Abstract
Sequence-defined nucleic acids and proteins with internal monomer sequences and arrangement are vital components in the living world, as a result of billions of years of molecular evolution. These natural hierarchical systems have inspired researchers to develop artificial hierarchical materials that can mimic similar functions such as replication, recognition, and information storage. In this Outlook, we describe the conceptual introduction of hierarchy into the design of metal–organic framework (MOF) materials. Starting with a history and background of hierarchical MOF synthesis and applications, we discuss further mesoscopic assembly strategies of MOF crystallites into hierarchical primary, secondary, tertiary, and quaternary architectures. This is followed by a highlight of the utilization of modular total synthesis for crafting MOFs with hierarchical compositions. The multiscale control over hierarchical MOF architecture formation can be rationally achieved by designing stepwise synthetic routes based on the knowledge from various fields including coordination chemistry, organic chemistry, reticular chemistry, and nanoscience. Altogether, this outlook is expected to shed light on these essential but embryonic materials and might offer inspiration for the development of the next generation of smart MOF materials with controllable heterogeneity and tailorable architectures.
Short abstract
Metal−organic frameworks with hierarchical porosity, architecture, and composition can be rationally designed and synthesized under principles of mesoscopic assembly and modular total synthesis.
Introduction
Metal–organic frameworks (MOFs) are a well-developed class of porous materials, and they are usually constructed by linking molecular building units (including organic linkers and inorganic clusters) through strong coordination bonds.1−5 Because of their high function tunability, porosity, and crystallinity, MOFs have shown great potential in fields including separation, storage, delivery, and catalysis.6−10
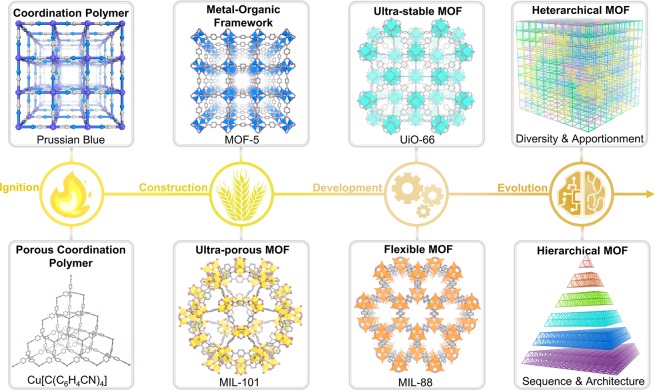
From this field’s start to the present, the simple extended coordination compound “Prussian Blue” was known since the early 18th century, a porous coordination polymer Cu[C(C6H4CN)4] was reported in 1990, and Yaghi and co-workers reported a robust MOF with permanent porosity, MOF-5, constructed from Zn4O clusters and linear dicarboxylate linkers in 1999 (Figure 1).1,11 Further development of MOFs includes the construction of ultraporous MOFs such as MIL-101, ultrastable MOFs such as UiO-66, and flexible MOFs such as MIL-88 (UiO stands for University of Oslo, MIL stands for Material Institut Lavoisier).12−14 Presently, research on the evolution of heterarchical, or multivariate, MOFs with enhanced diversity and hierarchical MOFs with controllable internal sequences and architectures has gained increasing attention because these MOF architectures have the potential to mimic living organisms that can conduct sequential behaviors and perform complicated functions.15−20 In most heterarchical MOFs, building units are distributed randomly within the framework lattice (Figure 2). In sequence-controlled hierarchical MOFs, these units are placed in a specific order within a lattice, which strongly influences the molecular and macroscopic properties of framework materials.21−24
Figure 1.
Advances in the development of metal–organic frameworks (MOFs). Important milestones include the development of an extended coordination compound “Prussian Blue” and the porous coordination polymer Cu[C(C6H4CN)4], the construction of robust MOF-5 and ultraporous MIL-101, the further development of ultrastable UiO-66 and flexible MIL-88, and the evolution of heterarchical MOFs with enhanced diversity and hierarchical MOFs with controllable internal sequences and architectures.
Figure 2.
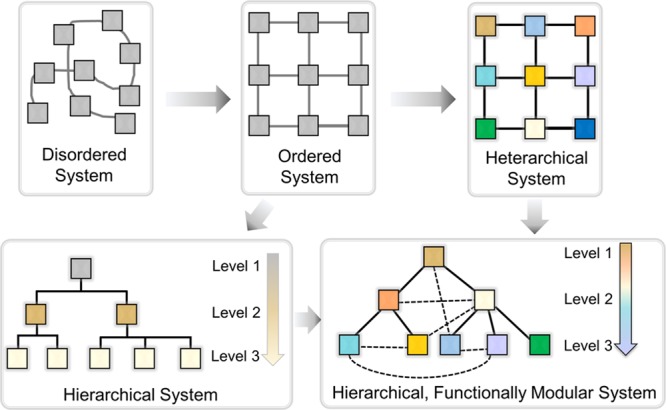
Conceptual introduction of heterarchy and hierarchy into the material design and development.
Analogous to the primary, secondary, tertiary, and quaternary structures observed in proteins, a hierarchy system can also be established in MOF superstructures, such as the mesoscopic architectures discovered by Kitagawa and co-workers.25 For instance, MOF primary architectures (unit cells) are constructed from basic building blocks including inorganic clusters and organic linkers, while secondary architectures such as MOF crystallites, tertiary architectures such as self-assemblies of MOF crystallites, and more complicated quaternary architectures can be further constructed.26 However, since their discovery, the development of MOF materials has mainly focused on molecular-level assembly. For example, to construct a MOF primary architecture, principles in coordination chemistry such as hard and soft acids and bases theory are utilized to guide the controllable synthesis. In addition, geometry and connectivity tunability in inorganic clusters and organic linkers have also been extensively explored, enhancing the fundamental diversity and functionalities of framework materials. Recent developments in MOF growth have focused on synthetic tools to introduce hierarchy into secondary MOF architectures, such as epitaxial growth, controlled assembly, and labilization approaches, generating MOF tertiary architectures with unusual complexity and properties. Yet, the current level of hierarchy and the functions of the artificial framework materials are far behind the complicated hierarchical systems found in nature, such as proteins and DNAs. The discovery of artificial framework superstructures with quaternary or more complicated architectures is rare in coordination chemistry, with little in the way of synthetic guidelines to direct research toward these structures.
This gap between the molecular assembly of MOFs and the construction of functional mesoscopic architectures has become a critical limitation, which has motivated researchers to study the multiscale control over MOF architecture formation and to design stepwise synthetic routes for the rational construction of hierarchical MOFs or MOF composites. In particular, improving the capability to tune these hierarchical structures on multiple scales will be necessary for the advancement of cooperative catalysis in MOFs, as it requires optimization of both the activity of the catalytic center and selectivity of the porous framework.27−30 Catalytic activity mainly focuses on the geometry and electronic properties of the active metal centers, with the design principle being primarily based upon activity effects found in homogeneous systems.31,32 The selectivity of the porous framework meanwhile tends to be based on larger scale effects, such as the pore environment, pore window, and crystal size, all of which enable the effective diffusion of the selected substrates to and from the active centers. The development of the next generation of cooperative catalysts requires both high activity centers and substrate partitioning, in order to truly reach breakthrough performance and utility.
Hierarchical MOFs have drawn increasing attention mainly because of their structural tunability in various scales and practical applications in promoting separation, storage, and catalytic transformation. Previous reviews have provided a comprehensive collection of hierarchical assembly of nanosized MOFs and the synthesis of hierarchically porous MOFs.33,34 This Outlook will not repeat these reviews but will briefly introduce the concept and scope of hierarchical MOFs, highlight their synthetic strategies, and discuss the emerging progresses and future opportunities in the field.
Hierarchy: Types and Levels
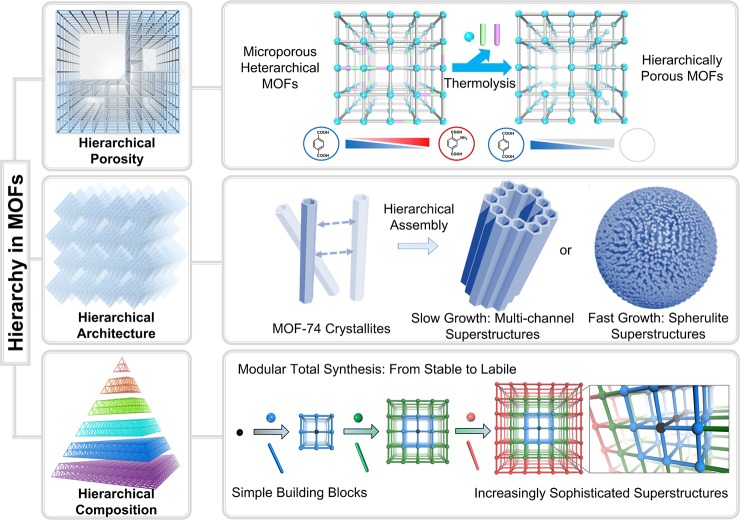
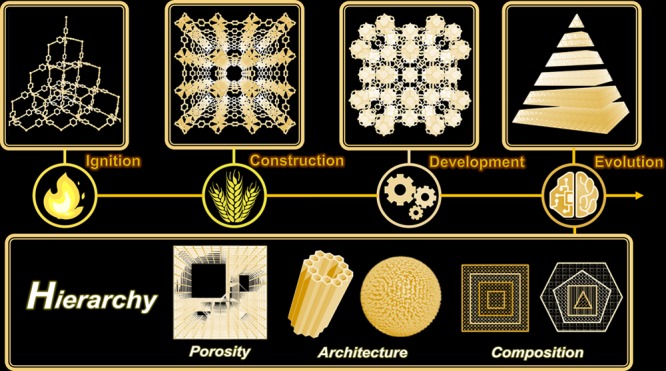
There are mainly three types of hierarchies in MOFs, including porous hierarchy, architectural hierarchy, and compositional hierarchy (Figure 3). The hierarchical pores in MOFs denote multiscale porosity within one framework, such as micro-, meso-, and macroporosity, which has been recently summarized.34−36 These hierarchical pores can be either intrinsically formed by the assembly of building blocks or postsynthetically formed by template etching. Hierarchically porous (HP) MOF structures have advantages such as facile tunability over pore sizes, the ability to alter reaction pathways and enhancement over diffusion kinetics and active site accessibility during reactions, which stimulate methodology advances in the synthesis of HP-MOFs. One typical example to demonstrate the beneficial integration of hierarchical porosity within one framework is the utilization of NU-1000 and HP-CYCU-3 for two-task applications: enzyme capture and catalytic conversion (NU stands for Northwestern University, CYCU stands for Chung-Yuan Christian University).29,37 The large mesopores in these MOFs can accommodate catalytically active enzymes, while the small micropores are used for diffusion of small molecular reactants and products. Traditional mesoporous MOFs could only complete one task at a time, which is enzyme capture, while the mesopores were blocked by large enzymes, limiting the diffusion of reactants into the catalytically active sites.
Figure 3.
Hierarchically structured MOFs from three different perspectives: hierarchical porosity (top), hierarchical architecture (middle), and hierarchical composition (bottom).
The need for designing one material for multiple tasks requires the hierarchical arrangement of compositions, architectures, and functionalities (Figure 4). The hierarchical architectures concern multilevel assembly of basic building units, usually involving primary, secondary, tertiary, and quaternary architectures; the hierarchical compositions in MOFs are related to the arrangement of varying components within selected domains in one MOF lattice at multiple length scales.38−40 Some recent reports have already shown the benefit of these hierarchical systems for multipurpose tasks. For instance, the integration of catalytically active porphyrinic MOFs and microporous MOFs with narrow pore windows leads to a hierarchical system for size selective catalysis, while the combination of porphyrinic MOFs exhibiting photothermal effects and MOFs with high guest storage capacity affords a hierarchical system for phototriggered guest delivery.21
Figure 4.
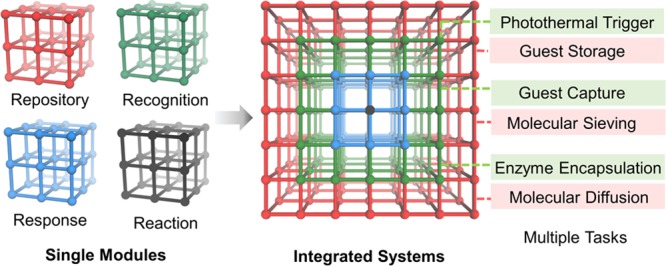
Integrating multiple modules into hierarchically structured MOFs for multitask applications.
Mesoscopic Assembly of Hierarchical MOF Architectures
The assembly of MOF crystallites into hierarchical MOF superstructures is considered a vital method to increase the packing density of frameworks and to fabricate multicomponent architectures for applications including capture, transportation, and catalysis.41−46 By tuning the kinetics of nucleation and growth, MOF superstructures with distinct morphologies can be synthesized, which then serve as blocks to assemble superior hierarchical architectures.47 Through tuning crystallization conditions, unique crystalline architectures can be prepared, with these structures often featuring tunable pore sizes and chemical environments.
The hierarchical assembly process requires elaborate control over MOF nucleation, orientational growth, and stability consideration. Our group recently discovered an unprecedented assembly case wherein hierarchically porous tubular superstructures with multiple levels of channel sizes can be assembled from a series of hollow tube crystallites during a facile one-pot synthesis at a suitable evolution temperature.48 MOF-74, a MOF well-known for its tunable one-dimensional channel sizes and great potential in gas storage and separation, was studied.49 Under 85 °C, the oriented assembly of well-defined MOF-74 tubes ensured the order of arrangement of both intrinsic MOF micropores and macropores formed from templates. Through self-healing and correction, the interfaces between neighboring tubes were fused, which integrated the individual MOF crystallites into a united superstructure. This evolution from MOF secondary to tertiary architectures demonstrates the power of self-assembly during the evolution process of coordination bond-based superstructures.
Common solvents used in MOF synthesis such as N,N-diethylformamide (DEF) and N,N-dimethylformamide (DMF) have different decomposition kinetics, which can be utilized to modulate the superstructure assembly. Under common solvothermal conditions, DEF decomposes more slowly than DMF into alkylamines. MOF-74 spherulite superstructures were formed within a few hours if DMF was selected as the reaction medium.50 The spherulite superstructures were radially assembled from crystalline MOF-74 nanofibrils with an external spherical envelope. They display a “‘Maltese-cross”’ extinction pattern under a polarized light, which is the initial example of this phenomena observed in porous materials. Different from the traditional crystal growth that produces a single crystal with a well-defined morphology from a primary crystallite, spherulites are unable to evolve in a discrete crystallographic direction as a result of small-angle branching. The crystallographic direction of each MOF-74 crystallite deviates slightly from that of its parent crystallite, leading to the formation of spherulites consisting of radial fibers.
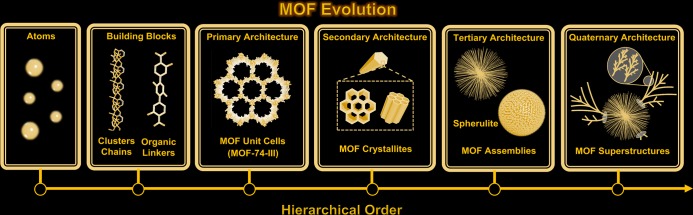
To fabricate more sophisticated architectures such as quaternary superstructures, tertiary seed architectures can be utilized during the assembly process (Figure 5).51 For example, we recently observed a secondary growth of MOF-74-II plumose superstructures when MOF-74-III spherulite superstructures were mixed with solutions containing organic linkers with different lengths. There are two sets of tertiary superstructures observed in the resulting quaternary heterosuperstructure: MOF-74-II dendrites and MOF-74-III spherulites. It should be noted that the MOF-74-II dendrites endow a multibranching tree-like fractal-type pattern, which is rarely observed in reticular chemistry. This seed-mediated study demonstrates a synthetic approach that produces complex heterostructured superstructures. The hierarchical MOF structures including atoms, building blocks, unit cells, crystallites, assemblies, and superstructures are quite similar to the multilevel arrangement of proteins which contain primary, secondary, tertiary, and quaternary structures. These superstructures contain precisely defined modules that are designed to execute specific tasks in sequence.
Figure 5.
Illustration of hierarchical MOF evolution from atoms, building blocks, unit cells, crystallites, assemblies to superstructures.
Owing to their controllable morphologies, porous framework materials are perfect prototypes for the development of hierarchical architectures such as core–shell structures, hollow structures, and hybrid composites with multiple functionalities. More mechanistic studies on the assembly of these superstructures are expected in the future, which can not only enhance our understanding of the nucleation and growth of MOF structures, but also guide us to fabricate more complicated architectures based on crystalline framework materials.
Modular Total Synthesis to Craft Hierarchical MOFs
In organic synthesis, the concept of modularity has been widely utilized to promote the synthesis of diverse natural products with complicated structures.52,53 By assembling various building blocks in sequences, stepwise synthesis of molecular compounds based on covalent bonds can be achieved.54,55 Recently, our group demonstrates that the conceptual scope of organic total synthesis can be further expanded into framework materials such as covalent organic frameworks (COFs) and MOFs, leading to the formation of varying composites including MOF-on-MOF, COF-on-MOF, MOF-on-polymer, and COF-on-polymer composites.21,56,57
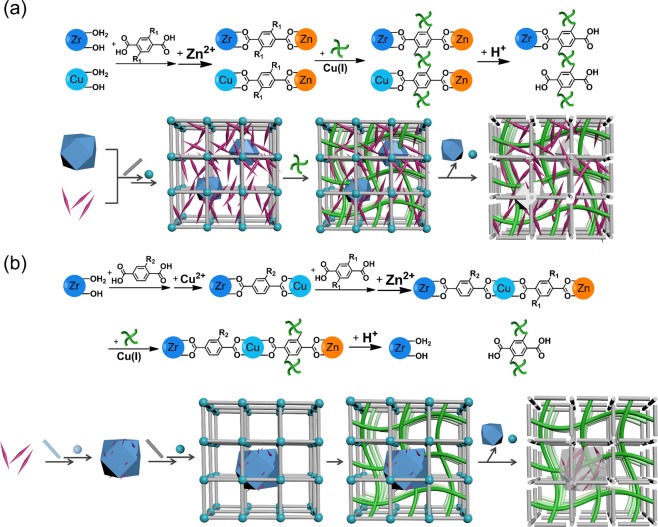
Two principles of hierarchical MOF synthesis, surface modification and retrosynthetic stability considerations, were reported.21 Surface modification allows for the formation of a secondary MOF phase on the seed MOFs more efficiently, while retrosynthetic stability considerations ensure the structural integrity during the secondary growth. Under the guidance of these principles, a series of hierarchical MOF-on-MOF structures form after the self-assembly of the shell MOF outside the core MOFs, even though they have mismatched lattices (Figure 6). These sophisticated MOF composites contain low-valent, metal-based carboxylate MOF shells that are synthesized under mild conditions and high-valent metal based carboxylate MOF cores that are presynthesized under harsher conditions. For example, a zirconium-MOF PCN-222 can be immobilized in the lattice of MOF-5 with controllable ratios and distributions through modular synthesis (PCN stands for porous coordination network). More modules such as three MOF modules can also be integrated into one system, as observed in the system of PCN-222@HKUST-1@Zn-MOF (HKUST stands for Hong Kong University of Science and Technology).
Figure 6.
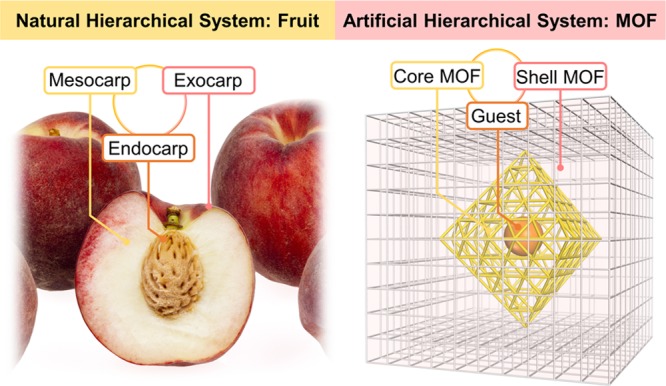
Schematic representation of a natural peach with a hierarchically arranged exocarp–mesocarp–endocarp system and an artificial MOF with a hierarchically arranged core–shell-guest system.
The application of modular total synthesis was further expanded into the construction of hierarchical MOF and COF composites.57 COFs are generally considered as a more robust framework material, due to the presence of covalent bonds that connect their building units.58 Modular synthesis progressively links simple blocks into increasingly complicated superstructures according to the assembly sequences determined by the strengths of coordination or covalent bonds. This leads to the formation of hierarchical COF-on-MOF structures, where architectural intricacy was achieved via sequence-defined connection of varying building blocks. Single-crystalline COF-303 was integrated into the frameworks, varying from Zn-MOFs to Zr-MOFs, while their tunability over spatial distribution and ratio was successfully achieved in these materials. An integrated three-module COF@MOF@MOF system with an internal sequence was further reported. In this case, a two-module COF-303@PCN-160 composite was first prepared, followed by the secondary growth of a more labile Zn-based MOF-5 as the outer shell. This successful synthesis route was achieved under the strength consideration of bonds within these three modules, in which the sequence is C=N > Zr–O > Zn–O. These examples indicate the generality of modular synthesis in the integration of multiple modules based on various bond types.
Multiple components within an integrated system can further be modified into functionalized modules independently, under the guidance of modular programming.56 To access composites with controllable compositions, ratios, and distributions, MOFs with hierarchical compositions can be utilized as templates. Followed by orthogonal modification including click reactions and acid treatments, three different MOF modules can be sequentially transformed into different phases, including a polymer phase, an original MOF phase, and a void phase (Figure 7). For example, catalytically active PCN-222 was immobilized into a MOF-templated polymer with controllable ratios and apportionment, leading to the formation of MOF@polymer composites with superior hierarchy and diversity. Note that the formation of these composites with such high tunability is extremely difficult to achieve through traditional direct synthesis. Similar to the preparation of templated MOF@polymer composites, COF@polymer composites can also be fabricated through the templated synthesis.
Figure 7.
Illustration of the concept of modular programming in the synthesis of hierarchical MOF@polymer composites with controllable apportionments.56 Scheme illustration of the stepwise transformation (a) from three-module MOF@(MOF@MOF) to hollow@(MOF@polymer) and (b) from three-module (MOF@MOF)@MOF to (MOF@hollow)@polymer. Copyright 2019, American Chemical Society.
Altogether, these results exemplify the power of modular total synthesis in the synthesis of hierarchical framework materials with superior tunability over hierarchy and diversity. This generalizable strategy may promote the discovery of multicomponent porous framework materials by bridging the gap between MOFs constructed from coordination bonds and COFs constructed from covalent bonds. The controlled assembly of tailored catalytic and porous materials with core–shell structures might also enable the biomimic cascade processes widely observed in cells. The multiple components in the integrated system are expected to work coordinately to complete diverse complex tasks, including delivery, movement, transformation, and response.59 The long-term goal is to design future porous materials that mimic a multifunctional living organism, where functions are carried out by individual and cooperative modules, such as functional polymers, MOFs, and COFs. The modular total synthesis provides synthetic guidance for future materials that can fast adapt and respond to variable environmental stimulus.
Crafting Crystals within Crystals
One important aspect of MOFs with hierarchical compositions is the status of interfaces between their multiple modules (Figure 8).60,61 For MOF-on-MOF structures with matched lattices, the interface configurations are precisely matched. For example, epitaxial growth of MOF-5 on its functionalized counterpart MOF-5-NH2 would generate MOF-5-NH2@MOF-5 core–shell structures with their interfaces directly connected.62 Moon, Kim, and co-workers reported that a metal cluster of one MOF can coordinately connect with the linker of a different MOF, forming a precisely matched interface configuration at the molecular level.63 For example, aided by computational simulation, single crystalline core–shell HKUST-1@MOF-5 crystals can be predesigned and synthesized, while HKUST-1 located within the center of MOF-5 crystals with seamless interfaces.
Figure 8.
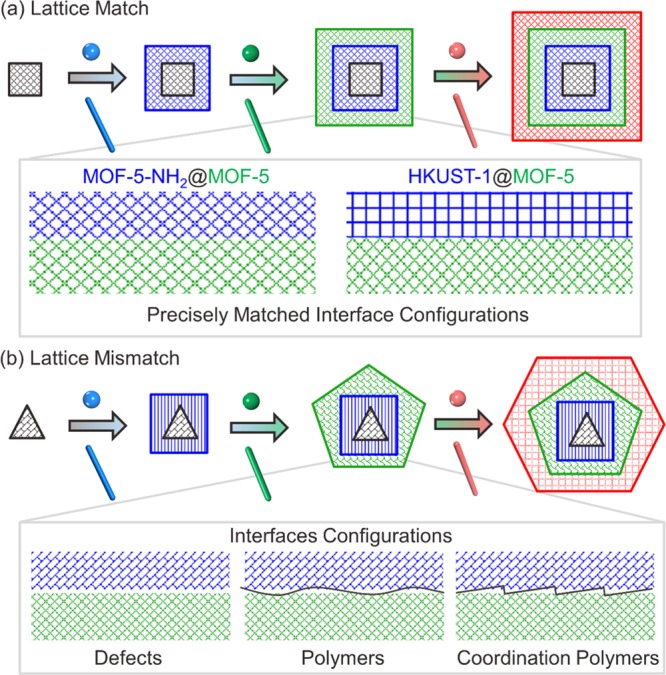
Constructing multicomponent framework materials with hierarchical compositions. (a) The interface configurations are precisely matched in MOF-on-MOF structures with matched lattices; (b) the interfaces between different MOF modules are connected by defects, polymers, and coordination polymers in MOF-on-MOF structures with mismatched lattices.57 Copyright 2020, American Chemical Society.
For MOF-on-MOF structures with mismatched lattices, the interfaces between different MOF modules are expected to have three possibilities: connection through defects, connection through polymer mediation, and connection through coordination polymers in hierarchical MOF structures. For instance, Imaz, Maspoch, and co-workers studied the hierarchical porosity of MOF@COF composites synthesized through spray-drying and unveiled that there exist a large number of defects, micropores and mesopores, at the MOF/COF interface as a result of fast crystallization.64 If a slow crystallization is adopted during the hierarchical MOF synthesis, coordination compounds or coordination polymers might form between two mismatch lattices, while the slow secondary growth of MOFs outside MOFs or COFs without the formation of mesoscopic defects would be observed, as indicated by the hierarchical MOF-on-MOF and COF-on-MOF examples synthesized under modular total synthesis.57 Additionally, polymers can also function as MOF-MOF interfaces and efficiently connect two different MOFs with mismatched lattices and diverse morphologies.65
Outlook
Overall, we summarize a set of synthetic strategies to design hierarchical MOF architectures with controllable morphologies and tailored functionalities. We show that synthetic chemistry can be used to prepare hierarchical MOFs with controlled sequences and diverse chemical structures. One beneficial factor to produce artificial hierarchical materials is that they might be able to be prepared in a larger scale, in comparison with the natural hierarchical systems such as protein and DNA technologies. Yet, it is more challenging to develop a general protocol for the fabrication of sequence-controlled artificial materials, compared with biotechnological materials. The visualization and analysis of sequence-controlled porous materials with hierarchical structures are also challenging, which might require the coupling of advanced characterization including crystallography, electron tomography, imaging tools such as fluorescent confocal microscopy and atomic-resolution transmission electron microscopy, and spectroscopic characterization including solid-state NMR spectroscopy and Raman spectroscopy.18,66−68
We envision that this research area will be continually expanded, focusing on improving the ability to control MOF architectures at molecular, supramolecular, and mesoscopic levels with unprecedented time and spatial precision. Advanced technologies such as lithography might be introduced to engineer patterns and pore environments in hierarchical MOFs. New assembly modes between multiple components are expected to be developed to bridge various functional framework materials, leading to a synergistic system with enhanced properties. Additionally, another direction is the systematic mechanistic studies over hierarchical structure formation, which shall introduce guiding principles to the design of functionalized architectures based on porous crystalline materials. The multiscale control over MOF architecture formation is anticipated to bring about new opportunities for hierarchical framework materials with integrated functionalities.
Acknowledgments
This work was supported by the Robert A. Welch Foundation through a Welch Endowed Chair to H.-C.Z. (A-0030).
Author Contributions
∥ L.F. and K.-Y.W. contributed equally to this work.
The authors declare no competing financial interest.
References
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402 (6759), 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Zhou H. C.; Long J. R.; Yaghi O. M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112 (2), 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Postsynthetic Methods for the Functionalization of Metal-Organic Frameworks. Chem. Rev. 2012, 112 (2), 970–1000. 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]
- Eddaoudi M.; Kim J.; Rosi N.; Vodak D.; Wachter J.; O’Keeffe M.; Yaghi O. M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295 (5554), 469–472. 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- Zhou H. C.; Kitagawa S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43 (16), 5415–5418. 10.1039/C4CS90059F. [DOI] [PubMed] [Google Scholar]
- Lee J.; Farha O. K.; Roberts J.; Scheidt K. A.; Nguyen S. T.; Hupp J. T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38 (5), 1450–1459. 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]
- Li J. R.; Kuppler R. J.; Zhou H. C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38 (5), 1477–1504. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Feng L.; Yuan S.; Qin J.-S.; Wang Y.; Kirchon A.; Qiu D.; Cheng L.; Madrahimov S. T.; Zhou H.-C. Lattice Expansion and Contraction in Metal-Organic Frameworks by Sequential Linker Reinstallation. Matter 2019, 1 (1), 156–167. 10.1016/j.matt.2019.02.002. [DOI] [Google Scholar]
- Yuan S.; Qin J. S.; Li J. L.; Huang L.; Feng L.; Fang Y.; Lollar C.; Pang J. D.; Zhang L. L.; Sun D.; Alsalme A.; Cagin T.; Zhou H. C. Retrosynthesis of multi-component metal-organic frameworks. Nat. Commun. 2018, 9, 808. 10.1038/s41467-018-03102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. M.; Martin C. R.; Galitskiy V. A.; Berseneva A. A.; Leith G. A.; Shustova N. B. Photophysics Modulation in Photoswitchable Metal–Organic Frameworks. Chem. Rev. 2019, 10.1021/acs.chemrev.9b00350. [DOI] [PubMed] [Google Scholar]
- Diercks C. S.; Kalmutzki M. J.; Diercks N. J.; Yaghi O. M. Conceptual Advances from Werner Complexes to Metal-Organic Frameworks. ACS Cent. Sci. 2018, 4 (11), 1457–1464. 10.1021/acscentsci.8b00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferey G.; Mellot-Draznieks C.; Serre C.; Millange F.; Dutour J.; Surble S.; Margiolaki I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309 (5743), 2040–2042. 10.1126/science.1116275. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130 (42), 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Serre C.; Mellot-Draznieks C.; Surblé S.; Audebrand N.; Filinchuk Y.; Férey G. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 2007, 315 (5820), 1828–1831. 10.1126/science.1137975. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Ahmad M.; Schug A.; Tsotsalas M. Rising Up: Hierarchical Metal–Organic Frameworks in Experiments and Simulations. Adv. Mater. 2019, 31, 1901744. 10.1002/adma.201901744. [DOI] [PubMed] [Google Scholar]
- Diring S.; Furukawa S.; Takashima Y.; Tsuruoka T.; Kitagawa S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22 (16), 4531–4538. 10.1021/cm101778g. [DOI] [Google Scholar]
- Deng H. X.; Doonan C. J.; Furukawa H.; Ferreira R. B.; Towne J.; Knobler C. B.; Wang B.; Yaghi O. M. Multiple Functional Groups of Varying Ratios in Metal-Organic Frameworks. Science 2010, 327 (5967), 846–850. 10.1126/science.1181761. [DOI] [PubMed] [Google Scholar]
- Kong X. Q.; Deng H. X.; Yan F. Y.; Kim J.; Swisher J. A.; Smit B.; Yaghi O. M.; Reimer J. A. Mapping of Functional Groups in Metal-Organic Frameworks. Science 2013, 341 (6148), 882–885. 10.1126/science.1238339. [DOI] [PubMed] [Google Scholar]
- Matyjaszewski K. Architecturally Complex Polymers with Controlled Heterogeneity. Science 2011, 333 (6046), 1104–1105. 10.1126/science.1209660. [DOI] [PubMed] [Google Scholar]
- Feng L.; Wang K.-Y.; Lv X.-L.; Powell J. A.; Yan T.-H.; Willman J.; Zhou H.-C. Imprinted Apportionment of Functional Groups in Multivariate Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141 (37), 14524–14529. 10.1021/jacs.9b06917. [DOI] [PubMed] [Google Scholar]
- Feng L.; Yuan S.; Li J. L.; Wang K. Y.; Day G. S.; Zhang P.; Wang Y.; Zhou H. C. Uncovering Two Principles of Multivariate Hierarchical Metal-Organic Framework Synthesis via Retrosynthetic Design. ACS Cent. Sci. 2018, 4 (12), 1719–1726. 10.1021/acscentsci.8b00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Wang K.-Y.; Day G. S.; Zhou H.-C. The chemistry of multi-component and hierarchical framework compounds. Chem. Soc. Rev. 2019, 48, 4823–4853. 10.1039/C9CS00250B. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Reboul J.; Diring S.; Sumida K.; Kitagawa S. Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43 (16), 5700–5734. 10.1039/C4CS00106K. [DOI] [PubMed] [Google Scholar]
- Kitagawa S.; Matsuda R. Chemistry of coordination space of porous coordination polymers. Coord. Chem. Rev. 2007, 251 (21–24), 2490–2509. 10.1016/j.ccr.2007.07.009. [DOI] [Google Scholar]
- Reboul J.; Furukawa S.; Horike N.; Tsotsalas M.; Hirai K.; Uehara H.; Kondo M.; Louvain N.; Sakata O.; Kitagawa S. Mesoscopic architectures of porous coordination polymers fabricated by pseudomorphic replication. Nat. Mater. 2012, 11 (8), 717–723. 10.1038/nmat3359. [DOI] [PubMed] [Google Scholar]
- Colfen H.; Antonietti M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem., Int. Ed. 2005, 44 (35), 5576–5591. 10.1002/anie.200500496. [DOI] [PubMed] [Google Scholar]
- Sun D.; Jang S.; Yim S. J.; Ye L.; Kim D. P. Metal Doped Core-Shell Metal-Organic Frameworks@Covalent Organic Frameworks (MOFs@COFs) Hybrids as a Novel Photocatalytic Platform. Adv. Funct. Mater. 2018, 28 (13), 1707110. 10.1002/adfm.201707110. [DOI] [Google Scholar]
- Peng Y. W.; Zhao M. T.; Chen B.; Zhang Z. C.; Huang Y.; Dai F. N.; Lai Z. C.; Cui X. Y.; Tan C. L.; Zhang H. Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core-Shell Hybrid Materials. Adv. Mater. 2018, 30 (3), 1705454. 10.1002/adma.201705454. [DOI] [PubMed] [Google Scholar]
- Li P.; Modica J. A.; Howarth A. J.; Vargas E.; Moghadam P. Z.; Snurr R. Q.; Mrksich M.; Hupp J. T.; Farha O. K. Toward design rules for enzyme immobilization in hierarchical mesoporous metal-organic frameworks. Chem. 2016, 1 (1), 154–169. 10.1016/j.chempr.2016.05.001. [DOI] [Google Scholar]
- Luo L.; Lo W.-S.; Si X.; Li H.; Wu Y.; An Y.; Zhu Q.; Chou L.-Y.; Li T.; Tsung C.-K. Directional Engraving within Single Crystalline Metal–Organic Framework Particles via Oxidative Linker Cleaving. J. Am. Chem. Soc. 2019, 141 (51), 20365–20370. 10.1021/jacs.9b10499. [DOI] [PubMed] [Google Scholar]
- Perez-Ramirez J.; Christensen C. H.; Egeblad K.; Christensen C. H.; Groen J. C. Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37 (11), 2530–2542. 10.1039/b809030k. [DOI] [PubMed] [Google Scholar]
- Schwieger W.; Machoke A. G.; Weissenberger T.; Inayat A.; Selvam T.; Klumpp M.; Inayat A. Hierarchy concepts: classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45 (12), 3353–3376. 10.1039/C5CS00599J. [DOI] [PubMed] [Google Scholar]
- Feng L.; Wang K.-Y.; Powell J.; Zhou H.-C. Controllable Synthesis of Metal-Organic Frameworks and Their Hierarchical Assemblies. Matter 2019, 1 (4), 801–824. 10.1016/j.matt.2019.08.022. [DOI] [Google Scholar]
- Feng L.; Wang K.-Y.; Lv X.-L.; Yan T.-H.; Zhou H.-C. Hierarchically porous metal–organic frameworks: synthetic strategies and applications. Natl. Sci. Rev. 2019, 10.1093/nsr/nwz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Yuan S.; Zhang L. L.; Tan K.; Li J. L.; Kirchon A.; Liu L. M.; Zhang P.; Han Y.; Chabal Y. J.; Zhou H. C. Creating Hierarchical Pores by Controlled Linker Thermolysis in Multivariate Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140 (6), 2363–2372. 10.1021/jacs.7b12916. [DOI] [PubMed] [Google Scholar]
- Shen K.; Zhang L.; Chen X. D.; Liu L. M.; Zhang D. L.; Han Y.; Chen J. Y.; Long J. L.; Luque R.; Li Y. W.; Chen B. L. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359 (6372), 206–210. 10.1126/science.aao3403. [DOI] [PubMed] [Google Scholar]
- Yuan S. A.; Zou L. F.; Qin J. S.; Li J. L.; Huang L.; Feng L. A.; Wang X. A.; Bosch M.; Alsalme A.; Cagin T.; Zhou H. C. Construction of hierarchically porous metal-organic frameworks through linker labilization. Nat. Commun. 2017, 8, 15356. 10.1038/ncomms15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K.; Furukawa S.; Kondo M.; Uehara H.; Sakata O.; Kitagawa S. Sequential Functionalization of Porous Coordination Polymer Crystals. Angew. Chem., Int. Ed. 2011, 50 (35), 8057–8061. 10.1002/anie.201101924. [DOI] [PubMed] [Google Scholar]
- Yang X. Y.; Yuan S.; Zou L. F.; Drake H.; Zhang Y. M.; Qin J. S.; Alsalme A.; Zhou H. C. One-Step Synthesis of Hybrid Core-Shell Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2018, 57 (15), 3927–3932. 10.1002/anie.201710019. [DOI] [PubMed] [Google Scholar]
- Szilagyi P. A.; Lutz M.; Gascon J.; Juan-Alcaniz J.; van Esch J.; Kapteijn F.; Geerlings H.; Dam B.; van de Krol R. MOF@MOF core-shell vs. Janus particles and the effect of strain: potential for guest sorption, separation and sequestration. CrystEngComm 2013, 15 (30), 6003–6008. 10.1039/c3ce40653a. [DOI] [Google Scholar]
- Zou L.; Kitta M.; Hong J.; Suenaga K.; Tsumori N.; Liu Z.; Xu Q. Fabrication of a Spherical Superstructure of Carbon Nanorods. Adv. Mater. 2019, 31 (24), 1900440. 10.1002/adma.201900440. [DOI] [PubMed] [Google Scholar]
- Dmitriev A.; Spillmann H.; Lin N.; Barth J. V.; Kern K. Modular assembly of two-dimensional metal-organic coordination networks at a metal surface. Angew. Chem., Int. Ed. 2003, 42 (23), 2670–2673. 10.1002/anie.200250610. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Hirai K.; Nakagawa K.; Takashima Y.; Matsuda R.; Tsuruoka T.; Kondo M.; Haruki R.; Tanaka D.; Sakamoto H.; Shimomura S.; Sakata O.; Kitagawa S. Heterogeneously Hybridized Porous Coordination Polymer Crystals: Fabrication of Heterometallic Core-Shell Single Crystals with an In-Plane Rotational Epitaxial Relationship. Angew. Chem., Int. Ed. 2009, 48 (10), 1766–1770. 10.1002/anie.200804836. [DOI] [PubMed] [Google Scholar]
- Avci C.; Imaz I.; Carne-Sanchez A.; Pariente J. A.; Tasios N.; Perez-Carvajal J.; Alonso M. I.; Blanco A.; Dijkstra M.; Lopez C.; Maspoch D. Self-assembly of polyhedral metal-organic framework particles into three-dimensional ordered superstructures. Nat. Chem. 2018, 10 (1), 78–84. 10.1038/nchem.2875. [DOI] [PubMed] [Google Scholar]
- Carne-Sanchez A.; Imaz I.; Cano-Sarabia M.; Maspoch D. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5 (3), 203–211. 10.1038/nchem.1569. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kwak J. H.; Choe W. Evolution of form in metal-organic frameworks. Nat. Commun. 2017, 8, 14070. 10.1038/ncomms14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai N.; Granick S. Directional Self-Assembly of a Colloidal Metal-Organic Framework. Angew. Chem., Int. Ed. 2012, 51 (23), 5638–5641. 10.1002/anie.201109132. [DOI] [PubMed] [Google Scholar]
- Feng L.; Li J. L.; Day G. S.; Lv X. L.; Zhou H. C. Temperature-Controlled Evolution of Nanoporous MOF Crystallites into Hierarchically Porous Superstructures. Chem. 2019, 5 (5), 1265–1274. 10.1016/j.chempr.2019.03.003. [DOI] [Google Scholar]
- Deng H.; Grunder S.; Cordova K. E.; Valente C.; Furukawa H.; Hmadeh M.; Gandara F.; Whalley A. C.; Liu Z.; Asahina S.; Kazumori H.; O'Keeffe M.; Terasaki O.; Stoddart J. F.; Yaghi O. M. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336 (6084), 1018–1023. 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- Feng L.; Wang K.; Yan T.-H.; Zhou H.-C. Porous Crystalline Spherulite Superstructures. Chem. 2020, 6 (2), 460–471. 10.1016/j.chempr.2019.12.001. [DOI] [Google Scholar]
- Feng L.; Wang K.; Yan T.-H.; Zhou H.-C. Seed-Mediated Evolution of Hierarchical Metal– Organic Framework Quaternary Superstructures. Chem. Sci. 2020, 11, 1643–1648. 10.1039/C9SC06064B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey E. J.; Cheng X.-M.. The Logic of Chemical Synthesis; Wiley: New York, 1995. [Google Scholar]
- Nicolaou K.; Vourloumis D.; Winssinger N.; Baran P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem., Int. Ed. 2000, 39 (1), 44–122. . [DOI] [PubMed] [Google Scholar]
- Wang F.; He S.; Wang H.; Zhang S.; Wu C.; Huang H.; Pang Y.; Tsung C.-K. F.; Li T. Uncovering Two Kinetic Factors in the Controlled Growth of Topologically Distinct Core-Shell Metal-Organic Frameworks. Chem. Sci. 2019, 10, 7755–7761. 10.1039/C9SC02576F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock N.; Biswas S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112 (2), 933–969. 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- Feng L.; Lv X.-L.; Yan T.-H.; Zhou H.-C. Modular Programming of Hierarchy and Diversity in Multivariate Polymer/Metal–Organic Framework Hybrid Composites. J. Am. Chem. Soc. 2019, 141 (26), 10342–10349. 10.1021/jacs.9b03707. [DOI] [PubMed] [Google Scholar]
- Feng L.; Wang K.-Y.; Lv X.-L.; Yan T.-H.; Li J.-R.; Zhou H.-C. Modular Total Synthesis in Reticular Chemistry. J. Am. Chem. Soc. 2020, 142 (6), 3069–3076. 10.1021/jacs.9b12408. [DOI] [PubMed] [Google Scholar]
- Ma T.; Kapustin E. A.; Yin S. X.; Liang L.; Zhou Z.; Niu J.; Li L.-H.; Wang Y.; Su J.; Li J.; Wang X.; Wang W. D.; Wang W.; Sun J.; Yaghi O. M. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 2018, 361 (6397), 48–52. 10.1126/science.aat7679. [DOI] [PubMed] [Google Scholar]
- Kitagawa S. Future porous materials. Acc. Chem. Res. 2017, 50 (3), 514–516. 10.1021/acs.accounts.6b00500. [DOI] [PubMed] [Google Scholar]
- Choi S.; Kim T.; Ji H.; Lee H. J.; Oh M. Isotropic and Anisotropic Growth of Metal-Organic Framework (MOF) on MOF: Logical Inference on MOF Structure Based on Growth Behavior and Morphological Feature. J. Am. Chem. Soc. 2016, 138 (43), 14434–14440. 10.1021/jacs.6b08821. [DOI] [PubMed] [Google Scholar]
- Luo T. Y.; Liu C.; Gan X. Y.; Muldoon P. F.; Diemler N. A.; Millstone J. E.; Rosi N. L. Multivariate Stratified Metal-Organic Frameworks: Diversification Using Domain Building Blocks. J. Am. Chem. Soc. 2019, 141 (5), 2161–2168. 10.1021/jacs.8b13502. [DOI] [PubMed] [Google Scholar]
- Koh K.; Wong-Foy A. G.; Matzger A. J. MOF@MOF: microporous core-shell architectures. Chem. Commun. 2009, (41), 6162–6164. 10.1039/b904526k. [DOI] [PubMed] [Google Scholar]
- Kwon O.; Kim J. Y.; Park S.; Lee J. H.; Ha J.; Park H.; Moon H. R.; Kim J. Computer-aided discovery of connected metal-organic frameworks. Nat. Commun. 2019, 10, 3620. 10.1038/s41467-019-11629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón-Tovar L.; Pérez-Carvajal J.; Yazdi A.; Hernández-Muñoz J.; Tarazona P.; Imaz I.; Zamora F.; Maspoch D. A MOF@ COF Composite with Enhanced Uptake through Interfacial Pore Generation. Angew. Chem. 2019, 131 (28), 9612–9616. 10.1002/ange.201904766. [DOI] [PubMed] [Google Scholar]
- Gu Y. F.; Wu Y. N.; Li L. C.; Chen W.; Li F. T.; Kitagawa S. Controllable Modular Growth of Hierarchical MOF-on-MOF Architectures. Angew. Chem., Int. Ed. 2017, 56 (49), 15658–15662. 10.1002/anie.201709738. [DOI] [PubMed] [Google Scholar]
- Boissonnault J. A.; Wong-Foy A. G.; Matzger A. J. Core–Shell Structures Arise Naturally During Ligand Exchange in Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139 (42), 14841–14844. 10.1021/jacs.7b08349. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Zhu Y.; Liu L.; Ying X.; Hsiung C.-E.; Sougrat R.; Li K.; Han Y. Atomic-resolution transmission electron microscopy of electron beam–sensitive crystalline materials. Science 2018, 359 (6376), 675–679. 10.1126/science.aao0865. [DOI] [PubMed] [Google Scholar]
- Qin X.; He S.; Wu J.; Fan Y.; Wang F.; Zhang S.; Li S.; Luo L.; Ma Y.; Lee Y.; Li T. Tracking and Visualization of Functional Domains in Stratified Metal–Organic Frameworks Using Gold Nanoparticles. ACS Cent. Sci. 2020, 6 (2), 247–253. 10.1021/acscentsci.9b01205. [DOI] [PMC free article] [PubMed] [Google Scholar]