Abstract
A Western-style diet (WD; high in fat and carbohydrates) increases vascular oxidative stress. We hypothesized that vascular cells adapt to a WD by developing resilience to oxidative stress. Male and female C57BL/6J mice (4 wk of age) were fed a control diet (CD) or a WD for 16–20 wk. Superior epigastric arteries (SEAs; diameter, ~125 µm) were isolated and pressurized for study. Basal reactive oxygen species production was greatest in SEAs from males fed the WD. During exposure to H2O2 (200 μM, 50 min), propidium iodide staining identified nuclei of disrupted endothelial cells (ECs) and smooth muscle cells (SMCs). For mice fed the CD, death of SMCs (21%) and ECs (6%) was greater (P < 0.05) in SEAs from males than females (9% and 2%, respectively). WD consumption attenuated cell death most effectively in SEAs from males. With no difference at rest, H2O2 increased intracellular Ca2+ concentration ([Ca2+]i) to the greatest extent in SEAs from males, as shown by fura 2 fluorescence. Selective disruption of the endothelium (luminal air bubble) increased [Ca2+]i and SMC death during H2O2 exposure irrespective of sex; the WD reduced both responses most effectively in males. Nonselective transient receptor potential (TRP) channel inhibition (ruthenium red, 5 μM) attenuated the rise of [Ca2+]i, as did selective inhibition of TRP vanilloid type 4 (TRPV4) channels (HC-067047, 1 μM), which also attenuated cell death. In contrast, inhibition of voltage-gated Ca2+ channels (diltiazem, 50 μM) was without effect. Thus, for resistance arteries during acute oxidative stress: 1) ECs are more resilient than (and can protect) SMCs, 2) vessels from females are inherently more resilient than those from males, and 3) a WD increases vascular resilience by diminishing TRPV4 channel-dependent Ca2+ entry.
Keywords: endothelial cells, hydrogen peroxide, smooth muscle cells, TRP channels
INTRODUCTION
Western-style diets (WDs), which are high in saturated fats and refined carbohydrates, lead to obesity, insulin resistance, and type 2 diabetes. Vascular complications include arterial stiffening and endothelial dysfunction (10, 12, 33) associated with an increase in systemic oxidative stress (12, 33). Elevated levels of reactive oxygen species (ROS), as exemplified by H2O2, disrupt the integrity of both smooth muscle cells (SMCs) (27) and endothelial cells (ECs) (43, 48), while vascular SMCs and ECs from females appear to be more resilient to ROS than those from males (37, 45). During advanced age, chronic oxidative stress results in adaptations that protect SMCs and ECs of resistance arteries during acute exposure to H2O2 (37, 43) in a manner analogous to the effect of ischemic preconditioning (8). However, it is unknown whether the elevation of ROS associated with a WD (33) can protect vascular cell integrity during acute oxidative stress. Furthermore, little is known of how diet and sex may interact to affect vascular cell resilience to ROS.
Aberrant increases in cytosolic Ca2+ concentration ([Ca2+]i) lead to cell death by initiating apoptosis (24, 37, 43). Influx of Ca2+ from the extracellular fluid is the primary source of elevated [Ca2+]i in ECs and SMCs of mouse superior epigastric arteries (SEAs) (37, 43) and in SMCs from rat mesenteric arteries (50) exposed to ROS. However the ion channels effecting Ca2+ influx may differ among ROS subtypes, their prevailing concentration, and the duration of exposure (21, 50). A reduction of Ca2+ entry underscores the adaptation that protects vascular ECs and SMCs from aged mice during acute H2O2 exposure (37, 43), but it is unknown whether consumption of a WD promotes resilience to oxidative stress in a similar manner. Therefore, to test the hypothesis that consumption of a WD increases the resilience of vascular cells to H2O2, we studied isolated SEAs from male and female mice fed a control diet (CD) or WD for 16–20 wk. We evaluated the viability of ECs and SMCs of intact pressurized SEAs, the SMC monolayer following selective endothelial disruption, and intact endothelial tubes following dissociation of SMCs. Complementary experiments tested the hypothesis that cellular resilience to oxidative stress is associated with diminished Ca2+ entry. Because oxidative stress can activate transient receptor potential (TRP) channels (1, 11, 48, 56), we tested for their effect on [Ca2+]i during H2O2 exposure.
METHODS
Animal Care and Use
All experimental procedures were reviewed and approved by the Animal Care and Use Committee of the University of Missouri (Columbia, MO). Male and female C57BL/6J mice (4 wk old; purchased from Jackson Laboratories, Bar Harbor, ME) were fed the WD (calories: 46% fat, 17.5% sucrose, 17.5% high-fructose corn syrup, 19% protein; catalog no. 58Y1, TestDiet, Richmond, IN) (12, 32, 33) or the CD (calories: 17% fat, 56% carbohydrate, 27% protein; Formulab Diet 5008, LabDiet, St. Louis, MO) for 16–20 wk. Mice were housed on a 12:12-h light-dark cycle at ∼23°C, with fresh water and food available ad libitum. A total of 76 male and 42 female mice were used, with animals from each sex and diet studied in random order. When used in experiments, mice were 4–6 mo of age, which corresponds to humans in their third decade (18). On the morning of an experiment, a mouse was anesthetized with isoflurane (4%) for harvesting of tissues and then killed by exsanguination.
Preparation of Isolated Superior Epigastric Arteries
Abdominal skin was cut along the midline from sternum to pubis and retracted. The entire abdominal musculature was excised as an intact sheet and pinned onto a 50-mm-diameter × 8-mm-thick circular silicon rubber pad (Sylgard 184, Dow Corning, Midland, MI) immersed in chilled (4°C) Ca2+-free physiological salt solution [PSS, pH 7.4; in mM: 140 NaCl (Thermo Fisher Scientific, Waltham, MA), 5 KCl (Fisher Scientific), 1 MgCl2 (Sigma-Aldrich, St. Louis, MO), 10 HEPES (Sigma), and 10 glucose (Fisher Scientific)]. While the musculature was viewed under a stereomicroscope, an unbranched segment (∼2 mm long) of the SEA was dissected from surrounding tissue. Sodium nitroprusside (10 µM; catalog no. 431451, Sigma) was added to the PSS only during dissection to relax SMCs (37, 43, 44). Individual SEAs were cannulated onto heat-polished glass micropipettes (∼100 µm outer diameter) and secured with a strand of silk suture. The cannulated vessel was immersed in a tissue chamber (catalog no. RC-27N, Warner Instruments, Hamden, CT), superfused at 3 mL/min with PSS containing 2 mM CaCl2 (Fisher Scientific; hereafter referred to as “standard” PSS), and pressurized to 100 cmH2O (~75 mmHg). All experiments, including preincubation with dyes, were performed at 37°C unless stated otherwise.
Endothelial disruption.
To evaluate responses inherent to SMCs, the endothelium was disrupted by perfusion of an air bubble through the lumen of the cannulated vessel (15) before H2O2 exposure. Endothelial damage was verified by staining with propidium iodide (PI, 2 µM; catalog no. P4170, Sigma) (37), which stains nuclei of cells with disrupted membranes (15), or by lack of vasodilation [intact = 87 ± 4%, disrupted = 7 ± 6% (n = 6–7)] to acetylcholine (10 µM; catalog no. A6625, Sigma) added to the superfusion solution following preconstriction with norepinephrine (NE; catalog no. A7256, Sigma) at its EC50 (170 nM) (3). After endothelial disruption, the lack of SMC staining with PI and maintenance of constriction to NE confirmed SMC integrity and the selectivity of endothelial damage. Vessel preparations were then washed for 15 min in standard PSS before introduction of H2O2.
Endothelial tubes.
As described in detail elsewhere (44), isolated SEAs were placed in PSS containing 0.62 mg/mL papain (catalog no. P4762, Sigma), 1.0 mg/mL dithioerythritol (catalog no. D8255, Sigma), and 1.5 mg/mL collagenase (catalog no. C8051, Sigma) and incubated for 25 min at 33°C. Vessels were placed in Ca2+-free PSS and then transferred to a tissue chamber on the stage of a standard bench microscope (Zeiss GFL) for trituration to remove SMCs. Trituration pipettes were pulled from borosilicate glass capillary tubes [product no. 1B100-4, World Precision Instruments (WPI), Sarasota, FL], heat-polished to a tip internal diameter (ID) of ~100 µm, and connected to a Nanoliter injector (WPI) for reproducible aspiration and ejection of the vessel segment. During trituration, preparations were observed at ×200 optical magnification to ensure complete dissociation of SMCs (44). The intact, freshly isolated endothelial tube was secured to the bottom of the tissue chamber (a 24 × 54-mm coverslip) and extended to approximate in situ length using heat-blunted pipettes (~80 μm diameter) secured in micromanipulators at each end of the tissue chamber (43, 44).
Vascular ROS Production
To evaluate ROS production, intact pressurized SEAs were loaded with dihydrorhodamine 123 (DHR; catalog no. D632, Fisher Scientific), a membrane-permeant dye that converts to cationic rhodamine 123 upon oxidation and then localizes to mitochondria (25). DHR was dissolved in DMSO, diluted to 10 µM in PSS (final DMSO = 0.5%) (30), preincubated for 10 min in a static bath, and remained in the superfusion solution throughout the experiment. Fluorescence images were acquired for 35 ms at 5-min intervals for 30 min with an MV PLAPO ×1 objective [numerical aperture (NA) = 0.25; Olympus, Tokyo, Japan] coupled to a megapixel charge-coupled device (CCD) camera (XR/Mega10, Stanford Photonics, Palo Alto, CA) on an Olympus MVX10 microscope (final magnification ×63). Illumination was provided by an X-Cite illuminator (model no. 120, Excelitas Technologies, Waltham, MA) with excitation at 472/30 nm and emission at 525/35 nm. Fluorescence intensity was quantified with ImageJ (National Institutes of Health) in a 100 µm × 400 µm region of interest located in the middle of a vessel following subtraction of background fluorescence.
To more specifically evaluate H2O2 production, intact pressurized SEAs were loaded with the cytosolic ROS indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCFH; catalog no. C6827, Fisher Scientific) (43). The DCFH was dissolved in DMSO and diluted to 15 µM in PSS (final DMSO = 0.5%; referred to as “vehicle”), and a vessel was preincubated in this solution for 30 min without flow (43). Restoration of superfusion with standard PSS removed excess DCFH, and fluorescence was evaluated as described for DHR.
As a positive control for generating ROS, the mitochondrial complex III inhibitor antimycin A (catalog no. sc-2022467A, Santa Cruz Biotechnology, Dallas, TX) (7) was added to the superfusion solution at a final concentration of 10 μM. To verify the sensitivity of DHR and DCFH to endogenous ROS production, experiments were repeated following 10 min of preincubation with the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl [1 mM, TEMPOL; catalog no. 3082, Tocris, Bristol, UK) in combination with polyethylene glycol (PEG)-catalase (500 U/mL; catalog no. C4963, Sigma) (43); respective reagents were present throughout the experiment.
Cell Death
Cannulation pipettes were preloaded with PSS containing the membrane-permeant nuclear dyes Hoechst 33342 (1 µM; catalog no. H1399, Fisher Scientific) to identify all cells and PI to identify dead and dying cells (15, 37). Respective dyes were introduced into the vessel lumen upon cannulation. After equilibration for 30 min, the vessel was exposed to H2O2 (catalog no. H1009, Sigma) for 50 min by addition of 200 μM H2O2 to the superfusion solution (37); this H2O2 concentration is consistent with levels reported during ischemic events in the rat brain (22) and has been validated in previous studies (37, 43). Superfusion under identical conditions in the absence of H2O2 served as the negative control. Our preliminary studies showed that higher concentrations of H2O2 killed nearly all cells within several minutes, while lower concentrations required several hours to produce such effects and, thereby, infringed on the viability of isolated vessels at 37°C (37). After 50 min of H2O2 exposure, superfusion with standard PSS was restored, and the vessel lumen was perfused with standard PSS (0.1 mL/min, 10 min) containing the nuclear dyes. The vessel lumen was not perfused during the experiment, because luminal flow was associated with a reduction of cell death (37).
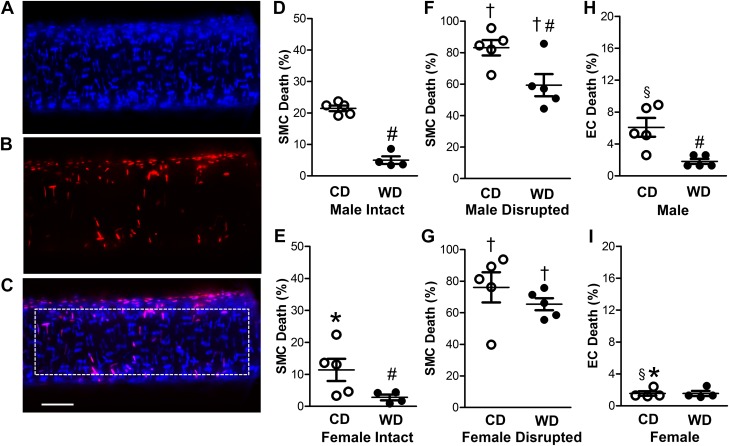
To evaluate cell death (37), fluorescence images of nuclear staining with Hoechst 33342 (blue) and PI (red) were acquired through appropriate filters using a water-immersion objective (×40 magnification, NA = 0.80) coupled to a DS-Qi2 camera with Elements software (version 4.51) on an E800 microscope (all from Nikon). Z stacks were acquired from the top half a vessel segment and analyzed using ImageJ software. Stained nuclei were counted manually within a defined region of interest (ROI, 150 × 500 µm; see Fig. 2C). Nuclei of ECs are oval-shaped and oriented parallel to the vessel axis, while SMC nuclei are thin and oriented perpendicular to the vessel axis (see Fig. 2, A–C). The small circular nuclei of interspersed adventitial cells were not counted.
Fig. 2.
Female sex and Western-style diet (WD) protect against H2O2-induced cell death in the arterial wall. A: Hoechst 33342 staining of nuclei from all cells in the wall of a SEA. B: propidium iodide staining of nuclei of dead cells in vessel wall. C: merged image of A and B. Dotted rectangle indicates region of interest for cell counts, which shows 14 dead and 94 total SMC nuclei with 3 dead and 59 total EC nuclei. Scale bar = 50 µm (applies to all images). D–G: percentage of dead SMCs for SEAs from male intact (D), female intact (E), male endothelium-disrupted (F), and female endothelium-disrupted (G) SEAs of mice fed the control diet (CD) or WD following exposure to H2O2 (200 µM, 50 min). For intact vessels, SMC death was greater in males than females and was reduced in WD vs. CD for both sexes. Endothelial disruption increased SMC death in all cases, with the protective effect of the WD persisting in males. H and I: EC death of intact SEAs was lower in females (I) than males (H), and the protective effect of the WD was manifest in males but not females. Note difference in y-axis scales across D–H and E–I. Values are means ± SE; n = 4–5 vessels per group. *P < 0.05, female vs. male. #P < 0.05, WD vs. CD. †P < 0.05, endothelium-disrupted vs. intact. §P < 0.05, EC vs. SMC of endothelium-disrupted within sex.
Ca2+ Photometry
A cannulated pressurized SEA was secured in a tissue chamber (as described above) and placed on an inverted microscope (Nikon Eclipse TS100). After superfusion at 37°C for 20 min with standard PSS, the vessel was incubated in fura 2-AM dye (catalog no. F14185, Fisher Scientific). The dye was dissolved in DMSO, diluted to 1 µM in PSS (final DMSO = 0.5%), and added to the tissue chamber to bathe the vessel for 40 min in the absence of flow. Superfusion with standard PSS was then resumed for 20 min to wash out excess dye. Fura 2 fluorescence was used to evaluate [Ca2+]i by alternately exciting the preparation at 340 and 380 nm while recording emissions at 510 nm through a ×20 objective (Nikon Fluor20; NA = 0.45) using IonWizard 6.3 software (IonOptix, Milford, MA) (36, 37). After fluorescence was recorded under baseline conditions (time 0), 200 µM H2O2 was added to the superfusion solution. Intracellular Ca2+ signals were measured for 30 s at 5-min intervals (to minimize photobleaching of fura 2 dye) during 50 min of H2O2 exposure and during the ensuing 30 min of superfusion with standard PSS. Under these conditions (dye loading from the bath), the [Ca2+]i signal originates primarily from SMCs (37).
Endothelial tubes.
For evaluation of [Ca2+]i responses in the endothelium, freshly isolated endothelial tubes were studied at 33°C to maintain their integrity (36, 43). An endothelial tube was incubated for 30 min with fura 2-AM dye in a static bath and then washed for 20 min during superfusion with standard PSS. After baseline ratio of fluorescence at 340 nm to fluorescence at 380 nm (F340/F380) was established, Ca2+ signals were measured for 30 s at 5-min intervals during 30 min of exposure to H2O2 and during 20 min of washout in standard PSS (43).
To test whether TRP channels contribute to the H2O2-induced Ca2+ response, the broad-spectrum TRP channel inhibitor ruthenium red (RuR; catalog no. R2751, Sigma) (5) was added to the superfusion solution (final concentration 5 µM with 0.1% DMSO) during a 20-min equilibration period following fura 2 loading (before H2O2 exposure) and maintained throughout the experiment (performed at 37°C for intact and endothelium-disrupted SEAs and at 33°C for endothelial tubes). In separate experiments, the TRP vanilloid type 4 (TRPV4) antagonist HC-067047 (catalog no. 4100, Tocris) (17) was added to the superfusion solution (final concentration 1 µM in 0.1% DMSO) for 40 min following fura 2 loading and maintained throughout the experiment. The extended equilibration time for HC-067047 was based on preliminary experiments performed to validate its effect. To test whether Ca2+ entry was a consequence of membrane depolarization, intact SEAs were treated with the L-type voltage-gated Ca2+ channel (VGCC) inhibitor diltiazem (50 µM; catalog no. 0685, Tocris) using the protocol described for RuR.
Vasoconstrictor Sensitivity
Changes in vessel ID were measured simultaneously with [Ca2+]i during H2O2 exposure using the edge-detection software of the IonOptix system. Images were acquired with a CCD camera. The initial maximal diameter was recorded in Ca2+-free PSS before beginning an experimental protocol. Superfusion with standard PSS was restored, and diameter was recorded for 30 s at 5-min intervals during and following H2O2 exposure. As a functional index of SMC viability, we evaluated adrenergic vasoconstriction to 170 nM NE (as described above) before and after 50 min of exposure to H2O2 + 30 min of washout in standard PSS.
Data Analysis
Values for DHR and DCFH fluorescence are expressed in arbitrary units for the change (Δ) from baseline within a ROI; linear regression was performed to determine the rate of fluorescence accumulation (43). Thus, ROS production was evaluated as follows:
Change (Δ) = (fluorescence at x min – fluorescence at 0 min), where x represents 5-min intervals during H2O2 exposure.
Rate is dF/dt, where F is fluorescence and t is time (min) during H2O2 exposure. Cell death in a ROI was calculated as follows:
% cell death = (no. of red nuclei/no. of blue nuclei) × 100. Vasomotor responses were calculated as follows:
% spontaneous baseline tone = [(IDmax − IDbase)/IDmax] × 100.
% peak tone during H2O2 = [(IDmax – IDmin)/IDmax] × 100.
% constriction to NE = [(IDbase – IDNE)/IDbase] × 100, where IDmax is ID under Ca2+-free conditions, IDbase is baseline ID during superfusion with standard PSS before addition of H2O2, IDmin is minimum ID during H2O2 exposure, and IDNE is ID during peak constriction to 170 nM NE before or following H2O2 exposure.
After subtraction of background fluorescence recorded before dye loading, [Ca2+]i values are expressed as the change in F340/F380 (Δ340/380) from baseline (0 min) at each 5-min interval.
Data were analyzed using unpaired Student’s t tests or ANOVA (Prism 5, GraphPad Software, La Jolla, CA) as appropriate. When significant main effects were detected with ANOVA, post hoc comparisons were performed using Bonferroni’s test. P < 0.05 was considered statistically significant. Summary data are presented as means ± SE, where n refers to the number of vessels (each from a different mouse) in an experimental group.
RESULTS
Effects of Sex and Diet on Vascular Oxidative Stress
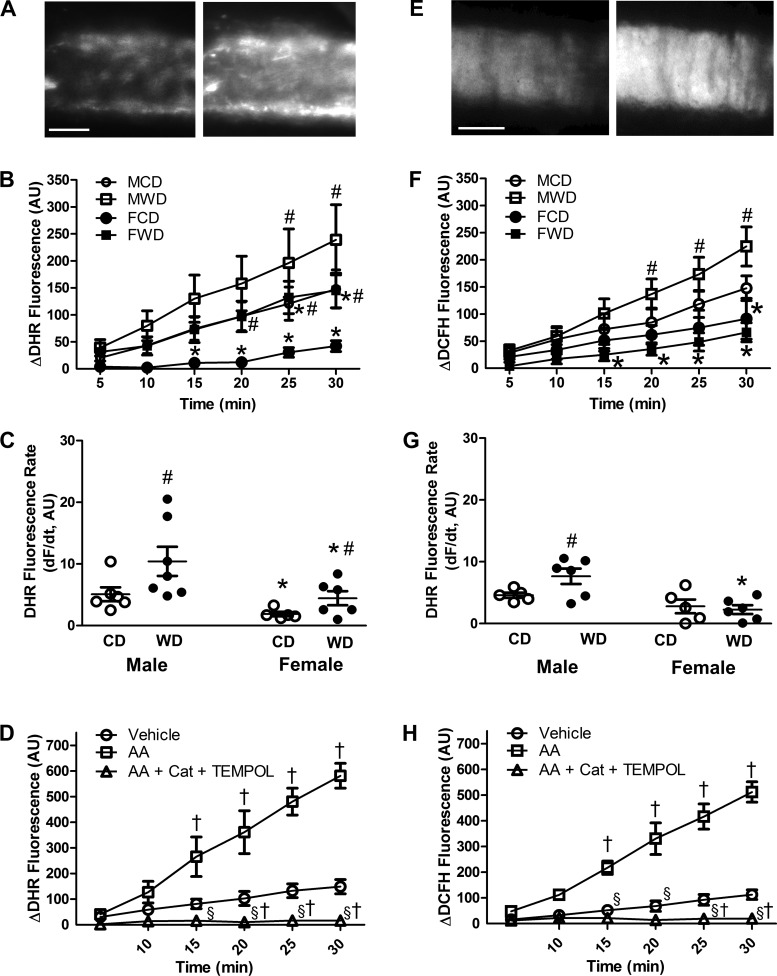
Consumption of a WD results in overnutrition, weight gain, vascular oxidative stress, and insulin resistance in mice (4, 12, 32, 33). In the present study, as shown by the accumulation of DHR fluorescence (Fig. 1A), ROS production at rest was significantly greater in intact pressurized SEAs from male than female mice (Fig. 1B). Furthermore, ROS production was increased by the WD in vessels from both sexes, with a mean rate that was approximately twofold greater in vessels from males than females (Fig. 1C). With DCFH, fluorescence accumulation (Fig. 1E) was also greater in vessels from males than females (Fig. 1F), and consumption of the WD elevated fluorescence accumulation in vessels from males (Fig. 1G). The difference in appearance of the fluorescence signal with respective dyes (Fig. 1A vs. Fig. 1E) is attributable to localization of DHR within mitochondria, while DCFH localizes in the cytosol (25).
Fig. 1.
ROS levels are greater in SEAs of male than female mice fed a Western-style diet (WD). A: representative image of initial (baseline) dihydrorhodamine 123 (DHR) fluorescence (left) and following 30 min of ROS accumulation (right) for intact pressurized SEA from a male mouse. B and C: summary data for changes in DHR fluorescence during 30 min and rate of DHR fluorescence accumulation [dF/dt, where F is fluorescence and t is time (min)]. WD augments ROS production in SEAs, which is greater in males than females. D: antimycin A (AA, 10 µM) applied to SEAs from male mice increased DHR fluorescence severalfold (note change in y-axis vs. y-axis in B); inclusion of PEG-catalase (Cat, 500 U/mL) and TEMPOL (1 mM) inhibited fluorescence accumulation. E: representative image of initial baseline 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCFH) fluorescence (left) and following 30 min of ROS accumulation (right) for an intact pressurized SEA from a male mouse. F and G: summary data for changes in DCFH fluorescence during 30 min and rate of DCFH fluorescence accumulation. WD augments H2O2 production in SEAs of males but not females. H: elevation of DCFH fluorescence antimycin A in SEAs from male mice was prevented by inclusion of PEG-catalase + TEMPOL (as shown in D). Values are means ± SE; n = 4–7 vessels per group. *P < 0.05, females vs. males receiving the same diet. #P < 0.05, WD vs. CD within the same sex. †P < 0.05 vs. vehicle. §P < 0.05 vs. AA alone. AU, arbitrary units; CD, control diet; MCD, male control diet; MWD, male Western-style diet; FCD, female control diet; FWD, female Western-style diet. In B, note overlap of data for MCD and FWD.
Stimulating ROS production with antimycin A increased fluorescence accumulation for both DHR (Fig. 1D) and DCFH (Fig. 1H). Both of these responses were blocked by the combination of TEMPOL and PEG-catalase, thereby validating respective optical sensors for ROS (particularly H2O2) production in our experiments. Arbitrary units of baseline fluorescence for DHR and DCFH were not significantly different (data not shown).
Arterial Cells of Females Are More Resilient to H2O2 than Those from Males
On average, each ROI contained ~120 SMC nuclei and ~80 EC nuclei, with no significant differences between sexes or diets. After 50 min of H2O2 exposure, SMC death in SEAs from females (Fig. 2E) was approximately half of that in vessels from males (Fig. 2D). For both sexes fed the CD, SMC death was severalfold greater than EC death (Fig. 2, H and I vs. Fig. 2, D and E), and EC death was severalfold greater for SEAs from males than females. Control experiments have verified that SEAs maintained under the same conditions in the absence of H2O2 exhibit minimal (<1%) cell death at the end of 50 min (37).
Western-Style Diet Increases Resilience of Vascular Cells to H2O2 Primarily in Males
In mice fed the WD vs. the CD, SMC death following H2O2 was reduced to ~5% vs. ~22% in vessels from males (Fig. 2D) and to ~3% vs. ~10% in vessels from females (Fig. 2E); EC death was reduced from ~7% to ~2% in males fed the WD (Fig. 2H) and was ~2% in females fed either diet (Fig. 2I). As observed for SMCs, ECs of vessels from females were inherently more resilient than those from males. Disruption of the endothelium increased H2O2-induced SMC death severalfold irrespective of sex or diet (Fig. 2, F and G vs. Fig. 2, D and E), with the protective effect of the WD on SMCs persisting in vessels from males. Control experiments have verified that, under the same conditions in the absence of H2O2, endothelial disruption does not result in PI staining of SMC nuclei (37).
Western-Style Diet Attenuates the Rise of Vascular [Ca2+]i Induced by H2O2
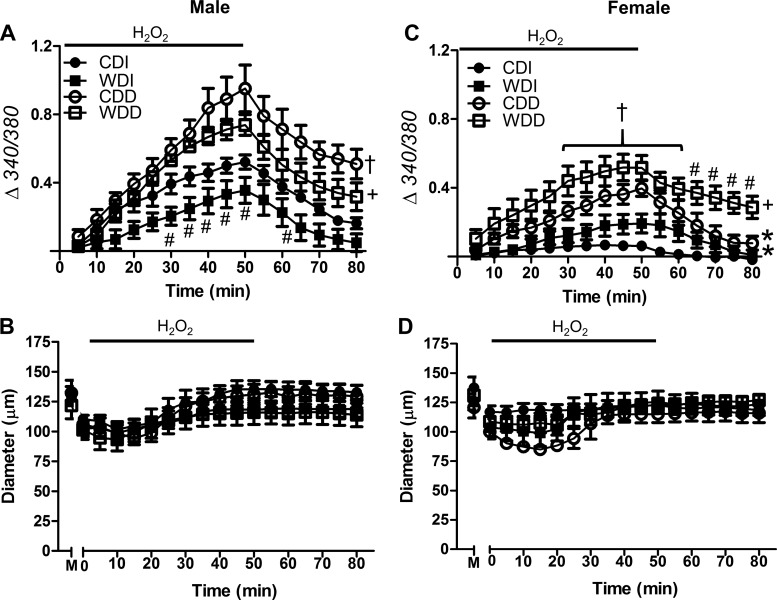
Excessive [Ca2+]i initiates cell death (24, 37, 43). We therefore assessed [Ca2+]i during and following H2O2 exposure. For intact SEAs from males fed the CD, [Ca2+]i increased progressively during H2O2 exposure and recovered during washout (Fig. 3A). Under the same conditions, the [Ca2+]i response to H2O2 was lower in intact SEAs from females than males fed the CD (Fig. 3C). WD consumption significantly reduced the [Ca2+]i response to H2O2 in vessels from males but not females. Consistent with its effect on cell death, endothelial disruption augmented [Ca2+]i responses to H2O2 in vessels irrespective of sex or diet. During the 30-min washout of H2O2, [Ca2+]i remained above baseline in endothelium-disrupted arteries from males irrespective of diet and in vessels from females fed the WD.
Fig. 3.
Effect of H2O2 exposure on cytosolic Ca2+ concentration ([Ca2+]i) in the vessel wall. A and C: [Ca2+]i responses with fura 2 (Δ340/380) during 50-min exposure to H2O2 (200 μM) followed by 30-min washout in standard PSS. Data are for intact and endothelium-disrupted SEAs from male (A) and female (C) mice fed the control diet (CD) or Western-style diet (WD). Compared with the CD, the WD reduced the [Ca2+]i response to H2O2 primarily in males; endothelial disruption increased [Ca2+]i responses in all groups and impaired recovery for each group except females fed the CD. B and D: internal diameters were not different between intact and endothelium-disrupted SEAs from males (B) or females (D). M, maximal diameter in Ca2+-free PSS. Values are means ± SE; n = 4–8 vessels per group. #P < 0.05, WD vs. respective CD. †P < 0.05, endothelium-disrupted vs. -intact within CD. +P < 0.05, endothelium-disrupted vs. -intact within WD. *P < 0.05, female vs. male for CDI and CDD. CDI, CD intact endothelium; WDI, WD intact endothelium; CDD, CD disrupted endothelium; WDD, WD disrupted endothelium.
In the absence of H2O2, vessel diameters, spontaneous tone, and [Ca2+]i remained constant throughout the ~90-min protocol (37) with no significant differences in baseline [Ca2+]i between groups (for reference, baseline F340/F380 of intact SEAs from males fed the CD = 0.83 ± 0.04; n = 7). After equilibration with standard PSS, spontaneous baseline tone averaged ~15% for intact SEAs across all groups (Table 1), with an average baseline ID of 111 ± 4 µm (at time 0). Exposure to H2O2 typically produced a transient vasoconstriction, with diameter remaining stable thereafter (Fig. 3, B and D).
Table 1.
Basal and peak tone during H2O2 exposure
| Male |
Female |
|||
|---|---|---|---|---|
| Treatment | Baseline | Peak | Baseline | Peak |
| CD intact | 16 ± 3 | 35 ± 3 | 15 ± 3 | 18 ± 3 |
| WD intact | 16 ± 3 | 26 ± 6 | 17 ± 3 | 27 ± 5 |
| CD disrupted | 18 ± 3 | 29 ± 7 | 14 ± 2 | 31 ± 4 |
| WD disrupted | 19 ± 3 | 26 ± 7 | 16 ± 5 | 27 ± 7 |
| RuR CD intact | 11 ± 2 | 12 ± 3 | 10 ± 2 | 14 ± 2 |
| RuR WD intact | 11 ± 3 | 12 ± 4 | 8 ± 2 | 17 ± 7 |
| HC CD intact | 15 ± 4 | 24 ± 7 | 13 ± 4 | 15 ± 6 |
| HC WD intact | 18 ± 3 | 23 ± 5 | 16 ± 2 | 24 ± 4 |
Values (%) are means ± SE; n = 4–6 vessels per group. Summary data for vasomotor tone: initial baseline and peak during H2O2 exposure. RuR, ruthenium red; HC, HC-067047.
Endothelial Protection of Adrenergic Vasoconstriction
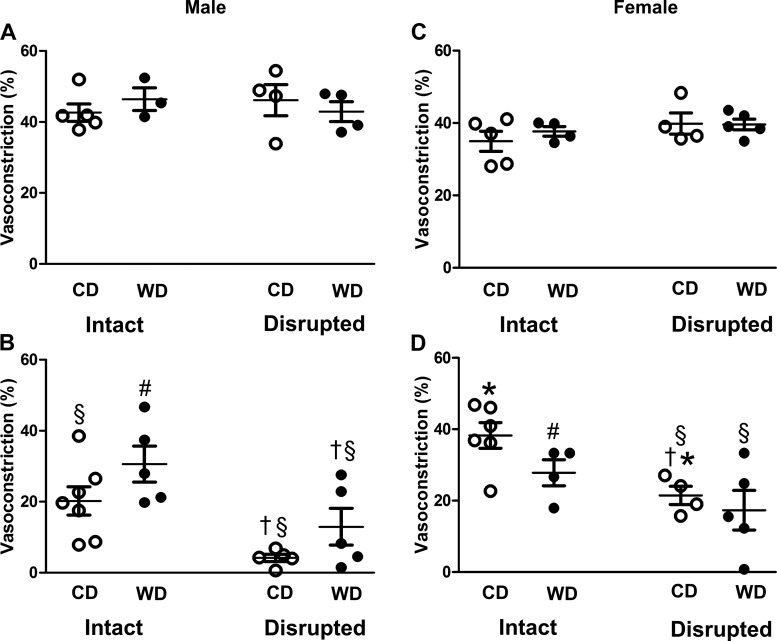
To evaluate the resilience of SMC function to acute oxidative stress, we assessed vasoconstriction to the adrenergic neurotransmitter NE before and after H2O2 exposure. Before H2O2 exposure, NE constricted all vessels by ~40–50% (Fig. 4, A and C). After 50 min of H2O2 exposure + 30 min of washout, vasoconstriction was blunted to a greater extent in intact vessels from male mice fed the CD than intact vessels from male mice fed the WD (Fig. 4B). Vasoconstriction of intact vessels from female mice fed either diet was not significantly different following H2O2 exposure (Fig. 4D). For vessels from both sexes, endothelial disruption before H2O2 exposure reduced adrenergic vasoconstriction following H2O2 exposure.
Fig. 4.
Integrity of endothelium protects adrenergic vasoconstriction. A–D: vasoconstrictor responses to NE (170 nM) before (A and C) and after (B and D) H2O2 exposure in intact and endothelium-disrupted SEAs from male (A and B) and female (C and D) mice fed the control diet (CD) or Western-style diet (WD). Values are means ± SE; n = 4–7 vessels per group. #P < 0.05, WD vs. respective CD. †P < 0.05, endothelium-disrupted vs. -intact. *P < 0.05, female vs. male. §P < 0.05, post- vs. pre-H2O2 exposure.
Mechanisms of Ca2+ Entry
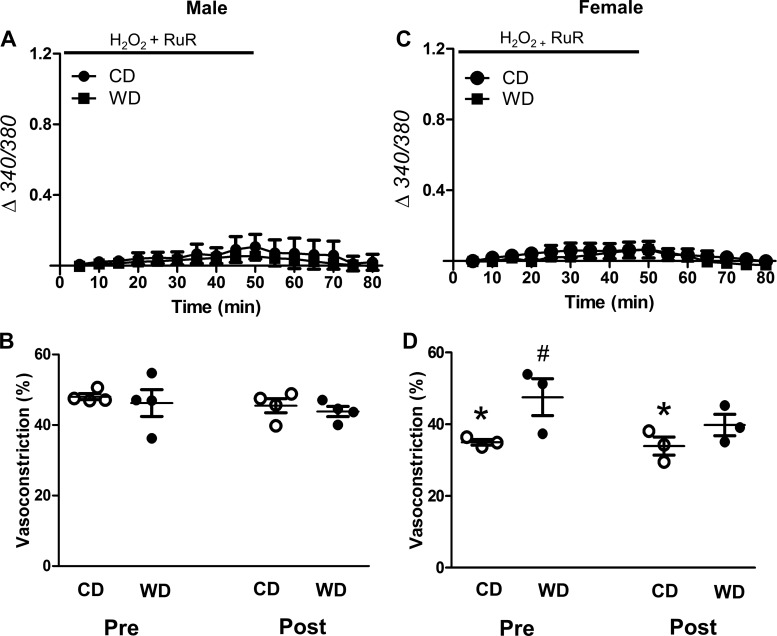
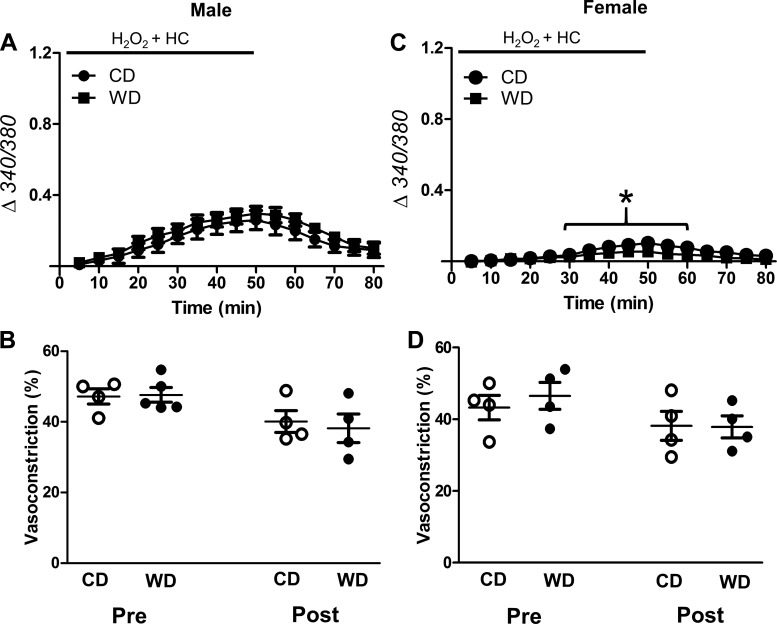
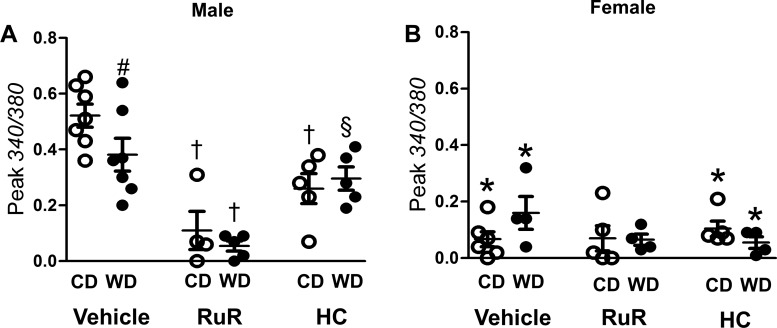
Oxidative stress activates multiple TRP channel isoforms (1, 11, 48, 56). We therefore tested whether H2O2 promotes Ca2+ entry by activating TRP channels. RuR (5 μM), a broad-spectrum TRP channel blocker, inhibited [Ca2+]i responses to H2O2 for SEAs from all experimental groups (Fig. 5, A and C) and preserved adrenergic vasoconstriction (Fig. 5, B and D). Specific inhibition of TRPV4 with HC-067047 (1 μM) eliminated the difference in [Ca2+]i responses between CD- and WD-fed groups (Fig. 6, A and C; cf. Fig. 3, A and C) and preserved adrenergic vasoconstriction (Fig. 6, B and D). In vessels from males, the effect of HC-067047 on peak [Ca2+]i was less than that of RuR (Fig. 7A; cf. Figs. 6A and 5A).
Fig. 5.
Ruthenium red (RuR) blocks H2O2-induced Ca2+ entry and preserves adrenergic vasoconstriction. A and C: changes in vessel wall [Ca2+]i with fura 2 (Δ340/380) in H2O2-exposed intact superior epigastric arteries from male (A) and female (C) mice fed the control diet (CD) or Western-style diet (WD) in the presence of RuR (5 μM). Baseline cytosolic Ca2+ concentration was not different between groups. B and D: vasoconstriction to norepinephrine [NE, 170 nM (EC50)] pre- and post-H2O2 exposure in vessels from males (B) and females (D). RuR prevented transient constriction to H2O2 and preserved vasoconstriction to NE. Vessel diameters averaged across experimental groups remained relatively constant throughout these experiments: 131 ± 7 µm (initial) and 121 ± 7 µm (final). Values are means ± SE; n = 3–5 vessels per group. #P < 0.05, WD vs. CD in female. *P < 0.05, female CD vs. male CD.
Fig. 6.
TRPV4 channel inhibition attenuates H2O2-induced Ca2+ entry and preserves adrenergic vasoconstriction. A and C: changes in vessel wall [Ca2+]i of intact SEAs exposed to H2O2 from male (A) and female (C) mice fed the control diet (CD) or Western-style diet (WD) in the presence of HC-067047 (HC, 1 μM). There were no differences in baseline [Ca2+]i between groups. B and D: vasoconstriction to NE (170 nM) was preserved following H2O2 exposure with HC present for intact vessels from males (B) and females (D) irrespective of diet. Values are means ± SE; n = 4–5 vessels per group. *P < 0.05, female vs. male.
Fig. 7.
Effects of TRP channel inhibitors on [Ca2+]i responses to H2O2. A and B: peak increases in fura 2 signal (340/380) during H2O2 exposure in the presence of vehicle (0.1% DMSO), ruthenium red (RuR; from Fig. 4), and HC-067047 (HC; from Fig. 5) in SEAs from males (A) and females (B). Values are means ± SE; n = 4–8 vessels per group. #P < 0.05, WD vs. CD. *P < 0.05, female vs. male. †P < 0.05, inhibitor vs. vehicle in males. §P < 0.05, HC vs. RuR for WD in males.
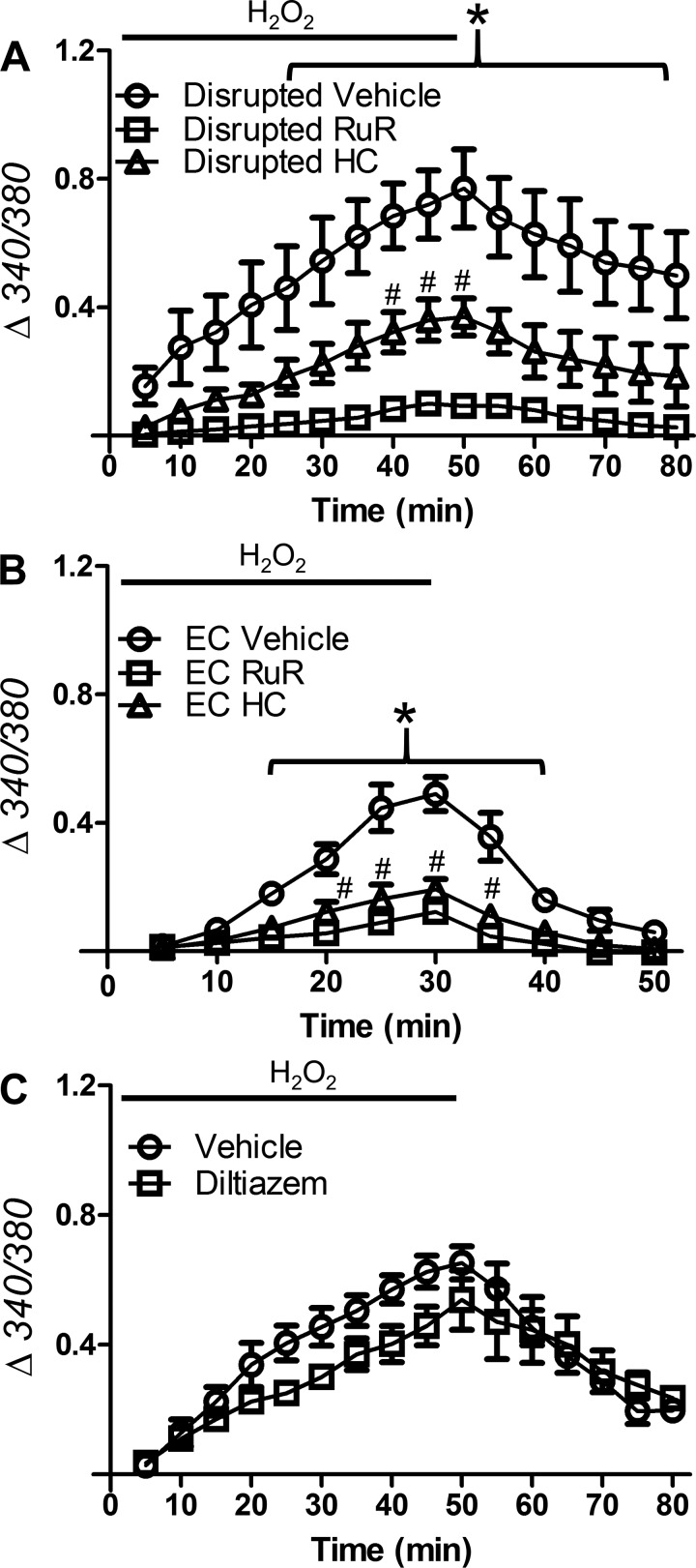
Of the four groups studied, SEAs from male mice fed the CD experienced the most cell death from H2O2 exposure. Therefore, vessels from males were used to evaluate the effect of our pharmacological interventions on cell type-specific [Ca2+]i responses to H2O2. These experiments were performed with endothelium-disrupted SEAs to resolve the response of SMCs and with endothelial tubes to resolve the response of ECs. We found that RuR nearly eliminated the rise of [Ca2+]i for both SMCs (Fig. 8A) and ECs (Fig. 8B); HC-067047 had a similar effect in ECs and an intermediate effect in SMCs. Thus, inhibition of TRPV4 channels was more effective in preventing the [Ca2+]i response to H2O2 in ECs than SMCs.
Fig. 8.
TRP channels mediate Ca2+ entry into SMCs and ECs during H2O2 exposure. A and B: [Ca2+]i responses of fura 2 (Δ340/380) to H2O2 in SMCs of endothelium-disrupted SEAs (A) or freshly isolated endothelial tubes (B) from male mice fed the control diet (CD) in the presence of vehicle (DMSO, 0.1%), ruthenium red (RuR; 5 μM), or HC-067047 (HC; 1 µM). RuR prevented the rise of [Ca2+]i for both cell types; HC had a similar effect in ECs and an intermediate effect in SMCs. C: for intact SEAs, blocking L-type voltage-gated Ca2+ channels with diltiazem (50 μM) had no significant effect on H2O2-induced Ca2+ entry. Values are means ± SE; n = 3–4 vessels per group. #P < 0.05, HC vs. vehicle. *P < 0.05, RuR vs. vehicle.
Opening TRP channels can depolarize cell membranes via nonselective cation entry (19). To test whether the vessel wall [Ca2+]i response to H2O2 was mediated through activation of VGCCs in SMCs (35), intact SEAs from male mice fed the CD were exposed to H2O2 in the presence of diltiazem (50 µM). Baseline [Ca2+]i before the addition of H2O2 was not different between conditions (F340/F380 = 0.72 ± 0.04 with vehicle and 0.67 ± 0.08 with diltiazem). Unlike RuR or HC-067047, diltiazem had no significant effect on vessel wall [Ca2+]i during H2O2 exposure (Fig. 8C). In separate experiments, diltiazem (50 μM) prevented SEA constriction to 100 mM KCl (77 ± 5% with vehicle and 2 ± 1% with diltiazem, n = 3).
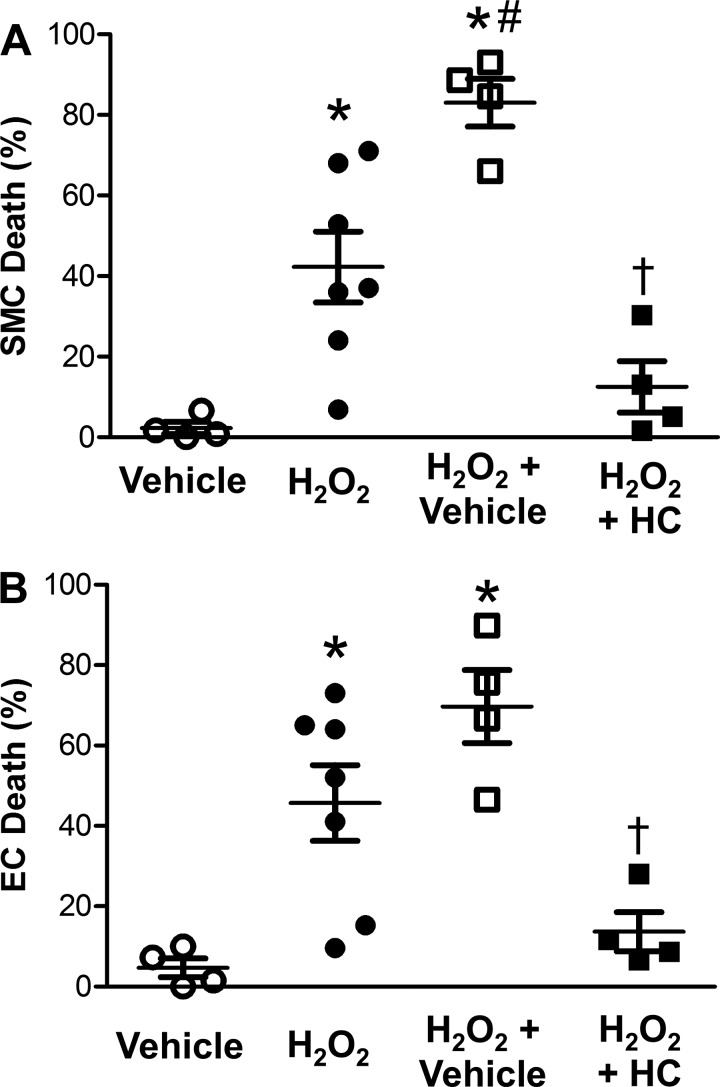
Finding that TRPV4 inhibition reduced [Ca2+]i responses to the greatest extent in SEAs from male mice fed the CD (Fig. 7), we tested whether inhibition of TRPV4 would protect cells from H2O2-induced death. Exposure to vehicle (0.1% DMSO in PSS) alone produced negligible cell death (Fig. 9). Cell death induced by H2O2 exposure was increased in the presence of DMSO, particularly in SMCs. Consistent with a role for TRPV4-dependent Ca2+ entry in cell death, the presence of HC-067047 reduced the death of both SMCs and ECs exposed to H2O2 (Fig. 9).
Fig. 9.
TRPV4 channels contribute to cell death induced by H2O2. Percentage of dead SMCs (A) and ECs (B) in intact SEAs from male mice fed the CD following 50-min exposure to H2O2 in the presence of vehicle (DMSO, 0.1%), H2O2, H2O2 + vehicle, or H2O2 + vehicle + HC-067047 (HC, 1 µM). Values are means ± SE; n = 4–7 vessels per group. *P < 0.05, H2O2 treatments vs. vehicle. #P < 0.05, H2O2 + vehicle vs. H2O2. †P < 0.05, H2O2 + HC vs. H2O2 + vehicle.
Cell death in SEAs from mice fed the CD was ~50% less in the present experiments than in our previous study in which we assessed the effects of advanced age (37). Because the mice used for these two studies were housed in separate facilities, this difference in control values emphasizes the importance of using animals that are housed under identical conditions across experimental groups within a given study.
DISCUSSION
Key findings in the present study are as follows: 1) WD elevates basal ROS levels in small resistance arteries; 2) in the intact vessel wall, SMCs are more susceptible than ECs to death induced by H2O2; 3) ECs protect SMCs during H2O2 exposure; 4) vessels of females are more resilient than vessels of males; and 5) WD increases resilience (primarily in SMCs) with diminished Ca2+ influx through TRP(V4) channels. We propose that resistance arteries, particularly those of males, develop resilience to oxidative stress during WD intake as an adaptation that preserves cellular structure and function to maintain blood flow regulation.
Oxidative stress and cell death are key events in the pathogenesis of vascular disease (13, 40, 47). However, little was known of whether SMCs or ECs differ in susceptibly to injury induced by acute oxidative stress, particularly in the wall of intact blood vessels. Nor was it known how sex may interact with diet to affect vascular cell resilience to oxidative stress. For resistance arteries of mouse skeletal muscle, the present findings demonstrate that SMCs are far more susceptible than ECs to disruption by H2O2, particularly when mice are fed the CD. In contrast, consumption of the WD (high in fat and refined carbohydrates) for 16–20 wk led to elevated ROS (particularly H2O2) levels in the vessel wall under resting conditions and was associated with greater resilience of vascular cells (especially SMCs) to H2O2 exposure. This protective effect of the WD was greater in vessels of males than females, because vessels from females were intrinsically more resilient to H2O2. Irrespective of sex or diet, cell death resulting from H2O2 exposure was associated with activation of Ca2+ entry through TRP channels, particularly TRPV4. Thus, for mice fed the CD, [Ca2+]i during H2O2 exposure increased to a greater extent in SEAs from males than females. While WD consumption diminished H2O2-induced cell death (primarily for SMCs) in SEAs from both sexes, WD-induced resilience to H2O2 was greater in vessels from males. In each case, greater protection from cell death was associated with a corresponding reduction of [Ca2+]i, thereby attenuating the induction of apoptosis (37).
Effects of Sex and Diet on Oxidative Stress and Cellular Resilience in Resistance Arteries
Basal ROS production for individual SEAs was greater in vessels from male than female mice (Fig. 1). Consistent with effects of a WD in large conduit arteries such as the aorta (26), consumption of the WD increased ROS production in small resistance arteries, particularly those of males. Western-style diets contribute to obesity, diabetes, and heart failure (31). The increase in oxidative stress is linked to arterial stiffening and impaired vasodilation with increased risk of cardiovascular disease (10, 12, 33, 53). A paradigm shift of the present findings is that adaptation to a WD protects vessels from ROS-induced damage.
Acute exposure to H2O2 initiates vascular cell death (27, 37, 43, 48). For intact SEAs from mice fed the CD, cell death induced by H2O2 was lower in females than males (Fig. 2). These findings are consistent with estrogen-dependent protection of both SMCs (34) and ECs (45). The greater resilience of vascular cells in females was associated with attenuated elevation of [Ca2+]i during H2O2 exposure (Fig. 3). Additional mechanisms of protection in females include elevated levels of antioxidants, increased expression of the antiapoptotic protein Bcl-2, and reduced activation of caspase-3 (6, 51, 52). These respective mechanisms are not mutually exclusive and may work synergistically. As shown for SEAs of aged vs. young mice, elevated levels of H2O2 were associated with a reduction of catalase activity (43). In turn, the present findings suggest that greater constitutive ROS production in vessels from males fed the WD underlies the adaptation that protects cells during acute H2O2 exposure. Thus, while cells in the vascular wall are intrinsically more resilient to oxidative stress in females than males (Fig. 2, D and E), the WD induced greater protection in males. This difference in protection between sexes in mice parallels the disproportionate incidence and severity of cardiovascular disease resulting from obesity and insulin resistance in women compared with men (38, 54).
SMCs were more susceptible than ECs to the adverse effects of H2O2, and disruption of the endothelium increased SMC lethality, even while the protective effect of the WD persisted in males (Fig. 2). These data confirm the protective effect of ECs in attenuating SMC death during H2O2 exposure (37). Thus, while EC integrity protects SMCs in vessels from both sexes, the finding that SMC death was no longer reduced in endothelium-disrupted arteries from female vs. male mice suggests that the enhanced protection from H2O2 in females is mediated via the endothelium. This effect is not mediated by nitric oxide (NO), because NO reacts with superoxide to form cytotoxic peroxynitrite under the conditions of these experiments (37). Nevertheless, other endothelium-derived gaseous molecules, e.g., carbon monoxide and hydrogen sulfide, can exert protective effects on vascular cells (28, 29) and may have contributed here; such protective effects of myoendothelial coupling through gap junctions have been reported as well (37). Functionally, WD consumption helped maintain adrenergic vasoconstriction following H2O2 exposure, particularly in arteries from males (Fig. 4). Thus, resilience to oxidative stress preserves vasomotor function in concert with cellular integrity of the vessel wall.
H2O2-Dependent Ca2+ Entry: Role of TRP Channels
In the present experiments, the extent of cell death was associated with an elevation of [Ca2+]i, which can increase mitochondrial Ca2+ content to trigger cytochrome c release and, thereby, initiate apoptosis (16, 37). Our recent findings illustrate that the rise of [Ca2+]i during H2O2 exposure is nearly abolished in the absence of extracellular Ca2+, pointing to Ca2+ entry as a key mediator of cell death induced by H2O2 (37). The progressive increases in [Ca2+]i during H2O2 exposure were attenuated in vessels from females vs. males and by consumption of the WD in males (Fig. 3) with corresponding reductions in cell death (Fig. 2). Consistent with its role in promoting SMC survival, integrity of the endothelium limited H2O2-induced Ca2+ entry in the vessel wall irrespective of sex or dietary regimen.
TRP channels contain six transmembrane domains with multiple sites for regulation and protein-protein interactions (9, 39). Consistent with a role for TRP channels in mediating Ca2+ entry during H2O2 exposure, the pan-TRP inhibitor RuR nearly abolished [Ca2+]i responses of SEAs exposed to H2O2 irrespective of sex or diet (Fig. 5). The TRPV4 isoform elicits H2O2-dependent Ca2+ influx in pulmonary microvascular endothelium (49), while blocking TRPV4 reduces apoptosis induced by ischemia-reperfusion in the myocardium (14). In SEAs from males fed the CD, which were those most adversely affected by H2O2, SMC and EC death was greatly attenuated by TRPV4 inhibition (Fig. 9), as were [Ca2+]i responses (cf. Figs. 3A and 6A). As shown for HEI-OC1 cells, elevated glucose concentration can lead to a reduction of TRPV4 expression (55), which may explain why Ca2+ entry during H2O2 exposure was reduced by the WD. However, because TRP channels form heteromers within cell membranes, inhibition of one channel subtype may have complex interactions with other subtypes, including alterations in trafficking and electrophysiological properties (9, 39, 46). Such interactions, along with our finding that TRPV4 inhibition with HC-067047 does not attenuate H2O2-induced Ca2+ influx to the same extent as pan-TRP inhibition with RuR (Fig. 7A), suggest that other TRP channel isoforms contribute to Ca2+ influx. These may include TRPM2, TRPA1, and TRPV1, which can also be activated by oxidative stress (1, 11, 39, 48, 56). Additional TRP channels, including TRPC1, TRPC3, and TRPC7, are implicated in apoptosis of cardiomyocytes and SMCs (23, 41, 42) and, thus, may also contribute to the H2O2-induced Ca2+ entry and cell death observed here.
Because TRP channels are expressed in both SMCs and ECs (23, 48, 57), we tested the effect of their inhibition in SMCs following endothelial disruption and in ECs of endothelial tubes. In each cell layer of SEAs from males, which exhibited the greatest rise of [Ca2+]i to H2O2, RuR eliminated this response (Fig. 8). Whereas HC-067047 was similarly effective in ECs, its intermediate effect in SMCs suggests variability in TRP isoform expression/activation compared with ECs. The nonselectivity of TRP channels to cations enables membrane depolarization, which can activate L-type VGCCs (35). In testing for such a role, we found that inhibition of L-type VGCCs had no significant effect on the [Ca2+]i response to H2O2 (Fig. 8C). These data lend additional support to the idea that TRP channels serve as the principal mediators of Ca2+ entry and are consistent with the lack of VGCC involvement in ROS-dependent death of human intestinal SMCs (2). Nevertheless, the differences observed here in cell death between sexes and diets are not fully linked to differences in [Ca2+]i responses. For example, intact vessels from female mice succumbed to cell death during H2O2 exposure (Fig. 2) despite exhibiting a relatively modest [Ca2+]i response compared with intact vessels from male mice (Fig. 3). Given the release of cytochrome c during H2O2 exposure to initiate intrinsic apoptosis (37), the effects of sex and diet on the role of mitochondria in ROS-dependent cell death are prime directions for future studies.
Conclusion
In resistance arteries of adult mice, this study demonstrates that sex and diet profoundly affect the resilience of SMCs and ECs during acute exposure to oxidative stress imposed by H2O2. Whereas both SMCs and ECs in SEAs from females are intrinsically more resilient than those from males, WD consumption increased resilience to a greater extent in vessels from males. The differences observed here between sexes are associated with constitutively elevated levels of ROS (i.e., chronic oxidative stress) in vessels of males, with vascular ROS production increased by WD consumption irrespective of sex. Inherent to the mechanism of adaptation to the WD is a reduction of Ca2+ entry through TRP channels during H2O2 exposure. As shown by the increase in SMC death following disruption of ECs, integrity of the endothelium is integral to protecting SMCs during acute exposure to ROS. When integrated with recent findings that advanced age and loss of IL-10 (both of which are associated with elevated ROS) protect the intima and media from H2O2-induced Ca2+ entry and cell death (37, 43), we propose that resilience to acute oxidative stress reflects adaptation to the prevailing level of ROS. In such manner, constitutively elevated oxidative stress can protect the vasculature during acute exposure to ROS analogous to ischemic preconditioning (8). Clinical events exhibiting such conditions include ischemic stroke, myocardial infraction, and organ transplantation. Remarkably, the incidence of ischemic stroke is reduced in men consuming high-fat (i.e., Western-style) diets (20). The apparently beneficial effects of a WD on the vascular wall represent a paradigm shift and contrast with the adverse effects of a WD on insulin resistance and obesity, which increase the risk for cardiovascular disease (31). While we do not advocate WD consumption, the preservation of vasoconstrictor sensitivity following H2O2 exposure illustrates that developing resilience to oxidative stress maintains the integrity of vascular function in resistance arteries and, thereby, preserves their ability to control tissue blood flow.
GRANTS
This research was supported by American Heart Association Grant 19TPA34850102 (S. S. Segal) and National Heart, Lung, and Blood Institute Grants 5K08 HL-129074 (C. Manrique-Acevedo), R01 HL-142770 (C. Manrique-Acevedo), and R37 HL-041026 (S. S. Segal).
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.E.N., C.M.-A., and S.S.S. conceived and designed research; C.E.N., N.L.J., and S.Y.S. performed experiments; C.E.N., N.L.J., S.Y.S., and S.S.S. analyzed data; C.E.N., N.L.J., S.Y.S., C.M.-A., and S.S.S. interpreted results of experiments; C.E.N. prepared figures; C.E.N. drafted manuscript; C.E.N., N.L.J., S.Y.S., C.M.-A., and S.S.S. edited and revised manuscript; C.E.N., N.L.J., S.Y.S., C.M.-A., and S.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Matthew Martin and Rebecca Shaw provided expert technical assistance with experimental protocols.
The data supporting the findings of this study are available upon request from the corresponding author.
REFERENCES
- 1.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28: 2485–2494, 2008. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Whiteis CA, Sharma RV, Abboud FM, Conklin JL. Reactive oxygen species and calcium homeostasis in cultured human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 272: G1439–G1450, 1997. doi: 10.1152/ajpgi.1997.272.6.G1439. [DOI] [PubMed] [Google Scholar]
- 3.Boerman EM, Segal SS. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J Physiol 594: 2323–2338, 2016. doi: 10.1113/JP270710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 15: 798–808, 2007. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 5.Cahusac PM. Effects of transient receptor potential (TRP) channel agonists and antagonists on slowly adapting type II mechanoreceptors in the rat sinus hair follicle. J Peripher Nerv Syst 14: 300–309, 2009. doi: 10.1111/j.1529-8027.2009.00242.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Delannoy M, Odwin S, He P, Trush MA, Yager JD. Enhanced mitochondrial gene transcript, ATP, Bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes. Toxicol Sci 75: 271–278, 2003. doi: 10.1093/toxsci/kfg183. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 8.Chlopicki S, Lomnicka M, Gryglewski RJ. Reversal of the postischaemic suppression of coronary function in perfused guinea pig heart by ischaemic preconditioning. J Physiol Pharmacol 50: 605–615, 1999. [PubMed] [Google Scholar]
- 9.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci 2: 387–396, 2001. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 10.Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ, Jaffe IZ. Sex-specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 7: e007675, 2018. doi: 10.1161/JAHA.117.007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DelloStritto DJ, Connell PJ, Dick GM, Fancher IS, Klarich B, Fahmy JN, Kang PT, Chen YR, Damron DS, Thodeti CK, Bratz IN. Differential regulation of TRPV1 channels by H2O2: implications for diabetic microvascular dysfunction. Basic Res Cardiol 111: 21, 2016. doi: 10.1007/s00395-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Díez J, Fortuño MA, Ravassa S. Apoptosis in hypertensive heart disease. Curr Opin Cardiol 13: 317–326, 1998. doi: 10.1097/00001573-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Dong Q, Li J, Wu QF, Zhao N, Qian C, Ding D, Wang BB, Chen L, Guo KF, Fu D, Han B, Liao YH, Du YM. Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Sci Rep 7: 42678, 2017. doi: 10.1038/srep42678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. doi: 10.1161/01.RES.86.1.94. [DOI] [PubMed] [Google Scholar]
- 16.Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol 38: 713–721, 2002. doi: 10.1016/S0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 17.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flurkey K, Currer J, Harrison D. The mouse in aging research. In: The Mouse in Biomedical Research (2nd ed.), edited by Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL.. Burlington, MA: Elsevier, 2007, p. 637–672. [Google Scholar]
- 19.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2: a003962, 2010. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA 278: 2145–2150, 1997. doi: 10.1001/jama.1997.03550240035030. [DOI] [PubMed] [Google Scholar]
- 21.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol 6: 260–271, 2015. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res 671: 181–186, 1995. doi: 10.1016/0006-8993(94)01291-O. [DOI] [PubMed] [Google Scholar]
- 23.Ingueneau C, Huynh UD, Marcheix B, Athias A, Gambert P, Nègre-Salvayre A, Salvayre R, Vindis C. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J Cell Mol Med 13, 8B: 1620–1631, 2009. doi: 10.1111/j.1582-4934.2008.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 29: 859–870, 1997. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 25.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer B, França LM, Zhang Y, Paes AMA, Gerdes AM, Carrillo-Sepulveda MA. Western diet triggers Toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am J Physiol Heart Circ Physiol 315: H1735–H1747, 2018. doi: 10.1152/ajpheart.00218.2018. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Li W, Su J, Liu W, Altura BT, Altura BM. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: possible relation to neurodegenerative diseases and strokes. Brain Res Bull 62: 101–106, 2003. doi: 10.1016/j.brainresbull.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Teng X, Jin S, Dong J, Guo Q, Tian D, Wu Y. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. J Hypertens 37: 1633–1643, 2019. doi: 10.1097/HJH.0000000000002101. [DOI] [PubMed] [Google Scholar]
- 29.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 55: 396–405, 2002. doi: 10.1016/S0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 30.Malorni W, Straface E, Matarrese P, Ascione B, Coinu R, Canu S, Galluzzo P, Marino M, Franconi F. Redox state and gender differences in vascular smooth muscle cells. FEBS Lett 582: 635–642, 2008. doi: 10.1016/j.febslet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci 92: 601–608, 2013. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology 154: 3632–3642, 2013. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Martinez-Lemus LA, Ramirez-Perez FI, Klein T, Meininger GA, DeMarco VG. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol 15: 94, 2016. doi: 10.1186/s12933-016-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maselli A, Matarrese P, Straface E, Canu S, Franconi F, Malorni W. Cell sex: a new look at cell fate studies. FASEB J 23: 978–984, 2009. doi: 10.1096/fj.08-114348. [DOI] [PubMed] [Google Scholar]
- 35.Nelson MT, Standen NB, Brayden JE, Worley JF III. Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature 336: 382–385, 1988. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 36.Norton CE, Segal SS. Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP channel activation. Am J Physiol Lung Cell Mol Physiol 315: L212–L226, 2018. doi: 10.1152/ajplung.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton CE, Sinkler SY, Jacobsen NL, Segal SS. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J Physiol 597: 3801–3816, 2019. doi: 10.1113/JP278255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JS, Nam JS, Cho MH, Yoo JS, Ahn CW, Jee SH, Lee HS, Cha BS, Kim KR, Lee HC. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause 17: 779–784, 2010. doi: 10.1097/gme.0b013e3181cd3d60. [DOI] [PubMed] [Google Scholar]
- 39.Pires PW, Earley S. Redox regulation of transient receptor potential channels in the endothelium. Microcirculation 24: e12329, 2017. doi: 10.1111/micc.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rössig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol 96: 11–22, 2001. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 41.Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, Mori Y, Makino N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem 294: 205–215, 2007. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- 42.Shan D, Marchase RB, Chatham JC. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am J Physiol Cell Physiol 294: C833–C841, 2008. doi: 10.1152/ajpcell.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL, Segal SS. Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J Physiol 593: 2155–2169, 2015. doi: 10.1113/JP270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Socha MJ, Segal SS. Isolation of microvascular endothelial tubes from mouse resistance arteries. J Vis Exp 81: e50759, 2013. doi: 10.3791/50759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spyridopoulos I, Sullivan AB, Kearney M, Isner JM, Losordo DW. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor. Circulation 95: 1505–1514, 1997. doi: 10.1161/01.CIR.95.6.1505. [DOI] [PubMed] [Google Scholar]
- 46.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001. doi: 10.1016/S0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 47.Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51: 978–992, 2011. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun L, Yau HY, Wong WY, Li RA, Huang Y, Yao X. Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. PLoS One 7: e43186, 2012. [Erratum in PLoS One 7: 43186, 2012.] doi: 10.1371/journal.pone.0043186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suresh K, Servinsky L, Reyes J, Baksh S, Undem C, Caterina M, Pearse DB, Shimoda LA. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am J Physiol Lung Cell Mol Physiol 309: L1467–L1477, 2015. doi: 10.1152/ajplung.00275.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol 44: 200–208, 2004. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol 66: 1411–1419, 2006. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 52.Viña J, Borrás C, Gambini J, Sastre J, Pallardó FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett 579: 2541–2545, 2005. doi: 10.1016/j.febslet.2005.03.090. [DOI] [PubMed] [Google Scholar]
- 53.Virdis A, Neves MF, Duranti E, Bernini G, Taddei S. Microvascular endothelial dysfunction in obesity and hypertension. Curr Pharm Des 19: 2382–2389, 2013. doi: 10.2174/1381612811319130006. [DOI] [PubMed] [Google Scholar]
- 54.Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, Williams B, Davies MJ. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 53: 1190–1198, 2010. doi: 10.1007/s00125-010-1689-9. [DOI] [PubMed] [Google Scholar]
- 55.Xing Y, Ming J, Liu T, Zhang N, Zha D, Lin Y. Decreased expression of TRPV4 channels in HEI-OC1 cell induced by high glucose is associated with hearing impairment. Yonsei Med J 59: 1131–1137, 2018. doi: 10.3349/ymj.2018.59.9.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ 13: 1815–1826, 2006. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- 57.Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L. Role of TRP channels in the cardiovascular system. Am J Physiol Heart Circ Physiol 308: H157–H182, 2015. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]