Abstract
Microbiology and cell biology both involve the study of cells, albeit at different levels of complexity and scale. Interactions between both fields during the past 25 years have led to major conceptual and technological advances that have reshaped the whole biology landscape and its biomedical applications.
Introduction
Microbiology and cell biology are disciplines that have grown side by side for almost 350 years. Indeed, the word ‘cell’ was coined in 1665 by Robert Hook in his book Micrographia [1], while the first bacteria were described in 1676 by Antonie van Leeuwenhoek [2]. The cell theory (‘the cell is the most basic unit of life’) was fully developed by 1850 after seminal contributions from Theodor Schwann, Matthias Jakob Schleiden, and Rudolf Virchow, while microbiology emerged as an independent field after Louis Pasteur's discoveries, which dismantled the spontaneous generation theory, and Robert Koch's postulates, which proved critical to determine the causality of a microbe in a given disease. Even though both microbiology and cell biology involve the study of ‘cells’ that share fundamental biological processes, such as replication, transcription, and translation, they do so at different levels of complexity (simpler in prokaryotic compared with eukaryotic cells) and at different scales (bacterial cells are on average ten times smaller than eukaryotic cells). Remarkably, over the years, cross-feeding between cell biology and microbial biology has significantly contributed to the generation of new concepts and new advances in both fields.
Cellular Microbiology
The understanding by pioneering microbiologists, such as Stanley Falkow, that pathogenic microorganisms are excellent ‘cell biologists’ that subvert cellular functions to promote disease, paved the way during the late 1980s for an emerging field named ‘cellular microbiology’ [3], in which microbiologists take advantage of the cell biology savoir faire to study the adaptations of pathogens to the intracellular environment, while cell biologists use microbial components to manipulate cells and uncover cellular functionalities. One of the most remarkable examples of cross-feeding between the two fields concerns the discovery of the first actin nucleator: the Listeria monocytogenes surface protein ActA, which is required for bacterial actin-based motility [4] (Figure 1), enabled the identification of the Arp2/3 complex as the first actin nucleator in eukaryotic cells [5], leading to numerous subsequent studies highlighting the contribution of Arp2/3 to cell migration, cell polarity, and membrane dynamics. Conversely, advances in the understanding of plasma membrane signaling, in the pathways that drive endocytosis and phagocytosis, as well as in the dynamics of intracellular vesicular trafficking, provided a working framework to investigate the diverse lifestyles of bacterial pathogens. Starting with the discovery of the use of integrins by Yersinia pseudotuberculosis to invade mammalian cells [6], many molecular infection strategies have been elucidated since, including remodeling of late endosomal compartments by Salmonella enterica, subversion of the host endoplasmic reticulum by Legionella pneumophila and Brucella abortus, interactions with the Golgi apparatus by Chlamydia trachomatis, as well as escape from their phagosomes by L. monocytogenes, Shigella flexneri, and Rickettsia conorii.
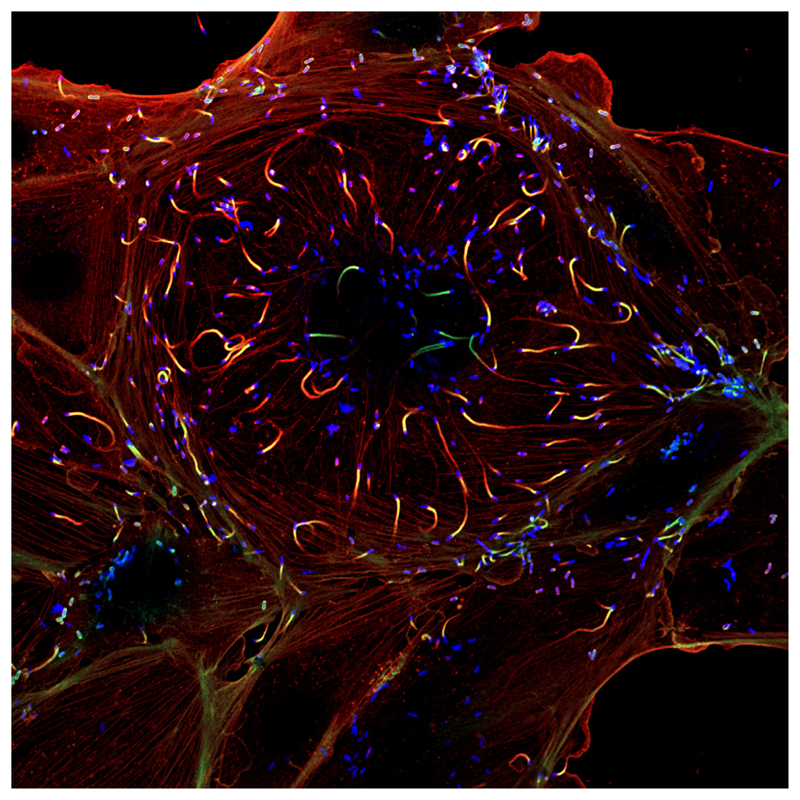
Figure 1. Mammalian Cell Infected with the Bacterial Pathogen Listeria monocytogenes.
The bacterial intracellular pathogen L. monocytogenes is able to invade mammalian epithelial cells within vacuolar compartments that are disrupted by bacterial exotoxins. In the host cell cytoplasm, L. monocytogenes uses an actin-based motility system to spread both intra- and intercellularly. In this micrograph, L. monocytogenes is fluorescently labeled in blue and the cellular actin in green and red: ‘actin comet tails’ are clearly distinguished at the rear poles of moving bacteria. The bacterial surface protein ActA, required for host actin polymerization [4], enabled the subsequent identification of the first eukaryotic actin nucleator, the Arp2/3 complex [5]. Image: courtesy of Edith Gouin and Matteo Bonazzi, Institut Pasteur.
Toxins
Bacterial toxins were historically among the first encounters between cell biology and microbiology. For instance, the diphtheria toxin produced by Corynebacterium diphtheriae was shown to transfer ADP ribose to the eukaryotic elongation factor 2 and to inhibit protein synthesis, thereby explaining the dramatic phenotype observed in intoxicated cells and in patients with diphtheria. The C3 exoenzyme from Clostridium botulinum is also an ADP ribosyltransferase toxin that targets small GTPases of the Rho family, and its use by cell biologists, such as Alan Hall, revealed the critical role of these GTPases in the control of actin cytoskeleton dynamics [7]. Neurotoxins from C. botulinum and Clostridium tetani have also been critical not only for investigating the molecular mechanisms of neurotransmission, but also for establishing the SNARE concept: botulinum neurotoxin A is a protease that blocks neurotransmitter release by selectively cleaving the synaptic protein SNAP-25 [8], while botulinum neurotoxin B and tetanus neurotoxin perform the same biological function by cleaving synaptobrevin [9]. These studies were crucial to the identification of SNAREs as the essential machinery driving vesicular docking and fusion [10]. In addition, hemolytic/poreforming toxins from L. monocytogenes and Streptococcus pyogenes, identified early on as critical virulence factors, have also been instrumental in the study of eukaryotic membrane stability.
Cytoskeleton
While the study of the eukaryotic cytoskeleton has long been a tradition in cell biology and has benefited from the use of bacterial tools, as mentioned above, the study of the bacterial cytoskeleton is more recent. Inspired by cell biology data, microscopical studies of the bacterial cell morphology allowed for the visualization of the assembly of the prokaryotic homologue of tubulin FtsZ at the division site [11]. Since then, the exquisite subcellular organization of prokaryotic cells and the complexity of their cytoskeleton have been increasingly revealed: MreB is analogous to eukaryotic actin, crescentin is equivalent to eukaryotic intermediate filaments, ParM has a similar structure to that of actin but behaves functionally like tubulin during plasmid partition between dividing cells, and bactofilin forms filaments in several bacteria. Many of these studies have benefited from advances in microscopy, in particular from the use of GFP as a microscopical reporter of protein expression, efficiently complementing the traditional lacZ enzymatic bacterial reporter, and from the development of super-resolution. Microscopy has completely revolutionized the field of cell biology by tracking the expression and dynamics of molecules in single living cells with optimal spatiotemporal resolution, or in millions of cells within the framework of high-throughput screens. The same is now occurring in microbial biology, where single molecules can be visualized in single bacteria [12].
Polarity
The unveiling of the complexity of the bacterial cytoskeleton, together with the observation that bacterial chemoreceptors and their associated signaling proteins localize to bacterial poles, demonstrated that membrane-bound vesicles, such as those observed in eukaryotes, are not a prerequisite for spatial organization of cells, and that bacteria are not unorganized bags of homogeneously distributed proteins, launching the study of cell polarity in bacteria. Many mechanisms involved in protein localization in bacteria have now been discovered. For example, positive and negative membrane curvatures represent geometrical cues that can influence the diffusion and capture of proteins, as in the case of the DivIVA protein of Bacillus subtilis, which recognizes membranes displaying high negative curvature and then forms a polymer that recruits other division-regulating molecules, such as MinJ. In Myxococcus xanthus, the bactofilin BacP polymerizes and forms large patches that guide the polar localization of PilB and PilT involved in type IV pili-dependent motility. The small Ras-like GTPases MglA and SofG participate in the recruitment of PilB and PilT. Lipid microdomains, first highlighted in eukaryotic cells, were also discovered in bacteria, and have been proposed as spatial cues to determine the polar localization of the transporter ProP in Escherichia coli.
Gene Silencing and Genome Editing
As detailed above, cell biology has led to the emergence of several new concepts in microbiology. Another important example of this conceptual influence is gene silencing: this area has completely changed the face of cell biology in recent years, while a related prokaryotic mechanism is now changing all areas in biology. The critical finding by Andrew Fire and Craig Mello concerning the inactivation of gene expression by RNA interference in the worm Caenorhabditis elegans [13] provided a pivotal molecular tool to inactivate gene expression in a specific and precise manner in eukaryotic cells. This milestone has had recent echoes in microbiology, in which researchers investigated defense mechanisms against invaders (phages and plasmids). The discovery of clustered regularly interspaced short palindromic repeats (CRISPRs) as ‘memory cassettes’ of the passage of invaders led to the identification of CRISPRs and CRISPR-associated Cas proteins as an adaptative immune system that can protect microbes against viral infections [14]. The identification that the CRISPR/Cas9 system targets DNA and creates double-stranded breaks [15] was the beginning of a molecular adventure that now allows the precise editing of mammalian cell genomes, with possible biomedical applications of a unprecedented nature in the near future.
Concluding Remarks and Perspectives
Concepts in eukaryotic and microbial cell biology are becoming ever more complementary, and several emerging fields are arising from this interaction. For example, it is increasingly recognized that bacteria can form microbial assemblies in which individual bacterial cells acquire properties that they did not have as single cells and, thus, behave as parts of a multicellular organism. With the advent of single-cell technologies, including microfluidics, it will be interesting to see how these events take place, not only in the bacterial cytosol, but also at the epigenetic level. Another emerging field of investigation concerns the study of symbiotic events: besides the well-known bacterial origin of mitochondria and chloroplasts in eukaryotic cells, an increasing number of studies show that microbes influence eukaryotic life in an unanticipated way. This includes not only the many spectacular studies on microbiotas and their influence on cells, organs, and whole organisms, but also studies on the influence of endosymbionts, such as Wolbachia, on the cell physiology and properties of host insects. It is clear that cell biologists and microbial biologists still have many subjects to share and long-lasting interactions to come.
Acknowledgments
We thank Edith Gouin and Matteo Bonazzi for the figure. We apologize to colleagues whose work could not be included in this article owing to space limitations. Work in the laboratory was supported by the Institut Pasteur, Institut National de la Santé et de la Recherche Médicale (INSERM Unité 604), Institut National de la Recherche Agronomique (INRA Unité Sous Contrat 2020), Institut Pasteur (PTR460 and PTR521 to J.P.C.), L’Agence Nationale de la Recherche (ANR) (ANR-15-CE15-0017 StopBugEntry to J.P.C.), Fondation Le Roch Les Mousquetaires, Fondation Balzan and European Research Council (ERC) Advanced Grant (670823 BacCellEpi to P.C.). P.C. is an International Senior Research Scholar of the Howard Hughes Medical Institute.
References
- 1.Hook R. Micrographia or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses. Royal Society; 1665. [Google Scholar]
- 2.van Leuwenhook A. Letter of 21 April 1676 to the Royal Society. London: Royal Society; 1676. [Google Scholar]
- 3.Cossart P, et al. Cellular microbiology emerging. Science. 1996;271:315–316. doi: 10.1126/science.271.5247.315. [DOI] [PubMed] [Google Scholar]
- 4.Kocks C, et al. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 5.Welch MD, et al. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 6.Isberg RR, et al. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 8.Blasi J, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo G, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo G, et al. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, et al. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci U S A. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson SR, et al. The positioning of cytoplasmic protein clusters in bacteria. Proc Natl Acad Sci U S A. 2006;103:8209–8214. doi: 10.1073/pnas.0600919103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 14.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 15.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]