Abstract
Cystic fibrosis (CF) lung disease persists and remains life-limiting for many patients. Elevated high-mobility group box-1 protein (HMGB-1) levels and epithelial sodium channel hyperactivity (ENaC) are hallmark features of the CF lung. The objective of this study was to better understand the pathogenic role of HMGB-1 signaling and ENaC in CF airway cells. We hypothesize that HMGB-1 links airway inflammation [via signaling to the receptor for advanced glycation end products (RAGE)] and airway surface liquid dehydration (via upregulation of ENaC) in the CF lung. We calculated equivalent short-current (Isc) and single-channel ENaC open probability (Po) in normal and CF human small airway epithelial cells (SAEC) in the presence and absence of human HMGB-1 peptide (0.5 μg/mL). In normal SAECs, HMGB-1 increased amiloride-sensitive Isc and elevated ENaC Po from 0.15 ± 0.03 to 0.28 ± 0.04 (P < 0.01). In CF SAECs, ENaC Po increased from 0.45 ± 0.06 to 0.73 ± 0.04 (P < 0.01). Pretreatment with 1 μM FPS-ZM1 (a RAGE inhibitor) attenuated all HMGB-1 effects on ENaC current in normal and CF SAECs. Confocal analysis of SAECs indicates that nuclear size and HMBG-1 localization can be impacted by ENaC dysfunction. Masson’s trichrome labeling of mouse lung showed that intraperitoneally injected HMGB-1 significantly increased pulmonary fibrosis. Bronchoalveolar lavage fluid from HMGB-1-treated mice showed significant increases in IL-1β, IL-10, IL-6, IL-27, IL-17A, IFN-β, and granulocyte-macrophage colony-stimulating factor compared with vehicle-injected mice (P < 0.05). These studies put forth a new model in which HMGB-1 signaling to RAGE plays an important role in perpetuating ENaC dysfunction and inflammation in the CF lung.
Keywords: airway inflammation, cystic fibrosis, cytokines, ENaC, HMGB-1, RAGE
INTRODUCTION
Cystic fibrosis (CF) is a disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulatory protein (CFTR). Although pancreatic enzyme therapy and airway mucolytic, inhaled antibiotic, and CFTR modulator therapies have rapidly increased survival of CF patients well beyond adolescence, CF lung disease remains life-limiting for most patients. In CF patients, airway inflammation begins early in life and progressively worsens, leading to tissue damage, bronchiectasis, and, eventually, lung failure. Despite availability of CF therapies that can slow the decline of lung function, many CF patients struggle with impaired host defenses, airway obstruction, and fibrotic damage to airway architecture throughout their lifetime. Little is known about the pathogenic mechanisms that lead to CF lung disease; therefore, increased understanding of these mechanisms is needed to develop more effective therapies for CF patients.
A possible mechanism contributing to development of the CF lung phenotype is hyperactivity of the epithelial sodium channel (ENaC). ENaC is an apically located, amiloride-sensitive channel that plays a critical role in generating the osmotic gradient needed for water absorption from the luminal to basolateral surfaces of the lung. It is clear that ENaC plays an important role in the pathogenesis of CF lung disease. Overexpression of either the α- or β-ENaC subunit results in CF-like lung disease in the absence of disease-causing CFTR mutations (9). Importantly, CF patients with loss-of-function mutations in the gene encoding the δ-ENaC subunit sodium channel epithelial 1δ (SCNN1D) exhibited a nonprogressive CF lung phenotype (1). Overactive ENaC makes major contributions to mucus stasis in CF and, in turn, airway inflammation (12). The exact mechanism leading to dysregulated ENaC in the CF lung is not fully understood.
In terms of developing new drug therapies, another potentially important target in the CF lung is high-mobility group box-1 protein (HMGB-1). After completing a single-center clinical trial, our research group identified elevated HMGB-1 levels in lung sputum from CF patients compared with healthy volunteers (15). As such, HMGB-1 is an important biomarker for severity of CF lung disease (5, 21). HMGB-1 has an established role as a nuclear protein that plays an important part in immune responses. In the nucleus, HMGB-1 enables transcription. Under conditions of tissue injury, infection, or inflammation, HMGB-1 translocates from the nucleus to the cytoplasm and, ultimately, is released into the extracellular space to influence inflammatory responses. Extracellular HMGB-1 is known to mediate inflammatory arthritis (2, 26), sepsis (24), acute lung injury (14), asthma (reviewed in Ref. 13), and chronic obstructive pulmonary disease (reviewed in 8). Notably, increased levels of HMGB-1 have been detected within the epithelial layer in airway biopsies and in the epithelial lining fluid (20, 25). Together, these studies indicate that HMGB-1 signaling may be an effective therapeutic target in the effort to improve lung function in CF patients. Generally, biomarkers that predict subsequent key outcomes or survival are candidates for being causal for progressive disease.
We propose that elevated levels of HMGB-1 and hyperactive ENaC in the CF lung may be linked via receptor for advanced glycation end product (RAGE) signaling. Secreted HMGB-1 can bind to various receptors, including Toll like receptors (TLR2, TLR4, and TLR9) and RAGE. Specific HMGB-1 binding to RAGE has been suggested to play an important role in airway inflammation in CF and CF-related diabetes (19). Because the CF lung is oxidatively stressed (3), oxidized Cys residues on HMGB-1 cause conformational changes in the protein structure that render HMGB-1 unable to bind to TLR4. It is widely regarded that HMGB-1 (Cys106) must be in the reduced form to bind to TLR4 (27). As such, oxidation prevents HMGB-1 binding to Toll-like receptors. Furthermore, it has been shown that HMGB-1 induces expression of RAGE, but not TLR2 or TLR4, in airway epithelial cells (4). It has been suggested that that HMGB-1 may signal via RAGE in the CF lung (19).
Based on evidence from the literature, we evaluated the role of HMGB-1 signaling via RAGE. Our hypothesis is that HMGB-1 signals via RAGE in the CF lung to increase inflammation and upregulate ENaC activity. We further hypothesize that upregulated ENaC, in turn, influences cellular secretion of HMGB-1, thus perpetuating inflammation and mucus dehydration in the lung. To test the hypothesis that HMGB-1 signals to ENaC via RAGE, we measured the effect of HMGB-1 on ENaC activity in the presence and absence of FPS-ZM1, a RAGE inhibitor. We then measured the effect of HMGB-1 on lung inflammation and cytokine levels. We also evaluated the effect of amiloride inhibition of ENaC activity, FPS-ZM1 inhibition of RAGE, and N-methyl-d-glucamine (NMDG) replacement of NaCl on HMGB-1 subcellular localization in CF lung cells. Outcomes from our study indicate that ENaC-RAGE cross talk may play a key role in the pathogenesis of CF.
MATERIALS AND METHODS
Reagents.
Human HMGB-1 peptide, rabbit anti-HMGB-1 antibody (catalog no. AB_1603373), and goat anti-rabbit IgG conjugated to Alexa Fluor 488 (catalog no. AB_955447) were purchased from Abcam Biotechnology (Eugene, OR). Amiloride and FPS-ZM1 were purchased from Sigma Aldrich Chemicals (St. Louis, MO).
Tissue culture.
Cryopreserved human small airway epithelial cells (SAECs) and diseased (CF) SAECs were purchased from Lonza Bioscience (Alpharetta, GA) and cultured in a 37°C humidified 5% CO2 incubator. The cells were cultured according to the supplier’s protocol using SAEC growth medium (SAGM, Lonza) and supplements. Basal culture medium lacked growth supplements. All experiments were performed between SAEC passages 1 and 4. For electrophysiological studies, cells were cultured to confluency on Costar Snapwell clear permeable supports with 0.4-μm polyester membrane (Kennebunk, ME). Prior to experimentation, Trypan blue exclusion tests were used to determine the number of viable cells in a cell suspension.
Single-channel patch-clamp analysis.
ENaC activity was recorded using standard single-channel patch-clamp analysis in the cell-attached configuration. Micropipettes were pulled with a two-stage vertical puller (model PC-10, Narishige International, Amityville, NY) from filamented borosilicate glass capillaries (World Precision Instruments, Sarasota, FL). Resistances of the micropipettes were between 5–9 MΩ when filled and immersed in patch solution [in mM: 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 HEPES; pH adjusted to 7.40 with NaOH]. Channel currents were sampled at 1 kHz with a patch amplifier (Axopatch 1B, Molecular Devices, San Jose, CA) and filtered at 200 Hz with a low-pass Bessel filter. Continuous single-channel activity was recorded and then analyzed using pClamp 10 software. Open probability (Po) and number (N) of active channels in SAEC apical membrane, as well as chord conductance (γ), were calculated from hyperpolarized and depolarized potentials clamped before and after treatment with HMGB-1 (0.5 μg/mL). SAECs were pretreated with FPS-ZM1 (1 μM) for 1 h, and amiloride (1 μM) was backfilled into the patch electrode in the single-channel patch-clamp studies.
Equivalent short-circuit measurements.
SAECs were seeded onto permeable membranes at a density of 1.5–2 × 105 cells/12-mm culture chamber, under air-liquid interface, until confluent monolayers were established (~5–7 days). At confluency, apical and luminal surfaces were bathed in cell culture medium and allowed to equilibrate in culture at 37°C in a humidified 5% CO2 incubator for 3 h before experimentation. Transepithelial voltage (V) and resistance (RTE) were measured using an epithelial volt-ohm meter (World Precision Instruments); equivalent short-circuit current (Isc) was calculated in accordance with Ohm’s law: V = (Isc)(RTE). All drug treatments were applied to the apical compartment.
Mice.
Wild-type (WT) C57BL/6J mice (6–8 wk old) were purchased from the Jackson Laboratory (Sacramento, CA). Scnn1β transgenic mice backcrossed to the C57BL/6N background (a kind gift from Dr. Alessandra Livraghi-Butrico, Marsico Lung Institute, Chapel Hill, NC) exhibit a lung phenotype similar to the pathologic descriptions of cystic fibrosis (16). Mice were given ad libitum access to food and water, housed under a normal light-dark cycle, and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press). The University of Utah Animal Care Committee approved all animal procedures. Male and female mice were randomly assigned to treatment groups. Experimental groups received an intraperitoneal injection of HMGB-1 (7 ng/kg body wt in a 50-μL injection volume) or vehicle control in the morning. A separate group of mice were nasally insufflated with 1 μM FPS-ZM1 (3 nL/kg body wt) in the morning. Animals received one dose daily over the course of 3 days. Animals were allowed to recover in home cages after each treatment. Studies were conducted ~84 h following the first dose. Mice were euthanized using isoflurane followed by exsanguination.
Cytokine and inflammation studies.
Tracheas were cannulated before bronchoalveolar lavage (BAL), which was performed with 50 μL of phosphate-buffered saline (PBS; Life Technologies, Grand Island, NY) per 1 g of body weight. BAL fluid was collected and then analyzed for cytokine levels using a commercially available enzyme-linked immunosorbent assay (Mouse Inflammation Panel 13 Plex Kit, BioLegend, San Diego, CA) following the manufacturer’s protocol. Flow cytometric analysis of BAL cytokines was performed using the FACScanto system (BD Biosciences, San Jose, CA) at the University of Utah Flow Cytometry Core (Salt Lake City, UT).
Histology and immunohistochemistry.
Lung tissue was fixed in formalin, embedded in paraffin, and stained with trichrome by ARUP Laboratories (Salt Lake City, UT). All SAECs were seeded at equal density on poly-d-lysine-coated 12-mm round glass coverslips (Fisher Scientific, Pittsburgh, PA) for immunohistochemistry. SAECs were fixed using 4% formaldehyde for 10 min, permeabilized with 0.5% Nonidet P-40 for 15 min, and then incubated in 0.5% BSA and 0.1% gelatin in PBS. After application of primary rabbit anti-HMGB-1 antibody (diluted 1:350 in PBS + 1× azide + 1% BSA) and incubation at room temperature for 1 h, the cells were labeled with the secondary goat anti-rabbit IgG conjugated to Alexa Fluor 488 (diluted 1:250 in PBS + 1× azide + 1% BSA) for 1 h at room temperature. Nuclear DNA was labeled with mounting medium containing 4′,6-diamidino-2-phenylindole (Abcam). Confocal imaging using a Nikon A1R confocal laser microscope system was performed at the University of Utah Health Science Center Cell Imaging Core. Wide-field imaging was performed using an IX71 microscope with associated DP2-BSW image-capture software (Olympus Life Science, Center Valley, PA). ImageJ software (National Institutes of Health) with Fiji plugin was used for image analysis of protein translocation and nuclear size.
Statistical analysis.
SigmaPlot and pCLAMP software were used for offline analysis of digitized data. All data are summarized as means ± SE, unless otherwise noted. Single comparisons were performed using paired Student’s t tests. Multiple comparisons were performed using one-way analysis of variance followed by Bonferroni’s post hoc test for pair-wise comparisons. P < 0.05 was considered statistically significant.
RESULTS
HMGB-1 increases ENaC Po.
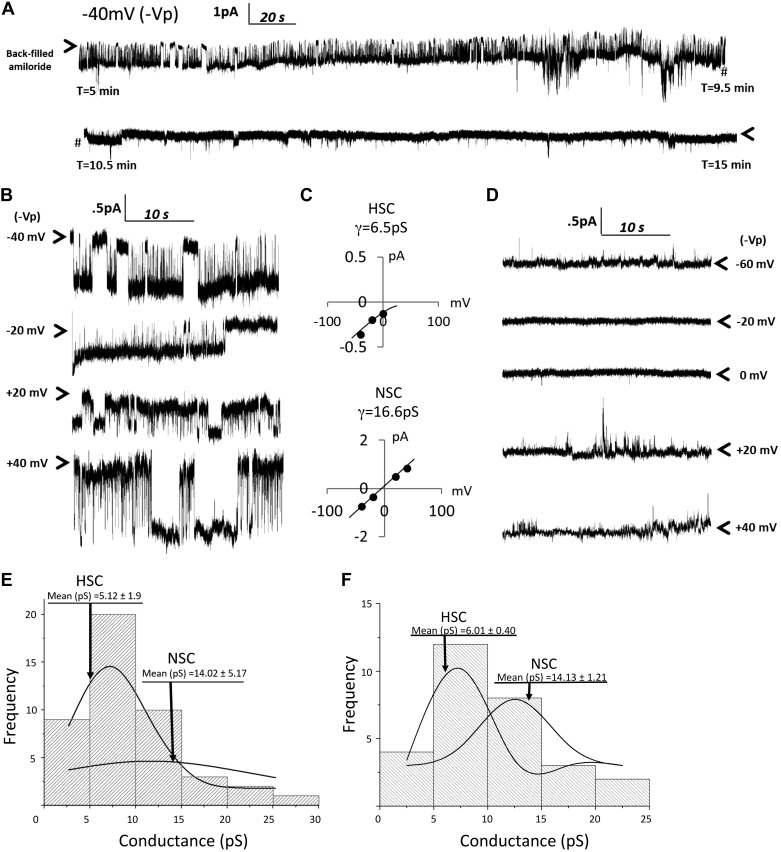
The biophysical transport properties of human SAECs in culture have not been clearly described. Therefore, we first tested the amiloride sensitivity of single-channel patch-clamp recordings conducted in SAECs. We backfilled patch electrodes with 1 μM amiloride and then formed a tight giga-ohm seal between the cell membrane and the glass electrode in the cell-attached configuration. Our prior experience indicates that ~10 min were required for diffusion of amiloride from the solution to the tip of the electrode to block channel pore openings (11). Figure 1A shows a portion of the recording at <10 min (before amiloride inhibition of channels) and >10 min (following amiloride inhibition of ENaC). Figure 1B shows channel activity before amiloride inhibition (<10 min) at hyperpolarizing and depolarizing potentials at which channel conductances were calculated and then shown in Fig. 1C. This segment of the recording shows that SAECs can have functional highly selective cation (HSC, γ = 6.5 pS) and nonselective cation (NSC, γ = 16.6pS) channels on the apical membrane. Figure 1D shows that inward and outward currents are still detectable at depolarizing and hyperpolarizing potentials following amiloride block of ENaC pore opening, as expected (although channel size is much smaller). Figure 1, E and F, shows the distribution of single-channel conductances from several different patch recordings of normal and CF SAECs. Conductances were obtained from a minimum of four holding potentials to calculate slope conductances. The mean conductance of normal SAEC HSC channels is 5.12 ± 1.9 pS, and that of NSC channels is 14.02 ± 5.17 pS. The mean conductances of HSC and NSC channels in CF SAECs were 6.01 ± 0.40 and 14.13 ± 1.21 pS, respectively.
Fig. 1.
Single Na+ channel characteristics in normal and cystic fibrosis (CF) human small airway epithelial cells (hSAECs). A: cell-attached patch-clamp analysis of normal hSAECs; 1 μM amiloride was backfilled into the patch electrode. Downward deflections from arrows (indicating closed-channel state) represent inward current; upward deflections from closed state represent outward current; # demarks a break in the continuous trace; near the 10.5-min mark, amiloride has reached the cell membrane and begins to block Na+ current. B and C: highly selective cation (HSC, 6.5 pS) and nonselective cation (NSC, 16.6 pS) channel conductances (γ) calculated from representative trace in A before amiloride inhibition. Both conductances have been shown to be amiloride-sensitive current in lung epithelia (7). D: complete amiloride inhibition of Na+ current at 0 and −20 mV (−Vp). Application of polarized potentials of +20, +40, and −60 mV (−Vp) induced current in the presence of amiloride, as expected, although current amplitudes were smaller. E and F: distribution of single-channel conductances from normal (E) and CF (F) hSAECs.
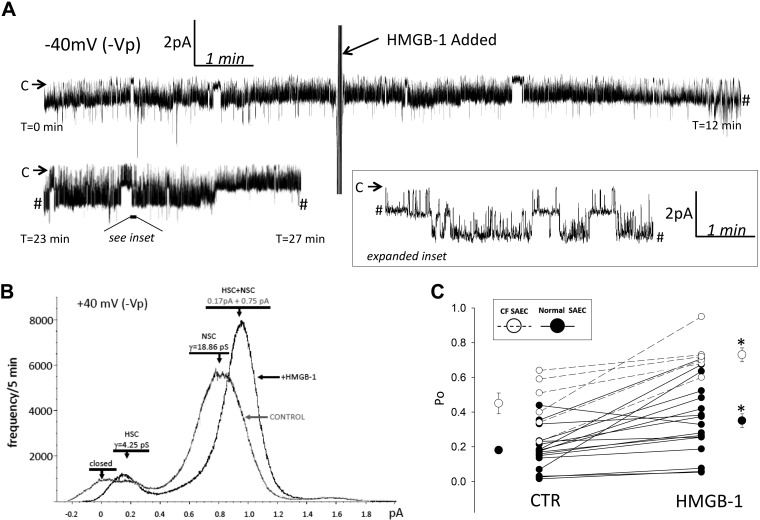
To determine if HMGB-1 regulates ENaC activity, we treated normal and CF SAECs with 0.5 μg/mL HMGB-1 and used cell-attached patch-clamp analysis to measure ENaC activity, as previously described (11). Figure 2A shows portions of a continuous recording at 0–12 and 23–27 min. HMGB-1 was applied to the extracellular bath solution of a cell-attached recording of a normal SAEC at 5 min; an increase in ENaC current was detected up to 27 min of the recording period. Figure 2B shows the frequency of HSC and NSC channel activity from the representative trace in Fig. 2A before and after HMGB-1 treatment. Figure 2C shows that, in 21 independent normal SAEC observations (from 5 different donor lungs), HMGB-1 increased ENaC Po from 0.15 ± 0. 03 to 0.28 ± 0.04 (P < 0.01). Single-channel analysis of CF SAECs after application of 0.5 μg/mL HMGB-1 to the extracellular bath is shown in Fig. 2C (n = 6). HMGB-1 increased CF SAEC Po from 0.45 ± 0.06 to 0.73 ± 0.04 (P < 0.01).
Fig. 2.
High-mobility group box-1 protein (HMGB-1) increases epithelial Na+ channel (ENaC) open probability (Po) in small airway epithelial cells (SAECs). A: representative trace of continuous single-channel recording before and after application of 0.5 μg/mL HMGB-1 to the extracellular bath. A portion of the single-channel recording was expanded between 23 and 27 min to show single-channel detail; # demarks a break in the continuous trace. Arrows indicate closed state (C); downward deflections from arrows indicate channel openings. B: highly selective cation (HSC, 4.25 pS) and nonselective cation (18.86 pS) channels under control condition and following HMGB-1 application to extracellular bath. C: HMGB-1 significantly increases ENaC Po in normal SAECs (n = 21 independent patch-clamp recordings from SAECs obtained from 5 different healthy male and female donor lungs) and CF SAECs (n = 6 independent patch-clamp recordings from SAECs obtained from 1 female CF donor lung). CTR, control. *P < 0.05, before vs. after HMGB-1 application (evaluated using the paired t-test).
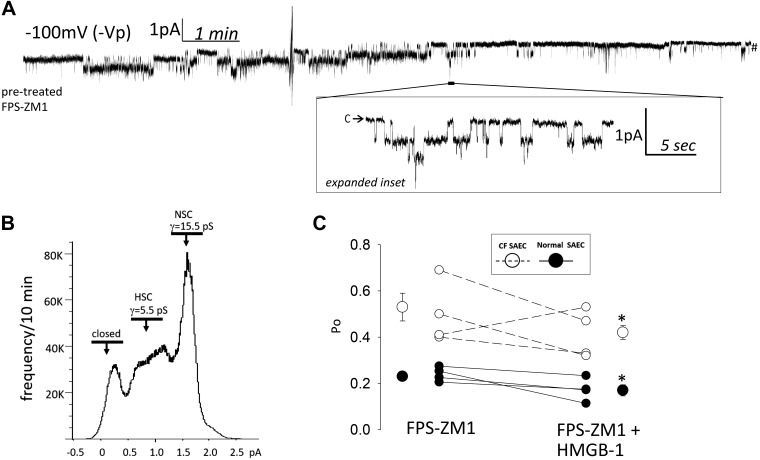
To determine if HMGB-1 signals to ENaC via RAGE, we pretreated SAECs with 1 μM FPS-ZM1 for 1 h and then with 0.5 μg/mL HMGB-1. Pretreatment of normal and CF SAECs with FPS-ZM1 significantly attenuated the previously reported HMGB-1-mediated increase in ENaC Po (Fig. 2). A representative continuous trace in Fig. 3A shows channel activity before and after HMGB-1 application following 1 h of pretreatment with 1 μM FPS-ZM1. Figure 3B shows a calculated γ and frequency of HSC (5.5 pS) and NSC (15.5 pS) channels following FPS-ZM1 pretreatment from the representative trace in Fig. 3A. No change in HSC and NSC channel frequency was observed following HMGB-1 treatment (data not shown). In four independent observations of normal SAECs, HMGB-1 failed to stimulate ENaC Po following FPS-ZM1 treatments. ENaC activity ran down in Fig. 3C experiments [as expected in continuous recordings conducted in the absence of agonist (10)]; Po changed from 0.24 ± 0.015 to 0.17 ± 0.024, with no change in channel number (data not shown). Figure 3C also includes single-channel analysis of CF SAECs pretreated with FPS-ZM1 and then treated with HMGB-1 in the same continuous recording (n = 4). Pretreatment of CF SAECs with FPS-ZM1 also attenuated HMBG-1 stimulation of ENaC Po, with associated rundown of channel activity. FPS-ZM1- and HMGB-1-treated CF SAEC Po values ran down from 0.53 ± 0.06 to 0.42 ± 0.03. Data from single-channel analysis of normal and CF SAECs indicate that inhibition of RAGE with FPS-ZM1 attenuates HMGB-1 signaling to ENaC.
Fig. 3.
Pretreatment with FPS-ZM1 (1 μM) attenuates high-mobility group box-1 protein (HMGB-1)-mediated increase in epithelial Na+ channel (ENaC) activity. A: small airway epithelial cells (SAECs) were pretreated with FPS-ZM1, an inhibitor of receptor for advanced glycation end products, 1 h before HMGB-1 (0.5 μg/mL) application to the extracellular bath. Inset: single-channel detail at −100 mV (–Vp) holding potential. B: calculated chord conductance (γ) and frequency histogram from representative trace in A. HSC, highly selective cation channel; NSC, nonselective cation channel. C: HMGB-1 fails to increase ENaC Po in SAECs pretreated with FPS-ZM1. Significant channel rundown was observed in both CF (n = 4) and normal (n = 4) SAECs. *P < 0.05, before vs. after HMGB-1 application in FPS-ZM1-pretreated SAECs (by paired t test).
HMGB-1 increases SAEC equivalent transepithelial current.
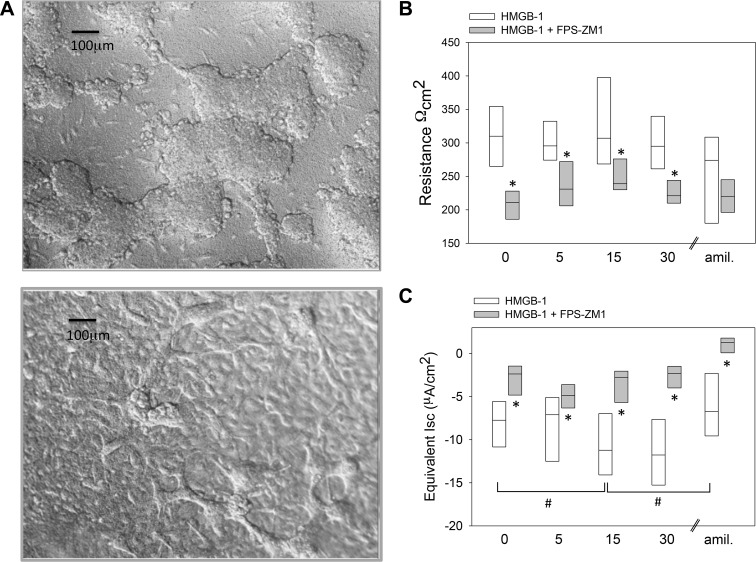
We also calculated ENaC Isc across a monolayer of normal SAECs cultured in basal medium under air-liquid interface. Figure 4A shows normal SAECs grown on permeable supports under an air-liquid interface on day 3 and at confluency (>10 days). CF SAECs grown in a similar manner would dome, but not form confluent monolayers for Isc evaluation (data not shown). Figure 4, B and C, shows RTE and calculated equivalent Isc values of normal SAECs following addition of HMGB-1 and HMGB-1 + FPS-ZM1 to the apical compartment. HMGB-1 significantly increased SAEC Isc within 15 min of treatment. Pretreatment of SAECs with FPS-ZM1 significantly attenuated the HMGB-1-mediated increase in Isc and decreased RTE in SAECs grown on a permeable support. Together with Fig. 3, the data indicate that HMGB-1 increases ENaC activity, in part, via FPS-ZM1-sensitive RAGE signaling.
Fig. 4.
High-mobility group box-1 protein (HMGB-1)-receptor for advanced glycation end products (RAGE) axis plays an important role in regulating transepithelial Na+ transport. A, top: normal small airway epithelial cells (SAECs) after 3 days under air-liquid interface and on permeable support culture system. A, bottom: culture of confluent normal SAECs on permeable support (10 days) allows for measurement of transepithelial resistance (RTE) and calculation of equivalent short-circuit current (Isc). B and C: SAEC RTE and equivalent Isc values following application of HMGB-1 (0.5 μg/mL, n = 8) and HMGB-1 + FPS-ZM1 (1 μM, n = 7). Amiloride (amil, 1 μM, n = 5) treatments were conducted separately, as indicated by //. FPS-ZM1 pretreatment attenuates HMGB-1-mediated increase in Isc. *P < 0.05, HMGB-1 vs. HMGB-1 + FPS-ZM1 (by paired t test). #P < 0.05 vs. respective control treatment group (t = 0 min) (by repeated-measures ANOVA followed by post hoc Holm’s t test).
HMGB-1 subcellular localization.
The data from our single-channel and Isc measurements show that HMBG-1 regulates ENaC. Next, we wanted to evaluate whether an increase in solute transport (via an increase in ENaC Po) can, in turn, regulate nuclear export of HMGB-1, since increased transcellular solute transport has been shown to increase intracellular fluid volume (ICF). Increased ICF expands the nucleoplasm and impacts the transport of nuclear protein such as HMGB-1 (18). To further support this paradigm, we modeled an increase in ICF with hyposmotic solution and evaluated cell viability, as well as HMGB-1 localization. First, we verified that hyposmotic (150 mosM) solution does not significantly affect SAEC viability (data not shown). Then we evaluated the effect of hyposmotic saline, FPS-ZM1, NMDG, and amiloride on nuclear size and protein export of HMGB-1 in normal and CF SAECs.
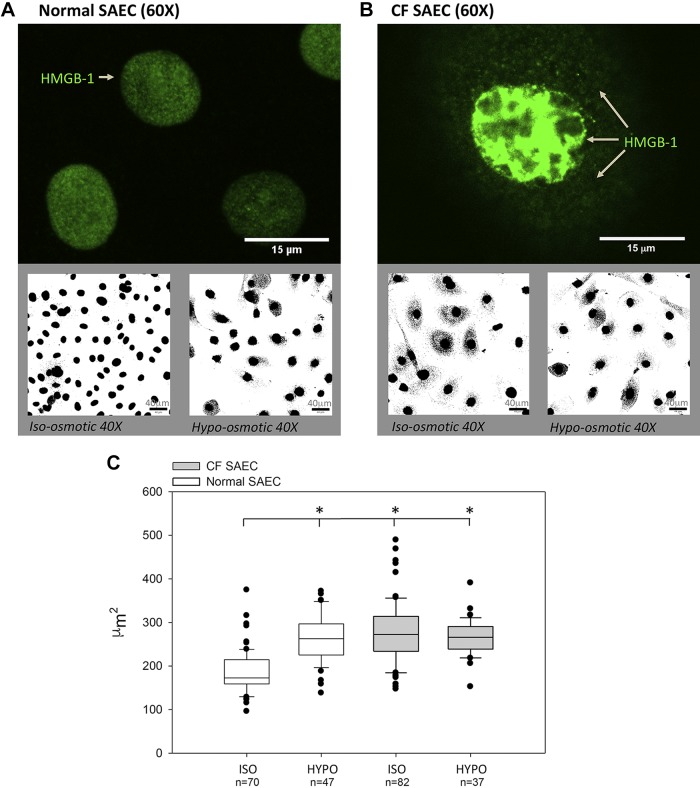
Figure 5A shows confocal HMGB-1 nuclear localization at ×60 magnification (using anti-HMGB-1 primary antibody and secondary conjugated Alexa Fluor 488 antibody). Normal SAECs exhibited nuclear HMGB-1 subcellular localization. The Alexa Fluor signal detected by confocal microscopy is shown in binary mode to contrast and highlight the shift in HMGB-1 subcellular localization from nuclear to cytoplasmic compartments following change from isosmotic to hyposmotic solutions. Figure 5B similarly shows HMGB-1 localization at ×60 magnification in CF SAECs. CF cells showed intense nuclear, as well as cytoplasmic, labeling of HMGB-1. As shown in Fig. 5B, bottom, incubation of CF SAECs in hyposmotic solution did not impact the subcellular localization of HMGB-1. Secondary antibody-labeling controls were negative and indicate that labeling in Fig. 5, A and B, is specific to the primary antibody (data not shown).
Fig. 5.
Osmotic stress in cystic fibrosis (CF) small airway epithelial cells (SAECs) results in enlarged nuclear structures and affects high-mobility group box-1 protein (HMGB-1) distribution. A and B, top: subcellular localization of HMGB-1 labeled with primary monoclonal rabbit anti-HMGB-1 and corresponding secondary goat anti-rabbit IgG conjugated with Alexa Fluor 488 (green) in normal and CF SAECs under isosmotic conditions. A and B, bottom: conjugated GFP secondary antibody signal was changed to binary (black and white) images to facilitate and contrast view of HMGB-1 subcellular location under isosmotic and hyposmotic solutions. C: hypotonic (HYPO) saline significantly increases nuclear size in normal SAECs (n = 47) vs. isotonic (ISO) control (n = 70). *P < 0.01. Nuclear size of CF SAECs did not change significantly in isotonic (n = 82) vs. hypotonic (n = 37) solution. Cell observations from 3 independent studies analyzing 4 regions of interest are reported. Statistical comparisons vs. normal SAECs in isotonic saline were conducted by ANOVA followed by post hoc testing (Holm’s t-test).
In Fig. 5C, we quantified normal and CF SAEC nuclear size under isomotic and hyposmotic saline solutions. The data show that 1) normal SAEC nuclear size (μm2) significantly increases in hyposmotic solution (150 mosM) compared with cells maintained in isosmotic saline (305 mosM); 2) in isosmotic saline, nuclear size is significantly greater in CF than normal SAECs; and 3) nuclear size does not change significantly when CF SAECs are placed in hyposmotic solution (presumably because of an innate increase in intracellular solute transport).
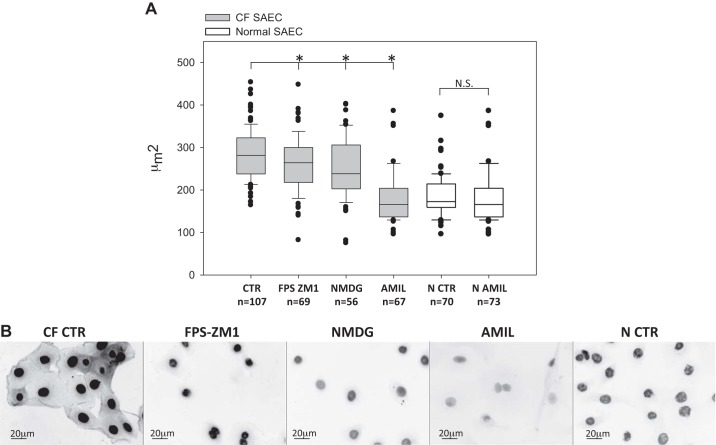
In Fig. 6, we extended observations of nuclear size and HMGB-1 subcellular localization to include the effect of FPS-ZM1 inhibition of RAGE, NMDG replacement of Na+, and amiloride inhibition of ENaC in CF SAECs. Each additional treatment was conducted in isosmotic saline solution. (Although a time course of 30 min, 3 h, and 24 h was evaluated in each group, we report the earliest time point that demonstrated significant change in nuclear size.) Figure 6A shows that 24 h of treatment with the RAGE inhibitor FPS-ZM1 (1 μM) significantly decreased CF SAEC nuclear size from 283.8 ± 5.6 μm2 (untreated control) to 260.6 ± 5.6 μm2. NMDG (109 mM) decreased nuclear size (252.3 ± 9.8 μm2) within 30 min of incubation. Sustained incubation in NMDG resulted in significant cell death at 3 and 24 h. After 3 h of amiloride (1 μM) treatment, nuclear size of CF SAECs decreased to 211.1 ± 10.31 μm2 and was similar to that of normal SAECs (187.12 ± 5.7 μm2). Fluorescence imaging of CF SAECs in Fig. 6B shows that FPS-ZM1, NMDG, and amiloride each affected cellular localization of HMGB-1 in CF cells.
Fig. 6.
Receptor for advanced glycation end products (RAGE)-mediated transport of Na+ plays an important role in nuclear size and high-mobility group box-1 protein (HMGB-1) localization in cystic fibrosis (CF) small airway epithelial cells (SAECs). A: FPS-ZM1, N-methyl-d-glucamine (NMDG), and amiloride (AMIL) treatments significantly decrease nuclear size of CF SAECs (gray bars; n = 3 independent studies with 4 regions of interest per slide analyzed). Nuclear size of normal (N) SAECs [control (CTR) and amiloride] is also shown (open bars). *P < 0.01 vs. CTR CF SAEC (by ANOVA and post hoc Holm’s t test). No significant difference (NS, by t-test) between normal SAEC treatment groups. B: binary wide-field images of HMGB-1 cellular localization in CF (control and FPS-ZM1-, NMDG-, and amiloride-treated) and normal SAECs.
HMBG-1 increases cytokine signaling and fibrosis in the mouse lung.
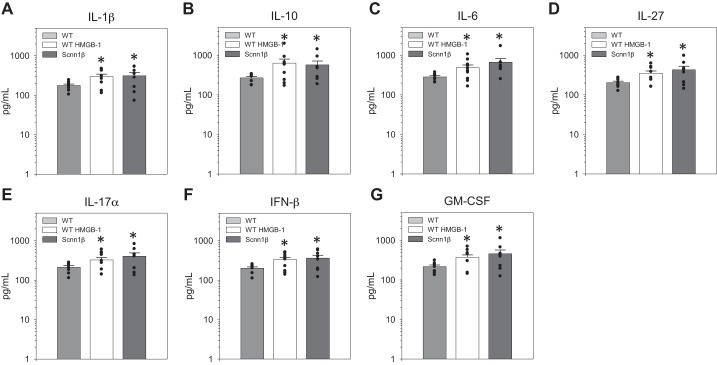
To better understand the pathophysiological consequence of perpetuating ENaC activity and sustaining high HMGB-1 levels, we studied cytokine levels in WT and Scnn1β mice. Scnn1β mice overexpress ENaC and have the CF lung phenotype (16). Importantly, levels of HMGB-1 in BAL fluid are significantly higher in Scnn1β than WT mice, which recapitulates high HMGB-1 levels measured in CF sputum (21). In the current study, Scnn1β mice and WT littermates were intraperitoneally injected with 1) sterile saline (vehicle control) or 2) HMGB-1 (7 ng/kg body wt). BAL fluid was collected and then analyzed for inflammatory markers using a commercially available 13-plex cytokine assay, which allowed simultaneous assessment of each cytokine by a flow cytometric approach. After collection of BAL fluid for cytokine study, lung tissue was harvested for histological evaluation. As shown in Fig. 7, levels of IL-1β, IL-10, IL-6, IL-27α, IL-17, IFN-β, and granulocyte-macrophage colony stimulating factor (GM-CSF) were significantly higher in saline-injected Scnn1β than saline-injected WT mice. Intraperitoneal injection of HMBG-1 in WT mice significantly increased the same cytokines that were elevated in the CF mouse lung model (IL-1β, IL-10, IL-6, IL-27α, IL-17, IFN-β, and GM-CSF). In Scnn1β mice, intraperitoneal injection of HMGB-1 did not significantly increase cytokines in collected BAL fluid (data not shown).
Fig. 7.
High-mobility group box-1 protein (HMGB-1) significantly increases cytokine signaling in C57BL/6 mouse lung. Cytokine [IL-1β, IL-10, IL-6, IL-27, IL-17α, IFN-β, and granulocyte-macrophage colony stimulating factor (GM-CSF)] profiles of WT C57BL/6 mice, WT C57BL/6 mice intraperitoneally injected with HMGB-1 (10 μg in a 50-μL injection volume), and Scnn1b mice are shown in log scale (A–G). Male and female cohorts of mice were studied; n = 3 independent observations with replicate measures of cytokines. *P < 0.05.
These data indicate that HMGB-1 is an important factor leading to development of the CF lung inflammation phenotype and identify the putative proinflammatory cytokines involved in the pathophysiological processes related to CF lung disorder. The finding that further intraperitoneal injection of HMGB-1 into ENaC-overexpressing mice did not lead to further increase in cytokine level suggests a linear pathway linking ENaC hyperactivity and HMGB-1-mediated cytokine upregulation.
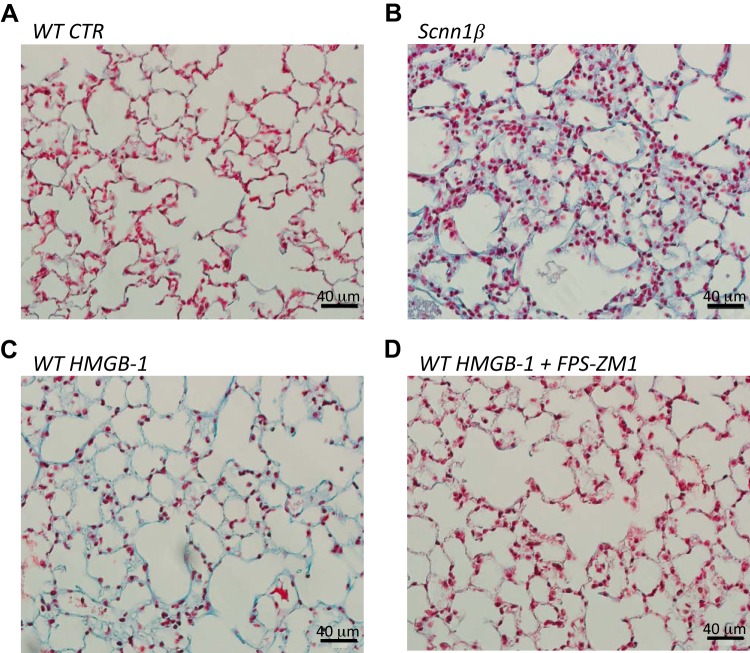
To determine whether HMGB-1 plays a role in fibrosis, we trichrome-labeled Scnn1β and WT mice injected with HMGB-1 with our without intratracheal instillation of FPS-ZM1. Masson’s trichrome labeling (Fig. 8) includes methyl blue labeling of collagen, which is deposited in excess in lung injury. Figure 8A shows trichrome labeling of healthy WT mouse lung. Figure 8, B and C, shows increased collagen labeling in Scnn1β mice and HMGB-1-injected WT mice compared with control WT mice. Figure 8D shows that intratracheal instillation of FPS-ZM1 protected mouse lung from HMGB-1-induced fibrotic injury. Together, Figs. 7 and 8 show the important role of HMGB-1 signaling to RAGE in proinflammatory cytokine release and lung fibrosis.
Fig. 8.
High-mobility group box-1 protein (HMGB-1) increases fibrosis in C57BL/6 mouse lung. A–D: Masson’s trichrome labeling in lung from age-matched wild-type (WT) control (CTR) mouse, lung from Scnn1β mouse, lung from WT mouse intraperitoneally injected with HMGB-1, and lung from WT mouse intraperitoneally injected with HMGB-1 and intratracheally instilled with FPS-ZM1. Male and female cohorts of mice were studied. Data represent results of 3 independent studies.
DISCUSSION
Biophysical properties of human SAECs.
The concurrent absorptive and secretory properties of SAECs were recently described by Shamsuddin and Quinton (23). In human and porcine SAECs (22, 23), amiloride inhibition of ENaC has been shown to significantly decrease equivalent Isc measurements. Using both single-channel and Isc measurements, we show that amiloride-sensitive ENaC current is a major contributor to the reabsorptive properties of human SAECs (hSAECs). Moreover, we report that hSAECs from normal healthy and CF donor lungs express amiloride-sensitive HSC and NSC channels; the molecular identity of NSCs has yet to be characterized. Together, these studies suggest that ENaC dysfunction in hSAECs can play a significant role in CF airway dehydration.
Molecular determinants of CF lung phenotype.
The molecular determinants of CF lung disease remain unclear and require further investigation. Loss of CFTR functional expression results in depolarizing shifts in membrane potentials that would electrogenically favor (excessive) Na+ reabsorption. An increase in ENaC activity could contribute to airway surface dehydration and, thus, subsequent impairment of mucociliary clearance. ENaC dysfunction alone, however, does not clearly explain defective innate immunity in the CF lung. Hyperinflammation in the CF lung is also known to occur in the absence of infection.
Our studies provide evidence linking airway surface dehydration to inflammation in the CF lung via a HMGB-1-RAGE and ENaC signaling pathway. Results from our study show that HMGB-1 binding to RAGE increases ENaC activity. This observation builds on our previous work showing an important role for RAGE in ENaC regulation in alveolar epithelial cells following cigarette smoke exposure (6). Our current results further support a role for HMGB-1-RAGE signaling to ENaC, although in hSAECs. Furthermore, our study also indicates that transcellular transport of solute across ENaC can lead to nuclear expansion. In airway epithelia, it appears that net solute transport across ENaC contributes to nuclear swelling and, consequently, translocation of HMGB-1 from the nucleus to cytoplasmic compartments. As such, the HMGB1-RAGE signaling to ENaC could be perpetuating its release and presence in the airway lumen of CF patients with hyperactive transport properties. We also showed that the interleukins, interferons, and GM-CSFs upregulated in the CF mouse model (with lung phenotype) can be similarly recapitulated by injection of HMGB-1 into a WT mouse. As such, our studies suggest that further evaluation of the HMGB-1-RAGE-ENaC signaling axis could lead to identification of potentially important targets that could attenuate lung inflammation and airway surface dehydration in CF patients.
Summary.
Our studies support a paradigm in which HMGB-1-RAGE signaling can perpetuate proinflammatory cytokine signaling by activating ENaC activity, which supports continued HMGB-1 translocation from the nucleus to cytoplasm for extracellular release. Because HMGB-1-RAGE signaling to ENaC contributes to both proinflammatory responses and airway inflammation, it is an intriguing signaling pathway to potentially drug and target in the CF lung.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL-137033 (M. N. Helms). R. Paine III is supported by VA Merit Grant 5I01BX001777. T.G. Liou received funding from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD (NHLBI Grant R01 HL-125520), the Cystic Fibrosis Foundation, Bethesda, MD (Grants LIOU13A0, LIOU14P0, LIOU14Y4, and LIOU15Y4), the Ben B. and Iris M. Margolis Family Foundation of Utah, and the Claudia Ruth Goodrich Stevens Endowment Fund at the University of Utah, Salt Lake City, UT.
DISCLOSURES
During the course of the study, T. G. Liou received other support for performing clinical trials from Abbvie, Inc; CFF Therapeutics, Inc.; Corbus Pharmaceuticals Holdings, Inc; Genentech, Inc; Gilead Sciences, Inc.; Laurent Pharmaceuticals, Inc; Nivalis Therapeutics, Inc; Novartis Pharmaceuticals; Proteostasis Therapeutics, Inc; Savara, Inc; and Vertex Pharmaceuticals, Inc. None of these sponsors were involved in any way with the performance of the current study. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.G.L. and M.N.H. conceived and designed research; G.J.G. and M.N.H. performed experiments; G.J.G. and M.N.H. analyzed data; G.J.G., R.P., and M.N.H. interpreted results of experiments; G.J.G. and M.N.H. prepared figures; G.J.G. and M.N.H. drafted manuscript; G.J.G., T.G.L., and M.N.H. edited and revised manuscript; G.J.G., T.G.L., R.P., and M.N.H. approved final version of manuscript.
REFERENCES
- 1.Agrawal PB, Wang R, Li HL, Schmitz-Abe K, Simone-Roach C, Chen J, Shi J, Louie T, Sheng S, Towne MC, Brainson CF, Matthay MA, Kim CF, Bamshad M, Emond MJ, Gerard NP, Kleyman TR, Gerard C. The epithelial sodium channel is a modifier of the long-term nonprogressive phenotype associated with F508del CFTR mutations. Am J Respir Cell Mol Biol 57: 711–720, 2017. doi: 10.1165/rcmb.2017-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson U, Erlandsson-Harris H. HMGB1 is a potent trigger of arthritis. J Intern Med 255: 344–350, 2004. doi: 10.1111/j.1365-2796.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- 3.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF Antioxidant Workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med 42: 15–31, 2007. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y-C, Statt S, Wu R, Chang H-T, Liao J-W, Wang C-N, Shyu W-C, Lee C-C. High mobility group box 1-induced epithelial mesenchymal transition in human airway epithelial cells. Sci Rep 6: 18815, 2016. doi: 10.1038/srep18815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, Di Dio G, Lionetti E, David A, Arrigo T, Salpietro C, La Rosa M. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 (HMGB1) between inflammation and infection. Clin Microbiol Infect 21: 368.e1–368.e9, 2015. doi: 10.1016/j.cmi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Downs CA, Kreiner LH, Johnson NM, Brown LA, Helms MN. Receptor for advanced glycation end-products regulates lung fluid balance via protein kinase C-gp91(phox) signaling to epithelial sodium channels. Am J Respir Cell Mol Biol 52: 75–87, 2015. doi: 10.1165/rcmb.2014-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 8.Gangemi S, Casciaro M, Trapani G, Quartuccio S, Navarra M, Pioggia G, Imbalzano E. Association between HMGB1 and COPD: a systematic review. Mediators Inflamm 2015: 1, 2015. doi: 10.1155/2015/164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579: 95–132, 2016. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with γ-ENaC. J Biol Chem 280: 40885–40891, 2005. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 11.Helms MN, Self J, Bao HF, Job LC, Jain L, Eaton DC. Dopamine activates amiloride-sensitive sodium channels in alveolar type I cells in lung slice preparations. Am J Physiol Lung Cell Mol Physiol 291: L610–L618, 2006. doi: 10.1152/ajplung.00426.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbalzano E, Quartuccio S, Di Salvo E, Crea T, Casciaro M, Gangemi S. Association between HMGB1 and asthma: a literature review. Clin Mol Allergy 15: 12, 2017. doi: 10.1186/s12948-017-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 288: L958–L965, 2005. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 15.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, Rogers SL, Lane C, Moore J, Sturrock A, Paine R III, Cox DR, Hoidal JR. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One 7: e42748, 2012. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 18.McDonough AA. Can ENaC regulate ICF as well as ECF volume? Focus on “osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes”. Am J Physiol Cell Physiol 275: C1179–C1181, 1998. doi: 10.1152/ajpcell.1998.275.5.C1179. [DOI] [PubMed] [Google Scholar]
- 19.Mulrennan S, Baltic S, Aggarwal S, Wood J, Miranda A, Frost F, Kaye J, Thompson PJ. The role of receptor for advanced glycation end products in airway inflammation in CF and CF related diabetes. Sci Rep 5: 8931, 2015. doi: 10.1038/srep08931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouwels SD, Hesse L, Faiz A, Lubbers J, Bodha PK, Ten Hacken NH, van Oosterhout AJ, Nawijn MC, Heijink IH. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol 311: L881–L892, 2016. doi: 10.1152/ajplung.00135.2016. [DOI] [PubMed] [Google Scholar]
- 21.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O’Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med 178: 822–831, 2008. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamsuddin AK, Quinton PM. Surface fluid absorption and secretion in small airways. J Physiol 590: 3561–3574, 2012. doi: 10.1113/jphysiol.2012.230714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamsuddin AKM, Quinton PM. Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human bronchioles. Am J Physiol Lung Cell Mol Physiol 316: L953–L960, 2019. doi: 10.1152/ajplung.00545.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep 7: 5850, 2017. doi: 10.1038/s41598-017-06205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suwara MI, Green NJ, Borthwick LA, Mann J, Mayer-Barber KD, Barron L, Corris PA, Farrow SN, Wynn TA, Fisher AJ, Mann DA. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol 7: 684–693, 2014. doi: 10.1038/mi.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi N, Kawakami Y, Maruyama I, Lotz M. HMGB proteins and arthritis. Hum Cell 31: 1–9, 2018. doi: 10.1007/s13577-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107: 11942–11947, 2010. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]