Opinion statement
Since the emergence of the 2003 severe acute respiratory syndrome (SARS), the 2003 reemergence of avian A/H5N1, the emergence of the 2009 pandemic influenza A/H1N1, the 2012 emergence of Middle East respiratory syndrome (MERS), the 2013 emergence of avian A/H7N9 and the 2014 Ebola virus outbreaks, the potential for the aerosol transmission of infectious agents is now routinely considered in the investigation of any outbreak. Although many organisms have traditionally been considered to be transmitted by only one route (e.g. direct/indirect contact and/or faecal-orally), it is now apparent that the aerosol transmission route is also possible and opportunistic, depending on any potentially aerosol-generating procedures, the severity of illness and the degree and duration of pathogen-shedding in the infected patient, as well as the environment in which these activities are conducted.This article reviews the evidence and characteristics of some of the accepted (tuberculosis, measles, chickenpox, whooping cough) and some of the more opportunistic (influenza, Clostridium difficile, norovirus) aerosol-transmitted infectious agents and outlines methods of detecting and quantifying transmission.
Keywords: Aerosol, Airborne, Droplet, Droplet nuclei, Infection, Transmission, Infection control, Influenza, Measles, Varicella, Chickenpox, Tuberculosis, Smallpox, SARS, Whooping cough, Bordetella pertussis, Clostridium difficile, Norovirus, Anthrax, Botulism, Tularaemia, Viral hemorrhagic fevers, Plague, Bioterrorism
Introduction
The transmission of infectious agents in human populations has been occurring in almost every conceivable situation since records began. Transmission of infectious agents can take place in the home, at work, on public transport or in crowded entertainment or shopping venues. Analysing and understanding the transmission of infection in a practical way may allow better interventions and prevention to be achieved.
In the context of public venues and transportation, the potential for the transmission of infectious agents has been investigated and documented by a number of researchers—with extensive studies being performed particularly during the Cold War era when several nations had covert biological weapons development programmes involving weaponised versions of anthrax, smallpox and tularaemia [1–3]. In the USA, military mock attacks were even staged for research purposes with what were then considered to be harmless bacteria, such as Serratia marcescens, without public knowledge—but which resulted in death in some of the individuals exposed [2, 4]. Despite the end of the Cold War many years ago and the 2001 terrorist attacks in the USA, such studies on the risk of infectious disease transmission on public transport have been revived in order to prepare for possible bioterrorist attacks; experiments have been conducted by the Lawrence Berkeley National Laboratory to test the dispersion of contaminants in Boston subway stations as part of the ongoing national bioterrorism preparedness [5].
Even in a civilian context, concerns about the potential for the transmission of airborne agents on transportation, particularly aircraft, have led to various investigations on specific pathogens, such as influenza [6] and, more recently, severe acute respiratory syndrome-associated coronavirus (SARS-CoV) [7], measles [8, 9], multiply-drug-resistant tuberculosis [10–12] and the 2009 pandemic influenza virus, A/H1N1p [13, 14]. Indeed, air travel is particularly problematic, as this allows infectious agents to be transported across the globe overnight [15–18]. Such concerns have led to more detailed studies being performed on assessing the risk of airborne infection within aircraft cabins from human exhalation airflows [19–21].
Specific examples in specific scenarios
Exploring this particular scenario of airborne transmission on an aircraft a little further will serve to illustrate how the various specialist disciplines can interact and cooperate to use their specific knowledge to understand the potential mechanisms of spread and thereby how best to implement preventative measures.
When a passenger infected with a respiratory pathogen coughs or sneezes on a passenger plane, who will be exposed to this potentially infectious aerosol? Just those passengers sitting immediately in front and perhaps those beside him/her? What about those sitting behind? What type of ventilation is present and how might this influence the path of such aerosols? And indeed, what does this aerosol consist of? Saliva and mucous? How many droplets of what sizes? What infectious agent could these droplets be carrying and how far could they spread? Will such infectious agents even survive in the airborne state for long enough to be transmitted to another passenger? Is the available, in-flight ventilation and filtration system sufficient to remove such infectious aerosols quickly enough and completely enough to prevent secondary transmission to other passengers? If not, will such a ventilation system at least be sufficient to dilute any such infectious aerosol to a harmless concentration? Would this dilution be sufficient to protect any immunocompromised passengers from acquiring a life-threatening infection?
Answers to such questions require the input and expertise from a multidisciplinary team of public health specialists, epidemiologists, microbiologists and engineers, as accurate travel and contact histories, a detailed understanding of the pathogen concerned and the making or estimating of the appropriate physical environment parameters, with or without additional modelling, are all required in order to understand how any transmission event may have occurred and how to intervene to prevent its reoccurrence in this context in the future. The necessity for a similar combination of microbiological and engineering expertise can be illustrated in a different context—that of airborne transmission within buildings.
The following describes two similar outbreak investigations, one on smallpox and one on severe acute respiratory syndrome (SARS). Although these investigations are separated by over 30 years, they ask very similar questions and use very similar principles, though the methods were obviously much more sophisticated in the more modern study.
Wehrle and colleagues [22] investigated an outbreak of smallpox in Meschede in the then Federal Republic of Germany. The index patient was isolated on the ground floor of a three-storey building, which was part of the local, large general hospital there. Once he was diagnosed with smallpox, he was moved to another hospital nearby that had been recently constructed, specifically, to house smallpox patients. However, after his relocation, secondary cases of smallpox started appearing within the same building in which he was initially housed at the hospital in Meschede. Out of a total of 17 first-generation contact cases, all were thought to have contracted smallpox from this index case, via the airborne route. The authors and the outbreak investigation team reached this conclusion because the other alternative routes direct (i.e. face-to-face) contact or via contaminated fomites were extremely unlikely whilst the index patient was housed in the hospital’s isolation room, with the carefully controlled access and disposal of contaminated linens and utensils that this would entail. Furthermore, a simple but effective smoke tracer experiment confirmed that the pattern of secondary cases could be readily explained by the route taken by the smoke as it leaked from within the patient’s isolation room. Within the building, the route traced by the smoke led to the nearby adjacent rooms then along the corridor to the entrance hall and up the building’s central stairwell to the first and second floors. The other contributory route of airborne dissemination was also shown to be via an open window in the patient’s isolation room, whereupon the smoke was shown to enter rooms on the first and second floors with open windows, as a result of convection currents set up within these rooms by the radiators positioned below these windows. All of these areas had secondary cases of smallpox within one incubation period (range 7–17, but typically around 12 days). Notably, the smoke experiments were performed retrospectively, in April when meteorological conditions were deemed to be similar to those at the time of the outbreak that had occurred 3 months earlier in January of the same year.
A similar approach using a retrospective engineering analysis with matching meteorological conditions was performed for a large outbreak of SARS in Hong Kong in March 2003 when a large housing estate, Amoy Gardens, reported several hundred cases of SARS within a 10-day period [23, 24]. The pattern of SARS cases within this housing estate was complex as there were seven tower blocks and the SARS cases were spread between four of them and at different levels within these affected towers. Although several competing hypotheses were suggested at the time to explain this complex pattern of SARS cases so early on in the 2003 outbreaks, remarkably, the most unifying hypothesis was that of the airborne spread of the virus excreted in faeces from a single index case with diarrhoea who had used a toilet in one of the apartments. In these apartments, floor drains in the bathrooms had a direct connection to the sewer pipes running down the outside of these buildings. However, when not filled with water, it was possible for sewage-contaminated aerosols to leak back into the bathroom—particularly if the extraction fans were on—to contaminate the apartment air and then to be extracted out into the gaps between the tower blocks [25]. Virus-contaminated aerosols could then be carried up on the up-welling air between the tower blocks and enter apartments in the adjacent towers through other open windows if kitchen or bathroom extraction fans were operating, creating a negative pressure sink within these apartments. An additional tier of evidence supporting an airborne transmission route is that not all of the tower blocks were affected in this housing estate—only four out of the seven towers reported SARS cases. An examination of the meteorological conditions for this period revealed that the wind direction during this period would have directed any such airborne contamination to these particular four towers, leaving the other three towers relatively safe. Computational fluid dynamics modelling taking these observations into account agreed with the pattern and distribution of the reported SARS cases very well, and the coating of biological material from the sewage probably protected the SARS virus from any desiccation or damaging exposure to ultraviolet radiation from the sun during the passage of the virus aerosols up between the towers. Indeed, a recent experimental study using surrogate microorganisms confirmed that bioaerosols can indeed be released from the sewage system into occupied rooms via depleted drain traps [26]. Despite various attempts to disprove this hypothesis over the years, since the end of the SARS outbreak, no other explanation has managed to explain the pattern of these SARS cases at the Amoy Gardens housing estate, so completely.
These two investigations are inspiring examples of how the disciplines of engineering, public health, microbiology and infectious diseases can work together to solve problems of direct benefit and impact to healthcare-related problems and the principles may have wider implications to the public and society in general. For example, from this investigation of the Amoy Gardens SARS outbreak, meteorological factors may now be routinely taken into account when considering patterns of airborne spread [27], and aerosol and airborne transmission has become a significant factor in individual outbreak, as well as epidemic and even pandemic investigations.
The impact of new technologies
Before describing which infectious agents are transmissible by the airborne route, it is important to understand how the various means of their detection have changed over the years. This has an important impact upon the strength of the evidence that is presented in the various studies for or against airborne transmission, since the sensitivity of the various diagnostic tests will determine whether the presence of the pathogen is reported or not.
Prior to the development of the polymerase chain reaction (PCR) in the late 1980s, and all the molecular detection techniques that followed, the detection of viruses and bacteria was limited to culture, microscopy, serology and antigen detection. At that time, many experimental laboratory-based studies on the airborne detection and viability of various infectious agents followed very similar protocols: first, generate a culture broth of the pathogen, quantify the concentration of pathogen in this broth, aerosolise the broth using some sort of nebuliser/atomiser system into a rotating chamber (to maintain the droplets produced, effectively airborne) and test the recovery of viable pathogen using culture after varying periods of time [28–30]. With this approach, mainly pathogen culture techniques were used, but not all pathogens of interest (particularly those viruses causing diarrhoea, i.e. rotaviruses and the then small round structured viruses—SRSVs–with noroviruses being the most prominent of these) were easily cultured, and even if they were, using culture to quantify the amount of virus at the input and recovery stages was very difficult to perform, accurately.
Other traditional methods for the detection of viral infection and demonstrating the presence of viruses were also impractical. Serology cannot be used for such pathogen detection studies as it only detects the host immune response (via antibody production) to infection with specific pathogens. Antigen detection was not available for many viruses, and where tests were available, the sensitivity was not particularly high. For most viruses, detection using electron microscopy (EM) was (and still is) inappropriate and EM cannot accurately quantify the number of viruses, particularly when its concentration threshold for the detection of any virus at all is in the order of 100,000 viruses per millilitre of sample medium (whether this be stool, urine, blood, etc.). The detection of airborne bacteria has been more successful, as culture could be reliably performed for most species of clinical importance and quantifying the number of input and recovered bacteria and counting the number of viable colony-forming units is relatively easy. However, some bacteria are thought to be damaged in the sampling process preventing their culture, and others where laboratory growth is very slow can result in drawn-out experiments that suffer with cross-contamination. A particularly difficult example is Mycobacterium tuberculosis which despite being arguably the best recognised airborne infection has never been successfully cultured from a room air sample.
Hence, given the lower sensitivity of these older detection methods, it is likely that earlier failures to detect the presence and number of specific pathogens in experimental air samples may have led to the belief that such pathogens may not have been transmissible by the airborne route. In addition, the conclusions of many older studies stating that the airborne route was responsible for explosive outbreaks were based only on traditional epidemiological methods (e.g. case definitions and case finding within a compatible timeline geographic location), without any sampling and laboratory testing at all.
So, it seems that prior to the availability of molecular methods, the evidence for or against airborne transmission of specific pathogens may not have been particularly accurate or reliable.However, one advantage of traditional culture methods over more modern molecular methods is now becoming important—the ability to detect viable microorganisms, which is discussed in greater detail in the succeeding paragraphs. In addition, it is always possible that some pathogens may be more transmissible by the airborne route in certain conditions, e.g. influenza in enclosed, crowded spaces like planes [6, 31], or schools [32], so an absolute statement about their ability to transmit by the airborne route may not be particularly useful and may, at times, be misleading.
For some of the more recently investigated organisms (e.g. severe acute respiratory syndrome-associated coronavirus [SARS-CoV] and influenza virus), the question of airborne transmission has been raised. However, even with the application of molecular detection and other sophisticated epidemiological methods in different scenarios and environments, the demonstration of airborne transmission is still not necessarily conclusive. This topic has been covered by numerous review articles [33–38], and from these, it is becoming clear that several laboratory and engineering criteria need to be assessed to provide acceptable evidence for the presence of clinically significant airborne transmission.
Thus, it is becoming clearer that the use of molecular methods have produced their own sets of criteria and raised additional questions about the nature and quality of the evidence, and therefore level of support that such results provide, in the ongoing debate about the capability of certain infectious agents to transmit at a clinically detectable and significant level via the airborne route. This in turn will have an impact on the guidelines advising on which form of personal protective equipment (PPE) should be used by healthcare workers (HCWs) when managing patients potentially or confirmed to be infected with such pathogens.
The PCR as a means to detect and identify the presence of organism RNA or DNA in air samples has been used in many studies to demonstrate the presence or absence of these organisms. The rationale is that if the genome of the organism is present in the air, then it is a reasonable assumption that the organism is also present in the air and therefore has the potential to be inhaled by a susceptible host. This is certainly a necessary condition, as all known infectious agents contain either RNA or DNA, but it is not necessarily sufficient. A PCR-only approach can be readily criticised, as it does not necessarily demonstrate a live, viable organism that is capable of replication and therefore causing clinical disease in susceptible individuals. However, neither does the presence of RNA/DNA necessarily preclude the viability of the airborne organism—and the emphasis placed on this requirement to demonstrate viability differs with the circumstances—perhaps not unreasonably so.
In clinical diagnostics, the use of PCR for diagnosing the presence of a causative agent has been well established and is in routine use in clinical diagnostics for various diseases, e.g. encephalitis (such as herpes simplex, varicella zoster, enterovirus, human herpesvirus 6, West Nile virus and JC virus, to name but a few) and blood-borne virus infections (such as with hepatitis B, hepatitis C, cytomegalovirus and Epstein-Barr virus, dengue virus and HIV), without the requirement to demonstrate independent viral viability in each case before a diagnosis is made and any necessary treatment initiated. Yet, increasingly, the emphasis has been on investigators to demonstrate the viability of the organisms whose RNA/DNA has been captured and identified in environmental (as opposed to clinical patient) samples, e.g. air samples, before any infection control-related interventions are to be implemented—and especially if the more expensive airborne infection control options (e.g. wearing N95 vs surgical masks).
Characterising the environment
A more subtle and indirect example of how culture or molecular detection methods can be used to infer the presence or absence of airborne transmission can be found when potential pathogen transmission routes are informed by environmental airflow patterns [37]. This additional investigation arm has been overlooked in many studies, often because many outbreak studies include public health/infectious disease physicians and microbiologists/virologists in the investigative teams but do not usually involve engineers who are able to define, characterise and assess the airflow dynamics of the environment in question. Including this expertise can have two valuable benefits: it can enable an investigation critically assess the feasibility of the airborne transmission route for disease causation, and if there is sufficient airflow data, it may enable quantification of the infectious dose.
Such careful airflow measurements cannot normally be conducted during the outbreak period itself when patient care takes priority, and often such outbreak investigations can only be performed retrospectively, using fluid dynamics modelling and/or any available data that was recorded at that time, e.g. meteorological data on wind speeds and direction if involving an outdoor environment [1, 22–24], or with airflow pattern measurements in indoor environments, such as clinics and hospitals [37, 39–43] summarise these and other studies where a convincing link was demonstrated between retrospectively measured airflow patterns and suspected airborne transmission had occurred in a healthcare environment. In contrast, air sampling of the environment in the presence of infected patients has more recently been performed with some useful results [44–46], as well as sampling airborne viruses contained in aerosolized droplets directly from coughing patients [47, 48]. Being able to perform both of these types of studies, i.e. airflow and air sampling, at the time of an outbreak would be ideal. This is virtually impossible in an outbreak, because by definition an outbreak will only reveal itself as such only after several days to weeks when sufficient numbers of cases become evident, by which time it is often too late to plan and carry out measurements and any investigation must be retrospective.
Thus, in many such investigations such engineering studies are never performed, either because the traditional epidemiological methods and evidence are deemed sufficient, or if the logistics of performing such studies retrospectively are too difficult and/or too uncertain and/or too costly—or, as might be the case for most studies, simply never considered. Traditional epidemiologists may argue that inferences based on traditional epidemiological methods do not require such engineering input. However, with the recent investigations into the possibility of airborne transmission of SARS-CoV and even ancient organisms like influenza, an engineering input is increasingly required to lend a certain degree of credibility for such investigations. There are also a very small number of planned transmission studies where the engineering input has proved hugely valuable.
Air sampling techniques—proof of presence of airborne pathogens
Microorganisms are present wherever air is sampled and are a potential source of transmission. Bacteria and fungi including spores can be carried as bioaerosols or individual particles. Small particles remain suspended for long periods whilst large particles fall to surfaces within a few minutes. Hospital infection control teams increasingly rely on engineering staff to provide evidence of adequate flow rates in the appropriate direction rather than bacterial counts. However, microbiological air sampling in the healthcare environment is used in commissioning new theatres and clean rooms and following refurbishment of ventilation systems [49, 50]. In areas where pharmaceuticals or medical devices are produced, air sampling is regulated and standards are published (ISO 14698-1/2). For infection control staff, ideally, for portable, real-time, mobile, environmental surveillance within a hospital or clinic setting, samplers need to be compact and simple to use and laboratories able to process the agar media used.
Bacteria-carrying droplets or particles can be detected by allowing them to passively settle on open agar plates or air can be drawn actively through a sampler at a fixed rate [49]. Settle plates are usually standard agar plates exposed for a fixed time and then incubated. They do not detect particles that remain airborne, and the volume of air sampled is not known, so there is no quantitative element. They may be affected by airflow rates and turbulence and can easily become contaminated. Another risk is that the agar can dry out. However, they can be useful to signal the need for quantitative sampling and can allow long-term sampling and are inexpensive. Hence, they are widely used in the food industry. Active samplers allow organisms to be collected onto a filter and eluted into solution (e.g. Institute of Medicine Personal Sampler and Airport MD8), impacted onto solid media (e.g. Andersen or Microbial Air Sampler-100), caught into a liquid medium (e.g. Biosampler, AGI-30 or Midget) or sampled using a centrifugal force (e.g. GK2-69, Reuter Centrifugal Sampler and Dorr-Oliver Cyclone). Some samplers use a rechargeable battery to power the pump to provide portability whilst others require a main power supply. Whilst operation of the sampler must be simple and efficient, counting and identification of airborne flora is a skilled task. Portable units for the clean room user (e.g. SAS Super 180) provide a high sampling rate to cover a cubic metre in 6 min onto an agar or contact plate.
Machines that pull air across solid media (impactors) use single or several stages. There are up to six stages in the Andersen Sampler (Anderson Instruments Inc., Smyma, Georgia) that fractionates into decreasing particle sizes. The Casella Slit Sampler used an agar plate mounted on a turntable but has largely been superseded. The Surface Air Systems (SAS) Sampler (Cherwell Laboratories, Bicester, UK) is still used. Centrifugal acceleration is used to impact organisms onto solid media in the Reuter Centrifugal Sampler (RCS) (Biotest UK Ltd., Birmingham, UK) or into liquid (Cyclone Sampler, Aimer Products Ltd., London) (Fig. 1). The solid media may dry out with prolonged sampling. Large volumes of air sampled in contaminated environments may result in difficulty counting the number of colonies and a loss of accuracy. Impactors are commonly used to test air in healthcare facilities and are volumetric. In a single-stage instrument, air is drawn through a narrow slit or perforated plate onto agar media. The media is at right angles to the air flow, and rate of impaction depends on the size of the particle and the rate of air flow. The velocity of the flow is determined by the size of the hole in a sieve sampler or the width of the slit. A high flow is needed for the smaller particles, but the large external pumps of early models have been replaced with battery-powered integral pumps. When a set volume of air has passed through the sampler, the agar is removed and incubated. The number of colony-forming units seen gives a quantitative estimate of the airborne bacterial load. Plates can be purchased ready for use or prepared in house. These samplers can sample at high rates and large volumes when air quality is likely to be good. However, very large air volumes can dry out the plates and mechanical stress may destroy some bacteria. Incubation may require several days to produce results depending on the organism. PCR can only be used if a water-soluble polymer is used as the base.
Fig. 1.
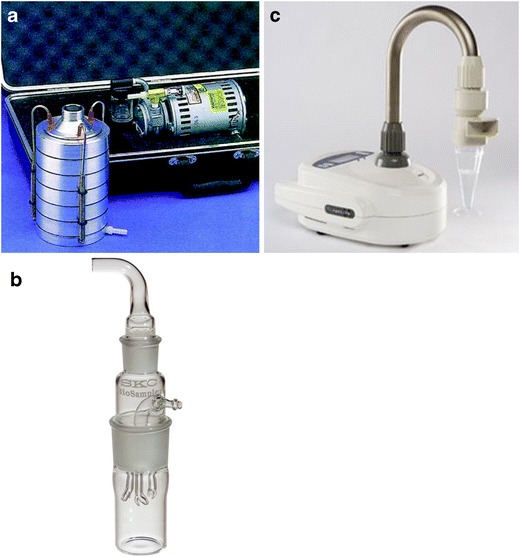
Some examples of air samplers: a Thermo Scientific™ Andersen Cascade Impactor http://www.thermoscientific.com/content/tfs/en/product/six-stage-viable-andersen-cascade-impactor-1.html; b SKC BioSampler Liquid Impinger, http://www.skcinc.com/catalog/index.php?cPath=400000000_401000000_401000050; c SAS Coriolis Cylcone Sampler, http://www.coriolis-airsampler.com/coriolis-micro.aspx. These images are for illustration purposes only, and their use should not be taken as an endorsement of any kind by the authors or the institutions that they represent.
A multistage instrument directs air through a series of sieve plates (e.g. the Thermo Scientific™ Andersen Cascade Impactor). Deposition occurs as the air flow changes direction on reaching the agar, but smaller particles continue to the next plate in the stack. The smaller holes increase the speed of air flow and deposit smaller particles. Particles from 0.3 to 15 μm are collected, the smaller representing those that would reach lung alveoli. The agar plates can be incubated without further manipulation. A calculation can be made to allow for some single colonies arising from more than one particle, but heavy growth has to be estimated by repeating with a smaller volume of air. Centrifugal samplers use an agar strip lining the housing of the impeller but are less accurate especially at small particle sizes. The SAS (Surface Air Systems) single-stage impactor uses an agar plate over which air is drawn through a perforated cover plate. The volume of sample is determined using a timer. It is portable and easier to use than older slit sampler models. However, it is less efficient at trapping small particle size (50 % at 4 μm).
Machines that pump air actively into liquid (impingers) achieve a very high efficiency (i.e. they capture a very high proportion of the airborne pathogens in the air volume sampled). Air is drawn through a narrow inlet to accelerate air towards the surface of the liquid where the change of direction deposits airborne particles. Flow rate, sample volume and sampling time enable a quantitative estimation to be made. Collection may be restricted by the relatively low rate of flow and rate of evaporation, e.g. single-stage micro-impingers (SKC Ltd, Dorset, UK), May liquid impinger (AW Dixon, Moolap, Victoria, Australia) and Ace all glass impinger-30 (Ace Glass NJ, USA). Some organisms may be damaged or could multiply in fluid if collection is delayed. The liquid medium allows PCR to be used [51]. Cyclone samplers use a liquid-air mix to collect particles on the inner wall before washing into a collection point (SAS-PCR, Coriolis Bertin technologies, Toulouse, France) (Fig. 1). Multistage impingers pass air through three liquid containers at different flow rates, impacting particles onto glass discs or into liquid. They are efficient because damage to the organisms is minimised by lower flow rates.
Filtration samplers use gelatin, polytetrafluoroethylene (PTFE), polycarbonate (PC) or mixed cellulose ester (Merck Millipore, Watford, UK; Nuclepore, Sterilin Ltd–Thermo Scientific, Waltham, MA, USA; Sartorius, Epsom, UK). Although some filters are highly efficient, the transfer from the filter results in some loss. Gelatin filters can be dissolved in suitable buffers which limits the loss of organisms. High- and low-volume pumps can be used. Organisms collected on the filters can be suspended in aqueous fluid before culture. Organisms on polycarbonate or cellulose filters can be counted under microscopy, e.g. acridine orange stain for viable counting under microscopy. Gelatin filters can be dissolved to release organisms and cultured.
Sampling air for viruses is less well developed than for bacteria. Collection into liquid medium has been used, but long sampling times may be needed. Viruses are subject to damage or there may be bacterial overgrowth. Molecular detection is increasingly used.
In immune compromised patients, exogenous infections are commonly acquired through airborne route [49]. Aspergillus air counts should be <5 colony-forming units (cfu)/m3 in protective isolation and preferably much lower. To detect spores requires their removal onto a surface. The particle size influences the likelihood of capture, but as the aerodynamic diameter varies widely, the efficiency of an air sampler can be difficult to predict. Depending on humidity, the aerodynamic diameter of Aspergillus fumigatus is around 3.1 μm. The sampling volume has to be sufficient for a representative sample and not so large that spore counts become excessive. This has to be determined by use.
Many countries have regulatory requirements for air sampling (e.g. ISO 14698-1/2). Samplers must be validated and calibrated regularly with respect to efficiency of particle collection and maintaining cell viability and flow rate. Usually, this is performed by an external company against membrane filtration or a Casella slit sampler for physical and biological efficiency and a certified flow meter for flow rate.
Air sampling in practice
Wards
In haematology/oncology wards, air sampling for fungal spores can be a useful measure of clinical risk for profoundly immune-suppressed hospital inpatients [52]. In one study of a HEPA-filtered ward, a third of monthly air samples were positive for Aspergillus compared with 95 % of outside air. Over a 10-year period, there were 48 spikes of Aspergillus-positive samples but a patient was infected only once when counts were high. In a comparison of six types of air sampler using six species of airborne bacteria and fungi, RCS High Flow and SAS Super 180 captured 80–90 % of fungal spores compared with 10–60 % of others [53]. The RCS Air Sampler, Andersen N6 single stage, SAS 90 and Air-o-Cell were all relatively consistent in capturing fungal spores [54].
Impactors such as RCS or BioStage are efficient for sampling of culturable bacteria, whilst impingers and cellulose acetate filter are better for total bacterial sampling [55]. RCS High Flow and MAS-100 acquired 20–30 % of bacterial particles compared with 10 % of other samples from other types [53].
Theatres
Despite evidence that ultraclean air is associated with low rates of infection in orthopaedic surgery, there is little evidence of a link between airborne bacterial counts and surgical wound infection when antibiotic prophylaxis is used. In plenum ventilated theatres, the air is filtered to 5 μm whilst ultraclean theatres have high-efficiency particulate air filters down to 0.3 μm. Vertical laminar flow is associated with a reduced number of airborne bacteria at the operative site. However, patients given antibiotic prophylaxis have similar infection rates whether operated in conventional or laminar flow-ventilated theatres [56]. Most organisms causing infection of clean wounds enter from the skin flora shed from patients or staff. Bacteria may deposit through direct contact or from airborne dust.
At 25 air changes per hour (UK standard), airborne counts are 50–150 cfu/m3 [50]. There are no agreed consensus limits on the number of organisms in air acceptable for use. During activity, UK recommendations are 180 cfu/m3 in a plenum theatre in use or 35 cfu/m3 in an empty theatre whilst in ultraclean theatre with conventional staff clothing the threshold is <10 cfu/m3. In Switzerland, the bacterial count within the laminar flow area is <1 and 5 cfu/m3 outside and 25 cfu/m3 in conventional theatres air filters having 95 % efficiency. Air sampling in 29 theatres over 3 years showed that lower bacterial air counts in empty theatres compared with those in use (median 12 cfu/m3 [interquartile range (IQR) 4–32] vs. 80 cfu/m3). Fungi were isolated in 39.13 % of samples collected in empty theatres and 56.95 % of samples collected in working theatres [57]. However, sampling methods are not standardised and results are valid only at the time of sampling. Annual engineering maintenance is preferable to routine sampling. Bacterial sampling is better limited to investigation of outbreaks, validation or commissioning after maintenance of the ventilation system. Particle size counters have been suggested as being more useful in giving immediate results than microbiological sampling [56]. However, correlation between particulate counts and numbers of airborne bacteria is poor or lacking [58].
Pharmacy production
In areas where drugs or medical devices are produced or packaged, microbiological monitoring of air is essential. In a systematic review of 19 studies, doses prepared in clinical environments were contaminated in 2–5 % of cases compared with 0–1.9 % in pharmaceutical environments [59]. Batch preparations were less likely to be contaminated than individual doses. UK and USA have set standards for compounding using laminar flow isolators. Good Manufacturing Practice for the EU also contains guidance. Both active and passive air sampling is used to ensure air quality at installation and during operation.
Selective examples of accepted and potentially airborne pathogens
Perhaps one indicator of whether an infectious organism is more or less likely to be transmitted via the airborne route is the value of its basic reproductive number, R o, i.e. the number of secondary cases of an infection produced from a single-index case in the presence of an otherwise fully susceptible population. The higher the R o, the more likely that the main route of transmission for that pathogen is via the airborne route (Table 1). Hence, from the R o values shown in Table 1, there are several organisms which are efficiently transmitted by the longer-range, droplet nuclei, airborne route, including measles (R o = 16–18) and chickenpox (R o = 10–12).
Table 1.
The basic reproductive number (R o) for some common infectious agents where the airborne transmission route is significant (derived from Anderson and May [60])
| Organism | Basic reproductive number (R o) | Critical proportion of population to be immunised for eradication (%) |
|---|---|---|
| Measles | 16–18 | 90–95 |
| Whooping cough | 16–18 | 90–95 |
| Chickenpox | 10–12 | 85–90 |
| Mumps | 11–14 | 85–90 |
| Rubella | 6–7 | 82–87 |
Tuberculosis (M. tuberculosis)
Studies by Riley et al. in 1957 demonstrated that tuberculosis (TB) is unequivocally an airborne infection. They showed exhaust air from a TB ward caused infection in guinea pigs [61, 62]. More recently, several studies recreated Well’s famous TB transmission studies from the 1950s [63], where air from a TB patient ward was extracted and passed over cages of guinea pigs, with and without the presence of UV air disinfection [64–66]. By using molecular techniques, the authors were able to relate particular guinea pig infections to patients and quantify the difference in cases between control and UV groups. In addition, the simultaneous measurement of ventilation flow rates enabled quantitative estimation of the generation of infectious doses (expressed as ‘quanta’) [61, 67], and therefore the infectiousness of different patients. Other well-documented outbreaks of TB such as that aboard the naval vessel ‘Richard R Byrd’ [68] and the spread of TB by wound irrigation [40] have provided ample evidence of airborne transmission of TB. Fennelly et al. reported on the size of the airborne infectious particle generated by cough from TB patients as measured by an air sampler. They found these particles to be within the respirable size range, further supporting the feasibility of airborne transmission [69].
For TB, R o values are rarely given, even though it is widely accepted that TB is an obligate airborne-transmitted pathogen, with inhalation being the main route of infection. One estimate of the R o for TB only puts it at 1–2 [70], which is similar to the more recent estimates for seasonal [71] and pandemic influenza A/H1N1pdm09 [72]. The main reason for this are the timescales involved and the major role played by reactivated (as opposed to primary, acute) infections within individuals from previous exposures that result in, possibly, subclinical infection, latency then reactivation to cause disease sometime later. As such, it is usually very hard to establish secondary cases associated with a particular TB cases.
Multiple examples of TB outbreaks have been reported over the years, using a combination of more traditional epidemiological and laboratory methods, as well as more modern methods to evaluate transmission. The airborne route has clearly played a major role in the dissemination of the agent [40, 73], and various risk assessment and modelling approaches [74–76] have been used to optimise interventions [77, 78] and develop guidelines to limit the spread of this pathogen [79–81]. Thus, there is no question about the presence and significance of the airborne route in the transmission of TB.
Varicella zoster virus (chickenpox)
For varicella zoster virus (VZV), a series of studies by Asano and colleagues from Japan demonstrated the rapid dissemination of VZV DNA, using the polymerase chain reaction (PCR), in environments in which there were index cases, by sampling fomites as well as the skin of household contacts [82, 83]. Stronger evidence for a more airborne route of dissemination was obtained when VZV DNA was detected in air-conditioning and air-purifier filters, which would not have been easily contaminated by direct contact [84–86]. A hospital outbreak also provides evidence for airborne transmission, with retrospective airflow assessments using SF6 tracer gas highlighting that patient rooms were inadvertently positively pressurised leading to contamination of corridor air [87]. Transient containment failures have also led to secondary infections with chickenpox [60]. Estimates of the basic reproductive number (R 0, i.e. the number of expected secondary cases arising from the presence of a single-index case introduced into a population of fully susceptible hosts) for VZV have been reported as 7–12 from data presented by Anderson and May [88] and more recently as 7.66 to 13.44 by Ogunjimi et al. [89], which shows a remarkable consistency over 20 years, using different methods. For R 0 values in this range, airborne transmission is likely to contribute significantly to the mode of transmission.
Measles (rubeola)
In contrast, for measles, which is another disease of antiquity, surprisingly, there are fewer studies directly investigating the evidence for airborne transmission, i.e. via environmental and/or air sampling. The work of Riley and colleagues [67, 90, 91] really brought home the concept of airborne transmission for measles as a public health problem in the 1970s and 1980s and reinvigorated the interest in this route of transmission for other pathogens, including TB [92]. However, due to almost universal, global and effective MMR (measles, mumps and rubella) immunisation, measles outbreaks in the modern era are limited to where vaccination is either refused or vaccine supply and administration is either inefficient and/or disrupted [93–96].
Influenza
The intense debate surrounding the potential for the aerosol transmission of influenza has been well documented elsewhere [34–36, 38, 97–99]. However, one historical observation is worth highlighting, where precautions to protect patients in hospitals from TB cross-infection led to an interesting outcome during the influenza season of 1957–1958. This event was documented in passing at the time and has now come to have more significance in recent years with the heightened interest in the various types and subtypes of influenza and their routes of transmission.
A hospital for war veterans in Livermore, CA, had a special wing for TB patients. This wing had been equipped with an ultraviolet germicidal irradiation system (UVGI) that was designed to keep the air free of airborne TB. The rest of this wing, which was free of this intervention, experienced a significantly higher incidence of influenza during the annual influenza season from November 1957 to March 1958, based on numbers of patients exhibiting acute respiratory symptoms and serological markers for influenza infection [100].
Although this study does appear to support the airborne route as the main transmission route for this particular strain of circulating influenza (the Asian pandemic A/H2N2 influenza strain at that time), there may be multiple confounders, which the authors acknowledge in their discussion. One of these is whether the mobility of the irradiated TB patients was similar to that of the patients in the non-irradiated wing, as contact transmission, both direct and indirect (i.e. via fomites), has also long been considered a significant route of transmission for influenza. Recently, modern researchers have been discussing whether this natural experiment can be repeated in a more rigorous manner. This would help to answer the question of whether influenza is truly transmitted by the airborne route in such in-patient hospitalised circumstances, and if so, how significant this proportion of secondary cases would be.
Other respiratory viruses
The other common respiratory viruses are not considered to be truly airborne by most researchers, clinicians or infection control teams—though opportunistic airborne transmission cannot always be ruled out and certain circumstances may occasionally allow airborne transmission [33, 101–103].
Rhinoviruses and coronaviruses which together are responsible for 60–70 % of all seasonal common colds or ‘influenza-like illness’ are mainly transmitted by large droplets over relatively short ranges or by direct contact, e.g. via infected mucous or saliva onto hands or fomites then to another individual’s hands and mucous membranes. This is also accepted to be the main mode of transmission for the other respiratory viruses: respiratory syncytial virus (RSV), parainfluenza viruses, adenoviruses and human metapneumovirus (hMPV, which is from the same family as RSV and clinically similar).
Bordetella pertussis (whooping cough)
Similar to TB, B. pertussis (also commonly known as whooping cough) is another respiratory, bacterial pathogen that is mainly transmitted by the airborne route. This is a highly contagious, acute respiratory illness and is a strict human pathogen with no known animal or environmental reservoir [104]. Maintenance of the organism within the human population is thought to require continuous transmission of the disease from infected to naive hosts [105]. Pertussis is widely described as being transmitted via aerosolized respiratory droplets; however, there have been few controlled studies that have documented airborne transmission of pertussis. Several retrospective hospital and school outbreak studies and prospective household contact studies have documented examples of transmission; however, these studies could not practically control for contact between individuals [106–108]. Therefore, the possibility of contact-mediated or indirect transmission confounding the evidence for airborne transmission could not be ruled out. The lack of earlier, effective animal models of pertussis transmission meant that carefully controlled laboratory studies to demonstrate airborne transmission could not be performed [109].
Then in 2012, Warfel and co-workers reported on a successful baboon model to study pertussis transmission. They demonstrated naïve baboons co-housed with infected baboons, either in the same cage or in separate cages located 7 ft away, became infected, demonstrating airborne transmission of pertussis. As expected for an airborne exposure, the rate of transmission was dependent upon distance between the infected and naive individual. Although pertussis is typically described as being highly infectious, efficient transmission (at least in this animal model) appeared to require close contact or prolonged exposure [105].
More recently, after a successful control for many years using infant immunisation strategies, this pathogen has seen a resurgence in the last decade, particularly in infants and adolescents. This may be due to a number of factors, including waning vaccine immunity, incomplete protection or undiagnosed primary vaccine failure, new introductions of source infections, emergence of new strains—with incomplete protection conferred by the existing vaccine. This may also be due to a possible increase in the identification of new cases due to better surveillance and more sensitive modern diagnostic techniques [110]. Estimates for R o for whooping cough are as high as those for measles at around 16–18 (Table 1), compatible with a predominantly airborne route of transmission.
Interestingly, there are a few gastrointestinal pathogens that are potentially transmitted by the airborne route, as well as via the more traditionally accepted oral-faecal route.
Clostridium difficile
C. difficile infection (CDI) is a major cause of hospital-associated infection in healthcare facilities worldwide [111]. An understanding of the mechanisms of transmission is essential to establish effective interventions to minimise spread in the healthcare environment. Studies have shown that a patient with CDI can excrete between 1 × 104 and 1 × 107 of C. difficile spores per gram of faeces [112]. The possibility that C. difficile could be an airborne pathogen has been suggested in a number of studies [113–117]. Early animal experiments using the hamster model have demonstrated the possibility of airborne transmission and environmental contamination of C. difficile [118]. Studies on hospitalised patients have been equivocal, with some workers providing evidence for the airborne route of transmission [115] and others failing to do so [117].
In 2010, Best and co-workers [119] reported on a study to determine the extent of C. difficile contamination in ward environments, by recovery of C. difficile from air and environmental surfaces in the immediate vicinity of patients with symptomatic CDI. They performed air sampling adjacent to 63 patients with CDI for 180 h in total and for 101 h in control settings. Environmental samples were obtained from surfaces adjacent to the patient and from communal areas of the ward. C. difficile isolates were characterised by ribotyping and multilocus variable-number tandem repeat analysis to determine relatedness. Of the first 50 patients examined (each for 1 h), only 12 % had positive air samples, most frequently those with active symptoms of CDI (10 %, vs 2 % for those with no symptoms). They then sampled the air around 10 patients with CDI symptoms, each for 10 h over 2 days, as well as a total of 346 surface sites. C. difficile was isolated (by culture) from the air in the majority of these cases (7 of 10 patients tested) and from the surfaces around 9 of the patients; 60 % of patients had both air and surface environments that were positive for C. difficile (Fig. 2).
Fig. 2.
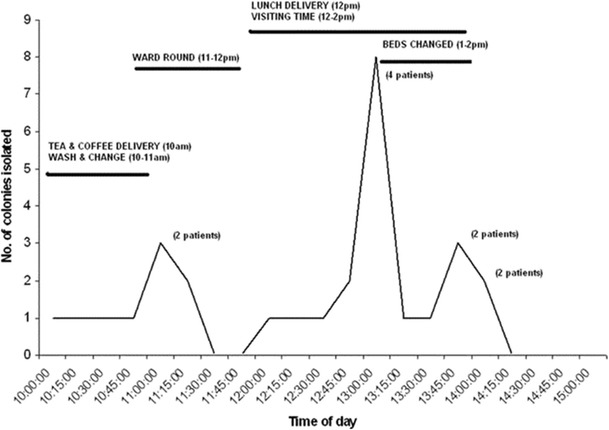
Line indicating the total number of Clostridium difficile colonies recovered at various times throughout the day (total of 10 patients tested for 2 days). The number of patients the colonies were isolated from is indicated in parentheses (reproduced, with permission, from [119]).
Molecular characterisation confirmed an epidemiological link between airborne dispersal, environmental contamination and CDI cases. They concluded the air within the patient’s immediate environment is contaminated with C. difficile spores either directly from symptomatic patients or from environmental surfaces and that people movement, including the opening and closing of doors, contributes to the circulation and dispersal of airborne C. difficile, and this could explain the widespread dissemination of epidemic strains. The infection control implications are that healthcare staff realise the importance of environmental cleaning and single-room isolation as soon as possible after the onset of diarrhoea to limit the dissemination of C. difficile [119]. More recently, this same team demonstrated that C. difficile could be aerosolised by the act of toilet flushing and recommended that toilet lids be used in wards where such patients are managed [120]
Norovirus
Vomiting due to infection with norovirus is a recognised risk factor for the airborne dispersion of the virus [121]. An analysis of an outbreak in a large hotel demonstrated that victims’ attack rates were inversely related to their distance from a woman who vomited during her meal [122]. An outbreak in an elementary school demonstrated that attack rates were directly related to the number of times pupils were exposed to vomiting episodes, suggesting that spread occurred by the inhalation and swallowing of viral particles [123]. An earlier study further suggested that sufficient numbers of viable virus remain suspended in the air to infect people walking through this contaminated air, as it reported evidence of transmission when individuals walked through an emergency department in which a vomiting patient was being evaluated [124].
Bioterrorist agents
Whilst this text is mainly focused on the potential for airborne transmission of naturally occurring infectious agents, a brief mention of agents of biological warfare is needed.
These agents have been classified by the US Centers for Disease Control and Prevention (US CDC) into categories A, B and C (http://www.bt.cdc.gov/agent/agentlist-category.asp), where category A indicates agents that can be easily disseminated or which transmit from person-to-person, with potentially high mortality and high public impact (including the inducement of panic and social disorder), and which require some form of special public health preparedness. The agents in this category are botulism (Clostridium botulinum toxin), plague (Yersinia pestis), anthrax (Bacillus anthracis), tularaemia (Francisella tularensis), smallpox (Variola major), viral haemorrhagic fevers (Filoviridae: Ebola and Marburg and Arenaviridae: Lassa, Junin, Machupo, Guanarito, etc.).
Of these, only smallpox has been shown to be definitively transmissible by the airborne route in its natural form and relatively effectively between people [22, 125], but this organism still remains effectively eradicated. Untreated, end-stage pneumonic plague can transmit between individuals by large droplets over short distances, but in the modern era with rapid access to effective treatment, person-to-person transmission with naturally occurring plague is not considered to be a serious risk [126]. Although the airborne transmission of anthrax spores have been reported from biological weapons factories [1] and more recently in letters [127], natural human infections of inhalational anthrax rarely transmit between individuals [128].
Inhalational tularaemia is not infrequently reported from endemic regions in North America and Europe, but person-to-person transmission is rare [129]. Natural botulinum toxin poisoning is usually acquired by ingestion. There have been attempts to weaponise it as an aerosol—as inhalational botulism—though it is not transmitted naturally by this route [130, 131].
For the viral haemorrhagic fevers, even though most naturally occurring secondary cases were thought to arise by direct contact with infected body fluids [132], there does appear to be the potential for aerosol transmission even in their natural form [133]. Concerns about weaponising such viruses as aerosols have been longstanding, and military research has been active in recent years in developing animal models to explore how such scenarios can be effectively countered [133, 134].
Such concerns about the potential for possible, opportunistic human-to-human airborne transmission have again been recently expressed for the massive Ebola virus outbreaks in West Africa [135, 136], despite this virus being traditionally considered to be only transmitted via the contact route [137], with only speculative concerns about the potential for airborne transmission suggested previously [138, 139]. Furthermore, Osterholm and colleagues have now advocated a new shift in the thinking around Ebola virus, to seriously consider the potential for aerosol transmission of this highly lethal virus, at least, in severe outbreak situations [140, 141].
Emerging and reemerging infections
Some of the main drivers for the recent resurgence of interest in airborne transmission over the past decade were the global outbreaks of severe acute respiratory syndrome (SARS) in 2003, particularly in Hong Kong, Singapore and Canada. During the SARS outbreaks, unusual clusters of cases were occurring in both hospital [41, 42] and community settings [23, 24], and even planes [7], which has led to multiple engineering computational fluid dynamics (CFD) modelling studies to determine how infected individuals may potentially seed the air with aerosol-transmitted pathogens and how they may then be potentially disseminated via ambient ventilation flows [19, 20, 37, 142–144].
The application of sensitive, quantitative PCR testing on both respiratory samples from patients [145–148] and environment air samples [44–46] demonstrated that viral RNA was present in the air and could be inhaled by other patients, healthcare workers and visitors working in those environments, perhaps explaining the observed widespread infection of healthcare workers seen during the 2003 SARS outbreaks.
In parallel with these quantitative molecular diagnostic tools, mostly initially stimulated by various SARS outbreaks in healthcare and community populations, techniques to visualise exhaled human airflows became very topical, using both naturally infected human volunteers [149–156] as well as human simulation models [157–160], as infected individuals were considered to be the source of such outbreaks, and, importantly, that the number of secondary cases becoming infected were seemingly too great and rising too rapidly to be due to just contact transmission. To this end, more exhaled airflow visualisation studies by interested engineers were conducted using various surrogates for infected humans, including life-size, human-like, thermal breathing manikins, as well as specifically designed ‘cough’ machines, to allow further characterisation of the potential dissemination of airborne pathogens by human respiratory airflows [161–166]. Importantly, several human volunteer studies examined the impact of masks or other forms of mouth coverings, to curtail potentially infectious exhalation flows. They all demonstrated that mouth coverings decreased the exit velocities of these exhaled flows, allowing them to flow upwards with the natural human thermal plume, thereby reducing the potential transmission risk of airborne infectious agents to anyone in their immediate surroundings [151–154, 166].
These quantitative molecular detection, environmental air sampling and airflow visualisation investigative methods continued to be developed and refined, within the context of the reemergence avian A/H5N1 influenza virus in Hong Kong and China, to the extent that when the 2009 influenza A/H1N1 pandemic emerged, they were all ready to be applied to this new pathogen [72, 167–170]. With all of this pre-pandemic preparedness (originally targeted at avian A/H5N1), stimulated by the 2003 SARS outbreaks, the 2009 A/H1N1 influenza pandemic was the most thoroughly investigated pandemic virus of all time. Quantitative PCR methods allowed comprehensive investigation and understanding of these new pathogens in various patient groups (children, adults, pregnant women, the immunocompromised), as well as its behaviour during various so-called ‘aerosol generating procedures’ (AGP) [171, 172]. Multiple studies have been performed to attempt to define the potential risk of aerosol transmission from infected patients using various air-sampling and quantitative PCR methods [48, 173–175].
These investigative techniques have been applied to various degrees in an attempt to characterise the aerosol transmission potential of various emerging viruses, including avian A/H5N1 influenza [176], seasonal and pandemic influenza A/H1N1pdm 2009 [177, 178], avian A/H7N9 influenza [179] and the Middle East respiratory syndrome-associated coronavirus (MERS-CoV) [180], leading, in some cases, to a reassessment of their airborne transmission potential [181].
Summary
Over the past 12 years, since the SARS-CoV outbreaks in 2003 followed by the emergence and reemergence of various novel human and animal influenza and coronaviruses, there has been much research into the role of the aerosol transmission route. As a result of this, now, the concept of aerosol transmission for various infectious agents that cause respiratory symptoms is now firmly established in the minds of clinical infectious diseases, public health, microbiology and virology specialists, worldwide. Infection control guidelines, including the use of negative pressure rooms and how personal protective equipment is used, need to take into account these findings—particularly where a strong precautionary principle is being advocated when dealing with infectious agents of high lethality.
Acknowledgments
Compliance with ethics guidelines
Conflict of interest
Nandini Shetty, Catherine Noakes and Julian Wei-Tze Tang have no relevant conflicts to report. Andrew Peter Wilson reports personal fees from Drug Safety Monitoring Board Quintiles, personal fees from Global Advisory Panel 3M, outside the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by the authors.
Footnotes
This article is part of the Topical Collection on Viral Infections
References and Recommended Reading
- 1.Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, et al. The Sverdlovsk anthrax outbreak of 1979. Science. 1994;266:1202–8. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 2.Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM., Jr Biological warfare. A historical perspective. JAMA. 1997;278:412–7. doi: 10.1001/jama.1997.03550050074036. [DOI] [PubMed] [Google Scholar]
- 3.Alibek, Ken and Steven Handelman (1999), Biohazard: the chilling true story of the largest covert biological weapons program in the world—told from inside by the man who ran it, Random House, ISBN 0-385-33496-6.
- 4.Carlton, Jim. Wall Street Journal 26 Oct 2001: Of microbes and mock attacks—51 years ago, the military sprayed germs on US cities. Available at: http://coloradoliberty.org/blog/2010/08/28/of-microbes-and-mock-attacks-51-years-ago-the-military-sprayed-germs-on-u-s-cities/. Accessed 14 June 2012.
- 5.US Department of Homeland Security. Environmental assessment for Bacillus subtilis particles to challenge bio-detection sensors in subway stations. Prepared for Department of Homeland Security Science and Technology Directorate. January 12, 2012 Version 15. Available at: http://www.dhs.gov/xlibrary/assets/st/st_dea_detect_to_protect.pdf. Accessed 4 June 2012.
- 6.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 7.Olsen SJ, Chang HL, Cheung TY, Tang AF, Fisk TL, Ooi SP, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416–22. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 8.Lasher LE, Ayers TL, Amornkul PN, Nakatab MN, Effler PV. Contacting passengers after exposure to measles on an international flight: implications for responding to new disease threats and bioterrorism. Public Health Rep. 2004;119(5):458–63. doi: 10.1016/j.phr.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman KP, Markey PG. Measles transmission in immunized and partially immunized air travellers. Epidemiol Infect. 2010;138(7):1012–5. doi: 10.1017/S0950268809991129. [DOI] [PubMed] [Google Scholar]
- 10.Martinez L, Blanc L, Nunn P, Raviglione M. Tuberculosis and air travel: WHO guidance in the era of drug-resistant TB. Travel Med Infect Dis. 2008;6(4):177–81. doi: 10.1016/j.tmaid.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Martinez L, Thomas K, Figueroa J. Guidance from WHO on the prevention and control of TB during air travel. Travel Med Infect Dis. 2010;8(2):84–9. doi: 10.1016/j.tmaid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin BJ, Hardiman MC. The international health regulations (2005), tuberculosis and air travel. Travel Med Infect Dis. 2010;8(2):90–5. doi: 10.1016/j.tmaid.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Ooi PL, Lai FY, Low CL, Lin R, Wong C, Hibberd M, et al. Clinical and molecular evidence for transmission of novel influenza A(H1N1/2009) on a commercial airplane. Arch Intern Med. 2010;170(10):913–5. doi: 10.1001/archinternmed.2010.127. [DOI] [PubMed] [Google Scholar]
- 14.Foxwell AR, Roberts L, Lokuge K, Kelly PM. Transmission of influenza on international flights, may 2009. Emerg Infect Dis. 2011;17(7):1188–94. doi: 10.3201/eid1707.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leder K, Newman D. Respiratory infections during air travel. Intern Med J. 2005;35(1):50–5. doi: 10.1111/j.1445-5994.2004.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365(9463):989–96. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowdall NP, Evans AD, Thibeault C. Air Travel and TB: an airline perspective. Travel Med Infect Dis. 2010;8(2):96–103. doi: 10.1016/j.tmaid.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Webster CH. Airline operating realities and the global spread of infectious diseases. Asia Pac J Public Health. 2010;22(3 Suppl):137S–43. doi: 10.1177/1010539510373130. [DOI] [PubMed] [Google Scholar]
- 19.Gupta JK, Lin CH, Chen Q. Transport of expiratory droplets in an aircraft cabin. Indoor Air. 2011;21(1):3–11. doi: 10.1111/j.1600-0668.2010.00676.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta JK, Lin CH, Chen Q. Inhalation of expiratory droplets in aircraft cabins. Indoor Air. 2011;21(4):341–50. doi: 10.1111/j.1600-0668.2011.00709.x. [DOI] [PubMed] [Google Scholar]
- 21.Gupta JK, Lin CH, Chen Q. Risk assessment of airborne infectious diseases in aircraft cabins. Indoor Air. 2012;22(5):388–95. doi: 10.1111/j.1600-0668.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- 22.Wehrle PF, Posch J, Richter KH, Henderson DA. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull World Health Organ. 1970;43(5):669–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731–9. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Duan S, Yu IT, Wong TW. Multi-zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air. 2005;15(2):96–111. doi: 10.1111/j.1600-0668.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 25.Gormley M, Swaffield JA, Sleigh PA, Noakes CJ. An assessment of, and response to, potential cross-contamination routes to defective appliance water trap seals in building drainage systems. BSER&T. 2011;33:203–22. [Google Scholar]
- 26.Gormley M, Aspray TJ, Kelly DA (2014) Bio-aerosol cross-transmission via the building drainage system. Indoor Air, Hong Kong, July 7th-July 12th 2014.
- 27.Yip C, Chang WL, Yeung KH, Yu IT. Possible meteorological influence on the severe acute respiratory syndrome (SARS) community outbreak at Amoy Gardens, Hong Kong. J Environ Health. 2007;70(3):39–46. [PubMed] [Google Scholar]
- 28.Songer JR. Influence of relative humidity on the survival of some airborne viruses. Appl Microbiol. 1967;15(1):35–42. doi: 10.1128/am.15.1.35-42.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benbough JE. Some factors affecting the survival of airborne viruses. J Gen Virol. 1971;10(3):209–20. doi: 10.1099/0022-1317-10-3-209. [DOI] [PubMed] [Google Scholar]
- 30.Ijaz MK, Karim YG, Sattar SA, Johnson-Lussenburg CM. Development of methods to study the survival of airborne viruses. J Virol Methods. 1987;18(2–3):87–106. doi: 10.1016/0166-0934(87)90114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker MG, Thornley CN, Mills C, Roberts S, Perera S, Peters J, et al. Transmission of pandemic A/H1N1 2009 influenza on passenger aircraft: retrospective cohort study. BMJ. 2010;340:c2424. doi: 10.1136/bmj.c2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Communicable Disease Surveillance Centre (Public Health Laboratory Service). Br Med J. 1978: 587. [DOI] [PMC free article] [PubMed]
- 33.Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100–14. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657–62. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl 6):S783–90. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(4):257–65. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Leung GM, Tang JW, Yang X, Chao CY, Lin JZ, et al. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air. 2007;17(1):2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 38.Seto WH. Airborne transmission and precautions: facts and myths. J Hosp Infect. 2015;89(4):225–8. doi: 10.1016/j.jhin.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloch AB, Orenstein WA, Ewing WM, Spain WH, Mallison GF, Herrmann KL, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75(4):676–83. [PubMed] [Google Scholar]
- 40.Hutton MD, Stead WW, Cauthen GM, Bloch AB, Ewing WM. Nosocomial transmission of tuberculosis associated with a draining abscess. J Infect Dis. 1990;161(2):286–95. doi: 10.1093/infdis/161.2.286. [DOI] [PubMed] [Google Scholar]
- 41.Wong TW, Lee CK, Tam W, Lau JT, Yu TS, Lui SF, et al. Cluster of SARS among medical students exposed to single patient. Hong Kong Emerg Infect Dis. 2004;10(2):269–76. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Huang X, Yu IT, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 43.Yu IT, Wong TW, Chiu YL, Lee N, Li Y. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40(9):1237–43. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. Infect Dis. 2005;191(9):1472–7. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48(4):438–40. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 46.Lindsley WG, Blachere FM, Davis KA, Pearce TA, Fisher MA, Khakoo R, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50(5):693–8. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 47.Stelzer-Braid S, Oliver BG, Blazey AJ, Argent E, Newsome TP, Rawlinson WD, et al. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81(9):1674–9. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 48.Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5(11):e15100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris G, Kokki MH, Anderson K, Richardson MD. Sampling of Aspergillus spores in air. J Hosp Infect. 2000;44:81–92. doi: 10.1053/jhin.1999.0688. [DOI] [PubMed] [Google Scholar]
- 50.Department of Health . Health Technical Memorandum HTM 03–01: specialised ventilation for healthcare premises, part a: design and validation. London: The Stationary Office; 2007. [Google Scholar]
- 51.Griffiths WD, Stewart IW, Futter SJ, Upton SL, Mark D. The development of sampling methods for the assessment of indoor bioaerosols. J Aerosol Sci. 1997;28:437–57. doi: 10.1016/S0021-8502(96)00446-6. [DOI] [Google Scholar]
- 52.Falvey DG, Streifel AJ. Ten-year air sample analysis of Aspergillus prevalence in a university hospital. J Hosp Infect. 2007;67:35–41. doi: 10.1016/j.jhin.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Yao M, Mainelis G. Analysis of portable impactor performance for enumeration of viable bioaerosols. J Occup Environ Hyg. 2007;4:514–24. doi: 10.1080/15459620701407388. [DOI] [PubMed] [Google Scholar]
- 54.Lee KS, Bartlett KH, Brauer M, Stephens GM, Black WA, Teschke K. A field comparison of four samplers for enumerating fungal aerosols I. Sampling characteristics. Indoor Air. 2004;14:360–6. doi: 10.1111/j.1600-0668.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- 55.Li K. Molecular comparison of the sampling efficiency of four types of airborne bacterial samplers. Sci Total Environ. 2011;409:5493–8. doi: 10.1016/j.scitotenv.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;5:79–84. doi: 10.1053/jhin.2002.1217. [DOI] [PubMed] [Google Scholar]
- 57.Pasquarella C, Vitali P, Saccani E, Manotti P, Boccuni C, Ugolotti M, et al. Microbial air monitoring in operating theatres: experience at the University Hospital of Parma. J Hosp Infect. 2012;81:50–7. doi: 10.1016/j.jhin.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Cristina ML, Spagnolo AM, Sartini M, Panatto D, Gasparini R, Orlando P, et al. Can particulate air sampling predict microbial load in operating theatres for arthroplasty? PLoS One. 2012;7(12):e52809. doi: 10.1371/journal.pone.0052809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austin P, Elia M. A systematic review and meta-analysis of the risk of microbial contamination of aseptically prepared doses in different environments. J Pharm Pharmaceut Sci. 2009;12:233–42. doi: 10.18433/j3jp4b. [DOI] [PubMed] [Google Scholar]
- 60.Tang JW, Eames I, Li Y, Taha YA, Wilson P, Bellingan G, et al. Door-opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: the importance of vorticity and buoyancy airflows. J Hosp Infect. 2005;61(4):283–6. doi: 10.1016/j.jhin.2005.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riley RL, et al. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc Pulm Dis. 1957;75:420–31. doi: 10.1164/artpd.1957.75.3.420. [DOI] [PubMed] [Google Scholar]
- 62.Riley RL, The J. Burns Amberson Lecture: aerial dissemination of pulmonary tuberculosis. Am Rev Tuber Pulm Dis. 1957;76:931–41. doi: 10.1164/artpd.1957.76.6.931. [DOI] [PubMed] [Google Scholar]
- 63.Wells WF. Airborne contagion and air hygiene: an ecological study of droplet infection. Cambridge: Harvard University Press; 1955. [Google Scholar]
- 64.Escombe AR, Oeser C, Gilman RH, Navincopa M, Ticona E, Martínez C, et al. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis. 2007;44:1349–57. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Escombe AR, Moore DAJ, Gilman RH, Pan W, Navincopa M, Ticona E, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5(9):1387–97. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Escombe AR, Moore DAJ, Gilman RH, Navincopa M, Ticona E, Mitchell B, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLOS Med. 2009;6(3):e1000043. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107(5):421–32. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 68.Houk V, et al. The epidemiology of tuberculosis in a closed environment. Arc Environ Health. 1968;16:26–52. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 69.Fennelly KP, et al. Isolation of viable airborne Mycobacterium tuberculosis; a new method to study transmission. Am J Respir Crit Care Med. 2004;169:604–9. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 70.Liao CM, Cheng YH, Lin YJ, Hsieh NH, Huang TL, Chio CP, et al. A probabilistic transmission and population dynamic model to assess tuberculosis infection risk. Risk Anal. 2012;32(8):1420–32. doi: 10.1111/j.1539-6924.2011.01750.x. [DOI] [PubMed] [Google Scholar]
- 71.Truscott J, Fraser C, Hinsley W, Cauchemez S, Donnelly C, Ghani A, et al. Quantifying the transmissibility of human influenza and its seasonal variation in temperate regions. PLoS Curr. 2009;1:RRN1125. doi: 10.1371/currents.RRN1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, WHO Rapid Pandemic Assessment Collaboration et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324(5934):1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houk VN. Spread of tuberculosis via recirculated air in a naval vessel. The Byrd study. In: Kundsin RB, Eds. Ann N Y Acad Sci. 1980;353:10–24. doi: 10.1111/j.1749-6632.1980.tb18901.x. [DOI] [PubMed] [Google Scholar]
- 74.Nicas M. Refining a risk model for occupational tuberculosis transmission. Am Ind Hyg Assoc J. 1996;57:16–22. doi: 10.1080/15428119691015179. [DOI] [PubMed] [Google Scholar]
- 75.Beggs CB, Noakes CJ, Sleigh PA, et al. The transmission of tuberculosis in confirmed spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis. 2003;7:1015–26. [PubMed] [Google Scholar]
- 76.Ko G, Thompson KM, Nardell EA. Estimation of tuberculosis risk on a commercial airliner. Risk Anal. 2004;24:379–88. doi: 10.1111/j.0272-4332.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 77.Rutala WA, Jones SM, Worthington JM, et al. Efficacy of portable filtration units in reducing aerosolized particles in the size range of Mycobacterium tuberculosis. Infect Control Hosp Epidemiol. 1995;16:391–8. doi: 10.2307/30141894. [DOI] [PubMed] [Google Scholar]
- 78.Miller–Leiden S, Lobascio C, Nazaroff WW, et al. Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. J Air Waste Manag Assoc. 1996;46:869–82. doi: 10.1080/10473289.1996.10467523. [DOI] [PubMed] [Google Scholar]
- 79.Nardell EA. Nosocomial tuberculosis in the AIDS era: strategies for interrupting transmission in developed countries. Bull Int Union Tuberc Lung Dis. 1991;66:107–11. [PubMed] [Google Scholar]
- 80.Hannan MM, Azadian BS, Gazzard BG, et al. Hospital infection control in an era of HIV infection and multi–drug resistant tuberculosis. J Hosp Infect. 2000;44:5–11. doi: 10.1053/jhin.1999.0651. [DOI] [PubMed] [Google Scholar]
- 81.Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm Rep. 2005;54(RR-17):1–141. [PubMed] [Google Scholar]
- 82.Asano Y, Yoshikawa T, Ihira M, Furukawa H, Suzuki K, Suga S. Spread of varicella-zoster virus DNA to family members and environments from siblings with varicella in a household. Pediatrics. 1999;103(5):e61. doi: 10.1542/peds.103.5.e61. [DOI] [PubMed] [Google Scholar]
- 83.Yoshikawa T, Ihira M, Suzuki K, Suga S, Tomitaka A, Ueda H, et al. Rapid contamination of the environments with varicella-zoster virus DNA from a patient with herpes zoster. J Med Virol. 2001;63(1):64–6. doi: 10.1002/1096-9071(200101)63:1<64::AID-JMV1009>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki K, Yoshikawa T, Tomitaka A, Suzuki K, Matsunaga K, Asano Y. Detection of varicella-zoster virus DNA in throat swabs of patients with herpes zoster and on air purifier filters. J Med Virol. 2002;66(4):567–70. doi: 10.1002/jmv.2182. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K, Yoshikawa T, Ihira M, Ohashi M, Suga S, Asano Y. Spread of varicella-zoster virus DNA to the environment from varicella patients who were treated with oral acyclovir. Pediatr Int. 2003;45(4):458–60. doi: 10.1046/j.1442-200X.2003.01746.x. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki K, Yoshikawa T, Tomitaka A, Matsunaga K, Asano Y. Detection of aerosolized varicella-zoster virus DNA in patients with localized herpes zoster. J Infect Dis. 2004;189(6):1009–12. doi: 10.1086/382029. [DOI] [PubMed] [Google Scholar]
- 87.Gustafson TL, Lavely GB, Brawner ER, Jr, Hutcheson RH, Jr, Wright PF, Schaffner W. An outbreak of airborne nosocomial varicella. Pediatrics. 1982;70(4):550–6. [PubMed] [Google Scholar]
- 88.Anderson RM, May RM. Infectious diseases of humans. Dynamics and control. Oxford: Oxford University Press; 1992. [Google Scholar]
- 89.Ogunjimi B, Hens N, Goeyvaerts N, Aerts M, Van Damme P, Beutels P. Using empirical social contact data to model person to person infectious disease transmission: an illustration for varicella. Math Biosci. 2009;218(2):80–7. doi: 10.1016/j.mbs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Riley RL. Airborne infection. Am J Med. 1974;57(3):466–75. doi: 10.1016/0002-9343(74)90140-5. [DOI] [PubMed] [Google Scholar]
- 91.Riley RL. Prevention and control of airborne infection in the community. Ann N Y Acad Sci. 1980;353:331–9. doi: 10.1111/j.1749-6632.1980.tb18936.x. [DOI] [PubMed] [Google Scholar]
- 92.Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med. 2004;350(17):1710–2. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 93.Moss WJ. Measles control and the prospect of eradication. Curr Top Microbiol Immunol. 2009;330:173–89. doi: 10.1007/978-3-540-70617-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takashima Y, Schluter WW, Mariano KM L, Diorditsa S, Quiroz Castro M, Ou AC, et al. Progress toward measles elimination—Philippines, 1998–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):357–62. [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi S, Metcalf CJ, Ferrari MJ, Moss WJ, Truelove SA, Tatem AJ, et al. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science. 2015;347(6227):1240–2. doi: 10.1126/science.aaa3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su Q, Zhang Y, Ma Y, Zheng X, Han T, Li F, et al. Measles imported to the United States by children adopted from China. Pediatrics. 2015;135(4):e1032–7. doi: 10.1542/peds.2014-1947. [DOI] [PubMed] [Google Scholar]
- 97.Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2(3):145–55. doi: 10.1016/S1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 98.Tang JW, Li Y. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(12):758. doi: 10.1016/S1473-3099(07)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hall CB. The spread of influenza and other respiratory viruses: complexities and conjectures. Clin Infect Dis. 2007;45(3):353–9. doi: 10.1086/519433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McLean RL. The effect of ultraviolet radiation upon the transmission of epidemic influenza in long-term hospital patients. Am Rev Respir Dis. 1961;83(2 Part 2):36–8. [Google Scholar]
- 101.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19:S97–102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 102.Goldmann DA. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg Infect Dis. 2001;7:249–53. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37(8):1094–101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 104.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infec Dis. 2012;206:902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mertsola J, Ruuskanen O, Eerola E, Viljanen MK. Intrafamilial spread of pertussis. J Ped. 1983;103:359–63. doi: 10.1016/S0022-3476(83)80403-X. [DOI] [PubMed] [Google Scholar]
- 107.Etkind P, Lett SM, Macdonald PD, Silva E, Peppe J. Pertussis outbreaks in groups claiming religious exemptions to vaccinations. Am J Dis Child. 1992;146:173–6. doi: 10.1001/archpedi.1992.02160140039017. [DOI] [PubMed] [Google Scholar]
- 108.Storsaeter J, Gustafsson L. Absolute efficacy of acellular pertussis vaccines in household settings. Dev Biolog Standard. 1997;89:153–9. [PubMed] [Google Scholar]
- 109.Elahi S, Holmstrom J, Gerdts V. The benefits of using diverse animal models for studying pertussis. Trends Microbiol. 2007;15:462–8. doi: 10.1016/j.tim.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008;9(3):201–11. doi: 10.1016/j.prrv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 111.Bauer MP, van Dissel JT, Kuijper EJ. Clostridium difficile: controversies and approaches to management. Curr Opin Infect Dis. 2009;22:517–24. doi: 10.1097/QCO.0b013e32833229ce. [DOI] [PubMed] [Google Scholar]
- 112.Mulligan ME, Rolfe RD, Finegold SM, George WL. Contamination of a hospital environment by Clostridium difficile. Curr Microbiol. 1979;3:173–5. doi: 10.1007/BF02601862. [DOI] [Google Scholar]
- 113.Larson HE, Price AB, Borriello SP. Epidemiology of experimental enterocecitis due to Clostridium difficile. J Infect Dis. 1980;142:408–13. doi: 10.1093/infdis/142.3.408. [DOI] [PubMed] [Google Scholar]
- 114.Toshniwal R, Silva J, Jr, Fekety R, Kim KH. Studies on the epidemiology of colitis due to Clostridium difficile in hamsters. J Infect Dis. 1981;143:51–4. doi: 10.1093/infdis/143.1.51. [DOI] [PubMed] [Google Scholar]
- 115.Fekety R, Silva J, Armstrong J, et al. Treatment of antibiotic-associated enterocolitis with vancomycin. Rev Infect Dis. 1981;3(suppl):S273–81. [PubMed] [Google Scholar]
- 116.Malamou-Ladas H, O’Farrell S, Nash JQ, Tabaqchali S. Isolation of Clostridium difficile from patients and the environment of hospital wards. J Clin Pathol. 1983;36:88–92. doi: 10.1136/jcp.36.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roberts K, Smith CF, Snelling AM, et al. Aerial dissemination of Clostridium difficile spores. BMC Infect Dis. 2008;8:7. doi: 10.1186/1471-2334-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larson HE. The experimental pathogenesis of antibiotic related colitis. Scand J Infect Dis Suppl. 1980; (suppl 22):7–10. [PubMed]
- 119.Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for airborne dispersal of clostridium difficile from symptomatic patients. Clin Infect Dis. 2010;50:1450–7. doi: 10.1086/652648. [DOI] [PubMed] [Google Scholar]
- 120.Best EL, Sandoe JA, Wilcox MH. Potential for aerosolization of Clostridium difficile after flushing toilets: the role of toilet lids in reducing environmental contamination risk. J Hosp Infect. 2012;80(1):1–5. doi: 10.1016/j.jhin.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 121.Said MA, Perl TM, Sears CL. Gastrointestinal flu: norovirus in health care and long-term care facilities. Clin Infect Dis. 2008;47:1202–8. doi: 10.1086/592299. [DOI] [PubMed] [Google Scholar]
- 122.Marks PJ, Vipond IB, Carlisle D, Deakin D, Fey RE, Caul EO. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol Infect. 2000;124:481–7. doi: 10.1017/S0950268899003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marks PJ, Vipond IB, Regan FM, Wedgwood K, Fey RE, Caul EO. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol Infect. 2003;131:727–36. doi: 10.1017/S0950268803008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sawyer LA, Murphy JJ, Kaplan JE, et al. 25- to 30-nm virus particle associated with a hospital outbreak of acute gastroenteritis with evidence for airborne transmission. Am J Epidemiol. 1988;127:1261–71. doi: 10.1093/oxfordjournals.aje.a114918. [DOI] [PubMed] [Google Scholar]
- 125.Milton DK. What was the primary mode of smallpox transmission? Impl Biodefense Front Cell Infect Microbiol. 2012;2:150. doi: 10.3389/fcimb.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kool JL. Risk of person-to-person transmission of pneumonic plague. Clin Infect Dis. 2005;40(8):1166–72. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 127.Allegra PC, Cochrane D, Dunn E, Milano P, Rothman J, Allegra J. Emergency department visits for concern regarding anthrax—New Jersey, 2001. MMWR Morb Mortal Wkly Rep. 2005;54(Suppl):163–7. [PubMed] [Google Scholar]
- 128.Doganay L, Welsby PD. Anthrax: a disease in waiting? Postgrad Med J. 2006;82(973):754–6. doi: 10.1136/pgmj.2005.044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Egan JR, Hall IM, Leach S. Modeling inhalational tularemia: deliberate release and public health response. Biosecur Bioterror. 2011;9(4):331–43. doi: 10.1089/bsp.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park JB, Simpson LL. Inhalational poisoning by botulinum toxin and inhalation vaccination with its heavy-chain component. Infect Immun. 2003;71(3):1147–54. doi: 10.1128/IAI.71.3.1147-1154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sobel J. Botulism. Clin Infect Dis. 2005;41(8):1167–73. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 132.Leffel EK, Reed DS. Marburg and Ebola viruses as aerosol threats. Biosecur Bioterror. 2004;2(3):186–91. doi: 10.1089/bsp.2004.2.186. [DOI] [PubMed] [Google Scholar]
- 133.Zumbrun EE, Bloomfield HA, Dye JM, Hunter TC, Dabisch PA, Garza NL, et al. A characterization of aerosolized Sudan virus infection in African green monkeys, cynomolgus macaques, and rhesus macaques. Viruses. 2012;4(10):2115–36. doi: 10.3390/v4102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zumbrun EE, Abdeltawab NF, Bloomfield HA, Chance TB, Nichols DK, Harrison PE, et al. Development of a murine model for aerosolized ebolavirus infection using a panel of recombinant inbred mice. Viruses. 2012;4(12):3468–93. doi: 10.3390/v4123468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schieffelin JS, Shaffer JG, Goba A, KGH Lassa Fever Program. Viral Hemorrhagic Fever Consortium. WHO Clinical Response Team et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371(22):2092–100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ebola Response Team WHO, Agua-Agum J, Ariyarajah A, Aylward B, Blake IM, Brennan R, et al. West African Ebola epidemic after one year—slowing but not yet under control. N Engl J Med. 2015;372(6):584–7. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(Suppl 1):S87–91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 138.Roels TH, Bloom AS, Buffington J, Muhungu GL, Mac Kenzie WR, Khan AS, et al. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis. 1999;179(Suppl 1):S92–7. doi: 10.1086/514286. [DOI] [PubMed] [Google Scholar]
- 139.CIDRAP. COMMENTARY: health workers need optimal respiratory protection for Ebola. http://www.cidrap.umn.edu/news-perspective/2014/09/commentary-health-workers-need-optimal-respiratory-protection-ebola. 2014a. Accessed 7 March 2015
- 140.CIDRAP. COMMENTARY: Ebola virus transmission via contact and aerosol—a new paradigm. http://www.cidrap.umn.edu/news-perspective/2014/11/commentary-ebola-virus-transmission-contact-and-aerosol-new-paradigm. 2014b. Accessed 7 March 2015
- 141.Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, Murphy FA, et al. Transmission of Ebola viruses: what we know and what we do not know. MBio. 2015;6(2):e00137–15. doi: 10.1128/mBio.00137-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beggs CB, Kerr KG, Noakes CJ, Hathway EA, Sleigh PA. The ventilation of multiple-bed hospital wards: review and analysis. Am J Infect Control. 2008;36(4):250–9. doi: 10.1016/j.ajic.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Nielsen PV. CFD and ventilation research. Indoor Air. 2011;21(6):442–53. doi: 10.1111/j.1600-0668.2011.00723.x. [DOI] [PubMed] [Google Scholar]
- 144.King MF, Noakes CJ, Sleigh PA. Modeling environmental contamination in hospital single- and four-bed rooms. Indoor Air. 2015 doi: 10.1111/ina.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ng S, Cowling BJ, Fang VJ, Chan KH, Ip DK, Cheng CK, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50(5):707–14. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee CK, Lee HK, Loh TP, Lai FY, Tambyah PA, Chiu L, et al. Comparison of pandemic (H1N1) 2009 and seasonal influenza viral loads. Singapore Emerg Infect Dis. 2011;17(2):287–91. doi: 10.3201/eid1702.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ip DK, Schutten M, Fang VJ, Fung RO, Dutkowski RT, Chan KH, et al. Validation of self-swab for virologic confirmation of influenza virus infections in a community setting. J Infect Dis. 2012;205(4):631–4. doi: 10.1093/infdis/jir803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Correia J, et al. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for the transmission of severe acute respiratory syndrome. Chest. 2004;125(3):1155–7. doi: 10.1378/chest.125.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang JW, Settles GS. Images in clinical medicine. Coughing and aerosols. N Engl J Med. 2008;359(15):e19. doi: 10.1056/NEJMicm072576. [DOI] [PubMed] [Google Scholar]
- 151.Tang JW, Settles G. Images in clinical medicine. Coughing and masks. N Engl J Med. 2009;361(26):e62. doi: 10.1056/NEJMicm0904279. [DOI] [PubMed] [Google Scholar]
- 152.Tang JW, Liebner TJ, Craven BA, Settles GS. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(Suppl 6):S727–36. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tang JW, Nicolle AD, Pantelic J, Jiang M, Sekhr C, Cheong DK, et al. Qualitative real-time schlieren and shadowgraph imaging of human exhaled airflows: an aid to aerosol infection control. PLoS One. 2011;6(6):e21392. doi: 10.1371/journal.pone.0021392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tang JW, Nicolle A, Pantelic J, Koh GC, Wang LD, Amin M, et al. Airflow dynamics of coughing in healthy human volunteers by shadowgraph imaging: an aid to aerosol infection control. PLoS One. 2012;7(4):e34818. doi: 10.1371/journal.pone.0034818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tang JW, Nicolle AD, Klettner CA, Pantelic J, Wang L, Suhaimi AB, et al. Airflow dynamics of human jets: sneezing and breathing—potential sources of infectious aerosols. PLoS One. 2013;8(4):e59970. doi: 10.1371/journal.pone.0059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nishimura H, Sakata S, Kaga A. A new methodology for studying dynamics of aerosol particles in sneeze and cough using a digital high-vision, high-speed video system and vector analyses. PLoS One. 2013;8(11):e80244. doi: 10.1371/journal.pone.0080244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hui DS, Ip M, Tang JW, Wong AL, Chan MT, Hall SD, et al. Airflows around oxygen masks: a potential source of infection? Chest. 2006;130(3):822–6. doi: 10.1378/chest.130.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ip M, Tang JW, Hui DS, Wong AL, Chan MT, Joynt GM, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35(10):684–9. doi: 10.1016/j.ajic.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hui DS, Chow BK, Ng SS, Chu LC, Hall SD, Gin T, et al. Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest. 2009;136(4):998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hui DS, Chow BK, Chu L, Ng SS, Lai ST, Gin T, et al. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology. 2011;16(6):1005–13. doi: 10.1111/j.1440-1843.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 161.Qian H, Li Y, Nielsen PV, Hyldgaard CE, Wong TW, Chwang AT. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air. 2006;16(2):111–28. doi: 10.1111/j.1600-0668.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 162.Chao CYH, Wan MP, Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, et al. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009;40:122–33. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pantelic J, Sze-To GN, Tham KW, Chao CY, Khoo YC. Personalized ventilation as a control measure for airborne transmissible disease spread. J R Soc Interface. 2009;6(Suppl 6):S715–26. doi: 10.1098/rsif.2009.0311.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gupta JK, Lin CH, Chen Q. Flow dynamics and characterization of a cough. Indoor Air. 2009;19(6):517–25. doi: 10.1111/j.1600-0668.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 165.Gupta JK, Lin CH, Chen Q. Characterizing exhaled airflow from breathing and talking. Indoor Air. 2010;20(1):31–9. doi: 10.1111/j.1600-0668.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 166.Chen C, Lin CH, Jiang Z, Chen Q. Simplified models for exhaled airflow from a cough with the mouth covered. Indoor Air. 2014;24(6):580–91. doi: 10.1111/ina.12109. [DOI] [PubMed] [Google Scholar]
- 167.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 168.Ng TC, Lee N, Hui SC, Lai R, Ip M. Preventing healthcare workers from acquiring influenza. Infect Control Hosp Epidemiol. 2009;30(3):292–5. doi: 10.1086/595690. [DOI] [PubMed] [Google Scholar]
- 169.Gralton J, McLaws ML. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Crit Care Med. 2010;38(2):657–67. doi: 10.1097/CCM.0b013e3181b9e8b3. [DOI] [PubMed] [Google Scholar]
- 170.Fineberg HV. Pandemic preparedness and response—lessons from the H1N1 influenza of 2009. N Engl J Med. 2014;370(14):1335–42. doi: 10.1056/NEJMra1208802. [DOI] [PubMed] [Google Scholar]
- 171.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, EASE Study Consortium et al. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic—the risk of aerosol generation during medical procedures. PLoS One. 2013;8(2):e56278. doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Noti JD, Lindsley WG, Blachere FM, Cao G, Kashon ML, Thewlis RE, et al. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis. 2012;54(11):1569–77. doi: 10.1093/cid/cis237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lindsley WG, King WP, Thewlis RE, Reynolds JS, Panday K, Cao G, et al. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. J Occup Environ Hyg. 2012;9(12):681–90. doi: 10.1080/15459624.2012.725986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207(7):1037–46. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 176.Imai M, Herfst S, Sorrell EM, Schrauwen EJ, Linster M, De Graaf M, et al. Transmission of influenza A/H5N1 viruses in mammals. Virus Res. 2013;178(1):15–20. doi: 10.1016/j.virusres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mubareka S, Lowen AC, Steel J, Coates AL, García-Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis. 2009;199(6):858–65. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Steel J, Staeheli P, Mubareka S, García-Sastre A, Palese P, Lowen AC. Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. J Virol. 2010;84(1):21–6. doi: 10.1128/JVI.01732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Richard M, Schrauwen EJ, de Graaf M, Bestebroer TM, Spronken MI, van Boheemen S, et al. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature. 2013;501(7468):560–3. doi: 10.1038/nature12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Azhar EI, Hashem AM, El-Kafrawy SA, Sohrab SS, Aburizaiza AS, Farraj SA, et al. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. MBio. 2014;5(4):e01450–14. doi: 10.1128/mBio.01450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.CIDRAP 2014c. COMMENTARY: Protecting health workers from airborne MERS-CoV—learning from SARS. http://www.cidrap.umn.edu/news-perspective/2014/05/commentary-protecting-health-workers-airborne-mers-cov-learning-sars.


