Abstract
Background
Seizures are a morbid complication of intracerebral hemorrhage (ICH) and increase the risk for herniation, status epilepticus, and worse patient outcomes. Prophylactic levetiracetam is administered to approximately 40% of patients with ICH. It is unclear which patients are consciously selected for treatment by physicians. We sought to determine how patients are selected for treatment with prophylactic levetiracetam after ICH.
Methods
We administered an adaptive conjoint analysis using decision making software to an NIH Stroke Trials Network (StrokeNet) Working Group. The adaptive conjoint analysis determines the most influential attributes for making a decision in an iterative, algorithm-driven process. We asked respondents which would most influence a decision to administer prophylactic levetiracetam. The attributes and their levels were taken from published phenotypes associated with prophylactic seizure medications and the likelihood of seizures after ICH: hematoma location (lobar or basal ganglia), hematoma volume (<=10ml or >10 mL), level of consciousness (Glasgow Coma Scale 5–12 or Glasgow Coma Scale 13–15), age (<65 or ≥65 years), and race (White or Caucasian or Black/African American). The algorithm terminated when the attributes were ranked from most to least influential.
Results
The study sample included 27 respondents who completed the adaptive conjoint analysis out of 42 who responded to the survey with a mean age of 43.4 ± 9.4 years. The attribute with the greatest weight was hematoma location (30%), followed by reduced level of consciousness (24%), hematoma volume (19%), race (14%), and age (13%). Ranks of attributes were different (P<0.001).
Conclusions
The decision to administer prophylactic levetiracetam to patients with ICH is driven by lobar hematoma location and depressed level of consciousness. Future research on prophylactic seizure medication could focus on patients most likely to receive it.
Keywords: Seizure medications, intracerebral hemorrhage, levetiracetam, decision making, adaptive conjoint analysis
Introduction
Seizures are a morbid and common complication of intracerebral hemorrhage (ICH). Seizures are associated with more midline shift, continued subclinical seizures, and, potentially, death.1 Preventing seizures with prophylactic seizure medication is a potentially clinically defensible therapy to improve patient outcomes for patients with ICH.
Prophylactic phenytoin was recommended by guidelines,2 however, after its association with more fever and worse patient outcomes, physicians largely switched to levetiracetam and guidelines were updated to discourage the use of prophylactic seizure medications.3–6 Currently, about 40% of patients with ICH in the United States receive prophylactic levetiracetam.6 The CAVE Score is a validated instrument for predicting seizures at least one week after ICH onset and highlights some criteria that increase the risk of seizures,7 however, it includes seizures within one week as a predictor of late seizures, which would mandate treatment.8 Indiscriminate use of prophylactic levetiracetam might also worsen patient outcomes, particularly quality of life in the domain of cognitive function,9 highlighting the need to precisely administer treatment to patients at highest risk for seizures. While we have not previously found associations between levetiracetam and delirium or agitation, levetiracetam is known to be associated with neurocognitive symptoms9,10 We tested if the decision to administer prophylactic seizure medications to patients with ICH was associated with clinically observable attributes at the time of presentation.
Methods
Human Subjects Protection
The Institutional Review Board approved the survey with a waiver of consent with requirements that respondents not be identifiable and restricted the demographic information that could be collected. Collection of self-reported age was permitted, but not sex, board certification (vascular neurology, neurocritical care), or the respondent’s email address.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request from the corresponding author.
Respondents
We surveyed members of the NIH StrokeNet Working Group as part of protocol development in June and July, 2017. A link was sent to respondents by NIH, and a link to a website which performed an adaptive conjoint analysis.
Adaptive Conjoint Analysis (ACA)
Questions for the ACA were adapted from the CAVE Score7 (age, hematoma location, hematoma volume) except early seizures (which would make the use of seizure medications therapeutic, not prophylactic). From the ICH Score,11 we also included level of consciousness (Glasgow Coma Scale) because it could indicate subclinical seizures. We included race because we previously identified its association with prophylactic seizure medications after ICH.12 We used the 1000Minds™ web-based, decision-making software.13 The ACA asks a respondent to select between pairs of criteria for making a medication prescribing decision. Importantly, each pair of criteria includes a trade-off and respondents are able to indicate that they favor them equally. Respondents choose which combination of attributes are most important (assuming all other attributes are equal) in the decision to prescribe prophylactic seizure medication after ICH for the prevention of early seizures. The following example illustrates the paired nature or the choice and the trade-off involved: Which of the following sets of characteristics are most important in the decision to prescribe prophylactic seizure medications after ICH? 1) lobar hematoma location and age ≤65 years versus 2) deep hematoma location and age >65. The survey is computer adapted and through iterative questioning 1000Minds™ determines ‘part-worth utilities’ (weights) representing the relative importance of all attributes for each participant using the Potentially All Pairwise RanKings of all possible Alternatives (PAPRIKA) method.14
Statistical Analysis
Data are provided as mean ± SD, median [25% – 75%], or N(%) as appropriate. Ranks for decision-making, with 1 indicating the most important, were compared between groups (hematoma volume, hematoma location, etc.) with Kruskal-Wallis H using R v3.4. The analysis was overseen by ET.
Results
There were 42 respondents to the demographic survey with age 43.4 ± 9.4 years.
Results of a survey asking which criteria would influence the decision to administer prophylactic levetiracetam are shown in Table 1. There was no association between these responses and completion of the ACA.
Table 1.
Respondents who indicated a criterion would independently influence their decision to administer prophylactic levetiracetam after ICH.
| Criterion | N(%) |
|---|---|
| Lobar hematoma location | 18 (43) |
| Depressed level of consciousness | 17 (40) |
| Hematoma volume >10 mL | 3 (7) |
| Age <65 years | 1 (2) |
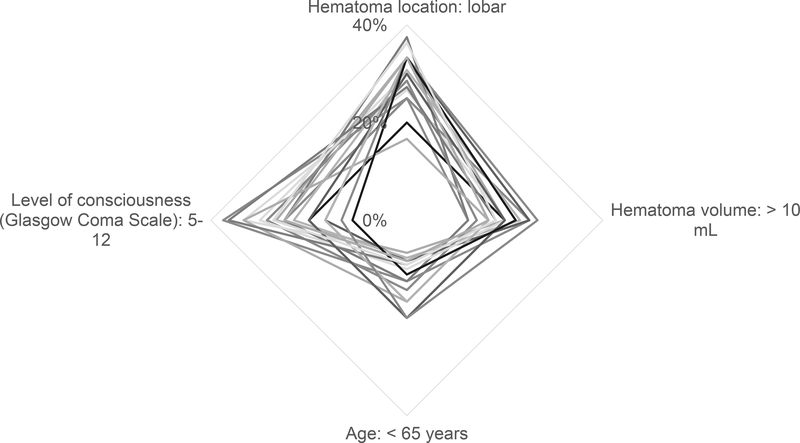
The results of the ACA are shown in Table 2, when respondents were asked to explicitly choose between competing criteria. Lobar hematoma location was the strongest predictor of the decision to administer prophylactic levetiracetam, followed by depressed level of consciousness. Ranks of relative importance for decision-making were different (P < 0.0001). A radar plot of the weight of each criterion is shown in the Figure.
Table 2.
Rankings of each attribute for administering prophylactic levetiracetam in the adaptive conjoint analysis, with 1 indicating greatest importance. Ranks were different between criteria (P<0.0001).
| Criteria | Median Rank | 25% – 75% Rank |
|---|---|---|
| Hematoma location | 1 | 1 – 2 |
| Level of Consciousness | 2 | 1.75 – 3 |
| Hematoma volume | 3 | 3 – 3.5 |
| Race | 4 | 3.5 – 4.25 |
| Age ≤65 years | 4 | 3.5 – 4.75 |
Figure.
Radar plot of the weights of criteria on the decision to prescribe prophylactic levetiracetam after ICH. Lobar hematoma location and depressed level of consciousness (Glasgow Coma Scale 5–12) influenced the decision most. Each line represents one response.
Discussion
By means of an adaptive survey of NIH StrokeNet Working Group members, we determined that the most important attribute for the decision to administer prophylactic levetiracetam was lobar hematoma location, followed by level of consciousness, and other attributes. Rather than studies of unselected cohorts of patients with ICH, these data allow future clinical care and research to focus on the subgroup of patients most likely to receive prophylactic levetiracetam for the prevention of early seizures.
We selected criteria for the survey from the published literature, which has not established prediction rules for seizures. The CAVE Score predicts late seizures from early seizures (within a week), age less than 65 years, hematoma volume at least 10 mL, and lobar hematoma location.7 Of the CAVE Score criteria, lobar hematoma location had the greatest preference weight associated with administering prophylactic levetiracetam. In contrast to the CAVE Score, respondents were less likely to administer prophylactic levetiracetam to patients ≤65 years. It is possible that respondents conflated older age is a predictor of greater severity of injury with older age as predictive of early seizures. We included race/ethnicity because we previously reported an association between levetiracetam use and race/ethnicity, with lobar hematoma location being the likely confounder.12 When confronted with a choice between the two, respondents prioritized lobar hematoma location. This underscores the ACA’s value as a method to determine how medical decisions are made as a complement to medication administration data.
Other criteria predict seizures as well. The 2HELPS2B15 score incorporates electroencephalography (EEG) interpretation and clinical seizures for the prediction of additional seizures. We did not include criteria from 2HELPS2B because it was not published when the survey was conducted, EEG interpretations are not available at the time of presentation with ICH, and clinical seizures would require treatment rather than prophylaxis.
There is a gap between current guideline recommendations (prophylactic seizure medications are not recommended) and clinical practice (clinicians may be more likely to treat patients with lobar hematomas and depressed level of consciousness, a potentially defensible judgment of a higher risk of seizures). The patients most likely to benefit from prophylactic seizure medications are those who are most likely to have seizures. Further research could elucidate if this subgroup of patients is likely to benefit from treatment with prophylactic seizure medications, using observational data, or a prospective trial.
The limitations to this study include the small cohort and the category of respondents as connected to NIH StrokeNet. It is possible that a cohort of physicians outside of the NIH StrokeNet would make different decisions regarding prophylactic levetiracetam, although this seems unlikely. A survey in a larger group could be worthwhile, although many societies have markedly decreased the number of surveys sent to members due to fatigue, and now require additional vetting, committee review, and fees in the thousands of dollars. Some physicians will not use prophylactic seizure medications at all, which may account for those who declined to complete the ACA, as it would not apply to their clinical practice.
Conclusions
Using an adaptive survey of decision making, we found that the phenotype of lobar hematoma and depressed consciousness were most important in deciding to administer prophylactic levetiracetam after ICH. Future research and patient care might focus on this phenotype for future research.
Acknowledgements
All those who participated in the manuscript are included as an author.
We gratefully acknowledge NIH StrokeNet for allowing us to survey Working Group members.
Sources of Funding
Dr. Naidech was supported, in part, by K18 NS023437 and R01 NS110779.
Dr. Pinto was supported, in part, by the Foundation for Physical Therapy Research’s Center on Health Services Training and Research Faculty Fellowship.
Footnotes
Conflict of Interest Disclosure
The authors are unaware of any conflict of interest.
References
- 1.Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–6. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. 2007 Update. Stroke 2007;38:2001–23. [DOI] [PubMed] [Google Scholar]
- 3.Naidech AM, Beaumont J, Prabhakaran S, Kho AN, Holl JL. Evolving Use of Seizure Medications After Intracerebral Hemorrhage: A Multi-Center Study. Neurology 2017;88:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant Use and Outcomes After Intracerebral Hemorrhage. Stroke 2009;40:3810–5. [DOI] [PubMed] [Google Scholar]
- 5.Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009;11:38–44. [DOI] [PubMed] [Google Scholar]
- 6.Sheth KN, Martini SR, Moomaw CJ, et al. Prophylactic Antiepileptic Drug Use and Outcome in the Ethnic/Racial Variations of Intracerebral Hemorrhage Study. Stroke 2015;46:3532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014;45:1971–6. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- 9.Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic Seizure Medication and Health-Related Quality of Life After Intracerebral Hemorrhage. Crit Care Med 2018;46:1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal LJ, Francis BA, Beaumont JL, et al. Agitation, Delirium, and Cognitive Outcomes in Intracerebral Hemorrhage. Psychosomatics 2017;58:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemphill J, Bonovich D, Besmertis L, Manley G, Johnston SC, Tuhrim S. The ICH Score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- 12.Naidech AM, Toledo P, Prabhakaran S, Holl JL. Disparities in the Use of Seizure Medications After Intracerebral Hemorrhage. Stroke: a Journal of the American Heart Association 2017;48:802–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberman AL, Pinto D, Rostanski SK, Labovitz DL, Naidech AM, Prabhakaran S. Clinical Decision-Making for Thrombolysis of Acute Minor Stroke Using Adaptive Conjoint Analysis. Neurohospitalist 2019;9:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen P, Ombler F. A new method for scoring additive multiattribute value models using pairwise rankings of alternative. J Multi-Criteria Decis Anal 2008;15:e107. [Google Scholar]
- 15.Struck AF, Ustun B, Ruiz AR, et al. Association of an Electroencephalography-Based Risk Score With Seizure Probability in Hospitalized Patients. JAMA Neurol 2017;74:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from the corresponding author.