Abstract
Albuminuria is frequently associated with proximal tubule (PT) cytotoxicity that can feed back to cause glomerular damage and exacerbate kidney disease. PT cells express megalin and cubilin receptors that bind to and internalize albumin over a broad concentration range. How the exposure to high concentrations of albumin leads to PT cytotoxicity remains unclear. Fatty acids and other ligands bound to albumin are known to trigger production of reactive oxygen species (ROS) that impair PT function. Alternatively or in addition, uptake of high concentrations of albumin may overload the endocytic pathway and elicit downstream responses. Here, we used a well-differentiated PT cell culture model with high endocytic capacity to dissect the effects of albumin versus its ligands on endocytic uptake and degradation of albumin, production of ROS, and cell viability. Cellular responses differed dramatically, depending on the preparation of albumin tested. Knockdown of megalin or cubilin failed to prevent ROS production mediated by albumin ligands, suggesting that receptor-mediated internalization of albumin was not necessary to trigger cellular responses to albumin ligands. Moreover, albumin induced cytotoxic responses when added to the basolateral surface of PT cells. Whereas overnight incubation with high concentrations of fatty acid-free albumin had no overt effects on cell function or viability, lysosomal degradation kinetics were slowed upon longer exposure, consistent with overload of the PT endocytic/degradative pathway. Together, the results of our study demonstrate that the PT responds independently to albumin and to its ligands and suggest that the consequences of albumin overload in vivo may be dependent on metabolic state.
Keywords: endocytosis, lipotoxicity, palmitate, proteinuria, proximal tubule, reactive oxygen species
INTRODUCTION
The glomerulus and proximal tubule (PT) of the kidney coordinately prevent the excretion of plasma proteins in the urine. The glomerular filtration barrier efficiently excludes most plasma proteins; those that normally escape filtration are recovered by PT cells. Disruption of the glomerular barrier, as occurs in nephrotic disease, results in high tubular concentrations of albumin and other serum proteins that can overwhelm the capacity for PT uptake and also trigger PT cell damage (7). In turn, injured PT cells can activate downstream inflammatory responses that further degrade glomerular function (15, 25).
Why is exposure of PT cells to serum proteins so damaging, when many cell types are continuously bathed in this fluid? At least some of the cytotoxicity can be recapitulated simply by incubating PT cells in high levels of albumin, the most abundant protein in serum. Albumin, present at ∼40 mg/mL, maintains oncotic pressure in the serum and is a carrier of numerous hydrophobic or amphipathic molecules, including steroid hormones, fatty acids, and heme (11). An emerging idea is that some of the ligands bound to albumin trigger toxic responses (1). Additionally, uptake of high concentrations of even free albumin (not complexed with any ligands) might impair the function of PT endocytic and degradative pathways. Understanding the differential cellular responses to albumin and its ligands is essential for determining approaches to treat proteinuric conditions initiated by different insults. However, dissecting the role of albumin versus its bound ligands in PT toxicity has proven challenging, and it is increasingly clear that the use of undefined albumin preparations and quantitation of different outcome measures can lead to discrepant conclusions (4, 11, 31, 35).
Albumin is normally taken up by the PT via megalin and cubilin receptors that are abundantly expressed in PT cells in vivo and believed to recycle constitutively (10, 29). Whether or how albumin uptake via these receptors contributes to PT cytotoxic responses is not known. Several studies have demonstrated that PTs in vivo and immortalized PT cells are sensitive to BSA-mediated inflammasome activation, apoptosis, oxidative stress, and impaired lysosomal function (7, 8, 20, 26). Moreover, Liu and colleagues (26) demonstrated that knockdown of megalin or cubilin blocked inflammasome activation and cytokine maturation in HK-2 cells exposed to fatty acid-free BSA (FAF-BSA).
Another unresolved question is whether albumin overload triggers compensatory changes in endocytosis and/or megalin/cubilin receptor expression. Immunohistochemical staining of human kidney sections suggest a small increase in cubilin receptor expression in tissue subject to nephrotic conditions (41). In concordance with this, Liu et al. (26) observed increased levels of megalin and cubilin in rats injected daily for 9 wk with FAF-BSA. In contrast, other studies in mice found no effect of megalin or cubilin expression under overload conditions (23, 30). Yet another study (37) reported disease-specific variations in megalin versus cubilin expression and distribution. Among many other possible reasons, temporal responses during the course of disease may contribute to these discrepant findings. Indeed, changes in the temporal response to albumin have been reported in vitro. HK-2 cells treated for up to 24 h with BSA expressed increased amounts of megalin and cubilin, whereas longer albumin exposure or treatment with concentrations above 20 mg/mL resulted in reduced receptor expression (26, 41). Understanding how the PT endocytic pathway responds to changes in lumenal protein content is important for designing therapies to limit kidney damage in proteinuric states.
We recently developed an opossum kidney (OK) cell model cultured under continuous shear stress that recapitulates key features of PT function, including well-differentiated apical and basolateral plasma membrane domains, increased energy utilization and ion transport, and dramatically expanded apical endocytic capacity (27). Similarly to the PT in vivo, these cells rely on oxidative metabolism for energy production, thus enabling us to better address the specific role of fatty acid ligands carried by albumin in overload responses (34). Using this model, we asked how exposure to nephrotic concentrations of human serum (HuSer), to FAF-BSA, or to BSA complexed with different ligands affects cellular outcomes known to be associated with proteinuria. Our results suggest that cell viability, endocytic responses, and increases in mitochondrial reactive oxygen species (ROS) production are discoordinately regulated by albumin ligand content. Moreover, knockdown of either megalin or cubilin did not prevent the toxic effects on ROS production, suggesting that endocytic uptake of albumin is not required to elicit cytotoxic responses. Our results are important for understanding how the PT responds to overload resulting from numerous pathological states, including diabetes, hemolysis, and heavy metal poisoning.
MATERIALS AND METHODS
Cell culture and albumin treatment.
OK cells (RRID:CVCL_0472) were cultured in DMEM-F-12 medium with 5% FBS (Atlanta Biologicals) and 5 mM GlutaMAX (GIBCO). For experiments, unless otherwise indicated, cells (4 × 105) were plated on 12-mm Transwell (0.4-µm pore) polycarbonate membrane inserts (no. 3401, Corning) in a 12-well plate with 0.5 mL of apical and 1.5 mL of basolateral culture medium. The following day, cells were transferred to an orbital shaker set at 146 rpm in the incubator and allowed to grow for an additional 3–4 days with daily medium changes, as previously described (27).
Lipid loading.
FAF-BSA (A7030, Sigma) was loaded with sodium palmitate (P9767, Sigma) or oleic acid (O1008, Sigma) at a 6:1 ratio of lipid-FAF-BSA. Briefly, 9.1 mM solutions of sodium palmitate or oleic acid and 1.5 mM FAF-BSA (BSA concentration of 100 mg/mL) were made in PBS+/+. Each lipid was incubated with equal volumes of FAF-BSA at 37°C for 1 h with intermittent agitation, resulting in a 6:1 solution of lipid-FAF-BSA that was 50 mg/mL for BSA. Cells were incubated as indicated with 6:1 sodium palmitate-FAF-BSA or 6:1 oleic acid-FAF-BSA at a final concentration of 20 mg/mL BSA.
Quantitation of endocytic uptake.
Cells cultured on Transwell supports as above and pretreated with 20 mg/mL albumin or normal HuSer (no. H4522, diluted 1:1 into serum-free medium, Sigma) as described above were incubated in serum-free medium with 40 µg/mL Alexa Fluor 647-albumin for 30–60 min at 37°C on an orbital shaker in the incubator and then washed with PBS and solubilized. Cell-associated fluorescence was quantified by spectrofluorimetry and normalized to protein recovery measured by the Lowry assay.
siRNA knockdown of megalin and cubilin.
OK cells (4 × 105) were transfected with 1 µg of megalin siRNA duplex (sense sequence 5′-GGAAAGAGAUGCCGGCAAAUU-3′, Dharmacon) or cubilin siRNA duplex (sense sequence 5′-CCAACGAACUUGUGGAAAAUU-3′, Dharmacon) using Lipofectamine RNAiMAX (no. 13778150, Invitrogen), following the manufacturer’s protocol, and seeded onto 12-mm (no. 3401, Corning) Transwell permeable supports. The following day, cells were transfected a second time and then shifted to an orbital shaker for 72 h with daily feeding. For aconitase measurements, 1.6 × 106 cells were transfected with 4 µg siRNA and cultured on 24-mm (no. 3412, Corning) Transwell permeable supports. Knockdown was confirmed by Western blot analysis using rabbit anti-megalin antibody [MC-220, gift from Daniel Biemesderfer, 1:20,000 dilution (43)] or a rabbit anti-cubilin antibody that we generated against a sequence within CUB domain 16 of Monodelphis domestica (rabbit no. 27445, 1:5,000 dilution).
Indirect immunofluorescence.
OK cells cultured on permeable supports were incubated with apically added 40 µg/mL Alexa Fluor 647-BSA for 30 min and then washed twice in warm PBS/+Ca2+/+Mg2+ (D8662, Sigma) and fixed in warm 4% paraformaldehyde-100 mM sodium cacodylate for 15 min at ambient temperature. After two washes in PBS, filters were quenched (PBS, 20 mM glycine, and 75 mM ammonium chloride) for 5 min and permeabilized for 5 min in quench solution containing 0.1% Triton X-100. After being washed with PBS, filters were blocked with PBS, 1% BSA, and 0.1% saponin for 30 min and incubated for 30 min with Acti-stain 488 phalloidin (PHDG1, Cytoskeleton) diluted in wash buffer [PBS, 0.5% BSA, and 0.025% (vol/vol) saponin]. Filters were washed three times in wash buffer and mounted onto glass slides with ProLong Gold antifade reagent (P36935, Molecular Probes). Filters were imaged on a Leica TCA SP5 confocal microscope using the PL APO CS ×63 objective.
Aconitase activity assay.
OK cells (1.6 × 106) were plated on 24-mm Transwell (0.4-µm pore) polycarbonate membrane inserts (no. 3412, Corning) in a six-well plate with 1.5 mL of apical medium and 2.5 mL of basolateral medium. The following day, cells were transferred to an orbital shaker set at 146 rpm in the incubator and allowed to grow for an additional 2 days with daily medium changes before exposure overnight to albumin or HuSer as indicated. Aconitase activity was measured using the Aconitase Activity Colorimetric Assay Kit (BioVision) according to the manufacturer’s instructions. Cells were washed twice with ice-cold PBS, scraped off the filters, and pelleted at 3,000 rpm for 10 min. Cell pellets were lysed in 150 µL assay buffer by passage through a 22-gauge needle 10 times. Lysates were sequentially centrifuged at 3,000 rpm (10 min) and 12,000 rpm (10 min), the supernatant was collected, and aconitase activity in each sample was normalized to total protein.
Uptake and fate of radioiodinated albumin.
FAF-BSA was radioiodinated to a specific activity of∼50 µCi/mg using the iodine monochloride method. Cells were incubated at 37°C with ∼40 µg 125I-labeled albumin (125I-albumin) added apically in serum-free medium for 15 min and then washed and incubated in fresh serum-free medium. At the indicated times, the apical and basolateral media were collected and combined, and the cells were solubilized. Samples were trichloroacetic acid (TCA) precipitated and counted in a γ-counter. Background counts were ≤0.5% of total internalized 125I-albumin under all conditions.
RESULTS
PT endocytic capacity is differentially modulated by albumin cargo.
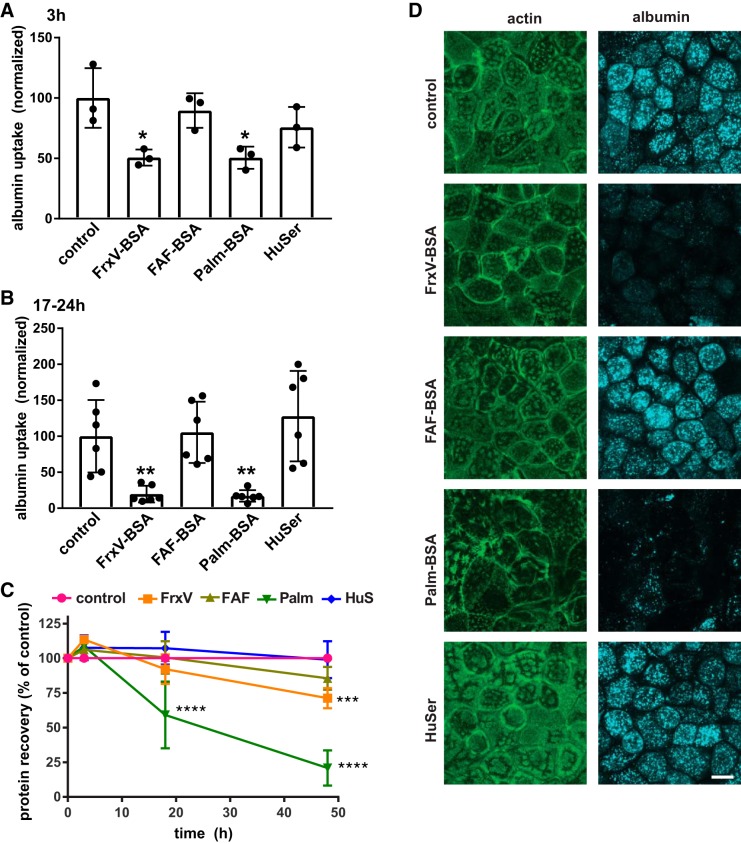
PT cells cultured overnight in high concentrations of BSA have previously been shown to have reduced endocytic capacity (21). To determine whether this effect is caused by albumin itself and/or dependent on the specific ligands complexed to albumin, we exposed OK cells cultured under our optimized PT differentiation conditions to nephrotic concentrations (20–25 mg/mL) of normal HuSer or different preparations of albumin and quantified the effects on cellular endocytic capacity. Cells were incubated for 3 h or overnight (17–24 h) with apically added fraction V BSA (FrxV-BSA; BP1600, Fisher Bioreagents), FAF-BSA, FAF-BSA complexed with palmitic acid at a 6:1 molar ratio (Palm-BSA), and normal HuSer diluted 1:1 in medium (so that the concentration of albumin was comparable with the other conditions). Cells were then washed extensively and incubated for 30 min with apically added 40 µg/mL Alexa Fluor-647 BSA, and cell-associated fluorescence was quantified by spectrofluorimetry, as described in materials and methods. An aliquot of lysate was used to quantify protein recovery in each sample as a measure of cell viability, and endocytic uptake was normalized accordingly. As shown in Fig. 1A, we observed a striking reduction in cellular uptake of albumin in cells pretreated with FrxV-BSA or Palm-BSA. The effects on endocytosis were rapid and could be observed after as little as 3 h of exposure. Preincubation for longer periods (17–24 h) resulted in greater effects on endocytosis (Fig. 1B). In contrast, pretreatment with FAF-BSA or HuSer had no effect on subsequent endocytic uptake of Alexa Fluor-647 BSA.
Fig. 1.
Differential effect of albumin ligands with proximal tubule (PT) endocytic capacity. Opossum kidney (OK) cells were incubated for 3 h (A) or 17–24 h (B) in serum-free medium without (control) or with apically added BSA [20 mg/mL fraction V BSA (FrV-BSA), fatty acid-free BSA (FAF-BSA), or palmitate-loaded BSA (Palm-BSA)] or 50% human serum (HuSer), as noted. Cells were then rinsed several times and incubated for 30 min with Alexa Fluor-647 albumin, and cell-associated fluorescence was quantified by spectrofluorimetry. Data were normalized to the protein recovered in each sample. The mean control uptake of all experiments was set to 100, and each point represents data from an independent experiment. *P < 0.02 and **P ≤ 0.01 by one-way ANOVA (Dunnett’s multiple-comparisons test). C: recovered protein concentrations (means ± SD, normalized to control) from cells treated for 3–48 h with albumin or HuSer as above. ***P ≤ 0.0005 and ****P ≤ 0.0001 by one-way ANOVA (Dunnett’s multiple-comparisons test): 3 h, n = 3; 17–24 h (combined), n = 9; 48 h, n = 5. D: control cells or cells treated overnight with albumin or HuSer and incubated for 30 min with Alexa Fluor-647 albumin (blue) were fixed and processed to detect actin (green). Maximum projections sections of confocal stacks are shown to confirm that exposure to FrxV-BSA or Palm-BSA reduces cellular albumin uptake and alters cell morphology but does not affect the integrity of the monolayer. Scale bar = 10 µm.
Surprisingly, although FrxV-BSA and Palm-BSA caused similarly rapid reductions in endocytic uptake, they had very different effects on cell viability (Fig. 1C). Neither treatment altered protein recovery after incubation for 3 h. Protein recovery in samples treated overnight with FrxV-BSA were similar to control (92 ± 11%); however, by 48 h of exposure, recovered protein was modestly reduced (to 71 ± 7.4% of control; Fig. 1C). In contrast, overnight treatment with Palm-BSA resulted in a significant loss of recovered protein (means ± SD: 59 ± 24% of control) that decreased further to 21 ± 13% of control after 48 h.
We assessed the effect of the various overnight treatments on monolayer integrity by confocal imaging of cells incubated for 30 min with Alexa Fluor-647 albumin and then fixed and processed to visualize actin. The reduction in albumin uptake was clearly evident in cells treated overnight with FrxV-BSA or Palm-BSA (Fig. 1D). Although the overall integrity of the cell monolayer was not compromised by overnight incubation with Palm-BSA or FrxV-BSA, some cells lacked the classic microvillar staining pattern observed in control cells (Fig. 1D). Moreover, cells treated with Palm-BSA appeared less densely packed compared with control, consistent with the reduction in protein recovery (Fig. 1D). We conclude that the ligands complexed to albumin, rather than albumin itself, can rapidly modulate endocytic capacity and cell viability in response to overload.
Albumin ligands dictate PT oxidative stress responses.
Cellular oxidative stress levels are known to be sensitive to serum metabolites typically carried by albumin, including heme and fatty acids. Therefore, we asked whether exposure with HuSer, FAF-BSA, or complexed albumin has different effects on ROS production in PT cells. To this end, we measured the enzymatic activity of aconitase, an enzyme in the tricarboxylic acid cycle localized primarily to mitochondria that converts citrate to isocitrate. Oxidation of the iron cluster within the active site of aconitase blocks its catalytic activity. As a consequence, reduced aconitase activity is a sensitive indicator of mitochondria-specific oxidative stress (12). Filter-grown OK cells were incubated overnight with HuSer or the different BSA preparations as above and then washed and solubilized. Equal volumes of lysate were incubated with substrate (citrate) for 1 h, and aconitase-mediated production of isocitrate was quantified and normalized to total protein. As shown in Fig. 2, exposure to FAF-BSA had no effect on mitochondrial ROS levels. In contrast, we observed a dramatic reduction in aconitase activity in cells exposed to FrxV-BSA. However, Palm-BSA and HuSer treatment caused only a small, albeit significant, decrease in aconitase. These data suggest that albumin ligands have dramatically different effects on cellular ROS responses. Moreover, the magnitude of ROS responses to HuSer and albumins do not correlate directly with the modulation we observed in endocytic capacity and protein recovery.
Fig. 2.
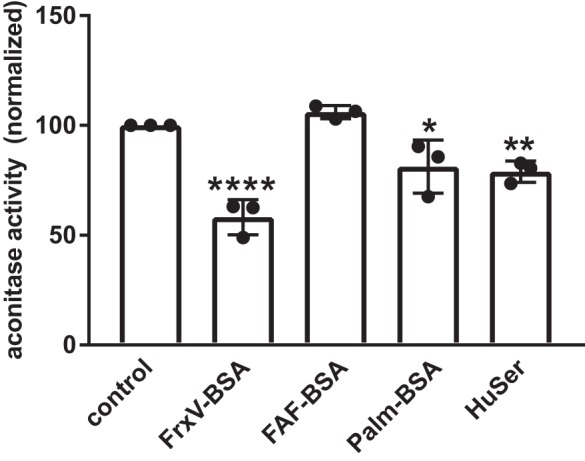
Albumin ligands differentially trigger mitochondrial oxidative stress. Opossum kidney (OK) cells were incubated for 17–24 h in serum-free medium without (control) or with apically added BSA [20 mg/mL fraction V BSA (FrV-BSA), fatty acid-free BSA (FAF-BSA), or palmitate-loaded BSA (Palm-BSA)] or 50% human serum (HuSer) and then lysed, and aconitase activity was measured and quantified as described in materials and methods. Data from 3 independent experiments performed in duplicate were normalized to untreated controls. Means and SDs are plotted, and each point represents data from a single experiment. *P ≤ 0.05, **P ≤ 0.02, and ****P < 0.0001 by one-way ANOVA (Dunnett’s multiple-comparisons test).
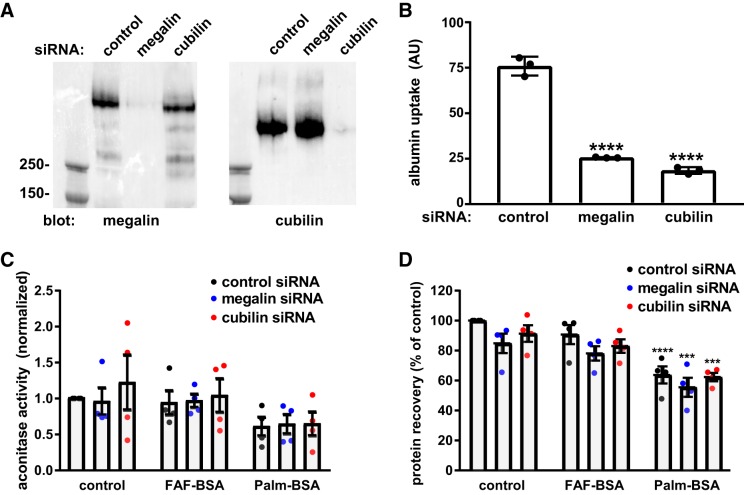
Megalin/cubilin-mediated albumin uptake is not required to induce cytotoxicity.
Because of the rapid effects of FrV-BSA and Palm-BSA on endocytic capacity, we asked whether albumin uptake via megalin and cubilin receptors is required to induce mitochondrial ROS and impair cell viability. OK cells were transfected as described in materials and methods with control siRNA or siRNA oligonucleotides directed against megalin or cubilin and cultured for an additional 72 h. Efficient knockdown was confirmed by Western blot analysis with anti-megalin and anti-cubilin antibodies (Fig. 3A). As expected, depletion of either receptor resulted in dramatically reduced uptake of albumin (Fig. 3B). Cells treated under similar conditions were then exposed overnight to FAF-BSA or Palm-BSA and protein recovery, and aconitase activity was quantified. As previously observed (Fig. 1C), treatment with Palm-BSA caused a significant reduction in protein recovery, whereas FAF-BSA had no effect (Fig. 3D). Similarly, pretreatment with Palm-BSA led to reduced aconitase activity (mean: 60.1 ± 13% of control, P = 0.02), as shown in Fig. 2, although the results did not reach statistical significance when the entire data set was analyzed by one-way ANOVA. Strikingly, neither the Palm-BSA-induced reduction in aconitase activity (Fig. 3C) nor protein recovery (Fig. 3D) was appreciably ameliorated by either megalin or cubilin knockdown. We conclude that receptor-mediated uptake of albumin is not needed to trigger ROS production in PT cells exposed to Palm-BSA.
Fig. 3.
Megalin and cubilin receptor knockdown impair albumin uptake but not reactive oxygen species (ROS) production in response to albumin ligands. A: equal amounts of lysate from cells transfected with control, megalin, or cubilin siRNA were blotted with antibodies against megalin or cubilin. B: albumin uptake (1-h incubation, corrected for protein recovery) was quantified in cells transfected with control, megalin, or cubilin siRNA uptake. Means ± SD of triplicate samples are plotted. ****P ≤ 0.0001 vs. control siRNA by one-way ANOVA (Dunnett’s multiple-comparisons test). C: aconitase activity was measured in cells transfected with the indicated siRNA and incubated for 18 h. Means and SDs are plotted; each point represents the mean of duplicate samples from 4 independent experiments. D: protein recovery was quantified in cells transfected with the indicated siRNA and incubated for 18 h in serum-free medium without (control) or with 20 mg/mL apically added fatty acid-free (FAF-BSA) or palmitate-loaded BSA (Palm-BSA). Means and SDs are plotted; each point represents the mean of duplicate samples from 4 independent experiments. ***P ≤ 0.0005 and ****P ≤ 0.0001 by two-way ANOVA (Dunnett’s multiple-comparisons test).
Does increased ROS production impair apical PT endocytosis of albumin?
Palmitate and other ligands carried by albumin may diffuse freely across plasma membranes and into cells or be actively transported by fatty acid transport mechanisms (18). Therefore, we asked whether increased oxidative stress triggered by exposure to FrxV-BSA and Palm-BSA impacts PT endocytic capacity. Prior studies have suggested that oxidative stress can impair endocytosis (42), but oxidative stress has also been reported to increase megalin expression, which would be expected to increase albumin uptake (22). To this end, we tested the hypothesis that exposure of filter-grown PT cells to ROS-producing molecules added to either the basolateral or apical compartment would similarly impair the subsequent apical uptake of fluorescent albumin. Control experiments using fluorescent 10K dextran confirmed that PT monolayers remained tight under all conditions (∼1% diffusion/h). Because unsaturated fatty acids are poor stimulators of ROS production (13), we also included BSA complexed to oleic acid (Olea-BSA; 6:1 molar ratio) as an additional negative control in these experiments. Cells were incubated for 3 h or overnight (17–20 h) with 20 mg/mL apical or basolateral FAF-BSA, FrxVBSA, Palm-BSA, or Olea-BSA and then washed, and uptake of Alexa Fluor-647 albumin was quantified as in Fig. 1. As predicted, exposure to apically or basolaterally added FAF-BSA or Olea-BSA had no effect on albumin uptake (Fig. 4, A and B). In contrast, FrxV-BSA reduced albumin uptake comparably regardless of whether it was added apically or basolaterally (Fig. 4, A and B). Interestingly, Palm-BSA had less of an effect on albumin uptake when added basolaterally for 3 h before albumin uptake was measured (Fig. 4A), whereas it inhibited endocytosis comparably with FrxV-BSA when added apically. However, after overnight exposure to either apically or basolaterally added Palm-BSA, endocytosis was profoundly inhibited (Fig. 4B). Together, these data suggest that megalin/cubilin-mediated uptake of FrxV-BSA or Palm-BSA is not required for their cytotoxic effects on PT cells. However, palmitate-induced responses occur more rapidly when the lipid is accessible via the lumenal surface.
Fig. 4.
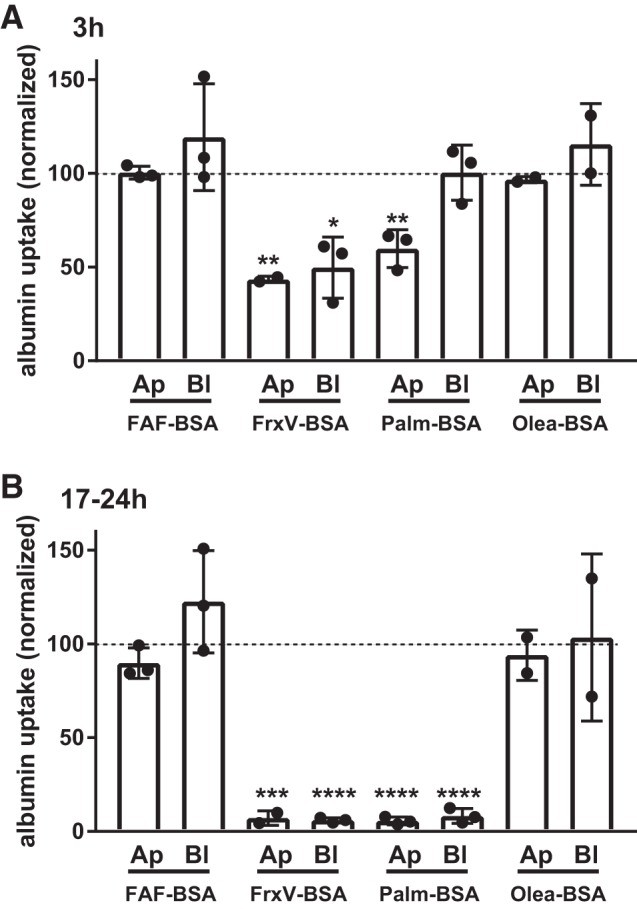
Basolateral exposure to BSA impairs apical endocytosis. Opossum kidney (OK) cells were incubated for 3 h (A) or 17–20 h (B) in serum-free medium without (control) or with apically (Ap) or basolaterally (Bl) added BSA [20 mg/mL fatty acid-free BSA (FAF-BSA) or palmitate-loaded BSA (Palm-BSA) or oleic acid-loaded BSA (Olea-BSA)]. Cells were then rinsed several times and incubated for 60 min with apically added Alexa Fluor-647 albumin, and uptake was quantified as described in materials and methods. Data were normalized for protein recovery. Each point represents the average uptake of duplicate samples (normalized to control), and the mean ± SD of 2–3 experiments for each condition is indicated by the bar. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.0005, and ****P ≤ 0.0001 by one-way ANOVA (Dunnett’s multiple-comparisons test).
Does overload with albumin affect endocytic pathway function?
PT cells in vivo have an extraordinary endocytic capacity, and under nephrotic conditions they accumulate high concentrations of lysosomal albumin (30, 40). Endocytic uptake of high concentrations of filtered proteins could overwhelm the endocytic pathway and/or degradation machinery and trigger downstream signaling responses (16). Because our OK cell model maintains robust apical endocytic capacity, we asked whether albumin degradation kinetics are affected by “overload” conditions upon chronic exposure to high concentrations of albumin. Additionally, we tested whether induction of ROS influences the processing of internalized albumin. Preliminary experiments to quantify the fate of internalized fluorescent conjugates of albumin yielded unsatisfactory results due to dequenching of the fluorescence upon albumin degradation. As an alternative, we used radioiodinated albumin (fatty acid free) as a readout of albumin fate. Cells were preincubated with apically added 20 mg/mL FAF-BSA for 72 h or with FrxV-BSA or Palm-BSA added basolaterally for 3 h (to impair endocytic capacity and/or induce oxidative stress without overload) and then washed extensively. Parallel confocal imaging experiments revealed no qualitative difference in lysosomal morphology under all conditions (not shown). Subsequently, cells were allowed to internalize apically added 125I-albumin (∼0.5 mg/mL) for 15 min, and the albumin fate was monitored over time. In contrast to shorter exposure times (Fig. 1), incubation for 72 h with FAF-BSA caused a modest (∼35%) reduction in the total amount of 125I-albumin taken up during the 15-min internalization period, suggesting that long-term exposure to high concentrations of albumin overloads the endocytic pathway (Fig. 5A). Similarly, and consistent with our results shown in Fig. 4, pretreatment for 3 h with basolaterally added FrxV-BSA resulted in a dramatic (∼70%) reduction in 125I-albumin uptake. In contrast, exposure for 3 h to basolaterally added Palm-BSA had little effect on subsequent apical uptake of 125I-albumin (Fig. 5A). Western blot analysis revealed an apparent reduction in megalin expression in cells treated for 3 h with basolateral FrxV-BSA but no decrease under the other treatment conditions (Fig. 5B).
Fig. 5.
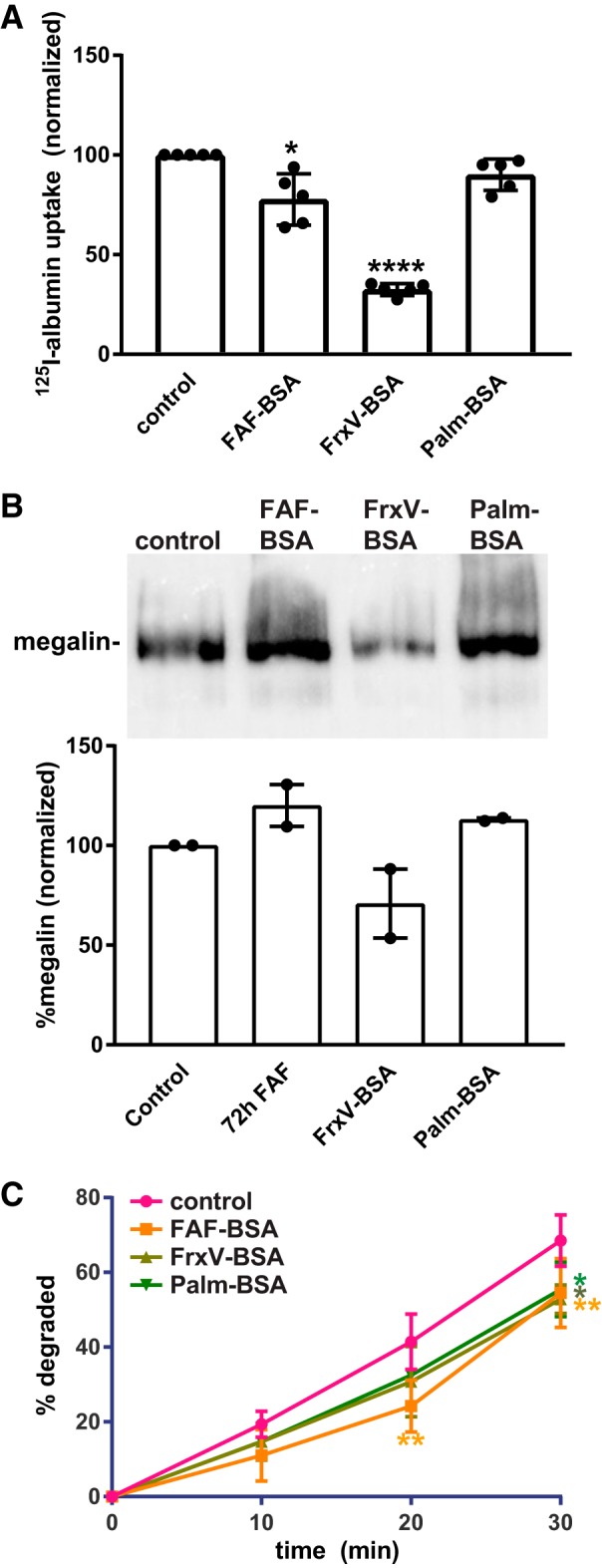
Chronic albumin overload slows degradation kinetics. Opossum kidney (OK) cells were pretreated with 20 mg/mL apical fatty acid-free BSA (FAF-BSA) for 72 h or 20 mg/mL Fraction V BSA (FrxV-BSA) or palmitate-loaded BSA (Palm-BSA) was added basolaterally for 3 h. After being washed, cells were incubated with 125I-labeled albumin (125I-albumin) for 15 min. A: total 125I-albumin internalized under each condition (normalized to control; mean ± SD of 5 experiments). *P ≤ 0.05 and ****P ≤ 0.0001 by Kruskal-Wallis test (Dunnett’s multiple-comparisons test). B: cells incubated with FAF-BSA, FrxV-BSA, or Palm-BSA as in A were solubilized, and equal protein loads were Western blotted with anti-megalin antibody. A representative blot is shown, and normalized intensities (means ± range) from 2 independent experiments are plotted. C: cells treated as above were returned to culture after internalization of 125I-albumin. Apical and basolateral medium from individual filters was collected, and cells were solubilized after 10, 20, or 30 min. Trichloroacetic acid-soluble 125I-dpm are plotted as the percent total for each condition. The mean ± SD of 3 experiments is plotted. *P ≤ 0.05 and **P ≤ 0.01 by two-way ANOVA (Dunnett’s multiple-comparisons test).
To assess degradation kinetics of endocytosed albumin, we incubated cells pretreated as above with 125I-albumin for 15 min and returned them to culture. The medium and lysates were collected from individual filters at 10, 20, or 30 min after internalization and precipitated with TCA to quantify intact versus degraded albumin. As shown in Fig. 5C, the kinetics of 125I-albumin degradation (quantified as the percentage of total counts per min that were TCA soluble in the medium plus supernatant) were significantly slower in cells pretreated for 72 h, with FAF-BSA compared with control cells. Similarly, albumin degradation was slower in cells treated for 3 h with basolaterally added FrxV-BSA or Palm-BSA (Fig. 5C). Despite the slower kinetics, the majority (>90%) of the internalized albumin was degraded under all conditions when the chase time was extended to 90 min (not shown). We conclude that long-term exposure of PT cells to high concentrations of free albumin can overwhelm the endocytic/degradation machinery.
DISCUSSION
Albuminuria induced by glomerular damage is clearly toxic to the PT, and unraveling cellular responses in vivo has proven challenging. Here, we examined the effects of albumin exposure on cytotoxicity and endosomal function using a highly differentiated PT culture model. We found remarkable differences in the cellular responses to different preparations of albumin carrying distinct ligands. Additionally, we observed that chronic exposure to free albumin impaired lysosomal degradation kinetics, suggesting that albumin itself can overload the endocytic pathway in the absence of other cytotoxic responses.
Our OK cell culture system provides an excellent model in which to dissect the specific functions of albumin and its ligands on PT function in different disease conditions. OK cells cultured under orbital shear stress develop an elaborate endocytic pathway and maintain the robust oxidative metabolism unique to the kidney PT and are thus likely to mimic in vivo responses to fatty acids and other potentially harmful molecules carried by albumin (27, 34). In contrast to in vivo studies, where the glomerular filtration barrier impedes direct access to the tubule lumen, the use of a culture model enables delivery of quantifiable levels of defined preparations of albumin to PT cells. Additionally, the absence of inflammatory responses and cross-talk from other nephron segments allows elucidation of the immediate and direct PT responses to albumin and its ligands. Using our model, we found that exposure of PT cells to high concentrations of normal human serum is not toxic per se and that specific ligands carried by albumin elicit distinct PT responses. Although these are impossible to test precisely in vivo, our scalable approach provides a means to perform more detailed studies of differential PT responses to serum from patients.
Our study demonstrates that the effects on cell toxicity of commercially available preparations of albumin (FrxV-BSA) or albumin complexed to palmitate are extreme compared with exposure to albumin in normal human serum. PT cells tolerated exposure to high concentrations of human serum with a modest increase in ROS production but no apparent change in endocytic capacity or cell viability. In contrast, exposure to Palm-BSA resulted in a similarly modest effect on ROS production but a dramatic reduction in endocytic capacity and cell viability. Finally, exposure to FrxV-BSA, used frequently by investigators to study overload responses to albumin, resulted in a profound and rapid loss of endocytic capacity and the greatest increase in ROS levels but only a slow decline in cell viability. FrxV-BSA was originally described as a preparation of albumin isolated using a cold ethanol precipitation; however, the original purification scheme has since been modified to include several variations, and the nomenclature no longer refers specifically to a precise purification strategy (32). Adding to the challenges of interpreting studies using FrxV-BSA is the observation that this preparation has been described to contain both growth-promoting and cytotoxic contaminants (19). The use of undefined (and unspecified) preparations of BSA in in vitro and in vivo albumin overload models likely explains much of the variability in previous studies of the sequelae of proteinuric insults to kidney function. Our results emphasize the need to carefully document the precise experimental reagents and conditions used and how the outcomes were assessed and reported. As in all studies where cytotoxicity is a potential outcome, documentation of cell viability and appropriate normalization of the data are essential to fully interpret the results.
Our data support the growing consensus that uptake of saturated nonesterified fatty acids (NEFAs) carried by albumin contributes significantly to PT toxicity during albumin overload (35). Albumin has seven binding sites for fatty acids, and in proteinuric conditions, NEFA levels reaching the tubule lumen are greatly increased. Cellular accumulation of saturated NEFAs stimulates production of ROS that trigger lipoapoptosis (17, 20, 36). However, metabolic status clearly has complex effects on PT responses, as unsaturated NEFAs may provide protection against ROS production and have also been reported to stimulate PT cell growth (13, 39). Although the fully loaded albumin used in our and other studies represents an unlikely pathological condition, our data support the emerging concept that metabolic disequilibria resulting in excessive serum lipids, as in obesity or diabetes, may exacerbate PT responses in nephrotic conditions (24).
Our data also suggest that free albumin itself does not trigger ROS production in cultured cells, and, indeed, previous studies have suggested that under some conditions it may be protective (2, 5, 11, 33). Moreover, we found that albumin uptake via megalin or cubilin receptors is not necessary to induce these cytotoxic PT responses. This makes sense, given that numerous cell types are sensitive to palmitate toxicity (9, 14). Passive uptake of high concentrations of albumin into internalized vesicles in endocytically active cells may provide a route of entry. Additionally, there are many ways for palmitate to enter cells, and there is an increasing awareness that fatty acid transport proteins are upregulated by metabolic cues, including a high-fat diet (24). Similar mechanisms may explain why megalin-deficient mice remain susceptible to proteinuria-induced tubular injury and why tubular cells downstream of the PT respond to proteinuric insults (6, 38).
Although exposure to free albumin did not apparently induce ROS production or impair cell viability, upon long-term incubation (72 h) with FAF-BSA, we observed a reduction in lysosomal degradation kinetics. This is consistent with the observed accumulation of albumin in swollen lysosomal compartments and its delayed proteolysis under nephrotic conditions in vivo (23, 30). Uptake of large amounts of albumin could increase the proton-buffering capacity within acidified compartments and thereby impair endosomal sorting and/or lysosomal function (26). Lysosomes are emerging as critical sensors of cell metabolism and signaling, and the accumulation of albumin and/or its degradation products likely initiates signaling pathways in addition to those triggered by albumin ligands (16). Indeed, previous studies have reported changes in inflammasome activation, cytokine maturation, and apoptosis in cells treated with FAF-BSA (8, 26).
The results of our study confirm the complex contributions of megalin and cubilin receptors to maintaining normal PT function and also to disease progression (3, 28). Additionally, they also reveal that the ligands carried by albumin enter PT cells via pathways independent of megalin/cubilin-mediated endocytosis. Although in vitro studies alone have limited direct impact, understanding specific PT responses to albumin and its various ligands is essential to inform the design and interpretation of more translational approaches to limit the progression of proteinuric disease. It is possible that therapeutic strategies to limit versus to enhance the PT uptake of filtered proteins will be differentially effective, depending on the specific metabolic conditions that accompany nephrosis. Our culture model is readily adapted to larger-scale screening approaches and may provide one avenue to further elucidate the various PT signaling pathways elicited by exposure to albumin and its ligands.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 Grants DK-101484, DK-100357, and DK-118726 (to O. A. Weisz). M. L. Gliozzi was supported by T32-DK-061296 and TL1-TR-001858. We are grateful for access to the cores of the Pittsburgh Center for Kidney Research funded by NIDDK Grant P30-DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.R.L., M.L.G., Q.R., and O.A.W. conceived and designed research; K.R.L., Y.R., M.L.G., and Q.R. performed experiments; K.R.L. and O.A.W. analyzed data; K.R.L. and O.A.W. interpreted results of experiments; K.R.L., Y.R., and O.A.W. prepared figures; O.A.W. drafted manuscript; K.R.L., Y.R., M.L.G., Q.R., and O.A.W. edited and revised manuscript; K.R.L., Y.R., M.L.G., Q.R., and O.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Catherine Baty for helpful comments on the manuscript and Alexander Sorkin for generous access to the γ-counter.
REFERENCES
- 1.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19: 393–402, 2010. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caruso-Neves C, Pinheiro AAS, Cai H, Souza-Menezes J, Guggino WB. PKB and megalin determine the survival or death of renal proximal tubule cells. Proc Natl Acad Sci USA 103: 18810–18815, 2006. doi: 10.1073/pnas.0605029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen EI, Nielsen R, Birn H. From bowel to kidneys: the role of cubilin in physiology and disease. Nephrol Dial Transplant 28: 274–281, 2013. doi: 10.1093/ndt/gfs565. [DOI] [PubMed] [Google Scholar]
- 4.Cobbs A, Chen X, Zhang Y, George J, Huang M-B, Bond V, Thompson W, Zhao X. Saturated fatty acid stimulates production of extracellular vesicles by renal tubular epithelial cells. Mol Cell Biochem 458: 113–124, 2019. doi: 10.1007/s11010-019-03535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon R, Brunskill NJ. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: implications for the pathophysiology of proteinuric states. J Am Soc Nephrol 10: 1487–1497, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Dizin E, Hasler U, Nlandu-Khodo S, Fila M, Roth I, Ernandez T, Doucet A, Martin P-Y, Feraille E, de Seigneux S. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am J Physiol Renal Physiol 305: F1053–F1063, 2013. doi: 10.1152/ajprenal.00006.2013. [DOI] [PubMed] [Google Scholar]
- 7.Erkan E, Devarajan P, Schwartz GJ. Apoptotic response to albumin overload: proximal vs. distal/collecting tubule cells. Am J Nephrol 25: 121–131, 2005. doi: 10.1159/000084888. [DOI] [PubMed] [Google Scholar]
- 8.Erkan E, Devarajan P, Schwartz GJ. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol 18: 1199–1208, 2007. doi: 10.1681/ASN.2006040407. [DOI] [PubMed] [Google Scholar]
- 9.Erkan E, Garcia CD, Patterson LT, Mishra J, Mitsnefes MM, Kaskel FJ, Devarajan P. Induction of renal tubular cell apoptosis in focal segmental glomerulosclerosis: roles of proteinuria and Fas-dependent pathways. J Am Soc Nephrol 16: 398–407, 2005. doi: 10.1681/ASN.2003100861. [DOI] [PubMed] [Google Scholar]
- 10.Eshbach ML, Weisz OA. Receptor-mediated endocytosis in the proximal tubule. Annu Rev Physiol 79: 425–448, 2017. doi: 10.1146/annurev-physiol-022516-034234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology 62: 1–16, 2010. doi: 10.1007/s10616-010-9263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol 349: 9–23, 2002. doi: 10.1016/S0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 13.Gehrmann W, Würdemann W, Plötz T, Jörns A, Lenzen S, Elsner M. Antagonism Between Saturated and Unsaturated Fatty Acids in ROS Mediated Lipotoxicity in Rat Insulin-Producing Cells. Cell Physiol Biochem 36: 852–865, 2015. doi: 10.1159/000430261. [DOI] [PubMed] [Google Scholar]
- 14.Girona J, Rosales R, Saavedra P, Masana L, Vallvé J-C. Palmitate decreases migration and proliferation and increases oxidative stress and inflammation in smooth muscle cells: role of the Nrf2 signaling pathway. Am J Physiol Cell Physiol 316: C888–C897, 2019. doi: 10.1152/ajpcell.00293.2018. [DOI] [PubMed] [Google Scholar]
- 15.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inpanathan S, Botelho RJ. The lysosome signaling platform: adapting with the times. Front Cell Dev Biol 7: 113, 2019. doi: 10.3389/fcell.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishola DA Jr, Post JA, van Timmeren MM, Bakker SJL, Goldschmeding R, Koomans HA, Braam B, Joles JA. Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int 70: 724–731, 2006. doi: 10.1038/sj.ki.5001629. [DOI] [PubMed] [Google Scholar]
- 18.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 1821: 852–857, 2012. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keenan J, Doherty G, Clynes M. Separation of growth-stimulating activity of BSA fraction V from the bulk of albumin using heparin sepharose chromatography. Cytotechnology 19: 63–72, 1995. doi: 10.1007/BF00749756. [DOI] [PubMed] [Google Scholar]
- 20.Khan S, Cabral PD, Schilling WP, Schmidt ZW, Uddin AN, Gingras A, Madhavan SM, Garvin JL, Schelling JR. Kidney Proximal Tubule Lipoapoptosis Is Regulated by Fatty Acid Transporter-2 (FATP2). J Am Soc Nephrol 29: 81–91, 2018. doi: 10.1681/ASN.2017030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koral K, Erkan E. PKB/Akt partners with Dab2 in albumin endocytosis. Am J Physiol Renal Physiol 302: F1013–F1024, 2012. doi: 10.1152/ajprenal.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurosaki Y, Imoto A, Kawakami F, Yokoba M, Takenaka T, Ichikawa T, Katagiri M, Ishii N. Oxidative stress increases megalin expression in the renal proximal tubules during the normoalbuminuric stage of diabetes mellitus. Am J Physiol Renal Physiol 314: F462–F470, 2018. doi: 10.1152/ajprenal.00108.2017. [DOI] [PubMed] [Google Scholar]
- 23.Lee D, Gleich K, Fraser SA, Katerelos M, Mount PF, Power DA. Limited capacity of proximal tubular proteolysis in mice with proteinuria. Am J Physiol Renal Physiol 304: F1009–F1019, 2013. doi: 10.1152/ajprenal.00601.2012. [DOI] [PubMed] [Google Scholar]
- 24.Li L-C, Yang J-L, Lee W-C, Chen J-B, Lee C-T, Wang P-W, Vaghese Z, Chen W-Y. Palmitate aggravates proteinuria-induced cell death and inflammation via CD36-inflammasome axis in the proximal tubular cells of obese mice. Am J Physiol Renal Physiol 315: F1720–F1731, 2018. doi: 10.1152/ajprenal.00536.2017. [DOI] [PubMed] [Google Scholar]
- 25.Liu B-C, Tang T-T, Lv L-L, Lan H-Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 93: 568–579, 2018. doi: 10.1016/j.kint.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC. Megalin/cubulin-lysosome-mediated albumin reabsorption is involved in the tubular cell activation of NLRP3 inflammasome and tubulointerstitial inflammation. J Biol Chem 290: 18018–18028, 2015. doi: 10.1074/jbc.M115.662064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long KR, Shipman KE, Rbaibi Y, Menshikova EV, Ritov VB, Eshbach ML, Jiang Y, Jackson EK, Baty CJ, Weisz OA. Proximal tubule apical endocytosis is modulated by fluid shear stress via an mTOR-dependent pathway. Mol Biol Cell 28: 2508–2517, 2017. doi: 10.1091/mbc.e17-04-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahadevappa R, Nielsen R, Christensen EI, Birn H. Megalin in acute kidney injury: foe and friend. Am J Physiol Renal Physiol 306: F147–F154, 2014. doi: 10.1152/ajprenal.00378.2013. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 89: 58–67, 2016. doi: 10.1016/j.kint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen R, Mollet G, Esquivel EL, Weyer K, Nielsen PK, Antignac C, Christensen EI. Increased lysosomal proteolysis counteracts protein accumulation in the proximal tubule during focal segmental glomerulosclerosis. Kidney Int 84: 902–910, 2013. doi: 10.1038/ki.2013.218. [DOI] [PubMed] [Google Scholar]
- 31.Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol 151: 580–590, 2007. doi: 10.1038/sj.bjp.0707251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raoufinia R, Mota A, Keyhanvar N, Safari F, Shamekhi S, Abdolalizadeh J. Overview of albumin and its purification methods. Adv Pharm Bull 6: 495–507, 2016. doi: 10.15171/apb.2016.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25: 119–146, 2016. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren Q, Gliozzi ML, Rittenhouse NL, Edmunds LR, Rbaibi Y, Locker JD, Poholek AC, Jurczak MJ, Baty CJ, Weisz OA. Shear stress and oxygen availability drive differential changes in opossum kidney proximal tubule cell metabolism and endocytosis. Traffic 20: 448–459, 2019. doi: 10.1111/tra.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggiero C, Elks CM, Kruger C, Cleland E, Addison K, Noland RC, Stadler K. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am J Physiol Renal Physiol 306: F896–F906, 2014. doi: 10.1152/ajprenal.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelling JR. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol 31: 693–706, 2016. doi: 10.1007/s00467-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Hultenby K, Axelsson J, Nordström J, He B, Wernerson A, Lindström K. Proximal tubular expression patterns of megalin and cubilin in proteinuric nephropathies. Kidney Int Rep 2: 721–732, 2017. doi: 10.1016/j.ekir.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theilig F, Kriz W, Jerichow T, Schrade P, Hähnel B, Willnow T, Le Hir M, Bachmann S. Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. J Am Soc Nephrol 18: 1824–1834, 2007. doi: 10.1681/ASN.2006111266. [DOI] [PubMed] [Google Scholar]
- 39.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol 13: 385–398, 1993. doi: 10.1159/000168653. [DOI] [PubMed] [Google Scholar]
- 40.Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 27: 482–494, 2016. doi: 10.1681/ASN.2014111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, He Y, Shen H, Ding H, Li K, Wang H. Expression of renal cubilin and its potential role in tubulointerstitial inflammation induced by albumin overload. Front Med China 2: 25–34, 2008. doi: 10.1007/s11684-008-0006-1. [DOI] [Google Scholar]
- 42.Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem 280: 4070–4078, 2005. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 43.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279: 34302–34310, 2004. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]