Abstract
Much environmental enrichment for laboratory animals is intended to enhance animal welfare and normalcy by providing stimulation to reduce ‘boredom’. Behavioural manifestations of boredom include restless sensation-seeking behaviours combined with indicators of sub-optimal arousal. Here we explored whether these signs could be reduced by extra daily play opportunity in laboratory ferrets. Specifically, we hypothesised that playtime would reduce restlessness, aggression, sensation-seeking and awake drowsiness, even 24h later in the homecage. Female ferrets (n = 14) were group housed in enriched multi-level cages. Playtime involved exploring a room containing a ball pool, paper bags, balls containing bells, and a familiar interactive human for 1h. This was repeated on three consecutive mornings, and on the fourth morning, homecage behaviour was compared between ferrets who had experienced the playtime treatment versus control cagemates who had not. Their investigation of stimuli (positive = mouse odour or ball; ambiguous = empty bottle or tea-strainer; and negative = peppermint or bitter apple odour) was also recorded. We then swapped treatments, creating a paired experimental design. Ferrets under control conditions lay awake with their eyes open and screeched significantly more, but slept and sat/stood less, than following playtime. They also contacted negative and ambiguous stimuli significantly more under control conditions than they did following playtime; contact with positive stimuli showed no effects. Attempts to blind the observer to treatments were unsuccessful, so replication is required, but the findings suggest that playtime may have reduced both sub-optimal arousal and restless sensation seeking behaviour, consistent with reducing boredom.
Keywords: Animal welfare, Boredom, Environmental enrichment, Exploration, Ferrets, Laboratory animals
Introduction
Many environmental enrichment (EE) attempts are intended at least partly to relieve boredom, either stated explicitly or implicitly, such as when the aim is to increase ‘stimulation’, ‘exploration’ or ‘cognitive challenge’ (e.g. Anderson & Wood 2001; Celli et al. 2003; Wells 2004; Meehan & Mench 2007; Puppe et al. 2007; Langbein et al. 2009). EE has been well defined before, and can encompass any environmental or husbandry modification that increases the welfare or biological functioning of a captive animal (e.g. Chamove 1989; Newberry 1995; Patterson-Kane 2001; Swaisgood & Shepherdson 2005). In the case of laboratory animals, EE is additionally important for increasing animal normalcy, to maximise the external validity of research (Bayne & Würbel 2014). The specific aims can vary, such as reducing fear (e.g. providing secluded shelters) or satisfying species-specific needs (e.g. providing perches for arboreal species), but it is those aimed at providing sensory or cognitive stimulation (e.g. novel objects, sensory stimuli, or exploration) that are particularly relevant for combating boredom (Meehan & Mench 2007; Manteuffel et al. 2009; Wells 2009; Meagher 2019). Opportunity to play generally could be effective, as play has been suggested as a mechanism for countering boredom (e.g. Burghardt 1984; Held & Špinka 2011; Burghardt 2014; Ahloy-Dallaire et al. 2018). However, until recently, objective indicators of boredom were lacking, so it was difficult to assess whether stimulating EE was ever successful in tackling it.
Boredom is a negative emotion, which is caused by monotony that fails to engage attention and to maintain optimal arousal levels (Wemelsfelder 2005; Eastwood et al. 2012; Burn 2017). It is associated with a motivation for almost anything different or more arousing than the stimuli available (Mason & Burn 2011; Meagher & Mason 2012; Meagher 2019). The motivation for general stimulation as being key to objectively indicating boredom was identified and used by Meagher and Mason (2012) who distinguished possible reasons why environmentally unenriched farmed mink were observed to lie awake with their eyes open more than enriched mink (Meagher et al. 2013). They suggested that if lying awake was due to boredom, the mink without EE would voluntarily interact with diverse stimuli ranging from pleasant to unpleasant, whereas this would not be the case for the alternative explanations of apathy or anhedonia. Mink lacking EE did indeed interact with ambiguous and negative stimuli more readily than enriched mink did, indicating that they sought general stimulation – even if it was not pleasant – which is consistent with boredom. Those results were largely replicated in a follow up study (Meagher et al. 2017).
Motivation for general stimulation is part of a more general aversion to a monotonous situation, so manifestations of boredom can present as stimulus-seeking (as in the mink), or as restlessness, risk taking, unprovoked aggression, or escape behaviour (Burn 2017). However, this set of behaviours is not entirely unique to boredom, because some of the manifestations of it could also occur in other states, such as excitement, exploration, frustration, pain or playfulness. Therefore, it is the seemingly paradoxical juxtaposition of these highly active behaviours versus low arousal states, such as lying awake and yawning, that seems to characterise boredom (Berlyne 1960; Wemelsfelder 2005; Fahlman et al. 2013). This is because boredom seems to occur when stimulation is of insufficient quality to maintain optimal arousal levels, making the animal drowsy but not tired, and motivating it to raise its arousal levels by whatever means possible (Burn 2017).
It is these two classes of indicators (drowsiness and arousal-seeking behaviours) that we chose to measure when assessing whether additional playtime could help reduce potential boredom in laboratory ferrets (Mustela putorius furo). Not all low arousal behaviours are relevant to boredom, because different types of inactivity can have very different implications for animal welfare, but lying awake with eyes open is one of the most relevant to boredom (Meagher et al. 2013).
Playtime, in ‘playrooms’ outside the home environment, has been used as putative EE in species including rats (Widman & Rosellini 1990), pigs (Casey et al. 2007), dogs (Adams et al. 2004), cats (Wilson et al. 1965), and primates (reviewed in Rennie & Buchanan-Smith 2006). Playing and exploration opportunities can enhance cognitive function (Wilson et al. 1965; Pereira et al. 2007) (but see Bennett et al. 2006) and encourage general exploration (Widman & Rosellini 1990) over the long term. On the other hand, in primates at least, EE within the homecage appeared more effective in terms of enhancing welfare than were regular playtimes, with primates performing increased abnormal behaviour upon being returned to barren cages after playtimes than without playtimes (reviewed in Rennie & Buchanan-Smith 2006). There could therefore be some concern that playtime benefits are only transient, and that there could even be a negative contrast effect: the playtime could increase homecage restlessness if the animal learns that the homecage is insufficiently stimulating compared with the playroom.
Playtimes have not yet been investigated in terms of their potential to reduce animal boredom specifically. If they are effective in this respect, they should ideally not just reduce boredom during the playtime itself, but also to some extent back in the homecage, indicating that the playtime has satisfied the motivation for greater stimulation. In the current study, we therefore aimed to investigate the hypothesis that, if playtime reduces boredom even back in the homecage, it would decrease behaviour indicating both stimulus-seeking and suboptimal arousal. We tested this in laboratory ferrets in their homecages one day after playtime. We used a playtime paradigm designed to offer all types of play: locomotor, social, object, and exploratory play (Burghardt 1984).
Materials and Methods
Animal housing and husbandry
Fourteen adult female pigmented ferrets were used. They were housed long term to participate in other studies. They had been obtained from Highgate Farms (UK) from 12-16 weeks old, and weighed between 670 and 1070g (mean±SD = 891±110g) at the time of testing. Nine of the ferrets were 1 year old, and five were 2-3 years old. For the purposes of other studies (e.g. Town et al. 2017) unrelated to the current paper, the five older ferrets were chronically implanted for bilateral electrophysiological recording from auditory cortex (Warp-16 microdrives (Neuralynx, MT), housing 16 independently moveable tungsten microelectrodes (WPI Inc., FL)). All animals were also trained on auditory discrimination tasks which required restricted access to water in their home cage during testing, but they participated in this study during their weeks off when they had unrestricted access to water in their home cage; they had a minimum of 65h ad lib water before participating.
Ferrets were housed in a room maintained at 15-24°C, with artificial lighting switched on according to their winter cycle at 8:00 and off at 18:00h. All ferrets had access to food (VitalinTM chicken and rice pellets, Grove Pet Foods, Lincoln) and water ad libitum. Ferrets were socially housed in multi-tier cages that could be interconnected via tunnels. During the data collection period of this study, ferrets were housed in groups of four in a single multi-level cage (175x90x74cm, four levels accessible via ramps, Tecniplast). Cages were provided with with woodshavings as bedding, paperwool, green plastic tunnels, small cardboard boxes and large paper bags.
All ferrets were allowed outside their cages to explore freely in their holding room every day at 12:30h, during cage cleaning. During this period (45-60 mins) they explored the floor of the room and could interact with conspecifics from and in other cages. The ferrets’ social groups were mixed and re-formed every week. The ferrets were also regularly handled and stroked by staff members.
This study was ethically approved by the Clinical Research and Ethical Review Board (CRERB) at the Royal Veterinary College, reference number URN 2017 1755-3.
Playtime treatment
For three consecutive days (Monday-Wednesday) two of the four ferrets within the experimental cage received 1h of extra playtime. This occurred at 10:00h-11:00h, on the basis of pilot observations that revealed this as the ferrets’ most active daytime period. The playtime treatment involved the ferrets being allowed out of their homecage in the holding room with two ferrets from another cage, similar to that occurring during cage-cleaning, but extra stimuli were provided, such as tunnels and balls (Table 1). The experimenter (JR) was also present to supervise and provide additional voluntary interaction with these ferrets. The remaining two cagemates stayed within the cage and acted as controls.
Table 1. Overview of the stimuli available to ferrets during the 1-hour of extra playtime.
| Playtime stimuli | Specifications |
|---|---|
| Rigid Tunnel | SnuggleSafe Way to Go Fun Tunnel 90cm x 15cm |
| Hard Brown Tube Piping | Short Plumbing Pipe |
| Ball with Bell (x4) | Bell Ball Cat Toys (Aimé) - Pack of 4, 10.7 x 3.6 x 15 cm |
| Plastic Ball (x6) | Marshall Pet Products Pop-N-Play Ball Pit Balls |
| Large Brown Empty Paper Sack | Previously contained ferret dry food (Vitalin pellets) |
These stimuli were partly on the basis of recommendations from a 7-chamber EE study investigating motivation in ferrets for different types of EE (Reijgwart et al. 2017).
Each week a different pair of ferrets was allocated to the playtime treatment, while their cagemates acted as controls. By the end of the 8-week study, all ferrets had experienced both treatments. This created a paired experimental design, unbalanced across cages because of the weekly mixing of social groups. Sampling was primarily opportunistic, based on which ferrets were off-study on a given week and whether animals had previously experienced been in the playtime or control group. Seven ferrets experienced playtime first, and seven control first.
Homecage Behavioural Observations
On the fourth day (Thursday, after three treatment days), an observation of homecage behaviour was conducted by the experimenter (JR), who stood quietly 1m away from the homecage. This occurred at 10:00-10:30h, i.e. at the same time as the playtime treatment had started on the preceding days, and 24h after the start of the most recent treatment.
Live behavioural observations were developed on the basis of a pilot study, which had been conducted over 1 week preceding the study and which also served as an attempt to habituate the ferrets to the observations. The behavioural ethogram is shown in Table 2. The 30 min protocol consisted of scan sampling of behaviour on a one-zero basis every 30 s for the four ferrets within the homecage, scanning from left to right, top to bottom (Martin & Bateson 2007). When a ferret performed multiple behaviours simultaneously, only the most fleeting behaviour was recorded so as not to miss it, based on a priority list (behaviours that occasionally occurred together, listed from highest priority to lowest were: Screeching > Biting > Chasing > Walk/Run > Standing).
Table 2. Ethogram of ferret behaviours and their relevance to the hypothesis.
| Behaviour | Definition | Hypothetical relevance |
|---|---|---|
| Biting | The animal bites another animal | Restlessness/stimulus-seeking |
| Chasing | The animal follows at a run another animal who is retreating | Restlessness/stimulus-seeking |
| Climbing | The animal moves along tunnel, or on a rope, cage bars or ramp | Restlessness/stimulus-seeking |
| Digging | The animal claws at the sawdust with paws/pushes the sawdust around with nose | Restlessness/stimulus-seeking |
| Drinking water | The animal is stationary consuming water | Restlessness/stimulus-seeking |
| Eating Food | The animal is stationary consuming food - mouth is chewing | Restlessness/stimulus-seeking |
| Allo-grooming | The animal strokes tongue/claws over another ferret's fur | Restlessness/stimulus-seeking |
| Lying with eyes open | The animal is lying down stationary with eyes open | Sub-optimal arousal |
| Out of Sight | The animal is out of sight for observation | Included for completeness |
| Screeching | The animal makes a vocal screeching noise | Restlessness/stimulus-seeking |
| Standing | The ferret stands stationary on all four feet for at least 2 seconds | Sub-optimal arousal |
| Sniffing Bars | The animal approaches the cage bars, sniffing and looking out with eyes open | Restlessness/stimulus-seeking |
| Self-grooming | The animal strokes tongue/claw over its fur | Restlessness/stimulus-seeking |
| Sitting | The animal is sitting stationary with head up and eyes open | Sub-optimal arousal |
| Sleeping | The animal is lying down stationary with head down and eyes closed | Sub-optimal arousal |
| Stretching | The animal is stretching | Sub-optimal arousal |
| Walking/running | The animal uses four limbs to locomote on a horizontal surface | Restlessness/stimulus-seeking |
| Yawning | The animal opens its mouth with head tilted backwards | Sub-optimal arousal |
The ethogram was based upon pilot investigations of homecage ferret behaviour patterns of interest. The pilot study consisted of instantaneous scans every 30s for a period of 1h starting at 10:00h and ending at 11:00h for a total of 1 week prior to commencing the behavioural observation study. The behaviours are separated according to whether they were hypothesised to signal restlessness/stimulus-seeking or suboptimal arousal aspects of behaviour, and thus decreased following playtime. They are all normal behaviours, so any differences would be relative between the two treatments rather than indicating that the behaviours always indicate restlessness/stimulus-seeking or suboptimal arousal.
Ideally, a person other than the experimenter would have administered the treatment, allowing the experimenter to remain blind to treatment during behavioural observations. However, due to personnel shortage, the experimenter had to both supervise the playtime treatment and conduct behavioural observations, so video recordings were taken to enable later blind scoring and testing of observer reliability. Despite this attempt, the video-recordings proved excessively dark, preventing identification of each ferret and observation of behaviour, so only the live-recordings could be analysed.
Stimulus Interaction
After completing the 30 min observation, all four ferrets were removed from their cage to explore the room for 5 min to awaken any who were drowsy. They were then placed back in their cage and presented with six different stimuli in a randomised order (Table 3). Each stimulus (aside from the ball with bell and empty plastic bottle) was presented inside a tea-leaf strainer, and each was attached to the outside of the cage for 2 min in the same position on the middle cage level, with approximately 15s between each stimulus. The ferrets’ interactions with the stimuli were entirely voluntary. The starting location of ferrets could not be controlled, but was noted and taken into account in analyses. The latency and duration of contact with the stimuli (the ferret physically touching the stimuli either with their nose or paws) was recorded live by the observer for each ferret for 2 min using a stopwatch. Again, video recordings were intended to provide data for later scoring, but these proved too dark for analysis.
Table 3. Overview of the stimuli presented to the ferrets.
| Stimuli | Effect | Rationale |
|---|---|---|
| Mouse bedding contained inside a tea-leaf strainer | Positive | Attractive to ferrets due to mice being prey in the wild |
| Ball with bell hung | Positive | Elicits a preference and a play response in ferrets (Reijgwart et al. 2017). |
| An empty tea-leaf strainer | Ambiguous | Novel with no apparent biological relevance |
| An empty plastic bottle | Ambiguous | Novel with no apparent biological relevance |
| Cotton wool soaked with 5ml of peppermint oil (Tisserand Aromatherapy® 100% extracted peppermint oil, Sayers Common, UK) contained inside a tea-leaf strainer | Negative | An aversive scent for the ferrets (as determined by headshakes and avoidance in the pilot study) |
| Cotton wool soaked with 5ml of bitter apple spray (Grannick’s Bitter Apple®) contained inside a tea-leaf strainer | Negative | Commercially available animal deterrent |
The stimuli were chosen following (Meagher & Mason 2012) and results of our pilot studies. They were hung on the outside bars in the central section of the homecage.
Statistical Analysis
Generalized Linear Mixed Models (GLMM) were used to analyse the data in SPSS, with Generalised Estimating Equations (GEE) being used in R when there were excessive zeroes (e.g. behaviours that most ferrets did not perform at all). For binary outcomes, models were checked for inflated standard errors; for continuous outcomes, models were checked for normality of residuals and homogeneity of variance, and the outcome transformed as necessary. Statistical significance is stated with two-tailed P-values < 0.05.
For the observations of unprovoked behaviour, many behaviours were too rare for statistical analysis, so the outcomes that could be tested were sleeping, lying with eyes open, sitting or standing stationary (sitting and standing summed together), walking/running, sniffing the bars, screeching, and aggression (screeching, biting and chasing summed together). The fixed factor predictors were treatment, time points, age/implant (considered together because animals with implants were older) and date/group (considered together because the groups of any four ferrets were each tested on unique dates), with ferret ID as a random factor. When there was complete separation of data (behaviour performed in one treatment and not at all in the other treatment), a non-parametric McNemar test was used.
For the stimulus interaction test, the effect of treatment was run in a GLMM across all ferrets, with whether or not the ferret investigated the stimuli as the outcome,. The fixed predictors were treatment, stimulus type (positive, ambiguous, or negative), their two-way interaction, ferret start position, and stimulus presentation order, with ferret ID, age/implant, and date/group as random factors. GLMMs were also run for only those ferrets who contacted the stimuli, and the measured outcomes tested were duration of, and latency to, contact. Latency was square root transformed to provide a normal distribution before running through the GLMM. The same predictors were used as with the previous GLMM. However, where insufficient degrees of freedom were observed to support the interaction, separate models were run per stimulus type (positive, ambiguous and negative stimuli). When a ferret did not contact a stimulus at all during the 2-minute observation, that data point was excluded as a missing value in the models of latency and duration of contact.
Results
Homecage Behavioural Observations
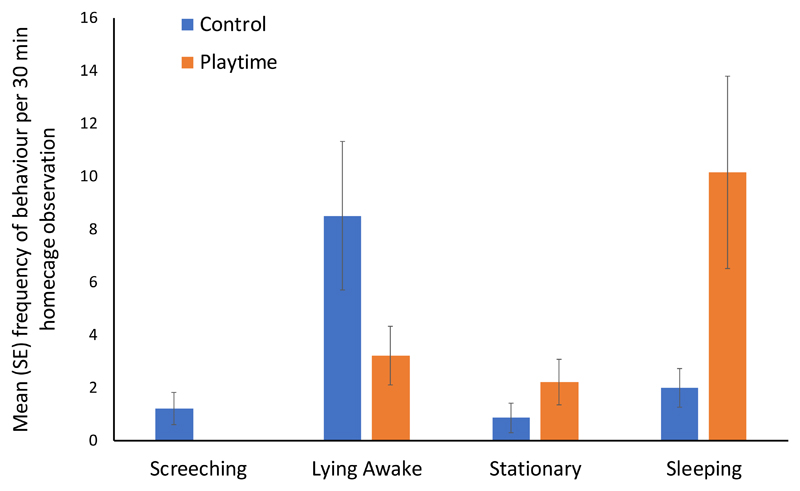
On the day after playtime, ferrets spent significantly more time sleeping (GEE: OD = 11.462; 95% CI [0.034, 0.227]; P < 0.001) and sitting (GEE: EO = 3.885; 95% CI [0.107, 0.619]; P = 0.002) than when in the control condition. In turn, ferrets in the control condition spent more time lying awake with eyes open (GMM: OD = 4.126; 95% CI [2.70, 6.260]; P < 0.001) and screeching (GEE: OD = 17.407; 95% CI [17.405, 17.405], P < 0.001). The statistically significant effects are shown in Figure 1. Walking/running and sniffing the bars showed no significant treatment effects. Signs of aggression other than screeching were too rare for analysis alone, but when combined with screeching to form an ‘overall aggression’ frequency, this showed no statistically significant effects.
Figure 1.
Mean ± SE frequency of screeching, lying awake with eyes open, sitting or standing stationary, and sleeping in ferrets who had and had not received extra playtime. The subjects were female ferrets (n = 14) in a paired experimental design. Behaviour was recorded every 30 s over a 30 min observation per ferret per treatment.
Younger ferrets without an implant spent more time screeching (GEE: OD = 3.427; 95% CI [3.427, 3.427], P < 0.001) and sitting (GEE: OD = 3.665; 96% CI [1.533, 8.760], P = 0.003) and less time sleeping (GEE: OD = 0.356; 95% CI [0.209, 0.606], P < 0.001) than older ferrets. They also exhibited increased frequencies of sniffing bars (GEE for SF: EO = 3.307; 95% CI [1.085, 10.086]; P = 0.035) and walking/running (GEE for WR: EO = 2.309; 95% CI [1.247, 4.275]; P = 0.008) than older animals. Time point and date/group showed no significant effect on behaviour.
Stimulus Interaction
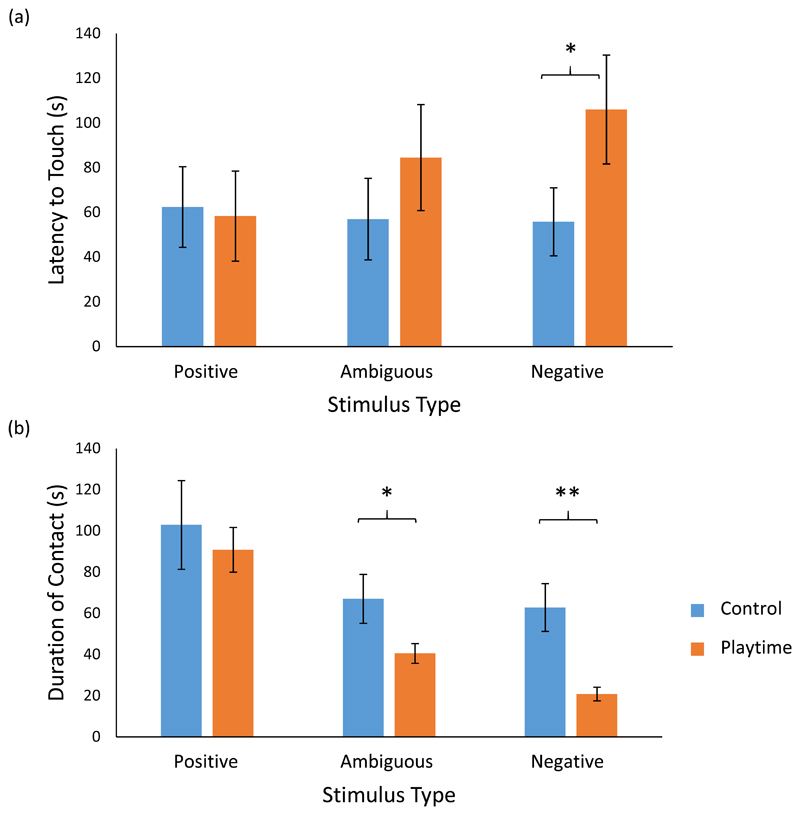
Ferrets in the control condition were more likely to contact stimuli than following the playtime treatment (GLMM: Odds +/- S.E. = 3.059 +/- 0.536, t = -2.217, P = 0.028). This effect was seen across stimulus types (Positive: Control = 12/14 ferrets vs Playtime = 10/14; Ambiguous: Control = 11/14 vs Playtime = 10/14; and Negative: Control = 11/14 vs Playtime = 9/14). Ferret starting position, stimulus type and order of presentation showed no effects on ferret interactions with the stimuli.
Of ferrets who did contact stimuli, playtime significantly increased latencies to contact the negative stimuli (GLMM: Coeff +/- S.E. = 0.974 +/- 0.376, t = 2.592, P = 0.012), with a non-significant trend in the same direction for ambiguous stimuli (Coeff +/- S.E. = 0.948 +/- 0.482; 1.967; P = 0.055; Figure 2). There was no significant difference or trend in latency to contact the positive stimuli.
Figure 2.
Interactions with stimuli presented to ferrets who had and had not been given extra playtime. The mean ± SE latency to contact the stimuli is shown in (a), and the mean ± SE duration of contact with stimuli is shown in (b). Positive stimuli = ball with bell and mouse bedding; ambiguous = empty plastic bottle and empty tea-leaf strainer; and negative = bitter apple spray and peppermint oil. The subjects were female ferrets (n = 14) in a paired experimental design, with control data in blue and extra playtime data in orange.
After playtime ferrets spent significantly less time interacting with the ambiguous (GLMM: Coeff +/- S.E. = -0.998 +/- 0.379; t = 2.637; P = 0.011) and negative (Coeff +/- S.E. = -1.733 +/- 0.278; t = 6.231; P < 0.001) stimuli, than under control conditions. Again, there was no significant treatment effect on duration interacting with the positive stimuli.
Discussion
The results suggest that playtime reduces behaviours consistent with boredom in laboratory ferrets, even measured 24h after the most recent play event. It seems that, just as boredom-like states sometimes appear to prompt play in animals (Burghardt 1984; Held & Špinka 2011; Ahloy-Dallaire et al. 2018), the inverse may also be true; play can reduce signs of boredom.
The increased interactions of the control ferrets with negative and ambiguous stimuli is entirely consistent with the aforementioned research in environmentally enriched versus standard-housed mink (Meagher & Mason 2012; Meagher et al. 2017), and this combined with aggressive screeching and lying awake further characterises a boredom-like state (Burn 2017). If playtime can help reduce general aggression in laboratory animals, this could be of great value for some species where aggression is a significant problem. However, in the ferrets, overall aggression was rare and showed no significant treatment effect, with only the screeching vocalisation being reduced after play. It is possible that the screeching was not truly aggressive, although it is described as occurring mainly in negative contexts (Boyce et al. 2001), so its reduction via playtime is consistent with improved welfare. In future, recording screeching alongside the other behaviours with which it occurs would help in interpreting its social context.
When ferrets had not had playtime in the current study, they chose to interact with even negative stimuli: scents that had made them gape, headshake and withdraw in our pilot studies. This is consistent with previous observations that animals in monotonous situations seemingly prefer even unpleasant experiences over their existing monotony, which perhaps confirms the aversive nature of boredom (Burn 2017). Examples include humans self-administering electric shocks when asked to think their own thoughts when alone for 15 min (Wilson et al. 2014), rats and hamsters choosing aversive food after eating solely their preferred food for several consecutive days (Galef & Whiskin 2003, 2005), and mink in barren cages choosing to interact with predator cues, handling gloves and sudden air puffs (Meagher & Mason 2012).
In the ferrets, playtime increased sleeping (i.e. lying down with eyes closed, not open), and sitting/standing stationary, neither of which we predicted. These are low arousal behaviours, but they do not suggest that arousal was sub-optimal after playtime, because they did not co-occur with obvious attempts to raise arousal. One possible explanation for these low arousal behaviours could be that the ferrets were simply tired out by the playtime. However, the fact that the ferrets responded just as readily to the positive stimuli after playtime as they did in the control condition, makes fatigue an unlikely explanation. Instead, their willingness to investigate positive stimuli, but not ambiguous or negative stimuli, suggests that they were more ‘choosy’ about their stimulation on the day after playtime than in the control condition. This choosiness suggests that the increased low arousal behaviour after playtime could indicate a form of satisfaction or relaxation; the playtime may thus exemplify EE that has satisfied the motivation for general stimulation (Meagher 2019).
Our attempts to blind the observer to the treatments were unsuccessful, which means that the results require replication under blinded conditions to eliminate the possibility of expectation bias (Tuyttens et al. 2014). We limited the potential for bias as much as possible before the experiment began, by discussing it explicitly and encouraging an impartial attitude; for example, whilst we hypothesised that playtime would reduce boredom, we discussed the possibility that instead we could find an equally noteworthy contrast effect if playtime caused the ferrets to perceive the homecage as more, rather than less, boring (as described in Rennie & Buchanan-Smith 2006). We also discussed how to interpret non-significant results to help counter publication bias towards significant outcomes (Fanelli 2010; Dwan et al. 2013). If we were thus successful in avoiding expectation bias, then the results do indeed suggest that playtime reduced behavioural indications both of sub-optimal arousal (lying awake with eyes open) and of motivation for greater stimulation (agonistic screeching, and interactions with negative and ambiguous stimuli) (Burn 2017).
It is worth noting that even the control ferrets here did have EE in their homecage and explored their holding room daily, and the results should not be interpreted as showing that their standard EE was ineffective. For ethical reasons, we did not compare the control treatment against a barren cage, and it is possible that we would have found many more signs of compromised welfare in the barren environment had we done so. It is also important to remember that the purpose of different EE varies, such as refuges to provide security, so not all beneficial EE functions to provide stimulation or reduce boredom.
Conclusion and animal welfare implications
In conclusion, subject to replication, the results here suggest that offering playtime to laboratory animals may be an effective refinement to reduce potential boredom and promote a more ‘relaxed’ state, even outside the playtime context.
Acknowledgements
We are grateful for funding for this project: JR was supported by a BBSRC funded London Interdisciplinary Doctoral Training Studentship; JKB was supported by Wellcome Trust (Grant Ref 098418/Z/12/Z). We would also like to thank Dr Yu-Mei Ruby Chang for statistical advice and Stephen Town and Joseph Sollini for support in testing the ferrets. The manuscript was approved for submission by RVC (manuscript number: PPS_01949).
References
- Adams KM, Navarro AM, Hutchinson EK, Weed JL. A Canine Socialization and Training Program at the National Institutes of Health. Lab Animal. 2004;33:32–37. doi: 10.1038/laban0104-32. [DOI] [PubMed] [Google Scholar]
- Ahloy-Dallaire J, Espinosa J, Mason G. Play and optimal welfare: Does play indicate the presence of positive affective states? Behavioural Processes. 2018;156:3–15. doi: 10.1016/j.beproc.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Anderson RC, Wood JB. Enrichment for Giant Pacific Octopuses: Happy as a Clam? Journal of Applied Animal Welfare Science. 2001;4:157–168. [Google Scholar]
- Bayne K, Würbel H. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Revue scientifique et technique (International Office of Epizootics) 2014;33:273–280. doi: 10.20506/rst.33.1.2282. [DOI] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiology of Learning and Memory. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal, and curiosity. McGraw-Hill; New York: 1960. pp. 144–192. [Google Scholar]
- Boyce SW, Zingg BM, Lightfoot TL. Behavior of Mustela Putorius Furo (The Domestic Ferret) Veterinary Clinics of North America: Exotic Animal Practice. 2001;4:697–712. doi: 10.1016/s1094-9194(17)30032-4. [DOI] [PubMed] [Google Scholar]
- Burghardt GM. On the origins of play. In: Smith PK, editor. Play in animals and humans. Blackwell; Oxford: 1984. pp. 5–41. [Google Scholar]
- Burghardt GM. A brief glimpse at the long evolutionary history of play. Animal Behavior and Cognition. 2014;1:90–98. [Google Scholar]
- Burn CC. Bestial boredom: a biological perspective on animal boredom and suggestions for its scientific investigation. Animal Behaviour. 2017;130:141–151. [Google Scholar]
- Casey B, Abney D, Skoumbordis E. A playroom as novel swine enrichment. Lab Animal. 2007;36:32. doi: 10.1038/laban0307-32. [DOI] [PubMed] [Google Scholar]
- Celli ML, Tomonaga M, Udono T, Teramoto M, Nagano K. Tool use task as environmental enrichment for captive chimpanzees. Applied Animal Behaviour Science. 2003;81:171–182. [Google Scholar]
- Chamove AS. Environmental enrichment: A review. Animal Technology. 1989;40:155–176. [Google Scholar]
- Dwan K, Gamble C, Williamson PR, Kirkham JJ. Systematic Review of the Empirical Evidence of Study Publication Bias and Outcome Reporting Bias — An Updated Review. PLoS ONE. 2013;8:e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood JD, Frischen A, Fenske MJ, Smilek D. The Unengaged Mind: Defining Boredom in Terms of Attention. Perspectives on Psychological Science. 2012;7:482–495. doi: 10.1177/1745691612456044. [DOI] [PubMed] [Google Scholar]
- Fahlman SA, Mercer-Lynn KB, Flora DB, Eastwood JD. Development and validation of the multidimensional state boredom scale. Assessment. 2013;20:68–85. doi: 10.1177/1073191111421303. [DOI] [PubMed] [Google Scholar]
- Fanelli D. Do Pressures to Publish Increase Scientists' Bias? An Empirical Support from US States Data. PLoS ONE. 2010;5:e10271. doi: 10.1371/journal.pone.0010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Jr, Whiskin EE. Preference for novel flavors in adult Norway rats (Rattus norvegicus) Journal of Comparative Psychology. 2003;117:96–100. doi: 10.1037/0735-7036.117.1.86. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Whiskin EE. Differences between golden hamsters (Mesocricetus auratus) and Norway rats (Rattus norvegicus) in preference for the sole diet that they are eating. Journal of Comparative Psychology. 2005;119:8–13. doi: 10.1037/0735-7036.119.1.8. [DOI] [PubMed] [Google Scholar]
- Held SDE, Špinka M. Animal play and animal welfare. Animal Behaviour. 2011;81:891–899. [Google Scholar]
- Langbein J, Siebert K, Nürnberg G. On the use of an automated learning device by group-housed dwarf goats: Do goats seek cognitive challenges? Applied Animal Behaviour Science. 2009;120:150–158. [Google Scholar]
- Manteuffel G, Langbein J, Puppe B. From operant learning to cognitive enrichment in farm animal housing: bases and applicability. Animal Welfare. 2009;18:87–95. [Google Scholar]
- Martin P, Bateson P. Measuring behaviour: an introductory guide. Cambridge University Press; Cambridge: 2007. pp. 1–171. [Google Scholar]
- Mason GJ, Burn CC. Chapter 7: Behavioural restriction. In: Appleby MC, Mench JA, Olsson A, Hughes BO, editors. Animal Welfare. CABI Publishing; 2011. pp. 98–119. [Google Scholar]
- Meagher RK, Mason GJ. Environmental enrichment reduces signs of boredom in caged mink. PLoS ONE. 2012;7:e49180. doi: 10.1371/journal.pone.0049180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RK, Campbell DLM, Dallaire JA, Díez-León M, Palme R, Mason GJ. Sleeping tight or hiding in fright? The welfare implications of different subtypes of inactivity in mink. Applied Animal Behaviour Science. 2013;144:138–146. [Google Scholar]
- Meagher RK, Campbell DL, Mason GJ. Effects of enrichment on boredom-like states in mink and their behavioural correlates: a replicate. Applied Animal Behaviour Science. 2017;197:112–119. [Google Scholar]
- Meagher RK. Is boredom an animal welfare concern? Animal Welfare. 2019;28:21–32. [Google Scholar]
- Meehan CL, Mench JA. The challenge of challenge: Can problem solving opportunities enhance animal welfare? Applied Animal Behaviour Science. 2007;102:246–261. [Google Scholar]
- Newberry RC. Environmental enrichment: Increasing the biological relevance of captive environments. Applied Animal Behaviour Science. 1995;44:229–243. [Google Scholar]
- Patterson-Kane EG. Environmental enrichment for laboratory rats: a review. Animal Technology. 2001;52:77–84. [Google Scholar]
- Pereira LO, Arteni NS, Petersen RC, da Rocha AP, Achaval M, Netto CA. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiology of Learning and Memory. 2007;87:101–108. doi: 10.1016/j.nlm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Puppe B, Ernst K, Schön PC, Manteuffel G. Cognitive enrichment affects behavioural reactivity in domestic pigs. Applied Animal Behaviour Science. 2007;105:75–86. [Google Scholar]
- Reijgwart ML, Vinke CM, Hendriksen CFM, van der Meer M, Schoemaker NJ, van Zeeland YRA. Ferrets' (Mustela putorius furo) enrichment priorities and preferences as determined in a seven-chamber consumer demand study. Applied Animal Behaviour Science. 2017;180:114–121. [Google Scholar]
- Rennie A, Buchanan-Smith H. Refinement of the use of non-human primates in scientific research. Part II: housing, husbandry and acquisition. Animal Welfare. 2006;15:215. [Google Scholar]
- Swaisgood RR, Shepherdson DJ. Scientific approaches to enrichment and stereotypies in zoo animals: what's been done and where should we go next? Zoo Biology. 2005;24:499–518. [Google Scholar]
- Town SM, Brimijoin WO, Bizley JK. Egocentric and allocentric representations in auditory cortex. PLoS Biology. 2017;15:e2001878. doi: 10.1371/journal.pbio.2001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyttens FAM, de Graaf S, Heerkens JLT, Jacobs L, Nalon E, Ott S, Stadig L, Van Laer E, Ampe B. Observer bias in animal behaviour research: can we believe what we score, if we score what we believe? Animal Behaviour. 2014;90:273–280. [Google Scholar]
- Wells DL. A review of environmental enrichment for kennelled dogs, Canis familiaris. Applied Animal Behaviour Science. 2004;85:307–317. [Google Scholar]
- Wells DL. Sensory stimulation as environmental enrichment for captive animals: A review. Applied Animal Behaviour Science. 2009;118:1–11. [Google Scholar]
- Wemelsfelder F. Animal boredom: understanding the tedium of confined lives. In: McMillan FD, editor. Mental Health And Well-Being In Animals. Blackwell Publishing; Oxford: 2005. pp. 79–93. [Google Scholar]
- Widman DR, Rosellini RA. Restricted daily exposure to environmental enrichment increases the diversity of exploration. Physiology & Behavior. 1990;47:57–62. doi: 10.1016/0031-9384(90)90042-3. [DOI] [PubMed] [Google Scholar]
- Wilson M, Warren J, Abbott L. Infantile stimulation, activity, and learning by cats. Child Development. 1965;36:843–853. [PubMed] [Google Scholar]
- Wilson TD, Reinhard DA, Westgate EC, Gilbert DT, Ellerbeck N, Hahn C, Brown CL, Shaked A. Just think: The challenges of the disengaged mind. Science. 2014;345:75–77. doi: 10.1126/science.1250830. [DOI] [PMC free article] [PubMed] [Google Scholar]