Abstract
Ruxolitinib is a Janus kinase (JAK) inhibitor used for the treatment of myelofibrosis with demonstrated efficacy for the alleviation of disease-related symptoms and splenomegaly. Anemia and thrombocytopenia are the main secondary effects. However, there are case reports of rare but serious adverse events following drug withdrawal. We present a case of a 76-year-old man diagnosed with primary myelofibrosis who presented with constitutional symptoms and symptomatic splenomegaly. Ruxolitinib was started (15 mg twice daily) and his disease-related symptoms disappeared. Six weeks later, he developed grade 4 thrombocytopenia and grade 3 anemia. Ruxolitinib was stopped and corticosteroid treatment (prednisone 1 mg/kg/day) was started to avoid a cytokine-rebound reaction. The patient then developed fever, chills, a biological inflammatory syndrome, and an acute respiratory disease syndrome. Full workup excluded an infection and we concluded that ruxolitinib withdrawal syndrome was the likely cause. Continued treatment with corticosteroids, as well as oxygen supply and continuous positive airway pressure, allowed an alleviation of his symptoms. This case report describes acute respiratory distress syndrome as another potential complication of ruxolitinib withdrawal syndrome.
Keywords: Ruxolitinib, Withdrawal syndrome, Myelofibrosis, Acute respiratory distress syndrome
Case report
A 76-year-old man was diagnosed with primary myelofibrosis (PMF) JAK2 V617F mutation positive (IPSS: intermediate-2; DIPSS: intermediate-1; DIPSSplus: intermediate-1). At the time of diagnosis, he reported constitutional symptoms (non-intentional weight loss and debilitating fatigue) and abdominal discomfort during the previous few months. Complete blood count (CBC) was: hemoglobin (Hb) 114 g/L; white blood cell count (WBC) 19 × 109/L (54 % segmented neutrophils, 22 % band neutrophils, 1.5 % eosinophils, 3 % myelocytes, 9.5 % monocytes, 8 % lymphocytes); 5 % erythroblasts; and platelets 179 × 109/L. Bone marrow was hypercellular (around 100 %) with trilineage hematopoiesis with the presence of clusters of megakaryocytes, and displayed a grade 2/4 fibrosis without excess blasts. Karyotype was 46 XY with an isolated deletion of the long arm of chromosome 20. Mutation of JAK2 V617F was positive (62.6 %). An abdominal computed tomography (CT) scan showed splenomegaly (20 × 9 × 16 cm).
A curative approach with hematopoietic stem cell transplantation was rejected due to his age and a treatment with ruxolitinib was started at 15 mg twice daily. Within 2 weeks, he experienced decreased fatigue, improved appetite, an increase in weight, and the resolution of abdominal discomfort. CBC was performed once a week in an outpatient setting and remained stable during the first 5 weeks of treatment. At week 6, the patient developed a grade 4 thrombocytopenia (platelet count 38 × 109/L) and grade 3 anemia (Hb 70 g/L) without bleeding and was hospitalized in our hematology clinic. Ruxolitinib was stopped and corticosteroids (prednisone 1 mg/kg/day) were started simultaneously to avoid a cytokine-rebound reaction. The day after ruxolitinib withdrawal, the patient developed fever >40 °C, chills, and a biological inflammatory syndrome (C-reactive protein, 134 mg/ml) without any clinical evidence for infection. The patient’s condition worsened and he developed an acute respiratory distress syndrome (ARDS) (PaO2/FiO2 < 26 kPa, bilateral pulmonary infiltrates) 3 days later (Fig. 1a–c). The main causes of ARDS were systematically excluded as follows. There was no suspicion of sepsis (all blood cultures were negative) or pneumonia (flexible broncoscopy showed non-inflammatory mucosa and serous fluids non compatible with infection. Microscopic examination of broncholoalveolar lavage and cultures were negative for pathogenic bacteria and mold yield; polymerase chain reaction for respiratory virus was negative). No traumatism, drug use or pancreatitis (amylase and lipase in normal range) were suspected. Transfusion-related lung injury was unlikely because hypoxemic respiratory insufficiency developed more than 6 h after the last blood cell pack transfusion. Biological analysis demonstrated also an increase in the lactate dehydrogenase level to 1996 UI/L [normal range (NR) 125–240] compared to the baseline level (740–928 UI/L) when treated with ruxolitinib, an increase of alkaline phosphates to 152 UI (NR 30–125; baseline level 77–103), and gamma-glutamyltransferase to 116 UI (NR 9–40; baseline level 38–58). A ruxolitinib withdrawal syndrome (RWS) was diagnosed. Corticosteroid treatment was continued, oxygen supply and continuous positive airway pressure were started, and the patient’s condition alleviated a few days later. In addition, 3 days after hospitalization, the patient developed a multi-sensitive Escherichia coli bacteremia associated with a septic superficial thrombosis on the site of the peripheral catheter, which was successfully treated with amoxicillin–clavulanate. During hospitalization, the patient required 6 packed red blood cells for anemia. Seven days after ruxolitinib withdrawal, the platelet count and WBC started to increase and he was discharged 18 days after hospitalization. Figure 2 shows the patient’s clinical course and laboratory values. Corticosteroids were weaned and discontinued without rebound of fever or biological inflammatory syndrome.
Fig. 1.
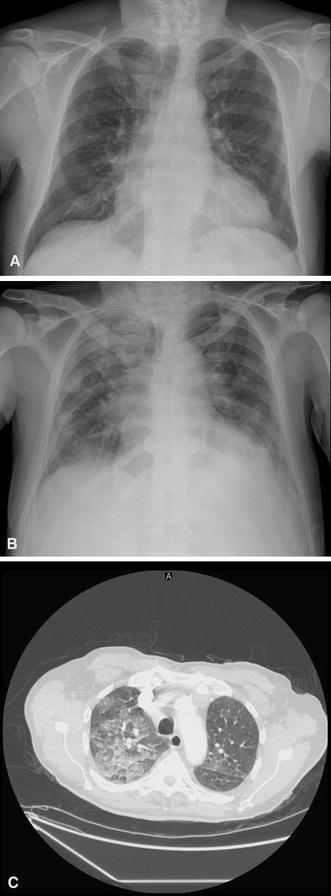
a Chest X-ray performed on the day of hospitalization. b Chest X-ray and c CT scan performed on the day of acute respiratory distress syndrome diagnosis with the presence of bilateral infiltrates and pleural effusion
Fig. 2.
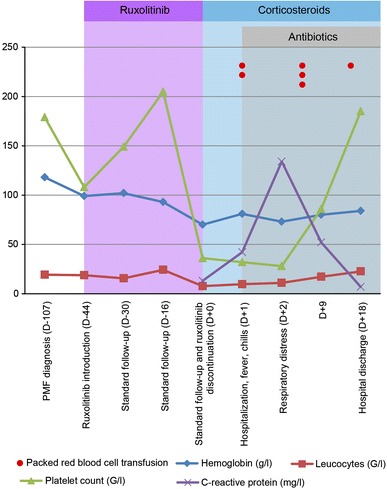
Patient’s clinical course and evolution of blood test levels over time
Discussion
According to the 2008 revised World Health Organization criteria, PMF is a myeloid Philadelphia-negative neoplasm classified among myeloproliferative neoplasms as essential thrombocythemia and polycythemia vera. PMF is associated with the JAK2-V617F mutation in approximately 50–60 % of the cases [1–3] and the MPL mutation in an additional 5–10 % [4, 5]. Recently, mutations of the calreticulin gene were found and associated with 80 % of negative JAK2-V617F and MPL mutations in PMF patients [6, 7]. Mutations of JAK2-V617F cause a constitutive activation of the STAT pathway. In addition to participation in cell differentiation, regulation and proliferation, the STAT pathway targets different genes, which are implicated in cytokines (IL-6, IL-10, IL-17, IL-23) and growth factor (vascular endothelial growth factor, fibroblast growth factor) productions [3]. Some of these proinflammatory cytokines are increased in PMF patients compared with healthy patients [8].
The median age of PMF onset is approximately 65 years [9, 10] with an estimated incidence of 0.21/100,000 [9]. Most symptoms are constitutional (fatigue, weight loss, night sweats or fever, pruritus) or due to splenomegaly (abdominal pain, loss of appetite) caused by extramedullary hematopoiesis and cytopenias. All are caused by myeloproliferation and high level or inflammatory cytokine production. Currently, the only curative treatment for PMF is allogeneic stem cell transplantation, but this approach can be proposed only to a minority of patients, mainly because of age and comorbidities [11, 12]. Thus, most treatments target PMF-related symptoms. Interferon alpha, followed by pegylated interferon, has been used for many years, as well as thalidomide or lenalidomide with or without cortiscosteroids [13]. Recently, ruxolitinib, a JAK inhibitor, was developed and demonstrated a very significant and persistent reduction of splenomegaly in around 60 % of the cases [14, 15], PMF-related symptoms in 50 % [14], and an improvement of quality of life [15]. Moreover, ruxolitinib was shown to result in a reduction of proinflammatory cytokines and inflammatory markers, such as C-reactive protein [16]. The main hematological secondary effects are anemia (grade 3–4 45 % [14]) and thrombocytopenia (grade 3–4 13 % [14]).
Even if ruxolitinib is a very efficient drug, the rapid onset of unforeseen anemia or thrombocytopenia are major secondary effects, which require dose reduction or treatment discontinuation. Current United States Food and Drug Administration recommendations are to adapt dosage according to the platelet count. If the platelet count decreases less than 50 × 103/µl, the drug should be discontinued. However, rapid drug discontinuation can cause severe reactions such as RWS. This syndrome is probably very rare and, to the best of our knowledge, only 7 cases have been reported to date (including our case) [17, 18]. RWS symptoms appeared less than 24 h after drug cessation in 3 reports. Respiratory distress (4 patients, 2 requiring intubation) and progression of splenomegaly (3 patients, 1 experienced splenic infarction) are the main symptoms described. Other reported symptoms range from recurrent PMF-related symptoms, such as fever or pruritus, to more severe complications, such as shock-like syndrome, pericardial effusion, or disseminated intravascular coagulation requiring hospitalization, intubation, or the use of vasopressors. Table 1 presents the main clinical characteristics of RWS patients in previously published case reports, including our report. As treatment with ruxolitinib decreases proinflamatory cytokines and inflammatory markers, a sudden discontinuation of the drug can cause an important rebound of cytokines, which may explain the different symptoms experienced by patients who developed RWS. Thus, weaning off the drug, rather than sudden discontinuation, should be preferred if possible. If not, patients should be closely monitored to assess CBC, recurrent splenomegaly, and the risk of respiratory distress. To avoid such cytokine rebound, corticosteroid treatment can be introduced preventively. In the case of very serious RWS despite corticosteroid treatment, the reintroduction of ruxolitinib should be considered.
Table 1.
Main clinical characteristics of 7 previously reported patients with ruxolitinib withdrawal syndrome, including this case report
| No | Sex, Age | Disease | Clinical characteristics of RWS |
|---|---|---|---|
| 0 | M, 76 | PMF | Recurrent fever, biological inflammatory syndrome and ARDS |
| 1 [17] | F, 59 | Post-PV MF | Respiratory distress, severe anemia requiring transfusion and symptomatic splenomegalia |
| 2 [17] | F, 69 | Post-PV MF | Respiratory distress with septic shock-like syndrome |
| 3 [17] | M, 44 | Post-PV MF | Respiratory distress associated with pleural and pericardial effusion |
| 4 [17] | M, 64 | PMF | Recurrent fever and recurrence of PMF symptoms (fatigue, pruritus, night sweats, splenomegalia with splenic infarction) |
| 5 [17] | F, 56 | Post-PV MF | Disseminated intravascular coagulation-like syndrome |
| 6 [18] | F, 70 | Post-PV MF | Recurrent fever, dyspnea, diarrhea and accelerated splenomegalia |
F female, M male, MF primary myelofibrosis, post-PV MF post polycythemia vera myelofibrosis, RWS ruxolibinib withdrawal syndrome, PMF primary myelofibrosis, ARDS acute respiratory distress syndrome
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The patient signed an informed consent for participation in this case report.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, Steensma DP, Elliott MA, Wolanskyj AP, Hogan WJ, McClure RF, Litzow MR, Gilliland DG, Tefferi A. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 5.Boyd EM, Bench AJ, Goday-Fernandez A, Anand S, Vaghela KJ, Beer P, Scott MA, Bareford D, Green AR, Huntly B, Erber WN. Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. Br J Haematol. 2010;149(2):250–257. doi: 10.1111/j.1365-2141.2010.08083.x. [DOI] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant’Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schonegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 7.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincorena I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O’Meara S, McLaren S, Bianchi M, Silber Y, Dimitropoulou D, Bloxham D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du MQ, Greaves M, Bowen D, Huntly BJ, Harrison CN, Cross NC, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 9.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 10.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. Am J Hematol. 1999;61(1):10–15. doi: 10.1002/(SICI)1096-8652(199905)61:1<10::AID-AJH3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Fleischman AG, Maziarz RT. Hematopoietic stem cell transplantation for myelofibrosis: where are we now? Curr Opin Hematol. 2013;20(2):130–136. doi: 10.1097/MOH.0b013e32835dd862. [DOI] [PubMed] [Google Scholar]
- 12.McLornan DP, Mead AJ, Jackson G, Harrison CN. Allogeneic stem cell transplantation for myelofibrosis in 2012. Br J Haematol. 2012;157(4):413–425. doi: 10.1111/j.1365-2141.2012.09107.x. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E, Thomas D, Kantarjian H, Zhou L, Pierce S, Cortes J, Verstovsek S. Comparison of thalidomide and lenalidomide as therapy for myelofibrosis. Blood. 2011;118(4):899–902. doi: 10.1182/blood-2010-12-325589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH, Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 16.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86(12):1188–1191. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai T, Friedman EW, Barta SK. Ruxolitinib withdrawal syndrome leading to tumor lysis. J Clin Oncol. 2013;31(29):e430–e432. doi: 10.1200/JCO.2012.47.6473. [DOI] [PubMed] [Google Scholar]


