Abstract
This cohort study examines medical records of 33 neonates born to women with COVID-19 to provide information on maternal-child transmission and infant outcomes.
The coronavirus disease 2019 (COVID-19) has spread rapidly across the world. With the sharp increase in the number of infections, the number of pregnant women and children with COVID-19 is also on the rise. However, only 19 neonates born to affected mothers have been investigated, and to our knowledge, no information on early-onset infection in newborns has been published in previous studies.1,2
Methods
In this cohort study, all neonates born to mothers with COVID-19 were recruited from Wuhan Children's Hospital, in Wuhan, Hubei Province, China. This study was approved by the local medical ethics committee. Written informed consent was obtained from the neonates’ parents. The diagnosis and management of newborns with or at risk of COVID-19 were in accordance with guidelines provided by the National Health Commission and the Chinese Perinatal-Neonatal SARS-CoV-2 Committee.3,4
Data regarding demographic, epidemiologic, and clinical features were obtained from the medical records system. In addition, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase–polymerase chain reaction tests (Novel Coronavirus PCR Fluorescence Diagnostic Kit [BGI]) were conducted using nasopharyngeal and anal swab samples. Data were collected from January 2020 to February 2020. All statistical analyses were performed in Stata version 15.0 (StataCorp).
Results
Thirty-three neonates born to mothers with COVID-19, including 3 neonates with COVID-19, were identified (Table). The most common symptom was shortness of breath (4 of 33 neonates). Radiographic findings were nonspecific. No deaths were reported.
Table. General Information and Clinical Features of 33 Newborns With Mothers With COVID-19 Pneumonia.
| Variable | Neonates with SARS-CoV-2, No. (%) | Patients with SARS-CoV-2 | |||
|---|---|---|---|---|---|
| No (n = 30) | Yes (n = 3) | Patient 1 | Patient 2 | Patient 3 | |
| Male | 16 (53) | 3 (100) | Yes | Yes | Yes |
| Preterm | 3 (10) | 1 (33) | GA: 40 wk | GA: 40 wk + 4 d | GA: 31 wk + 2 d |
| Small for gestational age | 2 (7) | 1 (33) | No; 3250 g | No; 3360 g | No; 1580 g |
| Asphyxia | 1 (3) | 1 (33) | No | No | Yes |
| Symptoms and complications | |||||
| Fever | 0 | 2 (67) | Yes | Yes | No |
| Pneumonia | 0 | 3 (100) | Yes | Yes | Yes |
| Respiratory distress syndrome | 3 (10) | 1 (33) | No | No | Yes |
| Shortness of breath | 3 (10) | 1 (33) | No | No | Yes |
| Cyanosis | 2 (7) | 1 (33) | No | No | Yes |
| Feeding intolerance | 2 (7) | 1 (33) | No | No | Yes |
| Laboratory test, median (range) | |||||
| White blood cell count, cells/μL | 9800 (6100-22 700) | 19 200 (8600-20 400) | 8600 | 19 200 | 20 400 |
| Lymphocyte count, cells/μL | 4300 (1500-10 700) | 2600 (800-3100) | 3100 | 2600 | 800 |
| Platelets, ×103/μL | 184 (116-303) | 245 (230-265) | 245 | 265 | 230 |
| Creatine kinase isoenzymes, U/L | 13 (22.5-43) | 31 (18-39) | 18 | 31 | 39 |
| Aspartate aminotransferase | 27.5 (12-45) | 24 (8-63) | 8 | 24 | 63 |
| Alanine aminotransferase | 21 (9-95) | 17 (11-88) | 11 | 17 | 88 |
| Treatment | |||||
| Mechanical ventilation | 0 | 1 (33) | No | No | Yes |
| Antibiotic | 6 (20) | 1 (33) | No | No | Yes |
| Duration of neonatal intensive care unit, median (range), d | 0 (0-6) | 4 (2-11) | 2 | 4 | 11 |
| Death | 0 | 0 | No | No | No |
| Maternal features | |||||
| Fever on admission | 7 (23) | 1 (33) | Yes | No | No |
| Postpartum fever | 4 (13) | 1 (33) | Yes | No | No |
| Cough | 9 (30) | 1 (33) | No | Yes | No |
| Intensive care unit admission | 0 | 0 | No | No | No |
| Pneumonia per computed tomography diagnosis | 30 (100) | 3 (100) | Yes | Yes | Yes |
| Nasopharyngeal swab | 30 (100) | 3 (100) | Yes | Yes | Yes |
| Delivered by cesarean delivery | 23 (77) | 3 (100) | Yes | Yes | Yes |
| Premature rupture of membranes | 2 (7) | 1 (33) | Yes | No | No |
Abbreviations: COVID-19, coronavirus disease 2019; GA, gestational age; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert the white blood cells and lymphocytes to cells × 109/L, multiply by 0.001; to convert platelets to cells × 109/L, multiply by 1.0; to convert creatinine, aspartate aminotransferase, and alanine aminotransferase to μkat/L, multiply by 0.0167.
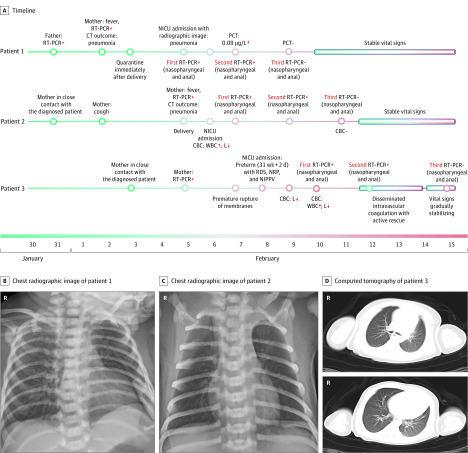
We provide details of the 3 infected neonates (Figure). Patient 1 was born at 40 weeks’ gestation. The delivery was by cesarean delivery because of meconium-stained amniotic fluid and confirmed maternal COVID-19 pneumonia. On day 2 of life, the infant experienced lethargy and fever, with unremarkable physical examination results, and was moved to the neonatal intensive care unit. A chest radiographic image showed pneumonia, but other laboratory tests (except procalcitonin) were normal. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 6.
Figure. Timeline and Imaging Findings of 3 Neonates Infected With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Normal ranges: lymphocytes (L), 3000 to 8000 cells/μL (to convert to cells × 109/L, multiply by 0.001); procalcitonin (PCT), <0.05 μg/L; white blood cell count (WBC), 8000-15000 cells/μL (to convert to cells × 109/L, multiply by 0.001). CBC indicates complete blood cell count; CT, computed tomography; NICU, neonatal intensive care unit; NIPPV, noninvasive positive-pressure ventilation; NRP, neonatal resuscitation program; RDS, respiratory distress syndrome; RT-PCR, reverse transcriptase–polymerase chain reaction.
Patient 2 was born at 40 weeks’ and 4 days’ gestation by cesarean delivery because of confirmed maternal COVID-19 pneumonia. He presented with lethargy, vomiting, and fever. A physical examination was unremarkable. Laboratory tests showed leukocytosis, lymphocytopenia, and an elevated creatine kinase–MB fraction. A chest radiographic image showed pneumonia. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 6.
Patient 3 was born at 31 weeks’ and 2 days’ gestation by cesarean delivery because of fetal distress and confirmed maternal COVID-19 pneumonia. Resuscitation was required. The infant’s Apgar scores were 3, 4, and 5 at 1, 5, and 10 minutes after birth. Neonatal respiratory distress syndrome and pneumonia confirmed by chest radiographic image on admission resolved on day 14 of life after treatment with noninvasive ventilation, caffeine, and antibiotics. He also had suspected sepsis, with an Enterobacter agglomerates–positive blood culture, leukocytosis, thrombocytopenia (11 cells × 103/μL; to convert to cells × 109/L, multiply by 1.0), and coagulopathy (prothrombin time, 21 seconds; activated partial thromboplastin time, 81.9 seconds), which improved with antibiotic treatment. Nasopharyngeal and anal swabs were positive for SARS-CoV-2 on days 2 and 4 of life and negative on day 7.
Discussion
Consistent with previous studies, the clinical symptoms from 33 neonates with or at risk of COVID-19 were mild and outcomes were favorable.1,2,5 Of the 3 neonates with symptomatic COVID-19, the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than SARS-CoV-2 infection.
In this cohort, 3 of 33 infants (9%) presented with early-onset SARS-CoV-2 infection. Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of SARS-CoV-2 in the neonates’ upper respiratory tracts or anuses were maternal in origin. Although 2 recent studies1,2 have shown that there were no clinical findings or investigations suggestive of COVID-19 in neonates born to affected mothers, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for SARS-CoV-2, the vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19.
Reference
- 1.Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51-60. doi: 10.21037/tp.2020.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-815. doi: 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Commission of China New coronavirus pneumonia prevention and control program (4th edition). Accessed March 9, 2020. http://www.gov.cn/zhengce/zhengceku/2020-01/28/5472673/files/0f96c10cc09d4d36a6f9a9f0b42d972b.pdf.
- 4.Wang L, Shi Y, Xiao T, et al. ; Working Committee on Perinatal and Neonatal Management for the Prevention and Control of the 2019 Novel Coronavirus Infection . Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (first edition). Ann Transl Med. 2020;8(3):47. doi: 10.21037/atm.2020.02.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020. doi: 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]