Abstract
Chemokines are a family of soluble cytokines that act as chemoattractants to guide the migration of cells, in particular of immune cells. However, chemokines are also involved in cell proliferation, differentiation, and survival. Chemokines are associated with a variety of human diseases including chronic inflammation, immune dysfunction, cancer, and metastasis. This review discusses the expression of CC and CXC chemokines in the tumor microenvironment and their supportive and inhibitory roles in tumor progression, angiogenesis, metastasis, and tumor immunity. We also specially focus on the diverse roles of CXC chemokines (CXCL9–11, CXCL4 and its variant CXCL4L1) and their two chemokine receptor CXCR3 isoforms, CXCR3-A and CXCR3-B. These two distinct isoforms have divergent roles in tumors, either promoting (CXCR3-A) or inhibiting (CXCR3-B) tumor progression. Their effects are mediated not only directly in tumor cells but also indirectly via the regulation of angiogenesis and tumor immunity. A full comprehension of their mechanisms of action is critical to further validate these chemokines and their receptors as biomarkers or therapeutic targets in cancer.
Keywords: angiogenesis, cancer, CC and CXC chemokines, CXCR3, tumor immunity, tumor invasion
INTRODUCTION
Chemokines are a family of soluble proteins with low molecular mass (8–15 kDa) that were originally identified as mediators of the inflammatory process and regulators of leukocyte trafficking (74, 75). Chemokines play important roles in development, homeostasis, and angiogenesis but also are involved in autoimmune diseases, in tumor-related inflammation and immunity, as well as tumor growth and metastasis (96, 135). Chemokines are divided into four distinct subfamilies according to the number and spacing of two conserved NH2-terminal cysteine residues. In the CXC and CX3C chemokines subfamilies, the two conserved NH2-terminal cysteines are separated by one and three amino acids, respectively. In the CC chemokines subfamily, the two conserved NH2-terminal cysteines are adjacent and in the C chemokines subfamily chemokines lack the second and fourth cysteines. The CXC chemokines subfamily is further classified on the basis of the presence or absence of a three amino acid sequence, glutamic acid-leucine-arginine (the “ELR” motif), immediately proximal to the CXC sequence. The ELR-positive CXC chemokines are potent promoters of angiogenesis, whereas the ELR-negative CXC chemokines are potent inhibitors of angiogenesis (71, 108). The CC and the CXC chemokines are the two major subfamilies of chemokines that are mainly expressed in the tumor microenvironment and that play a significant role in tumor progression, tumor-related inflammation and immunity, and tumor invasion (9, 17, 52, 117).
Most of the chemokines exert their biological effects through the activation of G protein-coupled seven transmembrane receptors (GPCRs) (79, 115). Similar to their ligands, chemokine receptors are classified into four groups, namely XCR, CCR, CXCR, and CX3CR. The aim of this review is to give a general overview of the importance in tumor biology of CC and CXC chemokines and their receptors with a particular focus on CXCR3 and CXCR3-interacting chemokines. We will highlight several aspects including direct effects on tumor cells or indirect control via the tumor microenvironment including the vascular and immune system.
OVERVIEW OF THE IMPORTANCE OF THE CC AND CXC CHEMOKINES IN THE TUMOR AND THE TUMOR MICROENVIRONMENT
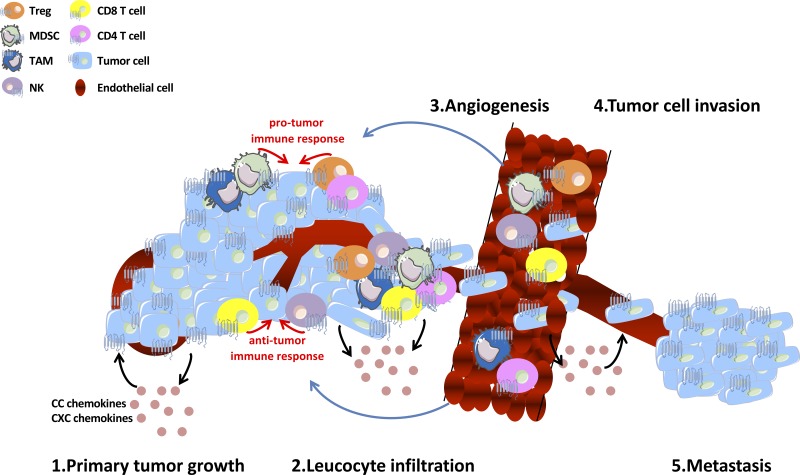
Several studies demonstrated that tumor cells secrete chemokines in an autocrine fashion to directly promote tumor cell growth, survival, and metastasis or in a paracrine fashion to activate stromal cells, which support tumor angiogenesis, metastatic spread, and immune escape. Indeed, the tumor microenvironment is composed of an extensive and varied mixture of CC and CXC chemokines, which modulate tumor growth, angiogenesis, invasion, and leukocyte infiltration into the tumor (9, 121) (Fig. 1 and Table 1). Several examples are given below.
Fig. 1.
Schema illustrating the multiple roles of CC and CXC chemokines network in the tumor and in the tumor microenvironment. Numerous host stromal cells types, such as endothelial cells and immune cells, are activated by these chemokines and modulate positively or negatively tumor cell proliferation, invasion, tumor angiogenesis and tumor immunity. MDSC, myeloid-derived suppressor cells; NK, natural killer cell; TAM, tumor-associated macrophage; Treg, regulatory T cell.
Table 1.
Classification of CC and CXC chemokines into functional subfamilies
| Tumor Type |
||||||
|---|---|---|---|---|---|---|
| Functions | Reproductive System | Breast | Skin | Lung | Digestive System | Kidney |
| Direct tumor-promoting functions | CCL2 (80); CXCL12 (101) | CCL1 (24); CXCL4, CXCL4L1 (94); CXCL12 (45, 77) | CCL1 (14, 24); CXCL1 (83); CXCL10 (27) | CCL25 (61); CXCL8 (85, 135) | CXCL4L1 (93); CXCL1, CXCL8 (112); CXCL12 (100) | CXCL7 (38) |
| Tumor angiogenic functions | CXCL8 (1) | CCL2 (13); CXC8 (21) | CXCL4L1 (110); CXCL8 (121) | CXCL2 (20); CXCL4 (132); CXCL4L1 (110); CXCL5 (5); CXCL10 (7) | CXCL4L1 (93) | CXCL7 (38); CXCL8 (44); CXCL9 (87) |
| Immune regulatory functions | CCL28 (30); CXCL1, CXCL2, CXCL5 (113) | CXCL10 (68, 126) | CCL2-3-4-5 (42, 73); CXCL9-10 (23, 27, 42, 73, 89, 128) | CCL2 (6); CXCL12 (123) | CXCL4 (26); CXCL9-10 (23); CXCL10 (62) | CXCL9 (87) |
Chemokines have been classified depending on the tumor type for their roles in 1) promoting tumor development and invasion (Direct tumor-promoting functions), 2) regulating tumor angiogenesis (Tumor angiogenic functions), and 3) regulating tumor immunity (Immune regulatory functions such as recruitment of myeloid derived suppressor cells, accumulation of highly cytotoxic natural killer cells, recruitment of tumor associated-macrophages, recruitment of regulatory or effector T cells). References are shown in parentheses.
Direct Tumor-Promoting Functions
Tumor-promoting effects have been described for these chemokines. The expression and secretion of CCL2 were reported to be upregulated in an established cabazitaxel-resistant prostate cancer cell line. The use of a CCR2 (specific receptor of CCL2) antagonist suppressed the proliferation of these chemoresistance prostate cancer cell line under treatments of cabazitaxel. Thus the CCL2-CCR2 axis appears as a key contributor to cabazitaxel resistance in prostate cancer cells (80). CCL1 is produced by lymph node lymphatic sinuses and promotes the development of metastasis through the recruitment of CCR8+ (specific receptor of CCL1) tumor cells to lymph node. Tumor cell migration to lymphatic endothelial cells in vitro is inhibited by blocking CCR8 or CCL1, and recombinant CCL1 promotes migration of CCR8-expressing tumor cells. In three mouse models of melanoma and breast cancer, blocking CCR8 or knockdown with shRNA significantly decreased lymph node metastasis (24). The CCL1-CCR8 axis has been identified as a critical checkpoint for the entry of metastases into the lymph nodes. In non-small cell lung cancer (NSCLC), CCR9 is highly expressed (61). Furthermore, the CCL25-CCR9 axis has been reported to induce NSCLC tumor progression through the suppression of tumor cell apoptosis (61).
As for CXC chemokines, CXCL1 was characterized as an autocrine growth factor in melanoma cells (14) and the use of specific antibodies targeting CXCL1 or its receptor CXCR2 inhibited the growth of melanoma cells in vitro (83). Another CXCR2 ligand, CXCL2 has been reported to be overexpressed in melanoma cell lines and to increase their ability to form colonies in soft agar and their tumorigenicity in nude mice (86). A few other studies have proposed a similar role for CXCL1 and CXCL8 in pancreas and non-small cell lung carcinoma (85, 112, 134). The CXCL12-CXCR4 axis is also of particular importance for the metastatic localization of many cancers (77, 101). In fact, CXCR4 is by far the most common chemokine receptor expressed in most cancers and the expression of its ligand CXCL12 is highest in sites of metastasis such as lung, liver, and lymph nodes and at lower levels in the brain (77).
A protumorigenic role in pancreatic ductal adenocarcinoma development for the endogenous CXCR3 ligand CXCL4L1 has been defined. In vivo administration of a blocking mouse monoclonal antibody against CXCL4L1 demonstrated inhibition of tumor growth when CXCR3 is expressed in tumor cells (93). On the other end, tumor cell invasion and metastasis are dependent on CXCR3 and may be inhibited by CXCR3 blockade (for details, see Role of CXCR3 in tumor cells later in this review).
In clear cell renal cell carcinomas (ccRCC), inhibition of the CXCL7 receptors CXCR1 and CXCR2 was sufficient to decrease both the tumor vasculature and tumor cell proliferation, suggesting that the CXCL7-CXCR1/CXCR2 axis may be a suitable target for the treatment of ccRCC (38).
Chemokine fragments have been reported to inhibit tumor growth by direct molecular interaction with other cytokines (CCL5, EGF, and FGF) (88, 94). For example, it has been reported that the COOH-terminal peptides of CXCL4 and CXCL4L1 (CXCL447–70 and CXCL4L147–70, respectively) have both direct EGF-dependent antiproliferative effects in MDA-MB-231 tumor cells. In addition, mostly CXCL447–70 exerted an antitumor immunity effect on EGF-dependent MDA-MB-231 tumor growth by multimerizing with the monocyte chemoattractant CCL5, consequently enhancing migration of monocytic cells (94).
Angiogenic Functions of Chemokines
With regard to angiogenesis, CXC chemokines are pivotal mediators that activate (ELR-positive CXC chemokines) or inhibit (ELR-negative CXC chemokines) this process through their interaction with cognate receptors expressed by endothelial cells. CXCL5 and CXCL8 exert potent angiogenic properties on endothelial cells through interaction with their cognate receptors CXCR1 and CXCR2. CXCL5 is an important regulator of human non-small cell lung cancer angiogenic activity (5). CXCL8 is involved in many cancers (breast, prostate, melanoma, renal cell carcinoma, glioblastoma), and several strategies blocking CXCL8 have been reported to induce significant damage to the tumor neo-vasculature (1, 21, 44, 105, 119). Angiostatic CXC chemokines include CXCL9, CXCL10, CXCL11, and CXCL4 and its variant CXCL4L1 that exert potent antiangiogenic properties on endothelial cells. They have been reported as potent inhibitors of tumor angiogenesis in several in vivo tumor models (breast cancer, melanoma, lung cancer, pancreatic cancer) (8, 93, 94, 109, 119). However, there is one exception to this rule, because CXCL2 (gro-β), a ELR-positive CXC chemokine, also exhibits antiangiogenic activities (20).
As for CC chemokines, CCL2 appears to indirectly promote tumor angiogenesis in breast cancer by recruiting macrophages (13, 67).
Immune Regulatory Functions
Effects of these chemokines on leukocyte trafficking and the immune system have also been described. The composition of the leukocyte infiltrate in many cancers is regulated by CC and CXC chemokines produced by tumor and stromal cells. CC and CXC chemokine expression may influence tumor progression by shaping the infiltrating immune cell population (Fig. 1). CCL2 is crucial in the recruitment of immune cells such as myeloid-derived suppressor cells (MDSCs) or tumor-associated macrophages (TAMs) (6, 113). Natural killer (NK) cells play an important role in antitumor immunity. During the progression of malignant melanoma to lymph node metastasis, highly cytotoxic NK cells become enriched in lymph nodes and recruitment of this NK cell population is induced by the release of CXCL8 in the tumor microenvironment (4). The CXCR3-CXCL10 axis is also a prerequisite for NK cell infiltration into tumors. Enforced CXCL10 expression in tumor cells increased the number of NK cells in the tumors and prolonged NK cell-dependent survival (126). Harlin et al. (42) have reported that a subset of six chemokines (CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10) is preferentially expressed in tumors that contained T cells. They showed that CXCL9 and CXCL10 are crucial chemokines in the tumor microenvironment of melanoma and are able to recruit CD8 effector T cells in a murine xenograft model (42). CCL5 could also be related to a more efficient antitumor immunity by recruiting T lymphocytes (82). In lung adenocarcinoma tumors, high CXCL12 expression correlated with increased tumor inflammation and increased recruitment of regulatory T cells (Tregs) (123). In ovarian cancer, Facciabene et al. (30) proposed that tumor hypoxia induces the expression of chemotactic factors such as CCL28. They showed that a direct link exists between tumor CCL28 upregulation and accelerated tumor growth, which is specifically attributable to Tregs recruitment in vivo through its cognate receptor CCR10.
Organotropism of Chemokines
The question may arise whether the different CC or CXC chemokines exhibit preferential organ expression and function. In general these chemokines are widely distributed and are unlikely to have specific organotropism. For example, CXCL10 has tumor-promoting and chemotactic function in both the brain (27) and the lung (90, 124). However, in breast carcinoma, the function may be inhibitory due to accumulation of NK cells (126). Furthermore, CXCL12 has tumor-promoting or inhibitory effects in the pancreas (100), the prostate (101), and the breast (45, 77).
CXCR3 AND ITS LIGANDS: BIOCHEMICAL PROPERTIES AND PRECISE ROLE IN TUMOR PROGRESSION
CXCR3 Structure
The chemokine receptor CXCR3, also known as G protein-coupled receptor 9 (GPR9) or CD183, is a GPCR first reported for its selective recruitment of effector T cells (65) and is now known to be a critical mediator of inflammation, vascular diseases, and cancer (95). CXCR3 belongs to the CXCR subfamily (CXCR1 to CXCR7). The CXCR3 gene is present on chromosome X (q13) (gene ID: 2833), which is in clear contrast to all other CXCR genes mainly localized on chromosome 2. CXCR3 receptor is composed of an extracellular NH2-terminal portion, six membrane-associated loops separated by seven transmembrane domains, and a cytoplasmic COOH-terminal segment. A conserved structural feature of chemokine receptors includes the presence of two conserved Cys, one in the NH2-terminal and the second in the third extracellular loop, which form a disulfide bridge essential for ligand binding and receptor activation (36, 49). The second intracellular loop has the conserved motif DRYLAIV, which is required for heterotrimeric GTP-binding proteins (G proteins) coupling. CXCR3 interacts with the interferon-γ (IFN-γ)-inducible CXCR3 ligands CXCL9, CXCL10 and CXCL11. CXCR3 has two distinct intracellular domains for activation: one is a COOH-terminal domain required by CXCL9 and CXCL10, and another is in the third intracellular loop required by CXCL11 (23a). The interaction with CXCR3 is not firmly established for CXCL4 since this molecule may also interfere with glycosaminoglycan and growth factor (11).
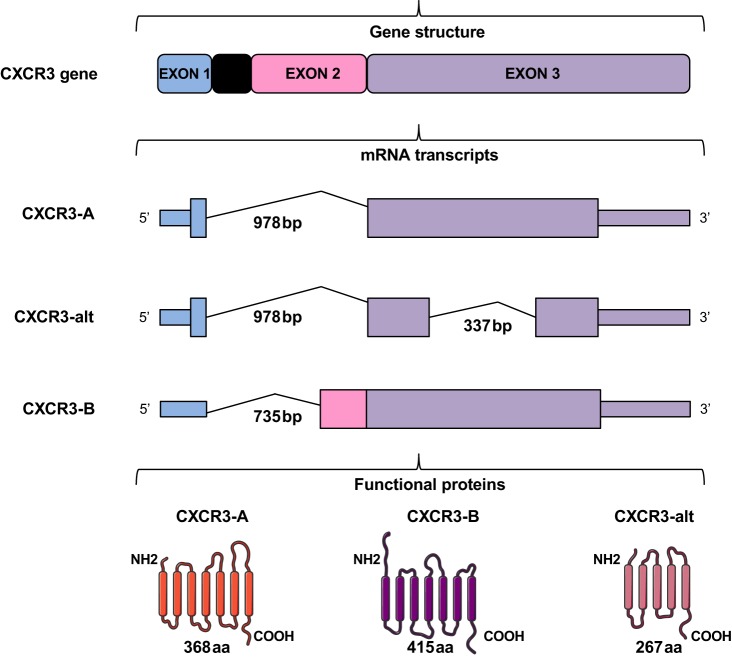
Human CXCR3 gene is alternatively spliced, generating three isoforms with unique characteristics differing either in their NH2 or in their COOH-terminal sequence: CXCR3-A, CXCR3-B and CXCR3-alt (Fig. 2). CXCR3-A isoform codes for a protein of 368 amino acids, and CXCR3-B isoform codes for a larger protein of 415 amino acids with an extension at the NH2 terminus of 52 amino acids compare with CXCR3-A (59). CXCL9, CXCL10, and CXCL11 are known to interact with both the splice variants CXCR3-A and CXCR3-B, whereas CXCL4 is claimed to selectively interact with CXCR3-B (59). If CXCL4 binding to CXCR3-B has been reported, CXCL4 can also interact directly with growth factors such as the fibroblast growth factor 2 (FGF-2) and impair its activity (88). Other mechanisms may also be involved. Indeed, chemotaxis-induced by CXCL4 in monocytes seems not dependent on CXCR3 but on CCR1 (32). These differences may be due to the different tissue/cell context as well as to different mechanisms involved. It has been reported that both CXCR3-A and CXCR3-B are implicated in the chemotactic effects of CXCL4L1 and the inhibition of the vasculature (111). CXCL4L1 exhibits a unique structure not found in any other CXC chemokine (57) with a modified COOH-terminal α-helix that swings out from the whole molecule. This will significantly decrease affinity to heparan sulfates. Interaction between CXCL4L1 and CXCR3-A seems more favorable, which, in part, is due to its lower glycan-binding affinity (57) that also alters the oligomeric state of the molecule (22).
Fig. 2.
Schematic representation of CXCR3 isoforms. The CXCR3 gene generates three chemokine receptors, resulting from alternative splicing. CXCR3-A results from splicing of a single intron. Exon 1 encodes 4 amino acids and exon 3 encodes the remaining 364 amino acids. CXCR3-alt results from posttranscriptional exon skipping and is predicted to have only four or five transmembrane domains. Its nucleotide sequence is identical with that of CXCR3-A except for missing bases 696–1032 in the exon 2 (i.e., 337 bp in the region common to CXCR3-A and CXCR3-B). CXCR3-B contains a unique NH2-terminal tail of 52 amino acids encoded by exon 2 and the rest sequences are common to CXCR3-A. GPCR structures of each CXCR3 isoform were modified from SMART (Servier Medical Art).
CXCR3-alt is a 101-amino acid-truncated version differing from CXCR3-A in its COOH-terminal sequence with a predicted 4–5 transmembrane domain structure (28).
CXCR3-alt lacks the third intracellular loop, which is also important for G protein interaction and subsequent activation of intracellular components (116). However, CXCR3-alt is still able to mediate CXCL11 but not CXCL9- or CXCL10-dependent chemotactic activity.
CXCR3 Signaling
To elicit signaling, GPCRs need to couple with G proteins composed of the α, β, and γ-subunits (81). The binding of a chemokine to its receptor leads to the activation of the regulatory α-subunit and results in the dissociation of the catalytic βγ-subunits complex. Upon activation of CXCR3, G proteins regulate a broad spectrum of signaling pathways such as adenylyl cyclase, phospholipase C (PLC), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinases (MAPK) that affect several cellular responses (calcium influx, proliferation, integrin activation, actin reorganization, and migration) (12, 18, 76, 104).
The CXCR3-A isoform is associated with the Gαi or Gαq subunit. Stimulation of the receptor leads to the activation of signaling pathways such as MAPK (ERK1/2, p38, and JNK) and PI3K/Akt, which induce elevation of intracellular calcium, DNA synthesis and cell proliferation or chemotaxis both in physiological cells (human airway epithelial cells and type II pneumocyte) as well as in cancer cells (3, 47, 70). In prostate carcinoma cells, CXCR3-A signaling mainly via Gαq subunits activates PLCβ, which induces cell motility and invasion via μ-calpain (130). CXCR3-alt isoform has been shown to elicit signaling such as ERK1/2 and Akt phosphorylation (28, 55) but has not been found to be involved in cell growth (3). The CXCR3-B isoform mediates antiproliferative, angiostatic, and proapoptotic signals. It has been shown that CXCR3-B activation inhibits endothelial cell proliferation and migration through the Gαs subunit that activates adenylyl cyclase (53).
Ligand-biased signaling, the ability of different ligands for the same receptor to selectively activate some signaling pathways while blocking others, is now an established paradigm for GPCR signaling (10, 107, 129). In the case of CXCR3, the concept of biased signaling is used by nature to fine-tune the CXCR3 biological responses. CXCR3 isoforms are known to induce activation of distinct pathways that couple to specific G proteins but also to additional downstream effectors such as the regulatory and scaffolding proteins β-arrestins. Several studies have tested whether different ligands targeting CXCR3 displayed bias between G-protein signaling, β-arrestin recruitment, ERK1/2 phosphorylation, and CXCR3 internalization.
Berchiche and Sakmar (10) have reported that both CXCL10 and CXCL11 activated CXCR3-A through Gαi signaling. Chemokines CXCL9 and CXCL4 failed to trigger significant Gαi activity even when CXCR3-A was stimulated with chemokine concentrations as high as 100 nM. CXCL10 and CXCL11 increased β-arrestin 1/2 recruitment to the receptor CXCR3-A and induced ERK1/2 phosphorylation with similar efficacy and potency. A small but statistically significant elevation in β-arrestin 2 recruitment to CXCR3-A after stimulation with CXCL9 or CXCL4 was also observed; however, only CXCL9 triggered modest ERK1/2 phosphorylation. CXCR3-B-mediated Gαi activation and β-arrestin recruitment were only detected after stimulation with 100 nM of CXCL11 but not at lower concentrations nor in the presence of the other CXC chemokines (10, 107). Smith et al. (107) focused on receptor coupling to two GRK receptor families, GRK2/3 and GRK5/6, and showed by siRNA knockdown that both GRK2/3 and GRK5/6 are involved in CXCL11-mediated β-arrestin 2 recruitment to CXCR3-A but that only GRK2/3 is involved in β-arrestin 2 recruitment to CXCR3-B. CXCR3-B acted as a β-arrestin-biased receptor relative to CXCR3-A, albeit with a less stable interaction with β-arrestin compared with CXCR3-A. This bias may be encoded at least in part by differential GRK recruitment, since siRNA knockdown of GRK5/6 attenuated β-arrestin recruitment to CXCR3-A but not to CXCR3-B (107). CXCL9 and CXCL11 induced ERK1/2 phosphorylation after stimulation of CXCR3-B, but CXCL4 stimulation seems only to lead to a weak response. All the chemokines tested induced internalization of CXCR3-A and CXCR3-B. CXCR3-alt failed to induce significant Gαi activation and β-arrestin recruitment in response to all chemokines tested. CXCR3-alt signaling was limited to modest ligand-induced receptor internalization and ERK1/2 phosphorylation in response to CXCL11, CXCL10, and CXCL9. CXCL11-mediated ERK1/2 phosphorylation through CXCR3-A was stronger and lasted longer than the one mediated through both CXCR3-B and CXCR3-Alt (10). Thus similar to ligand-mediated Gαi signaling and β-arrestin recruitment, CXCR3-mediated ERK1/2 phosphorylation intensity and signal duration depend on the CXCR3 isoform/chemokine pair assessed.
Ligand-mediated Gαi activation, β-arrestin recruitment, receptor internalization, and ERK1/2 phosphorylation may occur independently from each other. The differences in CXCR3 isoforms signaling support the idea that the interaction of a specific ligand with a CXCR3 isoform selectively determines receptor conformation. In fact, agonist activation resulted in totally different receptor conformational states for CXCR3-A when compared with CXCR3-B. Using the plasmon waveguide resonance (PWR) technique, Boyé et al. (15) showed that CXCR3-A ligand activation induced a receptor conformational change characterized by a receptor elongation and an increase in packing and ordering of the transmembrane helices. Strikingly, CXCR3-B ligand activation resulted in an opposite response with a conformational change leading to a looser and less ordered arrangement of the transmembrane helices accompanied by a slight decrease in length (15). Thus, depending on the CXCR3 isoform, agonist-mediated CXCR3 activation leads to opposite conformational changes. This may be related to different efficacy of Gαi activity and β-arrestin recruitment. The fact that CXCR3 isoforms activate different signaling pathways in response to different CXC chemokines supports the idea that CXCR3 ligands are not functionally redundant (72).
Ambivalent Roles of CXCR3 in Tumor Progression and Tumor Microenvironment
Emerging research and clinical evidence demonstrate that specific CXCR3 isoform expression and CXCR3 ligand-mediated signaling cascades on both tumor cells and stromal cells are central to regulate negatively or positively tumor progression, metastasis, angiogenesis, and tumor immunity. CXCR3-A and CXCR3-B are the most studied isoforms in tumor progression. CXCR3-A induces chemotaxis, cell proliferation, and migration, whereas CXCR3-B activation leads to apoptosis and inhibits cell migration. Therefore, CXCR3 ligands have opposing effects, depending on the splice variants and the localization of CXCR3 either in tumor cells or in the tumor stroma.
Role of CXCR3 in tumor cells.
The relative expression of the two splice variants CXCR3-A and CXCR3-B in cancer cells suggests different outcomes after CXCR3 activation. As a general rule, when CXCR3-A is predominant, the outcome is stimulation of various biological processes (proliferation, migration, etc.) whereas when CXCR3-B is predominant, the opposite occurs. For example, in papillary thyroid carcinoma, CXCR3-A and CXCL10 mRNA levels are increased and CXCR3-A expression remains greater than CXCR3-B, and this promotes tumor cell proliferation (117a). In primary human breast cancer as well as breast cancer cell lines, CXCR3-A dominates CXCR3-B expression. However, when CXCR3-B is overexpressed in the MDA-MB-231 breast cancer cell line, ligand-stimulated proliferation is inhibited via reduced activation of ERK1/2 and p38 kinases (63). In human renal cancer tissues, CXCR3-A is upregulated and the growth-inhibitory CXCR3-B is markedly downregulated. Overexpression of CXCR3-B in renal cancer cells significantly inhibits cell proliferation and promotes apoptosis (25). In multiple myeloma, CXCR3 expression is increased across the pathological stages, being higher in stage III than in stage I. The presence of both isoforms, CXCR3-A and CXCR3-B, was observed and is correlated with the cell cycle progression and apoptosis. The antiapoptotic effect of CXCL10 in myeloma cells is observed only when CXCR3-A is overexpressed, whereas the antiproliferative effect of CXCL10 only occurs when significant levels of CXCR3-B are expressed (37). High expression of CXCR3-A mRNA was seen in vitro and in vivo in the pancreatic ductal adenocarcinoma Panc-1 cells. The administration of an antibody specific to CXCL4L1 led to a reduction of tumor size and tumor weigh in both subcutaneously and orthotopically implanted Panc-1 tumors with no impact on tumor angiogenesis (93).
CXCR3 has also a role in tumor cell dissemination. In fact, CXCR3 may have a role in the homing of tumor cells toward sites where CXC chemokines are abundant. Several studies have reported that expression of CXCR3 in melanoma, colon, glioblastoma, and breast carcinoma accelerated tumor metastasis to lymph nodes (50, 51, 69, 91). In melanoma for instance, CXCL10 is upregulated in astrocytes and attracts melanoma cells into the brain (27). Furthermore, CXCR3 is upregulated in melanoma cells and invasion is inhibited by nano-particle siRNA delivery (27). These data are in agreement with the results obtained from our laboratory on the mechanism of glioblastoma invasion where CXCR3 is upregulated at the cell surface to promote invasion. Mechanistically, a cross talk between LRP1 and CXCR3 is involved in this process (16). Indeed, LRP1 forms a complex with CXCR3 and is implicated in CXCR3 trafficking. In the invasive front of glioblastoma, LRP1 is downregulated and localization of CXCR3 is increased at the cell surface, which promotes invasion of tumor cells. It should be mentioned that metastatic melanoma cells express reduced amounts of LRP1, which suggests that a similar CXCR3-dependent mechanism might be involved (122). Moreover, blockade of CXCR3 led to the inhibition of lung metastasis in murine osteosarcoma models (90). Indeed, it has been observed that tail vein injection of osteosarcoma cell lines treated with the CXCR3 inhibitor AMG487 led to an inhibition of metastasis formation in the lung (90). Thus CXCR3 and their ligands may intervene in the beginning of the dissemination of osteosarcoma cells to the lungs and also at a later stage by stimulating growth and expansion of metastatic foci. It has been further shown that CXCR3, when expressed in tumor cells, is implicated in metastasis to the bone (60). Neutralizing CXCL10 activity by monoclonal antibodies reduced migration of CXCR3 expressing tumor cells and bone metastasis. In colorectal cancers (CRC), CXCR3 expression in tumor cells may also contribute to metastatic spread. In murine and human CRC models, the use of the CXCR3 small inhibitor AMG487 led to the inhibition of lung and liver metastasis (19). CRC samples from patients expressed CXCR3 at significantly higher levels in metastatic foci within lymph nodes and liver compared with primary tumors (78). Knockdown of CXCR3 significantly reduced lymph nodes metastasis and dissemination in the liver and lungs.
When CXCR3 isoforms are distinguished, clear differences in the biological function are evidenced. A cooperative effect of CXCR3-A and CXCR4 has been observed, which indicates that CXCR3-A significantly reinforces the effects of CXCR4 on cell migration and invasion. CXCR3-A enhanced CXCR4 function in tumor cell invasion through forming heteromers with CXCR4 on cell surface and prevent CXCR4 internalization (48). In CXCR3-A-positive pancreatic ductal adenocarcinoma, the administration of an antibody specific to CXCL4L1 significantly reduced lung metastasis in orthotopically implanted tumors (93). In human prostate cancer, CXCR3 expression in tumor cells may contribute to metastatic spread. Indeed, both CXCR3 mRNA and protein were elevated in localized and metastatic human cancer biopsies compared with normal. Interestingly in these patients, CXCR3-A mRNA levels were upregulated while CXCR3-B mRNA was downregulated. Overexpression of CXCR3-B in human prostate cancer cells decreased cell movement and invasion (130). In gastric cancer cells and tissues, the CXCR3-A mRNA level was found to be upregulated while CXCR3-B mRNA level was downregulated. CXCR3-A acted as a positive regulator of in vitro cell growth, migration, and invasion of gastric cancer cells, whereas CXCR3-B exerted the opposite effects. Furthermore, in a nude mouse model, knockdown of CXCR3-A within gastric tumor cells led to a reduction in in vivo tumor cell growth and metastasis (133). Inhibition of cell migration has been mostly related to the CXCR3-B isoform. In prostate carcinoma cells, CXCR3-B overexpression blocked chemokine-induced cell motility and invasion via μ-calpain activation inhibition (125, 130). Furthermore, in human prostate cancer cells, overexpression of CXCR3-B decreased cell movement and invasion (130).
Taken together, these results indicate opposing effects of CXCR3-A and CXCR-B, where the A isoform promotes tumor growth, invasion, and metastasis and the B isoform exhibits clear antitumor properties.
Role of CXCR3 in the tumor microenvironment.
CXCR3 and CXC chemokines not only influence the sequential participation of inflammatory cells but also regulate the inflammatory reaction leading to angiostasis and inhibition of endothelial cell proliferation. CXCL9, CXCL10, and CXCL11 chemokines are induced by IFN-γ during infection, injury, inflammation, and cancer. CXCL9, CXCL10, CXCL11, and CXCL4L1 and its variant CXCL4L1 contribute, on one end, to angiostasis and, on the other end, to the inhibition or promotion of tumor progression and metastasis by attracting CXCR3-positive immune cells toward inflammatory and neoplastic regions.
CXC chemokines are angiostatic chemokines that affect the proliferation of differen t cell types such as endothelial cells, pericytes, and mesangial cells (12, 59, 99). CXCR3 is reported to mediate the angiostatic activity of CXCL9, CXCL10, CXCL11, and CXCL4 and its variant CXCL4L1 in endothelial cells (59, 111, 118). The vasculature has been shown to express CXCR3 and to respond to CXCR3 ligands. A level of complexity is added by the presence of the CXCR3 isoforms, which seem to be differentially expressed in the human vasculature.
What is the expression in different tissues? The distribution of CXCR3 in the different tissues is variable. Some tissues, like heart, kidney, liver, and skeletal muscle express both CXCR3 splice variants. Other tissues, such as placenta, express only CXCR3-A, whereas the human microvasculature express only CXCR3-B (98). The situation seems different in other species where only a single CXCR3 variant exists. In this case, blocking the function of the chemokine CXCL4L1 using anti-CXCR3 antibodies in the mouse inhibited corneal angiogenesis (111).
CXCL4 and its variant CXCL4L1 are unique and potent angiostatic chemokines. It has been shown that CXCL4 also inhibits angiogenesis by directly interacting with FGF-2, inhibiting its dimerization, and blocking FGF-2 binding to endothelial cells (88). A small peptide domain derived from the COOH-terminal sequence of CXCL4 (PF-447–70) was sufficient to maintain the antiangiogenic effect (41). Lasagni et al. (59) demonstrated by performing affinity binding assays in human microvascular endothelial cell line-1 (HMEC-1) transfected with CXCR3-A or CXCR3-B that CXCL4 exhibited high affinity and selectivity for CXCR3-B compared with CXCL9, CXCL10, and CXCL11. In HMEC-1 cells, interactions between CXCR3-B and CXCL4 were shown to induce apoptotic signals, but neither a chemotactic response toward a CXCL4 gradient nor induction of calcium flux was observed in CXCR3-B transfectants (59). Taken together, there is not yet clear consensus how CXCL4 inhibits angiogenesis.
In the context of tumor progression, Lewis lung carcinoma cells were transfected with the human CXCL4 and the ability of the transfected cells to form tumors and metastasis in vivo was evaluated by intravenously injecting transfected tumor cells in BALB/c nu/nu mice. The study showed that CXCL4 significantly reduces the number of lung metastasis and weight by inhibiting tumor-associated neovascularization (132). The same finding was also reported in a previous study that demonstrated that systemic administration of CXCL4 inhibited murine melanoma lung metastasis formation (54).
CXCL4L1 has been shown to be a more potent angiostatic chemokine in vitro compared with CXCL4 and more effective than CXCL4 in inhibiting FGF-2-induced angiogenesis in rat corneas (110). In several mouse xenograft models, tumor growth and formation of distant metastases are more efficiently inhibited by CXCL4L1 in comparison to CXCL4 (109, 119). In tumor model of melanoma (B16 melanoma orthotopically propagated in C57Bl/6 mice), Vandercappellen et al. (119) reported that the small peptide domain derived from the COOH-terminal sequence of CXCL4L1 (PF-4var47-70) impairs angiogenesis and suppresses B16 melanoma growth in vivo. Struyf et al. (109) showed that, in vitro, CXCL4L1 was more efficient than CXCL4 at inhibiting the chemotactic activity of both CXCL8 and FGF-2 in the endothelial cell migration assay using the Boyden chamber. In tumor models of melanoma (B16 melanoma cells orthotopically propagated in C57Bl/6 mice) and lung carcinoma (A549 adenocarcinoma and Lewis lung carcinoma cell lines orthotopically propagated in C57Bl/6 and SCID mice), they also demonstrated that CXCL4L1 was a stronger inhibitor of tumor growth and metastasis than CXCL4 by preventing tumor angiogenesis. While CXCL4L1 was more potent than CXCL10 in preventing tumor metastasis in immunocompromised mice, it had equal antitumoral activity as CXCL9 in immunocompetent mice. In a model of pancreatic ductal adenocarcinoma (BxPC3 CXCR3-negative cell line subcutaneously inoculated in RAG-γ/c mice), the injection of a monoclonal antibody specific to CXCL4L1 yielded to an increase in tumor development characterized by an increase in the density of small vessels. This effect only observed in treated tumors is compatible with an effect on the tumor stroma, which is angiogenesis related (93). All these data demonstrated that CXCL4L1 is a potent antiangiogenic chemokine that impairs tumor growth and development of metastasis.
Regarding the IFN-γ-inducible CXCR3 ligands CXCL9, CXCL10, and CXCL11, several studies have reported their activity in regulating tumor angiogenesis. In regressing Burkitt's lymphoma tumors, CXCL9 was found to be expressed at higher levels compared with progressively growing tumors. To support the idea that CXCL9 has antitumor activity in vivo, human recombinant CXCL9 was daily injected into Burkitt's tumors growing subcutaneously in BALB/c nu/nu mice, which led to tumor necrosis associated with extensive vascular damage (103). In non-small cell lung cancer (NSCLC), CXCL10 was reported to be an important endogenous angiostatic factor that regulates NSCLC-derived angiogenesis, tumor growth, and spontaneous metastasis. Using a model of human NSCLC tumorigenesis in SCID mice, Arenberg et al. (7, 8) showed that addition of CXCL10 in the tumor microenvironment resulted in a significant inhibition of tumor growth and tumor angiogenesis and that the inhibition of lung metastases was due to the angiostatic effect of CXCL10 on the primary tumor (. CXCL9 was also reported as an endogenous inhibitor of NSCLC tumor growth and metastasis in vivo via a decrease in tumor-derived vessel density. The reconstitution of CXCL9 in the tumor microenvironment by overexpression of CXCL9, using three different strategies including gene transfer, induced the inhibition of NSCLC tumor growth and metastasis by attenuation of tumor-derived angiogenesis (2). Using a mouse model of metastatic renal cell carcinoma (RENCA cells propagated in BALB/c mice), the injection of CXCL9 into the tumor also impaired RENCA tumor growth by reducing tumor-associated angiogenesis (87).
The molecular pathways of cancer-related inflammation have been largely studied with chemokines playing an important role in this context (9). CXCR3 is involved in the recruitment and homing of different leukocyte subsets, including T lymphocytes, monocytes, and NK cells, during tumor progression.
CXCR3 is predominantly expressed on activated T lymphocytes, NK cells, inflammatory dendritic cells, macrophages, and B cells (35, 65, 66). CXCR3 is rapidly induced on naïve T cells following activation and remains highly expressed on CD4(+) type-1 helper (Th1) cells, effector CD8(+) T cells and innate-type lymphocytes, such as NK and natural killer T (NKT) cells (39, 40). In colon cancer-bearing mice, it has been shown that CXCL4 promotes the activation of Tregs in a CXCR3-dependent manner (26). Activation of CXCR3 induces cellular responses that are involved in leukocyte trafficking, most notably integrin activation, cytoskeletal changes, and chemotactic migration, which considerably influence the course of inflammation process (39, 56).
Most of the studies have focused on the antitumor activity of CXCR3 and its ligands. In regressive melanoma, CXCR3 was expressed on the vast majority of infiltrating lymphocytes and a high expression of CXCL10, which can be induced by IFN-α, was seen when compared with samples from healthy control subjects (127). The lack of critical chemokines such as CXCL9 and CXCL10 in a subset of melanoma metastases may limit the migration of activated T cells, which in turn could limit the effectiveness of antitumor immunity (42). It has also been shown that advanced fibrosarcomas deficient in CXCL9 exhibited a decreased activation and recruitment of tumor-reactive T cells. These studies were extended to sarcomas and melanomas and demonstrated that the conversion of CXCL9-expressing tumor cells into CXCL9-deficient cells occurs during tumor growth in vivo. Accelerated growth in CXCL9-deficient tumors was not mediated by an increased ability to proliferate, but rather by resistance to antitumor immunity (89). A nonredundant requirement for the CXCR3-CXCL9/CXCL10 axis for CD8+ T-cell trafficking to murine and human melanoma was demonstrated. This study identifies CXCR3 interactions with cognate chemokines within the vessel wall as a critical checkpoint dictating the efficacy of T-cell-based cancer immunotherapy (73). In recent experiments, Chow et al. (23) have shown that tumor-bearing mice (D4M.3A.3 UV3 melanoma model and MC38 colon carcinoma model) deficient in CXCR3 responded poorly to anti-PD-1 treatment. Experiments using Cxcl9-null mice revealed that CXCL9 deficiency reduced the efficacy of PD-1 therapy and limited both proliferation and cytokine production of intratumoral CD8+ T cells. They showed that the CXCR3-CXCL9 axis was crucial for reinvigoration of intratumor CD8+ T-cell responses in response to PD-1 blockade.
A majority of studies assessing the role of CXCR3 in tumor immunity have relied on enforced expression of CXCR3 ligands in syngeneic tumors, xenograft, or therapeutic models. In a renal cell carcinoma model (RENCA cells propagated in BALB/c mice), the combined strategy of systemic IL-2 with intratumor CXCL9 was efficient for both reducing tumor-associated angiogenesis and augmenting tumor-associated immunity (87). Murine plasmacytoma and breast adenocarcinoma cell lines genetically modified to secrete high levels of murine CXCL10 were found to elicit a potent antitumor effect in normal mice, resulting in their failure to develop tumors. This effect of CXCL10 was considered T-cell dependent because it was not reproduced in athymic mice, and in euthymic mice it was accompanied by the induction of a brisk inflammatory response with lymphocytes, neutrophils, and monocytes (68). In a mouse model of glioblastoma (GL261 cell line subcutaneously implanted in C57BL/6 mice), the production of CXCL10 or TNF-α in the tumor microenvironment resulted in a significant delay of tumor growth. Complete tumor regression was observed when implanted glioma cells were expressing both CXCL10 and TNF-α, demonstrating synergistic antitumor activity by mediated immunostimulation rather than inhibition of angiogenesis (29). Mice challenged with EL4 T-cell lymphoma cells and genetically modified to produce murine CXCL11 showed a clear correlation between rejection of CXCL11-producing tumors and an increase of tumor-infiltrating CD8+ T cells. In vivo depletion of CD8+ T cells completely abrogated the antitumor effect of CXCL11 (43).
Besides T cells, monocytes and NK cells have also been recognized to be important in tumor immunity. CXCL4 has been reported to promote monocyte survival and macrophage activation (102) and may influence the development and activity of monocyte‐derived dendritic cells (131). In breast tumors treated with the COOH-terminal CXCL4 peptide CXCL447–70, macrophage markers are upregulated. CXCL447–70peptide could potentially maintain its effect on monocyte survival and activation, thereby leading to an expanding intratumoral macrophage population. This suggests that the CXCL447–70-specific immunological effect might be a driving force behind its in vivo induced tumor growth delay (94).
A high number of tumor-infiltrating NK cells is often associated with good prognosis in cancer patients. Wendel et al. (126) have shown that the expression of CXCR3 on NK cells is a prerequisite for NK cells infiltration into tumors. In CXCR3(−/−) mice, a lower number of tumor-infiltrating NK cells was detected and the accumulation of CXCR3(−/−) NK cells in the tumor was severely impaired. However, the reduction of NK cells in tumors of CXCR3(−/−) mice did not result in reduced survival, whereas enforced CXCL10 expression in tumor cells increased the numbers of NK cells in the tumors and prolonged NK cell-dependent survival (126).
Other studies provide support for a CXCR3-dependent protumor effect of the immune system. In a chemically inducible murine model of skin carcinoma, CXCR3 deletion in mice led to a twofold reduction in skin tumor development, associated with reduced epidermal thickness and proliferation. CD4+ and CD8+ T cells were the key immune cells able to rescue the proliferation defect in the epidermis of CXCR3(−/−) mice. Thus CXCR3 was shown to both promote skin tumor development and enhance T-cell-dependent keratinocyte proliferation (128).
Tregs are implicated in cancer immune evasion and escape and thus contribute to tumor development and progression. In human ovarian carcinomas, the higher prevalence of CXCR3+ Tregs was suggested to reduce T-cell responses. This results in the “collateral” limitation of efficient antitumor immunity thus favoring the progression of the tumor (97). This protumor effect of CXCR3+ Tregs was also observed in colon carcinoma and hepatocellular carcinoma (HCC). CXCL4 enhances the function of CXCR3+ Tregs in colon cancer mice (26). It was shown that the knockout of CXCL10 decreased hepatic recruitment of CXCR3+ Tregs. A correlation could be made between CXCL10-CXCR3-dependent Treg infiltration, increased tumor growth, and HCC recurrence after liver transplantation (62).
These studies paint a rather complicated picture of the role of CXCR3 in the tumor immune system. It seems that the net outcome of an anti- or protumor function of CXCR3-dependent tumor immunity is dependent on the respective tissue and tumor type. However, some reported data are difficult to reconcile because of completely opposing results with the same tumor type. Further clarification is thus needed.
THERAPEUTICAL TARGETING OF CC AND CXC CHEMOKINES
There has been some pharmaceutical development regarding the blockade of CC and CXC receptors. Several chemokine-based therapeutic strategies have been proposed with antibodies and small antagonist molecules being the most widely adopted approaches.
Given the role of CCL2 in the tumor microenvironment, inhibitors of the CCL2/CCR2 axis have been investigated. Neutralizing antibodies to CCL2 in combination with docetaxel in mice bearing prostate cancer resulted in significant tumor regression relative to initial tumor burden (64). CXCL8 is involved in tumors and angiogenesis, and different strategies were used to target CXCL8 and its receptors CXCR1/CXCR2. These strategies blocking the CXCL8-CXCR1/CXCR2 axis have been reported to induce significant damage to tumor neovasculature (1, 44). Antagonist for CXCR1 and CXCR2 inhibited melanoma growth and colon carcinoma liver metastasis in nude mice (106, 120).
The CXCL12-CXCR4 axis is of particular importance in the metastatic dissemination of many cancers. Small molecules antagonists are the bicyclam AMD3100 and analogues as well as peptides based on the amino-terminal region of the chemokines, such as T22, BKT140, and CTCE-9908 (58). Targeting CXCR4 in tumor models led to the inhibition of the primary tumor growth but also impaired the development of metastasis in several cancers (breast, melanoma) (45).
As described above, CXCR3 has a dual role in tumor progression and microenvironment and has been beside tumor growth involved in several other diseases such as rheumatoid arthritis, atherosclerosis, and inflammatory skin diseases (31, 92). Therefore, researchers are putting efforts in the development of both small CXCR3 antagonists and agonists. Animal models have been developed for testing several classes of small molecular compounds targeting CXCR3. In the murine model of breast cancer, a small molecular weight antagonist of CXCR3, AMG487, was tested and has the potential to inhibit tumor metastasis (124). Moreover, antagonism of CXCR3 by AMG487 results in the inhibition of lung metastasis in a murine osteosarcoma model (90) and in a metastatic colon carcinoma model (19). Another antagonist is TAK-779, which has affinity for CXCR3 and CCR5 and to a lesser extend for CCR2b, and has been investigated in the mouse rheumatoid arthritis model (34). In a murine model of pancreatic cancer, it has been shown that selective targeting of CCR5/CCL5 signaling by TAK-779 may represent a novel immunomodulatory strategy for the treatment of cancer (114). Among CXCR3 agonists, PS372424, an agonist specific for the human CXCR3, was tested in the humanized model of arthritic inflammation. Intravenous treatment with PS372424 prevented inflammatory migration of activated human T cells toward murine air pouches filled with chemokines or synovial fluid from patients with active rheumatoid arthritis (84). On the other end, SCH546738, a specific CXCR3 antagonist, has been shown to exhibit benefice in a rheumatoid arthritis model in mouse (46). These two latter observations seem contradictory, and this needs to be clarified. Nevertheless, blockade of CXCR3 may have a beneficial effect on tumor cell invasion since blockade by SCH546738 inhibits migration and invasion of glioblastoma cells (16).
CONCLUDING REMARKS
Chemokines have many regulatory functions and may act on both the tumor microenvironment and directly on tumor cells. Within the tumor microenvironment, chemokines act on three different levels, inflammation and immunity, the vasculature, and the nonvascular tumor stroma. Thus understanding their respective contribution in tumor development is rather complex because of its multitargeting properties and because many feed-forward and feed-back loops exist. Nevertheless, preclinical models have shown that they are especially involved in tumor invasion and metastasis. A very good example is represented by chemokines interacting with CXCR3. CXCR3 is present on tumor cells but also on the vasculature and immune cells and exists in humans as two main different isoforms with different molecular characteristics and signaling effects. These leads to completely opposite biological responses for CXCR3-A and CXCR3-B. Preclinical studies have demonstrated that blockade of CXCR3 may be useful in inhibiting tumor development, invasion, and metastasis. This has been corroborated in brain tumor and metastasis models such as osteosarcoma and melanoma. On the other end, activation of some CXCR3-expressing immune cells may lead to activation of antitumor-immunity and a more beneficial effect of PDL1/PD1 blockade. Other CXCR3-expressing immune cells behave the opposite. Thus the picture is unclear how the antitumor immune response is influenced by CXCR3 blockade. It seems critical to address which tumor type and tumor stage (initiation, dissemination, metastasis) will benefit best from CXCR3 inhibition. One consensus seems however to emerge, which is the requirement of CXCR3 for tumor cell invasion and metastasis and thus, inhibition of tumor cell-associated CXCR3 may be a potential useful strategy. However, clinical translation of these findings has been, until now, unsuccessful because of unfavorable pharmacology and toxicology of the drug formulations. Nevertheless, blocking CXCR3-A on tumor cells may be a promising strategy to halt tumor cells invasion and metastasis, which might be ultimately successful for clinical translation provided adequate pharmacological formulations are developed.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B. prepared figures; A.B. and C.B. drafted manuscript; A.B. and C.B. edited and revised manuscript; A.B. and C.B. approved final version of manuscript.
REFERENCES
- 1.Aalinkeel R, Nair B, Chen CK, Mahajan SD, Reynolds JL, Zhang H, Sun H, Sykes DE, Chadha KC, Turowski SG, Bothwell KD, Seshadri M, Cheng C, Schwartz SA. Nanotherapy silencing the interleukin-8 gene produces regression of prostate cancer by inhibition of angiogenesis. Immunology 148: 387–406, 2016. doi: 10.1111/imm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD, Strieter RM. The CXC chemokine, monokine induced by interferon-γ, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther 11: 247–261, 2000. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- 3.Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG. CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am J Physiol Lung Cell Mol Physiol 290: L909–L918, 2006. doi: 10.1152/ajplung.00430.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ali TH, Pisanti S, Ciaglia E, Mortarini R, Anichini A, Garofalo C, Tallerico R, Santinami M, Gulletta E, Ietto C, Galgani M, Matarese G, Bifulco M, Ferrone S, Colucci F, Moretta A, Kärre K, Carbone E. Enrichment of CD56(dim)KIR + CD57 + highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat Commun 5: 5639, 2014. doi: 10.1038/ncomms6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 102: 465–472, 1998. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SRB, Burdick MD, Iannettoni MD, Strieter RM. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother 49: 63–70, 2000. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 184: 981–992, 1996. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenberg DA, White ES, Burdick MD, Strom SR, Strieter RM. Improved survival in tumor-bearing SCID mice treated with interferon-γ-inducible protein 10 (IP-10/CXCL10). Cancer Immunol Immunother 50: 533–538, 2001. doi: 10.1007/s00262-001-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 357: 539–545, 2001. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 10.Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol 90: 483–495, 2016. doi: 10.1124/mol.116.105502. [DOI] [PubMed] [Google Scholar]
- 11.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim Biophys Acta 1836: 287–295, 2013. doi: 10.1016/j.bbcan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276: 9945–9954, 2001. doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]
- 13.Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515: 130–133, 2014. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 14.Bordoni R, Fine R, Murray D, Richmond A. Characterization of the role of melanoma growth stimulatory activity (MGSA) in the growth of normal melanocytes, nevocytes, and malignant melanocytes. J Cell Biochem 44: 207–219, 1990. doi: 10.1002/jcb.240440403. [DOI] [PubMed] [Google Scholar]
- 15.Boyé K, Billottet C, Pujol N, Alves ID, Bikfalvi A. Ligand activation induces different conformational changes in CXCR3 receptor isoforms as evidenced by plasmon waveguide resonance (PWR). Sci Rep 7: 10703, 2017. doi: 10.1038/s41598-017-11151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyé K, Pujol N, D Alves I, Chen YP, Daubon T, Lee YZ, Dedieu S, Constantin M, Bello L, Rossi M, Bjerkvig R, Sue SC, Bikfalvi A, Billottet C. The role of CXCR3/LRP1 cross-talk in the invasion of primary brain tumors. Nat Commun 8: 1571, 2017. doi: 10.1038/s41467-017-01686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis 19: 247–258, 2002. doi: 10.1023/A:1015587423262. [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 171: 1103–1108, 2005. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 19.Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer 100: 1755–1764, 2009. doi: 10.1038/sj.bjc.6605078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. gro-beta, a-C-X-C- chemokine, is an angiogenesis inhibitor that suppresses the growth of Lewis lung carcinoma in mice. J Exp Med 182: 2069–2077, 1995. doi: 10.1084/jem.182.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissière F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9: R15, 2007. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YP, Wu HL, Boyé K, Pan CY, Chen YC, Pujol N, Lin CW, Chiu LY, Billottet C, Alves ID, Bikfalvi A, Sue SC. Oligomerization state of CXCL4 chemokines regulates g protein-coupled receptor activation. ACS Chem Biol 12: 2767–2778, 2017. doi: 10.1021/acschembio.7b00704. [DOI] [PubMed] [Google Scholar]
- 23.Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50: 1498–1512.e5, 2019. doi: 10.1016/j.immuni.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Colvin RA, Campanella GS, Sun, Luster, AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem 279: 30219-30227, 2004. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 24.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, Condeelis J, Skobe M. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med 210: 1509–1528, 2013. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S. CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J Biol Chem 285: 36842–36848, 2010. doi: 10.1074/jbc.M110.170324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng S, Deng Q, Zhang Y, Ye H, Yu X, Zhang Y, Han GY, Luo P, Wu M, Yu Y, Han W. Non-platelet-derived CXCL4 differentially regulates cytotoxic and regulatory T cells through CXCR3 to suppress the immune response to colon cancer. Cancer Lett 443: 1–12, 2019. doi: 10.1016/j.canlet.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, Cohen N, Adler O, Hakim Z, Pozzi S, Scomparin A, Cohen J, Yassin M, Monteran L, Grossman R, Tsarfaty G, Luxenburg C, Satchi-Fainaro R, Pukrop T, Erez N. inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Rep 28: 1785–1798.e6, 2019. doi: 10.1016/j.celrep.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol 173: 6234–6240, 2004. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- 29.Enderlin M, Kleinmann EV, Struyf S, Buracchi C, Vecchi A, Kinscherf R, Kiessling F, Paschek S, Sozzani S, Rommelaere J, Cornelis JJ, Van Damme J, Dinsart C. TNF-alpha and the IFN-gamma-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastoma. Cancer Gene Ther 16: 149–160, 2009. doi: 10.1038/cgt.2008.62. [DOI] [PubMed] [Google Scholar]
- 30.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475: 226–230, 2011. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 31.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol 194: 398–405, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Fox JM, Kausar F, Day A, Osborne M, Hussain K, Mueller A, Lin J, Tsuchiya T, Kanegasaki S, Pease JE. CXCL4/platelet factor 4 is an agonist of CCR1 and drives human monocyte migration. Sci Rep 8: 9466, 2018. doi: 10.1038/s41598-018-27710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao P, Zhou XY, Yashiro-Ohtani Y, Yang YF, Sugimoto N, Ono S, Nakanishi T, Obika S, Imanishi T, Egawa T, Nagasawa T, Fujiwara H, Hamaoka T. The unique target specificity of a nonpeptide chemokine receptor antagonist: selective blockade of two Th1 chemokine receptors CCR5 and CXCR3. J Leukoc Biol 73: 273–280, 2003. doi: 10.1189/jlb.0602269. [DOI] [PubMed] [Google Scholar]
- 35.García-López MÁ, Sánchez-Madrid F, Rodríguez-Frade JM, Mellado M, Acevedo A, García MI, Albar JP, Martínez C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest 81: 409–418, 2001. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 36.Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273: 17979–17982, 1998. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 37.Giuliani N, Bonomini S, Romagnani P, Lazzaretti M, Morandi F, Colla S, Tagliaferri S, Lasagni L, Annunziato F, Crugnola M, Rizzoli V. CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica 91: 1489–1497, 2006. [PubMed] [Google Scholar]
- 38.Grépin R, Guyot M, Giuliano S, Boncompagni M, Ambrosetti D, Chamorey E, Scoazec JY, Negrier S, Simonnet H, Pagès G. The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Res 74: 873–883, 2014. doi: 10.1158/0008-5472.CAN-13-1267. [DOI] [PubMed] [Google Scholar]
- 39.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89: 207–215, 2011. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 317: 620–631, 2011. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagedorn M, Zilberberg L, Wilting J, Canron X, Carrabba G, Giussani C, Pluderi M, Bello L, Bikfalvi A. Domain swapping in a COOH-terminal fragment of platelet factor 4 generates potent angiogenesis inhibitors. Cancer Res 62: 6884–6890, 2002. [PubMed] [Google Scholar]
- 42.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69: 3077–3085, 2009. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother 28: 343–351, 2005. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 44.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70: 1063–1071, 2010. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang EH, Singh B, Cristofanilli M, Gelovani J, Wei C, Vincent L, Cook KR, Lucci A. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res 155: 231–236, 2009. doi: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Jenh CH, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC, Kinsley D, Qian S, Kim SH, Rosenblum S, Kozlowski J, Fine JS, Zavodny PJ, Lundell D. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 13: 2, 2012. doi: 10.1186/1471-2172-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji R, Lee CM, Gonzales LW, Yang Y, Aksoy MO, Wang P, Brailoiu E, Dun N, Hurford MT, Kelsen SG. Human type II pneumocyte chemotactic responses to CXCR3 activation are mediated by splice variant A. Am J Physiol Lung Cell Mol Physiol 294: L1187–L1196, 2008. doi: 10.1152/ajplung.00388.2007. [DOI] [PubMed] [Google Scholar]
- 48.Jin J, Zhang Z, Wang H, Zhan Y, Li G, Yang H, Fei Z, Xu Y, Li W. CXCR3 expression in colorectal cancer cells enhanced invasion through preventing CXCR4 internalization. Exp Cell Res 371: 162–174, 2018. doi: 10.1016/j.yexcr.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Karnik SS, Gogonea C, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol Metab 14: 431–437, 2003. doi: 10.1016/j.tem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26: 4679–4688, 2007. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- 51.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res 64: 4010–4017, 2004. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 52.Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res 106: 91–111, 2010. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest 110: 933–941, 2002. doi: 10.1172/JCI0214268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolber DL, Knisely TL, Maione TE. Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. J Natl Cancer Inst 87: 304–309, 1995. doi: 10.1093/jnci/87.4.304. [DOI] [PubMed] [Google Scholar]
- 55.Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 132: 503–515, 2011. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kouroumalis A, Nibbs RJ, Aptel H, Wright KL, Kolios G, Ward SG. The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate G alpha i-independent signaling and actin responses in human intestinal myofibroblasts. J Immunol 175: 5403–5411, 2005. doi: 10.4049/jimmunol.175.8.5403. [DOI] [PubMed] [Google Scholar]
- 57.Kuo JH, Chen YP, Liu JS, Dubrac A, Quemener C, Prats H, Bikfalvi A, Wu WG, Sue SC. Alternative C-terminal helix orientation alters chemokine function: structure of the anti-angiogenic chemokine, CXCL4L1. J Biol Chem 288: 13522–13533, 2013. doi: 10.1074/jbc.M113.455329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwong J, Kulbe H, Wong D, Chakravarty P, Balkwill F. An antagonist of the chemokine receptor CXCR4 induces mitotic catastrophe in ovarian cancer cells. Mol Cancer Ther 8: 1893–1905, 2009. doi: 10.1158/1535-7163.MCT-08-0966. [DOI] [PubMed] [Google Scholar]
- 59.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197: 1537–1549, 2003. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JH, Kim HN, Kim KO, Jin WJ, Lee S, Kim HH, Ha H, Lee ZH. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res 72: 3175–3186, 2012. doi: 10.1158/0008-5472.CAN-12-0481. [DOI] [PubMed] [Google Scholar]
- 61.Li B, Wang Z, Zhong Y, Lan J, Li X, Lin H. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway. Med Oncol 32: 66, 2015. doi: 10.1007/s12032-015-0531-0. [DOI] [PubMed] [Google Scholar]
- 62.Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol 65: 944–952, 2016. doi: 10.1016/j.jhep.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Reader JC, Ma X, Kundu N, Kochel T, Fulton AM. Divergent roles of CXCR3 isoforms in promoting cancer stem-like cell survival and metastasis. Breast Cancer Res Treat 149: 403–415, 2015. doi: 10.1007/s10549-014-3229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67: 9417–9424, 2007. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 65.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 184: 963–969, 1996. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol 28: 3696–3705, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 67.Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res 73: 662–671, 2013. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med 178: 1057–1065, 1993. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther 8: 490–498, 2009. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 70.Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, Romero IA. Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. J Neuroimmunol 199: 35–45, 2008. doi: 10.1016/j.jneuroim.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 71.Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost 97: 755–762, 2007. doi: 10.1160/TH07-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front Immunol 8: 1970, 2018. doi: 10.3389/fimmu.2017.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, Luster AD, Evans SS. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 6: 7458, 2015. doi: 10.1038/ncomms8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol 2: 123–128, 2001. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 75.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol 25: 75–84, 2004. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105: 3965–3971, 2005. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 77.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature 410: 50–56, 2001. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 78.Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, Sakai Y. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer 132: 276–287, 2013. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- 79.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52: 145–176, 2000. [PubMed] [Google Scholar]
- 80.Natsagdorj A, Izumi K, Hiratsuka K, Machioka K, Iwamoto H, Naito R, Makino T, Kadomoto S, Shigehara K, Kadono Y, Lin WJ, Maolake A, Mizokami A. CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells. Cancer Sci 110: 279–288, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neer EJ. G proteins: critical control points for transmembrane signals. Protein Sci 3: 3–14, 1994. doi: 10.1002/pro.5560030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, Conejo-Garcia JR. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res 69: 6331–6338, 2009. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norgauer J, Metzner B, Schraufstätter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol 156: 1132–1137, 1996. [PubMed] [Google Scholar]
- 84.O’Boyle G, Fox CR, Walden HR, Willet JD, Mavin ER, Hine DW, Palmer JM, Barker CE, Lamb CA, Ali S, Kirby JA. Chemokine receptor CXCR3 agonist prevents human T-cell migration in a humanized model of arthritic inflammation. Proc Natl Acad Sci USA 109: 4598–4603, 2012. [Erratum in Proc Natl Acad Sci USA 109: 7948.] doi: 10.1073/pnas.1118104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olbina G, Cieslak D, Ruzdijic S, Esler C, An Z, Wang X, Hoffman R, Seifert W, Pietrzkowski Z. Reversible inhibition of IL-8 receptor B mRNA expression and proliferation in non-small cell lung cancer by antisense oligonucleotides. Anticancer Res 16, 6B: 3525–3530, 1996. [PubMed] [Google Scholar]
- 86.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine β and γ proteins. Int J Cancer 73: 94–103, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 87.Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol 176: 1456–1464, 2006. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 88.Perollet C, Han ZC, Savona C, Caen JP, Bikfalvi A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood 91: 3289–3299, 1998. doi: 10.1182/blood.V91.9.3289. [DOI] [PubMed] [Google Scholar]
- 89.Petro M, Kish D, Guryanova OA, Ilyinskaya G, Kondratova A, Fairchild RL, Gorbachev AV. Cutaneous tumors cease CXCL9/Mig production as a result of IFN-γ-mediated immunoediting. J Immunol 190: 832–841, 2013. doi: 10.4049/jimmunol.1201906. [DOI] [PubMed] [Google Scholar]
- 90.Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Kleinerman ES, Schmid-Alliana A, Schmid-Antomarchi H. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer 125: 2586–2594, 2009. doi: 10.1002/ijc.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pu Y, Li S, Zhang C, Bao Z, Yang Z, Sun L. High expression of CXCR3 is an independent prognostic factor in glioblastoma patients that promotes an invasive phenotype. J Neurooncol 122: 43–51, 2015. doi: 10.1007/s11060-014-1692-y. [DOI] [PubMed] [Google Scholar]
- 92.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101: 746–754, 1998. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quemener C, Baud J, Boyé K, Dubrac A, Billottet C, Soulet F, Darlot F, Dumartin L, Sire M, Grepin R, Daubon T, Rayne F, Wodrich H, Couvelard A, Pineau R, Schilling M, Castronovo V, Sue SC, Clarke K, Lomri A, Khatib AM, Hagedorn M, Prats H, Bikfalvi A. Dual roles for CXCL4 chemokines and CXCR3 in angiogenesis and invasion of pancreatic cancer. Cancer Res 76: 6507–6519, 2016. doi: 10.1158/0008-5472.CAN-15-2864. [DOI] [PubMed] [Google Scholar]
- 94.Van Raemdonck K, Berghmans N, Vanheule V, Bugatti A, Proost P, Opdenakker G, Presta M, Van Damme J, Struyf S. Angiostatic, tumor inflammatory and anti-tumor effects of CXCL4(47-70) and CXCL4L1(47-70) in an EGF-dependent breast cancer model. Oncotarget 5: 10916–10933, 2014. doi: 10.18632/oncotarget.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev 26: 311–327, 2015. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res 317: 575–589, 2011. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 72: 4351–4360, 2012. doi: 10.1158/0008-5472.CAN-12-0579. [DOI] [PubMed] [Google Scholar]
- 98.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol 25: 201–209, 2004. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Romagnani P, Lazzeri E, Lasagni L, Mavilia C, Beltrame C, Francalanci M, Rotondi M, Annunziato F, Maurenzig L, Cosmi L, Galli G, Salvadori M, Maggi E, Serio M. IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and regulation by nitric oxide. J Am Soc Nephrol 13: 53–64, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Roy I, McAllister DM, Gorse E, Dixon K, Piper CT, Zimmerman NP, Getschman AE, Tsai S, Engle DD, Evans DB, Volkman BF, Kalyanaraman B, Dwinell MB. Pancreatic cancer cell migration and metastasis is regulated by chemokine-biased agonism and bioenergetic signaling. Cancer Res 75: 3529–3542, 2015. doi: 10.1158/0008-5472.CAN-14-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice. Cancer Res 77: 5158–5168, 2017. doi: 10.1158/0008-5472.CAN-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheuerer B, Ernst M, Dürrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 95: 1158–1166, 2000. doi: 10.1182/blood.V95.4.1158.004k31_1158_1166. [DOI] [PubMed] [Google Scholar]
- 103.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood 89: 2635–2643, 1997. doi: 10.1182/blood.V89.8.2635. [DOI] [PubMed] [Google Scholar]
- 104.Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol 291: C34–C39, 2006. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- 105.Sharma I, Singh A, Siraj F, Saxena S. IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion and vascular mimicry in glioblastoma. J Biomed Sci 25: 62, 2018. doi: 10.1186/s12929-018-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res 15: 2380–2386, 2009. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith JS, Alagesan P, Desai NK, Pack TF, Wu JH, Inoue A, Freedman NJ, Rajagopal S. C-X-C Motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways s. Mol Pharmacol 92: 136–150, 2017. doi: 10.1124/mol.117.108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Roczniak S, Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270: 27348–27357, 1995. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 109.Struyf S, Burdick MD, Peeters E, Van den Broeck K, Dillen C, Proost P, Van Damme J, Strieter RM. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res 67: 5940–5948, 2007. doi: 10.1158/0008-5472.CAN-06-4682. [DOI] [PubMed] [Google Scholar]
- 110.Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res 95: 855–857, 2004. doi: 10.1161/01.RES.0000146674.38319.07. [DOI] [PubMed] [Google Scholar]
- 111.Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S, Proost P, Opdenakker G, Parmentier M, Gerard C, Sozzani S, Strieter RM, Van Damme J. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood 117: 480–488, 2011. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas 21: 52–56, 2000. doi: 10.1097/00006676-200007000-00051. [DOI] [PubMed] [Google Scholar]
- 113.Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura E, Sugiyama A, Mandai M, Konishi I, Matsumura N. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun 9: 1685, 2018. doi: 10.1038/s41467-018-03966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182: 1746–1755, 2009. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thelen M. Dancing to the tune of chemokines. Nat Immunol 2: 129–134, 2001. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 116.Thelen M, Thelen S. CXCR7, CXCR4 and CXCL12: an eccentric trio? J Neuroimmunol 198: 9–13, 2008. doi: 10.1016/j.jneuroim.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 117.Thomas JK, Mir H, Kapur N, Bae S, Singh S. CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Sci Rep 9: 4014, 2019. doi: 10.1038/s41598-019-40514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117a.Urra S, Fischer MC, Martínez JR, Véliz L, Orellana P, Solar A, Bohmwald K, Kalergis A, Riedel C, Corvalán AH, Roa JC, Fuentealba R, Cáceres CJ, López-Lastra M, León A, Droppelmann N, González HE. Differential expression profile of CXCR3 splicing variants is associated with thyroid neoplasia. Potential role in papillary thyroid carcinoma oncogenesis? Oncotarget 9: 2445–2467, 2017. doi: 10.18632/oncotarget.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vandercappellen J, Van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev 22: 1–18, 2011. doi: 10.1016/j.cytogfr.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 119.Vandercappellen J, Liekens S, Bronckaers A, Noppen S, Ronsse I, Dillen C, Belleri M, Mitola S, Proost P, Presta M, Struyf S, Van Damme J. The COOH-terminal peptide of platelet factor-4 variant (CXCL4L1/PF-4var47-70) strongly inhibits angiogenesis and suppresses B16 melanoma growth in vivo. Mol Cancer Res 8: 322–334, 2010. doi: 10.1158/1541-7786.MCR-09-0176. [DOI] [PubMed] [Google Scholar]
- 120.Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett 300: 180–188, 2011. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev 13: 143–154, 2002. doi: 10.1016/S1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 122.de Vries TJ, Verheijen JH, de Bart AC, Weidle UH, Ruiter DJ, van Muijen GN. Decreased expression of both the low-density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor and its receptor-associated protein in late stages of cutaneous melanocytic tumor progression. Cancer Res 56: 1432–1439, 1996. [PubMed] [Google Scholar]
- 123.Wald O, Izhar U, Amir G, Avniel S, Bar-Shavit Y, Wald H, Weiss ID, Galun E, Peled A. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J Immunol 177: 6983–6990, 2006. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]
- 124.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 66: 7701–7707, 2006. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 125.Wells A, Chao YL, Grahovac J, Wu Q, Lauffenburger DA. Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis. Front Biosci 16: 815–837, 2011. doi: 10.2741/3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res 68: 8437–8445, 2008. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 127.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tüting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol 124: 37–48, 2005. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 128.Winkler AE, Brotman JJ, Pittman ME, Judd NP, Lewis JS Jr, Schreiber RD, Uppaluri R. CXCR3 enhances a T-cell-dependent epidermal proliferative response and promotes skin tumorigenesis. Cancer Res 71: 5707–5716, 2011. doi: 10.1158/0008-5472.CAN-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol 19: 638–653, 2018. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 130.Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer 11: 3, 2012. doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia CQ, Kao KJ. Effect of CXC chemokine platelet factor 4 on differentiation and function of monocyte-derived dendritic cells. Int Immunol 15: 1007–1015, 2003. doi: 10.1093/intimm/dxg100. [DOI] [PubMed] [Google Scholar]
- 132.Yamaguchi K, Ogawa K, Katsube T, Shimao K, Konno S, Shimakawa T, Yoshimatsu K, Naritaka Y, Yagawa H, Hirose K. Platelet factor 4 gene transfection into tumor cells inhibits angiogenesis, tumor growth and metastasis. Anticancer Res 25, 2A: 847–851, 2005. [PubMed] [Google Scholar]
- 133.Yang C, Zheng W, Du W. CXCR3A contributes to the invasion and metastasis of gastric cancer cells. Oncol Rep 36: 1686–1692, 2016. doi: 10.3892/or.2016.4953. [DOI] [PubMed] [Google Scholar]
- 134.Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer 91: 1970–1976, 2004. doi: 10.1038/sj.bjc.6602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity 36: 705–716, 2012. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]