Abstract
Permanent neonatal diabetes is caused by reduced β-cell number or impaired β-cell function. Understanding the genetic basis of this disorder highlights fundamental β-cell mechanisms.
We performed trio genome sequencing for 44 permanent neonatal diabetes patients and their unaffected parents to identify causative de novo variants. Replication studies were performed in 188 patients diagnosed with diabetes before 2 years of age without a genetic diagnosis.
EIF2B1 (encoding the eIF2B complex α subunit) was the only gene with novel de novo variants (all missense) in at least three patients. Replication studies identified 2 further patients with de novo EIF2B1 variants. In addition to diabetes, 4/5 patients had hepatitis-like episodes in childhood. The EIF2B1 de novo mutations were found to map to the same protein surface. We propose that these variants render the eIF2B complex insensitive to eIF2 phosphorylation which occurs under stress conditions and triggers expression of stress-response genes. Failure of eIF2B to sense eIF2 phosphorylation likely leads to unregulated unfolded protein response and cell death.
Our results establish de novo EIF2B1 mutations as a novel cause of permanent diabetes and liver dysfunction. These findings confirm the importance of cell stress regulation for β-cells and highlight EIF2B1’s fundamental role within this pathway.
Introduction
Permanent neonatal diabetes (PNDM) is a genetically and clinically heterogeneous condition diagnosed before the age of 6 months. A genetic cause is identified in 82% of cases, resulting in improved treatment in almost 40% (1).
Thirty-nine percent of patients with PNDM have a genetic aetiology resulting in development of at least one extra-pancreatic feature, alongside diabetes (1). The most common PNDM syndromic subtype is Wolcott-Rallison syndrome, which is caused by autosomal recessive mutations in the EIF2AK3 gene. Individuals with Wolcott-Rallison syndrome usually develop diabetes in the first year of life. They also present with repeated episodes of hepatitis-like liver dysfunction, which eventually results in fatal liver failure, and skeletal dysplasia. The EIF2AK3 mutations in these patients are thought to result in disruption of the unfolded protein response (UPR) and cell death due to unregulated endoplasmic reticulum (ER) stress (2; 3). Approximately 15% of individuals with syndromic PNDM do not have a mutation in one of the known causative genes, suggesting the existence of novel genetic subtypes (4).
Identifying novel genetic causes of PNDM is crucially important to gain insights into human β-cell development, function and survival and could identify potential targets for novel therapies.
We used a trio-based genome sequencing approach to identify heterozygous de novo EIF2B1 mutations as the cause of a novel genetic syndrome characterised by permanent neonatal/early onset diabetes and transient liver dysfunction.
Methods
Subjects
Individuals with PNDM and early onset diabetes were recruited by their clinicians for genetic analysis in the Exeter Molecular Genetics Laboratory. The study was conducted in accordance with the Declaration of Helsinki, and all subjects or their parents gave informed consent for genetic testing.
Genome sequencing
Genome sequencing was performed on DNA extracted from peripheral blood leukocytes of 44 probands diagnosed with PNDM before the age of 6 months and their unaffected, unrelated parents. Samples were sequenced on an Illumina HiSeq2500 with a mean read depth of 30. The sequencing data was analysed using an approach based on the GATK best practice guidelines. Briefly, the reads were aligned to the hg19/GRCh37 human reference genome with BWA mem, Picard used for duplicates removal, and GATK IndelRealigner for local re-alignment. GATK haplotypecaller was used to identify variants which were annotated using Alamut batch v1.8 (Interactive Biosoftware, Rouen, France). Variants which failed the QD2 VCF filter or had < 5 reads supporting the variant were excluded. CNVs were called by SavvyCNV (5) which uses read depth to predict copy number.
Targeted next generation sequencing
Replication studies were performed in a cohort of 188 patients diagnosed with diabetes before 2 years of age in whom the 25 known genetic causes of neonatal diabetes had been excluded. Patients were analysed using a targeted next generation sequencing assay, including the known monogenic diabetes genes and additional candidate genes, such as EIF2B1 (NM_001414.3). Variant confirmation and co-segregation in family members was performed by Sanger sequencing.
Sanger sequencing confirmation
EIF2B1 exon 3 (NM_001414.3) was amplified using in-house designed primers (F – TGTTCACTGATGTATCCCTAGCA, R – TCCTAGGAAGAAAAGAGCAAACT). PCR products were sequenced on an ABI3730 capillary machine (Applied Biosystems) and analysed using Mutation Surveyor v3.98 (SoftGenetics). The bioinformatics tools SIFT, PolyPhen-2 and Align GVGD were accessed through the Alamut Visual software (Interactive Biosoftware) to predict the effect of the variants.
Variants are reported using the HGVS nomenclature guidelines (6).
Molecular modelling of the EIF2B1 stop-loss variant
The predicted structure of the EIF2B1 stop-loss variant, c.915_916del, p.(*306Thrext*12) was modelled using the Phyre2 web server in intensive mode 3, with the full-length sequence of variant eIF2Bα as input. The resulting structure was modelled entirely on PDB entry 3ecs (crystal structure of human eIF2Bα 4) except for the C-terminal extension which was modelled ab initio. All structures were visualized in PyMOL (Molecular Graphics System v2.0, Schrödinger LLC).
Data and resource availability
EIF2B1 mutation details have been deposited in the Decipher database (https://decipher.sanger.ac.uk/). All other data sets generated and/or analysed for this study are available from the corresponding author upon reasonable request.
Results
Genetic analysis
EIF2B1 was the only gene identified with de novo variants in at least three patients. The three missense EIF2B1 variants identified, p.(Gly44Asp), p.(Gly44Val), and p.(Ser77Asn) (Table 1), were not listed in the gnomAD database (7) and affected residues which are highly conserved across species (up to Zebrafish for p.Gly44, up to Chicken for p.Ser77). No deletions or duplications involving EIF2B1 were detected.
Table 1. Patients’ clinical features.
| Case # | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Mutation | c.131G>A p.(Gly44Asp) |
c.131G>T p.(Gly44Val) |
c.230G>A p.(Ser77Asn) |
c.101T>G p.(Leu34Trp) |
c.915_916del, p.(*306Thrext*12) |
| De novo | Yes | Yes | Yes | Yes | Yes |
| Sex | Male | Male | Female | Male | Male |
| Current age (years) | 5 | 5 | Deceased | 13 | 18 |
| Country | Vietnam | Jordan | Chile | England | Israel |
| Birth Weight (g) | 2600 | 3000 | 2986 | 2920 | 3600 |
| Gestation (weeks) | 39 | N/A | 40 | 40 | 42 |
| Birth weight centile (SDS) | 4th (-1.74) | N/A | 18th (-0.90) | 7th (-1.44) | 20th (-0.84) |
| Diabetes features | |||||
| Age at onset (weeks) | 9 | 4 | 17 | 56 | 21 |
| Insulin treatment | from diagnosis, 0.46U/kg/day (at 2 months) | from diagnosis, 0.8U/kg/day | from diagnosis, 1.0U/kg/day | from diagnosis, 0.7U/kg/day | from diagnosis, 0.75U/kg/day |
| Hepatic features | |||||
| Age at first episode of liver dysfunction | 38 months | None reported | 16 months | 18 months | 5 months |
| Outcome of first episode | Resolved | N/A | Decease | Resolved | Resolved |
| Recurrent episodes | No | N/A | N/A | Yes, 8 episodes | Yes, 2 episodes |
| Age at last episode of liver dysfunction | N/A | N/A | N/A | 5.5 years | 5.5 months |
| Other features | |||||
| Neurological features | None | None | None | Mild learning disability | Attention deficit disorder |
| Additional Comments | Anaemia | Died at 16 months following respiratory infection and acute liver failure | Renal failure at 14 years | ||
Sequencing of EIF2B1 in 188 further patients without a known genetic diagnosis who had developed diabetes before the age of two years identified two additional novel heterozygous EIF2B1 variants, p.(Leu34Trp) and p.(*306Thrext*12). Parental testing showed that both variants had arisen de novo.
Clinical features
The clinical features of the 5 subjects with de novo EIF2B1 mutations are summarised in Table 1. All had been diagnosed with permanent diabetes between the age of 4 and 56 weeks and were treated with full replacement insulin doses (range 0.46-1 U/kg/day). All 4 patients for whom data was available had low birth weight (< 20th centile, SDS range -0.84 - -1.74) consistent with reduced insulin secretion in utero.
Four of the five patients had suffered episodes of hepatic dysfunction in childhood with hepatitis-like derangement of liver enzymes. The severity of these episodes was variable: in case 3 the first episode resulted in fatal acute liver failure, whilst in the other three cases it resolved with return to normal liver function and enzymes. Case 1 presented once with elevated liver enzymes at the age of 38 months. Cases 4 and 5 experienced multiple episodes requiring hospitalisation between the ages of 5 months and 5.5 years but have not experienced any further episodes (current ages 13 and 18).
No severe neurological features were reported, however Case 4 has mild learning disability and Case 5 has attention deficit disorder.
In silico protein analysis
EIF2B1 encodes for the α subunit of the heterodecameric eIF2B complex, which modulates the activity of the Eukaryotic Translation Initiation Factor 2 (eIF2) to regulate translation initiation and protein synthesis in basal and stress cell conditions. Under cell stress, serine 51 of eIF2α is phosphorylated by kinases of the Integrated Stress Response (ISR) (8). This phosphorylation results in altered interaction with eIF2B to inhibit the rate of translation of most mRNAs, with the exception of stress-response genes RNAs which are able to circumvent this block to allow cell recovery (9; 10). The recent publication of the structures of human eIF2B in complex with both phosphorylated and non-phosphorylated eIF2 (11; 12) showed that in its non-phosphorylated form, eIF2α makes contact with the eIF2B β, γ and δ subunits, but not with eIF2Bα. However, upon Ser51 phosphorylation, eIF2α binds tightly to eIF2Bα, resulting in a markedly different orientation of eIF2 in the complex with eIF2B (Figure 1A).
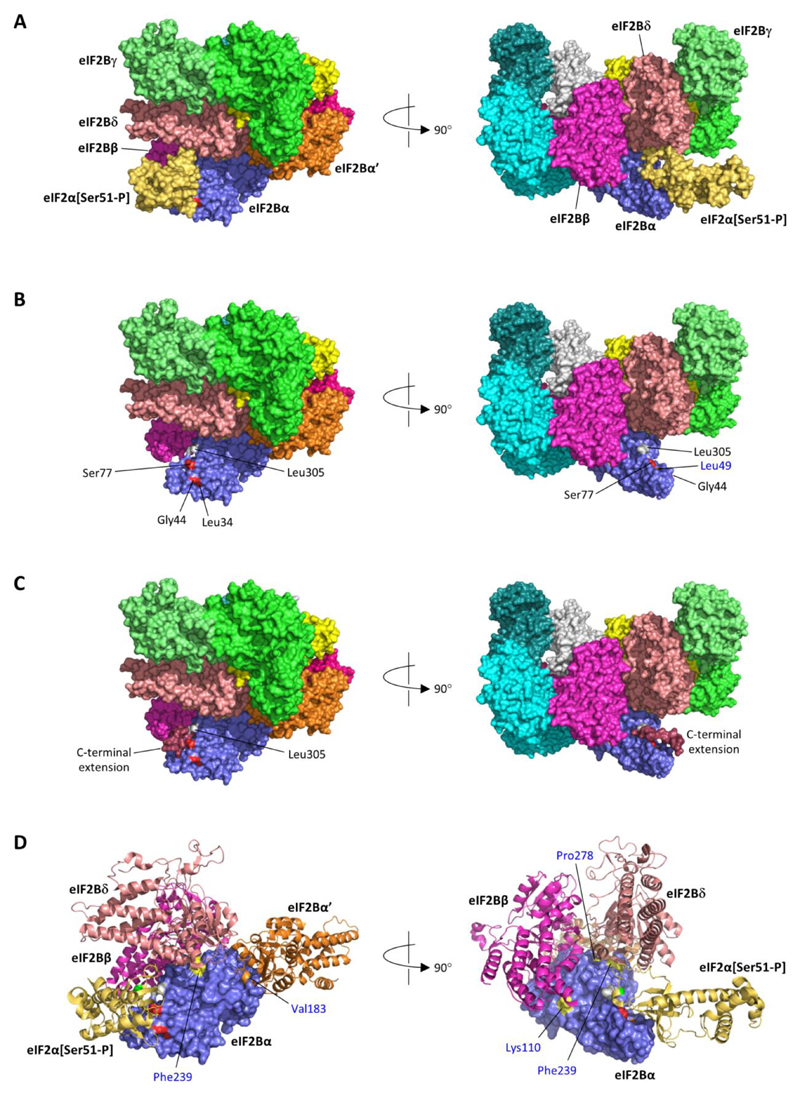
Figure 1. Heterozygous EIF2B1 variants in neonatal diabetes lie in the binding surface for phosphorylated eIF2α.
A) Structure of human eIF2B in complex with phosphorylated eIF2α (Ser51-P) (PDB id 6o9z (12)); the eIF2B complex is a heterodecamer comprised of two molecules each of subunits α, β, γ, δ and ε. B) As A, but shown without eIF2α (Ser51-P); positions of heterozygous missense variants in eIF2Bα identified in neonatal diabetes patients are coloured red and labelled in black font; the position of the homozygous p.(Leu49Arg) variant reported in a VWM patient is coloured orange and labelled in blue font; Leu305 (grey) is the C-terminal residue of eIF2Bα. C) The stop-loss variant c.915_916del, p.(*306Thrext*12) is expected to result in the addition of 12 novel amino acids (Thr-Cys-Glu-Pro-Phe-Pro-Ala-Lys-Val-Gln-Leu-Thr) to the C-terminal of eIF2B; this C-terminal extension (dark red) was predicted to form a short helix extending from Leu305 lying across the surface bound by eIF2α[Ser51-P]. D) As A, but zoomed and showing selected subunits (eIF2α[Ser51-P]; eIF2B subunits β, δ and α’) in ribbon format; surface positions of heterozygous missense variants identified in neonatal diabetes patients are coloured red, and the position of the homozygous p.(Leu49Arg) VWM variant orange, as in part B; positions of other homozygous variants identified in VWM patients are coloured yellow and labelled in blue font (Tyr275, the site of the p.Tyr275Cys variant, is not visible in these views but is surface-accessible at the junction of the interfaces with subunits δ and α’; Asn208, which was substituted by glutamate in a case of VWM, is not accessible at the eIF2Bα surface). The light green residue in the eIF2α ribbon indicates the position of phosphoserine 51.
Mapping the heterozygous EIF2B1 variants from our 5 cases shows that these all lie at the same surface as that occupied by phosphorylated eIF2α (Figure 1B). Moreover, one of these variants (identified in Case 3) occurs at p.Ser77 of eIF2Bα, which makes both hydrogen-bonded and non-bonded contacts with residues of phosphorylated eIF2α. In the stop-loss variant identified in Case 5, the C-terminal extension to eIF2Bα is likely to sterically hinder the interaction of eIF2α with eIF2Bα p.Leu305 as well as possibly occluding other parts of the interface (Figure 1C).
Discussion
We report the identification of EIF2B1 heterozygous de novo variants in 5 patients with permanent neonatal/early onset diabetes and transient liver dysfunction. The variants, 4 missense and one stop-loss, are all predicted to map to the same protein surface and in silico modelling suggests that they are likely to disrupt the interaction of the EIF2Bα subunit (encoded by EIF2B1) with phosphorylated EIF2α during the cell ISR.
Our patients’ clinical features are markedly different from those reported in patients with leukoencephalopathy with vanishing white matter (VWM), a rare paediatric neurological disease caused by recessive loss of function EIF2B1 mutations (13). VWM is a progressive condition, usually fatal in childhood. Extra-neurological features are generally not present, although diabetic ketoacidosis at 8 months was reported in one patient with a homozygous p.(Leu49Arg) variant in EIF2B1 (14). None of the five patients we report had severe neurological features, however Case 4 has mild learning disability and Case 5 has attention deficit disorder. The parents of individuals with VWM, who are heterozygous carriers for EIF2B1 loss of function mutations, are unaffected, thereby supporting our hypothesis that the mutations identified in our patients do not result in complete loss of function of the eIF2B complex.
eIF2B regulates translation initiation and protein synthesis by modulating eIF2activity. During protein synthesis under normal conditions, eIF2B acts to recycle initiation factor eIF2 for further rounds of translation by promoting its dissociation from GDP, allowing replacement by GTP and subsequent association of GTP-bound eIF2 with the initiator methionyl-tRNA. Under stress condition, specific kinases phosphorylate the serine 51 of eIF2α (8) resulting in inhibition of GDP dissociation, attenuating recycling of eIF2 to its active GTP-bound form through altered interaction with eIF2B and consequently inhibiting the rate of translation of most mRNAs (9; 10) (Figure 2A).
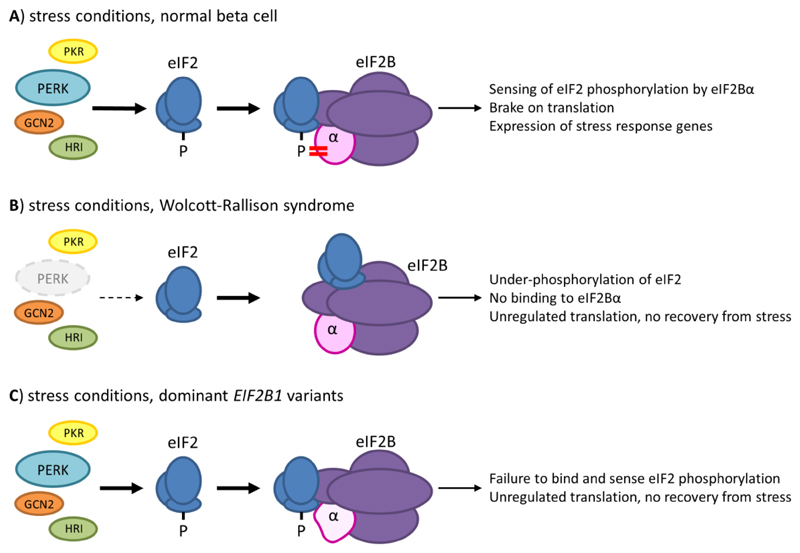
Figure 2. Different forms of monogenic neonatal diabetes affect steps in the Integrated Stress Response pathway.
A) Stress conditions induce activation of four kinases of the Integrated Stress Response (ISR); Pancreatic eIF2-alpha kinase (PERK) responds mainly to ER stress, and is of particular importance in maintaining liver and pancreatic β-cells; other kinases of the IRS are Protein kinase (PK) R, GCN2 and Heme-regulated eIF2α kinase (HRI), which respond to dsRNA, amino acid deprivation and heme deprivation respectively. Phosphorylation of Ser51 of eIF2α results in a tight interaction with eIF2Bα, which inhibits the GDP dissociation activity mediated primarily by the γ and ε subunits of eIF2B and thus arrests translation of most mRNAs, except for specific mRNAs associated with the stress response programme. B) In Walcott-Rallison syndrome, homozygous or compound heterozygous loss of function variants in EIF2AK3, the gene encoding PERK, prevent phosphorylation of eIF2α Ser51 in response to ER stress; the lack of an appropriate stress response ultimately leads to a prolonged unfolded protein response and cell death. C) The dominant eIF2B1 variants identified in neonatal patients all lie in the binding surface for phosphorylated eIF2α and likely prevent effective sensing of Ser51 phosphorylation by eIF2B. This defect would be expected to affect the response to all kinases of the ISR, potentially explaining differences in the phenotype compared to that of Wolcott Rallison syndrome and the dominant nature of the disease.
Mapping the heterozygous EIF2B1 variants identified in the 5 cases we report shows that these all localise at the same surface as that occupied by phosphorylated eIF2α (Figure 1B). Moreover one variant, p.(Ser77Asn), identified in Case 3 occurs at a residue which directly interacts with phosphorylated eIF2α. Two mutations, identified in cases 1 and 2, involve eIF2Bα p.Gly44. Previous experiments have shown that mutation of the equivalent residue in GCN3, the yeast orthologue of EIF2B1, results in full cell viability under normal conditions but failure to respond to eIF2α phosphorylation under stress (15). In contrast, most EIF2B1 variants which have been reported in patients with VWM occur at interfaces with other eIF2B subunits (Figure 1D), consistent with their reported effects on the formation and/or stability of the eIF2B complex (16). Taken together, this evidence strongly suggests that the EIF2B1 variants identified in our patients are likely to result in formation of the eIF2B complex and support translation under normal conditions, but impair eIF2B binding to phosphorylated eIF2α under stress conditions.
Impaired cellular stress response is a known β-cell dysfunction mechanism and is involved in other genetic subtypes of PNDM, including Wolcott-Rallison syndrome, the most common syndromic form of the disease (17). Wolcott-Rallison syndrome is characterised by permanent neonatal/early onset diabetes, severe liver dysfunction (presenting with transient episodes of acute liver failure) and skeletal dysplasia, and is caused by recessive mutations in the EIF2AK3 gene, encoding the Pancreatic eIF2-alpha kinase (PERK) (18). EIF2AK3 loss of function variants in Wolcott-Rallison syndrome disrupt the ISR thereby limiting the response to ER stress, impairing the cell’s ability to recover and leading to cell death (Figure 2B).
Our results are consistent with the hypothesis that the EIF2AK3 variants in Wolcott-Rallison syndrome and the novel EIF2B1 variants identified in our cases affect different steps within the same ER stress response pathway (Figure 2C), highlighting the importance of this pathway in pancreatic β and liver cell function and integrity (19). The convergence of both EIF2B1 and EIF2AK3 on the ISR provides an explanation for the phenotypic overlap between Wolcott-Rallison syndrome and the disease caused by dominant EIF2B1 variants, consisting of neonatal/early onset diabetes and liver dysfunction. However, whereas liver symptoms appear to resolve with age in the patients with EIF2B1 variants, prognosis in Wolcott-Rallison syndrome is poor and most patients die of liver failure. Additionally, individuals with Wolcott-Rallison syndrome have skeletal dysplasia, a feature not observed in the cases with EIF2B1 variants we report. It has been proposed that the skeletal manifestations in Wolcott-Rallison syndrome arise specifically from a developmental defect in osteoblasts due to absence of PERK activity (2; 3) rather than from the ER stress regulation defect which is thought to cause PNDM and liver failure. Another possible explanation for the differences in the extra-pancreatic phenotypes in cases with Wolcott-Rallison syndrome and the individuals with EIF2B1 de novo variants we report could be the existence of quantitative differences in strength of signal or in its specific relationship to ER stress. It is possible that the level of attenuation of the ISR and the context for the attenuation caused by heterozygous mutations in EIF2B1 are enough to cause β-cell dysfunction and diabetes, but not enough to result in the severe liver and skeletal phenotype.
In summary, we report a novel genetic syndrome of neonatal/early onset diabetes and transient liver dysfunction caused by dominant de novo EIF2B1 mutations which disrupt the ability of the eIF2B complex to sense eIF2 phosphorylation, and likely result in severe ER stress. These results further highlight the importance of ER stress regulation for β-cell function, adding another key gene to the list of ER stress regulators which, when mutated, cause syndromic forms of diabetes (4; 20–27). This knowledge is crucial to inform current efforts aimed at developing targeted therapies for patients with syndromic forms of diabetes caused by ER stress dysregulation (28; 29) but also for patients with type 2 (30) and type 1 diabetes (31), as ER stress is known to play a key role in the pathogenesis of these more common diabetes subtypes.
Acknowledgements
We would like to thank the families for participating in the study. We are also grateful to Rebecca Ward (Exeter Genomics Laboratory, RD&E Hospital) for technical assistance, Dr Thomas Laver (University of Exeter Medical School) for critical review of the manuscript, and Dr Ethel Codner (Institute for Mother and Child Research, University of Chile) for assistance with clinical data collection. E.D.F. is a Diabetes UK RD Lawrence Fellow (19/005971) and the recipient of an EFSD Rising Star Fellowship (2018). E.D.F. has also received funding from DRWF. A.T.H. and S.E. are the recipients of a Wellcome Trust Senior Investigator award (grant WT098395/Z/12/Z) and A.T.H. is employed as a core member of staff within the NIHR funded Exeter Clinical Research Facility and is an NIHR senior investigator. S.E.F. has a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant number 105636/Z/14/Z). D.R.’s research is supported by a Wellcome Trust Principal Research Fellowship (Wellcome Trust 200848/Z/16/Z). Whole genome data analysis was run on the University of Exeter ISCA high performance computing facility.
Footnotes
Duality of Interest.
No potential conflicts of interest relevant to this article were reported.
Author Contributions
EDF, ATH, SE, and SEF participated in study conception and design. EDF, SEF, MBJ and SE performed the genetic analysis. ATH analyzed the clinical data. MW and RC performed bioinformatic analysis. DR participated in data analysis and interpretation. EDF wrote the first draft of the manuscript. RC, ATH, and DR participated in manuscript improvement. AZ, VCD, CTBN, RG, MVJ, and MEK collected patients’ samples and clinical data. All authors reviewed the manuscript. EDF is the guarantor of this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, Ellard S, Hattersley AT. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386:957–963. doi: 10.1016/S0140-6736(15)60098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol. 2008;217:693–707. doi: 10.1002/jcp.21543. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Franco E, Flanagan SE, Yagi T, Abreu D, Mahadevan J, Johnson MB, Jones G, Acosta F, Mulaudzi M, Lek N, Oh V, et al. Dominant ER Stress-Inducing WFS1 Mutations Underlie a Genetic Syndrome of Neonatal/Infancy-Onset Diabetes, Congenital Sensorineural Deafness, and Congenital Cataracts. Diabetes. 2017;66:2044–2053. doi: 10.2337/db16-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laver TW, De Franco E, Johnson MB, Patel K, Ellard S, Weedon MN, Flanagan SE, Wakeling MN. SavvyCNV: genome-wide CNV calling from off-target reads. bioRxiv. 2019 doi: 10.1371/journal.pcbi.1009940. 617605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, Roux AF, Smith T, Antonarakis SE, Taschner PE. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 7.Konrad K, F LC, Grace T, C BB, Jessica A, Qingbo W, C RL, L KM, Andrea G, B DP, G LD, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-offunction intolerance across human protein-coding genes. bioRxiv. 2019 [Google Scholar]
- 8.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings MD, Pavitt GD. A new function and complexity for protein translation initiation factor eIF2B. Cell Cycle. 2014;13:2660–2665. doi: 10.4161/15384101.2014.948797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcik M. Could the eIF2alpha-Independent Translation Be the Achilles Heel of Cancer? Front Oncol. 2015;5:264. doi: 10.3389/fonc.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwagi K, Yokoyama T, Nishimoto M, Takahashi M, Sakamoto A, Yonemochi M, Shirouzu M, Ito T. Structural basis for eIF2B inhibition in integrated stress response. Science. 2019;364:495–499. doi: 10.1126/science.aaw4104. [DOI] [PubMed] [Google Scholar]
- 12.Kenner LR, Anand AA, Nguyen HC, Myasnikov AG, Klose CJ, McGeever LA, Tsai JC, Miller-Vedam LE, Walter P, Frost A. eIF2B-catalyzed nucleotide exchange and phosphoregulation by the integrated stress response. Science. 2019;364:491–495. doi: 10.1126/science.aaw2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Knaap MS, Leegwater PA, Konst AA, Visser A, Naidu S, Oudejans CB, Schutgens RB, Pronk JC. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264–270. doi: 10.1002/ana.10112. [DOI] [PubMed] [Google Scholar]
- 14.Alamri H, Al Mutairi F, Alothman J, Alothaim A, Alfadhel M, Alfares A. Clin Case Rep. England: 2016. Diabetic ketoacidosis in vanishing white matter; pp. 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavitt GD, Yang W, Hinnebusch AG. Homologous segments in three subunits of the guanine nucleotide exchange factor eIF2B mediate translational regulation by phosphorylation of eIF2. Mol Cell Biol. 1997;17:1298–1313. doi: 10.1128/mcb.17.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wortham NC, Proud CG. Biochemical effects of mutations in the gene encoding the alpha subunit of eukaryotic initiation factor (eIF) 2B associated with Vanishing White Matter disease. BMC Med Genet. 2015;16:64. doi: 10.1186/s12881-015-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 19.Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P. Endoplasmic reticulum stress and eIF2alpha phosphorylation: The Achilles heel of pancreatic beta cells. Mol Metab. 2017;6:1024–1039. doi: 10.1016/j.molmet.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulton CJ, Schot R, Kia SK, Jones M, Verheijen FW, Venselaar H, de Wit MC, de Graaff E, Bertoli-Avella AM, Mancini GM. Microcephaly with simplified gyration, epilepsy, and infantile diabetes linked to inappropriate apoptosis of neural progenitors. Am J Hum Genet. 2011;89:265–276. doi: 10.1016/j.ajhg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skopkova M, Hennig F, Shin BS, Turner CE, Stanikova D, Brennerova K, Stanik J, Fischer U, Henden L, Muller U, Steinberger D, et al. EIF2S3 Mutations Associated with Severe X-Linked Intellectual Disability Syndrome MEHMO. Hum Mutat. 2017;38:409–425. doi: 10.1002/humu.23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdulkarim B, Nicolino M, Igoillo-Esteve M, Daures M, Romero S, Philippi A, Senee V, Lopes M, Cunha DA, Harding HP, Derbois C, et al. A Missense Mutation in PPP1R15B Causes a Syndrome Including Diabetes, Short Stature, and Microcephaly. Diabetes. 2015;64:3951–3962. doi: 10.2337/db15-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amr S, Heisey C, Zhang M, Xia XJ, Shows KH, Ajlouni K, Pandya A, Satin LS, El-Shanti H, Shiang R. A homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram syndrome 2. Am J Hum Genet. 2007;81:673–683. doi: 10.1086/520961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnycastle LL, Chines PS, Hara T, Huyghe JR, Swift AJ, Heikinheimo P, Mahadevan J, Peltonen S, Huopio H, Nuutila P, Narisu N, et al. Autosomal dominant diabetes arising from a Wolfram syndrome 1 mutation. Diabetes. 2013;62:3943–3950. doi: 10.2337/db13-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, Rogers D, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20:143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 27.Synofzik M, Haack TB, Kopajtich R, Gorza M, Rapaport D, Greiner M, Schonfeld C, Freiberg C, Schorr S, Holl RW, Gonzalez MA, et al. Absence of BiP co-chaperone DNAJC3 causes diabetes mellitus and multisystemic neurodegeneration. Am J Hum Genet. 2014;95:689–697. doi: 10.1016/j.ajhg.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abreu D, Urano F. Current Landscape of Treatments for Wolfram Syndrome. Trends Pharmacol Sci. 2019 doi: 10.1016/j.tips.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryno LM, Wiseman RL, Kelly JW. Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases. Curr Opin Chem Biol. 2013;17:346–352. doi: 10.1016/j.cbpa.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 31.Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64:2–6. doi: 10.1097/JIM.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]