7, another key protein involved in autophagosome formation (49). It is possible that there is a biphasic effect in level of autophagy and in time course of its beneficial effects versus deleterious effects. Whether sustained high beclin 1 activity would cause more renal fibrosis in BK mice chronically after IRI needs to be explored in the future. On the other hand, extremely high beclin 1 activity could induce an Na+-K+-ATPase-regulated cell death called autosis, which is triggered by autophagy (48). Therefore, a proper level and duration of beclin 1 signaling activity are critical for preventing tissue damage and maintaining normal repair.
In addition to normal beclin 1 protein expression, low levels of the beclin 1/Bcl-2 complex are required for the kidney to maintain autophagy homeostasis and defend against renal insults. Mutation of the three phosphorylation sites on Bcl-2 stabilizes the beclin 1/Bcl-2 complex and lowers free beclin 1 both in vitro (60, 79) and in vivo (24, 61). As an antiapoptotic protein, high levels of free Bcl-2 are supposed to exert better cytoprotection (3, 17). Whether more released Bcl-2 from the beclin 1/Bcl-2 complex plays a role in renoprotection in an ischemic kidney injury model and in cytoprotection in an oxidative stress-induced cell injury model was not examined in the present study. Bcl2AAA cells were more sensitive to H2O2 insults, and Bcl2AAA mice demonstrated more severe AKI after IRI, supporting that the concept that high levels of the beclin 1/Bcl-2 complex impair cellular defense mechanisms against oxidative stress. The higher cell death induced by H2O2 in Bcl2AAA transfected cells and the higher kidney damage induced by IRI in Bcl2AAA mice may result from lower autophagy activity rather than from dysfunction of antiapoptotic activity of Bcl-2 because Bcl2AAA impairs the cellular autophagy response to an autophagy inducer (24, 60, 61). However, there were conflicting effects of Bcl2AAA on cell death. The in vitro experiments showed that Bcl2AAA protects cultured cell death (3, 17), but the in vivo experiment showed that Bcl2AAA mice do not have either higher or lower apoptosis in the heart, muscles, and other tissues after exposure to autophagy induction (24), indicating that Bcl2AAA-associated increased cell death may not be resultant from modulation of the antiapoptotic effect of Bcl-2. Furthermore, beclin 1 was shown to bind to Bcl-XL, a member of the Bcl-2 family (56); whether becn1FA could increase Bcl-XL and hence upregulate antiapoptosis remains to be examined.
We showed that Klotho is renoprotective, prevents AKI, and reduces kidney damage initiated by several renal insults (12, 28, 59, 67, 71). We also showed that Klotho upregulates autophagy activity in the kidney, protects the kidney against IRI, and retards AKI to CKD progression (68). The present study does not only confirm the renoprotection of soluble Klotho against IRI-induced kidney damage but also provides new findings to disclose the molecular mechanism whereby Klotho upregulates autophagy activity through disrupting the negative regulatory beclin 1/Bcl-2 complex (18, 24, 61) and releasing more beclin 1 protein. Klotho renoprotection was partially attenuated by haploinsufficient beclin 1 and was severely abolished by mutant Bcl2AAA, thus strongly supporting the concept that Klotho protects the kidney, at least in part, through modulation of beclin 1 release from the beclin 1/Bcl-2 complex. The molecular mechanisms of dissociation of beclin 1/Bcl-2 complex formation by Klotho remain to be deciphered. The present study adds a new mechanism for renoprotection by Klotho to the listed renoprotective mechanisms of Klotho, including antiapoptosis (52, 59, 72), antioxidation (65, 77), suppression of nitric oxide synthase (13, 77), antisenescence (8), modulation of endoplasmic reticulum stress (2), and suppression of proinflammatory and profibrotic cytokine production and/or bioactivities (15, 16, 29, 39, 54, 78, 81).
The limitations of this study were as follows. First, the present study was designed to examine the acute effect of beclin 1 and the beclin 1/Bcl-2 complex on kidney damage induced by IRI and to provide combined in vivo and in vitro evidence to test our hypothesis. The chronic effect of autophagy on renal outcome after AKI is worth exploring in the future. Second, we found low autophagy activity by the determination of lower LC3-I-to-II conversion and accumulation of p62 and LC3 punctas, but we still do not know whether low autophagy activity results from decreased autophagosome degradation in autolysosomes. To address this question, a LC3-red fluorescent protein/GFP reporter mouse line should be used in the future. Third, we also need to explore whether and how beclin 1 and beclin 1/Bcl-2 complexes modulate kidney recovery and fibrosis process at later time points. The present study confirmed the role of Klotho in the disruption of beclin 1/Bcl-2 complexes. More work is required to illustrate how Klotho modulates the complex stability. Finally, all murine models used in this study had global manipulation of target genes. To illustrate the role of beclin 1 in individual tubular segments in renoprotection, conditional knockout of beclin in specific renal tubules will be desirable.
In conclusion, both beclin 1 and Klotho are potent renoprotective proteins. Reduced beclin 1 abundance and/or a decrease in beclin 1 activity caused by stabilization of the beclin 1/Bcl-2 complex render the kidney more susceptible to IRI, whereas an increase in free active beclin 1 because of disruption of the beclin 1/Bcl-2 complex renders the kidney more resistant to IRI. Klotho is a multifaceted protein that exerts its cytoprotection through multiple pathways. As shown in Fig. 12, dissociation of the beclin 1/Bcl-2 complexes to release more beclin 1 is one of the mechanisms underlying Klotho’s renoprotection. Thus, strategies to increase beclin 1 protein or/and to release beclin 1 from the beclin 1/Bcl-2 complex should upregulate autophagy activity and protect against acute kidney damage induced by IRI.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-091392 and R01-DK-092461 (to O. W. Moe and M. C. Hu), the O’Brien Kidney Research Center at University of Texas Southwestern (NIH Grant P30-DK-07938, to O. W. Moe), the Pak Center Innovative Research Support, Endowed Professors Collaborative Research Support, and the Pak-Seldin Center for Metabolic Research (to O. W. Moe and M. C. Hu). P. Li was supported by a Visiting Research Scholar sponsored by Yantai Yu-Huang-Ding Hospital, Qingdao University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.W.M. and M.C.H. conceived and designed research; P.L., M.S., J.M., J.S., and S.Y. performed experiments; P.L., M.S., J.M., J.S., O.W.M., and M.C.H. analyzed data; P.L., M.S., J.M., J.S., O.W.M., and M.C.H. interpreted results of experiments; P.L., M.S., J.M., J.S., S.Y., O.W.M., and M.C.H. prepared figures; O.W.M. and M.C.H. drafted manuscript; O.W.M. and M.C.H. edited and revised manuscript; O.W.M. and M.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Noboru Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) for providing the GFP-LC3 plasmid and the transgenic GFP-LC3 reporter mouse line and Dr. Makoto Kuro-o (Jichi Medical University, Japan) for providing the Tg-Kl mouse line. We are most grateful to Dr. Beth Levine (University of Texas Southwestern Medical Center) for providing the Becn1F121A, Becn1+/−, and Bcl2AAA mouse lines as well as the plasmids of human wild-type (WT) and mutant beclin 1F123A and mouse WT and triple mutant Bcl-2 at T69A, S70A, and S87A (AAA) as well as for valuable suggestions during the experiment and critical reading of the manuscript. The authors thank Brianna Flores and Nancy Gillings for technical assistance and Sudeepa Bhattacharya for administrative assistance.
The results presented in this paper have not been previously published, but parts of them were presented in the American Society of Nephrology-Kidney Week 2017 in New Orleans, LA.
Present address of P. Li: Dept. of Nephrology, Yantai Yu-Huang-Ding Hospital, Qingdao Univ., Yantai, Shandong, People’s Republic of China.
REFERENCES
- 1.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Zhao Y, Sarkar PS, Rosenblatt KP, Tilton RG, Choudhary S. Klotho ameliorates chemically induced endoplasmic reticulum (ER) stress signaling. Cell Physiol Biochem 31: 659–672, 2013. doi: 10.1159/000350085. [DOI] [PubMed] [Google Scholar]
- 3.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J 23: 1207–1216, 2004. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian A, Neyra JA, Zhan M, Hu MC. Klotho, stem cells, and aging. Clin Interv Aging 10: 1233–1243, 2015. doi: 10.2147/CIA.S84978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian A, Shi M, Flores B, Gillings N, Li P, Yan SX, Levine B, Xing C, Hu MC. Downregulation of autophagy is associated with severe ischemia-reperfusion-induced acute kidney injury in overexpressing C-reactive protein mice. PLoS One 12: e0181848, 2017. doi: 10.1371/journal.pone.0181848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE II, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17: 839–849, 2007. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 8.Carracedo J, Buendía P, Merino A, Madueño JA, Peralbo E, Ortiz A, Martín-Malo A, Aljama P, Rodríguez M, Ramírez R. Klotho modulates the stress response in human senescent endothelial cells. Mech Ageing Dev 133: 647–654, 2012. doi: 10.1016/j.mad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16. . Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553: 461–466, 2018. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Tong H, Chen Y, Chen C, Ye J, Mo Q, Zhao G, Hong G, Zheng C, Lu Z. Klotho ameliorates sepsis-induced acute kidney injury but is irrelevant to autophagy. OncoTargets Ther 11: 867–881, 2018. doi: 10.2147/OTT.S156891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuchana P, Mausset-Bonnefont AL, Mathieu M, Espinoza F, Teigell M, Toupet K, Ripoll C, Djouad F, Noel D, Jorgensen C, Brondello JM. Secreted α-Klotho maintains cartilage tissue homeostasis by repressing NOS2 and ZIP8-MMP13 catabolic axis. Aging (Albany NY) 10: 1442–1453, 2018. doi: 10.18632/aging.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciechomska IA, Tolkovsky AM, Goemans CG. Why doesn’t beclin 1, a BH3-only protein, suppress the anti-apoptotic function of Bcl-2? Autophagy 5: 880–881, 2009. doi: 10.4161/auto.9096. [DOI] [PubMed] [Google Scholar]
- 15.Doi S, Masaki T. Klotho as a therapeutic target during the development of renal fibrosis. Contrib Nephrol 189: 178–183, 2017. doi: 10.1159/000450776. [DOI] [PubMed] [Google Scholar]
- 16.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichhorn JM, Sakurikar N, Alford SE, Chu R, Chambers TC. Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death. Cell Death Dis 4: e834, 2013. doi: 10.1038/cddis.2013.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, Hirsch JA, Stein R, Pinkas-Kramarski R. Differential interactions between beclin 1 and Bcl-2 family members. Autophagy 3: 561–568, 2007. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- 19.Farré JC, Krick R, Subramani S, Thumm M. Turnover of organelles by autophagy in yeast. Curr Opin Cell Biol 21: 522–530, 2009. doi: 10.1016/j.ceb.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández AF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, He C, Ting T, Liu Y, Chiang WC, Marciano DK, Schiattarella GG, Bhagat G, Moe OW, Hu MC, Levine B. Disruption of the beclin 1-Bcl2 autophagy regulatory complex promotes longevity in mice. Nature 558: 136–140, 2018. [Erratum in Nature 561: E30, 2018.] doi: 10.1038/s41586-018-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein SL, Jaber BL, Faubel S, Chawla LS; Acute Kidney Injury Advisory Group of American Society of Nephrology . AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 8: 476–483, 2013. doi: 10.2215/CJN.12101112. [DOI] [PubMed] [Google Scholar]
- 22.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network . Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 6: 966–973, 2011. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havasi A, Dong Z. Autophagy and tubular cell death in the kidney. Semin Nephrol 36: 174–188, 2016. doi: 10.1016/j.semnephrol.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481: 511–515, 2012. [Erratum in Nature 503: 146, 2013.] doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C, Levine B. The beclin 1 interactome. Curr Opin Cell Biol 22: 140–149, 2010. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsia CCW, Ravikumar P, Ye J. Acute lung injury complicating acute kidney injury: A model of endogenous αKlotho deficiency and distant organ dysfunction. Bone 100: 100–109, 2017. doi: 10.1016/j.bone.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, Hsu C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 37: 289–296, 2012. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol 8: 423–429, 2012. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-O M, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 26: 1290–1302, 2015. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91: 1104–1114, 2017. doi: 10.1016/j.kint.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016. doi: 10.1681/ASN.2014101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z. Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8: 1009–1031, 2012. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hum JM, O’Bryan L, Smith RC, White KE. Novel functions of circulating Klotho. Bone 100: 36–40, 2017. doi: 10.1016/j.bone.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176: 1181–1192, 2010. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int 89: 779–791, 2016. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krick S, Baumlin N, Aller SP, Aguiar C, Grabner A, Sailland J, Mendes E, Schmid A, Qi L, David NV, Geraghty P, King G, Birket SE, Rowe SM, Faul C, Salathe M. Klotho inhibits interleukin-8 secretion from cystic fibrosis airway epithelia. Sci Rep 7: 14388, 2017. doi: 10.1038/s41598-017-14811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo CJ, Hansen M, Troemel E. Autophagy and innate immunity: insights from invertebrate model organisms. Autophagy 14: 233–242, 2018. doi: 10.1080/15548627.2017.1389824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 42.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenoir O, Tharaux PL, Huber TB. Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int 90: 950–964, 2016. doi: 10.1016/j.kint.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Levine B, Liu R, Dong X, Zhong Q. Beclin orthologs: integrative hubs of cell signaling, membrane trafficking, and physiology. Trends Cell Biol 25: 533–544, 2015. doi: 10.1016/j.tcb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Zviti R, Ha C, Wang ZV, Hill JA, Lin F. Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J Biol Chem 292: 13774–13783, 2017. doi: 10.1074/jbc.M117.791483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Shoji-Kawata S, Sumpter RM Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PG, Puyal J, Levine B. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA 110: 20364–20371, 2013. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12: 976–998, 2016. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol 34: 17–26, 2014. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis (Basel) 3: 15–23, 2017. doi: 10.1159/000452880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11: 510–516, 2011. doi: 10.1111/j.1447-0594.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 53.Mei S, Livingston M, Hao J, Li L, Mei C, Dong Z. Autophagy is activated to protect against endotoxic acute kidney injury. Sci Rep 6: 22171, 2016. doi: 10.1038/srep22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mytych J, Romerowicz-Misielak M, Koziorowski M. Klotho protects human monocytes from LPS-induced immune impairment associated with immunosenescent-like phenotype. Mol Cell Endocrinol 470: 1–13, 2018. doi: 10.1016/j.mce.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone 100: 41–49, 2017. doi: 10.1016/j.bone.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-beclin 1 peptide complex: beclin 1 is a novel BH3-only protein. J Biol Chem 282: 13123–13132, 2007. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 57.Ologunde R, Zhao H, Lu K, Ma D. Organ cross talk and remote organ damage following acute kidney injury. Int Urol Nephrol 46: 2337–2345, 2014. doi: 10.1007/s11255-014-0766-2. [DOI] [PubMed] [Google Scholar]
- 58.Pabón MA, Patino E, Bhatia D, Rojas-Quintero J, Ma KC, Finkelsztein EJ, Osorio JC, Malick F, Polverino F, Owen CA, Ryter SW, Choi AM, Cloonan SM, Choi ME. Beclin-1 regulates cigarette smoke-induced kidney injury in a murine model of chronic obstructive pulmonary disease. JCI Insight 3: e99592, 2018. doi: 10.1172/jci.insight.99592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem 284: 2719–2728, 2009. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Pedro JM, Wei Y, Sica V, Maiuri MC, Zou Z, Kroemer G, Levine B. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy 11: 452–459, 2015. doi: 10.1080/15548627.2015.1017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 64.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112: 1809–1820, 2003. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW. α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307: L566–L575, 2014. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rocchi A, Yamamoto S, Ting T, Fan Y, Sadleir K, Wang Y, Zhang W, Huang S, Levine B, Vassar R, He C. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet 13: e1006962, 2017. doi: 10.1371/journal.pgen.1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol 303: F1641–F1651, 2012. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 27: 2331–2345, 2016. doi: 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi M, Flores B, Li P, Gillings N, McMillan KL, Ye J, Huang LJ, Sidhu SS, Zhong YP, Grompe MT, Streeter PR, Moe OW, Hu MC. Effects of erythropoietin receptor activity on angiogenesis, tubular injury, and fibrosis in acute kidney injury: a “U-shaped” relationship. Am J Physiol Renal Physiol 314: F501–F516, 2018. doi: 10.1152/ajprenal.00306.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi M, McMillan KL, Wu J, Gillings N, Flores B, Moe OW, Hu MC. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab Invest 98: 1105–1121, 2018. doi: 10.1038/s41374-018-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, Tsuchiya K. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 302: F1252–F1264, 2012. doi: 10.1152/ajprenal.00294.2011. [DOI] [PubMed] [Google Scholar]
- 72.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 73.Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 138: 2247–2262, 2018. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 75.Vega-Rubín-de-Celis S, Zou Z, Fernández AF, Ci B, Kim M, Xiao G, Xie Y, Levine B. Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc Natl Acad Sci USA 115: 4176–4181, 2018. doi: 10.1073/pnas.1717800115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 11: 410–417, 2012. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wehling-Henricks M, Welc SS, Samengo G, Rinaldi C, Lindsey C, Wang Y, Lee J, Kuro OM, Tidball JG. Macrophages escape Klotho gene silencing in the mdx mouse model of Duchenne muscular dystrophy and promote muscle growth and increase satellite cell numbers through a Klotho-mediated pathway. Hum Mol Genet, 27: 14–29, 2018. doi: 10.1093/hmg/ddx380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30: 678–688, 2008. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 100: 15077–15082, 2003. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]