Abstract
Sialic acids (Sias) are typically found as terminal monosaccharides attached to cell surface glycoconjugates. They play many important roles in many physiological and pathological processes, including microbe binding that leads to infections, regulation of the immune response, the progression and spread of human malignancies and in certain aspects of human evolution. This review will provide some examples of these diverse roles of Sias and briefly address immunohistochemical approaches to their detection.
Keywords: sialic acid, glycobiology, Siglec, glycomics
Main
All mammalian cells are covered by a dense glycocalyx, composed of glycolipids, glycoproteins, glycophospholipid anchors and proteoglycans.1, 2 More than 1% of the genome is involved in generating the developmentally regulated and tissue-specific glycosylation characteristic of each cell type in humans and other vertebrates. The biosynthesis of these glycan chains takes place mostly in compartments of the endoplasmic reticulum-Golgi pathway, in stepwise reactions involving specific nucleotide sugar transporters, glycosyltransferases, glycosidases and other glycan-modifying enzymes.1, 2 Expression of some of these gene products is altered in embryogenesis, cancer, injury and inflammation, resulting in altered glycan patterns. Sialic acids (sias) are typically the outermost monosaccharide units on the glycan chains of glycolipids and glycoproteins (Figure 1a) and are often part of recognition sites to which pathogens attach.3, 4, 5, 6, 7, 8, 9 On the other hand, they also serve important intrinsic functions and are required for normal development.10, 11 This brief review focuses on the many ways in which Sias can present themselves on cell surfaces, some examples from our own work of the resulting implications for normal biology and disease, and some immunohistological tools used to detect this diversity in situ.
Figure 1.
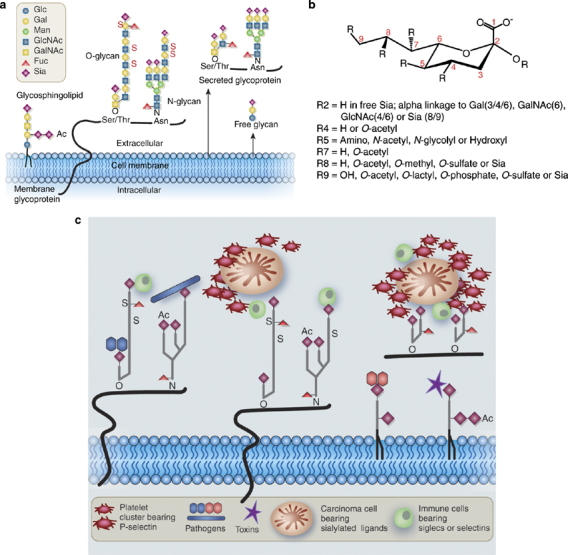
Natural diversity in Sia presentation, structure and function. (a) Diversity in presentation. Sias are typically found at the terminal position of N- and O-linked glycans attached to the cell surface and to secreted glycoproteins, as well as on glycosphingolipids expressed at the cell surface. Ac, O-acetyl ester; Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Man, mannose; S, sulfate ester. Reproduced with permission from Varki.11 (b) Structural diversity. All Sias share the common feature of having nine carbons, a carboxylic acid residue at the 1-position, and a variety of linkages to the underlying sugar chain from the 2-position. Various types of substitutions at the 4, 5, 7, 8 and 9 positions combine with the linkage variation to generate the diversity of Sias found in nature. Only a portion of this diversity is represented here. Some examples of the binding specificity of probes for detection of Sia types and linkages are discussed in the Box 1. (c) Examples of pathobiological interactions involving Sias. This cartoon shows examples of the types of interactions involving recognition of Sia diversity. The molecules and cells are obviously not drawn to scale.
Diversity in the types and linkages of sialic acids
Sias share a nine-carbon backbone and are among the most diverse sugars found on glycan chains of mammalian cell surfaces (Figure 1b). A variety of linkages to the underlying sugar chain from the 2-position and various types of substitutions at the 4, 5, 7, 8, and 9 positions combine to generate this diversity.4, 5 Given their terminal location on glycans, it is natural that this diversity has been utilized by a wide variety of Sia-binding proteins.11 Best known are the binding proteins of many viral and bacterial pathogens.5, 6, 7, 8, 9 In addition, there are intrinsic receptors within vertebrate cells that can recognize Sias, including the selectins and the Siglecs. Details regarding these binding proteins have been reviewed elsewhere.5, 9, 11, 12, 13
Examples of the pathobiological significance of sialic acid diversity
Examples of Sialic Acid Specificity in Pathogen Binding
Influenza A pathogenesis
Perhaps the earliest discovered ‘function’ of Sias was to serve as a receptor for the influenza A and B viruses (Figure 1c).14, 15 Most influenza viruses that infect and spread among wild and domesticated birds preferentially recognize Sias that are α2-3-linked to the underlying glycan chains (the most recent example being the H5N1 ‘bird flu’ virus). Humans are resistant to infection by such viruses at least partly because we instead display α2-6-linked Sias on the epithelium of upper airways (see Figure 2a). Thus, in order for avian influenza viruses to become human pathogens, certain specific mutations must occur in the Sia-binding pocket of the virus hemagglutinin. This is thought to occur in intermediate hosts such as the pig, which has both α2-3 and α2-6 linked Sias on its airways.14 However, there has been at least one instance (the 1918 influenza pandemic) wherein the virus adjusted to bind both types of linkages16, 17 and ‘jumped’ directly from birds into humans. Recent infections of a few humans with the bird flu have fortunately been traced not to such a change in virus-binding specificity, but likely exposure to a very large dose, which reached the lower airways and bound to α2-3-linked Sias present at that location.15 This can also explain why these patients did not easily pass on their infections to other humans.
Figure 2.
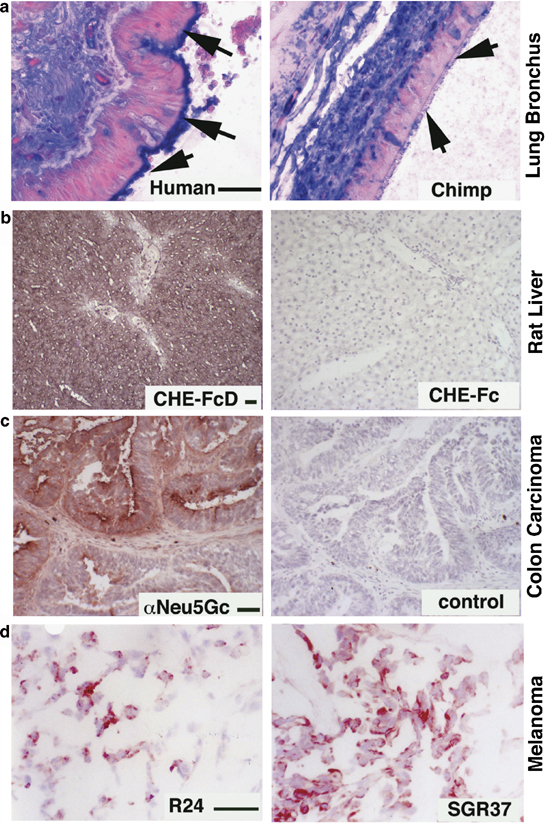
Some Examples of in situ detection of Sia types and linkages. (a) Differential display of α2-6-linked Sias on the ciliary border of lung bronchial epithelial cells in humans and chimpanzees. Paraffin sections were deparaffinized, rehydrated and blocked for endogenous biotin and overlaid with biotinylated Sambucus Nigra agglutinin (SNA), followed by Tris-buffered saline washes, alkaline phosphatase-labeled streptavidin and additional washes. Color detection used a Vector Labs blue substrate and nuclear fast red counterstain. Arrows point to the epithelial luminal edge, where staining is seen in humans, but not in chimpanzees or in other great apes.18 (b) Expression of 9-O-acetylated Sias in rat liver. Left panel: DFP-treated recombinant soluble influenza C Hemagglutinin-esterase (CHE-FcD). Right panel: non-DFP-treated version of the same probe (CHE-Fc, esterase active, negative binding control).19 Frozen sections of rat liver were blocked for endogenous peroxidases, fixed in formalin, washed, overlaid with the CHE-FcD or Che-Fc probes, which were pre-complexed with HRP anti-human antibodies at predetermined dilutions. After incubation at 4°C for 2 h, sections were washed in PBS and color developed using the AEC substrate with Mayer's hematoxylin for nuclear counterstain. (c) Detection of the nonhuman Sia Neu5Gc in human tumors, using an affinity-purified chicken IgY antibody. Left Panel: example of positive colon carcinoma. Frozen sections of a human colon carcinoma were blocked for endogenous peroxidases, fixed in formalin, washed and overlaid with a chicken anti-Neu5Gc antibody.38 Following washes and incubation with HRP-labeled donkey anti-chicken antibody and further washes, color was developed using the AEC substrate with Mayer's hematoxylin for nuclear counterstain. The right panel shows a negative control using a nonspecific chicken IgY. (d) Detection of GD3 and de-N-acetylated GD3 in human melanomas. Left Panel: detection of GD3 using MAb R24. Right Panel: detection of de-N-acetyl-GD3 using MAb SGR37. Unfixed frozen sections of a human melanoma were blocked for endogenous peroxidases and overlaid with monoclonal antibodies R24 or SGR37.33 Following PBS washes, incubation with HRP-labeled goat anti-mouse Ig antibodies and further washes, color was developed using the AEC substrate with Mayer's hematoxylin for nuclear counterstain. (Note: Scale bar=50 μm for each pair of panels is shown on the left side).
In this regard, when comparing the terminal glycans in organs from humans and the closely related great apes, we observed differences in expression of specific Sias and linkages, in multiple tissues.18 The most obvious difference was in the airway, where the prominent display of α2-6-linked Sias on the ciliary border of human columnar epithelial cells was not seen in the great apes, our closest evolutionary cousins (Figure 2a). This can help explain why chimpanzees have also not been a good model for human influenza A infection studies.
The common cold viruses and 9-O-acetyl-modified sialic acids
The addition of 9-O-acetyl esters to the side chain of Sias can block influenza A virus binding, but interestingly, this modification is required for binding of common cold viruses such as influenza C, and certain types of Coronaviruses.3, 7 About a third of human common cold viruses rely on this binding mechanism, which is mediated by a specific hemagglutinin distantly related to that of influenza A. This finding also allowed development of recombinant soluble forms of the influenza C hemagglutinin-esterase, which can be used as probes to detect the presence of 9-O-acetylated Sias in situ, after inactivation of the esterase activity (Figure 2b).7, 19
Malaria
In another example, invasion of red blood cells by the merozoite stage of Plasmodium falciparum is often dependent on the presence of Sia (although some Sia-independent binding mechanisms have emerged in this virulent pathogen). Different proteins on the Plasmodium merozoites govern the binding to red blood cells. We have found that the differential susceptibility of humans and chimpanzees to P. falciparum and P. reichnowii malarial parasites, respectively, might be explained by the differential preference of the parasite-binding proteins for human and nonhuman Sias.20 Thus, the change in Sias associated with human evolution (see below) was likely characterized by a phase in which our ancestors escaped from the common malaria of apes, only to later become the target for P. falciparum, which prefers the human Sia phenotype.
Examples of Roles of Sialic Acids in the Regulation of the Immune Response
Sias are a key component of the intrinsic ligands for the selectins, a family of receptors expressed on leukocytes, platelets and endothelium and playing critical roles in innate immunity, hemostasis and reperfusion injury.21, 22 Another recently discovered group of intrinsic Sia-binding proteins are the Siglecs (Sia-binding immunoglobulin-superfamily lectins), which are mostly found on cells of innate and adaptive immunity and appear to play important roles in these processes.13, 23 Multiple changes in Siglec biology seem to have occurred during human evolution.11 For example, the human-specific evolutionary loss of immune-regulating Siglec molecules on T cells may account for the hyperactivity of human T cells, and our apparent propensity to develop diseases mediated related T cell dysfunction.24 On the other hand, we have suggested that recent evolutionary changes in the binding specificity of human CD33-related Siglecs,25 might account for our propensity to be colonized and invaded by microorganisms that express human-like presentations of Sias.23
Examples of Roles of Sialic Acids in the Progression and Spread of Human Malignancies
Enhanced expression of terminal α2-6-linked Sias on cell surface N-linked glycans and of Sialyl-Lewis X on O-linked glycans (typically found on mucins) often correlates with poor prognosis of many human malignancies. The first example correlates with the upregulation of the expression of the ST6GAL1 gene, and has been described in carcinomas of the colon, breast, cervix, choriocarcinomas, acute myeloid leukemias and some malignancies of the brain as well.26 This may relate to the effects of α2-6-linked Sias on integrin function.27 In the case of Sialyl-Lewis X expression, it appears that tumor cells use this selectin ligand to facilitate interactions with the selectins. Tumor emboli and microthrombi result when carcinoma selectin ligands on hematogenously borne carcinoma cells interact with selectins on platelets, innate immune cells and endothelium, serving to propagate metastases.28, 29 The serendipitous finding that some clinically approved heparins can block many of these selectin-mediated processes at clinically acceptable levels might explain why heparins reduce the incidence of metastasis, when utilized during the ‘window of therapeutic opportunity’.28, 29, 30
Gangliosides are glycolipids carrying Sias, which are found in all tissues and cell types, but are particularly enriched in cells of neuroectodermal origin. Malignant melanomas express very high levels of the disialoganglioside GD3 as well as modified 9-O-acetylated31, 32 and 5-N-deacetylated33 forms of this ganglioside. Antibodies against all three forms are available and can be used to study their expression and distribution in melanomas (see example in Figure 2d). Enhanced expression of 9-O-acetyl-GD3 is also seen in basal cell carcinomas.34 There is evidence that whereas GD3 enhances apoptosis, 9-O-acetyl-GD3 has the opposite effect.35 There are multiple other examples of monoclonal antibodies recognizing specific gangliosides, which must be used on frozen sections, as the paraffin-embedding process results in extraction of gangliosides.33
Detection of a Nonhuman Sialic Acid Neu5Gc in Human Tissues
The most common mammalian Sias are N-acetylneuraminic acid (Neu5Ac) and N-glycolyneuraminic acid (Neu5Gc). Although Neu5Gc is abundant in many mammals, a human-specific genetic change eliminated our capacity to produce it.11, 36 Despite this, Neu5Gc was detected in human carcinomas and fetal tissues as an ‘oncofetal’ antigen, using a polyclonal monospecific antibody.37 An improved affinity-purified version of this antibody detected not only Neu5Gc expression in tumor cells (Figure 2c), but also small amounts in normal human tissues, despite the fact that humans are incapable of synthesizing it.38 This paradox is now explained by the fact that humans absorb Neu5Gc from dietary sources (principally red meat and milk products) and can metabolically incorporate it into certain cell types (in particular endothelium and epithelium).38 Meanwhile, healthy humans have significant and differing levels of circulating anti-Neu5Gc antibodies.38, 39 Our current hypothesis is that chronic inflammation related to this antigen–antibody reaction could be facilitating both carcinogenesis and atherogenesis.
A similar ‘contamination’ by Neu5Gc is apparently occurring throughout the biotechnology industry, arising from the use of animal cells, animal sera and other animal products during manufacture. For example, human embryonic stem cells are routinely grown on mouse embryonic fibroblast feeder layers and in media containing mammalian serum, or ‘serum substitutes’, which also contain components of animal origin.40 When such cells are exposed to human sera, they become coated with antibodies and complement,40 a process that in vivo would have marked them for immune attack.41 Similar contamination of many biotherapeutic products and cells with Neu5Gc is likely widespread, and could potentially play a part in immune responses against such agents.
Tools for In Situ Detection of Sialic Acid Types and Linkages, Using Glycan-Recognizing Probes
Recent efforts at ‘glycomics’ using mass spectrometry are defining the array of terminal glycan structures present in tissues of model organisms such as the mouse.42 However, such methods extract entire tissue samples, and do not take into account the diversity of cell types within a given tissue or organ—and can even miss major glycans on minor cell types. This approach to glycomics must thus be complemented with probing of tissue sections using natural and recombinant glycan-recognizing probes (GRPs) capable of detecting specific glycan structures in situ.43, 44 As indicated in the Box 1, there are several ways to detect Sias and their diversity in situ, each with their advantages and disadvantages. These approaches should be of particular interest to pathologists, whose expertise in the visual in situ detection of pathobiological changes will never likely be replaced by any computerized methods or mechanised approaches.
Box 1 Tools for In Situ Detection of Sialic Acid Types and Linkages, Using Glycan-Recognizing Probes.
Physicochemical approaches to glycomics must be complemented with probing of tissue sections using natural and recombinant glycan-recognizing probes capable of detecting specific glycan structures in situ. There are several simple yet powerful tools available for detecting Sia diversity on tissue sections.
Plant lectins. Lectins are glycan-binding proteins, which have traditionally been powerful tools to explore glycan structures.45 Their specificity is such that even isomeric glycans with identical sugar compositions can be distinguished. A well-known example is Sambucus nigra agglutinin (SNA), which binds in a highly selective fashion to Sias attached to either galactose or GalNAc units, via α2-6-linkages.46 In contrast to SNA, the family of lectins from Maackia amurensis seeds recognize α2-3-linked Sias. There is confusion in the literature regarding Maackia lectins, not only because there are more than one of differing specificity, but also because commercial sources sell them under different names. Preparations called ‘MAA’ are typically unspecified mixtures of two Maackia lectins, that is, one originally identified as a ‘leukoagglutinin’ (MAL) and a second one, a ‘hemagglutinin’ (MAH).47, 48, 49 As these two lectins have differing specificity, the significance of any published results using ‘MAA’ preparations must be interpreted cautiously. A further complication arises because the MAL lectin is sometimes sold as ‘MAL-I’ and MAH as ‘MAL-II’.49 Finally, MAL can also recognize a glycan wherein the Sia is replaced with a sulfate ester at the 3-position of galactose.50 Clearly, use of these lectins must be better controlled if interpretations are to be accurate. Also, such plant lectins are sometimes used with the assumption that they bind all forms of Sias, which is not the case. A lectin less often used that better recognizes multiple forms of Sias is the Limax flavus agglutinin.51
Recombinant soluble microbial proteins. A variety of microbial binding proteins and toxins specifically bind certain structures containing Sias.5, 6, 7, 8, 9 Thus, recombinant soluble versions of these molecules (or even intact virions bearing them) can be used to detect the relevant ligands in situ. Examples include cholera toxin, which binds selectively to GM1 ganglioside and the influenza C hemaggluttinin, which selectively recognizes 9-O-acetylated-Sia residues (see text).
Recombinant soluble mammalian receptors. Major classes of Sia-binding proteins found in vertebrates such as selectins and Siglecs,9, 11 can be converted to recombinant soluble chimeras with the Fc portion of Human Immunoglobin G1.52 These ‘pseudo-antibodies’ can be powerful tools for detecting specific types and arrangements of Sias in situ.
Antibodies. A variety of monoclonal antibodies recognize specific types of Sias and linkages, but typically only in the context of specific underlying sugar chains.53 In some cases, polyclonal antibodies have been generated that can recognize a specific type of Sia. For example, the nonhuman Sia N-glycolylneuraminic acid (Neu5Gc), can be recognized by an affinity-purified chicken polyclonal antibody (see text).37, 38
Future Perspectives
This brief survey of a vast area of research has focused on Sias and provided a few examples of their pathobiological significance, drawn from our own work. Combined with modern techniques of genomics and glycomics, the various tools that have been developed for the detection of the Sias in situ can reveal much about the roles of Sias in normal and pathological states. The future is bright for these and other aspects of sialobiology, and those with an interest in training in investigative pathology are urged to take advantage of these opportunities.
References
- 1.Varki A, Cummings R, Esko JD. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press: Plainview, NY; 1999. [PubMed] [Google Scholar]
- 2.Drickamer K, Taylor M. Introduction to Glycobiology. Oxford University Press: Oxford, UK; 2006. [Google Scholar]
- 3.Herrler G, Rott R, Klenk HD. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer R. Achievements and challenges of sialic acid research. Glycoconjugate J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 6.Ilver D, Johansson P, Miller-Podraza H. Bacterium–host protein–carbohydrate interactions. Methods Enzymol. 2003;363:134–157. doi: 10.1016/S0076-6879(03)01049-8. [DOI] [PubMed] [Google Scholar]
- 7.Strasser P, Unger U, Strobl B. Recombinant viral sialate-O-acetylesterases. Glycoconj J. 2004;20:551–561. doi: 10.1023/B:GLYC.0000043292.64358.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzkopf M, Knobeloch KP, Rohde E. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 12.Mandal C. Sialic acid binding lectins. Experientia. 1990;46:433–441. doi: 10.1007/BF01954221. [DOI] [PubMed] [Google Scholar]
- 13.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Ito T, Suzuki T. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/JVI.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinya K, Ebina M, Yamada S. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 16.Russell CJ, Webster RG. The genesis of a pandemic influenza virus. Cell. 2005;123:368–371. doi: 10.1016/j.cell.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Stevens J, Blixt O, Glaser L. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Gagneux P, Cheriyan M, Hurtado-Ziola N. Human-specific regulation of alpha2-6 linked sialic acids. J Biol Chem. 2003;278:48245–48250. doi: 10.1074/jbc.M309813200. [DOI] [PubMed] [Google Scholar]
- 19.Klein A, Krishna M, Varki NM. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc Natl Acad Sci USA. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin MJ, Rayner JC, Gagneux P. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci USA. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/S1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 22.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/S0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 23.Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DH, Hurtado-Ziola N, Gagneux P. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonnenburg JL, Altheide TK, Varki A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology. 2004;14:339–346. doi: 10.1093/glycob/cwh039. [DOI] [PubMed] [Google Scholar]
- 26.Dall’Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841–850. doi: 10.1023/A:1022288022969. [DOI] [PubMed] [Google Scholar]
- 27.Seales EC, Jurado GA, Brunson BA. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 28.Varki NM, Varki A. Heparin inhibition of selectin-mediated interactions during the hematogenous phase of carcinoma metastasis: rationale for clinical studies in humans. Semin Thromb Hemost. 2002;28:53–66. doi: 10.1055/s-2002-20564. [DOI] [PubMed] [Google Scholar]
- 29.Laubli H, Stevenson JL, Varki A. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006;66:1536–1542. doi: 10.1158/0008-5472.CAN-05-3121. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparins—correlation with selectin inhibition, not antithrombotic activity. Clin Cancer Res. 2005;11:7003–7011. doi: 10.1158/1078-0432.CCR-05-1131. [DOI] [PubMed] [Google Scholar]
- 31.Cheresh DA, Reisfeld RA, Varki A. O-Acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984;225:844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- 32.Kohla G, Stockfleth E, Schauer R. Gangliosides with O-acetylated sialic acids in tumors of neuroectodermal origin. Neurochem Res. 2002;27:583–592. doi: 10.1023/A:1020211714104. [DOI] [PubMed] [Google Scholar]
- 33.Chammas R, Sonnenburg JL, Watson NE. De-N-acetyl-gangliosides in humans: unusual subcellular distribution of a novel tumor antigen. Cancer Res. 1999;59:1337–1346. [PubMed] [Google Scholar]
- 34.Fahr C, Schauer R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J Invest Dermatol. 2001;116:254–260. doi: 10.1046/j.1523-1747.2001.01237.x. [DOI] [PubMed] [Google Scholar]
- 35.Malisan F, Franchi L, Tomassini B. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J Exp Med. 2002;196:1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varki Ajit. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. American Journal of Physical Anthropology. 2001;116(S33):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higashi H, Hirabayashi Y, Fukui Y. Characterization of N-glycolylneuraminic acid-containing gangliosides as tumor-associated Hanganutziu–Deicher antigen in human colon cancer. Cancer Res. 1985;45:3796–3802. [PubMed] [Google Scholar]
- 38.Tangvoranuntakul P, Gagneux P, Diaz S. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 40.Martin MJ, Muotri A, Gage F. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 41.Martin MJ, Muotri A, Gage F. Response to Cerdan et al: complement targeting of nonhuman sialic acid does not mediate cell death of human embryonic stem cells. Nat Med. 2006;12:1115. doi: 10.1038/nm1006-1115. [DOI] [PubMed] [Google Scholar]
- 42.Comelli EM, Head SR, Gilmartin T. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]
- 43.Martin LT, Marth JD, Varki A. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 44.Altheide TK, Hayakawa T, Mikkelsen TS. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: evidence for two modes of rapid evolution. J Biol Chem. 2006;281:25689–25702. doi: 10.1074/jbc.M604221200. [DOI] [PubMed] [Google Scholar]
- 45.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 46.Shibuya N, Goldstein IJ, Broekaert WF. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 47.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 48.Imberty A, Gautier C, Lescar J. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J Biol Chem. 2000;275:17541–17548. doi: 10.1074/jbc.M000560200. [DOI] [PubMed] [Google Scholar]
- 49.Brinkman-Van der Linden ECM, Sonnenburg JL, Varki A. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal Biochem. 2002;303:98–104. doi: 10.1006/abio.2001.5539. [DOI] [PubMed] [Google Scholar]
- 50.Bai XM, Brown JR, Varki A. Enhanced 3-O-sulfation of galactose in Asn-linked glycans and Maackia amurenesis lectin binding in a new Chinese hamster ovary cell line. Glycobiology. 2001;11:621–632. doi: 10.1093/glycob/11.8.621. [DOI] [PubMed] [Google Scholar]
- 51.Knibbs RN, Osborne SE, Glick GD. Binding determinants of the sialic acid-specific lectin from the slug Limax flavus. J Biol Chem. 1993;268:18524–18531. [PubMed] [Google Scholar]
- 52.Watson SR, Imai Y, Fennie C. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990;110:2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotani M, Kawashima I, Ozawa H. Differential distribution of major gangliosides in rat central nervous system detected by specific monoclonal antibodies. Glycobiology. 1993;3:137–146. doi: 10.1093/glycob/3.2.137. [DOI] [PubMed] [Google Scholar]


