Abstract
AMP-activated protein kinase (AMPK) activation promotes early stages of epithelial junction assembly. AMPK activation in MDCK renal epithelial cells facilitates localization of the junction-associated proteins aPKCζ and Par3 to the plasma membrane and promotes conversion of Cdc42, a key regulator of epithelial polarization and junction assembly, to its active GTP bound state. Furthermore, Par3 is an important regulator of AMPK-mediated aPKCζ localization. Both aPKCζ and Par3 serve as intermediates in AMPK-mediated junction assembly, with inhibition of aPKCζ activity or Par3 knockdown disrupting AMPK’s ability to facilitate zonula occludens (ZO-1) localization. AMPK phosphorylates the adherens junction protein afadin and regulates its interaction with the tight-junction protein zonula occludens-1. Afadin is phosphorylated at two critical sites, S228 (residing within an aPKCζ consensus site) and S1102 (residing within an AMPK consensus site), that are differentially regulated during junction assembly and that exert different effects on the process. Expression of phospho-defective mutants (S228A and S1102A) perturbed ZO-1 localization to the plasma membrane during AMPK-induced junction assembly. Expression of S228A increased the ZO-1/afadin interaction, while S1102A reduced this interaction during extracellular calcium-induced junction assembly. Inhibition of aPKCζ activity also increased the ZO-1/afadin interaction. Taken together, these data suggest that aPKCζ phosphorylation of afadin terminates the ZO-1/afadin interaction and thus permits the later stages of junction assembly.
Keywords: adherens junction, afadin, AMP-activated protein kinase, tight junction, zonula occludens-1
INTRODUCTION
Epithelial cells serve as barriers that separate different tissues and body compartments from one another. These barriers both protect an organism from its external environment and mediate the import and export of substances, often against steep concentration gradients. Epithelial cells are attached to one another through highly specialized protein complexes that constitute a variety of intercellular junctions, including tight junctions (TJs) and adherens junctions (AJs) (35). These junctions work together to not only facilitate adhesion between the lateral domains of adjacent cells but also to help to establish cell polarity (6).
The assembly of junction complexes is an early and critical step in the organization of individual epithelial cells into an intact, polarized, and functionally competent monolayer epithelium. Hundreds of transmembrane and cytoplasmic proteins have been identified that dynamically interact with each other to promote adherens and tight junction assembly and maturation (16). One obligate scaffolding protein includes zonula occludens-1 (ZO-1), which itself has been shown to potentially interact with more than 400 proteins (61). During the early stages of junction assembly, ZO-1 localizes to the nascent adherens junction, where it associates with the AJ scaffolding protein afadin, an interaction critical for the assembly and maturation of AJs and TJs (45). In association with junction maturation, ZO-1 dissociates from afadin and translocates to the tight junction, where it tethers transmembrane tight-junction proteins to the actin cytoskeleton (15, 45).
Much remains unknown about the molecular mechanisms that promote the formation of AJs and TJs, including the nature of the cellular machinery that regulates the ZO-1-afadin association. Previous observations from our group and others demonstrate that in the Madin-Darby canine kidney (MDCK) epithelial cell model, AMP-activated kinase (AMPK) plays an active role in initiating transient junction formation (39, 64–66). Our previous studies demonstrated that AMPK becomes phosphorylated and activated during Ca2+-induced junction formation and that pharmacological activation of AMPK promotes ZO-1 localization to the plasma membrane, while inhibition of AMPK kinase activity prevents junction assembly. We also found that AMPK specifically phosphorylates afadin in vitro and increases the level of afadin bound to ZO-1. AMPK plays important roles in the cellular response to energy deprivation and has been implicated in processes that contribute to epithelial polarization (5, 66).
Additional regulators of AJ and TJ formation include the atypical protein complex Cζ (aPKCζ)-Par3-Par6 complex proteins. aPKCζ phosphorylates several AJ and TJ proteins to promote their stable localization at the plasma membrane (24, 27, 57). aPKCζ interacts with the Par polarity proteins Par3 and Par6, which serve as scaffolding proteins to coordinate the proper localization of aPKCζ during junction assembly (23). Regulation of aPKCζ activation may occur via at least two different mechanisms: relief of Par6 suppression of aPKCζ activity through GTP-bound Cdc42 as well as through phosphorylation of a PKC by upstream kinases (20, 62). GTP-bound Cdc42 is important for junction assembly and inactivation of aPKCζ kinase activity during epithelial polarization (3, 9). The knockdown of aPKCζ, Par3, or Par6 have each been individually shown to prevent ZO-1 and afadin localization to sites of cell-cell contact (44).
Here, we sought to further elucidate the mechanisms through which AMPK regulates junction assembly and to explore the relationship between AMPK and aPKCζ-mediated events. We report for the first time that AMPK promotes both Par3 and aPKCζ localization to the plasma membrane during junction assembly and that aPKCζ inhibition prevents AMPK-mediated junction assembly by affecting the ZO-1/afadin interaction. We also analyzed afadin phosphorylation and identified residues that are important for the ZO-1/afadin interaction. Thus, our results demonstrate that AMPK-mediated junction assembly is dynamic and involves the participation of many junction proteins, with aPKCζ performing as a central player in this process.
EXPERIMENTAL PROCEDURES
Plasmids and Cell Lines
Par3 knockdown cells.
Gene transfer lentiviral plasmids were purchased from VectorBuilder (pLV U6 shRNA-hPGK puro, VB170922-1159qea). These plasmids allow expression of shRNA directed against PAR3 together with a puromycin resistance marker. Positive control (pLKO.1-puro-CMV-TagRFP, SHC012) and nontarget control shRNA (anti-luciferase, VB170922–1161yfy) were purchased from Sigma-Aldrich and VectorBuilder, respectively.
Lentiviral vectors were generated with the GIGA Viral Vectors platform. Briefly Lenti-X 293T cells (632180; Clontech) were co-transfected with gene transfer lentiviral plasmid, pSPAX2 (Addgene, Cambridge, MA) plasmid, and a VSV-G encoding plasmid (13). Viral supernatants were collected 48, 72, and 96 h posttransfection, filtered (0.2 µM), and concentrated ×100 by ultracentrifugation. The lentiviral vectors were then titrated with a qPCR Lentivirus Titration (Titer) Kit (LV900; ABM, Richmond, BC, Canada). MDCK cells were transduced with lentiviral vectors (50 TU/cell), allowing the dual expression of shRNA and puromycin resistance protein. Transduced cells were selected with 3 µg/mL puromycin (ant-pr-1; Invivogen). The absence of RCL and mycoplasma in the cell supernatant was confirmed with a qPCR Lentivirus Titration kit and MycoAlert PLUS Mycoplasma Detection Kit (Lonza, LT07-710). respectively.
Afadin S216A and S1083A cell lines.
The constructs encoding the wild-type mus muscularis afadin protein or its S1083A mutant cloned into the pcDNA3.1.neo vector were kindly provided by Dr. Yoshimi Takai. Sequence encoding a hemagglutinin (HA) epitope tag was added to the NH2 terminus of the encoded protein. A site-directed mutation at S216 was introduced by Quikchange Pfu turbo enzyme (Agilent Technologies, Santa Clara, CA). The PCR reaction mixture contained 45 ng of template DNA, 10× Pfu turbo reaction buffer, 0.26 mM of each dNTP, 0.26 μM of each primer, and 2.5 units of Pfu turbo enzyme in 50 μl. The mixture was heated at 95°C for 2 min and then subjected to thermal cycling (18 cycles of 95°C for 30 s, 55°C for 1 min, and 68°C for 11 min). Following subcloning, the gene was fully sequenced to ensure that the mutation was present and that no additional mutations were introduced by PCR. Stable cell lines were generated by transfection of MDCK cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and selection was achieved by culturing cells in medium supplemented with 400 mg/mL G418 (Invitrogen).
Antibodies
Rabbit anti-AMPKα1 and anti-pAMPK (T172) were purchased from Cell Signaling Technology (Danvers, MA). Both mouse and rabbit anti-aPKCζ were purchased from Santa Cruz Biotechnology (Dallas, TX). Rabbit anti-Par3 was purchased from Millipore (Billerica, MA). Both mouse and rabbit anti-ZO-1 were purchased from Invitrogen. Rabbit-anti-human-l-afadin was purchased from Sigma-Aldrich (St. Louis, MO). Mouse anti-αHA was purchased from Roche (Indianapolis, IN). Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG were purchased from Molecular Probes (Carlsbad, CA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG were purchased from Jackson ImmunoResearch (Westgrove, PA). Goat anti-mouse IRDye 800CW and anti-rabbit IRDye 680CW were purchased from Li-Cor BioSciences (Lincoln, NE).
Cell Culture
MDCK cells were maintained in α-MEM (Invitrogen) supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY), 2 mM l-glutamine (Gibco-BRL), 50 U/mL penicillin (Gibco-BRL), and 50 mg/mL streptomycin (Gibco-BRL). All cells were grown in a humidified incubator at 37°C and 5% CO2 atmosphere and were recently authenticated and tested for contamination.
Ca2+ Switch and Drug Exposure Experiments
MDCK cells were seeded on tissue culture plastic (for immunoblotting experiments) or on glass coverslips (for IF experiments) in α-MEM containing 1.8 mM Ca2+ (normal Ca2+ medium) until they formed a confluent monolayer. Cells were then washed four times with PBS before being incubated in Ca2+-free S-MEM (Gibco-BRL) supplemented with 5% dialyzed fetal bovine serum, 2 mM l-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin for 16 h. Cells were then returned to normal Ca2+-containing α-MEM medium or exposed to drugs for various time intervals as indicated. AICAR was purchased from Calbiochem (San Diego, CA), and aPKCζ pseudosubstrate (myristoylated) was purchased from Enzo Life Sciences (Farmingdale, NY). Another aPKCζ inhibitor, SC-3098, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence and Quantification of aPKCζ, Par3, and ZO-1 Staining
Cells grown on coverslips were washed twice with PBS and fixed in 100% methanol at room temperature for 7 min. Cells were then washed three times with PBS before being permeabilized in 0.3% Triton X-100-0.15% BSA (permeabilization buffer) in PBS for 15 min at room temperature. These cells were then blocked in GSDB (16% goat serum (Invitrogen), 20 mM sodium phosphate (pH 7.4), and 450 mM NaCl, 0.3% Triton X-100) for 30 min at room temperature. Cells were incubated in primary antibody diluted 1:100 in GSDB for 1 h at room temperature and then washed three times in permeabilization buffer. The corresponding secondary antibody and Hoechst reagent (Molecular Probes) were diluted 1:200 and 1:10,000 in GSDB, respectively. This mixture was added to the cells for a 1-h incubation. Cells were then washed three times in PBS before being mounted using Vectashield (Vector Laboratories, Burlingame, CA). The cells were visualized on a Zeiss LSM 780 confocal laser-scanning microscope. Brightness and contrast settings were chosen such that all pixels were in the linear range.
To quantify the average length of aPKCζ, Par3, or ZO-1 per cell, four fields were randomly selected from each coverslip, and the total length of aPKCζ, Par3, or ZO-1 localized at junction sites in each field was outlined manually and measured using ImageJ software. An investigator blinded to the conditions of each image performed a confirmatory experiment measuring ZO-1 lengths to ensure that there was no investigator bias. The total number of cell nuclei per field was counted, and the average aPKCζ, Par3, or ZO-1 length per cell was thus calculated.
Immunoblotting
Cells were lysed on ice in 20 mM Tris 7.4, 250 mM sucrose, 50 mM NaCl, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 2 mM fresh dithiothreitol (DTT), 1% Triton, and protease inhibitors (Roche) and sonicated for 30 s. Cell lysate was centrifuged at 14,000 rpm at 4°C for 30 min, and the supernatant was collected. The proteins were separated by SDS-PAGE using standard protocols and electrophoretically transferred to 0.2- or 0.45-µm nitrocellulose membranes (Bio-Rad, Hercules, CA). Proteins separated by Phos-tag SDS-PAGE (Wako, Richmond, VA) according to manufacturer’s protocols were transferred to PVDF membranes (Millipore). The membranes were blocked with milk solution [150 mM NaCl, 20 mM Tris, 5% milk (wt/vol), 1% bovine serum albumin (BSA), 0.1& Tween (vol/vol), pH 7.5] and probed with primary antibody (diluted 1:1000 in 5% BSA solution) and the corresponding IRDye- or HRP-conjugated secondary (diluted 1:10,000) antibodies. The immunoreactive bands were visualized with Odyssey Infrared Scanner (Li-Cor Biosciences) or an enhanced chemiluminescence detection kit (Amersham Biosciences, Pittsburgh, PA). For Par3 wild-type (WT) and knockdown (KD) cells, total protein quantification was obtained using a ChemiDoc MP gel imager (Bio-Rad). No membrane stripping for re-blotting was required, as proteins that were of similar molecular weights were probed with primary antibodies of different species and thus could be visualized with secondary antibodies conjugated to different IRDyes simultaneously.
Immunoprecipitation
Cells were lysed on ice in 20 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton, and protease inhibitors and sonicated for 15 s. Cell lysate was centrifuged at 14,000 rpm at 4°C for 30 min, after which the supernatant was collected. Lysates prepared for afadin immunoprecipitation were incubated with rabbit anti-I-afadin antibody and protein G agarose beads (Thermo Scientific, Waltham, MA) at 4°C overnight. Beads were collected by centrifugation at 2,000 rpm, and the pellets were washed three times with lysis buffer and two times with PBS for 5 min with rotation at 4°C. Lysates prepared for HA immunoprecipitation were incubated with anti-HA magnetic beads (Pierce, Waltham, MA) for 30 min at room temperature and washed according to the manufacturer’s protocol. Immunprecipitates and total cell lysates were resolved with 8% SDS-PAGE using standard protocols and analyzed by immunoblotting using the antibodies specified.
Cdc42 Activity Assay
Cdc42 Pak1-binding domain-GST tagged construct (kindly provided by Dr. Martin Schwartz, Yale University) was expressed and collected using glutathione-sepharose 4B beads (Amersham), as previously described (37). Cells were lysed on ice in GST-Fish buffer (10% glycerol, 50 mM Tris, pH 7.4, 100 mM NaCl, 1% NP-40, 2 mM MgCl2, 1 mM sodium orthovanadate, and protease inhibitors) and sonicated for 5 s. Cell lysates were centrifuged at 14,000 rpm at 4°C for 5 min, and extracts containing 500 μg of total proteins were incubated with 20 μg of glutathione-bound Pak1 beads (GST-PBD) on a rotator for 15 min at 4°C. Beads were collected by centrifugation at 2,000 rpm and washed twice with GST-Fish buffer. Cdc42 bound to GST-PBD, and total cell extracts were resolved by 15% SDS-PAGE using standard protocols and analyzed by immunoblotting using the antibodies specified.
Mass Spectrometry Sample Preparation
Immunoprecipitated afadin protein resolved by SDS-PAGE was visualized with Coomassie Blue staining and excised from the gel, and gel fragments were cut into 1-mm3 pieces. Gel pieces were sequentially washed with fresh solutions containing 50% (vol/vol) acetonitrile (ACN), 25 mM NH4HCO3, 50% (vol/vol) ACN, and 5 mM NH4HCO3. Proteins were digested overnight at 37°C in a solution containing 45 mM NH4HCO3, 5% ACN, and 13.33 ng/ul trypsin (Promega, Madison, WI). Peptides were extracted from gel pieces with 1.67% formic acid (FA) in 66.6% ACN. Samples were loaded onto stage tips assembled in house [2 × 1.06 mm punches of Empore C18 extraction disks (3M, Maplewood, Minnesota) in a 200-μL pipette tip], washed twice with 0.5% acetic acid, and eluted with 80% acetonitrile and 0.5% acetic acid. In-solution peptide labeling was performed using 100 mM TEAB, 4% formaldehyde (CH2O or CD2O), 0.6M cyanoborohydride, 1% (vol/vol) ammonia, and 5% (vol/vol) formic acid in water. Peptides from cells subjected to Ca2+ switch conditions were labeled using CD2O, and those from cells under LCM conditions were labeled using CH2O. Samples were then combined in a 1:1 ratio; 1 μL was taken from each sample and mixed with 19% formic acid, 27 mM sodium phosphate, pH 8.2 and 0.02% TFA for relative quantification of total afadin peptides (non-enriched sample). The remaining phosphopeptides were enriched by reconstitution in 20 μL of binding buffer (50% ACN, 0.5% TFA) and loaded onto stage tips assembled in-house [1 × 0.6 mm punch of Empore C18 extraction disks in a 200-μL pipette tip, loaded with 400 μg of Titanshere TiO2 microspheres (GL Sciences, Tokyo, Japan)]. Tips were washed twice with binding buffer and once with 80% ACN (0.1% FA). Peptides were eluted with 1% NH4OH, followed by 80% ACN (0.1% FA). Peptides were dried and reconstituted in a solution of 19% formic acid, 27 mM sodium phosphate, pH 8.2, and 0.02% TFA for liquid chromatography-mass spectrometry/mass spectrometry analysis.
Chromatographic separation was performed on an a Waters nanoAcquity system with a vented split configuration on a 32-mm, 150-μm ID trap column packed in-house with 3 μm of ReproSil-Pur C18-AQ 120A resin (Dr. Maisch) and a 200-mm, 75-μm ID PicoFrit analytical column (New Objective, Altamonte Springs, FL) packed in-house with 1.9 μm ReproSil-Pur C18-AQ 120A resin (Dr. Maisch) using a nonlinear, 90-min gradient from 5 to 95% ACN (with 0.1% FA) and analyzed on a LTQ Orbitrap Velos (Thermo Fisher Scientific). Peptides were detected in data-dependent mode using a Top 10 data dependent acquisition method and HCD dissociation. Data were searched using Maxquant version 1.5.1.0. The ratios of TiO2-enriched phosphopeptides were normalized according to the averages of all afadin nonphosphopeptide ratios from the corresponding nonenriched samples. Putative kinases were determined by motif scan according to MIT Scansite 4.0 (42).
Statistical Analysis
All data values are shown as means ± SE. Statistical analysis was performed with GraphPad Prism for Mac. For comparisons between two groups, a two-tailed Student’s t-test was performed. For multigroup comparisons, one-way or two-way ANOVA was performed as applicable. If the ANOVA indicated a difference, post hoc analysis with Tukey’s, Sidak’s, or Dunnett’s multiple-comparisons test was performed. Statistical significance was deemed if P < 0.05.
RESULTS
AMPK Regulates aPKCζ Localization During Junction Assembly
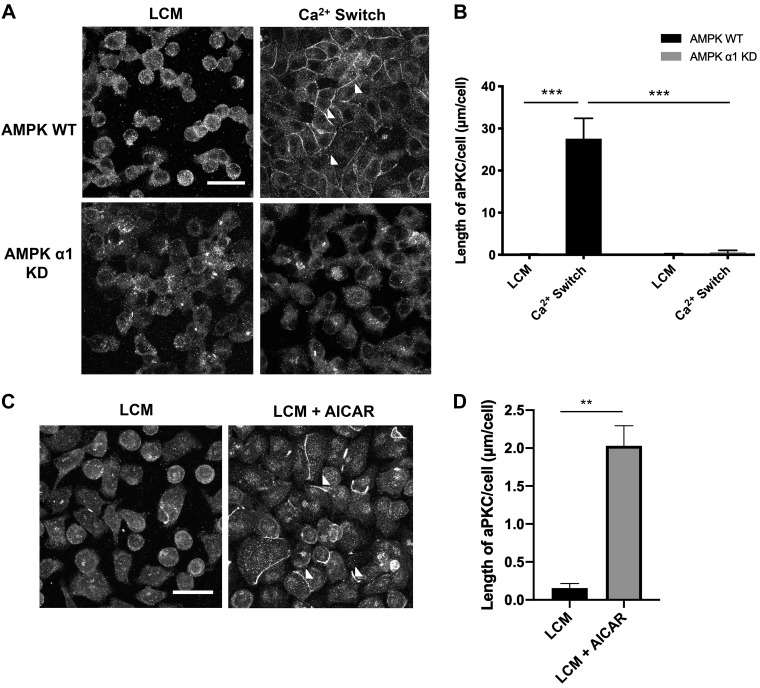
In the MDCK epithelial cell model system, the presence of extracellular Ca2+ is critical for the formation and maintenance of intercellular junctions. Exposure to low concentrations of extracellular Ca2+ disrupts intercellular junctions, while the restoration of Ca2+ to normal concentration levels leads to the deposition of junction proteins at sites of cell-cell attachment (6, 34). This manipulation has come to be known as the “Ca2+ switch.” Consistent with our previous findings, we were able to demonstrate that the Ca2+ switch promotes AMPK phosphorylation (Supplemental Fig. S1A; Supplemental Material is available at https://doi.org/10.6084/m9.figshare.11462001) (51, 64, 65). We employed this paradigm in experiments designed to assess whether AMPK regulates aPKCζ during junction formation. AMPK is a heterotrimer, requiring the proper assembly of all three subunits together to be functionally active. The knockdown of one subunit is thus sufficient to ablate AMPK activity. Expression of AMPK subunit isoforms differs among tissues, and we targeted the α1-subunit of AMPK, which is the predominant α-subunit isoform expressed in MDCK cells, to achieve effective knockdown of AMPK function (AMPKα1 KD) in our studies (59). A short-hairpin (sh) RNA construct targeting AMPK’s α1-subunit was stably transfected into MDCK cells. The efficacy of this approach has been demonstrated previously (51). Control cells were created by transfecting MDCK cells with an empty vector (referred to as AMPK WT cells). Previous studies from our laboratory have demonstrated that AMPK knockdown in cultured cells affects the rate of ZO-1 localization to the plasma membrane during tight-junction formation and decreases the magnitude of the transepithelial resistance (65).
We first examined whether AMPK activation could regulate aPKCζ localization by performing immunofluorescence (IF) studies to monitor aPKCζ deposition at the plasma membrane. We cultured AMPKα1 KD and AMPK WT cells to confluency in normal Ca2+ media [1.8 mM Ca2+ (NCM)] before incubating them in low-Ca2+ media (5 µM Ca2+, LCM) for 16 h. Following the reintroduction of NCM for 2 h (the Ca2+ switch), cells were fixed for IF studies. We found that, following incubation in LCM for 16 h, aPKCζ was diffusely localized throughout the cytoplasm in both AMPK WT and KD cells (Fig. 1A). We observed that following the Ca2+ switch, the quantity of aPKCζ that was distributed at the plasma membrane in control cells was significantly higher than that observed in AMPK KD cells (P < 0.001, ANOVA) (Fig. 1, A and B). We found that there was no difference in the quantity of aPKCζ protein expression in AMPK WT versus KD cells under steady state, LCM, or Ca2+ switch conditions (Supplemental Fig. S2, A and B). Examination of confocal z-stack images also demonstrated that aPKCζ and ZO-1 co-localized with one another during the Ca2+ switch, confirming previously published reports (Supplemental Fig. S3) (58). These results indicate that AMPK expression plays an important role in regulating aPKCζ localization during Ca2+ switch-mediated junction assembly.
Fig. 1.
AMPK regulates atypical protein complex Cζ (aPKCζ) localization during junction assembly. A: confluent Madin-Darby canine kidney (MDCK) cells were stably transfected with empty pSUPER vector [AMPK wild-type (WT)] or pSUPER containing shRNA directed against AMPKα1 [AMPKα1 knockdown (KD)] and were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM or media containing 1.8 mM Ca2+ (Ca2+ switch) were introduced for 2 h. Cells were fixed and immunostained for aPKCζ. Scale bar, 30 μm. B: quantification of aPKCζ localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of atypical protein complex Cζ (aPKCζ) per cell within each of the four selected fields of view. P values were calculated by two-way ANOVA with Sidak’s multiple-comparison test. ***Significant difference due to the Ca2+ switch (P < 0.001) compared with LCM conditions or AMPKα1 KD. C: confluent WT MDCK cells were incubated in media containing 5 μM Ca2+ (low-Ca2+ media, LCM) for 16 h. Fresh LCM with or without 2 mM AICAR was introduced for 1 h. Cells were fixed and immunostained for aPKCζ. Scale bar, 30 μm. Arrowheads indicate sites of junction assembly. D: quantification of aPKCζ localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of aPKCζ per cell within each of the four selected fields of view. **Significant difference due to the presence of AICAR (P < 0.0001) compared with LCM conditions based on the Student’s t-test.
To determine whether AMPK activity is involved in regulating aPKCζ localization, we monitored whether pharmacological activation of AMPK by 2 mM 5-aminoimidizole-4 carboxamide riboside (AICAR), an AMPK activator, for 2 h would also promote aPKCζ localization to the plasma membrane. We cultured MDCK cells to confluency in NCM before subjecting them to LCM for 16 h. AICAR (2 mM) was then introduced in LCM for 1 h (LCM + AICAR). Following AICAR treatment, we observed a significant increase in the number and length of strands of aPKCζ labeling located at the plasma membrane (P < 0.0001) without any changes in aPKCζ expression (Fig. 1, C and D, and Supplemental Fig. S2C). AMPK activation was verified by assessing the level of AMPK phosphorylation following AICAR treatment (Supplemental Fig. S1, A and B). Taken together, the AMPK KD and AICAR results indicate that AMPK activity is involved in regulating aPKCζ localization during junction assembly.
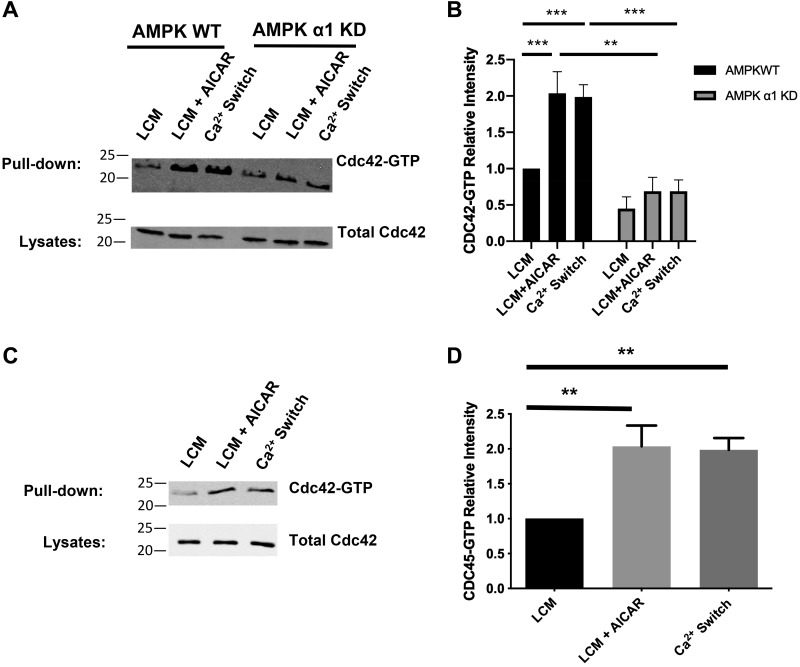
To probe how AMPK regulates aPKCζ localization and function, we monitored whether AMPK similarly controls the distributions and activities of two critical regulators of aPKCζ activity and localization, Cdc42 and Par3. When it is in its GTP-bound form, Cdc42 upregulates aPKCζ activity and promotes aPKCζ apical membrane localization (14, 18, 21). Consequently, we measured whether AMPK affects the level of Cdc42-GTP. We performed the Ca2+ switch manipulation on AMPKα1 KD and AMPK WT for 2 h before lysing the cells and measuring Cdc42-GTP levels by using pulldown assays that employ the GST-Pak1 effector domain as an affinity reagent that interacts specifically with GTP bound but not GDP bound Cdc42 (46, 56). We found that, despite similar levels of total Cdc42 expression at steady state and during the Ca2+ switch, control cells exhibited a robust increase in Cdc42-GTP levels in response to the Ca2+ switch at 2 h, whereas AMPK KD cells failed to display a similar increase (Fig. 2, A and B, Supplemental Fig. S2, A and B). In fact, Cdc42-GTP levels were similar between LCM and Ca2+ switch conditions in AMPK KD cells, suggesting that AMPK may be a significant regulator of the formation of Cdc42-GTP during Ca2+-induced junction assembly (Fig. 2, A and B). The increase in Cdc42-GTP levels in AMPK WT cells subjected to the Ca2+ switch (P < 0.001, ANOVA) was consistent to what we found in WT MDCK cells (P < 0.01, ANOVA) (Fig. 2, C and D).
Fig. 2.
AMP-activated protein kinase (AMPK) regulates the level of GTP-bound Cdc42. A: confluent Madin-Darby canine kidney (MDCK) cells were stably transfected with pSUPER containing shRNA against the AMPKα1 isoform [AMPKα1 knockdown (KD)] or the empty vector [AMPK wild-type (WT)] and incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM with or without 2 mM AICAR or media containing 1.8 mM Ca2+ (Ca2+ switch) were introduced for 2 h. Cell lysates from each condition were obtained and analyzed for GTP-bound Cdc42 via a pulldown assay as described in experimental procedures. Equal amounts of precipitates were then separated on SDS-PAGE and probed with anti-Cdc42 antibody. Cells lysates were analyzed by Western blotting with the Cdc42 antibody. B: quantification of the immunoreactive signal for Cdc42 in precipitates normalized to the level of Cdc42. Data were analyzed for each experimental condition based on three independent experiments and represent mean intensity relative to LCM (WT) level ± SE. ** or ***Significant difference due to the presence of AICAR (P < 0.01) or the Ca2+ switch (P < 0.001) compared with LCM conditions based on the two-way ANOVA and Sidak’s multiple comparison test. C: confluent MDCK cells were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM with or without 2 mM AICAR or media containing 1.8 mM Ca2+ (Ca2+ switch) were introduced for 2 h. Cell lysates from each condition were obtained and analyzed for GTP-bound Cdc42 via a pulldown assay, as described in experimental procedures. Equal amounts of precipitates were then separated on SDS-PAGE and probed with anti-Cdc42 antibody. Cell lysates were analyzed by Western blotting with the Cdc42 antibody. D: quantification of the immunoreactive signal for Cdc42 in precipitates normalized to the level of Cdc42. Data were analyzed for each experimental condition based on three independent experiments and represent mean intensity relative to LCM (WT) level ± SE. **Significant difference due to the presence of AICAR (P < 0.01) or the Ca2+ switch (P < 0.01) compared with LCM conditions based on the one-way ANOVA and with Dunnett’s multiple-comparisons test.
To further evaluate the role of AMPK in this process, we next analyzed whether AMPK activation in an LCM environment alone is sufficient to promote Cdc42 nucleotide exchange, leading to increases in the levels of GTP-bound Cdc42. WT MDCK cells grown to confluence in NCM were incubated in LCM for 16 h before being exposed to 2 mM AICAR for 2 h (LCM + AICAR). We found that AMPK activation resulted in a twofold increase in Cdc42-GTP levels without affecting total level of Cdc42 expression (P < 0.001, ANOVA) (Fig. 2, C and D and Supplemental Fig. S2C). The magnitude of the increase observed was comparable to that achieved during the Ca2+ switch (P < 0.001, ANOVA) (Fig. 2, C and D). To ensure that the effects of AICAR were mediated by AMPK, we analyzed the effect of AICAR on Cdc42-GTP levels in AMPK KD cells and found that these cells failed to exhibit an increase in Cdc42-GTP in response to AICAR treatment (Fig. 2, A and B). Taken together, these studies support the conclusion that AMPK expression and activity play important roles in promoting the formation of Cdc42-GTP during junction assembly.
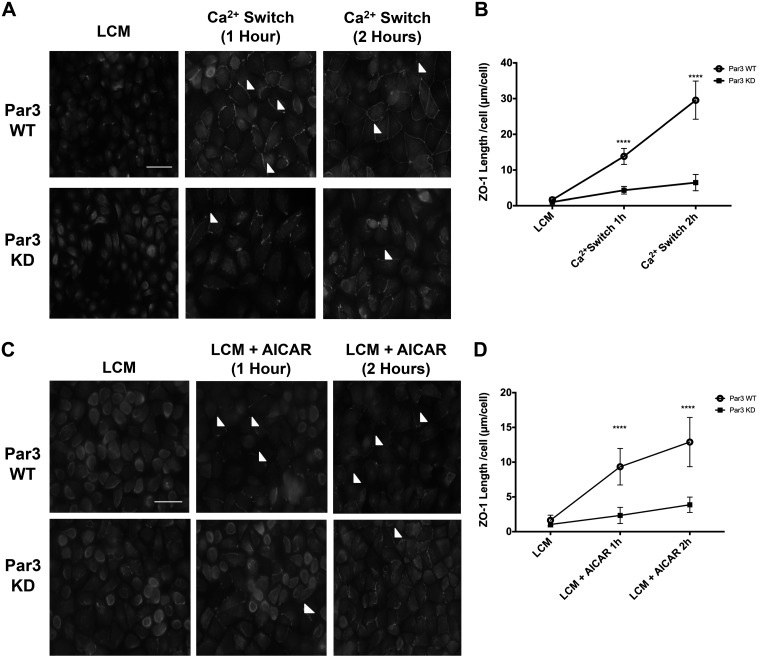
Par3 is an important scaffolding protein that localizes to the nascent adherens junction during the early stages of junction assembly, subsequently transitioning to a predominantly tight junction distribution when junctions have matured (32, 55). Following Cdc42-GTP-mediated aPKCζ activation, aPKCζ interacts with Par3, and the protein complex is subsequently recruited to primordial junctions (22, 24). Our confocal imaging experiments reveal that Par3 and ZO-1 co-localized with one another during the Ca2+ switch (Supplemental Fig. S3). We subsequently evaluated Par3’s role during junction assembly by monitoring the effects of shRNA-mediated Par3 knockdown (Par3 KD) on Ca2+ switch-mediated junction assembly (Supplemental Fig. S2D). Both Par3 KD cells and their WT counterparts were subjected to the Ca2+ switch, and ZO-1 localization was monitored by IF. We observed that the length of ZO-1 localization at the plasma membrane was significantly decreased in Par3 KD cells compared with control cells, demonstrating that Par3 contributes to ZO-1 localization during junction assembly (P < 0.0001 for both 1- and 2-h time points, ANOVA) (Fig. 3, A and B).
Fig. 3.
Par3 expression is important for both Ca2+ switch and AMPK-mediated zonula occludens-1 (ZO-1) localization to the plasma membrane. Madin-Darby canine kidney (MDCK) cells grown to confluency were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h before being introduced to fresh LCM, LCM containing 2 mM AICAR, or media containing 1.8 mM Ca2+ (Ca2+ switch) for 1 or 2 h as indicated. A and C: cells were fixed and immunostained for ZO-1. Scale bar, 30 μm. Arrowheads indicate sites of junction assembly. B and D: quantification of ZO-1 localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of Par3 per cell within each of the four selected fields of view. ****Significant difference due to the presence of AICAR (P < 0.0001) or the Ca2+ switch (P < 0.0001) compared with LCM conditions based on Student’s t-test. KD, knockdown; WT, wild type.
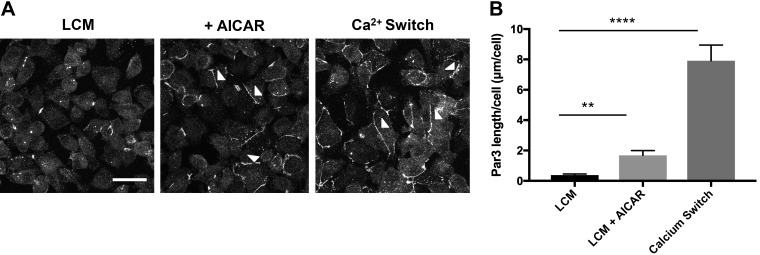
To determine whether AMPK could regulate Par3 localization, we performed the Ca2+ switch manipulation on AMPKα1 KD and AMPK WT cells for 2 h before monitoring Par3 deposition through IF analysis. We found that, following incubation in LCM for 16 h, Par3 was diffusely localized throughout the cytoplasm, whereas the level of total Par3 protein expression remained unchanged during both steady-state and Ca2+ switch conditions (Supplemental Figs. S2, A, and B, and S3). We also observed that, following the Ca2+ switch, Par3 was localized at the plasma membrane to a significantly lesser extent in AMPKα1 KD cells as compared with control cells (P < 0.0001, ANOVA) (Supplemental Fig. S4). These results indicate that AMPK expression is involved in regulating Par3 localization during Ca2+ switch-mediated junction assembly. The increase in Par3 localization at the plasma membrane in AMPK WT cells during the Ca2+ switch is consistent with what we found in WT MDCK cells (P < 0.0001, ANOVA) (Fig. 4). To determine whether AMPK activity plays a role regulating Par3 localization, we monitored whether activation of AMPK by 2 mM AICAR for 2 h could also promote Par3 localization to the plasma membrane in cells that were maintained in LCM (Fig. 4). MDCK cells were cultured to confluency in NCM before being subjected to LCM for 16 h. Two micromolar AICAR was introduced for 2 h (LCM + AICAR) before IF analysis. We found that, following AICAR treatment, there was a significant increase in the number and length of strand labeling for Par3 located at the plasma membrane without any changes in the level of Par3 protein expression (P < 0.01, ANOVA) (Fig. 4 and Supplemental Fig. S2C). These results confirm that AMPK activity plays an important role in regulating Par3 localization during junction assembly.
Fig. 4.
AICAR promotes the localization of Par3 to the plasma membrane. A: confluent Madin-Darby canine kidney (MDCK) cells were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM with or without 2 mM AICAR was introduced for 1 h. Cells undergoing the Ca2+ switch were introduced to media containing 1.8 mM Ca2+ for 1 h. Cells were fixed and immunostained for Par3. Scale bar, 30 μm. Arrowheads indicate sites of junction assembly. B: quantification of Par3 localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of Par3 per cell within each of the four selected fields of view. ** or ****Significant difference due to the presence of AICAR (P < 0.01) or the Ca2+ switch (P < 0.0001) compared with LCM conditions based on one-way ANOVA with Dunnett’s multiple-comparison test.
To assess whether Par3 acts as an intermediate in AMPK-mediated junction assembly, we first monitored the effects of Par3 KD on ZO-1 localization. We again promoted AMPK-mediated junction assembly by treating Par3 KD or control cells incubated in LCM for 16 h with 2 mM AICAR for 2 h. Compared with activity in control cells, we found that ZO-1 localization at the plasma membrane was significantly decreased in Par3 KD cells exposed to LCM + AICAR conditions (P < 0.0001 for both the 1- and 2-h time points, ANOVA) (Fig. 3, C and D). These results indicate that Par3 is an important intermediate in AMPK-mediated junction assembly.
aPKCζ Inhibition Prevents AMPK-Mediated Junction Assembly and Increases the Association Between ZO-1 and Afadin
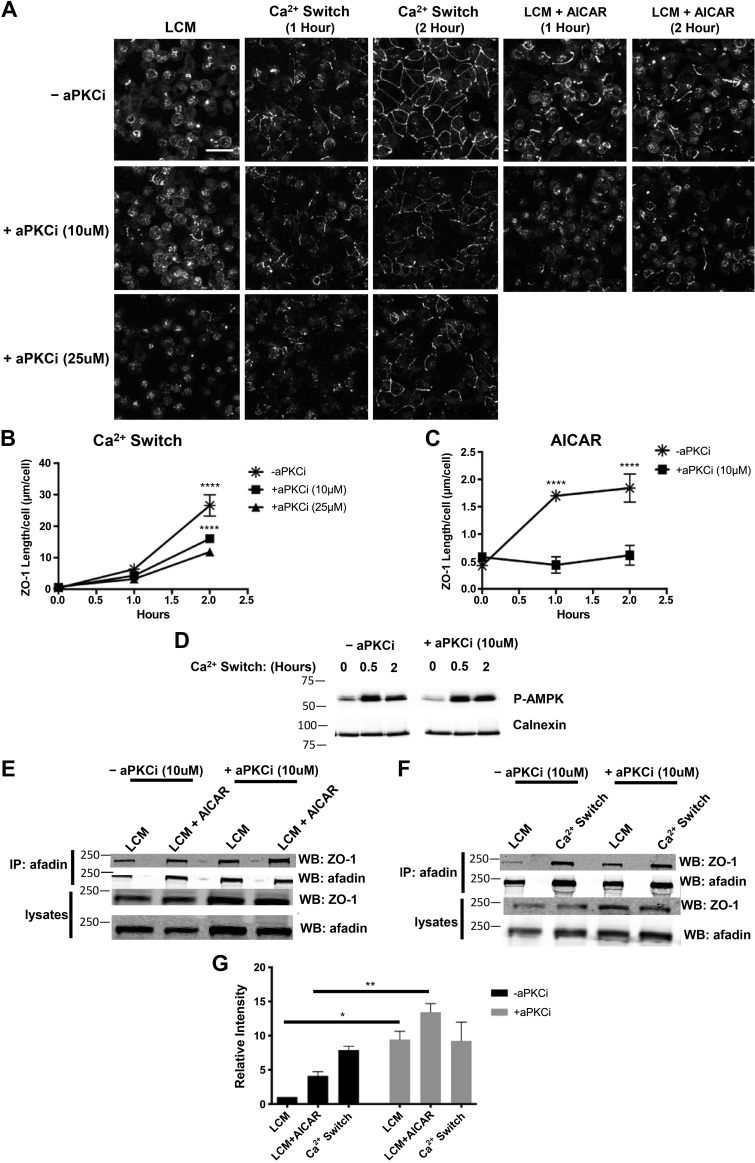
Because our studies show that AMPK regulates aPKCζ localization to the plasma membrane and may control aPKCζ activity through promotion of the formation of Cdc42-GTP, we next sought to ascertain whether aPKCζ plays a role in AMPK-mediated junction assembly by determining the effects of aPKCζ inhibition on this process. We used a cell-permeable myristoylated pseudosubstrate inhibitor of aPKCζ activity to pharmacologically inhibit aPKCζ (29, 31, 60). We first monitored the effects of aPKCζ inhibition during the Ca2+ switch to test the efficacy of the inhibitor. MDCK cells grown to confluence were incubated in LCM for 16 h before being pretreated for 2 h with or without the 10 or 25 μM aPKCζ inhibitor in LCM. NCM was introduced for another 2 h in the continued presence of the inhibitor. In concert with what has been previously reported, we found that aPKCζ inhibition at 10 μM was sufficient to partially prevent Ca2+ switch-mediated junction assembly and that this effect occurred in a dose-dependent manner (P < 0.0001 for both 10 and 25μM aPKCi at 2 h, ANOVA) (Fig. 5, A and B) (2, 27). We and others have previously found that the Ca2+ switch maneuver induces AMPK activation, as evidenced by its phosphorylation at Thr172 on its α-subunit (64, 66). Inhibition of aPKCζ activity did not affect this Ca2+ switch-induced AMPK phosphorylation (Fig. 5D). To monitor the role of aPKCζ in AMPK-mediated junction assembly, MDCK cells grown to confluency were again incubated in LCM for 16 h and then pretreated for 2 h with or without 10 μM aPKCζ inhibitor in LCM before being subjected to AMPK activation by 2 mM AICAR in the continued presence or absence of the aPKCζ inhibitor. We observed that, compared with cells treated with AICAR alone, those incubated in the presence of aPKCζ inhibitor with AICAR had a significantly lower level of ZO-1 distributed at the plasma membrane (P < 0.0001 at both 1 and 2 h, ANOVA) (Fig. 5, A and C). This result suggests that AMPK-mediated junction assembly requires aPKCζ activity.
Fig. 5.
Atypical protein complex Cζ (aPKCζ) inhibition decreases both Ca2+ switch- and AMP-activated protein kinase (AMPK)-mediated zonula occludens-1 (ZO-1) localization to sites of junction assembly and affects the level of interaction between ZO-1 and afadin during junction assembly. Confluent Madin-Darby canine kidney (MDCK) cells were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Cells without aPKCζ inhibition (−aPKCζi) were introduced to fresh LCM and switched to media containing 1.8 mM Ca2+ (NCM; Ca2+ switch) or 1 mM AICAR for the indicated time points (top). Cells subjected to aPKCζ inhibition (+aPKCζi) were pretreated with the indicated concentration of aPKCζ psuedosubstrate inhibitor for 2 h before being introduced to fresh LCM, NCM, or 1 mM AICAR containing aPKCζ inhibitor for the indicated quantity and time points. A: cells were fixed and immunostained for ZO-1. Scale bar, 30 μm. B and C: quantification of ZO-1 localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of ZO-1 per cell within each of the four selected fields of view. ****Significant difference in ZO-1 length following treatment with aPKCi (both 10 and 25 μM) after 2 h of the Ca2+ switch (P < 0.0001) and following aPKCi at 10 μM after 1 and 2 h of AICAR treatment (P < 0.0001). P values were calculated based on two-way ANOVA with Sidek’s multiple-comparison test. D: cell lysates were probed with the indicated antibodies by Western blot analysis. E and F: cell lysates from each condition were obtained and immunoprecipitated using antibody directed against afadin and protein G agarose beads. Equal amounts of immunoprecipitates were then separated on SDS-PAGE and probed with anti-ZO-1 antibody. Total cells lysates were simultaneously subjected to immunoblotting using anti-ZO-1 and afadin antibodies. G: quantification of the immunoreactive signal for ZO-1 in immunoprecipitates was normalized to the level of immunoprecipitated afadin. Data were analyzed for each experimental condition based on five independent experiments and represent mean intensity relative to LCM level ± SE. * or **Significant difference due to the presence of aPKCζ inhibition under LCM (P < 0.05) or LCM + AICAR (P < 0.01) conditions based on the two-way ANOVA with Sidek’s multiple-comparison test.
We have previously reported that AMPK activation promotes the formation of an interaction between ZO-1 and afadin (64). Because the data presented here indicate that aPKCζ acts downstream of AMPK to regulate junction assembly, we further probed whether these functional activities converge within one pathway. We specifically monitored whether aPKCζ is a regulator of the AMPK-mediated ZO-1-afadin interaction by assessing whether inhibition of aPKCζ activity alters the extent of this interaction. MDCK cells were grown to confluence before being incubated in LCM for 16 h. Cells were pretreated with or without 10 μM aPKCζ inhibitor for 2 h before 2 mM AICAR was introduced for another 2 h in the continued presence or absence of the inhibitor. To measure the level of ZO-1-afadin interaction, we performed co-immunoprecipitation studies in which endogenous afadin was immunoprecipitated using an antibody directed against afadin, and the quantity of co-precipitating ZO-1 was subsequently assessed by Western blotting using an antibody directed against the endogenous ZO-1 protein. In these studies, AMPK activation by AICAR led to an increase in the ZO-1-afadin interaction (P < 0.05, ANOVA) (Fig. 5, E and G). Inhibition of aPKCζ further increased the level of the ZO-1-afadin interaction during both the LCM incubation and following AMPK activation by AICAR as compared with that observed under similar conditions when aPKCζ activity was not inhibited (P < 0.05 and P < 0.01 respectively, ANOVA) (Fig. 5, E and G). These results indicate that aPKCζ inhibition increases the extent of the interaction between ZO-1 and afadin specifically when adherens and tight junctions are not present (LCM conditions) and during AMPK-mediated junction assembly (LCM + AICAR conditions). Inhibition of aPKCζ did not significantly affect the extent of the ZO-1-afadin interaction during the Ca2+ switch, suggesting that the interaction may reach its maximal level during the Ca2+ switch or that there may be additional mechanisms that also regulate the interaction and that are unaffected by aPKCζ inhibition (not significant (NS), ANOVA] (Fig. 5, F and G].
Afadin Phosphorylation at S228 and S1102 Are Regulators of Junction Assembly
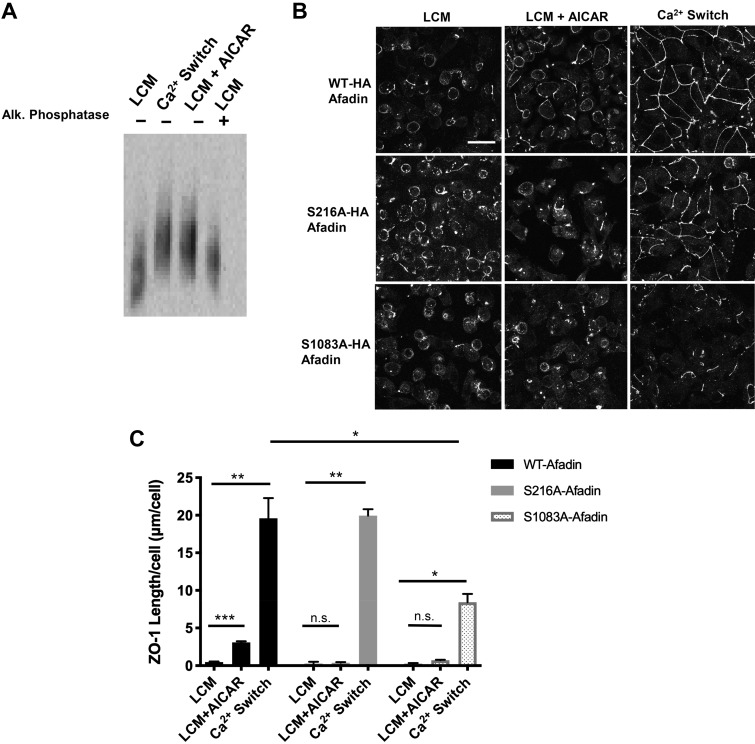
Because protein phosphorylation is a common regulator of protein activity, we investigated how the ZO-1-afadin interaction is controlled by afadin phosphorylation. We first probed for whether afadin phosphorylation is changed during the Ca2+ switch relative to LCM conditions by using Phos-tag acrylamide containing gels in concert with Western blotting. The Phos-tag reagent binds to phosphate groups, and its addition to acrylamide gels permits separation of proteins based on molecular weight as well as upon phosphorylation status (28). Proteins that are more heavily phosphorylated migrate at a slower rate in acrylamide gels containing the Phos-tag than their nonphosphorylated counterparts. We found that afadin phosphorylation robustly increased in response to the Ca2+ switch (Fig. 6A).
Fig. 6.
Afadin phosphorylation is an important component of AICAR- and Ca2+ switch-mediated junction assembly. A: confluent Madin-Darby canine kidney (MDCK) cells were incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM with or without 2 mM AICAR or media containing 1.8 mM Ca2+ (Ca2+ switch) were introduced for 2 h. Alkaline phosphatase treatment was performed at 37°C for 1 h. Cell lysates were separated by phos-tag acrylamide gel electrophoresis. Western blot analysis was used to probe for protein expression with the anti-Afadin antibody. B: confluent MDCK cells stably transfected with hemagglutinin (HA)-tagged afadin corresponding with the wild-type mus musculus cDNA sequence (top) or containing the S216A or S1083A mutations (bottom) were incubated in media containing 5 μM Ca2+ (LCM) for 16 h. Fresh LCM with or without 2 mM AICAR or media containing Ca2+ switch were introduced for 2 h. Cells were fixed and immunostained for zonula occludens-1 (ZO-1). Scale bar, 30 μm. C: quantification of ZO-1 localization at sites of junction assembly. Data were analyzed for each experimental condition based on four randomly obtained views from three independent coverslips. Error bars represent the SE of the length of ZO-1 per cell within each of the four selected fields of view. *, **, or ***Significant difference in ZO-1 length due to the Ca2+ switch (P < 0.01) or AICAR (P < 0.001) in cells with WT-afadin and in cells with S216A afadin (P < 0.01) or S1083A afadin (P < 0.05) due to the Ca2+ switch relative to their respective LCM conditions. There was also a significant difference in ZO-1 length in response to the Ca2+ switch in cells with S1083A afadin versus WT cells. P values were calculated based on the two-way ANOVA with Sidek’s multiple-comparisons test.
To identify the specific afadin residues phosphorylated during the Ca2+ switch and to assess whether phosphorylation at these residues is differentially regulated by this manipulation, we used a stable isotope dimethyl labeling quantitative proteomics strategy (43). Afadin was immunoprecipitated from cells maintained in LCM only or from LCM-incubated cells that were subjected to the Ca2+ switch, and the resulting immunoprecipitates were separated by SDS-PAGE. In-gel trypsin digestion followed by dimethyl labeling was performed, with samples subjected to the Ca2+ switch labeled with CD2O and LCM samples labeled with CH2O before being mixed in a 1:1 ratio (4). Phosphorylated peptides were enriched by TiO2 binding before analysis by mass spectrometry (36). Relative phosphopeptide abundance was normalized according to the median value of the relative abundance of all nonphosphorylated afadin peptides within the same sample. Although there were many sites on afadin that were found to be phosphorylated, two residues were of significant interest: S228 and S1102 (Supplemental Fig. S5). The peptide that includes the S1102 residue contains the consensus sequence for AMPK recognition (42). Whereas phosphorylation at this site was previously determined to be promoted by AMPK activation, in the present study we did not detect any changes in the phosphorylation level of S1102 in response to the Ca2+ switch manipulation (Table 1) (64). In contrast, S228 displayed an upregulation in its phosphorylation level in response to the Ca2+ switch by 1.703-fold (CI: 1.399–2.007). The peptide that contains the S228 residue also bears the consensus sequence for aPKCζ recognition (42).
Table 1.
Selected peptides from native afadin (uniprot: F1PSU6) identified by LC-MS/MS
| Position | Afadin Peptide (Dog) | m/z | CS/LCM Normalized Ratio (n=6) | CS/LCM Ratio 95% CI | Putative Kinase |
|---|---|---|---|---|---|
| S228 | 226-TISNPEVVMK-235 | 1196.551 | 1.703 | 1.399–2.007 | aPKC |
| S1102 | 1100-TSSVVTLEVAK-1110 | 1,212.6 | 0.9413 | 0.8695–1.013 | AMPK |
Confluent wild-type (WT) Madin-Darby canine kidney (MDCK) cells were incubated in media containing 5 μM Ca2+ (LCM) for 16 h. Cells undergoing the Ca2+ switch (CS) were introduced to media containing 1.8 mM Ca2+ for 2 h. Phosphopeptides were identified through LC-MS/MS analysis of afadin immunoprecipitated from MDCK cells subjected to LCM or Ca2+ switch conditions. Afadin peptides were subjected to titanium dioxide (TiO2) enrichment to isolate phosphopeptides. Unambiguous assignment of phosphorylation sites is indicated by S. Normalized ratios were obtained by comparing the phosphopeptide ratios of TiO2-enriched samples relative to the ratios of their nonphosphorylated counterparts (n = 6). Putative kinases were determined by searching MIT Scansite 4.0. aPKC, atypical protein complex C; CI, confidence interval; LCM, low-Ca2+ media.
Because phosphorylation at S228 and S1102 may be important for aspects of junction assembly, we sought to determine whether phosphorylation at either of these sites is important for regulating ZO-1 localization or for the interaction between afadin and ZO-1. We investigated the role of S228 and S1102 phosphorylation by generating phospho-defective mutant forms of afadin, in which these residues were individually replaced with alanine residues. These mutations were introduced into constructs containing the mus musculus cDNA sequence for hemagglutinin (HA)-tagged afadin at either the S216 or S1083 sites (which correspond with the S228 and S1102 sites in canis familiaris afadin), and the constructs were stably transfected into wild-type MDCK cells. As a control, a construct encoding the HA-tagged wild-type mus musculus cDNA sequence afadin was also stably transfected into wild-type MDCK cells. Under steady-state conditions, HA-tagged wild-type, S216A, and S1083A afadin localized to the plasma membrane (Supplemental Fig. S6A). The total level of afadin expression was increased by approximately 50% in cells expressing wild-type afadin and to a similar extent in cells expressing S216A mutant afadin compared with cells transfected with empty vector (Supplemental Fig. S6 B and C). Total afadin expression was increased by ∼200% in cells expressing the HA-tagged S1083A afadin mutant as compared with cells transfected with an empty vector (Supplemental Fig. 6, B and C).
To assess whether and how the absence of phosphorylation at afadin residues S216 and S1083 might impact junction assembly, we monitored whether expression of these mutant afadin proteins affected the localization of ZO-1 during the Ca2+ switch and AMPK-mediated junction assembly. Confluent MDCK cells stably transfected with constructs containing HA-tagged wild-type afadin or afadin carrying the S216A or S1083A mutations were subject to the Ca2+ switch or 2 mM AICAR treatment for 2 h, and ZO-1 localization was monitored by IF. We found that the introduction of the S216A mutation prevented an increase in ZO-1 translocation to the plasma membrane following AICAR treatment compared with the LCM condition but did not perturb ZO-1 localization in response to the Ca2+ switch compared with cells expressing WT afadin (NS and NS, respectively; ANOVA) (Fig. 6, B and C). The distribution of ZO-1 to the plasma membrane was found to be significantly lower in cells expressing S1083A HA-tagged afadin during the Ca2+ switch compared with those detected in cells expressing HA-tagged wild-type afadin (P < 0.05, ANOVA), and AICAR activation also failed to promote ZO-1 localization to the plasma membrane compared with LCM conditions (NS, ANOVA) (Fig. 6, B and C). Because the endogenous afadin is still expressed in these cells, it appears that the expression of the S216A or S1083A mutant afadin may exert a dominant-negative effect during junction assembly. However, additional mechanisms initiated by the Ca2+ switch may also be able to compensate for the dominant-negative properties of the S216A mutant protein. Together, these results suggest that phosphorylation at S216 or S1083A of mus musculus afadin plays modulatory roles during junction assembly.
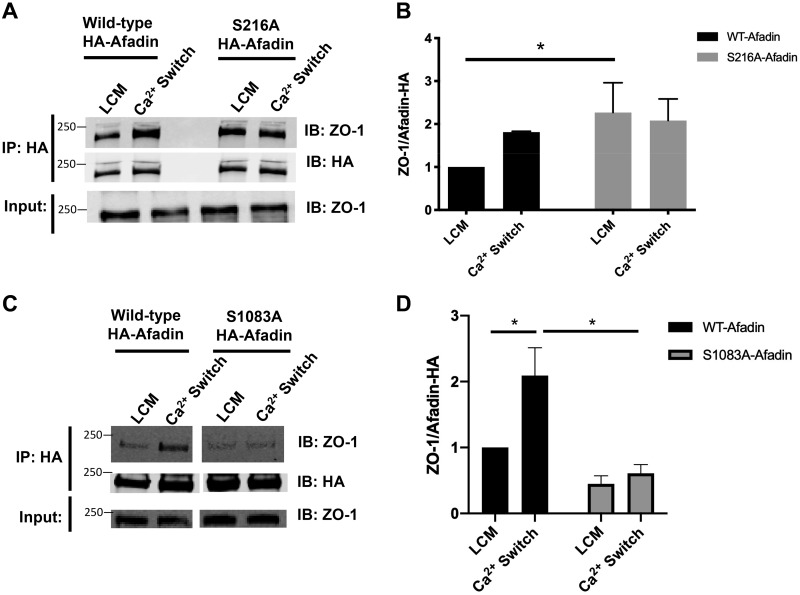
Because afadin’s interaction with ZO-1 is important for junction assembly, we also assessed whether S228 or S1102 phosphorylation affects afadin’s ability to interact with ZO-1. Using the same MDCK cells stably transfected with HA-tagged wild-type mus musculus afadin or afadin containing the S216A or S1083A mutation, we measured the extent of the ZO-1/HA-afadin interaction in response to the Ca2+ switch by performing co-immunoprecipitation experiments. In the following studies, HA-tagged afadin was immunoprecipitated using an antibody directed against the HA epitope tag, and the level of endogenous ZO-1 that interacts with the HA-tagged afadin was monitored by Western blotting. The Ca2+ switch was performed for 2 h in MDCK cells transfected with one of the HA-tagged constructs. In cells expressing the wild-type HA-tagged afadin construct, we observed an increase in the ZO-1/HA-tagged afadin interaction in response to the Ca2+ switch relative to LCM levels, consistent with the behavior of endogenous afadin (Fig. 7, A and B). Using the same experimental setup, we found that in MDCK cells expressing HA-tagged afadin that contains the S216A mutation, the extent of the ZO-1/HA-tagged afadin interaction was substantially larger during the LCM incubation relative to cells transfected with wild-type afadin (P < 0.05, ANOVA) (Fig. 7, A and B). The extent of this interaction was not further increased by the Ca2+ switch (Fig. 7, A and B). In contrast, in cells expressing S1083A afadin, we observed a low level of interaction between ZO-1 and afadin under LCM incubation conditions that remained low despite Ca2+ switch (P < 0.05, ANOVA) (Fig. 7, C and D). These results indicate that, during the Ca2+ switch, the absence of phosphorylation at the 216 residue in mus musculus afadin may promote the interaction between ZO-1 and afadin. Phosphorylation at S216 and S1083 appear to exert opposite effects on the interaction between afadin and ZO-1. While the ability to become phosphorylated at S1083 appears to be important in initiating a Ca2+ switch-induced association between ZO-1 and afadin, phosphorylation at S216 appears to be required for the termination of this association that accompanies the later stages of junction formation and maturation. Loss of the capacity to become phosphorylated at S216 results in the formation of a stable interaction between ZO-1 and afadin that persists independent of the presence of extracellular calcium and of the stimuli that induce junction assembly.
Fig. 7.
S216A and S1083A mutations in afadin influence its interaction with zonula occludens-1 (ZO-1). A and C: confluent Madin-Darby canine kidney (MDCK) cells stably transfected with hemagglutinin (HA)-tagged afadin corresponding with the wild-type (WT) mus musculus cDNA sequence; the S216A or the S1083A mutation was incubated in media containing 5 μM Ca2+ [low-Ca2+ media (LCM)] for 16 h. Fresh LCM or media containing 1.8 mM Ca2+ (Ca2+ switch) for 2 h. Cell lysates from each condition were obtained and immunoprecipitated with HA-antibody conjugated beads. Equal amounts of immunoprecipitates (IP) were then separated on SDS-PAGE and probed with anti-ZO-1 and anti-HA antibody. Total cells lysates were simultaneously subjected to immuoblotting (IB) using anti-ZO-1 antibody. B and D: quantification of the immunoreactive signal for ZO-1 in immunoprecipitates normalized to the level of immunoprecipitated HA-tagged afadin. Data were analyzed for each experimental condition based on three independent experiments and represent mean intensity relative to LCM (WT) level ± SE. *Significant difference in the amount of ZO-1 coimmunopreciptated with HA-afadin in cells with S216A HA-afadin compared with WT HA-afadin under LCM conditions (P < 0.05) and in cells with S1083A HA-afadin compared with WT HA-afadin under Ca2+ switch conditions (P < 0.05) based on the two-way ANOVA with Sidek’s multiple-comparisons test.
DISCUSSION
Epithelial adherens and tight-junction assembly is a complex and incompletely understood process that involves cooperation between numerous proteins and the coordination of many different signaling pathways. Because perturbations in junction structure and functions are important features of a number of pathophysiological conditions, including inflammatory bowel disease, ischemic acute kidney injury, and cancer metastasis (10, 11, 33), it is important to understand the processes that promote junction integrity. Although AMPK is a well-known regulator of cell polarity, the relevant signaling pathways that participate up- or downstream of AMPK in epithelial polarization are currently still unclear. The work presented here was designed to expand our understanding of the proteins that participate downstream of AMPK to regulate AMPK-mediated adherens and tight-junction assembly.
Because the aPKCζ/Par3/Cdc42 polarity complex proteins are central regulators of junction assembly and cell polarity, we explored whether and how AMPK influences and is influenced by these proteins in exerting its effects on junction formation (7). We found that AMPK serves as an upstream regulator of the aPKCζ/Par3/Cdc42 localizations and activities of components of this critical polarity complex. Furthermore, we found that AMPK and aPKCζ may phosphorylate afadin at S1102 and S228, respectively, to coordinate their antagonistic roles regulating the ZO-1-afadin interaction and to ultimately promote junction assembly. Taken together, these results not only reaffirm that AMPK plays a significant role regulating the development of the adherens and tight junction but that the signaling mechanism downstream of AMPK is complex and dynamically regulated.
Our data indicate that AMPK is responsible for promoting the localization of both aPKCζ and Par3 to the plasma membrane and for regulating the level of GTP-bound Cdc42. These results indicate that AMPK is an upstream regulator for both aPKCζ and Par3 localization to the plasma membrane during Ca2+ switch-induced junction assembly.
Several separate studies have shown that AMPK regulates the activities of members of the GTP-binding Rho family proteins. AMPK may interact directly with both RhoA and Rac1 or indirectly regulate Rac1 through its guanine nucleotide exchange factor Tiam1 (41, 63). Our results indicate that AMPK may play a similar role for Cdc42, through which it regulates the formation of Cdc42 GTP and thus modulates Cdc42 activity.
The adherens junction protein afadin interacts sequentially during junction assembly with many different junction proteins (25, 40, 45). It seems logical to suggest that afadin’s capacity to participate in these interactions must be dynamically regulated to define both spatially and temporally the complements of proteins with which it interacts during distinct phases of junction assembly. A common mechanism for regulating protein activity or assembly is through phosphorylation. We found by mass spectrometric analysis that afadin is phosphorylated at several different sites. Although there have been a number of studies investigating the phosphorylation status of afadin by upstream kinases, little is understood concerning how afadin phosphorylation specifically affects junction assembly and the ZO-1-afadin interaction (12, 47). In concert with previous studies, our data indicate that both AMPK and aPKCζ may regulate the phosphorylation status of afadin at S1102 and S228 respectively (64). S228 is located in the first Ras-associating domain, and S1102 is located in the PDZ domain, and both of these domains are important for protein-protein interactions and regulation. We demonstrated that the absence of phosphorylation at these positions not only perturbs AMPK-mediated junction assembly by decreasing the ability for ZO-1 to localize to the plasma membrane, but that these phosphorylation sites also specifically coordinate the level of ZO-1-afadin interaction. Phosphorylation at S228 and S1102 appears to decrease and increase the extent or stability of ZO-1-afadin interaction, respectively. These results are consistent with the idea that, through sequential phosphorylation and dephosphorylation, afadin is able to play a role that may evolve during the course of junction assembly.
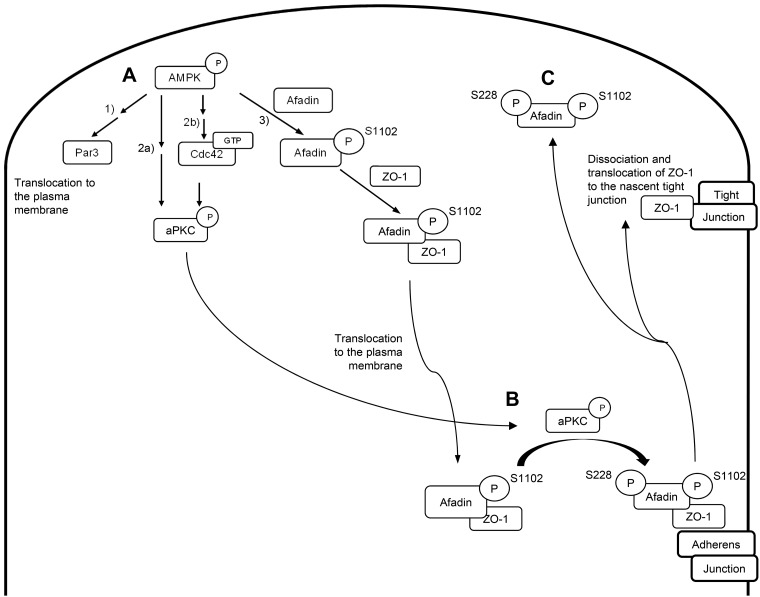
The data presented here are consistent with a model in which activation of AMPK promotes Par3 localization to the plasma membrane, which has been shown in the literature to in turn recruit aPKCζ. AMPK’s activation of Cdc42 further leads to aPKCζ activation. AMPK activity would also result in phosphorylation of afadin at S1102, which would promote its interaction with ZO-1 and thus drive the recruitment of ZO-1 to forming adherens junctions. Activation of aPKCζ would in turn phosphorylate afadin at S228 (which resides within a predicted aPKCζ phosphorylation site) to promote ZO-1’s dissociation from afadin, thus freeing ZO-1 to interact with transmembrane components of the tight junction and to participate in the maturation of assembling junctions (Fig. 8). It is interesting to note that a conceptually similar mechanism, in which phosphorylation regulates the interaction between the tight-junction protein ZO-2 and 14-3-3 proteins, has recently been shown to regulate the deposition of ZO-2 during junction formation (1).
Fig. 8.
Schematic diagram of proposed model for how AMP-activated protein kinase (AMPK) regulates Par polarity complex proteins. A: activation of AMPK leads to 1) Par3 translocation to the plasma membrane, 2) atypical protein complex Cζ (aPKCζ) translocation to the plasma membrane and phosphorylation either directly (2a) or via activation of GTP-bound Cdc42 (2b), and 3) afadin phosphorylation at S1102. B and C: phosphorylated afadin in turn interacts with zonula occludens-1 (ZO-1) to promote its localization to the nascent adherens junction (B), while activated aPKCζ phosphorylates afadin at S228 to promote the dissociation of afadin from ZO-1 such that ZO-1 may then translocate to the nascent tight junction (C).
Additional investigations are needed to evaluate more fully whether additional components are required downstream of AMPK to promote AMPK-mediated junction assembly. Because we found that the knockdown of AMPKα1 subunit prevents the redistribution of aPKCζ and Par3 to the plasma membrane during the Ca2+ switch, these findings suggest that AMPK plays an important role in regulating aPKCζ and Par3 localization in a way that cannot be compensated through other mechanisms activated by the Ca2+ switch. In light of the numerous reported regulators of Par3 and aPKCζ localization, including phosphoinositide-3-kinase, small GTPase Rap1, afadin, Willin, Morg1, and Crumbs3, it seems surprising that AMPK alone would have such a significant role controlling the distribution of Par3 and aPKCζ under these circumstances (8, 19, 26, 49, 54). This suggests the interesting possibility that other regulators of aPKCζ or Par3 localization may depend upon or act downstream of AMPK. Additional investigations may also help to determine whether AMPK is a direct or indirect regulator of Cdc42. Perhaps not surprisingly, we found that AMPKα1 KD leads to only a 50% reduction in the fraction of Cdc42 that is in the GTP-bound state in response to stimuli that initiate junction formation. Consequently, it appears that AMPK may be only one of many regulators of the GTP-bound status of Cdc42 during the process of junction assembly.
While the scheme presented in Fig. 8 encompasses the results of the present studies, additional work will be required to determine whether all of its predictions are observed and whether it is sufficient to account fully for the role of AMPK in junction assembly. It will be interesting in future studies to assess whether regulation of afadin’s phosphorylation at other sites may similarly help to define afadin’s participation in the initiation of junction assembly and in the maturation of the resultant junctional complexes. It will also be interesting to investigate whether afadin is subject to sequential phosphorylation by AMPK at S1102 and by aPKCζ at S228 that causes afadin to interact with ZO-1 and then to dissociate from this interaction, respectively.
While AMPK is widely understood to serve as an energy sensor and regulator of metabolism, its role as a promoter of adherens and tight-junction assembly has been largely considered separately from its energy-sensing functions (38, 64–66). AMPK activation during the Ca2+ switch occurs without any significant changes to cellular energetics, consistent with the paradigm that AMPK’s role as an energy sensor is distinct from its role as a regulator of junction assembly (65). The activation of AMPK decreases the activities of several energy-consuming cellular processes, such as cell growth and protein/fatty acid synthesis (52). Because junction formation and the maintenance of junction integrity requires dynamic and rapid molecular turnover, which very likely are energy-consuming processes, it thus seems somewhat counterintuitive that AMPK activation promotes junction assembly (17, 50, 53). It is important to note, however, that AMPK does not inhibit all energy-consuming processes. For example, it plays a significant role in activating glycolysis to increase cellular ATP levels and promote cell survival (38). Perhaps more importantly, although maintaining junction integrity maintenance may be energy consuming at a cellular level and thus unfavorable in the context of an individual cell’s energy stress, loss of junction integrity can lead to catastrophic effects at the levels of tissues, organs, and organisms. In the kidney, for example, disturbed junctional integrity induced by ischemic injury can lead to severe perturbations in renal tubular fluid and solute absorption, which has the potential to threaten an organism’s continued viability. Similarly, loss of junctional integrity as a consequence of intestinal hypoperfusion can lead to severe bacterial infections (10, 48). Faced with potentially life-threatening consequences for the organism, it may be advantageous for epithelial cells to expend some energy even in the face of their own energy deprivation so as to preserve junction integrity to preserve organ function and survival of the organism. Thus, AMPK’s role as an energy sensor may permit it to ensure that energy-requiring processes like junction maintenance continue even in the face of energy deficits.
There appears to be a growing and fascinating convergence between components of cellular machineries that participate in metabolic regulation and in orchestrating junction assembly, further supporting the idea that the two processes are inter-linked. For example, studies examining AMPK’s role in regulating metabolic processes demonstrate that aPKCζ acts downstream of AMPK to promote insulin-induced GLUT4 translocation (30). Our results indicate that aPKCζ also plays a role in AMPK-mediated junction assembly. It thus appears that AMPK may be able to concurrently increase both cellular glucose levels and to ensure the maintenance of junction integrity to promote cell and tissue survival by signaling through aPKCζ. It will be interesting to continue to explore both of these processes to elucidate further the extent of the overlap in the pathways that connect AMPK regulation of aPKCζ activity to metabolism and to junction assembly.
GRANTS
This work was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grants DK17433 and DK072612. J. Wu was supported in part by the American Association for University Women American Fellowship. B. M. Gassaway was supported by National Science Foundation Grant DGE1122492.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., P.R., F.J., B.M.G., J.R., and M.J.C. conceived and designed research; J.W., P.R., B.M.G., and V.R. performed experiments; J.W., P.R., F.J., B.M.G., J.R., and M.J.C. analyzed data; J.W., P.R., F.J., B.M.G., J.R., and M.J.C. interpreted results of experiments; J.W., P.R., and B.M.G. prepared figures; J.W. and M.J.C. drafted manuscript; J.W., P.R., F.J., B.M.G., J.R., and M.J.C. edited and revised manuscript; J.W., P.R., F.J., B.M.G., V.R., J.R., and M.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Yoshimi Takai (Kobe University Graduate School of Medicine) for providing the afadin construct. We thank all of the members of the Caplan laboratory for insightful advice and discussions.
REFERENCES
- 1.Amaya E, Alarcón L, Martín-Tapia D, Cuellar-Pérez F, Cano-Cortina M, Ortega-Olvera JM, Cisneros B, Rodriguez AJ, Gamba G, González-Mariscal L. Activation of the Ca2+ sensing receptor and the PKC/WNK4 downstream signaling cascade induces incorporation of ZO-2 to tight junctions and its separation from 14-3-3. Mol Biol Cell 30: 2377–2398, 2019. doi: 10.1091/mbc.E18-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreeva AY, Krause E, Müller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem 276: 38480–38486, 2001. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- 3.Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta 1778: 614–630, 2008. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc 4: 484–494, 2009. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 5.Caplan MJ, Seo-Mayer P, Zhang L. Epithelial junctions and polarity: complexes and kinases. Curr Opin Nephrol Hypertens 17: 506–512, 2008. doi: 10.1097/MNH.0b013e32830baaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cereijido M, Valdés J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol 60: 161–177, 1998. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 319: 1357–1364, 2013. doi: 10.1016/j.yexcr.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Choi W, Harris NJ, Sumigray KD, Peifer M. Rap1 and Canoe/afadin are essential for establishment of apical-basal polarity in the Drosophila embryo. Mol Biol Cell 24: 945–963, 2013. doi: 10.1091/mbc.e12-10-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citi S, Guerrera D, Spadaro D, Shah J. Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases 5: 1–15, 2014. doi: 10.4161/21541248.2014.973760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 11.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol 9: 715–720, 2009. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elloul S, Kedrin D, Knoblauch NW, Beck AH, Toker A. The adherens junction protein afadin is an AKT substrate that regulates breast cancer cell migration. Mol Cancer Res 12: 464–476, 2014. doi: 10.1158/1541-7786.MCR-13-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol 65: 1202–1207, 1991. doi: 10.1128/JVI.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106: 489–498, 2001. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 15.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci 1165: 113–120, 2009. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas AE, Capaldo CT, Nusrat A. Regulation of epithelial proliferation by tight junction proteins. Ann N Y Acad Sci 1258: 115–124, 2012. doi: 10.1111/j.1749-6632.2012.06556.x. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher SJ, Iqbal M, Jabbari S, Stekel D, Rappoport JZ. Analysis of occludin trafficking, demonstrating continuous endocytosis, degradation, recycling and biosynthetic secretory trafficking. PLoS One 9: e111176, 2014. doi: 10.1371/journal.pone.0111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, Takai Y. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J Cell Biol 166: 393–405, 2004. doi: 10.1083/jcb.200401093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayase J, Kamakura S, Iwakiri Y, Yamaguchi Y, Izaki T, Ito T, Sumimoto H. The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells. J Cell Biol 200: 635–650, 2013. doi: 10.1083/jcb.201208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133: 1–7, 2003. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 21.Honda T, Shimizu K, Kawakatsu T, Fukuhara A, Irie K, Nakamura T, Matsuda M, Takai Y. Cdc42 and Rac small G proteins activated by trans-interactions of nectins are involved in activation of c-Jun N-terminal kinase, but not in association of nectins and cadherin to form adherens junctions, in fibroblasts. Genes Cells 8: 481–491, 2003. doi: 10.1046/j.1365-2443.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 22.Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci 122: 1595–1606, 2009. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- 23.Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell 6: 845–854, 2004. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Iden S, Misselwitz S, Peddibhotla SS, Tuncay H, Rehder D, Gerke V, Robenek H, Suzuki A, Ebnet K. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol 196: 623–639, 2012. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol 146: 1117–1132, 1999. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol 13: 860–866, 2011. doi: 10.1038/ncb2274. [DOI] [PubMed] [Google Scholar]
- 27.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Cζ phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J 437: 289–299, 2011. doi: 10.1042/BJ20110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc 4: 1513–1521, 2009. doi: 10.1038/nprot.2009.154. [DOI] [PubMed] [Google Scholar]
- 29.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem 273: 30306–30315, 1998. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 30.Lee JO, Lee SK, Jung JH, Kim JH, You GY, Kim SJ, Park SH, Uhm KO, Kim HS. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. J Cell Physiol 226: 974–981, 2011. doi: 10.1002/jcp.22410. [DOI] [PubMed] [Google Scholar]
- 31.Lim S, Choi JW, Kim HS, Kim YH, Yea K, Heo K, Kim JH, Kim SH, Song M, Kim JI, Ryu SH, Suh PG. A myristoylated pseudosubstrate peptide of PKC-zeta induces degranulation in HMC-1 cells independently of PKC-zeta activity. Life Sci 82: 733–740, 2008. doi: 10.1016/j.lfs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Khaja AS, Bjartell A, Anderson KI, Malliri A. β2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol 14: 1169–1180, 2012. doi: 10.1038/ncb2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 1788: 872–891, 2009. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem 276: 9291–9296, 2001. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- 35.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4: 225–237, 2003. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 36.Mazanek M, Mituloviae G, Herzog F, Stingl C, Hutchins JR, Peters JM, Mechtler K. Titanium dioxide as a chemo-affinity solid phase in offline phosphopeptide chromatography prior to HPLC-MS/MS analysis. Nat Protoc 2: 1059–1069, 2007. doi: 10.1038/nprot.2006.280. [DOI] [PubMed] [Google Scholar]
- 37.Meller N, Irani-Tehrani M, Kiosses WB, Del Pozo MA, Schwartz MA. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol 4: 639–647, 2002. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- 38.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023, 2011. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda L, Carpentier S, Platek A, Hussain N, Gueuning MA, Vertommen D, Ozkan Y, Sid B, Hue L, Courtoy PJ, Rider MH, Horman S. AMP-activated protein kinase induces actin cytoskeleton reorganization in epithelial cells. Biochem Biophys Res Commun 396: 656–661, 2010. doi: 10.1016/j.bbrc.2010.04.151. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, Nusrat A, Parkos CA. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell 24: 2849–2860, 2013. doi: 10.1091/mbc.e13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon S, Han D, Kim Y, Jin J, Ho WK, Kim Y. Interactome analysis of AMP-activated protein kinase (AMPK)-α1 and -β1 in INS-1 pancreatic beta-cells by affinity purification-mass spectrometry. Sci Rep 4: 4376, 2014. doi: 10.1038/srep04376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat Protoc 1: 2650–2660, 2006. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 44.Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci 120: 2352–2365, 2007. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- 45.Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem 285: 5003–5012, 2010. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parsons M, Monypenny J, Ameer-Beg SM, Millard TH, Machesky LM, Peter M, Keppler MD, Schiavo G, Watson R, Chernoff J, Zicha D, Vojnovic B, Ng T. Spatially distinct binding of Cdc42 to PAK1 and N-WASP in breast carcinoma cells. Mol Cell Biol 25: 1680–1695, 2005. doi: 10.1128/MCB.25.5.1680-1695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radziwill G, Erdmann RA, Margelisch U, Moelling K. The Bcr kinase downregulates Ras signaling by phosphorylating AF-6 and binding to its PDZ domain. Mol Cell Biol 23: 4663–4672, 2003. doi: 10.1128/MCB.23.13.4663-4672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 7: 26–36, 2003. doi: 10.1016/S1091-255X(02)00090-2. [DOI] [PubMed] [Google Scholar]
- 49.Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20: 4652–4663, 2009. doi: 10.1091/mbc.e09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnittler H, Taha M, Schnittler MO, Taha AA, Lindemann N, Seebach J. Actin filament dynamics and endothelial cell junctions: the Ying and Yang between stabilization and motion. Cell Tissue Res 355: 529–543, 2014. doi: 10.1007/s00441-014-1856-2. [DOI] [PubMed] [Google Scholar]
- 51.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol 301: F1346–F1357, 2011. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9: 563–575, 2009. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695, 2008. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112: 63–75, 2003. doi: 10.1016/S0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 55.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 56.Shin YJ, Kim EH, Roy A, Kim JH. Evidence for a novel mechanism of the PAK1 interaction with the Rho-GTPases Cdc42 and Rac. PLoS One 8: e71495, 2013. doi: 10.1371/journal.pone.0071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CA, Lau KM, Rahmani Z, Dho SE, Brothers G, She YM, Berry DM, Bonneil E, Thibault P, Schweisguth F, Le Borgne R, McGlade CJ. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J 26: 468–480, 2007. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA 92: 6072–6076, 1995. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Théodore L, Derossi D, Chassaing G, Llirbat B, Kubes M, Jordan P, Chneiweiss H, Godement P, Prochiantz A. Intraneuronal delivery of protein kinase C pseudosubstrate leads to growth cone collapse. J Neurosci 15: 7158–7167, 1995. doi: 10.1523/JNEUROSCI.15-11-07158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K, Anderson JM. The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks. J Biol Chem 288: 13775–13788, 2013. doi: 10.1074/jbc.M113.466193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731, 2001. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 63.You GY, Lee JO, Kim JH, Kim N, Lee SK, Moon JW, Jie S, Lee HJ, Kim SJ, Park SH, Kim HS. Tiam-1, a GEF for Rac1, plays a critical role in metformin-mediated glucose uptake in C2C12 cells. Cell Signal 25: 2558–2565, 2013. doi: 10.1016/j.cellsig.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Jouret F, Rinehart J, Sfakianos J, Mellman I, Lifton RP, Young LH, Caplan MJ. AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3β (GSK-3β) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J Biol Chem 286: 16879–16890, 2011. doi: 10.1074/jbc.M110.186932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA 103: 17272–17277, 2006. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA 104: 819–822, 2007. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]