Abstract
Allergic disorders are the result of a complex pathophysiology, involving major cellular lineages and a multitude of humoral factors of the innate and adaptive immune system, and have the tendency to involve multiple organs. Consequently, even standard pharmacological treatment of allergies is rarely specific but usually targets more than one pathway/cellular system at a time. Accordingly, many of the classic anti-allergic drugs have a critical impact also on T helper cells, which are pivotal not only during the sensitization but also the maintenance phase of allergic diseases. Recent years have seen a dramatic increase of novel drugs with the potency to interfere, more or less specifically, with T lymphocyte function, which might, possibly together with classic anti-allergic drugs, help harnessing one of the central cellular players in allergic responses. A major theme in the years to come will be a thoughtful combination of previously established with recently developed treatment modalities.
Keywords: Anti-allergic drugs, T cells, allergy, pharmacological modulation, immunomodulation
1. Introduction
This review will focus on the T cell-directed effects of classic anti-allergic drugs and on established and novel immunomodulatory drugs, which have an intrinsic potential to interfere with undesired allergen-specific T cell reactions and offer treatment options especially in severe manifestations of allergic diseases. Since the first synthesis of histamine more than 100 years ago, the first clinical application of cortisone for auto-inflammatory and allergic diseases more than 60 years ago and the development of the first immuno-suppressive substances targeting calcineurin, such as cyclosporin A and tacrolimus (FK506) in the 1970ies and 1980ies respectively, the armamentarium of both classic anti-allergic and immunosuppressive drugs has grown steadily, nowadays ranging from targeted drugs impacting on very defined signaling pathways to the therapeutic application of oligonucleotides and monoclonal antibodies [1–4]. The T cell modulatory potential of classic anti-allergic drugs such as histamine receptor antagonists, β2-adrenoreceptor agonists, leukotriene receptor antagonists and corticoids will be discussed and contrasted with classic and novel immunosuppressive drugs. To restrict the scope of this review to a manageable volume, we here concentrate on the pharmacological options to interfere with allergic diseases but we refrain from discussing the multiple beneficial aspects of allergen-specific immunotherapy (AIT) [204] and of altered peptide ligands (APL) [205] on allergen-specific T lymphocytes.
2. Principal mode of action and effects on T lymphocyte function of classical anti-allergic drugs
This section describes the impact on T helper (Th) cells of systemically applied classic anti-allergic drugs but omits chromoglicic acid-based mast cell membrane stabilizers, which are almost exclusively applied locally.
2.1. Histamine and anti-histaminic drugs
Histamine, is a tissue hormone and neurotransmitter, which is ubiquitously present in the human body (with highest expression in the lung, skin and gut) and has been characterized already in 1907 by Windaus and Vogt [5]. Later, it was characterized by Dale and Laidlaw as a bodily substance in humans [6]. Already in 1937 Bovet and Straub developed first substances, which functioned as anti-histamines spearheading anti-histamine research and also the development of first neuroleptics [7]. Histamine is clearly one of the important mediators of allergic inflammation. Within the immune system, histamine is stored in mast cells and basophils from where it gets released either upon FceR-mediated triggers, engagement of the anaphylatoxin receptors C3a and/or C5a or other basic substances (e.g. bradykinin, substance P, mastoparan and others) resulting in immediate type allergic reactions, resulting in smooth muscle contraction and increased vascular permeability [10]. Histamine is also produced in non-immune cell types at high levels such as entero-chromaffine cells in the stomach, lymph nodes and thymus [10]. Genetic ablation of histidine decarboxylase (HDC) leading to complete absence of histamine resulted in a reduction of the overall numbers of mast cells and the appearance of abnormal mast cells but neither influenced viability nor fertility of mice [8]. However, HDC ablation had a major impact on the sleep-wake cycle of animals which was accompanied by changes in the cortical-electroencephalograms [9]. As of today, four different 7-span transmembrane and G-coupled receptors termed histamine-1-receptors (H1R) to H4R have been characterized (Fig. 1) [206,207]. Of note, these receptors reveal constitutive activity in the absence of agonists, which gets shifted by inverse agonists representing the classic ‘anti-histamines’ [208]. While the H3R is almost exclusively expressed in the brain, the other HRs are variably expressed on different leukocyte populations [10]. Apart from mast cells and basophils also CD4 Th cells express a whole set of different HRs. Consequently, histamine has been shown to also differentially regulate lymphocyte responses [11]. Engagement of the H1R leads to IFN-γ production and thus enhanced Th1 cell responses. In contrast, engagement of the H2R, which is predominantly expressed on Th2 cells, leads to suppression of Th1 and Th2 responses [12]. This fits to the observation that T cells of H2R ko mice produce increased levels of both Th1 and Th2 signature cytokines [13]. Compatible with these findings, ablation of either of the two receptors alters the susceptibility of mice to experimental allergic encephalomyelitis (EAE) [14].
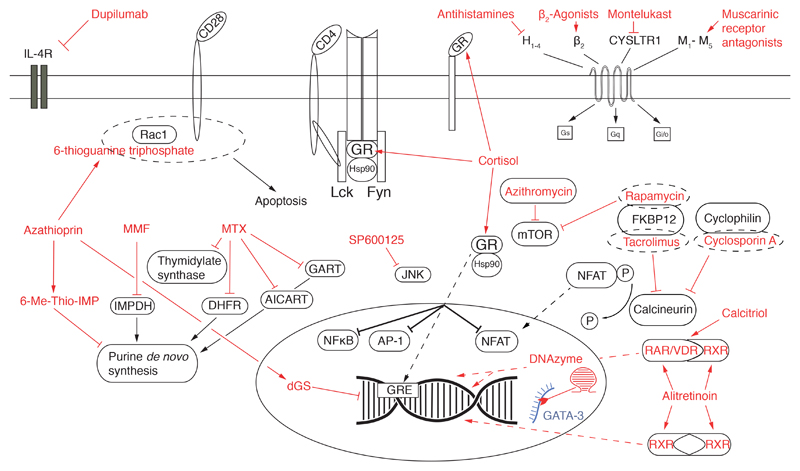
Fig. 1. Overview of targets typically modulated by anti-allergic drugs.
Figure shows a schematic T cell with relevant transmembrane receptors and ion channels inserted into the plasma membrane. The cytosol contains different signaling and metabolic pathways and a nucleus containing DNA and relevant transcription factors. The different anti-allergic and immunosupressive drugs and novel experimental substances (red font) and their targeted structures (red arrows) are depicted. Messenger RNA targeted by a DNAzyme is shown in blue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
This clear dichotomy between H1R and H2R is, however, discussed somewhat controversially in other reports, since it has been shown that engagement of H1R on T cells of rhinitic subjects enhanced Th2 cytokine production in one study, while it has the opposite effects in control individuals and asthmatics [15]. Apart from T cells, H2R-signaling exerts its inhibitory effects also on antigen presenting cells in which a whole collection of cytokines get inhibited [16,17]. The function of the H4R on T cells, which was identified by genomic homology screening much later than the other three receptors, is less well defined [18]. Recent investigations point to a role of the H4R in the induction and/or aggravation of pruritus in atopic dermatitis. In vitro polarized Th2 cells, which clearly express the H4R on the mRNA and protein level, up-regulated mRNA for IL-31 upon H4R ligation. IL-31 represents a pruritogenic cytokine [19]. Notably, in patients suffering from atopic dermatitis, H4R tends to be expressed at elevated levels and stimulation of PBMC of such patients with an H4R ligand and in the presence of the superantigen staphylococcal enterotoxin B (SEB) resulted in higher IL-31 levels. The molecular mechanism for H4R function seems to be related to the signal transducer molecule AP-1, which mediates and is required for the expression of Th2 related cytokines IL-4, IL-5 and IL-13. In line with these findings, H4R agonists have been shown to inhibit purified protein derivative (PPD)-induced IFN-γ production of peripheral blood mononuclear cells (PBMC) [20]. Of note, H4R antagonists such as JNJ-7777120 should strongly impact on Th cell functions including those of Th17 cells [21] and should be of benefit for the treatment of allergic symptoms. In fact, Cowden et al. have shown that treatment with a H4R antagonist reduces airway hyperreactivity along with the production of the Th2 cytokines IL-5 and IL-13 [22]. In contrast, H4R engagement was shown to reduce the production of inflammatory cytokines produced by plasmacytoid dendritic cells, such as TNF-α, IFN-γ and CXCL8 [23].
However, the situation seems to be more complicated, due to the pleiotropic functions of histamine since it has been shown in the past that H4R engagement by the bona fide H4R agonist 4-methyl-histamine, e.g. by intra-tracheal instillation, can also lead to the accumulation of Treg cells in lung tissues, which revealed increased IL-10 production [24]. In contrast, H4R agonists or histamine itself foster Th17 responses on the mRNA but also protein level since H4R is expressed on Th17 cells [25]. The impaired migratory and regulatory function of Treg might also be the reason for the increased susceptibility of H4R ko mice to EAE [26]. Due to its modulatory activity on CCR2 expression, H4R also impacts on the circulation of both Th cells but also antigen presenting cells such as epidermal dendritic cells and monocytes [27,28]. Because of the anti-inflammatory action of novel H4R antagonists, such as JNJ-7777120, but also due to their anti-pruritic activity, these substances represent prime drugs for the treatment of contact hypersensitivity [29]. Moreover, a more general regulatory role of H2R and H4R has been shown in γδ T cells, with H4R signal-ing increasing intracellular Ca2+ levels, actin polymerization and chemotaxis, while H2R signaling leads to cAMP accumulation and down-regulates cytotoxicity against tumor target cells [30].
2.2. β2 adrenergic-receptor agonists
β2-adrenergic receptor agonists (β2-ARA) are important drugs in asthma therapy [31]. While the primary intention for β2-ARA application in asthmatic patients is certainly their immediate bronchodilatatory effect, subtle effects of β2-ARA on immune cells have been described. In fact, β2-AR have been shown to be widely expressed on different subsets of leukocytes, their expression on T cells has also been clearly demonstrated [32–34]. While some studies suggested that β2-AR are differentially expressed on different Th cell subsets, with clear-cut expression on Th1 cell clones, but rather low to non-detectable expression on Th2 cell clones [32], others have shown that β2-AR are also regularly expressed and functional on Th2 cells [35]. Consequently, also the effects on T cells of β2-AR engagement have been controversially discussed during the last years depending on the responder T cell population studied and the stimuli used to activate them. Notably, some reports showed that β2-ARA monotherapy might increase the number of Th2 cells. By stimulating PB lymphocytes of healthy individuals or lupus patients with IL-2 in the presence of isoproterenol, a significant accumulation of Th2 cells producing IL-13 was observed in one study [36]. This effect was attributed to the protein kinase A (PKA)-activating properties of the G-protein coupled β2-AR [36]. In contrast, isoproterenol inhibited the growth of CD3/CD28 stimulated T cells, which was also accompanied by a reduction in the secretion of Th2 cytokines in another study [37]. Accordingly, β2-ARA treatment might increase cytokine production and secretion by type 2 T cells [36,38] upon antigen-independent but not necessarily upon antigen-dependent T cell stimulation. In contrast, other studies investigating the effect of the β2-ARA salbutamol on PHA plus PMA treated PB T cells of healthy subjects did not reveal inhibitory effects when compared to dexamethasone, which significantly suppressed the production of IL-4 and IL-5 and to a lesser degree also of IFN-γ [39].
Thus, studies investigating the effects on T cells of β2-ARA used as mono-substances have led to seemingly controversial results, which might, however, be explainable by the mode of cellular activation applied. However, the situation becomes much clearer when β2-ARA were combined with steroids and their combined effects were monitored. Along those lines, Loza et al. found that β2-ARA in combination with the steroids budesonid, tended to inhibit the generation of IL-13 producing Th2 cells in PB lymphocytes of asthmatics, while the numbers and percentages of IFN-γ producing cells remained unchanged [38]. This also fits to the observation by others that β2-ARA synergize with glucocorticoids in inducing enhanced production of IL-10 by human regulatory CD4+ T cells [40]. In the clinical setting this would indicate that dose escalation with long-acting β2-ARA might translate into additional clinical benefit in previous moderate responders to inhaled glucocorticoids and β2-ARA. Along those lines, allergen-specific T cells lines induced in the presence of fluticasone and salmeterol led to the generation of Tregs, which inhibited IL-5 and IL-13 production by Th2 cells when co-cultured at a ratio of 4:1 between effector and regulatory T cells [40]. This inhibition was clearly IL-10 dependent since it could be blocked by anti-IL-10 receptor antibodies. Consequently, treatment with β2-ARA might not only lead to bronchodilatation but, especially when combined with steroids, also seems to be proficient in inhibiting the production and release of effector cytokines from Th2 cells. Mechanistically, the activity of β2-AR is governed by the Gs-coupled adenylyl cyclase system leading to a rise in intracellular cAMP followed by an increase in PKA activity [41]. In fact, elevation of β2-AR signaling has been shown to induce Th2 cells with increased IL-4 production [42,43]. On top of the subtle cues regulating its activity, functionally relevant polymorphisms within the β2-AR system have been described recently. Especially the haplotype β2-AR (2/4) has been identified to be associated with severe asthma and poor bronchodilatator response [44]. Whether this polymorphism also affects signaling and function in immune cells remains to be shown in the future. Nevertheless, it is tempting to speculate that polymorphisms in β2-AR might impact on the generation of Treg cells, as discussed above, and might also differentially impact on other Th cell subtypes and thus contribute to the increased susceptibility to asthma and its exacerbation in different populations [44]. Apart from allelic variants, recent evidence suggests that the expression of β2-AR in Th1 and Th2 cells might also be regulated by epigenetic cues [43]. In fact, Th2 culturing conditions resulted in decreased β2-AR gene expression. This decrease is likely brought about by lower levels of H3 and H4 acetylation and H3K4 methylation, which are accompanied by higher levels in H3K9 and H3K27 methylation [43].
2.3. Muscarinic AChR
Apart from the above-described neurotransmitters, lymphocytes have been shown to express muscarinic cholinergic receptors [45,46]. In the Jurkat T cell line several muscarinic agonists, such as acetylcholine, carbachol, muscarine and oxotremorine-M, induced a transient elevation of cytosolic Ca2+ concentrations, which was insensitive to pertussis and botulinus toxin, i.e. not dependent on GTP-binding proteins [47], but inhibitable by phorbol ester triggered PKC activation. Notably, T cells of nasal polyposis samples released TNF-α upon exposure to increasing doses of methacholine [48].
2.4. Leukotrienes and leukotriene receptor-antagonists
Lipoxygenase-dependent arachidonic acid metabolites such as leukotrienes (LT) get synthesized by a large number of inflammatory cells, thrombocytes and reticulocytes [49,50]. The different cell types characteristically express different sets of lipoxygenases and thus produce a cell-type specific spectrum of LT [51,52]. Notably, many inflammatory stimuli amplify the production of cysteinyl LTs (LTC4, LTD4 and LTE4), which induce bronchoconstriction and mucus secretion in the lungs and inhibit tracheal mucus transport [53]. Two cysteinyl LT receptors with differential expression characteristics have been described in the past, Cys-LTR1, which is expressed in peripheral blood leukocytes, lung macrophages and airway smooth muscle cells, and Cys-LTR2, which is expressed in heart, adrenal glands, placenta and peripheral blood leukocytes [54]. A number of LT-specific antagonists for the treatment of asthma have been developed in the past [55]. While resting T lymphocytes express rather low levels of Cys-LTR1 and Cys-LTR2, they tend to up-regulate both receptors upon ligation of the TCR/CD3 complex with anti-CD3 mAbs [56]. Similarly, elevated Cys-LTR1 levels have also been observed on T cells of house dust mite allergic individuals upon stimulation with Der p, which could be prevented by co-incubation with the Cys-LTR1 inhibitor MK571 [57]. Notably, co-incubation of anti-CD3 mAb treated T cells with the selective Cys-LTR1 inhibitor montelukast increased IFN-γsecretion but also specific cell death of T cells while leaving IL-4 secretion levels unaltered. The increase in T cellular death was attributed to the gradual loss of the anti-apoptotic molecule Bcl-2 [56]. A more recent study demonstrated that, in fact, the effect of Cys-LT on Th2 cells is much broader than originally estimated [58]. In fact, highly purified human Th2 cells isolated from buffy coats revealed enhanced LTE4-dependent production of the pro-inflammatory proteins CCL3, CCL3L1, CCL3L3 and CCL4L2 and together with PGE2 increased the expression of the non-classical Th2 mediators IL-22, IL-8 and GM-CSF [58]. Thus apart from their bronchodilatatory effects, LTAs might also critically influence Th2 cell function.
2.5. Glucocorticoids: classical representatives of immunosuppressive drugs
Glucocorticoids regulate central aspects of cellular metabolism by driving gluconeogenesis, in addition, they exert strong antiinflammatory activity. Cortisone, the first immuno-suppressive drug for allergic conditions such as asthma and allergic dermatitis was already clinically applied in the 1950’s [59,60]. Since then, the development of a large number of synthetic glucocorticoids with increased potency and low systemic burden when applied locally, e.g. in the airways, paved their way into the clinician’s holster such as betamethasone, beclomethasone, budesonide and fluticasone [61].
Glucocorticoids exert pleiotropic effects via the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR), both of which are expressed ubiquitously on different cell types [62–64]. With regard to the GR, the mechanisms of action can be divided into classical genomic effects mediated upon binding to the cytosolic GR (cGR) and non-genomic effects also mediated through the cGR and by binding to a putative membrane GR, described in a single report to be a G-protein coupled receptor [65,66]. Notably, membrane expression of a GR (mGR) was also shown in one study in lymphocytes from leukemic patients and in a human T cell line [67]. Additionally, other reports suggested that glucocorticoids might also exert their function through non-specific interactions with the plasma membrane [66]. In principle, GRs reside in the cytosol where they are attached to a chaperone complex, which consists of heat-shock protein 90 (hsp90), hsp70, hsp90-binding protein p23 and immunophilins [68]. Upon binding of glucocorticoids to GRs, the such formed complexes translocate into the nucleus where they can either bind to glucocorticoid responsive elements (GRE) of the DNA, thereby facilitating or hindering gene transcription, or they directly interact with other transcription factors in an enhancing or suppressing fashion, like AP-1 or NFκB [68]. These genomic processes are termed transactivation and transrepression, respectively [69].
The basic principles of the GR also apply to the MR with the exception that a membrane receptor for mineralocorticoids has also been suggested but not sufficiently proven as of yet [70]. Very similar to the GR, also the MR usually resides in the cytoplasm and translocates to the nucleus after binding of its physiologic substrate, i.e. aldosterone, and is also capable of mediating rapid, non-genomic cellular changes [71,72].
In T lymphocytes, glucocorticoids have been shown to inhibit T-bet transcriptional activity by facilitating direct interaction of the GR with T-bet and thereby preventing it from binding to the DNA. Apart from T-bet, GCs were also shown to inhibit GATA-3 activity via inhibition of PKA-dependent phosphorylation of GATA-3 and p38 in murine splenocytes [73]. In principal, glucocorticoids reduce the expression of pro-inflammatory genes, such as e.g. cyclooxygenase 2 (COX2) mainly via inhibition of NFκB but also stimulate antiinflammatory cytokines such as IL-10 [74–76].
Notably, Foxp3+CD25+ T cells from atopic and non-atopic individuals showed increased suppressive activity on allergen-stimulated effector CD4+CD25− T cells via an IL-10 dependent mechanism when they were pre-incubated with fluticasone-propionate [77]. Along those lines, another study demonstrated that CD4+ T cells from asthmatic individuals receiving inhaled steroids or receiving inhaled plus systemic steroids had significantly increased mRNA expression of Foxp3 and IL-10 compared to asthmatic patients not treated with steroids or healthy individuals [78].
Thus, despite many other effects, GC treatment seems to favor regulatory T cell function while suppressing Th1 and Th2 cell differentiation and action. In addition, also a direct interaction between GCs and T cell receptor (TCR) signaling was suggested in one study that described rapid, immunosuppressive effects of dexamethasone pre-treatment on anti-CD3/CD28-stimulated human CD4+ T cells by GR-dependent inhibition of the lymphocyte-specific protein tyrosine kinases (PTKs) Lck and Fyn [79]. Mechanistically, this inhibitory activity of GCs was shown to be induced by their disruption of a complex formed between Lck, Fyn, Hsp90 and GR, which, under physiological conditions, associates with the TCR complex upon TCR ligation [80]. Apart from lymphocyte-specific PTKs, high-dose GC treatment also leads to phosphorylation of the tyrosine residues 315 and 492 of the ζ-chain-associated protein ZAP-70 and thereby accounts for further non-genomic inhibitory effects on the downstream TCR signaling cascade [81].
With regard to MCs, no direct effects on T lymphocytes upon ligand binding have been shown as of today. Nevertheless, MC treatment of mice led to a shift towards the Th1/Th17 cell lineage, however, this was due to indirect, agonistic effects by MR acting on dendritic cells (DCs) [82]. Notably, this Th17-promoting effects could be inhibited by co-treatment with either one of the aldosterone antagonizing substances eplerenone or spironolactone underscoring the T cell-specific immunomodulatory capacity of MR [83].
Prolonged GC treatment leads to a number of adverse side effects such as lipodystrophy, reduced insulin sensitivity, osteoporosis, candidiasis and psychological alterations [62]. Another problem of GC-treatment is represented by primary or secondary resistance to GCs, representing a major limitation when f.i. approaching the patient suffering from allergic asthma [84]. GC-resistance was found to be associated with increased expression of c-Fos, phosphorylated c-JUN and phosphorylated JUN N-terminal kinase (JNK) in immune-histochemically analyzed skin punch biopsies from GC-resistant individuals when compared to GC-sensitive individuals with regards to tuberculin-mediated cutaneous responses.
Consequently, failure of GC-mediated inhibition of JNK phosphorylation might explain GC resistance [85]. While certainly of interest, this study could, however, not show a T cell-specific difference in the degree of JNK phosphorylation or expression. That the JNK phosphorylation status might be critical for allergen-specific inflammation has been shown in another study in which inhibition of JNK by the ATP-competitive inhibitor SP600125, prior to allergen challenge, reduced the expression of Toll-like receptor 9 (TLR9) and significantly reduced the number of inflammatory cells and ovalbumin-specific IgE in a mouse model of acute asthma [86]. This study is in accordance with earlier work, which had described overexpression of c-Fos in T cells from GC-resistant but not GC-sensitive asthmatics. Thus, activity of both c-Fos and c-JUN, fine-tuned by up-stream kinases, which form the AP-1 transcription factor complex seem to be dysregulated in allergy and associated with GC resistance [87]. Studies performed with T cells from healthy individuals using SP600125 revealed specific inhibition of IL-2, IL-13 and IFN-γ but not of the major IgE-class switching factor IL-4 upon stimulation with anti-CD3/B7-H2 coated beads [88]. This implies that interference with JNK signaling might have different effects in health and disease. In summary, it will be interesting to study the effects of drugs targeting JNK or up-stream constituents of its signaling pathway with regard to their impact on Th effector functions such as the cytokines produced by them and the transcription factors activated, which can be studied in much more detail with the technologies available as of today. Apart from regulating AP-1-dependent gene expression GCs have been shown to inhibit IL-4 by interaction with the transcription factor nuclear factor of activated T cells (NFAT) [89].
Due to the severe side effects when administered systemically, there is a strong need for corticoids that only exert selected glucocorticoid actions such as immunosuppression or attenuation of inflammatory reactions while minimizing unwanted side effects. With this objective in mind, the development of selective glucocorticoid receptor agonists (SEGRAs) was encouraged. Similar to the classic representatives also the newly developed SEGRAs such as mapracorat (ZK 245186), which currently undergo clinical evaluation [90] and have significantly fewer side effects (e.g. skin atrophy, hyperglycemia and hepatotoxicity), show clear-cut inhibitory effects on T cells, which are equipotent to their parent drugs [91].
3. Principal mode of action and effects on T lymphocyte function of classical immunosuppressive drugs
This section describes immunosupressants used to treat severe forms of allergic diseases and their impact on T lymphocyte function.
3.1. Immunosuppressive drugs interfering with DNA metabolism
3.1.1. Azathioprine
Azathioprine is a pro-drug of 6-mercaptopurine (6-MP) and works by means of its various metabolites, e.g. deoxy-6-thioguanosine-5’-triphosphate (dGS) and S-methyl-thioinosine-5’-monophosphate (6-Me-Thio-IMP) through different mechanisms by inhibition of de novo synthesis of purines, protein synthesis and by induction of apoptosis [92]. The inhibitory capacity of purine analogues with a 6-mercapto group on the growth of Lactobacillus casei was first described by Elion et al. [93]. The relative selectivity of these drugs on lymphocytes was explained by their exclusive inhibition of de novo synthesis of purines, which represents the major route for providing nucleotides in lymphocytes compared to other cell types that can also sufficiently make use of the purine salvage pathway [94]. Care has to be taken with regard to the inter-individual differences in the activity of the enzymes thiopurine-methyltransferase (TPMT) and NUDT15 when treating patients with thiopurines, since both enzymes are important for inactivating cytotoxic metabolites [95]. Furthermore, the combination of allopurinol, a xanthine oxidase inhibitor, together with mercaptopurines can have hazardous effects due to the inhibition of the conversion of 6-MP to the metabolite thiouric acid [96]. Apart from limiting the purine pool, azathioprine metabolites, such as 6-thioguanine triphosphate, seem to directly affect T lymphocyte signaling, f.i. by converting the co-stimulatory signal provided by CD28 ligation into a pro-apoptotic signal in human CD4+ T cells by its interference with Rac1 via 6-Thio-GTP formation which replaces GTP and abrogates Rac1 downstream signaling [97,98]. While short-term in vivo treatment with thiopurines already blocks lymphocyte proliferation but still preserves their effector functions, only long-term treatment induces depletion of memory cells, which have a history of multiple antigen encounter [99]. The situation seems to be slightly different when activated (transformed) T cell types were interrogated, since one study found that azathioprine also inhibited the up-regulation of inflammatory genes such as α4-integrin, TNFRSF7 and TRAIL in Jurkat T lymphocytes upon stimulation [100]. According to a meta-analysis in the Cochrane database, the application of azathioprine as a corticosteroid sparing agent in asthma is lacking evidence for support due to the low numbers and types of studies available [101]. Nevertheless, with regard to atopic dermatitis, systemic TPMT-adjusted monotherapy with azathioprine appeared to be a useful alternative to systemic glucocorticoids in patients with symptoms insufficiently controlled by topical treatment [102].
3.1.2. Methotrexate (MTX)
Methotrexate is a folic acid analogue with minimal structural [124]. That MPA also alters the epigenetic status of CD4 T cells by differences inhibiting the enzyme dihydrofolate reductase (DHFR) [103]. The activity of folic acid analogues against different neoplasms and the ability to induce partial remission in acute leukemia in humans by the analogue 4-amino-N10-pteroylglutamic acid (Aminopterin) was first described by Farber in the 1940’s [104,105]. In animal studies amethopterin (Methotrexate, MTX) showed a ten times lower cytotoxicity than aminopterin which may explain why it became the clinical folate analogue of choice before newer folate antagonists such as i.a. pemetrexed or raltitrexed were designed [106,107]. Due to the inhibition of DHFR, folate metabolites are generated which, together with MTX, exert their antineoplastic and immunosuppressive effects via further mechanisms such as inhibition of thymidylate synthase, aminoimidazole carboxamide ribonucleotide transformylase (AICART) and glycinamide ribonucleotide transformylase (GART), enzymes also involved in the nucleotide synthesis pathway [108]. Since mammalian cells are not able to synthesize folic acid de novo and thus dependent on external folate supply, methotrexate treatment affects many different cell types and thus exerts considerable side effects, especially in frequently dividing cells, such as epithelial cells in the gut and cells of hematopoietic origin, such as immune cells. While rapidly dividing cells, which depend on de novo purine synthesis, are more prominently affected by MTX treatment, MTX also exerts additional immunomodulatory effects by inhibition of cytokine production, DNA and protein methylation and histone and protein acetylation [109]. Notably, the clinical success of MTX treatment in a collective of RA patients has been shown to positively correlate with high numbers of CD39+CD4+Treg cells and consequently high levels of ADO production [110]. Mechanistically, this is thought to be caused by MTX-mediated demethylation of the Foxp3 promoter, which has been shown to be also responsible for increased CTLA-4 expression on CD4+ T cells of patients undergoing MTX treatment [111]. Several studies, among them also a double-blind randomized placebo controlled study showed that low doses of MTX can be an effective steroid-sparing treatment for patients with steroid-dependent asthma and that overall tolerance was very good [112,113]. This effect is also reflected by the fact that ex vivo MTX treatment increases the effects of glucocorticoids on T cells from asthmatic patients [114].
3.1.3. Mycophenolate mofetil
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid, which was approved for the treatment of kidney transplant patients in the United States already in 1995 [115].
Mycophenolic acid (MPA) was first isolated from Penicillium stoloniferum and described for its antibiotic effects [116,117]. Soon, its potential as an anti-cancer drug became apparent and later on it was also evaluated for its capacity as an immuno-suppressant [118,119]. The main mechanism of action of MPA is the inhibition of inosinic acid dehydrogenase (IMPDH) and thereby the inhibition of purine nucleotide synthesis [120]. Human IMPDH is expressed in two isoforms whereby MPA is more potently inhibiting the type II isoform [121]. As outlined for thiopurines, interference with the de novo purine synthesis is supposed to affect lymphocytes more prominently than other cell types. Some authors suggested an additional selectivity for lymphocytes due to the increased expression of the type II isoform of IMPDH in stimulated T cells [122]. However, one study using PMA-stimulated PBMCs showed the upregulation of both isoforms and reasoned that type I and II IMPDH are responsible for nucleotide synthesis in activated T cells [123]. It was also shown that MPA, by inhibiting the de novo guanine nucleotide synthesis, resulted in a cell cycle block affecting the transition from G0 to S phase of activated normal human T cells and that inhibition of cyclin D/CDK6 kinase induction was responsible for this effect [124].
That MPA also alters the epigenetic status of CD4+ T cells by modulating HAT and HDAC function has been shown recently [125]. The application of MMF for patients with severe therapy-resistant asthma was described in a case study of 22 patients in which more than half of the patients benefitted from the treatment [126]. In an earlier study, the successful treatment of a patient suffering from non-allergic, therapy-resistant asthma with MMF had been reported [127]. Also, for severe cases of atopic dermatitis, which were not adequately controlled by topical treatment, MMF was successfully applied [128]. However, a definitive recommendation, whether or not to favor MMF compared to other immunosuppressants cannot be given as of yet due to the lack of randomized, placebo controlled clinical trials for this indication [129].
3.1.4. Calcineurin targeting drugs, classic immunosuppressive drugs
Calcineurin is a phosphatase responsible for the dephosphorylation of NFAT [130]. Dephosphorylated NFAT can enter the nucleus and promote transcription of genes such as IL-2, facilitating T cell activation and proliferation [131]. Tacrolimus (FK-506) and cyclosporin A (CsA) but also rapamycin (Sirolimus) are ligands of the so-called immunophilins, i.e. cyclophilin in the case of cyclosporine A and FK506-binding protein (FKBP) with regard to FK-506 (Tacrolimus) and sirolimus (Rapamycin) [132]. These immunophilin-drug complexes then mediate inhibition of calcineurin, which results in impaired de-phosphorylation and nuclear translocation of NFAT [133,134]. Although its name might suggest T cell specific expression, the transcription factor NFAT also exerts regulatory functions in other tissues such as the central nervous system, blood vessels, the heart and the kidneys [135]. This may also explain the pleiotropic side effects, which are associated with calcineurin inhibitor treatment, such as nephrotoxicity, neurotoxicity (i.e. tremor, headache, paresthesia) and metabolic disturbances such as dyslipidemia and diabetes [136].
Immunosuppressive drugs characterized by a macrolide structure, such as sirolimus (Rapamycin), also use FKBP to form an active complex [133,134], however, they exert their function by inhibiting the serine/threonine-kinase activity of the mammalian target of rapamycin (mTOR). Consequently, the activity of the downstream target of mTOR, i.e. p70SK6 gets impaired resulting in reduced activation of S6 and thus less activation of p34cdc2 kinase which is critical for cell cycle progression and thus proliferation. Interestingly, also other macrolides and macrolide-related compounds such as the antibiotics clarithromycin and azithromycin have been described to exert immunomodulatory effects [137]. In one study azithromycin inhibited proliferation and cytokine production of human CD4+ T cells upon stimulation with anti-CD3/anti-CD28 coated microbeads [138]. Notably, these effects were shown to be mediated by mTOR in an FKBP-independent fashion suggesting a direct interaction between azithromycin and mTOR [138]. Apart from its application in the post-transplant setting cyclosporin A has also a place as a corticosteroid-sparing drug in individuals suffering from corticosteroid-dependent asthma, i.e. individuals suffering from asthma and requiring continuous corticosteroid medication [139]. Furthermore, its application as an aerosol in asthmatic patients has been tested and was well-tolerated and, in fact, novel galenic formulations for this route of administration are under development [140,141]. Along those lines also entirely cell-nonpermeable analogues of CsA, such as MM218, have been developed, with the intention of blocking extracellular immunophilins functioning as important chemotactic agents attracting among other cell types CD4+ T cells [142]. In a mouse model of allergic asthma MM218 inhibited lung inflammation by 80% and directly inhibited the recruitment to the lungs of allergen-specific CD4+ T cells [142].
While no placebo-controlled clinical study investigating the application of tacrolimus in patients suffering from asthma could be found, occasional case reports describe the successful achievement of control over severe corticosteroid-dependent asthma with tacrolimus [143]. Of note, however, there is evidence for increased occurrence of allergic sensitizations among transplant patients treated with tacrolimus but not with cyclosporine A [144–146]. Whether these findings are due to pharmacodynamic or kinetic differences of the two drugs remains to be shown in future studies. More established is the use of tacrolimus and the structurally related pimecrolimus for the treatment of atopic dermatitis for mild to moderate disease activity, a treatment protocol, which has been already approved for children from 2 years onwards [147].
4. Principal mode of action and effects on allergen-specific T lymphocytes of selected vitamins known to impact on T cell function
4.1. Vitamin A (retinoic acid)
Vitamin A and its derivatives such as the metabolite alitretinoin (9-cis-retinoic acid) and isotretinoin (13-cis retinoic acid) are commonly used for the treatment of different dermatological conditions such as acne vulgaris and chronic hand eczema while all-trans retinoic acid (t-RA) is used to treat pro-myelocytic acute leukemia [148–151]. Several intracellular receptors have been identified as binding partners for alitretinoin such as the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), both representing nuclear receptors, the latter one being relatively specific for alitretinoin and not being bound by some other vitamin A derivatives, e.g. all-trans retinoic acid (t-RA) [152]. The action of retinoids via RAR or RXR requires heterodimerization of RAR with RXR or homodimerization of RXR and translocation into the nucleus although these two types of dimers bind to different DNA response elements [153]. Furthermore, RXR also binds to and functionally interacts with the vitamin D receptor (VDR) and the thyroid hormone receptor (TR) thereby stimulating DNA binding upon encountering their respective ligands [154].
Interestingly, vitamin A deficiency drives naïve CD4+ T cells towards the Th1 phenotype whereas treatment with alitretinoin can polarize these cells into Th2 cells via the RXR, both processes being independent of antigen presenting cells [155]. This was confirmed in another study in human PBMCs and purified human T cells in which t-RA and alitretinoin treatment resulted in increased mRNA expression levels of IL-4 and IL-5 as well as decreased expression of IFN-γ[156]. The same study also showed that t-RA treatment decreased expression of the Th1 master regulatory transcription factor T-bet but increased the Th2 lineage specific transcription factor GATA-3. Another study found that human peripheral blood T cells express the RAR isoforms a and γ but not 13 and that treatment of these cells with alitretinoin inhibited phytohemagglutinin (PHA) induced proliferation via ligation of RAR-γ and inhibition of janus kinase 3 (JAK3) expression [157]. Also, an anti-apoptotic effect of retinoid X receptor agonists such as alitretinoin on naïve CD4+ T cells via up-regulation of Bcl2a1 was described [158]. Moreover, Tóth et al. showed that interaction of an RARa/RXR heterodimer upon binding of alitretinoin interacted and altered the transcriptional activity of the ligated GR enhancing apoptosis of mouse thymocytes in vivo, which was independent of DNA binding of RAR or RXR [159]. The potential Th2-promoting effect of retinoic acid derivatives was confirmed in the follow-up of a randomized placebo-controlled trial investigating the effects of neonatal vitamin A supplementation, which was associated with an increased risk of atopy and wheeze in females but not boys [160].
All these findings raise the critical question whether retinoic acid derivatives could be created that, similar to the SEGRAs described above, would exert selected agonistic and antagonistic effects thereby altering T helper cell polarization towards a desired phenotype.
4.2. Vitamin D (1,25-dihydroxycholecalciferol, calcitriol)
Similar to the GR or the RAR also the vitamin D receptor (VDR) constitutes a nuclear receptor [161]. The vitamin D response elements either bind the VDR as a homodimer in the non-ligated state or as a heterodimer consisting of VDR and RXR upon binding of vitamin D [162]. With regard to its mechanism of action, it might be justified to call it a hormone rather than a vitamin. A tremendous amount of research regarding the involvement of vitamin D in a wide spectrum of diseases, predominantly autoimmune mediated diseases but also cancer has been performed within the last years. This is especially interesting with regard to the geographical distribution of certain diseases such as multiple sclerosis and their association with the level of sun exposure and vitamin D trough levels in individuals living at different latitudes. Examples for the immunomodulatory mechanisms of vitamin D are given by a study which showed that calcitriol inhibited Th9 differentiation via the aryl hydrocarbon receptor (AhR) through an IL-10 dependent mechanism in human T cells [163]. Other studies showed that calcitriol increases the expression of the purine ectonucleotidases CD39 and CD73 in human peripheral CD4+ T cells, which are supposed to downregulate inflammation by regulating extracellular levels of adenosine [164]. Another study performed in asthmatic individuals clearly showed that serum 1,25-dihydroxyvitamin D levels positively correlated with the number of CD4+CD25+Foxp3+CD127− Treg cells in peripheral blood and that, in fact, steroid-resistant asthmatics have significantly lower numbers of such Tregs [165]. Furthermore, 1,25-dihydroxyvitamin D3 also increased the immunomodulatory capacity of preexisting Tregs to suppress Th2-skewed immune responses and to regulate airway hyperresponsiveness [166]. Moreover, deficiency of dietary vitamin D in utero or perinatally was shown to shift the Th balance towards the Th2 phenotype, which was accompanied by reduced IL-10 secretion levels in mice challenged with house dust mite extracts [167]. However, when analyzed in T cell clones, some studies reached the conclusion, that vitamin D rather promoted the expansion of Th2 clones and their secretion of IL-4 and IL-13, especially when combined with IL-4 [168]. In humans, vitamin D reduced the expansion of Th17 cells in asthmatic children and reduced the number of IL-17-secreting T cells in adults with atopic conditions [169,170].
Notably, a recently closed randomized, double-blind, placebo-controlled trial investigating the role of oral vitamin D supplementation in individuals with asthma and vitamin D deficiency was unable to show a benefit with regard to first treatment failure and exacerbation rates [171]. In addition, also a meta-analysis of clinical trials investigating the role of oral vitamin D supplementation in asthmatic individuals could not confirm any improvement of disease-related symptoms or objective parameters such as lung function and exacerbation rates [172]. A second meta-analysis concluded that some evidence for a reduction of asthma exacerbations by vitamin D supplementation is provided but the role of supplementation for other asthma related outcomes is currently inconclusive [173].
5. Principal mode of action and effects on allergen-specific T lymphocytes of novel drugs targeting specific T cell functions
5.1. Biologics blocking the action of Th2 cytokines
The Th2 cytokines IL-4, IL-5 and IL-13 represent hallmarks of allergic immune reactions and play an important role in allergic asthma and atopic dermatitis [174]. They not only attract and activate a multitude of immediate effector cells but also represent important differentiation factors for T cells. In fact, the absence of IL-4 inhibits differentiation of Th0 cells towards Th2 cells [175,176]. Notably, the IL-4R and the IL-13R share the same high-affinity a-chain. Very recent clinical studies in atopic dermatitis patients have shown that targeting of the IL-4R α-chain with the monoclonal antibody dupilumab improves pruritus, symptoms of anxiety and depression as well as quality of life [177]. While separately targeting of IL-4 or IL-13 did not improve allergic asthma, beneficial effects of anti-IL-4Rα treatment were clearly evident in such patients [178,179]. Whether IL-4Ra blockade also impacted directly on Th cells expressing receptors for IL-4 and IL-13 or whether the beneficial effects observed are the results of targeting innate effector cells remains to be shown in future studies. The combination of Th2 cytokine receptor targeting drugs with other substance classes could represent very elegant, novel treatment options to reduce toxicity and increase treatment efficiency for difficult-to-treat patient collectives.
5.2. Kinase inhibitors and other targeted drugs
As anticipated for JNK in the previous section, another strategy to reduce GC-mediated side effects would be to lower the dose or even completely replace GC treatment by other substances, e.g. targeted drugs ideally exerting selected immunosuppressive effects on Th2 cells. Examples for clinically tested targeted drugs for the treatment of atopic conditions are the Syk-kinase inhibitor R112 for allergic rhinitis asthmatics [180,181], and masitinib, a c-kit/PDGF-receptor tyrosine kinase inhibitor for corticosteroid-dependent asthmatics [180,181]. The primary intention for the local application of a Syk-targeting drug was certainly the involvement of Syk in FceRI-mediated signaling in mast cells [182,183], however, Syk is also well known for its role in regulating TCR-dependent signaling where it promotes phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) within the TCR complex [184]. Similarly, masitinib was clinically evaluated because of its prominent role in inhibiting the c-Kit/PDGF signaling pathway, which regulates the function of mast cells and bronchial structural cells. Although, masitinib is regarded to be a highly-specific c-Kit/PDGF-inhibitor [185], some reports also suggested blocking activity for the T cell specific tyrosine kinase (TK) [186].
Inhibition of phosphoinositide kinase-3δ showed promising results in in vitro experiments performed with T cells from asthmatic patients with regard to inhibition of T cell activation and cytokine secretion and would be an interesting target since, apart from T cells, this kinase is also involved in B cell and mast cell activation [187,188]. Furthermore, the SHIP1 activator AQX-1125, which is supposed to enhance the endogenous inhibition of the phosphoinositide-3-kinase pathway, was well tolerated and led to significantly reduced late responses to allergen challenge in a randomized, double-blind, placebo-controlled, two-way crossover study [189]. Another target that has been shown to be reasonable to target in mouse models of asthma and in human cells from asthmatics in vitro is the Janus kinase 1/3 (JAK 1/3), which is involved in signaling important for Th2 immune responses [187,190]. Furthermore, the tyrosine kinase inhibitor dasatinib, which is known to efficiently inhibit SFK and Abl family kinases and which represents an important alternative for the effective treatment of imatinib-resistant or −intolerant CML-cases [191], was evaluated in a mouse model of asthma, which showed promising results with regard to lung inflammation and remodeling [192] and also had beneficial effects in patients with contact dermatitis [193]. These findings are not entirely surprising since Schade et al. have clearly shown that dasatinib is able to block the earliest events in TCR signaling even at single-digit nanomolar concentrations [194]. Besides its clear T cell modulatory capacity, dasatinib has also been shown to significantly impact on mast cell function by inhibiting FceRI-dependent mast cell degranulation [195]. Also, evaluated in in vivo experiments was the selective inhibition of IκB kinase (IKK) β by IMD-0354, which prevented mice from ovalbumin-induced airway inflammation through inhibition of activation and proliferation of CD4+ T cells in mediastinal lymph nodes [196].
That the IκB kinase 2 inhibitor IMD-0354 also inhibits human T cells has been shown in studies targeting adult T cell leukemia specimens, which are driven into growth arrest or even apoptosis by the inhibitor [197].
5.3. SiRNA and DNAzymes
Small interfering RNAs represent double-stranded RNA molecules, which have the ability to interfere with gene expression by interacting with complementary mRNA sequences of the targeted gene [198]. The use of small interfering (si) RNA to inhibit the lymphocyte-specific protein tyrosine kinase Lck in a mouse model of asthma reduced T cell specific mRNA expression of Lck and resulted in reduced levels of IL-4 and IgE in bronchoalveolar lavage fluids when these mice were pretreated with siRNA [199].
Another class of catalytically active, nucleotide-based molecules are represented by DNA enzymes (DNAzymes) [200]. DNA enzymes have first been described by Breaker and Joyce in 1994 and several representatives have been characterized by in vitro selection procedures from large sequence pools until today [200,201]. Along those lines, a highly interesting development represents the DNAzyme SB010, whose active product hgd40 is composed of a single stranded DNA of 34 bp length that forms GATA-3 mRNA-specific domains and a catalytically active center that allows for specific binding to and cleavage of GATA-3-specific mRNA, which represents the signature transcription factor of the majority of allergen-specific Th2 cells [3,202,203]. Applied locally as an inhalant it was tested in a randomized placebo controlled clinical trial showing promising results with significant reduction of early and late asthmatic responses [3]. The future will show, whether systemic application of targeted versions of allergen-specific DNAzymes can be realized.
Conclusions
Besides classic immunosuppressive drugs also a large number of the established, symptomatic anti-allergic drugs target various functions of T lymphocytes, which are known to be potent initiators but also drivers of the ‘allergic march’. Hypothesis-based combinations of such drugs with e.g. novel targeted drugs impacting on very specific signal-transduction pathways, might help to improve current treatment, especially of severely affected allergic individuals.
Acknowledgement
This work was supported by the Austrian Science Fund (FWF) projects SFB F4609 and DK W1248.
Abbreviations
- 6-MP
6-mercaptopurine
- AP-1
activator protein 1
- AChR
acetylcholine receptor
- AIT
Allergen-specific immunotherapy
- ARA
adrenergic receptor agonist
- AR
adrenergic receptor
- APL
altered peptide ligands
- AICART
aminoimidazole carboxamide ribonucleotide transformylase
- AhR
aryl hydrocarbon receptor
- cAMP
cyclicadenosine monophosphate
- CCL
chemokine ligand
- CML
chronic myelogenous leukemia
- COX2
cyclooxygenase 2
- cGR
cytosolic GR
- DC
dendritic cell
- dGS
deoxy-6-thioguanosine 5’ triphosphate
- DHFR
dihydrofolate reductase
- EAE
experimental auto-immune encephalomyelitis
- FcεR
Fcε receptor
- FKBP
FK506 binding protein
- GART
glucinamide ribonucleotide transformylase
- GR
glucocorticoid receptor
- GM-CSF
granulocyte macrophage colony stimulating factor
- Hsp90
heat-shock protein90
- HR
histamine receptor
- HDC
histidine decarboxylase
- HDAC
histone deacetylase
- HAT
Histone acetyltransferase
- IKK
IκB kinase
- ITAM
immuno-receptor tyrosine based activation motif
- IFN
interferon
- IL
interleukin
- JAK3
janus kinase 3
- JNK
JUN N-terminal kinase
- ko
knock-out
- LTR
leukotriene receptor
- LT
leukotriene
- Cys
cysteinyl
- mTOR
mammalian target of rapamycin
- mGR
membrane GR
- GRE
glucocorticoid response element
- MTX
methotrexate
- MR
mineralocorticoid receptor
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- IMPDH
inosinic acid dehydrogenase
- NFAT
nuclear factor of activated T cells
- PBMC
peripheral blood mononuclear cell
- PB
peripheral blood
- PMA
phorbol-myristate-12-acetate
- PHA
phytohemagglutinine
- PGE
prostaglandine E
- PKA
protein kinase A
- PKC
protein kinase C
- PPD
purifiedprotein derivative
- PTK
protein tyrosine kinase
- RAR
retinoic acid receptor
- RA
retinoic acid
- RXR
retinoid X receptor
- RA
rheumatoid arthritis
- 6-Me-Thio-IMP
S-methyl-thioinosine 5’-monophosphate
- SEGRA
selective glucocorticoid receptor agonist
- SH2
Src homology 2
- SHIP-1
SH2-containing inositol phosphatase-1
- SEB
staphylococcal enterotoxin B
- TCR
T cell receptor
- TPMT
thiopurine methyltransferase
- THR
thyroid hormone receptor
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TK
tyrosinekinase
- VDR
vitamin D receptor
- ZAP70
ζ-chain associated protein 70
Footnotes
Conflict of interest disclosure
Winfried F. Pickl: In the last five years consultant and lecture activity with and without relation to individual products: Novartis; Shareholder Biomay AG, Vienna, Austria. Peter A. Tauber declares no conflict of interest.
References
- [1].Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1987;40(9):1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- [2].Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Pentlow BD, Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2(8104–5):1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- [3].Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, Korn S, Ignatenko S, Timmer W, Rogon C, Zeitvogel J, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 2015;372(21):1987–1995. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
- [4].Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis rheumatic fever and certain other conditions. Arch Intern Med (Chic) 1950;85(4):545–666. doi: 10.1001/archinte.1950.00230100002001. [DOI] [PubMed] [Google Scholar]
- [5].Windaus A, Vogt W. Synthesis of imidazolyl ethyl amine. Ber Dtsch Chem Ges. 1907;40:3691–3695. [Google Scholar]
- [6].Dale HH, Laidlaw PP. Histamine shock. J Physiol. 1919;52(5):355–390. doi: 10.1113/jphysiol.1919.sp001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bovet D. Introduction to antihistamine agents and antergan derivative. Ann NY Acad Sci. 1950;50(9):1089–1126. doi: 10.1111/j.1749-6632.1950.tb39905.x. [DOI] [PubMed] [Google Scholar]
- [8].Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502(1–2):53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- [9].Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22(17):7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, Haas HL. International union of basic and clinical pharmacology. XCVIII. histamine receptors. Pharmacol Rev. 2015;67(3):601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413(6854):420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- [12].Jutel M, Klunker S, Akdis M, Malolepszy J, Thomet OA, Zak-Nejmark T, Blaser K, Akdis CA. Histamine upregulates Th1 and downregulates Th2 responses due to different patterns of surface histamine 1 and 2 receptor expression. Int Arch Allergy Immunol. 2001;124(1–3):190–192. doi: 10.1159/000053707. [DOI] [PubMed] [Google Scholar]
- [13].Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;4(6):735–740. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- [14].Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, Zachary JF, Blankenhorn EP. Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen-presenting cells. Am J Pathol. 2004;164(3):883–892. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Botturi K, Lacoeuille Y, Vervloet D, Magnan A. Histamine induces Th2 activation through the histamine receptor 1 in house dust mite rhinitic but not asthmatic patients. Clin Exp Allergy. 2010;40(5):755–762. doi: 10.1111/j.1365-2222.2010.03457.x. [DOI] [PubMed] [Google Scholar]
- [16].Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittmann M, Werfel T. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;74(9):5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- [17].Glatzer F, Mommert S, Kother B, Gschwandtner M, Stark H, Werfel T, Gutzmer R. Histamine downregulates the Th1-associated chemokine IP-10 in monocytes and myeloid dendritic cells. Int Arch Allergy Immunol. 2014;163(1):11–19. doi: 10.1159/000355960. [DOI] [PubMed] [Google Scholar]
- [18].Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279(2):615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- [19].Raap U, Wichmann K, Bruder M, Stander S, Wedi B, Kapp A, Werfel T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;22(2):421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- [20].Sugata Y, Okano M, Fujiwara T, Matsumoto R, Hattori H, Yamamoto M, Nishibori M, Nishizaki K. Histamine H4 receptor agonists have more activities than H4 agonism in antigen-specific human T-cell responses. Immunology. 2007;121(2):266–275. doi: 10.1111/j.1365-2567.2007.02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahmad SF, Zoheir KM, Abdel-Hamied HE, Alrashidi I, Attia SM, Bakheet SA, Ashour AE, Abd-Allah AR. Role of a histamine 4 receptor as an anti-inflammatory target in carrageenan-induced pleurisy in mice. Immunology. 2014;142(3):374–383. doi: 10.1111/imm.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cowden JM, Riley JP, Ma JY, Thurmond RL, Dunford PJ. Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respir Res. 2010;11:86. doi: 10.1186/1465-9921-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gschwandtner M, Mommert S, Kother B, Werfel T, Gutzmer R. The histamine H4 receptor is highly expressed on plasmacytoid dendritic cells in psoriasis and histamine regulates their cytokine production and migration. J Invest Dermatol. 2011;31(8):1668–1676. doi: 10.1038/jid.2011.72. [DOI] [PubMed] [Google Scholar]
- [24].Morgan RK, McAllister B, Cross L, Green DS, Kornfeld H, Center DM, Cruikshank WW. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol. 2007;78(12):8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- [25].Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol. 2012;180(1):177–185. doi: 10.1016/j.ajpath.2011.09.028. [DOI] [PubMed] [Google Scholar]
- [26].del Rio R, Noubade R, Saligrama N, Wall EH, Krementsov DN, Poynter ME, Zachary JF, Thurmond RL, Teuscher C. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol. 2012;88(2):541–547. doi: 10.4049/jimmunol.1101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dijkstra D, Leurs R, Chazot P, Shenton FC, Stark H, Werfel T, Gutzmer R. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J Allergy Clin Immunol. 2007;120(2):300–307. doi: 10.1016/j.jaci.2007.03.024. [DOI] [PubMed] [Google Scholar]
- [28].Dijkstra D, Stark H, Chazot PL, Shenton FC, Leurs R, Werfel T, Gutzmer R. Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol. 2008;28(7):1696–1703. doi: 10.1038/sj.jid.5701250. [DOI] [PubMed] [Google Scholar]
- [29].Seike M, Furuya K, Omura M, Hamada-Watanabe K, Matsushita A, Ohtsu H. Histamine H(4) receptor antagonist ameliorates chronic allergic contact dermatitis induced by repeated challenge. Allergy. 2010;65(3):319–326. doi: 10.1111/j.1398-9995.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- [30].Truta-Feles K, Lagadari M, Lehmann K, Berod L, Cubillos S, Piehler S, Herouy Y, Barz D, Kamradt T, Maghazachi A, Norgauer J. Histamine modulates gammadelta-T lymphocyte migration and cytotoxicity, via Gi and Gs protein-coupled signalling pathways. Br J Pharmacol. 2010;161(6):1291–1300. doi: 10.1111/j.1476-5381.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peters SP, Bleecker ER, Canonica GW, Park YB, Ramirez R, Hollis S, Fjallbrant H, Jorup C, Martin UJ. Serious asthma events with budesonide plus formoterol vs. budesonide alone. N Engl J Med. 2016;75(9):850–860. doi: 10.1056/NEJMoa1511190. [DOI] [PubMed] [Google Scholar]
- [32].Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158(9):4200–4210. [PubMed] [Google Scholar]
- [33].Ramer-Quinn DS, Swanson MA, Lee WT, Sanders VM. Cytokine production by naive and primary effector CD4+ T cells exposed to norepinephrine. Brain Behav Immun. 2000;14(4):239–255. doi: 10.1006/brbi.2000.0603. [DOI] [PubMed] [Google Scholar]
- [34].Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;66(1):232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- [35].Loza MJ, Foster S, Peters SP, Penn RB. Beta-agonists modulate T-cell functions via direct actions on type 1 and type 2 cells. Blood. 2006;107(5):2052–2060. doi: 10.1182/blood-2005-08-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loza MJ, Peters SP, Foster S, Khan IU, Penn RB. beta-Agonist enhances type 2 T-cell survival and accumulation. J Allergy Clin Immunol. 2007;119(1):235–244. doi: 10.1016/j.jaci.2006.09.019. [DOI] [PubMed] [Google Scholar]
- [37].Holen E, Elsayed S. Effects of beta2 adrenoceptor agonists on T-cell subpopulations. APMIS. 1998;106(9):849–857. [PubMed] [Google Scholar]
- [38].Loza MJ, Foster S, Peters SP, Penn RB. Interactive effects of steroids and beta-agonists on accumulation of type 2T cells. J Allergy Clin Immunol. 2008;121(3):750 e1–5 e3. doi: 10.1016/j.jaci.2007.10.036. [DOI] [PubMed] [Google Scholar]
- [39].Choy DK, Ko F, Li ST, lp LS, Leung R, Hui D, Lai KN, Lai CK. Effects of theophylline, dexamethasone and salbutamol on cytokine gene expression in human peripheral blood CD4+ T-cells. Eur Respir J. 1999;14(5):1106–1112. doi: 10.1183/09031936.99.14511069. [DOI] [PubMed] [Google Scholar]
- [40].Peek EJ, Richards DF, Faith A, Lavender P, Lee TH, Corrigan CJ, Hawrylowicz CM. Interleukin-10-secreting regulatory T cells induced by glucocorticoids and beta2-agonists. Am J Respir Cell Mol Biol. 2005;33(1):105–111. doi: 10.1165/rcmb.2005-0100OC. [DOI] [PubMed] [Google Scholar]
- [41].Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104(2 Pt (2)):S10–7. doi: 10.1016/s0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- [42].Fedyk ER, Adawi A, Looney RJ, Phipps RP. Regulation of IgE and cytokine production by cAMP: implications for extrinsic asthma. Clin Immunol Immunopathol. 1996;81(2):101–113. doi: 10.1006/clin.1996.0165. [DOI] [PubMed] [Google Scholar]
- [43].McAlees JW, Smith LT, Erbe RS, Jarjoura D, Ponzio NM, Sanders VM. Epigenetic regulation of beta2-adrenergic receptor expression in T(H)1 and T(H)2 cells. Brain Behav Immun. 2011;25(3):408–415. doi: 10.1016/j.bbi.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chung LP, Baltic S, Ferreira M, Temple S, Waterer G, Thompson PJ. Beta2 adrenergic receptor (ADRbeta2) haplotype pair (2/4) is associated with severe asthma. PLoS One. 2014;9(4):e93695. doi: 10.1371/journal.pone.0093695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gordon MA, Cohen JJ, Wilson IB. Muscarinic cholinergic receptors in murine lymphocytes: demonstration by direct binding. Proc Natl Acad Sci U S A. 1978;75(6):2902–2904. doi: 10.1073/pnas.75.6.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Strom TB, Lane MA, George K. The parallel, time-dependent, bimodal change in lymphocyte cholinergic binding activity and cholinergic influence upon lymphocyte-mediated cytotoxicity after lymphocyte activation. J Immunol. 1981;127(2):705–710. [PubMed] [Google Scholar]
- [47].Kaneda T, Kitamura Y, Nomura Y. Presence of m3 subtype muscarinic acetylcholine receptors and receptor-mediated increases in the cytoplasmic concentration of Ca2+ in Jurkat, a human leukemic helper T lymphocyte line. Mol Pharmacol. 1993;43(3):356–364. [PubMed] [Google Scholar]
- [48].Liu T, Xie C, Chen X, Zhao F, Liu AM, Cho DB, Chong J, Yang PC. Role of muscarinic receptor activation in regulating immune cell activity in nasal mucosa. Allergy. 2010;5(8):969–977. doi: 10.1111/j.1398-9995.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- [49].Bryant RW, Schewe T, Rapoport SM, Bailey JM. Leukotriene formation by a purified reticulocyte lipoxygenase enzyme. Conversion of arachidonic acid and 15-hydroperoxyeicosatetraenoic acid to 14, 15-leukotriene A4. J Biol Chem. 1985;260(6):3548–3555. [PubMed] [Google Scholar]
- [50].Panossian A, Hamberg M, Samuelsson B. On the mechanism of biosynthesis of leukotrienes and related compounds. FEBS Lett. 1982;50(2):511–513. doi: 10.1016/0014-5793(82)80801-6. [DOI] [PubMed] [Google Scholar]
- [51].Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;74(2):589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- [52].Conrad DJ. The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin Rev Allergy Immunol. 1999;17(1–2):71–89. doi: 10.1007/BF02737598. [DOI] [PubMed] [Google Scholar]
- [53].Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- [54].Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- [55].Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340(3):197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- [56].Spinozzi F, Russano AM, Piattoni S, Agea E, Bistoni O, de Benedictis D, de Benedictis FM. Biological effects of montelukast, a cysteinyl-leukotriene receptor-antagonist, on T lymphocytes. Clin Exp Allergy. 2004;34(12):1876–1882. doi: 10.1111/j.1365-2222.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- [57].Thivierge M, Turcotte S, Rola-Pleszczynski M, Stankova J. Enhanced cysteinyl-leukotriene type 1 receptor expression in T cells from house dust mite-allergic individuals following stimulation with Der p. J Immunol Res. 2015;2015 doi: 10.1155/2015/384780. 384780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xue L, Fergusson J, Salimi M, Panse I, Ussher JE, Hegazy AN, Vinall SL, Jackson DG, Hunter MG, Pettipher R, Ogg G, et al. Prostaglandin D2 and leukotriene E4 synergize to stimulate diverse TH2 functions and TH2 cell/neutrophil crosstalk. J Allergy Clin Immunol. 2015;135(5):1358–66, e1–11. doi: 10.1016/j.jaci.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Carryer HM, Koelsche GA, Prickman LE, Maytum CK, Lake CF, Williams HL. Effects of cortisone on bronchial asthma and hay fever occurring in subjects sensitive to ragweed pollen. Proc Staff Meet Mayo Clin. 1950;25(17):482–486. [PubMed] [Google Scholar]
- [60].Sternberg TH, Newcomer VD, Linden IH. Treatment of atopic dermatitis with cortisone. J Am Med Assoc. 1952;148(11):904–907. doi: 10.1001/jama.1952.02930110026007. [DOI] [PubMed] [Google Scholar]
- [61].Phillipps GH. Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respir Med. 1990;84(Suppl. A):19–23. doi: 10.1016/s0954-6111(08)80003-0. [DOI] [PubMed] [Google Scholar]
- [62].Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- [63].Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maier C, Runzler D, Schindelar J, Grabner G, Waldhausl W, Kohler G, Luger A. G-protein-coupled glucocorticoid receptors on the pituitary cell membrane. J Cell Sci. 2005;118(Pt. 15):3353–3361. doi: 10.1242/jcs.02462. [DOI] [PubMed] [Google Scholar]
- [66].Stahn C, Lowenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1–2):71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- [67].Gametchu B, Watson CS, Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J. 1993;7(13):1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- [68].Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic. 2012;13(3):364–374. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- [69].Krane SM. Some molecular mechanisms of glucocorticoid action. Br J Rheumatol. 1993;32(Suppl. 2):3–5. doi: 10.1093/rheumatology/32.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- [70].Ong GS, Young MJ. Mineralocorticoid regulation of cell function: the role of rapid signalling and gene transcription pathways. J Mol Endocrinol. 2017;58(1):R33–R57. doi: 10.1530/JME-15-0318. [DOI] [PubMed] [Google Scholar]
- [71].Rogerson FM, Brennan FE, Fuller PJ. Mineralocorticoid receptor binding, structure and function. Mol Cell Endocrinol. 2004;217(1–2):203–212. doi: 10.1016/j.mce.2003.10.021. [DOI] [PubMed] [Google Scholar]
- [72].Moura AM, Worcel M. Direct action of aldosterone on transmembrane 22Na efflux from arterial smooth muscle. Rapid and delayed effects. Hypertension. 1984;6(3):425–430. doi: 10.1161/01.hyp.6.3.425. [DOI] [PubMed] [Google Scholar]
- [73].Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009;23(5):1558–1571. doi: 10.1096/fj.08-121236. [DOI] [PubMed] [Google Scholar]
- [74].Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- [75].Tabardel Y, Duchateau J, Schmartz D, Marecaux G, Shahla M, Barvais L, Leclerc JL, Vincent JL. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery. 1996;119(1):76–80. doi: 10.1016/s0039-6060(96)80217-0. [DOI] [PubMed] [Google Scholar]
- [76].Visser J, van Boxel-Dezaire A, Methorst D, Brunt T, de Kloet ER, Nagelkerken L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 1998;91(11):4255–4264. [PubMed] [Google Scholar]
- [77].Dao Nguyen X, Robinson DS. Fluticasone propionate increases CD4CD25T regulatory cell suppression of allergen-stimulated CD4CD25T cells by an IL-10-dependent mechanism. J Allergy Clin Immunol. 2004;14(2):296–301. doi: 10.1016/j.jaci.2004.04.048. [DOI] [PubMed] [Google Scholar]
- [78].Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;14(6):1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- [79].Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, Peppelenbosch M, Hommes D. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106(5):1703–1710. doi: 10.1182/blood-2004-12-4790. [DOI] [PubMed] [Google Scholar]
- [80].Lowenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, van Deventer SJ, Hommes DW. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006;7(10):1023–1029. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Boldizsar F, Szabo M, Kvell K, Czompoly T, Talaber G, Bjorkan J, Bartis D, Nemeth P, Berki T. ZAP-70 tyrosines 315 and 492 transmit non-genomic glucocorticoid (GC) effects in T cells. Mol Immunol. 2013;53(1–2):111–117. doi: 10.1016/j.molimm.2012.07.007. [DOI] [PubMed] [Google Scholar]
- [82].Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38–45. doi: 10.1016/j.steroids.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF, Fardella CE, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184(1):191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- [84].Barnes PJ, Adcock IM. Steroid resistance in asthma. QJM. 1995;88(7):455–468. [PubMed] [Google Scholar]
- [85].Sousa AR, Lane SJ, Soh C, Lee TH. In vivo resistance to corticosteroids in bronchial asthma is associated with enhanced phosyphorylation of JUN N-terminal kinase and failure of prednisolone to inhibit JUN N-terminal kinase phosphorylation. J Allergy Clin Immunol. 1999;104(3 Pt (1)):565–574. doi: 10.1016/s0091-6749(99)70325-8. [DOI] [PubMed] [Google Scholar]
- [86].Wu HM, Fang L, Shen QY, Liu RY. SP600125 promotes resolution of allergic airway inflammation via TLR9 in an OVA-induced murine acute asthma model. Mol Immunol. 2015;67(2 Pt. B):311–316. doi: 10.1016/j.molimm.2015.06.016. [DOI] [PubMed] [Google Scholar]
- [87].Lane SJ, Adcock IM, Richards D, Hawrylowicz C, Barnes PJ, Lee TH. Corticosteroid-resistant bronchial asthma is associated with increased c-fos expression in monocytes and T lymphocytes. J Clin Invest. 1998;02(12):2156–2164. doi: 10.1172/JCI2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chialda L, Zhang M, Brune K, Pahl A. Inhibitors of mitogen-activated protein kinases differentially regulate costimulated T cell cytokine production and mouse airway eosinophilia. Respir Res. 2005;6:36. doi: 10.1186/1465-9921-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen R, Burke TF, Cumberland JE, Brummet M, Beck LA, Casolaro V, Georas SN. Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter. J Immunol. 2000;164(2):825–832. doi: 10.4049/jimmunol.164.2.825. [DOI] [PubMed] [Google Scholar]
- [90].Spinelli SL, Xi X, McMillan DH, Woeller CF, Richardson ME, Cavet ME, Zhang JZ, Feldon SE, Phipp RP. Mapracorat, a selective glucocorticoid receptor agonist, upregulates RelB, an anti-inflammatory nuclear factor-kappaB protein, in human ocular cells. Exp Eye Res. 2014;127:290–298. doi: 10.1016/j.exer.2014.07.013. [DOI] [PubMed] [Google Scholar]
- [91].Schacke H, Zollner TM, Docke WD, Rehwinkel H, Jaroch S, Skuballa W, Neuhaus R, May E, Zugel U, Asadullah K. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158(4):1088–1103. doi: 10.1111/j.1476-5381.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;4(8):753–767. doi: 10.1007/s00228-008-0478-6. [DOI] [PubMed] [Google Scholar]
- [93].Elion GB, Hitchings GH, Vanderwerff H. Antagonists of nucleic acid derivatives. VI. Purines. J Biol Chem. 1951;92(2):505–518. [PubMed] [Google Scholar]
- [94].Allison AC, Hovi T, Watts RW, Webster AD. Immunological observations on patients with Lesch-Nyhan syndrome, and on the role of de-novo purine synthesis in lymphocyte transformation. Lancet. 1975;2(7946):1179–1183. doi: 10.1016/s0140-6736(75)92661-6. [DOI] [PubMed] [Google Scholar]
- [95].Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K, Hofmann U, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;8(4):367–373. doi: 10.1038/ng.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Berns A, Rubenfeld S, Rymzo WT, Jr, Calabro JJ. Hazard of combining allopurinol and thiopurine. N Engl J Med. 1972;286(13):730–731. doi: 10.1056/nejm197203302861321. [DOI] [PubMed] [Google Scholar]
- [97].Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;11(8):1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shin JY, Wey M, Umutesi HG, Sun X, Simecka J, Heo J. Thiopurine prodrugs mediate immunosuppressive effects by interfering with rac1 protein function. J Biol Chem. 2016;291(26):13699–13714. doi: 10.1074/jbc.M115.694422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ben-Horin S, Goldstein I, Fudim E, Picard O, Yerushalmi Z, Barshack I, Bank I, Goldschmid Y, Meir SB, Mayer L, Chowers Y. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58(3):396–403. doi: 10.1136/gut.2008.157339. [DOI] [PubMed] [Google Scholar]
- [100].Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, McKean DJ, Lipsky JJ, Sandborn WJ, Egan LJ. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther. 2005;312(2):537–545. doi: 10.1124/jpet.104.074815. [DOI] [PubMed] [Google Scholar]
- [101].Dean T, Dewey A, Bara A, Lasserson TJ, Walters EH. Azathioprine as an oral corticosteroid sparing agent for asthma. Cochrane Database Syst Rev. 2004;1:CD003270. doi: 10.1002/14651858.CD003270.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367(9513):839–846. doi: 10.1016/S0140-6736(06)68340-2. [DOI] [PubMed] [Google Scholar]
- [103].Palmer DC, Skotnicki JS, Taylor EC. Synthesis of analogues of folic acid, aminopterin and methotrexate as antitumour agents. Prog Med Chem. 1988;25:85–231. doi: 10.1016/s0079-6468(08)70278-9. [DOI] [PubMed] [Google Scholar]
- [104].Farber S, Cutler EC, Hawkins JW, Harrison JH, Peirce EC, 2nd, Lenz GG. The action of pteroylglutamic conjugates on man. Science. 1947;106(2764):619–621. doi: 10.1126/science.106.2764.619. [DOI] [PubMed] [Google Scholar]
- [105].Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238(23):787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- [106].Ferguson FC, Jr, Thiersch JB, Philips FS. The action of 4-amino-N10-methyl-pteroylglutamic acid in mice, rats, and dogs. J Pharmacol Exp Ther. 1950;98(3):293–299. [PubMed] [Google Scholar]
- [107].Hagner N, Joerger M. Cancer chemotherapy: targeting folic acid synthesis. Cancer Manage Res. 2010;2:293–301. doi: 10.2147/CMR.S10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Baram J, Chabner BA, Drake JC, Fitzhugh AL, Sholar PW, Allegra CJ. Identification and biochemical properties of 10-formyldihydrofolate, a novel folate found in methotrexate-treated cells. J Biol Chem. 1988;263(15):7105–7111. [PubMed] [Google Scholar]
- [109].Sramek M, Neradil J, Veselska R. Much more than you expected: the non-DHFR-mediated effects of methotrexate. Biochim Biophys Acta. 2017;1861(3):499–503. doi: 10.1016/j.bbagen.2016.12.014. [DOI] [PubMed] [Google Scholar]
- [110].Peres RS, Liew FY, Talbot J, Carregaro V, Oliveira RD, Almeida SL, Franca RF, Donate PB, Pinto LG, Ferreira FI, Costa DL, et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2015;112(8):2509–2514. doi: 10.1073/pnas.1424792112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cribbs AP, Kennedy A, Penn H, Amjadi P, Green P, Read JE, Brennan F, Gregory B, Williams RO. Methotrexate restores regulatory t cell function through demethylation of the FoxP3 upstream enhancer in patients with rheumatoid arthritis. Arthritis Rheumatol. 2015;67(5):1182–1192. doi: 10.1002/art.39031. [DOI] [PubMed] [Google Scholar]
- [112].Nguyen VQ, Ulrik CS. Measures to reduce maintenance therapy with oral corticosteroid in adults with severe asthma. Allergy Asthma Proc. 2016;37(6):125–139. doi: 10.2500/aap.2016.37.4004. [DOI] [PubMed] [Google Scholar]
- [113].Comet R, Domingo C, Larrosa M, Moron A, Rue M, Amengual MJ, Marin A. Benefits of low weekly doses of methotrexate in steroid-dependent asthmatic patients. A double-blind, randomized, placebo-controlled study. Respir Med. 2006;100(3):411–419. doi: 10.1016/j.rmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [114].Corrigan CJ, Shiner R, Shakur BH, Ind PW. Methotrexate therapy in asthma increases T cell susceptibility to corticosteroid inhibition. Clin Exp Allergy. 2003;3(8):1090–1096. doi: 10.1046/j.1365-2222.2003.t01-1-01723.x. [DOI] [PubMed] [Google Scholar]
- [115].Nelson PH, Carr SF, Devens BH, Eugui EM, Franco F, Gonzalez C, Hawley RC, Loughhead DG, Milan DJ, Papp E, Patterson JW, et al. Structure-activity relationships for inhibition of inosine monophosphate dehydrogenase by nuclear variants of mycophenolic acid. J Med Chem. 1996;9(21):4181–4196. doi: 10.1021/jm9603633. [DOI] [PubMed] [Google Scholar]
- [116].Clutterbuck PW, Raistrick H. Studies in the biochemistry of micro-organisms: the molecular constitution of the metabolic products of Penicillium brevi-compactum Dierckx and related species. II. Mycophenolic acid. Biochem J. 1933;27(3):654–667. doi: 10.1042/bj0270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Abraham EP. The effect of mycophenolic acid on the growth of Staphylococcus aureus in heart broth. Biochem J. 1945;39(5):398–408. doi: 10.1042/bj0390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mitsui A, Suzuki S. Immunosuppressive effect of mycophenolic acid. J Antibiot (Tokyo) 1969;22(8):358–363. doi: 10.7164/antibiotics.22.358. [DOI] [PubMed] [Google Scholar]
- [119].Williams RH, Lively DH, DeLong DC, Cline JC, Sweeny MJ. Mycophenolic acid: antiviral and antitumor properties. J Antibiot (Tokyo) 1968;21(7):463–464. doi: 10.7164/antibiotics.21.463. [DOI] [PubMed] [Google Scholar]
- [120].Carter SB, Franklin TJ, Jones DF, Leonard BJ, Mills SD, Turner RW, Turner WB. Mycophenolic acid: an anti-cancer compound with unusual properties. Nature. 1969;223(5208):848–850. doi: 10.1038/223848a0. [DOI] [PubMed] [Google Scholar]
- [121].Natsumeda Y, Carr SF. Human type I and II IMP dehydrogenases as drug targets. Ann N Y Acad Sci. 1993;696:88–93. doi: 10.1111/j.1749-6632.1993.tb17144.x. [DOI] [PubMed] [Google Scholar]
- [122].Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- [123].Dayton JS, Lindsten T, Thompson CB, Mitchell BS. Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression. J Immunol. 1994;152(3):984–991. [PubMed] [Google Scholar]
- [124].Laliberte J, Yee A, Xiong Y, Mitchell BS. Effects of guanine nucleotide depletion on cell cycle progression in human T lymphocytes. Blood. 1998;91(8):2896–2904. [PubMed] [Google Scholar]
- [125].Yang Y, Tang Q, Zhao M, Liang G, Wu H, Li D, Xie Y, Tan Y, Dai Y, Yung S, Chan TM, et al. The effect of mycophenolic acid on epigenetic modifications in lupus CD4 + T cells. Clin Immunol. 2015;158(1):67–76. doi: 10.1016/j.clim.2015.03.005. [DOI] [PubMed] [Google Scholar]
- [126].Grainge C, Jayasekera N, Dennison P, Rupani H, Kurukulaaratchy R, Howarth P. Case series reporting the effectiveness of mycophenolate mofetil in treatment-resistant asthma. Eur Respir J. 2013;42(4):1134–1137. doi: 10.1183/09031936.00026413. [DOI] [PubMed] [Google Scholar]
- [127].Backer V, Hjardem E, Karlsmark T. Treatment with mycophenolat mofetil of steroid-dependent asthma-one case of severe asthma. J Allergy (Cairo) 2009;2009:821013. doi: 10.1155/2009/821013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Murray ML, Cohen JB. Mycophenolate mofetil therapy for moderate to severe atopic dermatitis. Clin Exp Dermatol. 2007;32(1):23–27. doi: 10.1111/j.1365-2230.2006.02290.x. [DOI] [PubMed] [Google Scholar]
- [129].Garritsen FM, Roekevisch E, van der Schaft J, Deinum J, Spuls PI, de Bruin-Weller MS. Ten years experience with oral immunosuppressive treatment in adult patients with atopic dermatitis in two academic centres. J Eur Acad Dermatol Venereol. 2015;29(10):1905–1912. doi: 10.1111/jdv.13064. [DOI] [PubMed] [Google Scholar]
- [130].Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;65(6444):352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- [131].Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- [132].Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- [133].Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- [134].Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- [135].Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- [136].Malvezzi P, Rostaing L. The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf. 2015;14(10):1531–1546. doi: 10.1517/14740338.2015.1083974. [DOI] [PubMed] [Google Scholar]
- [137].Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- [138].Ratzinger F, Haslacher H, Poeppl W, Hoermann G, Kovarik JJ, Jutz S, Steinberger P, Burgmann H, Pickl WF, Schmetterer KG. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. 2014;4:7438. doi: 10.1038/srep07438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lock SH, Kay AB, Barnes NC. Double-blind, placebo-controlled study of cyclosporin A as a corticosteroid-sparing agent in corticosteroid-dependent asthma. Am J Respir Crit Care Med. 1996;153(2):509–514. doi: 10.1164/ajrccm.153.2.8564089. [DOI] [PubMed] [Google Scholar]
- [140].Rohatagi S, Calic F, Harding N, Ozoux ML, Bouriot JP, Kirkesseli S, DeLeij L, Jensen BK. Pharmacokinetics, pharmacodynamics, and safety of inhaled cyclosporin A (ADI628) after single and repeated administration in healthy male and female subjects, and asthmatic patients. J Clin Pharmacol. 2000;40(11):1211–1226. [PubMed] [Google Scholar]
- [141].Wu X, Zhang W, Hayes D, Jr, Mansour HM. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery. Int J Nanomed. 2013;8:1269–1283. doi: 10.2147/IJN.S40904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, Bukrinsky MI, Fischer G, Constant SL. A cell-impermeable cyclosporine A derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. 2010;185(12):7663–7670. doi: 10.4049/jimmunol.1001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Taniguchi H, Tokui K, Iwata Y, Abo H, Izumi S. A case of severe bronchial asthma controlled with tacrolimus. J Allergy (Cairo) 2011;2011:479129. doi: 10.1155/2011/479129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gruber S, Tiringer K, Dehlink E, Eiwegger T, Mayer E, Konstantin H, Kikic Z, Graf A, Szepfalusi Z. Allergic sensitization in kidney-transplanted patients prevails under tacrolimus treatment. Clin Exp Allergy. 2011;41(8):1125–1132. doi: 10.1111/j.1365-2222.2011.03761.x. [DOI] [PubMed] [Google Scholar]
- [145].Prabhakaran K, Lau HT, Wise B, Schwarz K, Colombani PM. Incidence of allergic symptoms in pediatric liver transplant recipients treated with tacrolimus based immunosuppression. Pediatrics. 1999;104(Suppl. 4):786–787. [Google Scholar]
- [146].Granot E, Yakobovich E, Bardenstein R. Tacrolimus immunosuppression – an association with asymptomatic eosinophilia and elevated total and specific IgE levels. Pediatr Transplant. 2006;10(6):690–693. doi: 10.1111/j.1399-3046.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- [147].Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, Svensson A, Barbarot S, von Kobyletzki L, Taieb A, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30(5):729–747. doi: 10.1111/jdv.13599. [DOI] [PubMed] [Google Scholar]
- [148].Warrell RP, Jr, Frankel SR, Miller WH, Jr, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubowski A, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N Engl J Med. 1991;324(20):1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- [149].Jones H, Blanc D, Cunliffe WJ. 13-cis retinoic acid and acne. Lancet. 1980;2(8203):1048–1049. doi: 10.1016/s0140-6736(80)92273-4. [DOI] [PubMed] [Google Scholar]
- [150].Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, Butkus D, Arnaud-Battandier J. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300(7):329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- [151].Schindler M, Drozdenko G, Kuhl AA, Worm M. Immunomodulation in patients with chronic hand eczema treated with oral alitretinoin. Int Arch Allergy Immunol. 2014;65(1):18–26. doi: 10.1159/000365659. [DOI] [PubMed] [Google Scholar]
- [152].Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- [153].Lehmann JM, Jong L, Fanjul A, Cameron JF, Lu XP, Haefner P, Dawson MI, Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- [154].Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;68(9):4495–4503. doi: 10.4049/jimmunol.168.9.4495. [DOI] [PubMed] [Google Scholar]
- [156].Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ludanyi K, Nagy ZS, Alexa M, Reichert U, Michel S, Fesus L, Szondy Z. Ligation of RARgamma inhibits proliferation of phytohaemagglutinin-stimulated T-cells via down-regulating JAK3 protein levels. Immunol Lett. 2005;8(1):103–113. doi: 10.1016/j.imlet.2004.10.018. [DOI] [PubMed] [Google Scholar]
- [158].Rasooly R, Schuster GU, Gregg JP, Xiao JH, Chandraratna RA, Stephensen CB. Retinoid x receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. J Immunol. 2005;175(12):7916–7929. doi: 10.4049/jimmunol.175.12.7916. [DOI] [PubMed] [Google Scholar]
- [159].Toth K, Sarang Z, Scholtz B, Brazda P, Ghyselinck N, Chambon P, Fesus L, Szondy Z. Retinoids enhance glucocorticoid-induced apoptosis of T cells by facilitating glucocorticoid receptor-mediated transcription. Cell Death Differ. 2011;18(5):783–792. doi: 10.1038/cdd.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Aage S, Kiraly N, Da Costa K, Byberg S, Bjerregaard-Andersen M, Fisker AB, Aaby P, Benn CS. Neonatal vitamin A supplementation associated with increased atopy in girls. Allergy. 2015;0(8):985–994. doi: 10.1111/all.12641. [DOI] [PubMed] [Google Scholar]
- [161].Brumbaugh PF, Haussler MR. Nuclear and cytoplasmic receptors for 1 25-dihydroxycholecalciferol in intestinal mucosa. Biochem Biophys Res Commun. 1973;51(1):74–80. doi: 10.1016/0006-291x(73)90509-3. [DOI] [PubMed] [Google Scholar]
- [162].Cheskis B, Freedman LP. Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol. 1994;14(5):3329–3338. doi: 10.1128/mcb.14.5.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Takami M, Fujimaki K, Nishimura MI, Iwashima M. Cutting edge: AhR is a molecular target of calcitriol in human T cells. J Immunol. 2015;195(6):2520–2523. doi: 10.4049/jimmunol.1500344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Mann EH, Chambers ES, Chen YH, Richards DF, Hawrylowicz CM. 1alpha 25-dihydroxyvitamin D3 acts via transforming growth factor-beta to up-regulate expression of immunosuppressive CD73 on human CD4+ Foxp3-T cells. Immunology. 2015;146(3):423–431. doi: 10.1111/imm.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Chambers ES, Nanzer AM, Richards DF, Ryanna K, Freeman AT, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Serum 25-dihydroxyvitamin D levels correlate with CD4(+)Foxp3(+) T-cell numbers in moderate/severe asthma. J Allergy Clin Immunol. 2012;130(2):542–544. doi: 10.1016/j.jaci.2012.04.022. [DOI] [PubMed] [Google Scholar]
- [166].Gorman S, Judge MA, Burchell JT, Turner DJ, Hart PH. 1,25-dihydroxyvitamin D3 enhances the ability of transferred CD4+ CD25+ cells to modulate T helper type 2-driven asthmatic responses. Immunology. 2010;130(2):181–192. doi: 10.1111/j.1365-2567.2009.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, Hawrylowicz CM, Saglani S, Lloyd CM. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014;9(10):1380–1389. doi: 10.1111/all.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Thien R, Baier K, Pietschmann P, Peterlik M, Willheim M. Interactions of 1 alpha 25-dihydroxyvitamin D3 with IL-12 and IL-4 on cytokine expression of human T lymphocytes. J Allergy Clin Immunol. 2005;116(3):683–689. doi: 10.1016/j.jaci.2005.05.013. [DOI] [PubMed] [Google Scholar]
- [169].Hamzaoui A, Berraies A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219(11):873–879. doi: 10.1016/j.imbio.2014.07.009. [DOI] [PubMed] [Google Scholar]
- [170].Drozdenko G, Heine G, Worm M. Oral vitamin D increases the frequencies of CD38+ human B cells and ameliorates IL-17-producing T cells. Exp Dermatol. 2014;3(2):107–112. doi: 10.1111/exd.12300. [DOI] [PubMed] [Google Scholar]
- [171].Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA, Avila P, et al. Blood Institute’s, Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083–2091. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Luo J, Liu D, Liu CT. Can Vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma? A meta-analysis. Medicine (Baltimore) 2015;94(50):e2185. doi: 10.1097/MD.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0136841. doi: 10.1371/journal.pone.0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy. 2006;91:59–75. doi: 10.1159/000090230. [DOI] [PubMed] [Google Scholar]
- [175].Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Abehsira-Amar O, Gibert M, Joliy M, Theze J, Jankovic DL. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992;48(12):3820–3829. [PubMed] [Google Scholar]
- [177].Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP, Kingo K, et al. Investigators, two phase 3 trials of Dupilumab versus placebo in Atopic Dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- [178].Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, Wenzel SE, Chon Y, Dunn M, Weng HH, Lin SL. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- [179].Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- [180].Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005;15(4):791–796. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- [181].Humbert M, de Blay F, Garcia G, Prud’homme A, Leroyer C, Magnan A, Tunon-de-Lara JM, Pison C, Aubier M, Charpin D, Vachier I, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64(8):1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- [182].Niimi T, Orita M, Okazawa-Igarashi M, Sakashita H, Kikuchi K, Ball E, Ichikawa A, Yamagiwa Y, Sakamoto S, Tanaka A, Tsukamoto S, et al. Design and synthesis of non-peptidic inhibitors for the Syk C-terminal SH2 domain based on structure-based in-silico screening. J Med Chem. 2001;44(26):4737–4740. doi: 10.1021/jm010313k. [DOI] [PubMed] [Google Scholar]
- [183].Wong BR, Grossbard EB, Payan DG, Masuda ES. Targeting Syk as a treatment for allergic and autoimmune disorders. Expert Opin Investig Drugs. 2004;13(7):743–762. doi: 10.1517/13543784.13.7.743. [DOI] [PubMed] [Google Scholar]
- [184].Latour S, Fournel M, Veillette A. Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase. Mol Cell Biol. 1997;17(8):4434–4441. doi: 10.1128/mcb.17.8.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, Borge L, Hajem B, Lermet A, Sippl W, Voisset E, et al. Masitinib (AB1010) a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Marech I, Patruno R, Zizzo N, Gadaleta C, Introna M, Zito AF, Gadaleta CD, Ranieri G. Masitinib (AB1010), from canine tumor model to human clinical development: where we are? Crit Rev Oncol Hematol. 2014;91(1):98–111. doi: 10.1016/j.critrevonc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- [187].Southworth T, Plumb J, Gupta V, Pearson J, Ramis I, Lehner MD, Miralpeix M, Singh D. Anti-inflammatory potential of PI3Kdelta and JAK inhibitors in asthma patients. Respir Res. 2016;17(1):124. doi: 10.1186/s12931-016-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Rowan WC, Smith JL, Affleck K, Amour A. Targeting phosphoinositide 3-kinase delta for allergic asthma. Biochem Soc Trans. 2012;40(1):240–245. doi: 10.1042/BST20110665. [DOI] [PubMed] [Google Scholar]
- [189].Leaker BR, Barnes PJ, O’Connor BJ, Ali FY, Tam P, Neville J, Mackenzie LF, MacRury T. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin Exp Allergy. 2014;4(9):1146–1153. doi: 10.1111/cea.12370. [DOI] [PubMed] [Google Scholar]
- [190].Ashino S, Takeda K, Li H, Taylor V, Joetham A, Pine PR, Gelfand EW. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J Allergy Clin Immunol. 2014;133(4):1162–1174. doi: 10.1016/j.jaci.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, Hochhaus A, Guilhot F, Saglio G, Apperley J, Ottmann O, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- [192].da Silva AL, Magalhaes RF, Branco VC, Silva JD, Cruz FF, Marques PS, Ferreira TP, Morales MM, Martins MA, Olsen PC, Rocco PR. The tyrosine kinase inhibitor dasatinib reduces lung inflammation and remodelling in experimental allergic asthma. Br J Pharmacol. 2016;173(7):1236–1247. doi: 10.1111/bph.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Jung SH, Sun X, Ryu WS, Yang BS. Topical administration of the pan-Src kinase inhibitors, dasatinib and LCB 03-0110, prevents allergic contact dermatitis in mice. Br J Dermatol. 2013;168(1):112–119. doi: 10.1111/bjd.12069. [DOI] [PubMed] [Google Scholar]
- [194].Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111(3):1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Hadzijusufovic E, Peter B, Herrmann H, Rulicke T, Cerny-Reiterer S, Schuch K, Kenner L, Thaiwong T, Yuzbasiyan-Gurkan V, Pickl WF, Willmann M, et al. NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy. 2012;67(7):858–868. doi: 10.1111/j.1398-9995.2012.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Maslanka T, Otrocka-Domagala I, Zuska-Prot M, Mikiewicz M, Przybysz J, Jasiecka A, Jaroszewski JJ. IkappaB kinase beta inhibitor IMD-0354, prevents allergic asthma in a mouse model through inhibition of CD4(+) effector T cell responses in the lung-draining mediastinal lymph nodes. Eur J Pharmacol. 2016;775:78–85. doi: 10.1016/j.ejphar.2016.02.023. [DOI] [PubMed] [Google Scholar]
- [197].Uota S, Zahidunnabi Dewan M, Saitoh Y, Muto S, Itai A, Utsunomiya A, Watanabe T, Yamamoto N, Yamaoka S. An IkappaB kinase 2 inhibitor IMD-0354 suppresses the survival of adult T-cell leukemia cells. Cancer Sci. 2012;103(1):100–106. doi: 10.1111/j.1349-7006.2011.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101(3):235–238. doi: 10.1016/s0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- [199].Zhang S, Yang R, Zheng Y. The effect of siRNA-mediated lymphocyte-specific protein tyrosine kinase (Lck) inhibition on pulmonary inflammation in a mouse model of asthma. Int J Clin Exp Med. 2015;8(9):15146–15154. [PMC free article] [PubMed] [Google Scholar]
- [200].Silverman SK. In vitro selection, characterization, and application of deoxyribozymes that cleave RNA. Nucl Acids Res. 2005;33(19):6151–6163. doi: 10.1093/nar/gki930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Breaker RR, Joyce GF. A DNA enzyme that cleaves RNA. Chem Biol. 1994;1(4):223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- [202].Garn H, Renz H. GATA-3-specific DNAzyme – A novel approach for stratified asthma therapy. Eur J Immunol. 2017;7(1):22–30. doi: 10.1002/eji.201646450. [DOI] [PubMed] [Google Scholar]
- [203].Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23(7):415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137(2):351–357. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Candia M, Kratzer B, Pickl WF. On Peptides and Altered Peptide Ligands: From Origin, Mode of Action and Design to Clinical Application (Immunotherapy) Int Arch Allergy Immunol. 2016;170(4):211–233. doi: 10.1159/000448756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. Classification of histamine receptors. Pharmacol Rev. 1997;49(3):253–278. [PubMed] [Google Scholar]
- [207].Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001;59(3):415–419. [PubMed] [Google Scholar]
- [208].Bakker RA, Wieland K, Timmerman H, Leurs R. Constitutive activity of the histamine H(1) receptor reveals inverse agonism of histamine H(1) receptor antagonists. Eur J Pharmacol. 2000;387(1):R5–R7. doi: 10.1016/s0014-2999(99)00803-1. [DOI] [PubMed] [Google Scholar]