Abstract
Recent neuroimaging studies in OCD have reported structural alterations in the brain, not limited to frontostriatal regions. While Diffusion Tensor Imaging (DTI) is typically used to interrogate WM microstructure in OCD, additional imaging metric, such as Magnetization Transfer Imaging (MTI), allows for further identification of subtle but important structural changes across both GM and WM. In this study, both MTI and DTI were utilised to investigate the structural integrity of the brain, in OCD in relation to healthy controls. 38 adult OCD patients were recruited, along with 41 age- and gender-matched controls. Structural T1, MTI and DTI data were collected. Case-control differences in Magnetization Transfer Ratio (MTR) and DTI metrics (FA, MD) were examined, along with MTR/DT-related associations with symptom severity in patients. No significant group differences were found across MTR, FA, and MD. However, OCD symptom severity was positively correlated with MTR in a distributed network of brain regions, including the striatum, cingulate, orbitofrontal area and insula. Within the same regions, OCD symptoms were also positively correlated with FA in WM, and negatively correlated with MD in GM. These results indicate a greater degree of myelination in certain cortical and subcortical regions in the more severe cases of OCD.
Keywords: Magnetization transfer imaging, Obsessive-Compulsive disorder, Diffusion tensor imaging, MTI, OCD, DTI
1. Introduction
Obsessive Compulsive Disorder (OCD) is characterized by unwanted, intrusive, and recurrent thoughts (obsessions); and/or repetitive mental actions or behaviours (compulsions) that are performed in response to obsessions or according to rigid rules (APA, 2013). OCD symptoms are generally characterized by themes (or content) including the fear of contamination and washing/cleaning; “shameful” obsessions and checking; and symmetry and arranging compulsions (Abramowitz et al., 2010; Yagi et al., 2017). The condition affects approximately 3.1% of the general population (Fontenelle and Hasler, 2008), leading to impairment of functioning and quality of life of the individuals, also impacting families and wider society (Fontenelle and Hasler, 2008; Huppert et al., 2009).
Reviews on the neurobiological mechanism of OCD propose the cortico-striatal-thalamic-cortical (CSTC) model to explain disturbances in the reward system (Frydman et al., 2016; Hazari et al., 2019). Currently available biological models of OCD emphasise involvement of dysfunctional communication between frontal cortical regions (especially orbitofrontal cortex, OFC), and the striatum (Chamberlain et al., 2005; Frydman et al., 2016; Graybiel and Rauch, 2000; Oscar F. Gonçalves et al., 2017; van Velzen et al., 2014; Whiteside et al., 2004). More recent whole brain analyses further indicated that widespread neural networks, including additional parietal, occipital, insular, and cerebellar regions, are also involved in the pathophysiology of OCD (Burguière et al., 2015; Eng et al., 2015; Gan et al., 2017; Menzies et al., 2008a; Piras et al., 2015).
DTI is a commonly used neuroimaging technique to investigate white matter integrity where fractional anisotropy (FA) and mean diffusivity (MD) are the most common indexes to study (Basser et al., 1994; Frydman et al., 2016; Gonçalves et al., 2017). FA reflects the degree of water anisotropy diffusion and is linked to fibre orientation, while MD indicates average diffusivity in all directions, which is associated with tissue density (Eng et al., 2015; Gonçalves et al., 2017; Zhou et al., 2018). Individual DTI studies and meta-analyses findings in OCD have revealed structural abnormalities in widespread regions mainly including OFC, ACC, and striatum (Hazari et al., 2019). However, DTI studies in OCD using either Voxel Based or Tract-Based Spatial Statistics (TBSS) have revealed rather heterogeneous results. For instance, FA values in OCD have been reported to be increased (Cannistraro et al., 2007; Gong et al., 2011; Nakamae et al., 2008; Yoo et al., 2007; Zarei et al., 2011) or decreased (Bora et al., 2011; Chiu et al., 2011; Fan et al., 2016; Fontenelle et al., 2011; Gan et al., 2017; Garibotto et al., 2010; Nakamae et al., 2011; Oh et al., 2012; Zhou et al., 2018) or either increased or decreased in different brain regions (Hartmann et al., 2016; Lochner et al., 2012; Menzies et al., 2008b) such as corpus callosum (CC), cingulum bundle, and anterior and posterior limb of the internal capsule (ALIC and PLIC). These inconsistent results could be due to limited power to detect small effect sizes, heterogeneous symptom dimensions across recruited samples or DTI specific challenges. For instance, obtained DTI values can be influenced by other factors such as inflammation, and complex crossing fibres, which may complicate interpretation (Alexander et al., 2011). As such, it is suggested that the combination of DTI with other complementary measures sensitive to myelination, may be useful to help overcome inconsistences, and assist with interpretation. One popular complementary measure is Magnetization Transfer Ratio (MTR), derived from Magnetization Transfer (MT) imaging, (Alexander et al., 2011; Mandl et al., 2015).
MT is an indirect myelin imaging technique used to detect magnetization transfer that occurs between free and bound water pools in GM and WM through chemical exchange and dipolar coupling (Alexander et al., 2011; Kit and Stephenson, 2016; Mandl et al., 2015; Mossahebi, 2013; Varma et al., 2015). More technical information is explained in detail elsewhere (Alexander et al., 2011; Kit and Stephenson, 2016; Mossahebi, 2013; Varma et al., 2015). Increased MTR values are associated with higher concentrations of lipids and proteins in myelinated axons in white matter (Alexander et al., 2011; Mossahebi, 2013). MTR has been used in a wide range of neurological diseases such as multiple sclerosis (MS) (Bodini et al., 2014; Brown et al., 2013; Jonkman et al., 2016; Nantes et al., 2017), Schizophrenia (Mandl et al., 2015, 2013), Alzheimer’s Disease and mild cognitive impairment MCI (Alexander et al., 2011; Bouhrara et al., 2018), brain development and aging (Armstrong et al., 2004; Van Buchem et al., 2001), Supranuclear Palsy (Saini et al., 2014), and major depressive disorder MDD (Kumar et al., 2004), as a sensitive measure of gray and white matter abnormalities.
To the best of our knowledge, there has been only one study that used combined MTR and DTI in OCD (Glahn et al., 2015). This prior study evaluated 14 unmedicated male OCD patients compared to 20 age- and sex-matched healthy controls. Compared to control subjects, patients with OCD had increased MTR in the left middle frontal gyrus and gray matter of right inferior frontal gyrus. On the other hand, OCD patients showed reduced FA in bilateral OFC and white matter of left medial frontal gyrus as well as increased apparent diffusion coefficient (ADC) in bilateral OFC, para-hippocampal regions and in left middle temporal lobe. However, this prior study did not correct findings for multiple comparisons and comprised a relatively small number of patients (14 male). Our objectives were thus to interrogate brain changes in OCD using the combined application of MTR and DTI in a larger sample; and to assess the association between brain structural measures and OCD symptom severity.
2. Methods
Participant eligibility was determined through a detailed telephone screening interview which included the Mini International Neuropsychiatric Interview (MINI) screener (Sheehan et al., 1998), questions relating to the exclusion criteria, general demographics, general medical and mental health history, history of alcohol and drug use, and MRI contraindications. All participants provided inform consent. This work has been carried out in accordance to the Declaration of Helsinki. The data used in this project is not available to public due to the sensitive nature of the questions asked, participants were assured that raw data would remain confidential.
Inclusion criteria for OCD and control groups involved the following: aged between 18-55 years, having normal to corrected to normal vision, and being fluent in English. A confirmation of OCD diagnosis using the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) was an inclusion criterion for the OCD group (First et al., 1998). Exclusion criteria for all participants included history of neurological disease or seizures, significant head injury or concussion, standard MRI contraindications, significant or sustained steroid use, history of alcohol abuse or dependence, and use of cannabis or other illicit drug use > 50 times.
Additional exclusion criteria for the OCD group included presence of a primary psychiatric disorder other than OCD (the assessment of 'primary' was based on a semi-structured general medical history questionnaire). Any ambiguous cases were resolved through consultation with the clinical team (including a psychiatrist specialising in OCD). Additional exclusion criteria for the control group included having a lifetime history of psychiatric illness. All subjects were assessed for the severity of obsessive-compulsive symptoms, depression and anxiety symptoms using the Obsessive-Compulsive Inventory Revised (OCI-R) (Foa et al., 2002), the Beck Depression Inventory (BDI) (Beck et al., 1988), and the State and Trait Anxiety Inventory (STAI) respectively (Marteau and Bekker, 1992).
2.1. Magnetic Resonance Imaging
Images were acquired at Monash Biomedical Imaging using a 3T SIEMENS Skyra scanner with a 32-channel head coil. Anatomical T1-weighted images were acquired using a Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequence with parameters: repetition time (TR) = 2300ms, echo time (TE) = 2.07ms, inversion time (TI) = 900ms, 192 slices, 1x1x1 mm isotropic.
Magnetization transfer (MT) images were obtained using a Siemens 3d Flash Low Angle Shot (3dFLASH) sequence, one of spoiled gradient echo sequences with MT saturated (MT-ON) and MT unsaturated (MT-OFF) and the following parameters: TR = 38ms, TE = 4.04ms, 60 slices, field of view (FOV read) = 238mm, 2.5x2.5x2.5 mm isotropic. Diffusion scans were also conducted with TR = 8800ms, TE = 110ms, 2.5x2.5x2.5 mm isotropic. Each scan obtained 67 volumes (60 b3000 s/mm2 directions with seven volumes of b0 evenly interspersed throughout the scan).
2.2. Image Processing
All the image processing performed in the Multi-modal Australian ScienceS Imaging and Visualisation Environment (MASSIVE) high performance infrastructure (Goscinski et al., 2014).
2.2.1. MTR
Individual brain masks were created using FSL (Jenkinson et al., 2005) and applied on the MT-ON and the MT MT-OFF images to remove non-brain tissues. Then, we computed the MTR maps by subtracting MT-ON images from their corresponding MT-OFF images and divided by MT-OFF (as equation below) using the qMRLab (Cabana et al., 2015). MTR value in each voxel was presented in percent unit where 0 means no signal loss and 100 represents total signal reduction due to magnetization transfer.
The MTR images were aligned to T1-weighted images. Then, T1-weighted were normalized to anatomical standard (i.e., MNI152) space along with the aligned MTR images. Finally, the MTR images were smoothed with an 8mm kernel using SPM12 (Statistical Parametric Mapping). All the pre-processed MTR maps were visually checked to confirm correct registration without any artefacts. In order to detect potential MTR outliers, the average of global MTR values for all participants (healthy controls and OCDs) were calculated individually. Any individual global MTR having greater or smaller value than ± 2 standard deviation were considered as an outlier and removed from the analysis. One healthy control subject was removed due to this quality checking step).
2.2.2. DTI
DTI data were processed using MRtrix/3.0_rc3 software and FMRIB library in FSL version 5.0.11 (https://mrtrix.readthedocs.io/, www.fmrib.ox.ac.uk/fsl/). Initially, we denoised and corrected data for eddy current distortions and head motion artefacts. Then we extracted brain tissue in FSL (Jenkinson et al., 2005). Subsequently, fractional anisotropy FA and mean diffusivity MD images as well as axial diffusivity (AD) and radial diffusivity (RD) were computed by fitting diffusion to the data tensors using FMRIB Diffusion Toolbox (FDT). Once all the DTI measures were computed, TBSS was used for the following steps (Smith et al., 2006): firstly, the most typical subject from our healthy controls was selected as target image instead of using default template. Then, all participants’ FA images were nonlinearly transformed to the target image and then affine transformed to MNI152 space (combined transformation process). Finally, MD, AD, and RD images were similarly aligned to the MNI space using FNIRT.
2.3. Statistical Analysis
Primary analyses: To determine group differences of whole brain MTR between controls and OCD patients, a non-parametric permutation approach (10,000 permutations) with threshold free cluster enhancement (TFCE) method was run for each contrast (CON > OCD and CON < OCD) using FSL’s randomise tool (Winkler et al., 2014), while controlling for depression (BDI), anxiety (STAI), age and gender as independent variables. To determine relationships between MTR and symptom severity in OCD, FSL’s randomise was again adopted (10,000 permutations, TFCE method) at the whole brain level, with OCI-R total scores as the covariate of interest, again controlling for BDI, STAI, age, and gender. Multiple comparison was corrected using family wise error (FWR) correction at significance level of p <.05 (Winkler et al., 2014). Following this, the significant regions from the MTR analysis were masked to standard GM and WM masks to create GM and WM clusters for further DTI analysis. The WM/GM masks we used were generated from a standard SPM plug-in tool, WFU_PickAtlas with zero mm dilation (https://www.fil.ion.ucl.ac.uk/spm/ext/#WFU_PickAtlas). Then, mean values of voxels within WM cluster were extracted for MTR, FA and RD maps, while the mean values within GM cluster were extracted for MTR and MD maps. Post-hoc analyses and plots were generated using GraphPad Prism version 7.0b (www.graphpad.com). Secondary exploratory analyses: To explore potential influences of medication, the OCD group was split into medicated sub-group (M=10 / F=10) and non-medicated sub-group (M=7 / F=9). OCI-R and MTR outcomes in both WM and GM clusters were tested between two groups using t-test.
3. Results
In total, 38 OCD patients and 41 healthy controls completed the study. One subject was excluded due to missing clinical data, two subjects were removed as failure of MTR quality standard described above. Therefore, the final analysis was conducted based on 36 OCD patients and 40 control subjects. Demographic and clinical data for the two study groups are presented in Table 1. The groups did not significantly differ in terms of age or gender. It is notable that mean OCI-R scores in patients were consistent with moderate severity of symptoms (Gönner et al., 2008; Wootton et al., 2015). As expected, depression and anxiety scores were significantly higher in the OCD group compared to controls.
Table 1.
Demographic and clinical measures (mean (SD)) of healthy controls (HC) and patients with OCD.
| Variables | HC (n=40) | OCD (n=36) | p | |
|---|---|---|---|---|
| Age, years | 35 (9.47) | 31.83 (9.14) | .14 | |
| Gender (M/F) | 20/20 | 17/19 | .89 | |
| BDI | 3.05 (4.81) | 21.13 (12.56) | <.001* | |
| STAI | 28.32 (7.80) | 47.77 (14.00) | <.001* | |
|
OCI-R Washing Obsessing Hoarding Ordering Checking Neutralizing |
NA |
31.47 (11.08) 5.13 (4.12) 7.97 (3.22) 3.3 (3.57) 4.72 (3.24) 5.8 (3.51) 4.52 (3.79) |
NA | |
| OCD Onset, years | NA | 19.86 (9.51) | NA | |
| OCD Duration, years | NA | 11.7 (7.62) | NA | |
|
Medication Fluoxetine Fluvoxamine Sertraline Paroxetine Escitalopram Quetiapine |
NA | Sample size 2 4 2 1 6 4 |
Dose, mg 40 (28.28) 122.5 (119) 200 40 41.6 (24.01) 53 (36.09) |
|
BDI=Beck Depression Index, STAI=State-Trait Anxiety Inventory, OCI-R=Obsessive-Compulsive Inventory Revised. Age, BDI, and STAI are compared using t-test, and gender using chi-square test. Seven patients were using Escitalopram but the mean (SD) across six of them is reported here because the dose of this medication was not available for one of the patients.
Patients with OCD did not show any significant differences in MTR compared to their healthy counterparts, after correction for multiple comparison (CON>OCD p=0.68, OCD>CON p=0.47). However, there was a significant positive correlation between total OCI-R scores and MTR in the patients, after statistical correction (Figure 1). We separated the significant regions based on MTR results to WM and GM clusters.
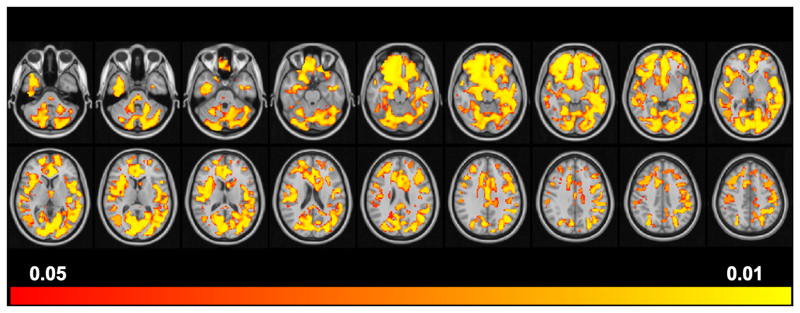
Figure 1.
Positive Correlation between MTR and OCI-R scores in the 36 OCD patients. The starting volume is -40 with 5 slice-spacing increments in Z space and ending in volume +45.
The WM cluster (k=36,900, p=0.012) included, according to the JHU (Johns Hopkins University) atlas, the genu, body and splenium of corpus callosum, bilateral forceps minor and major, cingulum (hippocampal), inferior and superior longitudinal fasciculus, posterior thalamic radiation, inferior fronto-occipital fasciculus, left anterior thalamic radiation, and right uncinate fasciculus. Meanwhile according to Harvard-Oxford Cortical and Subcortical Structural Atlas, the GM differences included three large clusters (k1=7500, p1=0.014; k2=4500, p2=0.012; k3=2000, p3=0.014) of brain regions, covering bilateral hippocampus and amygdala, left para-hippocampal and thalamus, right putamen and pallidum; bilateral frontal orbital & medial cortex, frontal pole; left temporal pole, inferior temporal gyrus, fusiform gyrus and temporooccipital part, right insular; bilateral intracalcarine and supracalcarine cortex, right lingual gyrus, lateral occipital cortex and occipital pole; bilateral cingulate gurus (anterior and posterior), precuneous; left postcentral gyrus and left cuneus.
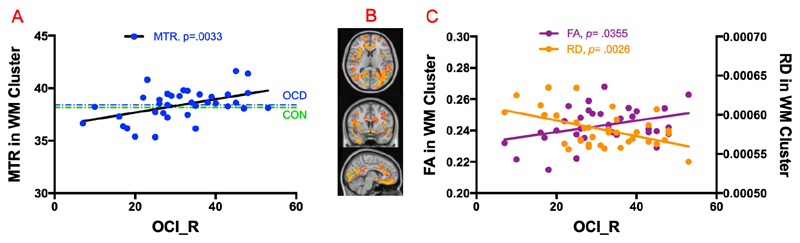
The post-hoc analysis found the MTR value within WM cluster mentioned above was significantly correlated with total OCI-R scores (r=0.46, p=0.0033, n=36), as illustrated in Figure 2A, although mean MTR values in this cluster did not differ between OCD patients and controls (38.41 ± 1.47; 38.16 ± 1.44; p=0.47). Within this WM cluster, we extracted the common DTI measurements FA and RD, and found a significant positive correlation with total OCI-R for FA (r= 0.34, p=0.0355, n=36), but negative correlation for RD (r= 0.47, p=0.0026, n=36), as shown in Figure 2C.
Figure 2.
(A) Positive correlation of MTR with OCI_R scores in WM cluster in OCD group. The mean of MTR in healthy controls in the same cluster is shown in green color for display purpose only. (B) Axial, coronal, and sagittal view of brain with WM cluster in orange, and GM cluster in light blue in the background. (C) FA and RD correlations with OCI_R scores in WM cluster in OCD group.
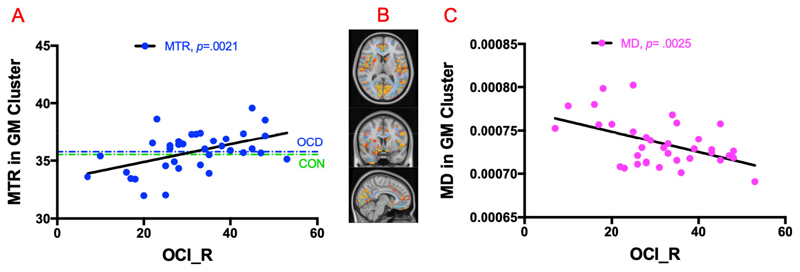
Similar analysis in GM clusters showed a significantly positive correlation between MTR value and OCI-R scores (r=0.48, p=0.0021, n=36) as shown in Figure 3A. Again, the mean MTR value did not differ between OCD patients and controls in these clusters (35.77 ± 1.72; 35.53 ± 1.62; p=0.54). For the post-hoc DTI analysis, MD values were extracted within these regions, and showed a significant negative correlation with OCI-R scores, (r=0.47, p=0.0025, n=36) as depicted in Figure 3C.
Figure 3.
(A) Positive correlation of MTR with OCI_R scores in GM cluster in OCD population. The MTR mean of healthy controls is shown in green color only for display purpose. (B) Axial, coronal, and sagittal view of brain with GM cluster in orange color and WM cluster in light blue in background. (C) Negative correlation of MD with OCI_R scores in GM cluster in OCD cohort.
There was no significant difference between medicated and non-medicated OCDs, in terms of OCI-R (p=0.7), MTR values in the GM cluster (p=0.26), nor in the WM cluster (p=0.25).
4. Discussion
In this multimodal study using a whole-brain approach, with strict correction for multiple comparisons, we examined brain structure (Magnetization Transfer Ratio, MTR; and Diffusion Tensor Imaging, DTI) in 36 OCD patients and 40 healthy controls. We evaluated (i) whether patients differed from controls in MTR and DTI measures; and (ii) whether symptom severity in the OCD patients correlated with these measures. The main findings were that groups did not differ significantly on the imaging parameters, but that symptom severity in the OCD group had a number of significant correlations with MTR and DTI. Our results were in line with the only previous study in this area reporting that OCD severity positively correlated with MTR in the left parietal-temporal-occipital association cortex and inferior parietal lobule, while negatively correlated with ADC maps, which was similar as MD in our study, in the left insula, striatum and cingulate cortex (Glahn et al., 2015). However, our findings can be regarded as being particularly robust, as they survived whole brain voxel-wise multiple comparison correction. Another robust aspect of the current study is that in addition to age and gender, we statistically controlled for two main common comorbidities (i.e., depression and anxiety) apart from demographic information, as these comorbidities are extremely common in OCD patients and can influence brain structure (Fontenelle and Hasler, 2008; Hartmann et al., 2016; Reess et al., 2016).
Further complementary DTI analysis helped to interpret MTR as a sensitive biomarker of myelination in WM. OCD symptom severity was positively correlated with Fractional Anisotropy (FA), whilst negatively correlating with Radial Diffusion (RD), in WM regions implicated by our MTR findings. It is notable that higher FA values represent more water diffusivity along the fibre, suggesting better WM integrity (Gonçalves et al., 2017). Additionally, radial diffusivity (RD) is another surrogate index of myelination given it measures perpendicular diffusion on WM tracts (Frydman et al., 2016; Gonçalves et al., 2017). These lines of evidence from multi-modal MR results consistently suggest that patients with higher symptom severity scores had increased myelination compared to less severe OCD patients in numerous regions including OFC, corpus callosum, and striatum. The regions identified linked to symptom severity accord fairly well with regions previously implicated more generally in the pathophysiology of the disorder (Burguière et al., 2015; Chamberlain et al., 2005; Eng et al., 2015; Frydman et al., 2016; Gan et al., 2017; Graybiel and Rauch, 2000; Menzies et al., 2008a; Oscar F. Gonçalves et al., 2017; Piras et al., 2015; van Velzen et al., 2014; Whiteside et al., 2004). The increased myelination related to OCD severity herein may relate to a decrease conduction velocity along WM axons, according to an “Inverted-U” function (Chomiak and Hu, 2009; Walsh et al., 2017; Wu et al., 2012). Functional MR studies have shown decreased functional connectivity in OCD cohort among numerous brain regions including OFC (Anticevic et al., 2014), cerebellum and occipital regions (Hou et al., 2014), posterior temporal areas (Hou et al., 2014; Zhang et al., 2011), hypoconnectivity between midbrain and ventral striatum as well as between lateral PFC and dorsal striatum (Harrison et al., 2009). More importantly, results of our recent study using rest-state fMRI data from same cohort revealed that OCI-R severity was associated with decreased effective connectivity from left anterior thalamus to the left dorsal anterior cingulate cortex (Parkes et al., 2018). However, further verification of this hypothesis will be necessary to understand the structural-functional relationship between myelination and functional connectivity in patients.
In terms of the GM integrity, the regions in which OCD symptom severity was positively associated with MTR also showed significant negative correlations with MD in several GM regions. MD is indicative of average diffusivity in all directions that is linked to tissue density (Frydman et al., 2016; Zhou et al., 2018), whereby increase in MD is thought to accompany neurodegeneration (Scola et al., 2010; Weston et al., 2015). In this study, patients with higher OCD severity showed relatively lower MD level in GM, which may imply an opposite direction to normal neurodegeneration process. A plausible explanation of this decreased MD could be related to neuroplastic changes induced by repetitive behaviour. For example, a novel longitudinal multi-modal study showed significant decrease of hippocampal MD in GM in healthy cohort followed by a two hour repetition of a learning task compared to the control group doing several different tasks (Sagi et al., 2012). Therefore, these findings may reflect neuroplastic changes stemming from the repetitive habitual behaviours that are central to OCD, occurring as a function of disease severity (Luigjes et al., 2019).
Several limitations should be considered in this study. Although MTR is a potential biomarker of myelination the signal is a complex function of radiofrequency (RF) parameters, which may be affected by overall macromolecular content as well as inflammation (Mossahebi, 2013); hence the symptom severity - MTR relationships in this study are open to several interpretations. Also, we acknowledge that there are several quantitative methods available in the literature in regard to computing MT ratio (Cercignani et al., 2005; Kit and Stephenson, 2016; Sled, 2018), in this study we employed the classic MTR which have been used in many clinical studies (Bouhrara et al., 2018; Glahn et al., 2015; Jonkman et al., 2016; Lin et al., 2015). We also opted to replicate the methods of the original MTR paper conducted in OCD population (Glahn et al., 2015), which smoothed MTR maps with an 8mm kernel. While spatial smoothing is a necessary step to reduce the influence of anatomical variability across subjects and improve signal-to-noise ratio (Mikl et al., 2008), it should be noted that image smoothing may blur the boundaries between WM and GM boundary. As such, effects that we observe along WM and GM boundary may be conflated by anatomical differences across structures and should be interpreted carefully. Further, we acknowledge that the diffusion sequence with b3000 we used to measure DTI parameters may not be an optimum protocol compared to b1000. Contrary to expectation, we did not find significant differences in brain structure between patients and controls. This could be due to symptom severity effect of OCD in this study. Since symptom severity was associated with changes in brain parameters, group-level differences in MTR/DTI may have been apparent if we had included a more severely affected group of patients. For measurement of OCD severity in patients, a potential limitation is that we used a self-report scale (OCI-R) rather than clinical assessment using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). Another limitation is that some of the OCD patients were receiving psychotropic medication(s), and some patients had comorbidities (for example, the level of depression in the patients was ‘moderate severity’ on average, based on the BDI). Nonetheless, we did not find evidence that medication had a significant effect on the brain parameters, and we also statistically controlled for anxiety and depression scores in all the analyses.
In this study we investigated brain differences among 36 OCD patients and 40 healthy controls at whole brain level using multimodal MRI approach. The results demonstrated that OCD symptom severity was associated with MTR and DTI measures in multiple white and gray matter regions indicating higher myelination in more severely ill patients. Our findings suggest that longitudinal multimodal neuroimaging research would be valuable in OCD, in order to evaluate the potential effects of symptom repetition and chronicity on brain structure and function, including potential neuroplastic changes.
Supplementary Material
Acknowledgement
This work was supported by the MASSIVE HPC facility (www.massive.org.au).
Funding and Disclosures
Murat Yücel has received funding from Monash University, and Australian Government funding bodies such as the National Health and Medical Research Council (NHMRC; including Fellowship #APP1117188), the Australian Research Council (ARC), and the Department of Industry, Innovation and Science. He has also received philanthropic donations from the David Winston Turner Endowment Fund, Wilson Foundation, as well as payment from law firms in relation to court and/or expert witness reports. The funding sources had no role in the design, management, data analysis, or interpretation and write-up of the data. Dr Chamberlain’s role in this study was funded by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z). Dr Chamberlain consults for Cambridge Cognition, Shire, Promentis, and Ieso Digital Health. Dr Chamberlain receives a stipend for his work as Associate Editor at Neuroscience and Biobehavioral Reviews; and at Comprehensive Psychiatry. Dr. Fontenelle supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (308237/2014-5), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, (203590); the D’Or Institute of Research and Education (no grant number available); and the David Winston Turner Endowment Fund (no grant numbers available). Dr Fontenelle receives a stipend for his work as Associate Editor at Journal of Obsessive-Compulsive and Related Disorders. Dr Chye is supported by the Monash Postdoctoral Bridging Fellowship.
Footnotes
Declaration of interest: None
References
- Abramowitz JS, Deacon BJ, Olatunji BO, Wheaton MG, Berman NC, Losardo D, Timpano KR, McGrath PB, Riemann BC, Adams T, Björgvinsson T, et al. Assessment of Obsessive-Compulsive Symptom Dimensions: Development and Evaluation of the Dimensional Obsessive-Compulsive Scale. Psychol Assess. 2010;22:180–98. doi: 10.1037/a0018260. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Pasha A, Mossahebi P, Tromp DPM, Zakszewski E, Field AS, Hosseinbor AP, Mossahebi P, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1:423–46. doi: 10.1089/brain.2011.0071.Characterization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. American Journal of Psychiatry. 5th ed. 2013. Diagnostic and statistical manual of mental disorders. [DOI] [Google Scholar]
- Armstrong CL, Traipe E, Hunter JV, Haselgrove JC, Ledakis GE, Tallent EM, Shera D, Van Buchem MA. Age-related, regional, hemispheric, and medial-lateral differences in myelin integrity in vivo in the normal adult brain. Am J Neuroradiol. 2004;25:977–84. [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- Bodini B, Cercignani M, Toosy A, De Stefano N, Miller DH, Thompson AJ, Ciccarelli O. A novel approach with “skeletonised MTR” measures tract-specific microstructural changes in early primary-progressive MS. Hum Brain Mapp. 2014;35:723–33. doi: 10.1002/hbm.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Fornito A, Cocchi L, Pujol J, Fontenelle LF, Velakoulis D, Pantelis C, Yücel M. White matter microstructure in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:42–6. doi: 10.1503/jpn.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, Spencer RG. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer’s Dement. 2018;14:998–1004. doi: 10.1016/j.jalz.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Narayanan S, Arnold DL. Segmentation of magnetization transfer ratio lesions for longitudinal analysis of demyelination and remyelination in multiple sclerosis. Neuroimage. 2013;66:103–9. doi: 10.1016/j.neuroimage.2012.10.059. [DOI] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana JF, Gu Y, Boudreau M, Levesque IR, Atchia Y, Sled JG, Narayanan S, Arnold DL, Pike GB, Cohen-Adad J, Duval T, et al. Quantitative magnetization transfer imaging made easy with qMTLab: Software for data simulation, analysis, and visualization. Concepts Magn Reson Part A Bridg Educ Res. 2015;44A:263–277. doi: 10.1002/cmr.a.21357. [DOI] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety. 2007;24:440–6. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Cercignani M, Symms MR, Schmierer K, Boulby PA, Tozer DJ, Ron M, Tofts PS, Barker GJ. Three-dimensional quantitative magnetisation transfer imaging of the human brain. Neuroimage. 2005;27:436–41. doi: 10.1016/j.neuroimage.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chiu CH, Lo YC, Tang HS, Liu IC, Chiang WY, Yeh FC, Jaw FS, Tseng WYI. White matter abnormalities of fronto-striato-thalamic circuitry in obsessive-compulsive disorder: A study using diffusion spectrum imaging tractography. Psychiatry Res - Neuroimaging. 2011;192:176–82. doi: 10.1016/j.pscychresns.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Chomiak T, Hu B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One. 2009;4:e7754. doi: 10.1371/journal.pone.0007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng GK, Sim K, Chen SHA. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neurosci Biobehav Rev. 2015;52:233–57. doi: 10.1016/j.neubiorev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Fan S, van den Heuvel OA, Cath DC, van der Werf YD, de Wit SJ, de Vries FE, Veltman DJ, Pouwels PJW. Mild white matter changes in un-medicated obsessive-compulsive disorder patients and their unaffected siblings. Front Neurosci. 2016;9:495. doi: 10.3389/fnins.2015.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1998. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) for DSMIV. [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The obsessive-compulsive inventory: Development and validation of a short version. Psychol Assess. 2002;14:485–496. doi: 10.1037/1040-3590.14.4.485. [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Bramati IE, Moll J, Medlowicz MV, de Oliveira-Souza R, Tovar-Moll F. White Matter Changes in OCD Revealed by Diffusion Tensor Imaging. CNS Spectr. 2011;16:101–9. doi: 10.1017/S1092852912000. [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Hasler G. The analytical epidemiology of obsessive-compulsive disorder: Risk factors and correlates. Prog Neuro-Psychopharmacology Biol Psychiatry. 2008;32:1–15. doi: 10.1016/j.pnpbp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Frydman I, de Salles Andrade JB, Vigne P, Fontenelle LF. Can Neuroimaging Provide Reliable Biomarkers for Obsessive-Compulsive Disorder? A Narrative Review. Curr Psychiatry Rep. 2016;18:90. doi: 10.1007/s11920-016-0729-7. [DOI] [PubMed] [Google Scholar]
- Gan J, Zhong M, Fan J, Liu W, Niu C, Cai S, Zou L, Wang Ya, Wang Yi, Tan C, Chan RCK, et al. Abnormal white matter structural connectivity in adults with obsessive-compulsive disorder. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D. Disorganization of anatomical connectivity in obsessive compulsive disorder: A multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol Dis. 2010;37:468–76. doi: 10.1016/j.nbd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Glahn A, Prell T, Grosskreutz J, Peschel T, Müller-Vahl KR. Obsessive-compulsive disorder is a heterogeneous disorder: Evidence from diffusion tensor imaging and magnetization transfer imaging. BMC Psychiatry. 2015;15:135. doi: 10.1186/s12888-015-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves ÓF, Carvalho S, Leite J, Fernandes-gonçalves A. Morphometric and Connectivity White Matter Abnormalities in Obsessive Compulsive Disorder. 2017;3 [Google Scholar]
- Gong Q, Li F, Huang X, Wu Q, Zhang T, Yang Y, Lui S, Kemp GJ, Li B. Microstructural Brain Abnormalities in Patients with Obsessive-Compulsive Disorder: Diffusion-Tensor MR Imaging Study at 3.0 T. Radiology. 2011;260:216–23. doi: 10.1148/radiol.11101971. [DOI] [PubMed] [Google Scholar]
- Gönner S, Leonhart R, Ecker W. The Obsessive-Compulsive Inventory-Revised (OCI-R): Validation of the German version in a sample of patients with OCD, anxiety disorders, and depressive disorders. J Anxiety Disord. 2008;22:734–49. doi: 10.1016/j.janxdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Goscinski WJ, McIntosh P, Felzmann U, Maksimenko A, Hall CJ, Gureyev T, Thompson D, Janke A, Galloway G, Killeen NEB, Raniga P, et al. The multi-modal Australian ScienceS Imaging and Visualization Environment (MASSIVE) high performance computing infrastructure: applications in neuroscience and neuroinformatics research. Front Neuroinform. 2014;8 doi: 10.3389/fninf.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/S0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, Deus J, Alonso P, Yücel M, Pantelis C, Menchon JM, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Vandborg S, Rosenberg R, Sørensen L, Videbech P. Increased fractional anisotropy in cerebellum in obsessive-compulsive disorder. Acta Neuropsychiatr. 2016;28:141–8. doi: 10.1017/neu.2015.57. [DOI] [PubMed] [Google Scholar]
- Hazari N, Narayanaswamy J, Venkatasubramanian G. Neuroimaging findings in obsessive–compulsive disorder: A narrative review to elucidate neurobiological underpinnings. Indian J Psychiatry. 2019;61:S9–S29. doi: 10.4103/psychiatry.indianjpsychiatry_525_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, Zhou DQ, Xie B, He M, Guo JW, Qu W, et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 2014;39:304–11. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert JD, Simpson HB, Nissenson KJ, Liebowitz MR, Foa EB. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26:39–45. doi: 10.1002/da.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces. Elev Annu Meet Organ Hum brain Mapp. 2005 [Google Scholar]
- Jonkman LE, Fleysher L, Steenwijk MD, Koeleman JA, De Snoo TP, Barkhof F, Inglese M, Geurts JJG. Ultra-high field MTR and qR2* differentiates subpial cortical lesions from normal-appearing gray matter in multiple sclerosis. Mult Scler. 2016;22:1306–14. doi: 10.1177/1352458515620499. [DOI] [PubMed] [Google Scholar]
- Kit WC, Stephenson MC. Implementation, optimization, and application of quantitative magnetization transfer ratio (qMTR) 2016 [Google Scholar]
- Kumar A, Gupta RC, Thomas MA, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res - Neuroimaging. 2004;130:131–40. doi: 10.1016/j.pscychresns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lin YC, Daducci A, Meskaldji DE, Thiran JP, Michel P, Meuli R, Krueger G, Menegaz G, Granziera C. Quantitative analysis of myelin and axonal remodeling in the uninjured motor network after stroke. Brain Connect. 2015 doi: 10.1089/brain.2014.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner C, Fouché JP, du Plessis S, Spottiswoode B, Seedat S, Fineberg N, Chamberlain SR, Stein DJ. Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 2012;37:193–9. doi: 10.1503/jpn.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, Lorenzetti V, de Haan S, Youssef GJ, Murawski C, Sjoerds Z, van den Brink W, Denys D, Fontenelle LF, Yücel M. Defining Compulsive Behavior. Neuropsychol Rev. 2019;29:4–13. doi: 10.1007/s11065-019-09404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RCW, Pasternak O, Cahn W, Kubicki M, Kahn RS, Shenton ME, Hulshoff Pol HE. Comparing free water imaging and magnetization transfer measurements in schizophrenia. Schizophr Res. 2015;161:126–132. doi: 10.1016/j.schres.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RCW, Rais M, van Baal GCM, van Haren NEM, Cahn W, Kahn RS, Pol HEH. Altered white matter connectivity in never-medicated patients with schizophrenia. Hum Brain Mapp. 2013;34:2353–65. doi: 10.1002/hbm.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008a;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian BJ, Robbins TW, Bullmore ET. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008b;165:1308–15. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Mikl M, Mareček R, Hluštík P, Pavlicová M, Drastich A, Chlebus P, Brázdil M, Krupa P. Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging. 2008;26:490–403. doi: 10.1016/j.mri.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mossahebi P. Quantitative Magnetization Transfer Imaging Techniques and Applications. 2013 [Google Scholar]
- Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Nishimura T, Fukui K. Diffusion tensor imaging and tract-based spatial statistics in obsessive-compulsive disorder. J Psychiatr Res. 2011;45:687–90. doi: 10.1016/j.jpsychires.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Nakamae T, Narumoto J, Shibata K, Matsumoto R, Kitabayashi Y, Yoshida T, Yamada K, Nishimura T, Fukui K. Alteration of fractional anisotropy and apparent diffusion coefficient in obsessive-compulsive disorder: A diffusion tensor imaging study. Prog Neuro-Psychopharmacology Biol Psychiatry. 2008;32:1221–6. doi: 10.1016/j.pnpbp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Nantes JC, Proulx S, Zhong J, Holmes SA, Narayanan S, Brown RA, Hoge RD, Koski L. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis. Neuroimage. 2017;157:705–715. doi: 10.1016/j.neuroimage.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Oh JS, Jang JH, Jung WH, Kang DH, Choi JS, Choi CH, Kubicki M, Shenton ME, Kwon JS. Reduced fronto-callosal fiber integrity in unmedicated OCD patients: A diffusion tractography study. Hum Brain Mapp. 2012;33:2441–54. doi: 10.1002/hbm.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves Oscar F, Sousa Sonia, Carvalho Sandra, Leite Jorge, Ganho Ana, Fernandes-Gonçalves Ana, Pocinho Fernando, Carracedo Angel, Sampaio Adriana. Alterations of gray and white matter morphology in obsessive compulsive disorder. Psicothema. 2017;29:35–42. doi: 10.7334/psicothema2016.86. [DOI] [PubMed] [Google Scholar]
- Parkes L, Tiego J, Aquino K, Braganza L, Chamberlain SR, Fontenelle L, Harrison BJ, Lorenzetti V, Paton B, Razi A, Fornito A, et al. Transdiagnostic variations in impulsivity and compulsivity in obsessive-compulsive disorder and gambling disorder correlate with effective connectivity in cortical-striatal-thalamic-cortical circuits. bioRxiv. 2018 doi: 10.1101/389320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras Federica, Piras Fabrizio, Chiapponi C, Girardi P, Caltagirone C, Spalletta G. Widespread structural brain changes in OCD: A systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Reess TJ, Rus OG, Schmidt R, De Reus MA, Zaudig M, Wagner G, Zimmer C, Van Den Heuvel MP, Koch K. Connectomics-based structural network alterations in obsessive-compulsive disorder. Transl Psychiatry. 2016;6:e882. doi: 10.1038/tp.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the Fast Lane: New Insights into Neuroplasticity. Neuron. 2012;73:1195–203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Saini J, Pal P, Sandhya M, Yadav R, Pasha S. A voxel based comparative analysis using magnetization transfer imaging and T1-weighted magnetic resonance imaging in progressive supranuclear palsy. Ann Indian Acad Neurol. 2014;17:193–8. doi: 10.4103/0972-2327.132626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola E, Bozzali M, Agosta F, Magnani G, Franceschi M, Sormani MP, Cercignani M, Pagani E, Falautano M, Filippi M, Falini A. A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. J Neurol Neurosurg Psychiatry. 2010;81:798–805. doi: 10.1136/jnnp.2009.189639. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1988;59:22–33. [PubMed] [Google Scholar]
- Sled JG. Modelling and interpretation of magnetization transfer imaging in the brain. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2017.11.065. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Van Buchem MA, Steens SCA, Vrooman HA, Zwinderman AH, McGowan JC, Rassek M, Engelbrecht V. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: A preliminary study. Am J Neuroradiol. 2001;22:762–6. [PMC free article] [PubMed] [Google Scholar]
- van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response Inhibition and Interference Control in Obsessive-Compulsive Spectrum Disorders. Front Hum Neurosci. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73:614–22. doi: 10.1002/mrm.25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Landman KA, Hughes BD. What is the optimal distribution of myelin along a single axon? Neurosci Lett. 2017;658:97–101. doi: 10.1016/j.neulet.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Weston PSJ, Simpson IJA, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: A potential marker of early neurodegeneration. Alzheimer’s Res Ther. 2015;7:47. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res - Neuroimaging. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton BM, Diefenbach GJ, Bragdon LB, Steketee G, Frost RO, Tolin DF. A contemporary psychometric evaluation of the Obsessive Compulsive Inventory-Revised (OCI-R) Psychol Assess. 2015;27:874–82. doi: 10.1037/pas0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LMN, Williams A, Delaney A, Sherman DL, Brophy PJ. Increasing internodal distance in myelinated nerves accelerates nerve conduction to a flat maximum. Curr Biol. 2012;22:1957–61. doi: 10.1016/j.cub.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Hirano Y, Nakazato M, Nemoto K, Ishikawa K, Sutoh C, Miyata H, Matsumoto J, Matsumoto K, Masuda Y, Obata T, et al. Relationship between symptom dimensions and white matter alterations in obsessive-compulsive disorder. Acta Neuropsychiatr. 2017;29:153–163f. doi: 10.1017/neu.2016.45. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS. White matter abnormalities in drug-naïve patients with obsessive-compulsive disorder: A Diffusion Tensor Study before and after citalopram treatment. Acta Psychiatr Scand. 2007;116:211–9. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, Winmill L, Nijhawan S, Matthews PM, James A. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–90. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, Yue Q, Tang H, Yan C, Lui S, Huang X, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Xu J, Ping L, Zhang F, Chen W, Shen Z, Jiang L, Xu X, Cheng Y. Cortical thickness and white matter integrity abnormalities in obsessive–compulsive disorder: A combined multimodal surface-based morphometry and tract-based spatial statistics study. Depress Anxiety. 2018;35:724–751. doi: 10.1002/da.22758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.