Abstract
Granzymes (Grs), serine proteases present in granules of effector lymphocytes, are involved in several host immune responses, including the activation of cell death and inflammatory pathways. The main goal of this study was to determine whether the local cell-mediated Gr pathway is activated during severe respiratory syncytial virus (RSV) lower respiratory tract illness (LRTI) in children. Tracheal aspirates (TA) from 23 children with RSV-LRTI and 12 controls without pulmonary disease were analyzed for Gr A and B. Bronchoalveolar lavage fluid samples from seven children with RSV-LRTI were analyzed for cellular expression of GrB. Levels of GrA and GrB in TA were significantly increased in RSV patients compared with controls and both Grs showed preserved activity. Gr levels correlated with the total leukocyte counts and IL-8 levels in the airways at several time points. However, no correlation between Gr levels and release of caspase-cleaved cytokeratin-18 was found. There was evidence for marked expression of GrB by both CD8+ and CD4+ T cells and natural killer cells in the respiratory tract. These findings suggest activation of the cell-mediated Gr pathway during severe RSV-LRTI in children.
Main
Respiratory syncytial virus (RSV) is a major respiratory pathogen among infants and young children (1). Although in general, RSV infection is limited to the upper respiratory tract, the disease may progress to the lower airways leading to acute hypoxemic respiratory failure. The most severe cases of RSV-lower respiratory tract illness (RSV-LRTI) fulfill the criteria of acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) (2). Despite decades of research, the exact mechanisms that determine the development of severe RSV-LRTI in previously healthy children remain unclear.
One hypothesis proposes that activation of cell death pathways directed against RSV-infected cells and/or uninfected bystander cells contributes to disease severity (3). Although regulated cell death or apoptosis seems an important mechanism for RSV clearance, Welliver et al. showed marked expression of the apoptosis marker caspase-3 in airway epithelium of children with fatal RSV-LRTI, suggesting an imbalance in this process (4,5). Interestingly, increasing evidence implicates epithelial apoptosis in the pathogenesis of ALI/ARDS (6). Several studies demonstrated increased expression of classical apoptotic mediators such as FasL and granzymes (Grs) in the lungs of adults with ALI/ARDS correlating with disease severity (7,8). These findings implicate enhanced activation of apoptotic pathways in the pathogenesis of acute inflammatory lung diseases and may be relevant for severe RSV-LRTI in children.
In antiviral host immune response an important cell death pathway is the granule exocytosis pathway involving the serine proteases GrA and GrB, which is exploited by CD8+ T-lymphocytes (CTLs) and natural killer (NK) cells [reviewed elsewhere (9)]. Grs induce rapid cell death when directed into a target cell, and this mechanism is facilitated by the protein perforin but may also occur independently from perforin (10). Detection of free extracellular GrA and GrB is considered to reflect cytotoxic activation of the cell-mediated immune response (11,12). However, proteolytic active extracellular Grs may also contribute to the activation of pro-inflammatory cytokine release and degradation of the extracellular matrix, indicating that Grs may be involved in several mechanisms of the host immune response (13).
Studies investigating the cell-mediated immune response in the lungs of children with RSV-LRTI have reported limited numbers of effector lymphocytes (5,14), although in rodents both CTLs and NK cells are recruited to the lungs upon primary RSV infection where they appear to contribute to disease pathogenesis (15–17). In the present study, we hypothesized that severe RSV-LRTI in children is associated with local activation of the Gr pathway by the cell-mediated host immune response. To test this, we investigated extracellular GrA and GrB and cellular expression of GrB in the respiratory tract of infants with RSV-LRTI.
METHODS
Patients and sample collection.
All protocols were approved by the Academic Medical Center ethical committee and informed consent was obtained from parents. Tracheal aspirate (TA) samples were obtained from 23 children with RSV-LRTI and 12 age-matched controls without a pulmonary condition. All patients were admitted to the intensive care unit for mechanical ventilation (MV) between November 2003 and March 2006. Infection with RSV was proven by direct immunofluorescence assay (Imagen, DakoCytomation, UK) of nasopharyngeal aspirate. As part of an international randomized placebo-controlled trial on the use of glucocorticosteroids during RSV-LRTI (www.star-trial.com), RSV patients received dexamethasone 0.15 mg/kg/dose, i.v. or placebo (QID, eight doses in total) starting within 24 h after start of MV. Oxygenation index calculated as (FiO2 × mean airway pressure (cmH2O) × 100)/Pao2 (kPa), during the first 24 h was used to assess severity of oxygenation anomaly. If more than one arterial blood gas was obtained, the best index was chosen. Pediatric risk of mortality (PRISM) scores were used as a measure of disease severity.
TA was collected as described before (18). Aspirate was collected without previous installation of fluids on the day of start of MV and in the RSV-patients on day 2 and 4 as long as the patient was intubated. An arterial blood sample was obtained from 15 RSV-patients on the day of start of MV.
Sample processing.
After determination of the volume of the aspirate sample, an equal volume of cold 10 mM DTT (Sigma Chemical Co.-Aldrich, St. Louis, MO) in 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 8.0 was added at 4°C followed by 15 min mixing. If the aspirate remained mucoid this process was repeated once with a similar amount of DTT. Remaining cellular aggregates were cleared by adding DNAse (Sigma Chemical Co.-Aldrich) at 4°C. Cells in processed aspirate were collected by centrifugation at 450g for 10 min. Supernatant was aspirated, aliquoted, and stored at −80°C. The cell pellet was resuspended in PBS, and total white blood cells (WBC) were counted in a Bürker bright line counting chamber. Air-dried cytospins were stained with Romanovsky (Diff-Quick) and differential WBC counts were obtained by counting 300 leukocytes using a standard light microscope. Reliable differential counts could not be obtained in 18% of the samples because of debris and some degeneration of cells.
Immunoassays.
Extracellular GrA and GrB were measured by immunoassays (Sanquin, the Netherlands) according to the manufacturer's description. Interleukin (IL)-8 was measured by immunoassay using monoclonal antibody (MAb) against IL-8 (R&D Systems) for capture and detection. Extracellular caspase-cleaved cytokeratin-18 (CK18), a marker of epithelial cell apoptosis, was measured using the M30-Apoptosense sandwich immunoassay (Peviva AB, Sweden). The M30 antigen levels are expressed as Units/mL, where 1 U corresponds to 1.24 pmol of recombinant M30-containing peptide. Assay lower limit: 0.7 U/mL.
TA samples were diluted minimally 1:100 (GrA/B and IL-8) and 1:20 (cleaved CK18) to overcome interference of DTT with the assays and serial dilutions were tested. All reported antigen levels in TA are corrected for the sample processing dilution factor.
Granzyme activity.
Activity of GrA was measured by a recently developed immunoassay as described elsewhere (19). For measurement of GrB activity, GrB in samples was captured on microtiter plates coated with anti-GrB MAb GB-11 (2 μg/mL in 0.1 M sodium carbonate/bicarbonate buffer, pH 5.5). The plates were then washed and incubated with GrB-specific chromogenic substrate Ac-Ile-Glu-Thr-Asp-pNA [Alexis Biochemicals, Lausen, Switzerland; 0.4 mM in 50 mM Tris, 100 mM NaCl and 0.1% Tween (vol/vol), pH 7.4]. GrB activity was measured for 4 h at 37°C at an absorbance (A) of 405 nm on a Titer-Tek Multiscan (Labsystems, Helsinki, Finland) and is expressed as deltaA per hour.
Fluorescent-activated cell sorting (FACS) analysis.
FACS analysis was performed in bronchoalveolar lavage fluid (BALF) samples from mechanically ventilated children with RSV-LRTI in the winters of 2006–2008. BALF was obtained by three subsequent instillations of 1 mL/kg of 0.9% saline through a wedged suction catheter passed through the endotracheal tube. After each instillation, fluid was suctioned (mean ± SE recovery: 36% ± 4). The last two samples were pooled and 10 min centrifuged at 450g. The pooled BALF cells in FACS buffer (0.5% BSA, 0.02% potassium-EDTA in PBS) containing 10% normal human serum were labeled with APC-labeled anti-CD3, PerCPCy5-labeled CD8 or -CD4 (BD Pharmingen, San Jose, CA) and FITC-labeled anti CD16 (Sanquin, the Netherlands) and −56 (BD Pharmingen). For intracellular GrB staining cells were fixed and permeabilized with fixation/permeabilization solution (BD Pharmingen) and stained with PE-labeled anti-GrB MAb (Sanquin) or a PE-labeled isotype control (BD Pharmingen). Cells were analyzed using a FACSCalibur flow cytometer and CellQuest Pro software (BD Biosciences).
Statistical analysis.
Not normally distributed data are expressed as medians and were analyzed by using the Mann-Whitney U test for differences between groups. For normally distributed data, a Student t test was used to compare group means. Spearman's correlation coefficient was calculated to assess the degree of association between Grs and studied markers. Proportions in the patient groups were compared by Fisher's exact test. A two-sided p value of <0.05 was considered statistically significant.
RESULTS
Baseline patient characteristics.
The baseline characteristics of the patients are shown in Table 1. Ten RSV patients (43%) had received dexamethasone as part of the aforementioned randomized placebo-controlled trial. Total WBC counts and differentials in TA samples of controls and RSV patients are shown in Table 2.
Table 1.
Baseline patient characteristics
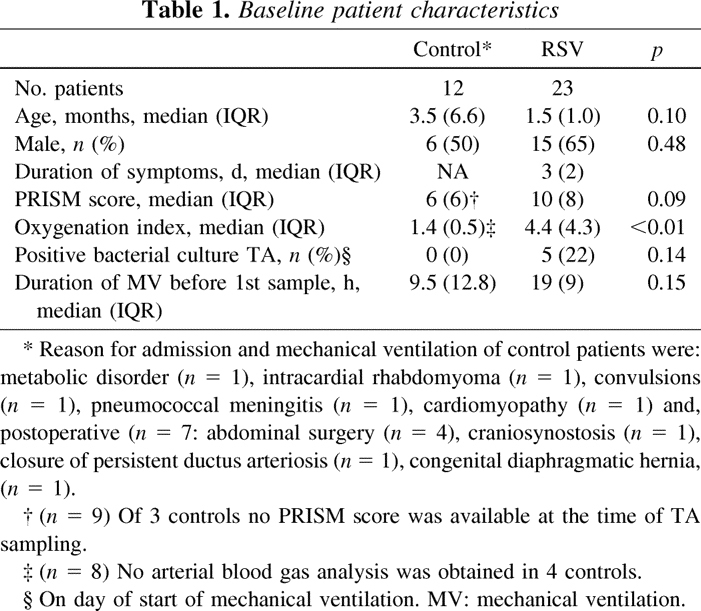
Table 2.
Total white blood cell (WBC) counts and differentials in TA samples of mechanically ventilated patients without pulmonary disease (controls) and RSV patients on the day of start of mechanical ventilation
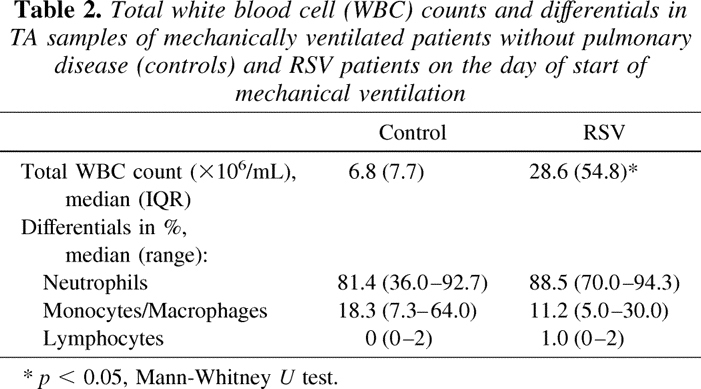
Elevated GrA and GrB in TA during severe RSV-LRTI.
The median (range) level of GrA in TA on the day of start of MV was 0.6 (0.3–14.3) ng/mL and 11.1 (0.3–98.4) ng/mL in controls and RSV-patients respectively (p < 0.01, Fig. 1A). Likewise, the median (range) level of GrB in TA was higher in the RSV patients (69.0, 3.1–728.0, ng/mL) compared with the controls (1.7 ng/mL, 0.5–39.6, ng/mL) (p < 0.01, Fig. 1B). There was a significant correlation between the levels of GrA and GrB in TA (Spearman r = 0.67, p < 0.001). In the RSV patients, the plasma levels of GrA (median 63.0, range 3.0–180.0, pg/mL) and GrB (median 35.0, range 13.0–92.0, pg/mL) on the first day of MV were significantly lower than the levels in TA (p < 0.001 for both comparisons, data not shown).
Figure 1.
(A–B) Levels of GrA and GrB (ng/mL) in TA from children without pulmonary condition (controls, n = 12) or with RSV-LRTI (n = 23) on the day of start of mechanical ventilation (MV). Bars represent median values. Dotted lines represent lowest detectable levels. *p < 0.01. (C–D) Relative change of median levels of GrA and GrB in TA on day 2 (n = 23) and day 4 (n = 14) from baseline (day of start of MV, n = 23) in RSV patients who received placebo (□, n = 13) or dexamethasone (▪, n = 10) treatment. Error bars show the 25th or 75th percentile. p = n.s.
GrA and GrB in TA were detected throughout the course of RSV-LRTI, but no effect of dexamethasone as compared with placebo on the Gr levels was found (Fig. 1C and D). No difference in Gr levels was found between RSV patients with or without a positive bacterial culture of TA.
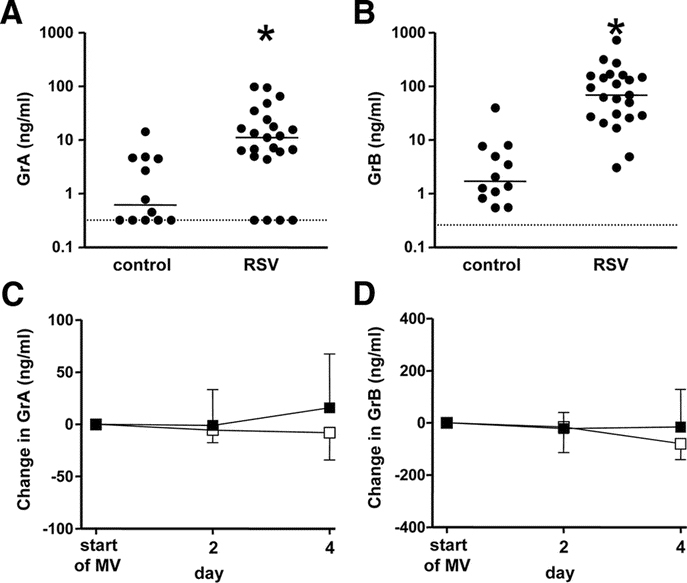
GrA and GrB in TA have retained activity.
To ascertain biologic activity of GrA and GrB in the airways of RSV patients we measured active GrA and GrB with enzyme capture assays. Because the sensitivity of these assays was 50 pg/mL and 1 ng/mL respectively, only 10 samples of TA containing high levels of GrA and GrB were measured. There was a significant correlation between the levels of active GrA and total GrA antigen (Spearman r = 0.82, p < 0.01) (Fig. 2A). In addition, we found a positive correlation between GrB activity and total GrB antigen (Spearman r = 0.62, p = 0.05) (Fig. 2B).
Figure 2.
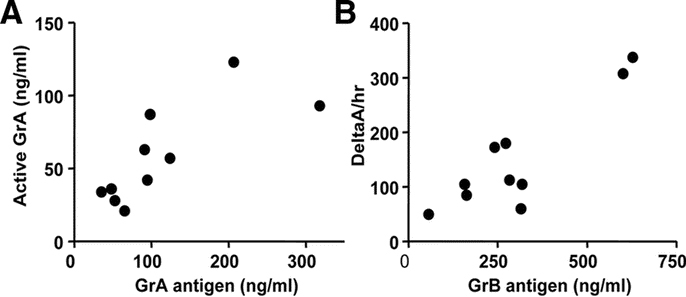
(A) Correlation between the levels (ng/mL) of GrA antigen and active GrA in TA (Spearman r = 0.82, p < 0.01). (B) Correlation between the level of total GrB antigen (ng/mL) and GrB proteolytic activity (deltaA per hr) in TA (Spearman r = 0.62, p = 0.05).
Granzymes in TA correlate with inflammatory markers, but not with cleaved-CK18.
There was a positive correlation between GrA and GrB and total WBC counts and IL-8 in TA in the RSV patients and this correlation tended to be stronger after the first days of MV (Fig. 3). However, no correlation between Gr levels and disease severity at baseline, as determined by PRISM scores and oxygenation index, was found (data not shown).
Figure 3.
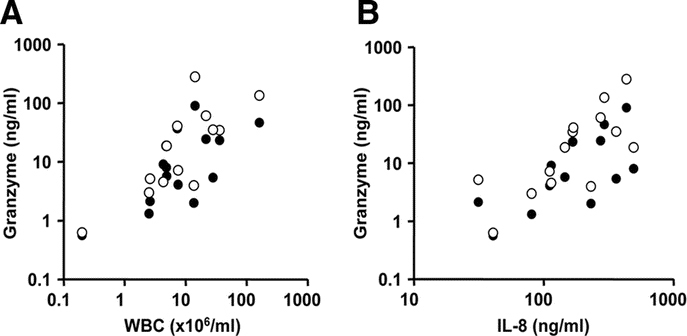
(A) Correlation between the levels of GrA (•) or GrB (○) and total white blood cell counts (A) and IL-8 levels (B) in TA of RSV patients on day 4 after start of MV. Spearman r > 0.63 (p < 0.05) for all comparisons.
To study lung epithelial apoptosis in relation to extracellular Grs, we analyzed the correlation between GrB and cleaved-CK18 in TA. CK-18 is an epithelium-specific intermediate filament protein which is cleaved by caspases early during apoptosis and may be subsequently released from cells into the extracellular space (20). Caspases are activated by GrB, but not by GrA. There was no significant correlation between GrB and cleaved-CK18 in TA at any time point. Also, no statistically significant difference between cleaved-CK18 levels (U/mL) of controls (median 14.5, IQR 39.2) compared with the RSV patients (median 8.4, IQR 17.7) was found (p = 0.20).
GrB expression by lymphocytes in BALF.
To find a potential source of Grs in the lungs of children with severe RSV-LRTI, we performed FACS analysis in BALF samples in an additional cohort of mechanically ventilated children (mean age 1.4 ± 0.2 mo, n = 7) with RSV-LRTI. These patients did not differ from the original cohort in terms of age, gender distribution, and duration of illness (data not shown). T cells and NK cells were detected in low numbers throughout the course of RSV-LRTI (Table 3). GrB-positive cells were found predominantly among cells within the side/forward scatter region of the lymphocyte population (Fig. 4A–E). In addition to CD8+ T cells and NK cells, CD8− T cells were found to express GrB (Fig. 4F–J). This latter observation suggests that besides CD4−CD8−T cells (which may include gamma/delta T cells) CD4+ T cells may express GrB, which was confirmed in an additional FACS analysis of four RSV patients (Fig. 4K). The percentages of GrB-positive T cells and NK cells and corresponding mean fluorescence intensity are shown in Figure 5.
Table 3.
Mean percentages ± SE (of total cells) of cells expressing CD3 + , CD3 + CD8 + and CD3 + CD16/56 + (NK cells) in BALF of mechanically ventilated children with severe RSV infection, as determined by FACS analysis
Figure 4.
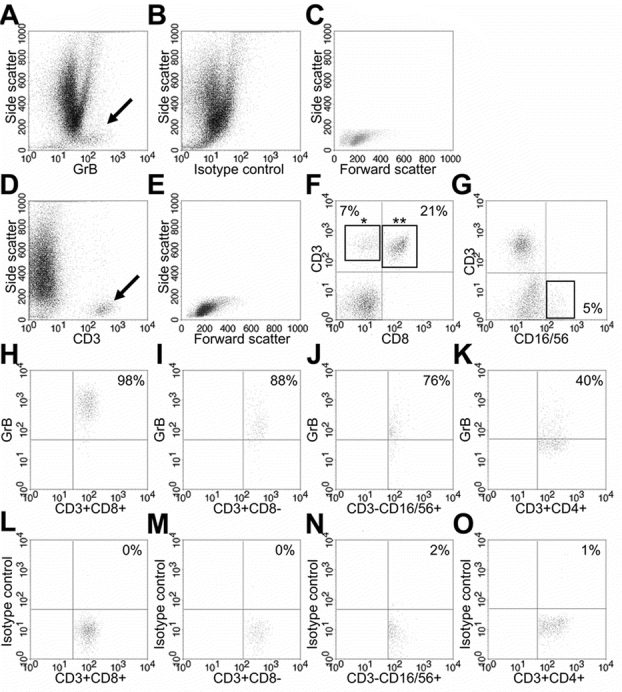
Representative examples of FACS scatter plots of RSV patients. (A) Side/anti-GrB MAb scatter plot showing the GrB-positive cell population (arrow), which is not detected in the same sample stained with an isotype control for GrB (B). (C) Side/forward scatter plot of the gated GrB-positive cell population. (D) Side/anti-CD3 MAb scatter plot showing a CD3-positive population (arrow). (E) Side/forward scatter plot of the gated CD3-positive cell population. Note that the GrB- positive cell population (C) is detected in the same side/forward region as the CD3-positive cell population. (F) Anti-CD3/anti-CD8 MAb scatter plot of a gated lymphocyte region (see E) showing CD3+CD8− cells (*) and CD3+CD8+ cells (**). (G) Anti-CD3/anti-CD16/56 MAb scatter plot of a gated lymphocyte region (see E) showing CD3−CD16/56+ (NK) cells. (H) Anti-GrB/anti-CD8 MAb scatter plot of a gated CD3+CD8+cell population [(L) Corresponding GrB isotype control plot]. (I) Anti-GrB/anti-CD3 MAb plot of a gated CD3+CD8− cell population [(M) Corresponding GrB isotype control plot]. (J) Anti-GrB/anti-CD16/56 MAb plot of a gated CD3−CD16/56+ cell population [(N) Corresponding GrB isotype control plot]. (K) Anti-GrB/anti-CD4 MAb plot of a gated CD3+CD4+ cell population [(O) Corresponding GrB isotype control plot].
Figure 5.
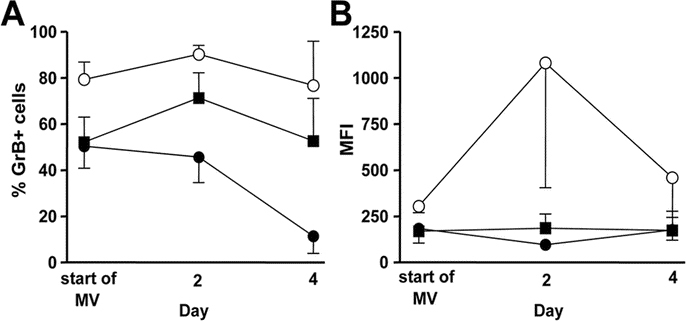
Mean percentages (A) and mean fluorescence intensity (B) of GrB-positive CTLs (CD3+CD8+: ○), CD3+CD8− cells (▪) and NK cells (CD3−CD16/56+: •) in BALF throughout the course of severe RSV-LRTI. First day of mechanical ventilation: n = 7, day 2: n = 6, day 4: n = 4. Error bars show the SE.
DISCUSSION
The main goal of this study was to determine whether severe RSV infection in children is associated with local activation of the Gr pathway by the cell-mediated host immune response. We report high levels of proteolytic active extracellular GrA en GrB and marked expression of GrB by T-lymphocytes and NK cells in the respiratory tract of children during the course of severe RSV-LRTI. The levels of extracellular GrA and GrB correlated with total WBC counts and IL-8 levels in the airways after the acute-onset of RSV disease, but no association with the epithelium apoptosis marker cleaved-CK18 could be demonstrated.
The present study extends our understanding of cell-mediated cytotoxic responses during severe RSV-LRTI in children. Numerous rodent studies have shown that both NK cells and CTLs are recruited to the lungs during primary RSV infection (15–17). Graham et al. reported that although CTLs are involved in the clearance of RSV, depletion of these lymphocytes diminishes clinical illness upon exposure to RSV (15). Treatment of RSV-infected CTL depleted mice with high dose RSV-specific CTLs results in viral clearance, but augments lung injury by causing severe hemorrhage and neutrophilic infiltration (21). These findings suggest that cell-mediated cytotoxic responses play an important role in RSV disease pathogenesis in mice, but the relevance in humans is unclear. In vitro studies show that CTLs isolated from peripheral blood of RSV infected infants lyse infected autologous target cells (22,23). However, Welliver et al. recently reported the near absence of CTLs and NK cells in lung tissues from nine infants who died of RSV-LRTI (5). Previous analysis of BALF samples of RSV-infected children showed that a small percentage of the total cells in the lungs is CD8-positive (14). Similarly, in the present study we found low numbers of T-lymphocytes and NK cells in the lungs. However, despite these low cell counts, we report high levels of extracellular Grs and marked GrB expression in these cell populations in the respiratory tract.
The activation of the cell-mediated Gr response in children with severe RSV-LRTI raises the possibility that this cell death pathway is involved in RSV disease pathogenesis. Direct killing of infected cells by Grs is considered a major host defense mechanism against viruses in general. In mice, both GrA and GrB are essential in controlling ectromelia and cytomegalovirus infection (24,25). Interestingly, viruses have been reported to encode proteins that inhibit Grs, suggesting pathogen immune evasive adaptations (26,27). On the other hand, enhanced death of infected cells and/or bystander cells may contribute to the development of disease by causing tissue dysfunction as has been suggested for severe inflammatory lung diseases (6,7,28). Welliver et al. found marked expression of caspase-3 in airway epithelium of children with fatal RSV-LRTI, a finding consistent with studies performed in adult patients with ALI/ARDS (5,29). The study of Welliver et al. suggests an imbalance in apoptosis during severe RSV-LRTI, but the involved cell death pathways remain unclear. Our findings may point toward a role for the Gr pathway in apopotic cell death during severe RSV-LRTI, but contrary to our expectations, we found no correlation between the levels of extracellular GrB and caspase-cleaved CK18 in the airways. The release of caspase cleaved CK18 is a surrogate marker of epithelial apoptosis but has not been studied extensively in vivo. The detection of cleaved CK18 in serum was found to correlate well with tissue damage in patients with hantavirus infection, but it is unclear how this protein behaves and reflects epithelial injury in other body fluids, including BALF (30). Histologic analysis of apoptosis to confirm our data are highly preferred but could not be performed in our study for obvious ethical reasons.
In addition to CTLs and NK cells, CD4+ T cells and basophils may express Grs (31,32). Recently, neutrophils also were shown to express Grs (33), although this has been debated by others (34). In the present study, GrB-positive cells were only found among lymphocytes. CTLs seemed to contribute to GrB expression the most, but actual degranulation and subsequent Gr release may have differed between the studied cell populations. The highest GrB expression by NK cells occurred on the first day of admission, before the peak of GrB expression by T cells. Similarly, in RSV infected mice the cytotoxic activity of NK cells is highest before CTLs are recruited to the lungs (16). Remarkably, Gr levels in the airways during severe RSV-LRTI were not affected by treatment with dexamethasone, although several studies in vitro have shown suppression of CTL and NK cytotoxic activity by corticosteroids (35,36).
Our finding of GrB expression by CD4+ T-lymphocytes is interesting because it suggests that the Gr pathway may be used to modulate immune responses to RSV. Devadas et al. have shown that TH2 cells express GrB which induces their own cell death (37). Furthermore, they reported that GrB deficient mice have augmented TH2 cytokine responses and lung cellular infiltrations in a model of allergic inflammation. Grossman et al. have shown that the Gr pathway is exploited by natural T-regulatory cells (CD4+CD25+) against autologous activated CD8+ and CD4+ T cells (38). These findings suggest that Grs function to control immune responses, in addition to their role in direct virus-infected cell killing. In contrast, another line of research proposes that Grs actively participate in the activation of pro-inflammatory responses. For example, studies in vitro have shown that proteolytic active GrA induces the release of IL-6 and IL-8 from epithelial cells and lung fibroblasts (13). In the present study, Gr levels significantly correlated with IL-8 levels in the airways during severe RSV-LRTI and with total WBC counts after the acute phase of respiratory failure. Moreover, we found evidence that active forms of GrA as well as GrB were present, confirming the potential to exert a biologic function.
This study has three potential limitations. First, all patients had severe acute hypoxemic respiratory failure necessitating MV and thus represent the end of the spectrum of RSV disease. This withholds us from drawing firm conclusions when relating the extent of activation of the Gr pathway with markers of disease severity such as PRISM scores and oxygenation index. Second, because all patients (including controls) were subjected to MV and supplemental oxygen it is possible that this might have had (synergistic) effects on Gr levels, inflammation or apoptosis. Third, soluble Grs were measured in TA that may reflect more localized (and thus variable) proximal epithelial lining fluid.
In conclusion, severe RSV-LRTI in children is associated with high levels of proteolytic active extracellular GrA and GrB and expression of GrB by lymphocytes in the respiratory tract. These findings suggest that the Gr pathway is activated by the local cell-mediated host immune response to RSV. Further studies should elucidate the exact role of Grs in the lungs during RSV disease.
Acknowledgements
We thank B. Smids, T. Dekker and M. Snoek for their excellent technical support, and T.A. Out and P.J.E.J van de Berg for expert discussions on FACS analysis. We thank Peviva AB for providing some ELISA materials.
Glossary
- BALF
bronchoalveolar lavage fluid
- CTL
cytotoxic T-lymphocyte
- Gr
granzyme
- MV
mechanical ventilation
- NK
natural killer
- RSV-LRTI
respiratory syncytial virus lower respiratory tract illness
- TA
tracheal aspirate
Footnotes
Supported by a grant from the Dutch Cancer Society and the Association for International Cancer Research (to M.B., J.P.M.) and the Landsteiner Foundation for Blood Research (LSBR, grant 0212, to A.M.W.).
References
- 1.Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 2.Hammer J, Numa A, Newth CJ. Acute respiratory distress syndrome caused by respiratory syncytial virus. Pediatr Pulmonol. 1997;23:176–183. doi: 10.1002/(SICI)1099-0496(199703)23:3<176::AID-PPUL2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Bem RA, Bos AP, Matute-Bello G, van Tuyl M, van Woensel JB. Lung epithelial cell apoptosis during acute lung injury in infancy. Pediatr Crit Care Med. 2007;8:132–137. doi: 10.1097/01.PCC.0000257207.02408.67. [DOI] [PubMed] [Google Scholar]
- 4.Viuff B, Tjornehoj K, Larsen LE, Rontved CM, Uttenthal A, Ronsholt L, Alexandersen S. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol. 2002;161:2195–2207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc. 2005;2:214–220. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 8.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 9.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 10.Pinkoski MJ, Hobman M, Heibein JA, Tomaselli K, Li F, Seth P, Froelich CJ, Bleackley RC. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998;92:1044–1054. [PubMed] [Google Scholar]
- 11.Takayama H, Trenn G, Humphrey W, Jr, Bluestone JA, Henkart PA, Sitkovsky MV. Antigen receptor-triggered secretion of a trypsin-type esterase from cytotoxic T lymphocytes. J Immunol. 1987;138:566–569. [PubMed] [Google Scholar]
- 12.Spaeny-Dekking EH, Hanna WL, Wolbink AM, Wever PC, Kummer AJ, Swaak AJ, Middeldorp JM, Huisman HG, Froelich CJ, Hack CE. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–3616. [PubMed] [Google Scholar]
- 13.Buzza MS, Bird PI. Extracellular granzymes: current perspectives. Biol Chem. 2006;387:827–837. doi: 10.1515/BC.2006.106. [DOI] [PubMed] [Google Scholar]
- 14.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussell T, Openshaw PJ. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 17.Openshaw PJ. Flow cytometric analysis of pulmonary lymphocytes from mice infected with respiratory syncytial virus. Clin Exp Immunol. 1989;75:324–328. [PMC free article] [PubMed] [Google Scholar]
- 18.van Woensel JB, Lutter R, Biezeveld MH, Dekker T, Nijhuis M, van Aalderen WM, Kuijpers TW. Effect of dexamethasone on tracheal viral load and interleukin-8 tracheal concentration in children with respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22:721–726. doi: 10.1097/01.inf.0000078165.62923.15. [DOI] [PubMed] [Google Scholar]
- 19.Spaeny-Dekking EH, Kamp AM, Froelich CJ, Hack CE. Extracellular granzyme A, complexed to proteoglycans, is protected against inactivation by protease inhibitors. Blood. 2000;95:1465–1472. [PubMed] [Google Scholar]
- 20.Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, Nap M, Bjorklund V, Bjorklund P, Bjorklund B, Lane EB, Omary MB, Jornvall H, Ramaekers FC. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacs D, Bangham CR, McMichael AJ. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;2:769–771. doi: 10.1016/S0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 23.Mbawuike IN, Wells J, Byrd R, Cron SG, Glezen WP, Piedra PA. HLA-restricted CD8+ cytotoxic T lymphocyte, interferon-gamma, and interleukin-4 responses to respiratory syncytial virus infection in infants and children. J Infect Dis. 2001;183:687–696. doi: 10.1086/318815. [DOI] [PubMed] [Google Scholar]
- 24.Mullbacher A, Waring P, Tha HR, Tran T, Chin S, Stehle T, Museteanu C, Simon MM. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci USA. 1999;96:13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riera L, Gariglio M, Valente G, Mullbacher A, Museteanu C, Landolfo S, Simon MM. Murine cytomegalovirus replication in salivary glands is controlled by both perforin and granzymes during acute infection. Eur J Immunol. 2000;30:1350–1355. doi: 10.1002/(SICI)1521-4141(200005)30:5<1350::AID-IMMU1350>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Andrade F, Bull HG, Thornberry NA, Ketner GW, Casciola-Rosen LA, Rosen A. Adenovirus L4–100K assembly protein is a granzyme B substrate that potently inhibits granzyme B-mediated cell death. Immunity. 2001;14:751–761. doi: 10.1016/S1074-7613(01)00149-2. [DOI] [PubMed] [Google Scholar]
- 27.Jerome KR, Chen Z, Lang R, Torres MR, Hofmeister J, Smith S, Fox R, Froelich CJ, Corey L. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J Immunol. 2001;167:3928–3935. doi: 10.4049/jimmunol.167.7.3928. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki H, Kuwano K, Yoshida K, Maeyama T, Yoshimi M, Fujita M, Hagimoto N, Yoshida R, Nakanishi Y. The perforin mediated apoptotic pathway in lung injury and fibrosis. J Clin Pathol. 2004;57:1292–1298. doi: 10.1136/jcp.2003.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingstrom J, Hardestam J, Stoltz M, Zuber B, Lundkvist A, Linder S, Ahlm C. Loss of cell membrane integrity in puumala hantavirus-infected patients correlates with levels of epithelial cell apoptosis and perforin. J Virol. 2006;80:8279–8282. doi: 10.1128/JVI.00742-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruth U, Sinigaglia F, Schlesier M, Kilgus J, Kramer MD, Simon MM. A novel serine proteinase (HuTSP) isolated from a cloned human CD8+ cytolytic T cell line is expressed and secreted by activated CD4+ and CD8+ lymphocytes. Eur J Immunol. 1987;17:1625–1633. doi: 10.1002/eji.1830171116. [DOI] [PubMed] [Google Scholar]
- 32.Tschopp CM, Spiegl N, Didichenko S, Lutmann W, Julius P, Virchow JC, Hack CE, Dahinden CA. Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood. 2006;108:2290–2299. doi: 10.1182/blood-2006-03-010348. [DOI] [PubMed] [Google Scholar]
- 33.Wagner C, Iking-Konert C, Denefleh B, Stegmaier S, Hug F, Hansch GM. Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood. 2004;103:1099–1104. doi: 10.1182/blood-2003-04-1069. [DOI] [PubMed] [Google Scholar]
- 34.Grossman WJ, Ley TJ. Granzymes A and B are not expressed in human neutrophils. Blood. 2004;104:906–907. doi: 10.1182/blood-2004-03-0858. [DOI] [PubMed] [Google Scholar]
- 35.Schleimer RP, Jacques A, Shin HS, Lichtenstein LM, Plaut M. Inhibition of T cell-mediated cytotoxicity by anti-inflammatory steroids. J Immunol. 1984;132:266–271. [PubMed] [Google Scholar]
- 36.Zhou J, Olsen S, Moldovan J, Fu X, Sarkar FH, Moudgil VK, Callewaert DM. Glucocorticoid regulation of natural cytotoxicity: effects of cortisol on the phenotype and function of a cloned human natural killer cell line. Cell Immunol. 1997;178:108–116. doi: 10.1006/cimm.1997.1138. [DOI] [PubMed] [Google Scholar]
- 37.Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, Moore PA, Das G, Shi Y. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]


