Abstract
Background:
The ratios of tricuspid annular plane systolic excursion (TAPSE)/echocardiographically measured systolic pulmonary artery pressure (PASP), fractional area change/invasively measured mean pulmonary artery pressure, right ventricular (RV) area change/end-systolic area, TAPSE/pulmonary artery acceleration time, and stroke volume/end-systolic area have been proposed as surrogates of RV-arterial coupling. The relationship of these surrogates with the gold standard measure of RV-arterial coupling (invasive pressure-volume loop-derived end-systolic/arterial elastance [Ees/Ea] ratio) and RV diastolic stiffness (end-diastolic elastance) in pulmonary hypertension remains incompletely understood. We evaluated the relationship of these surrogates with invasive pressure-volume loop-derived Ees/Ea and end-diastolic elastance in pulmonary hypertension.
Methods:
We performed right heart echocardiography and cardiac magnetic resonance imaging 1 day before invasive measurement of pulmonary hemodynamics and single-beat RV pressure-volume loops in 52 patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. The relationships of the proposed surrogates with Ees/Ea and end-diastolic elastance were evaluated by Spearman correlation, multivariate logistic regression, and receiver operating characteristic analyses. Associations with prognosis were evaluated by Kaplan-Meier analysis.
Results:
TAPSE/PASP, fractional area change/mean pulmonary artery pressure, RV area change/end-systolic area, and stroke volume/end-systolic area but not TAPSE/pulmonary artery acceleration time were correlated with Ees/Ea and end-diastolic elastance. Of the surrogates, only TAPSE/PASP emerged as an independent predictor of Ees/Ea (multivariate odds ratio: 18.6; 95% CI, 0.8–96.1; P=0.08). In receiver operating characteristic analysis, a TAPSE/PASP cutoff of 0.31 mm/mm Hg (sensitivity: 87.5% and specificity: 75.9%) discriminated RV-arterial uncoupling (Ees/Ea <0.805). Patients with TAPSE/PASP <0.31 mm/mm Hg had a significantly worse prognosis than those with higher TAPSE/PASP.
Conclusions:
Echocardiographically determined TAPSE/PASP is a straightforward noninvasive measure of RV-arterial coupling and is affected by RV diastolic stiffness in severe pulmonary hypertension.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03403868.
Keywords: acceleration, echocardiography, hypertension, magnetic resonance imaging, pressure
Clinical Perspective.
The gold standard for assessment of right ventricular (RV)-arterial coupling is measurement of the end-systolic/arterial elastance (Ees/Ea) ratio from invasive pressure-volume loops. However, this approach is technically demanding, expensive, and unpractical at the bedside, and noninvasive surrogates of Ees/Ea are therefore being sought. The ratio of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP, measured by echocardiography) has been used as a surrogate of Ees/Ea but has not yet been validated against invasive pressure-volume loop-derived Ees/Ea. The current study is the first to evaluate the relationship of TAPSE/PASP and other proposed surrogates of Ees/Ea with invasive pressure-volume loop-derived Ees/Ea and RV diastolic stiffness (end-diastolic elastance). In 52 patients with severe pulmonary hypertension, TAPSE/PASP and other echocardiographic surrogates were correlated with Ees/Ea and end-diastolic elastance but only TAPSE/PASP emerged as an independent predictor of Ees/Ea in multivariate analysis. TAPSE/PASP <0.31 mm/mm Hg predicted RV-arterial uncoupling (defined as Ees/Ea <0.805) in the study cohort and was associated with a poor prognosis in both the study cohort and an external validation cohort of 193 patients. These results show that TAPSE/PASP is a clinically relevant, straightforward, noninvasive measure of RV-arterial coupling that also provides information on RV diastolic stiffness in severe pulmonary hypertension. Both TAPSE and PASP are easily measured by a standard bedside echocardiographic examination. Further studies are needed to evaluate the possible added value of TAPSE/PASP in current risk assessment strategies for severe pulmonary hypertension.
Introduction
See Editorial by Bashline and Simon
Right ventricular (RV) function is the main determinant of symptomatology and outcome in severe pulmonary hypertension (PH).1,2 The RV adapts to increased afterload in PH by increasing contractility to preserve RV-arterial coupling and flow output response to peripheral demand. When this homeometric (ie, contractility) adaptation is exhausted, the RV relies on a heterometric (ie, dimension or Starling law) adaptation, resulting in increased filling pressures, dilatation, negative ventricular interaction, and systemic congestion.3 The gold standard metric of contractility is pressure-volume loop-derived end-systolic elastance (Ees), and RV-arterial coupling is assessed as a ratio of end-systolic to arterial elastances (Ees/Ea).1–3 We recently showed that RV-arterial coupling has considerable reserve, as the Ees/Ea ratio has to decrease from 1.5 to 2 to 0.8 before the RV volume increases above normal and right heart failure may be diagnosed.4 Thus the Ees/Ea metric could help to anticipate and possibly prevent right heart failure in severe PH.1,2
However, measuring Ees and Ea via pressure-volume loops is invasive, technically demanding, and expensive. Therefore, simpler noninvasive surrogates are being sought. One surrogate is the Doppler echocardiography measurement of the tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP) ratio.5 TAPSE/PASP is a potent independent predictor of precapillary PH and prognosis in heart failure,5–10 with a prognostic cutoff value of 0.36 mm/mm Hg.11 TAPSE/PASP is also an independent predictor of outcome in pulmonary arterial hypertension (PAH).12 Initially regarded as an indirect assessment of the ventricular length-tension relationship,5 TAPSE/PASP has been considered a surrogate of Ees/Ea based on the assumption that TAPSE estimates contractility and PASP estimates afterload.6–12 To what extent this assumption is correct has not yet been investigated.
We, therefore, assessed the relationship of echocardiographic TAPSE/PASP with Ees/Ea in severe PH. We also evaluated other suggested surrogates, including the ratios of RV fractional area change (FAC) to mean pulmonary artery pressure (mPAP, invasively measured),13–15 RV area change to RV end-systolic area (ESA),16 TAPSE to pulmonary artery acceleration time (PAAT),17 and stroke volume (SV) to ESA (derived by dividing PASP/ESA as a surrogate of Ees18,19 by PASP/SV as a surrogate of Ea).9 Because RV diastolic function may be altered independently of Ees or Ees/Ea and is associated with disease severity and outcome in PAH,20,21 we also assessed the relationship of the surrogates with end-diastolic elastance (Eed).
Methods
The raw data that underpin this study are available from the corresponding author on reasonable request.
Study Design and Patients
The current analysis included 52 consecutive patients with PAH and chronic thromboembolic PH who were prospectively enrolled into the Right Heart I study and the Giessen PH Registry22 between January 2016 and June 2018 (study cohort; Figure I in the Data Supplement). Forty-two of the patients have been reported previously4,23,24 and were reanalyzed for the present study. The patients were diagnosed according to current guidelines,25 with a multidisciplinary board (including pulmonologists and radiologists) assessing each diagnosis before enrollment. All patients underwent right heart echocardiography and cardiac magnetic resonance imaging 1 day before pressure-volume/Swan-Ganz catheterization and received targeted PAH therapies based on clinical evaluation and best standard of care. All participating patients gave written informed consent for enrollment into the Right Heart I study.
Only for survival analysis, a separate group of 193 patients with idiopathic PAH (from a previously reported cohort of 290 patients with PAH)12 was analyzed retrospectively as an external validation cohort.
The investigation conforms with the principles of the Declaration of Helsinki and was approved by the ethics committee of the Faculty of Medicine at the University of Giessen (Approval No. 108/15). All authors had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Imaging
Doppler echocardiography was performed according to current guidelines,26 using Vivid E9 and Vivid S5 systems (GE Healthcare, Wauwatosa, WI). RV area change/ESA was calculated as (end-diastolic area−ESA)/ESA.16 Cardiac magnetic resonance imaging of RV volumes was performed with the Avanto 1.5 Tesla scanner system (Siemens Healthineers, Erlangen, Germany; gradient strength and slew rate: SQ-Engine (45mT/m @ 200 T/m/s).27
Right Heart Catheterization
All patients underwent right heart catheterization by insertion of a Swan-Ganz catheter via the internal jugular vein using an 8F introducer sheath. Pressure values were continuously assessed. Cardiac index was measured using the direct or indirect Fick method as available. Pulmonary vascular resistance was calculated as (mPAP−pulmonary artery wedge pressure)/cardiac output.
Pressure-Volume Catheterization
We inserted a 4F pressure-volume catheter (CA-Nr 41063, CD Leycom, Zoetermeer, the Netherlands) via the same 8F introducer sheath as above, and positioned the tip of the catheter in the RV apex with guidance from transthoracic echocardiography and online pressure-volume loops.4 An intracardiac analyzer (Inca, CD Leycom, Zoetermeer, the Netherlands) was used to display real-time, beat-to-beat pressure-volume loops. Ees and Ea were calculated using the RV single-beat method.28 RV Eed was calculated from a curvilinear adjustment of end-systolic and end-diastolic pressure/volume ratios to generate a β-coefficient of stiffness.20,29 We calibrated volume measurements with cardiac magnetic resonance imaging.
Outcomes
The study cohort was followed prospectively until March 20, 2019. We evaluated clinical worsening in the study cohort and overall survival in the external validation cohort. Clinical worsening was defined as any of the following: (1) a reduction in exercise capacity (−15% compared with the baseline 6-minute walk test); (2) worsening in World Health Organization functional class; or (3) clinical deterioration requiring hospital admission (need for new PAH therapies, intravenous diuretics, lung transplantation, or death).
Statistical Analyses
Adherence to a gaussian distribution was determined using the Kolmogorov-Smirnov test and visual assessment of histograms. Variables with a non-normal distribution were ln-transformed and then rechecked with the Kolmogorov-Smirnov test. Differences in echocardiographic parameters dichotomized at an Ees/Ea cutoff of 0.805 (defined as the threshold at which RV-arterial uncoupling begins)4 were evaluated using the independent t test or independent Mann-Whitney U test. Interobserver and intraobserver variability of echocardiographic measurements were assessed in 10 randomly selected patients using intraclass correlation coefficients. Coefficient of variation was defined as the SD of the difference between the 2 measurements (or observers) divided by their mean value times 100.30 TAPSE/PASP (measured echocardiographically) was compared with TAPSE/invasive systolic pulmonary arterial pressure (assessed by right heart catheterization) using Bland-Altman analysis.31
Associations of surrogates for RV-arterial coupling (TAPSE/PASP, FAC/mPAP, RV area change/ESA, TAPSE/PAAT, and SV/ESA, all measured by echocardiography except mPAP [right heart catheterization] and SV [cardiac magnetic resonance]) with pressure-volume variables (Ees, Ees/Ea, Eed, and Ea) were measured with Spearman rank correlation coefficient; trend lines were least-squares fits of straight-line models. The relationship of Eed with TAPSE/PASP was also evaluated by stratifying data according to TAPSE/PASP tertile, as done previously9,12; between-group differences were analyzed with the Kruskal-Wallis test. For these analyses, P<0.05 was considered statistically significant.
To determine which surrogate is most strongly related to Ees/Ea, all surrogates were included in a univariate logistic binary regression analysis as continuous variables. Surrogates that were significant in the univariate analysis were added into a multivariate model (P<0.10 was considered statistically significant for this analysis). Multicollinearity was assessed using the variance inflation factor. Receiver operating characteristic curves were used to identify the most powerful surrogate for discriminating RV-arterial uncoupling (Ees/Ea <0.805). Area under the curves were compared using the methodology of Delong and coworkers.32 Cox proportional-hazards regression models and Kaplan-Meier analyses with log-rank tests were used to assess the prognostic relevance of the TAPSE/PASP ratio. For the multivariate prognostic models, variable selection was based on clinical significance.
MedCalc version 18.11.6 (MedCalc Software, Belgium) was used to compare receiver operating characteristic area under the curves. SPSS, version 23.0 (IBM, Armonk, NY) was used for all other statistical analyses.
Results
Study Cohort
Most of the 52 included patients presented with idiopathic PAH (Table 1). Pulmonary hemodynamics were impaired, and most of the patients presented in World Health Organization functional class III (n=28). Pressure-volume loop measurements, surrogates for RV-arterial coupling, and other echocardiographic and cardiac magnetic resonance data are shown in Table 2. Cardiac magnetic resonance measurements revealed substantial RV dilatation and hypertrophy, with decreased ejection fraction. Echocardiography revealed a preserved TAPSE and RV systolic free wall myocardial velocity, substantially elevated PASP, impaired FAC, and high right atrial and RV dimensions. Patients with Ees/Ea <0.805 showed significantly increased right atrial and RV dilatation, impaired TAPSE, and lower values for all surrogates of RV-arterial coupling except TAPSE/PAAT compared with patients with Ees/Ea ≥0.805 (Table I in the Data Supplement).
Table 1.
Clinical Characteristics of the Study Cohort
| Patients With PH (n=52) | |
|---|---|
| Male/female | 26/26 |
| Age, y | 54±14 |
| PH subtype | |
| Idiopathic pulmonary arterial hypertension | 34 (65) |
| Heritable pulmonary arterial hypertension | 2 (4) |
| Pulmonary arterial hypertension associated with | 9 (17) |
| HIV infection | 1 |
| Portal hypertension | 4 |
| Connective tissue disease | 3 |
| Congenital heart disease | 1 |
| Chronic thromboembolic PH | 6 (12) |
| Pulmonary veno-occlusive disease | 1 (2) |
| Treatment | |
| Phosphodiesterase type 5 inhibitor | 26 (50) |
| Endothelin receptor antagonist | 30 (58) |
| Soluble guanylate cyclase stimulator | 16 (31) |
| Prostanoid | 14 (27) |
| Combination therapy | |
| Dual therapy | 16 (31) |
| Triple therapy | 13 (25) |
| World Health Organization functional class* | |
| I | 3 (6) |
| II | 17 (34) |
| III | 28 (56) |
| IV | 2 (4) |
| Right heart catheterization | |
| Mean pulmonary artery pressure, mm Hg | 47 [35–54] |
| Right atrial pressure, mm Hg | 8±4 |
| Pulmonary vascular resistance, Wood Units | 6.9 [4.4–10.2] |
| Cardiac index, L/min per m2 | 2.8±0.7 |
| Pulmonary artery wedge pressure, mm Hg | 9±3 |
Values represent n, n/n, n (%), mean±SD or median [interquartile range]. PH indicates pulmonary hypertension.
n=50.
Table 2.
RV Pressure-Volume Loop Measurements and Echocardiographic and Cardiac Magnetic Resonance Imaging-Derived Parameters in the Study Cohort
| Patients With PH (n=52) | |
|---|---|
| RV pressure-volume loop measurements | |
| Ea, mm Hg/mL | 0.77 [0.46–1.09] |
| Ees, mm Hg/mL | 0.49 [0.32–0.73] |
| Ees/Ea ratio | 0.70 [0.47–1.02] |
| Eed, mm Hg/mL | 0.14 [0.06–0.24] |
| Surrogates for RV-arterial coupling and contractility | |
| TAPSE/PASP, mm/mm Hg* | 0.28 [0. 19–0.42] |
| FAC/mPAP, %/mm Hg† | 0.45 [0.25–0.77] |
| RV area change/ESA† | 0.26 [0.14–0.41] |
| TAPSE/PAAT, mm/ms† | 0.27[0.25–0.37] |
| PASP/ESA, mm Hg/cm2 | 3.0 [1.4–5.8] |
| SV/ESA, mL/cm2† | 3.5 [2.3–4.1] |
| Cardiac magnetic resonance measurements | |
| RV end-diastolic volume/body surface area, mL/m2 | 111 [88–144] |
| RV mass diastolic/body surface area, g/m2 | 38 [28–52] |
| RV ejection fraction, % | 37±13 |
| SV, mL | 71 [59–95] |
| Echocardiographic data | |
| TAPSE, mm | 21 [18–25] |
| PASP, mm Hg* | 75±24 |
| Right atrial size, cm2† | 21 [17–26] |
| RV basal diameter, cm† | 4.9 [4.3–5.4] |
| RV systolic free wall myocardial velocity, cm/s‡ | 12 [10–13] |
| FAC, %† | 21±11 |
| PAAT, ms† | 74 [59–89] |
| ESA, cm2† | 24 [19–30] |
Values represent mean±SD or median [interquartile range]. Ea indicates arterial elastance; Eed, end-diastolic elastance; Ees, end-systolic elastance; ESA, end-systolic area; FAC, fractional area change; mPAP, mean pulmonary artery pressure (assessed by right heart catheterization); PAAT, pulmonary artery acceleration time; PASP, systolic pulmonary artery pressure; PH, pulmonary hypertension; RV, right ventricular; SV, stroke volume (assessed by cardiac magnetic resonance); and TAPSE, tricuspid annular plane systolic excursion.
n=45.
n=47.
n=50.
Intraclass correlation coefficients for interobserver and intraobserver comparisons in key echocardiographic measurements are shown in Table II in the Data Supplement. The overall agreement between TAPSE/PASP (echocardiographic) and TAPSE/systolic pulmonary arterial pressure (invasive) was good (mean bias: 0.0176 mm/mm Hg; 95% CI, −0.0087 to 0.043 mm/mm Hg; Figure II in the Data Supplement).
Surrogates of RV-Arterial Coupling and Their Association With Pressure-Volume Loop Measurements
Ees/Ea, Ea, and Eed were correlated with TAPSE/PASP, FAC/mPAP, RV area change/ESA, and SV/ESA (Figures 1 and 2) but not with TAPSE/PAAT (transformed or not; data not shown). None of the surrogates were correlated with Ees. Eed increased substantially from TAPSE/PASP tertile 3 to tertile 1 (Figure 3).
Figure 1.
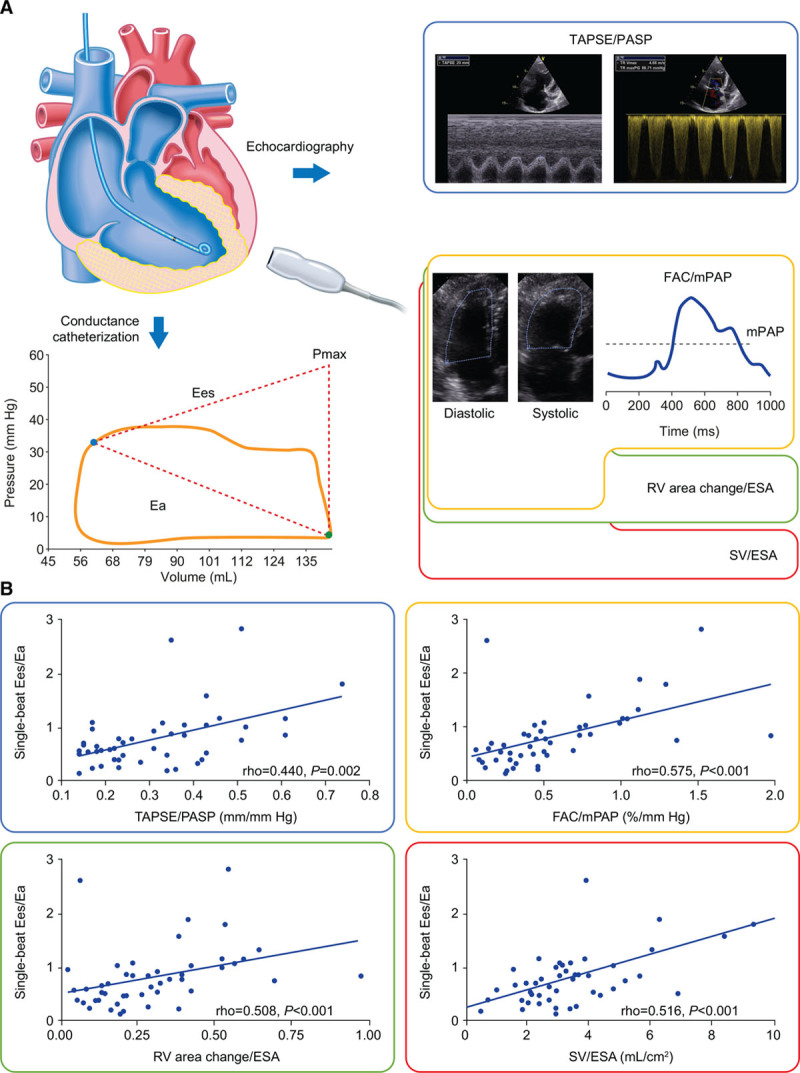
Surrogates for right ventricle (RV)-arterial coupling and their association with single-beat end-systolic elastance (Ees)/arterial elastance (Ea) in patients with pulmonary hypertension. A, Surrogates were compared with data obtained from invasively measured single-beat pressure-volume loops in the study cohort. B, Tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP), fractional area change (FAC)/mean pulmonary artery pressure (mPAP), RV area change/end-systolic area (ESA), and stroke volume (SV)/ESA (all measured by echocardiography except mPAP [right heart catheterization] and SV [cardiac magnetic resonance]) showed significant associations with invasively measured single-beat Ees/Ea. No association of Ees/Ea with TAPSE/pulmonary artery acceleration time (ρ=−0.016; P=0.926) and PASP/ESA (ρ=−0.015; P=0.924) was evident. Pmax indicates maximum pressure of an isovolumic beat.
Figure 2.
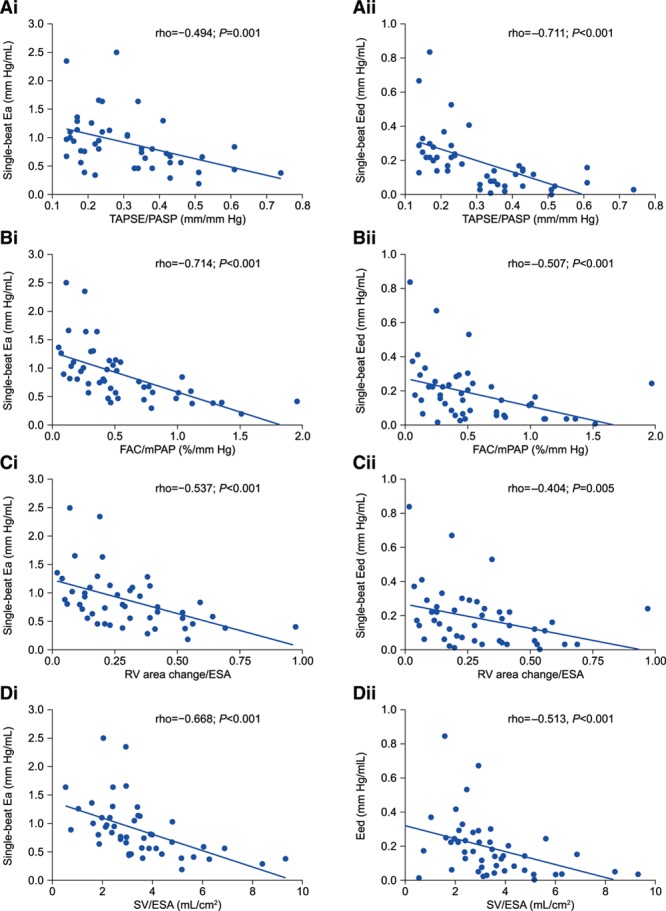
Association of surrogates for right ventricle (RV)-arterial coupling with RV single-beat arterial elastance (Ea) and end-diastolic elastance (Eed) in patients with pulmonary hypertension. A, Tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP), (B) Fractional area change (FAC)/mean pulmonary artery pressure (mPAP), (C) RV area change/end-systolic area (ESA), and (D) stroke volume (SV)/ESA (all measured by echocardiography except mPAP [right heart catheterization] and SV [cardiac magnetic resonance]) showed significant associations with i, Ea and ii, Eed in the study cohort. Ea and Eed showed no significant association with TAPSE/pulmonary artery acceleration time (ρ=−0.072, P=0.631 and ρ=−0.051, P=0.734, respectively) and PASP/ESA (ρ=0.118, P=0.452 and ρ=0.085, P=0.589, respectively).
Figure 3.

Stratification of end-diastolic elastance (Eed) according to tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP) tertile in patients with pulmonary hypertension. Eed showed significant differences across tertiles of the echocardiographic TAPSE/PASP ratio in the study cohort (Kruskal-Wallis test). Boxes show median and interquartile range; whiskers show minimum to maximum.
Relevance of Surrogates for Prediction of RV-Arterial Coupling and Prognosis
In univariate logistic regression analysis, TAPSE/PASP, FAC/mPAP, RV area change/ESA, and SV/ESA were associated with Ees/Ea dichotomized at 0.805 (Table 3). Using multivariate analysis, only TAPSE/PASP (β-coefficient: 2.154) remained associated (Table 3). Using receiver operating characteristic analyses and the Youden index, we identified cutoffs of 0.31 mm/mm Hg for TAPSE/PASP, 0.71%/mm Hg for FAC/mPAP, and 2.93 mL/cm2 for SV/ESA to discriminate RV-arterial uncoupling (Ees/Ea <0.805; Figure 4). We found no significant differences in area under the curve between the surrogates (TAPSE/PASP versus FAC/mPAP, P=0.5334; TAPSE/PASP versus SV/ESA, P=0.3405; and FAC/mPAP versus SV/ESA, P=0.7462).
Table 3.
Logistic Binary Regression Analysis of the Association of Surrogates for RV-Arterial Coupling and Contractility with Ees/Ea Dichotomized at 0.805 in the Study Cohort
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| TAPSE/PASP | 27.0 | 3.5–205.9 | 0.001 | 8.6 | 0.8–96.1 | 0.080 |
| FAC/mPAP | 4.2 | 1.5–11.5 | 0.005 | 9.0 | 0.2–318.0 | 0.251 |
| RV area change/ESA | 2.8 | 1.1–7.1 | 0.028 | 6.9 | 0.2–250.0 | 0.292 |
| TAPSE/PAAT | 3.4 | 0.4–33.0 | 0.288 | … | … | … |
| PASP/ESA | 1.1 | 0.6–2.0 | 0.882 | … | … | … |
| SV/ESA | 1.9 | 1.2–3.1 | 0.010 | 1.1 | 0.7–2.0 | 0.632 |
Displayed parameters were measured by echocardiography except mPAP (right heart catheterization) and SV (cardiac magnetic resonance). Ea indicates arterial elastance; Ees, end-systolic elastance; ESA, end-systolic area; FAC, fractional area change; mPAP, mean pulmonary artery pressure; PAAT, pulmonary artery acceleration time; PASP, systolic pulmonary artery pressure; RV, right ventricular; SV, stroke volume; and TAPSE, tricuspid annular plane systolic excursion.
Figure 4.
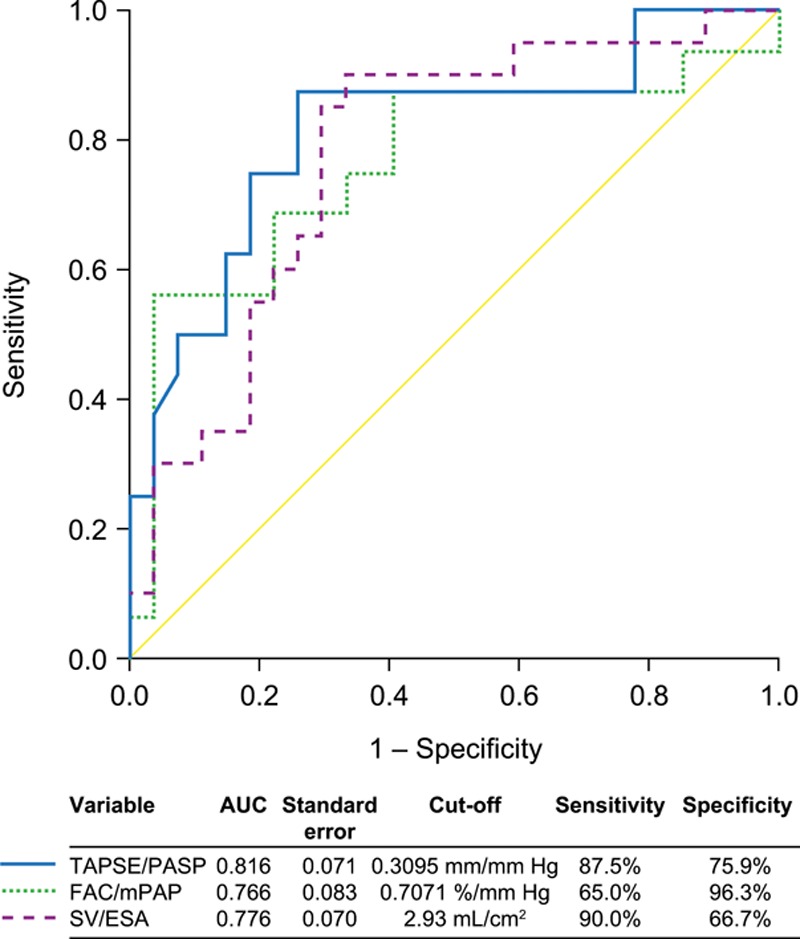
Receiver operating characteristic curve analysis of tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP), fractional area change (FAC)/mean pulmonary artery pressure (mPAP), and stroke volume (SV)/end-systolic area (ESA) for discriminating right ventricle (RV)-arterial uncoupling in patients with pulmonary hypertension. TAPSE, PASP, FAC, and ESA were assessed by echocardiography in the study cohort; mPAP was assessed by right heart catheterization; and SV by cardiac magnetic resonance. RV-arterial uncoupling was defined as single-beat end-systolic elastance (Ees)/arterial elastance (Ea) <0.805. Diagonal segments were produced by ties. AUC indicates area under the curve.
In the study cohort, 26 clinical worsening events were observed during a median follow-up time of 13 (interquartile range: 5.3–24.8) months. Kaplan-Meier analysis revealed that patients with PH and TAPSE/PASP <0.31 mm/mm Hg had a significantly worse prognosis than those with higher TAPSE/PASP (log-rank P=0.019; Figure 5A). This was supported by multivariate Cox regression analysis including age, sex, and TAPSE/PASP dichotomized at 0.31 mm/mm Hg; TAPSE/PASP showed a hazard ratio of 2.73 (95% CI, 1.14–6.54) as a predictor of clinical worsening.
Figure 5.
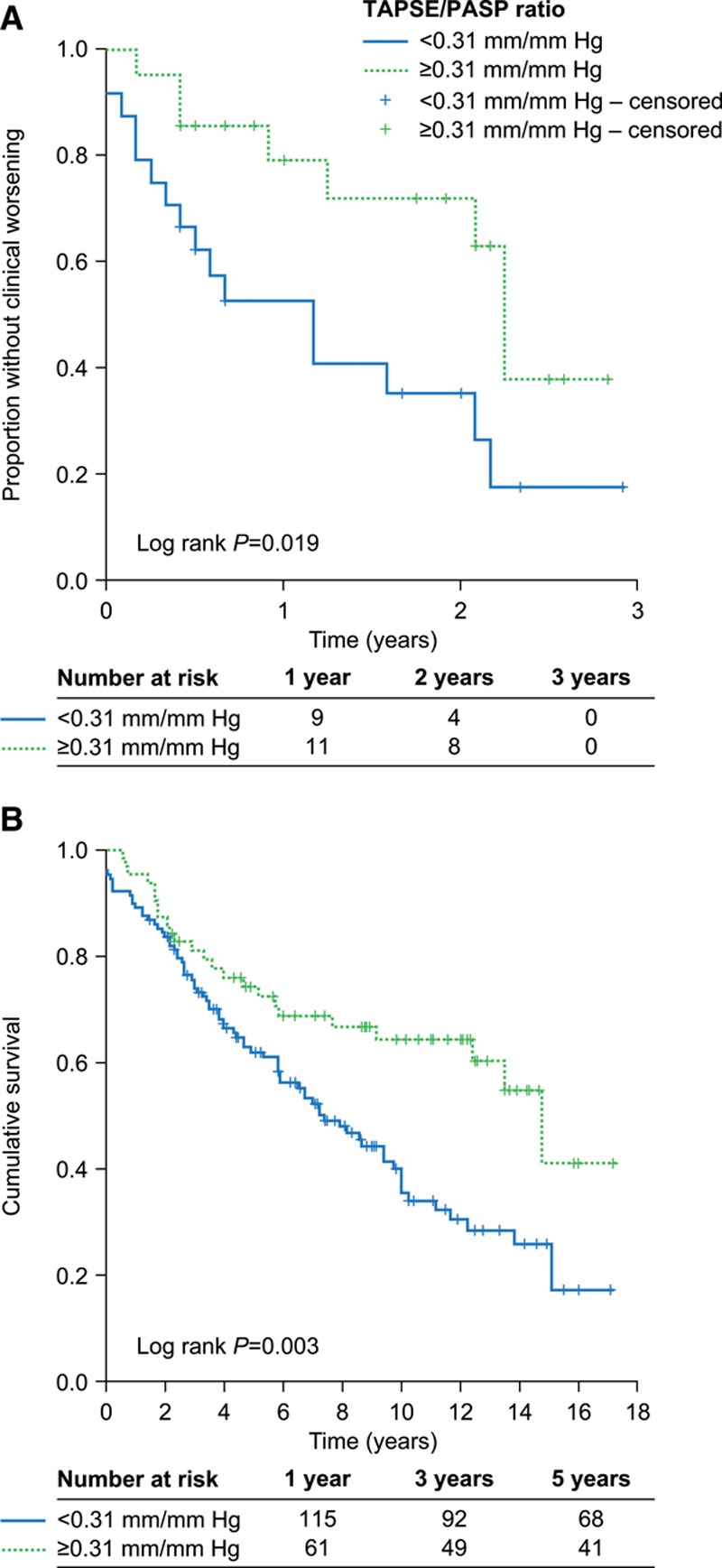
Kaplan-Meier plots of outcomes in patients stratified by tricuspid annular plane systolic excursion (TAPSE)/systolic pulmonary artery pressure (PASP). Stratification by echocardiographic TAPSE/PASP (≥0.31 mm/mm Hg and <0.31 mm/mm Hg) revealed significant differences in A, clinical worsening in patients with pulmonary hypertension (study cohort; estimates of mean and median time to clinical worsening are shown in Table III in the Data Supplement), and (B) overall survival in patients with idiopathic pulmonary arterial hypertension (external validation cohort; subset of a previously published cohort12).
In the external validation cohort, patients with idiopathic PAH and TAPSE/PASP <0.31 mm/mm Hg showed significantly worse overall survival than those with higher TAPSE/PASP (log-rank P=0.003; Figure 5B). TAPSE/PASP also predicted overall mortality (hazard ratio: 1.85; 95% CI, 1.16–2.95) in multivariate Cox regression analysis including World Health Organization functional class, age, sex, pulmonary vascular resistance, and TAPSE/PASP dichotomized at 0.31 mm/mm Hg.
Discussion
The present results show that the echocardiographic TAPSE/PASP ratio is a clinically relevant and valid surrogate of invasively measured Ees/Ea to assess RV-arterial coupling and provides information on RV diastolic stiffness but not RV contractility.
Several other ratios have been proposed to estimate RV-arterial coupling. FAC/mPAP showed relationships with pulsatile RV afterload14 and biomarkers of RV dysfunction.13,15 RV area change/ESA was introduced as a surrogate for RV-arterial coupling in a small cohort of patients with scleroderma-associated PAH.16 In that study, neither RV area change/ESA nor TAPSE/PASP had prognostic relevance.16 TAPSE/PAAT was introduced as a substitute for TAPSE/PASP in children in whom a sufficient quality of Doppler velocity signal of tricuspid regurgitation could not be obtained,33 and relied on the argument that PAAT would be a better measure of pulmonary vascular impedance (or afterload) than PASP.34 However, none of these surrogates was evaluated against the gold standard Ees/Ea metric for RV-arterial coupling. Only echocardiographic TAPSE/PASP and TAPSE/PAAT were evaluated against simplified calculations of Ees/Ea based on single RV pressure curve analysis7,17 or echocardiographic SV/ESA.9 The present study shows that only TAPSE/PASP is independently related to invasively measured Ees/Ea.
All considered surrogates of RV-arterial coupling except TAPSE/PAAT were associated with RV afterload (Ea) and diastolic stiffness (Eed) but not RV contractility (Ees). The observed associations with Ea provide the missing link between the physiological concept of assessing RV load adaptability indirectly via echocardiography and the direct invasive measurement. It is unclear whether RV systolic and diastolic dysfunction can evolve separately in heart failure or severe PH because some studies showed tight correlations between Ees and Eed35,36 while others found Ees and Eed to be dissociated.20,21 The correlation of surrogates with Eed but not Ees in our study is compatible with dissociated adaptation of inotropic and lusitropic function in severe PH.
The absence of correlations between surrogates of RV-arterial coupling and Ees agrees with the previously reported association of echocardiographic indices of RV function with RV-arterial coupling but not contractility in an experimental model of chronic pressure overload.37 The absence of a correlation between TAPSE/PAAT and Ees/Ea is more surprising, as PAAT is a better measure of afterload than PASP.38 This is possibly explained by the curvilinearity of the relationship between PAAT and PASP, with a smaller range of PAAT than PASP covered in the present study making PAAT less sensitive to the observed range of RV afterload.
Based on our receiver operating characteristic and univariate analyses of TAPSE/PASP, FAC/mPAP, and SV/ESA to significantly discriminate RV-arterial uncoupling, all these surrogates might be used in the clinic based on local preferences. Nevertheless, the information content of TAPSE/PASP is superior as this metric was the only independent predictor of gold standard Ees/Ea in severe PH.
In the present study, the TAPSE/PASP cutoff value for prediction of RV-arterial uncoupling (Ees/Ea <0.805)4 was 0.31, which is lower than the cutoff value of 0.36 previously associated with survival in heart failure.11 This difference may be related to better-preserved contractility adaptation to higher levels of afterload in severe PH compared with heart failure. In addition, our study showed the prognostic and clinical relevance of the proposed cutoff in PAH and, therefore, highlighted the association between clinical utility and RV pathophysiology.
Limitations
We used the single-beat method to estimate Eed, Ees, and Ea; it is unclear if the multiple-beat approach would yield similar results, although there is a recent report of agreement between the 2 approaches.39 Limitations of echocardiographic analysis include observer dependency, reliability, and 2-dimensional plane measurement. In general, echocardiographic measurements have shown favorable interobserver and intraobserver variability in PH,40 but some variability must be considered in clinical practice. Moreover, as RV dysfunction progresses, TAPSE reaches a minimum and shows no further decrease.41 Most of the patients in our cohort were already in a state of uncoupling (median Ees/Ea: 0.70). Other reasons for the somewhat unexpected absence of correlation between TAPSE and Ees in our study may be that TAPSE is afterload-dependent, plane-dependent, and falsely increased in the presence of severe tricuspid regurgitation. The reliability of TAPSE as a parameter of RV function (and therefore the utility of TAPSE/PASP) may thus be reduced in the later stages of RV failure or after heart surgery. In addition, echocardiographic PASP is influenced by the quality of acquisition and signal of the peak tricuspid regurgitation velocity.42 An important limitation is the partial dependency of the Eed calculation29 as well as the TAPSE/PASP ratio on volume, which might explain the higher correlation of TAPSE/PASP with Eed than with Ees/Ea. The comprehensive and demanding methodology limited the size of the study cohort. The relatively short prospective observational period, the study cohort sample size, and the retrospective analysis of the external validation cohort limit the interpretation of the results; the prognostic value of the identified TAPSE/PASP cutoff must be confirmed in a large prospective study.
Conclusions
The echocardiographic TAPSE/PASP ratio is a straightforward surrogate of invasively measured Ees/Ea to assess RV-arterial coupling. Both TAPSE and PASP are easily measured by a standard bedside echocardiographic examination. Further studies are needed to evaluate the possible added value of TAPSE/PASP in current risk assessment strategies for severe PH.
Supplementary Material
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.119.009047.
Acknowledgments
We thank Claire Mulligan, PhD, (Beacon Medical Communications Ltd, Brighton, United Kingdom) for editorial support, funded by the University of Giessen.
Sources of Funding
This work was funded by the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the Collaborative Research Center (SFB) 1213—Pulmonary Hypertension and Cor Pulmonale, grant number SFB1213/1, project B08 (German Research Foundation, Bonn, Germany).
Disclosures
Dr Tello has received speaking fees from Actelion and Bayer. Dr Ghofrani has received consultancy fees from Bayer, Actelion, Pfizer, Merck, GSK, and Novartis; fees for participation in advisory boards from Bayer, Pfizer, GSK, Actelion, and Takeda; lecture fees from Bayer HealthCare, GSK, Actelion, and Encysive/Pfizer; industry-sponsored grants from Bayer HealthCare, Aires, Encysive/Pfizer, and Novartis; and sponsored grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research. Dr Naeije has relationships with drug companies including AOPOrphan Pharmaceuticals, Actelion, Bayer, Reata, Lung Biotechnology Corporation, and United Therapeutics. In addition to being an investigator in trials involving these companies, relationships include consultancy service, research grants, and membership of scientific advisory boards. Dr Seeger has received speaker/consultancy fees from Pfizer and Bayer Pharma AG. Dr Sommer has received fees from Actelion. Dr Gall has received fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics. Dr Richter has received support from United Therapeutics and Bayer; speaker fees from Bayer, Actelion, Mundipharma, Roche, and OMT; and consultancy fees from Bayer. The other authors report no conflicts.
References
- 1.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM; American Thoracic Society Assembly on Pulmonary Circulation. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. an official american thoracic society research statement. Am J Respir Crit Care Med. 2018;198:e15–e43. doi: 10.1164/rccm.201806-1160ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53:1801900 doi: 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 4.Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Sommer N, Wilhelm J, Gall H, Richter MJ. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12:e005512 doi: 10.1161/CIRCHEARTFAILURE.118.005512 [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 6.Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi: 10.1002/ejhf.873 [DOI] [PubMed] [Google Scholar]
- 7.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 8.Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. doi: 10.1093/ehjci/jex133 [DOI] [PubMed] [Google Scholar]
- 9.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV Contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10(10 pt B):1211–1221. doi: 10.1016/j.jcmg.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 10.Guazzi M, Naeije R, Arena R, Corrà U, Ghio S, Forfia P, Rossi A, Cahalin LP, Bandera F, Temporelli P. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest. 2015;148:226–234. doi: 10.1378/chest.14-2065 [DOI] [PubMed] [Google Scholar]
- 11.Guazzi M. Use of TAPSE/PASP ratio in pulmonary arterial hypertension: An easy shortcut in a congested road. Int J Cardiol. 2018;266:242–244. doi: 10.1016/j.ijcard.2018.04.053 [DOI] [PubMed] [Google Scholar]
- 12.Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, Seeger W, Gall H, Richter MJ. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229–235. doi: 10.1016/j.ijcard.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 13.Prins KW, Archer SL, Pritzker M, Rose L, Weir EK, Sharma A, Thenappan T. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37:376–384. doi: 10.1016/j.healun.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prins KW, Weir EK, Archer SL, Markowitz J, Rose L, Pritzker M, Madlon-Kay R, Thenappan T. Pulmonary pulse wave transit time is associated with right ventricular-pulmonary artery coupling in pulmonary arterial hypertension. Pulm Circ. 2016;6:576–585. doi: 10.1086/688879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter MJ, Ghofrani HA, Gall H. Beyond interleukin-6 in right ventricular function: evidence for another biomarker. J Heart Lung Transplant. 2018;37:674–675. doi: 10.1016/j.healun.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 16.French S, Amsallem M, Ouazani N, Li S, Kudelko K, Zamanian RT, Haddad F, Chung L. Non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension. Pulm Circ. 2018;8:2045894018788268 doi: 10.1177/2045894018788268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy PT, El Khuffash A, Woo KV, Hauck A, Hamvas A, Singh GK. A novel noninvasive index to characterize right ventricle pulmonary arterial vascular coupling in children [published online December 6, 2018]. J Am Coll Cardiol Img. doi: 10.1016/j.jcmg.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 18.Claessen G, La Gerche A, Voigt JU, Dymarkowski S, Schnell F, Petit T, Willems R, Claus P, Delcroix M, Heidbuchel H. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging. 2016;9:532–543. doi: 10.1016/j.jcmg.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 19.Pratali L, Allemann Y, Rimoldi SF, Faita F, Hutter D, Rexhaj E, Brenner R, Bailey DM, Sartori C, Salmon CS, Villena M, Scherrer U, Picano E, Sicari R. RV contractility and exercise-induced pulmonary hypertension in chronic mountain sickness: a stress echocardiographic and tissue Doppler imaging study. JACC Cardiovasc Imaging. 2013;6:1287–1297. doi: 10.1016/j.jcmg.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 20.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmüller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128:2016–2025, 2021. doi: 10.1161/CIRCULATIONAHA.113.001873 [DOI] [PubMed] [Google Scholar]
- 21.Trip P, Rain S, Handoko ML, van der Bruggen C, Bogaard HJ, Marcus JT, Boonstra A, Westerhof N, Vonk-Noordegraaf A, de Man FS. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J. 2015;45:1603–1612. doi: 10.1183/09031936.00156714 [DOI] [PubMed] [Google Scholar]
- 22.Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36:957–967. doi: 10.1016/j.healun.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 23.Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Wilhelm J, Gall H, Richter MJ. Cardiac magnetic resonance imaging-based right ventricular strain analysis for assessment of coupling and diastolic function in pulmonary hypertension [published online March 8, 2019]. J Am Coll Cardiol Img. doi: 10.1016/j.jcmg.2018.12.032 [DOI] [PubMed] [Google Scholar]
- 24.Tello K, Richter MJ, Axmann J, Buhmann M, Seeger W, Naeije R, Ghofrani HA, Gall H. More on single-beat estimation of right ventriculoarterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;198:816–818. doi: 10.1164/rccm.201802-0283LE [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 26.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 27.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–508. doi: 10.1161/CIR.0b013e3181d44a8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol. 2003;284:H1625–H1630.. doi: 10.1152/ajpheart.01023.2002 [DOI] [PubMed] [Google Scholar]
- 29.Vanderpool RR, Puri R, Osorio A, Wickstrom K, Desai A, Black S, Garcia JGN, Yuan J, Rischard F. Express: surfing the right ventricular pressure waveform: methods to assess global, systolic and diastolic RV function from a clinical right heart catheterization [published online April 2, 2019]. Pulm Circ. doi: 10.1177/2045894019850993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, Roos-Hesselink JW. Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:656–664. doi: 10.1093/ejechocard/jer107 [DOI] [PubMed] [Google Scholar]
- 31.Giavarina D. Understanding bland altman analysis. Biochem Med (Zagreb). 2015;25:141–151. doi: 10.11613/BM.2015.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 33.Levy PT, El Khuffash A, Woo KV, Singh GK. Right ventricular-pulmonary vascular interactions: an emerging role for pulmonary artery acceleration time by echocardiography in adults and children. J Am Soc Echocardiogr. 2018;31:962–964. doi: 10.1016/j.echo.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Rudski L, Gargani L, Naeije R, Bossone E. Authors’ reply: pulmonary flow wave morphology characteristics of pulmonary hypertension. J Am Soc Echocardiogr. 2018;31:964–965. doi: 10.1016/j.echo.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 35.Vanderpool RR, Desai AA, Knapp SM, Simon MA, Abidov A, Yuan JX, Garcia JGN, Hansen LM, Knoper SR, Naeije R, Rischard FP. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700764 doi: 10.1183/13993003.00764-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC, Simon MA. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. doi: 10.1136/heartjnl-2014-306142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guihaire J, Haddad F, Boulate D, Decante B, Denault AY, Wu J, Hervé P, Humbert M, Dartevelle P, Verhoye JP, Mercier O, Fadel E. Non-invasive indices of right ventricular function are markers of ventricular-arterial coupling rather than ventricular contractility: insights from a porcine model of chronic pressure overload. Eur Heart J Cardiovasc Imaging. 2013;14:1140–1149. doi: 10.1093/ehjci/jet092 [DOI] [PubMed] [Google Scholar]
- 38.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, Abe H. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302 [DOI] [PubMed] [Google Scholar]
- 39.Inuzuka R, Hsu S, Tedford RJ, Senzaki H. Single-beat estimation of right ventricular contractility and its coupling to pulmonary arterial load in patients with pulmonary hypertension. J Am Heart Assoc. 2018;7:e007929 doi: 10.1161/JAHA.117.007929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Tsujino I, Ohira H, Oyama-Manabe N, Yamada A, Ito YM, Goto C, Watanabe T, Sakaue S, Nishimura M. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J Am Soc Echocardiogr. 2012;25:280–286. doi: 10.1016/j.echo.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 41.Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson. 2010;12:35 doi: 10.1186/1532-429X-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amsallem M, Sternbach JM, Adigopula S, Kobayashi Y, Vu TA, Zamanian R, Liang D, Dhillon G, Schnittger I, McConnell MV, Haddad F. Addressing the controversy of estimating pulmonary arterial pressure by echocardiography. J Am Soc Echocardiogr. 2016;29:93–102. doi: 10.1016/j.echo.2015.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


