Abstract
Purpose
The purpose of this study was to perform a systematic review to assess current evidence for association between various risk factors and the prevalence or incidence of early childhood caries (ECC).
Methods
Two reviewers searched various databases until January 2019. The Newcastle-Ottawa scale was used to perform risk of bias assessment.The included studies were categorized according to the World Bank classification. Data were summarized in a meta-analysis using fixed and random effects inverse-generic meta-analyses.
Results
A total of 7,034 records involving 89 studies that evaluated 1,352,097 individuals were included; 23 were high, 46 were moderate, and 20 were of low quality. A total of 123 risk factors were found. Meta-analysis revealed that the strongest risk factors found in the high-income countries were presence of dentinal caries (dmft greater than zero; odds ratio [OR] equals 4.21 [2.18 to 8.16]) and high levels of mutans streptococci (OR equals 3.83 [1.81 to 8.09]). In upper-middle-income countries, presence of enamel defects (OR equals 14.62 [6.10 to 35.03]) was found to be the strongest risk factor.
Conclusion
The strongest risk factors associated with early childhood caries was the presence of enamel defects, presence of dentinal caries and high levels of mutans streptococci.
Keywords: Dental Caries, Risk Factors, Infant, Child, Cohort Studies, Case Control Studies
Early Childhood Caries (ECC) remains the most prevalent chronic disease in children, with significant impact on society.1,2 Numerous studies have observed the increasingly skewed distribution of carious lesions.3–6 Most carious lesions or restorations are found in a small number of disadvantaged individuals. ECC is disproportionately found in certain segments of the childhood population.7,8 Although the key factors causing dental caries in adults and children are similar, there are certain unique risk factors present in young children, probably because oral microbial flora and host defense mechanisms are in the developing stage. Also, newly erupted tooth surfaces may have hypoplastic defects associated with higher risk for caries. In addition, parents must understand the dietary changes from liquids to solids through breastfeeding/bottle feeding.
Several studies have evaluated and categorized the risk factors of ECC, such as sociodemographic factors, dietary factors, oral hygiene factors, and factors related to oral bacterial flora and breastfeeding/bottle feeding.1,2,6,8,9 However, the degree to which different risk factors are associated with ECC remains unclear.
Significant gaps have been observed in the collective evidence on risk factors known to cause ECC. Until now, only two systematic reviews have examined the evidence on multiple risk factors associated with ECC. Harris et al. in 20049 systematically reviewed the literature and identified 106 risk factors associated with ECC. Nevertheless, more than 50 percent of the included studies were cross-sectional, thereby lacking robustness for the evaluation of risk factors and for conclusions to be drawn. In addition, there were few studies of a high quality, defined as those using validated and standardized measures for oral hygiene and dietary habits. The other systematic review10 studied risk factors for ECC only in the first year of life and suggested further clarification to identify and quantify the main risk factors. Neither of the two systematic reviews presented a quantitative analysis. Furthermore, recently reported risk factors—namely, increased body mass index, maternal cognitive disorders, increased enamel permeability, enamel composition, and the influence of parental attitudes, were not included. Finally, the search for the review by Harris8 was conducted over a decade ago, in 2004; hence, an update is indicated.
Therefore, the purpose of this study was to conduct a systematic review and a meta-analysis of cohort and case control studies for possible associations between various risk factors and early childhood caries.
Methods
Guidelines from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) were followed in the present review, which was registered at PROSPERO before the initial screening stage. We deviated from the original protocol by adding a category of included studies based on the World Bank Classification. In addition, we also searched for another database—LILACS—which was not mentioned in the original protocol.
Search strategy
The identification of included studies, which began on July 1, 2016 and was updated until January 2019, was based on a search strategy performed for each electronic database: MEDLINE; EMBASE; Cochrane Central Database; Cochrane Oral Health Group’s Specialised Register; CINAHL via EBSCO; LILACS; and IndMED. The MeSH terms used were “dental caries,” “preschool child,” “infant,” and “risk factors.” The following strategy was used to search MEDLINE: (“dental caries”[MeSH terms] OR (“dental”[all fields] AND “caries”[all fields]) OR “dental caries”[all fields]) AND ((“infant”[MeSH terms] OR “infant”[all fields]) OR ((“child”[MeSH terms] OR “child”[all field AND preschool [all fields]) OR (“child”[MeSH terms] OR “child”[all fields] OR “children”[all fields])) AND (“risk factors”[MeSH terms] OR (“risk”[all fields] AND “factors”[all fields]) OR “risk factors”[all fields] OR (“risk”[all fields] AND “factor”[all fields]) OR “risk factor”[all fields]). The search strategies for CENTRAL (Cochrane Central Register of Controlled Trials), EMBASE, EBSCO, LILACS, and IndMED were comparable to those used in the MEDLINE search. We identified and synthesized all relevant studies, up to June 2016, to reduce selection bias. In addition, the reference lists of existing systematic and narrative reviews and of all included studies were reviewed for studies that might have been missed. We hand searched some key journals in this field (from 2005)—such as Community Dental Health, International Journal of Paediatric Dentistry, Journal of Public Health Dentistry, Community Dentistry and Oral Epidemiology, Pediatrics, Pediatric Dentistry, European Archives of Pediatric Dentistry, European Journal of Pediatric Dentistry, Pediatric Dental Journal, Journal of Dentistry for Children, Journal of Clinical Pediatric Dentistry, and International Journal of Clinical Pediatric Dentistry—to identify those publications that could have been missed from the electronic database and searches of the reference lists.10 Hand searches were performed from 2005 to June 2016. This was because there was already an update on hand searches by Harris et al.9 until 2004. This has further been updated to January 2019. This also helped us identify very recent articles. Attempts to obtain grey literature were performed by screening a national database for dissertation abstracts (i.e., SHODHGANGA).
Selection of studies
A reference management system (Mendeley Desktop 1.17.13, Elsevier, Atlanta, Ga., USA) was used to upload all the potentially eligible studies and remove duplicate studies. Two trained reviewers independently assessed for inclusion of all the eligible studies on the basis of the title, abstract, and keywords. Full texts of papers or reports, for those studies that required more information to determine relevance or in cases where abstracts were unclear/unavailable, were obtained through electronic mail or communication through Research Gate. In addition, the full text of each study considered for inclusion was also obtained. Blinding of the articles was not performed regarding the journals published, authors, or institutions. Disagreements among the reviewers were resolved by discussion. Where agreement could not be reached, a third reviewer arbitrated to reach consensus. All excluded studies at this stage were documented in an Excel spreadsheet (Excel 10, Microsoft Corp., Redwood City, Calif., USA), along with the reasons for exclusion.
Selection criteria
We included prospective cohort, retrospective cohort, and case control studies that investigated the association between risk factors and ECC prevalence, experience, or incidence. Case series, case reports, and cross-sectional studies were excluded. Randomized controlled trials (RCTs) were also excluded because an interventional study is not the ideal study design in which to evaluate the association between the risk factor and disease occurrence. Our study followed the PECO format.
All preschool children, regardless of gender, race, health status, geographical location, or socioeconomic status (SES), from birth until six years of age (less than 72 months old) were included. Children with special health care needs were excluded. Exposure included socio demographic factors, dietary factors, factors related to oral hygiene, factors related to breastfeeding and bottle feeding, and other factors. In case control studies, individuals without ECC are the matched control group. Presence of ECC was the outcome. However, any method of assessment of the outcome (ECC) was considered.
Data extraction and quality assessment
For all studies that met the inclusion criteria, data extraction was performed independently by two reviewers using piloted electronic Excel 10 spreadsheets. Wherever possible, appropriate translators were used for data extraction from papers in languages not known by the review authors. Review authors discussed disagreements in data extraction. A third review author resolved discrepancies, and lead authors of the respective studies were contacted to obtain missing data, if necessary. Data were recorded in accordance with the guidelines outlined by the Cochrane Collaboration and categorized as study characteristics, participant characteristics, adjusted effects, and absolute effects estimates.
The Newcastle-Ottawa scale (NOS), modified for observational studies,12 was used to perform the risk of bias assessment of the included studies. The domains of the scale include selection of cases and controls, comparability of the groups, and measurement of exposure and outcomes. The scale has two parts, one pertinent to case control studies and one for cohort studies. Studies were categorized as having low, moderate, and high methodological quality, according to NOS scores under five, from five to seven, and above seven, respectively. This quality assessment was used only for the descriptive part and not for statistical evaluation.
Data synthesis and analysis
Although there is a need for controlling confounders in observational studies, we used unadjusted measures as the primary effect estimates when they were provided. Odds ratio (OR) is considered an appropriate effect estimate for cohort and case control studies. Only those studies that reported or allowed the calculation of OR and error estimates (P-values, confidence intervals [CIs], and standard deviation) were used for quantitative data synthesis. When investigators used multivariate models to adjust for potential confounders, we did not consider the measures, since they would usually involve adjusted ORs. If unadjusted measures were not given as a part of the primary analysis, we calculated the same wherever possible.
The results of the included studies were evaluated Review Manager 2012 statistical software (Revman 5.3, The Cochrane Collaboration, London, UK). Forest plots were used to visualize the estimate effect sizes and 95 percent (95%) CIs of individual studies. Inverse-variance weighted averages and 95% CIs were used to represent the summary estimates for the entire sample. Data were summarized in a meta-analysis when they were sufficiently homogeneous. We combined data from studies if they had comparable risk factors, follow-ups, and outcome measures and organized the results by the particular type of exposure examined in the study. For ease of categorization, the studies retrieved were categorized according to the World Bank classification into lower-income (LI), lower-middle-income (LMI), upper-middle-income (UMI), and high-income (HI) countries.
We assessed clinical heterogeneity (e.g., participant characteristics, risk factors, and study settings) by investigating the pertinent criteria. The chi-square and I-square tests were used for the assessment of heterogeneity.13 An I-square value between 50 percent and 100 percent was considered for statistical heterogeneity to be present. A random-effects model for meta-analysis was used if there was evidence of substantial or considerable heterogeneity. To estimate effect sizes and their 95% CIs, both random and fixed-effects generalized linear models were used.
Results
Study selection and characteristics
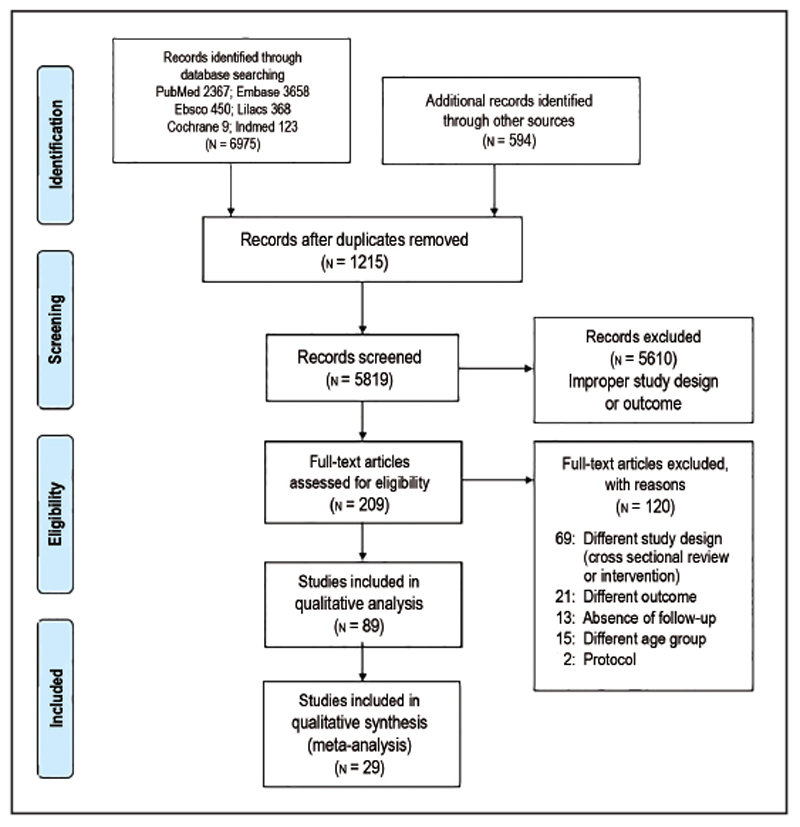
The search revealed that 7,034 studies were relevant to the present systematic review. Following the removal of 1,215 duplicates, 5,819 records were screened based on the title, abstract, and keywords. Of these, 5,610 records were eliminated based on improper study design or outcome. The remaining 209 papers were assessed for complete examination. The reason for exclusion of the 120 articles at this stage was different study design—including review, cross sectional or interventional-based studies, outcomes other than dental caries, or the absence of follow-up, as described in Figure 1. After a full text review, 89 studies1,2,14–101 with a 1,352,097 total participants, were included in the present review. Of these, five articles were translated to English by Google Translate. Further, six authors were contacted requesting full texts through Research G or electronic mail. Figure 1 summarizes the study identification process in the form of PRISMA flow diagram. The study participants’ ages ranged from birth to six years. Publication years of included studies ranged from 1981 to Jan 2019. Among the included studies, 64 were prospective cohort,1,2,17–21,23,25,27,29–35,37,39–47,49,51–77,89–91,93–97 four were retrospective cohort,14,15,16,92 and 21 were case control.22,24,26,28,36, 38,48,78–87,98–101 Among the 68 cohort studies, 50 studies1,2,14,15,17, 20,21,23,25,29,30,32–35,37,40,42–47,52–56,58,59,61,63,65–77,88–90,92,97 belonged to the HI category, 1616,18,19,27,31,49,51,57,60,62,64,69,91,93,94,96 studies belonged to the UMI category, one study41 belonged to the LMI category, and one study95 belonged to the LI category. Among the 21 case control studies, 10 studies36,38,48,78,81,83–86,99 belonged to the HI category, eight studies22,24,79,80,82,87,98,101 belonged to the UMI category, three belonged to the LMI category26,28,100 and no studies were present in the LI category.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram.
Risk of bias in included studies
The NOS was used for the quality assessment of included studies (Table 1). This is a star rating system, with eight questions, that assigns a maximum of nine stars within three domains: selection (four stars); comparability (two stars); and measurement of exposure (risk factor) in case control studies or outcome (dental caries) in cohort studies (three stars). A high risk of bias was considered for those studies with less than five stars. Quality varied greatly among studies, with 20 studies of low quality, 46 studies showing moderate quality, and 23 studies demonstrating high quality. Overall, five studies were rated with low risk of bias and high methodological quality in all three NOS risk of bias categories (i.e., four prospective cohort studies and one case control study. All four cohort studies studied different risk factors and were conducted in various parts of the world, including England (HI),17 Brazil (UMI),18 Thailand (UMI),19 and the United States (HI).20 The study by Lai et al.20 was a case-controlled prospective study conducted in the United States (HI) to learn if the enamel hypoplasia seen in very low birthweight children predisposed them to increased dental caries risk; it concluded that no significant association existed. Studies with a high risk of bias and low methodological quality in all three NOS risk of bias categories included one case control82 and three cohort studies,21,46,67 as seen in Table 1. Three of the four studies were based on the same cohort in Japanese preschool children (HI country), with data collected prospectively.21,46,67 The fourth study was a retrospective study in which risk factors—namely consumption of cariogenic food, oral hygiene habits, topical application of fluoride, and annual oral evaluation—were studied.82
Table 1. Quality of Evidence of included Studies based on the Newcastle-Ottawa Scale.
| Studies graded with high methodological quality | Studies graded with moderate methodological quality | Studies graded with low methodological quality | |
|---|---|---|---|
| Peltzer and Mongkochali (2015)51 | Ostberg et al. (2016)2 | Nelson et al. (2005)84 | Ghazal et al. (2015)29 |
| Yokomichi H et al. (2015)52 | Shantinath et al. (1996)86 | Warren et al. (2016)1 | Zaror et al. (2014)14 |
| Winter et al. (2015)53 | Mahesh et al. (2013)28 | Tanaka et al. (2015)40 | Gao et al. (2014)55 |
| Peltzer et al. (2014)27 | Tanaka et al. (2015)88 | Watanabe et al. (2014)54 | Almeida et al. (2012)60 |
| Majorana et al. (2014)15 | Campus et al. (2007)85 | Hong et al. (2014)56 | Mattila et al. (1998)70 |
| Zhou et al. (2012)31 | Schroth et al. (2014)76 | Moimaz et al. (2014)57 | Sanders and Slade (2010)33 |
| Kay et al. (2010)17 | Law and Seow (2006)39 | Tanaka et al. (2013)58 | Ismail et al. (2009)23 |
| Hong et al. (2009)47 | Wigen and Wang (2011)77 | Kato et al. (2015)34 | Yonezu and Yakushiji (2008)21 |
| Teanpaisan et al. (2007)18 | Peretz and Kafka (1997)78 | Tanaka et al. (2013)59 | Lim et al. (2008)66 |
| Oliveira et al. (2006)19 | Slade et al. (2006)83 | Chankanka et al. (2015)25 | Yonezu et al. (2006)67 |
| Van Palenstein Henderman et al. (2006)41 | Nunes et al. (2012)16 | Tanaka et al. (2012)32 | Yonezu et al. (2006)46 |
| Ansai et al. (2000)45 | Grytten et al. (1988)75 | Grindefjord et al. (1996)73 | Tada et al. (1999)35 |
| Lai et al. (1997)20 | Levy et al. (2003)68 | Bankel et al. (2011)61 | O’ Sullivan et al. (1996)44 |
| Wendt et al. (1996)72 | Rodrigues and Sheiham (2000)69 | Parisoto et al. (2011)62 | Al Mendalwi and Karam (2014)24 |
| Wendt et al. (1995)74 | Ollila et al. (1998)37 | Targino et al. (2011)49 | Seow et al. (2009)48 |
| Aaltonen et al. (1994)43 | Thibodeau and O’ Sullivan (1996)71 | Wigen et al. (2011)63 | Yu et al. (2015)79 |
| Menon et al. (2013)26 | Meruman and Pienihakkihen (2010)30 | Ismail et al. (2008)65 | Evans et al. (2013)81 |
| Dantas Cabral de Melo et al. (2015)80 | Warren et al. (2009)42 | Feldens et al. (2010)64 | Del Rosario Garcia et al. (2011)82 |
| Melo et al. (2011)22 | Nishide et al. (2018)89 | Peres et al. (2017)94 | Lulic Dukic et al. (2001)38 |
| Qin et al. (2008)87 | Cabral et al. (2017)91 | Bernabe et al. (2017) | Marino et al. (1989)36 |
| Boustedt et al. (2018)90 | Jean et al. (2018)92 | Fan et al. (2016)98 | |
| Birungi et al. (2017)95 | Feldens et al. (2018)93 | Paglia et al. (2016)99 | |
| Nirunsittirat (2016)97 | Dabawala et al. (2017)100 | Roberts et al. (1994)101 | |
Assessment of the outcome
Most studies evaluated dental caries using the decayed, filled, and missing primary teeth (dmft) index and decayed, filled, and missing primary surfaces (dmfs) index, according to the World Health Organization23; a few studies determined both noncavitated and cavitated teeth and surfaces, according to the International Caries Detection and Assessment System (ICDAS). Only one study used a five egrade caries diagnostic system, from the most superficial (grade one) to the most profound (grade five). Grades one and two constituted enamel carious lesions (initial caries), and grades three to five were diagnosed when the carious lesions had reached the dentin (manifest caries). Initial and/or manifest carious lesions (grades one to five) constituted all carious lesions of different depths.2
Narrative review
Most of the included studies examined a wide range of exposures. Information about these exposures was obtained predominantly from parents through interviews,28,30 self-reports,14,24,52 or questionnaires.1,2,15,22,27,29,40,51,53–55–60 In total, the number of risk factors found to be associated with ECC among the 76 included studies were 123. These could be grouped as 19 sociodemographic factors, 28 factors related to diet, 10 factors related to oral hygiene habits, 10 factors related to breastfeeding, 15 related to bottle feeding, three related to oral bacteria flora, and 38 related to other factors such as genetic mutation and parental smoking (Table 2). The results of the studies, according to each category (sociodemographic factors, dietary factors, factors related to oral hygiene, factors related to breastfeeding and bottle feeding, and other factors), are summarized next.
Table 2. Factors Related to the Prevalence AND/OR Incidence of Primary Teeth Caries in Children Age 6 Years and Younger.
| Sociodemographic factors | Dietary factors | Oral hygiene |
|---|---|---|
| Gender (male)27,51,52,81 Residence (urban) 24 Age65,79,81 Non-Hispanic Caucasian81 Low socioeconomic status2,26,85,101 Low education of the caregiver22 Low parental education24,81,56 No schooling of mother51 Low maternal education1,22 Greater household size 1,22,81 Young maternal age1,63 Birth order (3 or more)33,54,80,101 Drinking water in household1,33 Ethnicity30,33,84 Mother unemployed28,48 Single mother27 Low household income 27,33,40,48,51 Single parenting household36 First born child28 |
Daily sweet snacks17,54 High sugar foods >1x/day15,29,74,101 Cariostat 3 or more54 Daily consumption of fruit juice79 Added sugar beverage intake1,42 Consumption of beverages/carbonated drinks daily54 Sweet food index >2453 Presweetened cereal consumption at meals 25 No milk consumption at meals 25 Use of thirst quenchers other than water30 Added sugar22,30 High density of sugar at 12 months64 Very frequent sugar consumption75 Cariogenic food consumption82 Sweet drinks1,87,100 Regular exposure to sweet drinks in the first 6 months83 Nighttime consumption of sweet beverages after 24 months38 Eating sweets several times a day87 Added sugar at snacks25 Pre-chewed food87 Juice in bottle during day-time86 Snack more than 3x/day28 Solid sugar consumption79 Consumption of sweets between meals80 Low levels of Vitamin D during pregnancy32,76 Low levels of calcium during pregnancy32 Low levels of dairy products during pregnancy32 Low levels of curd during pregnancy32 Low levels of cheese during pregnancy32 |
Daily frequency of toothbrushing at <1 year old24,27,33,53,87 No daily toothbrushing by parents2,54 Age brushing started >12,33,38,53 Visible plaque31,39,42,48,89 Parental indulgence while toothbrushing2 Lack of fluoride toothpaste28,49,53,101 Poor oral hygiene exam at 18 months26,46 Low Oral Hygiene Index score84 Trouble with toothbrushing48 Visible plaque index79 |
| Factors related to breastfeeding/bottle feeding |
Oral bacterial flora | Other factors* | |
|---|---|---|---|
| Breastfeeding | Bottle feeding | ||
| Duration of breastfeeding <6 months56 No breastfeeding15,54 Prolonged breastfeeding >12 months14,46,64,101 Breastfeeding at least 6 months34 Nocturnal breastfeeding30,31,46 Breastfeeding35 Daily breastfeeding frequency at 12 months64 >15 minutes/feeding at night41 ≥2 nocturnal breastfeeding41 Breastfeeding =24 months94 |
Sleep with bottle at 30 months 1-6x/week51 Nocturnal bottle feeding64 Nighttime bottle use at 2 months64 Bottle feeding38,84,85 Slept at night with bottle containing sweet drink33,101 Feeding to help them sleep86 On-demand feeding86 Feeding associated with nap time86 Age of weaning from bottle36,86 Formula in bottle at night86 Child held bottle while falling asleep (propping)86 Prolonged bottle feeding, especially at night36,93 Added sugar in bottle48 Sleeping while feeding after 12 months87 Feeding habits before 6 months87 |
Presence of Streptococcus mutans48 Increased baseline salivary S. mutans levels30,31,42–45,71 Presence of LB37,55 |
Presence of enamel defects19,31,48,47,49 Smoking by family members27,54 1 parent born abroad2 2 parents born abroad2,73 Parent’s dental attendance2 Parent’s negative attitude2 High chance locus of control2 Drinking water in household/home water fluoride level27,28,56 Low birthweight56,96 History of previous dental visit at age 3 years29 Previous dental experience14 Regular dental check ups <553 Late bedtime36,54 Low body mass index31 One or both parents of non-western origin63 Blue collar occupation of caretaker30 Reported poor oral health of father30,33 Teeth erupted at 18 months >633 Low Apgar score33 High density of lipids at 12 months64 Soda consumption 2-6x/day65 Mother missing teeth75 Incidence of caries (DMFT>0)48,70 Parental stress26 Reason for dental visits84,101 Complication during pregnancy78 Delivery (instrument/Caesarean)78,90 Tantrums/strong temper36 Parental smoking24,96 Ear infection84 No previous dental visit28 Day care person28 Visible abscess48 Mutation in the locus79 Inappropriate fluoride supplementation36 Mothers knowledge of when to clean the child’s mouth and brush the child’s teeth48 Oral thrush92 |
Sociodemographic factors
Of the 19 sociodemographic factors, gender (male) and low household income were found to be frequently implicated in most studies.27,33,40,48,51,52,81 Factors such as low SES, low maternal education, and unemployed mother have been investigated and were found to be significant in only a few studies.1,2,26–28,48 The reason for the inconsistent results with the SES factor could be the different scales used in different studies, based either on only household income24 or mother’s education at recruitment and family income,25 per capita monthly income,26 or based on the parent’s occupation status, with social class level based on the higher occupation status of the father or mother85. The factors studied in a single study were residence of the child (urban/rural),24 low education of the caregiver,22 presence of a single mother,27 and the child being firstborn.28
Dietary factors
There were many dietary factors associated with ECC. Most of these factors were related either to the frequency, amount, or timing of sugar consumption.17,29,30 Among all the dietary factors, the most commonly investigated risk factor was frequency of eating foods high in sugar more than once per day. Although this factor was found to have a significant association in some studies,5,29,74 one study reported31 that this association was not significant when adjusted for confounders (unadjusted OR equals 2.5; 95% CI equals 1.2 to 5.2; adjusted ORs not provided). Another study32 was conducted on the association between calcium intake and dairy products during pregnancy and dental caries in children; it concluded that the increased maternal intake of cheese during pregnancy may significantly decrease the risk of developing dental caries in children (P=0.001). Weaning after 18 months as a risk factor was assessed in another study33 and found to be not significant (P=0.291).
Factors related to breastfeeding/bottle feeding
The number of included studies that investigated breastfeeding and bottle feeding as a risk factor are 15 and 13, respectively. According to Kato et al. in 2015,34 breastfeeding for six to seven months or more might increase dental caries risk due to simultaneous events that occur during the same period, such as the eruption of primary teeth. The same study reported breastfeeding and bottle feeding as risk factors for ECC; in that study, breastfeeding was specifically associated with caries in maxillary anterior teeth and bottle feeding was associated with caries in molars.34 That study also mentioned that this association became attenuated through the follow-up period and was no longer statistically significant beyond the age of 42 months for the partially breastfed group and beyond the age of 54 months for the exclusively breastfed group. Another case control study involving South African children compared a group with nursing caries to those without it. They found no statistically significant differences for feeding patterns between the groups in relation to the prevalence of nursing caries.101 Most studies counted on parental recall in the form of questionnaires or interviews,14,23, 31,35–40 and very few studies used standardized validated questions or previous dental records, which are more reliable.14,41
Factors related to oral hygiene
Past studies collected data by means of self-reports or more directly via the use of a plaque or oral hygiene index for oral hygiene habits. It is interesting to note that, in one of the included studies, parental indulgence (when parents neglected to help the child brush twice daily or when they did not have the time to brush) was reported as one of the most important risk factors for ECC.2 Among all the factors studied, visible plaque42,48 and toothbrushing less than once daily24,27,33,53,87 were the two most important oral hygiene factors related to ECC. The other less important factors are age at which toothbrushing was started,3,38 not having teeth brushed at bedtime, using nonfluoridated toothpaste,28,49 and parental supervision of toothbrushing.2
Factors related to oral bacteria flora
Streptococcus mutans is known to be the main bacterium in the aetiology of dental caries. An association between ECC and the colonization of mutans streptococci (MS) in saliva or plaque has been demonstrated. The age at which MS is detectable in a child’s oral cavity is said to be an important indicator of caries risk, although it may not be detectable in the infant’s mouth prior to tooth eruption (Table 2). One study31 suggested that the earlier S. mutans colonizes in a child, the greater the risk of developing caries. Another study18 observed MS in 1.78 percent of predentate infants as young as three months and studied the presence of dental caries in nine- and 24-month-old children. Most studies assessed how the individual’s baseline caries risk influenced the development of caries in children aged six months to six years. Almost all the studies in this area observed an increase in the caries experience, with increased salivary MS levels at baseline.30,42–46 However, whatever the ethnic group may be, if MS is present in the oral cavity, it appears to be an important indicator of caries risk. Ethnic differences in the prevalence of dental caries can, to an extent, be explained by differences in the acquisition of cariogenic bacteria.
Other factors
There were 38 factors which belonged to this category. Among them, enamel hypoplasia was the most commonly studied. All studies that included the presence of enamel hypoplasia as a potential risk factor for ECC concluded that the risk of developing dental caries was significantly increased.19,31,47–49 One study19 observed a total of 224 children, with enamel defects from the age of 12 to 54 months, for the presence of ECC. At 12 months, none of the infants showed the presence of dental caries. At 42 months, 9.2 percent of children presented with carious teeth; at 54 months, 48.4 percent of the children with dental caries showed the presence of enamel defects. The study also concluded that enamel hypoplasia was the most common category of enamel defect associated with dental caries. On the contrary, another study47 concluded that the type of enamel defect with the most frequently associated risk factor with dental caries in children aged 36 months was opacity with enamel hypoplasia (42.7 percent), followed by hypoplasia (42.7 percent) and diffuse opacity (6.4 percent).
A recent study assessed whether there is an association between oral thrush or other Candida-related conditions in infancy and ECC diagnosed by pediatricians. The study design was a retrospective cohort using electronic health records from six national children’s hospitals. There were 1,012,668 children included in the study, with one visit at ages one to 12 months and another visit at ages 13 to 71 months. This study concluded that oral thrush may be a risk factor for ECC.92
Quantitative analysis
Among the 89 included studies, 68 are cohort studies and 21 are case control studies. Of the 68 cohort studies 50 studies1,2,14,15,17, 20,21,23,25,29,30,32–35,37,40,42–47,52–56,58,59,61,63,65–77,88–90,92,97 belonged to the HI category, 16 studies16,18,19,27,31,49,51,57, 60,62,64,69,91,93,94,96 fit in the UMI category, one study41 was categorized as LMI, one study95 belonged to the LI category. From the 68 cohort studies, only 29 studies contributed for quantitative analysis. Among these 29 studies, 23 studies1,2,17,25,29,30,37,39,42,45,53–56,58,63, 66–68,70,72,73,75 fit within the HI category and six studies18,19,31,49,51,64 belonged to the UMI category. No studies from the LMI and the LI category were included. The remaining 30 studies14–16,20,21,23,27,32–35,40,41,43,44,46,47,52,57,59,60–62,65,69, 71,74,76,77,88–97 were excluded, either because the data were missing or heterogeneous.
None of the risk factors among the 21 case control studies22,24,26,28,36,38,48,78,79,80–87,98–101 was eligible for quantitative analysis. Either the factors could not be combined, due to missing data, or they belonged to a different country classification based on income.
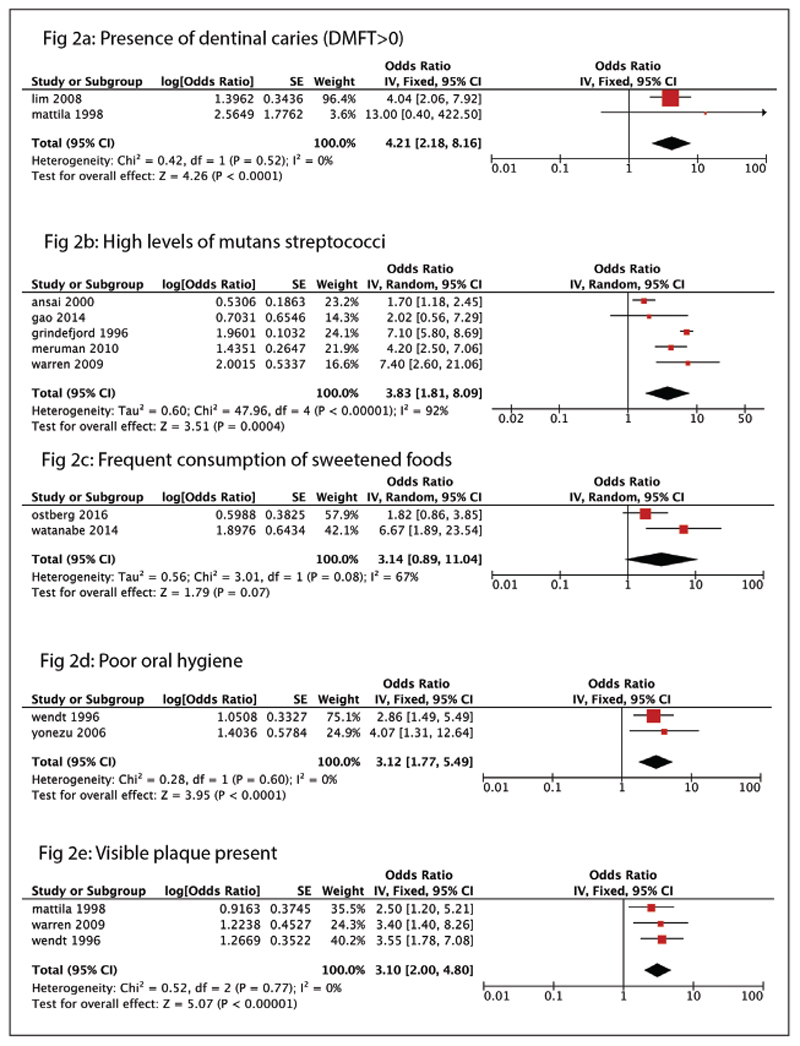
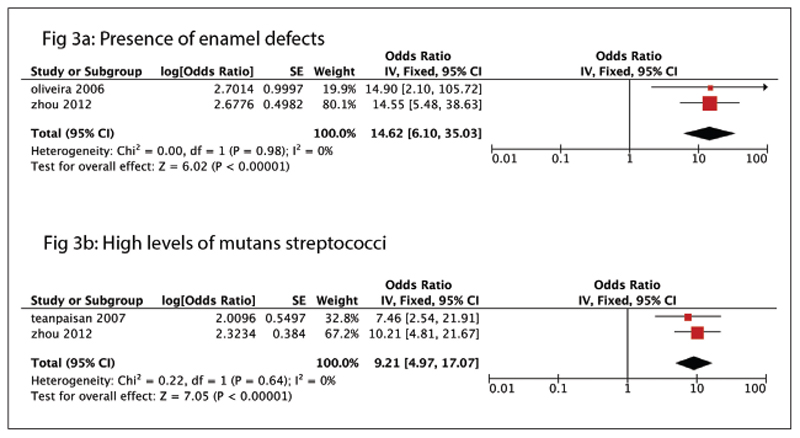
Figures 2 and 3 show the significant risk factors found in the HI and UMI categories, respectively. The forest plots represent only those with an OR greater than three (Figures 2 and 3). Figures 4 to 9 represent the re-maining risk factor forest plots (see Electronic Appendix). Table 3 shows an overview of the meta-analysis of the included cohort studies, categorized as UMI and HI countries.
Figure 2.
Risk factors found in the high-income category. (a) Forest plot showing presence of dentinal caries (decayed, filled, and missing primary teeth [dmft] index score greater than zero) as a risk factor for early childhood caries. (b) Forest plot showing presence of mutans streptococci as a risk factor for ECC. (c) Forest plot showing frequent consumption of sweetened foods as a risk factor for ECC. (d) Forest plot showing poor oral hygiene as a risk factor for ECC. (e) Forest plot showing visible plaque present as a risk factor for ECC.
Figure 3.
Risk factors found in the upper-middle-income category. (a) Forest plot showing presence of enamel defects as a risk factor for ECC. (b) Forest plot showing presence of mutans streptococci as a risk factor for ECC.
Table 3. Overview of the Meta-Analysis of the Included Cohort Studies Categorized as Upper-Middle-Income and Upper-Income Countries*.
| Risk factor | n | K | Pooled odds ratio (95% Cl) | Chi-square2 value | I2 value |
|---|---|---|---|---|---|
| Upper-middle-income countries | |||||
| Low birthweight31,51 | 822 | 2 | 0.83 (0.49, 1.41) | 1.83 | 45 |
| Increased baseline salivary levels of Streptococcus mutans18,31 | 394 | 2 | 9.21 (4.97, 17.07) | 0.22 | 0 |
| Presence of enamel defects19,31 | 453 | 2 | 14.62 (6.10, 35.03) | 0.00 | 0 |
| Night bottle feeding49,64 | 564 | 2 | 0.62 (0.49, 0.78) | 1.91 | 48 |
| Night breastfeeding31,49 | 449 | 2 | 1.28 (1.11, 1.47) | 1.54 | 35 |
| Gender (male)51,64 | 937 | 2 | 1.26 (0.85, 1.88) | 3.45 | 71 |
| Toothbrushing at least once a day31,49 | 449 | 2 | 1.36 (1.08, 1.72) | 0.60 | 0 |
| Brushing with fluoride toothpaste51,64 | 937 | 2 | 1.03 (0.75, 1.42) | 0.47 | 0 |
| Sugar snacks at least once a day31,49 | 449 | 2 | 0.69 (0.16, 3.00) | 0.95 | 84 |
| Low maternal age (<25 years)31,51 | 822 | 2 | 0.65 (0.45, 0.94) | 1.75 | 43 |
| High-income countries | |||||
| Low maternal education (≤9 years)2,17,56,63,66,70,75 | 5,885 | 8 | 1.84 (1.14, 2.08) | 31.49 | 78 |
| Low birthweight (<2,500 g)56,63 | 1,857 | 2 | 1.70 (0.89, 3.23) | 0.00 | 0 |
| Smoking during pregnancy1,63 | 1,580 | 2 | 1.33 (0.74, 2.39) | 2.53 | 60 |
| Increased baseline salivary levels of S. mutans30,42,45,55,73 | 2,812 | 5 | 3.83 (1.81, 8.09) | 47.96 | 92 |
| Increased consumption of soda pop25,56 | 886 | 2 | 1.12 (1.03, 1.23) | 0.18 | 0 |
| Maternal age (<25 years)1,17,63 | 2,565 | 3 | 1.26 (0.65, 2.45) | 17.43 | 89 |
| Toothbrushing at least once a day2,25,66,67 | 2,328 | 4 | 0.91 (0.55, 1.51) | 6.69 | 55 |
| Visible plaque present42,70,72 | 1,106 | 3 | 3.1 (2.00, 4.80) | 0.52 | 0 |
| Poor oral hygiene67,72 | 394 | 2 | 3.12 (1.77, 5.49) | 0.28 | 0 |
| Night bottle feeding37,42,58 | 592 | 3 | 1.15 (0.44, 3.04) | 14.91 | 87 |
| Age at dental exam >1 year2,25,66,67 | 2,328 | 2 | 1.68(1.06, 2.66) | 0.78 | 0 |
| Liquids in bottle other than milk58,68 | 421 | 2 | 1.27 (0.83, 1.94) | 2.04 | 51 |
| Presence of lactobacilli37,55 | 1,728 | 2 | 2.18 (2.03, 2.34) | 1.13 | 11 |
| Gestational age <37 weeks29,63 | 1,445 | 2 | 0.67 (0.14, 3.12) | 3.50 | 71 |
| Gender (males)17,30,39,63 | 2,727 | 4 | 0.98 (0.80, 1.19) | 2.01 | 0 |
| Age started brushing ≥12,53 | 836 | 2 | 2.12 (1.49, 3.01) | 0.00 | 0 |
| Brushing <1x/day17,53,54,75 | 32,984 | 4 | 1.08 (0.61, 1.92) | 12.39 | 76 |
| Dentinal caries (dmft >0)66,70 | 2,268 | 2 | 4.21 (2.18, 8.16) | 0.42 | 0 |
| No topical fluoride application53,54 | 31,768 | 2 | 1.50 (1.39, 1.63) | 1.22 | 18 |
| Frequent consumption of sweetened foods2,54 | 31,472 | 2 | 3.14 (0.89, 11.04) | 3.01 | 67 |
| Intake of sugar snacks daily2,30,54 | 31,831 | 3 | 1.56 (1.42, 1.71) | 0.68 | 0 |
| Intake of sugar beverages2,42,67,68,73 | 1,298 | 5 | 1.67 (0.25, 3.92) | 46.18 | 91 |
| Socioeconomic status25,39 | 412 | 2 | 0.46 (0.28, 0.74) | 0.05 | 0 |
n=number of participants; K=number of studies; dmft=decayed, filled, and missing primary teeth.
The important risk factors (OR greater than one) amid HI countries were: low maternal education; low birth weight (less than 2,500 g); smoking during pregnancy; the presence of MS; increased daily soda pop intake; maternal age younger than 25 years; visible plaque present; bad oral hygiene; night bottle feeding; age at first dental examination younger than one year; liquids other than milk in bottles; the presence of lactobacilli; tooth brushing less than once daily; age when brushing began at one year of age or older; negative parental attitudes; the presence of dentinal caries (dmft greater than zero); topical fluoride application; frequent consumption of sweetened foods; daily intake of sugary snacks; and intake of sugary beverages. The strongest risk factors associated were the: presence of dentinal caries (dmft greater than zero; OR equals 4.21 [2.18 to 8.16]); high levels of MS (OR equals 3.83 [1.81 to 8.09]); frequent consumption of sweetened foods (OR equals 3.14 [0.89 to 11.04]); poor oral hygiene (OR equals 3.12 [1.77 to 5.49]); and visible plaque present (OR equals 3.10 [2.0 to 4.80]; Figure 2).
Among the studies grouped under UMI countries, the factors found to have a positive association with ECC (OR greater than one) were high levels of MS counts, the presence of enamel defects, nighttime breastfeeding, gender (male), brushing with fluoride toothpaste, and brushing at least once a day. The strongest risk factors associated with ECC, among the studies, were the presence of enamel defects (OR equals 14.62 [6.10 to 35.03]) and high levels of MS (OR equals 9.21 [4.97 to 17.07]; Figure 3).
Discussion
To the best of our knowledge, this study is the first systematic review and meta-analysis, including case control and cohort studies, examining possible associations between various risk factors and ECC. The objective of a systematic review is to identify, evaluate, and synthesize evidence from previously conducted studies to provide informative empirical answers to unanswered research questions. The key question of the present review is, what are the main risk factors for early childhood caries?
To answer this, we undertook a structured approach to identify pertinent literature and to minimize bias in the selected studies.9 The only way to understand the relationship between etiological factors and disease in the population is through observational studies, since randomization is impossible. Nevertheless, the confounding factors may mask the exact association between a risk factor and ECC, in the absence of randomization. The description of a risk factor clearly indicates that the exposure has occurred prior to the outcome. Hence, longitudinal studies are needed to study risk factors. In a cross-sectional study, an exposure associated with an outcome can be considered a risk indicator only. Hence, we included only cohort and case control studies in the present systematic review, which is the ideal study design to examine risk factors.50 This evidence can have key implications for the development of prevention strategies for common risk factors associated with ECC.
The present review used the NOS to assess risk of bias of individual studies. Modification of this scale for two questions was needed to suit the present research question. First, in the rating system for ascertainment of exposure, one star was allocated not only for the structured interview (as in the original scale) but also for questionnaire or medical records. This item was modified for both the cohort and case control studies. Second, under the rating of comparability for cases and controls, it was not possible to determine the main confounder, as the present systematic review studied the role of multiple etiological factors. Therefore, it was decided to give two stars if the study adjusted for confounders using multiple logistic regression analysis and one star if the study controlled for at least one potential confounder (e.g., age, gender, income, or SES). In the present systematic review, 76 out of the 89 studies adjusted for at least one of the confounding variables, which can be considered a major strength of the included studies.
However, the present review used only the studies that provided unadjusted ORs for the meta-analysis, since there was no standardization of confounders adjusted in various studies. This probably led to the fewer number of studies included under each risk factor category.
Limitations
Overall, there are three major limitations with the included studies of risk factors for ECC. The first is the absence of adjustment for confounding factors. A known constraint of observational studies is the ability of confounding factors to exaggerate or diminish the significance of some factors, since randomization is not possible. This is usually compensated by using multiple logistic regression analysis, which is almost compulsory in these studies. This analysis depends on the use of dichotomized data, which means that the categorizations used in each study may be as significant as the numbers of exposures tested. For example, one study22 might investigate toothbrushing frequency by comparing once, twice, or thrice daily versus less than once daily, whereas another study might compare one or more times daily53 and reach different conclusions. Although most studies performed some form of adjustment for confounders, this was often poorly reported or not described. Moreover, the values—namely, adjusted or unadjusted P-values and odds ratios were not provided. Furthermore, 11 included studies in this review did not perform any method to account for the confounding factors.
The ideal selection of a confounder in the present study is based on existing evidence of an accepted association with the risk factor studied (exposure) and ECC (outcome). The second is the lack of consistency and detail among the categories of risk factors studied, which restricts comparison between and among the studies. Also, it is possible that the mothers of the study participants, who completed questionnaires regarding their children’s various risk factors, were provided with some basic information regarding the same. Hence, the accuracy of their answers could be questionable. This could be explained by the wide range of risk factors evaluated across the included studies. However, specific definitions of risk factors studied are necessary to ensure the accuracy of the data collected. Further standardization among the studies to measure oral health outcomes (dental caries) and the risk factors in children is required to facilitate a more accurate knowledge base of the risk factors for ECC. In addition to the shortcomings of the included studies, our statistical analysis has caveats, as we pooled estimates from various study designs, detection cutoffs, caries measures, and statistical models. The third limitation of the included studies was that, among the 89 included studies, quality varied greatly among studies—with 20 studies of low quality, 46 studies showing moderate quality, and 23 studies demonstrating high quality. Overall, only five studies were rated high in all three categories. These findings carry implications for future research.
Among the 89 included studies, using World Bank classification for categorizing the countries: 60 studies (10 case control studies, 50 cohort studies) were from the HI category; 24 studies (eight case control studies, 16 cohort studies) were categorized as UMI; four studies (three case control studies, one cohort study) fell into the LMI category; and one study was categorized as LI. Of the 76 studies only 29 cohort studies contributed for quantitative analysis. Evaluation of the population studied in the 29 cohort studies (HI equals 23; UMI equals six; LMI equals zero; LI equals zero) showed that various SES children were included in each study. Among the 23 studies in the HI category, SES profiles of the population studied were low, high, all profiles, and not mentioned in three, one, one, and 18 studies, respectively. In the six UMI categorized studies, the SES profiles were low in four studies and not mentioned in two studies. As low SES is associated with greater risk of acquiring ECC, it is imperative that future studies should mention the population studied for better understanding of this association. The categorization further revealed that only one study was performed in the LMI category (Myanmar)41 and one study was performed in the LI category (Uganda).95 Therefore, future studies are required mainly in LMI and LI country category groups, using standardized data collection and outcome measures with appropriate adjustment of potential confounders.
Meta-analysis of UI countries showed that presence of dentinal caries, high levels of MS, frequent consumption of sweetened foods, poor oral hygiene, and visible plaque present are major risk factors (each with an OR above three) associated with ECC. In UMI countries, high levels of MS and presence of enamel defects were the major risk factors. However, the readers are advised to interpret these findings with caution, because the population studied might belong to a low, moderate, or high SES in UI or UMI countries, as previously discussed (Table 4). Further studies in HI, UMI, LMI, and LI countries, including all SES populations, are needed to better understand the various risk factors associated with ECC in different countries and among people from different SES.
Table 4. Overview of the Socioeconomic Status (SES) of the Population Studied in Each Study in the Meta-Analysis, Based on World Bank Classification.
| High-income countries | Upper-middle-income countries | Lower-middle-income countries | Lower-income countries | ||||
|---|---|---|---|---|---|---|---|
| Low SES (n=3) | High SES (n=1) | Not mentioned (n=18) | All SES profiles (n=1) | Low SES (n=4) | Not mentioned (n=2) | — | — |
| Lim et al. (2008)66; Warren et al.(2009)42; Ghazal et al. (2015)29 |
Hong et al. (2014)56 | Gao et al. (2014)55; Watanabe et al. (2014)54; Wigen and Wang (2011)77; Tanaka et al. (2013)58; Levy et al. (2003)68; Kay et al. (2010)17; Yonezu T et al. (2006)67; Ansai et al. (2000)45; Mattila et al. (1998)70; Ollila et al. (1998)37; Wendt et al. (1996)72; Grindefjord et al. (1996)73; Grytten et al. (1988)75; Law and Seow (2006)39; Gao et al. (2014)55; Watanabe et al. (2014)54; Wigen and Wang (2011)77; Levy et al. (2003)68 |
Warren et al. (2016)1 | Feldens et al. (2010)64; Oliveira et al. (2006)19; Teanpaisan et al. (2007)18; Targino et al. (2011)49 |
Peltzer and Mongkochali (2015)51; Zhou et al. (2012)31 |
||
Regardless of the heterogeneous nature of the included studies, when it comes to study design and the statistical tests used, the accuracy and magnitude of our estimates strongly support the presence of an association between certain risk factors and ECC. In the HI category, the presence of dentinal caries, high levels of MS, frequent consumption of sweetened foods, poor oral hygiene, and the presence of visible plaque were the significant risk factors. This can be attributed to the fact that sugar consumption is usually higher and more equally distributed in HI countries versus LI countries. In UMI countries, the presence of enamel defects and high levels of MS were found to be significant. This may be because malnutrition and increased rates of infection in early life are more prevalent in these countries and are predisposing factors for enamel defects. It is noteworthy that no longitudinal study was found that evaluated host factors, such as enamel permeability, enamel composition, contact areas, and types of pits and fissures, as risk factors for ECC. Their role in the etiology of ECC remains unclear and requires further investigation.
Conclusions
Based on this study’s results, the following conclusions can be made:
The two strongest risk factors associated with early childhood caries in high- or upper-middle-income categories were: (a) the presence of enamel defects; and (b) high levels of mutans streptococci.
Significant secondary risk factors in the high-income category were the presence of dentinal caries, frequent consumption of sweetened foods, poor oral hygiene, and the presence of visible plaque.
Supplementary Material
Supplemental material available in tihe online version.
Acknowledgment
The authors wish to thank Amit Agarwal, MSc,PhD, Senior Research officer, Department of Telemedicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India, for his help with the EMBASE database search.
References
- 1.Warren JJ, Blanchette D, Dawson DV, Marshall TA, Phipps KR, Starr D, Drake DR. Factors associated with dental caries in a group of American Indian children at age 36 months. Community Dent Oral Epidemiol. 2016;44(2):154–61. doi: 10.1111/cdoe.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostberg AL, Skeie MS, Skaare AB, Espelid I. Caries increment in young children in Skaraborg, Sweden: associations with parental sociodemography, health habits, and attitudes. Int J Paediatr Dent. 2017;27(1):47–55. doi: 10.1111/ipd.12225. [DOI] [PubMed] [Google Scholar]
- 3.Marthaler TM. Changes in dental caries 1953-2003. Caries Res. 2004;38(3):173–81. doi: 10.1159/000077752. [DOI] [PubMed] [Google Scholar]
- 4.Geyer S, Schneller T, Micheelis W. Social gradients and cumulative effects of income and education on dental health in the Fourth German Oral Health Study. Community Dent Oral Epidemiol. 2010;38(2):120–8. doi: 10.1111/j.1600-0528.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 5.Pitts N, Amaechi B, Niederman R, et al. Global oral health inequalities: dental caries task group—research agenda. Adv Dent Res. 2011;23(2):211–20. doi: 10.1177/0022034511402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do LG. Distribution of caries in children: variations between and within populations. J Dent Res. 2012;91(6):536–43. doi: 10.1177/0022034511434355. [DOI] [PubMed] [Google Scholar]
- 7.Macek MD, Heller KE, Selwitz RH, Manz MC. Is 75 percent of dental caries really found in 25 percent of the population? J Public Health Dent. 2004;64(1):20–5. doi: 10.1111/j.1752-7325.2004.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 8.Weston-Price S, Copley V, Smith H, Davies GM. A multi-variable analysis of four factors affecting caries levels among five-year-old children; deprivation, ethnicity, exposure to fluoridated water and geographic region. Community Dent Health. 2018;35(4):217–222. doi: 10.1922/CDH_4383Weston-Price06. [DOI] [PubMed] [Google Scholar]
- 9.Harris R, Nicoll AD, Adair PM, Pine CM. Risk factors for dental caries in young children: a systematic review of the literature. Community Dent Health. 2004;21(1 Suppl):71–85. [PubMed] [Google Scholar]
- 10.Leong PM, Gussy MG, Barrow SY, de Silva-Sanigorski A, Waters E. A systematic review of risk factors during first year of life for early childhood caries. Int J Paediatr Dent. 2013;23(4):235–50. doi: 10.1111/j.1365-263X.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- 11.De Silva AM, Hegde S, Akudo Nwagbara B, et al. Community-based population-level interventions for promoting child oral health. Cochrane Database Syst Rev. 2016;9:CD009837. doi: 10.1002/14651858.CD009837.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonran-domised studies in meta-analyses. [Accessed January 15, 2016]; Available at: “ http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp”. (Archived by WebCite® at: “ http://www.webcitation.org/77CGOluNC”)
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Zaror SC, Sapunar ZJ, Muñoz NS, González CD. Association between overweight and early childhood caries. Rev Chil Pediatr. 2014;85(4):455–61. doi: 10.4067/S0370-41062014000400008. [DOI] [PubMed] [Google Scholar]
- 15.Majorana A, Cagetti MG, Bardellini E, et al. Feeding and smoking habits as cumulative risk factors for early childhood caries in toddlers, after adjustment for several behavioural determinants: a retrospective study. BMC Pediatr. 2014;14:45. doi: 10.1186/1471-2431-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunes AM, Alves CM, Borba de Araújo F, et al. Association between prolonged breastfeeding and early childhood caries: a hierarchical approach. Community Dent Oral Epidemiol. 2012;40(6):542–9. doi: 10.1111/j.1600-0528.2012.00703.x. [DOI] [PubMed] [Google Scholar]
- 17.Kay EJ, Northstone K, Ness A, Duncan K, Crean SJ. Is there a relationship between birthweight and subsequent growth on the development of dental caries at 5 years of age? A cohort study. Community Dent Oral Epidemiol. 2010;38(5):408–14. doi: 10.1111/j.1600-0528.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 18.Teanpaisan R, Thitasomakul S, Piwat S, Thearmontree A, Pithpornchaiyakul W, Chankanka O. Longitudinal study of the presence of mutans streptococci and lactobacilli in relation to dental caries development in 3-24 month old Thai children. Int Dent J. 2007;57(6):445–51. doi: 10.1111/j.1875-595x.2007.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira AF, Chaves AM, Rosenblatt A. The influence of enamel defects on the development of early childhood caries in a population with low socioeconomic status: a longitudinal study. Caries Res. 2006;40(4):296–302. doi: 10.1159/000093188. [DOI] [PubMed] [Google Scholar]
- 20.Lai PY, Seow WK, Tudehope DI, Rogers Y. Enamel hypoplasia and dental caries in very-low birthweight children: a case controlled, longitudinal study. Pediatr Dent. 1997;19(1):42–9. [PubMed] [Google Scholar]
- 21.Yonezu T, Yakushiji M. Longitudinal study on influence of prolonged non-nutritive sucking habits on dental caries in Japanese children from 1.5 to 3 years of age. Bull Tokyo Dent Coll. 2008;49(2):59–63. doi: 10.2209/tdcpublication.49.59. [DOI] [PubMed] [Google Scholar]
- 22.Melo MM, Souza WV, Lima ML, Braga C. Factors associated with dental caries in preschoolers in Recife, Pernambuco State, Brazil. Cad Saude Publica. 2011;27(3):471–85. doi: 10.1590/s0102-311x2011000300008. [DOI] [PubMed] [Google Scholar]
- 23.Ismail AI, Sohn W, Lim S, Willem JM. Predictors of dental caries progression in primary teeth. J Dent Res. 2009;88(3):270–5. doi: 10.1177/0022034508331011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mendalawi MD, Karam NT. Risk factors associated with deciduous tooth decay in Iraqi preschool children. Avicenna J Med. 2014;4(1):5–8. doi: 10.4103/2231-0770.127414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chankanka O, Levy SM, Marshall TA, et al. The associations between dietary intakes from 36 to 60 months of age and primary dentition non-cavitated caries and cavitated caries. J Public Health Dent. 2015;75(4):265–73. doi: 10.1111/j.1752-7325.2012.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon I, Nagarajappa R, Ramesh G, Tak M. Parental stress as a predictor of early childhood caries among pre-school children in India. Int J Paediatr Dent. 2013;23(3):160–5. doi: 10.1111/j.1365-263X.2012.01238.x. [DOI] [PubMed] [Google Scholar]
- 27.Peltzer K, Mongkolchati A, Satchaiyan G, Rajchagool S, Pimpak T. Sociobehavioral factors associated with caries increment: a longitudinal study from 24 to 36 months old children in Thailand. Int J Environ Res Public Health. 2014;11(10):10838–50. doi: 10.3390/ijerph111010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahesh R, Muthu MS, Rodrigues SJ. Risk factors for early childhood caries: a case control study. Eur Arch Paediatr Dent. 2013;14(5):331–7. doi: 10.1007/s40368-013-0089-5. [DOI] [PubMed] [Google Scholar]
- 29.Ghazal T, Levy SM, Childers NK, et al. Factors associated with early childhood caries incidence among high cariesrisk children. Community Dent Oral Epidemiol. 2015;43(4):366–74. doi: 10.1111/cdoe.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meurman PK, Pienihakkinen K. Factors associated with caries increment: a longitudinal study from 18 months to 5 years of age. Caries Res. 2010;44(6):519–24. doi: 10.1159/000320717. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Yang JY, Lo EC, Lin HC. The contribution of life course determinants to early childhood caries: a 2-year cohort study. Caries Res. 2012;46(2):87–94. doi: 10.1159/000335574. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Miyake Y, Sasaki S, Hirota Y. Dairy products and calcium intake during pregnancy and dental caries in children. Nutr J. 2012;11:33. doi: 10.1186/1475-2891-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders AE, Slade GD. Apgar score and dental caries risk in the primary dentition of five year olds. Aust Dent J. 2010;55(3):260–7. doi: 10.1111/j.1834-7819.2010.01232.x. [DOI] [PubMed] [Google Scholar]
- 34.Kato T, Yorifuji T, Yamakawa M, Inoue S, Saito K, Doi H, Kawachi I. Association of breastfeeding with early childhood dental caries: Japanese population-based study. BMJ Open. 2015;5(3):e006982. doi: 10.1136/bmjopen-2014-006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tada A, Ando Y, Hanada N. Caries risk factors among three year old children in Chiba, Japan. Asia Pacif J Public Health. 1999;11(2):109–112. doi: 10.1177/101053959901100210. [DOI] [PubMed] [Google Scholar]
- 36.Marino RV, Bomze K, Scholl TO, Anhalt H. Nursing bottle caries: characteristics of children at risk. Clin Pediatr (Phila) 1989;28(3):129–31. doi: 10.1177/000992288902800305. [DOI] [PubMed] [Google Scholar]
- 37.Ollila P, Niemelä M, Uhari M, Larmas M. Prolonged pacifier-sucking and use of a nursing bottle at night: possible risk factors for dental caries in children. Acta Odontol Scand. 1998;56(4):233–7. doi: 10.1080/00016359850142853. [DOI] [PubMed] [Google Scholar]
- 38.Lulic-Dukic O, Juric H, Dukic W, Glavina D. Factors predisposing to early childhood caries (ECC) in children of pre-school age in the city of Zagreb, Croatia. Coll Antropol. 2001;25(1):297–302. [PubMed] [Google Scholar]
- 39.Law V, Seow WK. A longitudinal controlled study of factors associated with mutans streptococci infection and carious lesion initiation in children 21 to 72 months old. Pediatr Dent. 2006;28(1):58–65. [PubMed] [Google Scholar]
- 40.Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25(8):620–5. doi: 10.1016/j.annepidem.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Van Palenstein Helderman WH, Soe W, van’t Hof MA. Risk factors of early childhood caries in a Southeast Asian population. J Dent Res. 2006;85(1):85–8. doi: 10.1177/154405910608500115. [DOI] [PubMed] [Google Scholar]
- 42.Warren JJ, Weber-Gasparoni K, Marshall TA, et al. A longitudinal study of dental caries risk among very young low SES children. Community Dent Oral Epidemiol. 2009;37(2):116–22. doi: 10.1111/j.1600-0528.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aaltonen AS, Tenovuo J. Association between mother-infant salivary contacts and caries resistance in children: a cohort study. Pediatr Dent. 1994;16(2):110–6. [PubMed] [Google Scholar]
- 44.O’Sullivan DM, Thibodeau EA. Caries experience and mutans streptococci as indicators of caries incidence. Pediatr Dent. 1996;18(5):371–4. [PubMed] [Google Scholar]
- 45.Ansai T, Tahara A, Ikeda M, Katoh Y, Miyazaki H, Takehara T. Influence of colonization with mutans streptococci on caries risk in Japanese preschool children: 24-month survival analysis. Pediatr Dent. 2000;22(5):377–80. [PubMed] [Google Scholar]
- 46.Yonezu T, Ushida N, Yakushiji M. Longitudinal study of prolonged breast- or bottle feeding on dental caries in Japanese children. Bull Tokyo Dent Coll. 2006;47(4):157–60. doi: 10.2209/tdcpublication.47.157. [DOI] [PubMed] [Google Scholar]
- 47.Hong L, Levy SM, Warren JJ, Broffitt B. Association between enamel hypoplasia and dental caries in primary second molars: a cohort study. Caries Res. 2009;43(5):345–53. doi: 10.1159/000231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seow WK, Clifford H, Battistutta D, Morawska A, Holcombe T. Case control study of early childhood caries in Australia. Caries Res. 2009;43(1):25–35. doi: 10.1159/000189704. [DOI] [PubMed] [Google Scholar]
- 49.Targino AG, Rosenblatt A, Oliveira AF, Chaves AM, Santos VE. The relationship of enamel defects and caries: a cohort study. Oral Dis. 2011;17(4):420–6. doi: 10.1111/j.1601-0825.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- 50.Silva MJ, Scurrah KJ, Craig JM, Manton DJ, Kilpatrick N. Etiology of molar incisor hypomineralization: a systematic review. Community Dent Oral Epidemiol. 2016;44(4):342–5. doi: 10.1111/cdoe.12229. [DOI] [PubMed] [Google Scholar]
- 51.Peltzer K, Mongkolchati A. A Severe early childhood caries and social determinants in three-year-old children from Northern Thailand: a birth cohort study. BMC Oral Health. 2015;15:108. doi: 10.1186/s12903-015-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokomichi H, Tanaka T, Suzuki K, Akiyama T. Macrosomic neonates carry increased risk of dental caries in early childhood: findings from a cohort study, the Okinawa Child Health Study, Japan. PLoS One. 2015;10(7):e0133872. doi: 10.1371/journal.pone.0133872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter J, Glaser M, Heinzel-Gutenbrunner M, Pieper K. Association of caries increment in preschool children with nutritional and preventive variables. Clin Oral Investig. 2015;19(8):1913–9. doi: 10.1007/s00784-015-1419-2. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe M, Wang DH, Ijichi A, et al. The influence of lifestyle on the incidence of dental caries among 3-year-old Japanese children. Int J Environ Res Public Health. 2014;11(12):12611–22. doi: 10.3390/ijerph111212611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao X, Hsu CY, Loh T, Hwarng B, Koh D. Role of microbiological factors in predicting early childhood caries. Pediatr Dent. 2014;36(4):342–7. [PubMed] [Google Scholar]
- 56.Hong L, Levy SM, Warren JJ, Broffitt B. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36(4):348–54. [PMC free article] [PubMed] [Google Scholar]
- 57.Moimaz SA, Garbin AJ, Lima AM, Lolli LF, Saliba O, Garbin CA. Risk factors in the mother-child relationship that predispose to the development of early childhood caries. Eur Arch Paediatr Dent. 2014;15(4):245–50. doi: 10.1007/s40368-014-0108-1. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K, Miyake Y, Sasaki S, Hirota Y. Infant feeding practices and risk of dental caries in Japan: the Osaka Maternal and Child Health Study. Pediatr Dent. 2013;35(3):267–71. [PubMed] [Google Scholar]
- 59.Tanaka K, Miyake Y, Sasaki S, Hirota Y. Socioeconomic status and risk of dental caries in Japanese preschool children: the Osaka Maternal and Child Health Study. J Public Health Dent. 2013;73(3):217–23. doi: 10.1111/jphd.12016. [DOI] [PubMed] [Google Scholar]
- 60.Almeida TF, Vianna MI, Cabral MB, Cangussu MC, Floriano FR. Family context and incidence of dental caries in preschool children living in areas covered by the Family Health Strategy in Salvador, Bahia State, Brazil. Cad Saude Publicas. 2012;28(6):1183–95. doi: 10.1590/s0102-311x2012000600017. [DOI] [PubMed] [Google Scholar]
- 61.Bankel M, Robertson A, Kohler B. Carious lesions and caries risk predictors in a group of Swedish children 2 to 3 years of age. One year observation. Eur J Paediatr Dent. 2011;12(4):215–9. [PubMed] [Google Scholar]
- 62.Parisotto TM, King WF, Duque C, Mattos-Graner RO, Steiner-Oliveira C, Nobre-Dos-Santos M, Smith DJ. Immunological and microbiologic changes during caries development in young children. Caries Res. 2011;45(4):377–85. doi: 10.1159/000330230. [DOI] [PubMed] [Google Scholar]
- 63.Wigen TI, Espelid I, Skaare AB, Wang NJ. Family characteristics and caries experience in preschool children: a longitudinal study from pregnancy to 5 years of age. Community Dent Oral Epidemiol. 2011;39(4):311–7. doi: 10.1111/j.1600-0528.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 64.Feldens CA, Giugliani ER, Vigo Á, Vítolo MR. Early feeding practices and severe early childhood caries in four-year-old children from southern Brazil: a birth cohort study. Caries Res. 2010;44(5):445–52. doi: 10.1159/000319898. [DOI] [PubMed] [Google Scholar]
- 65.Ismail AI, Lim S, Sohn W, Willem JM. Determinants of early childhood caries in low-income African American young children. Pediatr Dent. 2008;30(4):289–96. [PubMed] [Google Scholar]
- 66.Lim S, Sohn W, Burt BA, et al. Cariogenicity of soft drinks, milk and fruit juice in low-income African-American children: a longitudinal study. J Am Dent Assoc. 2008;139(7):959–67. doi: 10.14219/jada.archive.2008.0283. [DOI] [PubMed] [Google Scholar]
- 67.Yonezu T, Yotsuya K, Yakushiji M. Characteristics of breastfed children with nursing caries. Bull Tokyo Dent Coll. 2006;47(4):161–5. doi: 10.2209/tdcpublication.47.161. [DOI] [PubMed] [Google Scholar]
- 68.Levy SM, Warren JJ, Broffitt B, Hillis SL, Kanellis MJ. Fluoride, beverages and dental caries in the primary dentition. Caries Res. 2003;37(3):157–65. doi: 10.1159/000070438. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues CS, Sheiham A. The relationships between dietary guidelines, sugar intake and caries in primary teeth in low-income Brazilian 3-year-olds: a longitudinal study. Int J Paediatr Dent. 2000;10(1):47–55. doi: 10.1046/j.1365-263x.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 70.Mattila ML, Paunio P, Rautava P, Ojanlatva A, Sillanpaa M. Changes in dental health and dental health habits from 3 to 5 years of age. J Public Health Dent. 1998;58(4):270–4. doi: 10.1111/j.1752-7325.1998.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 71.Thibodeau EA, O’Sullivan DM. Salivary mutans streptococci and dental caries patterns in pre-school children. Community Dent Oral Epidemiol. 1996;24(3):164–8. doi: 10.1111/j.1600-0528.1996.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 72.Wendt LK, Hallonsten AL, Koch G, Birkhed D. Analysis of caries-related factors in infants and toddlers living in Sweden. Acta Odontol Scand. 1996;54(2):131–7. doi: 10.3109/00016359609006019. [DOI] [PubMed] [Google Scholar]
- 73.Grindefjord M, Dahllöf G, Nilsson B, Modéer T. Step-wise prediction of dental caries in children up to 3.5 years of age. Caries Res. 1996;30(4):256–66. doi: 10.1159/000262333. [DOI] [PubMed] [Google Scholar]
- 74.Wendt LK, Birkhed D. Dietary habits related to caries development and immigrant status in infants and toddlers living in Sweden. Acta Odontol Scand. 1995;53(6):339–44. doi: 10.3109/00016359509005998. [DOI] [PubMed] [Google Scholar]
- 75.Grytten J, Rossow I, Holst D, Steele L. Longitudinal study of dental health behaviors and other caries predictors in early childhood. Community Dent Oral Epidemiol. 1988;16(6):356–9. doi: 10.1111/j.1600-0528.1988.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 76.Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt ME. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133(5):e1277–e1284. doi: 10.1542/peds.2013-2215. [DOI] [PubMed] [Google Scholar]
- 77.Wigen TI, Wang NJ. Maternal health and lifestyle, and caries experience in preschool children: a longitudinal study from pregnancy to age 5 years. Eur J Oral Sci. 2011;119(6):463–8. doi: 10.1111/j.1600-0722.2011.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peretz B, Kafka I. Baby bottle tooth decay and complications during pregnancy and delivery. Pediatr Dent. 1997;19(1):34–6. [PubMed] [Google Scholar]
- 79.Yu LX, Tao Y, Qiu RM, Zhou Y, Zhi QH, Lin HC. Genetic polymorphisms of the sortase A gene and social-behavioural factors associated with caries in children: a case control study. BMC Oral Health. 2015;15:54. doi: 10.1186/s12903-015-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dantas Cabral de s MM, de Souza WV, Tavares MC, de Lima ML, Jamelli S, Couto GB. Social conditions and high levels of dental caries in five-year-old children in Brazil. J Dent Child (Chic) 2015;82(1):29–35. [PubMed] [Google Scholar]
- 81.Evans EW, Hayes C, Palmer CA, Bermudez OI, Cohen SA, Must A. Dietary intake and severe early childhood caries in low-income, young children. J Acad Nutr Diet. 2013;113(8):1057–61. doi: 10.1016/j.jand.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Del Rosario Garcia-Garcia M, Villarreal-Ríos E, Galicia-Rodríguez L, Martínez-González L. Risk factors and the probability of developing dental decay in four-year-old children. Rev Med Inst Mex Seguro Soc. 2011;49(1):9–12. [PubMed] [Google Scholar]
- 83.Slade GD, Sanders AE, Bill CJ, Do LG. Risk factors for dental caries in the five-year-old South Australian population. Aust Dent J. 2006;51(2):130–9. doi: 10.1111/j.1834-7819.2006.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 84.Nelson S, Nechvatal N, Weber J, Canion S. Dental caries and ear infections in preschool-aged children. Oral Health Prev Dent. 2005;3(3):165–71. [PubMed] [Google Scholar]
- 85.Campus G, Solinas G, Sanna A, Maida C, Castiglia P. Determinants of ECC in Sardinian preschool children. Community Dent Health. 2007;24(4):253–6. [PubMed] [Google Scholar]
- 86.Shantinath SD, Breiger D, Williams BJ, Hasazi JE. The relationship of sleep problems and sleep-associated feeding to nursing caries. Pediatr Dent. 1996;18(5):375–8. [PubMed] [Google Scholar]
- 87.Qin M, Li J, Zhang S, Ma W. Risk factors for severe early childhood caries in children younger than 4 years old in Beijing, China. Pediatr Dent. 2008;30(2):122–8. [PubMed] [Google Scholar]
- 88.Tanaka S, Shinzawa M, Tokumasu H, Seto K, Tanaka S, Kawakami K. Second-hand smoke and incidence of dental caries in deciduous teeth among children in Japan: population-based retrospective cohort study. BMJ. 2015;351:h6009. doi: 10.1136/bmj.h5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishide R, Mizutani M, Tanimura S, Kudo N, Nishii T, Hatashita H. Homecare protective and risk factors for early childhood caries in Japan. Environ Health Prev Med. 2018;23(1):57. doi: 10.1186/s12199-018-0746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boustedt K, Roswall J, Twetman S, Dahlgren J. Influence of mode of delivery, family and nursing determinants on early childhood caries development: a prospective cohort study. Acta Odontol Scand. 2018;76(8):595–9. doi: 10.1080/00016357.2018.1490965. [DOI] [PubMed] [Google Scholar]
- 91.Cabral MBBS, Mota ELA, Cangussu MCT, Vianna MIP, Floriano FR. Risk factors for caries-free time: longitudinal study in early childhood. Rev Saude Publica. 2017;51:118. doi: 10.11606/S1518-8787.2017051006558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jean J, Goldberg S, Khare R, et al. Retrospective Analysis of Candida-related conditions in infancy and early childhood caries. Pediatr Dent. 2018;40(2):131–5. [PMC free article] [PubMed] [Google Scholar]
- 93.Feldens CA, Rodrigues PH, de Anastácio G, Vítolo MR, Chaffee BW. Feeding frequency in infancy and dental caries in childhood: a prospective cohort study. Int Dent J. 2018;68(2):113–21. doi: 10.1111/idj.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peres KG, Nascimento GG, Peres MA, et al. Impact of prolonged breastfeeding on dental caries: a population-based birth cohort study. Pediatrics. 2017;140(1) doi: 10.1542/peds.2016-2943. pii e20162943. [DOI] [PubMed] [Google Scholar]
- 95.Birungi N, Fadnes LT, Kasangaki A, et al. PROMISE-EBF study group. Assessing causal effects of early life-course factors on early childhood caries in 5-year-old Ugandan children using directed acyclic graphs (DAGs): a prospective cohort study. Community Dent Oral Epidemiol. 2017;45(6):512–21. doi: 10.1111/cdoe.12314. [DOI] [PubMed] [Google Scholar]
- 96.Bernabé E, MacRitchie H, Longbottom C, Pitts NB, Sabbah W. Birthweight, breastfeeding, maternal smoking and caries trajectories. J Dent Res. 2017;96(2):171–8. doi: 10.1177/0022034516678181. [DOI] [PubMed] [Google Scholar]
- 97.Nirunsittirat A, Pitiphat W, McKinney CM, et al. Breast-feeding duration and childhood caries: a cohort study. Caries Res. 2016;50(5):498–507. doi: 10.1159/000448145. [DOI] [PubMed] [Google Scholar]
- 98.Fan C, Wang W, Xu T, Zheng S. Risk factors of early childhood caries among children in Beijing: a case control study. BMC Oral Health. 2016;16(1):98. doi: 10.1186/s12903-016-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paglia L, Scaglioni S, Torchia V, et al. Familial and dietary risk factors in Early Childhood Caries. Eur J Paediatr Dent. 2016;17(2):93–9. [PubMed] [Google Scholar]
- 100.Dabawala S, Suprabha BS, Shenoy R, Rao A, Shah N. Parenting style and oral health practices in early childhood caries: a case control study. Int J Paediatr Dent. 2017;27(2):135–44. doi: 10.1111/ipd.12235. [DOI] [PubMed] [Google Scholar]
- 101.Roberts GJ, Cleaton-Jones PE, Fatti LP, et al. Patterns of breast and bottle feeding and their association with dental caries in 1- to 4-year-old South African children. 2. A case control study of children with nursing caries. Community Dent Health. 1994;11(1):38–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.