Abstract
Septate junctions (SJs) are occluding cell-cell junctions that have roles in paracellular permeability and barrier function in the epithelia of invertebrates. Arthropods have two types of SJs, pleated SJs and smooth SJs (sSJs). In Drosophila melanogaster, sSJs are found in the midgut and Malpighian tubules, but the functions of sSJs and their protein components in the tubule epithelium are unknown. Here we examined the role of the previously identified integral sSJ component, Mesh, in the Malpighian tubule. We genetically manipulated mesh specifically in the principal cells of the tubule at different life stages. Tubules of flies with developmental mesh knockdown revealed defects in epithelial architecture, sSJ molecular and structural organization, and lack of urine production in basal and kinin-stimulated conditions, resulting in edema and early adult lethality. Knockdown of mesh during adulthood did not disrupt tubule epithelial and sSJ integrity but decreased the transepithelial potential, diminished transepithelial fluid and ion transport, and decreased paracellular permeability to 4-kDa dextran. Drosophila kinin decreased transepithelial potential and increased chloride permeability, and it stimulated fluid secretion in both control and adult mesh knockdown tubules but had no effect on 4-kDa dextran flux. Together, these data indicate roles for Mesh in the developmental maturation of the Drosophila Malpighian tubule and in ion and macromolecular transport in the adult tubule.
Keywords: drosokinin, Drosophila, Malpighian tubule, Mesh, paracellular permeability, smooth septate junctions
INTRODUCTION
In multicellular organisms, epithelia form sheets that cover external and internal surfaces, serving as barriers and regulating the passage of solutes and water between compartments. Transepithelial solute and water transport can occur through the transcellular pathway (i.e., across cells) or the paracellular pathway (i.e., between cells). The permeability of the paracellular pathway in the epithelia of vertebrates is controlled by tight junctions (TJs; 1, 22, 24, 27). Invertebrate epithelia generally lack TJs and instead possess septate junctions (SJs) as their occluding junctions (32). SJs form circumferential belts around the lateral regions of epithelial cells and, in cross-section electron microscopy, display a ladderlike structure between adjacent cells with septa spanning a 15–20-nm intercellular space (26). SJs are subdivided into several morphological variants that exist across invertebrate phyla, and some animals possess multiple types of SJs depending on the developmental origin of the epithelial cells (26, 33). In arthropods, two types of SJs have been described: pleated (pSJs) and smooth SJs (sSJs). In tangentially cut sections, pSJs reveal undulating rows of septa, whereas septa in the sSJs show regularly spaced parallel lines (26, 38, 47). pSJs are observed in ectodermally derived epithelia such as the epidermis, foregut, hindgut, trachea, and salivary glands, whereas sSJs are found in endodermally derived midgut and gastric ceca (37, 69). sSJs are also present in Malpighian tubules, although their epithelial cells are ectodermal and mesodermal derivatives (16, 18, 37).
More than 20 pSJ-associated proteins have been identified in Drosophila melanogaster (15, 30, 33). pSJs consist of transmembrane and cytoplasmic scaffolding proteins, linked to the actin/spectrin cytoskeleton, and loss-of-function mutations in most of these genes disrupt epithelial barrier function and tracheal tube morphogenesis [for review, see Izumi and Furuse (30) and Jonusaite et al. (33)]. Furthermore, the pSJ-associated scaffolding proteins Discs large (Dlg), Lethal giant larvae, and Scribble are involved in the establishment of epithelial polarity and regulation of cell growth and proliferation as Drosophila tumor suppressors (5, 6, 12, 43, 76). These observations suggest that pSJ components play an important role during epithelial morphogenesis in addition to forming paracellular barriers. Compared with pSJs, far less is known about the molecular architecture and function of sSJs. In Drosophila, only three transmembrane proteins, Snakeskin (Ssk), Mesh, and tetraspanin 2A (Tsp2A), have been shown to localize to the sSJs in the midgut and Malpighian tubules (23, 31, 32, 81). Ssk is a protein with four membrane-spanning domains and appears to be conserved within arthropod species (81). Mesh is a single-pass transmembrane protein with a large extracellular region containing a nidogen-like domain (NIDO domain), an Ig-like E set domain, an adhesion-associated domain in mucin-4 and other proteins (AMOP domain), a von Willebrand factor type D domain (vWD domain), and a Sushi domain, and has orthologs in other invertebrates such as Caenorhabditis elegans and sea urchin as well as mouse (31, 67). Ectopic expression of Mesh in cultured Drosophila S2 cells that lack SJs induces cell-cell adhesion, suggesting that it may work as an adhesion molecule at sSJs (31). Tsp2A belongs to the tetraspanin family of proteins, which have four transmembrane domains and are widely conserved in metazoans (32). Ssk, Mesh, and Tsp2A form a complex with each other and show mutually dependent localization at sSJs in larval and adult Drosophila midgut (23, 29, 31, 32). Compromised expression of ssk, mesh, or tsp2A causes ultrastructural defects in larval midgut sSJs, such as reduced septa and frequent intercellular gaps (31, 32, 81). Furthermore, first-instar larvae with RNA interference (RNAi)-mediated reduction of ssk, mesh, or tsp2A show leakage of a 10-kDa fluorescent tracer from the midgut to the body cavity, suggesting that these proteins play a crucial role in the intestinal barrier function in larval Drosophila (31, 32, 81). More recent studies have demonstrated the necessity of Mesh, Ssk, and Tsp2A for the maintenance of midgut barrier function and homeostasis in adult flies (29, 57, 79, 80). However, the contribution of sSJ proteins to the morphology and physiological function of insect Malpighian tubules remains unknown.
Similar to the vertebrate kidney, insect Malpighian tubules play an essential role in the maintenance of internal homeostasis, achieved through regulated transepithelial ion and water transport (54). Adult Drosophila have four Malpighian tubules, a longer anterior pair and a shorter posterior pair, which join the alimentary canal through common ureters (65). The tubules are divided into four morphological segments and are made up of large principal cells and thin intercalating stellate cells (65). Primary urine is generated in the main segment by isosmotic transepithelial secretion of a KCl-rich fluid. K+ is transported through the principal cell, whereas transcellular Cl− flux occurs through stellate cells and is stimulated by kinin peptide hormones and tyramine (7, 8, 10, 11, 19, 21, 41, 48, 50). Studies of ion and water transport mechanisms in Drosophila Malpighian tubules have focused on transcellular pathways. Experiments in other insect Malpighian tubules suggest important roles for the paracellular pathway as well. For example, the Malpighian tubules of Rhodnius prolixus are permeable to paracellular permeability markers, such as sucrose, inulin, and polyethylene glycol (PEG) of ~4,000 Da, and have higher permeability to negatively charged molecules than positively charged ones (49, 66). In the Malpighian tubules of the adult yellow fever mosquito, Aedes aegypti, the sSJs are permselective to Cl− (4, 51). This junctional Cl− permeability provides a paracellular pathway for Cl−, allowing Cl− to serve as a counterion for Na+ and K+ secreted across the epithelium by energy-dependent transcellular pathways (3). Paracellular Cl− permeability in adult A. aegypti tubules increases in the presence of a diuretic peptide hormone, leucokinin, which in turn increases transepithelial Na+, K+, and fluid secretion (51, 82, 83). It has also been shown that leucokinin changes the Malpighian tubule of adult A. aegypti from a moderately tight epithelium (58 Ω·cm2) to a leaky epithelium (10 Ω·cm2) and increases paracellular permeability to inulin and sucrose (51, 73). A recent study demonstrated that the Drosophila Malpighian tubule is also moderately tight and that Drosophila leucokinin (drosokinin) results in a chloride-dependent decrease in transepithelial resistance (2). Additional recent studies in larval insects have demonstrated that changes in sSJ protein transcript abundance correlate with alterations in the paracellular permeability of the Malpighian tubules, but the functions of individual sSJ components in this process have not been examined (34, 36).
The goal here was to investigate whether there is a role for Mesh in epithelial morphogenesis and ion and water transport in the Drosophila Malpighian tubule. We used Drosophila transgenics to induce mesh knockdown in a cell- and time-specific manner. We found that mesh knockdown throughout development in the tubule principal cells leads to compromised urine production and early lethality in young adult flies. The tubules of these flies exhibit defects in epithelial cell and sSJ architecture. Furthermore, we demonstrate that knockdown of mesh during adulthood in the principal cells reduces tubule transepithelial fluid and ion transport and lumen-positive voltage as well as paracellular macromolecule permeability. These data indicate that Mesh is essential for the development and maintenance of a functional Drosophila Malpighian tubule.
MATERIALS AND METHODS
Fly stocks and genetics.
Fly rearing and crosses were done on a 12:12-h light-dark cycle on standard cornmeal/yeast/molasses food (prepared in a central kitchen at the University of Utah). The following Drosophila melanogaster strains were used: w−Berlin; w;c42-GAL4, expressing GAL4 in the principal cell of the Malpighian tubule (55; obtained from Julian Dow and Shireen Davies, University of Glasgow, Glasgow, United Kingdom) and outcrossed for five generations to the Rodan laboratory w−Berlin; w;tub-GAL80ts20, a temperature-sensitive GAL4 repressor, allowing temporal manipulation of GAL4 activity (45; obtained from Bloomington Drosophila Stock Center (BDSC), Indiana University, Bloomington, IN, stock no. 7019); w;UAS-meshRNAi, which drives expression of interfering RNA against Drosophila mesh [31; obtained from Fly Stocks of National Institutes of Genetics, Japan, stock no. NIG12074-R1, and line 6867, obtained from the Vienna Drosophila Resource Center (VDRC), Vienna, Austria, Fig. 1]; and w;tsh04319, which drives lacZ expression under the control of a teashirt (tsh) enhancer (18; obtained from BDSC, stock no. 11370). Crosses for mesh knockdown during development were performed at 18 and 28°C. Crosses for mesh knockdown in adulthood using tub-GAL80ts20 were performed at 18°C, and 1-day-old female flies were shifted to 28°C for 14 days before experimentation. In all crosses, 25 virgin female and 25 male flies of desired genotype were set up in a bottle, with care taken to prevent overcrowding. For the electrophysiological measurements (performed in Osnabrück), crosses of 10 virgin females with 10 males were performed in vials at 18°C, and 1-day-old female flies were shifted to 27°C for 14–28 days before experimentation. Heterozygous GAL4 and upstream activation sequence (UAS) controls were obtained by crossing GAL4-containing males to w−Berlin virgins (Osnabrück stock) or UAS-containing virgins to w−Berlin males. For Tsh-LacZ immunostaining experiments (performed in Japan), crosses of 20 virgin females with 10 males were performed in vials at 28°C.
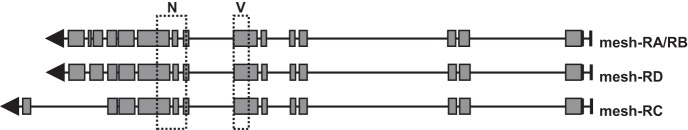
Fig. 1.
Diagram depicting mesh transcripts and target sites of the two different RNA interference lines used in this study. The introns are shown as solid lines, and the exons are shown as rectangular boxes. The arrows indicate the direction of transcription. The target regions of the RNAi lines are shown as dashed boxes for National Institute of Genetics (N) and Vienna Drosophila Resource Center (V). The Malpighian tubules express three splice variants of mesh as predicted by FlyAtlas 2 (40).
Abdominal volume measurement.
Seven-day-old adult female flies of desired genotype (21 flies per genotype) were anesthetized with CO2, and the length of cross-sectional and longitudinal axes of their abdomens was measured with an ocular micrometer. The volume of the abdomen was calculated assuming it to be a spheroid as follows: abdominal volume = (4/3π)a2c, where a and c are the radii of cross-sectional and longitudinal axes of the abdomen, respectively.
Immunofluorescence microscopy.
Immunohistochemistry was performed according to previously described protocols (34, 35). Briefly, whole guts (with Malpighian tubules still attached) of nonwandering third-instar larvae, 1-day-old or 14-day-old adult female flies of desired genotype (5–15 samples per genotype) were dissected in Drosophila saline (composition in mM: 117.5 NaCl, 20 KCl, 2 CaCl2, 8.5 MgCl2, 10.2 NaHCO3, 4.3 NaH2PO4, 15 HEPES, and 20 glucose, pH 7.0) and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 h at room temperature (RT). Fixed tissues were thoroughly washed in PBS and blocked for 2 h at RT with 10% antibody dilution buffer (ADB; 10% goat serum, 3% BSA in PBS) and 0.3% Triton X-100. The tissues were then thoroughly rinsed in PBS and incubated overnight at 4°C with primary antibodies. The following antibodies diluted in ADB with 0.05% Triton X-100 were used: rabbit anti-Mesh (1:1,000; 31), rabbit anti-Ssk (1:1,000; 81), rabbit anti-Tsp2A (1:1,000; 32), mouse anti-Dlg [1:50; 4F3, Developmental Studies Hybridoma Bank (DSHB); 52], mouse anti-Na+/K+-ATPase (NKA; 1:10; a5, DSHB; 60), and rat anti-secretory chloride channel (secCl; 1:1,000; a kind gift from Dr. Joseph A. Dent, Department of Biology, McGill University, Montreal, QC, Canada; 21). As negative controls, tissues were incubated overnight at 4°C in ADB alone. Following incubation, samples were washed in PBS and probed for 2 h at RT with either Alexa Fluor 594-conjugated goat anti-rabbit (111-585-003; Jackson ImmunoResearch Laboratories), Alexa Fluor 488-conjugated sheep anti-mouse (515-545-003; Jackson ImmunoResearch Laboratories), DyLight 650-conjugated goat anti-rabbit (84546; Thermo Scientific), or Alexa Fluor 647-conjugated donkey anti-rat (150155; Abcam) secondary antibodies at 1:400 or 1:1,000. To visualize F-actin, samples were incubated for 2 h at RT in 0.5 µM Alexa Fluor 568-conjugated phalloidin (A12380; Invitrogen). Tissues were rinsed in PBS and mounted in ProLong Gold Antifade reagent (Invitrogen). Images were acquired using a Nikon A1R laser-scanning confocal microscope with its accompanying NIS-Elements multiplatform acquisition software. All images were assembled using Adobe Photoshop CS2 software.
For immunostaining of Tsh-LacZ, 1-day-old adult female flies of desired genotype (3 samples per genotype) were dissected in Hanks’ balanced salt solution, and the Malpighian tubules were fixed with 4% paraformaldehyde in PBS-0.2% Tween 20 for 30 min at RT. The fixed specimens were washed three times with PBS-0.4% Triton X-100 and blocked with 5% skim milk in PBS-0.2% Tween 20. Thereafter, the samples were incubated with anti-rabbit β-galactosidase antibody (55976; Cappel/ICN/MP Biomedicals) at 1:1,000 overnight at 4°C. Following incubation, samples were washed in PBS-0.2% Tween 20 and probed for 3 h at RT with Alexa Fluor 488-conjugated donkey anti-rabbit (A21206; Thermo Fisher, Waltham, MA) secondary antibody at 1:400. Nuclei were stained for DNA with TOTO-3 (T3604; Thermo Fisher, Waltham, MA). After another three washes, the samples were mounted in Fluoro-KEEPER (12593-64; Nakalai Tesque, Kyoto, Japan). Images were acquired with a confocal microscope (model TCSSPE; Leica Microsystems, Wetzlar, Germany) using its accompanying software and HC PLAN Apochromat ×20 numerical aperture (NA) 0.7 and HCX PL objective lenses (Leica Microsystems).
Electron microscopy.
Pairs of anterior Malpighian tubules of 1-day-old (developmental knockdown) or 14-day-old (adult knockdown) adult female flies of desired genotype (3 samples per genotype) were dissected in Drosophila saline and fixed overnight at 4°C in 2.5% glutaraldehyde in 50 mM cacodylate buffer. Following fixation, tubules were washed in cacodylate buffer and post-fixed in 2% osmium tetroxide for 1 h at RT. The tubules were then washed in cacodylate buffer and stained with 4% uranyl acetate for 0.5 h at RT. Subsequently, tubules were rinsed in distilled water, transferred to 50% ethanol and infiltrated with 2% agarose to prevent them from breaking. The samples were dehydrated in a graded ethanol series (1 × 15 min in 50%, 1 × 15 min in 70%, 2 × 15 min in 95%, and 4 × 15 min in 100% ethanol) and acetone (3 × 15 min). Following dehydration, samples were infiltrated overnight at RT with 1:1 solution of Embed 812 resin [Electron Microscopy Sciences (EMS)] and acetone. The samples were further embedded in 3:1 mixture of resin-acetone for 8 h and 100% resin for 2 days. The tubules were sectioned at 60-nm thickness using Leica UC 6 ultramicrotome (Leica Microsystems, Vienna, Austria) and mounted on Formvar and carbon-coated 2 × 1-mm slot copper grids (EMS). The sections were stained with saturated uranyl acetate for 20 min and with lead citrate for 10 min. Tubule main segment sections were viewed using a Hitachi 7100 transmission electron microscope and photographed using a Gatan digital camera. Images were assembled using ImageJ.
Measurement of transepithelial fluid secretion and K+ flux.
The Malpighian tubule secretion assay and measurement of secreted fluid K+ concentration using ion-specific electrodes was performed as previously described (19, 59). Briefly, pairs of anterior tubules of 1-day-old (developmental knockdown) or 14-day-old (adult knockdown) adult female flies of desired genotype (22–38 tubules per genotype) were dissected in Drosophila saline and transferred to a dish with individual 20-µL drops of standard bathing medium [SBM; 1:1 mixture of Drosophila saline and Schneider’s medium (Life Technologies)] suspended in water-saturated mineral oil (Fisher BioReagents). After 2 h, the diameter of secreted fluid droplets was measured for the determination of volume and rate of fluid secretion (fluid droplet volume per secretion time). Levels of K+ in the droplets of secreted fluid were measured using a liquid state K+-selective microelectrode as described previously (59). Briefly, ion-specific and reference electrodes were prepared using unfilamented and filamented, respectively, borosilicate glass capillaries (Harvard Apparatus, Holliston, MA). Pipettes were pulled to a tip size of 1–2 μm using a vertical pipette puller (Narishige, East Meadow, NY). Ion-selective electrodes were silanized with vapors of dichlorodimethylsilane (Sigma) at 300°C for 1 h. K+-selective electrodes were back-filled with 0.5 M KCl and front-loaded via negative pressure with a short column length of potassium ionophore I cocktail B (Sigma). To prevent displacement of the ionophore cocktail by the mineral oil, microelectrodes were dipped in a solution of polyvinylchloride (PVC; Fluka) in tetrahydrofuran (Fluka), before use. The reference electrode was back-filled with 3 M KCl. To determine K+ activity, calibration drops consisting of 15, 75, 150, and 200 mM KCl were measured by immersing the reference and ion-specific electrodes into the fluid drop and recording the potential using a Digidata 1200 amplifier (Axon Instruments, Union City, CA) and a FD223a dual-channel electrometer (World Precision Instruments, Sarasota, FL). Slope change in K+ concentration was calculated using the Nernst equation for the difference between 15 and 150 mM, 75 and 150 mM, and 150 and 200 mM, and the average slope was then calculated. K+ activity in the experimental fluid was measured, and concentration was calculated using the following equation: [K+]e = [K+]c × 10(V/s), where [K+]e is the potassium concentration of the experimental drop, [K+]c is the potassium concentration of the calibration drop, V is the change in potential (mV) between the experimental drop and the calibration drop, and s is the slope (mV) for a 10-fold change in K+ concentration, determined by measurements from the calibration drops. Transepithelial K+ flux was calculated by multiplying fluid secretion rate by K+ concentration.
Measurement of transepithelial FITC-dextran flux and permeability.
Fluorescently labeled dextran has been used to quantify paracellular permeability in Drosophila midgut (31, 42, 81) and other insect Malpighian tubules (36). To examine dextran permeability in Drosophila Malpighian tubules, anterior tubules of 14-day-old adult female flies of desired genotype (31–34 tubules per genotype) were set up for secretion assay as described above (see materials and methods, Measurement of transepithelial fluid secretion and K+ flux) and bathed in SBM containing 2.5 mg/mL fluorescein isothiocyanate-labeled dextran (FITC-dextran, 3–5 kDa; Sigma) for 4 h. Following this, the droplets of secreted fluid were collected and sized using an ocular micrometer. Droplets were then added to 50 μL of SBM. Samples were loaded into a 96-well polystyrene black plate (VWR International, LLC) along with 50-µL standards diluted in SBM. The fluorescence intensity of each sample was measured using Synergy HTX Multi-Mode microplate reader (BioTek Instruments, Inc.) with excitation and emission filters at 485 and 528 nm, respectively, and analyzed using Gen5 version 3.03 software. FITC-dextran concentration in the secreted droplets was calculated by accounting for the dilution factor (original droplet volume + 50 μL)/original droplet volume. Transepithelial FITC-dextran flux was determined by multiplying fluid secretion rate by FITC-dextran concentration.
Drosokinin treatment.
The Drosophila kinin peptide (NSVVLGKKQRFHSWG-amide), drosokinin, used in the studies described in materials and methods, Measurement of transepithelial fluid secretion and K+ flux, and materials and methods, Measurement of transepithelial FITC-dextran flux and permeability, was synthesized by the University of Texas Southwestern Protein Chemistry Core Facility and purified by reverse-phase HPLC to 98.6%. The peptide was dissolved in water at a concentration of 10−4 M and diluted in SBM to a final concentration of 10−6 M. Tubules were bathed in the peptide-containing SBM for the entirety of the experiment (2 or 4 h). As controls, tubules were bathed in SBM containing water alone at the same concentration.
Electrophysiology.
Two microelectrodes were used to measure the transepithelial voltage (Vt) and the basolateral membrane voltage (Vbl) of the principal cells of anterior tubules from 14–28-day-old adult female flies of desired genotype (6–20 tubules per genotype). Electrode resistance ranged from 25 to 65 MΩ when filled with 3 M KCl. Ag/AgCl bridges were used in the electrodes. Voltages were measured with respect to ground in the peritubular bath via Drosophila saline 4% agar bridge. Once both microelectrodes were placed into the saline bath, asymmetries between sensing microelectrodes and the ground electrode were nulled at the P-Clamp 500B (Axon Instruments). One sensing microelectrode was advanced into the tubule lumen (passing it through a stellate cell) to measure Vt. The other sensing microelectrode impaled a principal cell for the measurement of Vbl. The apical membrane voltage, Va, was calculated as the difference between Vt and Vbl. After stable control values of Vt and Vbl were obtained with the tubule in Drosophila saline (identical to the previously used basic Ringer solution; 2), the bath was changed to a solution with 10-fold reduced Cl− concentration (composition in mM: 117.5 Na-gluconate, 20 K-gluconate, 2 CaCl2, 5.95 MgCl2, 3.83 MgSO4, 10.2 NaHCO3, 15 HEPES, 4.3 NaH2PO4, and 20 glucose) for the measurement of transepithelial Cl− diffusion potential (DPCl). Bath volume was <500 µL in order to change the peritubular medium within a few seconds for measurements of DPCl in response to step [Cl−] changes. The bath was returned to normal [Cl−] by washout with Drosophila saline. Next, drosokinin (1 µM; JPT Peptides, Berlin, Germany) was added to the peritubular bath. The Vt response was immediate, producing a step depolarization of Vt. The transepithelial DPCl was measured again via the bath change described above. After returning the bath to saline plus drosokinin, the electrodes were withdrawn to record electrode offsets, usually <3 mV. The data were corrected for these offsets, and for the change (+8 mV) in ground electrode voltage upon the step change from saline to 1/10 Cl− solution. Note that whereas measurements of transepithelial fluid, K+, and 4-kDa dextran secretion were performed in SBM (Drosophila saline + Schneider’s medium, see above), electrophysiological experiments were performed using Drosophila saline alone to allow the 10-fold reduction in Cl− concentration required to measure DPCl.
Statistical analysis.
Data are expressed as means ± SE (n = number of tubules), except in Table 4, where data are expressed as means ± SD. Data were compared using one- or two-way ANOVA followed by Tukey’s or Sidak’s multiple comparisons of means with a significance level of P < 0.05, adjusted for multiple comparisons where appropriate. Fisher’s exact test was used to compare the number of secreting tubules with and without drosokinin in the developmental mesh knockdown experiment with mesh RNAi line 6867. Repeated measures two-way ANOVA was used for the electrophysiological measurements since the same tubules were analyzed with and without drosokinin. All statistical analyses were conducted using GraphPad Prism, version 7 or 8.
Fig. 4.
Developmental principal cell mesh knockdown in the larval Drosophila Malpighian tubule. A and B: bright-field images of the anterior Malpighian tubules from a nonwandering third-instar control larva (w;c42-GAL4/+; A) and developmental principal cell mesh knockdown larva (w;UAS-meshRNAi/+;c42-GAL4/+; B). The mesh knockdown tubules reveal some dilation compared with control tubules. C and D: Mesh immunolocalizes to the smooth septate junctions between the epithelial cells in control tubules. E and F: decreased Mesh immunofluorescence is observed in mesh knockdown tubules. Five samples per genotype were examined. Scale bars, 100 µm (A and B) and 50 μm (C–F).
RESULTS
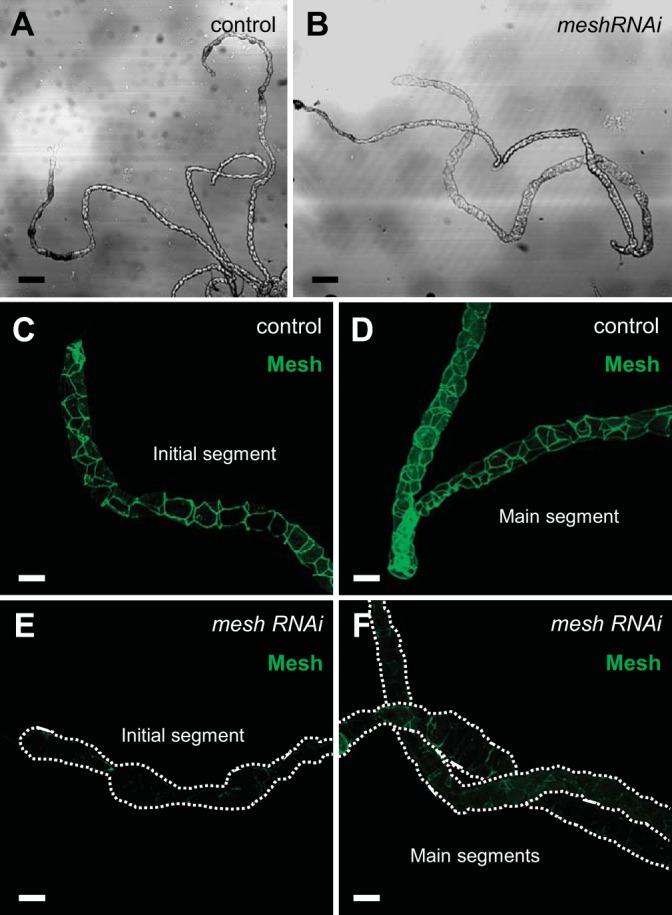
Mesh is required for Malpighian tubule morphogenesis.
Mesh is expressed in the Drosophila Malpighian tubule from the late-stage embryo through adulthood (31). Mesh loss-of-function mutants die as first-instar larvae (31). To gain insight into the functional role of Mesh in the tubule, we induced mesh RNAi (12074-R1 generated by NIG-Fly; Fig. 1) in the principal cells using the principal cell-specific c42-GAL4 driver, which is expressed from the embryonic stage (25, 56). These animals emerged into adult flies but did not survive more than 24 h posteclosion (data not shown). We therefore examined the Malpighian tubules from ~1-day-old adult control and mesh knockdown female flies, focusing on the longer anterior pair. We found that developmental mesh knockdown tubules (c42-GAL4>UAS-mesh RNAi) exhibited abnormal gross morphology, appearing distended, especially in the distal initial/transitional and lower tubular segments, compared with control tubules (c42-GAL4/+; Fig. 2, A and B). In addition, bright-field microscopy revealed that in contrast to the clear initial segments of control tubules, the initial segments of mesh knockdown tubules contained opaque concretions (arrows in Fig. 2B). The initial segments of anterior tubules of larval and adult Drosophila sequester excess hemolymph Ca2+ as biomineralized luminal granules (9, 20, 74, 75); clearance of these granules appears to be impaired by the loss of Mesh. Next, following immunofluorescence staining, we verified that the tubules of control flies had Mesh expression at the sSJs between all epithelial cells throughout the tubule (Fig. 2, C–E). In contrast, little to no Mesh immunoreactivity was detected in the initial/transitional, main, and lower segments of developmental mesh knockdown tubules (Fig. 2, F–H), confirming a significant knockdown in overall tubule expression of mesh. Similar effects were observed using an independently generated UAS-mesh RNAi line targeting a distinct region of the gene [VDRC, 6867, labeled as UAS-mesh RNAi (II); Figs. 1 and 3]. Notably, the efficiency of the latter mesh RNAi line was lower compared with line 12074-R1 since trace amounts of Mesh were still seen in the knockdown tubules (Fig. 3, F–H), and these flies survived up to 7 days posteclosion (data not shown). Together, these results suggest that developmental loss of Mesh impairs gross tubule morphogenesis.
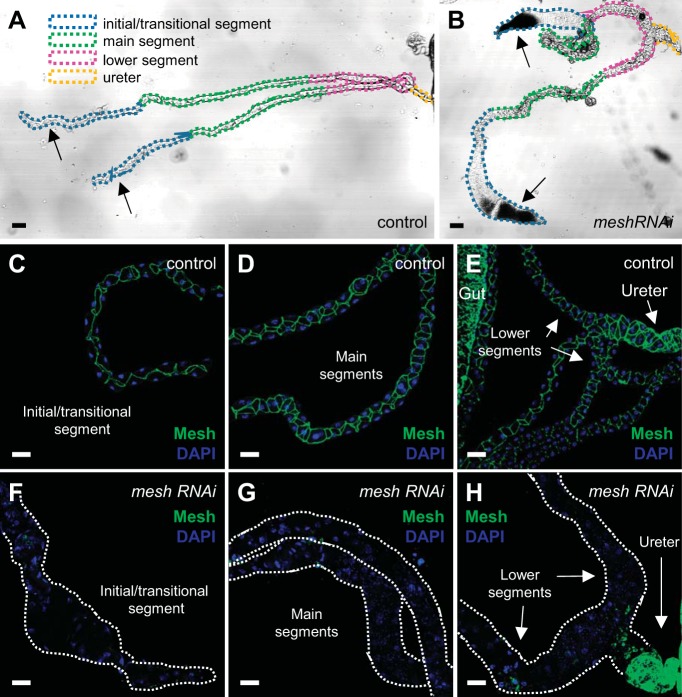
Fig. 2.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule using mesh RNA interference (RNAi) line 12074-R1 results in morphological defects in adult tubules. A and B: bright-field images with depiction of the major segments of the anterior Malpighian tubules from a 1-day-old adult control female fly (w;c42-GAL4/+; A) and developmental principal cell mesh knockdown fly (w;UAS-meshRNAi/+;c42-GAL4/+; B). The mesh knockdown tubules appear dilated compared with control tubules and accumulate opaque concretions in the initial segments. C–H: Mesh immunolocalizes to the smooth septate junctions (sSJs) between the epithelial cells throughout all segments in control tubules (C–E), but little to no Mesh immunoreactivity is observed in the initial/transitional, main, and lower segments and upper ureter of mesh knockdown tubules (F-H). Nuclei were counterstained blue with DAPI in C–H. Ten samples per genotype were examined. Scale bars, 100 µm (A and B) and 50 μm (C–H). The mesh RNAi line 12074-R1 was used in subsequent figures unless otherwise noted.
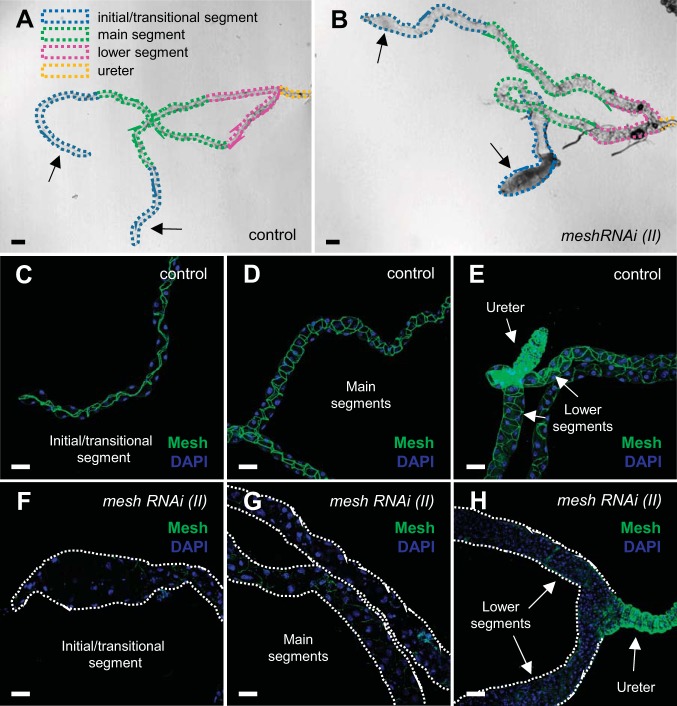
Fig. 3.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule using mesh RNA interference (RNAi) line 6867 causes morphological defects in adult fly tubules. A and B: bright-field images with depiction of major morphological segments of the anterior Malpighian tubules from a 1-day-old adult control female fly [w;UAS-meshRNAi (II)/+; A] and developmental principal cell mesh knockdown fly [w;UAS-meshRNAi (II)/+;c42-GAL4/+; B]. The mesh knockdown tubules appear enlarged compared with control tubules, and their initial segments contain opaque concretions (A and B, arrows). C–E: in control tubules, Mesh immunolocalizes to the smooth septate junctions between the epithelial cells throughout all segments. F–H: in mesh knockdown tubules, only trace levels of Mesh immunofluorescence are observed in the initial/transitional, main, and lower segments. Nuclei were counterstained blue with DAPI in C–H. Ten samples per genotype were examined. Scale bars, 100 µm (A and B) and 50 μm (C–H).
Given the abnormal appearance of Malpighian tubules in newly eclosed adult flies, we also examined tubules at an earlier developmental time point. In tubules from nonwandering third-instar larvae, we observed apparent tubular dilation in multiple tubule segments, similar to adult tubules (Fig. 4, A and B). However, unlike in adult tubules, in which Mesh expression is largely abolished (Fig. 2), we observed some residual Mesh immunostaining in larval knockdown tubules (Fig. 4, E and F). Given the correlations we observe in adult tubules between residual Mesh immunostaining, tubule fluid secretion and ion transport (described below), and adult viability, the residual Mesh immunostaining in larval tubules may explain larval survival with subsequent metamorphosis and eclosion of adult flies, although it is also possible that mesh is dispensable for larval Malpighian tubule function.
Mesh is required for proper localization of other sSJ proteins in developing tubules.
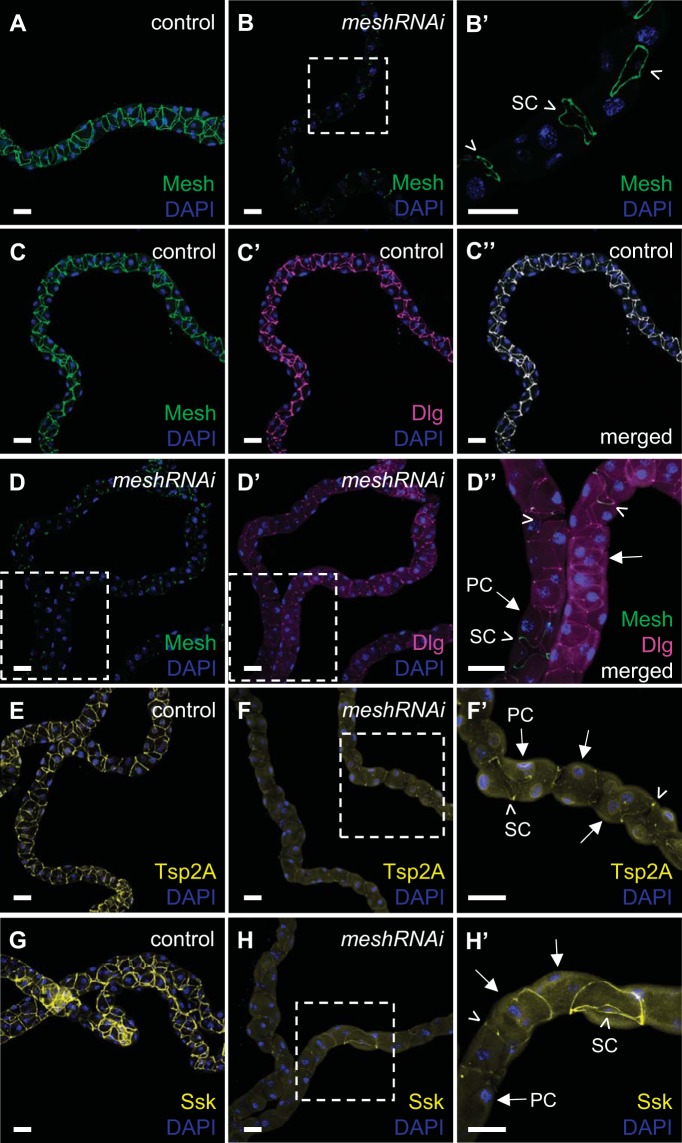
To examine the role of Mesh in the molecular organization of sSJs in the Malpighian tubules, we analyzed the localization of other sSJ components in developmental mesh knockdown tubules. We focused on Dlg, Tsp2A, and Ssk, the only other known proteins shown to be localized to the fly tubule sSJs (17, 32, 44, 81). Co-immunofluorescence staining of Mesh and Dlg showed their overlapping expression at sSJs in control tubule main segments (Fig. 5, A–A′′). Tsp2A and Ssk also localized to the sSJs in control tubules (Fig. 5, C and E). However, Dlg, Tsp2A, and Ssk revealed a more diffuse intracellular distribution in mesh knockdown tubule epithelium (Fig. 5, B′, B′′, D, D′, F, and F′), although some residual expression of Dlg at sSJs was still present (Fig. 5B′′). These observations suggest that Mesh is required for the proper localization of other sSJ proteins in developing tubules.
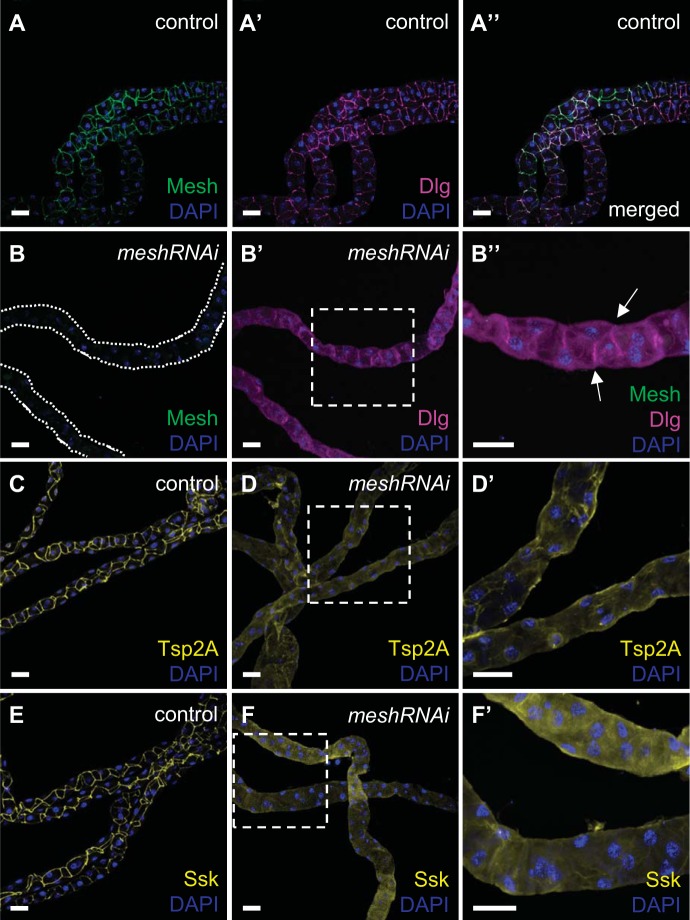
Fig. 5.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule results in mislocalization of other smooth septate junction (sSJ) proteins. In the main segment of 1-day-old adult control female fly anterior tubules (w;c42-GAL4/+), Mesh colocalizes with Discs large (Dlg; A–A′′), tetraspanin 2A (Tsp2A; C), and Snakeskin (Ssk; E) at sSJs. In the main segment of principal cell mesh knockdown tubules (w;UAS-meshRNAi/+;c42-GAL4/+), in which Mesh signal is not detected (B), Dlg (B′ and B′′), Tsp2A (D and D′), and Ssk (F and F′) are distributed diffusely throughout the tubule epithelial cells. Some Dlg expression is still seen at sSJs (arrows in B′′). B′′, D′, and F′ show higher-magnification images of dashed-box areas in B′, D, and F, respectively. Nuclei were counterstained blue with DAPI. RNAi, RNA interference. Ten samples per genotype were examined. Scale bars, 50 μm.
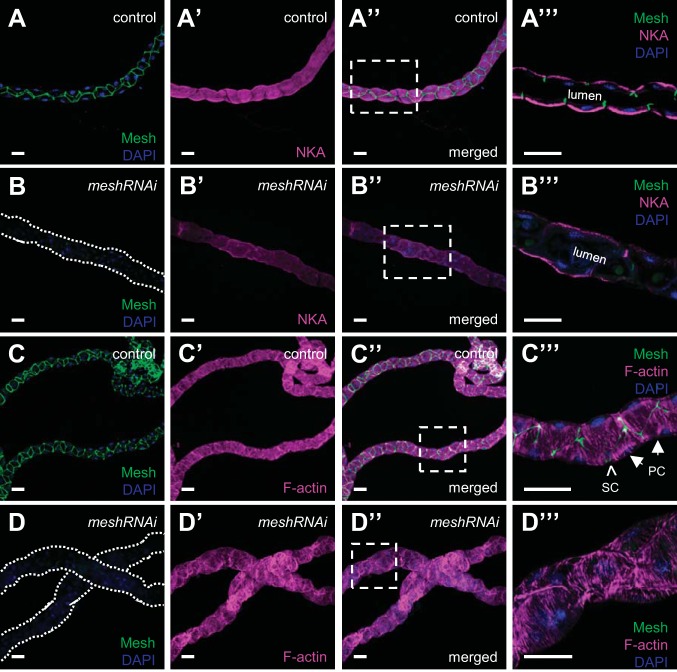
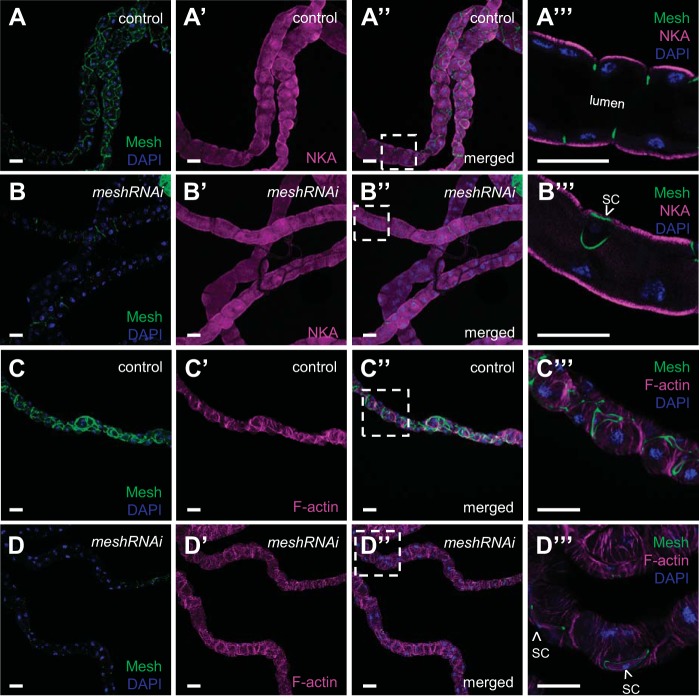
Dlg is expressed before sSJ formation in Drosophila tubules and is a component of the Scribble polarity complex required for the initial establishment of tubule cell polarity (12, 69). Dlg mislocalization in developmental mesh knockdown tubules prompted us to ask whether there is a requirement for Mesh to maintain tubule epithelial cell polarity. To address this question, we analyzed the expression of the principal cell basolateral membrane marker NKA (41, 55, 60, 61) in developmental mesh knockdown tubules. We found that localization of NKA in mesh knockdown tubules of 1-day-old adult flies was not altered, as the protein was still present on the basolateral membrane of the principal cells (Fig. 6, A–A′′′ and B–B′′′), although expression levels of NKA appeared reduced. We also examined the effect of developmental mesh knockdown on the tubule cell cytoskeleton. We found that F-actin distribution in the principal cells of mesh knockdown tubules (Fig. 6, D–D′′′) was largely intact (Fig. 6, C–C′′′), with striated F-actin bundles oriented perpendicularly to the tubule long axis.
Fig. 6.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule does not alter the localization of Na+/K+-ATPase (NKA) or the organization of the actin cytoskeleton. The main segment of 1-day-old adult control female fly anterior tubules (w;c42-GAL4/+; A–A′′′ and C–C′′′) shows high expression of Mesh at bicellular contacts and NKA at the principal cells’ basolateral membrane and F-actin bundles that lie perpendicularly to the tubule long axis. A′′′ and C′′′ show single-confocal plane images indicated by dashed-box areas in A′′ and C′′, respectively. In principal cell mesh knockdown tubule main segments (w;UAS-meshRNAi/+;c42-GAL4/+; B–B′′′ and D–D′′′), NKA expression appears reduced but remains basolateral, and the expression and distribution of F-actin appear largely intact. B′′′ and D′′′ show single-confocal plane images indicated by dashed-box areas in B′′ and D′′, respectively. Nuclei were counterstained blue with DAPI. PC, principal cell; RNAi, RNA interference; SC, stellate cell. Ten samples per genotype were examined. Scale bars, 50 μm.
Developmental loss of Mesh compromises tubule epithelial architecture and sSJ organization.
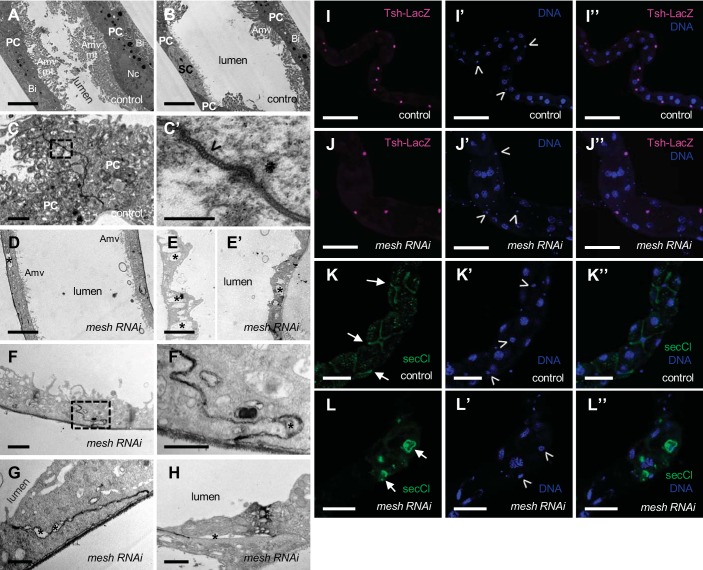
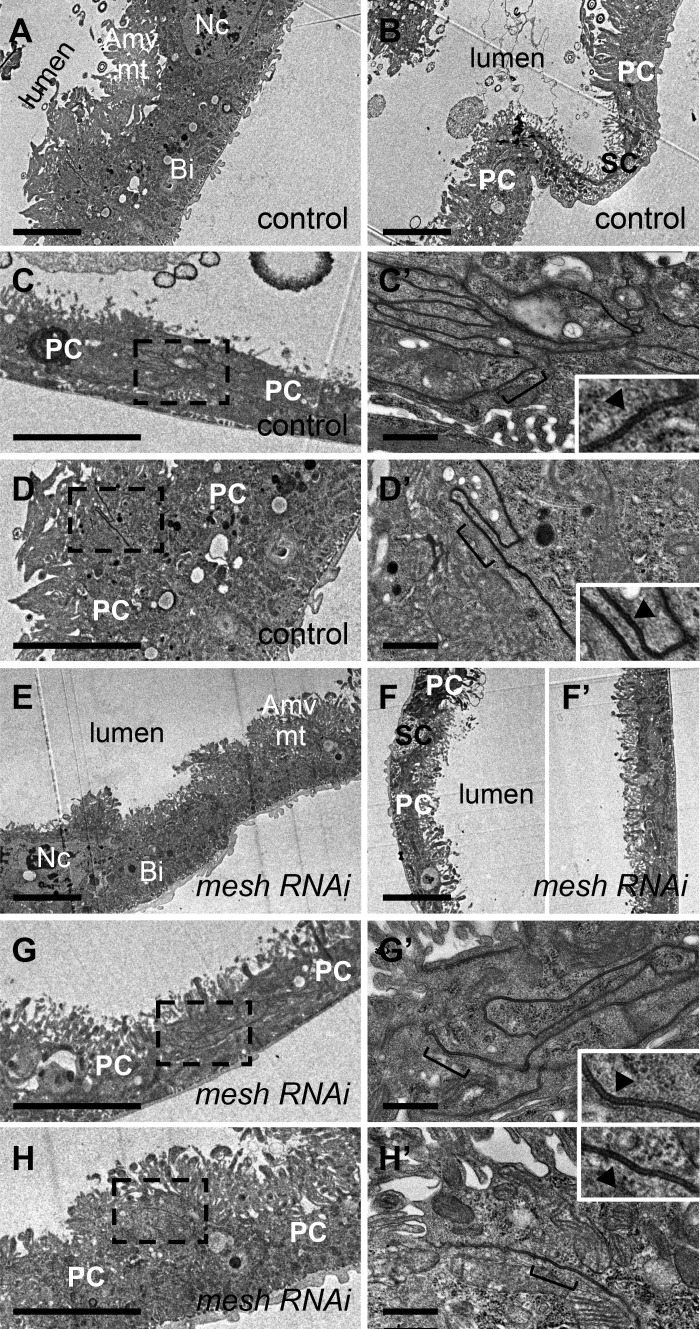
To further examine the effects of mesh knockdown during development, we performed electron microscopy analysis of main segment ultrathin sections from 1-day-old adult fly anterior tubules. In control tubules, the principal cells revealed features of actively transporting epithelial cells, such as a large number of mitochondria associated with extensive apical membrane microvilli and basal membrane infoldings, which increase membrane area available for transmembrane transport (Fig. 7, A and B; 28). In addition, the sSJs were observed as uniformly spaced parallel plasma membranes between adjacent cells with ladderlike septa in control tubules (Fig. 7, C and C′). Unlike control tubule epithelium, which shows clear morphological differences between the principal and stellate cells (Fig. 7, A and B), there were no morphologically distinct cells in the ultrathin sections of developmental mesh knockdown tubule epithelium (Fig. 7, D, E, and E′). The epithelium of mesh knockdown tubules appeared thinner, and the cells exhibited reduced or absent apical membrane microvilli, mitochondria, and basal membrane infoldings as well as the presence of empty vacuoles in the cytoplasm (Fig. 7, D, E, and E′). The sSJs between the epithelial cells of mesh knockdown tubules also showed defects. Specifically, the lateral plasma membranes of adjacent cells were often less parallel, and large gaps between the cells were present (Fig. 7, F, F′, G, and H).
Fig. 7.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule results in disruption of epithelial architecture and smooth septate junction (sSJ) organization. A–H: transmission electron micrographs of 1-day-old adult female fly anterior tubule main segment epithelial cells. Compared with a control tubule main segment (w;c42-GAL4/+; A and B), the epithelial cells of a principal cell mesh knockdown tubule main segment (w;UAS-meshRNAi/+;c42-GAL4/+; D–H) reveal a cytoplasm containing empty vacuoles (*), reduced or absent apical membrane microvilli (Amv) and associated mitochondria (mt), and reduced basal membrane infoldings (Bi). The sSJs between the principal cells (PCs) in a control tubule main segment (C and C′) show parallel plasma membranes and ladderlike septa (open arrowhead). C′ shows a higher-magnification image of dashed-box area in C. In mesh knockdown tubule main segment sSJs (F–H), the plasma membranes of adjacent cells are less parallel, and frequent large intercellular gaps are observed (*). F′ shows a higher-magnification image of dashed-box area in F. I–I′′ and J–J′′: confocal images of a 1-day-old adult female fly anterior tubule main segment expressing teashirt (tsh)-lacZ without (control; w;tsh04319/+;c42-GAL4/+) or with upstream activation sequence (UAS)-mesh RNA interference (mesh RNAi; w;tsh04319/+;c42-GAL4/UAS-meshRNAi) and stained for β-galactosidase (magenta) and DNA (blue, TOPO-3). Both control and mesh knockdown tubules express Tsh-LacZ restricted to the stellate cells with smaller nuclei (open arrowheads in I′ and J′). K–K′′ and L–L′′: confocal images of a 1-day-old adult female fly anterior tubule main segment stained for secretory chloride channel (secCl, green) and DNA (blue, DAPI). Control (w;c42-GAL4/+; K–K′′) and mesh knockdown (w;UAS-meshRNAi/+;c42-GAL4/+; L–L′′) tubules show secCl expression in the stellate cells with smaller nuclei (arrows and open arrowheads in K, K′, L, and L′). However, compared with control tubule main segment, the stellate cells of mesh knockdown tubules are abnormally shaped. Nc, nucleus; SC, stellate cell. Three samples per genotype were examined in A–J; 10 samples per genotype were examined in K and L. Scale bars, 100 µm (I–I′′ and J–J′′), 50 µm (K–K′′ and L–L′′), 5 μm (A, B, D, E, and E′), 1 μm (C, F, G, and H), 500 nm (F′), and 200 nm (C′).
Expression of NKA in developmental mesh knockdown tubules (see Fig. 6, B′–B′′′) suggests the presence of principal cells. However, we were unable to distinguish the principal and stellate cells in developmental mesh knockdown tubules by their morphology on electron microscopy. To determine whether stellate cells are also present, we examined the expression of the stellate cell markers teashirt (tsh, a nuclear transcription factor) and the chloride channel secCl (17, 19, 21). In agreement with previous reports, we observed Tsh-lacZ and secCl expression in control adult tubule stellate cells (Fig. 7, I–I′′ and K–K′′; 17, 21). The main segment of mesh knockdown tubules also showed Tsh-lacZ- and secCl-positive stellate cells, but the cells appeared cuboidal rather than star shaped (Fig. 7, J–J′′ and L–L′′). Taken together, these results suggest that Mesh is required for the establishment of epithelial architecture and sSJ organization in the tubules but that despite their abnormal appearance, the epithelial cells express markers of principal and stellate cells.
Developmental loss of Mesh abolishes tubule fluid and ion transport and response to kinin stimulation.
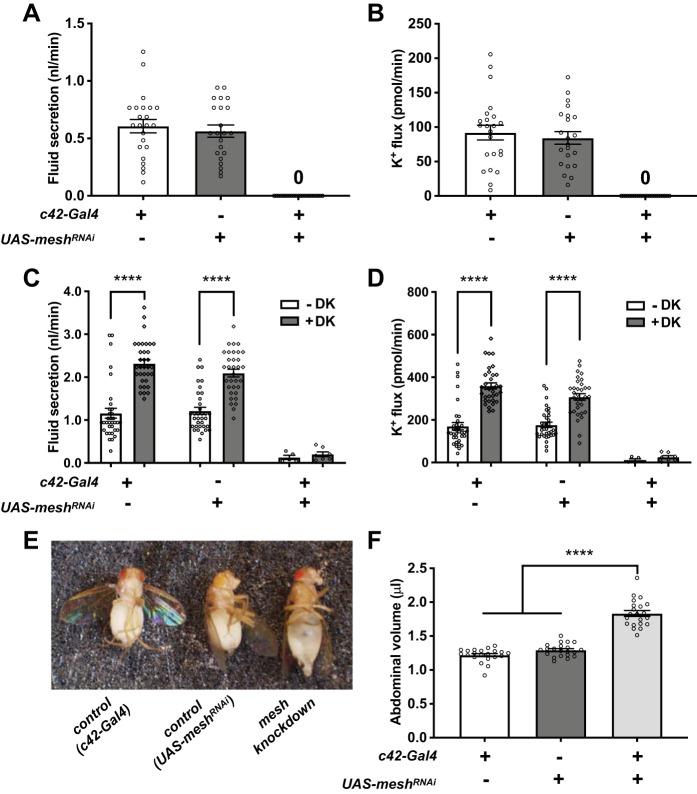
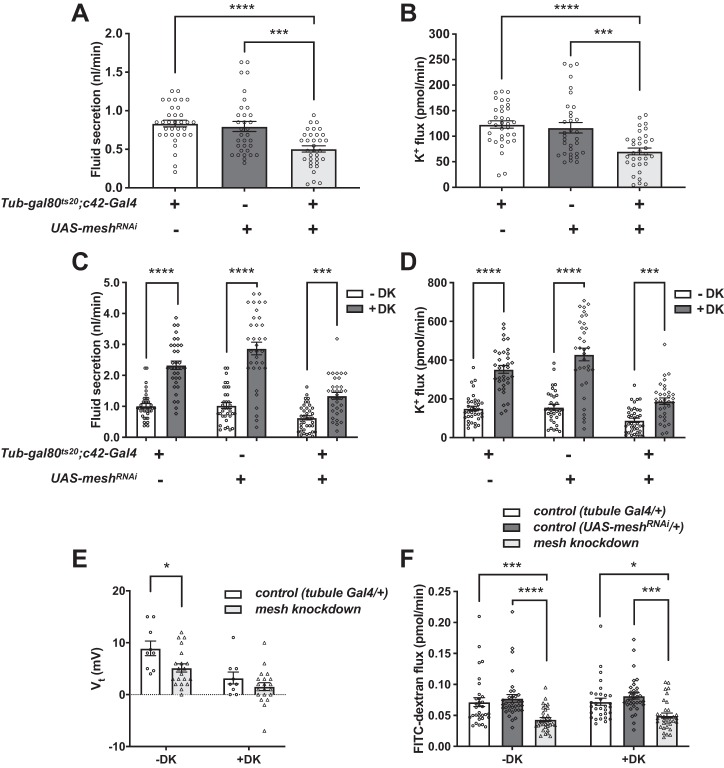
To determine whether developmental mesh knockdown in the principal cells affects tubule transport function, we measured transepithelial fluid secretion and K+ flux in the absence or presence of drosokinin, a diuretic peptide known to stimulate fluid and Cl− secretion in the fly tubule (11, 50, 71). Developmental depletion of Mesh in tubules from flies reared at 28°C abolished main segment transepithelial basal fluid and K+ flux compared with controls (c42-GAL4/+ and UAS-meshRNAi/+; Fig. 8, A and B, and Table 1). Since these flies also die within 24 h posteclosion, we reduced the rearing temperature to 18°C, which is known to lower c42-GAL4 activity. These flies survived up to 7 days (data not shown), and a small percentage (~17%) of tubules showed very low levels of basal fluid and K+ secretion but failed to respond to drosokinin (1 µM), although statistical power was limited because of the low number of secreting tubules (Fig. 8, C and D, and Table 1). Compromised transepithelial fluid and K+ transport and lack of response to drosokinin stimulation were also observed in developmental mesh knockdown tubules from flies reared at 28°C using a second UAS-meshRNAi line (Figs. 1 and 9 and Table 2). Developmental principal cell mesh knockdown flies also exhibited inflated abdomens, resulting in significantly larger abdominal volume compared with control animals (Fig. 8, E and F), presumably due to reduced fluid secretion and edema. Together, these observations suggest that Mesh is required for normal ion and water transport function in the Malpighian tubule.
Fig. 8.
Developmental principal cell mesh knockdown in the Drosophila Malpighian tubule abolishes transepithelial fluid and K+ transport and response to drosokinin and causes a bloated abdomen phenotype. A and B: main segment fluid secretion (A) and K+ transport (B) is detected in 1-day-old adult control female fly tubules (w;c42-GAL4/+ and w;UAS-meshRNAi/+), but not the developmental principal cell mesh knockdown tubules (w;UAS-meshRNAi/+;c42-GAL4/+). Flies were reared at 28°C. Here, n = 22–30 tubules per genotype, one-way ANOVA P < 0.0001. C and D: Drosophila kinin (DK, 1 µM) treatment resulted in increased transepithelial fluid secretion and K+ flux in control tubules but had no effect on mesh knockdown tubules. Flies were reared at 18°C. Here, n = 33–34 control tubules per condition (100% of analyzed tubules secreting), and n = 5–7 mesh knockdown tubules per condition (~18% of analyzed tubules secreting); two-way ANOVA P < 0.0001 for the effects of genotype and DK and P = 0.0055 (C) and 0.0028 (D) for the interaction. ****P < 0.0001. E and F: mesh knockdown flies have distended abdomens with significantly larger abdominal volume. Flies were reared at 18°C. Here, n = 21 flies per genotype, one-way ANOVA P < 0.0001. Data are expressed as means ± SE; ****P < 0.0001. RNAi, RNA interference; UAS, upstream activation sequence. Adjusted P values for all multiple comparisons testing are shown in Table 1.
Table 1.
Secretion rate, [K+], potassium flux, and abdominal volume in developmental principal cell mesh knockdown using mesh RNAi line 12074-R1
| Genotype | Secretion Rate, nL/min | [K+], mM | K+ Flux, pmol·min−1·tubule−1 | n | Slope | Abdominal Volume, µL |
|---|---|---|---|---|---|---|
| Developmental principal cell mesh knockdown | ||||||
| GAL4 control | 0.61 ± 0.05 | 145 ± 8.2 | 92 ± 10.7 | 23 | 53.5 | 1.22 ± 0.02 |
| UAS control | 0.56 ± 0.05 | 148 ± 7.0 | 84 ± 9.2 | 22 | 1.29 ± 0.02 | |
| mesh Knockdown | 0 | 0 | 0 | 25 | 1.83 ± 0.04 | |
| One-way ANOVA P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Tukey’s multiple comparison adjusted P values | ||||||
| GAL4 control vs. UAS control | 0.7828 | 0.9697 | 0.7801 | 0.2057 | ||
| GAL4 control vs. mesh knockdown | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| UAS control vs. mesh knockdown | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Developmental principal cell mesh knockdown and drosokinin | ||||||
| GAL4 control, −DK | 1.16 ± 0.12 | 146 ± 3.5 | 170 ± 17.5 | 34 | 53.2 | |
| GAL4 control, +DK | 2.32 ± 0.09 | 154 ± 2.5 | 359 ± 14.4 | 34 | ||
| UAS control, −DK | 1.22 ± 0.08 | 146 ± 3.6 | 176 ± 12.2 | 34 | ||
| UAS control, +DK | 2.10 ± 0.09 | 146 ± 3.6 | 308 ± 15.3 | 33 | ||
| mesh Knockdown, −DK | 0.13 ± 0.05 | 79 ± 11.0 | 12 ± 5.3 | 5/35 | ||
| mesh Knockdown, +DK | 0.20 ± 0.05 | 118 ± 13.0 | 26 ± 6.9 | 7/35 | ||
| Two-way ANOVA P values | ||||||
| Genotype | <0.0001 | <0.0001 | <0.0001 | |||
| Drosokinin | <0.0001 | 0.0007 | <0.0001 | |||
| Interaction | 0.0055 | 0.0109 | 0.0028 | |||
| Sidak’s multiple comparison adjusted P values | ||||||
| GAL4 control, −DK vs. +DK | <0.0001 | 0.3021 | <0.0001 | |||
| UAS control, −DK vs. +DK | <0.0001 | 0.9997 | <0.0001 | |||
| mesh Knockdown, −DK vs. +DK | 0.9938 | 0.0033 | 0.9901 | |||
Values are means ± SE; n is the number of tubules analyzed. Genotypes are as follows (developmental principal cell mesh knockdown): GAL4 control, w;c42-GAL4/+; upstream activation sequence (UAS) control, w;UAS-meshRNAi/+; and mesh knockdown, w;UAS-meshRNAi/+;c42-GAL4/+. For mesh knockdown with and without drosokinin, the number of tubules secreting fluid and the total number of tubules analyzed are shown. “Slope” is the mean slope per 10-fold difference in [K+] of the electrodes used for each experiment. For abdominal volume, 21 flies per genotype were analyzed. DK, drosokinin; RNAi, RNA interference.
Fig. 9.
Impaired transepithelial fluid and K+ transport in the developmental principal cell mesh knockdown tubule using mesh RNA interference (RNAi) line 6867. A and B: transepithelial basal fluid secretion (A) and K+ transport (B) are significantly reduced in developmental mesh knockdown tubules from 3–4-day-old adult female flies [w;UAS-meshRNAi (II)/+;c42-GAL4/+] compared with control groups [w;c42-GAL4/+ and w;UAS-meshRNAi (II)/+]. Here, n = 17 control tubules per genotype (100% of analyzed tubules secreting), and n = 8 mesh knockdown tubules (~44% of analyzed tubules secreting); one-way ANOVA P < 0.0001 (A) and 0.0003 (B). ***P ≤ 0.001, ****P < 0.0001. P values for Tukey’s multiple comparisons test are shown in Table 2. C and D: compared with increased control tubule fluid and K+ transport, Drosophila kinin (DK, 1 µM) treatment has no effect on mesh knockdown tubule transport. Here, n = 20–24 control tubules per condition (100% of analyzed tubules secreting), and n = 7–12 mesh knockdown tubules per condition (~29% of analyzed tubules secreting in −DK and 50% of analyzed tubules secreting in +DK); two-way ANOVA P < 0.0001 for the effects of genotype, DK treatment, and interaction. ****P < 0.0001. P values for Sidak’s multiple comparisons test are shown in Table 2. Data are expressed as means ± SE.
Table 2.
Secretion rate, [K+], and potassium flux in developmental principal cell mesh knockdown using mesh RNAi line 6867
| Genotype | Secretion Rate, nL/min | [K+], mM | K+ Flux, pmol·min−1·tubule−1 | n | Slope |
|---|---|---|---|---|---|
| Developmental principal cell mesh knockdown | |||||
| GAL4 control | 0.84 ± 0.06 | 135 ± 4.4 | 115 ± 9.5 | 17 | 51.9 |
| UAS control | 0.90 ± 0.04 | 134 ± 5.4 | 121 ± 7.8 | 17 | |
| mesh Knockdown | 0.35 ± 0.15 | 96 ± 14.9 | 45 ± 21.9 | 8/17 | |
| One-way ANOVA P value | <0.0001 | 0.0021 | 0.0003 | ||
| Tukey’s multiple comparison adjusted P values | |||||
| GAL4 control vs. UAS control | 0.8083 | 0.9906 | 0.9127 | ||
| GAL4 control vs. mesh knockdown | 0.0002 | 0.0029 | 0.001 | ||
| UAS control vs. mesh knockdown | <0.0001 | 0.0039 | 0.0004 | ||
| Developmental principal cell mesh knockdown and drosokinin | |||||
| GAL4 control, −DK | 0.75 ± 0.08 | 142 ± 7.8 | 102 ± 11.8 | 20 | 53.9 |
| GAL4 control, +DK | 1.93 ± 0.13 | 150 ± 4.5 | 288 ± 19.8 | 24 | |
| UAS control, −DK | 0.77 ± 0.09 | 147 ± 5.0 | 112 ± 14.9 | 23 | |
| UAS control, +DK | 1.44 ± 0.11 | 147 ± 6.1 | 212 ± 18.3 | 22 | |
| mesh Knockdown, −DK | 0.47 ± 0.11 | 132 ± 12.9 | 65 ± 17.6 | 7/24 | |
| mesh Knockdown, +DK | 0.42 ± 0.10 | 127 ± 13.5 | 57 ± 17.3 | 12/24 | |
| Two-way ANOVA P values | |||||
| Genotype | <0.0001 | 0.1097 | <0.0001 | ||
| Drosokinin | <0.0001 | 0.8378 | <0.0001 | ||
| Interaction | <0.0001 | 0.6918 | <0.0001 | ||
| Sidak’s multiple comparison adjusted P values | |||||
| GAL4 control, −DK vs. +DK | <0.0001 | <0.0001 | |||
| UAS control, −DK vs. +DK | <0.0001 | <0.0001 | |||
| mesh Knockdown, −DK vs. +DK | 0.9925 | 0.9945 | |||
| Contingency analysis | |||||
| Fisher’s exact test P value | 0.2375 |
Values are means ± SE; n is the number of tubules analyzed. See Table 1 legend for genotypes. For mesh knockdown with and without drosokinin, the number of tubules secreting fluid and the total number of tubules analyzed are shown. Fisher’s exact test indicates the P value for the difference between the number of secreting mesh knockdown tubules with and without drosokinin. “Slope” is the mean slope per 10-fold difference in [K+] of the electrodes used for each experiment. DK, drosokinin; RNAi, RNA interference; UAS, upstream activation sequence.
Mesh knockdown in adulthood affects the expression of other tubule sSJ components.
To examine the role of Mesh in mature Malpighian tubules, we induced the expression of mesh RNAi in the principal cells of 1-day-old adult female flies for 14 days by using a combination of the c42-GAL4 driver and temperature-sensitive tub-GAL80ts20, which allows temporal manipulation of GAL4 activity (45). Following immunofluorescence staining, Mesh expression was observed to be confined to the borders of stellate cells with little to no immunoreactivity at sSJs between the principal cells in mesh knockdown tubule main segments (tub-GAL80ts20/+;c42-GAL4>UAS-meshRNAi) compared with control tubules (tub-GAL80ts20/+; c42-GAL4/+; Fig. 10, A, B, and B′). We found no gross morphological abnormalities in the tubules subjected to adult onset of mesh knockdown; however, Dlg, Tsp2A, and Ssk localization at sSJs was diminished, and instead, they were distributed diffusely in the cytoplasm of the principal cells of the mesh knockdown tubule main segment compared with control tubules (Fig. 10, C′, D′, D′′, F, F′, H, and H′).
Fig. 10.
Principal cell mesh knockdown in the adult Drosophila Malpighian tubule results in mislocalized expression of other smooth septate junction (sSJ) proteins. The main segment of a 14-day-old adult control female fly anterior tubule (w;tub-GAL80ts20/+;c42-GAL4/+) shows Mesh expression at sSJs between all cells (A). Mesh colocalizes with Discs large (Dlg; C–C′′), tetraspanin 2A (Tsp2A; E), and Snakeskin (Ssk; G) at sSJs in control tubules. In the main segment of tubules subjected to 14-day adult principal cell mesh knockdown (w;tub-GAL80ts20/UAS-meshRNAi;c42-GAL4/+), Mesh immunoreactivity at sSJs between the principal cells is either greatly reduced or undetectable and is mostly confined to the borders of the stellate cells (B, arrowheads in B′). B′ shows a higher-magnification image of dashed-box area in B. The mesh knockdown tubules also show reduced levels of Dlg at sSJs and its spread into the cytoplasm of the principal cells (D–D′′, arrows in D′′). D′′ shows a higher-magnification image of merged dashed-box areas in D and D′. Tsp2A (F and F′) and Ssk (H and H′) are mostly expressed along the edges of the stellate cells (arrowheads in F′ and H′) and inside the principal cells of mesh knockdown tubules (arrows in F’ and H’). F′ and H′ show higher-magnification images of the dashed-box areas in F and H, respectively. Nuclei were counterstained blue with DAPI. PC, principal cell; RNAi, RNA interference; SC, stellate cell. Ten samples per genotype were examined. Scale bars, 50 μm.
We also analyzed the effects of Mesh depletion in adult tubules on principal cell polarity and actin cytoskeleton. We found that immunofluorescence of the principal cell basolateral membrane marker NKA and F-actin in mesh knockdown tubules resembled their expression and distribution in control tubules (Fig. 11).
Fig. 11.
Principal cell mesh knockdown in the adult Drosophila Malpighian tubule does not alter Na+/K+-ATPase (NKA) expression and actin cytoskeleton. NKA is expressed in the basolateral membrane of the principal cells of a 14-day-old adult control female fly tubule main segment (w;tub-GAL80ts20/+;c42-GAL4/+; A′′ and A′′′) and in the main segment of the tubule subjected to adult onset of principal cell mesh knockdown (w;tub-GAL80ts20/UAS-meshRNAi;c42-GAL4/+; B′′ and B′′′) where Mesh expression at smooth septate junctions (sSJs) between the principal cells is greatly reduced and is mostly restricted to the stellate cells (B and B′′′). A′′′ and B′′′ show single-confocal plane images indicated by dashed-box areas in A′′ and B′′, respectively. F-actin staining in a control tubule main segment (C′–C′′′) is also similar to its staining in a mesh knockdown tubule main segment (D′–D′′′). C′′′ and D′′′ show single-confocal plane images indicated by dashed-box areas in C′′ and D′′, respectively. Nuclei were counterstained blue with DAPI. RNAi, RNA interference; SC, stellate cell. Ten samples per genotype were examined. Scale bars, 50 μm.
Mesh knockdown in adulthood does not alter tubule epithelial morphology and sSJ integrity.
We next examined whether principal cell mesh knockdown in adulthood had an effect on tubule epithelial architecture and sSJ structure. Electron microscopy analysis revealed that the main segment of 14-day-old control female fly tubules exhibited morphological characteristics of actively transporting epithelium, including apical membrane microvilli associated with mitochondria and basal membrane infoldings (Fig. 12, A and B). The sSJs between the principal cells of control tubules showed a typical ladderlike structure of electron-dense septa spanning the intercellular space between the parallel plasma membranes of adjacent cells (Fig. 12, C, C′, D, and D′). Overall, epithelial and sSJ architecture in mesh knockdown tubules appeared similar to control tubules (Fig. 12, E, F, F′, G, G′, H, and H′).
Fig. 12.
Principal cell mesh knockdown in the adult Drosophila Malpighian tubule does not affect epithelial integrity and smooth septate junction (sSJ) organization. Transmission electron micrographs of 14-day-old adult female fly anterior tubule main segment epithelial cells. Compared with control tubule main segment (w;tub-GAL80ts20/+;c42-GAL4/+; A and B), the morphology of the main segment tubule epithelium of 14-day adult principal cell mesh knockdown (w;tub-GAL80ts20/UAS-meshRNAi;c42-GAL4/+) is intact (E and F). Similarly, the sSJs between the principal cells of mesh knockdown tubule main segments (G, G′, H, and H′) appear normal with parallel plasma membranes and ladderlike septa (triangles in insets in G′ and H′) compared with control tubule sSJs (C, C′, D, and D′, triangles in insets in C′ and D′). Amv, apical microvilli; Bi, basal infoldings; mt, mitochondria; Nc, nucleus; PC, principal cell; RNAi, RNA interference; SC, stellate cell. Three samples per genotype were examined. Scale bars, 5 μm (A–C, D, E, F, F′, G, and H), 500 nm (C′, D′, G′, and H′).
Mesh is required for the maintenance of tubule transport and increased permeability in adulthood.
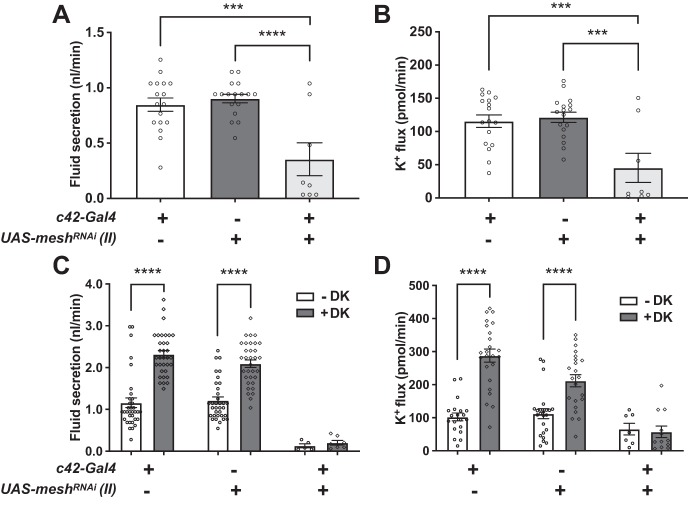
We next examined tubule ion and water transport and electrophysiological properties. Basal transepithelial fluid secretion and K+ flux rates were significantly lower for mesh adult knockdown tubules compared with control groups (Fig. 13, A and B), but drosokinin (1 µM) stimulated fluid secretion and K+ flux in both control and mesh knockdown tubules (Fig. 13, C and D). We further characterized the defect in mesh adult knockdown tubules by measuring transepithelial potential (Vt) and the diffusion potential of chloride (DPCl) in the presence or absence of drosokinin. A lumen-positive Vt of ~30–40 mV is generated by the principal cell apical vacuolar H+-ATPase and depolarizes (becomes less positive) with the addition of kinin or tyramine, because of increased chloride permeability through the stellate cell (2, 7, 8, 11, 21, 48, 50, 77). Unexpectedly, control tubules (tub-GAL80ts20/+; c42-GAL4/+) demonstrated low Vt of 8.9 mV (Fig. 13E) compared with prior studies and our own measurement of w1118 flies reared at 22°C (Vt = 31.6 ± 4.0 mV, mean ± SE, n = 11 tubules; 2). This was surprising given that transepithelial fluid and K+ secretion rates in the same genotype (Fig. 13, A–D, and Table 3) were not reduced compared with previous studies (11, 21, 48, 55, 68, 78). A possible explanation for this is differences in rearing conditions or the w−Berlin flies used in the control crosses (see materials and methods). Nevertheless, there were statistically significant reductions in Vt and increases in DPCl with the addition of drosokinin, although compared with prior studies (2, 7, 48), the magnitude of increase in DPCl was blunted because of the low basal Vt (Fig. 13E and Table 4). Compared with controls, Vt was decreased in adult mesh knockdown tubules, and the ~40–50% decrease in Vt (Fig. 13E and Table 4) correlated with the ~40–50% decrease in fluid secretion (Fig. 13C and Table 3) in the absence and presence of drosokinin. There was no statistically significant effect of genotype on DPCl (Table 4). Overall, these results suggest that mesh knockdown in adult tubules impairs transepithelial ion transport by decreasing Vt.
Fig. 13.
Principal cell mesh knockdown in the adult Drosophila Malpighian tubule decreases transepithelial fluid and ion transport, transepithelial potential (Vt), and paracellular permeability. A and B: transepithelial basal fluid secretion (A) and K+ transport (B) are significantly lower in the tubules subjected to 14-day adult-onset principal cell mesh knockdown (w;tub-GAL80ts20/UAS-meshRNAi;c42-GAL4/+) compared with control female fly tubules (w;tub-GAL80ts20/+;c42-GAL4/+ and w;UAS-meshRNAi/+). Here, n = 33–34 tubules per genotype, one-way ANOVA P < 0.0001. ***P < 0.001, **** P < 0.0001. Adjusted P values in multiple comparisons testing are shown in Table 3. C and D: fluid and K+ secretion is stimulated by Drosophila kinin (DK, 1 µM) in control and mesh knockdown tubules. Here, n = 33–38 tubules per genotype/condition, two-way ANOVA P < 0.0001 for the effects of genotype, DK treatment, and interaction. ***P < 0.001, ****P < 0.0001. Adjusted P values in multiple comparisons testing are shown in Table 3. E: Vt is diminished in mesh knockdown tubules compared with control tubules (w;tub-GAL80ts20/+;c42-GAL4/+) and is further reduced in both groups in the presence of DK. Here, n = 9–20 tubules per genotype, repeated measures two-way ANOVA P = 0.0204 for the effect of genotype, P < 0.0001 for the effect of DK, and P = 0.2686 for the interaction. *P < 0.05. Adjusted P values in multiple comparisons testing are shown in Table 4. F: Mesh knockdown tubules have reduced paracellular flux of FITC-labeled 3–5-kDa dextran (FITC-dextran). FITC-dextran flux in control and mesh knockdown tubules is not affected by DK. Here, n = 31–34 tubules per genotype, two-way ANOVA P < 0.0001 for the effect of genotype, P = 0.4122 for the effect of DK, and P = 0.8692 for the interaction. *P < 0.05, ***P < 0.001, ****P < 0.0001. Adjusted P values in multiple comparisons testing are shown in Table 5. RNAi, RNA interference; UAS, upstream activation sequence. All data are expressed as means ± SE.
Table 3.
Secretion rate, [K+], and potassium flux in mesh knockdown in adulthood
| Genotype | Secretion Rate, nL/min | [K+], mM | K+ Flux, pmol·min−1·tubule−1 | n | Slope |
|---|---|---|---|---|---|
| mesh Knockdown in adult principal cells | |||||
| GAL4 control | 0.83 ± 0.04 | 146 ± 3.3 | 123 ± 7.1 | 35 | 51.8 |
| UAS control | 0.80 ± 0.07 | 145 ± 3.0 | 117 ± 10.3 | 34 | |
| mesh Knockdown | 0.51 ± 0.04 | 133 ± 4.6 | 70 ± 6.6 | 34 | |
| One-way ANOVA P value | <0.0001 | 0.0289 | <0.0001 | ||
| Tukey’s multiple comparison adjusted P values | |||||
| GAL4 control vs. UAS control | 0.8605 | 0.9888 | 0.8462 | ||
| GAL4 control vs. mesh knockdown | <0.0001 | 0.0446 | <0.0001 | ||
| UAS control vs. mesh knockdown | 0.0003 | 0.0654 | 0.0003 | ||
| mesh Knockdown in adult principal cells and drosokinin | |||||
| GAL4 control, −DK | 1.01 ± 0.07 | 148 ± 3.4 | 150 ± 11.8 | 36 | 54.7 |
| GAL4 control, +DK | 2.33 ± 0.14 | 152 ± 2.5 | 352 ± 20.2 | 34 | |
| UAS control, −DK | 1.03 ± 0.09 | 153 ± 3.2 | 157 ± 14.9 | 34 | |
| UAS control, +DK | 2.87 ± 0.21 | 149 ± 3.7 | 429 ± 32.7 | 34 | |
| mesh Knockdown, −DK | 0.63 ± 0.07 | 138 ± 5.4 | 89 ± 10.7 | 38 | |
| mesh Knockdown, +DK Two-way ANOVA P values |
1.34 ± 0.11 | 138 ± 4.1 | 188 ± 16.7 | 33 | |
| Genotype | <0.0001 | 0.0016 | <0.0001 | ||
| Drosokinin | <0.0001 | 0.9623 | <0.0001 | ||
| Interaction | <0.0001 | 0.5389 | <0.0001 | ||
| Sidak’s multiple comparison adjusted P values | |||||
| GAL4 control, −DK vs. +DK | <0.0001 | 0.8193 | <0.0001 | ||
| UAS control, −DK vs. +DK | <0.0001 | 0.8151 | <0.0001 | ||
| mesh Knockdown, −DK vs. +DK | 0.0001 | 0.9999 | 0.0008 |
Values are means ± SE; n is the number of tubules analyzed. Genotypes are as follows (mesh knockdown in adult principal cells): GAL4 control, w;tub-GAL80ts20/+;c42-GAL4/+; upstream activation sequence (UAS) control, w;UAS-meshRNAi/+; and mesh knockdown, w;tub-GAL80ts20/UAS-meshRNAi;c42-GAL4/+. The meshRNAi line used is 12074-R1. “Slope” is the mean slope per 10-fold difference in [K+] of the electrodes used for each experiment. DK, drosokinin.
Table 4.
Transepithelial and principal cell basolateral and apical membrane voltages and Cl− diffusion potential
| Genotype | Transepithelial Voltage, mV | Principal Cell Basolateral Membrane Voltage, mV | Principal Cell Apical Membrane Voltage, mV | Transepithelial Cl− Diffusion Potential, mV |
|---|---|---|---|---|
| mesh Knockdown in adult principal cells, −DK | ||||
| GAL4 control | 8.9 ± 4.2 (9) | −47.8 ± 8.2 (6) | 54.7 ± 7.0 (4) | 8.8 ± 7.8 (10) |
| mesh Knockdown | 5.2 ± 3.6 (20) | −49.9 ± 4.3 (9) | 55.1 ± 5.3 (8) | 7.9 ± 8.0 (15) |
| mesh Knockdown in adult principal cells, +DK | ||||
| GAL4 control | 3.2 ± 3.5 (9) | −49.3 ± 5.5 (6) | 50.8 ± 5.9 (4) | 17.0 ± 9.3 (10) |
| mesh Knockdown | 1.6 ± 3.4 (20) | −47.5 ± 1.8 (9) | 49.3 ± 5.8 (8) | 11.8 ± 10.4 (15) |
| Two-way ANOVA P values | ||||
| Genotype | 0.0204 | 0.7372 | 0.8915 | 0.3747 |
| Drosokinin | <0.0001 | 0.5260 | 0.0635 | 0.0005 |
| Interaction | 0.2686 | 0.1613 | ||
| Sidak’s multiple comparison adjusted P values | ||||
| −DK | ||||
| GAL4 control vs. mesh knockdown | 0.0231 | 0.9655 | ||
| +DK | ||||
| GAL4 control vs. mesh knockdown | 0.4402 | 0.2998 |
Values are means ± SD; number of tubules n is in parentheses. See Table 3 legend for genotypes. The meshRNAi line used is 12074-R1. Paired electrophysiological measurements (with and without 1 µM drosokinin) were made of control and adult principal cell mesh knockdown tubules. The apical membrane voltage was calculated as transepithelial voltage minus basolateral membrane voltage in tubules in which both values were measured. P values for repeated measures 2-way ANOVA are shown. DK, drosokinin.
The Vt reflects the sum of the apical and basolateral membrane potentials. To determine which of these parameters was affected by mesh knockdown, the basolateral membrane potential was measured in a subset of tubules, and the apical membrane potential was calculated from tubules in which measurements of both transepithelial and basolateral membrane potentials were available. No significant effects of genotype or drosokinin treatment were observed, but because of the technical difficulty of these experiments, only a limited number of measurements were obtained (Table 4).
To assess the paracellular permeability to macromolecules (the so-called “leak” pathway; 62), we examined main segment transepithelial permeability of 4-kDa FITC-dextran and found that mesh knockdown tubules had reduced permeability to FITC-dextran compared with controls (Fig. 13F and Table 5). Since studies in other insects have implicated kinins in the regulation of tubule paracellular permeability to dextran and other macromolecules (36, 73), we also examined the effect of drosokinin on control and mesh knockdown tubule dextran permeability. Neither control nor mesh knockdown tubule permeability of FITC-dextran was altered by drosokinin treatment of isolated tubules (Fig. 13F).
Table 5.
Secretion rate, [FITC-dextran], and FITC-dextran flux
| Genotype | Secretion Rate, nL/min | [FITC-Dextran], ng/nL | FITC-Dextran Flux, pmol·min−1·tubule−1 | n |
|---|---|---|---|---|
| mesh Knockdown in adult principal cells and drosokinin | ||||
| GAL4 control, −DK | 1.10 ± 0.11 | 0.36 ± 0.03 | 0.071 ± 0.007 | 32 |
| GAL4 control, +DK | 1.92 ± 0.11 | 0.19 ± 0.01 | 0.072 ± 0.006 | 31 |
| UAS control, −DK | 1.20 ± 0.09 | 0.37 ± 0.03 | 0.077 ± 0.006 | 34 |
| UAS control, +DK | 1.99 ± 0.11 | 0.21 ± 0.02 | 0.081 ± 0.005 | 33 |
| mesh Knockdown, −DK | 0.57 ± 0.06 | 0.44 ± 0.04 | 0.043 ± 0.003 | 33 |
| mesh Knockdown, +DK | 1.04 ± 0.09 | 0.29 ± 0.03 | 0.049 ± 0.004 | 33 |
| Two-way ANOVA P values | ||||
| Genotype | <0.0001 | 0.0115 | <0.0001 | |
| Drosokinin | <0.0001 | <0.0001 | 0.4122 | |
| Interaction | 0.1212 | 0.9556 | 0.8692 | |
| Sidak’s multiple comparison adjusted P values | ||||
| −DK | ||||
| GAL4 control vs. UAS control | 0.7500 | 0.9947 | 0.8192 | |
| GAL4 control vs. mesh knockdown | 0.0004 | 0.1643 | 0.001 | |
| UAS control vs. mesh knockdown | <0.0001 | 0.1882 | <0.0001 | |
| +DK | ||||
| GAL4 control vs. UAS control | 0.8742 | 0.8693 | 0.5330 | |
| GAL4 control vs. mesh knockdown | <0.0001 | 0.0891 | 0.0128 | |
| UAS control vs. mesh knockdown | <0.0001 | 0.2320 | 0.0001 |
Values are means ± SE; n is number of tubules analyzed. See Table 3 legend for genotypes. The meshRNAi line used is 12074-R1. DK, drosokinin; UAS, upstream activation sequence.
DISCUSSION
Here we demonstrate that the sSJ protein Mesh plays an essential role in the physiological maturation and function of the Drosophila Malpighian tubule epithelium. We show that depletion of Mesh in developing tubules leads to epithelial cell and sSJ deformities and a lack of transport activity, which has a profound effect on urine generation and survival in adult flies. In contrast, knocking down mesh in adult tubules did not appear to affect the morphology of the fluid-secreting main segment but was required for normal transepithelial fluid and ion transport and paracellular permeability to dextran.
Mesh expression in Drosophila Malpighian tubules starts at around embryonic stage 16 and is maintained in both larvae and adults (31). In the larval midgut, Mesh is required for proper localization of the other integral sSJ components, Tsp2A and Ssk, for sSJ formation at the ultrastructural level, and for paracellular barrier function (31, 32). Observations made in our study indicate that Mesh similarly plays a role in proper localization of Tsp2A and Ssk and assembly and/or maturation of sSJs in developing Drosophila Malpighian tubules. In larval fly midgut, Mesh localization to sSJs is equally dependent on Tsp2A and Ssk, implying that these three proteins work together to organize and maintain the sSJ complex (31, 32). However, the nature of the interplay and hierarchy among Mesh, Tsp2A, and Ssk during sSJ formation in the midgut and Malpighian tubules remains to be clarified. Interestingly, of the latter proteins, only Mesh shows cell-cell adhesion activity when expressed in cultured Drosophila cells that have no SJs, suggesting that it might be one of the components of the septa observed in ultrathin section electron microscopy (23, 31; Fig. 7C′). This is in agreement with the observed reduction in septa and appearance of intercellular gaps between the cells of developmental mesh knockdown tubules (Fig. 7G). On the other hand, septa were present in tubules in which mesh was knocked down during adulthood (Fig. 12, G′ and H′). Possible explanations for these findings are that Mesh, or one or more associated proteins, is required during development but not during adulthood for septa formation or that residual Mesh in adult knockdown tubules is sufficient to form septa.
We further demonstrate that developmental loss of Mesh affects tubule epithelial integrity and localization of Dlg. Dlg is a component of the Scribble polarity complex (5, 6), and Scribble is required for embryonic Drosophila tubule polarization (12). We observed normal basolateral localization of NKA, but studies of additional membrane markers and polarity proteins will be required to investigate whether epithelial polarization is impaired in mesh knockdown tubules. The pSJ proteins are required for epithelial cell cytoskeletal organization (43, 66). Observations made in our study revealed that F-actin distribution appeared largely intact in the cells of developmental mesh knockdown tubules. Since some Mesh expression is detected in third-instar larval tubules after developmental mesh knockdown (Fig. 4), it is possible that residual Mesh expression during development is sufficient for tubule epithelial polarization and cytoskeletal organization. Alternatively, Mesh may not be required for these processes. We further observed that contrary to the characteristic starlike morphology, the shape of mesh knockdown tubule main segment stellate cells was altered, despite expression of tsh (Fig. 7, I–I′′, J–J′′, K–K′′, and L–L′′), which is required for stellate cell differentiation and morphogenesis (16, 17). These findings suggest that Mesh also contributes to tubule cell development, and further investigation will help clarify the molecular mechanisms by which Mesh influences this process.
It has recently been demonstrated that Mesh, as well as Ssk and Tsp2A, are necessary for the maintenance of midgut homeostasis and barrier integrity in adult Drosophila (13, 29, 57, 79, 80). Similar to larval midgut, all three proteins show mutually dependent localizations at sSJs in adult fly midgut (29, 32). We extend these observations in the present study and show that Mesh is required for the proper localization of Ssk and Tsp2A in adult fly tubules. Mesh depletion in adult midgut is also associated with aberrant Dlg distribution (29), which is in agreement with our findings of Dlg mislocalization in adult-onset mesh knockdown tubules. However, unlike defects in epithelial organization and polarization in mesh-deficient adult midguts (13, 29), we find that Mesh function in mature adult tubules appears dispensable for the maintenance of epithelial architecture and polarity. The tissue-specific phenotypes might be due to the differences in Mesh function in these epithelia. Although both the midgut and Malpighian tubules form sSJs, these epithelia derive from developmentally distinct embryonic layers (16, 46). Alternatively, the adult Drosophila midgut might require more Mesh activity because of the higher rates of turnover compared with the Malpighian tubules (63). We cannot exclude the possibility that some Mesh activity is still present during the maintenance of epithelial integrity in adult tubules, since we used RNAi knockdown and knocking down mesh specifically in the principal cells does not silence its expression associated with the stellate cells (Fig. 10B′). Nevertheless, observations made in the present study support temporal and tissue-specific complexity of the regulation of epithelial morphology and polarization in Drosophila (5, 13, 39, 44, 58, 70).
We also show here that Mesh activity in developing tubules is essential for basal as well as drosokinin-induced urine production in isolated tubules from young adult female flies (Figs. 8, A–D, and 9), as also suggested by the accumulation of luminal granules that would normally be flushed away by urine flow (9; Figs. 2, A and B, and 3, A and B). Compromised fluid homeostasis in these flies is also indicated by their bloated abdomens, a phenotype that has previously been observed in flies with impaired tubule development and/or water transport (11, 17, 44, 58). The lack of fluid secretion in developmental mesh knockdown tubules is likely due to the profound abnormalities in tubule morphology. For example, the apical membrane brush borders and basal membrane infoldings, which serve to increase the membrane surface area available to the active transport machinery, were greatly reduced or completely absent in the epithelial cells of developmental mesh knockdown tubules. Consistent with this, mesh knockdown tubules appeared to have reduced levels of basolateral membrane NKA (Fig. 6B′′′), and transport capacity of the Drosophila tubule epithelium has been shown to correlate with the length of the apical microvilli (28). In addition, loss of mitochondria could contribute to the lack of fluid secretion, as altered mitochondrial localization and function are associated with decreased fluid secretion and mitochondrial poisons collapse the transepithelial voltage required for fluid secretion (2, 71). Additionally, mesh knockdown tubules had morphologically aberrant stellate cells, which also contribute to basal and kinin-simulated transport function (17).
In contrast to tubules in which mesh was knocked down throughout development, principal cell knockdown of mesh for 14 days during adulthood did not result in apparent morphological abnormalities or lethality, but decreased proportionately the transepithelial fluid and K+ secretion and lumen-positive Vt in the urine-producing main segment (Fig. 13). In contrast, no statistically significant effect of mesh knockdown was observed on transepithelial DPCl, and drosokinin decreased Vt and increased fluid secretion in both control and mesh knockdown tubules (Fig. 13 and Tables 3 and 4). This is consistent with prior studies showing that drosokinin (and tyramine) stimulate an increase in DPCl through a rise in intracellular calcium concentrations and transepithelial chloride secretion in the stellate cell mediated by the chloride channels chloride channel-a (ClC-a) and secCl (7, 8, 10, 11, 21, 50, 72) and suggests that these processes are not impaired by principal cell mesh knockdown. Further studies are required to determine the mechanisms by which Vt is decreased in adult mesh knockdown tubules.
Studies in other insects have demonstrated paracellular flux of macromolecules in the Malpighian tubules (34, 36, 49, 64, 73), but whether this occurs in Drosophila tubules is unknown. Here we demonstrate that 4-kDa dextran diffuses across the Malpighian tubule main segment in a Mesh-dependent manner, suggesting that Mesh plays a role in the epithelial leak pathway of the Malpighian tubule. This is in apparent contrast to the observation that in adult Drosophila midgut, mesh knockdown causes increased midgut-to-hemolymph leak of the 800-Da organic anion, Brilliant Blue FCF, commonly used as a marker of the midgut barrier integrity (13, 29, 53, 57, 79, 80). This, together with our findings of reduced tubule main segment permeability upon mesh knockdown, further supports the idea of tissue-specific functions for Mesh, perhaps reflecting differences in the functions of the tubule main segment and midgut epithelia. These include water and ion absorption by the midgut versus secretion in the tubule main segment and varied environmental exposures requiring differences in barrier function. Tissue-specific roles for Mesh have also been suggested in the Malpighian tubules and midgut of larval A. aegypti (34). An interesting question is whether Mesh interacts with different proteins in these two epithelia, since present understanding of the Mesh interactome is limited. We also observe that unlike tubules of other insects, in which paracellular permeability to macromolecules is modulated by kinins, drosokinin had no effect on dextran permeability in the Drosophila Malpighian tubule, suggesting species-specific roles for this hormone (36, 73).
In summary, we have demonstrated a role for the sSJ protein Mesh in the development and maintenance of a functional Drosophila renal tubule epithelium, adding to its importance in midgut homeostasis (29, 31). The presence of Mesh-homologous proteins in other invertebrates as well as vertebrates implies that this family of proteins may share functions conserved among species, especially in epithelia whose performance depends on integrated transcellular and paracellular transport. Interestingly, the Mesh mammalian ortholog, Sushi domain-containing 2 (Susd2), is also highly expressed in mouse renal tubules (67) and displays regulatory activity in the growth of renal cancer cells (14). Further investigations on the functions of Mesh family proteins in other species will be an exciting avenue for exploration.
GRANTS
This project was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-110358 to A. R. Rodan), by Deutsche Forschungsgemeinschaft grants to A. Paululat (SFB 944 TP7, SFB 944 Z-Project) and to H. Meyer (SFB 944 TP21), and by a Grant-in-Aid for Scientific Research (C) (19K06650) to Y. Izumi from the Japan Society for the Promotion of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J., K.W.B., Y.I., and A.R.R. conceived and designed research; S.J., K.W.B., H.M., A.P., and Y.I. performed experiments; S.J., K.W.B., Y.I., and A.R.R. analyzed data; S.J., K.W.B., Y.I., and A.R.R. interpreted results of experiments; S.J. and Y.I. prepared figures; S.J. drafted manuscript; S.J., K.W.B., Y.I., M.F., and A.R.R. edited and revised manuscript; S.J., K.W.B., H.M., A.P., Y.I., M.F., and A.R.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Julian Dow and Shireen Davies for the generous gift of fly lines as well as the Bloomington Drosophila Stock Center at Indiana University (supported by NIH Grant P40-OD-018537), the National Institute of Genetics (NIG-Fly), and the Vienna Drosophila Resource Center for fly stocks. We also thank Dr. Joseph Dent for the anti-secCl antibody. Finally, we thank Gaëlle Mercenne, Linda Nikolova, and the University of Utah Electron Microscopy Core Laboratory for assistance with transmission electron microscopy and the University of Osnabrück for providing space and equipment under the auspices of K. W. Beyenbach’s guest professorship in the Department of Biology/Chemistry.
REFERENCES
- 1.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyenbach KW. Voltages and resistances of the anterior Malpighian tubule of Drosophila melanogaster. J Exp Biol 222: jeb201574, 2019. doi: 10.1242/jeb.201574. [DOI] [PubMed] [Google Scholar]
- 3.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf) 202: 387–407, 2011. doi: 10.1111/j.1748-1716.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 55: 351–374, 2010. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 5.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289: 113–116, 2000. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 6.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680, 2000. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal EM. Characterization of transepithelial potential oscillations in the Drosophila Malpighian tubule. J Exp Biol 204: 3075–3084, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 284: C718–C728, 2003. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 9.Browne A, O’Donnell MJ. Segment-specific Ca2+ transport by isolated Malpighian tubules of Drosophila melanogaster: a comparison of larval and adult stages. J Insect Physiol 87: 1–11, 2016. doi: 10.1016/j.jinsphys.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Cabrero P, Richmond L, Nitabach M, Davies SA, Dow JA. A biogenic amine and a neuropeptide act identically: tyramine signals through calcium in Drosophila tubule stellate cells. Proc Biol Sci 280: 20122943, 2013. doi: 10.1098/rspb.2012.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JA. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci USA 111: 14301–14306, 2014. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell K, Knust E, Skaer H. Crumbs stabilises epithelial polarity during tissue remodelling. J Cell Sci 122: 2604–2612, 2009. doi: 10.1242/jcs.047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Sayadian AC, Lowe N, Lovegrove HE, St Johnston D. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol 16: e3000041, 2018. doi: 10.1371/journal.pbio.3000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y, Wang X, Wang P, Li T, Hu F, Liu Q, Yang F, Wang J, Xu T, Han W. SUSD2 is frequently downregulated and functions as a tumor suppressor in RCC and lung cancer. Tumour Biol 37: 9919–9930, 2016. doi: 10.1007/s13277-015-4734-y. [DOI] [PubMed] [Google Scholar]
- 15.Deligiannaki M, Casper AL, Jung C, Gaul U. Pasiflora proteins are novel core components of the septate junction. Development 142: 3046–3057, 2015. doi: 10.1242/dev.119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denholm B. Shaping up for action: the path to physiological maturation in the renal tubules of Drosophila. Organogenesis 9: 40–54, 2013. doi: 10.4161/org.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denholm B, Hu N, Fauquier T, Caubit X, Fasano L, Skaer H. The tiptop/teashirt genes regulate cell differentiation and renal physiology in Drosophila. Development 140: 1100–1110, 2013. doi: 10.1242/dev.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol 13: 1052–1057, 2003. doi: 10.1016/S0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- 19.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Dube K, McDonald DG, O’Donnell MJ. Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophila melanogaster: roles of sequestration and secretion. J Insect Physiol 46: 1449–1460, 2000. doi: 10.1016/S0022-1910(00)00069-X. [DOI] [PubMed] [Google Scholar]
- 21.Feingold D, Knogler L, Starc T, Drapeau P, O’Donnell MJ, Nilson LA, Dent JA. secCl is a cys-loop ion channel necessary for the chloride conductance that mediates hormone-induced fluid secretion in Drosophila. Sci Rep 9: 7464, 2019. doi: 10.1038/s41598-019-42849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2: a002907, 2010. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuse M, Izumi Y. Molecular dissection of smooth septate junctions: understanding their roles in arthropod physiology. Ann N Y Acad Sci 1397: 17–24, 2017. doi: 10.1111/nyas.13366. [DOI] [PubMed] [Google Scholar]
- 24.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol 16: 181–188, 2006. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Gautam NK, Verma P, Tapadia MG. Ecdysone regulates morphogenesis and function of Malpighian tubules in Drosophila melanogaster through EcR-B2 isoform. Dev Biol 398: 163–176, 2015. doi: 10.1016/j.ydbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Green CR, Bergquist PR. Phylogenetic relationships within the invertebrates in relation to the structure of septate junctions and the development of ‘occluding’ junctional types. J Cell Sci 53: 279–305, 1982. [Google Scholar]
- 27.Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halberg KA, Rainey SM, Veland IR, Neuert H, Dornan AJ, Klämbt C, Davies SA, Dow JA. The cell adhesion molecule Fasciclin2 regulates brush border length and organization in Drosophila renal tubules. Nat Commun 7: 11266, 2016. doi: 10.1038/ncomms11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumi Y, Furuse K, Furuse M. Septate junctions regulate gut homeostasis through regulation of stem cell proliferation and enterocyte behavior in Drosophila. J Cell Sci 132: jcs232108, 2019. doi: 10.1242/jcs.232108. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Furuse M. Molecular organization and function of invertebrate occluding junctions. Semin Cell Dev Biol 36: 186–193, 2014. doi: 10.1016/j.semcdb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Izumi Y, Yanagihashi Y, Furuse M. A novel protein complex, Mesh-Ssk, is required for septate junction formation in the Drosophila midgut. J Cell Sci 125: 4923–4933, 2012. doi: 10.1242/jcs.112243. [DOI] [PubMed] [Google Scholar]
- 32.Izumi Y, Motoishi M, Furuse K, Furuse M. A tetraspanin regulates septate junction formation in Drosophila midgut. J Cell Sci 129: 1155–1164, 2016. doi: 10.1242/jcs.180448. [DOI] [PubMed] [Google Scholar]
- 33.Jonusaite S, Donini A, Kelly SP. Occluding junctions of invertebrate epithelia. J Comp Physiol B 186: 17–43, 2016. doi: 10.1007/s00360-015-0937-1. [DOI] [PubMed] [Google Scholar]
- 34.Jonusaite S, Donini A, Kelly SP. Salinity alters snakeskin and mesh transcript abundance and permeability in midgut and Malpighian tubules of larval mosquito, Aedes aegypti. Comp Biochem Physiol A Mol Integr Physiol 205: 58–67, 2017. doi: 10.1016/j.cbpa.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Jonusaite S, Kelly SP, Donini A. Tissue-specific ionomotive enzyme activity and K+ reabsorption reveal the rectum as an important ionoregulatory organ in larval Chironomus riparius exposed to varying salinity. J Exp Biol 216: 3637–3648, 2013. doi: 10.1242/jeb.089219. [DOI] [PubMed] [Google Scholar]
- 36.Kolosov D, Jonusaite S, Donini A, Kelly SP, O’Donnell MJ. Septate junction in the distal ileac plexus of larval lepidopteran Trichoplusia ni: alterations in paracellular permeability during ion transport reversal. J Exp Biol 222: jeb204750, 2019. doi: 10.1242/jeb.204750. [DOI] [PubMed] [Google Scholar]
- 37.Lane NJ, Dallai R, Martinucci G, Burighel P. Electron microscopic structure and evolution of epithelial junctions. In: Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease, edited by Citi S. Austin, TX: Landes, 1994, p. 23–43. [Google Scholar]
- 38.Lane NJ, Skaer HB. Intercellular junctions in insect tissues. In: Advances in Insect Physiology, edited by Berridge MJ, Treherne JE, Wigglesworth VB. London: Academic, 1980, p. 35–213. [Google Scholar]
- 39.Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 459: 1141–1145, 2009. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 40.Leader DP, Krause SA, Pandit A, Davies SA, Dow JA. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res 46: D809–D815, 2018. doi: 10.1093/nar/gkx976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linton SM, O’Donnell MJ. Contributions of K+:Cl− cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 202: 1561–1570, 1999. [DOI] [PubMed] [Google Scholar]
- 42.MacMillan HA, Yerushalmi GY, Jonusaite S, Kelly SP, Donini A. Thermal acclimation mitigates cold-induced paracellular leak from the Drosophila gut. Sci Rep 7: 8807, 2017. doi: 10.1038/s41598-017-08926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manfruelli P, Arquier N, Hanratty WP, Sémériva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development 122: 2283–2294, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Corrales G, Cabrero P, Dow JA, Terhzaz S, Davies SA. Novel roles for GATAe in growth, maintenance and proliferation of cell populations in the Drosophila renal tubule. Development 146: dev178087, 2019. doi: 10.1242/dev.178087. [DOI] [PubMed] [Google Scholar]
- 45.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004: pl6, 2004. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 46.Miguel-Aliaga I, Jasper H, Lemaitre B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 210: 357–396, 2018. doi: 10.1534/genetics.118.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noirot-Timothée C, Noirot C. Septate and scalariform junctions in arthropods. Int Rev Cytol 63: 97–140, 1980. doi: 10.1016/S0074-7696(08)61758-1. [DOI] [PubMed] [Google Scholar]
- 48.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 199: 1163–1175, 1996. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell MJ, Maddrell SH, Gardiner BO. Passage of solutes through walls of Malpighian tubules of Rhodnius by paracellular and transcellular routes. Am J Physiol Regul Integr Comp Physiol 246: R759–R769, 1984. doi: 10.1152/ajpregu.1984.246.5.R759. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 51.Pannabecker TL, Hayest TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol 132: 63–76, 1993. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- 52.Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron 32: 415–424, 2001. doi: 10.1016/S0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 53.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 109: 21528–21533, 2012. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodan AR. The Drosophila Malpighian tubule as a model for mammalian tubule function. Curr Opin Nephrol Hypertens 28: 455–464, 2019. doi: 10.1097/MNH.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodan AR, Baum M, Huang CL. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol 303: C883–C894, 2012. doi: 10.1152/ajpcell.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosay P, Davies SA, Yu Y, Sözen MA, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Salazar AM, Resnik-Docampo M, Ulgherait M, Clark RI, Shirasu-Hiza M, Jones DL, Walker DW. Intestinal Snakeskin limits microbial dysbiosis during aging and promotes longevity. iScience 9: 229–243, 2018. doi: 10.1016/j.isci.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxena A, Denholm B, Bunt S, Bischoff M, VijayRaghavan K, Skaer H. Epidermal growth factor signalling controls myosin II planar polarity to orchestrate convergent extension movements during Drosophila tubulogenesis. PLoS Biol 12: e1002013, 2014. doi: 10.1371/journal.pbio.1002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schellinger JN, Rodan AR. Use of the Ramsay assay to measure fluid secretion and ion flux rates in the Drosophila melanogaster Malpighian tubule. J Vis Exp 105: 53144, 2015. doi: 10.3791/53144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schubiger M, Feng Y, Fambrough DM, Palka J. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron 12: 373–381, 1994. doi: 10.1016/0896-6273(94)90278-X. [DOI] [PubMed] [Google Scholar]
- 61.Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001. doi: 10.1152/ajpcell.2001.281.2.C449. [DOI] [PubMed] [Google Scholar]
- 62.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skaer H. The alimentary canal. In: The Development of Drosophila Melanogaster, edited by Bate M, Martinez A. New York: Cold Spring Harbor Lab, 1993, p. 941–1012. [Google Scholar]
- 64.Skaer HL, Maddrell S. How are invertebrate epithelia made tight? J Cell Sci 88: 139–141, 1987. [Google Scholar]
- 65.Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol 127: 1345–1360, 1994. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugahara T, Yamashita Y, Shinomi M, Yamanoha B, Iseki H, Takeda A, Okazaki Y, Hayashizaki Y, Kawai K, Suemizu H, Andoh T. Isolation of a novel mouse gene, mSVS-1/SUSD2, reversing tumorigenic phenotypes of cancer cells in vitro. Cancer Sci 98: 900–908, 2007. doi: 10.1111/j.1349-7006.2007.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Q, Wu Y, Jonusaite S, Pleinis JM, Humphreys JM, He H, Schellinger JN, Akella R, Stenesen D, Krämer H, Goldsmith EJ, Rodan AR. Intracellular chloride and scaffold protein Mo25 cooperatively regulate transepithelial ion transport through WNK signaling in the Malpighian tubule. J Am Soc Nephrol 29: 1449–1461, 2018. doi: 10.1681/ASN.2017101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol 161: 563–596, 1994. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 70.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol 28: 655–685, 2012. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 71.Terhzaz S, Cabrero P, Chintapalli VR, Davies SA, Dow JA. Mislocalization of mitochondria and compromised renal function and oxidative stress resistance in Drosophila SesB mutants. Physiol Genomics 41: 33–41, 2010. doi: 10.1152/physiolgenomics.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terhzaz S, O’Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JA. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 202: 3667–3676, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Wang S, Rubenfeld A, Hayes T, Beyenbach K. Leucokinin increases paracellular permeability in insect Malpighian tubules. J Exp Biol 199: 2537–2542, 1996. [DOI] [PubMed] [Google Scholar]
- 74.Wessing A, Zierold K. Metal-salt feeding causes alterations in concretions in Drosophila larval Malpighian tubules as revealed by X-ray microanalysis. J Insect Physiol 38: 623–632, 1992. doi: 10.1016/0022-1910(92)90114-S. [DOI] [Google Scholar]
- 75.Wessing A, Zierold K, Hevert F. Two types of concretions in Drosophila Malpighian tubules as revealed by X-ray microanalysis: a study on urine formation. J Insect Physiol 38: 543–554, 1992. doi: 10.1016/0022-1910(92)90080-W. [DOI] [Google Scholar]
- 76.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol 134: 1469–1482, 1996. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, Baum M, Huang CL, Rodan AR. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am J Physiol Regul Integr Comp Physiol 309: R747–R756, 2015. doi: 10.1152/ajpregu.00148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Y, Schellinger JN, Huang CL, Rodan AR. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem 289: 26131–26142, 2014. doi: 10.1074/jbc.M114.577767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao X, Yang L, Pang X, Zhang R, Zhu Y, Wang P, Gao G, Cheng G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat Microbiol 2: 17020, 2017. doi: 10.1038/nmicrobiol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu C, Tang HW, Hung RJ, Hu Y, Ni X, Housden BE, Perrimon N. The septate junction protein Tsp2A restricts intestinal stem cell activity via endocytic regulation of aPKC and Hippo signaling. Cell Reports 26: 670–688.e6, 2019. doi: 10.1016/j.celrep.2018.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanagihashi Y, Usui T, Izumi Y, Yonemura S, Sumida M, Tsukita S, Uemura T, Furuse M. Snakeskin, a membrane protein associated with smooth septate junctions, is required for intestinal barrier function in Drosophila. J Cell Sci 125: 1980–1990, 2012. doi: 10.1242/jcs.096800. [DOI] [PubMed] [Google Scholar]
- 82.Yu M, Beyenbach KW. Leucokinin and the modulation of the shunt pathway in Malpighian tubules. J Insect Physiol 47: 263–276, 2001. doi: 10.1016/S0022-1910(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 83.Yu MJ, Beyenbach KW. Effects of leucokinin-VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol 207: 519–526, 2004. doi: 10.1242/jeb.00772. [DOI] [PubMed] [Google Scholar]