Abstract
Purpose:
The long-term implications of premature birth on autonomic nervous system (ANS) function are unclear. Heart rate recovery (HRR) following maximal exercise is a simple tool to evaluate ANS function and is a strong predictor of cardiovascular disease. Our objective was to determine whether HRR is impaired in young adults born preterm (PYA).
Methods:
Individuals born between 1989 and 1991 were recruited from the Newborn Lung Project, a prospectively followed cohort of subjects born preterm weighing <1500g with an average gestational age of 28 weeks. Age-matched term-born controls were recruited from the local population. HRR was measured for two minutes following maximal exercise testing on an upright cycle ergometer in normoxia and hypoxia, and maximal aerobic capacity (VO2max) was measured.
Results:
Preterms had lower VO2max than controls (34.88±5.24 v 46.15±10.21 ml/kg/min respectively, p<0.05), and exhibited slower HRR compared to controls after one and two minutes of recovery in normoxia (absolute drop of 20±4 v 31±10 and 41±7 v 54±11 beats per minute (bpm), respectively, p<0.01) and hypoxia (19±5 v 26±8 and 39±7 v 49±13 bpm, respectively, p<0.05). After adjusting for VO2max, HRR remained slower in preterms at one and two minutes of recovery in normoxia (21±2 v 30±2 and 42±3 v 52±3 bpm respectively, p<0.05), but not hypoxia (19±3 v 25±2 and 40±4 v 47±3 bpm, respectively, p>0.05).
Conclusions:
Autonomic dysfunction as seen in this study has been associated with increased rates of cardiovascular disease in non-preterm populations, suggesting further study ofthe mechanisms of autonomic dysfunction after preterm birth.
Keywords: Preterm birth, Autonomic function, Heart rate recovery, Cardiovascular disease, Autonomic dysfunction, Prematurity, Premature birth, Exercise testing, Maximal aerobic capacity, Preterm birth
Introduction
One in every 10 infants born in the United States is born preterm, or before 37 completed weeks of gestation (Centers for Disease Control and Prevention 2015). Premature birth can lead to a number of acute and chronic cardiovascular comorbidities. Young adult survivors of prematurity are more likely to develop hypertension (Norman 2010), right ventricular (RV) and left ventricular hypertrophy, and lower RV ejection fraction compared to term-born controls (Lewandowski et al. 2013). Given the known long-term effects of prematurity on the cardiovascular system, a better understanding of chronic alterations in other regulators of cardiovascular function is of substantial importance for identifying the cardiovascular risk in young adults with a history of preterm birth.
While evidence in the literature is growing about long-term cardiovascular effects of prematurity, relatively little is known about cardiac autonomic regulation in this population. The autonomic nervous system (ANS) is made up of the sympathetic and parasympathetic branches, each working in tandem with the other in regulating several internal body processes to maintain equilibrium, and plays a significant role in regulating blood pressure, heart rate and ventilation (Joyner et al. 2010; Kleiger et al. 2005). The ANS develops significantly in the third trimester of pregnancy (Porges and Furman 2011), and cardiovascular autonomic development is temporarily disrupted by preterm birth, which has lasting effects on autonomic regulation of heart rate during infancy (de Meautsart et al. 2016; Patural et al. 2004; Yiallourou et al. 2013). While ANS development continues after birth (Porges and Furman 2011), preterm infants have lower parasympathetic modulation of the heart compared to term-born controls (Yiallourou et al. 2013), and children born preterm have lower heart rate variability compared to term born controls (Yiallourou et al. 2017). Autonomic function regulates a number of physiologic responses to reductions in arterial oxygen tension during hypoxic exposure, including increased ventilation and increases in heart rate (Amann and Kayser 2009). Young adults born preterm have a blunted ventilatory response to inhaled hypoxic air (Bates et al. 2014). The blunted ventilatory response to hypoxic air suggests long-term alterations in the autonomic response to changes in blood gas tensions in adults born preterm. Globally, deficient vagal tone (parasympathetic regulation) is associated with all-cause mortality and cardiovascular disease (Thayer and Lane 2007), and evidence that vagal tone in particular may be reduced in this population is cause for further investigation.
Heart rate recovery (HRR) following maximal exercise is primarily due to the reactivation of the parasympathetic nervous system and sympathetic withdrawal (Savin et al. 1982; Pierpont et al. 2013), and is recognized as a marker of autonomic function (Davrath et al. 2006). Slower HRR following exercise has been shown to be a predictor of cardiovascular disease(Cole et al. 1999; Qiu et al. 2017), pulmonary arterial hypertension severity (Minai et al. 2012), insulin resistance (Kuo and Gore 2015), heart failure (Bilsel et al. 2006), and all-cause mortality (Cole et al. 1999; Watanabe et al. 2001).
In this study, we sought to determine whether young adults born preterm exhibit abnormal autonomic function by assessing HRR following maximal exercise, and whether this autonomic response is affected by hypoxia. We hypothesized that preterm adults would exhibit slower HRR in normoxia compared to term-born controls, and that hypoxia would further blunt their HRR compared to controls.
Methods
Ethical approval:
The protocol was approved by the Institutional Review Boards at the University of Wisconsin Madison. Each subject was informed of the purpose and risks associated with the study and written consent was obtained from all subjects in accordance with the standards set by the Declaration of Helsinki. All study procedures were approved by the University of Wisconsin- Madison Institutional Review Board.
Participants:
Preterm participants were recruited from the Newborn Lung Project (Palta et al. 2001; Palta et al. 1998; Palta et al. 2000; Palta et al. 1994; Weinstein et al. 1994), a cohort established at the University of Wisconsin (Madison, WI) that enrolled individuals born preterm with very low birth weight (<1,500 g) between 1988 and 1991 in Wisconsin and Iowa. Control subjects were recruited from the local community and were born full-term from 1988 to 1991. Inclusion criteria for all participants was ability to complete a maximal exercise test, non-smoking, free of mental, physical, visual or neurological disabilities, and no diagnosed adult cardiovascular or respiratory disease. Subjects’ height was measured using a mechanical measuring rod to the nearest 0.5 cm (Seca; Hamburg, Germany), and weight was measured using a digital scale to the nearest 0.1 kg (Taylor; Oak Brook, IL) and recorded at the beginning of the study visit.
Baseline Physical Activity Questionnaire:
Subjects verbally completed a baseline physical activity questionnaire, the Global Physical Activity Questionnaire (GPAQ) (Bull et al. 2009). The GPAQ consists of 16 questions that assess the participants’ physical activity in three main domains: work, transport and leisure time, and how much time is spent in sedentary behavior. The scores from the questionnaire were calculated and used to determine subjects’ metabolic equivalent (MET) minutes per week.
Graded Exercise Testing:
Participants performed two incremental exercise tests on an upright cycle ergometer (Velotron; RacerMate; Seattle, WA) while breathing room air (0.21 FIO2, AirGas; Radnor, PA) during the first test and hypoxic air (0.12 FIO2, AirGas; Radnor, PA) during the second test. Participants rested for a minimum of 45 minutes between exercise tests, or until HR returned to pre-exercise resting values preceding the maximal exercise test in normoxia. There was a wash-in period preceding the hypoxia exercise test where participants breathed the hypoxic gas at rest for eight minutes in order to obtain resting HR and metabolic measurements (Kennedy et al. 2008). Participants cycled at 60–70 revolutions per minute (rpm) starting at 65W for one minute, and wattage was increased by 15W every minute until subjects were no longer able to maintain 55 rpm for more than five seconds. Heart rate was continuously monitored using forehead pulse oximetry (OxiMax N-595; Nellcor, Mansfield, MA), expired gases were collected in a breath-by-breath manner (Gemini; CWE, Ardmore, PA) and ventilatory and metabolic parameters were continuously recorded and analyzed in PowerLab (ADInstruments; Colorado Springs, CO). Maximal power (Pmax) was determined as the wattage of the highest stage maintained for more than 30 seconds. Time to exhaustion (Tmax) was calculated as total time from the start of the incremental test to the start of recovery. Maximal aerobic capacity (VO2max) was determined using a 30 second rolling average of the VO2 (ml O2/kg/min). In order for a test to be a VO2max, the primary criteria of a plateau in VO2 defined as a change of < 2 mL/kg/min in O2 consumption over the last 60 seconds of the test had to be met, in addition to one of the following secondary criteria: 1) a maximal heart rate (HRmax) of more than 90% age predicted HRmax (220-age), or 2) a respiratory exchange ratio (VCO2/VO2) of ≥ 1.1 (Midgley et al. 2007).
Ventilatory Threshold:
To account for fluctuations in breath-by-breath measurements of minute ventilation, a 30 second rolling average of minute ventilation was used to determine ventilatory threshold (VT). VT was determined as the time at which an upward deflection was noted in the slope of total ventilation over time, and oxygen consumption at VT was determined for each subject.
Heart Rate Recovery:
After reaching maximal volitional exhaustion, subjects were instructed to stop pedaling and sit completely still and quietly on the bicycle while HR was recorded for two minutes. HRR was calculated as the absolute drop in HR (bpm) from HRmax for two minutes at ten second intervals (HRRabs).
Statistical Analysis:
Data was initially evaluated for normality using descriptive statistics and histogram analysis. Wilcoxon Rank Sum tests were used to compare demographic and metabolic variables between the control and preterm groups. Given the unequal distribution of sex between the groups and in order to account for the influence of sex on metabolic variables, VO2max, Pmax, Tmax and VTVO2 were compared between groups using least squares means from linear regression models including birth status and sex as covariates in the model. Cohen’s d was calculated to determine the effect size between the preterm and control groups (Cohen 1988), where a higher number indicates a greater effect size.
HRRabs and HRabs were compared between groups at each 10-second interval using least squares means from separate linear regression models. To evaluate the effect of cardiorespiratory fitness on the difference in HRR between the groups, similar comparisons of least squares means at each time point were made using separate linear models including birth status and VO2max as covariates. For both the unadjusted and adjusted comparisons of least squares means, -p-values were adjusted for multiple pairwise comparisons using the method previously described by Holm (Holm 1979). Finally, for both the control and preterm groups separately, HRR at each timepoint was compared between the normoxia and hypoxia conditions with paired t-tests with adjustment for multiple comparisons. 2-way ANOVAs were used to evaluate the interaction of sex and birth status on VO2max, Pmax, and Tmax. Data analyses were conducted with R (R Development Core Team 2010). All tests were two-tailed and p<0.05 was used to define statistical significance.
Results
Characteristics of the subjects at baseline:
Twelve preterm (28.5 ± 2.7 weeks gestation at birth) and 16 term-born (39.5 ± 0.6 weeks gestation at birth) subjects completed the study in normoxia. Due to pre-syncopal symptoms while breathing hypoxic air, four preterm subjects (one male and three females) were unable to complete the hypoxia portion of the study. Controls were slightly younger than preterm subjects and had greater gestational age compared to control subjects, but otherwise the two groups were well matched (Table 1).
Table 1.
Physical Characteristics
| Control (n=16) | Preterm (n=12) | p-value | Cohen’s d | |
|---|---|---|---|---|
| Sex (n, % female) | 6, 38% | 6, 50% | 0.379 | |
| Age (years) | 25.6 ± 0.7 | 26.9 ± 1.1 | <0.001 | 1.86 |
| Height (cm) | 176.1 ± 8.6 | 171.1 ± 9.8 | 0.109 | 0.50 |
| Weight (kg) | 77.6 ± 14.9 | 70.0 ± 13.3 | 0.123 | 0.57 |
| BMI (kg/m2) | 24.9 ± 3.0 | 23.8 ± 3.3 | 0.340 | 0.32 |
| Gestational age (weeks) | 39.5 ± 0.6 | 28.5 ± 2.7 | <0.001 | 20.6 |
| G-PAQ (MET-min/week) | 3515 ± 2707 | 3282 ± 1953 | 0.875 | 0.10 |
Data are expressed as mean ± SD. BMI, body mass index; G-PAQ, global physical activity questionnaire; MET, metabolic equivalent.
Physical Activity:
There was no difference in MET minutes/week in control compared to preterm subjects as assessed by the GPAQ (Table 1).
Exercise capacity:
Preterm subjects attained significantly lower VO2max than controls, expressed absolutely and relative to body weight in both normoxia and hypoxia, and a lower oxygen consumption relative to body weight at VT in normoxia and hypoxia. Similarly, Pmax and Tmax were significantly lower and shorter, respectively, in preterm adults compared to controls in both normoxia and hypoxia (Table 2).
Table 2.
Aerobic fitness, metabolic and heart rate variables
| Normoxia (FIO2 = 0.21) | Hypoxia (FIO2 = 0.12) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (n=16) | Preterm (n=12) | p-value | Cohen’s d | Control (n=16) | Preterm (n=8) | p-value | Cohen’s d | |
| VO2max (L/min)* | 3.46 ± 0.62 | 2.43 ± 0.70 | <0.001 | 1.63 | 2.58 ± 0.50 | 1.79 ± 0.70 | 0.002 | 1.79 |
| VO2max (mL/kg/min)* | 45.79 ± 8.71 | 34.88 ± 9.26 | 0.003 | 2.15 | 34.47 ± 7.52 | 26.49 ± 10.52 | 0.029 | 1.25 |
| Pmax (watts)* | 231 ± 40 | 175 ± 45 | 0.002 | 1.42 | 186 ± 28 | 154 ± 39 | 0.020 | 1.53 |
| Tmax (minutes)* | 13.48 ± 4.16 | 9.79 ± 3.36 | 0.021 | 0.99 | 9.06 ± 1.75 | 6.98 ± 2.45 | 0.016 | 0.94 |
| HRrest (bpm) | 70 ± 11 | 80 ± 16 | 0.115 | 0.74 | 97 ± 12 | 113 ± 11 | 0.008 | 1.39 |
| HRmax (bpm) | 182 ± 12 | 182 ± 8 | 0.953 | 0.10 | 179 ± 10 | 180 ± 6 | 0.851 | 0.15 |
| HRR1min (bpm) | 31 ± 10 | 20 ± 4 | 0.001 | 2.55 | 26 ± 8 | 19 ± 5 | 0.039 | 1.47 |
| HRR2min (bpm) | 54 ± 11 | 41 ± 7 | 0.001 | 1.88 | 49 ± 13 | 39 ± 7 | 0.028 | 1.47 |
| VTVO2 (ml/kg/min)* | 37.86 ± 5.82 | 28.50 ± 6.60 | <0.001 | 0.274 | 28.23 ± 6.51 | 21.88 ± 9.11 | 0.043 | 0.081 |
Data are expressed as mean ± standard deviation (SD). Variables defined: VO2max, maximal aerobic capacity expressed absolutely (L/min) and relative to body weight (ml/kg/min); Pmax, maximal wattage attained for more than one half of final stage of incremental maximal exercise test; Tmax, time to exhaustion during maximal exercise test; HRmax, maximal HR attained during maximal exercise testing; HRR1min, HRR at one minute of resting recovery; HRR2min, HRR at two minutes of resting recovery; VTVO2 (ml/kg/min), oxygen consumption per kg of body weight at ventilatory threshold.
Sex-adjusted estimates.
HRR in normoxia:
HRmax was not different between the groups (Table 2). Absolute HR during recovery in normoxia was not significantly different between groups (p>0.05). HRR was slower in preterm subjects throughout the 2-minute recovery period, with the difference achieving statistically significance at 30 seconds through 2 minutes (Figure 1a). In order to evaluate the effect of differences in VO2max on HRR, VO2max was included as a covariate in a secondary analysis of HRR. After adjustment for VO2max, HRR was slower in preterm subjects compared to controls at one and two minutes of recovery in normoxia (21±2 v 30±2 and 42±3 v 52±3 bpm respectively, p<0.05, Figure 1b). Although the differences in least squares means between the groups appeared to be attenuated slightly by the inclusion of VO2max within the model, VO2max was not a significant predictor of HRR at any timepoint (p>0.05 for all).
Figure 1:
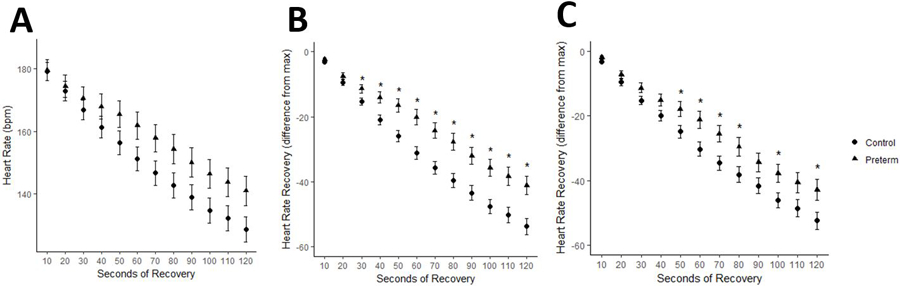
Heart rate recovery in normoxia
A. HRR (bpm; absolute drop in HR from max) in control (circles) and preterm (triangles) subjects. B. HRR adjusted for VO2max in control and preterm subjects. Data are expressed as mean ± SEM. *p<0.05 adjusted for pairwise comparison between control and preterm groups at each time point.
HRR in hypoxia:
Comparing control and preterm-born adults in hypoxia, preterm subjects had slower HRR compared to controls (Figure 2a). After adjusting for VO2max in hypoxia, HRR was slower in preterm subjects, but was only significantly different at early time points in recovery (Figure 2b). As in normoxia, however, VO2max was not a significant predictor of HRR at any timepoint (p>0.05 for all).
Figure 2:
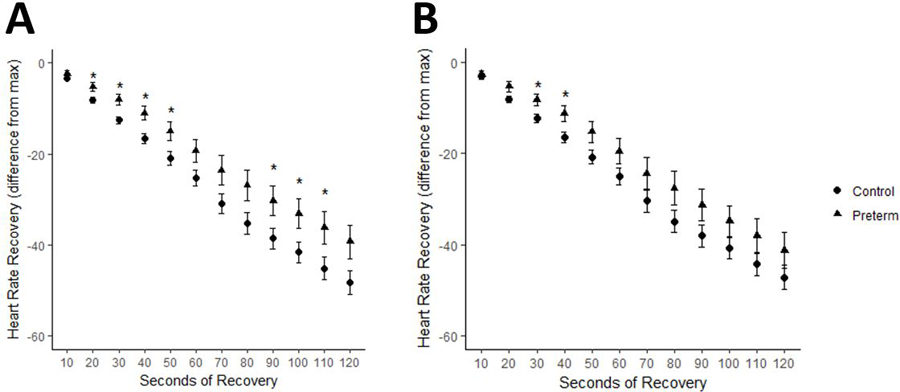
Heart rate recovery in hypoxia
A. HRR (bpm; absolute drop in HR from max) in control (circles) and preterm (triangles) subjects. B. HRR adjusted for VO2max in control and preterm subjects. Data are expressed as mean ± SEM. *p<0.05 adjusted for pairwise comparison between control and preterm groups at each time point.
Among control subjects, HRR was slower in hypoxia compared to normoxia throughout the recovery period (Figure 3a). HRR in preterm adults was slower in hypoxia early on in recovery, but no significant differences were identified later during recovery at individual time points (Figure 3b).
Figure 3:
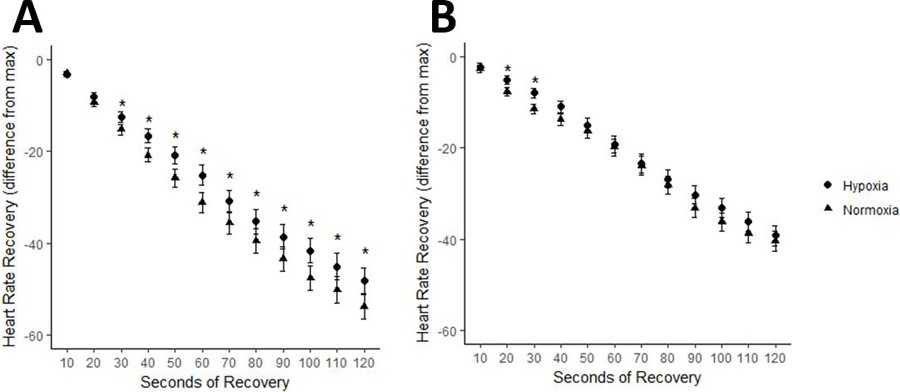
Heart rate recovery response between normoxia and hypoxia in control and preterm subjects
A. HRR (bpm; absolute drop in HR from max) in normoxia (triangles) and hypoxia (circles) in control subjects. B. HRR in normoxia (triangles) and hypoxia (circles) in preterm subjects. Data are expressed as mean ± SEM. *p<0.05 adjusted for pairwise comparison between hypoxia and normoxia conditions at each time point.
Effects of sex on VO2max, Pmax, and HRR:
Consistent with the NIH goal to evaluate sex as an independent biologic variable, we also evaluated the effect of sex on multiple parameters. Preterm females and males had significantly lower HRR1min compared to control females and males (Figure 4a). There was no difference between preterm and control females in VO2max and Pmax, but preterm males had significantly lower VO2max and Pmax compared to controls (p<0.01 for both). There was no interaction between birth status and sex in HRR1min, Pmax or VO2max.
Figure 4:
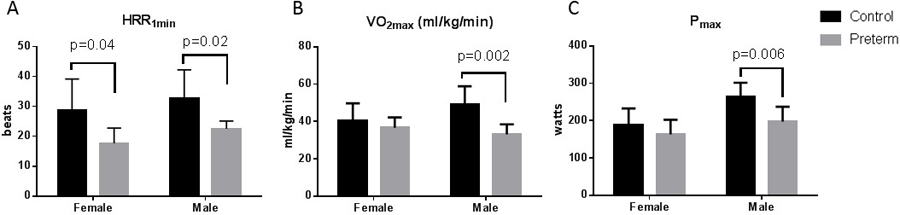
Sex effects on HRR, VO2max and Pmax
A. HRR1min (bpm; absolute drop in HR from max after 1 minute of recovery) sex-birth status interaction, p=0.91; B. VO2max sex-birth status interaction, p=0.06; C. Pmax sex-birth status interaction, p=0.21. Data are expressed as mean ± SD. HRR1min, heart rate recovery after one minute of recovery; VO2max, maximal aerobic capacity (ml/kg/min); Pmax, maximal power attained during maximal exercise test.
Discussion
In this study, we sought to determine whether young adults born preterm exhibit signicantly different autonomic function by assessing HRR following maximal exercise, and whether this autonomic response is affected by hypoxia. The key finding in this study is that HRR following maximal exercise is slower in healthy young adults born preterm compared to term-born controls in normoxia and hypoxia. This suggests that autonomic function is impaired in healthy young adults with a history of preterm birth free of known cardiovascular disease or neurological impairment. With strong evidence in the literature suggesting a correlation between slow HRR and cardiovascular disease risk (Cole et al. 1999; Qiu et al. 2017), our results suggest that otherwise healthy young adults born preterm may be at an increased risk of developing cardiovascular disease.
To our knowledge, this is the first study to investigate autonomic function in young adults born preterm using HRR following maximal exercise. Much of ANS development, particularly the parasympathetic branch, occurs in the third trimester of pregnancy (Gagnon et al. 1987). As the average gestational age in the preterm group in our study was 28.5 ± 2.7 weeks (range: 24–31 weeks), this critical window of development was largely completed out of the womb in our preterm adult group. Although the effects of such altered autonomic development on cardiovascular function in adults born preterm are not fully understood, autonomic dysfunction is correlated with several negative cardiovascular outcomes (Florea and Cohn 2014; Evrengul et al. 2006; Kishi 2012).
Adolescents and young adults born preterm have lower heart rate variability at rest, suggesting altered autonomic regulation, particularly lower high frequency heart rate variability, which is a marker of reduced parasympathetic regulatory capacity (Mathewson et al. 2015; Haraldsdottir et al. 2018). HR response to exercise is mediated by the ANS, where initiation of exercise signals vagal withdrawal followed by increased sympathetic activation, and exercise cessation conversely signals parasympathetic reactivation and sympathetic withdrawal (Mitchell 1985; Savin et al. 1982). Failure of HR to fall quickly during recovery following exercise has been identified as an independent predictor of all-cause mortality, clinical worsening of pulmonary arterial hypertension, carotid atherosclerosis, and higher risk of cardiovascular disease and coronary heart disease (Cole et al. 1999; Cole et al. 2000; Morshedi-Meibodi et al. 2002; Minai et al. 2012; Ramos et al. 2012; Jae et al. 2008; Watanabe et al. 2001).
The use of HRR as an indicator of disease or mortality risk is appealing due to its non-invasive nature, and there is emerging evidence that there is a threshold for HRR and adverse health outcomes. HRR after one minute of recovery (HRR1min) of ≤ 18 beats following peak exercise has been described as an independent predictor of all-cause mortality in older patients referred for exercise stress echocardiography (Watanabe et al. 2001) and for those with pulmonary arterial hypertension (Ramos et al. 2012). In our study, HRR1min in the control group was similar to that seen in healthy young adults in other studies (Hautala et al. 2006; Hargens et al. 2008), but was strikingly lower in young adults born preterm.
We also found that preterm adults had a lower exercise capacity compared to controls in both normoxia and hypoxia, including lower VO2max, VTVO2, Pmax, and Tmax, which is consistent with previously published reports in the literature (Farrell et al. 2015; Duke et al. 2014). While VO2max is positively correlated with HRR in endurance athletes (Ostojic et al. 2010), it is also affected by autonomic function and disease status, and HRR is faster in athletes and slower in patients with heart failure (Imai et al. 1994). While there is data to suggest that lower cardiorespiratory fitness is correlated with slower HRR (Watson et al. 2017; Imai et al. 1994), after adjusting HRR in normoxia and hypoxia for VO2max, HRR was still found to be significantly slower in preterm subjects compared to control adults. Furthermore, physical activity levels in the preterm group were similar to those in the control group, and the GPAQ has been shown to have a strong correlation with accelerometer data and moderate-to-vigorous activity levels (Cleland et al. 2014). Therefore, we do not believe that fitness status is primarily responsible for the blunted HRR in the preterm group.
We found that preterm males had significantly lower VO2max and Pmax compared to control males, whereas there was no significant difference between females. To our knowledge, this is the first report of sex-specific effects on VO2max after preterm birth. This is not altogether surprising though, given that males born preterm often have worse neonatal outcomes (Peacock et al. 2012). Despite this sex-specific difference in VO2max, in the current study we found that HRR was equally impaired in males and females born preterm. The finding that HRR1min is similarly impaired in both preterm males and females, while maximal aerobic capacity is impaired more in males than females born preterm, supports our hypothesis that there is an intrinsic impairment in autonomic function in young adults born prematurely, irrespective of fitness or sex.
To our knowledge, this is also the first study to compare HRR following maximal exercise in both normoxia and hypoxia in healthy young adults. HR response to hypoxia is well documented at rest, where exposure to hypoxia results in a drop in arterial oxygen content (Amann et al. 2007), resulting in a physiological response to reverse the hypoxemia. The sympathetic nervous system is activated in order to combat the drop in arterial oxygen content and results in an increase in HR, greater cardiac contractility, hyperpnea and peripheral vasoconstriction (Amann and Kayser 2009).
Interestingly, we found that HRR was not significantly different in preterm young adults between normoxic and hypoxic conditions at the one minute of recovery, a time point with clinical implications in the literature (Watanabe et al. 2001; Cole et al. 1999). The lack of difference in HRR1min in preterm adults between normoxia and hypoxia is curious, and suggests that the autonomic response to to hypoxia is blunted in this group. In conjunction with the impaired HRR in normoxia, this suggests a blunted parasympathetic reactivation and impaired sympathetic withdrawal following maximal exercise in adults born preterm. Interestingly, young adults born preterm also have a blunted ventilatory response in hypoxia compared to term-born controls (Bates et al. 2014), suggestive of a dysfunctional autonomic ventilatory response. Though the mechanism is unknown, it has been suggested that desensitized carotid bodies become insensitive to hypoxia, resulting in blunted hypoxic ventilatory sensitivity (Prabhakar and Peng 2004). Prior data from this same cohort of young adults born preterm demonstrate evidence of a blunted ventilatory response to hypoxia (Bates et al. 2014) and our current findings of an impaired HRR recovery following maximal exercise suggest that young adults born preterm may have autonomic dysfunction that affects both respiratory and cardiac autonomic innervation.
In the preterm group, only 8 of the 12 participants were able to complete maximal exercise testing in hypoxia, while all full-term control subjects were able to complete exercise testing in both conditions. The participant dropout was due to inability to tolerate the wash-in period of hypoxic gas as a result of symptoms including dizziness, lightheadedness, or presyncope. While the low number in the preterm hypoxia group is admittedly a limitation in the study, it provides additional insight into the autonomic dysfunction in this population. Our lab has previously shown that young adults born preterm exhibit a blunted ventilatory drive during hypoxic exposure (Bates et al. 2014), and significantly lower SpO2 and SaO2 at maximal exercise in hypoxia compared to controls (Farrell et al. 2015). This evidence, combined with the inability of 33% of the preterm participants to tolerate 8 minutes of hypoxia wash-in, supports the notion that young adults born preterm have an inadequate autonomic response to hypoxic conditions.
The mechanisms driving a slower HRR in this population of young adults born preterm is beyond the scope of this study. Past research investigating autonomic function in subjects with a history of prematurity have focused mainly on measurements taken at rest. Children born preterm have augmented sympathoadrenal activity at rest as determined by elevated urinary catecholamines (Johansson et al. 2007), and young adults born preterm have reduced high frequency heart rate variability, indicative of lower parasympathetic activity (Mathewson et al. 2015). Our finding that HRR, which is due to a combination of sympathetic withdrawal and vagal reactivation, is slower in preterm adults supports the notion that they may have imbalanced autonomic function, suggesting less effective sympathetic withdrawal and/or blunted parasympathetic reactivation during recovery.
This study had several strengths including a true resting period immediately following maximal exercise, use of two types of physiologic stress: hypoxia and exercise, and inclusion of groups with similar physical activity and BMI, both of which can affect HRR. Furthermore, our adjustment of HRR for VO2max gave us the ability to control for a variable that can be a significant confounder in the measurement of HRR. Our study was limited by the relatively homogeneous populations in the groups, where the experimental cohort was based out of Iowa and Wisconsin, and may not be representative of a more diverse population. Furthermore, we included a moderate to severe preterm-born population, and whether these results will be applicable to young adults born mildly premature remains unknown. Because the study was conducted at a single location, there may have been biases with respect to subject recruitment, where those from the local area were enrolled. Our analysis of autonomic function only studied HRR, and additional studies evaluating resting heart rate variability and muscle sympathetic nervous activity, an overall marker of sympathetic outflow (Joyner et al. 2010), would be helpful. Furthermore, our study design did not allow us to differentiate between the mechanisms altering HRR from maximal exercise in this population.
In conclusion, our study demonstrates that HRR is significantly slower in healthy young adults born preterm compared to age-matched, term-born controls. Although preterm adults demonstrated lower aerobic fitness, the difference in HRR persisted after adjusting for differences in VO2max. Autonomic dysfunction as seen in this study is associated with significantly increased rates of cardiovascular disease and mortality. With nearly half a million preterm births annually in the United States and improving neonatal survival rates, there are a growing number of adults born premature in the general population who may lack typical cardiovascular risk factors yet present with autonomic dysfunction reflected by slower HRR following maximal exercise. Further study into the mechanisms of autonomic dysfunction following preterm birth is warranted.
Acknowledgements
KH, AMW, KNG and MWE conceptualized and designed the study and are the guarantor of the content of the manuscript, including the data and analysis. KH, AMW, KNG, AGB, DFP, LHT, MDB, RMC and MWE assisted with data collection. KH, AMW, KNG, AGB, MP, LHT, MDB, RMC and MWE contributed to the analysis and interpretation of data. AMW conducted statistical analysis. KH and AMW prepared figures. KH drafted the initial manuscript. All authors reviewed, revised, and approved the final manuscript as submitted.
Sources of Funding:
National Institutes of Health: NIH-NHLBI R01–HL115061, NIH-NHLBI R01Supplement–HL1150613 (PI Eldridge), T32- HL 07936 (Haraldsdottir)
Abbreviations:
- ANS
autonomic nervous system
- GPAQ
Global physical activity questionnaire
- HR
Heart rate
- HRmax
Maximal heart rate
- HRR
Heart rate recovery
- MET
metabolic equivalent
- Pmax
maximal power
- Tmax
maximal time to exhaustion
- VO2max
maximal aerobic capacity
- VTVO2
oxygen consumption per kg of body weight at ventilatory threshold
References
- Amann M, Kayser B (2009) Nervous System Function during Exercise in Hypoxia. High Altitude Medicine & Biology 10 (2):149–164. doi: 10.1089/ham.2008.1105 [DOI] [PubMed] [Google Scholar]
- Amann M, Pegelow DF, Jacques AJ, Dempsey JA (2007) Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 293 (5):R2036–R2045. doi: 10.1152/ajpregu.00442.2007 [DOI] [PubMed] [Google Scholar]
- Bates ML, Farrell ET, Eldridge MW (2014) Abnormal Ventilatory Responses in Adults Born Prematurely. New England Journal of Medicine 370 (6):584–585. doi: 10.1056/NEJMc1311092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsel T, Terzi S, Akbulut T, Sayar N, Hobikoglu G, Yesilcimen K (2006) Abnormal heart rate recovery immediately after cardiopulmonary exercise testing in heart failure patients. International Heart Journal 47 (3):431–440. doi: 10.1536/ihj.47.431 [DOI] [PubMed] [Google Scholar]
- Bull FC, Maslin TS, Armstrong T (2009) Global Physical Activity Questionnaire (GPAQ): Nine Country Reliability and Validity Study. Journal of Physical Activity & Health 6 (6):790–804 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) Preterm Birth. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm Accessed June 21 2017
- Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA (2014) Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. Bmc Public Health 14. doi: 10.1186/1471-2458-14-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences. 2nd edn. L. Erlbaum Associates, Hillsdale, N.J. [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS (1999) Heart-rate recovery immediately after exercise as a predictor of mortality. New England Journal of Medicine 341 (18):1351–1357. doi: 10.1056/nejm199910283411804 [DOI] [PubMed] [Google Scholar]
- Cole CR, Foody JM, Blackstone EH, Lauer MS (2000) Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Annals of Internal Medicine 132 (7):552–555 [DOI] [PubMed] [Google Scholar]
- Davrath LR, Akselrod S, Pinhas I, Toledo E, Beck A, Elian D, Scheinowitz M (2006) Evaluation of autonomic function underlying slow postexercise heart rate recovery. Medicine and Science in Sports and Exercise 38 (12):2095–2101. doi: 10.1249/01.mss.0000235360.24308.c7 [DOI] [PubMed] [Google Scholar]
- de Meautsart CC, Dyson RM, Latter JL, Berry MJ, Clifton VL, Wright IMR (2016) Influence of sympathetic activity in the control of peripheral microvascular tone in preterm infants. Pediatric Research 80 (6):793–799. doi: 10.1038/pr.2016.160 [DOI] [PubMed] [Google Scholar]
- Duke JW, Elliott JE, Laurie SS, Beasley KM, Mangum TS, Hawn JA, Gladstone IM, Lovering AT (2014) Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J Appl Physiol (1985). doi: 10.1152/japplphysiol.00307.2014 [DOI] [PubMed] [Google Scholar]
- Evrengul H, Tanriverdi H, Kose S, Amasyali B, Kilic A, Celik T, Turhan H (2006) The relationship between heart rate recovery and heart rate variability in coronary artery disease. Annals of Noninvasive Electrocardiology 11 (2):154–162. doi: 10.1111/j.1542-474X.2006.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell ET, Bates ML, Pegelow DF, Palta M, Eickhoff JC, O’Brien MJ, Eldridge MW (2015) Pulmonary Gas Exchange and Exercise Capacity in Adults Born Preterm. Ann Am Thorac Soc 12 (8):1130–1137. doi: 10.1513/AnnalsATS.201410-470OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea VG, Cohn JN (2014) The Autonomic Nervous System and Heart Failure. Circulation Research 114 (11):1815–1826. doi: 10.1161/circresaha.114.302589 [DOI] [PubMed] [Google Scholar]
- Gagnon R, Campbell K, Hunse C, Patrick J (1987) Patterns of human-fetal heart-rate accelerations from 26 weeks to term. American Journal of Obstetrics and Gynecology 157 (3):743–748 [DOI] [PubMed] [Google Scholar]
- Haraldsdottir K, Watson A, Goss K, Beshish A, Pegelow D, Palta M, Tetri L, Barton G, Brix M, Centanni R, Eldridge M (2018) Impaired autonomic function in adolescents born preterm. Physiological Reports [DOI] [PMC free article] [PubMed]
- Hargens TA, Guill SG, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG (2008) Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep 31 (1):104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala AJ, Rankinen T, Kiviniemi AM, Makikallio TH, Huikuri HV, Bouchard C, Tulppo MP (2006) Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. American Journal of Physiology-Heart and Circulatory Physiology 291 (1):H459–H466. doi: 10.1152/ajpheart.01193.2005 [DOI] [PubMed] [Google Scholar]
- Holm S (1979) A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6 (2):65–70 [Google Scholar]
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T (1994) Vagally mediated heart-rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart-failure. Journal of the American College of Cardiology 24 (6):1529–1535 [DOI] [PubMed] [Google Scholar]
- Jae SY, Carnethon MR, Heffernan KS, Choi YH, Lee MK, Park WH, Fernhall B (2008) Slow heart rate recovery after exercise is associated with carotid atherosclerosis. Atherosclerosis 196 (1):256–261. doi: 10.1016/j.atherosclerosis.2006.10.023 [DOI] [PubMed] [Google Scholar]
- Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpee M (2007) Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. Journal of internal medicine 261 (5):480–487. doi: 10.1111/j.1365-2796.2007.01776.x [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG (2010) Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56 (1):10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MD, Warburton DE, Boliek CA, Esch BT, Scott JM, Haykowsky MJ (2008) The oxygen delivery response to acute hypoxia during incremental knee extension exercise differs in active and trained males. Dynamic medicine : DM 7:11. doi: 10.1186/1476-5918-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T (2012) Heart failure as an autonomic nervous system dysfunction. Journal of Cardiology 59 (2):117–122. doi: 10.1016/j.jjcc.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT (2005) Heart rate variability: Measurement and clinical utility. Annals of Noninvasive Electrocardiology 10 (1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Gore JM (2015) Relation of heart rate recovery after exercise to insulin resistance and chronic inflammation in otherwise healthy adolescents and adults: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Clinical Research in Cardiology 104 (9):764–772. doi: 10.1007/s00392-015-0843-2 [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K, Leeson P (2013) Right Ventricular Systolic Dysfunction in Young Adults Born Preterm. Circulation 128 (7):713–720. doi: 10.1161/circulationaha.113.002583 [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Van Lieshout RJ, Saigal S, Morrison KM, Boyle MH, Schmidt LA (2015) Autonomic Functioning in Young Adults Born at Extremely Low Birth Weight. Glob Pediatr Health 2:2333794X15589560. doi: 10.1177/2333794X15589560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley AW, McNaughton LR, Polman R, Marchant D (2007) Criteria for determination of maximal oxygen uptake - A brief critique and recommendations for future research. Sports Medicine 37 (12):1019–1028. doi: 10.2165/00007256-200737120-00002 [DOI] [PubMed] [Google Scholar]
- Minai OA, Gudavalli R, Mummadi S, Liu XB, McCarthy K, Dweik RA (2012) Heart Rate Recovery Predicts Clinical Worsening in Patients with Pulmonary Arterial Hypertension. American journal of respiratory and critical care medicine 185 (4):400–408. doi: 10.1164/rccm.201105-0848OC [DOI] [PubMed] [Google Scholar]
- Mitchell JH (1985) Cardiovascular control during exercise- central and reflex neural mechanisms. American Journal of Cardiology 55 (10):D34–D41. doi: 10.1016/0002-9149(85)91053-7 [DOI] [PubMed] [Google Scholar]
- Morshedi-Meibodi A, Larson MG, Levy D, O’Donnell CJ, Vasan RS (2002) Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (the Framingham Heart Study). American Journal of Cardiology 90 (8):848–852. doi: 10.1016/s0002-9149(02)02706-6 [DOI] [PubMed] [Google Scholar]
- Norman M (2010) Preterm Birth-An Emerging Risk Factor for Adult Hypertension? Seminars in Perinatology 34 (3):183–187. doi: 10.1053/j.sempen.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Ostojic SM, Markovic G, Calleja-Gonzalez J, Jakovljevic DG, Vucetic V, Stojanovic MD (2010) Ultra short-term heart rate recovery after maximal exercise in continuous versus intermittent endurance athletes. European Journal of Applied Physiology 108 (5):1055–1059. doi: 10.1007/s00421-009-1313-1 [DOI] [PubMed] [Google Scholar]
- Palta M, Sadek M, Barnet JH, Evans M, Weinstein MR, McGuinness G, Peters ME, Gabbert D, Fryback D, Farrell P (1998) Evaluation of criteria for chronic lung disease in surviving very low birth weight infants. Newborn Lung Project. The Journal of pediatrics 132 (1):57–63 [DOI] [PubMed] [Google Scholar]
- Palta M, Sadek-Badawi M, Evans M, Weinstein MR, McGuinnes G (2000) Functional assessment of a multicenter very low-birth-weight cohort at age 5 years. Newborn Lung Project. Arch Pediatr Adolesc Med 154 (1):23–30 [PubMed] [Google Scholar]
- Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G, Peters ME (2001) Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol 154 (6):521–529 [DOI] [PubMed] [Google Scholar]
- Palta M, Weinstein MR, McGuinness G, Gabbert D, Brady W, Peters ME (1994) A population study. Mortality and morbidity after availability of surfactant therapy. Newborn Lung Project. Arch Pediatr Adolesc Med 148 (12):1295–1301 [DOI] [PubMed] [Google Scholar]
- Patural H, Barthelemy JC, Pichot V, Mazzocchi C, Teyssier G, Damon G, Roche F (2004) Birth prematurity determines prolonged autonomic nervous system immaturity. Clinical Autonomic Research 14 (6):391–395. doi: 10.1007/s10286-004-0216-9 [DOI] [PubMed] [Google Scholar]
- Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A (2012) Neonatal and infant outcome in boys and girls born very prematurely. Pediatric Research 71 (3):305–310. doi: 10.1038/pr.2011.50 [DOI] [PubMed] [Google Scholar]
- Pierpont GL, Adabag S, Yannopoulos D (2013) Pathophysiology of Exercise Heart Rate Recovery: A Comprehensive Analysis. Annals of Noninvasive Electrocardiology 18 (2):107–117. doi: 10.1111/anec.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Furman SA (2011) The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behaviour: A Polyvagal Perspective. Infant and Child Development 20 (1):106–118. doi: 10.1002/icd.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ (2004) Peripheral chemoreceptors in health and disease. Journal of Applied Physiology 96 (1):359–366. doi: 10.1152/japplphysiol.00809.2003 [DOI] [PubMed] [Google Scholar]
- Qiu SH, Cai X, Sun ZL, Li L, Zuegel M, Steinacker JM, Schumann U (2017) Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Journal of the American Heart Association 6 (5). doi: 10.1161/jaha.117.005505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2010) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Ramos RP, Arakaki JSO, Barbosa P, Treptow E, Valois FM, Ferreira EVM, Nery LE, Neder JA (2012) Heart rate recovery in pulmonary arterial hypertension: Relationship with exercise capacity and prognosis. American Heart Journal 163 (4):580–588. doi: 10.1016/j.ahj.2012.01.023 [DOI] [PubMed] [Google Scholar]
- Savin WM, Davidson DM, Haskell WL (1982) Autonomic contribution to heart-rate recovery from exercise in humans. Journal of Applied Physiology 53 (6):1572–1575 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD (2007) The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology 74 (2):224–242. doi: 10.1016/j.biopsycho.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS (2001) Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality - The case of stress echocardiography. Circulation 104 (16):1911–1916 [PubMed] [Google Scholar]
- Watson AM, Brickson SL, Prawda ER, Sanfilippo JL (2017) Short-Term Heart Rate Recovery is Related to Aerobic Fitness in Elite Intermittent Sport Athletes. Journal of Strength and Conditioning Research 31 (4):1055–1061. doi: 10.1519/jsc.0000000000001567 [DOI] [PubMed] [Google Scholar]
- Weinstein MR, Peters ME, Sadek M, Palta M (1994) A new radiographic scoring system for bronchopulmonary dysplasia. Newborn Lung Project. Pediatr Pulmonol 18 (5):284–289 [DOI] [PubMed] [Google Scholar]
- Yiallourou SR, Wallace EM, Whatley C, Odoi A, Hollis S, Weichard AJ, Muthusamy JS, Varma S, Cameron J, Narayan O, Horne RSC (2017) Sleep: A Window Into Autonomic Control in Children Born Preterm and Growth Restricted. Sleep 40 (5). doi: 10.1093/sleep/zsx048 [DOI] [PubMed] [Google Scholar]
- Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RSC (2013) The development of autonomic cardiovascular control is altered by preterm birth. Early Human Development 89 (3):145–152. doi: 10.1016/j.earlhumdev.2012.09.009 [DOI] [PubMed] [Google Scholar]


