Abstract
Background
This study examined the effects of abdominal draw-in lumbar stabilization exercises (ADIM) with respiratory resistance on women ages 40–49 years with low back pain.
Material/Methods
Forty-four women ages 40–49 years were screened for participation and were randomly assigned to either a respiratory with resistance exercise group (n=22) or a control group (n=22). Abdominal draw-in lumbar stabilization exercises were administered to both groups, but only the respiratory with resistance exercise group received the respiratory resistance training. The exercise training lasted 50 min per session, 3 sessions per week for 4 weeks. The assessment methods used were the quadruple visual analogue scale (QVAS), Oswestry disability index-Korean version (ODI-K), diaphragm thickness and contraction rate, and lung capacity test.
Results
Both groups showed significant differences in the QVAS, ODI-K, maximum voluntary ventilation (MVV), and diaphragm thickness and contraction rate before and after the intervention (p<0.05). In the respiratory resistance exercise group, the ODI-K, forced vital capacity (FVC), forced expiratory volume in one second (FEV1), MVV, and diaphragm thickness and contraction rate showed significantly better improvement than the control group (p<0.05).
Conclusions
A lumbar stabilization exercise program consisting of ADIM and respiratory resistance resulted in decreased pain, reduced dysfunctions, and increased muscle thickness in contraction, contraction rate, and pulmonary function. Strong contraction of the diaphragm and deep abdominal muscles through breathing resistance increased the pressure in the abdominal cavity. Therefore, this may be an effective clinical exercise method for patients with lumbar instability.
MeSH Keywords: Airway Resistance, Diaphragm, Low Back Pain, Patient Care, Respiration, Respiratory Function Tests
Background
Low back pain (LBP) is a musculoskeletal disorder experienced by 90% of adults at least once in their lifetime. Severe LBP affects the ability to perform everyday activities of daily living of patients and imposes psychological, economic, and social burdens [1]. Unbalanced muscle patterns in patients with low back pain can cause motility in one joint and excessive stress or hypermotility in the other, resulting in instability if not appropriately maintained [2].
An unstable lumbar spine, along with weakening of muscle strength, leads to structural inconsistencies and imbalances, causing persistent irritation and stress on the lumbar region [3]. This will result in difficulties in functional motor control abilities, such as walking or sitting, as well as physical and psychological problems [4–6].
Stabilization exercise of the lumbar spine is an effective intervention for back pain patients with spinal instability by increasing the abdominal pressure through mutual activation of the pelvic floor, diaphragm, and abdominal muscles and reducing the pressure on the lumbar spine [7–10]. Recently, the effects of exercise methods on the regulation and stability of the lumbar segments and strengthening of the interbody deep muscles have been reported [11,12]. Among the methods, the abdominal drawing-in maneuver (ADIM) decreases the excessive lumbar lordosis or anterior tilting of the pelvis through abdominal hollowing [13] and simultaneously induces selective contraction of the diaphragm and transversus abdominis to contribute to lumbar stabilization [14].
During stabilization exercise, coordinated contraction of primary respiratory muscles and synergist muscles have been emphasized in inhalation and exhalation [15]. In particular, in the case of adding resistance to air flow during inhalation and exhalation, the respiratory muscles and deep muscles, such as the internal obliques, multifidus, and pelvic floor muscles, are under resistance, resulting in postural alignment and structural stabilization [15–18].
ADIM is undoubtedly an effective exercise method [19]. In addition, accurate timing of respiration and appropriate respiratory resistance may relieve much of the pain in patients with LBP. Barton and Kellie [20] highlighted the need for ongoing research to strengthen the quality of evidence supporting respiratory interventions for chronic nonspecific low back pain, including randomized controlled trials examining the differences between the type, frequency, and duration of respiratory movements. Therefore, the present study administered ADIM to female patients ages 40–49 years and investigated the effects of respiratory resistance on pain level, dysfunction level, lung capacity, and diaphragm contraction rate.
Material and Methods
Participants
This study recruited 60 female LBP patients ages 40–49 years who were admitted to P hospital in D city, Korea. The inclusion criteria were patients who had experienced LBP at least within the past 6 weeks, had a score of 3 or higher in the quadruple visual analogue scale (QVAS) and 3 or more positives among the 5 lumbar instability tests [21], and had no history of back surgery. The exclusion criteria were patients who had difficulties in motor performance due to pain, participated in less than 85% of the sessions, and had systemic and respiratory diseases such as cancer. All participants understood the purpose and process of the study, and they all confirmed their voluntary participation in the study. The Ethics Committee of Daejeon University approved the study, which was registered in the International Clinical Trials Registry Platform (KCI0004372).
Procedures
This research was a single-blinded, randomized, control trial study. G-power 3.19 software was used to calculate the sample size. The effect size was set at 0.8 based on a previous study [22]. The significance level was set to α=0.05 and power (1–β)=0.8, resulting in a minimum of 15 participants per group [22].
A lumbar instability test was given as a screening test to 60 participants. This consists of 5 separate tests: (1) Prone instability test – If the pain appears when the examiner applies manual compression on the lumbar segments of the participants, continue the test. After the participant lifts both legs, the examiner applies manual compression to the lumbar segment. The test is positive when the pain disappears. (2) Passive lumbar extension test – Pain occurring when both legs are lifted in the prone position and pain relief when the legs are returned to the starting position is indicated as positive. (3) Posterior-anterior mobility test – Positive if the lumbar spine moves excessively or abnormally during spinal compression. (4) Straight leg raising test – Bends the hip joint to the maximum and the average of the left and right moving range exceeds 90 degrees, and mean flexed on both sides is indicated as positive. (5) Under 40 years of age – Patients were classified as unstable lumbar when 3 or more test results were positive [21].
After the lumbar instability screening tests, were excluded 14 patients who had positive results in 2 or fewer tests (n=10) and a score of 3 or lower in QVAS (n=4) [23]. To compare the effects of the interventions, 46 participants were randomly assigned to the respiratory with resistance exercise group (n=23) and control group (n = 23) using a random-number generation program [24]. The participants were not given information about the group that they had been assigned to. The respiratory with resistance exercise group received a lumbar stabilization exercise program that consisted of respiratory resistance along with ADIM, and the control group received the same program without respiratory resistance. All interventions were provided 50 min a session, 3 sessions a week, for 4 weeks. The QVAS, Oswestry disability index-Korean version (ODI-K), pulmonary function test (PFT), and diaphragm thickness ratio during respiration were compared within the groups for intervention methods, and the differences in the changed values between the groups were compared. One participant from each group dropped out due to discharge from the hospital (n=2), leading to statistical analysis using the data of 22 participants from the respiratory with resistance exercise group and 22 participants from the control group.
Intervention
Abdominal draw-in maneuver
All participants received ADIM with a lumbar stabilization exercise program. ADIM is an important respiratory exercise method that selectively contracts the diaphragm and transversus abdominis among the abdominal wall muscles to increase the abdominal pressure for stabilization [25]. In supine position, the participant inhales through the mouth for 5 s to expand the abdomen to the maximal level, minimizing movement of the thorax, and performs a long exhalation to maintain a lower abdominal contraction and retroversion of the pelvis [26]. Stabilizer pressure biofeedback (Chattanooga, USA) was used to maintain the same pelvic retroversion and exhalation intensity to 40 mmHg. All interventions were controlled by 2 physical therapists with more than 3 years of experience.
Lumbar spine stabilization exercise program
A lumbar stabilization program was provided to both groups after modifying the intervention method of Boucher et al. [27]. Through selective contraction of the abdominal muscles and strengthening of the surrounding muscles, this exercise program induces stabilization by appropriately controlling the outside pressure, and it consists of the following exercises: curl up, dead bug, bridge, bird dog, and side flank with knee flexion. Before and after the intervention, 5 min of stretching to warm up and cool down was performed, and 3 by 5 sets with each lasting for 20 s were performed for each exercise program. Between the sets, a 1-min break was allowed. The program lasted for 4 weeks, 3 sessions per week, and 50 min each session.
Lumbar stabilization exercise with respiratory resistance
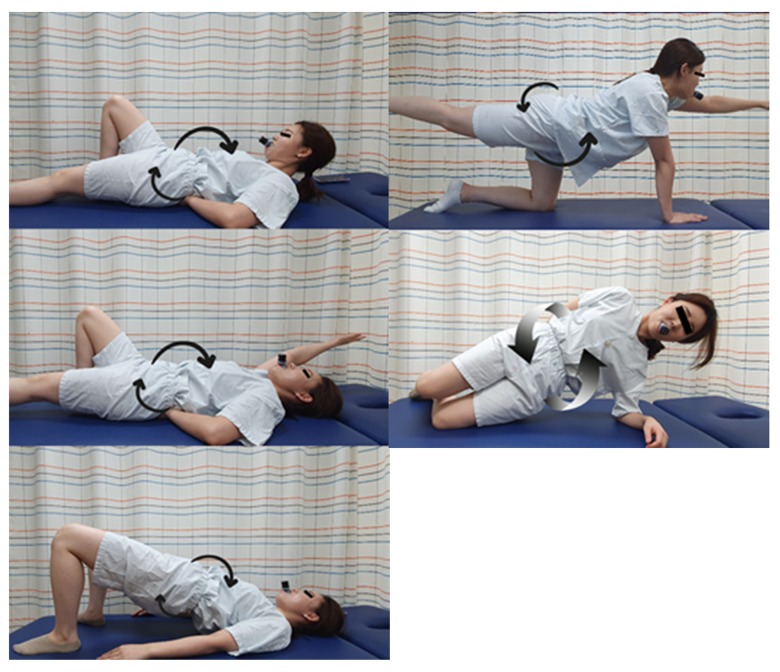
The respiratory with resistance exercise group performed lumbar stabilization exercises with respiratory resistance training. Before the intervention, the participants received training in respiratory resistance. The participants were provided with sufficient education on how to perform ADIM. Respiratory resistance (Expand a lung, Miami, USA) allows control of ventilation during inhalation and exhalation to strengthen respiratory muscles by resisting the flow of air. When using respiratory resistance during the intervention, resistance was controlled to maintain the scale of the perceived force (RPE) of the participants to less than 14 [28]. The participants were educated about dizziness and dyspnea that may occur during the intervention and pre-trained to stop the intervention immediately in the event of an abnormal situation. Figure 1 illustrates the lumbar stabilization exercise program with respiratory resistance.
Figure 1.
Lumbar stabilization exercise with respiratory resistance. Curl up; dead bug; bridge; bird dog; side flank.
Outcome measures
Quadruple visual analogue scale
The pain level of the participants was assessed before and after the intervention using a 4-item QVAS. This assessment consists of questions on 4 items, with each item composed of: (1) current pain level, (2) mean level of current pain, (3) pain level at the mildest, and (4) pain level at the most severe. Each question has a score ranging from zero (no pain) to 10 (worst pain). The reliability is high (r=.76~.84) [23].
Oswestry Disability Index-Korean version
The ODI-K suggested by Kim et al. (2005) [29] was used to assess the dysfunction level due to LBP. This assessment consists of 9 items: pain level, personal hygiene, lifting objects, walking, sitting, standing, sleeping, social participation, travel, and mobility. The scores range from zero to 5, and the highest possible score is 45. The reliability of ODI-K is high (r=.92) [29].
Diaphragm thickness
The diaphragm thickness was assessed using ultrasound while performing ADIM and maintaining pelvic retroversion in a knee-bent supine position. The maximum inhalation and exhalation were performed when measuring the thickness in millimeters after being in contact with a 3.5 MHz linear convex transducer in a vertical position with the mid-axillary line between rib bones 8 and 9 (Figure 2). The distance between the 2 parallel lines of the mid-pleura and the light-colored peritoneum was measured 3 times during contraction and relaxation to record the mean value, and the contraction thickness was divided by the relaxed thickness to calculate the contraction rate [30]. A physical therapist with 3 years of clinical experience and proficient in use of the diaphragm thickness test performed the measurement using ultrasound. The measurements of the diaphragm thickness using ultrasound showed high inter-rater reliability (r=.99) [31].
Figure 2.
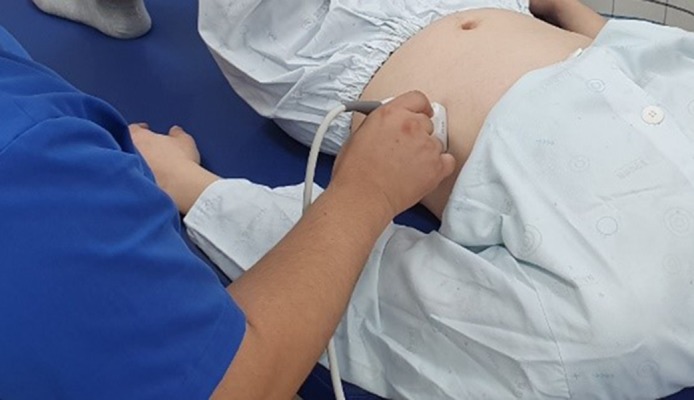
Diaphragm thickness measurement using ultrasound.
Pulmonary function test
Lung capacity measurement was performed using Microquark (COSMED, Rome, Italy). In an upright standing position and with the nose closed with the mouthpiece held in the mouth, the participant performed the maximal inhalation followed by a quick and strong exhalation. The exhalation was measured for the forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and forced expiratory volume in one second/forced vital capacity (FEV1%), and the maximum voluntary ventilation (MVV) was measured. For accurate measurements, the participants were given full explanations with a demonstration, and an experienced physical therapist made the assessment.
Statistical analysis
All data collected in this study were analyzed using the Statistical Package for the Social Sciences ver. 25.0 (SPSS Inc., Chicago, IL, USA). The general characteristics of the participants are reported as the mean and standard deviation through descriptive statistics. A Schapiro-Wilk test was used for the normality test. The homogeneity between the groups was confirmed using a chi-square test and independent-samples t test. Paired-samples t tests were performed to compare the dependent variables within the groups before and after the intervention. Repeated-measures ANOVA was used to identify the effects of the time-dependent changes and time*group interactions. The statistical significance level (α) was set to.05.
Results
Data from 22 subjects in the experimental group and 22 subjects in the control group were collected, but 1 patient was discharged early from the respiratory with the resistance exercise group and 1 patient was discharged early from the control group. The general characteristics of the participants between the groups before the intervention were homogenous (Table 1). In both groups, the previous values of the dependent variables were homogenous. Significant differences in QVAS, KODI, contraction thickness, and contraction rate before and after the intervention were observed (p<.05). In addition, the experimental group showed significant differences in KODI, muscle thickness at contraction, and shrinkage compared to the control group (p<.05) (Table 2). In pulmonary function, FVC, FEV1, and MVV showed significant differences in the experimental group (p<.05), but there were only significant differences in the MVV in the control group (p<.05). In addition, the experimental group showed a significant difference in the FVC, FEV1, and MVV compared to the control group (p<.05) (Table 3).
Table 1.
General characteristics.
| Respiratory with resistance exercise group (n=22) | Control group (n=22) | t/χ2 | p | |
|---|---|---|---|---|
| Age (years) | 46.14±2.59* | 44.45±2.54 | 0.882 | 0.383 |
| Height (cm) | 159.95±2.82 | 159.91±4.67 | 0.039 | 0.969 |
| Weight (kg) | 57.05±4.66 | 56.18±4.99 | 0.593 | 0.556 |
| BMI (kg/m2) | 22.24±1.58 | 21.96±1.55 | 0.595 | 0.555 |
| Onset (months) | 16.91±3.50 | 16.82±3.05 | 0.092 | 0.927 |
Mean±standard deviation;
BMI – body mass index.
Table 2.
Comparison of before and after the intervention between the 2 groups.
| Respiratory with resistance exercise group (n=22) | Control group (n=22) | t(p) | Time F(p) |
Time*group F(p) |
||
|---|---|---|---|---|---|---|
| QVAS (point) | Pre | 6.44±0.42# | 6.40±0.47 | −0.645 (.523) | 394.827 (.000) | 0.792 (.379) |
| Post | 4.58±0.46 | 4.45±0.42 | ||||
| Post-pre | −1.94±0.69 | −1.86±0.48 | −1.090 (.288) | |||
| t(p) | −18.206 (.000) | −13.214 (.000) | ||||
| ODI-K (score) | Pre | 17.32±5.90# | 16.09±4.24 | 0.792 (.433) | 122.536 (.000) | 5.393 (.025 |
| Post | 9.45±3.92 | 10.95±4.36 | ||||
| Post-pre | −7.86±3.77 | −5.13±4.02 | 2.295 (.032) | |||
| t(p) | −9.782 (.000) | −6.000 (.000) | ||||
| Diaphragm thickness expiration (mm) | Pre | 0.24±0.02# | 0.25±0.02 | 0.843 (.155) | 0.036 (.850 | 0.904 (.347) |
| Post | 0.24±0.02 | 0.25±0.02 | ||||
| Post-pre | 0.00±0.01 | −0.00±0.01 | −1.000 (.329) | |||
| t(p) | 0.826 (.418) | −0.526 (.605) | ||||
| Diaphragm thickness inspiration (mm) | Pre | 0.29±0.02 | 0.30±0.02 | 0.991 (.108) | 1644.876 (.000) | 379.672 (.000) |
| Post | 0.51±0.03 | 0.38±0.02 | ||||
| Post-pre | 0.22±0.03 | 0.08±0.02 | −23.103 (.000) | |||
| t(p) | 36.364 (.000) | 21.914 (.000) | ||||
| Thickness ratio (%) | Pre | 1.20±0.04 | 1.19±0.04 | 0.818 (.846) | 1215.410 (.000) | 280.366 (.000) |
| Post | 2.07±0.14 | 1.50±0.10 | ||||
| Post-pre | 0.88±0.13 | 0.31±0.09 | 19.356 (.000) | |||
| t(p) | 31.362 (.000) | 15.938 (.000) |
Mean±standard deviation;
QVAS – quadruple visual analogue scale; ODI-K – Korean version of Oswestry disability index.
Table 3.
Comparison of before and after the intervention between the groups.
| Respiratory with resistance exercise group (n=22) | Control group (n=22) | t(p) | Time F(p) |
Time*group F(p) |
||
|---|---|---|---|---|---|---|
| FVC(L) | Pre | 4.08±0.83 | 4.00±0.84 | 0.628 (.733) | 98.904 (.000) | 51.126 (.000) |
| Post | 4.51±0.83 | 4.07±0.83 | ||||
| Post-pre | 0.43±0.09 | 0.07±0.22 | −8.128 (.000) | |||
| t(p) | 23.218 (.000) | 1.503 (.148) | ||||
| FEV1(L) | Pre | 3.07±0.95 | 2.84±0.86 | 0.374 (.000) | 444.294 (.000) | 469.533 (.000) |
| Post | 3.40±0.94 | 2.83±0.86 | ||||
| Post-pre | 0.33±0.05 | −0.01±0.05 | −19.339 (.000) | |||
| t(p) | 29.325 (.000) | −.431 (.671) | ||||
| FEV1/FVC (%) | Pre | 87.50±9.73 | 83.09±13.68 | 0.130 (.223) | .059 (.809) | .015 (.904) |
| Post | 87.50±9.72 | 83.10±13.72 | ||||
| Post-pre | −0.01±0.19 | −0.01±0.29 | 0.138 (.892) | |||
| t(p) | −0.335 (.741) | −0.073 (.943) | ||||
| MVV(L) | Pre | 87.15±31.25 | 89.32±30.53 | 0.667 (.817) | 606.599 (.000) | 67.069 (.000) |
| Post | 121.69±29.42 | 117.62±27.4 | ||||
| Post-pre | 34.55±7.14 | 17.3±6.82 | −8.758 (.000) | |||
| t(p) | 22.685 (.000) | 11.905 (.000) |
FVC – forced vital capacity; FEV1 – forced expiratory volume in 1 second; FEV1/FVC – forced expiratory volume in 1 second/forced vital capacity; MVV – maximum voluntary ventilation in 1 minute.
Discussion
This study compared the effects of the lumbar stabilization exercise program with and without respiratory resistance in female LBP patients in their 40s. We found that the stabilization program with respiratory resistance effectively decreased the low back pain and increased the lung capacity and diaphragm contraction rate.
The QVAS of the respiratory with resistance exercise and control groups showed a mean of 6.44 and 6.39, respectively, resulting in a moderate level of pain. The effects of various lumbar stabilization exercise programs for pain and dysfunction improvements have been reported in various studies [12]. Park et al. (2018) [32] provided a stabilization exercise program using a sling for 12 weeks to chronic LBP patients and found a significant decrease in the pain level (p<0.5, effect size, d=4.54). Lee et al. (2015) [33] provided a stabilization program that applied abdominal expansion maneuver (AEM) for 4 weeks, resulting in a significant decrease in pain (p<0.5, effect size, d=2.64). This study demonstrated a significant decrease in pain after administering 4 weeks of a lumbar stabilization exercise program with ADIM (effect size, d=4.37). The group that only received ADIM also showed a significant decrease in pain (effect size, d=4.22), but the difference in change in pain between the groups was not significant (p>.05, effect size d=0.29).
Lumbar pain causes difficulties performing activities of daily living, leading to poor quality of life and dysfunction [15]. In addition to the pain level, the lumbar dysfunction was assessed with ODI-K. Due to the nature of Korean culture, sensitive questions, including those about sexual activities, were excluded to make the maximum score of 45. When conversions were made with the scores, the respiratory with resistance exercise group showed a significant change in dysfunction (from 38.48% to 21%) and the control group also showed a significant change (from 35.75% to 34.33%) (p<0.05). In particular, the respiratory with resistance exercise group showed a change from severe dysfunction before the intervention to minimum dysfunction after the intervention, which is a more statistically significant increase compared to that in the control group (p<0.05).
Patients with lumbar instability have increased lordosis and weakened abdominals, leading to LBP. To improve the structural stability, ADIM methods that induce abdominal muscles and deep pelvic floor muscle strengthening have been applied in a range of clinical settings. In the present study, participants performed stabilization exercises that included curl up, dead bug, bridge, bird dog, and side flank with knee flexion to all participants, resulting in decreased pain and dysfunction levels. In the respiratory with resistance exercise group, respiratory resistance was added to ADIM. The resistance in airflow during respiration induced forced inhale and exhale, and at the same time the diaphragm and deep abdominals had strong contractions. Strong contractions of the abdominal muscles increased the intra-abdominal pressure, which affected the lumbar area to contribute to a decrease in lordotic curve. The pressure applied in a vertical direction decreased markedly, and it assisted in proper postural control. This may have contributed to the improvement of lumbar dysfunction.
To examine the effects of respiratory resistance along with a lumbar stabilization exercise program, the changes in diaphragm contraction rate and lung capacity were explored. The diaphragm maintains a dome shape in relaxation and contracts downwards to a flat position in inhalation. Ultrasound equipment is useful for measuring the diaphragm by a non-invasive method [34]. The location that could be detected most accurately was the midpoint of the rib bones between 8 and 9, and the mid-axillary line is the point of the transverse plane of the diaphragm. During inhalation in the intervention, the thickness of the diaphragm was increased by 75% in the respiratory with resistance exercise group and by 26% in the control group (p<0.05), and the respiratory with resistance exercise group showed greater improvement than the control group (p<0.05). During exhalation, the diaphragm thickness proportion increased by 88% in the respiratory with resistance exercise and by 31% in the control group, and the respiratory with resistance exercise group showed a more significant change.
Respiratory resistance performed by the respiratory with resistance exercise group increased the load on the diaphragm and the synergist muscles, and it contributed to improved muscle strength and endurance [16–18]. This also affected the increase in lung capacity in the participants. In particular, the FVC, FEV1, and MVV values of the respiratory with resistance exercise group increased significantly before and after the intervention (p<0.05), and it was significantly different from the control group (p<0.05).
The respiratory resistance that was applied in this study was provided based on the principle of particularity, the principle of overload, and the principle of reversibility. The expansion ability of the lung due to diaphragm strengthening in inhalation and strengthening of a contraction on the abdominals in exhalation might have been due to the increase in forced exhalation. The improvement of these 2 functions may have affected the improvement of MVV. Therefore, the stabilization exercise program with respiratory resistance stabilized the lumbar region and also strengthened the diaphragm to increase the expansion ability, thus enhancing lung functions. These results are in line with reports by Clini et al. (2006) [35] and Park et al. (2019) [15], who demonstrated changes in ventilation and lung capacity achieved by respiratory resistance training.
The present study examined the effects of respiratory resistance with the existing exercise program to decrease pain and increase function in LBP patients. This study may have methodological value because it explored the change in muscles that contribute to respiration by measuring the diaphragm contraction rate and lung capacity using ultrasound. In conclusion, our study showed that respiration is just as important as accurate exercise movement for LBP patients. While conducting this study, there were some difficulties in applying accurate movements, muscle contraction, and respiratory resistance. Due to experiencing pain and unfamiliarity with the movements, the participants required 1–2 days of prior practice to maintain the draw-in of the abdominals and to perform the stabilization exercise program. In addition, breathing simultaneously with both the nose and mouth with the resistance yielded inaccurate respiration resistance. Therefore, the participants were required to breathe through the mouth. This study had some limitations. First, the hand pressure and direction of the ultrasonic probe was not the same during the measurement using ultrasound. Secondly, the participants were all females ages 40–49 years. Hence, the study results cannot be generalized to males of the same age group or to different age groups. The third limitation is that the study only investigated changes in diaphragm thickness and pulmonary functions; the abdominal contraction ability or maximal inspiratory pressure and maximal expiratory pressure were not assessed. Fourth, the participants were patients admitted to a hospital, making it difficult to control the social participation, physical activity, and medical treatments. Finally, the psychological characteristics of the outcome measures could not be assessed. Future studies will need to compensate for these limitations and modify the difficulty levels of respiratory resistance training, as well as to encourage interest in the stabilization exercise program to effectively increase respiratory functions and decrease LBP.
Conclusions
The study examined the results of ADIM respiratory resistance movement in patients with lumbar pain ages 40–49 years. The pain decreased, with a noticeable improvement in the diaphragm thickness of contraction and breathing function. A strong contraction of the diaphragm and abdominal deep muscles through respiratory resistance is believed to increase the pressure in the abdominal cavity. In particular, the pressure on the lumbar spine was reduced in the vertical direction, making postural adjustment easier. The short application period (4 weeks) and the lack of complete control over treatment are limitations of this study. With these results, ADIM with respiratory resistance can be proposed as a positive exercise program for female LBP patients ages 40–49 years. Nevertheless, more research is needed to explain the effects of respiratory resistance and lumbar stabilization exercise programs and relevant psychometric properties.
Footnotes
Source of support: Departmental sources
References
- 1.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–71. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Edmondston S, Singer K. Thoracic spine: Anatomical and biomechanical considerations for manual therapy. Man Ther. 1997;2:132–43. doi: 10.1054/math.1997.0293. [DOI] [PubMed] [Google Scholar]
- 3.Lee H-O. Activation of trunk muscles during stabilization exercises in four-point kneeling. The Journal of Korean Physical Therapy. 2010;22:33–38. [Google Scholar]
- 4.Comerford MJ, Mottram SL. Movement and stability dysfunction – contemporary developments. Man Ther. 2001;6:15–26. doi: 10.1054/math.2000.0388. [DOI] [PubMed] [Google Scholar]
- 5.Mcconnell J. Recalcitrant chronic low back and leg pain – a new theory and different approach to management. Man Ther. 2002;7:183–92. doi: 10.1054/math.2002.0478. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JM, Cleland JA, Speckman M, et al. Physical therapy for acute low back pain: Associations with subsequent healthcare costs. Spine. 2008;33:1800–5. doi: 10.1097/BRS.0b013e31817bd853. [DOI] [PubMed] [Google Scholar]
- 7.Neumann P, Gill V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(2):125–32. doi: 10.1007/s001920200027. [DOI] [PubMed] [Google Scholar]
- 8.Sapsford R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man Ther. 2004;9:3–12. doi: 10.1016/s1356-689x(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 9.Hodges PW, Eriksson AM, Shirley D, Gandevia SC. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38:1873–80. doi: 10.1016/j.jbiomech.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Loukas M, Shoja MM, Thurston T, et al. Anatomy and biomechanics of the vertebral aponeurosis part of the posterior layer of the thoracolumbar fascia. Surg Radiol Anat. 2008;30:125–29. doi: 10.1007/s00276-007-0291-4. [DOI] [PubMed] [Google Scholar]
- 11.Akuthota V, Nadler SF. Core strengthening. Archi Phys Med Rehabil. 2004;85:86–92. doi: 10.1053/j.apmr.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Behm DG, Drinkwater EJ, Willardson JM, Cowley PM. The use of instability to train the core musculature. Appl Physiol Nutr Metab. 2010;35:91–108. doi: 10.1139/H09-127. [DOI] [PubMed] [Google Scholar]
- 13.Kisner C, Colby LA. Therapeutic exercise: Foundations and techniques. 2002. [Google Scholar]
- 14.Beazell JR, Grindstaff TL, Hart JM, et al. Changes in lateral abdominal muscle thickness during an abdominal drawing-in maneuver in individuals with and without low back pain. Res Sports Med. 2011;19:271–82. doi: 10.1080/15438627.2011.608053. [DOI] [PubMed] [Google Scholar]
- 15.Park S-H, Lee M-M. Effects of a progressive stabilization exercise program using respiratory resistance for patients with lumbar instability: A randomized controlled trial. Med Sci Monit. 2019;25:1740–48. doi: 10.12659/MSM.913036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moodie L, Reeve J, Elkins M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: A systematic review. J Physiother. 2011;57:213–21. doi: 10.1016/S1836-9553(11)70051-0. [DOI] [PubMed] [Google Scholar]
- 17.Jo M-R, Kim N-S, Jung J-H. The effects of respiratory muscle training on respiratory function, respiratory muscle strength, and cough capacity in stroke patients. Journal of Korean Society of Physical Medicine. 2014;9:399–405. [Google Scholar]
- 18.Kim C-B, Choi J-D. Effects of chest expansion resistance exercise on chest expansion and maximal inspiratory pressure in patients with stroke. Journal of the Korean Society of Physical Medicine. 2015;10:15–21. [Google Scholar]
- 19.Macedo LG, Maher CG, Latimer J, Mcauley JH. Motor control exercise for persistent, nonspecific low back pain: A systematic review. Phys Ther. 2009;89:9–25. doi: 10.2522/ptj.20080103. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BE, Bliven KCH. The use of breathing exercises in the treatment of chronic, nonspecific low back pain. J Sport Rehabil. 2017;26:452–58. doi: 10.1123/jsr.2015-0199. [DOI] [PubMed] [Google Scholar]
- 21.Hicks GE, Fritz JM, Delitto A, Mishock J. Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84:1858–64. doi: 10.1016/s0003-9993(03)00365-4. [DOI] [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 23.Boonstra M, Malefijt MDW, Verdonschot N. How to quantify knee function after total knee arthroplasty? Knee. 2008;15:390–95. doi: 10.1016/j.knee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kisner C, Colby L. Therapeutic exercise: Foundations and techniques (Jang JH. Trans.) Yeong Mun Publishing Company. :2010. [Google Scholar]
- 26.Carolin K, Allen CL. Therapeutic exercise. Philadelphia: FA Davis; Company: 2007. pp. 231–50. [Google Scholar]
- 27.Boucher J-A, Preuss R, Henry SM, et al. The effects of an 8-week stabilization exercise program on lumbar movement sense in patients with low back pain. BMC Musculoskelet Disord. 2016;17:23. doi: 10.1186/s12891-016-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollander DB, Durand RJ, Trynicki JL, et al. RPE, pain, and physiological adjustment to concentric and eccentric contractions. Med Sci Sports Exerc. 2003;35:1017–25. doi: 10.1249/01.MSS.0000069749.13258.4E. [DOI] [PubMed] [Google Scholar]
- 29.Kim D-Y, Lee S-H, Lee H-Y, et al. Validation of the Korean version of the oswestry disability index. Spine. 2005;30:E123–27. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 30.Enright SJ, Unnithan VB, Heward C, et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther. 2006;86:345–54. [PubMed] [Google Scholar]
- 31.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Moon JH, Shin YA. Change of pain, lumbar sagittal alignment and multifidus after sling exercise therapy for patients with chronic low back pain. The Journal of Korean Physical Therapy. 2018;30:173–80. [Google Scholar]
- 33.Lee H-J, Kim S-Y. Comparison of the effects of abdominal draw-in and expansion maneuvers on trunk stabilization in patients with low back pain and lumbar spine instability. Physical Therapy Korea. 2015;22:37–48. [Google Scholar]
- 34.Hodges P, Pengel L, Herbert R, Gandevia S. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve. 2003;27:682–92. doi: 10.1002/mus.10375. [DOI] [PubMed] [Google Scholar]
- 35.Clini E, Costi S. Inspiratory muscle training: A way to breathe more easily. Respiration. 2006;73:143–44. doi: 10.1159/000091529. [DOI] [PubMed] [Google Scholar]