Abstract
Purpose
Respiratory syncytial virus (RSV) is a common cause of lower respiratory tract infection in young children. However, there are limited data on severe RSV infection requiring pediatric intensive care unit (PICU) admission. This retrospective study described features of RSV-associated PICU admissions in Hong Kong and investigated factors for mortality and duration of PICU stay.
Methods
Children with laboratory-confirmed RSV infection and admitted to the PICUs of all eight government hospitals in Hong Kong between January 2009 and June 2011 were identified from computerized auditing systems and PICU databases. RSV in respiratory samples was detected by direct immunofluorescence and/or viral culture. The relationships between mortality and PICU duration and demographic and clinical factors were analyzed.
Results
A total of 118 (2.4 %) PICU admissions were identified among 4,912 RSV-positive pediatric cases in all hospitals. Sixty-five (55.6 %) patients were infants. PICU admissions were higher between October and March. Eight (6.8 %) patients died, but only two were infants. RSV-associated mortality was related to prior sick contact, presence of older siblings, neurodevelopmental conditions, chromosomal and genetic diseases, and bacterial co-infections, but none was significant following logistic regression analyses (odds ratio 9.36, 95 % confidence interval 0.91–96.03 for prior sick contact, p = 0.060). Chronic lung disease was the only risk factor for the duration of PICU admission (β = 0.218, p = 0.017).
Conclusions
The majority of RSV-infected children do not require PICU support. There is winter seasonality for RSV-associated PICU admission in Hong Kong. Prior sick contact is the only risk factor for RSV-associated mortality, whereas the presence of chronic lung disease is associated with longer PICU stay. The current risk-based approach of RSV prophylaxis may not be effective in reducing severe RSV infections.
Keywords: Mortality, Pediatric intensive care, Respiratory syncytial virus, Seasonality
Introduction
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections such as bronchiolitis and bronchopneumonia, as well as hospitalizations in children aged <2 years [1]. RSV infections led to the intermediate or intensive care admission of approximately 1–2 % of each annual birth cohort in Switzerland [2]. In a national registry in France, 467 young children hospitalized for bronchiolitis from 24 pediatric intensive care units (PICUs) were reviewed [3]. Seventy-six percent of them were RSV-positive. Over one-third of these children required non-invasive ventilation and/or mechanical ventilation, and six infants died. A single-center study in Hong Kong found that 1.5 % of RSV-related hospitalizations required PICU support, which accounted for 2.4 % of annual PICU admissions. RSV exceeded influenza, parainfluenza, and other respiratory viruses as the most common viral pathogens in the PICU of a regional hospital in Hong Kong [4, 5]. Infants with underlying cardiopulmonary compromise such as surgical congenital cardiac disease and infants born prematurely with chronic lung disease are considered to be more susceptible to RSV infection, with resultant increased mortality and morbidity.
Although no RSV vaccine is available, two products have been approved for passive immunization against RSV infections: RSV immunoglobulin (RSV-IGIV), prepared from donors possessing high serum titers of RSV-neutralizing antibody, and palivizumab, a humanized monoclonal antibody against the fusion protein of RSV [6, 7]. These effective treatments are expensive and usually given to at-risk infants during the winter months when RSV infection is more prevalent. However, seasonality may pose a problem in subtropical and tropical climates. Among Asian cities, Lee et al. [8] reported a biennial pattern of RSV-related hospitalization in Taiwan, whereas a retrospective study of RSV cases in a PICU in Hong Kong did not identify any definite seasonality [5]. Our PICU study suggested younger age, bacterial co-infections, history of chronic lung disease, acyanotic heart disease, and neurodevelopmental conditions (e.g., mental retardation, cerebral palsy, neuromuscular diseases) to be risk factors for severe RSV infection [5]. The medical costs for managing RSV-infected children with underlying diseases are high in our region [8]; thus, any prophylactic measures against RSV are potentially cost-saving. Nonetheless, the above two options of RSV prophylaxis have not yet been introduced among public hospitals in Hong Kong, mainly due to the unavailability of data regarding their cost-effectiveness in southern Chinese children. Despite this, the local Hospital Authority approved the subsidization of palivizumab prophylaxis to high-risk groups from 2012. The aim of this study was to describe features of RSV-positive children who required PICU admission, as well as to identify factors associated with mortality and duration of PICU stay in Hong Kong before the introduction of RSV prophylaxis to the region.
Patients and methods
Study design and patients
Hong Kong has a population of over 7.1 million people, of which 1.1 million are under 18 years of age (http://www.censtatd.gov.hk/hkstat/sub/so20_tc.jsp). There is a dual public and private system for pediatric inpatient service in a total of ten public and ten private hospitals. Dedicated PICUs are available in only eight public hospitals, designated as hospitals A–H in this study, which admitted >90 % of all sick children in Hong Kong. Different PICUs have different admission policies, with the upper age limit ranging from 12 to 18 years old. The criteria for PICU admissions include cardiopulmonary insufficiency/failure, neurological deterioration, and concerns of emergency care and/or general pediatric staff.
This territory-wide retrospective review collected inpatient data during a 30-month period between January 2009 and June 2011. The RSV Concern Group was formed under the Hong Kong Society of Paediatric Respirology (HKSPR), the only local professional organization for pediatric pulmonologists, initiated this study. Two to three coordinators from all eight PICUs, with a total of 44 beds, participated in data retrieval. The total numbers of RSV-positive patients aged <18 years in participating hospitals during the review period were retrieved from hospitals’ microbiology databases and RSV-associated PICU admissions were identified from both computerized auditing systems and PICU databases of participating departments. The respective data in one of the hospitals were previously reported [4]. The case records of all RSV-positive children hospitalized in these PICUs during the study period were reviewed. RSV was detected using direct immunofluorescence testing on respiratory samples (nasopharyngeal aspirate, tracheal aspirate, or bronchoalveolar lavage) and/or viral culture. The results of all other bacterial cultures on respiratory specimens, urine, mucous membranes, cerebrospinal fluid, and blood were also recorded. The coordinators of participating PICUs recorded patients’ demographic and clinical data anonymously using a common data collection form. Ethical approval for this multicenter study was obtained from the Joint Chinese University of Hong Kong–New Territories East Cluster (NTEC) Clinical Research Ethics Committee (CREC).
Statistical analysis
Data were expressed as number and percentage or median and interquartile range (IQR), as appropriate. The relationship between mortality and these clinical and laboratory variables was analyzed by Fisher’s exact or χ2 tests for categorical variables and the Mann–Whitney U-test for numerical variables. The relationship between fatality and factors with suggestive significance by univariate analyses (p < 0.15) was analyzed by logistic regression, adjusted for age and hospital identity as covariates. The association between the length of PICU admission and possible personal and clinical factors was evaluated by the Mann–Whitney U-test and confirmed by stepwise linear regression. Comparisons were made one-tailed for risk factors known to be associated with RSV severity and two-tailed for other variables using SPSS v.18 (SPSS Inc., Chicago, IL). p-values <0.05 were considered statistically significant.
Results
Clinical characteristics
A total of 4,912 RSV-positive pediatric cases were identified during the 30-month study period. Table 1 describes their distribution among the eight PICUs. One hundred and eighteen (2.4 %) RSV-associated PICU admissions in eight participating hospitals were identified in 117 children, including one patient who was admitted twice in March 2009 and October 2010. All these cases were confirmed by the review of hospitals’ microbiology databases. Sixty-five (55.6 %) and 112 (95.7 %) patients were under 1 and 5 years of age, respectively. Fourteen (11.9 %) of 118 admissions involved infants <30 days of age.
Table 1.
Distribution of respiratory syncytial virus (RSV)-positive cases and RSV-associated pediatric intensive care unit (PICU) admissions among eight government hospitals in Hong Kong between January 2009 and June 2011
| Hospital | RSV-positive cases | PICU admissionsa |
|---|---|---|
| A | 722 | 18 (2.5) |
| B | 619 | 18 (2.9) |
| Cb | 1,498 | 16 (1.1) |
| D | 481 | 16 (3.3) |
| E | 366 | 16 (4.4) |
| F | 533 | 15 (2.8) |
| G | 145 | 11 (7.6) |
| H | 548 | 8 (1.5) |
| Total | 4,912 | 118 (2.4) |
aExpressed in absolute numbers (percentages of RSV-positive cases) for individual hospitals
bHospital C provides microbiology laboratory services for two hospitals within a Hospital Authority Cluster in Hong Kong, which also contains a five-bed PICU servicing both hospitals
Chronic lung disease was defined in infants who were oxygen-dependent at 28 days of life. Cyanotic heart diseases in our patients included Tetralogy of Fallot, pulmonary atresia, transposition of great arteries, interrupted aortic arch, and total anomalous pulmonary venous drainage; and acyanotic heart diseases included atrial sepal defect, ventricular septal defect, endocardial cushion defect, patent ductus arteriosus, and coarctation of the aorta.
Concerning the clinical features on PICU admission, wheeze, cough, dyspnea, rhinorrhea, and fever (≥38 °C) were found in 45, 100, 92, 59, and 85 % of patients, respectively. Vomiting was reported in 16 patients and diarrhea in four patients. The presence of all these features was not different between the death and survived cases (p > 0.1).
Figure 1 describes the seasonality pattern of RSV-associated PICU admissions, which tended to be higher during the winter months (October to March). Bacterial co-infections were present in 14 (11.9 %) episodes of PICU admissions. Table 2 provides the details of these bacterial isolates.
Fig. 1.
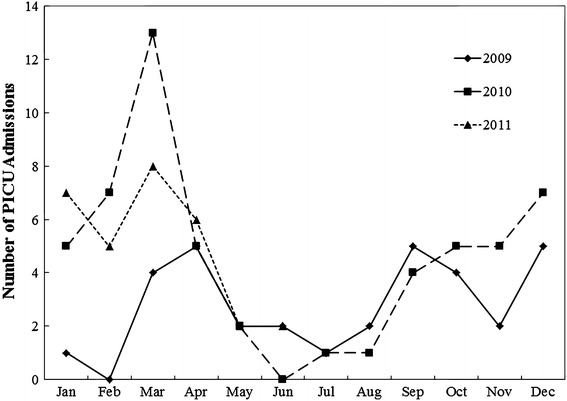
Seasonality pattern of respiratory syncytial virus (RSV)-associated pediatric intensive care unit (PICU) admissions between January 2009 and June 2011
Table 2.
Spectrum of bacterial co-infections detected in 14 of 117 PICU patients
| Hospital | Sex/age | Isolated bacteria (source of culture) | Death |
|---|---|---|---|
| A | M/1.4 years | Stenotrophomonas maltophilia (TA) | No |
| A | F/1.6 years | Acinetobacter, Chryseobacterium, and Malassezia species (TA) | Yes |
| A | F/2.2 years | Pseudomonas aeruginosa (TA) | Yes |
| A | F/9 months | Staphylococcus aureus, ESBL Enterobacter aerogenes, and Pseudomonas pickettii (TA) | No |
| A | M/11 months | Branhamella catarrhalis and Haemophilus influenzae (TA) | No |
| A | M/1.9 years | Staphylococcus aureus and Haemophilus influenzae (TA) | No |
| B | M/4 months | Klebsiella and Acinetobacter (TA) | No |
| C | F/2.1 years | Beta-lactamase-producing Moraxella catarrhalis (TA) | Yes |
| C | M/2 months | Staphylococcus aureus (blood) | No |
| C | M/7 months | Streptococcus pyogenes (TA) | No |
| D | M/1 month | Streptococcus pneumoniae (blood) | No |
| D | F/4 months | Staphylococcus aureus, Escherichia coli, and Acinetobacter (TA) | No |
| D | M/1.6 years | Moraxella species (blood) and ESBL Klebsiella (TA) | No |
| E | F/1.3 years | Streptococcus pneumoniae (TA) | No |
ESBL extended-spectrum beta-lactamase, TA tracheal aspirate
Mortality and duration of RSV-associated PICU admissions
Eight (6.8 %) patients died, and their median (IQR) age was 1.8 (0.6–2.8) years. Figure 2 describes the underlying diseases of these death cases. Only two of them were aged below 1 year old. Mortality did not differ among the eight hospitals (p = 0.42). Table 3 summarizes the distribution of clinical and disease-related factors between the death and survived patients. Prior sick contact, presence of older siblings, neurodevelopmental conditions, chromosomal and genetic diseases, and bacterial co-infections were associated with RSV-associated mortality. Although none of these remained significant following multivariate logistic regression analysis, we observed a trend for mortality to be associated with prior sick contact (odds ratio 9.36, 95 % confidence interval 0.91–96.03, p = 0.06). Table 4 illustrates the relationship between the duration of PICU admission and different personal and clinical factors. A number of factors were individually associated with the duration of PICU admission. Nonetheless, regression analyses revealed that the presence of chronic lung disease was the only significant factor for the duration of PICU admission (β = 0.218, p = 0.017).
Fig. 2.
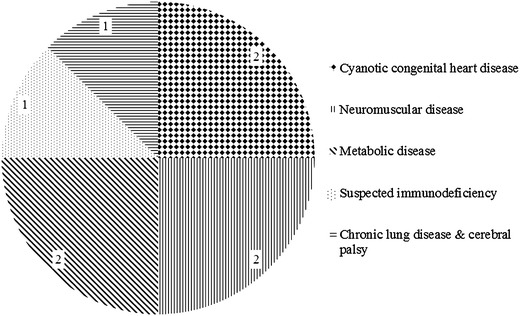
Underlying diseases of our eight death cases
Table 3.
Relationship between mortality and patients’ clinical and treatment factors
| Parameter | Death cases (n = 8) | Survived cases (n = 110) | p valuea |
|---|---|---|---|
| Age, years | 1.8 (0.6–2.8) | 0.7 (0.2–1.8) | 0.13 |
| Male, n (%) | 3 (37.5) | 65 (59.1) | 0.28* |
| Duration of PICU stay (days) | 19.5 (8.3–33.0) | 5.0 (4.0–10.3) | 0.019 |
| Possible risk factors | |||
| Sick contact, n (%)b | 6 (75.0) | 44 (40.0) | 0.06 |
| Passive smoking exposure, n (%) | 0 | 9 (8.2) | 0.52* |
| Presence of older siblings, n (%) | 0 | 42 (38.2) | 0.049* |
| Multiple pregnancy, n (%) | 1 (12.5) | 11 (10.0) | 0.59* |
| Prematurity <37 weeks, n (%) | 3 (37.5) | 32 (29.1) | 0.44* |
| Chronic lung disease, n (%) | 2 (25.0) | 25 (22.7) | 0.59* |
| Congenital heart disease, n (%) | 2 (25.0) | 20 (18.2) | 0.46* |
| Neurodevelopmental conditions, n (%) | 6 (75.0) | 21 (19.1) | 0.002 |
| Chromosomal and genetic diseases, n (%) | 5 (62.5) | 13 (11.8) | 0.002 |
| Bacterial co-infections, n (%) | 3 (37.5) | 11 (10.0) | 0.05* |
| Treatments | |||
| IPPV, n (%) | 8 (100) | 23 (20.9) | <0.001 |
| NIV, n (%) | 3 (37.5) | 46 (41.8) | 0.56* |
| Systemic corticosteroids, n (%) | 4 (50.0) | 32 (29.1) | 0.25* |
IPPV intermittent positive pressure ventilation, NIV non-invasive ventilation
aAnalyzed by Fisher’s exact test (indicated by *) or χ2 tests for categorical variables and the Mann–Whitney U-test for numerical variables
bSick contact was defined as the exposure of our patients to subjects with symptoms of respiratory tract infections in the same household, class, or inpatient ward within one week from their onset of RSV infection
Table 4.
Relationship between the duration of PICU admission and clinical and treatment factors
| Factor | Duration, median and IQR (days) | p valuea | |
|---|---|---|---|
| Presence of factor | Absence of factorb | ||
| Male gender | 5.5 (3.0–9.0) | 7.0 (4.0–14.0) | 0.18 |
| Clinical features | |||
| Cough | 6.0 (4.0–10.8) | 7.0 (3.0–16.5) | 0.64 |
| Wheeze | 6.0 (4.0–9.0) | 6.0 (4.0–12.0) | 0.82 |
| Dyspnea | 6.5 (4.0–12.0) | 4.0 (3.0–7.5) | 0.17 |
| Rhinorrhea | 5.0 (3.0–8.0) | 7.0 (4.0–14.0) | 0.014 |
| Fever | 7.0 (3.0–14.0) | 5.0 (4.0–7.0) | 0.24 |
| Vomiting | 4.5 (3.0–5.8) | 7.0 (4.0–12.0) | 0.024 |
| Diarrhea | 8.5 (2.3–19.3) | 6.0 (4.0–11.0) | 0.88 |
| Possible risk factors | |||
| Sick contact | 5.0 (4.0–10.3) | 6.0 (4.0–12.0) | 0.54 |
| Passive smoking exposure | 4.0 (3.0–7.5) | 6.0 (4.0–12.0) | 0.11 |
| Presence of older siblings | 5.0 (4.0–8.3) | 6.5 (4.0–13.8) | 0.38 |
| Multiple pregnancy | 7.0 (4.2–7.8) | 6.0 (4.0–11.5) | 0.75 |
| Prematurity <37 weeks | 7.0 (4.0–14.0) | 5.0 (3.0–11.0) | 0.13 |
| Chronic lung disease | 7.0 (5.0–12.0) | 5.0 (3.0–11.0) | 0.030 |
| Congenital heart disease | 6.0 (3.0–14.3) | 6.0 (4.0–9.8) | 0.70 |
| Neurodevelopmental conditions | 8.0 (4.0–18.0) | 5.0 (4.0–10.0) | 0.28 |
| Chromosomal and genetic diseases | 9.5 (3.8–20.0) | 5.5 (4.0–9.0) | 0.18 |
| Bacterial co-infections | 11.5 (4.8–27.8) | 5.0 (4.0–9.0) | 0.021 |
| Treatments | |||
| IPPV | 13.0 (8.0–25.0) | 5.0 (3.0–7.0) | <0.001 |
| NIV | 7.0 (4.0–14.0) | 5.0 (3.0–9.0) | 0.017 |
| Systemic corticosteroid | 8.0 (5.3–14.8) | 5.0 (3.0–9.5) | 0.002 |
IQR interquartile range
aAnalyzed by the Mann–Whitney U-test
bA factor that was either documented to be absent or unrecorded in the case note was treated as the absence of that factor
Discussion
This is the first surveillance study of RSV-associated PICU admissions in the Chinese population. A total of 118 (2.4 %) PICU admissions were identified among 4,912 RSV-positive pediatric cases in all eight government hospitals. There is an apparent winter seasonality for these PICU admissions in Hong Kong. Eight (6.8 %) patients died, and factors associated with mortality were prior sick contact, presence of older siblings, neurodevelopmental conditions, chromosomal and genetic diseases, and bacterial co-infections. This study identified chronic lung disease as the only risk factor for the duration of PICU admission.
Eight (6.8 %) of our 117 sick RSV-infected patients died. This mortality rate was similar to the published data in the UK (8.6 %) [9] but lower than those in Spain and Japan (over 10 %) [10, 11]. Prior sick contact might be a risk factor for RSV-associated mortality among our children who required PICU admission. Nonetheless, the retrospective nature of this study did not allow us to distinguish whether our patients acquired RSV from hospitals or their household or institutional members.
In the present territory-wide study, the presence of conventional risk factors for severe RSV infection such as history of prematurity [2, 12, 13], chronic lung disease [2, 8, 9, 14], airway abnormalities [9, 14], congenital heart disease [2, 8, 9, 13, 14], and neurodevelopmental conditions [8, 9, 13, 15] were not associated with RSV-associated mortality in Hong Kong children. Purcell and Fergie [13] reported that young age <6 weeks, history of prematurity, congenital heart disease, and neurologic disease were risk factors for severe RSV infection. Two-thirds of their patients with three or more risk factors were admitted to the PICU, and nearly half of these children required mechanical ventilation. A Taiwanese study found that underlying diseases including congenital heart and chronic lung diseases and neurological disorders were risk factors for endotracheal intubation [8]. Zhang et al. [16] reported, in a recent prospective study of 10,836 Chinese children hospitalized for community-acquired pneumonia, that fatality was associated with the presence of congenital heart disease, trisomy 21, and immunodeficiency.
The duration of PICU stay is an important outcome indicator of the severity of RSV infection. Chronic lung disease was a risk factor for longer RSV-associated PICU admission in Hong Kong, which was found in another PICU study of 89 RSV-infected infants younger than 2 years old [14]. Other risk factors reported in that study included congenital heart diseases, airway abnormalities, and non-cardiac congenital malformations. Purcell and Fergie [13] identified, through a retrospective chart review of 3,308 patients discharged with a diagnosis of RSV lower respiratory tract infection, that history of prematurity, congenital heart disease, and neurologic disease were risk factors for longer hospital stay. In another study, male sex, lower body weight, and higher viral load on any day were independent predictors of longer duration of hospitalization in American children [17]. However, the authors did not report data specifically for RSV-associated PICU stay. DeVincenzo et al. [18] prospectively studied 141 previously healthy and naturally RSV-infected children, and reported that congenital anomaly, lower weight on admission, and higher nasal RSV load were associated with longer hospitalization, respiratory failure, and requirement for PICU admission.
Our results cast doubt on the case selection for RSV preventive programs. In the initial randomized, double-blind, placebo-controlled trial, palivizumab prophylaxis resulted in lower rate and shorter duration of RSV-related hospitalization and less PICU admissions among children with prematurity (≤35 weeks) or bronchopulmonary dysplasia [7]. A meta-analysis suggested that prophylactic administration of both palivizumab and RSV-IGIV reduced RSV-related hospitalization and PICU admission [6]. The American Academy of Pediatrics (AAP) guidelines recommended a targeted approach for RSV prophylaxis based on risk factors such as prematurity, as well as chronic lung and congenital heart diseases [19]. This guideline was set to reduce RSV-related hospitalization instead of the more serious outcomes such as mortality and PICU admissions associated with RSV infection. The majority (70.2 %) of RSV-infected children being admitted to the PICU had no underlying medical illness, and over 88 % of all PICU admissions would not qualify for RSV prophylaxis according to the Canadian guideline [20]. A number of studies yielded similar findings among children from different western countries [2, 14, 21, 22]. Besides, we identified chronic lung disease to be an independent risk factor for longer PICU stay, supporting the importance of including this medical condition in the relevant guidelines. Nonetheless, the lack of a significant association between serious RSV outcomes and all other known risk factors such as prematurity and congenital heart diseases challenged the validity of such a risk stratification approach. In addition, RSV prophylaxis should be administered not only to infants because only two of our eight death cases were below 1 year of age. Lastly, a nationwide surveillance of 1,568 RSV infections from 14 German pediatric hospitals revealed that patients with neuromuscular impairment were at much higher risks for PICU admissions, mechanical ventilation, and mortality [15]. Our data also suggested neurodevelopmental conditions to be a risk factor for RSV-associated mortality, although such an association was not confirmed following multivariate analysis. Overall, these observations strongly imply that the decision algorithm of RSV prophylaxis should not be restricted only to high-risk patients, as defined by prematurity and premorbid cardiopulmonary diseases.
Regarding the timing of starting RSV prophylaxis, the American guideline recommended to initiate palivizumab during the peak RSV season between November and March in the temperate climates of North America [23]. Substantial variation in the timing of community outbreaks of RSV disease from year to year exists within and between communities in the same year, even in the same region. The overall pattern of RSV outbreaks usually begins in November or December, peaks in January or February, and ends by the end of March or some time in April. The RSV-associated PICU admissions in Hong Kong (Fig. 1) appeared to follow a similar seasonality pattern. Nonetheless, RSV seasonality patterns vary and may occur throughout the year in equatorial countries. Among Asian countries, a seasonal peak was observed for RSV in September–December in Malaysia and Korea [24, 25]. RSV was detected most frequently in the fall and winter, but continued at a lower level throughout the year in India [26], whereas RSV infections started to increase in the early winter and declined in the spring in Japan [27]. There are inconsistent data on RSV seasonality in Asian countries. A previous single-center PICU study of RSV cases in Hong Kong failed to identify any definite seasonality [5], whereas a Taiwanese study found a biennial pattern of RSV-related hospitalization which peaked in the spring and fall [8].
This study has a number of limitations. Firstly, this retrospective study found bacteria in blood or tracheal aspirate in 14 (11.9 %) episodes of PICU admissions. Bacterial co-infection was present in three of our eight death cases (Table 3). Two recent studies in Asia also reported bacterial co-infections to be associated with severe RSV infections [11, 28]. The presence of bacteria in tracheal aspirate might indicate true infection or colonization, but infection biomarkers such as peripheral leucocyte count, lactate dehydrogenase, and C-reactive protein, which were associated with severe RSV infection [16], were not recorded in the case notes from most of our patients. This territory-wide surveillance of severe RSV infections relied on the retrospective collection of patient data. Our study was, thus, liable to recall and sampling biases. Although our coordinators tried their best to identify eligible PICU cases through different databases, we could not exclude the possibility of missing patients from any of the eight participating units. Besides, this multicenter study cannot evaluate the relationship between RSV-associated mortality and RSV viral load and genotypes because most hospitals did not archive respiratory samples or RSV isolates for future research purposes. In addition, our group, as well as many others, reported a number of emerging respiratory viruses such as human coronaviruses, metapneumovirus, and rhinoviruses to be important causes of severe respiratory tract infections in young children [29–33]. Patients co-infected with RSV and another virus might have more severe disease, but the present study did not investigate possible co-infections in our PICU patients.
In conclusion, most Hong Kong children hospitalized for RSV infection did not need PICU admission. The majority of these admissions occurred during the winter months. Prior sick contact appears to be a risk factor for RSV-associated mortality, whereas chronic lung disease was the major determinant for the duration of PICU stay.
Acknowledgments
The full list of members (hospitals) of the Hong Kong Society of Paediatric Respirology (HKSPR) RSV Concern Group in alphabetical order is as follows: P.Y. Chow and D.K.K. Ng (Kwong Wah Hospital), S.W. Ku and T. Leung (Pamela Youde Nethersole Eastern Hospital), K.L. Hon, A.S.Y. Leung, and T.F. Leung (Prince of Wales Hospital), Q.U. Lee and R.S.Y. Lee (Princess Margaret Hospital), T.Y. Miu (Queen Elizabeth Hospital), C.S.K. Chau, S.S.S. Chiu, and N.S. Tsoi (Queen Mary Hospital), D.S.Y. Lam and T.K.F. Lau (Tuen Mun Hospital), and W.K. Chiu (United Christian Hospital). Funding There was no pharmaceutical financial support or research grant for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Full members of the Hong Kong Society of Paediatric Respirology (HKSPR) RSV Concern Group are listed in the “Acknowledgment” at the end of this manuscript.
References
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger TM, Aebi C, Duppenthaler A, Stocker M, Swiss Pediatric Surveillance Unit Prospective population-based study of RSV-related intermediate care and intensive care unit admissions in Switzerland over a 4-year period (2001–2005) Infection. 2009;37:109–116. doi: 10.1007/s15010-008-8130-z. [DOI] [PubMed] [Google Scholar]
- 3.Soilly AL, Ferdynus C, Desplanches O, Grimaldi M, Gouyon JB. Paediatric intensive care admissions for respiratory syncytial virus bronchiolitis in France: results of a retrospective survey and evaluation of the validity of a medical information system programme. Epidemiol Infect. 2012;140:608–616. doi: 10.1017/S0950268811001208. [DOI] [PubMed] [Google Scholar]
- 4.Hon KL, Leung E, Tang J, Chow CM, Leung TF, Cheung KL, Ng PC. Premorbid factors and outcome associated with respiratory virus infections in a pediatric intensive care unit. Pediatr Pulmonol. 2008;43:275–280. doi: 10.1002/ppul.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hon KL, Leung TF, Cheng WY, Ko NM, Tang WK, Wong WW, Yeung WH, Chan PK. Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis. J Crit Care. 2012;27:464–468. doi: 10.1016/j.jcrc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Morris SK, Dzolganovski B, Beyene J, Sung L. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect Dis. 2009;9:106. doi: 10.1186/1471-2334-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.[No authors listed] Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 8.Lee JT, Chang LY, Wang LC, Kao CL, Shao PL, Lu CY, Lee PI, Chen JM, Lee CY, Huang LM. Epidemiology of respiratory syncytial virus infection in northern Taiwan, 2001–2005—seasonality, clinical characteristics, and disease burden. J Microbiol Immunol Infect. 2007;40:293–301. [PubMed] [Google Scholar]
- 9.Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child. 2009;94:99–103. doi: 10.1136/adc.2008.139188. [DOI] [PubMed] [Google Scholar]
- 10.Medrano López C, García-Guereta L, CIVIC Study Group Community-acquired respiratory infections in young children with congenital heart diseases in the palivizumab era: the Spanish 4-season civic epidemiologic study. Pediatr Infect Dis J. 2010;29:1077–1082. doi: 10.1097/INF.0b013e3181efdac5. [DOI] [PubMed] [Google Scholar]
- 11.Ito H, Osamura T, Nakajima F, Fujiwara D, Kuwabara Y, Yamamoto T, Yasuno T, Komatsu H, Kizaki Z, Kishida K, Akiyama Y, Oomae T, Nakajima K, Nakamura A, Kiyosawa N, Nisikomori R. Survey of severe respiratory syncytial virus infection in Kyoto Prefecture from 2003 to 2007. Pediatr Int. 2010;52:273–278. doi: 10.1111/j.1442-200X.2009.02962.x. [DOI] [PubMed] [Google Scholar]
- 12.Papenburg J, Hamelin MÈ, Ouhoummane N, Carbonneau J, Ouakki M, Raymond F, Robitaille L, Corbeil J, Caouette G, Frenette L, De Serres G, Boivin G. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purcell K, Fergie J. Driscoll Children’s Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J. 2004;23:418–423. doi: 10.1097/01.inf.0000126273.27123.33. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham SC, Quasney MW, Bush AJ, DeVincenzo JP. Respiratory syncytial virus infections in the pediatric intensive care unit: clinical characteristics and risk factors for adverse outcomes. Pediatr Crit Care Med. 2001;2:318–323. doi: 10.1097/00130478-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wilkesmann A, Ammann RA, Schildgen O, Eis-Hübinger AM, Müller A, Seidenberg J, Stephan V, Rieger C, Herting E, Wygold T, Hornschuh F, Groothuis JR, Simon A, DSM RSV Ped Study Group Hospitalized children with respiratory syncytial virus infection and neuromuscular impairment face an increased risk of a complicated course. Pediatr Infect Dis J. 2007;26:485–491. doi: 10.1097/INF.0b013e31805d01e3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Guo Z, Bai Z, MacDonald NE. A 4 year prospective study to determine risk factors for severe community acquired pneumonia in children in southern China. Pediatr Pulmonol. 2013;48:390–397. doi: 10.1002/ppul.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Committee on Infectious Diseases Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 20.Butt ML, Symington A, Janes M, Elliott L, Steele S, Paes BA. The impact of prophylaxis on paediatric intensive care unit admissions for RSV infection: a retrospective, single-centre study. Eur J Pediatr. 2011;170:907–913. doi: 10.1007/s00431-010-1376-3. [DOI] [PubMed] [Google Scholar]
- 21.Prais D, Danino D, Schonfeld T, Amir J. Impact of palivizumab on admission to the ICU for respiratory syncytial virus bronchiolitis: a national survey. Chest. 2005;128:2765–2771. doi: 10.1378/chest.128.4.2765. [DOI] [PubMed] [Google Scholar]
- 22.Prais D, Schonfeld T, Amir J, Israeli Respiratory Syncytial Virus Monitoring Group Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics. 2003;112:548–552. doi: 10.1542/peds.112.3.548. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Respiratory syncytial virus activity—United States, July 2007–December 2008. MMWR Morb Mortal Wkly Rep. 2008;57:1355–1358. [PubMed] [Google Scholar]
- 24.Khor CS, Sam IC, Hooi PS, Quek KF, Chan YF. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of 27 years. BMC Pediatr. 2012;12:32. doi: 10.1186/1471-2431-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CK, Choi J, Callaway Z, Kim HB, Chung JY, Koh YY, Shin BM. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in Seoul, Korea, 2003–2008. J Korean Med Sci. 2010;25:342–347. doi: 10.3346/jkms.2010.25.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broor S, Parveen S, Bharaj P, Prasad VS, Srinivasulu KN, Sumanth KM, Kapoor SK, Fowler K, Sullender WM. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato M, Saito R, Sakai T, Sano Y, Nishikawa M, Sasaki A, Shobugawa Y, Gejyo F, Suzuki H. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J Clin Microbiol. 2005;43:36–40. doi: 10.1128/JCM.43.1.36-40.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki A, Lupisan S, Furuse Y, Fuji N, Saito M, Tamaki R, Galang H, Sombrero L, Mondoy M, Aniceto R, Olveda R, Oshitani H. Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect Dis. 2012;12:267. doi: 10.1186/1471-2334-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung TF, To MY, Yeung AC, Wong YS, Wong GW, Chan PK. Multiplex molecular detection of respiratory pathogens in children with asthma exacerbation. Chest. 2010;137:348–354. doi: 10.1378/chest.09-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee W-M, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung TF, Li CY, Lam WY, Wong GW, Cheuk E, Ip M, Ng PC, Chan PK. Epidemiology and clinical presentations of human coronavirus NL63 infections in Hong Kong children. J Clin Microbiol. 2009;47:3486–3492. doi: 10.1128/JCM.00832-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peiris JS, Tang WH, Chan KH, Khong PL, Guan Y, Lau YL, Chiu SS. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak RK, Tse LY, Lam WY, Wong GW, Chan PK, Leung TF. Clinical spectrum of human rhinovirus infections in hospitalized Hong Kong children. Pediatr Infect Dis J. 2011;30:749–753. doi: 10.1097/INF.0b013e31821b8c71. [DOI] [PubMed] [Google Scholar]


