The coronary sinus (CS) Reducer (Neovasc, Inc, Richmond, BC, Canada) is a percutaneous device aiming at symptoms control in patients suffering from refractory angina. Its clinical effect1 may be exerted through flow redistribution toward ischemic territories as a consequence of increased coronary drainage pressure resulting from CS narrowing.2 Current imaging evidences are limited.1,2 We evaluated the impact of the Reducer upon regional myocardial ischemia in patients with refractory angina using stress Cardiac Magnetic Resonance (stress-CMR).
Consecutive patients with RA1 and evidence of inducible ischemia involving at least 1 myocardial segment at stress-CMR were included. The study was approved by the institutional review committee, and all patients gave informed consent. Study database is available from the corresponding author on request.
Stress-CMR was performed at baseline and 4 months after reducer implantation at 1.5T (Philips Ingenia, Best, The Netherlands). First-pass perfusion was performed using a saturation-recovery prepared balanced steady-state free precession for 3 short-axis slices within each cardiac cycle (45-dynamics). Stress was induced with dipyridamole (0.56–0.84 mg/kg in 4–6 minutes). Ischemic burden was defined as the percentage of LV wall involved by inducible perfusion defect (IPD). Visual inducible perfusion defect was scored according to AHA 16-segment model and transmurality (1=1%–25%; 2=25%–50%; 3=51%–75%; 4=>75%).
Segmental myocardial perfusion reserve index (MPRI) was calculated (CVI42, Circle Cardiovascular Imaging, Inc, Canada) according to myocardial layers (subendocardial, mesocardial, subepicardial, and transmural). MPRI<1.3 defined severe ischemia.
Patient-level comparisons were carried by paired Wilcoxon signed-rank test. Segment-level ΔMPRI data were analyzed using linear mixed-effects models with random intercept per patient. Two models were defined: to compare ΔMPRI between ischemic and nonischemic segments and among the 3 myocardial layers.
Two patients were excluded for poor image quality at baseline CMR. Final population included 15 patients (93.3% males; age 66 [IQR, 58.5–74] years; ejection fraction 57% [55.0–62.5]; 3-vessel disease 86.7%; previous percutaneous (66.6%); and surgical (93.3%) coronary revascularization; Canadian Cardiovascular Society class 3 [3-3]; number of anti-ischemic drugs 3 [3-3]).
Four months following reducer implantation, 13 (86.7%) patients improved by at least 1 Canadian Cardiovascular Society class (from 3 [3-3] to 1 [1–2]; P=0.001). At CMR, median per-patient ischemic burden reduced from 13.00% to 10.88% (P=0.0092) and the number of segments with inducible perfusion defect from 6 (2–9) to 5 (2–6; P=0.0138). The overall number of segments with inducible perfusion defect reduced from 92/240 (38%) to 69/240 (29%; P<0.001, by logistic mixed-effects model). Reducer implantation led to a significant increase in transmural MPRI (P<0.001; Figure A and B), driven by the ischemic segments (predicted ΔMPRI=0.355 in segments with baseline MPRI<1.3 and =−0.036 in segments with baseline MPRI≥1.3, P<0.001; Figure C and D).
Figure.
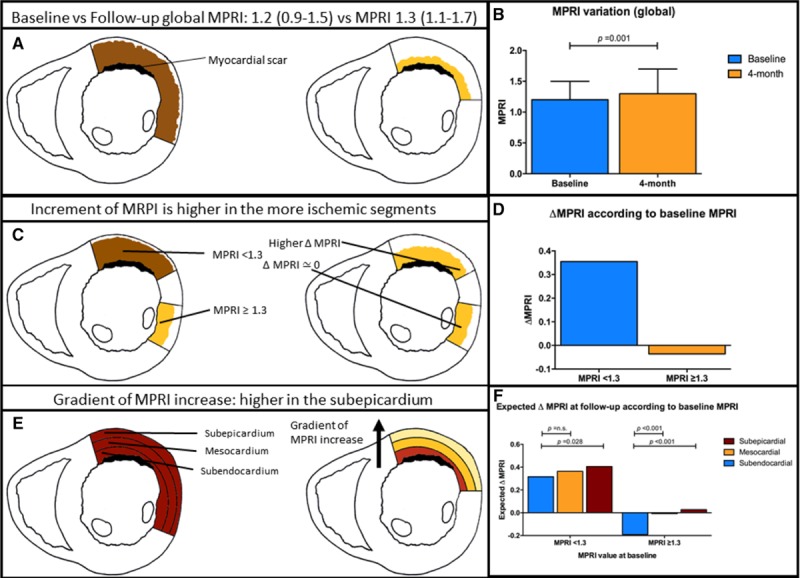
The impact of the coronary sinus reducer upon myocardial perfusion. A and B, Improvement of global myocardial perfusion reserve index (MPRI). C and D, Gradient of ischemia improvement across myocardial layers. E and F, Improvement according to the ischemic status of a segment.
The magnitude of the MPRI increase in the ischemic segments followed a transmural gradient (Figure E and F): ΔMPRI=0.3156, 0.3634, and 0.4057 for the subendocardial, mesocardial, and subepicardial layers (ΔMPRI endocardial versus mesocardial: P=n.s.; ΔMPRI endocardial versus subepicardial: P=0.0282).
These results demonstrate that Reducer decreases the ischemic burden, providing a strong physiological base underlying its clinical efficacy.
This is the first study to examine the physiological effects of Reducer in refractory angina patients using stress-CMR, which allows to subanalyze by-layers perfusion patterns, potentially providing new insight into Reducer’s mechanisms of action.
The most accepted mechanism of action of Reducer is blood redistribution from the less ischemic subepicardium to the more ischemic subendocardium.2
In the present study, improvement in myocardial ischemia was present in all myocardial layers, and more pronounced in the subepicardium as compared with the subendocardium. This observation calls for different mechanisms underlying Reducer effects. The blood redistribution mechanism may still be prevalent, though occurring from less ischemic segments to more ischemic ones, within the same layer, rather than with a by-layers pattern. The observation of slight, even if nonsignificant, MPRI reduction in nonischemic segments is possibly consistent with this concept.
Several mechanisms may potentially explain the observed transmural gradient in MPRI improvement: (1) the pressure increase established by CS narrowing may be greater in the subepicardium because of the progressive pressure dissipation backward from the site of Reducer implant; (2) the extravascular pressure load to which the ischemic subendocardium is subject may counteract the intravascular pressure boost established by the Reducer; (3) the Thebesian venous system, which is more represented in the subendocardium, may short-circuit the CS through direct drainage in the left ventricle, potentially blunting Reducer effect.3 Last, in segment with acutely induced ischemia, resting myocardial blood flow was demonstrated to substantially increase in the subendocardium (and only mildly in other layers) during intermittent CS occlusion.4 If this would be occur also in chronic CS narrowing and chronic ischemia, it could have an impact on the measured subendocardial MPRI.
While limited by the small sample size, our hypothesis-generating study provides the basis for further mechanistic analyses aimed at better characterization of Reducer functioning, potentially translating in better patient selection and clinical outcomes.
Disclosures
Dr Giannini is a consultant for Neovasc, Inc. The other authors report no conflicts.
References
- 1.Giannini F, Baldetti L, Konigstein M, Rosseel L, Ruparelia N, Gallone G, Colombo A, Banai S, Verheye S. Safety and efficacy of the reducer: a multi-center clinical registry - REDUCE study. Int J Cardiol. 2018;269:40–44. doi: 10.1016/j.ijcard.2018.06.116 [DOI] [PubMed] [Google Scholar]
- 2.Konigstein M, Bazan S, Revivo M, Banai S. Coronary Sinus Reducer implantation improves symptoms, ischaemia and physical capacity in patients with refractory angina unsuitable for myocardial revascularisation: a single-centre experience. EuroIntervention. 2018;14:e452–e458. doi: 10.4244/EIJ-D-18-00102 [DOI] [PubMed] [Google Scholar]
- 3.Ansari A. Anatomy and clinical significance of ventricular Thebesian veins. Clin Anat. 2001;14:102–110. doi: 10.1002/1098-2353(200103)14:2<102::AID-CA1018>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 4.Ido A, Hasebe N, Matsuhashi H, Kikuchi K. Coronary sinus occlusion enhances coronary collateral flow and reduces subendocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H1361–H1367. doi: 10.1152/ajpheart.2001.280.3.H1361 [DOI] [PubMed] [Google Scholar]


