Abstract
Background
Depression is a long-term complex psychiatric disorder, and its etiology remains largely unknown. Valeriana jatamansi Jones ex Roxb (V. jatamansi) is used in the clinic for the treatment of depression, but there are insufficient reports of its antidepressive mechanisms and a poor understanding of its endogenous substance-related metabolism. The objective of this study was to identify biomarkers related to depression in serum samples and evaluate the antidepressive effects of the iridoid-rich fraction of V. jatamansi (IRFV) in a chronic unpredictable mild stress (CUMS) mouse model.
Methods
Here, CUMS was used to establish a mouse model of depression. Behavioral and biochemical indicators were investigated to evaluate the pharmacodynamic effects. A comprehensive serum metabolomics study by nuclear magnetic resonance (NMR) approach was applied to investigate the pharmacological mechanism of IRFV in CUMS mouse. Subsequently, we used multivariate statistical analysis to identify metabolic markers, such as principal component analysis (PCA) and orthogonal projection to latent structure with discriminant analysis (OPLS-DA), to distinguish between the CUMS mouse and the control group.
Results
After IRFV treatment, the immobility time, sucrose preference, and monoamine neurotransmitter were improved. PCA scores showed clear differences in metabolism between the CUMS group and control group. The PLS-DA or OPLS-DA model exhibited 26 metabolites as biomarkers to distinguish between the CUMS mice and the control mouse. Moreover, IRFV could significantly return 21 metabolites to normal levels.
Conclusion
The results confirmed that IRFV exerted an antidepressive effect by regulating multiple metabolic pathways, including the tricarboxylic acid cycle, the synthesis of neurotransmitters, and amino acid metabolism. These findings provide insights into the antidepressive mechanisms of IRFV.
Keywords: Valeriana jatamansi Jones, iridoids, depression, 1H NMR, metabolomics
Introduction
Depression, a common mental illness characterized by sadness, severely affects a patient's quality of life (Gong et al., 2019). Patients with depression exhibit symptoms including unhappiness, insomnia, anorexia, loss of interest, low mood, and anxiety (Avenevoli et al., 2015). The etiology of depression is assumed to be associated with genetics, the noradrenergic, dopaminergic, and serotoninergic systems, and stress (Drago et al., 2011). In recent years, one suffering from depression would have been primarily treated by synthetic antidepressants with emerging adverse effects, such ascardiotoxicity, hypertensive crisis, and sleep disorders (Read et al., 2014). Thus, greater therapeutic efficacy and fewer adverse effects are required. Traditional Chinese medicine (TCM) has been treating depression for more than 2,000 years. Its toxicity, safety, effectiveness, and multitargeted characteristics have received widespread attention from scholars both here and abroad in the field of depression treatment research (Yeung et al., 2012; Zhang and Cheng, 2019)
Valeriana jatamansi (V. jatamansi), originating from the herbs of Diannan written by Lan Mao (1396–1476) (Zhang et al., 2018), is a famous TCM used for relieving malaise, neurovegetation, and insomnia for more than 2,000 years (Chen et al., 2003; Wang et al., 2012). In recent years, studies have reported that V. jatamansi has significant antidepressive effects (Sah et al., 2011). Tagara, in which V. jatamansi is used as the key ingredient for the treatment of depression-type insomnia, has been marketed abroad (Singhal and Neetu, 2013). V. jatamansi contains IRFV, flavonoids, alkaloids, and volatile oils (Toolika et al., 2015). IRFV is the primary sedative and active component of V. jatamansi. In addition, IRFV exhibits potential therapeutic effects on Parkinson's disease, anxiety, and Alzheimer's disease (Jugran et al., 2019). However, there are few reports on the antidepressive effects and mechanism of IRFV, and there is a poor understanding of the endogenous substance-related metabolism in the IRFV antidepressive state.
Metabolomics is interrelated with disease phenotype (Gao et al., 2019). Metabolomics is a nonselective and universally applicable comprehensive analytical method for the qualitative and quantitative analyses of metabolites (Zheng et al., 2011; Wolfender et al., 2013). The occurrence and development of any disease will affect the metabolism of the body. Using a metabolomics approach, biomarkers of depression can be found, which can also describe metabolic abnormalities in the pathological process of depression. NMR-based metabolomics is widely used to analyze natural extracts (Choi et al., 2006; Wolfender et al., 2015). Hence, a 1H NMR integrated metabolomic approach was applied to screen and identify biomarkers associated with CUMS mice and understand the antidepressive influences of IRFV in a depressive mouse model. The primary goal of this work was to elucidate the antidepressive effects of IRFV on metabolic profiles, which can be used to better understand the underlying mechanism of treating depression.
In this study, an integrated 1H NMR-based metabolomic approach was applied to screen and identify biomarkers associated with CUMS mice and understand the antidepressive influences of IRFV in a depressive mouse model. The primary goal of this work was to elucidate the antidepressive effects of IRFV on metabolic profiles in order to better understand the underlying mechanism of treating depression.
Materials and Methods
Reagents
The roots and rhizomes of V. jatamansi were purchased from the Lotus Pond Chinese herbal medicine market in Chengdu, Southwest China. The sample was identified by Professor Liangke Song according to the pharmacognostic standard documented in the Chinese Pharmacopoeia. A voucher specimen (No. 20181003) was deposited in the herbarium of the Laboratory, School of Life Science and Engineering, Southwest Jiaotong University, China. Fluoxetine (Flu) was purchased from Suzhou Lilai Pharmaceuticals Co., Ltd. (Chengdu, China).
A phosphate buffer solution (0.1 M, K2HPO4/NaH2PO4, pH 7.4) was prepared as the extraction solvent, which contained 10% D2O (99.9% D) to provide a field lock for the NMR spectrometer. Distilled water was used for the preparation of all solutions.
IRFV Preparation
According to the previous extraction preparation process in our laboratory (Ke-Ke et al., 2014), a total of 10.4 kg of V. jatamansi dry plant rhizome powdered material was soaked in 70% aqueous ethanol at room temperature with occasional shaking and extracted three times for 24 h each time. The extracts were filtered through a muslin cloth and then acquired from the filtrate by reduced pressure evaporation at 45°C to yield the ethanol extract (2.32 kg) and underwent ethanol–water gradient elution using a D101 macroporous resin column, first eluted with pure water and 60% ethanol. Then, the eluate was eluted with 95% ethanol, and the eluent was collected. The ethanol was concentrated and recovered under vacuum to obtain the extract (IRFV) (0.19 kg; purity: 76.58%). Furthermore, our group established a quality control standard for IRFV, and the chlorovaltrate content was 8.91 mg/g (Zhu et al., 2016). IRFV was preserved at 4°C without light.
Experiment Animals and Drug Administration
Kunming (KM) mice (6 weeks old; weighing 20–24 g), with certificate number: SCXK (Chuan) 2015-030, were purchased from Dashuo Biological Technology Company in Chengdu and fed in the animal house of the pharmacological lab of Southwestern Jiao tong University. The mice were housed in propylene cages for an acclimatization period of 7 days prior to the experiments under controlled laboratory conditions with temperature of 25 ± 2°C, a relative humidity of 45 ± 15%, and a 12 h light/dark cycle. All animals were provided food and water. All mice were randomly divided into 6 groups (n = 7): the control group, CUMS model group, positive control group (Flu), and low, middle, and high dose IRFV treatment groups (IRFV-L, IRFV-M, IRFV-H, respectively). The experiments were approved by the Animal Ethics Committee of Southwest Jiaotong University (March 19, 2018, No. S20190319002).
Mice from the three IRFV treatment groups (IRFV-L, IRFV-M, IRFV-H) were orally administered 5.73, 11.47, and 22.94 mg/kg IRFV. Flu (2.5 mg/kg) was administered to mice in the positive control group. Mice in the control and model groups were fed an equal volume of 5% carboxymethylcellulose sodium water solution (CMC-Na, Chengdu Kelong Chemical Reagent Factory, China). All of the drugs were administered 30 min before stress exposure.
CUMS is the most extensively validated antidepressive screening (Willner, 2017) model and was used in this experiment. Mice were subjected to constant exposure to varying mild stressors for 4–8 weeks, resulting in a loss of responsiveness to rewards. The following stressors were conducted in a random order once a day for four weeks without repeating the stressors for two consecutive days: fed in individual cages including 24 h of food or water deprivation, damp sawdust for 24 h (50 ml of water per individual cage, which is enough to make the sawdust bed-ding wet), swimming in cold water at 4–8°C for 5 min, tail clamp for 1 min, and constraint for 2 h. The CUMS procedure was conducted for four weeks on all of the animals except for the mice in the control group.
Behavioral Tests and Biochemical Indicator Test
After two weeks of drug administration, the tail suspension test (TST) and sucrose consumption test (SPT) were performed as previously described (Klein et al., 2015). In the TST, the mice were suspended individually using a suspended tail instrument (Chengdu Taimeng Software Co. Ltd). After a period of struggle, the mice would appear to tail on the instrument without struggling. This period of time was considered the immobility time. The test was performed for a total of 6 min, and the immobility time during the last 4 min was recorded using a video tracking system. In the SPT, two bottles with a 1% sucrose solution were placed in each cage for the first 24 h. Then, one bottle was replaced with a bottle of tap water for the next 24 h. The mice were deprived of water and food for 23 h and then given a bottle with 100 ml of a 1% sucrose solution and another bottle with 100 ml of tap water. The percent volume of sucrose solution consumed was calculated (sucrose preference (%) = sucrose consumption/(water consumption + sucrose consumption) × 100%). The mice were sacrificed after the behavioral tests and the hippocampal tissues were collected for homogenization. The hippocampal tissue homogenate was centrifuged (3,000 g, 5 min, 4°C). Moreover, the concentrations of corticotropin releasing factor (CRF), 5-hydroxytryptamine (5-HT) and noradrenalin (NE) in hippocampal tissues were determined using enzyme-linked immunosorbent assay (ELISA) kits.
Sample Collection and Preparation for NMR Analysis
The whole blood of all mice was collected from the orbit, and the serum samples were acquired by centrifugation at 3,500 g and 4°C for 15 min, quickly frozen in liquid nitrogen and then stored at −80°C for further analysis. A volume of 200 μl of serum was mixed with 400 μl of a 90 mM phosphate buffer (NaH2PO4 and K2HPO4, pH 7.4) in 0.9% saline solution (100% D2O) and then centrifuged at 13,000 g and 4°C for 15 min. The supernatants were transferred into a 5 mm NMR tube for NMR analysis.
The serum samples were analyzed at 298 K using a Varian VNMRS 600 MHz NMR spectrometer operating at 25°C by using the Carr–Purcell–Meiboom–Gill (CPMG) spin-echo pulse sequence, where a total spin–spin relaxation delay 2 (nτ) of 320 ms was applied to attenuate the broad NMR signals from the slowly tumbling proteins and lipoproteins due to their long transverse relaxation time. The free induction decays (FIDs) were collected with 64 k data points with a spectral width of 12,000 Hz and 128 scans. The FIDs were zero-filled to double size and multiplied by an exponential line-broadening factor of 1.0 Hz before Fourier transformation (FT). In addition, standard COSY, TOCSY, HMBC and J-resolved spectra were acquired for metabolite identification purposes for the selected plasma samples.
Multivariate Data Analysis
All of the 1H NMR spectra were manually phased and corrected for baseline distortion by MestReNova 7.1.0 software (Mestrelab Research, Spain). All the spectra were referenced to the methyl group of creatinine at δ 3.039. In order to exploit all metabolites in formation embedded in the spectra, all NMR spectra (0.5–9.0) were segmented into equal widths of both 0.01 ppm and 0.002 ppm. Spectral regions of δ 4.68–5.20, δ 3.35–3.38, and δ 2.06–2.08 were excluded to eliminate variations caused by imperfect water suppression, methyl alcohol, and n-acetylcysteine. The centralized data were imported into SIMCA-P 12.0 for multivariate data analysis. Principal component analysis (PCA) was applied to detect group clustering and identify outliers. Additionally, an orthogonal partial least squares discriminate analysis (OPLS-DA) algorithm was further constructed using unit-variance scaling with a seven-fold internal cross-validation and CV-ANOVA approach (p < 0.05). Color-coded loading plots with their absolute value of coefficients (r) were generated with MATLAB (http://www.mathworks.com) with some in-house modifications, which were performed to identify significantly altered metabolites. The main parameters of the verification model (Q2 and R2) were calculated on SIMCA-P 12.0. R2 indicates the goodness of fit of the model, whereas Q2 estimates its prediction ability (Liu et al., 2012). The variable importance in the projection (VIP) values, which expresses the significance in discriminating between groups, was used to select the biomarkers. In this study, the data with VIP > 1 were selected for independent samples, and a t-test using SPSS Statistics Base 17.0 (SPSS Inc., USA) was used to compare significant differences between two groups. Fold change (FC) was calculated as the average peak area between the two groups. After that, P values were obtained for the analysis of data statistics and the determination of the metabolites in the next step. The data with p < 0.05 were considered important, and data with p < 0.01 were considered to be very significant. The final data to identify the biomarkers were those with a VIP value greater than 1 and a P value less than 0.05 (Xiong et al., 2016). The spectra were compared with the Human Metabolome Database (HMDB) (http://www.hmdb.ca/) and related literature to finalize the metabolite species. After that, metabolites and their metabolic pathways were studied by searching the KEGG database (https://www.kegg.jp/kegg/pathway.html). The metabolite-correlation network and metabolic networks were constructed using Cytoscape software (v.3.6.) and Gene Cards (http://www.genecards.org), which were matched with the genes from the disturbed metabolic pathways (Zhou et al., 2016).
Results
Body Weight
The statistical analysis of body weight of mice is shown in (Figure 1). The 1st, 2nd, 3rd, and 4th weeks after CUMS intervention and two weeks after drug administration, the body weights of all mice treated with CUMS were slightly lower than the mice in the control group. During the CUMS intervention period, there was no significant difference in weight between each group (p > 0.05). During the dosing period, the body weights in the control group showed a natural increase, while the body weights in the IRFV dosage and Flu groups significantly increased compared to the model group (p < 0.05 or p < 0.01). However, no significant difference was observed for the weights of the different treatment groups (p > 0.05).
Figure 1.
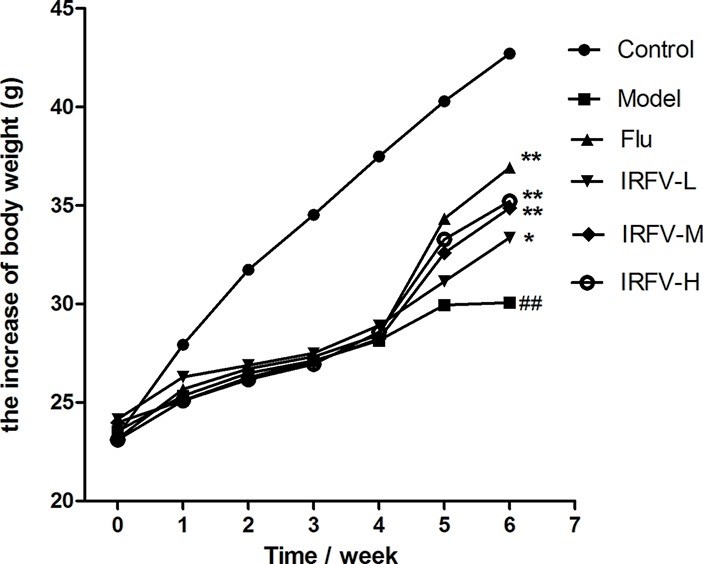
The 1st, 2nd, 3rd, and 4th weeks after CUMS intervention and two weeks after drug administration, all mice treated with CUMS weighed slightly less than the mice in the control group. During the CUMS intervention period, there was no significant difference in body weight between the different groups (p > 0.05). During the dosing period, the mouse body weights of the control group showed a natural increase, while the body weights of the IRFV dose and Flu groups significantly increased compared to the model group (p < 0.05 or p < 0.01). However, no significant difference was observed among the weights of the different treatment groups (p > 0.05). ##p < 0.01 vs the control group; *p < 0.05, **p < 0.01 vs the model group.
Effect of IRFV on Behavior
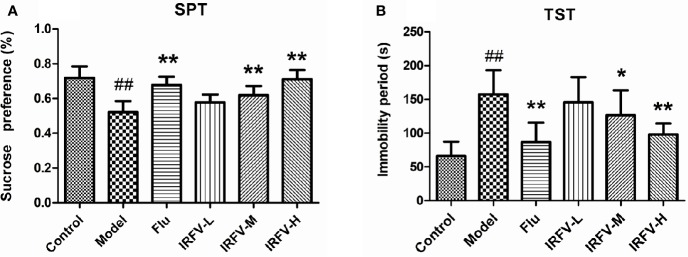
The immobility time of the TST in all groups is shown in Figure 2A. In the model group, the immobility times were dramatically longer than those in the control group (p < 0.01). However, after administration of Flu and IRFV, the immobility time shortened significantly (p < 0.05 or p < 0.01), but no obvious difference was observed in the IRFV-L group (p > 0.05). The SPT results are shown in Figure 2B. The sucrose preference of the model group decreased (p < 0.01), while both Flu and IRFV treatments increased the sucrose preference (p < 0.05 or p < 0.01). However, the sucrose preference of IRFV-L also showed no obvious difference (p > 0.05).
Figure 2.
The immobility time of the TST in all groups (A). In the model group, the immobility times were dramatically longer than those in the control group (p < 0.01). However, after administration of Flu and IRFV, the immobility time was shortened significantly (p < 0.05 or p < 0.01), but no obvious difference was observed in the IRFV-L group (p > 0.05). SPT results (B). The sucrose preference of the model group decreased (p < 0.01), while both Flu and IRFV treatments increased the sucrose preference (p < 0.05 or p < 0.01). However, the sucrose preference of IRFV-L also showed no obvious difference (p > 0.05). ##p < 0.01 vs the control group; *p < 0.05, **p < 0.01 vs the model group.
Effects of the Hippocampal Tissue Contents of NE, 5-HT, and CRF
As shown in Figure 3, we observed that the concentrations of NE and 5-HT were significantly decreased in the model group compared to the control group (p < 0.01). When the CUMS mice were treated with Flu and IRFV, the levels of 5-HT observably increased (p < 0.01, p < 0.05). NA only significantly increased in the IRFV-H group (p < 0.05). In contrast, CRF increased significantly in the model group compared to the control group (p < 0.01). After treatment with Flu and IRFV, the levels of CRF significantly increased compared to the model group (p < 0.01, p < 0.05), but no obvious difference was shown for the IRFV-L group (p > 0.05).
Figure 3.
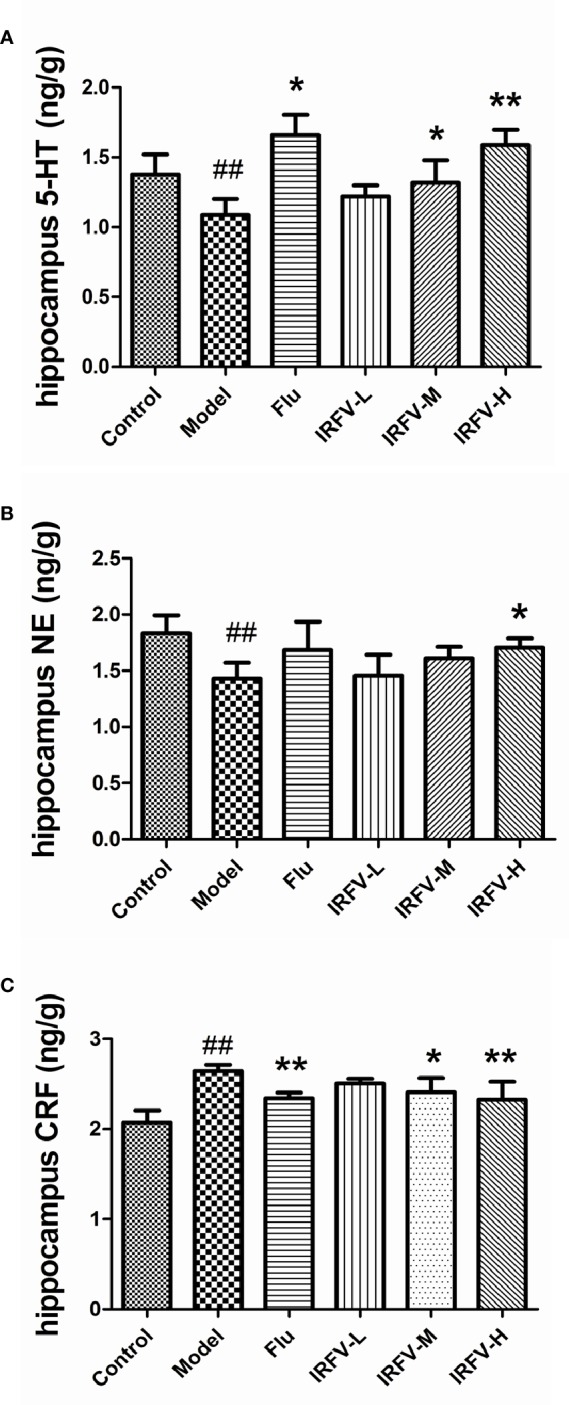
The concentrations of NE and 5-HT were significantly decreased in the model group compared to the control group (p < 0.01). When the CUMS mice were treated with Flu and IRFV, their levels of 5-HT observably increased (p < 0.01, p < 0.05). NA levels significantly increased only in the IRFV-H group (p < 0.05). In contrast, CRF increased significantly in the model group compared to the control group (p < 0.01). After treatment with Flu and IRFV, the levels of CRF significantly increased compared to the model group (p < 0.01, p < 0.05), but no obvious difference was observed in the IRFV-L group (p > 0.05). ##p < 0.01 vs the control group; *p < 0.05, **p < 0.01 vs the model group.
1H NMR Analysis of Serum
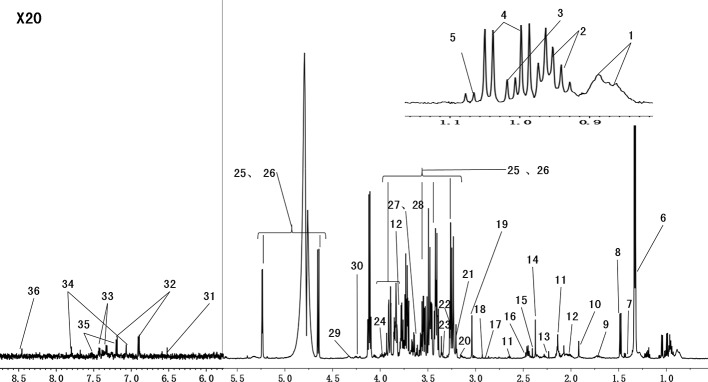
Serum metabolites may change with CUMS intervention and drug treatment. Figure 4 shows the representative 1H NMR spectra of serum from the control group. The spectra illustrated the majority of the metabolites in this experiment. We marked the metabolites from relevant peaks with different chemical shifts, which were based on previous literature (Tian et al., 2016) and an in-house NMR database, and 36 metabolites were finally identified in the serum samples.
Figure 4.
Representative 600 MHz 1H-CPMG NMR spectra (δ 0.5–4.6, δ 5.2–9.0) of serum from the normal groups. The spectra illustrate the majority of the metabolites in this experiment. We marked the metabolites from the relevant peaks of different chemical shifts, which were based on the previous literature and an in-house NMR database. There were 36 metabolites in the serum samples (1, HDL\LDL; 2, isoleucine; 3, leucine; 4, valine; 5, isobutyrate; 6, lactate; 7, lysine; 8, alanine; 9, arginine; 10, acetate; 11, glutamate; 12, glutamine; 13, acetone; 14, pyruvate; 15, succinate; 16, methionine; 17, N,N-dimethylglycine; 18, creatinine; 19, creatine; 20, choline; 21, phosphorylcholine; 22, malate; 23, taurine; 24,betaine; 25, β-glucose; 26, α-glucose; 27, glycine; 28, glycerol; 29, threonine; 30, myoinositol; 31, fumarate; 32, tyrosine; 33, phenylalanine; 34, histidine; 35, trypotophan; 36, formate).
IRFV Induced Metabolomic Variations in CUMS Mice
To evaluate the effects of IRFV on the metabolic profiles, we applied unsupervised principal component analysis to explore the separation of the model, Flu, IRFV-L, IRFV-M, IRFV-H, and control groups. As shown in Figure 5, the PCA score plot of the serum of the mice was found to be clearly separated among the six groups. The metabolic features of the model group were clearly separated from those of the control and drug-treated groups. Moreover, with increasing doses of IRFV, the metabolic features were closer to those of the control mice. These data indicated a remarkable distinction in the score plots between the model group and the other groups along the PC1 and PC2 axes, with the values of PC1 = 86.7% and PC2 = 9.88% in the serum.
Figure 5.
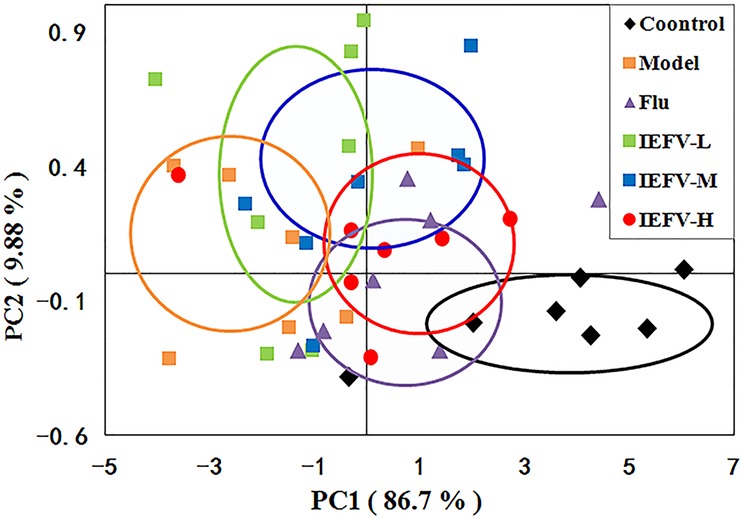
PCA score plots based on the 1H NMR spectra of serum samples (A) following different dosages of IRFV. In the plot, each dot represents NMR profiling data from an individual sample and colors indicate samples in different treatment groups.
Metabolic Changes and Biomarker Identification
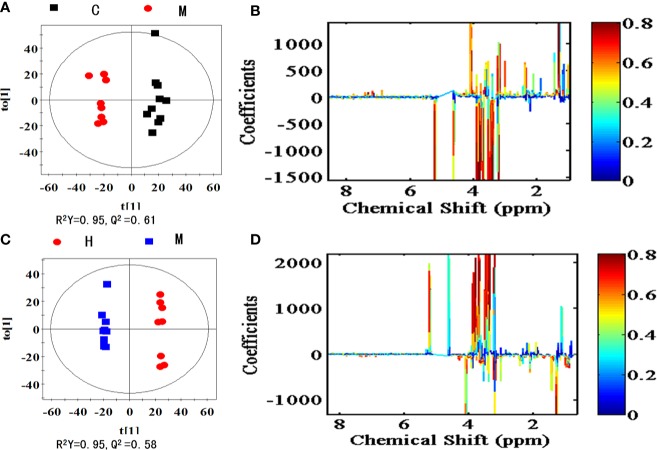
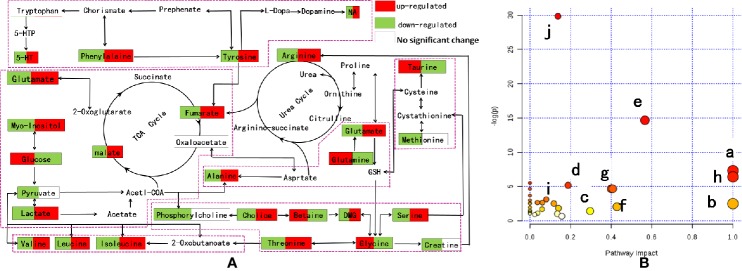
To explore the metabolites responsible for the separation, OPLS-DA score plots and the corresponding loading plots of serum samples were generated between the model vs control group as shown in Figure 6A and the IRFV-H vs model groups as shown in Figure 6B. The comparison of the model and control groups was R2Y = 95% and Q2Y = 0.61, while the IRFV-H group and model group were R2X = 95% and Q2Y = 0.58, which presented the explained variance and a high predictive capability, respectively (Westerhuis et al., 2010; Surmacki et al., 2015). The color-coded coefficient plots demonstrating metabolite changes in detail after CUMS intervention are shown in Figure 6C, and the IRFV-H-treated results are shown in Figure 6D. In this study, potential biomarkers may be identified with VIP values > 1.0 and p < 0.05. Compared with the control group, the key biomarkers in the model samples were as follows: decreased levels of fumarate, malate, pyruvate, proline, phenylalanine, arginine, threonine, alanine, lactate, tyrosine, methionine, valine, lysine, isoleucine, myoinositol, N,N-dimethylglycine (DMG), phosphorylcholine, choline, serine, and leucine were found in the model group; and increased levels of glycine, glutamine, betaine, taurine, α-glucose and β-glucose were found in the model group. The related information (chemical shift, fold change, Components assignment, P value, etc.) of the potential metabolites derived from the serum samples is summarized in Table 1. With IRFV-H treatment, 21 metabolites returned to normal levels in the serum samples. Additionally, the metabolic biomarkers that were obtained from the serum samples can be summarized as branched amino acids (isoleucine, leucine, valine), other amino acids (tyrosine, glutamate, arginine, etc.), organic acids (malate, fumarate, myoinositol), and energy storage compounds (lactate, glucose, fumarate, etc.).
Figure 6.
OPLS-DA score plots (left panel) and corresponding coefficient loading plots (right panel) derived from 1H NMR spectra of the model group and control group (A, B) and the IRFV-H group and model group (C, D). The correlation coefficients are color coded in the coefficient plot, which shows the significance of metabolite variations. Signals with a positive direction indicate that the number of metabolites is higher in the control group than in the model group, and vice versa. The metabolites are assigned in Table 1.
Table 1.
Quantitative comparison of metabolites derived from the 1H NMR of serum samples.
| Metabolite | Chemical shift (ppm) | Component assignment | FCa | VIP score | FCb | VIP score |
|---|---|---|---|---|---|---|
| Leucine | 0.96(d) | CH2 | 0.88# | 1.39 | 1.26** | 1.56 |
| Valnine | 1.03 (d) | CH3 | 0.75## | 1.59 | 1.62** | 1.66 |
| Lactate | 1.33 (d) | CH3 | 0.70## | 1.66 | 1.27** | 1.58 |
| Alanine | 1.47(d) | CH3 | 0.73### | 1.41 | 1.51** | 1.61 |
| Arginine | 1.70 (m) | δ-CH2 | 0.75# | 1.52 | 1.49** | 1.62 |
| Lysine | 1.74 (m) | β-CH2 | 0.75## | 1.37 | — | — |
| glutamate | 2.34 (m) | γ-CH2 | 0.71## | 1.75 | 1.37* | 1.71 |
| Pyruvate | 2.38 (s) | CH3 | 0.84# | 1.38 | — | — |
| Methionine | 2.66 (m) | β-CH2 | 0.71# | 1.49 | — | — |
| N,N-dimethylglycine | 2.94 (s) | CH3 | 0.78## | 1.33 | 1.32* | 1.55 |
| Creatine | 3.03(s) | CH3 | 0.75## | 1.25 | — | — |
| Choline | 3.21 (s) | N(CH3)3 | 0.78### | 1.80 | 1.31* | 1.77 |
| Taurine | 3.25 (s) | NCH2 | 1.30### | 2.01 | 0.80** | 2.05 |
| β-glucose | 3.41 (d) | CH4 | 1.34## | 1.99 | 0.78** | 2.03 |
| Glycine | 3.56 (s) | CH2 | 1.17### | 1.66 | 0.89* | 1.51 |
| α-Glucose | 3.71 (d) | CH3 | 1.35### | 1.93 | 0.80** | 2.01 |
| phosphorylcholine | 3.31 (d) | β-CH3 | 0.76## | 1.47 | 1.48** | 1.65 |
| Glutamine | 3.77 (t) | α-CH | 1.19## | 1.82 | 0.90** | 1.66 |
| Betaine | 3.91 (s) | N(CH3)3 | 1.23# | 1.85 | 0.84** | 1.66 |
| Serine | 3.97(m) | β-CH | 0.85# | 1.21 | 1.30* | 1.57 |
| Myo-inositol | 4.06 (t) | H2 | 0.84## | 1.58 | — | — |
| Threonine | 4.25(m) | β-CH | 0.73# | 1.19 | 1.46** | 1.56 |
| Malate | 4.31(m) | CH | 0.71# | 1.56 | 1.29* | 1.37 |
| Fumarate | 6.52(s) | CH | 0.14## | 1.65 | 6.75* | 1.51 |
| Tyrosine | 6.91 (m) | H3/H5 | 0.74# | 1.26 | 1.45* | 1.38 |
| Phenylalanine | 7.33 (m) | H2/H6 | 0.73## | 1.58 | 1.69** | 1.65 |
FCa: fold change. FC >1 indicates that the amount of metabolites is higher in the model group than in the control group, and vice versa.
FCb: fold change. FC >1 indicates that the amount of metabolites is higher in the treated group than in the model group, and vice versa.
compared with control group, #p < 0.05, ##p < 0.01, ###p < 0.001; compared with model group,*p < 0.05, **p < 0.01.
Cytoscape to Explore the Biomarker Metabolic Network
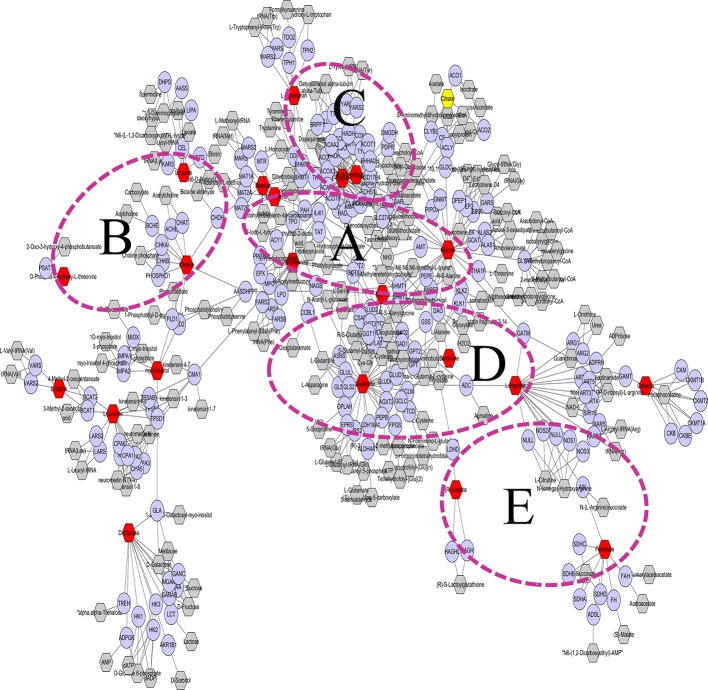
The metabolic networks involved in some enzymes and genes were constructed with Cytoscape to better understand the internal correlation of the potential biomarkers in terms of the enzyme or gene levels (Tian et al., 2018). The metabolic networks that were established based on the markedly different metabolites are shown in Figure 7. Glycine, serine, methionine and threonine metabolisms are shown in Figure 7A. Some enzymes and genes were also found to be involved in the TCA cycle and lysine metabolism (Figure 7B) such as phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH), and tryptophan hydroxylase (TPH) is involved in tryptophan and tyrosine metabolism (Figure 7C). Glutamic acid decarboxylase (GAD) and gamma-glutamyl transferase (GGT) are involved in arginine, proline, glutamate, aspartate and asparagine metabolism (Figure 7D). d-Amino-acid oxidase (DAO), thyroid peroxidase (TPO), and fumarate hydratase (FH) are involved in glycosphingolipid, methionine, and cysteine metabolism (Figure 7E).
Figure 7.
Glycine, serine, methionine, and threonine metabolism are shown (A). Some enzymes and genes were also found to be involved in the TCA cycle and lysine metabolism (B) such as phenylalanine hydroxylase (PAH) and tyrosine hydroxylase (TH); tryptophan hydroxylase (TPH) of genes is involved in tryptophan and tyrosine metabolism (C). Glutamic acid decarboxylase (GAD) and gamma-glutamyl transferase (GGT) are involved in arginine, proline, glutamate, aspartate, and asparagine metabolism (D). d-Amino-acid oxidase (DAO), thyroid peroxidase (TPO), and fumarate hydratase (FH) are involved in glycosphingolipid metabolism and methionine and cysteine metabolism (E).
Metabolic Pathway Analysis
Based on the above biological tests and multiple analyses, potential biomarkers were finally identified in the model and IRFV-treated rats. Subsequently, the relevant metabolic pathways were found using MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/MetaboAnalyst/) (Lauri et al., 2016) and are associated with the series of metabolic responses to the metabolites that were obtained after IRFV treatment. As shown in Figure 8A, different points represent different metabolic pathways, and the size of the points represents the impact value that was calculated from the pathway topology analysis. Pathways with an impact value above 0.1 were screened out as potential target pathways. In total, there were eight metabolic pathways involved in this study, including isoleucine, leucine, valine biosynthesis; arginine and proline metabolism; d-glutamine and d-glutamate metabolism; glycine, lysine, threonine, choline, and serine metabolism; the tricarboxylic cycle (TCA cycle); phenylalanine metabolism; tryptophan, phenylalanine, and tyrosine metabolism; and taurine and hypotaurine biosynthesis. The details of the pathways are shown in Figure 8B.
Figure 8.
(A) Metabolic pathway analysis of IRFV-treated mice from MetaboAnalyst 3.0. The impact is the pathway impact value calculated from pathway topology analysis. Phenylalanine, tyrosine, and tryptophan biosynthesis (a); d-glutamine and d-glutamate metabolism (b); alanine, aspartate, and glutamate metabolism (c); arginine and proline metabolism (d); glycine, serine, and threonine metabolism (e); taurine and hypotaurine metabolism (f); phenylalanine metabolism (g); and valine, leucine, and isoleucine biosynthesis (h). The details of the pathways are shown in panel B.
Discussion
Depression affects more than 300 million people worldwide, sometimes leading to a deadly fate in suicide (World Health Organization, 2018). V. jatamansi is a famous TCM herb that has been wildly used in Asia and Europe for thousands of years. A previous study from our laboratory reported that IRFV possesses various effects, such as neuroprotective effects, antifree radicals, anti-inflammatory, and even antianxiety (Yan et al., 2010) (Zhang et al., 2018). It may also have antidepressant effects. Thus, the focus of this paper was to investigate the potential effects and mechanisms of IRFV on CUMS-induced depression. A 1H NMR-based metabolomic approach was conducted to demonstrate the metabolic differences between normal and CUMS mice with different syndromes in this study and evaluate the effect of IRFV on CUMS mice.
The CUMS model was established to evaluate the activity of antidepressants and has been proven to have high repeatability and consistency with the results of depressive disorders (Raya et al., 2018). The behaviors of the mice after chronic stimulation are consistent with the clinical behaviors of patients with depression. Behavioral tests after CUMS, including the SPT (Klein et al., 2015) and TST (Peng et al., 2007), were applied to investigate the effects of Flu and IRFV. The results demonstrated CUMS hyposensitivity to reward stimulations of sucrose and anhedonia, while the immobility time in the TST reflects the degree of despair. Both Flu and IRFV could relieve the depressive state induced by CUMS interventions, and the effects of IRFV-H were similar to those of Flu.
The CUMS model results in behavioral and physiological abnormalities in animals that may relate to an imbalance of monoamine neurotransmitters in the brain. Our biochemical findings indicated that IRFV could regulate the levels of monoamine neurotransmitters (5-HT, NA, CRF) and exert antidepressant activity.
Amino Acid Metabolism and Synthesis of Neurotransmitters
Tyrosine, phenylalanine, and tryptophan have been widely reported in the study of depression models (Eskelund et al., 2016; Keegan et al., 2016). Phenylalanine is an essential amino acid, and its metabolic process is to produce tyrosine with PAH in the liver. Tyrosine can be further metabolized into catecholamine neurotransmitters, such as DA and NE, by TH, which are closely related to depression (Zhao et al., 2015b; Du et al., 2016; Chen et al., 2019). Moreover, tryptophan can be further metabolized to 5-HT by TPH (Pompili et al., 2019). In this study, the concentrations of phenylalanine and tyrosine significantly decreased (McKean et al., 1968; Du et al., 2016; Strasser et al., 2017) in the model group, which may have further affected the synthesis of catecholamine neurotransmitters. We found that the levels of NE and 5-HT significantly decreased in mouse hippocampal tissues, which was consistent with the above result. TH, PAH, and TPH were found in Cytoscape to explore biomarker metabolism, and their enzyme expression was reduced in depression model rats or patients with depression (Guo et al., 2018; Scherer et al., 2018; Lu et al., 2019). This result may indicate that CUMS leads to a decrease in TH, PAH, and TPH activity and that the concentrations of synthetic catecholamine neurotransmitters, tyrosine, and phenylalanine are reduced, resulting in the symptoms of depression.
Glutamate is an important excitatory neurotransmitter of the nervous system in CUMS-induced depression (O'Connor and Cryan, 2013). Interestingly, glutamate and glutamine can interconvert mutually in the body, and glutamine is a precursor of gamma amino butyric acid (GABA) synthesis, which has been found to be deficient in a depression model (Heckers et al., 2002; Ma et al., 2019). Moreover, GAD is the key synthetic enzyme for GABA, which has been found to be deficient in depression models (Heckers et al., 2002; Gao et al., 2013). In addition, glutamine and glutamate can be interconnected through enzyme catalysis, and they are both precursors of glutathione (GSH). As GGT (Yamada et al., 2013) activity decreases, this may influence GSH metabolism. GSH concentrations in the blood serum, plasma, or the brain have also been identified to be significantly decreased in depression (de Souza et al., 2006; Kodydkova et al., 2009; Maes et al., 2011). The glutamate system also regulates HPA axis function, further regulating CRF concentrations in depression (Zafir and Banu, 2009; Davis et al., 2018). In the current study, the level of glutamate significantly decreased, and glutamine significantly increased in the model group. We also found that the levels of CRF were significantly increased in mouse hippocampal tissues. These results may indicate that CUMS leads to decreased Gad1 and GGT activity and reduced or increased concentrations of CRF, glutamate, and glutamine, resulting in symptoms of depression.
Glycine is biosynthesized in the body from the amino acids serine and threonine. In this study, the levels of glycine, serine, and threonine in the CUMS model group were markedly changed, which may indicate that glycine, serine, and threonine metabolism was disturbed. Changes in glycine, serine, and threonine metabolism have been reported to be associated with depression (Maes et al., 1998; Song et al., 2019). Moreover, glycine is crucial for controlling synaptic plasticity and is an inhibitory neurotransmitter in the central nervous system (Altamura et al., 1995). In addition, glycine is currently a favored therapeutic target for rapid antidepressive action.
Previously reported levels of other amino acids, such as isoleucine, leucine, valine (Jia et al., 2013), arginine, alanine (Hu et al., 2018), threonine, and methionine (Benelli et al., 1999), decreased in the CUMS mice which is consistent with our results. Additionally, isoleucine, leucine, and valine are proteinogenic amino acids with aliphatic side chains and are called branched-chain amino acids (BCAAs). BCAAs can be quickly transported across the blood–brain barrier as major amino group donors for the synthesis of glutamate and 5-HT in the brain (Auer et al., 2000), and the biosynthesis of BCAAs is believed to play a crucial role in the development of depression. Furthermore, we found that arginine and proline metabolism was significantly disturbed, which has been reported in the prefrontal cortex of a learned helplessness rat model in a metabolomics study (Zhou et al., 2017). These amino acid changes may be related to the pathogenesis of CUMS mice.
Energy Metabolism
Previous studies have indicated that sucrose intake greatly affects alterations in serum metabolites. The tricarboxylic acid (TCA) (Zhao et al., 2015a) cycle is correlated with CUMS-induced depression, and glucose can be metabolized by glycols to produce pyruvate, ATP and lactate, which act as energy substrates. Glycine can also be converted into pyruvate and then into acetyl-coenzyme A (acetyl-CoA), which enters the TCA cycle. The changes in the levels of valine and glycine in the model group indicated that pyruvate metabolism was disturbed (Du et al., 2017). Moreover, fumarate can be further metabolized into L-malate by FH (Mescam et al., 2011). These changes weakened the strength of glycolysis, leading to further energy deficiency. In this experiment, reduced levels of lactate, myoinositol, fumarate, and malate and increased levels of α-glucose, β-glucose, and glycine indicated that energy production was disturbed in CUMS mice.
Other Metabolites and Enzymes
Choline can synthesize betaine and phosphatidylcholine, and betaine is further converted into N,N-dimethylglycine (DMG). First, they have an important role in maintaining protein structure and cell membrane integrity. Second, they participate in the body's oxidative stress response (Sussulini et al., 2009). Several previous studies have indicated that depressed patients display a disturbance of gut microflora, including metabolites of dimethylglycine and DMG (Zhao et al., 2015d). Moreover, studies have shown that the levels of unsaturated phosphorylcholine, choline, and betaine in depression model animals show an enhanced oxidative effect, which is a typical feature of depression (Liu et al., 2015). It has been reported that choline and betaine change the content of phosphorylcholine and indicate that the integrity of the cell membrane has been damaged after depression, which is consistent with the results of this study (Zhao et al., 2015c). This damage may be related to increased oxidative stress in the body and a decreased immune response (Lever et al., 2017). In our study, the concentrations of choline, phosphorylcholine, betaine, and DMG were obviously changed in CUMS mice. These changes may indicate that oxidative stress destroys the cell membrane structure or gut microflora in CUMS mice.
Taurine and myoinositol (Zhang et al., 2009) are the major controllers of osmolarity in the brain. The increasing concentration of taurine and decreasing concentration of myoinositol might disturb osmotic regulation in CUMS mice. Lactate participates in synaptic plasticity, and has been viewed as a good biomarker for brain status (Aalling et al., 2018). Decreased levels of lactate have been found in CUMS mice (Chen et al., 2017). In addition, DAO is a peroxisomal enzyme that is known to oxidize d-serine and may play a regulatory role in N-methyl-d-aspartate-type (NMDA) receptor function (Corvin et al., 2007). The NMDA receptor has a major role in the neurophysiology of depression (Sattar et al., 2018). Moreover, hypothyroidism was determined, and TPO levels were significantly decreased with a higher risk of depression, which was expressed during gestation as a marker for subsequent postpartum depression (Dama et al., 2016).
Over all, IRFV downregulated fumarate, malate, phenylalanine, arginine, threonine, alanine, lactate, tyrosine, valine, isoleucine, DMG, choline, serine, and leucine and upregulated glycine, glutamine, betaine, taurine, and glucose in mouse serum. In addition, it is worth noting that whether the activities of some enzymes, such as PAH, TH, TPH, GAD, GGT, DAO, TPO, and FH, were affected by IRFV requires further investigation.
Conclusions
In this study, a metabolomics approach based on 1H NMR was applied to investigate the metabolomics changes in CUMS mouse serum samples. Twenty-six metabolites were identified as biomarkers that were closely related to CUMS mice. Moreover, we paid more attention to the effects of IRFV on CUMS mice. CUMS was used to establish a depressed mouse model, and SPT, TST, 5-HT, NA, and CRF were investigated to evaluate the pharmacodynamic effects. Administration of IRFV could return the behavioral and biochemical indicators to normal and restore metabolic disturbance. Our results suggested that IRFV may protect mice from depression damage via regulation of multiple metabolic pathways primarily involving amino acids, energy metabolism and the synthesis of neurotransmitters. These findings provide new perspective insight to better understand the pathophysiological mechanism underlying the CUMS model and are also supported as the basis for follow-up research on the antidepressive mechanism of IRFV.
Data Availability Statement
All datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Animal Ethical and Welfare of Southwest Jiaotong University (March 19, 2018, No. S20190319002).
Author Contributions
ZY conceived and designed the experiments. YL, LWu, XZ, TZ, and LWa performed the experiments. CC, CG, YL, and XW analyzed the data. AL guided the experiments. YL wrote the paper. CC revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the Sichuan Province Academic and Technical Leaders Cultivate Support Funds, Key Project of Research and Development Plan of Science and Technology Department of Sichuan Province (Nos. 2018ZR0368 and 2018SZ0078), the Major Scientific and Technological Special Project for “Significant New Drugs Creation” (Nos.2018ZX09201010-001-003, 2019ZX09201005, 2018ZX09735-002) and the National Natural Science Foundation of China (No. 81703948).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aalling N. N., Nedergaard M., DiNuzzo M. (2018). Cerebral Metabolic Changes During Sleep. Curr. Neurol. Neurosci. Rep. 18, 57–73. 10.1007/s11910-018-0868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura C., Maes M., Dai J., Meltzer H. Y. (1995). Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 5 Suppl, 71–75. 10.1016/0924-977x(95)00033-l [DOI] [PubMed] [Google Scholar]
- Avenevoli S., Swendsen J., He J. P., Burstein M., Merikangas K. R. (2015). Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child. Adolesc. Psychiatry 54 (1), 37–44. e32. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer D. P., Putz B., Kraft E., Lipinski B., Schill J., Holsboer F. (2000). Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 47, 305–313. 10.1016/s0006-3223(99)00159-6 [DOI] [PubMed] [Google Scholar]
- Benelli A., Filaferro M., Bertolini A., Genedani S. (1999). Influence of S-adenosyl-L-methionine on chronic mild stress-induced anhedonia in castrated rats. Br. J. Pharmacol. 127, 645–654. 10.1038/sj.bjp.0702589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Kang L., Qin L., Zheng H., Guo C. (2003). The study on quality standard and sedative-hypnotic activity of valepotriate. Chin. Tradit. Patent Med. 25, 663–665. 10.3969/j.issn.1001-1528.2003.08.023 [DOI] [Google Scholar]
- Chen J. J., Zhou C. J., Zheng P., Cheng K., Wang H. Y., Li J., et al. (2017). Differential urinary metabolites related with the severity of major depressive disorder. Behav. Brain Res. 332, 280–287. 10.1016/j.bbr.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Chen B., Li J., Xie Y., Ming X., Li G., Wang J., et al. (2019). Cang-ai volatile oil improves depressive-like behaviors and regulates DA and 5-HT metabolism in the brains of CUMS-induced rats. J. Ethnopharmacol. 244, 112088. 10.1016/j.jep.2019.112088 [DOI] [PubMed] [Google Scholar]
- Choi Y. H., Kim H. K., Linthorst H. J., Hollander J. G., Lefeber A. W., Erkelens C., et al. (2006). NMR Metabolomics to Revisit the Tobacco Mosaic Virus Infection in Nicotiana t abacum Leaves. J. Natural Products 69 (5), 742–748. 10.1021/np050535b [DOI] [PubMed] [Google Scholar]
- Corvin A., Donohoe G., McGhee K., Murphy K., Kenny N., Schwaiger S., et al. (2007). D-amino acid oxidase (DAO) genotype and mood symptomatology in schizophrenia. Neurosci. Lett. 426, 97–100. 10.1016/j.neulet.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Dama M., Steiner M., Lieshout R. V. (2016). Thyroid peroxidase autoantibodies and perinatal depression risk: A systematic review. J. Affect. Disord. 198, 108–121. 10.1016/j.jad.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Davis E. G., Keller J., Hallmayer J., Pankow H. R., Murphy G. M., Jr., Gotlib I. H., et al. (2018). Corticotropin-releasing factor 1 receptor haplotype and cognitive features of major depression. Transl. Psychiatry 8, 5–13. 10.1038/s41398-017-0051-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza F. G., Rodrigues M. D., Tufik S., Nobrega J. N., D'Almeida V. (2006). Acute stressor-selective effects on homocysteine metabolism and oxidative stress parameters in female rats. Pharmacol. Biochem. Behav. 85, 400–407. 10.1016/j.pbb.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Drago A., Crisafulli C., Sidoti A., Serretti A. (2011). The molecular interaction between the glutamatergic, noradrenergic, dopaminergic and serotoninergic systems informs a detailed genetic perspective on depressive phenotypes. Prog. Neurobiol. 94 (4), 418–460. 10.1016/j.pneurobio.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Du H., Wang K., Su L., Zhao H., Gao S., Lin Q., et al. (2016). Metabonomic identification of the effects of the Zhimu-Baihe saponins on a chronic unpredictable mild stress-induced rat model of depression. J. Pharm. BioMed. Anal. 128, 469–479. 10.1016/j.jpba.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Du H., Zhao H., Lai X., Lin Q., Zhu Z., Chai Y., et al. (2017). Metabolic profiles revealed synergistically antidepressant effects of lilies and Rhizoma Anemarrhenae in a rat model of depression. BioMed. Chromatogr. 31 (7), 487–493. 10.1002/bmc.3923 [DOI] [PubMed] [Google Scholar]
- Eskelund A., Budac D. P., Sanchez C., Elfving B., Wegener G. (2016). Female Flinders Sensitive Line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 329, 337–348. 10.1016/j.neuroscience.2016.05.024 [DOI] [PubMed] [Google Scholar]
- Gao S. F., Klomp A., Wu J. L., Swaab D. F., Bao A. M. (2013). Reduced GAD(65/67) immunoreactivity in the hypothalamic paraventricular nucleus in depression: a postmortem study. J. Affect. Disord. 149, 422–431. 10.1016/j.jad.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Gao J., Wang T., Wang C., Wang S., Wang W., Ma D., et al. (2019). Effects of Tianshu Capsule on Spontaneously Hypertensive Rats as Revealed by 1H-NMR-Based Metabolic Profiling. Front. Pharmacol. 10, 989. 10.3389/fphar.2019.00989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W., Zhu S., Chen C., Yin Q., Li X., Du G., et al. (2019). The anti-depressioneffect of Angelicae Sinensis Radix is related to the pharmacological activity of modulating the hematological anomalies. Front. Pharmacol. 10, 192. 10.3389/fphar.2019.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Zhang S., Sun H., Xu X., Hao Z., Mu C., et al. (2018). Tyrosine hydroxylase down-regulation after loss of Abelson helper integration site 1 (AHI1) promotes depression via the circadian clock pathway in mice. J. Biol. Chem. 293, 5090–5101. 10.1074/jbc.RA117.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S., Stone D., Walsh J., Shick J., Koul P., Benes F. M. (2002). Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch. Gen. Psychiatry 59, 521–529. 10.1001/archpsyc.59.6.521 [DOI] [PubMed] [Google Scholar]
- Hu Q., Shen P., Bai S., Dong M., Liang Z., Chen Z., et al. (2018). Metabolite-related antidepressant action of diterpene ginkgolides in the prefrontal cortex. Neuropsychiatr. Dis. Treat 14, 999–1011. 10.2147/NDT.S161351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. M., Feng Y. F., Liu Y. T., Chang X., Chen L., Zhang H. W., et al. (2013). Integration of (1)H NMR and UPLC-Q-TOF/MS for a comprehensive urinary metabonomics study on a rat model of depression induced by chronic unpredictable mild stress. PloS One 8, e63624. 10.1371/journal.pone.0063624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugran A. K., Rawat S., Bhatt I. D., Rawal R. S. (2019). Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 33, 482–503. 10.1002/ptr.6245 [DOI] [PubMed] [Google Scholar]
- Keegan M. R., Chittiprol S., Letendre S. L., Winston A., Fuchs D., Boasso A., et al. (2016). Tryptophan Metabolism and Its Relationship with Depression and Cognitive Impairment Among HIV-infected Individuals. Int. J. Tryptophan Res. 9, 79–88. 10.4137/IJTR.S36464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke-Ke X. U., Shao-Hua L. I., Zuo C. Y., Chen C. Y. (2014). Content Determination of Chlorovaltrate and Valjatrate B in Total Iridoids of Valerianae Jatamansi Rhizoma et Radix. Chin. J. Exp. Tradit. Med. Formulae 20, 64–66. 10.13422/j.cnki.Syfjx.2014160064 [DOI] [Google Scholar]
- Klein S., Bankstahl J. P., Loscher W., Bankstahl M. (2015). Sucrose consumption test reveals pharmacoresistant depression-associated behavior in two mouse models of temporal lobe epilepsy. Exp. Neurol. 263, 263–271. 10.1016/j.expneurol.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Kodydkova J., Vavrova L., Zeman M., Jirak R., Macasek J., Stankova B., et al. (2009). Antioxidative enzymes and increased oxidative stress in depressive women. Clin. Biochem. 42, 1368–1374. 10.1016/j.clinbiochem.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Lauri I., Savorani F., Iaccarino N., Zizza P., Pavone L. M., Novellino E., et al. (2016). Development of an Optimized Protocol for NMR Metabolomics Studies of Human Colon Cancer Cell Lines and First Insight from Testing of the Protocol Using DNA G-Quadruplex Ligands as Novel Anti-Cancer Drugs. Metabolites 6 (1), 4–18. 10.3390/metabo6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M., McEntyre C. J., George P. M., Chambers S. T. (2017). Is N, N-dimethylglycine N-oxide a choline and betaine metabolite? Biol. Chem. 398 (7), 775–784. 10.1515/hsz-2016-0261 [DOI] [PubMed] [Google Scholar]
- Liu X. J., Zhou Y. Z., Li Z. F., Cui J., Li Z. Y., Gao X. X., et al. (2012). Anti-depressant effects of Xiaoyaosan on rat model of chronic unpredictable mild stress: a plasma metabonomics study based on NMR spectroscopy. J. Pharm. Pharmacol. 64, 578–588. 10.1111/j.2042-7158.2011.01412.x [DOI] [PubMed] [Google Scholar]
- Liu C.-C., Wu Y.-F., Feng G.-M., Gao X.-X., Zhou Y.-Z., Hou W.-J., et al. (2015). Plasma-metabolite-biomarkers for the therapeutic response in depressed patients by the traditional Chinese medicine formula Xiaoyaosan: A 1H NMR-based metabolomics approach. J. Affect. Disord. 185, 156–163. 10.1016/j.jad.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Lu Q., Mouri A., Yang Y., Kunisawa K., Teshigawara T., Hirakawa M., et al. (2019). Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 372, 112053. 10.1016/j.bbr.2019.112053 [DOI] [PubMed] [Google Scholar]
- Ma K., Zhang H., Wang S., Wang H., Wang Y., Liu J., et al. (2019). The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J. Cell Mol. Med. 23 (2), 7021–7028. 10.1111/jcmm.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Verkerk R., Vandoolaeghe E., Lin A., Scharpe S. (1998). Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr. Scand. 97, 302–308. 10.1111/j.1600-0447.1998.tb10004.x [DOI] [PubMed] [Google Scholar]
- Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. (2011). Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis / chronic fatigue syndrome: another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol. Lett. 32, 133–140. 10.1159/000326838 [DOI] [PubMed] [Google Scholar]
- McKean C. M., Boggs D. E., Peterson N. A. (1968). The influence of high phenylalanine and tyrosine on the concentrations of essential amino acids in brain. J. Neurochem. 15, 235–241. 10.1111/j.1471-4159.1968.tb06202.x [DOI] [PubMed] [Google Scholar]
- Mescam M., Vinnakota K. C., Beard D. A. (2011). Identification of the catalytic mechanism and estimation of kinetic parameters for fumarase. J. Biol. Chem. 286, 21100–21109. 10.1074/jbc.M110.214452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R. M., Cryan J. F. (2013). The effects of mGlu(7) receptor modulation in behavioural models sensitive to antidepressant action in two mouse strains. Behav. Pharmacol. 24, 105–113. 10.1097/FBP.0b013e32835efc78 [DOI] [PubMed] [Google Scholar]
- Peng W. H., Lo K. L., Lee Y. H., Hung T. H., Lin Y. C. (2007). Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 81, 933–938. 10.1016/j.lfs.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Pompili M., Lionetto L., Curto M., Forte A., Erbuto D., Montebovi F., et al. (2019). Tryptophan and Kynurenine Metabolites: Are They Related to Depression? Neuropsychobiology 77, 23–28. 10.1159/000491604 [DOI] [PubMed] [Google Scholar]
- Raya J., Girardi C. E. N., Esumi L. A., Ferreira L. B. T., Hipolide D. C. (2018). Multiple trial inhibitory avoidance acquisition and retrieval are resistant to chronic stress. Behav. Proces. 147, 28–32. 10.1016/j.beproc.2017.12.008 [DOI] [PubMed] [Google Scholar]
- Read J., Cartwright C., Gibson K. (2014). Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 216 (1), 67–73. 10.1016/j.psychres.2014.01.042 [DOI] [PubMed] [Google Scholar]
- Sah S. P., Mathela C. S., Chopra K. (2011). Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii Patchouli alcohol chemotype. Phytomedicine 18, 1269–1275. 10.1016/j.phymed.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Sattar Y., Wilson J., Khan A. M., Adnan M., Azzopardi Larios D., Shrestha S., et al. (2018). A Review of the Mechanism of Antagonism of N-methyl-D-aspartate Receptor by Ketamine in Treatment-resistant Depression. Cureus 10, 2652–2660. 10.7759/cureus.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T., Allegri G., Sarkissian C. N., Ying M., Grisch-Chan H. M., Rassi A., et al. (2018). Tetrahydrobiopterin treatment reduces brain L-Phe but only partially improves serotonin in hyperphenylalaninemic ENU1/2 mice. J. Inherit Metab. Dis. 41, 709–718. 10.1007/s10545-018-0150-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal H. K., Neetu (2013). A comprehensive review on Tagara (Valeriana wallichii). Ayurpharm Int. J. Ayur. Alli. Sci. 2 (5), 144–150. [Google Scholar]
- Song J., Ma W., Gu X., Zhao L., Jiang J., Xu Y., et al. (2019). Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J. Transl. Med. 17, 224. 10.1186/s12967-019-1970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B., Sperner-Unterweger B., Fuchs D., Gostner J. M. (2017). Mechanisms of Inflammation-Associated Depression: Immune Influences on Tryptophan and Phenylalanine Metabolisms. Curr. Top. Behav. Neurosci. 31, 95–115. 10.1007/7854_2016_23 [DOI] [PubMed] [Google Scholar]
- Surmacki J., Brozek-Pluska B., Kordek R., Abramczyk H. (2015). The lipid-reactive oxygen species phenotype of breast cancer. Raman spectroscopy and mapping, PCA and PLSDA for invasive ductal carcinoma and invasive lobular carcinoma. Molecular tumorigenic mechanisms beyond Warburg effect. Analyst 140, 2121–2133. 10.1039/c4an01876a [DOI] [PubMed] [Google Scholar]
- Sussulini A., Prando A., Maretto D. A., Poppi R. J., Tasic L., Banzato C., et al. (2009). Metabolic Profiling of Human Blood Serum from Treated Patients with Bipolar Disorder Employing\\r 1\\r H NMR Spectroscopy and Chemometrics. Anal. Chem. 81 (23), 9755–9763. 10.1021/ac901502j [DOI] [PubMed] [Google Scholar]
- Tian J. S., Xia X. T., Wu Y. F., Zhao L., Xiang H., Du G. H., et al. (2016). Discovery, screening and evaluation of a plasma biomarker panel for subjects with psychological suboptimal health state using (1)H-NMR-based metabolomics profiles. Sci. Rep. 6, 33820. 10.1038/srep33820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J. S., Liu S. B., He X. Y., Xiang H., Chen J. L., Gao Y., et al. (2018). Metabolomics studies on corticosterone-induced PC12 cells: a strategy for evaluating an in vitro depression model and revealing the metabolic regulation mechanism. Neurotoxicol. Teratol. 69, 27–38. 10.1016/j.ntt.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Toolika E., Bhat N. P., Shetty S. K. (2015). A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia). Ayu 36, 46–49. 10.4103/0974-8520.169008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Shi J. L., Yong L., Ren Z., Zhai Y. J., Guo J. Y. (2012). Anxiolytic-like effects of compound zhi zhu xiang in rats. Evid. Based Complement. Alternat. Med. 2012, 701289. 10.1155/2012/701289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhuis J. A., van Velzen E. J., Hoefsloot H. C., Smilde A. K. (2010). Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 6, 119–128. 10.1007/s11306-009-0185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. (2017). The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 6, 78–93. 10.1016/j.ynstr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfender J.-L., Rudaz S., Hae Choi Y., Kyong Kim H. (2013). Plant metabolomics: from holistic data to relevant biomarkers. Curr. Med. Chem. 20 (8), 1056–1090. 10.2174/0929867311320080009 [DOI] [PubMed] [Google Scholar]
- Wolfender J.-L., Marti G., Thomas A., Bertrand S. (2015). Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 1382, 136–164. 10.1016/j.chroma.2014.10.091 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2018). Depression: Key Facts. Retrieved from http://www.who.int/en/news-room/fact-sheets/detail/depression [Google Scholar]
- Xiong Z., Yang J., Huang Y., Zhang K., Bo Y., Lu X., et al. (2016). Serum metabonomics study of anti-depressive effect of Xiao-Chai-Hu-Tang on rat model of chronic unpredictable mild stress. J. Chromatogr. B. Analyt. Technol. BioMed. Life Sci. 1029-1030, 28–35. 10.1016/j.jchromb.2016.06.044 [DOI] [PubMed] [Google Scholar]
- Yamada K., Tsuji T., Kunieda T. (2013). Phenotypic characterization of Ggt1(dwg/dwg) mice,a mouse model for hereditary gamma-glutamyltransferase deficiency. Exp. Anim. 62, 151–157. 10.1538/expanim.62.151 [DOI] [PubMed] [Google Scholar]
- Yan Z., Zhang T.E., Xiao T., Pan L., Qin J., Zhang Z., et al. (2010). Anti-anxiety effect of Valeriana jatamansi Jones extract via regulation of the hypothalamus-pituitary-adrenal axis. Neural Regen Res. 5 (14), 1071–1075. 10.3969/j.issn.1673-5374.2010.14.006 [DOI] [Google Scholar]
- Yeung W. F., Chung K. F., Poon M. M., Ho F. Y., Zhang S. P., Zhang Z. J., et al. (2012). Prescription of chinese herbal medicine and selection of acupoints in pattern-based traditional chinese medicine treatment for insomnia: a systematic review. Evid. Based Complement. Alternat. Med. 2012, 902578. 10.1155/2012/902578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafir A., Banu N. (2009). Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J. Biochem. Biophys. 46, 53–58. 10.1007/s00249-008-0372-2 [DOI] [PubMed] [Google Scholar]
- Zhang Y.-W., Cheng Y.-C. (2019). Challenge and prospect of traditional chinese medicine in depression treatment. Front. Neurosci. 13, 190. 10.3389/fnins.2019.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu H., Wu J., Zhang X., Liu M., Wang Y. (2009). Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem. Int. 54, 481–487. 10.1016/j.neuint.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Zhu J. L., Sun Y., Dai Y. L., Chen X., Cao J. H., et al. (2018). Anxiolytic potency of iridoid fraction extracted from Valeriana jatamansi Jones and its mechanism: a preliminary study. Nat. Prod. Res. 32, 2071–2075. 10.1080/14786419.2017.1360881 [DOI] [PubMed] [Google Scholar]
- Zhao J., Jung Y. H., Jang C. G., Chun K. H., Kwon S. W., Lee J. (2015. a). Metabolomic identification of biochemical changes induced by fluoxetine and imipramine in a chronic mild stress mouse model of depression. Sci. Rep. 5, 8890. 10.1038/srep08890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Xiong Z., Lu X., Zheng S., Wang F., Ge L., et al. (2015. b). Metabonomic Evaluation of Chronic Unpredictable Mild Stress-Induced Changes in Rats by Intervention of Fluoxetine by HILIC-UHPLC/MS. PloS One 10, e0129146. 10.1371/journal.pone.0129146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Xiong Z., Lu X., Zheng S., Wang F., Ge L., et al. (2015. c). Metabonomic evaluation of chronic unpredictable mild stress-induced changes in rats by intervention of fluoxetine by HILIC-UHPLC/MS. PloS One 10 (6), 1–13. 10.1371/journal.pone.0129146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zeisel S. H., Zhang S. (2015. d). Rapid LC-MRM-MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N-oxide, and creatinine in human plasma and urine. Electrophoresis 36, 2207–2214. 10.1002/elps.201500055 [DOI] [PubMed] [Google Scholar]
- Zheng S., Zhang S., Yu M., Tang J., Lu X., Wang F., et al. (2011). An 1 H NMR and UPLC–MS-based plasma metabonomic study to investigate the biochemical changes in chronic unpredictable mild stress model of depression. Metabolomics 7 (3), 413–423. 10.1007/s11306-010-0261-4 [DOI] [Google Scholar]
- Zhou C., Jia H. M., Liu Y. T., Yu M., Chang X., Ba Y. M., et al. (2016). Metabolism of glycerophospholipid, bile acid and retinol is correlated with the early outcomes of autoimmune hepatitis. Mol. Biosyst. 12, 1574–1585. 10.1039/c6mb00092d [DOI] [PubMed] [Google Scholar]
- Zhou X., Liu L., Zhang Y., Pu J., Yang L., Zhou C., et al. (2017). Metabolomics identifies perturbations in amino acid metabolism in the prefrontal cortex of the learned helplessness rat model of depression. Neuroscience 343, 1–9. 10.1016/j.neuroscience.2016.11.038 [DOI] [PubMed] [Google Scholar]
- Zhu J., Xu K., Zhang X., Cao J., Jia Z., Yang R., et al. (2016). Studies on the regulation of lipid metabolism and its mechanism of the iridoids rich fraction in Valeriana jatamansi Jones. Biomed. Pharmacother. 84, 1891–1898. 10.1016/j.biopha.2016.10.099 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are available on request to the corresponding author.