Abstract
Vitamin D deficiency has been linked to a reduction in skeletal muscle function and oxidative capacity; however, the mechanistic bases of these impairments are poorly understood. The biological actions of vitamin D are carried out via the binding of 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) to the vitamin D receptor (VDR). Recent evidence has linked 1α,25(OH)2D3 to the regulation of skeletal muscle mitochondrial function in vitro; however, little is known with regard to the role of the VDR in this process. To examine the regulatory role of the VDR in skeletal muscle mitochondrial function, we used lentivirus-mediated shRNA silencing of the VDR in C2C12 myoblasts (VDR-KD) and examined mitochondrial respiration and protein content compared with an shRNA scrambled control. VDR protein content was reduced by ~95% in myoblasts and myotubes (P < 0.001). VDR-KD myoblasts displayed a 30%, 30%, and 36% reduction in basal, coupled, and maximal respiration, respectively (P < 0.05). This phenotype was maintained in VDR-KD myotubes, displaying a 34%, 33%, and 48% reduction in basal, coupled, and maximal respiration (P < 0.05). Furthermore, ATP production derived from oxidative phosphorylation (ATPOx) was reduced by 20%, suggesting intrinsic impairments within the mitochondria following VDR-KD. However, despite the observed functional decrements, mitochondrial protein content, as well as markers of mitochondrial fission were unchanged. In summary, we highlight a direct role for the VDR in regulating skeletal muscle mitochondrial respiration in vitro, providing a potential mechanism as to how vitamin D deficiency might impact upon skeletal muscle oxidative capacity.
Keywords: adaptation, metabolism, mitochondria, skeletal muscle, vitamin D
INTRODUCTION
Vitamin D deficiency is characterized by serum 25-hydroxyvitamin D (25(OH)D) levels of <50 nmol/L (15). On the basis of these numbers, it has been reported that ~40% of adults in the United States can be classified as deficient (7). The classical actions of vitamin D are well established, primarily functioning to maintain calcium and phosphate balance to prevent bone-related disease (1, 13). Vitamin D carries out its actions via its active metabolite, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), which binds to the ubiquitously expressed vitamin D receptor (VDR) (14). The VDR, together with its binding partner retinoid x receptor alpha (RXRα), recruit transcriptional cofactors to regulate genomic transcription (17, 21).
In addition to its role in bone biology, vitamin D has also been shown to play a role in skeletal muscle development (11, 20) and regeneration (20). Given that vitamin D exerts its biological actions through binding to the VDR, multiple studies have sought to elucidate the role of the VDR within skeletal muscle (9, 10, 12). For example, whole body VDR knockout mice (VDRKO) present muscle weakness, muscle fiber atrophy, and hypernuclearity (8), which is also present in skeletal muscle-specific VDR knockout (VDR-mKO) mice (9). Collectively these studies suggest a specific role for the VDR in skeletal muscle regulation (4, 10).
In addition to regulating skeletal muscle mass and function, evidence also suggests that vitamin D may regulate skeletal muscle mitochondrial function (2, 26). For example, treating human primary myoblasts with 1α,25(OH)2D3 resulted in an improvement in mitochondrial function and an increase in ~80 mRNAs encoding for mitochondrial proteins (23). In addition, the VDR appeared to be critical in mediating the effects of 1α,25(OH)2D3, as siRNA targeted toward the VDR blocked mitochondrial adaptation. Therefore, the aim of the present work was to further examine the regulatory role of the VDR for mitochondrial function in skeletal muscle. To achieve this, we generated a stable VDR loss-of-function C2C12 cell line model and examined mitochondrial respiration and protein content in myoblasts and fully differentiated myotubes.
METHODS
Generation of VDR-KD and control cell lines.
The lentiviral plasmid used (pLKO.1 backbone) was designed in-house and was based on (Clone ID: RMM3981-201757375) and targeted the (3′ UTR) mouse sequence 5′-TTA AAT GTG ATT GAT CTC AGG-3′ of the mouse Vdr gene; the scramble shRNA was used as a negative control, as previously reported (16), with a hairpin sequence: CCT AAG GTT AAG TCG CCC TCG CTC TAG CGA GGG CGA CTT AAC CTT AGG (Addgene plasmid 1864, Cambridge, MA). Oligos were obtained from ITDDNA USA (Integrated DNA Technologies, Iowa City, IA) and suspended, annealed, and cloned into pLKO.1 at EcoRI and AgeI restriction sites, as per the pLKO.1 protocol from Addgene. The resultant plasmids were transformed in DH5α cells for amplification and isolated. The actual DNA sequence was confirmed at the Pennsylvania State University College of Medicine DNA sequence core facility. Packaging plasmids psPAX2 and envelope protein plasmid pMD2.G were a gift from Prof. Didier Trono, available as Addgene plasmids 12260 and 12259, respectively. HEK293FT cells (Invitrogen, Carlsbad, CA) were grown in DMEM; 80–85% confluent plates were rinsed once with Opti-MEM (Invitrogen) and then incubated with Opti-MEM for 4 h before transfections. psPAX2 and pMD2.G along with either scramble or pLKO.1 clones targeting mouse Vdr. Three clones were added after mixing with Lipofectamine 2000 as per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Opti-MEM was changed after overnight incubation with DMEM containing 10% FBS without antibiotics to allow cells to take up the plasmids and recover. Culture media were collected at 36 and 72 h posttransfection for viral particles. Viral particles present in the supernatant were harvested after a 15-min spin at 1,500 g to remove cellular debris. The supernatant was further filtered using a 0.45-μm syringe filter. Supernatant-containing virus was either stored at −80°C for long-term storage or at 4°C for immediate use. C2C12 myoblasts (American Type Culture Collection, Manassas, VA) at 60% confluence were infected twice overnight with 3 ml of viral supernatant containing 8 μg/ml polybrene in serum-free–antibiotic-free DMEM. Fresh DMEM media containing 10% FBS, 1% penicillin-streptomycin and 2 μg/ml puromycin dihydrochloride (Sigma, St. Louis, MO) were added the next day. Cells that survived under puromycin selection were harvested as stable VDR knockdown (VDR-KD) myoblasts or controls and stored in liquid N2 until further analysis.
Extracellular flux analysis.
Both control and VDR-KD (n = 9 or 10 wells/group) cells were seeded in XFe24-well cell culture microplates (Seahorse Bioscience, North Billerica, MA) at 3.0 × 105 cells/well in 100 μL of growth medium. For myoblast experiments, cells were incubated at 37°C and 5% CO2 for 3 h to allow sufficient time for adherence and subsequently assayed. For myotube experiments, cells were incubated for a period of 24 h, and medium was changed to differentiation medium (DMEM, 2% horse serum, and 1% penicillin-streptomycin). Differentiation medium was changed every other day for 7 days. Prior to the assay, cells were washed and placed in 500 µl of Seahorse XF Base Medium (glucose 10 mM, sodium pyruvate 1 mM, glutamine 1 mM, pH 7.4) prewarmed to 37°C. The plate was then transferred to a non-CO2 incubator for 1 h. Following calibration, cell respiratory control, and associated extracellular acidification were assessed following the sequential addition of oligomycin (1 µM), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (1 µM), and a combination of antimycin A and rotenone (1 µM). Upon completion of the assay, cells were collected in sucrose lysis buffer (50 mM Tris pH 7.5; 270 mM sucrose; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 50 mM sodium fluoride; 5 mM sodium pyrophosphate decahydrate; 25 mM β-glycerolphosphate; 1 cOmplete protease inhibitor cocktail EDTA-free tablet) and protein concentrations determined using the DC protein assay (Bio-Rad, Hercules, CA). Oxygen consumption rate (OCR) is reported relative to protein content (picomoles per minute per microgram). Estimations of ATP production derived from both oxidative phosphorylation and glycolysis were performed, as previously described (18).
Mitochondrial membrane potential.
Control and VDR-KD (n = 5 wells/group) cells were plated at 1.0 × 105 cells/well in 100 μL of growth medium in a black 96-well plate with a clear bottom (Corning, Costar, NY). Cells were subsequently incubated for 30 min with 100 nM of tetramethylrhodamine ethyl ester (TMRE). Following incubation, cells were washed with PBS-0.2% BSA and then read at 549 nm using a CLARIOstar microplate reader (BMG Labtech, Germany) in 100 μL of PBS-0.2% BSA.
Immunoblotting.
Control and VDR-KD (n = 5–6 wells/group) cells were plated at 1.0 × 1010 cells/well in 2 ml of growth medium in six-well plates (Nunc, Roskilde, Denmark). Both myoblasts and myotubes were maintained and harvested, as described previously, with protein concentrations determined using the DC protein assay (Bio-Rad, Hercules, CA). Total protein lysates of a known concentration were mixed 3:1 with 4× Laemmli sample loading buffer. Prior to gel loading, samples were boiled for 5 min unless probing for MitoProfile OXPHOS antibody cocktail, in which case nondenatured samples were used. The immunoblotting procedure was performed, as previously described (27).
Antibodies.
All primary antibodies were used at a concentration of 1:1,000 in TBS-Tween. Antibody for dynamin-1-like protein (DRP1;8570) was from Cell Signaling Technology; MitoProfile OXPHOS antibody cocktail (110413) and mitofilin (110329) were from Abcam; Optic Atrophy-1/dynamin-like 120 kDa protein (OPA1; CPA3687) was from BD Biosciences; citrate synthase (CS; SAB2701077) and mitochondrial fission protein 1 (FIS1; HPA017430) were from Sigma Aldrich; vitamin D receptor (D-6) (VDR; 13133) was from Santa Cruz Biotechnology. Secondary antibodies were used at a concentration of 1:10,000 in TBS-T. Anti-mouse (7076) and anti-rabbit (7074) were from Cell Signaling Technology.
Statistical analysis.
Statistical analysis was performed using the Statistical Package for the Social Sciences, version 24.0. Differences between control and VDR-KD C2C12s were determined by independent t tests. All data are presented as mean ± SD. Statistical significance was set at P < 0.05.
RESULTS
Successful generation of VDR-KD myoblasts.
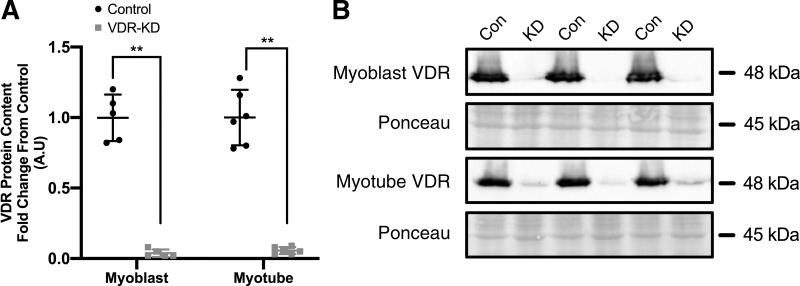
Following shRNA interference, VDR protein content was reduced by 96% (P < 0.001) and 95% (P < 0.001) in VDR-KD C2C12 myoblasts (Fig. 1A) and myotubes (Fig. 1B), respectively.
Fig. 1.
Generation of vitamin D receptor (VDR) loss of function C2C12 myoblasts. A: quantification of VDR protein content in VDR-knockdown (KD) compared with control myoblasts and myotubes. B: representative immunoblot images of VDR protein content in VDR-KD myoblasts and myotubes. **P < 0.005, independent t test. Data are means ± SD (n = 5-6 lanes/group) and represented as a fold change from control.
VDR-KD results in reduced mitochondrial respiration in C2C12 myoblasts and myotubes.
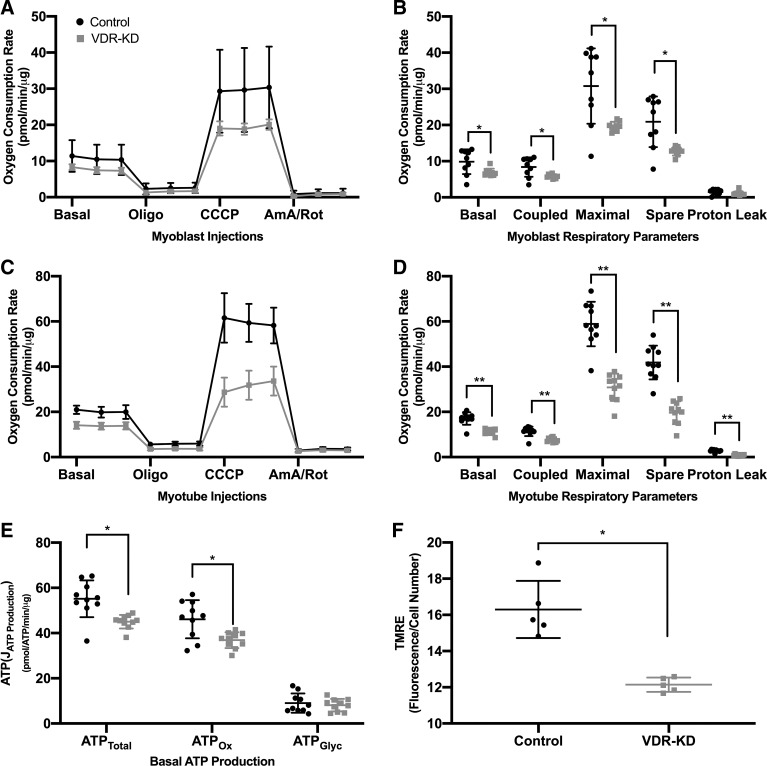
To determine the effects of VDR-KD upon mitochondrial function, extracellular flux analysis was performed in VDR-KD myoblasts and myotubes. VDR-KD myoblasts displayed a 30% reduction in basal respiration compared with control (P = 0.034; Fig. 2B). In addition, coupled and maximal respiration was reduced by 30% (P = 0.023) and 36% (P = 0.013), respectively. Furthermore, the spare respiratory capacity was also reduced by 39% (P = 0.008; Fig. 2B). This deficit was retained following differentiation, with VDR-KD myotubes displaying a 34% reduction in basal respiration (P < 0.001) and a 33% reduction in coupled respiration (P < 0.001) (Fig. 2D). Furthermore, maximal respiration was reduced by 48% (P < 0.001), and the spare respiratory capacity by 53% (P < 0.001; Fig. 2D) in VDR-KD. While proton leak remained unchanged in VDR-KD myoblasts (Fig. 2B), VDR-KD myotubes displayed a 67% decrease in proton leak (P < 0.001; Fig. 2D). To establish where mitochondrial impairments originated, we estimated oxidative phosphorylation (ATPOx) and glycolysis (ATPGlyc) using recently described equations (18). Accordingly, total ATP production and ATPOx were reduced by 18% (P = 0.002) and 20% (P = 0.007), respectively, in VDR-KD myoblasts (Fig. 2E). Finally, mitochondrial membrane potential assessed via TMRE fluorescence was reduced by 25% in VDR-KD (P = 0.001; Fig. 2F).
Fig. 2.
Vitamin D receptor-knockdown (VDR-KD) myoblasts display reduced mitochondrial respiration compared with control. A: oxygen consumption rate (OCR) during analysis of respiratory control in control and VDR-KD myoblasts. B: respiratory control parameters from control and VDR-KD myoblasts. C: OCR during analysis of respiratory control in control and VDR-KD myotubes. D: respiratory control parameters from control and VDR-KD myotubes. E: estimations of total ATP production (ATPTotal), oxidative phosphorylation (ATPOx), and glycolysis (ATPGlyc) in control and VDR-KD myoblasts. F: mitochondrial membrane potential assessed via TMRE (tetramethylrhodamine ethyl ester) fluorescence in control and VDR-KD myoblasts. *P < 0.05, **P < 0.005, independent t test. Data are means ± SD (A–E: n = 9–10 wells/group. F: n = 5 wells/group).
No change in mitochondrial-related protein content in VDR-KD myoblasts and myotubes.
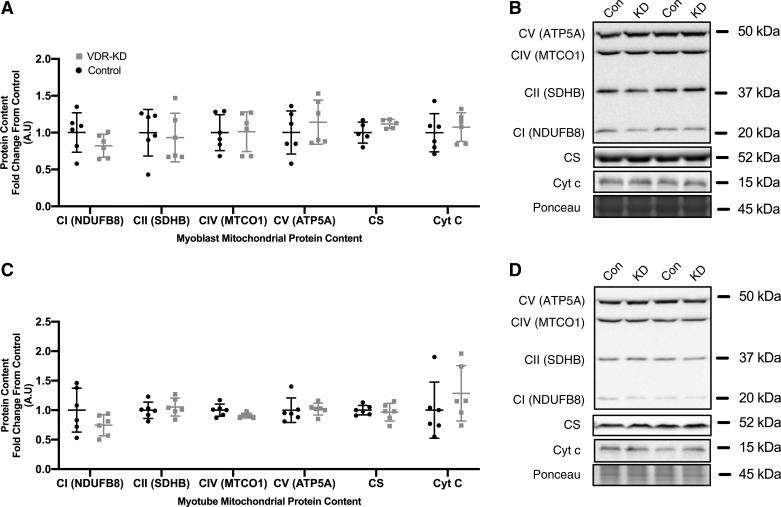
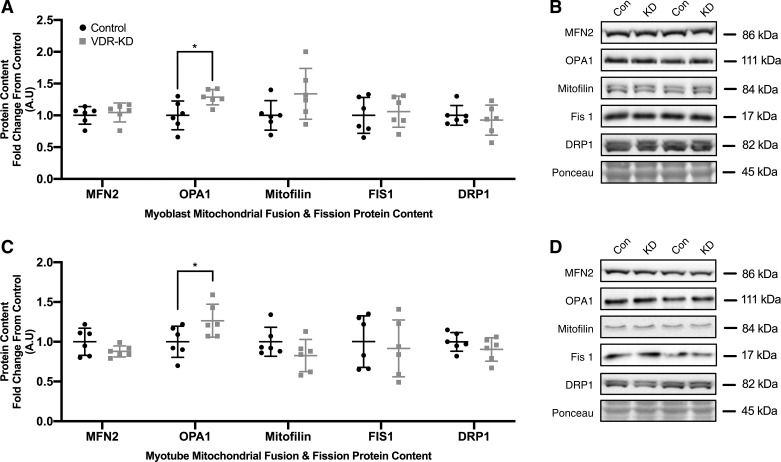
Given the observed decrements in mitochondrial respiration in both VDR-KD myoblasts and myotubes, we sought to determine whether a reduction in mitochondrial-related protein content might underlie this phenotype. However, no differences were observed in mitochondrial ETC subunit I–V, citrate synthase (CS) or cytochrome-c (Cyt-c) protein content in either VDR-KD myoblasts (Fig. 3A) or myotubes (Fig. 3C). To further explore the potential influence of mitochondrial dynamics in mediating the observed decrements in mitochondrial function, multiple proxy markers of mitochondrial fusion and fission were probed. MFN2 remained unchanged although, OPA1 increased by 15% in both VDR-KD myoblasts (P = 0.021; Fig. 4A) and myotubes (P = 0.046; Fig. 4C). Furthermore, mitofilin, FIS1, and DRP1 all remained unchanged in VDR-KD myoblasts (Fig. 4A) and myotubes (Fig. 4C).
Fig. 3.
No change in markers of mitochondrial protein content in vitamin D receptor-knockdown (VDR-KD) myoblasts and myotubes compared with control. A–D: protein abundance of mitochondrial subunits complex I (CI; NDUFB8), complex II (CII; SDHB), complex IV (CIV; MTCO1), complex V (CV; ATP5A), as well as citrate synthase (CS) and cytochrome c (Cyt c) in control and VDR-KD myoblasts (A and B) and myotubes (C and D). Data are expressed as means ± SD (n = 6 lanes/group) and represented as a fold change from control.
Fig. 4.
Markers of mitochondrial fission remain unchanged while optic atrophy-1 (OPA1) protein abundance is increased in vitamin D receptor-knockdown (VDR-KD) myoblasts and myotubes compared with control. A–D: protein abundance of markers of mitochondrial fusion (MFN2 and OPA1) and fission [mitofilin, fission protein 1 (Fis1), and dynamin-like protein 1 (DRP1)] in control and VDR-KD myoblasts (A and B) and myotubes (C and D). *P < 0.05, independent t tests. Data are means ± SD (n = 6 lanes/group) and represented as a fold change from control.
DISCUSSION
The role of vitamin D within skeletal muscle has received considerable interest in recent years, with current evidence, suggesting that vitamin D-related metabolites promote mitochondrial function within skeletal muscle (22–26). Building upon previous studies, we demonstrate that loss of VDR function results in significant reductions in mitochondrial respiration in both myoblasts and myotubes (Fig. 2, A–D). Furthermore, we report that impairments were specifically observed in respiration derived from oxidative phosphorylation (ATPOx) (Fig. 2E) and were not as a result of decreased mitochondrial-related protein content (Fig. 3, A–D).
Previously, it has been reported that mitochondrial protein content remains unchanged in both human skeletal muscle myoblasts treated with 1α,25(OH)2D3 and within the quadriceps of VDR-mKO mice (10, 23). Similarly, we also observed no change in mitochondrial protein content in both VDR-KD myoblasts and myotubes. Despite this, it has been reported that the treatment of both human primary and C2C12 myoblasts with vitamin D metabolites resulted in an increase in mitochondrial function (22, 23, 25). Although the observed increases in respiration were abolished following siRNA silencing of the VDR in human primary myoblasts (23), the role of the VDR in basal mitochondrial regulation is unknown. Therefore, our results build upon previous findings and indicate that the VDR is required for the maintenance of optimal mitochondrial respiration in myoblasts and myotubes. Furthermore, our results demonstrating that VDR-KD cells have significant reductions in ATPOx suggest that impairments are intrinsic to the mitochondria following VDR loss-of-function and are not mediated by decreases in mitochondrial protein content per se. Despite in vitro evidence indicating vitamin D and the VDR regulate skeletal muscle mitochondrial function (22, 23, 25), in vivo evidence is currently lacking. Given that the supplementation of vitamin D has been shown to improve symptoms of fatigue and indirect measures of mitochondrial function (26), further examination of the role of vitamin D and the VDR in vivo is warranted.
The mitochondria exist in a reticulated network within skeletal muscle (28) and, therefore, we also examined multiple markers of mitochondrial dynamics to ascertain whether loss of VDR function may alter mitochondrial morphology. Although we observed no differences in the abundance of MFN2, we did observe small, but significant (∼15%), increase in OPA1 protein abundance in both VDR-KD myoblasts and myotubes. OPA1 is known to modulate fusion of the inner mitochondrial membrane, cristae remodeling and reduce mitochondrial fragmentation in protection from apoptosis (5, 6, 8). Given the observed impairments in mitochondrial function and membrane potential following VDR-KD, an increase in OPA1 may be a compensatory mechanism to try to rescue mitochondrial dysfunction. Interestingly, OPA1 was also shown to be responsive to 1α,25(OH)2D3 treatment in human skeletal muscle myoblasts, suggesting mitochondrial dynamics within skeletal muscle may be influenced by vitamin D status (23). Further examination of the mitochondrial network in VDR-KD cell lines via mitochondrial labeling techniques may shed light upon VitD-VDR-OPA1 interactions in this context.
In summary, we report a requirement for the VDR to maintain optimal mitochondrial respiration in C2C12 myoblasts and myotubes. The observed reductions in mitochondrial function were a result of reduced ATPOx, although in contrast, markers of mitochondrial protein content were unchanged. The regulatory role of the VDR within skeletal muscle mitochondrial function in vivo remains largely underexplored. Given the observed reduction in mitochondrial function in vitro, the examination of mitochondrial function within the skeletal muscle of VDR-mKO mice may reveal similar impairments in respiration (4, 10). Furthermore, it is possible that reductions in mitochondrial respiration may be linked to dysregulation of mitochondrial organization, membrane permeability, or calcium homeostasis. With regard to the latter, the treatment of skeletal muscle cell lines with vitamin D-related metabolites has been shown to increase calcium flux (3, 19); however, this has not been previously linked to mitochondrial respiration. Overall, given the significant reduction in mitochondrial respiration displayed following VDR deletion, our results suggest that the VDR plays a fundamental regulatory role in skeletal muscle mitochondrial function.
GRANTS
The MRC-ARUK Centre for Musculoskeletal Aging Research was funded through grants from the Medical Research Council (Grant MR/K00414X/1) and Arthritis Research UK (Grant 19891) awarded to the Universities of Birmingham and Nottingham. S. P. Ashcroft was funded by a MRC-ARUK Doctoral Training Partnership studentship, joint funded by the College of Life and Environmental Sciences, University of Birmingham.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.A., J.J.B., A.A.K., P.J.A., and A.P. conceived and designed research; S.P.A., J.J.B., and A.A.K. performed experiments; S.P.A. analyzed data; S.P.A. and A.P. interpreted results of experiments; S.P.A. and A.P. prepared figures; S.P.A. and A.P. drafted manuscript; S.P.A., J.J.B., P.J.A., and A.P. edited and revised manuscript; S.P.A., J.J.B., A.A.K., P.J.A., and A.P. approved final version of manuscript.
REFERENCES
- 1.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am 39: 321–331, 2010. doi: 10.1016/j.ecl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Bouillon R, Verstuyf A. Vitamin D, mitochondria, and muscle. J Clin Endocrinol Metab 98: 961–963, 2013. doi: 10.1210/jc.2013-1352. [DOI] [PubMed] [Google Scholar]
- 3.Buitrago CG, Arango NS, Boland RL. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J Cell Biochem 113: 1170–1181, 2012. doi: 10.1002/jcb.23444. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Villalta SA, Agrawal DK. FOXO1 mediates vitamin D deficiency-induced insulin resistance in skeletal muscle. J Bone Miner Res 31: 585–595, 2016. doi: 10.1002/jbmr.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932, 2004. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155: 160–171, 2013. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31: 48–54, 2011. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189, 2006. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M, Clifton-Bligh RJ, Gunton JE. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int 97: 602–610, 2015. doi: 10.1007/s00223-015-0054-x. [DOI] [PubMed] [Google Scholar]
- 10.Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, Schindeler A, Stokes R, Swarbrick MM, Evesson FJ, Cooper ST, Gunton JE. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle 10: 1228–1240, 2019. doi: 10.1002/jcsm.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155: 347–357, 2014. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 12.Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155: 3227–3237, 2014. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham AW, Lewis MD. Hypervitaminosis D rickets: the action of vitamin D. Br J Exp Pathol 15: 228–234, 1934. [Google Scholar]
- 14.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int 92: 77–98, 2013. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930, 2011. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 16.Kazi AA, Lang CH. PRAS40 regulates protein synthesis and cell cycle in C2C12 myoblasts. Mol Med 16: 359–371, 2010. doi: 10.2119/molmed.2009.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 83: 841–850, 1995. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 18.Mookerjee SA, Gerencser AA, Nicholls DG, Brand MD. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J Biol Chem 292: 7189–7207, 2017. [Erratum in J Biol Chem 293: 12,649-12,652, 2018.] doi: 10.1074/jbc.M116.774471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morelli S, de Boland AR, Boland RL. Generation of inositol phosphates, diacylglycerol and calcium fluxes in myoblasts treated with 1,25-dihydroxyvitamin D3. Biochem J 289: 675–679, 1993. doi: 10.1042/bj2890675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens DJ, Sharples AP, Polydorou I, Alwan N, Donovan T, Tang J, Fraser WD, Cooper RG, Morton JP, Stewart C, Close GL. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am J Physiol Endocrinol Metab 309: E1019–E1031, 2015. doi: 10.1152/ajpendo.00375.2015. [DOI] [PubMed] [Google Scholar]
- 21.Pike JW, Meyer MB, Bishop KA. Regulation of target gene expression by the vitamin D receptor—an update on mechanisms. Rev Endocr Metab Disord 13: 45–55, 2012. doi: 10.1007/s11154-011-9198-9. [DOI] [PubMed] [Google Scholar]
- 22.Romeu Montenegro K, Carlessi R, Cruzat V, Newsholme P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J Steroid Biochem Mol Biol 193: 105423, 2019. doi: 10.1016/j.jsbmb.2019.105423. [DOI] [PubMed] [Google Scholar]
- 23.Ryan ZC, Craig TA, Folmes CD, Wang X, Lanza IR, Schaible NS, Salisbury JL, Nair KS, Terzic A, Sieck GC, Kumar R. 1α,25-dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem 291: 1514–1528, 2016. doi: 10.1074/jbc.M115.684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan ZC, Craig TA, Wang X, Delmotte P, Salisbury JL, Lanza IR, Sieck GC, Kumar R. 1α,25-dihydroxyvitamin D3 mitigates cancer cell mediated mitochondrial dysfunction in human skeletal muscle cells. Biochem Biophys Res Commun 496: 746–752, 2018. doi: 10.1016/j.bbrc.2018.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnell DM, Walton RG, Vekaria HJ, Sullivan PG, Bollinger LM, Peterson CA, Thomas DT. Vitamin D produces a perilipin 2-dependent increase in mitochondrial function in C2C12 myotubes. J Nutr Biochem 65: 83–92, 2019. doi: 10.1016/j.jnutbio.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab 98: E509–E513, 2013. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 27.Stocks B, Dent JR, Joanisse S, McCurdy CE, Philp A. Skeletal muscle fibre-specific knockout of p53 does not reduce mitochondrial content or enzyme activity. Front Physiol 8: 941, 2017. doi: 10.3389/fphys.2017.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta 1817: 1833–1838, 2012. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]