Abstract
Prior studies have implicated myosin light chain kinase (MLCK) in the regulation of aquaporin-2 (AQP2) in the renal collecting duct. To discover signaling targets of MLCK, we used CRISPR-Cas9 to delete the MLCK gene (Mylk) to obtain MLCK-null mpkCCD cells and carried out comprehensive phosphoproteomics using stable isotope labeling with amino acids in cell culture for quantification. Immunocytochemistry and electron microscopy demonstrated a defect in the processing of AQP2-containing early endosomes to late endosomes. The phosphoproteomics experiments revealed that, of the 1,743 phosphopeptides quantified over multiple replicates, 107 were changed in abundance by MLCK deletion (29 decreased and 78 increased). One of the decreased phosphopeptides corresponded to the canonical target site in myosin regulatory light chain. Network analysis indicated that targeted phosphoproteins clustered into distinct structural/functional groups: actomyosin, signaling, nuclear envelope, gene transcription, mRNA processing, energy metabolism, intermediate filaments, adherens junctions, and tight junctions. There was significant overlap between the derived MLCK signaling network and a previously determined PKA signaling network. The presence of multiple proteins in the actomyosin category prompted experiments showing that MLCK deletion inhibits the normal effect of vasopressin to depolymerize F-actin, providing a potential explanation for the AQP2 trafficking defect. Changes in phosphorylation of multiple proteins in the nuclear envelope prompted measurement of nuclear size, showing a significant increase in average nuclear volume. We conclude that MLCK is part of a multicomponent signaling pathway in both the cytoplasm and nucleus that includes much more than simple regulation of conventional nonmuscle myosins through myosin regulatory light chain phosphorylation.
Keywords: Aquaporin-2, endosomal trafficking, mass spectrometry, nuclear membrane, stimulated emission depletion microscopy, vasopressin
INTRODUCTION
Renal water excretion is regulated by the peptide hormone arginine vasopressin, in part through control of trafficking of membrane vesicles containing the water channel aquaporin-2 (AQP2) to and from the apical plasma membrane in collecting duct principal cells, thus regulating water reabsorption (28). Vasopressin acts by binding to the vasopressin V2 receptor, which signals via the heterotrimeric G protein α-subunit Gαs, resulting in the activation of adenylyl cyclase 6- and cAMP-mediated activation of PKA (14). Downstream from PKA is a broad signaling network associated with secondary activation of multiple protein kinases and intracellular Ca2+ mobilization (14, 22). Changes in intracellular Ca2+ concentration trigger numerous downstream signaling responses, in part through Ca2+/calmodulin-dependent effectors. One such effector is the protein kinase myosin light chain kinase (protein: MLCK and gene: Mylk). In isolated perfused collecting ducts, MLCK inhibitors, calmodulin inhibitors, Ca2+ chelators, and the nonmuscle myosin inhibitor blebbistatin have been shown to markedly attenuate vasopressin’s effect to increase osmotic water transport (6–8). Consequently, MLCK is believed to be required for control of AQP2 trafficking and water transport in kidney collecting duct cells.
Among the 21,000 protein coding genes in mammalian genomes are ~500 protein kinase genes (24). Some of these kinases, such as PKA, have many substrates and regulate a broad network of proteins (18). Others, such as PRKR-like endoplasmic reticulum kinase (PERK), have only one key substrate [eukaryotic translation initiation factor 2A (Eif2a) for PERK] and a highly specific function (negative regulation of protein translation in response to endoplasmic reticulum stress for PERK) (33). MLCK is generally considered to be in the second category. To address whether MLCK selectively phosphorylates myosin regulatory light chain in AQP2-expressing renal epithelial cells, we used genome editing (CRISPR-Cas9) to ablate Mylk gene expression in mpkCCD cells followed by large-scale phosphoproteomics analysis using protein mass spectrometry to identify downstream signaling that is dependent on MLCK-mediated phosphorylation.
The canonical target for MLCK, myosin regulatory light chain, is represented by three paralogous genes (Myl9, Myl12a, and Myl12b) that regulate conventional nonmuscle myosins, which are critical for maintenance of cell shape, size, and motility in a variety of nonmuscle cell types (19, 37). Two conventional nonmuscle myosins are expressed in collecting duct principal cells: nonmuscle myosin II-A (gene: Myh9) and nonmuscle myosin II-B (gene: Myh10) (5). Classically, phosphorylation of myosin regulatory light chain by MLCK induces conformational changes in the heavy chains (i.e., myosin II-A or II-B), which increase their ability to bind filamentous actin (F-actin) to generate “stress fibers.” Prior studies have demonstrated that vasopressin decreases the density of stress fibers in collecting duct cells, which was implicated in the regulation of AQP2 trafficking (21). Phosphorylation changes at the COOH-terminal ends of the heavy chains (distal tail domain) are also thought to regulate nonmuscle myosin function (27, 31).
Genome editing, here achieved by introduction of mutations in the Mylk gene using the CRISPR-Cas9 technique, provides a facile means to investigate the roles of protein kinases in particular cell types. In contrast to siRNA knockdown, CRISPR-Cas9 allows complete ablation of protein expression of target genes. Deletion of individual protein kinases, combined with modern large-scale phosphoproteomics, allows mapping of phosphorylation networks that can include direct targets of the deleted kinase as well as secondary targets that can result from modification of other protein kinases and phosphatases (18). Here, CRISPR-Cas9 combined with phosphoproteomics revealed just such a network for MLCK, extending known actions of MLCK beyond its canonical role in myosin regulatory light chain phosphorylation.
MATERIALS AND METHODS
Cell culture.
Mouse mpkCCD clone 11 (mpkCCDC11) cells and subclones were cultured as previously described (47). Cells were grown on membrane supports (catalog nos. 3419, 3460, and 3450, Corning) in DMEM-F-12 medium containing 2% FCS and 0.1 nM 1-desamino-8-d-arginine-vasopressin (dDAVP) and supplemented with 5 μg/mL insulin, 50 nM dexamethasone, 1 nM triiodothyronine, 10 ng/mL epidermal growth factor, 60 nM sodium selenite, and 5 μg/mL transferrin for 4–7 days. The medium was changed to serum-free medium with 0.1 nM dDAVP and maintained for 3 days to ensure complete polarization. Transepithelial resistance was measured using an epithelial volt ohmmeter (EVOM, WPI), and growth medium was changed daily. For short-term experiments, dDAVP-treated cells were incubated in the absence of dDAVP for 2 h and then exposed to either 0.1 nM dDAVP or vehicle for 30 min.
Generation of MLCK-null and MLCK-intact cell lines.
Lipofectamine 3000 (Invitrogen) was used, according to the manufacturer’s instructions, to transfect mpkCCDC11–38 cells with pCMV-Cas9-green fluorescent protein (GFP) plasmids (Sigma) containing guide RNAs (gRNAs) specific for the Mylk gene. A cell sorter (FACSAria II, BD) was used to sort GFP-expressing cells into 96-well plates (∼1 cell/well). These cells were cultured for 1–2 wk and then transferred to 24-well plates. Target gene expression was evaluated by Western blot analysis for MLCK, and genomic indel mutations were further identified by genomic sequencing (see below). MLCK-intact (control) lines were made from cells that were subjected to this protocol but continued to express the Mylk gene with unmutated sequence, as confirmed by Sanger sequencing. The sequences of the four gRNAs were as follows: 5′-GACGTTGACTGCACGCACTCGG-3′ for gRNA1, 5′-GGACAAGTCTTCCGACTTGTGG-3′ for gRNA2, 5′-TTCAAGGCCTATTCCGCCAAGG-3′ for gRNA3, and 5′-TTTGAGCGTATCATTGACGAGG-3′ for gRNA4.
Genomic sequencing.
Genomic DNA of cultured cells was extracted using the DNeasy Blood & Tissue Kit (Qiagen). A ~400-bp region flanking the target sites was amplified by PCR and cloned into pGEM-T Easy plasmid (Promega) using JM109 competent cells. At least six plasmids were sequenced for each cell line.
Immunoblot analysis and antibodies.
Samples were prepared for immunoblot analysis as previously described by Isobe et al. (18). After SDS-PAGE on 12% polyacrylamide or gradient minigels, proteins were transferred electrophoretically to nitrocellulose membranes, blocked, and probed with primary antibodies. Blocking buffer and infrared fluorophore-conjugated secondary antibodies were obtained from LI-COR (Lincoln, NE). Fluorescence images were visualized using the LI-COR Odyssey system. Band intensities were analyzed by LI-COR Image Studio software. Rabbit polyclonal antibodies for AQP2 (15) and phosphorylated (S269) AQP2 (15) were generated in our laboratory. In the Western blots, the AQP2 antibodies recognized strong bands at 37 kDa (glycosylated) and 29 kDa (nonglycosylated) (see results), as is typically seen for AQP2 in both mouse mpkCCD cells (47) and native rat inner medullary collecting duct cells (15). Rabbit anti-MLCK antibody was a gift from Dr. Primal deLanerolle (University of Illinois, Chicago, IL) and was characterized in our laboratory by Chou et al. (6) using preadsorption controls with turkey gizzard MLCK.
Immunofluorescence microscopy.
Immunofluorescence labeling was performed as previously described (35). All antibodies were diluted at 1:100 except for the anti-AQP2 antibody, which was diluted at 1:250. Confocal fluorescence images were obtained using the LSM 780 confocal microscope system (Carl Zeiss, Light Microscopy Core Facility at the National Heart, Lung, and Blood Institute). Stimulated emission depletion (STED) images were obtained using the Leica SP8 STED ×3/confocal microscope (Leica Microsystems, Light Microscopy Core Facility at the National Heart, Lung and Blood Institute). Images were analyzed by Imaris software (Oxford Instruments, Zurich, Switzerland).
AQP2 surface retrieval assay.
Surface biotinylation procedures were carried out as recommended by the manufacturer (Fisher Scientific) and as previously described by Loo et al. (23) and Moeller et al. (26) with minor modifications. Cells were grown on six-well Transwell supports for 10 days with 0.1 nM dDAVP, washed with ice-cold medium, and placed on ice for 20 min. All the following steps were performed on ice. The apical medium was rinsed with ice-cold GlycoLink coupling buffer (catalog no. 88944, ThermoFisher Scientific). The apical medium was changed to oxidation buffer (GlycoLink coupling buffer + 20 mM NaIO4) and incubated for 30 min on ice in darkness. After the cells had been washed three times with GlycoLink coupling buffer, 900 μL of GlycoLink coupling buffer containing 0.9% aniline and 100 μL of 50 mM alkoxyamine-PEG4-SS-PEG4-biotin (catalog no. 26138, ThermoFisher Scientific) were added, and the cells were incubated for 40 min. After removal of the biotinylation buffer, the apical medium was switched to stabilization buffer [PBS + 50 mM NH4Cl (pH 7.4)] for 5 min. After removal of the stabilization buffer, the apical and basolateral wells were washed three times with serum-free medium (37°C), both wells were switched to serum-free medium (37°C), and the wells were incubated for 10, 20, or 40 min at 37°C. To strip surface biotin, the apical well was washed with ice-cold PBS (pH 7.4), treated with ice-cold biotin stripping solution [100 mM sodium 2-mercaptoethanesulfonate, 100 mM NaCl, 1 mM EDTA, 50 mM Tris (pH 8.6), and 0.2% BSA], and incubated on ice for 20 min. The apical wells were washed with ice-cold PBS (pH 7.4), and the cells were lysed with 400 μL of 0.5% Laemmli buffer [0.5% SDS and 10 mM Tris (pH 6.8)] containing a protein phosphatase and protease inhibitor cocktail (catalog no. 78440, ThermoFisher Scientific). Then, 150 μL of Dynabeads (MyOne Streptavidin T1, catalog no. 65601, ThermoFisher Scientific) in a 1.5-mL tube were washed with 0.5% SDS-Laemmli buffer. After removal of 40 μL of cell lysate, the remaining cell lysate was added to the beads and incubated overnight at 4°C with gentle rotation. After removal of the unbound fraction, the beads were washed five times with 1 mL of 0.5% SDS-Laemmli buffer. Bound proteins on the beads were eluted in 60 μL of 2% SDS sample buffer containing 200 mM dithiothreitol at 95°C for 15 min.
Stable isotope labeling with amino acids in cell culture.
All stable isotope labeling with amino acids in cell culture (SILAC) reagents were purchased from Invitrogen. All three pairs of MLCK-intact (Mylk+) and MLCK-null (Mylk–) cells were grown in culture medium containing the amino acids arginine and lysine labeled with stable isotopes: [12C6,14N4]arginine and [12C6]lysine (“light”) and [13C6,15N4]arginine and [13C6]lysine (“heavy”), respectively, for 17 days (5 passages). Cells were grown on membrane supports in DMEM-F-12 medium with serum, 0.1 nM dDAVP, and light and heavy amino acids for 4 days. The serum was then removed and maintained for 3 days. Protein lysates (2 mg) were mixed equally and subjected to proteomics procedures.
Sample preparation for LC-MS/MS and phosphopeptide purification.
Phosphoproteomic analysis was carried out as previously described with modifications (32). Cells were lysed and sonicated with 8 M urea buffer [8 M urea, 50 mM Tris·HCl, 75 mM NaCl, and protease and phosphatase inhibitors (catalog no. 78440, ThermoFisher Scientific)]. Samples were reduced with 20 mM dithiothreitol (catalog no. 20291, ThermoFisher Scientific) for 1 h at 25°C and then alkylated with iodoacetamide (final concentration: 40 mM, catalog no. 90034, ThermoFisher Scientific) for 1 h at 25°C in darkness. Samples were further diluted to a final concentration of <1 M urea with 20 mM triethylammonium bicarbonate buffer (pH 8.5, catalog no. T7408, Sigma) and digested in solution with trypsin/Lys-C (1:20 by weight, catalog no. V5072, Promega) overnight at 37°C. The peptides were desalted using hydrophilic-lipophilic-balanced extraction cartridges (catalog no.WAT094225, Oasis, Milford, MA). Samples were vacuum dried and subjected to high-pH reversed-phase fractionation (12 fractions/sample).
Peptides were fractionated across an XBridge BEH C18 reversed-phase column (4.6 × 250 mm, Waters). Fractions were collected every minute and pooled into 12 samples, which were dried for further analysis. Each fraction was divided into three parts for total peptide analysis (2%) and two phosphopeptide enrichment analyses (49% each) using Fe-NTA and TiO2 columns (catalog nos. 88300 and 88301, ThermoFisher Scientific) according to the manufacturer’s protocols and then vacuum dried and stored at −80°C. Before mass spectrometry analysis, samples were dissolved in 0.1% formic acid (catalog no. 28905, ThermoFisher Scientific) in LC-MS-grade water (catalog no.9831, J. T. Baker Chemicals).
LC-MS/MS analysis.
Total peptides and phosphopeptides were analyzed using an Eksigent NanoLC system connected to an Orbitrap Elite mass spectrometer (ThermoFisher Scientific). Precursor mass spectra (MS1) were acquired in the orbitrap at 60,000 full width at half-maximum. Product mass spectra (MS2) were obtained with the ion trap. MS spectra were analyzed using Proteome Discoverer 1.4 (ThermoFisher Scientific). To obtain comprehensive spectra-peptide matches, we used Mascot and SEQUEST to integrate the results of peptide-spectra matching. The search criteria were set as follows: 1) 20-ppm precursor mass tolerance, 2) 0.05-Da fragment mass tolerance, and 3) two missed cleavages per peptide. Specific amino acid modifications were as follows: 1) isotope labeling of lysine (K + 6.020 Da) and arginine (R + 10.008 Da), 2) carbamidomethylation of cysteine (C + 57.021 Da), 3) oxidation of methionine (M + 15.995 Da), 4) deamination on glutamine and asparagine (N, Q + 0.984 Da), and 5) phosphorylation of serine, threonine, and tyrosine (S, T, Y + 79.966 Da). The mouse Swiss-Prot database (July 10, 2016) was used. The false discovery rate was calculated by the target-decoy algorithm (12). The results from SEQUEST and Mascot searches were integrated in Proteome Discoverer 1.4 with the following filters: false discovery rate < 0.01 and peptide rank = 1. Relative quantification of peptides and phosphopeptides was performed using the quantification module within Proteome Discoverer 1.4 for SILAC data, which calculates relative peptide abundance ratios from light and heavy channels using the areas under the curve for reconstructed MS1 ion chromatograms corresponding to each peptide. The maximum ratio is set to 1,000, and the minimum ratio is set to 0.001. The missing value of a SILAC channel is replaced by the minimum intensity detectable for the run. The probabilities of the phosphorylation site localizations were calculated based on the given MS2 data using the phosphoRS 3.1 (42) module within Proteome Discoverer 1.4. Peptides with a phosphorylation site probability of >80% were selected for further analysis. The mass spectrometry data are available on a publicly accessible webpage (https://hpcwebapps.cit.nih.gov/ESBL/Database/MYLKPhospho/MLCK.html).
Bioinformatics.
We used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING; https://string-db.org/) to organize regulated phosphoproteins into a network built on functional interactions. The undirected graph generated by STRING was expanded by manual curation of interactions from UniProt records and the literature. The resulting network was converted to a publication-quality figure using Cytoscape (https://cytoscape.org/) and Inkscape (https://inkscape.org/).
Electron microscopy.
Polarized monolayers of cells grown on six-well Transwell plates were fixed in 2.5% glutaraldehyde, 1% formaldehyde, and 0.1 M sodium cacodylate buffer (pH 7.4) for conventional transmission electron microscopy. Specimens were rinsed in cacodylate buffer, postfixed with 1% OsO4 in the same buffer on ice, en bloc stained with 1% uranyl acetate, dehydrated in an ethanol series, and embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections were cut parallel to the adherent surface, stained with uranyl acetate and lead citrate, and viewed with an electron microscope (JEM1400, JEOL USA, Peabody, MA) equipped with a digital camera (model XR-111, Advanced Microscopy Techniques, Woburn, MA).
Statistics.
We used simple unpaired t tests, with P < 0.005 as a significance threshold, to compare various measures (band density on Western blots, vesicle diameter, surface biotinylation, apical surface area of cells, and nuclear volumes) for MLCK-intact and MLCK-null cells.
Data availability.
Protein mass spectrometry data (raw files, search results, and spectra) have been uploaded to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD014985.
RESULTS
Cultured mpkCCD cells replicate key regulatory mechanisms seen in native collecting ducts.
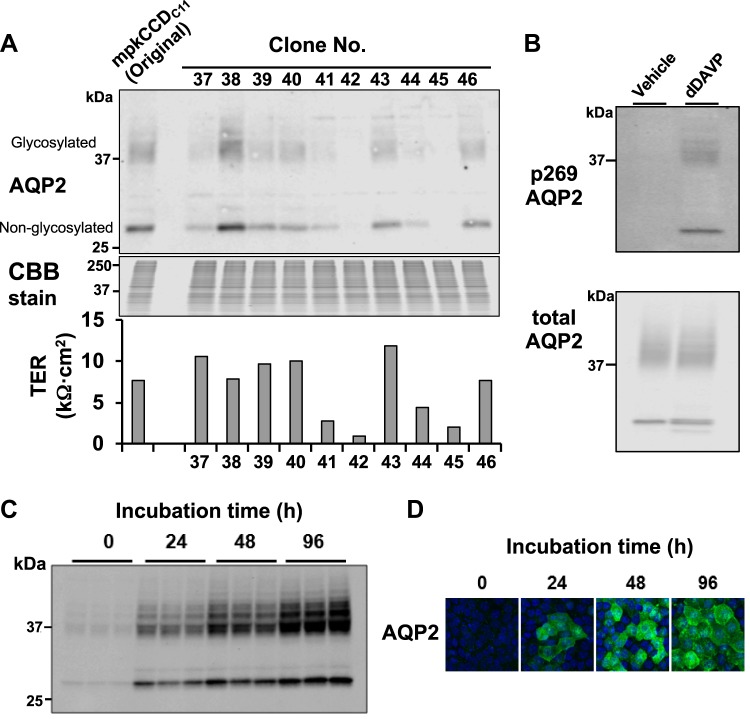
We have observed that as mpkCCD cells are passaged, they become more heterogeneous, requiring recloning of the cells to maximize AQP2 protein abundance and minimize cell-to-cell variability (47). Here, we again recloned mpkCCD cells and, using Western blot analysis, found highly variable AQP2 expression among the clones, as observed previously (47) (Fig. 1A). [On Western blots, AQP2 is typically found at both 37 kDa (glycosylated) and 29 kDa (nonglycosylated), matching the bands seen here.] We chose the clone expressing AQP2 at the highest level (clone 38) for further study. This clone, grown on permeable supports in the presence of vasopressin, was found to maintain a high resistance, similar to the original cells (Fig. 1A). To ensure that these cells continue to be an appropriate physiological model of the renal collecting duct, we investigated the short-term (Fig. 1B) and long-term (Fig. 1, C and D) responses to vasopressin. Short-term (30-min) exposure to the vasopressin analog dDAVP (0.1 nM) resulted in a marked increase in AQP2 phosphorylation at S269 (Fig. 1B), consistent with prior observations in native collecting ducts (15, 25). The protein kinase responsible for S269 phosphorylation remains unknown. Long-term exposure to vasopressin resulted in a marked increase in AQP2 protein abundance (Fig. 1, C and D), mimicking responses in the native collecting duct (11). Based on these observations, we conclude that mpkCCD clone 38 mimics the most important aspects of AQP2 regulation in the native collecting duct and, therefore, is an appropriate cell model relevant to the native kidney.
Fig. 1.
Isolation and characterization of vasopressin-responsive mpkCCD clones expressing a high level of aquaporin-2 (AQP2). A, top: Western blot showing highly variable AQP2 expression among various mpkCCD clones. Glycosylated and nonglycosylated AQP2 bands are indicated. A, bottom: corresponding transepithelial resistances (TERs) for each clone. Clone 38 was selected and used in all subsequent experiments. CBB, Cooomassie brilliant blue. B: Western blot confirming expected increases in S269 phosphorylation (p269) in response to short-term (30-min) exposure to 0.1 nM 1-desamino-8-d-arginine-vasopressin (dDAVP). C and D: Western blot (C) and immunofluorescence (D) images confirming expected increases in AQP2 protein abundance at 0–96 h after the introduction of dDAVP (0.1 nM). mpkCCD clone 38 mimicked the typical responses to vasopressin in native collecting ducts.
CRISPR-Cas9 deletion of the Mylk gene.
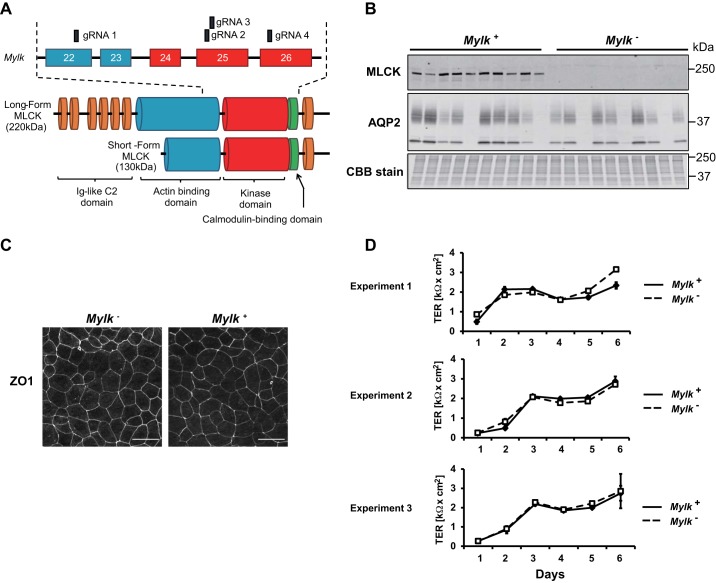
We used CRISPR-Cas9 to introduce mutations that ablate Mylk gene expression in mpkCCD clone 38 cells. We used gRNAs targeting four different sites corresponding to exons 22, 25, and 26 (independently transfected), coding for the kinase domain (catalytic region) of the MLCK protein or the region just upstream (Fig. 2A). As shown in Fig. 2B, numerous single cell clones shown by immunoblot analysis to be MLCK null were retrieved, while others had intact MLCK expression, due presumably to gRNA mistargeting. Note that the long form of MLCK (220 kDa) (19) was the major MLCK isoform. These latter clones were used as controls (“MLCK-intact cells”) for further experiments. We chose a total of three separate MLCK-null-MLCK-intact pairs for phosphoproteomic analysis. The Mylk gene was sequenced by the Sanger method for all clones used in further experiments, confirming frame-shifting mutations in MLCK-null cells and a lack of Mylk mutations in control MLCK-intact cells. Both MLCK-intact (Mylk+) and MLCK-null (Mylk−) lines formed polarized epithelia with organized tight junctions, as evidenced by zonula occludens 1 (tight junction protein 1) labeling (Fig. 2C). They developed high transepithelial resistances that appeared to be unaffected by MLCK deletion (Fig. 2D).
Fig. 2.
Generation and characterization of myosin light chain kinase (MLCK)-null and MLCK-intact cells. A: domain architecture of the Mylk gene and MLCK protein. Exons targeted by guide RNAs (gRNAs) are indicated. B: immunoblot of multiple MLCK-intact (Mylk+) and MLCK-null (Mylk−) clones showing MLCK protein levels along with aquaporin-2 (AQP2) protein levels and a Coomassie brilliant blue (CBB)-stained gel of total protein. C: MLCK-intact and MLCK-null cells formed polarized monolayers, as shown by zonula occludens 1 (ZO-1) staining, when grown on permeable membrane supports. D: development of transepithelial resistances (TERs) for three different pairs of MLCK-intact and MLCK-null cells.
MLCK deletion affects AQP2 trafficking.
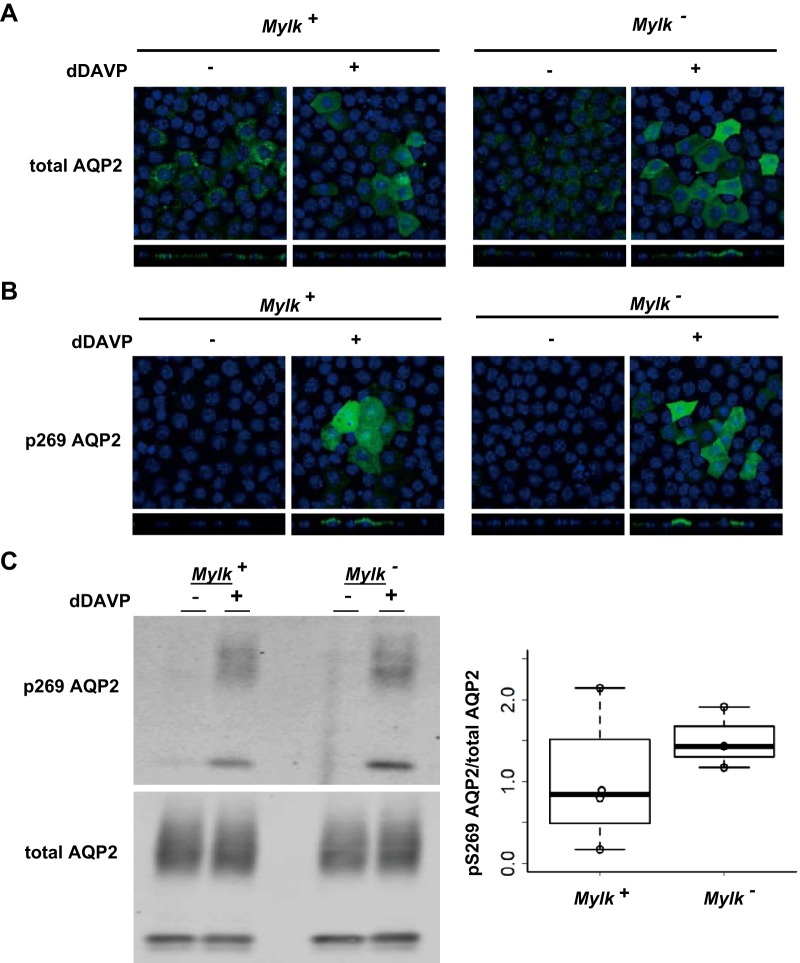
Studies in isolated perfused collecting ducts have provided evidence that MLCK is involved in short-term regulation of osmotic water transport (6–8), presumably through effects on membrane trafficking of AQP2. To assess AQP2 trafficking in MLCK-null versus MLCK-intact cells, we used immunofluorescence immunocytochemistry. Figure 3A shows that AQP2 redistribution to the apical region of the cell in response to 30 min of exposure to dDAVP was not blocked by MLCK deletion. Furthermore, both MLCK-intact and MLCK-null cells showed an increase in AQP2 phosphorylation at S269 in response to dDAVP (Fig. 3B). S269 phosphorylation is thought to be a key determinant of AQP2 trafficking through inhibition of AQP2 endocytosis (15, 25). The lack of an effect of MLCK deletion on the vasopressin-induced increase in phosphorylation of AQP2 at S269 was confirmed by Western blot analysis (Fig. 3C). Thus, MLCK does not appear to be necessary for AQP2 phosphorylation at S269, and the protein kinase responsible remains unknown.
Fig. 3.
Apical distribution and phosphorylation of aquaporin-2 (AQP2) after exposure to 1-desamino-8-d-arginine-vasopressin (dDAVP). A and B: representative confocal images of myosin light chain kinase (MLCK)-intact (Mylk+) and MLCK-null (Mylk−) cells labeled with anti-AQP2 and anti-phosphorylated (S269) AQP2 (p269 AQP2) antibodies. Note redistribution of AQP2 from intracellular location to apical region in response to 30 min of exposure to 0.1 nM dDAVP. C: typical Western blot (left) and ratio of phosphorylated (S269) AQP2 to total AQP2 (right). Note the increased S269 phosphorylation of AQP2 after 30 min of treatment with dDAVP (0.1 nM) for both MLCK-intact and MLCK-null cells.
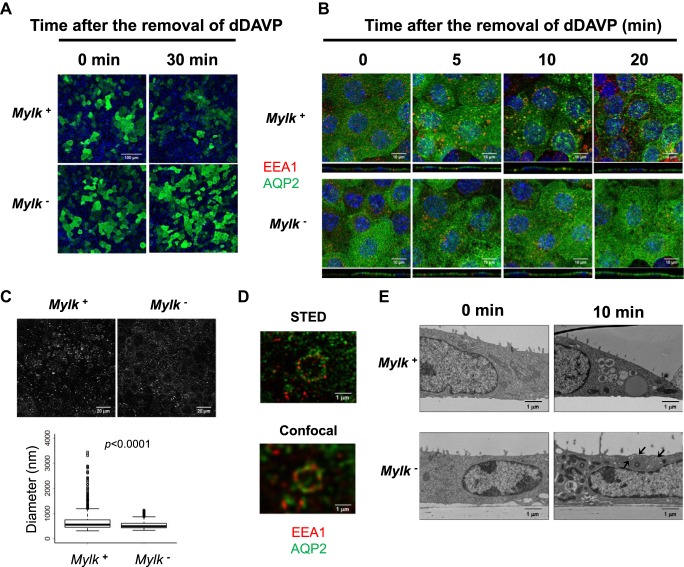
In contrast to findings with addition of dDAVP, we observed substantial differences in the distribution of AQP2 during washout of dDAVP from the basolateral bathing solution between MLCK-null and MLCK-intact cells (Fig. 4A). In MLCK-intact cells, AQP2 was redistributed into a punctate pattern 30 min after dDAVP washout, typical of the response in native collecting duct cells (29). In contrast, there was no obvious redistribution with dDAVP washout in MLCK-null cells. This suggests that either endocytosis occurred or was inhibited, but the endocytic vesicles remained just under the apical plasma membrane due to failure of normal endosomal processing. To look more deeply, using simultaneous labeling of AQP2 and the endosomal marker early endosome antigen 1 (EEA1), we repeated the experiments at 0, 5, 10, and 20 min (Fig. 4B). In MLCK-intact cells, with dDAVP washout, AQP2 appeared rapidly in EEA1-positive vesicles, many of which were very large, consistent with multivesicular bodies, as previously seen with vasopressin withdrawal in isolated perfused collecting ducts (29). In contrast, the very large EEA1-positive vesicles failed to appear in MLCK-null cells. This finding is consistent with a general decrease in endosomal processing to late endosomes in MLCK-null cells. Quantification of EEA1-positive vesicle size (Fig. 4C) statistically confirmed the lack of large vesicles in MLCK-null cells.
Fig. 4.
Subcellular distribution of aquaporin-2 (AQP2) and early endosome antigen 1 (EEA1) after 1-desamino-8-d-arginine-vasopressin (dDAVP) washout. A: representative immunofluorescence images of AQP2 (green) in the presence of dDAVP (0 min) and 30 min after dDAVP washout in myosin light chain kinase (MLCK)-intact (Mylk+) and MLCK-null (Mylk−) cells. B: confocal images of AQP2 (green) and EEA1 (red) at 0, 5, 10, and 20 min after dDAVP removal. Nuclei are blue. C: representative image of EEA1-containing vesicles 5 min after dDAVP removal (top) and box plot of diameter of EEA1 vesicles (bottom). D: high-resolution stimulated emission depletion (STED) and confocal images of a large endosome in MLCK-intact cells. Note colocalization of AQP2 (green) and EEA1 (red) within the same endosome. E: electron microscope image of MLCK-intact and MLCK-null cells in the presence of dDAVP (left) and 10 min after washout (right). Note the large multivesicular bodies after washout in MLCK-intact, but not MLCK-null, cells. Arrows at bottom right point to small subapical vesicles seen in MLCK-null, but not MLCK-intact, cells.
The large EEA1-positive vesicles were visualized by superresolution microscopy (STED) (Fig. 4D). The AQP2 labeling in the lumens of these vesicles (green) supports the conclusion that the large vesicles are multivesicular bodies. The presumptive identification of these vesicles as multivesicular bodies was confirmed directly by electron microscopy (Fig. 4E), showing large multivesicular bodies 10 min after dDAVP withdrawal in MLCK-intact, but not MLCK-null, cells. In addition, after vasopressin washout, there appeared to be abundant small vesicles just beneath the apical plasma membrane in MLCK-null, but not MLCK-intact, cells. This is presumably responsible for sustained labeling in the apical region after vasopressin washout in MLCK-null cells. The general picture is therefore consistent with an effect of MLCK deletion on endosomal processing, but not on endocytosis per se.
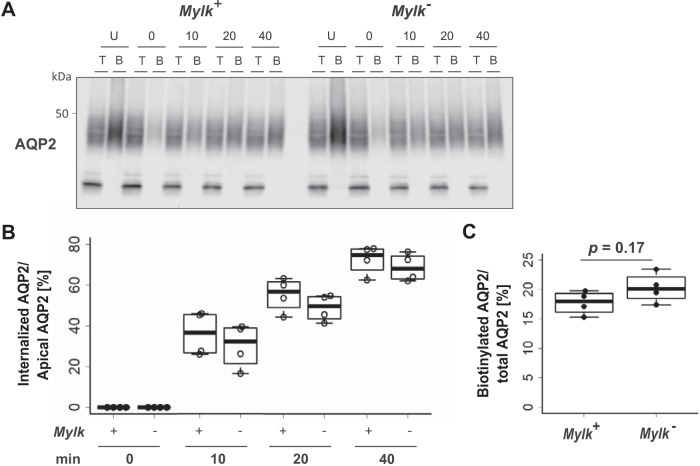
To measure endocytosis directly, we carried out surface biotinylation protection experiments using a derivatizing agent that allows biotin to be cleaved from surface proteins with 2-mercaptoethanesulfonate, while intracellular biotin is protected and remains attached. A representative experiment is shown in Fig. 5A. Here, the protected (intracellular) fraction of AQP2 increased with time in both MLCK-null and MLCK-intact cells (see columns labeled “B”). The rate of internalization of AQP2 in MLCK-null cells was not significantly different from that in MLCK-intact cells (Fig. 5B). These results show that the deletion of MLCK does not appreciably affect endocytosis of AQP2, despite the demonstrated changes in endosomal processing. Furthermore, there was no difference in the ratio of apical AQP2 to total AQP2 at time 0 between MLCK-null and MLCK-intact cells (Fig. 5C).
Fig. 5.
Time course of surface retrieval of aquaporin-2 (AQP2) after 1-desamino-8-d-arginine-vasopressin (dDAVP) washout. A: immunoblot analysis of surface-labeled AQP2 at 0, 10, 20, and 40 min. T, total lysate; B, eluted biotinylated proteins; U, samples not treated with sodium 2-mercaptoethanesulfonate (MesNa). Cells were treated with MesNa at 0, 10, 20, and 40 min after dDAVP removal and lysed. B: ratio of internalized AQP2 to apical AQP2 in myosin light chain kinase (MLCK)-intact (Mylk+) and MLCK-null (Mylk−) cells at 0, 10, 20, and 40 min after dDAVP removal. Values are means ± SE; n = 4. C: quantitative analysis of apical AQP2 normalized by total AQP2.
Phosphoproteomics.
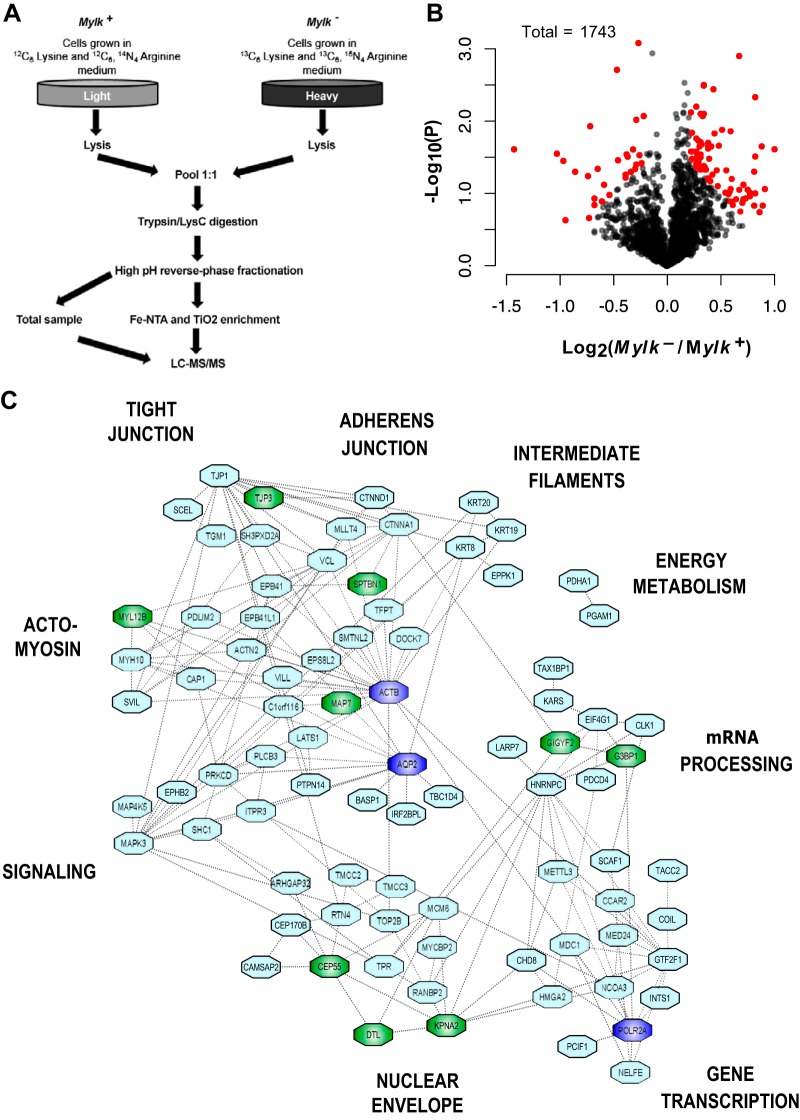
The main goal of this work was to identify phosphorylation events that are dependent on MLCK in mpkCCD cells. We used SILAC for quantification in large-scale phosphoproteomic analysis (Fig. 6A). Proteins from MLCK-null and MLCK-intact cells are separately labeled by culture in amino acids with different stable carbon and nitrogen isotopes in the amino acids lysine and arginine (30). Each replicate was done with a different pair of CRISPR clones and is a true biological replicate. All cells were grown in the presence of dDAVP (0.1 nM dDAVP was present continuously in the growth medium). With SILAC, the MLCK-null and MLCK-intact cell lines are then combined into a single sample, allowing each peptide to be “seen” twice by the mass spectrometer at two slightly different molecular masses. The relative amounts of a given phosphopeptide from MLCK-intact versus MLCK-null cells are calculated from their respective peak heights in the MS1 spectra integrated over time.
Fig. 6.
Phosphoproteomic analysis of myosin light chain kinase (MLCK)-null (Mylk−) versus MLCK-intact (Mylk+) mpkCCD cells. A: MLCK-intact and MLCK-null cells were grown in medium containing light ([12C6]lysine and [12C6,14N4]arginine) or heavy ([13C6]lysine and [13C6,15N4]arginine) amino acids for ≥17 days. Cells were grown on membrane supports in 1-desamino-8-d-arginine-vasopressin (dDAVP)-containing medium for 7 days. Equal amounts of protein from cell lysates were mixed, digested with trypsin/Lys-C, and fractionated using high-pH reversed-phase fractionation. Phosphorylated peptides were enriched using Fe-NTA and TiO2 columns and analyzed by LC-MS/MS. An aliquot not enriched for phosphopeptides was also analyzed to quantify each protein, i.e., “total proteins.” B: volcano plot of 1,743 proteins quantified in all 3 pairs of samples. Red points indicate phosphopeptides with Pjoint < 0.025 and log2(Mylk−/Mylk+) > 0.2. C: regulated phosphoproteins cluster into specific functional and structural groups. The undirected graph shows functional interactions (dashed lines) among phosphoproteins altered in response to Mylk gene deletion. Relationships were identified using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and UniProt annotations. Putative direct targets of MLCK are indicated in green. Three extra nodes (dark blue), namely, AQP2, RNA polymerase II (Polr2a), and β-actin (Actb), were inserted to increase dimensionality of the network. Peripheral labels provide descriptive identifiers for each major cluster.
The general results are shown as a “volcano plot” in Fig. 6B. Most phosphopeptides did not change substantially in abundance with MLCK deletion. However, based on stringent criteria [Pjoint < 0.025 and absolute value of log2(Mylk−/Mylk+) > 0.2] with observations in three independent clones with independent controls, we found demonstrable changes in 107 of 1,743 peptides quantified in all 3 pairs [Fig. 6B; see Supplemental Data Set S1 (Supplemental Data are available online at https://hpcwebapps.cit.nih.gov/ESBL/Database/MLCK-Data/index.html)]. Of these 107 peptides, 29 were decreased and 78 were increased. These data have been made available to users on a publicly accessible webpage (https://hpcwebapps.cit.nih.gov/ESBL/Database/MYLKPhospho/MLCK.html). Extended phosphoproteomic data reporting all phosphopeptides quantified in at least two replicates (n = 3238) are provided as Supplemental Data Set S3.
The total protein abundances (independent of phosphorylation status) were also quantified in samples taken before the phosphopeptide enrichment step (see Supplemental Data Set S2). These data showed that none of the phosphopeptides identified as changed in abundance were changed because of an effect on total protein abundance. Supplemental Table S1 provides the 16 proteins (of 2,732 proteins quantified in all 3 MLCK-null-MLCK-intact pairs) that changed in association with Mylk deletion. These data also confirmed that the MLCK-null clones indeed lack the MLCK protein.
Table 1 shows the 29 phosphorylation sites that showed decreases in abundance in MLCK-null cells relative to MLCK-intact cells, which include putative direct targets of MLCK. As expected, the canonical target for MLCK (S20 of myosin regulatory light chain, Myl12b, with amino acid numbering based on UniProt record Q3THE2) showed a significant decrease in phosphorylation. At the same time, eight other sites that were decreased showed a motif similar to that of S20 of Myl12b, i.e., -X-(S/T)-p(S/T)-X, which we refer to below as a “double-S/T” motif (indicated in Table 1). Thus, the phosphoproteins Myl12b, zonula occludens-3 (tight junction proten 3), denticleless (Dtl), ensconsin (Map7), importin-α1 (Kpna2), centrosomal protein of 55 kDa (Cep55), spectrin β-chain, nonerythrocytic 1 (Sptbn1), Ras GTPase-activating protein-binding protein 1 (G3bp1), and PERQ amino acid-rich with GYF domain-containing 2 (Gigyf2) can be regarded as putative direct MLCK targets at the amino acid sites shown in Table 1. Among these targets are two proteins (G3bp1 and Gigyf2) critical to the formation of mRNA storage organelles, or “stress granules.” It is noteworthy that S20 phosphorylation of Myl12b (the canonical target of MLCK) did not fall nearly as much as many of the other double-S/T motif sites (Table 1). The limited fall in phosphorylation at this site was not unexpected, since several protein kinases other than MLCK, such as Rho-associated coiled coil-containing kinase (ROCK), death-associated protein kinase 3 (DAPK3), and CDC42-binding kinase (MRCK, also known as CDC42BP), are known to phosphorylate it (44).
Table 1.
Phosphopeptides decreased in MLCK-null versus MLCK-intact cells
| Log2(MLCK null/MLCK intact) | |||||||
|---|---|---|---|---|---|---|---|
| UniProt ID | Gene Symbol | Site(s) | Annotation | Mean | SD | Centralized Sequence | Pjoint |
| Q9ERU9 | Ranbp2 | S954 | E3 SUMO-protein ligase RanBP2 | −1.43 | 0.40 | PATGILS*PRGDDY | 0.00003 |
| P40124 | Cap1 | S34 | Adenylyl cyclase-associated protein 1 | −1.03 | 0.31 | HCGYGDS*PSKGAV | 0.00056 |
| Q3TLR7 | Dtl | S694 | Denticleless protein homolog† | −0.97 | 0.33 | RQSGKTS*PGPVTI | 0.00101 |
| Q99MN1 | Kars | S393 | Lysine-tRNA ligase | −0.86 | 0.35 | PPFRRIS*MVEELE | 0.00249 |
| O88735 | Map7 | T243 | Ensconsin† | −0.72 | 0.14 | LARSKST*AALSGD | 0.00121 |
| Q8VDQ9 | Kri1 | S142 | Protein KRI1 homolog | −0.74 | 0.32 | YVDEDNS*DGETVD | 0.00552 |
| P52293 | Kpna2 | S62 | Importin subunit α1† | −0.95 | 0.98 | FPDDATS*PLQENR | 0.00750 |
| Q8BT07 | Cep55 | S40 | Centrosomal protein of 55 kDa† | −0.65 | 0.25 | IAAFKTS*LDEITS | 0.00637 |
| Q8CI12 | Smtnl2 | S339 | Smoothelin-like protein 2 | −0.68 | 0.44 | KLKRSQS*FGVASA | 0.01479 |
| P97855 | G3bp1 | S231 | Ras GTPase-activating protein-binding protein 1† | −0.68 | 0.51 | DVQKSTS*PAPADV | 0.01796 |
| Q9QXY1 | Tjp3 | S157;S161 | Tight junction protein ZO-3† | −0.73 | 0.71 | GRRSS*GGGS*EANG | 0.02147 |
| Q5U4C3 | Scaf1 | S518 | Splicing factor, arginine/serine-rich 19 | −0.61 | 0.42 | DPAPPDS*PSWEAK | 0.02178 |
| Q8BU14 | Sec62 | S356 | Translocation protein SEC62 | −0.59 | 0.30 | DDRSQHS*SGNGND | 0.01391 |
| Q3UKC1 | Tax1bp1 | S632 | Tax1-binding protein 1 homolog | −0.54 | 0.33 | RKLEGQS*PQQVSR | 0.02274 |
| Q80U49 | Cep170b | S709 | Centrosomal protein of 170-kDa protein B | −0.47 | 0.04 | PSGRADS*PAGLEA | 0.00057 |
| Q9JJG0 | Tacc2 | S140 | Transforming acidic coiled-coil-containing protein 2 | −0.46 | 0.15 | EPRSSDS*EEAFET | 0.01053 |
| Q99P72 | Rtn4 | S857 | Reticulon-4 | −0.39 | 0.17 | VSAKDDS*PKEYTD | 0.02062 |
| Q5PSV9 | Mdc1 | S168 | Mediator of DNA damage checkpoint protein 1 | −0.38 | 0.11 | VLLAADS*EEEGDF | 0.01100 |
| Q6NZJ6 | Eif4g1 | S1081 | Eukaryotic translation initiation factor 4 γ1 | −0.39 | 0.17 | KITKPGS*IDSNNQ | 0.02284 |
| Q61879 | Myh10 | S1956 | Conventional nonmuscle myosin II-B | −0.37 | 0.12 | GASLELS*DDDTES | 0.01263 |
| Q62261 | Sptbn1 | S2340 | Spectrin β-chain, nonerythrocytic 1† | −0.34 | 0.14 | TITSESS*PGKREK | 0.02207 |
| P97311 | Mcm6 | S689 | DNA replication licensing factor MCM6 | −0.32 | 0.09 | VNGHADS*PAPVNR | 0.01139 |
| Q3THE2 | Myl12b | S20 | Myosin regulatory light chain 12B† | −0.30 | 0.12 | RPQRATS*NVFAMF | 0.02282 |
| Q8CG79 | Tp53bp2 | S479 | Apoptosis-stimulating of p53 protein 2 | −0.29 | 0.05 | TLRKNQS*SEDILR | 0.00489 |
| A2A7S8 | Kiaa1522 | S838;S842 | Uncharacterized protein KIAA1522 | −0.29 | 0.10 | RRALS*GRAS*PVTA | 0.02043 |
| P22518 | Clk1 | S139 | Dual specificity protein kinase CLK1 | −0.27 | 0.01 | RRKRSRS*VEDDEE | 0.00045 |
| Q6Y7W8 | Gigyf2 | S26 | PERQ amino acid-rich with GYF domain-containing 2† | −0.26 | 0.08 | SGGSITS*PPLSPA | 0.01650 |
| Q9DBJ1 | Pgam1 | S118 | Phosphoglycerate mutase 1 | −0.24 | 0.08 | VKIWRRS*YDVPPP | 0.02228 |
| Q8BYR2 | Lats1 | T246 | Serine/threonine-protein kinase LATS1 | −0.22 | 0.04 | PQVRSVT*PPPPPR | 0.00528 |
Proteins phosphorylated at sites with X-(T/S)-p(T/S)-X motif and potential myosin light chain kinase (MLCK) target sites. E3-SUMO, E3-type small ubiquitin-like modifier; RanBP2, ras-related nuclear protein-binding protein-2; ZO-3, zonula occludens 3; LATS1, large-tumor suppressor kinase 1.
Phosphorylated amino acid.
Table 2 shows the 78 phosphorylation sites in 64 proteins that were increased in abundance in MLCK-null relative to MLCK-intact cells. Since the experiment involved deletion of a protein kinase, MLCK, one would expect increases in phosphorylation only via secondary mechanisms, such as a secondary increase in activity of some protein kinase or a secondary decrease in activity of some protein phosphatase in response to MLCK deletion. This indicates that MLCK’s action is not specific to a single substrate, myosin regulatory light chain, but that MLCK is a component of a fairly broad regulatory network involving both direct and indirect effects of MLCK-mediated phosphorylation. Among the proteins with increased phosphorylation in response to MLCK deletion is the protein kinase Mapk3, better known as ERK1, which undergoes an increase in phosphorylation in its activation loop (Y205). ERK1 is a “proline-directed” kinase that preferentially phosphorylates serines and threonines that are immediately followed by a proline. There are 19 such phosphorylation sites among the 78 phosphorylation sites that increase in response to MLCK deletion (Table 2).
Table 2.
Phosphopeptides increased in MLCK-null versus MLCK-intact cells
| Log2(MLCK null/MLCK intact) | |||||||
|---|---|---|---|---|---|---|---|
| UniProt ID | Gene Symbol | Site(s) | Annotation | Mean | SD | Centralized Sequence | Pjoint |
| P54763 | Ephb2 | S776 | Ephrin type B receptor 2 | 1.00 | 0.28 | FLEDDTS*DPTYTS | 0.00056 |
| P35486 | Pdha1 | S232 | Pyruvate dehydrogenase E1 component subunit-α, somatic form, mitochondrial | 0.91 | 0.50 | RYGMGTS*VERAAA | 0.00335 |
| Q8K3X4 | Irf2bpl | S498 | Interferon regulatory factor 2-binding protein-like | 0.89 | 0.67 | SLSRAPS*APPGTG | 0.00665 |
| Q7TPH6 | Mycbp2 | S2905 | E3 ubiquitin-protein ligase MYCBP2 | 0.88 | 0.23 | PRERSKS*DSYTLD | 0.00102 |
| Q61823 | Pdcd4 | S457 | Programmed cell death protein 4 | 0.86 | 0.74 | GRKRFVS*EGDGGR | 0.00941 |
| Q9JLF6 | Tgm1 | S94 | Protein-glutamine γ-glutamyltransferase K | 0.82 | 0.10 | RPESRGS*GVNAAG | 0.00029 |
| Q9D312 | Krt20 | S11 | Keratin, type I cytoskeletal 20 | 0.82 | 0.49 | RQSFHRS*LSSSSQ | 0.00641 |
| Q8R1G6 | Pdlim2 | S210 | PDZ and LIM domain protein 2 | 0.82 | 0.25 | SSPRFSS*LDLEED | 0.00197 |
| Q63844 | Mapk3 | Y205 | Mitogen-activated protein kinase 3 | 0.81 | 0.33 | TGFLTEY*VATRWY | 0.00331 |
| P35486 | Pdha1 | T231 | Pyruvate dehydrogenase E1 component subunit-α, somatic form, mitochondrial | 0.81 | 0.60 | NRYGMGT*SVERAA | 0.00994 |
| P19426 | Nelfe | S51 | Negative elongation factor E | 0.77 | 0.49 | GVKRSLS*EQPVVD | 0.00872 |
| Q9D312 | Krt20 | S11 | Keratin, type I cytoskeletal 20 | 0.77 | 0.45 | RQSFHRS*LSSSSQ | 0.00771 |
| Q5SU73 | Coil | T122 | Coilin | 0.75 | 0.41 | LMEDEET*DQGYKS | 0.00815 |
| Q99K30 | Eps8l2 | S204 | Epidermal growth factor receptor kinase substrate 8-like protein 2 | 0.73 | 0.47 | ILPPPQS*PAPIPF | 0.01145 |
| Q8BPM2 | Map4k5 | T400 | Mitogen-activated protein kinase kinase kinase kinase 5 | 0.71 | 0.47 | PKPRVNT*YPEDSL | 0.01288 |
| Q9QZQ1 | Mllt4 | S1795 | Afadin | 0.71 | 0.36 | ERQRLFS*QGQDVS | 0.00829 |
| Q9D312 | Krt20 | S13 | Keratin, type I cytoskeletal 20 | 0.70 | 0.50 | SFHRSLS*SSSQGP | 0.01492 |
| Q9D312 | Krt20 | S16 | Keratin, type I cytoskeletal 20 | 0.68 | 0.58 | RSLSSSS*QGPALS | 0.02174 |
| P48193 | Epb41 | S543 | Protein 4.1 | 0.67 | 0.37 | SKRASRS*LDGAAA | 0.01129 |
| Q91YD6 | Vill | S763 | Villin-like protein | 0.67 | 0.04 | LQAFKGS*QDSPEN | 0.00016 |
| Q8K4L3 | Svil | S857 | Supervillin | 0.64 | 0.43 | GDLRKLS*VDNNTS | 0.01769 |
| Q9EQG3 | Scel | S398 | Sciellin | 0.61 | 0.45 | PSANRSS*QHSLDE | 0.02426 |
| Q9Z1K8 | Slc7a7 | S19 | Y+L amino acid transporter 1 | 0.60 | 0.27 | HEADDGS*ALGDGA | 0.01092 |
| P70227 | Itpr3 | S2669 | Inositol 1,4,5-trisphosphate receptor type 3 | 0.59 | 0.33 | DVQNCMS*R_____ | 0.01565 |
| Q8K4L3 | Svil | S960 | Supervillin | 0.59 | 0.41 | VVLRRGS*LELGNP | 0.02343 |
| Q9Z2H5 | Epb41l1 | S544 | Band 4.1-like protein 1 | 0.59 | 0.12 | LPSSPAS*PSPKGT | 0.00252 |
| Q9Z204 | Hnrnpc | S268 | Heterogeneous nuclear ribonucleoproteins C1/C2 | 0.58 | 0.33 | EAGADDS*AEEGDL | 0.01841 |
| Q64511 | Top2b | S1537;S1539 | DNA topoisomerase 2β | 0.57 | 0.34 | KKRKAS*GS*ENEG | 0.01963 |
| Q99K74 | Med24 | S860 | Mediator of RNA polymerase II transcription subunit 24 | 0.55 | 0.22 | KLMRLLS*SSDDDA | 0.00992 |
| P11679 | Krt8 | S485 | Keratin, type II cytoskeletal 8 | 0.54 | 0.32 | KLVSESS*DVVSK_ | 0.02106 |
| Q9JI91 | Actn2 | S147 | α-Actinin-2 | 0.51 | 0.10 | FAIQDIS*VEETSA | 0.00325 |
| Q8BYJ6 | Tbc1d4 | S761 | TBC1 domain family member 4 | 0.48 | 0.12 | RTSSTCS*NESLNA | 0.00596 |
| Q8VDP4 | Ccar2 | S612 | Cell cycle and apoptosis regulator protein 2 | 0.47 | 0.25 | TAAESDS*PLKEDG | 0.02250 |
| Q8R310 | Tmcc3 | S242 | Transmembrane and coiled-coil domains protein 3 | 0.46 | 0.18 | PRAYGGS*ATIVNK | 0.01417 |
| Q05CL8 | Larp7 | S253 | La-related protein 7 | 0.44 | 0.09 | KRPRTAS*EGSEAE | 0.00468 |
| Q8R1A4 | Dock7 | S1428 | Dedicator of cytokinesis protein 7 | 0.43 | 0.05 | SGSAFGS*QENLRW | 0.00118 |
| Q8C1B1 | Camsap2 | S439 | Calmodulin-regulated spectrin-associated protein 2 | 0.42 | 0.18 | GIIRSVS*NEGLTL | 0.01870 |
| Q811P8 | Arhgap32 | S856 | Rho GTPase-activating protein 32 | 0.41 | 0.11 | ASSEPVS*PVQEKL | 0.00777 |
| O89032 | Sh3pxd2a | S1029 | SH3 and PX domain-containing protein 2A | 0.40 | 0.19 | LAERAAS*QGSESP | 0.02415 |
| Q8BI29 | Sarg | S79 | Specifically androgen-regulated gene protein | 0.39 | 0.12 | ESEPATS*PRSFRA | 0.01267 |
| P26231 | Ctnna1 | S652 | Catenin-α1 | 0.38 | 0.17 | EDFDVRS*RTSVQT | 0.02345 |
| F6ZDS4 | Tpr | S2223 | Nucleoprotein TPR | 0.38 | 0.10 | FAEAIHS*PQVAGV | 0.00940 |
| P51432 | Plcb3 | S537 | 1-Phosphatidylinositol 4,5-bisphosphate phosphodiesterase-β3 | 0.38 | 0.10 | SLEPQKS*LGEESL | 0.00877 |
| Q7TQG1 | Plekha6 | T899 | Pleckstrin homology domain-containing family A member 6 | 0.38 | 0.09 | GIVPPRT*KSPAEE | 0.00789 |
| Q09XV5 | Chd8 | S2520 | Chromodomain-helicase-DNA-binding protein 8 | 0.36 | 0.14 | TTGYPSS*PATTTS | 0.01916 |
| Q8C3P7 | Mettl3 | S48 | N6-adenosine-methyltransferase subunit METTL3 | 0.35 | 0.14 | LSPTFRS*DSPVPT | 0.01971 |
| Q3U1J1 | Tfpt | S180 | TCF3 fusion partner homolog | 0.35 | 0.13 | ATLDPTS*PAPGEG | 0.01830 |
| P30999 | Ctnnd1 | S864 | Catenin-δ1 | 0.34 | 0.13 | RSQSSHS*YDDSTL | 0.01801 |
| P19001 | Krt19 | S22 | Keratin, type I cytoskeletal 19 | 0.34 | 0.03 | GGTGGGS*VRIGSG | 0.00141 |
| Q56A10 | Znf608 | S626 | Zinc finger protein 608 | 0.34 | 0.03 | QTKAPGS*PGAGNP | 0.00141 |
| Q8C5R2 | Proser2 | S223 | Proline- and serine-rich protein 2 | 0.34 | 0.05 | EALSPTS*PSKEGR | 0.00355 |
| P59114 | Pcif1 | S116 | Phosphorylated CTD-interacting factor 1 | 0.33 | 0.05 | SRKRQLS*EEQPSG | 0.00390 |
| O09000 | Ncoa3 | S847 | Nuclear receptor coactivator 3 | 0.32 | 0.08 | PYNRAVS*LDSPVS | 0.00975 |
| Q9EQG3 | Scel | S343 | Sciellin | 0.31 | 0.12 | NMKRGKS*LDNLIK | 0.02008 |
| Q9EQG3 | Scel | S264 | Sciellin | 0.31 | 0.10 | LDKRAQS*LESLIY | 0.01502 |
| Q3TQI7 | BC005624 | S15;S17 | Uncharacterized protein C9orf78 homolog | 0.31 | 0.09 | RRRADS*ES*EEDE | 0.01372 |
| P52927 | Hmga2 | S104 | High-mobility group protein HMGI-C | 0.31 | 0.05 | ETSSQES*AEED__ | 0.00380 |
| Q61823 | Pdcd4 | S76 | Programmed cell death protein 4 | 0.31 | 0.12 | DSGRGDS*VSDNGS | 0.02285 |
| Q9ESE1 | Lrba | S1000;S1003 | Lipopolysaccharide-responsive and beige-like anchor protein | 0.31 | 0.07 | GRASS*IDS*ASNTE | 0.00943 |
| Q9R1R2 | Trim3 | S7 | Tripartite motif-containing protein 3 | 0.30 | 0.08 | MAKREDS*PGPEVQ | 0.01102 |
| E1U8D0 | Soga1 | S1300 | Protein SOGA1 | 0.29 | 0.10 | VCGRAPS*PTTAAG | 0.01891 |
| Q3THK3 | Gtf2f1 | T389 | General transcription factor IIF subunit 1 | 0.29 | 0.07 | GTSRPGT*PSAEAA | 0.00959 |
| P52927 | Hmga2 | S101 | High-mobility group protein HMGI-C | 0.28 | 0.08 | ETEETSS*QESAEE | 0.01562 |
| Q8R310 | Tmcc3 | S4 | Transmembrane and coiled-coil domains protein 3 | 0.28 | 0.08 | ___MPGS*DTALTV | 0.01547 |
| Q7TQG1 | Plekha6 | S901 | Pleckstrin homology domain-containing family A member 6 | 0.27 | 0.04 | VPPRTKS*PAEEEL | 0.00339 |
| P30999 | Ctnnd1 | S268;S269 | Catenin-δ1 | 0.27 | 0.09 | RVGGS*S*VDLHRF | 0.01735 |
| Q80W04 | Tmcc2 | S435 | Transmembrane and coiled-coil domains protein 2 | 0.27 | 0.06 | IRNKFGS*ADNIAH | 0.00927 |
| P28867 | Prkcd | T505 | Protein kinase Cδ type | 0.27 | 0.10 | GEGRAST*FCGTPD | 0.02110 |
| Q62130 | Ptpn14 | S314 | Tyrosine-protein phosphatase nonreceptor type 14 | 0.27 | 0.10 | CTEQSNS*PPPIRR | 0.02283 |
| Q91XV3 | Basp1 | T36 | Brain acid-soluble protein 1 | 0.27 | 0.08 | GTEEEGT*PKESEP | 0.01445 |
| Q64727 | Vcl | S290 | Vinculin | 0.27 | 0.08 | LRDPNAS*PGDAGE | 0.01419 |
| Q8K3X4 | Irf2bpl | S636;S638 | Interferon regulatory factor 2-binding protein-like | 0.23 | 0.08 | ARRNS*SS*PVSPA | 0.01979 |
| Q6P4S8 | Ints1 | T83 | Integrator complex subunit 1 | 0.23 | 0.05 | RPKLSST*PPLSAL | 0.00866 |
| P98083 | Shc1 | Y423 | SHC-transforming protein 1 | 0.23 | 0.07 | LFDDPSY*VNIQNL | 0.01558 |
| Q8R0W0 | Eppk1 | S1537 | Epiplakin | 0.22 | 0.06 | GLRKQVS*AGDLFR | 0.01600 |
| Q9Z2H5 | Epb41l1 | T685 | Band 4.1-like protein 1 | 0.22 | 0.03 | DRGACST*PEMPQF | 0.00469 |
| Q80X80 | C2cd2l | S662 | C2 domain-containing protein 2-like | 0.21 | 0.07 | EAGLSQS*HDDLSN | 0.02255 |
| P39447 | Tjp1 | S617 | Tight junction protein ZO-1 | 0.20 | 0.03 | KRNLRKS*REDLSA | 0.00579 |
MLCK, myosin light chain kinase; ZO-1, zonula occludens 1.
Phosphorylated amino acid.
Figure 6C shows the functional interactions between the phosphoproteins altered in MLCK-null versus MLCK-intact cells as identified using STRING (https://string-db.org/). (This includes phosphoproteins corresponding to all 107 phosphorylation sites that were affected by Mylk gene deletion.) These proteins clustered into distinct functional and structural groups labeled around the periphery of Fig. 6C. Based on the direction of phosphorylation change and sequence motif, several of the phosphoproteins are plausible direct targets of MLCK (labeled in green). Interestingly, despite evidence for altered endosomal trafficking in MLCK-null cells, none of the phosphoprotein groups appear to relate directly to membrane trafficking. Instead, it seems more likely that the reported changes in AQP2 trafficking are due to changes within the cluster referred to as “actomyosin” in Fig. 6C (43). Beyond the actomyosin category, which might be expected based on the canonical role of MLCK in the regulation of conventional myosins, proteins in several other functional and structural categories underwent changes in phosphorylation. This includes proteins in a category referred to as “signaling” that contains five additional protein kinases [Mapk3 (ERK1), Map4k5 (an upstream regulator of Jnk), Ephb2 (ephrin type B receptor 2), Prkcd (PKC-δ), and Lats1 (a key mediator of Hippo signaling)] that can have their own effects on protein phosphorylation. Thus, the STRING network analysis reinforces the conclusion that the role of MLCK is not limited to regulation of myosins; instead, MLCK appears to have a broader role as part of a signaling network involving several other protein kinases.
Of interest also was the number of nuclear proteins that underwent changes in phosphorylation (lower aspects of Fig. 6C), including those related to the structure of the nuclear membrane (nuclear envelope) and those involved in initiation and elongation phases of gene transcription (gene transcription). Vasopressin regulates Aqp2 gene transcription (34), in part through regulation of nuclear import of regulatory proteins through the nuclear envelope (36). MLCK, although generally associated with actions in the cytoplasm, is also present in the nucleus of mpkCCD cells (36), where it undergoes a marked decrease in phosphorylation at S364 in response to vasopressin (centralized sequence: PAIGSFS*PGEDRK) (1), a putative ERK phosphorylation target. MLCK has also been detected in nuclei of nonepithelial cells (45).
We carried out gene set enrichment analysis in the Database for Annotation, Visualization, and Integrated Discovery (DAVID, version 6.8) using Gene Ontology Biological Process terms (Table 3). In general, the overrepresented terms are consistent with the analysis shown in Fig. 6C and suggest that the major effects of MLCK deletion are on processes related to the actin cytoskeleton, cell morphogenesis, nuclear import, and the MAPK signaling pathway. As previously noted, the phosphorylation of Mapk3 (ERK1) at its activation site was markedly increased, compatible with MAPK pathway activation in MLCK-null cells relative to MLCK-intact cells. A similar increase in active site ERK1 phosphorylation was previously seen with PKA deletion in mpkCCD cells (18), and a decrease in active site phosphorylation in both ERK1 and ERK2 was seen with vasopressin stimulation (32), suggesting a possible interaction between vasopressin/PKA signaling and MLCK signaling at the level of MAPKs.
Table 3.
GO Biological Process terms enriched in the list of all phosphopeptides with phosphorylation sites altered in MLCK-null versus MLCK-intact cells
| GO Biological Process Term | Count | Fold Enrichment | P Value (Fisher’s exact test) |
|---|---|---|---|
| Actin cytoskeleton organization | 11 | 3.4 | 0.0003 |
| Cell morphogenesis | 11 | 2.4 | 0.0042 |
| Protein import into nucleus | 6 | 3.8 | 0.0043 |
| Regulation of mitotic cell cycle | 6 | 3.6 | 0.0059 |
| Cell surface receptor signaling pathway | 12 | 2.1 | 0.0080 |
| MAPK cascade | 6 | 3.2 | 0.0093 |
| Signal transduction by protein phosphorylation | 6 | 3.2 | 0.0098 |
| Negative regulation of transcription, DNA templated | 8 | 2.2 | 0.0250 |
Database for Annotation, Visualization, and Integrated Discovery (v6.8) (https://david.ncifcrf.gov/) was used for analysis. GO, Gene Ontology; MLCK, myosin light chain kinase.
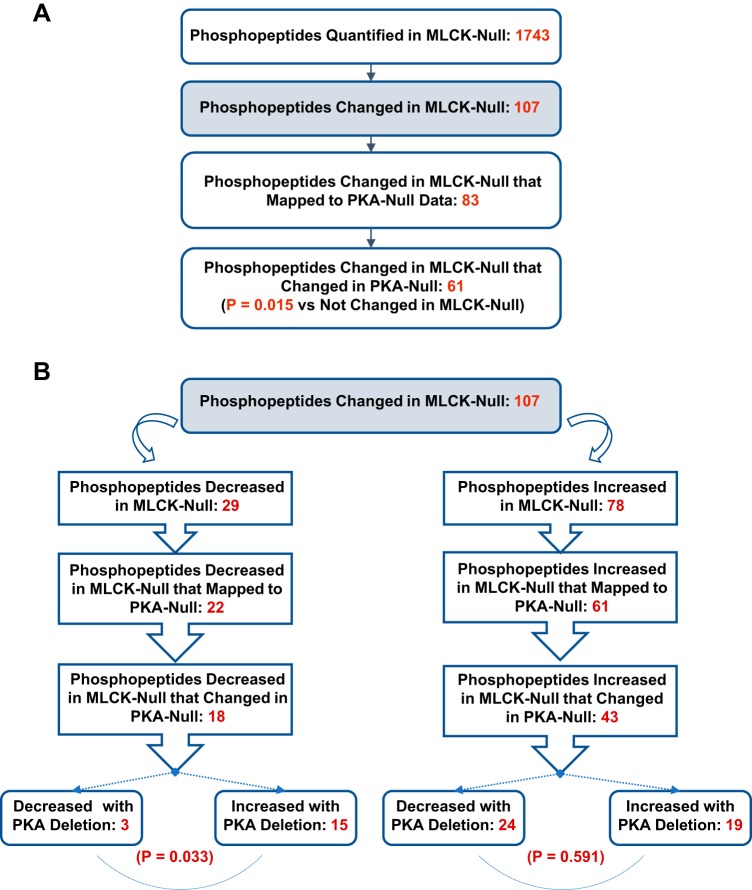
Vasopressin signals in collecting duct cells via cAMP-mediated activation of PKA. To identify common elements of PKA- and MLCK-dependent signaling, we mapped phosphopeptide quantification data from our recent study in which PKA was deleted from mpkCCD cells (18) to the present data with MLCK deletion (Fig. 7A). Of the 107 phosphopeptides that were found to have changed with MLCK deletion, 83 were also successfully quantified in PKA-null cells. Of these, 61 had previously been found to change in PKA-null cells [P = 0.015 vs. unchanged in MLCK-null cells (by χ2-analysis)]. Thus, the MLCK signaling network and the PKA signaling network overlap and are interdependent. As shown in Fig. 7B, the 18 phosphopeptides decreased in MLCK-null cells that changed in PKA-null cells were divided unequally between those increased in PKA-null cells (n = 15) and those decreased in PKA-null cells (n = 3). The specific phosphopeptides among the 15 that changed in opposite directions are shown in Table 4. Nine of the fifteen oppositely regulated sites have the motif X-p(S/T)-P; this is indicative of opposite regulation of some proline-directed protein kinase (i.e., a kinase of the MAPK or CDK families) or opposite regulation of a phosphatase that is selective for X-p(S/T)-P sites (10).
Fig. 7.
Mapping phosphorylation changes with myosin light chain kinase (MLCK) deletion to phosphorylation changes with PKA deletion. A: general details of mapping showing significant overlap between MLCK signaling and PKA signaling. P values were determined by χ2-analysis. B: breakdown in mapping with regard to direction of change. There was a strong preference for phosphopeptides decreased with MLCK deletion to have been increased with PKA deletion (bottom left). P values were determined by χ2-analysis.
Table 4.
Phosphopeptides that showed abundance changes in opposite directions in response to deletion of MLCK versus PKA
| Gene Symbol | Site(s) | Centralized Sequence | Log2(MLCK null/MLCK intact) | Log2(PKA null/PKA intact) |
|---|---|---|---|---|
| Ranbp2 | S954† | PATGILS*PRGDDY | −1.43 | 0.71 |
| Cap1 | S34† | HCGYGDS*PSKGAV | −1.03 | 3.73 |
| Kpna2 | S62† | FPDDATS*PLQENR | −0.95 | 0.81 |
| Smtn12 | S339 | KLKRSQS*FGVASA | −0.68 | 0.49 |
| Cep55 | S40 | IAAFKTS*LDEITS | −0.65 | 1.51 |
| Scaf1 | S518† | DPAPPDS*PSWEAK | −0.61 | 0.42 |
| Tax1bp1 | S632† | RKLEGQS*PQQVSR | −0.54 | 0.62 |
| Cep170b | S709† | PSGRADS*PAGLEA | −0.47 | 1.23 |
| Rtn4 | S857† | VSAKDDS*PKEYTD | −0.39 | 0.67 |
| Eif4g1 | S1081 | KITKPGS*IDSNNQ | −0.39 | 0.29 |
| Mdc1 | S168 | VLLAADS*EEEGDF | −0.38 | 0.74 |
| Myh10 | S1956 | GASLELS*DDDTES | −0.37 | 0.54 |
| Sptbn1 | S2340† | TITSESS*PGKREK | −0.34 | 1.04 |
| Kiaa1522 | S838, S842† | RRALS*GRAS*PVTA | −0.29 | 0.69 |
| Pgam1 | S118 | VKIWRRS*YDVPPP | −0.24 | 0.36 |
Sites with the motif X-p(S/T)-P. MLCK, myosin light chain kinase; Ranbp2, RAN-binding protein 2; Cap1, cyclase-associated actin cytoskeleton regulatory protein 1; Kpna2, karyopherin subunit α2; Smtn12, smoothelin-like protein 2; Cep55, centrosomal protein 55; Scaf1, splicing factor, arginine/serine-rich 19; Tax1bp1, Tax1-binding protein 1; Cep170b, centrosomal protein 170B; Rtn4, reticulon 4; Eif4g1, eukaryotic translation initiation factor 4γ; Mdc1, mediator of DNA damage checkpoint 1; Myh10, myosin heavy chain 10; Sptbn1, spectrin β-chain, brain 1; Pgam1, phosphoglycerate mutase 1.
Phosphorylated amino acid.
Effect of MLCK deletion on the actin cytoskeleton.
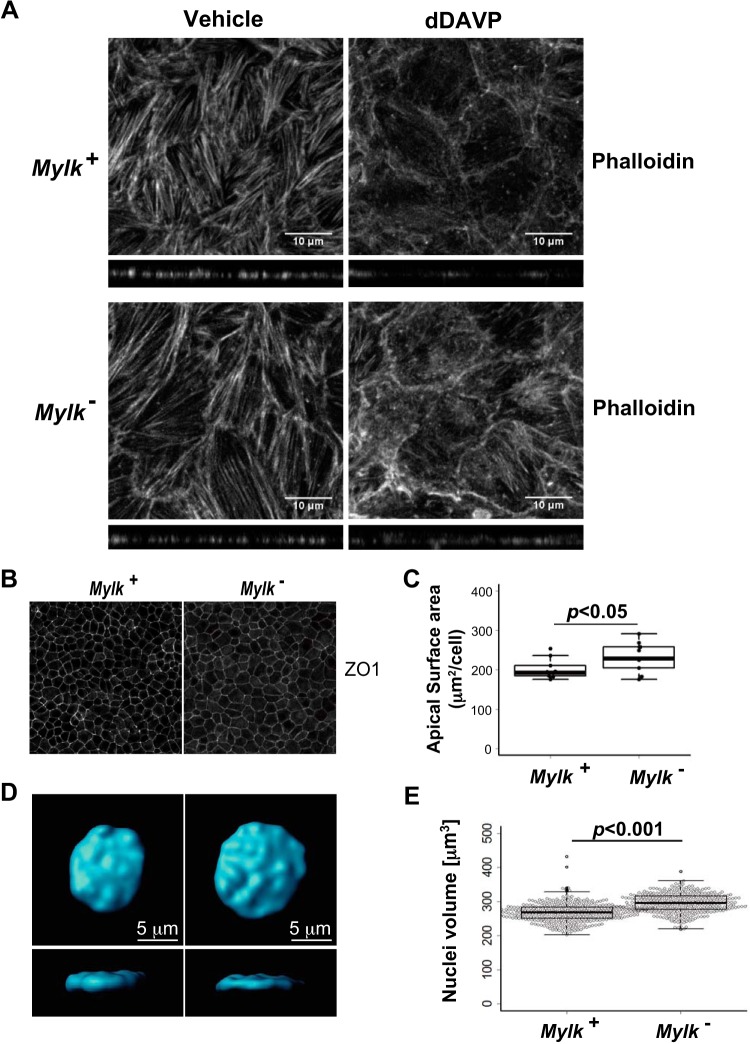
The processes related to actin organization in the cell were prominent in the STRING-based network analysis in Fig. 6C and in the gene enrichment analysis in Table 3. To address the state of the actin cytoskeleton, we used phalloidin labeling to assess the distribution of F-actin in MLCK-null versus MLCK-intact cells (Fig. 8). Normally, vasopressin causes a loss of F-actin stress fibers in renal collecting duct cells (6, 23, 38, 40), a response that is believed to be a crucial element of vasopressin regulation of AQP2 trafficking. Loss of F-actin stress fibers with vasopressin addition for 30 min was confirmed in MLCK-intact cells, but this response was attenuated in MLCK-null cells (Fig. 8A). This finding points to the possibility of morphological effects of MLCK deletion, which was suggested also by the enrichment of the Gene Ontology biological process term “cell morphogenesis” (regulated phosphoproteins: RTN4, EPB41, KRT8, MAP7, MYL12B, DOCK7, CAP1, HMGA2, TOP2B, MYH10, and VCL) shown in Table 3. Indeed, analysis of cell density at confluence revealed that the average cross-sectional area per cell was significantly greater in MLCK-null cells than in MLCK-intact cells (Fig. 8, B and C).
Fig. 8.
Vasopressin-induced redistribution of F-actin and defects in cellular morphology of myosin light chain kinase (MLCK)-null (Mylk−) cells. A: representative maximum-intensity projection images of Alexa Fluor 594-phalloidin-labeled MLCK-intact (Mylk+) and MLCK-null cells treated with vehicle or 1-desamino-8-d-arginine-vasopressin (dDAVP) for 30 min. B: representative confocal images of MLCK-intact and MLCK-null cells labeled with anti-zonula occludens-1 (ZO1) antibodies. C: box plot of cross-sectional areas of MLCK-intact and MLCK-null cells. n = 10. D: representative image of a nucleus of a MLCK-intact cell and a MLCK-null cell. Top: x-y; bottom: x-z. Confocal images were rendered and analyzed using Imaris software. E: box plot of nuclear volume of MLCK-intact and MLCK-null cells and overlaid with individual data points. Numbers of nuclei were quantified as follows: 362 for MLCK-intact cells and 336 for MLCK-null cells.
Effect of MLCK deletion on nuclear morphology.
One surprising aspect of the phosphoproteomic analysis was that many of the regulated phosphoproteins are elements of the nuclear envelope (Fig. 6C) (responsible for enriched Gene Ontology term “protein import into nucleus”). These observations suggest that nuclear structure could be altered in MLCK-null cells. To measure nuclear volume, cells grown on permeable supports were stained with DAPI and imaged by confocal microscopy, and their nuclear volume was measured by numerical integration through the confocal image stack (Fig. 8, D and E). The nuclear volume of MLCK-null cells was significantly larger than that of MLCK-intact cells. In addition, the nuclei appeared to be broader and flatter in MLCK-null cells than controls. Thus, the morphometric analysis showed that MLCK is an important determinant of size and shape of mpkCCD cells and their nuclei.
DISCUSSION
This study combines genome-editing techniques (CRISPR-Cas9) with quantitative phosphoproteomics to identify signaling events downstream from MLCK in renal collecting duct cells. From the data, we conclude the following. First, MLCK-mediated phosphorylation is upstream of a multicomponent signaling pathway that includes much more than simple regulation of conventional nonmuscle myosins through myosin light chain phosphorylation. Second, there is significant overlap between MLCK- and PKA-dependent signaling. Third, MLCK plays an important role in vasopressin-induced actin depolymerization. Fourth, MLCK does not function only in the cytoplasm; it also can be seen to evoke phosphorylation changes in many nuclear proteins. Finally, MLCK plays a critical role in endosomal trafficking of AQP2 at the level of transition from early to late endosomes.
The basis of the interaction between PKA signaling and MLCK signaling is of interest. Prior studies have identified two processes by which vasopressin, acting through PKA, could alter MLCK activity. First, working through the V2 receptor, vasopressin causes an increase in intracellular Ca2+ in collecting duct principal cells (4, 39). Ca2+ mobilization has been shown to consist of an increase in the “frequency” of aperiodic Ca2+ spikes (7, 46) in response to vasopressin. Ca2+ mobilization is also seen in response to addition of cAMP (7). An increase in intracellular Ca2+ would be expected to increase MLCK activity via Ca2+/calmodulin binding (41). Second, in the nuclei of mpkCCD cells, vasopressin has been observed to produce a very large decrease in phosphorylation of MLCK at S364 (1), a site phosphorylated by ERK (17, 20). Importantly, ERK-mediated phosphorylation increases MLCK activity (17, 20). Therefore, the effect of vasopressin would be to decrease MLCK activity. Under these circumstances, PKA activation would be expected to produce phosphorylation changes in the opposite direction from that of MLCK activation, as seen for many phosphorylation sites altered by MLCK deletion and PKA deletion (Fig. 7 and Table 4). These findings support the idea that vasopressin-mediated PKA activation could decrease MLCK activity in collecting duct cells, at least in some PKA subdomains, by reducing ERK activity in that subdomain. This seems likely in the nucleus. Indeed, 9 of the 15 MLCK/PKA counterregulated phosphorylation sites in Table 4 are in proteins associated with the nuclear envelope, gene transcription, or mRNA processing (Fig. 6). Furthermore, it seems possible that PKA activation increases MLCK activity via Ca2+ mobilization in different subdomains, including the cytosol, accounting for phosphorylation changes that occur in the same direction with MLCK deletion and PKA deletion. The fact that PKA evokes localized subcellular effects in cells is well documented (9).
The roles of MLCK in the nucleus are also of considerable interest. Phosphoproteomic analysis of the response to MLCK deletion identified phosphorylation changes in 14 proteins involved in transcriptional initiation and elongation (Fig. 6C). A previous study (16) has demonstrated a role for β-actin in the preinitiation complex and the regulation of RNA polymerase II. In addition, 14 phosphoproteins altered with MLCK deletion are related to the nuclear envelope (Fig. 6C), providing a possible explanation for the demonstrated changes in nuclear size and shape (Fig. 8, D and E). A previous study (3) has shown that overexpression of constitutively activated MLCK reduces nuclear size in conjunction with an overall increase in cell stiffness. Given the overlap between PKA signaling and MLCK signaling in collecting duct cells, it is of interest to note that vasopressin markedly reduces cell stiffness in collecting duct cells (13). Of course, from our experiments, it is difficult to tell whether the changes in phosphorylation of nuclear envelope proteins were mediated by nuclear or cytoplasmic MLCK.
MLCK deletion had a distinct effect on endosomal processing, either blocking or markedly slowing movement of endocytosed AQP2 into late endosomes/multivesicular bodies (Fig. 4), which directs AQP2 to the normal route of AQP2 disposal in lysosomes. A steady state is presumably achieved by rerouting AQP2 into other degradation pathways. Measurements of AQP2 trafficking using a surface biotinylation/debiotinylation protection assay revealed no evidence for either an impairment of endocytosis or a change in the steady-state amount of AQP2 at the cell surface. This conclusion seems at odds with the finding that the MLCK inhibitors ML-7 and ML-9 decreased the vasopressin-induced increase in water permeability in the collecting duct (6), which was presumably a result of a decrease in AQP2 in the plasma membrane with either a reduction in AQP2 exocytosis or an increase in AQP2 endocytosis (2). The solution to this conundrum is unclear. One likely possibility is that ML-7 and ML-9 decrease water permeability in the collecting duct by inhibiting other kinases. Indeed, large-scale profiling of ML-7 and ML-9 with regard to inhibition of a library of kinases demonstrates such lack of specificity and even showed a substantial inhibition of PKA by ML-7 or ML-9 at the concentrations used in the collecting duct experiments [International Centre for Kinase Profiling: http://www.kinase-screen.mrc.ac.uk/kinase-inhibitors], providing a potential explanation for its effect on water permeability.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.R., C.-L.C., C.-R.Y., and M.A.K. conceived and designed research; K.I., V.R., and C.-R.Y. performed experiments; K.I., V.R., L.K., C.-R.Y., and M.A.K. analyzed data; V.R., C.-R.Y., and M.A.K. interpreted results of experiments; K.I., V.R., L.K., and M.A.K. prepared figures; K.I., V.R., and M.A.K. drafted manuscript; K.I., V.R., C.-L.C., C.-R.Y., and M.A.K. edited and revised manuscript; K.I., V.R., L.K., C.-L.C., C.-R.Y., and M.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Guangwei Wang for expert advice concerning the mass spectrometry and Daniela Malide for instructions on the use of Imaris software. The work was funded by Division of Intramural Research, National Heart, Lung, and Blood Institute (NHLBI) Projects ZIA-HL001285 and ZIA-HL006129 (to M. A. Knepper). We used the NHLBI Flow Cytometry Core Facility (P. McCoy, Director), NHLBI Electron Microscopy Core Facility (Christopher Bleck, Director), NHLBI Proteomics Core Facility (Marjan Gucek, Director), and NHLBI Light Microscopy Core Facility (Christian Combs, Director) for these experiments. K. Isobe was supported in part by a Japan Society for the Promotion of Science Research Fellowship.
REFERENCES
- 1.Bolger SJ, Hurtado PA, Hoffert JD, Saeed F, Pisitkun T, Knepper MA. Quantitative phosphoproteomics in nuclei of vasopressin-sensitive renal collecting duct cells. Am J Physiol Cell Physiol 303: C1006–C1020, 2012. doi: 10.1152/ajpcell.00260.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 3.Cai S, Pestic-Dragovich L, O’Donnell ME, Wang N, Ingber D, Elson E, De Lanerolle P. Regulation of cytoskeletal mechanics and cell growth by myosin light chain phosphorylation. Am J Physiol Cell Physiol 275: C1349–C1356, 1998. doi: 10.1152/ajpcell.1998.275.5.C1349. [DOI] [PubMed] [Google Scholar]
- 4.Champigneulle A, Siga E, Vassent G, Imbert-Teboul M. V2-like vasopressin receptor mobilizes intracellular Ca2+ in rat medullary collecting tubules. Am J Physiol Renal Physiol 265: F35–F45, 1993. doi: 10.1152/ajprenal.1993.265.1.F35. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou CL, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de Lanerolle P, Nielsen S, Knepper MA. Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J Biol Chem 279: 49026–49035, 2004. doi: 10.1074/jbc.M408565200. [DOI] [PubMed] [Google Scholar]
- 7.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 8.Chou CL, Yu MJ, Kassai EM, Morris RG, Hoffert JD, Wall SM, Knepper MA. Roles of basolateral solute uptake via NKCC1 and of myosin II in vasopressin-induced cell swelling in inner medullary collecting duct. Am J Physiol Renal Physiol 295: F192–F201, 2008. doi: 10.1152/ajprenal.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol 9: 216–221, 1999. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande V, Kao A, Raghuram V, Datta A, Chou CL, Knepper MA. Phosphoproteomic identification of vasopressin V2 receptor-dependent signaling in the renal collecting duct. Am J Physiol Renal Physiol 317: F789–F804, 2019. doi: 10.1152/ajprenal.00281.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214, 2007. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 13.Grantham JJ. Vasopressin: effect on deformability of urinary surface of collecting duct cells. Science 168: 1093–1095, 1970. doi: 10.1126/science.168.3935.1093. [DOI] [PubMed] [Google Scholar]
- 14.Hoffert JD, Chou CL, Knepper MA. Aquaporin-2 in the “-omics” era. J Biol Chem 284: 14683–14687, 2009. doi: 10.1074/jbc.R900006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann WA, Stojiljkovic L, Fuchsova B, Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA, Lessard JL, Hope TJ, Hozak P, de Lanerolle P. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat Cell Biol 6: 1094–1101, 2004. doi: 10.1038/ncb1182. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 117: 4619–4628, 2004. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 18.Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, Burg MB, Raghuram V, Knepper MA. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci USA 114: E8875–E8884, 2017. doi: 10.1073/pnas.1709123114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 20.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 137: 481–492, 1997. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 22.Knepper MA. Systems biology in physiology: the vasopressin signaling network in kidney. Am J Physiol Cell Physiol 303: C1115–C1124, 2012. doi: 10.1152/ajpcell.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo CS, Chen CW, Wang PJ, Chen PY, Lin SY, Khoo KH, Fenton RA, Knepper MA, Yu MJ. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci USA 110: 17119–17124, 2013. doi: 10.1073/pnas.1309219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 298: 1912–1934, 2002. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 25.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009. doi: 10.1038/ki.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeller HB, Praetorius J, Rützler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussavi RS, Kelley CA, Adelstein RS. Phosphorylation of vertebrate nonmuscle and smooth muscle myosin heavy chains and light chains. Mol Cell Biochem 127-128: 219–227, 1993. doi: 10.1007/BF01076773. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S, Muller J, Knepper MA. Vasopressin- and cAMP-induced changes in ultrastructure of isolated perfused inner medullary collecting ducts. Am J Physiol Renal Physiol 265: F225–F238, 1993. doi: 10.1152/ajprenal.1993.265.2.F225. [DOI] [PubMed] [Google Scholar]
- 30.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1: 376–386, 2002. doi: 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Pecci A, Ma X, Savoia A, Adelstein RS. MYH9: structure, functions and role of non-muscle myosin IIA in human disease. Gene 664: 152–167, 2018. doi: 10.1016/j.gene.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3882–3887, 2010. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest 110: 1383–1388, 2002. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA. Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep 6: 34863, 2016. doi: 10.1038/srep34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24: 1793–1805, 2013. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, Hoffert JD, Pisitkun T, Knepper MA. Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23: 1008–1018, 2012. doi: 10.1681/ASN.2011070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta 1496: 3–22, 2000. doi: 10.1016/S0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 38.Simon H, Gao Y, Franki N, Hays RM. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am J Physiol Cell Physiol 265: C757–C762, 1993. doi: 10.1152/ajpcell.1993.265.3.C757. [DOI] [PubMed] [Google Scholar]
- 39.Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81: 1879–1888, 1988. doi: 10.1172/JCI113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001. doi: 10.1152/ajprenal.0091.2001. [DOI] [PubMed] [Google Scholar]
- 41.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem 269: 9912–9920, 1994. [PubMed] [Google Scholar]
- 42.Taus T, Köcher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K. Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res 10: 5354–5362, 2011. doi: 10.1021/pr200611n. [DOI] [PubMed] [Google Scholar]
- 43.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- 44.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Dreger M, Madrazo E, Williams CJ, Samaniego R, Hodson NW, Monroy F, Baena E, Sánchez-Mateos P, Hurlstone A, Redondo-Muñoz J. WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proc Natl Acad Sci USA 115: 8581–8586, 2018. doi: 10.1073/pnas.1719405115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002. doi: 10.1113/jphysiol.2001.012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. doi: 10.1073/pnas.0813002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Protein mass spectrometry data (raw files, search results, and spectra) have been uploaded to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD014985.