Abstract
CD148 is a transmembrane protein tyrosine phosphatase (PTP) that is expressed in the renal vasculature, including the glomerulus. Previous studies have shown that CD148 plays a role in the negative regulation of growth factor signals (including epidermal growth factor and vascular endothelial growth factor), suppressing cell proliferation and transformation. However, the role of CD148 in kidney disease remains unknown. Here, we generated an agonistic anti-CD148 antibody and evaluated its effects in murine diabetic nephropathy (DN). Monoclonal antibodies (mAbs) against the mouse CD148 ectodomain sequence were generated by immunizing CD148 knockout (CD148KO) mice. The mAbs that increased CD148 activity were selected by biological (proliferation) and biochemical (PTP activity) assays. The mAb (18E1) that showed strong agonistic activity was injected (10 mg/kg ip) in streptozotocin-induced wild-type and CD148KO diabetic mice for 6 wk, and the renal phenotype was then assessed. The effects of 18E1 mAb in podocyte growth factor signals were also assessed in culture. Compared with control IgG, 18E1 mAb significantly decreased albuminuria and mesangial expansion without altering hyperglycemia and blood pressure in wild-type diabetic mice. Immunohistochemical evaluation showed that 18E1 mAb significantly prevented the reduction of podocyte number and nephrin expression and decreased glomerular fibronectin expression and renal macrophage infiltration. The 18E1 mAb showed no effects in CD148KO diabetic mice. Furthermore, we demonstrated that 18E1 mAb reduces podocyte epidermal growth factor receptor signals in culture and in diabetic mice. These findings suggest that agonistic anti-CD148 mAb attenuates DN in mice, in part by reducing epidermal growth factor receptor signals in podocytes. This antibody may be used for the treatment of early DN.
Keywords: agonistic antibody, CD148, diabetic nephropathy, podocyte, protein tyrosine phosphatase
INTRODUCTION
CD148 (also named DEP-1, PTPRJ, PTPη, Byp) is a transmembrane protein tyrosine phosphatase (PTP) that is expressed in the renal vasculature, including the glomerulus (47). CD148 is expressed in a wide range of cell types, including epithelial cells, endothelial cells, and hematopoietic lineages (2, 12, 47). Previous studies have shown that CD148 interacts with and dephosphorylates growth factor receptors and their signaling proteins and negatively regulates their activities, inhibiting epithelial or endothelial cell proliferation and transformation. These include epidermal growth factor receptor (EGFR) (43, 44, 51), VEGF receptor-2 (VEGFR-2) (5, 43, 44), platelet-derived growth factor receptor-β (PDGFRβ) (23, 27), ERK1/2 (30, 37), phospholipase C (PLC)-γ1 (3, 53), and the p85 subunit of phosphatidylinositol 3-kinase (55). CD148 was shown to be downregulated in malignant tumor cells, and their neoplastic phenotype is suppressed when CD148 expression is restored (52, 53). Thus, there is substantial evidence demonstrating that CD148 plays an important role in the negative regulation of growth factor signals; however, its role in kidney disease remains unknown.
Diabetic nephropathy (DN) is the leading course of end-stage renal disease in many countries. Although multiple cell types and mechanisms are involved, recent studies have shown that excessive and prolonged growth factor signals serve as a key driver in advancing diabetic glomerular injury. EGFR activation in podocytes was shown to induce podocyte injury and loss and advance DN (1, 7, 60). Furthermore, excessive VEGF production in the glomerulus has been shown to advance DN (57, 58), whereas VEGF inhibitors attenuate DN (13, 39). These findings indicate that inhibition of growth factor signaling by CD148 activation may be a strategy for the treatment of DN. Based on this hypothesis, in the present study, we created an agonistic anti-CD148 monoclonal antibody (18E1 mAb) by immunizing CD148 knockout (CD148KO) mice using the recombinant protein of the CD148 ectodomain and asked if CD148 activation attenuates DN by treating diabetic mice with the antibody. Our data demonstrate that agonistic anti-CD148 antibody attenuates DN in mice in part by decreasing EGFR signals in podocytes.
MATERIALS AND METHODS
Antibodies and reagents.
The antibodies for immunoblot analysis and immunoprecipitation were as follows. Anti-phospho-EGFR (Tyr1173), anti-EGFR (1005), and anti-actin (C-2) were from Santa Cruz Biotechnology (Dallas, TX). Anti-hemagglutinin (HA)-peroxidase was from Sigma-Aldrich (St. Louis, MO). Anti-phopho-ERK1/2 (Thr202/Tyr204), anti-phospho-Akt (Ser473), and anti-Akt were from Cell Signaling Technology (Danvers, MA). Anti-ERK1/2 was from Millipore Sigma (Burlington, MA). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG were from GE Healthcare Bio-Sciences (Pittsburgh, PA).
The antibodies for immunofluorescence stain and immunohistochemistry were as follows. Anti-CD31 was from HistoBioTec (Miami Beach, FL). Anti-F4/80 (clone CI-A3-1) was from Bio-Rad (Hercules, CA). Anti-Wilms’ tumor-1 (WT1; C-19), anti-nephrin (N-20), and anti-phospho-EGFR (Tyr1173) were from Santa Cruz Biotechnology. Anti-phospho-ERK1/2 (Thr202/Tyr204) was from Cell Signaling Technology. Anti-fibronectin was from Novus Biologicals (Centennial, CO). Anti-podocin was from Sigma-Aldrich.
The secondary antibodies for immunofluorescence stain were as follows. Alexa Fluor 488-conjugated donkey anti-goat IgG and Alexa Fluor 546-conjugated donkey anti-rabbit IgG were from ThermoFisher Scientific (Grand Island, NY). Rabbit anti-mouse CD148 polyclonal antibody was raised using mouse CD148 ectodomain-Fc fusion protein and affinity purified at ThermoFisher Scientific. Its specificity was assessed by immunoblot analysis using A431D cells lacking or transiently overexpressing mouse CD148 and used for cell sorting. Human IgG Fc fragment and class-matched mouse IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and OriGene Technologies (Rockville, MD), respectively.
Plasmids.
The mouse CD148 (Byp) expression plasmid was a gift from Dr. Tadashi Yamamoto (University of Tokyo, Tokyo, Japan) (28), and the full-length mouse CD148 sequence (amino acids 29–1238, GenBank Accession No. Q64455) was subcloned into BamHI/EcoRI sites in LZRS-IRES-Zeo retroviral vector (a gift from Dr. Al Reynolds, Vanderbilt University) (22) to generate stable cells. The ectodomain sequence of mouse CD148 (amino acids 29–876, GenBank Accession No. Q64455) was amplified by PCR and subcloned into NheI/XbaI sites in pYD11 vector and HindIII/BamHI sites in pTT5 vector to produce CD148-Fc fusion and 8xHis-tagged CD148 ectodomain proteins, respectively. These expression plasmids (4, 14) were provided by Vanderbilt Antibody and Protein Resource (VAPR). Subcloning was performed by Custom DNA Constructs (University Heights, OH), and all constructs were confirmed by DNA sequencing.
Recombinant proteins.
Mouse CD148-Fc or Fc proteins were produced by transient transfection of the expression plasmids to human embryonic kidney-293 cells and purified using a HiTrap protein A HP column (GE Healthcare Life Sciences, Marlborough, MA) as previously described (43). 8xHis-tagged CD148 ectodomain protein was prepared using a HisTrap Excel column (GE Healthcare) as previously described (44). The purity and quality of the proteins were assessed by immunoblot analysis and Colloidal blue stain as previously described (43).
Generation of anti-mouse CD148 mAbs.
Mouse hybridoma antibodies against the mouse CD148 ectodomain sequence were generated by immunizing CD148KO mice (Deltagen, San Mateo, CA) using the mouse CD148-Fc fusion protein. The immunization, hybridoma production, and initial screening were carried out at VAPR according to their protocols. Hybridomas were screened by the ELISA against 8xHis-tagged mouse CD148 ectodomain protein. The 8xHis-tagged CD148 protein was used to eliminate the hybridomas producing antibodies against the Fc sequence. The positive clones (907 clones) were further screened by immunoblot analysis using cell lysates from A431D cells stably expressing mouse CD148 (A431D/m-CD148 cells) as well as by immunostaining of A431D cells that were transiently transfected with mouse CD148. The transient transfection was performed using the FuGENE transfection reagent (Promega, Fitchburg, WI) according to the manufacturer’s protocol. One hundred sixty clones were positive in immunoblot analysis and 270 clones in cell immunostaining. The top 10 clones that showed strong immunoreactivity in all three tests were subjected to small-scale antibody production, and a cell proliferation assay was then conducted using A431D/m-CD148 cells to assess their agonistic activity. The mAb that showed the strongest biological activity [clone 18E1 (isotype IgG2c)] was subcloned and used for this study. Antibody production, purification, subcloning, and isotyping were carried out at VAPR according to their protocols.
Cell culture and stable cell preparation.
A431D cells were cultured as previously described (43, 44). A431D cells stably expressing HA-tagged mouse CD148 were prepared using the LZRS-IRES-Zeo retroviral vector as previously described (43). Zeocin-selected cells were stained with anti-mouse CD148 rabbit polyclonal antibody and sorted using a BD FACS Aria II flow cytometer (BD Biosciences, San Jose, CA). Mouse glomerular endothelial cells were purchased from Cell Biologics (Chicago, IL) and cultured according to the manufacturer’s protocol. Immortalized mouse podocytes and mouse inner medullary collecting duct (IMCD) cells were provided by Dr. Ray Harris (Vanderbilt University) and Dr. Roy Zent (Vanderbilt University) and cultured as previously described (6, 7).
Immunoprecipitation and immunoblot analysis.
Cells were lysed in Triton lysis buffer [20 mM Tris (pH 7.5), 1% Triton X-100, 150 mM NaCl, and 2 mM EDTA] containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Kidneys from wild-type (WT) and CD148KO mice were lysed in RIPA buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS] containing protease inhibitor cocktail from Roche Applied Science. Cell or tissue protein lysates (40 or 200 μg) were subjected to the immunoblot analysis using 18E1 mAb (1.0 µg/mL). Proteins were separated by SDS-PAGE on 6.0% gels under reducing conditions, and immunoblot analysis was carried out as previously described (41, 43, 44).
The phosphorylation of EGFR, ERK1/2, and Akt was assessed as previously described (7, 44). Briefly, podocytes were plated in 100-mm dishes at 30% density, and serum was then reduced to 0.5% FBS for 18 h. Cells were treated with 20 ng/mL EGF (R&D Systems, Minneapolis, MN) with either 10 µg/mL 18E1 mAb or control IgG for 30 min, washed, and lysed in lysis buffer [20 mM HEPES (pH 7.5), 1% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 5 mM NaF, 5 mM iodoacetic acid, 1 mM Na3VO4, and protease inhibitor cocktail (Roche Life Science)]. EGFR was immunoprecipitated from clarified cell lysates (500 µg) using anti-EGFR antibody and immunoblotted using phosphospecific EGFR (Tyr1173) antibody. For ERK1/2 and Akt phosphorylation, crude lysates (5 µg) were immunoblotted using phosphospecific ERK1/2 (Thr202/Tyr204) or Akt (Ser473) antibody. The antibodies were stripped, and membranes were reblotted using antibodies to total EGFR, ERK1/2, or Akt.
Cell proliferation assay.
Cell proliferation of A431D and A431D/m-CD148 cells was assessed as previously described (44). Briefly, 1.5 × 103 cells were plated in 96-well plates, and serum was reduced to 0.1% FBS overnight (day 0). Next, cells were cultured in growth medium (supplemented with 2.5% FBS) with 18E1 mAb or control IgG at the indicated concentrations. Cell number was assessed at days 2 and 4 using the Cy-QUANT NF cell proliferation assay kit (ThermoFisher Scientific).
shRNA-mediated CD148 knockdown.
CD148 was knocked down in A431D/m-CD148 cells using mouse CD148-targeting shRNA lentiviral particles (Sigma-Aldrich) and subjected to the cell proliferation assay as previously described (43). Scrambled shRNA lentivirus (Sigma-Aldrich) was used as a control.
PTPase activity assay.
CD148 PTPase activity was assessed in 18E1 mAb or control IgG-treated A431D/m-CD148 cells as previously described (43). Briefly, serum was reduced to 2.5% FBS for 12 h, and cells were then incubated with 10 µg/mL 18E1 mAb or control IgG for 30 min in the presence or absence of CD148-Fc (10 μg/mL) or equal molar (1.2 μg/mL) of control Fc. CD148 was immunoprecipitated using Anti-HA Affinity Matrix (Sigma-Aldrich), and its catalytic activity was measured using 5 mM para-nitrophenyl phosphate (pNPP) in the presence or absence of 0.1 mM Na3VO4. The amounts of CD148 in immunoprecipitates were assessed by immunoblot analysis using anti-HA antibody as previously described (43).
Animals.
Mice of the DBA/2J strain were purchased from Jackson Laboratory (Bar Harbor, ME). CD148KO (CD148tlacZ/tlacZ) mice of the C57BL/6N strain were purchased from Deltagen (San Mateo, CA) and backcrossed on DBA/2J strain mice for 10 generations. Mice were genotyped according to the protocol provided by Deltagen. All animal experiments were performed under the approval of the Institutional Animal Care and Use Committee of Vanderbilt University and conducted in accordance with institutional guidelines. Mice were euthanized by inhalation of CO2 overdose and subsequent cervical dislocation.
β-Galactosidase histochemistry.
Kidneys were sampled from intact or diabetic [6 wk after streptozotocin (STZ) injections] heterozygous CD148 mice, and β-galactosidase histochemistry was performed and photographed as previously described (41, 46). Immunohistochemistry for CD31 was superimposed on β-galactosidase histochemistry as previously described (41).
Antibody treatment experiments of diabetic mice.
Diabetes was induced in WT or CD148KO mice of the DBA/2J strain at the age of 8 wk by intraperitoneal injections of low-dose STZ (50 mg/kg, 5 consecutive days, Sigma-Aldrich), as previously described (24). Nondiabetic control mice were generated by intraperitoneal injections of sodium citrate buffer (0.1 mol/L). The development of diabetes was confirmed by measurements of blood glucose at 2 wk after STZ injections, as previously described (24). The mice whose blood glucose levels exceeded 300 mg/dL were considered diabetic and used for the study. 18E1 mAb or control IgG were intraperitoneally injected to the mice (10 mg/kg, 3 times/wk) for a total 6 wk (see Fig. 3A). The titer of anti-CD148 antibody in serum was measured by ELISA using CD148-Fc protein as the antigen. The serum titer obtained with this dosage and injection protocol corresponded to 10–15 µg/mL of 18E1 mAb.
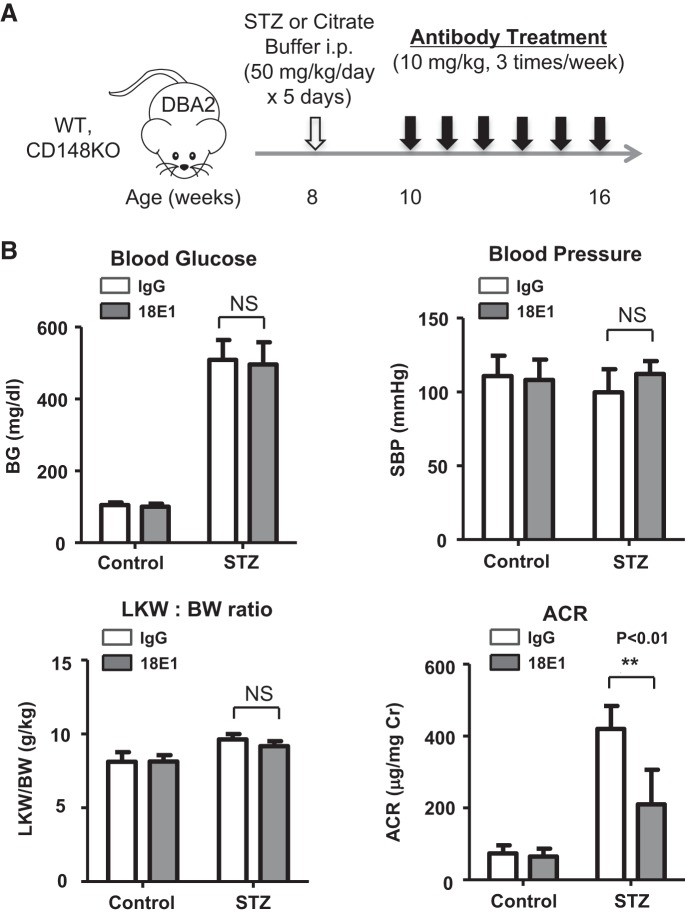
Fig. 3.
Effects of 18E1 monoclonal antibody (mAb) in murine diabetic nephropathy (DN). A: experimental protocol of the 18E1 mAb treatment study. Diabetes was induced in male mice of the DBA/2J strain at the age of 8 wk by multiple low-dose streptozotocin (STZ) injections. Mice injected with citrate buffer were used as nondiabetic controls. 18E1 mAb or control IgG was injected (10 mg/kg ip, 3 times/wk) in diabetic (STZ) or nondiabetic (control) mice for 6 wk, starting 2 wk after STZ injections. Measurements and tissue sampling were performed before (data not shown) and 6 wk after antibody treatment (8 wk after STZ or citrate buffer injections). B: effects of 6 wk 18E1 mAb or control IgG treatment on blood glucose (BG), systolic blood pressure (SBP), left kidney weight-to-body weight ratio (LKW/BW), and urinary albumin-to-creatinine ratio (ACR) were assessed in diabetic (STZ) and nondiabetic (control) mice as described in materials and methods. n = 8 mice/group. Data are presented as means ± SE. **P < 0.01 vs. control IgG-treated mice.
Measurements of blood glucose, blood pressure, and urinary albumin excretion.
Fasting blood glucose, systolic blood pressure, and urinary albumin excretion were measured as previously described (24, 60). In brief, blood was sampled by saphenous vein puncture after a 6-h fast, and blood glucose was measured using Accu-Chek test strips (Roche Applied Science). Systolic blood pressure was measured in conscious trained mice at room temperature using a tail-cuff monitor (Visitech BP-2000 Series II Blood Pressure Analysis System). Urinary albumin excretion was assessed by the determination of the albumin-to-creatinine ratio (ACR) of 24-h urine. Twenty four-hour urine was collected from individually caged mice using polycarbonate metabolic cages (Tecniplast, Buguggiatte, Italy). Urinary albumin and creatinine were measured using the Albuwell M Microalbuminuria ELISA kit (Exocell, Philadelphia, PA) and the Creatinine Companion kit (Exocell), respectively.
Renal histopathology.
Mice were anesthetized, and the kidneys were perfused with PBS via the left ventricle together with the incision of the renal vein. Kidneys were removed, and the right kidney was processed for histological examinations. For light microscopic examination, kidneys were fixed in 4% paraformaldehyde in PBS overnight at 4°C, and paraffin sections were processed by standard procedures. Sections were stained using the periodic acid-Schiff stain kit (Sigma-Aldrich) and photographed by light microscopy (Zeiss Axioskop 40). Mesangial expansion score of glomeruli was assessed as previously described (15, 35). At least 50 cortical glomeruli were examined in each mouse.
Immunofluorescence stain and immunohistochemistry.
Kidney tissues were placed in OCT compound (Sakura Finetec, Torrance, CA) and snap frozen in a dry ice-acetone bath. Cryostat sections were fixed in 100% acetone for 10 min at −20°C, washed three times in cold PBS, and blocked with 5% normal donkey serum (Jackson ImmunoResearch Laboratories) for 20 min at room temperature. For fibronectin and nephrin immunofluorescence staining, sections were incubated with primary antibodies (diluted to 1:100 in PBS) overnight at 4°C, washed, and incubated with Alexa Fluor 488-conjugated donkey anti-rabbit IgG (2 µg/mL) for 30 min at room temperature. For phospho-EGFR and podocin double immunostaining, kidney sections were incubated with primary antibodies (diluted to 1:50 in PBS) overnight at 4°C, washed, and then incubated with Alexa Fluor 488-donkey goat IgG and Alexa Fluor 546-donkey rabbit IgG antibodies. Washed sections were mounted (FLUORO-GEL with Tris buffer, Electron Microscopy Sciences, Hatfield, PA) and photographed using an OLYMPUS BX51 fluorescence microscope. Immunohistochemistry for F4/80, WT1, and phospho-ERK1/2 was carried out on paraffin sections of 4% paraformaldehyde-fixed kidneys. For F4/80, dewaxed paraffin sections were treated with 0.1% trypsin solution (0.1% calcium chloride in PBS, pH7.6, Sigma Aldrich) for 30 min at 37°C. For WT1 and phospho-ERK1/2, paraffin sections were heated in Retrieve-All solution (Signet Pathology Systems, Dedham, MA) for 15 min at 97°C using a microwave and then cooled down for at least 30 min. Sections were blocked, incubated with primary antibodies overnight at 4°C, and washed, and immunoreactions were detected using a VECTASTATIN Rabbit IgG ABC Kit (Vector Laboratories, Burlingame, CA) and visualized using the Peroxidase DAB Substrate Kit (Vector Laboratories). Dehydrated tissue sections were mounted (Cytoseal XYL, ThermoFisher Scientific) and photographed by light microscopy (Zeiss Axioskop 40). Macrophage infiltration was assessed by measuring F4/80-immunostained cells in the field containing the cortical glomerulus as previously described (60). At least 60 areas were assessed with a fixed color threshold, and the results were expressed as percentages in the glomerular area. Podocyte density was assessed by counting WT1-positive cells per cortical glomerulus. At least 50 glomeruli were assessed in each mouse. The intensity of nephrin immunofluorescense was quantified using ImageJ software (National Institutes of Health, Bethesda, MD). At least 40 glomerular areas were evaluated, and relative fluorescence intensity was calculated as previously described (59).
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed using Prism4 (GraphPad Software, La Jolla, CA). For two-group comparisons, an unpaired Student’s t test was used to calculate the P value. ANOVA was used for multiple-group comparisons. P < 0.05 was considered as statistically significant.
RESULTS
CD148 expression in the mouse kidney.
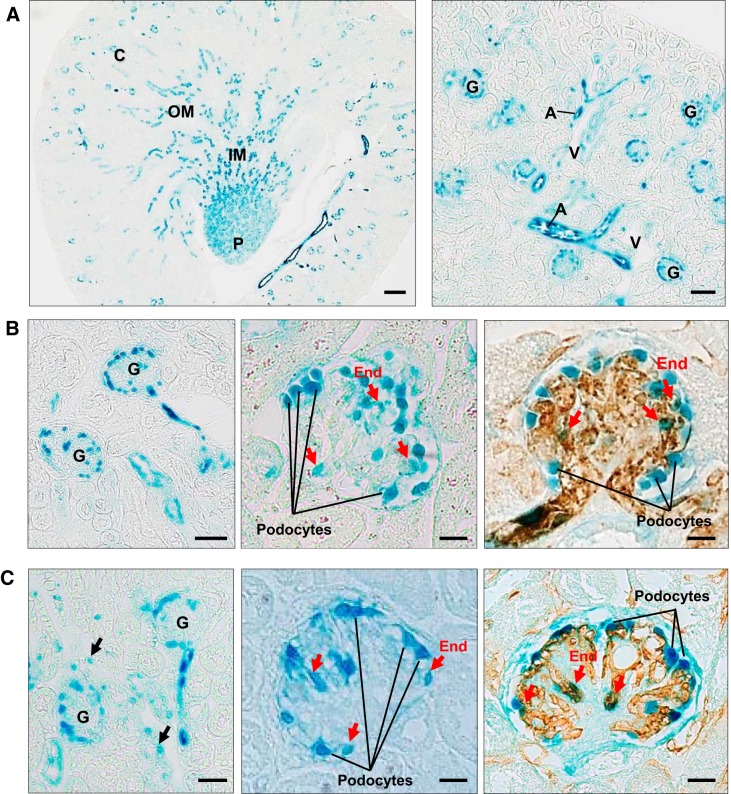
First, we examined the expression of CD148 in normal and diabetic mouse kidneys. Previous studies have investigated CD148 expression in human and mouse tissues by immunostaining. However, the results were quite different in different studies (2, 47), although these anti-CD148 antibodies specifically recognized CD148 in Western blot analysis. CD148 is a highly glycosylated membrane protein (20, 28); therefore, this may be because the antibodies recognize the glycosylation status or genetic variants (36) of CD148. For this reason, in this study, we assessed CD148 expression using heterozygous CD148 mice and β-galactosidase histochemistry. In these heterozygous mice, the LacZ gene, which has a nuclear localization sequence, is driven under the endogenous CD148 promoter (44). As shown in Fig. 1A, evident β-galactosidase activity, demonstrating CD148 promoter activity, was observed in the arterial endothelium, glomerular microvasculature, distal tubules (subpopulations), collecting ducts, and tubular segments in the inner medulla and papilla. In glomeruli, evident CD148 promoter activity was observed in podocytes and subpopulations of glomerular endothelial cells in both normal and diabetic mice (Fig. 1, B and C). CD148 expression was limited in the peritubular capillaries and venous vasculature (Fig. 1). The expression pattern of CD148 in diabetic mouse kidneys was similar to that in normal mouse kidneys, yet increased glomerular endothelial CD148 expression was observed in some (~10%) diabetic glomeruli. The frequency of CD148 expression in proximal tubules was increased more in diabetic mice (~5%), whereas its expression was highly limited (~1%) in normal mice (Fig. 1, B and C). The β-galactosidase histochemistry combined with anti-aquaporin 2 or anti-Tamm-Horsfall protein immunohistochemistry showed that CD148 was expressed in all collecting ducts labeled with aquaporin-2, whereas its expression was absent in the thick ascending limb of Henle’s loop, which was labeled with Tamm-Horsfall protein (data not shown).
Fig. 1.
CD148 expression in the adult mouse kidney. A: β-galactosidase histochemistry of the adult heterozygous CD148tlacZ/+ mouse kidney. Evident β-galactosidase activity indicating CD148 promoter activity was observed in the arterial vasculature (A), glomerular vasculature, distal tubules, collecting ducts, and tubular segments in the inner medulla (IM) and papilla. C, cortex; OM, outer medulla; P, papilla; G, glomerulus, V, vein. B: CD148 promoter activity in adult heterozygous CD148tlacZ/+ mouse glomeruli. CD148 promoter activity was observed in podocytes and subpopulations of glomerular endothelial cells (red arrows). Endothelial β-galactosidase activity (red arrows) was confirmed by superimposing CD31 immunohistochemistry (brown) on β-galactosidase histochemistry (right). C: CD148 promoter activity in diabetic heterozygous CD148tlacZ/+ mouse glomeruli [DBA2 strain, 6 wk after streptozotocin (STZ) injections]. Like the nondiabetic mouse kidney (B), CD148 promoter activity was observed in podocytes and subpopulations of glomerular endothelial cells (red arrows). Right, colocalization of β-galactosidase activity and CD31 immunoreactivity (brown) in glomerular endothelial cells (red arrows). Note that CD148 promoter activity was also observed in proximal tubules (black arrows) in the diabetic mouse kidney. Representative data of 5 mice kidneys are shown. Scale bar = 200 µm in A, left, 50 µm in A, right, 25 µm in B, left, and C, left, and 10 µm in B, middle and right, and C, middle and right.
Generation of an agonistic anti-mouse CD148 mAb.
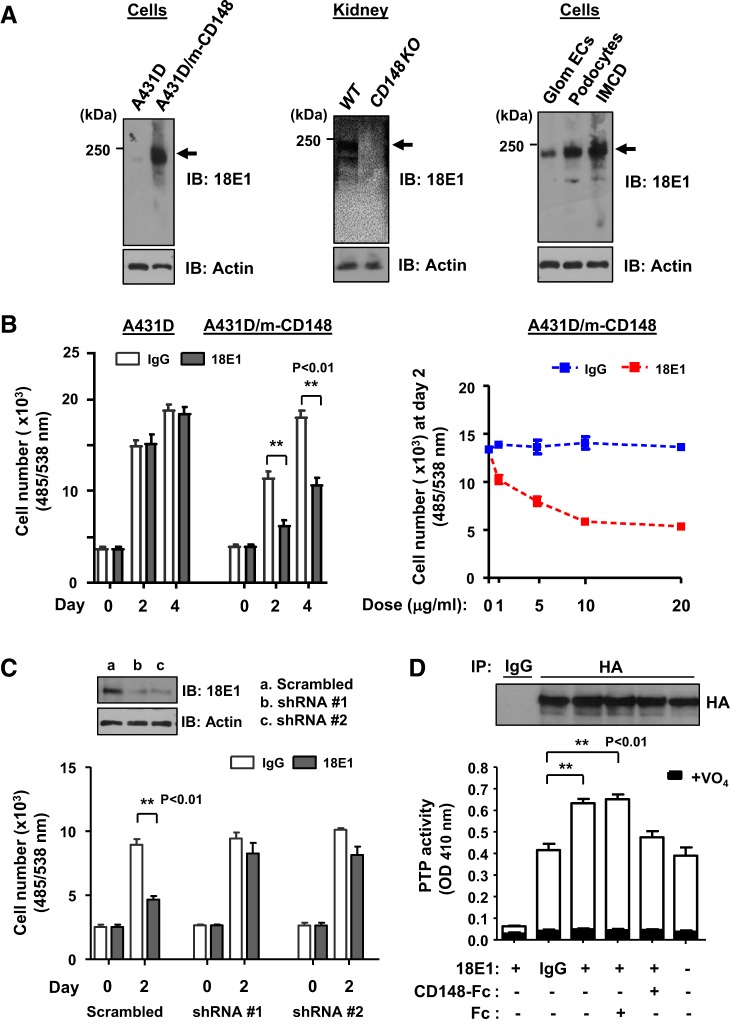
We have previously shown that a bivalent mAb raised against the human CD148 ectodomain sequence serves as a CD148 agonist (48). Because this antibody has low affinity to the mouse CD148 sequence, here we created an agonistic mAb against the mouse CD148 sequence to investigate the role of CD148 in mouse kidney disease. For this, five CD148KO mice were immunized using mouse CD148 ectodomain-Fc fusion protein, and the mouse whose serum showed the highest titer against the immunogen was subjected to mAb production. These hybridomas were screened by ELISA using the immunogen (907 clones positive), Western blot analysis with the cell lysates from mouse CD148-transfected cells (160 clones positive), and immunostaining of these transfected cells (270 clones positive). The top 10 clones that showed strong immunoreactivity in all three tests were further investigated for their activity to activate CD148. Among them, three clones (18E1, 10G1, and 24E11) inhibited cell proliferation of the A431D cells that was stably transfected with mouse CD148 (A431D/m-CD148 cells), whereas they showed no effects in A431D cells that lack CD148 expression (A431D cells). Clone 18E1 (isotype: IgG2c), which showed the strongest growth inhibitory activity, was further evaluated.
As shown in Fig. 2A, 18E1 mAb specifically recognized mouse CD148 (~230 kDa) that is expressed in A431D/m-CD148 cells, WT mouse kidneys, and cultured mouse glomerular endothelial cells, podocytes, and the IMCD. Furthermore, the 18E1 mAb dose dependently inhibited cell proliferation in A431D/m-CD148, but not A431D, cells (maximum inhibition, ~65%; Fig. 2B). This activity was largely diminished by shRNA-mediated CD148 knockdown, whereas scrambled shRNA did not alter the activity of 18E1 mAb to inhibit A431D/m-CD148 cell proliferation (Fig. 2C). Finally, we asked if 18E1 mAb increases the catalytic activity of CD148 using a PTPase activity assay. A431D/m-CD148 cells were treated with 10 μg/mL 18E1 mAb or control IgG for 30 min, CD148 (HA tagged) was immunoprecipitated using anti-HA antibody, and vanadate-sensitive phosphatase activity in the immunoprecipitates was then measured using pNPP as a substrate as previously described (43, 44). As shown in Fig. 2D, 18E1 mAb, but not control IgG, significantly increased (~50%) the catalytic activity of immunoprecipitable CD148, and this effect was antagonized by the incubation of the antibody with mouse CD148-Fc but not Fc alone. Collectively, these data demonstrate that 18E1 mAb serves as an agonistic antibody for mouse CD148.
Fig. 2.
Anti-CD148 monoclonal antibody (mAb; clone 18E1) inhibits cell proliferation of mouse CD148-expressing A431D cells, accompanied by an increase in CD148 catalytic activity. A: immunoblot analysis using 18E1 mAb. 18E1 mAb recognizes mouse CD148 (m-CD148, arrows), which is stably expressed in A431D cells (A431D/m-CD148) and wild-type (WT) kidneys, whereas its immunoreactivity is absent in control A431D cells (lacking CD148) and CD148 knockout (KO) mouse kidneys (left and middle). 18E1 mAb demonstrated CD148 expression in cultured mouse glomerular endothelial cells (Glom ECs), podocytes, and inner medullary collecting duct (IMCD) cells (right). The antibody was stripped, and the membranes were immunoblotted for actin (bottom) to assess the loading of protein lysates. Representative results of four independent experiments are shown. B: dose-dependent growth inhibition of A431D/m-CD148 cells by 18E1 mAb. A431D/m-CD148 or A431D cells were plated in 96-well plates and starved, and 10 μg/mL (left) or 1, 5, 10, or 20 μg/mL (right) of 18E1 mAb or class-matched IgG were then added to the medium. Cell number was assessed at days 0, 2, and 4. Data are means ± SE of quadruplicate determinations. Representative results of five independent experiments are shown. C: effects of CD148 knockdown in 18E1 mAb inhibition of A431D/m-CD148 cell proliferation. CD148 was knocked down in A431D/m-CD148 cells using lentivirus encoding mouse CD148-targeting shRNA (shRNA #1 and shRNA #2). Lentivirus encoding scrambled shRNA was used as a control. Their effects on 18E1 (10 μg/mL) inhibition of A431D/m-CD148 cell proliferation were assessed by a cell proliferation assay as in B. CD148 knockdown was confirmed by immunoblot analysis (top). Data show means ± SE of quadruplicate determinations. Representative data of four independent experiments are shown. **P < 0.01. Note that CD148 knockdown largely diminished 18E1’s growth inhibitory activity in A431D/m-CD148 cells. D: effects of 18E1 mAb on CD148 protein tyrosine phosphatase (PTP) activity. A431D/m-CD148 cells were treated with 10 μg/mL 18E1 or control IgG for 30 min with or without CD148-Fc (10 μg/mL) or Fc alone (1.2 μg/mL). CD148 was immunoprecipitated using anti-hemagglutinin (HA) antibody. As a control, immunoprecipitation was also conducted using control IgG. The washed immunocomplexes were subjected to a PTP activity assay with or without 1 mM sodium orthovanadate (VO4). The amount of CD148 in the immunocomplexes was evaluated by immunoblot analysis using anti-HA antibody (top). Data show means ± SE of quadruplicate determinations. Representative data of five independent experiments are shown. **P < 0.01 vs. control IgG-treated cells. Note that CD148-Fc, but not control Fc, blocked 18E1 activity to increase CD148 catalytic activity.
Effects of an agonistic anti-CD148 mAb in murine DN.
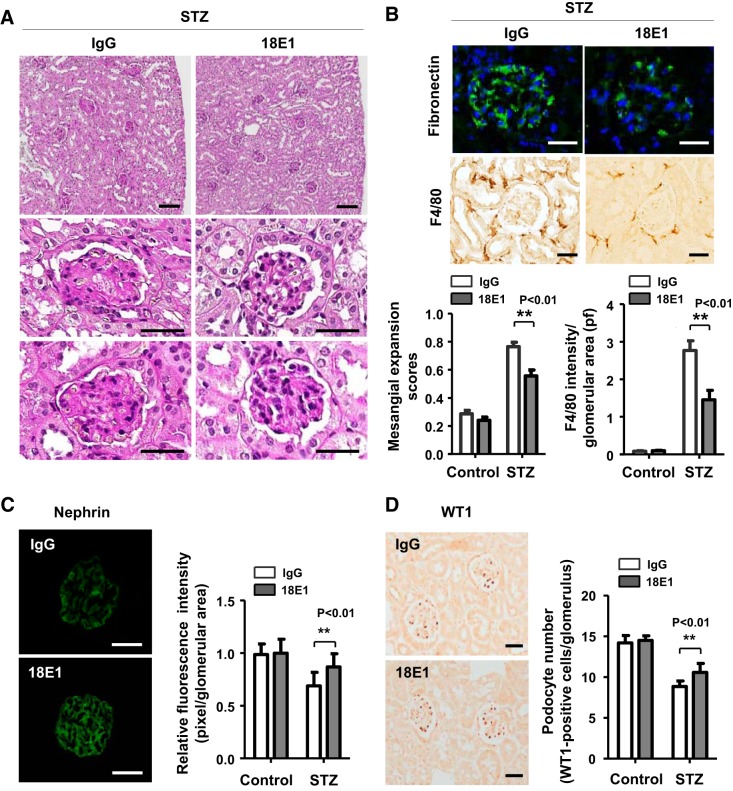
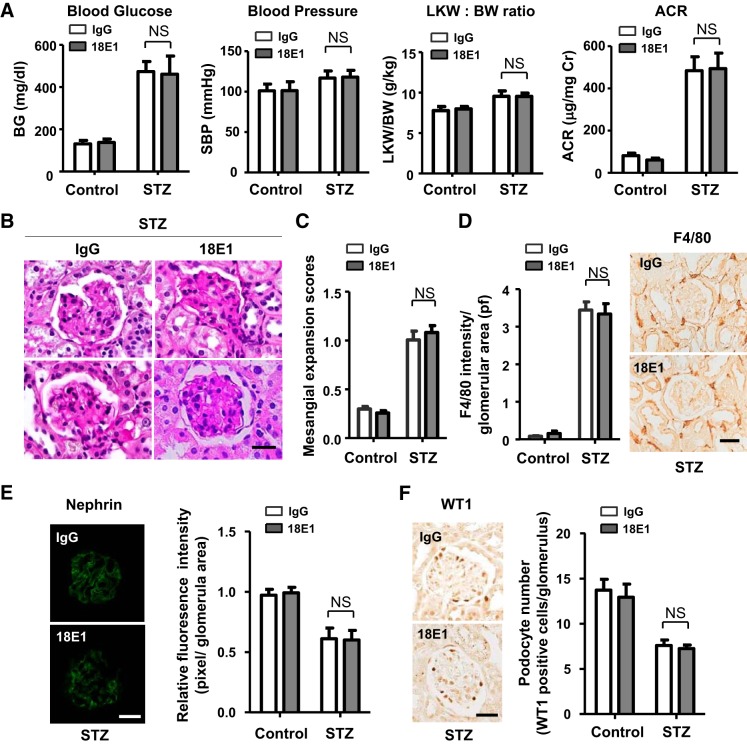
We next evaluated the effects of 18E1 mAb in murine DN using STZ-induced diabetic mice. Because the DBA/2J strain of mice has been shown to develop more advanced DN compared with other strains (35), we conducted this study with male mice of the DBA/2J strain. Diabetes was induced by multiple low-dose STZ injections (50 mg/kg ip for 5 days), and its development was confirmed at 2 wk after STZ injections (24, 35). Mice injected with citrate buffer were used as nondiabetic controls. These mice were treated with either 18E1 mAb or control IgG (10 mg/kg ip, 3 times/wk) for 6 wk, and their renal effects were investigated (Fig. 3A). Although 18E1 mAb did not alter blood glucose, blood pressure, or the left kidney-to-body weight ratio, it significantly reduced (~45%) albuminuria in diabetic mice (Fig. 3B). Albuminuria was decreased by ~20% (ACR: 338.3 ± 45.3, n = 4) and ~50% (ACR: 208.8 ± 64.4, n = 4) with 5 and 30 mg/kg dosages, respectively, suggesting that the effects are dose dependent. A similar level (~50%) of albuminuria reduction (ACR: 204.6 ± 71.6, n = 5) was observed with captopril treatment (24 mg/L, drinking water). There was no difference in plasma creatinine level between 18E1 mAb- and control IgG-treated diabetic mice (0.114 ± 0.019 vs. 0.116 ± 0.011 mg/dL, n = 8 mice/group, P = 0.5501). There was no difference in these parameters between the two groups, 18E1 mAb versus control IgG, before antibody treatment (data not shown). There was also no difference between control IgG- and PBS-treated diabetic mice (data not shown). 18E1 mAb showed no effects in nondiabetic mice (Fig. 3B). Histological, immunohistochemical, or immunofluorescent evaluation of these kidneys showed that 18E1 mAb significantly reduced mesangial expansion (~27%; Fig. 4, A and B), glomerular fibronectin expression (~35%; Fig. 4B), and macrophage infiltration (~48%; Fig. 4B). Furthermore, 18E1 mAb significantly prevented the reduction of nephrin expression (~62%) and podocyte number (~37%) in diabetic glomeruli (Fig. 4, C and D). Finally, we assessed the specificity of the effects of 18E1 mAb using CD148KO mice. As in WT mice, diabetes was induced in CD148KO mice of the DBA/2J strain by low-dose STZ injections. Mice were treated with either 18E1 mAb or control IgG (10 mg/kg ip, 3 times/wk) for 6 wk, starting from 2 wk after STZ injections, and their renal effects were then investigated (Fig. 3A). CD148KO mice injected with citrate buffer were used as nondiabetic controls. As shown in Fig. 5A, 18E1 mAb did not alter albuminuria as well as blood glucose, blood pressure, or the left kidney-to-body weight ratio in CD148KO diabetic mice. Furthermore, 18E1 mAb showed no effects in histological, immunohistochemical, and immunofluorescent readouts in CD148KO diabetic mice, including mesangial expansion, macrophage (F4/80) infiltration, nephrin immunofluorescense, and podocyte number (Fig. 5, B–F). These results demonstrate that 18E1 mAb attenuates murine DN through CD148. It is of note that CD148KO diabetic mice, treated with either 18E1 mAb or control IgG, showed a more advanced renal phenotype than WT diabetic mice treated with control IgG, yet there was no difference in renal phenotype between WT and CD148KO nondiabetic mice treated with these antibodies. These include albuminuria (~15% increase vs. WT counterparts, P < 0.01), mesangial expansion (~40% increase vs. WT counterparts, P < 0.01), and macrophage (F4/80) infiltration (~20% increase vs. WT counterparts, P < 0.01).
Fig. 4.
18E1 monoclonal antibody (mAb) attenuates renal histopathological changes in diabetic mice. A: representative renal histopathology in diabetic mice treated with either 18E1 mAb or control IgG for 6 wk. Periodic acid-Schiff (PAS) stainining is shown. B, top and middle: representative glomerular fibronectin immunofluorescence stain and F4/80 immunohistochemical stain in diabetic mice treated with either 18E1 mAb or control IgG for 6 wk. B, bottom: mesangial expansion score (left) and F4/80 macrophage infiltration in glomerular field (right) in diabetic [streptozotocin (STZ)] and nondiabetic (control) mice treated with either 18E1 or IgG for 6 wk. n = 8 mice/group. C: representative nephrin immunofluorescence stain (left) and its fluorescence intensity in glomeruli (right) in diabetic (STZ) and nondiabetic (control) mice treated with 18E1 or IgG for 6 wk. n = 8 mice/group. D: representative Wilms’ tumor 1 (WT1) immunostain (left) and number of WT1-positive cells in the glomerulus (right). n = 8 mice/group. Data are presented as means ± SE. **P < 0.01 vs. control IgG-treated mice. Scale bar = 100 µm in A, top, and 25 µm in A, middle and bottom, B, top and middle, C, and D.
Fig. 5.
Renal effects of 18E1 monoclonal antibody (mAb) in CD148 knockout (CD148KO) diabetic mice. A: blood glucose (BG), systolic blood pressure (SBP), left kidney weight-to-body weight ratio (LKW/BW), and urinary albumin-to-creatinine ratio (ACR) were measured in CD148KO diabetic [streptozotocin (STZ)] or nondiabetic (control) mice treated with 18E1 mAb or control IgG for 6 wk. n = 8 mice/group. Data are presented as means ± SE. Note that 18E1 mAb did not decrease albuminuria in CD148KO diabetic mice. B–F: renal histopathology [B; periodic acid-Schiff (PAS) stain], mesangial expansion scores (C), F4/80 macrophage infiltration (D), nephrin immunoreactivity (E), and podocyte number (F) were assessed in CD148KO diabetic (STZ) and nondiabetic (control) mice treated with 18E1 mAb or control IgG for 6 wk as in Fig. 4. n = 8 mice/group. Data are presented as means ± SE. Scale bar = 25 µm in B and D−F.
CD148 is expressed in other organs, such as the spleen (hematopoietic cells), brain, lung, liver, and heart (28), and binding of 18E1 mAb was observed in these organs (assessed by the injection of Alexa Fluor 546-labeled 18E1 mAb); however, evident histological changes were not observed in 18E1 mAb-injected diabetic or nondiabetic mice compared with control IgG or PBS-treated mice (data not shown). Body weight loss was also not observed in 18E1 mAb-injected mice, suggesting limited tissue toxicity of the antibody.
Effects of an agonistic anti-CD148 mAb in EGFR signaling in podocytes.
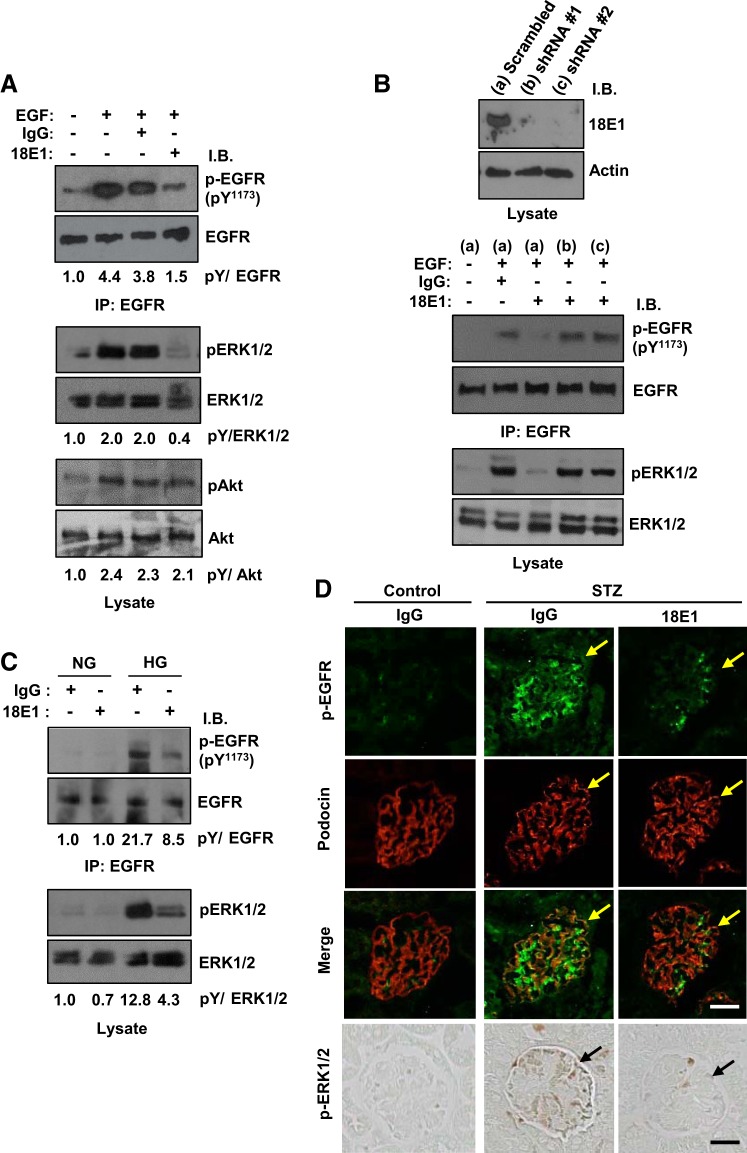
Recent studies have shown that EGFR activation in podocytes advances DN (1, 7). Given the fact that CD148 is expressed in podocytes (Fig. 1) and that CD148 strongly dephosphorylates EGFR and inhibits EGFR signaling (37, 51), we next asked if 18E1 mAb reduces EGFR signaling in podocytes using cultured mouse podocytes. As shown in Fig. 6A, 18E1 mAb, but not control IgG, reduced the phosphorylation of EGFR (~66%) and ERK1/2 (~80%) that was induced with EGF stimulation in podocytes, whereas its effects were limited in Akt phosphorylation. EGFR Tyr1068 phosphorylation was also suppressed by 18E1 mAb (data not shown). These effects were largely diminished by CD148 knockdown (Fig. 6B). It has been recently shown that hyperglycemia induces EGFR activation through reactive oxygen species-induced Src kinase activation (7). Therefore, we asked if 18E1 mAb also suppresses hyperglycemia-induced EGFR activation in podocytes. As shown in Fig. 6C, high glucose culture induced EGFR and ERK1/2 phosphorylation in podocytes, and they were suppressed (phospho-EGFR: ~61% and phospho-ERK1/2: ~67%) by 18E1 mAb compared with control IgG. There was no difference in EGFR and ERK1/2 phosphorylation level in the presence or absence of control IgG (data not shown). Finally, we assessed the effects of 18E1 mAb in podocyte EGFR and ERK1/2 phosphorylation in diabetic mice by immunofluorescense stain or immunohistochemistry using phosphospecific antibodies. As shown in Fig. 6D, reduced podocyte EGFR and ERK1/2 phosphorylation was noted in 18E1 mAb-treated diabetic mice compared with control IgG-treated diabetic mice. It is of note that EGFR phosphorylation is observed in mesangial cells and in podocytes in diabetic mice and that it was also reduced by 18E1 mAb, yet β-galactosidase histochemistry showed the limited CD148 expression in mesangial cells (Fig. 1). This may be the secondary effect. Collectively, these findings suggest that 18E1 mAb suppresses podocyte EGFR activity in diabetic podocytes.
Fig. 6.
18E1 monoclonal antibody (mAb) reduces epidermal growth factor (EGF) receptor (EGFR) and ERK1/2 phosphorylation in podocytes. A: cultured mouse podocytes were treated with EGF (20 ng/mL) for 15 min with or without 10 µg/mL 18E1 mAb or control IgG. Phosphorylation of EGFR (immunoprecipitated), ERK1/2, and Akt was assessed by immunoblot analysis using phosphospecific antibodies against EGFR (Tyr1173), ERK1/2 (Thr202/Tyr204), and Akt (Ser473). The antibodies were stripped, and membranes were reblotted with antibodies to total EGFR, ERK1/2, or Akt. The ratios of phosphorylated protein to total protein are shown. The ratio in the unstimulated condition is expressed as 1.0. B: specificity of the 18E1 mAb effects was assessed by CD148 knockdown. CD148 knockdown was carried out using lentivirus encoding mouse CD148-targeting shRNA (shRNA #1 and shRNA #2). Lentivirus encoding scrambled shRNA was used as a control. CD148 knockdown was confirmed by immunoblot analysis (top). Effects of 18E1 mAb in EGFR and ERK1/2 phosphorylation were assessed in control (a) and CD148 knocked down (b and c) cells as in A. Note that CD148 knockdown largely diminished the 18E1 mAb suppression of EGFR and ERK1/2 phosphorylation. C: mouse podocytes were cultured in normal glucose (NG; 5.6 mM) and high glucose (HG; 25 mM) conditions for 48 h with either 18E1 or control IgG (10 µg/mL). Phosphorylation of EGFR and ERK1/2 was assessed as in A. Note that 18E1 mAb reduced HG-induced EGFR and ERK1/2 phosphorylation compared with control IgG. Representative data of five independent experiments are shown in A−C. D: immunostaining of EGFR and ERK1/2 phosphorylation in 18E1 mAb- or control IgG-treated diabetic mouse glomeruli. The glomeruli in nondiabetic control mouse treated with control IgG are also shown. Immunofluorescence staining of podocin (red) was used to identify podocytes. Arrows indicate podocytes. Representative data of eight mice are shown. Note that treatment with 18E1 mAb reduced EGFR and ERK1/2 phosphorylation in podocytes in diabetic mice. Scale bar = 25 µm.
DISCUSSION
The present study provides the first evidence that agonistic anti-CD148 antibody attenuates DN. CD148 was shown to interact with growth factor receptors and their signaling proteins and negatively regulate their activities through dephosphorylation. These include EGFR (43, 44, 51), VEGFR-2 (5, 43, 44), PDGFRβ (23, 27), ERK1/2 (30, 37), PLC-γ1 (3, 53), and the phosphatidylinositol 3-kinase p85 subunit (55). On the other hand, recent studies have implicated excessive and prolonged growth factor signals as a key pathogenic mechanism in DN. EGFR activation in diabetic podocytes was shown to induce podocyte injury and loss as evidenced by reduced synaptopodin expression and podocyte number and cleaved caspase-3 expression (1, 7), whereas VEGFR-2 activation in diabetic glomerular endothelial cells was shown to induce albuminuria, abnormal glomerular angiogenesis, vascular leak, nodular lesion, and macrophage infiltration (13, 32, 39, 57, 58). As shown in Fig. 1, CD148 is expressed in these glomerular cells, and our data demonstrate that CD148 agonism reduces EGFR signals in podocytes. Furthermore, we have previously shown that CD148 activation reduces VEGFR-2 signals in glomerular endothelial cells and inhibits their proliferation (43, 44, 48). Thus, the protective effects of agonistic CD148 antibody in DN could be in part explained by reduced growth factor signals in podocytes and glomerular endothelial cells.
Besides negative regulation of growth factor signals, CD148 was shown to be expressed in inflammatory cells, such as macrophages, T cells, and neutrophils, and inhibit their activation, yet the detailed mechanisms of this are not fully understood (3, 10, 49, 62). Furthermore, anti-CD148 mAb was shown to inhibit colony stimulating factor-1-induced macrophage spreading and chemotaxis (11). More recently, CD148 was shown to negatively regulate CD98hc, which has been implicated in macrophage activation (9, 56). In support of these reports, 18E1 mAb-treated diabetic mice showed decreased macrophage infiltration, especially in the interstitial space. The macrophage infiltration in glomeruli was still limited in the present diabetic mice, perhaps because of the short period (6 wk) of diabetes and their young age, yet 18E1 mAb-treated diabetic mice showed less glomerular macrophage infiltration. Thus, inhibition of inflammatory cells may be another mechanism by which agonistic anti-CD148 antibody attenuates DN. The investigations of its detailed mechanisms are warranted for future studies. Compared with the evident effects of 18E1 mAb, the effects of CD148 deficiency were mild in this pathological condition. This may be because of the functional redundancy of PTPs (61).
In the past decades, inhibitors that target growth factors or their receptors have been developed, especially for cancer therapeutics. These include EGFR inhibitors and VEGFR-2 inhibitors. Furthermore, experimental studies have shown that EGFR or VEGFR-2 inhibitors attenuate kidney diseases, including DN, hypertensive nephropathy, obstructive kidney disease, and polycystic kidney diseases (8, 13, 29, 39, 40, 60). However, it was also shown that long-term treatment of these inhibitors induces side effects. It is well known that EGFR inhibitors exhibit serious skin toxicity by inducing kerationocyte injury, abnormal differentiation, and barrier dysfunction, leading to inflammation and bacterial infection (17, 19). Although agonistic anti-CD148 antibody inhibits EGFR signals, its skin toxicity may be less for the following reasons. First, CD148 expression is limited in skin (The Human Protein Atlas, https://www.proteinatlas.org/). Second, CD148 activation does not reduce Akt cell survival signals; therefore, keratinocyte injury may be less. CD148 has been shown to increase Src activity by dephosphorylating the COOH-terminal suppressive tyrosine residue, while it reduces its activity when Src is fully activated (31, 33, 38). This Src activation preserves Akt activity and promotes cell-matrix adhesion and cell-cell contacts (5, 33, 42). Third, CD148 has been shown to inhibit inflammatory cell activation, as described above. Finally, CD148 promotes physiological cell differentiation (25). Thus, CD148 agonist could work differently from EGFR inhibitors. On the other hand, long-term treatment of VEGFR-2 inhibitor has been shown to cause glomerular endothelial injury by reducing Akt-endothelial nitric oxide synthase signals and aggravate DN or cause thrombotic microangiopathy (26, 34). Because CD148 activation does not reduce endothelial Akt activity upon VEGF stimulation (5), this side effect should also be less with CD148 agonists.
Another benefit with CD148 agonism may be that CD148 inhibits multiple growth factor signals. CD148 directly interacts with ERK1/2 and reduces its activity (37). CD148 dephosphorylates fully activated Src by dephosphorylating the tyrosine residue in the activation loop and reduces its activity (31, 38). Furthermore, a more recent study (54) has shown that CD148 reduces transforming growth factor-β signaling in fibroblasts and suppresses lung fibrosis. Given the fact that these pathways have been implicated in the pathogenesis of DN and other kidney diseases (21, 50, 63), this CD148 activity may be beneficial. In this context, it is of note that CD148 is expressed in renal fibroblasts in fibrotic mouse renal disease (T. Takahashi, unpublished observations). Finally, given the fact that the neutralizing anti-TGF-α antibody that inhibits EGFR activation exhibited additive effects on renin-angiotensin-aldosterone system blockade (18), this antibody may also be combined with renin-angiotensin-aldosterone system blockade or effectively used in the condition of aldosterone escape that induces EGFR activation (16). Thus, agonistic anti-CD148 antibody may enable less toxic and potent (broad) growth factor signal inhibition and, therefore, could effectively be used for the treatment of DN and other kidney diseases. Further investigation to determine the renal effects of agonistic CD148 antibody should provide new therapeutics for DN and other kidney diseases.
GRANTS
Monoclonal antibodies and recombinant proteins were produced and purified by the Vanderbilt Antibody and Protein Resource, which is supported by the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center [National Institutes of Health (NIH) Grant P30-CA-68485]. This work was facilitated by the Vanderbilt O’Brien Kidney Research Core Center (NIH Grant P30-DK-114809). This work was supported by NIH Grant DK-97332 and the Nippon Medical School Grant-in-Aid for Overseas Training Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.T. conceived and designed research; K.T., R.H.K., L.P., L.H., S.N., D.K., T.M., A.S., R.C.H., R.L.M., and T.T. performed experiments; K.T., R.H.K., R.L.M., and T.T. analyzed data; K.T., R.H.K., R.C.H., R.L.M., and T.T. interpreted results of experiments; K.T., R.H.K., and T.T. prepared figures; K.T., T.M., and T.T. drafted manuscript; K.T., R.H.K., L.H., and T.T. edited and revised manuscript; K.T., R.H.K., L.P., L.H., S.N., D.K., T.M., A.S., R.C.H., R.L.M., and T.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Fenghua Zeng for help.
REFERENCES
- 1.Advani A, Wiggins KJ, Cox AJ, Zhang Y, Gilbert RE, Kelly DJ. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 16: 573–581, 2011. doi: 10.1111/j.1440-1797.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 2.Autschbach F, Palou E, Mechtersheimer G, Rohr C, Pirotto F, Gassler N, Otto HF, Schraven B, Gaya A. Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue Antigens 54: 485–498, 1999. doi: 10.1034/j.1399-0039.1999.540506.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol Cell Biol 21: 2393–2403, 2001. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher C, St-Laurent G, Loignon M, Jolicoeur M, De Crescenzo G, Durocher Y. The bioactivity and receptor affinity of recombinant tagged EGF designed for tissue engineering applications is defined by the nature and position of the tags. Tissue Eng Part A 14: 2069–2077, 2008. doi: 10.1089/ten.tea.2008.0037. [DOI] [PubMed] [Google Scholar]
- 5.Chabot C, Spring K, Gratton JP, Elchebly M, Royal I. New role for the protein tyrosine phosphatase DEP-1 in Akt activation and endothelial cell survival. Mol Cell Biol 29: 241–253, 2009. doi: 10.1128/MCB.01374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol 287: F602–F611, 2004. doi: 10.1152/ajprenal.00015.2004. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol 26: 1115–1125, 2015. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Agostino S, Lanzillotta D, Varano M, Botta C, Baldrini A, Bilotta A, Scalise S, Dattilo V, Amato R, Gaudio E, Paduano F, Palmieri C, Iuliano R, Perrotti N, Indiveri C, Fusco A, Gaspari M, Trapasso F. The receptor protein tyrosine phosphatase PTPRJ negatively modulates the CD98hc oncoprotein in lung cancer cells. Oncotarget 9: 23334–23348, 2018. doi: 10.18632/oncotarget.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dave RK, Dinger ME, Andrew M, Askarian-Amiri M, Hume DA, Kellie S. Regulated expression of PTPRJ/CD148 and an antisense long noncoding RNA in macrophages by proinflammatory stimuli. PLoS One 8: e68306, 2013. doi: 10.1371/journal.pone.0068306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave RK, Hume DA, Elsegood C, Kellie S. CD148/DEP-1 association with areas of cytoskeletal organisation in macrophages. Exp Cell Res 315: 1734–1744, 2009. doi: 10.1016/j.yexcr.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 12.de la Fuente-García MA, Nicolás JM, Freed JH, Palou E, Thomas AP, Vilella R, Vives J, Gayá A. CD148 is a membrane protein tyrosine phosphatase present in all hematopoietic lineages and is involved in signal transduction on lymphocytes. Blood 91: 2800–2809, 1998. doi: 10.1182/blood.V91.8.2800.2800_2800_2809. [DOI] [PubMed] [Google Scholar]
- 13.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 12: 993–1000, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30: E9, 2002. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 85: 579–589, 2014. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 16.Grossmann C, Gekle M. Non-classical actions of the mineralocorticoid receptor: misuse of EGF receptors? Mol Cell Endocrinol 277: 6–12, 2007. doi: 10.1016/j.mce.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Guttman-Yassky E, Mita A, De Jonge M, Matthews L, McCarthy S, Iwata KK, Verweij J, Rowinsky EK, Krueger JG. Characterisation of the cutaneous pathology in non-small cell lung cancer (NSCLC) patients treated with the EGFR tyrosine kinase inhibitor erlotinib. Eur J Cancer 46: 2010–2019, 2010. doi: 10.1016/j.ejca.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Heuer JG, Harlan SM, Yang DD, Jaqua DL, Boyles JS, Wilson JM, Heinz-Taheny KM, Sullivan JM, Wei T, Qian HR, Witcher DR, Breyer MD. Role of TGF-α in the progression of diabetic kidney disease. Am J Physiol Renal Physiol 312: F951–F962, 2017. doi: 10.1152/ajprenal.00443.2016. [DOI] [PubMed] [Google Scholar]
- 19.Holcmann M, Sibilia M. Mechanisms underlying skin disorders induced by EGFR inhibitors. Mol Cell Oncol 2: e1004969, 2015. doi: 10.1080/23723556.2015.1004969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda H, Inazawa J, Nishida J, Yazaki Y, Hirai H. Molecular cloning, characterization, and chromosomal localization of a novel protein-tyrosine phosphatase, HPTP eta. Blood 84: 4186–4194, 1994. doi: 10.1182/blood.V84.12.4186.bloodjournal84124186. [DOI] [PubMed] [Google Scholar]
- 21.Hong Z, Hong Z, Wu D, Nie H. Specific MAPK inhibitors prevent hyperglycemia-induced renal diseases in type 1 diabetic mouse model. Mol Cell Biochem 419: 1–9, 2016. doi: 10.1007/s11010-016-2722-1. [DOI] [PubMed] [Google Scholar]
- 22.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol 159: 465–476, 2002. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jandt E, Denner K, Kovalenko M, Ostman A, Böhmer FD. The protein-tyrosine phosphatase DEP-1 modulates growth factor-stimulated cell migration and cell-matrix adhesion. Oncogene 22: 4175–4185, 2003. doi: 10.1038/sj.onc.1206652. [DOI] [PubMed] [Google Scholar]
- 24.Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473–1484, 2007. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane MM, Lowrey GA, Ettenberg SA, Dayton MA, Lipkowitz S. The protein tyrosine phosphatase DEP-1 is induced during differentiation and inhibits growth of breast cancer cells. Cancer Res 56: 4236–4243, 1996. [PubMed] [Google Scholar]
- 26.Kim HW, Lim JH, Kim MY, Chung S, Shin SJ, Chung HW, Choi BS, Kim YS, Chang YS, Park CW. Long-term blockade of vascular endothelial growth factor receptor-2 aggravates the diabetic renal dysfunction associated with inactivation of the Akt/eNOS-NO axis. Nephrol Dial Transplant 26: 1173–1188, 2011. doi: 10.1093/ndt/gfq610. [DOI] [PubMed] [Google Scholar]
- 27.Kovalenko M, Denner K, Sandström J, Persson C, Gross S, Jandt E, Vilella R, Böhmer F, Ostman A. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem 275: 16219–16226, 2000. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 28.Kuramochi S, Matsuda S, Matsuda Y, Saitoh T, Ohsugi M, Yamamoto T. Molecular cloning and characterization of Byp, a murine receptor-type tyrosine phosphatase similar to human DEP-1. FEBS Lett 378: 7–14, 1996. doi: 10.1016/0014-5793(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 29.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massa A, Barbieri F, Aiello C, Iuliano R, Arena S, Pattarozzi A, Corsaro A, Villa V, Fusco A, Zona G, Spaziante R, Schettini G, Florio T. The phosphotyrosine phosphatase eta mediates somatostatin inhibition of glioma proliferation via the dephosphorylation of ERK1/2. Ann N Y Acad Sci 1030: 264–274, 2004. doi: 10.1196/annals.1329.033. [DOI] [PubMed] [Google Scholar]
- 31.Mori J, Nagy Z, Di Nunzio G, Smith CW, Geer MJ, Al Ghaithi R, van Geffen JP, Heising S, Boothman L, Tullemans BME, Correia JN, Tee L, Kuijpers MJE, Harrison P, Heemskerk JWM, Jarvis GE, Tarakhovsky A, Weiss A, Mazharian A, Senis YA. Maintenance of murine platelet homeostasis by the kinase Csk and phosphatase CD148. Blood 131: 1122–1144, 2018. doi: 10.1182/blood-2017-02-768077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes 58: 1471–1478, 2009. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pera IL, Iuliano R, Florio T, Susini C, Trapasso F, Santoro M, Chiariotti L, Schettini G, Viglietto G, Fusco A. The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue. Oncogene 24: 3187–3195, 2005. doi: 10.1038/sj.onc.1208510. [DOI] [PubMed] [Google Scholar]
- 34.Perazella MA, Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int 87: 909–917, 2015. doi: 10.1038/ki.2015.30. [DOI] [PubMed] [Google Scholar]
- 35.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- 36.Ruivenkamp CA, van Wezel T, Zanon C, Stassen AP, Vlcek C, Csikós T, Klous AM, Tripodis N, Perrakis A, Boerrigter L, Groot PC, Lindeman J, Mooi WJ, Meijjer GA, Scholten G, Dauwerse H, Paces V, van Zandwijk N, van Ommen GJ, Demant P. Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat Genet 31: 295–300, 2002. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 37.Sacco F, Tinti M, Palma A, Ferrari E, Nardozza AP, Hooft van Huijsduijnen R, Takahashi T, Castagnoli L, Cesareni G. Tumor suppressor density-enhanced phosphatase-1 (DEP-1) inhibits the RAS pathway by direct dephosphorylation of ERK1/2 kinases. J Biol Chem 284: 22048–22058, 2009. doi: 10.1074/jbc.M109.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepanek O, Kalina T, Draber P, Skopcova T, Svojgr K, Angelisova P, Horejsi V, Weiss A, Brdicka T. Regulation of Src family kinases involved in T cell receptor signaling by protein-tyrosine phosphatase CD148. J Biol Chem 286: 22101–22112, 2011. doi: 10.1074/jbc.M110.196733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol 17: 3093–3104, 2006. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 40.Sweeney WE Jr, Chen Y, Nakanishi K, Frost P, Avner ED. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int 57: 33–40, 2000. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Kim R, Lauhan C, Park Y, Nguyen NG, Vestweber D, Dominguez MG, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW, Takahashi T. Expression of receptor-type protein tyrosine phosphatase in developing and adult renal vasculature. PLoS One 12: e0177192, 2017. doi: 10.1371/journal.pone.0177192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Matafonov A, Sumarriva K, Ito H, Lauhan C, Zemel D, Tsuboi N, Chen J, Reynolds A, Takahashi T. CD148 tyrosine phosphatase promotes cadherin cell adhesion. PLoS One 9: e112753, 2014. doi: 10.1371/journal.pone.0112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi K, Mernaugh RL, Friedman DB, Weller R, Tsuboi N, Yamashita H, Quaranta V, Takahashi T. Thrombospondin-1 acts as a ligand for CD148 tyrosine phosphatase. Proc Natl Acad Sci USA 109: 1985–1990, 2012. doi: 10.1073/pnas.1106171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi K, Sumarriva K, Kim R, Jiang R, Brantley-Sieders DM, Chen J, Mernaugh RL, Takahashi T. Determination of the CD148-interacting region in thrombospondin-1. PLoS One 11: e0154916, 2016. doi: 10.1371/journal.pone.0154916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Takahashi K, Gerety S, Wang H, Anderson DJ, Daniel TO. Temporally compartmentalized expression of ephrin-B2 during renal glomerular development. J Am Soc Nephrol 12: 2673–2682, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T, Takahashi K, Mernaugh R, Drozdoff V, Sipe C, Schoecklmann H, Robert B, Abrahamson DR, Daniel TO. Endothelial localization of receptor tyrosine phosphatase, ECRTP/DEP-1, in developing and mature renal vasculature. J Am Soc Nephrol 10: 2135–2145, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T, Takahashi K, Mernaugh RL, Tsuboi N, Liu H, Daniel TO. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood 108: 1234–1242, 2006. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tangye SG, Wu J, Aversa G, de Vries JE, Lanier LL, Phillips JH. Negative regulation of human T cell activation by the receptor-type protein tyrosine phosphatase CD148. J Immunol 161: 3803–3807, 1998. [PubMed] [Google Scholar]
- 50.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L, Masson EA, Momen A, Shikatani EA, John R, Husain M, Fantus IG. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 62: 3874–3886, 2013. doi: 10.2337/db12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarcic G, Boguslavsky SK, Wakim J, Kiuchi T, Liu A, Reinitz F, Nathanson D, Takahashi T, Mischel PS, Ng T, Yarden Y. An unbiased screen identifies DEP-1 tumor suppressor as a phosphatase controlling EGFR endocytosis. Curr Biol 19: 1788–1798, 2009. doi: 10.1016/j.cub.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trapasso F, Iuliano R, Boccia A, Stella A, Visconti R, Bruni P, Baldassarre G, Santoro M, Viglietto G, Fusco A. Rat protein tyrosine phosphatase eta suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1). Mol Cell Biol 20: 9236–9246, 2000. doi: 10.1128/MCB.20.24.9236-9246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Trapasso F, Yendamuri S, Dumon KR, Iuliano R, Cesari R, Feig B, Seto R, Infante L, Ishii H, Vecchione A, During MJ, Croce CM, Fusco A. Restoration of receptor-type protein tyrosine phosphatase eta function inhibits human pancreatic carcinoma cell growth in vitro and in vivo. Carcinogenesis 25: 2107–2114, 2004. doi: 10.1093/carcin/bgh224. [DOI] [PubMed] [Google Scholar]
- 54.Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, El-Chemaly S, Perrella MA, Rosas IO. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol 58: 208–215, 2018. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuboi N, Utsunomiya T, Roberts RL, Ito H, Takahashi K, Noda M, Takahashi T. The tyrosine phosphatase CD148 interacts with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J 413: 193–200, 2008. doi: 10.1042/BJ20071317. [DOI] [PubMed] [Google Scholar]
- 56.Tsumura H, Ito M, Li XK, Nakamura A, Ohnami N, Fujimoto J, Komada H, Ito Y. The role of CD98hc in mouse macrophage functions. Cell Immunol 276: 128–134, 2012. doi: 10.1016/j.cellimm.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Veron D, Aggarwal PK, Velazquez H, Kashgarian M, Moeckel G, Tufro A. Podocyte-specific VEGF-a gain of function induces nodular glomerulosclerosis in eNOS null mice. J Am Soc Nephrol 25: 1814–1824, 2014. doi: 10.1681/ASN.2013070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 54: 1227–1241, 2011. doi: 10.1007/s00125-010-2034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, Li W, Wen J, Yang Z. Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling. J Cell Mol Med 21: 1315–1328, 2017. doi: 10.1111/jcmm.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang MZ, Wang Y, Paueksakon P, Harris RC. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63: 2063–2072, 2014. doi: 10.2337/db13-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity 28: 183–196, 2008. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu JW, Doan K, Park J, Chau AH, Zhang H, Lowell CA, Weiss A. Receptor-like tyrosine phosphatases CD45 and CD148 have distinct functions in chemoattractant-mediated neutrophil migration and response to S. aureus. Immunity 35: 757–769, 2011. doi: 10.1016/j.immuni.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]